Abstract
Background
A core symptom of posttraumatic stress disorder is persistent fear memory, which can be defined as fear memory that is resistant to updating, inhibition, or extinction. posttraumatic stress disorder emerges after traumatic stress exposure, but neurobiological mechanisms via which traumatic stress leads to persistent fear memory are not well defined. Akt signaling within the amygdala (Amy) is enhanced with traumatic stress, and phosphatidylinositol kinase 3 (PI3K) activation of Akt within the basolateral Amy (BLA) has been implicated as critical to fear memory formation. These findings raise the possibility that traumatic stress enhances PI3K→Akt signaling in the BLA, which leads to persistent fear memory.
Methods
To test this hypothesis, rats were exposed to traumatic stress using the single prolonged stress model, and changes in Akt phosphorylation were assayed in the Amy at 0 and 30 minutes after fear conditioning (FC). In a separate experiment, we inhibited PI3K→Akt signaling in the BLA prior to FC and observed the effect this had on acquisition, expression, and extinction of FC in stressed and control rats.
Results
Enhanced Akt phosphorylation in the Amy at both time points was observed in stressed rats, but not in control rats. PI3K→Akt inhibition in the BLA had no effect on freezing in control rats but decreased freezing during extinction training and testing in stressed rats.
Conclusion
These findings suggest that PI3K→Akt signaling in the BLA could be a mechanism via which traumatic stress leads to fear memory that is resistant to extinction.
Keywords: Basolateral amygdala, extinction, fear memory, PTSD, single prolonged stress
Significance Statement.
Previous studies have observed that Akt phosphorylation (i.e., activation of Akt) is enhanced with traumatic stress exposure, and PI3K→Akt signaling in the basolateral amygdala (BLA) is necessary for fear memory formation. This suggests that traumatic stress may enhance fear memory by enhancing PI3K→Akt signaling in the BLA. The results of this study suggests that PI3K→Akt signaling in the BLA is not important for fear memory per se but is a molecular signaling pathway that facilitates traumatic stress-induced changes in fear memory. In turn, these findings raise the possibility that PI3K→Akt signaling might contribute to enhancements in fear memory observed in traumatic stress-induced disorders such as posttraumatic stress disorder.
Introduction
Posttraumatic stress disorder (PTSD) is a debilitating disorder that has a lifetime prevalence rate of 7%–9% of the US population (Kessler et al., 1995). A core PTSD symptom is the persistence of memories that accompany traumatic events that initiated PTSD (i.e., traumatic fear memory) (Kessler et al., 1995; Milad et al., 2009; American Psychiatric Association, 2013). Persistent fear memory can be defined as memory that is resistant to inhibition, updating, or extinction (Giustino and Maren, 2015; Bergstrom, 2016; Careaga et al., 2016). Persistent fear memory in PTSD can emerge because of enhanced fear memory (Morgan et al., 1995; Jovanovic et al., 2011; Inslicht et al., 2013) or deficits in inhibitory extinction memory (Milad et al., 2008; Pitman et al., 2012). Examining neurobiological mechanisms via which trauma leads to persistent fear memory can lead to the discovery of drugs and treatments for PTSD.
Animal models of PTSD/traumatic stress are useful in identifying neurobiological mechanisms via which traumatic stress exposure leads to specific PTSD symptoms (Armario et al., 2008; Bowers and Ressler, 2015; Deslauriers et al., 2018). Akt signaling in key nodes of the fear circuit is sensitive to the effects of traumatic stress in different animal models of PTSD/traumatic stress. Previous reports have observed that inescapable shock enhances phosphorylation of Akt (pAkt) in the dorsal hippocampus (dHipp) and amygdala (Amy) (Dahlhoff et al., 2010; Chen et al., 2015). Single prolonged stress (SPS) enhances pAkt in the Hipp (Eagle et al., 2013) but may decrease pAkt in the medial prefrontal cortex (mPFC) (Liu et al., 2018). Aggressor models of traumatic stress have reported a decrease in pAkt in the Hipp (Sun et al., 2020) and ventral tegmental area (Krishnan et al., 2008). Other studies have reported that activation of Akt by phosphatidylinositol kinase 3 (PI3K) (i.e., PI3K→Akt signaling) is critical for fear memory formation [though it should be noted that previous findings raise the possibility that PI3K→Akt signaling in the BLA renders contextual fear memory sensitive to fear extinction (Slouzkey and Maroun, 2016)]. Mice bred for high anxious behavior show enhanced pAkt accompanied by enhanced conditioned fear and avoidance (Yen et al., 2012). In the Amy, pAkt is enhanced with fear potentiated startle, and inhibiting PI3K→Akt signaling in the basolateral Amy (BLA) during fear conditioning (FC) reduces fear potentiated startle (Lin et al., 2001; Ou and Gean, 2006). Together, these findings raise the possibility that persistent fear memory emerges after traumatic stress, because traumatic stress enhances PI3K→Akt signaling in the BLA during fear memory formation. However, whether PI3K→Akt signaling in the BLA during FC is a pathway through which traumatic stress leads to fear memory persistence has been insufficiently explored. This is especially so because other studies have observed that inhibiting PI3K→Akt signaling in the BLA enhances, not reduces, persistent fear memory (Kritman and Maroun, 2013).
In this study, we examined how PI3K→Akt signaling contributes to persistent fear memory brought on by traumatic stress. We employed the SPS model of PTSD/traumatic stress, because it is a validated animal model (Armario et al., 2008; Yamamoto et al., 2009; Bowers and Ressler, 2015; Deslauriers et al., 2018; Lisieski et al., 2018). We first assayed FC-induced changes in pAkt in multiple nodes of the fear circuit in SPS and control rats. These nodes included the mPFC, dHipp, Amy, and ventral Hipp (vHipp). SPS selectively enhanced pAkt in the Amy in rats undergoing FC, but there was no enhancement in pAkt in the Amy of control rats undergoing FC. We then inhibited PI3K→Akt signaling in the BLA during FC in another set of stressed and control animals. We observed the effect this treatment had on conditioned freezing during FC (i.e., acquisition of fear memory), extinction training (i.e., expression and extinction of fear memory), and extinction testing (i.e., retention of extinction memory). Inhibiting PI3K→Akt signaling in the BLA prior to FC had no effect on conditioned freezing in control rats but lowered conditioned freezing during extinction training and testing in SPS rats.
