Abstract
Purpose:
Although cisplatin plus radiotherapy is a standard treatment of locally advanced head and neck squamous cell carcinoma (LA-HNSCC), cisplatin contraindication is common. Radiation elicits and promotes tumor-directed immune-stimulation, which may potentiate anti-PD-1 therapy. We provide the first efficacy report of combined pembrolizumab and definitive radiotherapy in LA-HNSCC.
Experimental Design:
This single-arm, multi-institution, phase II study (NCT02609503) enrolled 29 cisplatin ineligible patients. Patients received radiotherapy concurrently with 3 cycles of pembrolizumab 200mg q3 weeks followed by 3 adjuvant cycles. The primary endpoint was a PFS of ≥16 months. Correlative studies included peripheral blood flow cytometry and Luminex cytokine profiling.
Results:
Reasons for cisplatin ineligibility included otopathy (69.0%), nephropathy (20.7%), and neuropathy (6.9%). With median follow-up of 21 months, estimated twenty-four month PFS and OS rates were 71% (95% CI 49–84) and 75% (51–88). The primary PFS endpoint has exceeded the hypothesis and its median has not been reached. Toxicities were typical of radiotherapy; however high rates of grade 3/4 lymphopenia (58.6%) were observed. Flow cytometry revealed a relative decline in CD4 T cells and B cells, but not CD8 T cells. Upon treatment, frequencies of transitional B cells and tissue-like memory B cells increased while resting memory B cells decreased. Patients with progression had greater percentages of baseline naïve B cells and fewer marginal zone B cells.
Conclusions:
Pembrolizumab and radiotherapy is efficacious in LA-HNSCC and should be evaluated in a randomized trial. The observed changes in B-cell markers deserve further study both as potential biomarkers and as therapeutic targets.
Introduction:
A standard treatment for locally advanced squamous cell carcinoma of the head and neck (HNSCC) is the combination of radiation and cisplatin. However, cisplatin causes numerous toxicities including hearing loss, tinnitus, neuropathy and nephropathy that can lead to contradictions to its use. Thus, there is a great clinical unmet need for an effective systemic agent for combination with radiation in cisplatin-ineligible patients with potentially curable disease.
Pembrolizumab is a fully humanized IgG monoclonal antibody against the programmed death receptor 1 (PD1) that is active against head and neck cancer. At the time of study design and initiation, multiple studies had shown activity in platinum-refractory recurrent/metastatic disease, leading to approval. Recently, based on data reported in Keynote 0481, pembrolizumab was approved in first line metastatic/recurrent disease. However, in contrast to other cancers, such as non-small cell lung cancer (NSCLC), most head and neck cancer (~90%) does not present with metastatic disease, but rather with local or loco-regional disease. Because of this curability, as well as the large number of quality life years gained when cure is achieved, this population is particularly important.
We hypothesized that pembrolizumab and radiation could provide synergistic benefit in patients with locally advanced HNSCC. Radiation results in type I interferon induction through a stimulator of interferon genes (STING)-dependent pathway. Further, it can increase HLA expression, upregulate HLA-associated tumor antigen complexes, and increase both T cell receptor (TCR) clonality and diversity. Critically for the combination of a PD-1 agent, radiation therapy can increase PD1 and PDL1 expression as well as improve T-cell trafficking to the tumor micro-environment2. Preclinically, concurrent PD-L1 blockade has been shown to sensitize cancer cells to the effects of ionizing radiation3 and ionizing radiation has been shown to sensitize tumors to PD-L1 inhibition4. We hypothesized that additional pembrolizumab subsequent to the last dose of radiotherapy could provide ongoing sensitization to radiation’s effects and, based on the preceding rationale, could be particularly effective just after radiotherapy.
We therefore embarked upon a single-arm, phase II study for patients with locally advanced HNSCC for whom chemoradiotherapy would be a standard of care, except with a contraindication to cisplatin. Patients were treated with three cycles of pembrolizumab concurrent with standard of care, definitive radiation (70Gy), followed by three additional adjuvant cycles of pembrolizumab. In the context of more than 150 studies exploring the addition of immunotherapy to standard full dose radiotherapy (with or without chemotherapy)5 we herein present data on toxicity, PFS (primary endpoint), OS, correlative studies, quality of life measures, and patient reported outcomes.
Methods:
Patients
Patient were required to have untreated stage III-IV (non-metastatic) HNSCC which was histologically or cytologically confirmed as per American Joint Committee on Cancer (AJCC) version 7.0, which was current staging criteria at time of study initiation. Nasopharyngeal carcinoma was excluded. Patients were required to have a contraindication to cisplatin as defined by: abnormal renal function (GFR< institutional lower limit of institutional normal (<LLN)); abnormal hearing (patient or audiology defined); pre-existing tinnitus; neuropathy (bilateral paresthesias or loss of deep tendon reflexes in upper and/or lower extremities); diabetes mellitus; oncologist-certification that patient would not be considered eligible for high dose cisplatin when given as standard of care (for example, due to age or another medical problem, reason was required to be documented); patient refusal for high dose cisplatin. Of note, other than for GFR as defined above, thresholds for other indications for cisplatin ineligibility were provider-defined consistent with standard of care practice, without specific thresholds. Patients were required to have ECOG PS 0–1 and normal organ function. Patients with active autoimmune disease, a requirement for immunomodulating medications, active infection or non-infectious pneumonitis were excluded. Full details of inclusion criteria may be found in the protocol, provided as supplementary appendix 1.
