Abstract
To determine whether lifestyle intervention programs comprising dietary intervention and prescribed, unsupervised exercise improve outcomes for people with metabolic syndrome. A systematic review and meta-analysis of randomised controlled trials. Online databases CINAHL, MEDLINE, PubMed and Embase were searched from the earliest date available to October 2020. Post-intervention data were pooled to calculate mean differences (MD) or standardised mean differences (SMD) and 95% confidence intervals (CI) using inverse variance methods and random effects models. Trial methodological quality was assessed using the Physiotherapy Evidence Database (PEDro) scale and overall quality of each meta-analysis was assessed using the Grading of Recommendation Assessment, Development and Evaluation approach. Eleven studies from 9 randomised controlled trials with 1,835 participants were included. There was high quality evidence that lifestyle intervention programs with unsupervised exercise reduced waist circumference (MD -2.82 cm, 95%CI -5.64 to 0.00, I2 91%) and blood pressure (systolic: MD -3.89 mmHg, 95%CI -5.19 to -2.58, I2 4%; diastolic: MD -3.16 mmHg, 95%CI -4.83 to -1.49, I2 50%) and increased physical activity levels (SMD 0.47, 95%CI 0.24 to 0.70, I2 45%) when compared to usual care. There was low quality evidence that they improved quality of life (SMD 0.59, 95%CI 0.05 to 1.13, I2 84%). Unsupervised programs had no significant effect on fasting blood glucose (unless > 3 months duration), metabolic syndrome prevalence or cholesterol. Lifestyle intervention programs with prescribed, unsupervised exercise are a practical alternative to supervised programs for people with metabolic syndrome when time, access or resources are limited or when social distancing is required.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11154-021-09644-2.
Keywords: Metabolic syndrome, Lifestyle intervention, Exercise, Diet
Introduction
Metabolic syndrome is an increasingly prevalent condition worldwide [1] characterised by a clustering of risk factors including abdominal obesity, hypertension, impaired glucose tolerance and dyslipidaemia. Metabolic syndrome is associated with increased healthcare costs [2], a 1.5-fold increase in all-cause mortality [3], a twofold increase in risk of cardiovascular disease and a fivefold increase in risk of type 2 diabetes [4].
A recent systematic review found low to moderate quality evidence that multi-disciplinary lifestyle modification programs including both dietary intervention and supervised exercise are effective in managing the individual risk factors for metabolic syndrome and reducing prevalence of metabolic syndrome [5]. However, multidisciplinary lifestyle intervention programs with supervised exercise components are resource-intensive and may not be cost-effective in the long-term [6]. These programs can be difficult to access for people living in rural or remote areas and people without ready access to transport and, of great consequence at this critical moment due to the global COVID-19 pandemic, these programs cannot be conducted due to social distancing requirements. Lifestyle interventions with unsupervised exercise components could be considered a worthwhile alternative as they can be administered from flexible locations (e.g. home based or in a primary care clinic, via telephone or online service) and may be more accessible and cost-efficient.
There is conflicting evidence when comparing unsupervised exercise interventions to supervised exercise for people with other chronic diseases. For people with diabetes [7] and peripheral vascular disease [8], supervised exercise appears to be superior. However unsupervised, home-based training may be equally or more beneficial for people with chronic obstructive pulmonary disease [8] and older adults with metabolic risk factors such as obesity and hypertension [9]. To date, no review has analysed the effect of lifestyle interventions programs with only unsupervised exercise components in metabolic syndrome populations. Therefore, the aim of this systematic review was to determine whether multi-factorial (diet and exercise) lifestyle intervention programs that include only unsupervised exercise improve outcomes for people with metabolic syndrome when compared to usual care.
Method
This review was prospectively registered with the PROSPERO database of systematic reviews (CRD42020157091) and is reported according to PRISMA guidelines [10].
Information sources, search, and study design
The search strategy was based on key words and MeSH headings related to the two main constructs of metabolic syndrome and lifestyle intervention (Appendix A). Four databases were searched from the earliest date possible until October 2020: CINAHL, MEDLINE, PubMed and Embase. Additional searches were conducted by scanning the reference lists and citations (via Google Scholar) of included trials to ensure all relevant trials were identified.
Two authors independently screened titles and abstracts using pre-defined inclusion and exclusion criteria to determine which articles could be conclusively excluded. All other articles were obtained in full text for further evaluation. The same process was followed independently on full-text articles to determine which trials would be included in the final review. Authors then met to discuss any discrepancies until consensus was reached. If consensus was not reached, the third reviewer was consulted. Agreement between authors was assessed using the kappa statistic where a kappa of 0.21 to 0.40 indicates fair agreement; 0.41 to 0.60 is moderate agreement; 0.61 to 0.80 is substantial agreement; and 0.81 to 0.99 shows almost perfect agreement [11].
Inclusion and exclusion criteria
To be eligible for the review, trials had to be randomised controlled trials published in English, that evaluated the effect of a lifestyle intervention program with an unsupervised exercise component on outcomes for adults (18 + years) with metabolic syndrome. Accepted definitions of metabolic syndrome included the International Diabetes Federation [12] and the National Cholesterol Education Program criteria [13] as well as country-specific adaptations. Essentially, the diagnosis of metabolic syndrome needed to be based on the participant having at least 3 of the 5 metabolic risk factors: central obesity, impaired fasting glucose, hypertension, high triglycerides and/or low high-density lipoprotein cholesterol.
For the purpose of this review, lifestyle interventions were those that included both diet and exercise interventions at a minimum but could include other interventions such as counselling, stress management, smoking cessation etc. Because we were interested in less resource-intensive interventions, only those with unsupervised exercise components were included, such as education and advice to exercise, behaviour change and telehealth interventions to promote exercise and physical activity. Education, counselling and other components of the intervention could be delivered face-to-face as long as the exercise component was unsupervised. Control group participants could have received usual care, no treatment or general lifestyle advice. Trials were excluded if not all participants had metabolic syndrome, if the intervention included supervised exercise or was not multifactorial (i.e. did not include both diet and exercise).
