Abstract
Keloids are abnormal fibroproliferative scars with aggressive dermal growth expanding beyond the borders of the original injury. Different therapeutic modalities, such as corticosteroids, surgical excision, topical silicone gel sheeting, laser therapy, cryotherapy, photodynamic therapy and radiotherapy, have been used to treat keloids; however, none of these modalities has proven completely effective. Recently, researchers have devised several promising anti-keloid therapies including anti-hypertensive pharmaceuticals, calcineurin inhibitors, electrical stimulation, mesenchymal stem cell therapy, microneedle physical contact and ribonucleic acid-based therapies. The present review summarises emerging and novel treatments for keloids. PubMed® (National Library of Medicine, Bethesda, Maryland, USA), EMBASE (Elsevier, Amsterdam, Netherlands) and Web of Science (Clarivate Analytics, Philadelphia, Pennsylvania, USA) were searched for relevant literature published between January 1987 to June 2020. A total of 118 articles were included in this review. A deeper understanding of the molecular mechanisms underlying keloid scarring pathogenesis would open further avenues for developing innovative treatments.
Keywords: Keloid, Treatment, Fibroblast, Scar, Dermatology
Keloids are fibroproliferative scars that undergo aggressive dermal growth expanding beyond the borders of the original injury and do not regress spontaneously.1,2 Keloids often form within a year of injury, tend to persist over time and are among the most perplexing challenges facing physicians.3 Keloids can arise from burns, deep dermal injury, post-elective surgery or trauma and can result in deformity and even restricted joint mobility.4
Homo sapiens is the only species that develops keloids.5 The recorded history of keloids dates back to 3,000 BCE, where Egyptian surgical techniques were described on the Smith papyrus.6,7 Rooted in African folklore, the ancient Yoruba people of Western Nigeria recorded features of the keloid diathesis and depicted them in sculptures of the 10th century AD.8 The term keloid stems from the Greek cheloide which is derived from chele, meaning crab claw, and was initially coined by Alibert in 1816 because the lesions resemble crayfish legs penetrating the skin.6
Keloids are equally prevalent in both genders, with the greatest incidence in the second decade of life.9 It seems that some genetic predispositions can affect the occurrence rate of keloid scarring. In this context, individuals with darker skin tones are more prone to keloid development than fairer-skinned individuals.10 Keloids are described as the fifth most frequent skin disease among black adults in the United Kingdom.11 The incidence of keloid scarring in high-risk populations including African, Hispanic and Asian people is approximately 5% to 16%.12
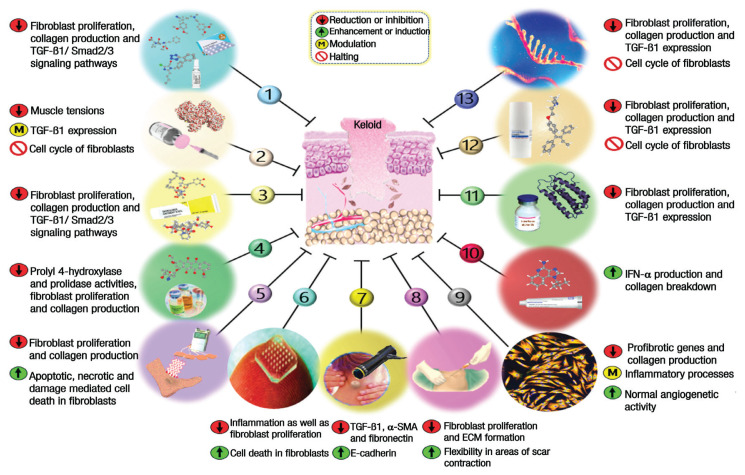
Various therapeutic modalities have been described for keloid treatment. Conventional treatment options include silicone gel sheeting, compressive therapy, intralesional steroids, topical mitomycin C, intralesional or topical 5-fluorouracil (5-FU), surgical excision, cryotherapy, radiotherapy, laser therapy and photodynamic therapy, etc.13,14 Nevertheless, no single effective therapeutic regimen has been hailed as the gold standard, mainly owing to the high recurrence rates of keloids and a dearth of extensive research evaluating available treatments.9 In recent decades, advances in understanding molecular mechanisms of diseases have led to a new era of drug design and development. This compendious review aims to summarise emerging and novel anti-keloid therapies. Due to the scope of this review and space constraints, however, conventional therapies are not discussed herein; interested readers should seek out reviews provided by others.1,15,16 Also available within this article are grades of recommendations and levels of evidence, which have been established for each therapy according to the National Institute for Health and Clinical Excellence guidelines [Tables 1–3].17 A schematic overview of possible anti-keloid mechanisms of action with regard to emerging and novel treatments is also presented [Figure 1].