MATERIALS AND METHODS
Animals
A total 114 male Sprague-Dawley rats (approximately 150–250 g on arrival) obtained from Charles River Inc. were used in this study. This strain and sex was used because the SPS model is well characterized in this strain and sex of animal (Yamamoto et al., 2009; Lisieski et al., 2018). On arrival, rats were housed in pairs during a 5-day acclimation period with ad libitum access to food and water. Experiments commenced following the animals’ acclimation period. Following SPS and control procedures, rats were individually housed and restricted to 23 g/d of standard rat chow per the manufacturer’s recommendation (LabDiet, St. Louis, MO) with ad libitum access to water. The rats were on a 12-hour-light/-dark cycle, and all experimental procedures were performed in the animals’ light cycle between the hours of 9:00 am and 4:00 pm. All experiments were approved by the University of Delaware Institutional Animal Care and Use committee following guidelines established by the NIH.
SPS and Behavioral Procedures
All rats were randomly assigned to SPS or control stress. SPS was conducted as previously described (Liberzon et al., 1999; Knox et al., 2010) and consisted of 2 hours of restraint followed by 20 minutes of forced swim, then ether exposure until general anesthesia was induced. For control stress, rats were placed into a novel room in their home cages for the duration of SPS. A post-stress incubation period of 7–10 days was allowed to elapse prior to experimental testing, because this window of time is necessary to observe SPS effects (Liberzon et al., 1999; Knox et al., 2012b).
FC sessions were conducted as previously described (Knox et al., 2012a, 2012b) using 6 MedAssociates (Fairfax, VT) operant boxes. Briefly, FC consisted of 5 presentations of a 10-second auditory conditioned stimulus (CS; 2 kHz, 80 dB) that co-terminated with a 1-second, 1-mA footshock unconditioned stimulus. In some experiments, animals were also subjected to extinction training (30 CS-only presentations) and extinction testing (1 CS-only presentations). FC, extinction training, and testing sessions were conducted in unique contexts, and all sessions began with a 210-second baseline period and had inter-stimulus intervals of 60 seconds.
Western Blot
SPS and control rats were randomly assigned to 1 of 3 groups: baseline, FC0, or FC30. Rats in the baseline treatment were removed from the housing colony and immediately killed to determine basal Akt and pAkt levels in brain regions. All other rats were removed from the housing colony, subjected to FC, and removed from the operant boxes and killed either immediately (FC0) or 30 minutes (FC30) after FC. These timelines were selected because a previous report suggests maximal pAkt is observed 30 minutes after FC (Lin et al., 2001). All animals were killed via rapid decapitation, and their brains were immediately extracted and flash frozen in isopentane chilled on dry ice. Brains were then stored in a −80°C freezer until further processing. To dissect brain regions, brains were thawed to −13°C in a cryostat (Leica CM1350), and 300-µm coronal sections through the mPFC, Amy, dHipp, and vHipp were taken and these brain regions dissected out and placed into 1.5-mL microtubes. Dissected brain regions were then stored in a −80°C freezer until further processing.
At the start of sample preparation, brain regions were homogenized in 250 μL of buffer (50 mM Tris buffer, 10% sucrose, 1 mM ethylenediaminetetraacetic acid, 0.5 mM dithiothreitol, 1 mM benzamidine, 0.3 mM phenylmethylsulfonyl fluoride) by a motor-driven homogenizer (Fisher Scientific, PowerGen125). The homogenate was then centrifuged at 14 800 × g for 45 minutes at 4°C and the supernatant treated as the total cellular extract of brain tissue. The protein concentration of samples was then determined using Bradford assay per the manufacturer’s instructions (Pierce BCA Protein Assay Kit). Also 0.5× Laemmli sample buffer was mixed with approximately 15 µg of protein from each sample. These samples were stored in a −80°C until western blot.
At the start of western blot, protein samples were heated at 70°C for 7 minutes before being loaded into 10% Tris-HCl polyacrylamide gels and separated by SDS polyacrylamide gel electrophoresis. Separated proteins were electrophoretically transferred from gels to nitrocellulose membranes. The membranes were subsequently left to dry for 30 minutes at 37°C followed by rehydration washes in 0.5 M Tris-buffered saline (TBS). Membranes were blocked for 1 hour at room temperature in TBS containing 5% nonfat milk. Nitrocellulose membranes were first probed for pAkt and β-actin (reference protein) by incubating overnight at 4°C with a polyclonal rabbit pAkt antibody (1:500, Cell Signaling, #4060, ser473 site) and a mouse β-actin antibody (1:2000, Cell Signaling Inc. #8H10D10) in TBS. After 18–20 hours, the membranes were subjected to several washes in 0.5 M TBS with 0.1% Tween-20 and then a 2-hour incubation at room temperature with polyclonal goat anti-rabbit (800CW, 1:2000, LI-COR Lincoln, NE) and anti-mouse IgG (680RD, 1:5000, LI-COR) secondary antibodies in 0.5M TBS containing 0.1% Tween and 5% nonfat milk. Nitrocellulose membranes were then washed in TBS and scanned in the Li-cor Odyssey Clx scanner under the following settings: resolution, 169 µm; quality, lowest; focus offset, 0.0 mm. This procedure was then repeated again in all membranes to image Akt bands using a polyclonal rabbit pan-Akt antibody (1:500, Cell Signaling, #4691) and the 800CW goat anti-rabbit antibody.
PI3K-akt Inhibition
For experiments involving PI3K-Akt inhibition prior to FC, the drug treatments were adapted from previous studies that show infusion of wortmannin into the BLA decreases phosphorylation of Akt at the ser273 site (Lin et al., 2001; Ou and Gean, 2006). Rats were first implanted with 26-gauge guide cannulas (PI technologies, Roanoke, VA) 11 mm long. Cannulas were aimed at the BLA (AP: −2.3 mm, ML: ±5.1 mm, DV: −7.1 mm) using previously described techniques (Fitzpatrick et al., 2011). Guide cannulas were secured to the skulls of rats using dental acrylic. After 5 days of surgical recovery, rats were subjected to either SPS or control stress. Fifteen minutes prior to the start of FC, 0.2 µL/hemisphere of the selective PI3K inhibitor wortmannin (TOCRIS Inc.; 5 µg/µL) or vehicle was infused into the BLA at a rate of 0.2 µL/min. The dose of wortmannin was selected because it inhibits pAkt at the ser473 site (Lin et al., 2001; Ou and Gean, 2006). Wortmannin has a short half-life (10–15 minutes) and thus can only alter acquisition or consolidation of FC. Infusion was accomplished by using a micropump (Harvard Apparatus, Holliston, MA), infusion cannula (33 gauge projecting 1 mm beyond the guides), and PE-tubing connecting infusion cannula to 5-µL Hamilton syringes. Animals were then subjected to FC, extinction training, and extinction testing.
