Abstract
Objective
To examine trajectories of psychological distress in mothers of children born very preterm (VPT, <30 weeks gestation) and full term from 2 to 13 years after the birth, and examine predictors of maternal psychological distress over time within the VPT group.
Methods
Mothers of children born VPT (n = 159) and full term (n = 71) completed questionnaires assessing their psychological distress when their child was 2, 7, and 13 years of age. Mixed models were used to examine differences between groups in maternal psychological distress over time. Family social risk, child neonatal medial risk, child sex, multiple pregnancy, and child’s neurodevelopmental impairment in early childhood were examined as potential predictors of maternal psychological distress within the VPT group.
Results
Mothers of children born VPT displayed elevated psychological distress compared with mothers of full-term children, and this difference was consistent over time. Higher family social risk was associated with elevated maternal psychological distress throughout childhood across all time-points. There was evidence that mothers of children at higher neonatal medical risk displayed increasing psychological distress over time.
Conclusions
Mothers of children born VPT show prolonged psychological distress. Mothers from socially disadvantaged background and those whose child has neonatal medical complications may require extensive support to prevent prolonged psychological distress and promote optimal outcomes for children and families.
Keywords: anxiety, depression, prematurity, psychological distress, social risk
Introduction
Preterm birth is associated with long-lasting neurodevelopmental and social-emotional difficulties, with those born very preterm (<32 weeks’ gestation) being at greater risk (Anderson, 2014; Aylward, 2014; Hayes & Sharif, 2009; Larroque et al., 2008; Reijneveld et al., 2006). The experience of preterm birth and its consequences may affect parents’ well-being and mental health (Treyvaud, 2014). The infant’s stay in the neonatal intensive care unit (NICU) can be traumatic and stressful for the parents, including the initial shock of having a baby earlier than expected, and can be associated with prolonged feelings of grief and loss (Shah, Clements, & Poehlmann, 2011; Yaari et al., 2017). The psychological distress may continue after hospital discharge, with uncertainties regarding the child’s prognosis and the stress associated with raising a child who may have poor health and additional needs (Moster, Lie, & Markestad, 2008).
It is widely accepted that parental mental health is an important factor affecting child development (Mensah & Kiernan, 2010; Smith, 2004). Maternal depression, for example, has been associated with poor child cognitive, language and social-emotional outcomes, particularly when prolonged and chronic (Goodman et al., 2011; Grace, Evindar, & Stewart, 2003; Sohr-Preston & Scaramella, 2006). The caregiving environment has been reported to be more influential on the development of infants born preterm compared with those born full-term (Gueron-Sela, Atzaba-Poria, Meiri, & Marks, 2015; Poehlmann et al., 2011; Shah, Robbins, Coelho, & Poehlmann, 2013). It is therefore important to identify and support parents of children born preterm who are at risk of prolonged psychological distress.
The emotional impact of preterm birth on the parents, especially mothers, has been documented in numerous studies. Studies that focus on psychological distress in the weeks following birth and the first months of life consistently report that parents of infants born preterm experience elevated symptoms of depression, anxiety, and post-traumatic stress in comparison to new parents of healthy term-born infants (Bener, 2013; Carson, Redshaw, Gray, & Quigley, 2015; Helle et al., 2015; Henderson, Carson, & Redshaw, 2016; Pace et al., 2016; Padovani, Carvalho, Duarte, Martinez, & Linhares, 2009; Vigod, Villegas, Dennis, & Ross, 2010). There is also cross-sectional evidence of elevated psychological distress among mothers of preschool children born preterm (Donohue, Maurin, Kimzey, Allen, & Strobino, 2008; Pierrehumbert, Nicole, Muller-Nix, Forcada-Guex, & Ansermet, 2003; Treyvaud et al., 2010). Only a few studies have assessed parental psychological distress in later childhood and adolescence, and these have yielded mixed results, with some reporting elevated levels of distress among mothers of preterm born children (Saigal, Burrows, Stoskopf, Rosenbaum, & Streiner, 2000; Taylor, Klein, Minich, & Hack, 2001; Treyvaud, Lee, Doyle, & Anderson, 2014) and others documenting no difference (Saigal, Pinelli, Streiner, Boyle, & Stoskopf, 2010; Singer et al., 2007).
To better understand the long-term effects of preterm birth on the parent and how these effects may change over time, longitudinal studies are needed. Only a small number of longitudinal studies on parental psychological distress after preterm birth have been reported, and most are restricted to the first few months of life (e.g., Pace et al., 2016). Of the few longitudinal studies that have continued to the preschool years and beyond, Misund, Nerdrum, Braten, Pripp, and Diseth (2013) and Miles, Holditch-Davis, Schwartz, and Scher (2007) reported an initial decline in maternal psychological distress around hospital discharge, followed by relative stability over the first 2 years of life (Miles et al., 2007; Misund et al., 2013). Moore et al. examined family burden and parental distress of children born at very low birthweight compared with children born with normal birthweight between the children’s age of 11 and 14 years. Results from this study indicated elevated distress and family burden in parents across all time-points, with family resources, socioeconomic status, and child’s medical risk moderating this association (Moore, Taylor, Klein, Minich, & Hack, 2006; Taylor et al., 2001). While psychological distress of parents of children born preterm may persist years after hospital discharge, there is a paucity of long term, longitudinal data, from early years to adolescence.
Prolonged distress and mental health difficulties in mothers after preterm birth have indeed been associated with poorer child cognitive and emotional-behavioral outcomes (Holditch-Davis et al., 2009, 2015; Woodward et al., 2014), which may then further influence parental functioning and mental health. It is therefore important to examine potential early risk factors associated with long-term maternal distress, in order to identify and provide timely extended support to the more vulnerable mothers. Parent, family, and child characteristics have been previously examined as predictors of maternal psychological distress in cohorts of children born preterm (Barlow, Cullen‐Powell, & Cheshire, 2006; Moore et al., 2006; Ong, Chandran, & Boo, 2001; Polic et al., 2016; Singer et al., 2010; Taylor et al., 2001; Witt et al., 2012). The predictors considered commonly included demographics (e.g., parental education, family income, and social disadvantage), child neonatal medical condition, and the child having a neurological impairment (sensory, motor, or cognitive disabilities). These studies vary in their design, sample characteristics, and in the construct of maternal psychological distress, and include measures of maternal anxiety, depression, and post-traumatic symptoms, parenting stress, and quality of life.
Family social risk has been documented as a predictor of distress among mothers of school-aged children born preterm, with lower maternal education, and higher family social disadvantage associated with higher maternal distress levels (Moore et al., 2006; Ong et al., 2001; Polic et al., 2016; Singer et al., 2010; Taylor et al., 2001; Witt et al., 2012). Child neonatal medical risk has also consistently been shown to be associated with distress in mothers of children born preterm, with evidence of prolonged elevated stress among mothers of children who were at higher medical neonatal risk (Moore et al., 2006; Polic et al., 2016; Singer et al., 2007, 2010; Witt et al., 2012).