Table 1.
Strength of recommendation and level of evidence for anti-keloid therapies17
| Treatment | Strength of recommendation | Level of evidence |
|---|---|---|
| Anti-hypertensive pharmaceuticals | B | 1+ |
| Botulinum toxin A | A | 1++ |
| Calcineurin inhibitors | D | 3 |
| Doxorubicin | D (GPP) | 4 |
| Electrical stimulation | D | 2+ |
| Microneedle physical contact | C | 2+ |
| Extracorporeal shockwave therapy | C | 2+ |
| Fat grafting | C | 2+ |
| Stem cell therapy | D (GPP) | 4 |
| Imidazoquinolines | D | 3 |
| Interferons | B | 1++ |
| Tamoxifen | D | 3 |
| RNA-based therapies | D (GPP) | 4 |
GPP = good practice point.
Table 2.
Strength of recommendation for anti-keloid therapies17
| Grade | Evidence |
|---|---|
| A |
|
| B |
|
| C |
|
| D |
|
| D (GPP) |
|
RCT = randomised controlled trial; NICE = National Institute for Health and Clinical Excellence; GPP = good practice point.
Table 3.
Level of evidence for anti-keloid therapies17
| Level of evidence | Type of evidence |
|---|---|
| 1++ |
|
| 1+ |
|
| 1− |
|
| 2++ |
|
|
|
| 2+ |
|
| 2− |
|
| 3 |
|
| 4 |
|
RCT = randomised controlled trial.
Figure 1.
Possible mechanisms of action for emerging and novel treatments of keloids.
1 = Anti-hypertensive pharmaceuticals; 2 = Botulinum toxin A; 3 = Calcineurin inhibitors; 4 = Doxorubicin; 5 = Electrical stimulation; 6 = Microneedle physical contact; 7 = Extracorporeal shockwave therapy; 8 = Fat grafting; 9 = Stem cell therapy; 10 = Imidazoquinolines; 11 = Interferons; 12 = Tamoxifen; 13 = RNA-based therapies; TGF-β = transforming growth factor beta; α-SMA= alpha-smooth muscle actin; ECM = extracellular matrix; IGF = insulin-like growth factor; IFN-α = interferon-alpha.
Methods
PubMed® (National Library of Medicine, Bethesda, Maryland, USA), EMBASE (Elsevier, Amsterdam, Netherlands) and Web of Science (Clarivate Analytics, Philadelphia, Pennsylvania, USA) databases were searched for articles published from January 1987 to June 2020. The following terms were used: ‘keloid’ AND ‘treatments’ OR ‘therapies’ OR ‘anti-hypertensive pharmaceuticals’ OR ‘botulinum toxin A’ OR ‘calcineurin inhibitors’ OR ‘doxorubicin’ OR ‘electrical stimulation’ OR ‘microneedle physical contact’ OR ‘extracorporeal shockwave therapy’ OR ‘fat grafting’ OR ‘mesenchymal stem cell therapy’ OR ‘imidazoquinolines’ OR ‘interferons’ OR ‘tamoxifen’ OR ‘RNA-based therapies’. Reference lists of selected articles, other related studies and review articles were also examined. The inclusion criteria comprised all articles referring to different methods of treatment for keloids in the English language literature. Following an initial screening of 5,351 titles and abstracts, 167 potentially relevant articles were retrieved for full-text review. Of these potentially relevant articles, 49 were excluded as they failed to meet the inclusion criteria. A total of 118 articles were selected for final inclusion in this review.