After extinction testing, animals were infused with 0.2 µL of a 2% pontamine sky blue solution in physiological saline, under 2% isoflurane anesthesia, to locate infusion cannula tips. Animals were then killed by rapid decapitation and brains extracted and sliced in a cryostat at 30 µm. Sections were then Nissl stained as previously described (Knox and Keller, 2016).
Data and Statistical Analysis
Freezing behavior was analyzed using ANY-maze (Stoelting Inc., Wood Dale, IL) as previously described (Knox et al., 2012b). For FC involving western-blot experiments, baseline freezing was analyzed using a stress (SPS vs control) × condition (FC0 vs FC30) factor design. Conditioned freezing during FC trials was analyzed using a stress × condition × trial (1–5) factor design. For all PI3K-Akt inhibition experiments, conditioned freezing during behavioral sessions was analyzed at select critical points. For FC, this was baseline and trial 5 (to measure acquisition of fear memory), extinction training during baseline, the first 4 CS trials (to measure fear memory retrieval [FMR]), and the last 2 trials of extinction training (to measure acquisition of extinction), and extinction testing during baseline and the single CS trial. To maximize the power of statistical analyses for the PI3K-Akt inhibition experiments, the effects of infusing wortmannin into the BLA was analyzed separately in SPS and control rats using drug (wortmannin vs vehicle) × trial factor designs.
To reduce variability in western-blot data, representative rats from each independent factor (i.e., stress and condition) were always included in each protein assay and western blot. The integrated intensity of all bands were scored using ImageStudio. The profile curves of all bands were inspected to ensure that there was significant integrated intensity signal above the background within each lane in every western. For all statistical analyses, pAkt and Akt were expressed relative to β-actin to yield relative pAkt and Akt levels. Relative pAkt and Akt levels from FC experiments were normalized relative to baseline and subjected to a stress × condition (FC0 vs FC30) factor design.
For all factor designs, main and simple effects were analyzed using ANOVA, while main and simple comparisons were analyzed using independent, paired sample, or 1 sample t test with a Bonferroni correction applied where appropriate. The reference value for all 1 sample t tests was set to 100. Statistical significance was assumed with P < .05 for all statistical tests. Outliers were excluded from data analysis and were defined as numbers that were 2 SDs away from a group mean.
RESULTS
Western Blot
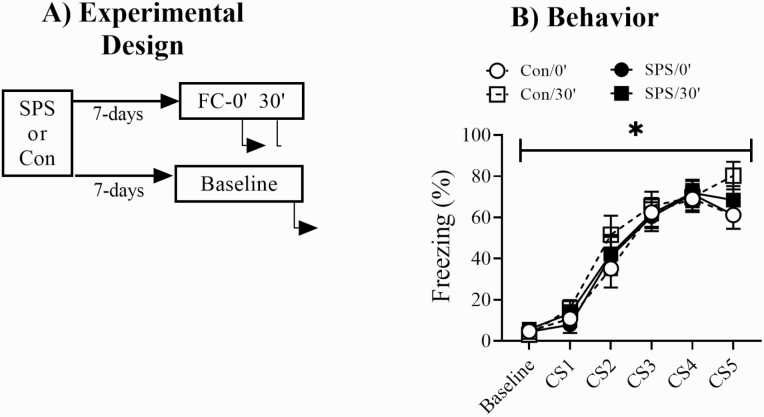
Figure 1 shows the experimental design and freezing behavior during FC. There were no significant effects of stress or condition for baseline freezing (Ps > .05). There was a main effect of trial [F(4,128) = 95.825, P < .001] for conditioned freezing during FC but no significant effects of stress or condition (Ps > .05). This suggested rats in all groups acquired fear memory in an equivalent manner.
Figure 1.
(A) Experimental design used for western-blot experiments. Underneath arrows indicate time points when rats were killed. (B) All animals in fear conditioning (FC) groups showed equivalent levels of conditioned freezing during FC. FC-0’/SPS = 9, FC-0’/control = 9, FC-30’/SPS = 9, FC-30’/control = 9. *Main effect of time. Error bars represent SEM.
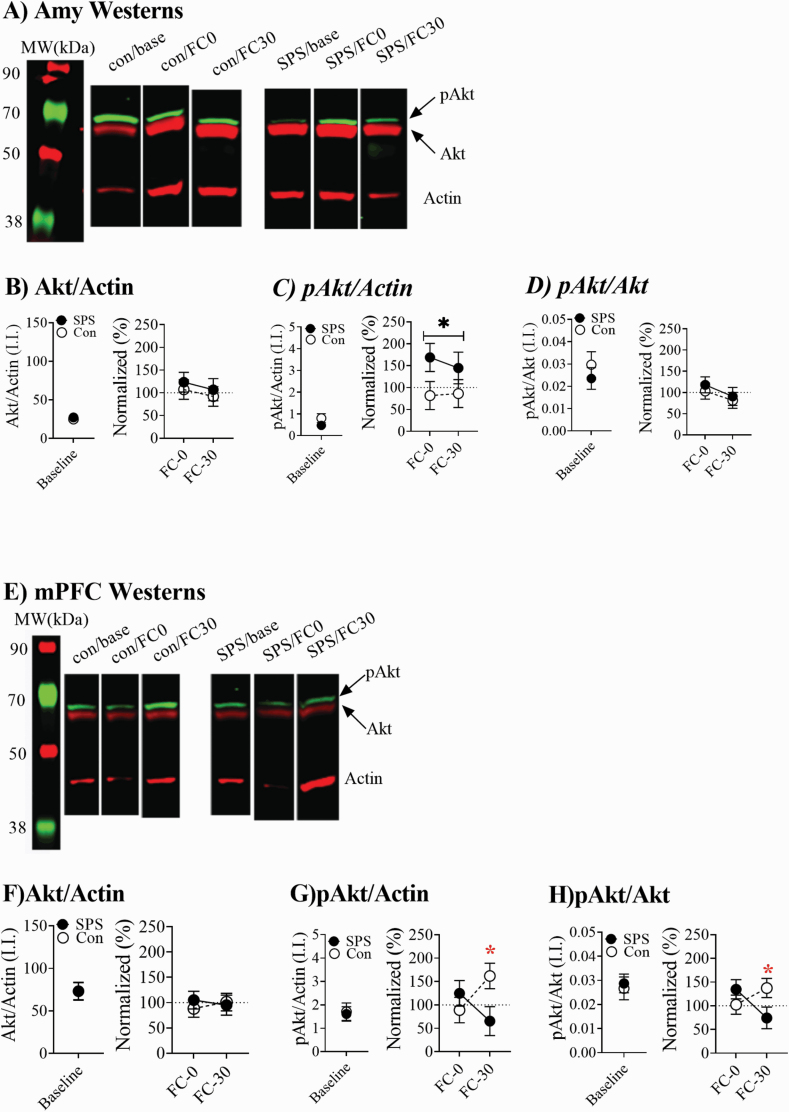
The use of our western-blot protocol to separately measure Akt and pAkt bands is illustrated in supplementary Figure 1. Figure 2A shows representative westerns for Amy homogenates. SPS enhanced pAkt levels during FC in Amy homogenates without having any effect on Akt levels (Figure 2B–C). This was revealed by a significant main effect of stress for pAkt/actin ratios [F(1,34) = 4.84, P = .036] but not Akt/actin ratios (P > .05). This effect was most pronounced at the FC0 time point. pAkt/Akt ratios from Amy homogenates were not affected by any treatment (Ps > .05; Figure 2D). Figure 2E shows representative westerns for mPFC homogenates. In the mPFC, Akt levels were unaffected by any treatments (Ps > .05). PAkt levels were enhanced at FC30 in control rats but decreased in SPS rats. This resulted in a stress × condition effect for pAkt/actin and pAkt/Akt ratios [pAkt/actin: F(1,34) = 5.493, P = .026; pAkt/Akt: F(1,30) = 5.264, P = .029; Figure 2F–H].