Methodological quality / risk of bias
Trails were assessed for risk of bias using the valid and reliable 11-item Physiotherapy Evidence Database (PEDro) scale [14, 15]. This scale assesses whether the trial reports eligibility criteria, random allocation, concealed allocation, similarity at baseline, participant blinding, therapist blinding, assessor blinding, > 85% retention, intention-to-treat analysis, between-group statistical comparisons and point measures, and measures of variability [14]. A total score is given out of 10 (as the first item is not scored) with a higher score indicating that more criteria were satisfied. Trials were assessed independently by two reviewers using the PEDro scale. Agreement was recorded and discrepancies were resolved through discussion between the two reviewers until consensus was reached. Because it is not possible to blind participants and therapists in lifestyle intervention programs the maximum total score achievable was 8 out of 10. A score of < 4 was considered low quality, 4–6 was moderate quality and ≥ 7 was high quality [5].
Data extraction
A previously developed data extraction form based on the Cochrane Collaboration template was used [5]. One reviewer extracted data on study design (methods, setting, quality), participant characteristics (age, sex, metabolic risk factors), interventions (type, content, frequency, duration, method of delivery), control group conditions (usual care, general advice), outcomes assessed, results and adverse events. The second reviewer checked the data extraction forms for accuracy and any discrepancies were resolved by referring back to the trial report.
Outcomes of interest
Primary outcomes were related to metabolic risk factors (central obesity, fasting glucose, hypertension, triglycerides, high-density lipoprotein cholesterol) and presence or absence of metabolic syndrome. Secondary outcomes included other measures of behaviour change, physical function, quality of life and adverse events.
Data analysis
Mean differences (MD) and/or standardised mean differences (SMD) and 95% confidence intervals (CI) were calculated using post-intervention means and standard deviations using RevMan 5 [16]. Meta-analyses were conducted using random-effects models and inverse variance methods using Hedges’ g. Strength of the SMD was reported according to Cohen (1962) where 0.2 is considered a small effect, 0.5 a moderate effect and 0.8 a large effect [17]. Risk Ratios (RR)and 95% CIs were calculated using random-effects models to determine differences in prevalence of metabolic syndrome. Statistical heterogeneity was assessed using the I2 statistic with an I2 value > 50% representing significant heterogeneity [18]. Where significant heterogeneity was present in a meta-analysis, sensitivity analyses were conducted to determine the source of heterogeneity and confirm results.
Risk of bias across trials
The quality of the body of evidence in each meta-analysis was determined by applying the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach [19]. Because all included trials were randomised controlled trials, the quality rating starts at ‘high’. The quality of each meta-analysis is then downgraded or upgraded depending on the pre-determined criteria. Evidence was downgraded one place if there was evidence of: 1. Risk of bias (majority of trials scored < 6 on PEDro); 2. Unexplained inconsistency (I2 > 50%); 3. Indirectness in intervention or outcome; 4. Imprecision of results (wide 95%CI > 0.8 for SMD and > minimal clinically important difference for MD); or 5. Publication bias (visual inspection of funnel plots when there were at least 10 trials in the meta-analysis). Evidence was downgraded two places if the majority of trials scored ≤ 4 on the PEDro scale and was upgraded one place if the effect size was large (SMD ≥ 0.8 or MD > clinically important difference). Meta-analyses were subsequently graded as representing high-, moderate-, low-, or very low-quality evidence.
Results
Study selection
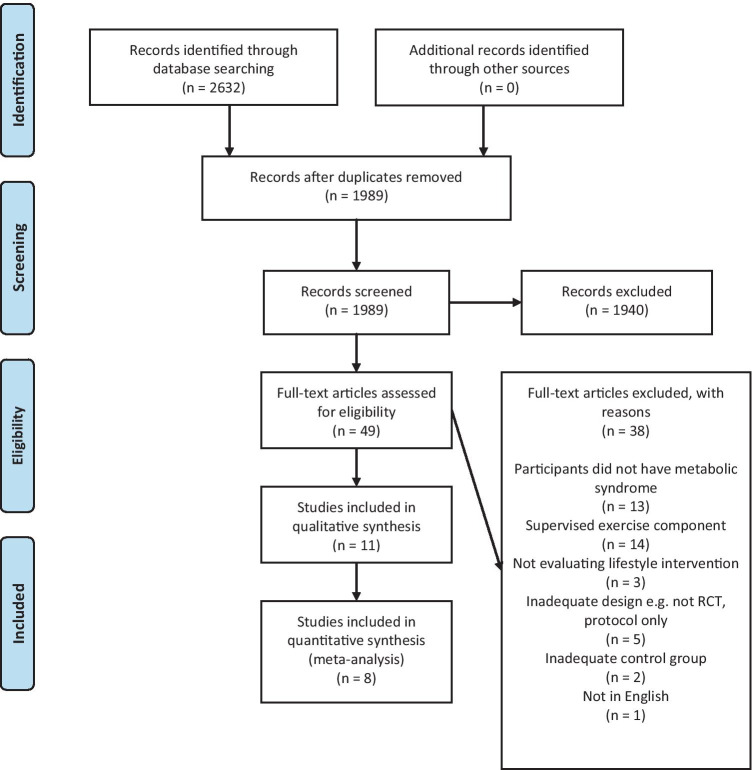
Searching identified 2,632 potentially eligible studies. After removal of duplicates, 1,989 were screened on title and abstract independently by two reviewers. Of these, 1,940 were excluded and 49 were evaluated in full-text. Agreement between reviewers was substantial (kappa 0.74, 95%CI 0.63 to 0.85). After evaluation of full-text, 11 papers from 9 randomised controlled trials were included for review (Fig. 1) [20–30]. Four published papers [24, 25, 27, 30] presented results from two trials. Two papers by Jahangiry et al. [24, 25] report data from the Red Ruby Study with one reporting metabolic outcomes [24] and the second reporting behaviour change and quality of life outcomes [25]. In another pair of papers, Wang et al. [27] reported metabolic outcomes and Zheng et al. [30] reported cardiovascular risk, self-efficacy and behaviour change outcomes from the same trial. The first papers published (which report metabolic outcomes) [24, 27] are considered the primary trials throughout.
Fig. 1.
Flow of trials through the review
Study characteristics
The mean PEDro score of the included trials was 6.1 out of 10, ranging from 5 [23] to 7 [27, 29] (Table 1, Appendix B). All included trials were randomized controlled trials but only three reported concealment of allocation [24, 27, 29], six reported intention-to-treat analysis [21–24, 26, 27] and all reported between group differences. Overall, seven trials were categorized as being moderate quality (scoring 4–6) and two were considered high (scoring ≥ 7) [27, 29]. Agreement between reviewers when assessing quality was substantial (kappa 0.78, 95%CI 0.66 to 0.91).
Table 1.