A child’s neurosensory, motor, cognitive, or neurobehavioral impairments, which are more common among children born preterm (Anderson, 2014; Aylward, 2014) may also contribute to prolonged maternal distress. It is well-established from previous cohorts of children with disabilities that mothers of children with cerebral palsy, intellectual and developmental disabilities show elevated levels of psychological distress (Barlow et al., 2006; Brehaut et al., 2004; Singer, 2006). However, studies examining this association in cohorts of children born preterm vary in their findings. An association between the child’s neurodevelopmental impairment and maternal psychological distress has been documented in cohorts of children born preterm at the child age of 2 and 11 years (Cacciani et al., 2013; Lakshmanan et al, 2017; Taylor et al., 2001). On the other hand, in two long-term follow-up studies of young adults who were born preterm, at extremely low birthweight, the presence of a child’s disability was not associated with elevated maternal distress (Saigal et al., 2010; Wolke, Baumann, Busch, & Bartmann, 2017), suggesting a potential decrease over time in parental distress. Thus, evidence regarding the association between child’s impairment and long-term maternal distress is rather limited and mixed.
Finally, there is conflicting evidence regarding multiple versus singleton birth in relation to parental psychological distress. Spinelli, Poehlmann, and Bolt (2013) reported higher stress among mothers of multiples versus mothers of singletons at 4 months; however, stress levels in mothers of multiples decreased more over time than stress levels in mothers of singletons (Spinelli et al., 2013). Multiple birth was associated with elevated distress among mothers of children born at VLBW at the age of 8 years (Singer et al., 2007), but not earlier (Singer et al., 1999). Treyvaud et al. (2016) reported similar levels of psychological distress among mothers of multiple and singletons born VPT at 2 and 7 years. However, amongst mothers of multifetal pregnancies, those who experienced loss of a fetus reported higher levels of distress compared with mothers who had not experienced bereavement (Treyvaud et al., 2016).
Parental emotional state is an important factor in a child’s development, directly and indirectly (via parenting), influencing long-term developmental outcome. However, individual distress responses vary among parents and over time, and it is important to identify and support those who are more vulnerable and prone to prolonged or increasing distress. Notably, most of the studies described above, examining predictors of maternal distress, are cross-sectional, and limited to child’s first years of life. Longitudinal studies are needed to shed light on how these early characteristics are associated with long-term trajectories in maternal psychological distress over time.
We have previously reported increased psychological distress measured by levels of mental health symptoms (referred to hereafter as psychological distress), in parents of children born VPT (<30 weeks gestation) at 2 and 7 years of age compared with their term-born peers (Treyvaud et al., 2010, 2014). In the current study, we aimed to (a) investigate the trajectories in maternal distress from 2 to 13 years, and (b) examine potential predictors of the trajectory of maternal distress within the VPT group. We hypothesized that the level of distress in mothers of children born VPT would decrease with time since birth but remain elevated compared with mothers of term-born children. We also hypothesized that among mothers of children born VPT, higher social risk, higher child’s neonatal medical risk, and child’s neurodevelopmental impairment at 2 years would be associated with increasing maternal distress over time.
Methods
Participants and Procedure
Eligible participants were families in the Victorian Infant Brain Studies (VIBeS) longitudinal study. The VPT group comprised 185 mothers of 224 infants (including 39 mothers with twins or triplets) born at <30 weeks’ gestation or with a birth weight <1,250 g at the Royal Women’s Hospital, Melbourne, Australia, between 2001 and 2003. The comparison group comprised 77 mothers of 79 term-born children (>36 weeks’ gestation, including two mothers with twins) who were recruited at birth from the Royal Women’s Hospital maternity wards between 2001 and 2003 (n = 46) or at 2 years from maternal-child health centers (n = 31). At 2-years’ corrected age children underwent a neurodevelopmental assessment; these outcomes have been reported elsewhere (Roberts et al., 2008; Spittle et al., 2009). Parent mental health and functioning were also assessed at this time. Participants and their parents underwent further assessment when the child was 7 and 13 years of age, corrected for prematurity. Caregivers were sent paper-versions of the questionnaires to complete at home before the assessment. Some completed them during or after the assessment.
This longitudinal study was approved by the Human Research Ethics Committees of the Royal Women’s Hospital and the Royal Children’s Hospital, and informed written consent was obtained from parents for all children.
Measures
Maternal Distress
The General Health Questionnaire (GHQ; Goldberg, 1988) was used to assess maternal distress at 2 years. The GHQ is a self-report questionnaire designed to assess mental health symptoms in adults. It includes 28 items on four scales: anxiety, depression, social dysfunction and somatic symptoms, and a total score. Item scores range from 0 to 3, with a total score ranging from 0 to 84, higher scores indicating more symptoms. The recommended cut-off to indicate mental health disorders is a total score >23, the sensitivity and specificity of which has been reported as 79.8 and 78.5, respectively (Goldberg et al., 1997). The Hospital Anxiety and Depression Scale (HADS; Zigmond & Snaith, 1983) was used to assess maternal distress at 7 and 13 years. The HADS is at 14-item questionnaire, commonly used for screening depression and anxiety symptoms. Item scores range from 0 to 3, the scores for anxiety and depression scales range between 0 and 21, with higher scores indicating more symptoms. The recommended cut-off to indicate moderate or severe depression or anxiety is a score above 10 on the depression or anxiety scales. The sensitivity and specificity of the HADS scales in identifying cases with mental health disorders have been reported to be both approximately 0.8 (Bjelland, Dahl, Haug, & Neckelman, 2002). A total score, summing the anxiety and depression scales ranging from 0 to 42, was calculated to measure overall distress.
Familial Social Risk
Family social risk was calculated based on main caregiver report at 2 years, with a composite measure designed to capture a range of social factors known to be associated with child development, and based on other composite social risk scales that have been used in follow-up studies of preterm infants (Hack et al., 1992; Whitaker, Feldman, & Schonfeld, 1996). The measure includes six social risk factors: (a) family structure (two caregivers, separated parents with dual custody, single caregiver); (b) education of primary caregiver (tertiary educated, 11–12 years of education, less than 11 years); (c) occupation of primary income earner (skilled/professional, semi-skilled, unskilled); (d) employment status of primary income earner (full time, part-time, unemployed); (e) language spoken at home (English only, some English, no English); and (f) maternal age when the child was born (above 21 years, 18–21 years, less than 18 years). Each factor is scored on a three-point scale, where zero represented lowest risk and 2 represented highest risk, summed to give a total score (range: 0–12). Each family was categorized as lower (social risk score of 0 or 1) or higher social risk (social risk score >1) around the median score of 1. This scale and its cut-points have been used and validated in previous reports ((Treyvaud et al., 2009, 2011).
Child’s Neonatal Medical Risk
Child’s medical risk was defined as “higher” if one or more of the following conditions occurred during the neonatal period (in either child in mothers of multiple births): grade 3 or 4 intraventricular hemorrhage, cystic periventricular leukomalacia, bronchopulmonary dysplasia (defined as the requirement for oxygen at 36 weeks of postmenstrual age), postnatal corticosteroid treatment, or infection (either proven sepsis or necrotizing enterocolitis). Children with none of these conditions were assigned to the “lower medical risk” category.
Child’s Neurodevelopmental Impairment at 2 Years
Neurodevelopmental impairment was defined as meeting one of the following conditions: cognitive or motor delay (based on the Mental or Psychomotor Development Index score <70 of the Bayley Scales of Infant Development, BSID-II), moderate to severe cerebral palsy based on a neurological examination, blindness (visual acuity worse than 20/200 in the better eye) or significant hearing loss (requiring hearing aids or worse). Mothers with twins and triplets were categorized as having a child with a neurodevelopmental impairment if criteria were met for any of their children.