Figure 2.
Changes in phosphorylated Akt (pAkt) and Akt in the amygdala (Amy) and medial prefrontal cortex (mPFC). (A) Representative Amy westerns. (B) Single prolonged stress (SPS) had no effect on Akt levels, but (C) enhanced pAkt/actin ratios in the Amy. (D) There was no effect of SPS on pAkt/Akt ratios. (E) Representative mPFC westerns. (F) In control animals, pAkt/actin ratios were enhanced 30’ after fear conditioning (FC), but in SPS rats these ratios were decreased 30’ after FC. (G) SPS had no effect on Akt/actin ratios. (H) In control animals, pAkt/Akt ratios were enhanced 30’ after FC, but in SPS rats these ratios were decreased 30’ after FC. Baseline levels (SPS = 18, control = 18) of Akt and pAkt in the Amy and mPFC were not affected by SPS. One SPS animal’s datum point was dropped from the Amy analysis, because it was more than 2 SDs away from the group mean. Error bars represent SEM, black * indicates main effect of stress, red * indicates stress × time interaction.
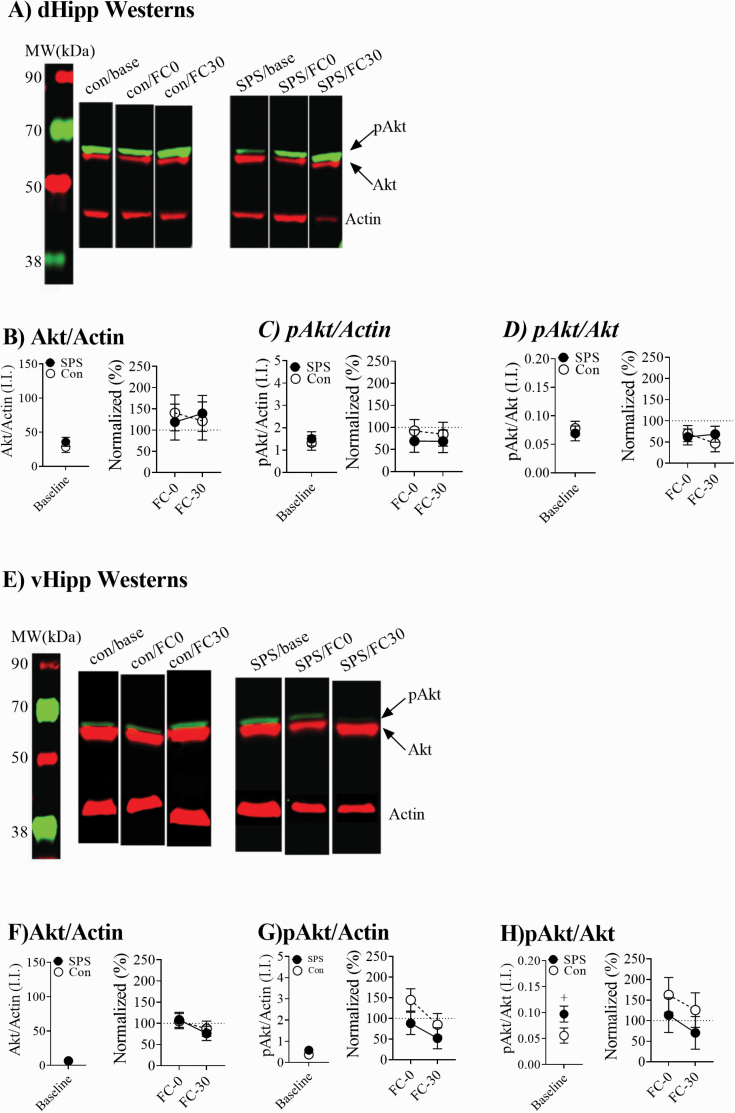
Figure 3A show representative westerns for dHipp homogenates. Neither stress nor condition had any effect on pAkt or Akt ratios in the dHipp (Ps > .05; Figures 3B–D). Figure 3E shows representative westerns for vHipp homogenates. Akt/actin and pAkt/actin ratios from vHipp homogenates were unaffected by any treatments (Ps > .05; Figures 3F and 3G). SPS enhanced pAkt/Akt ratios at baseline in vHipp homogenates and this effect approached significance (t(33) = 1.945, P = .06; Figure 3H). However, there was no significant effects of stress on pAkt/Akt ratios in the vHipp during FC (Ps > .05; Figure 3H).
Figure 3.
Changes in pAkt and Akt in the dorsal hippocampus (dHipp) and ventral hippocampus (vHipp). (A) Representative dHipp westerns. (B–D) Single prolonged stress (SPS) had no effect on Akt or phosphorylated Akt (pAkt) levels. (E) Representative vHipp westerns. (F and G) There were no effects observed for pAkt/actin or Akt/actin levels. (E) SPS rats had enhanced pAkt/Akt ratios at baseline (SPS = 18, control = 18), but this effect only approached significance (+ represents P = .06). SPS had no effect on pAkt/Akt ratios during fear conditioning.