Study characteristics
| Study (country) |
Population | PEDro Score | Participants (Int: Con) |
Male: Female (Int/Con) |
Age range (mean ± SD) | Outcomes | Metabolic syndrome criteria used | Timing of outcomes |
|---|---|---|---|---|---|---|---|---|
| Avram et al. [20] (Romania) | Otherwise healthy adults < 80y/o | 6 | 133:120 | 82:51 / 77:43 |
Int: 56 ± 8 Con: 57 ± 8 |
Behaviour: Physical activity, dietary intake | NCEP-ATP III |
Baseline 18 months |
| Chirinos et al. [21] (USA) | Low-income, minority adults 30 -70 y/o | 6 | 60:60 | 30:30 / 23:37 |
Int: 53 ± 8 Con: 51 ± 9 |
Physical: Waist circumference, cholesterol, blood pressure, fasting glucose, weight, prevalence of metabolic syndrome, biomarkers | NCEP-ATP III |
Baseline 6 months 12 months |
| Fappa et al. [22] (Greece) | Otherwise healthy patients attending a lipid clinic | 6 | 29:29 | 50:37 (total) | 49 ± 12 (total) |
Physical: Waist circumference, cholesterol, blood pressure, fasting glucose, weight, prevalence of metabolic syndrome Behaviour: dietary intake, physical activity |
NCEP-ATP III |
Baseline 6 months |
| Gomez-Huelgas et al. [23] (Spain) | Volunteers from an epidemiological study, 18—80 y/o | 5 | 298:303 | 165:133 / 166:137 |
Int: 54 ± 114 Con: 54 ± 14 |
Physical: Waist circumference, cholesterol, blood pressure, fasting glucose, weight, prevalence of metabolic syndrome Behaviour: dietary intake, physical activity Psychological: quality of life |
IDF 2005 |
Baseline 3 years |
| Jahangiry et al. [24] & [25] (Iran) | Volunteers ≥ 20 y/o who registered on a website | 6 | 80:80 | 50:30 / 56:24 |
Int: 43 ± 10 Con: 45 ± 10 |
Physical: Waist circumference, cholesterol, blood pressure, fasting glucose, weight, prevalence of metabolic syndrome Behaviour: dietary intake, physical activity Psychological: quality of life |
NCEP-ATP III |
Baseline 3 months 6 months |
| Nanri et al. [26] (Japan) | Male employees from one company | 6 | 49:53 | 100% male |
Int: 54 ± 6 Con: 53 ± 7 |
Physical: Waist circumference, cholesterol, blood pressure, fasting glucose, weight, prevalence of metabolic syndrome Behaviour: dietary intake, physical activity |
Japanese adaptation of IDF/NCEP-ATP# |
Baseline 6 months |
| Wang et al. [27] & Zheng et al. [30] (China) | Previously hospitalized adults ≥ 18 y/o | 7 | 86:87 | 40:46 / 45:42 |
Int: 55 ± 11 Con: 56 ± 10 |
Physical: Waist circumference, cholesterol, blood pressure, fasting glucose, weight, prevalence of metabolic syndrome Psychological: depression, health-related quality of life Behaviour: self-efficacy, health responsibility |
IDF 2005 |
Baseline 1 month 3 months |
| Zhang et al. [28] (China) | Otherwise healthy faculty members | 6 | 153:153 | 63:90 / 65:88 |
Int: 56 ± 6 Con: 56 ± 6 |
Physical: Waist circumference, cholesterol, blood pressure, fasting glucose, weight, prevalence of metabolic syndrome Behaviour: dietary intake, physical activity |
NCEP-ATP III |
Baseline 12 months |
| Zhang et al. [29] (China) | Volunteers ≥ 18y/o | 7 | 31:31 | 13:18 / 14:17 |
Int: 58 ± 5 Con: 57 ± 5 |
Physical: Waist circumference, triglycerides, blood pressure Behaviour: dietary intake, physical activity Psychological: health-related quality of life |
IDF 2005 |
Baseline 12 weeks |
int intervention, con control, y/o years old, IDF International Diabetes Federation, NCEP-ATP National Cholesterol Education Program-Adult Treatment Panel
# Japanese metabolic syndrome criteria, waist circumference ≥ 85 cm plus 2 or more, fasting blood glucose ≥ 110 mg/dl and/or on medications, HDL-cholesterol < 40 mg/dl and/or triglycerides > 15 mg/dl or on medications; systolic blood pressure > 130 and/or diastolic blood pressure > 85 or on antihypertensives
Participants
Included trials comprised 1,835 participants (45% female) of whom 919 participated in a lifestyle intervention program with unsupervised exercise. Five trials were conducted in Asia [24, 26–29], three in Europe [20, 22, 23] and one in North America [21] (Table 1). Metabolic syndrome was diagnosed according to NCEP-ATP III criteria in 5 trials, IDF criteria in 3 and the Japanese adaptation of these in one trial [26].
Intervention
All trials evaluated the effects of lifestyle intervention programs which primarily comprised dietary intervention and unsupervised exercise. Interventions contained mostly education and advice on healthy lifestyle change [20, 21, 23, 24, 27] and in four trials, interventions were based on behaviour change principles using counselling techniques [22, 26, 28, 29]. Interventions ranged in duration from three months [27, 29] to three years [23] and varied in intensity from one session every three months [28] to two sessions per week [29]. In one trial the intervention was delivered entirely online [24], two trials utilised both face-to-face education/counselling and telephone interventions [20, 27] and the remainder delivered face-to-face education/counselling. None of the interventions included supervised exercise. Most trials held individual sessions; two trials were comprised solely of group sessions [21, 29] and two trials utilised both group and individual sessions [23, 28]. Interventions were most commonly delivered by medical practitioners [20, 23, 28, 29], nurses [26, 27] and dietitians [22, 24]. In some trials, medical practitioners worked in collaboration with nurses [23], nutritionists [28], psychologists [29], physiotherapists [20] and diabetes specialists [20].
All intervention group participants were provided with education and advice on exercise prior to commencing unsupervised exercise. Exercise interventions included general education on exercise [24], advice to increase exercise [20], prescribed walking [21, 23, 28, 29] and individualised goal setting [22, 26, 27]. In three trials [21, 26, 27] participants were also encouraged to self-monitor their daily physical activity.
In all but one trial [27] the dietary components focussed primarily on weight loss. The Mediterranean diet was utilised in two trials [22, 23]. Comparison groups received general advice and written information (Table 2).
Table 2.