EMERGING AND NOVEL TREATMENTS
Anti-hypertensive pharmaceuticals
Angiotensin-converting enzyme (ACE) is a peptidyl-dipeptide hydrolase which converts angiotensin I to angiotensin II. This enzyme is involved in both blood pressure regulation and fibrous remodeling.18,19 Given that severe keloids seem to be associated with hypertension, ACE inhibition should be considered as a potential treatment against keloids.20,21 Angiotensin II elevates the level of transforming growth factor beta (TGF-β) and plays a prominent role in collagen biosynthesis and wound healing.22,23 For instance, an ACE-inhibitor lisinopril weakened in vitro proliferation of mouse NIH 3T3 fibroblasts and collagen expression by suppressing phosphorylation of SMAD2/3 and TAK1.24 In rats with acute dermal wounds, scars treated with ramipril (an ACE-inhibitor) were narrower compared to the control groups, which had their wounds treated with water; the treated wounds also exhibited augmented neovascularisation and re-epithelialisation. 24 Interestingly, complete recovery from keloid scars was reported in a 54-year-old female patient following daily oral administration of the ACE-inhibitor enalapril (10 mg).25 In a second case, satisfactory results were reported in a 70-year-old female diabetic patient who received the same dose of enalapril.25 Uzun et al. showed that oral administration of enalapril following dermal injury reduced formation of hypertrophic scars in rabbit ear wounding models.26 Moreover, six weeks of twice-daily application of captopril cream (5%) in an 18-year-old female patient lessened not only the height of scar but also the redness and itchiness, without cutaneous and systemic side effects.23 One study evaluated the efficacy of losartan (an angiotensin II receptor blocker) on hypertrophic scars and keloids.22 In this study, 5% losartan ointment twice a day for three months resulted in a significant reduction in vascularity and pliability compared to patients receiving a placebo (P <0.05). No allergic or hypotensive symptoms were observed in patients who underwent the ointment treatment.22
Verapamil is a member of the phenylalkylamine family, which is a class of calcium antagonists. It is capable of blocking both L-type and T-type calcium channels, thereby lowering blood pressure.27 Verapamil triggers synthesis of procollagenase in keloids and normal human cultured fibroblasts, causing reduced production of fibrous tissue. For example, an intralesional injection of verapamil (2.5 mg/mL) led to a decrement in pliability, vascularity, height and width of scars after three weeks of treatment.28 A double-blind randomised controlled trial (RCT) revealed that intralesional verapamil injections (2.5 mg/mL) at monthly intervals (four doses) in half of the wounds were safe but not as effective as a similar regimen (10 mg/mL) of triamcinolone (TAC) for prevention of keloid scar recurrence following excision removal.29 It has been suggested that increasing the dose or frequency of injections would improve treatment outcomes when verapamil is exploited as an adjunct to surgical excision.29 In a recent retrospective study, combination of verapamil and intralesional TAC led to a pronounced improvement of keloid scars with a long-lasting result.30 Lawrence also demonstrated that intralesional verapamil hydrochloride after earlobe surgical keloid excision had a 52% cure rate in 31 African-American patients.31 Similarly, one study revealed that keloidectomy with core fillet flap and intralesional verapamil injection is a reliable and cost-effective method in the treatment of earlobe keloids with a low rate of recurrence.32 Overall, keloid recurrence rates for verapamil have been found to range from 1.4–48%.29
Botulinum toxin A
Botulinum toxin, a potent neurotoxin produced by Clostridium botulinum, has a wide range of applications in medicine including the United States Food and Drug Administration (FDA)-approved treatments for strabismus, blepharospasm, hemifacial spasm, cervical dystonia, axillary hyperhidrosis, chronic migraine, neurogenic detrusor overactivity and for cosmetic use.33 With regard to keloids, intralesional injection of the toxin abates scar proliferation by reducing muscle tension during wound healing, halting fibroblast cell cycles in the non-proliferative stage and modulating TGF-β1 expression.34,35 Patients have also reported higher satisfaction rates and improvements in erythema, pain, pliability and itching.36,37