PI3K-akt Inhibition
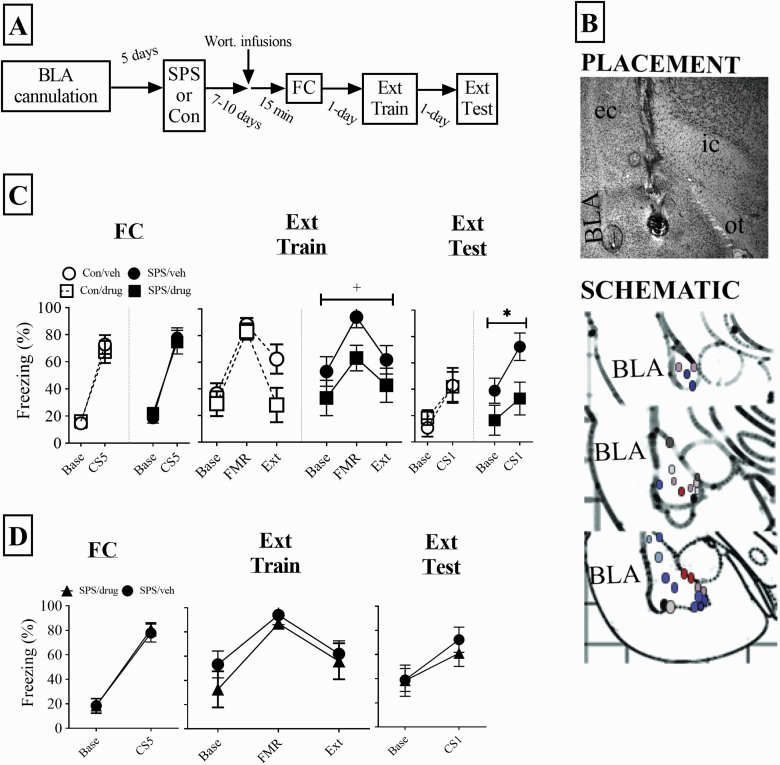
Figure 4A shows the design for this experiment. Figure 4B shows placements of cannulas for the drug condition for SPS (red/brown) and control (blue/purple) animals. Qualitative analysis revealed that irrespective of cannula placement, vehicle-treated animals within a stress group did not differ significantly regarding behavior. As a result, all SPS and control animals in the vehicle treatment were used for statistical analysis.
Figure 4.
Effect of inhibiting PI3K→Akt signaling in the basolateral amygdala (BLA) during FC on fear memory. (A) experimental design. (B) Representative placement of infusion tip in the BLA along with schematic of all BLA placements. Red/brown circles are single prolonged stress (SPS) rats and blue/purple circles are control rats. (C) Infusing wortmannin into the BLA prior to fear conditioning reduced conditioned freezing during extinction training (approached significance with P = .06, indicated with a + sign) and testing (P < .05, indicated with an asterisks). These effects were not observed in control rats. SPS/vehicle = 10, SPS/drug = 7, control/vehicle = 11, control/drug = 8. (D) Infusing wortmannin outside of the BLA had no effect on conditioned fear in SPS rats (n = 6). The SPS/vehicle group from C is plotted in this graph for comparison. One SPS/vehicle animal was excluded from all behavioral analyses because its mean for extinction testing was greater than 2 SDs away from the group mean. Error bars represent SEM.
There was a main effect of trial (SPS: F(1,15) = 73.028, P < .001; control: F(1,17) = 91.521, P < .001), but no drug effects for SPS or control rats (Ps > .05) for conditioned freezing during FC (Figure 4C). These results indicated all animals acquired fear memory in an equivalent manner. There was a main effect of trial for the quadratic trend component for extinction training (SPS: F(1,15) = 17.287, P = .001; control: F(1,17) = 45.164, P < .001) for SPS or control rats (Figure 4C). These findings suggest all animals expressed fear and acquired extinction in an equivalent manner. The main term of drug for analysis of SPS animals approached significance (F(1,15) = 4.073, P = .065), which may have reflected enhanced freezing in SPS rats during the FMR trial. Conditioned freezing during extinction testing was reduced in drug-treated SPS rats (F(1,15) = 4.778, P = .045), but not drug-treated control rats (P > .05). These results are illustrated in Figure 4C. Furthermore, drug effects in SPS rats were not observed when wortmannin was infused outside of the BLA (Ps > .05; Figure 4D). Incorrect infusion sites included the striatum, central nucleus of the Amy, basal forebrain, and ventral CA3.
Discussion
SPS enhanced pAkt/actin ratios in the Amy of rats undergoing FC for up to 30 minutes after FC, but there was no enhancement in pAkt/actin ratios at baseline. This suggests SPS did not enhance pAkt levels on its own but enhanced pAkt signaling in SPS rats during fear memory formation. There was no change in the ratios of pAkt/Akt in Amy homogenates (see Results). The reason for this is not clear. One possibility could be a combined enhancement in Akt (too small to attain statistical significance in this study) with an enhancement in phosphorylation of Akt. Irrespective, the enhancement in pAkt/actin ratios in Amy homogenates suggests an enhancement in pAkt levels. Inhibiting PI3K→Akt signaling in the BLA reduced conditioned freezing in SPS rats during extinction training and testing. Importantly, inhibition of PI3K→Akt signaling had no effect on conditioned freezing at baseline or during CS presentation in FC. These findings suggest that wortmannin had no effect on locomotor activity or acquisition of fear memory. Overall, these findings suggest that PI3K→Akt signaling in the BLA during FC enhances fear memory in SPS rats and renders fear memory resistant to extinction. There was also an enhancement in conditioned freezing during extinction training in SPS/vehicle rats compared with SPS/drug rats that approached significance. This observation too is consistent with SPS enhancing fear memory. In control rats, there was no change in pAkt in the Amy with FC, and inhibiting PI3K→Akt signaling in the BLA had no effect on conditioned freezing in control rats. Thus, it would appear that PI3K→Akt signaling in the BLA during FC selectively modulates fear memory in stressed rats.
SPS decreased pAkt in the mPFC 30 minutes after FC. In control rats, FC increased pAkt at the same time point. The mPFC is not necessary for cued fear memory formation (Maren, 2001; Paré et al., 2004; Quirk et al., 2006; Corcoran and Quirk, 2007; Orsini and Maren, 2012). The mPFC has been implicated in contextual fear memory (Beeman et al., 2013; Rozeske et al., 2015), but previous studies have observed that inhibiting PI3K→Akt signaling in the mPFC during FC has no effect on contextual fear memory (Sui et al., 2008). We used cued FC to examine fear memory, which makes it unlikely that changes in pAkt observed in the mPFC 30 minutes after FC contributed to changes in fear memory in SPS or control animals. SPS may have enhanced pAkt in the vHipp at baseline, which is consistent with a previous finding (Eagle et al., 2013), but SPS had no effect on pAkt levels in the dHipp or vHipp during FC.