Study Design
This study was conducted as single arm, multicenter phase II study at the University of North Carolina, Fox Chase Cancer Center and Johns Hopkins. Patients were treated with 70Gy of intensity modulated radiation therapy (IMRT) in 35 fractions. To ensure appropriate and uniform treatment across sites, details of this treatment were specified in the protocol and can be seen in detail in supplementary appendix 1. Patients were treated with 3 cycles of pembrolizumab at a flat dose of 200mg IV concurrent with radiation, at time points 0, 3 weeks and 6 weeks and for three adjuvant cycles following. Toxicity was assessed via CTCAE v4.0 and PRO-CTCAE6. Continuous safety monitoring7 was conducted to ensure that patients received at least 95% of the intended radiation dose. The primary endpoint was PFS. Secondary endpoints included survival, toxicity, and quality of life (via FACT-HN). Correlative endpoints were exploratory.
Study Oversight
This trial was approved by the Lineberger Comprehensive Cancer Center Protocol Review Committee and the University of North Carolina Institutional Review Board and the relevant regulatory bodies at each collaborating site. It was conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent. The study was registered at clinicaltrials.gov (NCT02609503). The study was designed and the manuscript was written by the authors, who vouch for the accuracy and completeness of the data reported and adherence to the study protocol. No one who is not an author contributed to the writing of the manuscript. Merck supplied pembrolizumab and funds for the conduct of the study. Additional funding for correlative analysis was supplied by Lineberger Comprehensive Cancer Center.
Statistical Analysis
We hypothesized a median PFS of at least 16 months and assumed a median null hypothesis of 10 months, uniform accrual, no loss to follow-up, exponentially distributed times, a one-sided alpha of 0.1, 24-month accrual and follow-up time of 12 months to accrue 29 patients to achieve 80% power. The null hypothesis of 10 months was derived from the Bonner study8,9, which compared radiation therapy alone to radiation therapy plus cetuximab. In that study, which did not select patients for cisplatin ineligibility, PFS was improved from 12.4 months to 17.1 months with the addition of cetuximab to radiotherapy. We adjusted the null hypothesis to 10 months to account for the accrual of less fit patients due to the inclusion requirement of cisplatin ineligibility and sought to provide a greater benefit than that seen with the addition of cetuximab to radiotherapy.
Sequential boundaries were used to monitor for unacceptable toxicity, defined as failing to receive at least 95% of the intended dose of XRT--that is, if the number of patients is equal to or exceeds bn out of n patients (see supplementary table 3). This was a Pocock-type stopping boundary7 that yields the probability of crossing the boundary at most 0.05 when the rate of failing to receive at least 95% of the intended dose of XRT is equal to the acceptable rate of 10%.
The Kaplan Meier method was used to estimate overall survival (OS) and progression free survival (PFS). PFS was defined as the time from the start of treatment to the first occurrence of progressive disease (RECIST or clinically defined) or death as a first event and subjects without an event were censored at their last evaluation date. OS was defined as the time from the start of treatment to death from any cause, with censoring of those alive and the last date known to be alive. All patients, regardless of total treatment exposure, were included in analyses of both safety and efficacy. Patients who withdrew from treatment were followed for progression or survival if they gave permission, or censored at the time of withdrawal of permission.
Correlative Analysis
PDL1 staining was conducted by Qualtek Molecular Laboratories (Newtown, PA) as previously described5 and required by Merck in supported investigator initiated trials. For both the modified H-score (MHS) and modified percent score (MPS), the percent of tumor with membrane-specific staining was directly estimated at four intensity levels [negative (<1), low (1+), moderate (2+), and high (3+)] and reported as a percentage quantitatively as any one of the following: 0, 1, 2, 5, 10, 15, 20, 30, 40, 50, 60, 70, 80, 85, 90, 93, 95, 99, or 100%. To calculate MPS, the percent of tumor staining at low, moderate, and high levels are summed. To calculate MHS, the percent of membrane staining at low levels was multiplied by a factor of 1, the percent of staining at moderate levels was multiplied by 2, and percent of staining at high levels was multiplied by 3. The three products are then summed to arrive at a final modified H-score. The abundance of tumor infiltrating lymphocytes (TILs) present was also noted as a number between 0–3 with 0 being the absence of TILs and 3 indicating high profusion of TILs.
Peripheral blood was obtained for flow cytometry at baseline, week 20 and week 40. Flow cytometry gates are provided in supplementary figures 1 and 2. Briefly, CD19+ B cells were focused by removing T/NK/Moncytic cell lineages and divided into mature (CD10−) and immature (CD10+) subsets. Transtional B cells were defined as IgD+CD38+ under immature subsets. The mature B cell subset was subsequently divided into plasmablasts, unswitched B cells, and memory B cells based on IgD and CD38 expression. Marginal zone B cells and naïve B cells were distinguished by CD27 expression in unswitched B cell subset. Memory B cells were subcategorized into four subsets based on CD21 and CD27 expression under IgD-CD38− gates, including active memory (AM), resting memory (RM), tissue-like memory (TLM), and intermediate memory (IM) B cells. T cells were split into naïve (CD45RA+) and memory/effector (CD45RA−) subsets. T regulatory cells (Treg) were defined by transcription factor Foxp3 and CD25+CD127− surface phenotypes. Exhaustion markers (TIGIT, PD1, CD39, and CTLA4) were monitored in CD4 T cells, whereas only PD1 were analyzed in CD8 T cells.
Blood was obtained for cytokine and chemokine analysis at baseline, week 4, week 20 and week 40. Plasma cytokine and chemokine concentrations were determined with the Bio-Rad Bio-Plex Pro Cytokine, Chemokine, and Growth Factor Assays 27-Plex Panel™ (Cat. No. M50–0KCAFOY). Plasma samples cryopreserved at −80°C were thawed and centrifuged 1000 × g for 15 minutes at 4°C before transferring the cell and platelet-free supernatants to the 96 well assay plate. The bead-bound immunoassay was performed according to manufacturer’s instruction and data was acquired on the Bio-Plex 200 fluorescent plate reader. Cytokine, chemokine, and growth factor concentrations were acquired and analyzed by the Bio-Plex Manager. ™ Among 27 cytokines, 12 were detected whereas other 15 cytokines were found either out of range of the standard curve for each protein. Mixed-effects analysis plus Tukey’s multiple comparisons test were performed at alpha=0.05 by GraphPad analyses.