A prospective, uncontrolled study revealed that intralesional injections of botulinum toxin A (70–140 units per session every three months) into keloids resulted in peripheral regression and lesion flattening without recurrence after one year.38 This finding is in line with an RCT in which intralesional administration of botulinum toxin A (5 IU/cm3 every eight weeks) caused significant decrements in both the volume and height of keloid lesions (P <0.01 each) as well as substantial softening of the lesions compared to baseline.39 By contrast, another study found that intralesional botulinum toxin A (70–140 Speywood units per session every two months) did not lead to regression of keloid tissue; neither cell proliferation nor metabolism of keloid fibroblasts were influenced by botulinum toxin A.35 In a double-blinded study, botulinum toxin A was not superior to corticosteroids for preventing earlobe or sternal keloid recurrence at one- and three-month follow-up.40 However, the advantage of using botulinum toxin A is that it is a single injection compared to steroid therapy which requires monthly injections.40
A recent study demonstrated adjuvant properties of botulinum toxin in treating keloids.41 In this context, intralesional TAC plus botulinum toxin A led to significant symptomatic improvement of pain and pruritus in comparison to intralesional TAC alone (P <0.001). Likewise, botulinum toxin A in combination with surgery was also successful in treating post-otoplasty keloids in 16 patients.42 A systematic review and meta-analysis of 15 RCTs also revealed that injection of intralesional botulinum toxin A was more effective in treating keloids than injecting intralesional corticosteroid or a placebo.43 Although intralesional injection of the toxin has shown satisfactory results in patients with keloids, further large-scale studies with comparative designs and long-term follow-up are warranted to delineate the value of this therapy in keloid management protocols.44
Calcineurin inhibitors
The cyclic depsipeptide tacrolimus (FK-506) produced by Streptomyces tsukubaensis inhibits calcineurin complex with FK-binding proteins.45 As a potent immunosuppressor, tacrolimus has been applied widely to prevent organ rejection and treat autoimmune diseases as well as skin disorders including atopic dermatitis, keloids, erosive mucosal lichen planus, psoriasis and pyoderma gangrenosum.46–48 Tacrolimus interdicts keloidal fibroblast proliferation, migration and collagen production. Additionally, it inhibits TGF-β/SMAD signalling pathways in keloid fibroblasts through down-regulation of TGF-β receptors.49 Expression of glioma-associated oncogene 1 (GLI1) has been shown to be elevated in keloids. Because tacrolimus is able to inhibit signalling from the GLI1, Kim et al. suggested that tacrolimus may have anti-keloid properties.50 Another study demonstrated the beneficial effects of topical tacrolimus ointment in preventing hypertrophic scars in rabbit models.51 In an open-label pilot study, the majority of patients exhibited a decrement in induration, erythema, pruritus and tenderness after topical application of tacrolimus ointment (0.1%) twice a day for 12 weeks, yet no statistically significant benefits as a therapeutic agent were observed.52 Another study showed the preventive role of topical tacrolimus against keloids in 25 patients after surgery.53 Nevertheless, further clinical investigations involving topical tacrolimus are warranted for delineating its efficacy in keloid treatment.
Sirolimus (rapamycin) is a cyclic depsipeptide produced by Streptomyces hygroscopius; it has anti-fungal, immunosuppressive and anti-tumour/anti-proliferative properties that have been ascribed to sirolimus.45,54 This medication hampers the mammalian target of rapamycin (mTOR), a serine/threonine kinase that regulates both metabolic processes and translation rates. Levels of total endogenous mTOR have been reported to be increased in keloids.55 It is worth noting that mTOR regulates the expression of collagen type I in human dermal fibroblasts.56 When applied to cultured normal and keloidal fibroblasts, sirolimus down-regulated the expression of cytoplasmic proliferating cell nuclear antigen, cyclin D1, collagen fibronectin and alpha-smooth muscle actin (α-SMA) in a dose- and time-dependent manner, indicating the anti-proliferative effects and therapeutic potential of sirolimus in keloid treatment.55 Inhibition of extracellular matrix (ECM) deposition, prevention of platelet-derived growth factor-induced collagen synthesis and reduction in over-expression of collagen I and III in keloid fibroblasts are other functions of sirolimus.57
Doxorubicin
Doxorubicin, which was first extracted from Streptomyces peucetius, is an anthracycline antibiotic with a DNA intercalating property that is routinely exploited in cancer chemotherapy.58,59 Poor wound healing as a consequence of impaired collagen biosynthesis has been acknowledged as an adverse effect associated with doxorubicin administration, suggesting the potential role of this medication in treating hypertrophic scars and keloids.60 Doxorubicin has been demonstrated to decrease fibroblast proliferation in vitro.61 It inhibits enzymes prolyl 4-hydroxylase and prolidase in human skin fibroblast cultures, thereby reducing collagen synthesis.60,61 No published trials regarding the anti-keloidal effects of doxorubicin are available in the literature, warranting further studies to evaluate its efficacy in keloid treatment.62