Intervention Characteristics
| Study | Intervention (delivered by) | Duration and frequency of sessions | Exercise component | Dietary component | Comparison |
|---|---|---|---|---|---|
| Avram et al. [20] |
Lifestyle counselling program for weight reduction (GP) |
18 months 30-min GP visits (6-monthly) Monthly phone calls (3 face-to-face sessions, 18 phone calls) |
Advice to increase daily physical activity (GPs advised by physiotherapists) | Emphasis on weight loss, decreasing fat intake, portion control and healthy food (GPs advised by dietitians) | One-page written information on the importance of a healthy lifestyle |
| Chirinos et al. [21] | Enhanced lifestyle intervention program of education (‘clinicians’) |
12 months 90-min group face-to-face sessions × 8 in the first 3 months then monthly (17 group sessions) |
Unsupervised brisk walking progressing to 30 min, 5 × week by week 5. Self-monitored using pedometers | Changing dietary habits to achieve weight reduction through calorie restriction | Standard care: laboratory results provided at each timepoint. Lifestyle modification advice at baseline and 6 months by their medical provider |
| Fappa et al. [22] | Lifestyle intervention based on motivational and behaviour strategies and goal setting (dietitian) |
6 months 60-min individual face-to-face counselling every 2 weeks for 2 months then monthly for 4 months (7 individual sessions) |
Individualized physical activity goal setting | Hypocaloric Mediterranean-style diet | Instructions regarding hypocaloric Mediterranean-style diet and physical activity goals at initial assessment only |
| Gomez-Huelgas et al. [23] | Long-term lifestyle intervention program (GP and nurse) |
3 years 15–30-min individual and group face-to-face. 6 visits in first 3 months, then once every 3 months for the remainder of the 1st year, then 6-monthly (20 individual, 7 group sessions) |
Education about exercise. Aiming for a minimum of 150 min/week of walking | Mediterranean diet plus calorie restriction for those who were overweight. Education on food and practical concepts related to cooking and shopping | General advice on heart-healthy diet and exercise. 4 × 10-min nursing and 4 × 10-min medical appointments |
| Jahangiry et al. [24] & [25] | Interactive web-based lifestyle educational program (dietitian) |
6 months Online, individually led (mean 5 logins per person) |
Education on exercise | Education and calorie-restricted diet tailored by a dietitian | Email with encouragement to make appropriate diet and activity changes every 3 weeks |
| Nanri et al. [26] | Lifestyle behavioural modification program based on behavioural theory (nurse) |
6 months Face-to-face individual sessions at baseline, 1 month and 3 months (3 individual sessions) |
Exercise goal setting and self-monitoring walking using pedometers | Goal setting for weight loss and aims to increase fruit, vegetable and dairy intake and limit alcohol | Standard health guidance at baseline only |
| Wang et al. [27] & Zheng et al. [30] | Lifestyle intervention program based on health promotion model (nurse) |
3 months 1 × 30–40-min face-to-face session followed by 6 bi-weekly 20–30-min telephone calls (1 face-to-face session, 6 phone calls) |
Education, assessment of behaviour, advice on regular exercise, making behaviour modification plans and self-monitoring | Education, assessment of behaviour, advice on healthy diet, making behaviour modification plans and self-monitoring | Usual care and 10-min brief discharge advice |
| Zhang et al. [28] | Lifestyle intervention program utilizing behavioural counselling (doctor) |
1 year 5 × 60-min face-to-face sessions (1 individual, 4 group sessions) |
Individualized advice suggesting moderate exercise (e.g. brisk walking) for 150 min/week | Individually prescribed diet based on best practice weight loss | General verbal information on healthy lifestyle |
| Zhang et al. [29] | Lifestyle intervention using patient-centered cognitive behavioural therapy (doctor and psychologist) |
12 weeks 90–120-min group workshops 2 × week for 12 weeks (24 group sessions) |
Guided and encouraged to adopt and maintain 150 min of moderate exercise per week. Walking was encouraged | Guided and encouraged to adopt a 200–300 kcal reduction in daily dietary calories. Eat less fat and more fruit and vegetables | Written basic lifestyle advice and general information on risk factors plus weekly text messages about standard care |
GP general practitioner
Outcome measures
Triglycerides and systolic blood pressure were measured in eight trials and HDL-cholesterol, diastolic blood pressure and fasting blood glucose were measured in seven trials (Table 1). Anthropometric measures of waist circumference were reported in seven trials and body weight in five trials. The prevalence of metabolic syndrome following intervention was reported in five trials, whilst quality of life was measured in four trials and behaviour change in relation to physical activity and diet were reported in eight trials (Table 1). Presence or absence of adverse events were not reported in any trials.
Metabolic outcomes
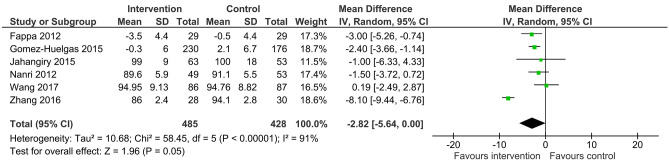
When compared to usual care, six of the included trials with 913 participants revealed high quality evidence that lifestyle intervention with unsupervised exercise reduced waist circumference by a mean of 2.82 cm (95%CI -5.64 to 0.00, I2 91%) (Fig. 2). There was a high degree of heterogeneity in the analysis. When two trials with the shortest duration intervention (3 months) [27, 29] were removed in a sensitivity analysis, results remained similar and heterogeneity was reduced (MD -2.29 cm, 95%CI -3.26 to -1.32, I2 0%).
Fig. 2.
Mean difference (95% confidence interval) for the effect of unsupervised lifestyle intervention programs on waist circumference
There was high quality evidence from four trials with 797 participants that lifestyle intervention with unsupervised exercise had no effect on body weight (MD -0.94 kg, 95%CI -2.49 to 0.60, I2 19%) (Table 3).
Table 3.