Analyses
Analyses were performed at the family level; the outcome was mother’s distress, with higher medical risk and presence of neurodevelopmental impairment defined as having a child in that category. Total scores of the GHQ and HADS were converted to Z-scores based on the mean and standard deviation (SD) of the full-term group’s scores at each time-point. Changes over time in maternal distress levels (aim 1) were compared between the VPT and full-term groups using linear mixed models. Group and time (corrected age) were included as fixed effects and a group × time interaction was included to examine differences between groups in rates of change over time. The models included a random intercept and slope, as this model had a better fit for the data than including a random intercept only. Higher social risk was included as covariate the model to control for its potential confounding effect.
To examine whether family and child characteristics were associated with the trajectory of distress in mothers of children born VPT (aim 2), the variables of interest (namely higher social risk, higher neonatal medial risk, child sex, multiple vs. singleton pregnancy and presence of child’s neurodevelopment impairment at 2 years) were entered into linear mixed models where the outcome was the level of maternal distress. Each variable was assessed in a separate model, which included a main effect, to assess whether the variable was associated with outcome across all three time-points, as well as interaction with time to assess whether the variable was associated with a change in the outcome over time. Following the univariable regression, multivariable models were fitted, including variables for which there was evidence (p < .05) for an association with maternal distress.
Results
Twenty out of 185 families of children born VPT withdrew/were lost to follow-up between recruitment and the 13-year assessment, and six had data only from the father or other family member; thus questionnaires from mothers for at least one time-point were available for 159 (86%) families. Four out of 75 families of children born at term withdrew/were lost to follow-up; thus, questionnaires from mothers for at least one time-point were available for 71 (95%) families. Attrition rates were higher among families of children born VPT, families with higher social risk and families with a child with neurodevelopmental impairment (Supplementary Table 1). These attrition rates and potential factors associated with attrition, are similar to those reported in other studies (e.g. Hack et al., 2002; Hille, Elbertse, Gravenhorst, Brand, & Verloove-Vanhorick, 2005; Johnson et al., 2009; Moore et al., 2012), with evidence shoeing only marginal effects on the validity of the prediction models (Powers & Loxton, 2010; Wolke et al., 2009). Of the 159 VPT and 71 term-born children included in the analysis, questionnaires from all three time-points were available for 139 mothers, two time-points for were available for 55 mothers, and one time-point for 36 mothers.
Demographic and medical characteristics of the families included in this analysis are presented in Table I. There were fewer mothers of singletons, more families with higher social risk, and more families with children who had neurodevelopmental impairment at 2 years in the VPT group compared with controls. Of note, social risk scores in the cohort were relatively stable from 2 to 13 years, spearman’s Rho (215) = 0.71. Within the VPT group, 70% of the children had higher neonatal medical risk and 22% had neurodevelopmental impairment; 25% of the children with higher medical risk had also a neurodevelopmental impairment. Descriptive statistics and rates of mothers scoring above the clinical cut-offs at the different time-points are detailed in Table II.
Table II.
Scores and Rates of Women Experiencing Clinical Levels of Distress
| Very preterm (n = 159) | Full term (n = 71) | |
|---|---|---|
| Scale M (SD) | ||
| 2 years—GHQ | 18.3 (10.5) | 15.5 (7.3) |
| 7 years HADS—anxiety | 7.1 (4.0) | 6.1 (3.2) |
| 7 years HADS—depression | 3.9 (3.7) | 2.9 (2.6) |
| 13 years HADS—anxiety | 6.6 (4.1) | 5.0 (2.8) |
| 13 years HADS—depression | 3.4 (3.3) | 2.5 (2.8) |
| Mothers with scores in the clinical range (%) | ||
| 2 years—GHQa | 25 | 11 |
| 7 years HADS—anxietyb | 21 | 8 |
| 7 years HADS—depressionb | 6 | 0 |
| 13 years HADS—anxietyb | 18 | 2 |
| 13 years HADS—depressionb | 3 | 2 |
Note. GHQ = General Health Questionnaire; HADS = Hospital Anxiety and Depression Scales; m = mean; SD = standard deviation.
Total scores range 0–84, clinical range >23.
Scale scores range 0–21, moderate or severe clinical range >10.
Table I.
Characteristics of Participants Included in Analyses
| Very preterm (n = 159 families) | Full term (n = 71 families) | |
|---|---|---|
| Demographic characteristics, n (%) | ||
| Female | 75 (47) | 38 (53) |
| Main caregiver’s education (tertiary; secondary school; <secondary school) | 40 (27); 91(60); 20(13) | 41 (59); 28(40); 1(1) |
| Main caregiver’s profession (skilled/ professional; semi-skilled; unskilled/unemployed) | 62 (39); 49 (31); 47(30) | 38 (54); 20 (28); 3(18) |
| Main caregiver’s employment status (part-time; full time; unemployed) | 117 (74); 16 (10); 25(16) | 65 (91); 4 (6); 2(3) |
| Main language spoken at home (English; some English; no English) | 144 (91); 10 (6); 5(3) | 65 (92); 5 (7); 1(1) |
| Maternal age <21 years | 10 (6) | 2(3) |
| Family structure (two caregivers; separated/dual custody; single parent) | 138 (87); 4 (3); 17(10) | 68 (96); 0; 3(4) |
| Higher social risk at 2 years | 94 (63) | 23 (33) |
| Medical characteristics, n (%) | ||
| Birth weight (g), M (SD) | 933 (223) | 3344 (503) |
| Birth weight SD score, M (SD) | −0.64 (0.96) | 0.13 (0.89) |
| Gestational age (weeks), M (SD) | 27.3 (2.0) | 39.3 (1.3) |
| Singleton | 113 (72%) | 69 (97%) |
| Oxygen at 36 weeks | 56 (35%) | 0 |
| Intraventricular hemorrhage grade III or IV | 7 (4%) | 0 |
| Cystic periventricular leukomalacia | 5 (3%) | 0 |
| Postnatal corticosteroids | 16 (10%) | 0 |
| Higher medical risk | 111 (70%) | 0 |
| MDI <70 at 2 years | 25 (16%) | 2 (3%) |
| PDI <70 at 2 years | 20 (13%) | 1 (1%) |
| Cerebral palsy at 2 years (mild; moderate; severe) | 8 (5%); 3 (2%); 2 (1%) | 0 |
| Blindness | 1 (0.5%) | 0 |
| Hearing deficit requiring aid | 4 (3%) | 0 |
| Neurodevelopmental impairment at 2 years | 35 (22%) | 2 (3%) |
Note. M = mean; MDI = mental developmental index; PDI = psychomotor developmental index; SD = standard deviation.
Table III.
Regression Coefficients From Linear Mixed Models for Maternal Psychological Distress Z-Scores
| Estimate (SE) | 95% CI | p-Value | |
|---|---|---|---|
| Model with main effects only | |||
| Main effect of group | 0.34 (0.16) | 0.03 to 0.66 | .03 |
| Main effect of time | 0.01 (0.01) | −0.01 to 0.03 | .47 |
| Social risk | 0.32 (0.16) | 0.02 to 0.62 | .04 |
| Model including a group × time interaction | |||
| Effect of time in VPT group | 0.02 (0.01) | −0.004 to 0.04 | .12 |
| Effect of time in full-term group | −0.01 (0.01) | −0.04 to 0.01 | .32 |
| Group × time interaction effect | 0.02 (0.02) | −0.02 to 0.06 | .41 |
Note. CI = confidence interval; SE = standard error; VPT = very preterm.