Quality of Life (QoL), Patient Reported Outcomes (PRO) and G-tube use:
FACT-HN and PRO-CTCAE were evaluated at baseline, week 10, and week 20. Of note, higher FACT scores indicate superior quality of life. The ranges for physical, social and functional were 0–28, emotion is 0–24, and head/neck is 0–40. PRO-CTCAE symptoms were asked as a combination of severity, frequency, and interference items on a 0–4 scale. A composite score (0–3) for each symptom was created combining information from the three items10. G-tube placement “yes/no” and duration of use (weeks) was recorded.
Results:
Demographics:
Between May 2016 and July 2018, we accrued 29 patients. At the time of data cutoff, median follow up time for PFS was 21 months (range 10 months to 40 months). Baseline characteristics describe a population typical for platinum ineligible head and neck cancer (table 1). Median age was 63, and most patients were male. The most common primary contraindication to cisplatin was otopathy, due to pre-existing hearing loss (48.3%) and tinnitus (20.7%). Various anatomic locations were represented. Of 20 patients with oropharynx cancer, 14 were p16 status positive, of whom 8 also had ≤ 10 pack year smoking history. The stage matrix (supplementary table 1) shows the population to advanced stage, consistent with the inclusion criteria requiring stage III or IV disease in the AJCC7 system. The updated AJCC version 8 staging system incorporates site of origin, TNM stage, and HPV status to provide an integrated risk of death. Therefore, we also provided staging of study patients based on AJCC8 with 21/29 patients (72.4%) having stage III or IV disease. Additional patient details are provided in supplementary table 2.
Table 1:
Demographics
| Demographic | Number | Percent |
|---|---|---|
| Age (yrs) | 63.1 (mean) | 39–86 (range) |
| Male | 28 | 96.6% |
| Race | ||
| White | 25 | 86.2% |
| American Indian or | 1 | 3.4% |
| Alaskan native | 3 | |
| Unknown | 10.3% | |
| Smoking Status | ||
| Current | 3 | 10.3% |
| Former | 15 | 51.7% |
| Never | 11 | 37.9% |
| Smoking Pack Yrs (for former/current) | ||
| < 10 pack yrs | 13 | 44.8% |
| ≥ 10 pack yrs | 16 | 55.2% |
| ECOG PFS | ||
| PS0 | 12 | 41.4% |
| PS1 | 17 | 58.6% |
| Reason for cisplatin ineligibility | ||
| Hearing | 14 | 48.3% |
| Tinnitus | 6 | 20.7% |
| Renal function | 5 | 17.2% |
| Diabetes | 2 | 6.9% |
| Neuropathy | 2 | 6.9% |
| CCMI | ||
| 0 | 6 | 20.6% |
| 1 | 6 | 20.6% |
| 2 | 11 | 37.9% |
| 3 | 2 | 6.9% |
| 4 | 3 | 10.4% |
| 5 | 1 | 3.4% |
| Primary Site | ||
| Base of Tongue | 10 | 34.5% |
| Tonsil | 10 | 34.5% |
| Oropharynx only | ||
| P16+ | 14 | |
| <=10pkyr | 8 | |
| >10pkyr | 6 | |
| P16− | 6 | |
| Supraglottic Larynx | 3 | 10.3% |
| Hypopharynx | 2 | 6.9% |
| Unknown primary | 2 | 6.9% |
| Oral tongue | 1 | 3.4% |
| Uvula | 1 | 3.4% |
| AJCC v8 stage | ||
| I | 5 | 17.2% |
| II | 3 | 10.3% |
| III | 11 | 37.9% |
| IVA | 8 | 27.6% |
| IVB | 2 | 6.9% |
| PDL1 MPS | 60 (median) | 0–100 (range) |
| PDL1 MHS | 105 (median) | 0–280 (range) |
| TIL | 3+ (median) | 0–3 (range) |
Toxicities and Peripheral Blood Measurements:
Toxicities were mostly grade 1–2 and consistent with those expected from radiation alone (table 2). The major exception was grade 3–4 lymphopenia, experienced in 17 patients (58.6%). These were not associated with infections typically associated with lymphopenia. Median absolute lymphocyte count (ALC) on C1D1 was .1.5/L (.8–3.3), .3/L (.1–2.1) at the end of radiation, .6/L (.2–4.3) at week 20 and .6/L (.2–2.7) at week 40 (figure 2A).
Table 2:
Toxicities noted in >2 patients or at grade 3–4 in at least 1 patient.