GRADE risk of bias within meta-analyses
| Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Rating | ||
|---|---|---|---|---|---|---|---|
|
Waist circumference 6 RCTs (n = 913) |
MD -2.82 cm, 95%CI -5.64 to 0.00, I2 91% | 0 | 0 | 0 | 0 | 0^ | 4—high |
|
Weight 4 RCTs (n = 797) |
MD -0.94, 95%CI -2.49 to 0.60, I2 19% | 0 | 0 | 0 | 0 | 0^ | 4—high |
|
HDL cholesterol 6 RCTs (n = 1160) |
SMD 0.07, 95%CI -0.05 to 0.18, I2 0% | 0 | 0 | 0 | 0 | 0^ | 4—high |
|
Triglycerides 7 RCTs (n = 1219) |
SMD -0.39, 95%CI -0.80 to 0.02, I2 91% | 0 | 0 | 0 | -1+ | 0^ | 4—high |
|
Systolic blood pressure 7 RCTs (n = 1219) |
MD -3.89 mmHg, 95%CI -5.19 to -2.58, I2 4% | 0 | 0 | 0 | 0 | 0^ | 4 – high |
|
Diastolic blood pressure 6 RCTs (n = 1161) |
MD -3.16 mmHg, 95%CI -4.83 to -1.49, I2 50% | 0 | 0 | 0 | 0 | 0^ | 4 – high |
|
Fasting glucose 6 RCTs (n = 1161) |
SMD -0.13, 95%CI -0.35 to 0.09, I2 68% | 0 | 0 | 0 | 0 | 0^ | 4—high |
|
Prevalence 5 RCTs (n = 974) |
RR 0.8, 95%CI 0.62 to 1.03, I2 93% | 0 | -1# | 0 | 0 | 0^ | 3—moderate |
|
Quality of Life 3 RCTs (n = 391) |
SMD 0.59, 95%CI 0.05 to 1.13, I2 84% | 0 | -1# | 0 | -1+ | 0^ | 2—low |
|
Physical activity 3 RCTs (n = 668) |
SMD 0.47, 95%CI 0.24 to 0.70, I2 45% | 0 | 0 | 0 | 0 | 0^ | 4 – high |
|
Energy intake 4 RCTs (n = 930) |
SMD -0.10, 95%CI -0.23 to 0.03, I2 0% | 0 | 0 | 0 | 0 | 0^ | 4—high |
NB 0 not downgraded
#Downgraded one place due to unexplained heterogeneity,
+ Downgraded one place due to wide confidence interval,
^ Funnel plots not completed due to < 10 studies in meta-analysis
There was high quality evidence that lifestyle intervention with unsupervised exercise had no effect on HDL-cholesterol when compared to usual care in six trials with 1,160 participants (SMD 0.07, 95%CI -0.05 to 0.18, I2 0%) or triglycerides in seven trials with 1,219 participants (SMD -0.39, 95%CI -0.80 to 0.02, I2 91%) (Table 3). There was a high degree of heterogeneity in the analysis of triglycerides. When the trials with the shortest intervention duration [27, 29] were removed in a sensitivity analysis, there was no change in the results; findings remained non-significant and heterogeneity was reduced (SMD -0.12, 95%CI -0.29 to 0.04, I2 34%).
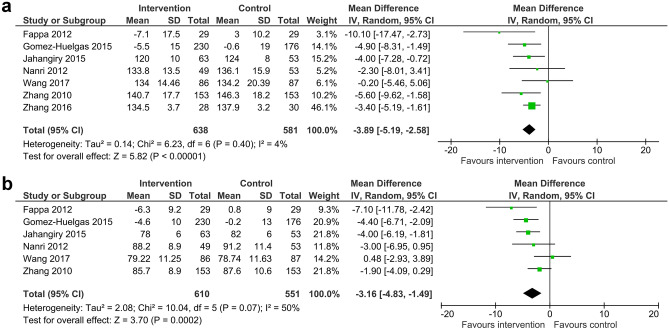
Meta-analysis of systolic blood pressure included seven trials (1,219 participants) and diastolic blood pressure included six trials (1,161 participants). There was high quality evidence to suggest that lifestyle intervention programs with unsupervised exercise reduced systolic blood pressure by 3.89 mmHg (95%CI -5.19 to -2.58, I2 4%) (Fig. 3a) and diastolic blood pressure by 3.16 mmHg (95%CI -4.83 to -1.49, I2 50%) (Fig. 3b).
Fig. 3.
a Mean difference (95% confidence interval) for the effect of unsupervised lifestyle intervention programs on systolic blood pressure. b Mean difference (95% confidence interval) for the effect of unsupervised lifestyle intervention programs on diastolic blood pressure
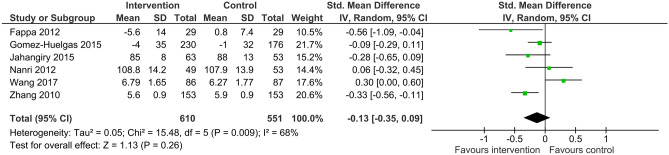
In six trials with 1,161 participants, lifestyle intervention programs with unsupervised exercise did not change fasting glucose levels when compared to usual care (SMD -0.13, 95%CI -0.35 to 0.09, I2 68%) (Fig. 4). On sensitivity analysis, where the trial with the shortest duration [27] was removed, heterogeneity was reduced and meta-analysis indicated there was high quality evidence that unsupervised lifestyle intervention reduced fasting glucose by a small amount (SMD -0.21, 95%CI -0.38 to -0.06, I2 37%).
Fig. 4.
Standardised mean difference (95% confidence interval) for the effect of unsupervised lifestyle intervention programs on fasting blood glucose levels
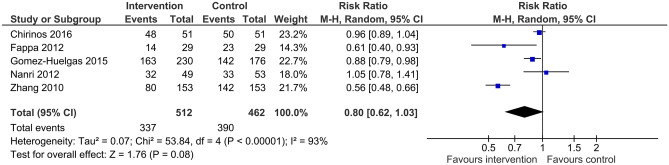
Prevalence of metabolic syndrome at follow-up was reported in five trials with a total of 974 participants. Based on this data, there is moderate quality evidence that when compared to usual care, lifestyle intervention with unsupervised exercise did not significantly reduce the prevalence of metabolic syndrome (RR 0.8, 95%CI 0.62 to 1.03, I2 93%) (Fig. 5). There was a large degree of unexplained heterogeneity in the data.
Fig. 5.
Risk ratio (95% confidence interval) for the effect of unsupervised lifestyle intervention programs on prevalence of metabolic syndrome
Behaviour change
All but one trial [21] reported lifestyle behaviour change outcomes relating to diet, alcohol intake and physical activity levels. Four trials [22–24, 28] reported on behaviour change in energy intake in 930 participants with high quality evidence that lifestyle intervention with unsupervised exercise did not change self-reported energy intake (SMD -0.10, 95%CI -0.23 to 0.03, I2 0%) (Table 3). Significant reduction in alcohol intake and intake of cereals, sugars and sweeteners were reported by intervention group participants in one trial [26]. Another trial reported dietary targets in relation to fruit, vegetable and saturated fat intakes were more often achieved by the intervention group when compared to control [29].