Maternal Distress over Time: VPT versus Full Term
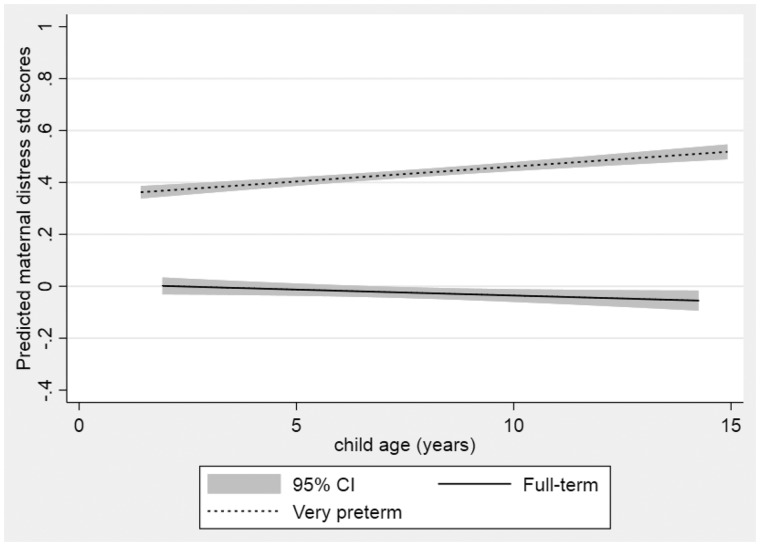
There was evidence of higher distress levels across all time-points among mothers of children born VPT compared with mothers of children born full term (mean difference 0.27; 95% CI [confidence interval] 0.03, 0.66; p = .03) (Table III and Figure 1). The hypothesis that maternal distress levels change over time across all children was not supported by the data (main effect for time 0.01 (95% CI −0.01, 0.03; p = .44), nor was the hypothesis that the change over time differed by group (interaction p = .42).
Table IV.
Regression Coefficients for Predictors of Maternal Distress in the Very Preterm Group—Univariable Models
| Variable estimate (95% CI), p-value | Main effect of predictora (no interaction) | Main effect of time (no interaction)a | Effect of time in referenceb groupc | Effect of time in comparison groupc | Interaction effect |
|---|---|---|---|---|---|
| Higher social risk | 0.47 (0.09 to 0.86) | 0.012 (−0.01 to 0.04) | −0.02 (−0.06 to 0.02) | 0.03 (−0.001 to 0.06) | p = .07 |
| p = .02 | p = .35 | p = .40 | p = .06 | ||
| Higher neonatal medical risk | 0.13 (−0.25 to 0.51) | 0.01 (−0.01 to 0.04) | −0.03 (−0.07 to 0.01) | 0.04 (0.01 to 0.07) | p = .01 |
| p = .52 | p = .29 | p = .15 | p = .01 | ||
| Sex | 0.01 (−0.36 to 0.39) | 0.01 (−0.03 to 0.04) | 0.003 (−0.03 to 0.04) | 0.02 (−0.01 to 0.06) | p = .42 |
| p = .94 | p = .29 | p = .84 | p = .19 | ||
| Multiple birth | −0.16 (−0.57 to 0.25) | 0.01 (−0.01 to 0.04) | 0.02 (−0.004 to 0.05) | −0.01 (−0.06 to 0.03) | p = .18 |
| p = .44 | p = .27 | p = .10 | p = .60 | ||
| Child impairment at 2 years | 0.25 (−0.19 to 0.70) | 0.01 (−0.11 to 0.04) | 0.01 (−0.02 to 0.03) | 0.04 (−0.01 to 0.1) | p = .24 |
| p = .26 | p = .28 | p = .68 | p = .12 |
Models models including main effects of time and main effect of predictor.
Reference group = lower social risk, lower neonatal medical risk, males, singleton, no impairment.
Models including main effects of time, main effect of predictor, and an interaction.
Figure 1.
Estimated level of maternal distress in very preterm and full-term groups with child’s age.
Predictors of Maternal Distress in the VPT Group
Within the VPT group, the hypothesis that maternal distress levels changed over time was not supported by the data (Table IV). There was, however, evidence of higher distress levels across all time-points among mothers in the higher social risk group (mean difference 0.47; 95% CI 0.09, 0.86; p = .02). There was weak evidence that the change over time varied by group (slope estimate for the higher social risk group 0.03, 95% CI −0.001, 0.06; for the lower risk group −0.02, 95% CI −0.06, 0.02; interaction p = .07). See also Supplementary Table 2 for actual scores among the subgroups.
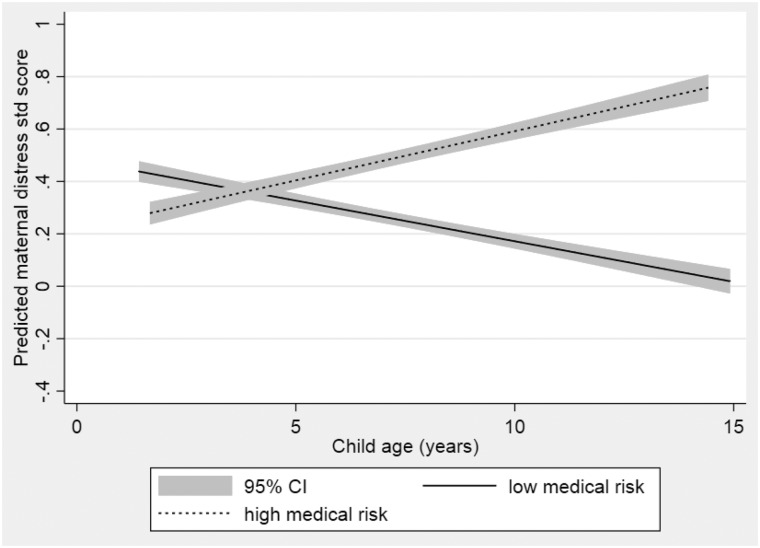
The data did not support the hypothesis that there was an association between higher neonatal medical risk and higher maternal psychological distress levels across all time-points. However, there was evidence that the trajectory of distress for mothers varied in those classified as higher versus lower medical risk, with an increase over time in distress levels for mothers in the higher neonatal medical risk group, but little change over time in mothers in the lower neonatal medical risk group (slope estimate for the higher medical risk group 0.04, 95% CI 0.01, 0.07, for the lower medical risk group −0.03, 95% CI −0.07, 0.01; interaction p = .01) (see Figure 2).
Figure 2.
Estimated level of maternal distress in the very preterm group with child’s age according to child neonatal medical risk.
The data did not support the hypothesis that there was an association between child’s sex, multiple birth, or child neurodevelopmental impairment at 2 years and maternal psychological distress levels across all time-points, or that changes in maternal distress levels over time differed within subgroups. The multivariable models, including the time, social risk main effect and child neonatal medical risk main and interaction effects yielded similar results to the univariable analyses (see Supplementary Table 3).