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Radiation dermatitis | 8 | 6 | . | . |
| Dry mouth | 13 | 11 | . | . |
| Oral mucositis | 2 | 15 | 7 | . |
| Dysgeusia | 17 | 6 | . | . |
| Lymphocyte count decreased | 2 | 4 | 15 | 2 |
| Fatigue | 15 | 3 | . | . |
| Nausea | 10 | 1 | 2 | . |
| Dysphagia | 2 | 8 | 2 | . |
| Anemia | 10 | 1 | . | . |
| Weight loss | 6 | 5 | . | . |
| Maculopapular rash | 9 | 1 | . | . |
| Sore throat | 6 | 4 | . | . |
| Decreased WBC | 7 | 3 | . | . |
| Mucosal infection | 6 | 1 | . | . |
| Anorexia | 3 | 1 | 1 | . |
| Increased bilirubin | 5 | . | . | . |
| AST increased | 3 | 1 | . | . |
| Constipation | 3 | 1 | . | . |
| GERD | 4 | . | . | . |
| ALT increased | 2 | 1 | . | . |
| Alkphos increased | 3 | . | . | . |
| Chills | 3 | . | . | . |
| Hoarseness | 3 | . | . | . |
| Lymphedema | 3 | . | . | . |
| Oral pain | 2 | 1 | . | . |
| Pain | 3 | . | . | . |
| Decreased platelet count | 2 | 1 | . | . |
| Dehydration | . | 1 | 1 | . |
| Aspiration | . | . | 1 | . |
| Esophagitis | . | . | 1 | . |
Figure 2:
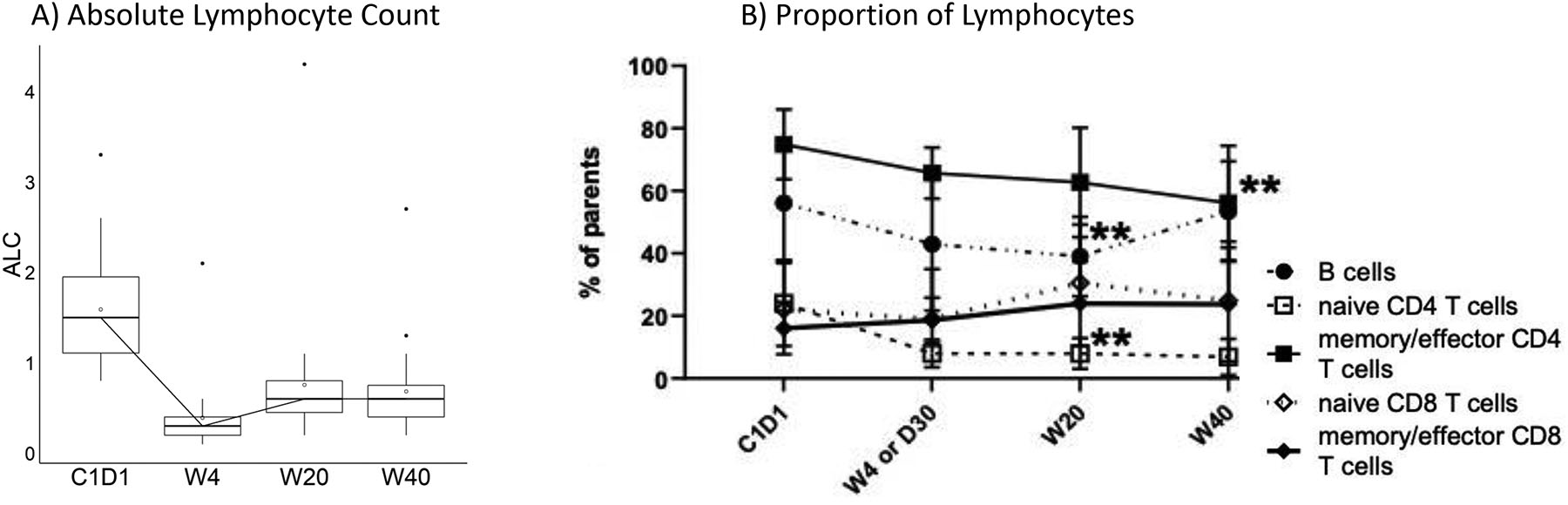
Lymphopenia
A) Absolute lymphocyte count over time
B) Proportions of lymphocytes in periphery. Declined frequencies of memory/effector CD4 T cells at week 40 (p=0.0022); naïve CD4 T cells at week 20 and 40 respectively (p=0.0079 and p=0.0061). B cells were also decreased at week 20 (p=0.0033) though significantly recovered by week 40 (p=0.04) (2-way ANOVA and Tukey’s multiple comparisons tests were performed at α=0.05; error bar=SD).
We further characterized the nature of the lymphopenia through peripheral blood flow cytometry (figure 2B). Memory/effector CD4 T cells, naïve T cells, and B cells declined while CD8 T cells, both memory/effector and naïve were preserved. Within B cells, significant but modest increases were observed in tissue-like memory B cells and transitional B cells while decline was observed in resting memory cells (figure 3B). Within memory/effector T cells, the greatest declines observed, as expected, were in CD4+PD1+ and CD8+PD1+ cells (figure 3C); a significant decline in naïve CD8+PD1 cells was also observed (figure 3D). Although an increase in CD4+CD39+ cells was observed, we note an absence of change in CD4+TIGIT+. Although CD4+CD25+ cell increased from baseline through week 20, with subsequent decline by week 40, we note the absence of significant change in CD4+CD25+CD127−.