In relation to change in physical activity levels, three trials [23, 24, 26] with 668 participants reported subjective physical activity levels using questionnaires, with high quality evidence that lifestyle intervention programs with unsupervised exercise increased physical activity levels by a moderate amount (SMD 0.47, 95%CI 0.24 to 0.70, I2 45%) (Table 3). Two additional trials reported that those in the intervention groups were significantly more active than control group participants on completion of the lifestyle intervention program [20, 29]. Intervention group participants in one trial reported significant increases in self-efficacy and participation in health promoting behaviours related to diet and exercise compared to control group participants [30].
Quality of life
Quality of life was reported in four trials. One trial utilised a quality of life scale specific to weight loss in people with obesity and found small but significant (p = 0.01) differences in quality of life favouring the intervention group at three years [23]. The remaining three trials used the SF-12 [27] and the SF-36 [24, 29] to measure quality of life. There was low quality evidence from three trials with 391 participants that lifestyle intervention programs with unsupervised exercise improved quality of life by a moderate amount (SMD 0.59, 95%CI 0.05 to 1.13, I2 84%) (Table 3). There was a large degree of unexplained heterogeneity in the data.
Discussion
This systematic review including nine randomised controlled trials with 1,835 participants, found high quality evidence to support the use of lifestyle intervention programs with unsupervised exercise to reduce waist circumference, systolic blood pressure, diastolic blood pressure and to increase daily physical activity levels in people with metabolic syndrome. The review also found low quality evidence that the programs improved quality of life. Unsupervised programs had no significant effect on body weight, cholesterol, fasting blood glucose (unless > 3 months duration), metabolic syndrome prevalence or energy intake. Adverse events were not reported.
Our meta-analysis reports clinically significant findings in relation to some of the individual components of the metabolic syndrome. With reference to previous research showing that for every 1 cm increase in waist circumference there is a 2% increase in risk of cardiovascular events [31], the 3 cm reduction in waist circumference reported in this meta-analysis is likely to be clinically significant. Reductions in blood pressure reported in this analysis (-4 mmHg systolic; -3 mmHg diastolic) compare favourably with those caused by blood pressure lowering medication, which have been linked to a decrease in total risk of cardiovascular events [32]. Based on these findings, it is likely that lifestyle intervention programs with unsupervised exercise will result in clinically meaningful reductions in waist circumference and blood pressure for people with metabolic syndrome.
Significant changes in cholesterol levels were not found in this review. Previous reviews also found no improvements in HDL-cholesterol following lifestyle intervention for people with metabolic syndrome [5, 33]. The absence of improvement in HDL-cholesterol in the current review may be attributed to the fact that the baseline mean HDL-cholesterol levels of the majority of participants were already within normal ranges or that exercise was not of sufficient volume [34]. Previous reviews found significant reductions in triglyceride levels following lifestyle interventions [5, 33], however, there was no significant change to participants’ triglyceride level in the current review despite the fact participants were above threshold levels at baseline. Research has previously shown that exercise intensity has to be sufficiently high to change triglyceride levels [35]. It is possible the unsupervised exercise was of insufficient intensity to instil these changes as the focus was mostly on duration and not intensity in the included studies. The previous reviews included exercise components that were mostly supervised [5, 33].
When comparing the results of the current review that focuses on lifestyle interventions with unsupervised exercise to our previous systematic review of lifestyle interventions with supervised exercise [5], improvements appear to be smaller in magnitude for unsupervised programs (Appendix C). On meta-analysis of interventions with a supervised exercise component, prevalence, fasting blood glucose, weight and triglyceride levels were significantly reduced and the magnitude of improvements in waist circumference, systolic blood pressure and quality of life were greater [5]. The reviews were of similar size and included similar dietary components primarily focusing on weight loss. There are, however, two noticeable differences between the exercise interventions: supervision and mode of exercise.
Although both supervised and unsupervised exercise are beneficial when compared to no exercise, supervised exercise appears to have a more widespread and pronounced impact on metabolic and anthropometric outcomes in populations with similar characteristics to metabolic syndrome. A recent systematic review for people with type 2 diabetes found that supervised exercise was superior to unsupervised exercise for glycaemic control and weight loss [7]. Stefanov and colleagues [36] found that although unsupervised exercise did improve cardiometabolic risk factors in overweight adults, improvements were greater following supervised exercise. A possible rationale for the improved outcomes is that under supervision, exercises and exercise intensity can be monitored, progressed and adhered to appropriately.
Mode of exercise may have also impacted on outcomes of the reviews. Over 50% of the trials (six out of 10) in our previous review [5] of supervised exercise interventions utilised combined (aerobic and strengthening) circuit-based training. The previous meta-regression analysis identified that a single mode intervention such as walking (n = 3) may have been less effective than combined training at producing significant differences between groups [5]. In this current review, over 50% of the included studies (five out of nine) specified walking-based interventions with the remainder reporting non-specific exercise that likely included walking. Whilst walking at a moderate intensity is just as effective as other exercise to increase physical activity levels and decrease risk of cardiovascular events in sedentary healthy adults [37], there is convincing evidence to support the use of combined exercise programmes for the prevention and management of Type 2 diabetes [7, 38] and to improve anthropometric outcomes and cardiovascular disease risk factors in overweight and obese adults [39, 40]. Due to the close association between diabetes, obesity and the metabolic syndrome, combined training may also be more clinically effective than single mode interventions for people with metabolic syndrome. Of course, there may be many other possible contributing factors to the difference in results, however the inclusion of supervised group exercise utilising combined training at sufficient intensity appears to result in greater improvements for people with metabolic syndrome.
On the contrary, there are other advantages of unsupervised lifestyle intervention programs. For the exercise component of a lifestyle intervention to be supervised, substantial time commitment (from both practitioners and patients) as well as high economic costs (facility, equipment, staff and travel costs) make the sustainability of such programs questionable for long-term management in the general population [6]. In addition, due to these time, personnel and cost barriers, supervised programs may not be accessible in all areas and for all people in need. Unsupervised programs may be both more accessible and more cost-effective, allowing wider implementation for population health. Furthermore, long term adherence to exercise programs is often a problem following completion of lifestyle intervention programs as the effects of supervised short-term exercise programs diminish over time. Unsupervised programs in this review were of longer duration and appeared to result in true behaviour change (as evidenced by increased physical activity levels over a period greater than 6 months) and therefore may be more effective for long-term behaviour change [41, 42]. However, further research is required to support this.