Discussion
In this study, mothers of children born VPT reported consistently higher levels of psychological distress compared with mothers of full terms from 2 to 13 years, even after adjusting for social risk, but the data did not support the hypothesis that this changed over time. This resonates with previous studies in which parents of children born preterm reported higher levels of psychological distress compared with parents of children born at term (Treyvaud, 2014); indeed it extends our previous findings at 2 and 7 years in this cohort to 13 years of age (Treyvaud et al., 2010, 2014). Evidence from previous longitudinal studies suggests high initial psychological distress in the first weeks and months after birth, followed by a stable decline over the years, therefore it was anticipated that group differences would decrease over time. However, in our study, we found maternal psychological distress to be relatively stable over time. One reason for this may be that the initial time-point in this study was at 2 years, a point in time in which psychological distress related to the preterm birth may have already stabilized, compared with the first months of life in which acute stress is likely to be present (Miles et al., 2007; Misund et al., 2013; Pace et al., 2016). By the age of 2 years, there may be more clarity regarding the child’s health and development, and appropriate support and services may already be arranged if needed, therefore it is expected to see more stability from this time on. We did not observe an anticipated greater decline in maternal psychological distress over time among mothers of children born VPT compared with children born full term. The lack of change in maternal psychological distress may reflect the ongoing challenges of parenting a preterm born child, facing school transitions and increasing social and academic demands, particularly as children born VPT have considerably lower academic performance than children born at term (Joseph et al., 2016; Twilhaar, de Kieviet, Aarnoudse-Moens, van Elburg, & Oosterlaan, 2018).
There may be a number of mechanisms for ongoing effects of preterm birth contributing to elevated psychological distress. Having unresolved feelings of loss and grief over the lost wished-for, healthy, pregnancy and child may contribute to depression symptoms over time. The potentially traumatic experience of the preterm birth and hospitalization in the NICU, if not addressed, may lead to untreated post-traumatic stress and anxiety symptoms. Moreover, the daily burden of raising a child with medical and special needs may also contribute to prolonged distress. It is important to continue to study maternal psychological distress after preterm birth to shed light on the adaptation processes of parents to stressful events related to their children’s health.
Previous studies have suggested that the longitudinal association between preterm birth and parental distress is influenced by child and family characteristics (Moore et al., 2006; Ong et al., 2001; Singer et al., 2010). As anticipated, and in line with previous reports (e.g., (Lakshmanan et al., 2017; Moore et al., 2006)), higher family social disadvantage was associated with elevated maternal psychological distress. The impact of preterm birth on maternal psychological distress thus seems to be exacerbated by social risk, as it has more impact on the distress for those who are initially having more social risk. Moreover, preterm birth may be associated with higher rates of family burden and conflict, and more difficulties in employment and attaining education (Singer et al., 2007), contributing to accumulating risk for the family and the child. Although all mothers of children born preterm may require some support, there is a need of enhanced support for this vulnerable group of socially disadvantaged families.
As expected, higher neonatal medical risk was associated with increasing distress over time in the VPT group compared with, if anything, slightly reducing distress in mothers of children born VPT with lower medical risk, also resonating with previous reports (Northrup, Evans, & Stotts, 2013; Polic et al., 2016; Taylor et al., 2001). Contrary to predictions, the data did not support the hypothesis that child neurodevelopmental impairment, as assessed at the age of 2 years, was associated with elevated maternal distress. Variation in definitions of impairment between studies may account for the discrepancy in our findings compared with previous studies (Cacciani et al., 2013; Huhtala et al., 2011; Singer et al., 2007).
The discrepancy in the results between medical risk and neurodevelopmental impairment, which may be considered related variables, requires further exploration. In this sample, these two variables are rather independent, with only 25% of the children at higher medical risk having a neurodevelopmental impairment. Our definition of impairment may have not captured later-onset learning, social-emotional and behavioral difficulties, which are associated with higher medical risk (Miceli et al., 2000). Importantly, the subgroup of children with higher medical risk, who are free of neurodevelopmental disability, may differ from those with a diagnosed disability in their developmental and behavioral difficulties and their impact on maternal psychological distress.
Severe impairments are usually identified early in life and are generally considered to be stable over time. Mothers whose child had received a formal diagnosis of impairment in the early years may have clarity regarding their child’s outcomes and are likely to have accessed early intervention services, which can attenuate the stress of raising a child with additional needs (Lakshmanan et al., 2017). On the other hand, developmental, social-emotional, and behavioral difficulties associated with high medical risk may be identified only later in life. These difficulties may intensify over time with transitions to school and adolescence and increasing social and academic demands and have bidirectional influence on maternal mental health—thus potentially contributing to the increase in maternal psychological distress over time. Indeed, in the Wolke et al. study, while maternal well-being was not associated with the child’s neurosensory impairment, it was associated with the child’s mental health and peer relationships (Wolke et al., 2017). Finally, studies following young adults who were born preterm, provide evidence to suggest that in the long term, mothers of children born preterm and have a neurosensory impairment, experience personal growth, and feel good about themselves for having managed their child’s health (Saigal et al., 2010; Wolke et al., 2017). Thus, in the future it would be important to account for the child’s later-onset developmental and behavioral difficulties, and maternal personal growth and examine their associations with psychological distress.
In the current study, maternal psychological distress was measured by a variety of mental health symptoms. The literature on postnatal psychological distress following preterm birth to date has mainly focused on postnatal depression. However, mothers giving birth preterm birth may also experience anxiety, post-traumatic symptoms, daily burden, and parenting stress. Therefore, in the case of preterm birth it may be better to conceptualize maternal psychological distress more broadly across a range of symptoms, and to acknowledge its unique presentation, that should be assessed and addressed more broadly, and potentially in different ways than in the general population (Henderson et al., 2016; Segre, McCabe, Chuffo-Siewert, & O’Hara, 2014), hence we see this as a strength of our study.
Given the evidence from the current manuscript that maternal psychological distress after VPT birth can last through child school years and early adolescence, this has implications for clinical practice, emphasizing the importance of addressing maternal psychological distress beyond the first few months after months. It is important to persist with screening for maternal psychological distress and provide support for those who are experiencing high distress. The decision as to which families should be offered support should be based on maternal and family risk factors and not only on the child’s medical condition and developmental risk. Special attention should be given, and additional support considered, to mothers and families experiencing social disadvantage, and those whose child was born at high medical risk, who may be susceptible to elevated psychological distress over time.
Limitations
The first time-point in which data were collected on maternal psychological distress in this study was at 2 years of age. Thus, information on maternal distress following birth and during infancy, which would have been valuable, was lacking. Although scores were standardized, the use of two different tools to measure the same construct of psychological distress in the study should be noted as a potential limitation. In particular, the use of different measures precluded applying the questionnaires’ cut-off scores to assess the rates of mothers whose levels of psychological distress were in the clinically significant range. It also limits the examination of patterns of distress at the individual level (i.e., whether the same women have clinically significant elevated distress levels at the different time-points). Rates of attrition should also be noted as a potential limitation; with 14% and 5% attrition in the VPT and full-term groups, respectively. Loss to follow-up was not completely at random, as rates were higher among families of children born VPT, families with higher social risk and families with a child with neurodevelopmental impairment. The current study was limited to mothers’ reports as the majority of questionnaires was completed by mothers. There is evidence that preterm birth may affect fathers, yet perhaps with a different presentation of symptoms and trajectories over time (Pace et al., 2016), and it is important in future studies to include both parents. Prenatal mental health, which is associated with higher risk of preterm birth as well as risk for postnatal mental health problems, was not assessed or accounted for in the study, thus somewhat limiting interpretation of the causality relationship of preterm birth on maternal psychological distress. The group of children born at term was smaller than those born VPT, which limits the power of this study to identify potential relationships. “Higher social risk” in this study was defined as scores above the median score, and therefore does not necessarily represent a “true” high-risk population. Finally, in future studies it would be beneficial to examine how ongoing maternal psychological distress is associated with children’s cognitive and social-emotional outcomes over the longer term, and the potential role of parenting behaviors and parent–child relationship in mediating this association.