Figure 3:
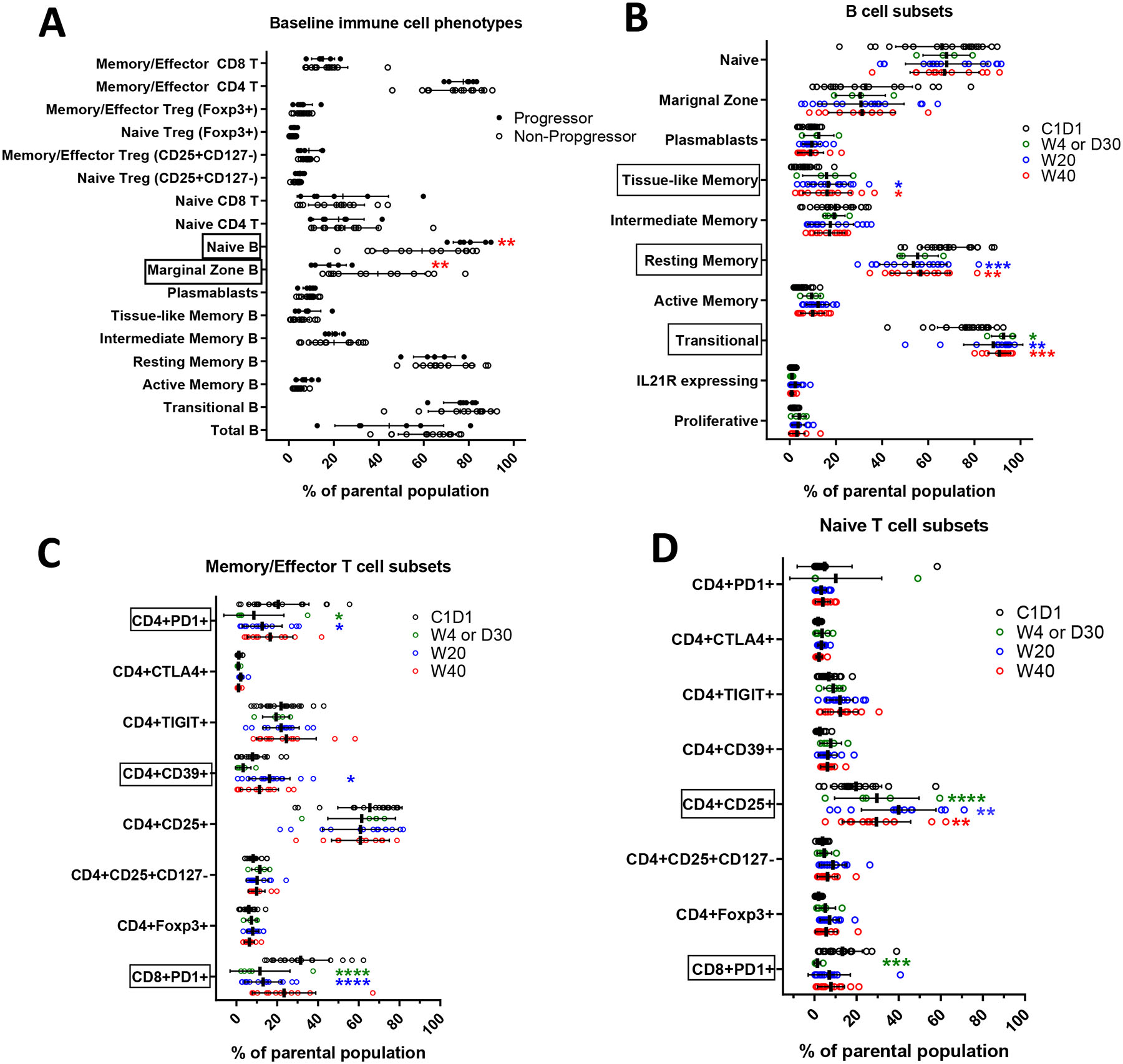
Peripheral Blood Flow Cytometry
A. Comparison of baseline lymphocyte phenotypes between progressors and non-progressors. Patients with progression had greater percentages of baseline naïve B cells (p=0.0077) and fewer marginal zone B cells (p=0.0088) (2-way ANOVA and Sidak’s multiple comparisons were determined by 6 of progressors and 13 of non-progressors; α=0.05; error bar= SD). Patients without progression are represented with open circles and patients with progression in closed circles.
B. B cells subsets post XRT. Significantly increased transitional B cells at week 4 (p=0.0246), week 20 (p=0.0051), and week 40 (p=0.0008); increased tissue-like memory (TLM) B cells at week 20 (p=0.0173) and week 40 (p=0.0398). On the other hand, frequencies of resting memory (RM) B cells significantly declined at week 20 (p=0.0002) and week 40 (p=0.0097) (2-way ANOVA and Tukey’s multiple comparisons tests were performed at α=0.05; error bar=SD).
C. Memory T cells subsets post XRT. PD1 expression was significantly down regulated at week 4 and 20 on both CD4 (p=0.0373 and p=0.0495) and CD8 T cells (p<0.0001). PD1 expression was significantly resumed on CD8 T cells at week 40 when patients were off treatment (p=0.0134). Frequencies of T regulatory cells showed no changes by looking into Foxp3 expression and CD25+CD127− phenotypes. Among all three exhaustion markers (CTLA4, TIGIT, and CD39), CD39 expression on CD4 memory T cells were up regulated at week 20 (p=0.0359) (2-way ANOVA and Tukey’s multiple comparisons tests were performed at α=0.05; error bar=SD).
D. Naïve T cells subsets post XRT. Pembrolizumab showed limited effect on naïve T cells. Decreased PD1 expression was observed on naïve CD8 T cells at week 4 and 20 (p=0.0009 and 0.0178). The percentage of CD25 expression on CD4 naïve T cells was significantly increased at week 20 and week 40 comparing to baseline (p<0.0001 and p=0.0047). Once the patients were off treatment (week 40), the CD25 expression significantly dropped comparing to week 20 (p=0.0031) (2-way ANOVA and Tukey’s multiple comparisons tests were performed at α=0.05; error bar=SD).
Absolute lymphopenia did not predict for progression. In comparing changes in cell types in patients with and without progression (figure 3A), we note that patients with progression had higher baseline naïve B cells and lower circulating marginal zone B cells.
No major changes were seen in peripheral blood cytokines (measured by Luminex) over time (supplementary figure 3) and peripheral blood cytokines did not predict for progression (data not shown).
Clinical Efficacy:
With a median follow-up of 21 months, one year PFS and OS rates were 76% (95% CI 56–88) and 86% (67–95), respectively. Estimated 2-year PFS was 71% (49%–84%) and estimate 2-year OS was 75% (51%–88%). The primary PFS endpoint has exceed the hypothesized median of 16 months and its median has not been reached. (figure 1). PDL1 did not predict for progression. Patients with p16 associated oropharynx cancer had a 1 year PFS of 86% (54–96%) and OS of 93% (59–99%) while others (non-oroparhynx plus p16− oropharynx) had 1 year PFS of 67% (38%-85%) and 1 year OS of 80% (50–93%). In evaluating p16+ oropharynx cancers, patients with ≤10 pack year smoking history had a 1 year PFS of 88% (39%–98%) and 1 year OS of 100% while those with a ≥ 10 pack year smoking history had a 1 year PFS of 83% (27%–97%) and 1 year OS of 83% (27%–97%). Although accrual was defined by AJCC v.7, we re-categorized subjects by AJCC v.8 to provide a measure of outcomes by integrated stage-based and biologic risk. As shown in supplementary figure 4, subjects with lower stage had numerically superior PFS and OS.