As a result of the current COVID-19 global pandemic, many countries have introduced urban confinement or social distancing measures to contain the spread of COVID-19. Consequently, many group-based health programs have been cancelled and many health organisations have had to modify their service delivery to reduce face-to-face contact with clients. A major benefit of including unsupervised exercise in lifestyle interventions to manage metabolic syndrome is that programs can continue even with restrictions related to social distancing and urban confinement. This review provides reassurance that after appropriate instruction and education, exercise interventions can still be effective for people with metabolic syndrome even when unsupervised. Other components of lifestyle interventions, such as dietary intervention and counselling, can also be delivered remotely allowing people with metabolic syndrome to access the resources they need in order to make behaviour changes to improve outcomes.
There was a large amount of heterogeneity present in a number of the meta-analyses. To account for this, sensitivity analyses were conducted by removing trials with shorter duration lifestyle interventions as they were often the cause of heterogeneity. There was also a significant amount of clinical heterogeneity in the included interventions. While the included trial interventions varied in terms of content, duration, frequency and delivery, they all included the core components of diet and unsupervised exercise. No studies reported on adverse events of unsupervised exercise making it difficult to draw conclusions on safety. Finally, while we did report comparisons to our previous review on lifestyle interventions with supervised exercise, this review did not analyse direct comparisons within randomised controlled trials, hence results should be interpreted with caution. In spite of these limitations, a number of strengths must be noted. Our review is reported in accordance to PRISMA guidelines, assesses the quality of each meta-analysis using GRADE and includes moderate to high quality randomised controlled trials.
Conclusion
There is high quality evidence that multi-factorial, lifestyle intervention programs with prescribed, unsupervised exercise components reduce waist circumference and blood pressure in people with metabolic syndrome. They also cause positive behaviour change in relation to physical activity and improve quality of life. While it may be preferable to run supervised exercise programs as part of a lifestyle intervention, where this is not possible due to social distancing or time, personnel and resource restrictions, unsupervised exercise could be considered an appropriate alternative.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
CP contributed significantly to conception, data collection, data analysis, data interpretation and manuscript preparation. MvN contributed significantly to database searching, data collection, and manuscript preparation. GO’D contributed significantly to quality analysis, data analysis, data interpretation and manuscript preparation.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Casey L. Peiris, Email: C.Peiris@latrobe.edu.au
Maria van Namen, Email: Maria.VanNamen@easternhealth.org.au.
Gráinne O’Donoghue, Email: grainne.odonoghue@ucd.ie.
References
- 1.O'Neill S, O'Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015;16:1–12. doi: 10.1111/obr.12229. [DOI] [PubMed] [Google Scholar]
- 2.Boudreau DM, Malone DC, Raebel MA, Fishman PA, Nichols GA, Feldstein AC, et al. Health care utilization and costs by metabolic syndrome risk factors. Metab Syndr Relat Disord. 2009;7:305–314. doi: 10.1089/met.2008.0070. [DOI] [PubMed] [Google Scholar]
- 3.Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, et al. The metabolic syndrome and CV risk: A systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113–1132. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 4.Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on Scientific Issues Related to Definition. Circulation 2004;109:433–38. [DOI] [PubMed]
- 5.van Namen M, Prendergast L, Peiris C. Supervised lifestyle intervention for people with metabolic syndrome improves outcomes and reduces individual risk factors of metabolic syndrome: A systematic review and meta-analysis. Metabolism. 2019;101:153988. doi: 10.1016/j.metabol.2019.153988. [DOI] [PubMed] [Google Scholar]
- 6.Eddy DM, Schlessinger L, Kahn R. Clinical outcomes and cost-effectiveness of strategies for managing people at high risk for diabetes. Ann Intern Med. 2005;143(4):251–264. doi: 10.7326/0003-4819-143-4-200508160-00006. [DOI] [PubMed] [Google Scholar]
- 7.Pan B, Ge L, Xun Y-Q, Chen Y-J, Gao C-Y, Han X, et al. Exercise training modalities in patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. Int J Behav Nutr Phys Act. 2018;15:72. doi: 10.1186/s12966-018-0703-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashworth NL, Chad KE, Harrison EL, Reeder BA, Marshall SC. Home versus center based physical activity programs in older adults. Cochrane Database Syst Rev 2005 Jan 25;(1):CD004017. [DOI] [PMC free article] [PubMed]
- 9.Fisher KL, Reeder BA, Harrison EL, Bruner BG, Ashworth NL, Pahwa P, et al. Comparing Class-Based and Home-Based Exercise for Older Adults With Chronic Health Conditions: 12-Month Follow-Up of a Randomized Clinical Trial. J Aging Phys Act. 2018;26:471–485. doi: 10.1123/japa.2016-0285. [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. BMJ 2009;339:b2535, 10.1136/bmj.b2535 [PMC free article] [PubMed]
- 11.Landis RJ, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 12.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome - a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med 2006;23:469–80. [DOI] [PubMed]
- 13.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung and Blood Institute scientific statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 14.Centre for Evidence-Based Physiotherapy (2010) The Physiotherapy Evidence Database (PEDro) [cited 2019 Nov 6]. Available from: www.pedro.org.au
- 15.de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. 2009;55:129–133. doi: 10.1016/S0004-9514(09)70043-1. [DOI] [PubMed] [Google Scholar]
- 16.RevMan 5. 3 ed. Copenhagen: The Nordic Cochrane Centre: The Cochrane Collaboration; 2014.