Conclusions
This study, reporting on trajectories in maternal psychological distress 2–13 years after very preterm birth, supports the evidence on the long-term effects of preterm birth on maternal mental health. Mothers of children born preterm may require additional support not only in the first months after birth, but also later in the child’s development, to support their own well-being and functioning, as well as buffer the potential effects of the psychological distress on their relationship with the child. Mothers from a socially disadvantaged background and those whose child has neonatal medical complications may require more extensive support to prevent prolonged psychological distress and promote optimal outcomes for the children and their families.
Supplementary Data
Supplementary data can be found at: https://academic.oup.com/jpepsy.
Funding
This study was supported by the Australian National Health and Medical Council [Centre for Research Excellence 1060733; Project grants 237117, 491209, and 1066555; Senior Research Fellowship (1081288 to PJA)]; US National Institutes of Health (HD058056); The Hebrew University (Postdoctoral Research Fellowship for Women, to MY); the Israel Science Foundation (50/18 to MY); the Murdoch Children’s Research Institute and the Royal Children’s Hospital Foundation; and the Victorian Government’s Operational Infrastructure Support Program.
Conflicts of interest: None declared.
Supplementary Material
References
- Anderson P. J. (2014). Neuropsychological outcomes of children born very preterm. Seminars in Fetal and Neonatal Medicine, 19, 90–96. doi:10.1016/j.siny.2013.11.012 [DOI] [PubMed] [Google Scholar]
- Aylward G. P. (2014). Neurodevelopmental outcomes of infants born prematurely. Journal of Developmental and Behavioral Pediatrics, 35, 392–393. doi:10.1097/01.DBP.0000452240.39511.d4 25007063 [DOI] [PubMed] [Google Scholar]
- Barlow J., Cullen‐Powell L., Cheshire A. (2006). Psychological well‐being among mothers of children with cerebral palsy. Early Child Development and Care, 176(3–4), 421–428. [Google Scholar]
- Bener A. (2013). Psychological distress among postpartum mothers of preterm infants and associated factors: A neglected public health problem. Revista Brasileira de Psiquiatria, 35, 231–236. doi:10.1590/1516-4446-2012-0821 [DOI] [PubMed] [Google Scholar]
- Bjelland I., Dahl, A. A., Haug, T. T., & Neckelmann, D. (2002). The validity of the Hospital Anxiety and Depression Scale. An updated literature review. Journal of Psychosomatic Research, 52(2), 69–77. [DOI] [PubMed] [Google Scholar]
- Brehaut J. C., Kohen D. E., Raina P., Walter S. D., Russell D. J., Swinton M., Rosenbaum P. (2004). The health of primary caregivers of children with cerebral palsy: how does it compare with that of other Canadian caregivers? Pediatrics, 1142, e182–e191. [DOI] [PubMed] [Google Scholar]
- Cacciani L., Di Lallo D., Piga S., Corchia C., Carnielli V., Chiandotto V., Cuttini M. (2013). Interaction of child disability and stressful life events in predicting maternal psychological health. Results of an area-based study of very preterm infants at two years corrected age. Research in Developmental Disabilities, 34, 3433–3441. doi:10.1016/j.ridd.2013.07.018 [DOI] [PubMed] [Google Scholar]
- Carson C., Redshaw M., Gray R., Quigley M. A. (2015). Risk of psychological distress in parents of preterm children in the first year: Evidence from the UK Millennium Cohort Study. BMJ Open, 5, e007942. doi:10.1136/bmjopen-2015-007942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue P. K., Maurin E., Kimzey L., Allen M. C., Strobino D. (2008). Quality of life of caregivers of very low-birthweight infants. Birth-Issues in Perinatal Care, 35, 212–219. doi:10.1111/j.1523-536X.2008.00242.x [DOI] [PubMed] [Google Scholar]
- Goldberg D. P. (1988). User’s guide to the general health questionnaire. NFER-Nelson, Windsor, Berks. [Google Scholar]
- Goldberg D. P., Gater R., Sartorius N., Ustun T. B., Piccinelli M., Gureje O., Rutter C. (1997). The validity of two versions of the GHQ in the WHO study of mental illness in general health care. Psychological Medicine, 271, 191–197. [DOI] [PubMed] [Google Scholar]
- Goodman S. H., Rouse M. H., Connell A. M., Broth M. R., Hall C. M., Heyward D. (2011). Maternal depression and child psychopathology: A meta-analytic review. Clinical Child and Family Psychology Review, 14, 1–27. doi:10.1007/s10567-010-0080-1 [DOI] [PubMed] [Google Scholar]
- Grace S. L., Evindar A., Stewart D. E. (2003). The effect of postpartum depression on child cognitive development and behavior: A review and critical analysis of the literature. Archives of Womens Mental Health, 6, 263–274. doi:10.1007/s00737-003-0024-6 [DOI] [PubMed] [Google Scholar]
- Gueron-Sela N., Atzaba-Poria N., Meiri G., Marks K. (2015). The caregiving environment and developmental outcomes of preterm infants: Diathesis stress or differential susceptibility effects? Child Development, 86, 1014–1030. doi:10.1111/cdev.12359 [DOI] [PubMed] [Google Scholar]
- Hack M., Breslau N., Aram D., Weissman B., Klein N., Borawski-Clark E. (1992). The Effect of Very Low Birth Weight and Social Risk on Neurocognitive Abilities at School Age. Journal of Developmental & Behavioral Pediatrics, 136, 412–420. doi:10.1097/00004703-199212000-00005. [PubMed] [Google Scholar]
- Hack M., Flannery D. J., Schluchter M., Cartar L., Borawski E., Klein N. (2002). Outcomes in young adulthood for very-low-birth-weight infants. New England Journal of Medicine, 3463, 149–157. [DOI] [PubMed] [Google Scholar]
- Hayes B., Sharif F. (2009). Behavioural and emotional outcome of very low birth weight infants—Literature review. The Journal of Maternal-Fetal & Neonatal Medicine, 22, 849–856. [DOI] [PubMed] [Google Scholar]
- Helle N., Barkmann C., Bartz-Seel J., Diehl T., Ehrhardt S., Hendel A., Bindt C. (2015). Very low birth-weight as a risk factor for postpartum depression four to six weeks postbirth in mothers and fathers: Cross-sectional results from a controlled multicentre cohort study. Journal of Affective Disorders, 180, 154–161. doi:10.1016/j.jad.2015.04.001 [DOI] [PubMed] [Google Scholar]
- Henderson J., Carson C., Redshaw M. (2016). Impact of preterm birth on maternal well-being and women’s perceptions of their baby: A population-based survey. BMJ Open, 6, e012676. doi:10.1136/bmjopen-2016-012676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille E., Elbertse L., Gravenhorst J. B., Brand R., Verloove-Vanhorick S. (2005). Nonresponse bias in a follow-up study of 19-year-old adolescents born as preterm infants. Pediatrics, 1165, e662–e666. [DOI] [PubMed] [Google Scholar]
- Holditch-Davis D., Miles M. S., Weaver M. A., Black B., Beeber L., Thoyre S., Engelke S. (2009). Patterns of distress in African-American mothers of preterm infants. Journal of Developmental and Behavioral Pediatrics, 30, 193–205. doi:10.1097/DBP.0b013e3181a7ee53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holditch-Davis D., Santos H., Levy J., White-Traut R., O’Shea T. M., Geraldo V., David R. (2015). Patterns of psychological distress in mothers of preterm infants. Infant Behaviour and Development, 41, 154–163. doi:10.1016/j.infbeh.2015.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhtala M., Korja R., Lehtonen L., Haataja L., Lapinleimu H., Munck P., … PIPARI Study Group. (2011). Parental psychological well‐being and cognitive development of very low birth weight infants at 2 years. Acta Paediatrica, 100, 1555–1560. [DOI] [PubMed] [Google Scholar]