Electrical stimulation
Electrical stimulation (ES) has been used to treat abnormal scars in the past.63 A novel device called Fenzian system, which produces degenerate waves, was developed recently and shows promise in curing keloids and hypertrophic scars.64 For instance, in a study conducted by Ud-din et al., the effectiveness of the Fenzian system on symptomatic raised dermal scars was assessed in 18 patients using full-field laser perfusion imaging to evaluate changes in dermal blood flow.65 A significant reduction in pain scores (P = 0.007) and pruritus scores (P = 0.002) was observed over one month. The researchers also found that symptoms in patients with long-standing sternal scars needed more time to diminish compared to patients with scars in less stress-prone anatomical locations such as the breast or abdomen.65
Perry et al. showed that the Fenzian system successfully reduced pain, itching and scar scores (P <0.05) in 30 patients with 140 scars, most of which were keloids and hypertrophic scars on the sternum, breast and shoulder girdle.63 It seems that suppression of excessive collagen I formation is a major mechanism behind the anti-keloid properties of ES.63 Another investigation revealed that the cytotoxic effects of photodynamic therapy on keloid fibroblasts can be enhanced significantly (P <0.05) when combined with degenerate electrical waveform stimulation.66 In one study, combining the local treatment of mature scars with low-intensity electromagnetic and electric stimulation with negative pressure exhibited a satisfactory synergic effect on scar collagen and elastic fibre remodelling in 20 patients.67 Nevertheless, further large-scale controlled studies are needed to elucidate the overall efficacy of ES.
Microneedle physical contact
Microneedles have been studied by many researchers over the past decade primarily for transdermal drug delivery. In contrast to conventional needles that are at least 1 mm in width or have even larger dimensions, microneedle devices consist of needles with sizes expressed in microns. Microneedles are able to penetrate the stratum corneum without contacting the nerves in the dermis. Various types of microneedles, such as solid, coated, dissolving and hollow forms, exist. On the whole, these devices cause less pain, infection and injury compared with conventional injections.68
In a controlled clinical trial, Tan et al. found that once-daily application of dissolving TAC-embedded microneedle patches markedly diminished the volume of keloids on the chest, arms and shoulders.69 Similarly, Yeo et al. tested FDA-approved liquid crystalline polymer-based microneedles to determine its ability to inhibit keloid fibroblast proliferation.70 After a 12- hour treatment, the non-viable proportion of keloid fibroblasts in cell cultures increased to 83.8 ± 11.96%. They also showed that microneedle treatment in rabbit ear hypertrophic scar models prevented dermis tissue thickening in 83.33% (n = 15) of wounds.70 In the same study, the microneedle patch was evaluated on a patient suffering from a single post-surgical hypertrophic scar on the dorsum region; after treatment, the patient’s redness, pruritus and chronic inflammation reduced.70
The efficiency of a microneedle patch comprising polyethylene glycol diacrylate and encapsulating 5-FU for transdermal delivery has been examined in its use with keloids. The microneedle patch effectively abolished keloid fibroblast proliferation in a cell culture. Although soluble 5-FU delivery was able to reduce keloid fibroblast proliferation (6.9 ± 3.6-fold expansion), the extent of inhibition was even higher when microneedle-assisted delivery was applied (2.0 ± 0.4-fold expansion; P <0.05).71 Surprisingly, even drug-free microneedles induced an inhibitory effect, with an approximately 10-fold reduction in cell viability compared to controls.71 A preliminary analysis of cell morphology demonstrated that keloid fibroblasts treated with blank microneedles decreased in size and had higher nucleus-to-cytoplasm ratios, while the non-treated keloid fibroblasts were flat and spindle-shaped. However, the mechanism of this unexpected phenomenon in which the physical presence of microneedles inhibits the proliferation of keloid fibroblasts remains unclear.71 Transdermal delivery of antimicrobial peptides using microneedle stamping devices or drug-loaded microneedle patches has recently been proposed as a new strategy for treating keloids and hypertrophic scars.72,73 Future studies should involve clinical subjects to validate the efficiency and safety of microneedle drug delivery systems for treating keloids.