The results of this study are consistent with previous findings that have observed enhancing pAkt is accompanied by enhanced conditioned fear responding (Dahlhoff et al., 2010; Yen et al., 2012). Previous findings have observed PI3K→Akt signaling is critical for fear memory in nonstressed rats (Lin et al., 2001; Ou and Gean, 2006), which at first seems inconsistent with the results of this study. Studies that have implicated PI3K→Akt signaling in fear memory have used fear potentiated startle paradigms, which involve presentation of startle probes prior to FC (Lin et al., 2001; Ou and Gean, 2006). Startle probes have an anxiogenic property (Smith et al., 2011; Furlong et al., 2016) and may lead to higher levels of acute stress in animals prior to FC compared with behavioral paradigms that use changes in freezing to measure emotional memory. This higher level of stress could have engaged PI3K→Akt signaling in the BLA and rendered this signaling pathway important for fear memory in the fear-potentiated startle paradigm.
How might SPS lead to enhanced PI3K→Akt signaling? PI3K can be activated by growth factors (e.g., BDNF), hormones (e.g., CRH), and G-protein coupled receptor activation (Brunet et al., 2001; Hauger et al., 2012; Borrie et al., 2017; Roux and Topisirovic, 2018). Enhanced BDNF and CRH expression and/or signaling in the BLA is observed with FC (Rattiner et al., 2004; Ou and Gean, 2007; Ou et al., 2010; Bijlsma et al., 2011; Mountney et al., 2011; Chou et al., 2014). SPS enhances BDNF signaling in the Hipp during contextual FC (Takei et al., 2011) and enhances CRH expression in the central nucleus of the Amy (Sabban et al., 2015), but the effect of SPS on BDNF, CRH, or other hormone and growth factor signaling in the BLA during FC is not clear. Previous research has observed that SPS leads to a tonic decrease in locus coeruleus-norepinephrine (LC-NE) system activity but enhances phasic activity of the LC-NE system (George et al., 2012). Postsynaptic adrenergic receptor (β-ARs and α-ARs) expression increases with a decrease in NE (U’Prichard et al., 1980; Woo et al., 1996). A tonic decrease in LC-NE activity could lead to an enhancement in postsynaptic AR expression in BLA neurons that receive input from the LC. An increase in AR expression in the BLA coupled with a phasic increase in LC-NE activity during FC could enhance PI3K→Akt signaling in the BLA.
Enhanced PI3K→Akt signaling in the BLA of SPS rats during FC could activate mTOR, which can have widespread effects on cellular function by altering protein transcription and translation (Brunet et al., 2001; Borrie et al., 2017; Roux and Topisirovic, 2018). Via this mechanism, SPS may alter protein expression that ultimately enhances fear memory. SPS conducted prior to FC consistently renders fear memory resistant to extinction (i.e., enhances fear memory persistence) even when SPS does not enhance fear memory expression (i.e., enhanced freezing during FMR portion of extinction training) (Yamamoto et al., 2008; Knox et al., 2012b, 2016; Keller et al., 2015a, 2015b; RaiseAbdullahi et al., 2019). These findings raise the possibility that SPS may have a selective effect on a neurobiological function that is somewhat independent of fear memory expression/retrieval. The magnitude of fear memory (often inferred by the magnitude of fear conditioned responses) can be independent of the resistance of fear memory to change or inhibition. Perineuronal nets within the Amy are critical for the resistance of fear memory to extinction but not FMR (Gogolla et al., 2009). The formation of perineuronal nets is a neurobiological mechanism that can account for why fear memory persistence is different in animals at different developmental stages (Gogolla et al., 2009; Baker et al., 2017). If traumatic stress could alter properties of perineuronal nets within the BLA, this could be a mechanism through which traumatic stress leads to persistent fear memory. While speculative, this hypothesis would be interesting to explore as it would provide a relatively novel mechanism through which traumatic stress alters emotional memory.
CONCLUSION
The results of this study suggest that enhancements in PI3K→Akt signaling within the BLA could be a mechanism via which traumatic stress exposure renders fear memory resistant to extinction. However, changes in PI3K→Akt signaling with traumatic stress have been observed in other brain regions (see Introduction and Results), and PI3K→Akt signaling is also critical for extinction memory (Kritman and Maroun, 2013), a process that is sensitive to traumatic stress (Yamamoto et al., 2008; Milad et al., 2009; Knox et al., 2012b, 2016, 2018). These findings suggest that SPS effects on PI3K→Akt signaling during extinction memory formation could also lead to persistent fear memory.
Given that traumatic stress has a number of effects on psychological function mediated by distinct neural circuits, traumatic stress-induced changes in PI3K→Akt signaling may represent a molecular signaling pathway through which multiple traumatic stress effects manifest. Further research elucidating the relationship between traumatic stress-induced changes in PI3K→Akt signaling in specific brain regions and manifestation of specific PTSD-like symptoms is needed.
Supplementary Material
Acknowledgments
We thank all of the undergraduates who helped with imaging and classification of Nissl stained sections.
This work was supported by grant 1P20GM103653 from the National Institutes of General Medicine of the National Institutes of Health.
Statement of Interest
None.