- Johnson S., Fawke J., Hennessy E., Rowell V., Thomas S., Wolke D., Marlow N. (2009). Neurodevelopmental disability through 11 years of age in children born before 26 weeks of gestation. Pediatrics, 1242, e249–e257. [DOI] [PubMed] [Google Scholar]
- Joseph, R. M., Oã Shea, T. M., Allred, E. N., Heeren, T., Hirtz, D., Jara, H., . . . Kuban, K. C. K. (2016). Neurocognitive and academic outcomes at age 10 years of extremely preterm newborns. Pediatrics, 137(4), e20154343. doi:10.1542/peds.2015–4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmanan A., Agni M., Lieu T., Fleegler E., Kipke M., Friedlich P. S., Belfort M. B. (2017). The impact of preterm birth <37 weeks on parents and families: A cross-sectional study in the 2 years after discharge from the neonatal intensive care unit. Health and Quality of Life Outcomes, 15, 38. doi:10.1186/s12955-017-0602-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larroque B., Ancel P.-Y., Marret S., Marchand L., André M., Arnaud C., Kaminski M. (2008). Neurodevelopmental disabilities and special care of 5-year-old children born before 33 weeks of gestation (the EPIPAGE study): A longitudinal cohort study. Lancet, 371, 813–820. doi:10.1016/S0140-6736(08)60380-3 [DOI] [PubMed] [Google Scholar]
- Mensah F. K., Kiernan K. E. (2010). Parents’ mental health and children’s cognitive and social development: Families in England in the Millennium Cohort Study. Social Psychiatry and Psychiatric Epidemiology, 45, 1023–1035. doi:10.1007/s00127-009-0137-y [DOI] [PubMed] [Google Scholar]
- Miceli P. J., Goeke M. M. C., Whitman T. L., Kolberg K. S., Miller L. C., White R. D. (2000). Brief report: Birth status, medical complications, and social environment: Individual differences in development of preterm, very low birth weight infants. Journal of Pediatric Psychology, 25, 353–358. doi:10.1093/jpepsy/25.5.353 [DOI] [PubMed] [Google Scholar]
- Miles M. S., Holditch-Davis D., Schwartz T. A., Scher M. (2007). Depressive symptoms in mothers of prematurely born infants. Journal of Developmental and Behavioural Pediatrics, 28, 36–44. doi:10.1097/01.DBP.0000257517.52459.7a [DOI] [PubMed] [Google Scholar]
- Misund A. R., Nerdrum P., Braten S., Pripp A. H., Diseth T. H. (2013). Long-term risk of mental health problems in women experiencing preterm birth: A longitudinal study of 29 mothers. Annals of General Psychiatry, 12, 33. doi:10.1186/1744-859X-12-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M., Taylor G. H., Klein N., Minich N., Hack M. (2006). Longitudinal changes in family outcomes of very low birth weight. Journal of Pediatric Psychology, 31, 1024–1035. doi:10.1093/jpepsy/jsj075 [DOI] [PubMed] [Google Scholar]
- Moore T., Hennessy E. M., Myles J., Johnson S. J., Draper E. S., Costeloe K. L., Marlow N. (2012). Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: the EPICure studies. BMJ 345, e7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moster D., Lie R. T., Markestad T. (2008). Long-term medical and social consequences of preterm birth. New England Journal of Medicine, 359, 262–273. doi:10.1056/NEJMoa0706475 [DOI] [PubMed] [Google Scholar]
- Northrup T. F., Evans P. W., Stotts A. L. (2013). Depression among mothers of high-risk infants discharged from a neonatal intensive care unit. MCN: The American Journal of Maternal-Child Nursing, 38, 89–94. doi:10.1097/NMC.0b013e318270f8b8 [DOI] [PubMed] [Google Scholar]
- Ong L. C., Chandran V., Boo N. Y. (2001). Comparison of parenting stress between Malaysian mothers of four-year-old very low birthweight and normal birthweight children. Acta Paediatrica, 90, 1464–1469. [DOI] [PubMed] [Google Scholar]
- Pace C. C., Spittle A. J., Molesworth C. M.-L., Lee K. J., Northam E. A., Cheong J. L. Y., Anderson P. J. (2016). Evolution of depression and anxiety symptoms in parents of very preterm infants during the newborn period. JAMA Pediatrics, 170, 863–870. doi:10.1001/jamapediatrics.2016.0810 [DOI] [PubMed] [Google Scholar]
- Padovani F. H., Carvalho A. E., Duarte G., Martinez F. E., Linhares M. B. (2009). Anxiety, dysphoria, and depression symptoms in mothers of preterm infants. Psychological Reports, 104, 667–679. doi:10.2466/pr0.104.2.667-679 [DOI] [PubMed] [Google Scholar]
- Pierrehumbert B., Nicole A., Muller-Nix C., Forcada-Guex M., Ansermet F. (2003). Parental Post-Traumatic Reactions after Premature Birth: Implications for Sleeping and Eating Problems in the Infant. Archives of Disease in Childhood. Fetal and Neonatal Edition, 88, F400–F404. doi:10.1136/fn.88.5.F400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poehlmann J., Schwichtenberg A. J., Shlafer R. J., Hahn E., Bianchi J. P., Warner R. (2011). Emerging self-regulation in toddlers born preterm or low birth weight: Differential susceptibility to parenting? Developmental Psychopathology, 23, 177–193. doi:10.1017/S0954579410000726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polic B., Bubic A., Mestrovic J., Markic J., Kovacevic T., Juric M., Kolcic I. (2016). Late preterm birth is a strong predictor of maternal stress later in life: Retrospective cohort study in school‐aged children. Journal of Paediatrics and Child Health, 52, 608–613. [DOI] [PubMed] [Google Scholar]
- Powers J., Loxton D. (2010). The impact of attrition in an 11-year prospective longitudinal study of younger women. Annals of Epidemiology, 204, 318–321. [DOI] [PubMed] [Google Scholar]
- Reijneveld S. A., de Kleine M. J., van Baar A. L., Kollee L. A., Verhaak C. M., Verhulst F. C., Verloove-Vanhorick S. P. (2006). Behavioural and emotional problems in very preterm and very low birthweight infants at age 5 years. Archives of Disease in Childhood. Fetal and Neonatal Edition, 91, F423–F428. doi:10.1136/adc.2006.093674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts G., Howard K., Spittle A. J., Brown N. C., Anderson P. J., Doyle L. W. (2008). Rates of early intervention services in very preterm children with developmental disabilities at age 2 years. Journal of Paediatric and Child Health, 44, 276–280. doi:10.1111/j.1440-1754.2007.01251.x [DOI] [PubMed] [Google Scholar]
- Saigal S., Burrows E., Stoskopf B. L., Rosenbaum P. L., Streiner D. (2000). Impact of extreme prematurity on families of adolescent children. Journal of Pediatrics, 137, 701–706. doi:10.1067/mpd.2000.109001 [DOI] [PubMed] [Google Scholar]
- Saigal S., Pinelli J., Streiner D. L., Boyle M., Stoskopf B. (2010). Impact of extreme prematurity on family functioning and maternal health 20 years later. Pediatrics, 126, e81–e88. doi:10.1542/peds.2009-2527 [DOI] [PubMed] [Google Scholar]