Extracorporeal shockwave therapy
Extracorporeal shockwave therapy (ESWT) was first introduced in 1982 for urinary stone lithotripsy.74 In recent decades, the success of ESWT in addressing urinary stones has made it a first-line, cost-effective, non-invasive treatment. ESWT has also been used to treat musculoskeletal maladies including plantar fasciitis and chronic lateral epicondylitis.75 Multiple lines of evidence exist in the literature regarding ESWT’s effectiveness in enhancing healing in patients with acute and chronic wounds and diabetic foot ulcers.76,77
A few studies have evaluated ESWT for treating post-burn, hypertrophic and keloid scars.78–80 In one study, 17 patients with burn scars on their extremities underwent ESWT sessions once a week for six weeks. The visual analogue scale (VAS) was used to quantify pruritus and pain, while the Vancouver Scar Scale (VSS) was used to evaluate scar appearance. Both VAS and VSS scores were significantly diminished after treatment and during follow-ups (P <0.001).78 Another study assessed the effects of ESWT used twice a week for six weeks on 16 patients with post-burn scar contractures, hypertrophic scars and keloids.79 The scars were located on patients’ extremities, their faces or mentosternal regions or on their trunks. The study found that the scars appeared more pliable and colour mismatch was lessened after the first session. At the end of the treatment, all of the scars had more acceptable appearances.79 Wang et al. also compared the efficiency of ESWT and intralesional steroid injection with TAC in treating keloid scars.80 The ESWT group received three ESWT treatments in six weeks whereas the steroid group received three intralesional TAC injections in six weeks. Both groups demonstrated substantial improvements in keloid appearance with less discolouration, greater flattening, a softer consistency and greater elasticity. Moreover, the ESWT group displayed comparable functional outcomes and a remarkable reduction in collagen fibres as well as increasing matrix metalloproteinase-13 degrading enzyme levels compared to the intralesional steroid injection group.80
The exact mechanism underlying the observed beneficial effects of ESWT remains unknown. When primary dermal fibroblasts derived from human post-burn hypertrophic scars were exposed to shockwave pulses, TGF-β1, α-SMA, collagen-I, fibronectin and TWIST1 levels were significantly reduced, while expression of E-cadherin was increased.81 It was suggested that suppressed epithelial-mesenchymal transition might be responsible for the anti-scarring effects of ESWT.81 The acoustic waves also mechanically disrupt tissue by cavitation.78 Indeed, shock waves yield microscopic injuries in scar tissue and disintegrate collagen fibres which results in scar remodelling. Two mechanisms for ESWT have been explained: (1) shock waves affect pain receptor physiology and (2) these waves generate micro-trauma and release cytokines, promoting tissue repair.78
Fat grafting
Autologous fat grafting (i.e. lipotransfer) for patients with keloids has been exemplified in several studies.82–84 In three clinical cases, fat injection at the dermal-hypodermal junction in hemifacial hypertrophic scars and keloids led to substantial improvement in skin texture, softness, thickness and elasticity after a six-month follow-up period.82 Another study also demonstrated that autologous fat grafting in 18 patients with post-burn hypertrophic scars and keloids improved texture, colour, softness, thickness and elasticity of the treated skin.83 New collagen deposition, dermal hyperplasia and neoangiogenesis were also evident based on histological evaluation.83 The procedure causes less fibrosis, pain reduction and more flexibility in areas of scar contraction. It seems that transfer of adipose tissue-derived stem cells (ADSCs) into wounds plays a key role in inhibiting keloid fibroblast proliferation and ECM formation.84
Stem cell therapy