Figure 1.
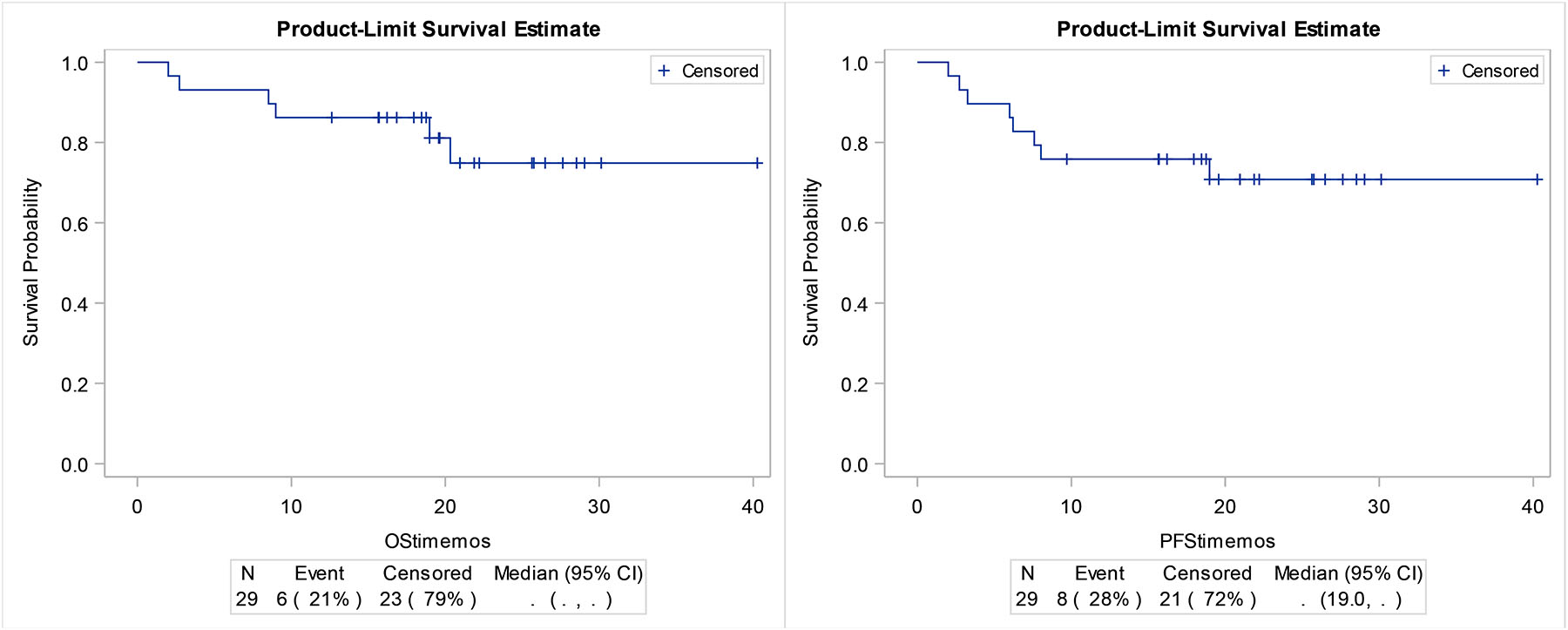
A)PFS and B)OS in the total study population
Quality of Life and Patient Reported Outcomes:
The patient experience was further characterized via FACT-HN and PRO-CTCAE. Key symptoms of pain, decreased appetite, swallowing difficulty, dry mouth, and fatigue climaxed at ten weeks and improved from climax at week 20 (figure 4). A similar time course was seen in FACT subscales including the head and neck subscore.