- 17.Cohen J. The statistical power of abnormal-social psychological research: a review. J Abnorm Soc Psych. 1962;65:145–153. doi: 10.1037/h0045186. [DOI] [PubMed] [Google Scholar]
- 18.Higgins J, Thompson S, Deeks J, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avram C, Iurciuc M, Craciun L, Avram A, Iurciuc S, Oancea C, Gaita D. Dietary and physical activity counseling in high-risk asymptomatic patients with metabolic syndrome - A primary care intervention. J Food Agric Environ. 2012;9:16–19. [Google Scholar]
- 21.Chirinos DA, Goldberg RB, Llabre MM, Gellman M, Gutt M, McCalla J, et al. Lifestyle modification and weight reduction among low-income patients with the metabolic syndrome: the CHARMS randomized controlled trial. J Behav Med. 2016;39:483–492. doi: 10.1007/s10865-016-9721-2. [DOI] [PubMed] [Google Scholar]
- 22.Fappa E, Yannakoulia M, Ioannidou M, Skoumas Y, Pitsavos C, Stefanadis C. Telephone counseling intervention improves dietary habits and metabolic parameters of patients with the metabolic syndrome: a randomized controlled trial. Rev Diabet Stud. 2012;9:36–45. doi: 10.1900/RDS.2012.9.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomez-Huelgas R, Jansen-Chaparro S, Baca-Osorio AJ, Mancera-Romero J, Tinahones FJ, Bernal-López MR. Effects of a long-term lifestyle intervention program with Mediterranean diet and exercise for the management of patients with metabolic syndrome in a primary care setting. Eur J Intern Med. 2015;26:317–323. doi: 10.1016/j.ejim.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Jahangiry L, Shojaeizadeh D, Farhangi MA, Yaseri M, Mohammad K, Najafi M, Montazeri A. Interactive web-based lifestyle intervention and metabolic syndrome: findings from the Red Ruby (a randomized controlled trial) Trials. 2015;16:418. doi: 10.1186/s13063-015-0950-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jahangiry L, Montazeri A, Najafi M, Yaseri M, Farhangi MA. An interactive web-based intervention on nutritional status, physical activity and health-related quality of life in patient with metabolic syndrome: a randomized-controlled trial (The Red Ruby Study) Nut Diabet. 2017;7(1):e240. doi: 10.1038/nutd.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nanri A, Tomita K, Matsushita Y, Ichikawa F, Yamamoto M, Nagafuchi Y, et al. Effect of six months lifestyle intervention in Japanese men with metabolic syndrome: randomized controlled trial. J Occup Health. 2012;54:215–222. doi: 10.1539/joh.11-0238-OA. [DOI] [PubMed] [Google Scholar]
- 27.Wang Q, Chair SY, Wong EM. The effects of a lifestyle intervention program on physical outcomes, depression, and quality of life in adults with metabolic syndrome: A randomized clinical trial. Int J Cardiol. 2017;230:461–467. doi: 10.1016/j.ijcard.2016.12.084. [DOI] [PubMed] [Google Scholar]
- 28.Zhang GL, Guo G, Cheng YP. Efficacy of early lifestyle intervention on metabolic syndrome. J Geriatr Cardiol. 2010;7:14–20. [Google Scholar]
- 29.Zhang Y, Mei S, Yang R, Chen L, Gao H, Li L. Effects of lifestyle intervention using patient-centered cognitive behavioral therapy among patients with cardio-metabolic syndrome: a randomized, controlled trial. BMC Cardiovasc Disord. 2016;16:227. doi: 10.1186/s12872-016-0398-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng X, Yu H, Qiu X, Chair SY, Wong EM, Wang Q. The effects of a nurse-led lifestyle intervention program on cardiovascular risk, self-efficacy and health promoting behaviours among patients with metabolic syndrome: Randomized controlled trial. Int J Nurs Stud. 2020;109:103638. doi: 10.1016/j.ijnurstu.2020.103638. [DOI] [PubMed] [Google Scholar]
- 31.Katzmarzyk PT, Hu G, Cefalu WT, Mire E, Bouchard C. The importance of waist circumference and BMI for mortality risk in diabetic adults. Diabet Care. 2013;36:3128–3130. doi: 10.2337/dc13-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turnbull F, Blood Pressure Lowering Treatment Trialists' Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet 2003;362:1527–35. [DOI] [PubMed]
- 33.Lin CH, Chiang SL, Tzeng WC, Chiang LC. Systematic review of impact of lifestyle-modification programs on metabolic risks and patient-reported outcomes in adults with metabolic syndrome. Worldviews Evid Based Nurs. 2014;11:361–368. doi: 10.1111/wvn.12069. [DOI] [PubMed] [Google Scholar]
- 34.Kodama S, Tanaka S, Saito K, Shu M, Sone Y, Onitake F, et al. Effect of Aerobic Exercise Training on Serum Levels of High-Density Lipoprotein Cholesterol: A Meta-analysis. Arch Intern Med. 2007;167:999–1008. doi: 10.1001/archinte.167.10.999. [DOI] [PubMed] [Google Scholar]
- 35.Mann S, Beedie C, Jimenez A. Differential Effects of Aerobic Exercise, Resistance Training and Combined Exercise Modalities on Cholesterol and the Lipid Profile: Review Synthesis and Recommendations. Sports Med. 2014;44:211–221. doi: 10.1007/s40279-013-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stefanov T, Vekova A, Bonova I, Tzvetkov S, Kurktschiev D, Bluher M, et al. Effects of supervised vs non-supervised combined aerobic and resistance exercise programme on cardiometabolic risk factors. Cent Eur J Public Health. 2013;21:8–16. doi: 10.21101/cejph.a3801. [DOI] [PubMed] [Google Scholar]
- 37.Jeon CY, Lokken P, Hu FB, van Dam RM. Physical Activity of Moderate Intensity and Risk of Type 2 Diabetes A systemaric review. Diabetes Care. 2007;30:744–752. doi: 10.2337/dc06-1842. [DOI] [PubMed] [Google Scholar]
- 38.Schwingshackl L, Missbach B, Dias S, König J, Hoffmann G. Impact of different training modalities on glycaemic control and blood lipids in patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetologia. 2014;57:1789–1797. doi: 10.1007/s00125-014-3303-z. [DOI] [PubMed] [Google Scholar]
- 39.Schwingshackl L, Dias S, Strasser B, Hoffmann G. Impact of Different Training Modalities on Anthropometric and Metabolic Characteristics in Overweight/Obese Subjects: A Systematic Review and Network Meta-Analysis. PLoS ONE. 2013;8:e82853. doi: 10.1371/journal.pone.0082853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schroeder EC, Franke WD, Sharp RL, Lee D-C. Comparative effectiveness of aerobic, resistance, and combined training on cardiovascular disease risk factors: A randomized controlled trial. PLoS ONE. 2019;14:e0210292. doi: 10.1371/journal.pone.0210292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leung A, Chan R, Sea M, Woo J. An Overview of Factors Associated with Adherence to Lifestyle Modification Programs for Weight Management in Adults. Int J Environ Res Public Health. 2017;14:922. doi: 10.3390/ijerph14080922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Middleton KR, Anton SD, Perri MG. Long-Term Adherence to Health Behavior Change. Am J Lifestyle Med. 2013;7:395–404. doi: 10.1177/1559827613488867. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.