References
- American Psychiatric Association, Force DSMT (2013) Diagnostic and statistical manual of mental disorders: DSM-5. Arlington, VA: American Psychiatric Association. [Google Scholar]
- Armario A, Escorihuela RM, Nadal R (2008) Long-term neuroendocrine and behavioural effects of a single exposure to stress in adult animals. Neurosci Biobehav Rev 32:1121–1135. [DOI] [PubMed] [Google Scholar]
- Baker KD, Gray AR, Richardson R (2017) The development of perineuronal nets around parvalbumin gabaergic neurons in the medial prefrontal cortex and basolateral amygdala of rats. Behav Neurosci 131:289–303. [DOI] [PubMed] [Google Scholar]
- Beeman CL, Bauer PS, Pierson JL, Quinn JJ (2013) Hippocampus and medial prefrontal cortex contributions to trace and contextual fear memory expression over time. Learn Mem 20:336–343. [DOI] [PubMed] [Google Scholar]
- Bergstrom HC (2016) The neurocircuitry of remote cued fear memory. Neurosci Biobehav Rev 71:409–417. [DOI] [PubMed] [Google Scholar]
- Bijlsma EY, van Leeuwen ML, Westphal KG, Olivier B, Groenink L (2011) Local repeated corticotropin-releasing factor infusion exacerbates anxiety- and fear-related behavior: differential involvement of the basolateral amygdala and medial prefrontal cortex. Neuroscience 173:82–92. [DOI] [PubMed] [Google Scholar]
- Borrie SC, Brems H, Legius E, Bagni C (2017) Cognitive dysfunctions in intellectual disabilities: the contributions of the Ras-MAPK and PI3K-AKT-mTOR pathways. Annu Rev Genomics Hum Genet 18:115–142. [DOI] [PubMed] [Google Scholar]
- Bowers ME, Ressler KJ (2015) An overview of translationally informed treatments for posttraumatic stress disorder: animal models of Pavlovian fear conditioning to human clinical trials. Biol Psychiatry 78:E15–E27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Datta SR, Greenberg ME (2001) Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol 11:297–305. [DOI] [PubMed] [Google Scholar]
- Careaga MBL, Girardi CEN, Suchecki D (2016) Understanding posttraumatic stress disorder through fear conditioning, extinction and reconsolidation. Neurosci Biobehav Rev 71:48–57. [DOI] [PubMed] [Google Scholar]
- Chen C, Ji M, Xu Q, Zhang Y, Sun Q, Liu J, Zhu S, Li W (2015) Sevoflurane attenuates stress-enhanced fear learning by regulating hippocampal BDNF expression and Akt/GSK-3β signaling pathway in a rat model of post-traumatic stress disorder. J Anesth 29:600–608. [DOI] [PubMed] [Google Scholar]
- Chou D, Huang CC, Hsu KS (2014) Brain-derived neurotrophic factor in the amygdala mediates susceptibility to fear conditioning. Exp Neurol 255:19–29. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ (2007) Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci 27:840–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlhoff M, Siegmund A, Golub Y, Wolf E, Holsboer F, Wotjak CT (2010) AKT/GSK-3beta/beta-catenin signalling within hippocampus and amygdala reflects genetically determined differences in posttraumatic stress disorder like symptoms. Neuroscience 169:1216–1226. [DOI] [PubMed] [Google Scholar]
- Deslauriers J, Toth M, Der-Avakian A, Risbrough VB (2018) Current status of animal models of posttraumatic stress disorder: behavioral and biological phenotypes, and future challenges in improving translation. Biol Psychiatry 83:895–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle AL, Knox D, Roberts MM, Mulo K, Liberzon I, Galloway MP, Perrine SA (2013) Single prolonged stress enhances hippocampal glucocorticoid receptor and phosphorylated protein kinase B levels. Neurosci Res 75:130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick CJ, Knox D, Liberzon I (2011) Inactivation of the prelimbic cortex enhances freezing induced by trimethylthiazoline, a component of fox feces. Behav Brain Res 221:320–323. [DOI] [PubMed] [Google Scholar]
- Furlong TM, Richardson R, McNally GP (2016) Habituation and extinction of fear recruit overlapping forebrain structures. Neurobiol Learn Mem 128:7–16. [DOI] [PubMed] [Google Scholar]
- George SA, Knox D, Curtis A, Valentino R, Liberzon I (2012) Altered locus coeruleus activity following single prolonged stress, a rodent model of PTSD. Eur J Neurosci 37:901–909. [DOI] [PubMed] [Google Scholar]
- Giustino TF, Maren S (2015) The role of the medial prefrontal cortex in the conditioning and extinction of fear. Front Behav Neurosci 9:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogolla N, Caroni P, Lüthi A, Herry C (2009) Perineuronal nets protect fear memories from erasure. Science 325:1258–1261. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Olivares-Reyes JA, Dautzenberg FM, Lohr JB, Braun S, Oakley RH (2012) Molecular and cell signaling targets for PTSD pathophysiology and pharmacotherapy. Neuropharmacology 62:705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inslicht SS, Metzler TJ, Garcia NM, Pineles SL, Milad MR, Orr SP, Marmar CR, Neylan TC (2013) Sex differences in fear conditioning in posttraumatic stress disorder. J Psychiatr Res 47:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Phifer JE, Sicking K, Weiss T, Norrholm SD, Bradley B, Ressler KJ (2011) Cortisol suppression by dexamethasone reduces exaggerated fear responses in posttraumatic stress disorder. Psychoneuroendocrinology 36:1540–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller SM, Schreiber WB, Staib JM, Knox D (2015a) Sex differences in the single prolonged stress model. Behav Brain Res 286:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller SM, Schreiber WB, Stanfield BR, Knox D (2015b) Inhibiting corticosterone synthesis during fear memory formation exacerbates cued fear extinction memory deficits within the single prolonged stress model. Behav Brain Res 287:182–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB (1995) Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 52:1048–1060. [DOI] [PubMed] [Google Scholar]
- Knox D, George SA, Fitzpatrick CJ, Rabinak CA, Maren S, Liberzon I (2012a) Single prolonged stress disrupts retention of extinguished fear in rats. Learn Mem 19:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D, Keller SM (2016) Cholinergic neuronal lesions in the medial septum and vertical limb of the diagonal bands of Broca induce contextual fear memory generalization and impair acquisition of fear extinction. Hippocampus 26:718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D, Nault T, Henderson C, Liberzon I (2012b) Glucocorticoid receptors and extinction retention deficits in the single prolonged stress model. Neuroscience 223:163–173. [DOI] [PubMed] [Google Scholar]
- Knox D, Perrine SA, George SA, Galloway MP, Liberzon I (2010) Single prolonged stress decreases glutamate, glutamine, and creatine concentrations in the rat medial prefrontal cortex. Neurosci Lett 480:16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D, Stanfield BR, Staib JM, David NP, DePietro T, Chamness M, Schneider EK, Keller SM, Lawless C (2018) Using c-Jun to identify fear extinction learning-specific patterns of neural activity that are affected by single prolonged stress. Behav Brain Res 341:189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D, Stanfield BR, Staib JM, David NP, Keller SM, DePietro T (2016) Neural circuits via which single prolonged stress exposure leads to fear extinction retention deficits. Learn Mem 23:689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Mazei-Robison M, Iñiguez SD, Ables JL, Vialou V, Berton O, Ghose S, Covington HE 3rd, Wiley MD, Henderson RP, Neve RL, Eisch AJ, Tamminga CA, Russo SJ, Bolaños CA, Nestler EJ (2008) AKT signaling within the ventral tegmental area regulates cellular and behavioral responses to stressful stimuli. Biol Psychiatry 64:691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritman M, Maroun M (2013) Inhibition of the PI3 kinase cascade in corticolimbic circuit: temporal and differential effects on contextual fear and extinction. Int J Neuropsychopharmacol 16:825–833. [DOI] [PubMed] [Google Scholar]