- Segre L. S., McCabe J. E., Chuffo-Siewert R., O’Hara M. W. (2014). Depression and anxiety symptoms in mothers of newborns hospitalized on the neonatal intensive care unit. Nursing Research, 63, 320–332. doi:10.1097/NNR.0000000000000039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P. E., Clements M., Poehlmann J. (2011). Maternal resolution of grief after preterm birth: Implications for infant attachment security. Pediatrics, 127, 284–292. doi:10.1542/peds.2010-1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P. E., Robbins N., Coelho R. B., Poehlmann J. (2013). The paradox of prematurity: The behavioral vulnerability of late preterm infants and the cognitive susceptibility of very preterm infants at 36 months post-term. Infant Behaviour and Development, 36, 50–62. doi:10.1016/j.infbeh.2012.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer L. T., Salvator A., Guo S., Collin M., Lilien L., Baley J. (1999). Maternal psychological distress and parenting stress after the birth of a very low-birth-weight infant. Jama, 2819, 799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer G. H. (2006). Meta-analysis of comparative studies of depression in mothers of children with and without developmental disabilities. American journal on mental retardation, 1113, 155–169. [DOI] [PubMed] [Google Scholar]
- Singer L. T., Fulton S., Kirchner H. L., Eisengart S., Lewis B., Short E., Baley J. E. (2007). Parenting very low birth weight children at school age: Maternal stress and coping. Journal of Pediatrics, 151, 463–469. doi:10.1016/j.jpeds.2007.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer L. T., Fulton S., Kirchner H. L., Eisengart S., Lewis B., Short E., Baley J. E. (2010). Longitudinal predictors of maternal stress and coping after very low-birth-weight birth. Archives of Pediatrics & Adolescent Medicine, 164, 518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. (2004). Parental mental health: Disruptions to parenting and outcomes for children. Child & Family Social Work, 9, 3–11. doi:10.1111/j.1365-2206.2004.00312.x [Google Scholar]
- Sohr-Preston S. L., Scaramella L. V. (2006). Implications of timing of maternal depressive symptoms for early cognitive and language development. Clinical Child and Family Psychology Review, 9, 65–83. doi:10.1007/s10567-006-0004-2 [DOI] [PubMed] [Google Scholar]
- Spinelli M., Poehlmann J., Bolt D. (2013). Predictors of parenting stress trajectories in premature infant-mother dyads. Journal of Family Psychology, 27, 873–883. doi:10.1037/a0034652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spittle A. J., Treyvaud K., Doyle L. W., Roberts G., Lee K. J., Inder T. E., Anderson P. J. (2009). Early emergence of behavior and social-emotional problems in very preterm infants. Journal of the American Academy of Child and Adolescent Psychiatry, 48, 909–918. doi:10.1097/CHI.0b013e3181af8235 [DOI] [PubMed] [Google Scholar]
- Taylor H. G., Klein N., Minich N. M., Hack M. (2001). Long-term family outcomes for children with very low birth weights. Archives of Pediatrics & Adolescent Medicine, 155, 155–161. doi:10.1001/archpedi.155.2.155 [DOI] [PubMed] [Google Scholar]
- Treyvaud K. (2014). Parent and family outcomes following very preterm or very low birth weight birth: A review. Seminars in Fetal and Neonatal Medicine, 19, 131–135. doi:10.1016/j.siny.2013.10.008 [DOI] [PubMed] [Google Scholar]
- Treyvaud K., Aldana A. C., Scratch S. E., Ure A. M., Pace C. C., Doyle L. W., Anderson P. J. (2016). The influence of multiple birth and bereavement on maternal and family outcomes 2 and 7 years after very preterm birth. Early Human Development, 100, 1–5. doi:10.1016/j.earlhumdev.2016.04.005 [DOI] [PubMed] [Google Scholar]
- Treyvaud K., Anderson V. A., Howard K., Bear M., Hunt R. W., Doyle L. W., Anderson P. J. (2009). Parenting behavior is associated with the early neurobehavioral development of very preterm children. Pediatrics, 123, 555–561. doi:10.1542/peds.2008-0477 [DOI] [PubMed] [Google Scholar]
- Treyvaud K., Anderson V. A., Lee K. J., Woodward L. J., Newnham C., Inder T. E., Anderson P. J. (2010). Parental mental health and early social-emotional development of children born very preterm. Journal of Pediatric Psychology, 35, 768–777. doi:10.1093/jpepsy/jsp109 [DOI] [PubMed] [Google Scholar]
- Treyvaud K., Doyle L. W., Lee K. J., Roberts G., Cheong J. L., Inder T. E., Anderson P. J. (2011). Family functioning, burden and parenting stress 2 years after very preterm birth. Early Human Development, 87, 427–431. doi:10.1016/j.earlhumdev.2011.03.008 [DOI] [PubMed] [Google Scholar]
- Treyvaud K., Lee K. J., Doyle L. W., Anderson P. J. (2014). Very preterm birth influences parental mental health and family outcomes seven years after birth. Journal of Pediatrics, 164, 515–521. doi:10.1016/j.jpeds.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twilhaar E. S., de Kieviet J. F., Aarnoudse-Moens C. S., van Elburg R. M., Oosterlaan J. (2018). Academic performance of children born preterm: A meta-analysis and meta-regression. Archives of Disease in Childhood. Fetal and Neonatal Edition, 103, F322–F330. doi:10.1136/archdischild-2017-312916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigod S. N., Villegas L., Dennis C. L., Ross L. E. (2010). Prevalence and risk factors for postpartum depression among women with preterm and low-birth-weight infants: A systematic review. BJOG: An International Journal of Obstetrics & Gynaecology, 117, 540–550. doi:10.1111/j.1471-0528.2009.02493.x [DOI] [PubMed] [Google Scholar]
- Whitaker A., Feldman, J., & Schonfeld, I. (1996). Neonatal cranial ultrasound abnormalities in low birth weight infants: Relation to cognitive outcomes at six years of age. Pediatrics, 98(4), 719. [PubMed] [Google Scholar]
- Witt W. P., Litzelman K., Spear H. A., Wisk L. E., Levin N., McManus B. M., Palta M. (2012). Health-related quality of life of mothers of very low birth weight children at the age of five: results from the newborn lung project statewide cohort study. Quality of Life Research, 219, 1565–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolke D., Waylen A., Samara M., Steer C., Goodman R., Ford T., Lamberts K. (2009). Selective drop-out in longitudinal studies and non-biased prediction of behaviour disorders. The British Journal of Psychiatry, 1953, 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolke D., Baumann N., Busch B., Bartmann P. (2017). Very preterm birth and parents’ quality of life 27 years later. Pediatrics, e20171263. [DOI] [PubMed] [Google Scholar]
- Woodward L. J., Bora S., Clark C. A., Montgomery-Honger A., Pritchard V. E., Spencer C., Austin N. C. (2014). Very preterm birth: Maternal experiences of the neonatal intensive care environment. Journal of Perinatology, 34, 555–561. doi:10.1038/jp.2014.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaari M., Millo I., Harel-Gadassi A., Friedlander E., Bar-Oz B., Eventov-Friedman S., Yirmiya N. (2017). Maternal resolution of preterm birth from 1 to 18 months. Attachment and Human Development, 19, 487–503. doi:10.1080/14616734.2017.1324499 [DOI] [PubMed] [Google Scholar]
- Zigmond A. S., Snaith R. P. (1983). The Hospital Anxiety and Depression Scale. Acta Paediatrica, 67, 361–370. doi:10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.