Figure 4:
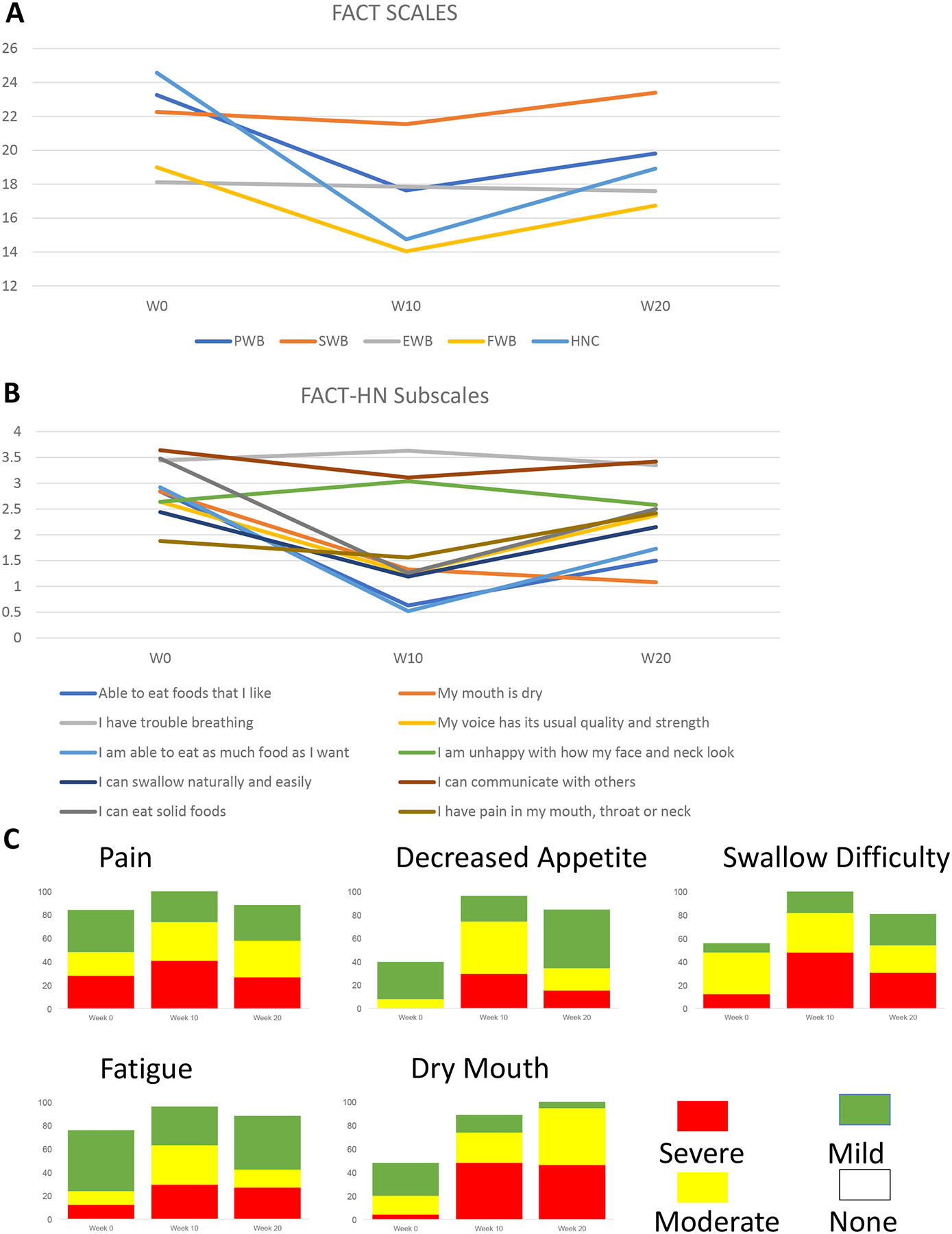
Quality of Life
A: Changes in Global and Functional indexes over time: Global, PWB, SFWB, EWB, FWB, HN
B: Changes in individual HN subscales over time
C: PROs
Of 24 patients with data on G-tube use, 4 (16.7%) had G-tubes placed with a median duration of 50 days. Minimum radiation dose was 69.75Gy; all patients received 70Gy except for one. Only four patients received less than the 6 planned doses of pembrolizumab with 2 patients receiving 5 doses and 2 patients receiving 3 doses. Reasons for treatment discontinuation included adverse events, progression on study, death on study, and patient preference. In patients with progression disease, subsequent therapies included salvage surgery (1), carboplatin/nab-paclitaxel/cetuximab (1) and carboplatin/paclitaxel (1).
Discussion:
In this phase II study of pembrolizumab concurrent with radiation for locally advanced head and neck cancer in cisplatin-ineligible patients, the primary endpoint of a median PFS of at least 16 months was met. To our knowledge, this represents the first published efficacy data on definitive immunoradiotherapy in this population. Treatment was safe with few grade 3–4 events. The most common toxicities, including radiation dermatitis, dry mouth, oral mucositis and dysgeusia are expected from radiation therapy and not worsened by pembrolizumab. We did not observe any immune related adverse events.
The major toxicity observed was lymphopenia. In this study, 59% of patients experienced grade 3–4 lymphopenia. Lymphopenia was not reported with pembrolizumab alone in the keynote-048 study11. When radiotherapy was combined with cetuximab or with cisplatin in the RTOG 1016 study11 the rate of grade 3–4 events was 17% with either regimen, although other studies have shown that the radiation fractionation used in this study can result in severe lymphopenia12,13. Safety data on HPV+ patients in a phase II trial of pembrolizumab, cisplatin and radiotherapy reported a 94% rate of grade 3–4 lymphopenia14. Given that the mechanism of action of pembrolizumab is checkpoint inhibition of T cells, this toxicity is relevant to efficacy, safety, and future directions. We therefore further characterized the lymphopenia through flow cytometry. While B cells and CD4 T cells declined, CD8 T cells were relatively preserved. Peripheral blood flow cytometry sub-categorized changes in lymphocyte subsets over the course of treatment. We observed an increase in tissue-like memory B cells and transitional B cells while resting memory cells declined. In comparing baseline immune phenotypes, we observed more naïve B cells and fewer marginal zone B cells in patients with progression.
The patient perspective on non-hematologic toxicity and on QoL were further characterized with select PRO-CTCAE measures and the FACT-HN subscales. Both measures showed decline at 10 weeks compared to baseline, with incomplete recovery by 20 weeks. These patient-derived outcomes confirm CTCAE results that the acute toxicity of pembrolizumab and radiation is acceptable for the definitive treatment of HNSCC. The absence of long-term data is a weakness of this work and would be of use in future studies.
We acknowledge multiple additional limitations of this study. First, the study is small and non-randomized. We have therefore avoided comparison of efficacy to landmark randomized studies. However, we do believe that the favorable efficacy data observed in this phase II study are sufficient to justify phase III study. The PRO and CTCAE data provide a more comprehensive characterization of the patient experience than is typically provided in small phase II studies. We caution against over interpretation of this data, but note the consistency between toxicity, PRO-CTCAE and FACT and believe that this data will be useful in phase III design considerations.