- Liberzon I, López JF, Flagel SB, Vázquez DM, Young EA (1999) Differential regulation of hippocampal glucocorticoid receptors mRNA and fast feedback: relevance to post-traumatic stress disorder. J Neuroendocrinol 11:11–17. [DOI] [PubMed] [Google Scholar]
- Lin CH, Yeh SH, Lin CH, Lu KT, Leu TH, Chang WC, Gean PW (2001) A role for the PI-3 kinase signaling pathway in fear conditioning and synaptic plasticity in the amygdala. Neuron 31:841–851. [DOI] [PubMed] [Google Scholar]
- Lisieski MJ, Eagle AL, Conti AC, Liberzon I, Perrine SA (2018) Single-prolonged stress: a review of two decades of progress in a rodent model of post-traumatic stress disorder. Front Psychiatry 9:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Feng D, Wang J, Zhang H, Peng Z, Cai M, Yang J, Zhang R, Wang H, Wu S, Tan Q (2018) rTMS ameliorates PTSD symptoms in rats by enhancing glutamate transmission and synaptic plasticity in the ACC via the PTEN/Akt signalling pathway. Mol Neurobiol 55:3946–3958. [DOI] [PubMed] [Google Scholar]
- Maren S (2001) Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci 24:897–931. [DOI] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK (2008) Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res 42:515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL (2009) Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry 66:1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CA 3rd, Grillon C, Southwick SM, Davis M, Charney DS (1995) Fear-potentiated startle in posttraumatic stress disorder. Biol Psychiatry 38:378–385. [DOI] [PubMed] [Google Scholar]
- Mountney C, Anisman H, Merali Z (2011) In vivo levels of corticotropin-releasing hormone and gastrin-releasing peptide at the basolateral amygdala and medial prefrontal cortex in response to conditioned fear in the rat. Neuropharmacology 60:410–417. [DOI] [PubMed] [Google Scholar]
- Orsini CA, Maren S (2012) Neural and cellular mechanisms of fear and extinction memory formation. Neurosci Biobehav Rev 36:1773–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou LC, Gean PW (2006) Regulation of amygdala-dependent learning by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol-3-kinase. Neuropsychopharmacology 31:287–296. [DOI] [PubMed] [Google Scholar]
- Ou LC, Gean PW (2007) Transcriptional regulation of brain-derived neurotrophic factor in the amygdala during consolidation of fear memory. Mol Pharmacol 72:350–358. [DOI] [PubMed] [Google Scholar]
- Ou LC, Yeh SH, Gean PW (2010) Late expression of brain-derived neurotrophic factor in the amygdala is required for persistence of fear memory. Neurobiol Learn Mem 93:372–382. [DOI] [PubMed] [Google Scholar]
- Paré D, Quirk GJ, Ledoux JE (2004) New vistas on amygdala networks in conditioned fear. J Neurophysiol 92:1–9. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, Milad MR, Liberzon I (2012) Biological studies of post-traumatic stress disorder. Nat Rev Neurosci 13:769–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, González-Lima F (2006) Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry 60:337–343. [DOI] [PubMed] [Google Scholar]
- Raise Abdullahi P, Vafaei AA, Ghanbari A, Dadkhah M, Rashidy-Pour A (2019) Time-dependent protective effects of morphine against behavioral and morphological deficits in an animal model of posttraumatic stress disorder. Behav Brain Res 364:19–28. [DOI] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, French CT, Ressler KJ (2004) Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. J Neurosci 24:4796–4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux PP, Topisirovic I (2018) Signaling pathways involved in the regulation of mRNA translation. Mol Cell Biol 38:e00070-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozeske RR, Valerio S, Chaudun F, Herry C (2015) Prefrontal neuronal circuits of contextual fear conditioning. Genes Brain Behav 14:22–36. [DOI] [PubMed] [Google Scholar]
- Sabban EL, Laukova M, Alaluf LG, Olsson E, Serova LI (2015) Locus coeruleus response to single-prolonged stress and early intervention with intranasal neuropeptide Y. J Neurochem 135:975–986. [DOI] [PubMed] [Google Scholar]
- Slouzkey I, Maroun M (2016) PI3-kinase cascade has a differential role in acquisition and extinction of conditioned fear memory in juvenile and adult rats. Learn Mem 23:723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Meloni EG, Myers KM, Van’t Veer A, Carlezon WA Jr, Rudolph U (2011) Reduction of fear-potentiated startle by benzodiazepines in C57BL/6J mice. Psychopharmacology (Berl) 213:697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui L, Wang J, Li BM (2008) Role of the phosphoinositide 3-kinase-Akt-mammalian target of the rapamycin signaling pathway in long-term potentiation and trace fear conditioning memory in rat medial prefrontal cortex. Learn Mem 15:762–776. [DOI] [PubMed] [Google Scholar]
- Sun L, Cui K, Xing F, Liu X (2020) Akt dependent adult hippocampal neurogenesis regulates the behavioral improvement of treadmill running to mice model of post-traumatic stress disorder. Behav Brain Res 379:112375. [DOI] [PubMed] [Google Scholar]
- Takei S, Morinobu S, Yamamoto S, Fuchikami M, Matsumoto T, Yamawaki S (2011) Enhanced hippocampal BDNF/TrkB signaling in response to fear conditioning in an animal model of posttraumatic stress disorder. J Psychiatr Res 45:460–468. [DOI] [PubMed] [Google Scholar]
- U’Prichard DC, Reisine TD, Mason ST, Fibiger HC, Yamamura HI (1980) Modulation of rat brain alpha- and beta-adrenergic receptor populations by lesion of the dorsal noradrenergic bundle. Brain Res 187:143–154. [DOI] [PubMed] [Google Scholar]
- Woo CC, Wilson DA, Sullivan RM, Leon M (1996) Early locus coeruleus lesions increase the density of beta-adrenergic receptors in the main olfactory bulb of rats. Int J Dev Neurosci 14:913–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Morinobu S, Fuchikami M, Kurata A, Kozuru T, Yamawaki S (2008) Effects of single prolonged stress and D-cycloserine on contextual fear extinction and hippocampal NMDA receptor expression in a rat model of PTSD. Neuropsychopharmacology 33:2108–2116. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Morinobu S, Takei S, Fuchikami M, Matsuki A, Yamawaki S, Liberzon I (2009) Single prolonged stress: toward an animal model of posttraumatic stress disorder. Depress Anxiety 26:1110–1117. [DOI] [PubMed] [Google Scholar]
- Yen YC, Mauch CP, Dahlhoff M, Micale V, Bunck M, Sartori SB, Singewald N, Landgraf R, Wotjak CT (2012) Increased levels of conditioned fear and avoidance behavior coincide with changes in phosphorylation of the protein kinase B (AKT) within the amygdala in a mouse model of extremes in trait anxiety. Neurobiol Learn Mem 98:56–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.