Similarly, the small sample size of the peripheral blood flow cytometry data and cytokine/chemokine data, together with the absence of tissue studies and functional studies, precludes our ability to define the functional B cells differences observed in peripheral blood. However, given emerging evidence for the role of B cells in the antineoplastic response15, we consider these findings worthy of further study. While neither PDL1 nor cytokine/chemokine profiling of peripheral blood predicted recurrence (the former consistent with results from the PACIFIC16 trial in lung cancer) we again caution against over-interpretation given small patient numbers and encourage their evaluation in larger studies. Third, the use of non-clinical staining conditions and scoring precludes meaningful analyses or interpretation of PDL1 expression; we note only that PDL1+ TILs were observed at high level in most patients. Finally, the optimal number of pembrolizumab cycles was not defined. Currently trials are evaluating one year of maintenance immunotherapy and it is possible that longer treatment could have resulted in superior efficacy.
Multiple clinical studies are already underway to further define the role of immunoradiotherapy in HNSCC. Most relevant to this work is GORTEC-2015-01, which randomizes patients ineligible for cisplatin to radiotherapy with either cetuximab or pembrolizumab. Other future work should investigate immune modulations concurrent with radiation, evaluate optimal sequence and timing, and consider the role of immune cells other than T cells.
Supplementary Material
Translational Relevance:
PD-1 inhibition is an effective treatment against cancer, but efficacy data on concurrent therapy with radiation are lacking. Preclinical data suggest both that radiation can increase sensitivity to immunotherapy and that immunotherapy can sensitize cancer cells to radiation. We conducted a phase II study of concurrent combined therapy with radiation and the PD-1 inhibitor pembrolizumab in patients not eligible for cisplatin. The study met its primary PFS endpoint, in addition to showing acceptable safety profile and favorable preliminary survival. Lymphopenia was common, driven by CD4 T cells and B cells, but not CD8 T cells. Changes in tissue-like memory B cells and resting memory cells were observed and progression was associated with greater percentages of baseline naïve B cells and fewer marginal zone B cells. Phase III study of pembrolizumab with radiotherapy is indicated and further translational work is required to understand the meaning of the B cell signals.
Footnotes
Disclosures:
Jared Weiss discloses consulting honorarium from Astrazeneca, EMD Serono, Genentech, Inivata, Celgene, G1 Therapeutics, Jounce Therapeutics, Abbvie, Rakuten, Nanobiotix, Azitra, Eli Lilly, Blueprint Medicines and Pfizer. Jared Weiss has received research grants (to institution) from Celgene, Pfizer, Merck, Astrazeneca, Amgen, Carefusion, G1 Therapeutics, Immunicum and Loxo/Lilly. Jared Weiss has stock in Nektar.
Mark Weissler discloses stock ownership of in Merck and Pfizer.
Shetal Patel has received research grants (to institution) from Astrazeneca.
Jessica Bauman has received honorarium for consulting from Pfizer, Bayer and Astrazeneca and travel to an investigator meeting from Turning Point Therapeutics.
Benjamin Vincent has equity in and has consulted for Genecentric Therapeutics.
Citations:
- 1.Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet (London, England). 2019. [DOI] [PubMed] [Google Scholar]
- 2.Karam SD, Raben D. Radioimmunotherapy for the treatment of head and neck cancer. Lancet Oncol. 2019;20(8):e404–e416. [DOI] [PubMed] [Google Scholar]
- 3.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74(19):5458–5468. [DOI] [PubMed] [Google Scholar]
- 4.Oweida A, Lennon S, Calame D, et al. Ionizing radiation sensitizes tumors to PD-L1 immune checkpoint blockade in orthotopic murine head and neck squamous cell carcinoma. Oncoimmunology. 2017;6(10):e1356153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deutsch E, Chargari C, Galluzzi L, Kroemer G. Optimising efficacy and reducing toxicity of anticancer radioimmunotherapy. Lancet Oncol. 2019;20(8):e452–e463. [DOI] [PubMed] [Google Scholar]
- 6.Basch E, Rogak LJ, Dueck AC. Methods for Implementing and Reporting Patient-reported Outcome (PRO) Measures of Symptomatic Adverse Events in Cancer Clinical Trials. Clinical therapeutics. 2016;38(4):821–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ivanova A, Qaqish BF, Schell MJ. Continuous toxicity monitoring in phase II trials in oncology. Biometrics. 2005;61(2):540–545. [DOI] [PubMed] [Google Scholar]
- 8.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567–578. [DOI] [PubMed] [Google Scholar]
- 9.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11(1):21–28. [DOI] [PubMed] [Google Scholar]
- 10.Basch EBC, Rogak LJ, Schrag D, Reeve BB, Spears P, Smith ML, Gounder MM, Mahoney MM, Schwartz GK, Bennett AV, Mendoza TR, Cleeland CS, Sloan JA, Abernethy AP, Bruner DW, Schwab G, Atkinson TM, Thanarajasingam G, Bertagnolli MM, Dueck AC. Development of a Composite Scoring Algorithm for the National Cancer Institute’s Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). Paper presented at: 26th Annual Meeting of the International Society for Quality of Life Research. 2019; San Diego, CA. [Google Scholar]
- 11.Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915–1928. [DOI] [PubMed] [Google Scholar]
- 12.Campian JL, Sarai G, Ye X, Marur S, Grossman SA. Association between severe treatment-related lymphopenia and progression-free survival in patients with newly diagnosed squamous cell head and neck cancer. Head & neck. 2014;36(12):1747–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verastegui EL, Morales RB, Barrera-Franco JL, Poitevin AC, Hadden J. Long-term immune dysfunction after radiotherapy to the head and neck area. International immunopharmacology. 2003;3(8):1093–1104. [DOI] [PubMed] [Google Scholar]
- 14.Powell S. Pembrolizumab plus Chemoradiotherapy in HPV-Positive Head and Neck Cancer. Paper presented at: SITC2018
- 15.Griss J, Bauer W, Wagner C, et al. B cells sustain inflammation and predict response to immune checkpoint blockade in human melanoma. Nature communications. 2019;10(1):4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med. 2017;377(20):1919–1929. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