The possible mechanisms underlying mesenchymal stem cell (MSC) therapy are modulation and prevention of inflammatory processes as well as anti-fibrosis effects by reducing collagen production while enhancing normal angiogenetic activity.36 Various delivery methods, including systemic injection, local injection at the site of wound, intradermal or subcutaneously and engineered MSC-seeded tissue scaffolds have been used.85 For instance, Zhang et al. demonstrated that intralesional injection of ADSCs decreased hypertrophic scarring in rabbits by reducing the gene expression of collagen type I, α-SMA and collagen deposition.86 In another study, ADSC-conditioned medium (ADSC-CM) not only decreased the expression of collagen type I and III and α-SMA but also suppressed collagen deposition and scar formation through the inhibition of the p38/mitogen-activated protein kinase (MAPK) signalling pathway in hypertrophic scar-derived fibroblasts in vitro.87 In a recent study, ADSC-CM has been reported to attenuate the gene expression of plasminogen activator inhibitor-1, tissue inhibitor of metalloproteinases 1 (TIMP-1) and collagen type I in keloid fibroblasts.88 Notably, 24-hour incubation of keloid fibroblasts with ADSC-CM significantly inhibited cell proliferation in the G2/M phase compared to the control group (P <0.05). ADSC-CM was also capable of reducing invasive abilities of keloid fibroblasts in vitro.88 Furthermore, bone marrow-derived stem cells-conditioned medium (BMSC-CM) has been shown to inhibit cell proliferation and migration of hypertrophic scar and keloid fibroblasts.89 At both transcriptional and translational levels, BMSC-CM reduced expression of profibrotic genes in hypertrophic scar and keloid fibroblasts.89 Additionally, this novel strategy has been investigated in several fibrotic diseases such as myocardial infarctions, renal fibrosis, and liver cirrhosis. Although promising, MSC therapy requires further in vivo studies in order to apply these results to clinical practice.36
Imidazoquinolines
Imidazoquinolines, including imiquimod and resiquimod, are toll-like receptors 7 and 8 agonists with potent immune modulator activities. Resiquimod is up to 100-fold more potent in vitro and in vivo than imiquimod.90 They stimulate production of interferon-alpha (IFN-α) at the site of application, which intensifies collagen breakdown. Imiquimod 5% cream is approved for the treatment of genital warts, superficial basal cell carcinoma and actinic keratosis.91 Imiquimod can be applied post-surgery using different treatment regimens, starting on the night of surgery with daily treatments or two weeks after the operation on alternate nights for eight weeks.92–94
In a pilot study, post-surgical use of imiquimod 5% cream was effective in treating earlobe keloids without recurrence after 12 months’ follow-up.95 In addition, alleviation of symptoms such as pruritus and pain within the first post-operative month was reported in all patients.95 Likewise, Berman et al. showed the effectiveness of imiquimod 5% cream in preventing recurrence of earlobe keloids after excision.96 In their study, four patients with a total of eight large pedunculated earlobe keloids were successfully treated with debulking by tangential shave excision followed by daily application of imiquimod 5% cream for six weeks.96 In another study, post-surgical use of imiquimod 5% cream in 35 patients with keloids indicated a recurrence rate of 28.6%.93 However, a high recurrence rate (88.9%) was reported in one study in which nine patients with trunk keloids underwent surgery followed by daily application of imiquimod 5% cream for eight weeks.97 The imiquimod-poly(2-(2-methoxyethoxy)ethyl methacrylate) hydrogel dressing has also been shown to inhibit the proliferation of keloid fibroblasts in vitro.98
Interferons
Interferons are potent cytokines that possess anti-proliferative, anti-fibrotic and anti-viral effects. They are extensively employed in a variety of maladies including condylomata accuminata, basal cell carcinoma, high risk melanoma, Kaposi’s sarcoma and viral hepatitis. All interferon isoforms—especially INF-α2b and INF-γ—have been shown to attenuate collagen synthesis together with fibroblast proliferation and to induce TGF-β1 down-regulation.36,99,100 Some evidence showed that INF-γ enhances myofibroblast apoptosis and prevents its differentiation, whereas INF-α2b inhibits wound contraction in vitro.101,102 Nonetheless, some clinical trials have revealed contrasting results.103–105 For this reason, more RCTs should be performed to enrich existing data. Complications of therapy with interferon injection are flu-like symptoms, fever, headaches, fatigue and myalgia.36,99
In Berman and Flores’ study, post-operative injections of INF-α2b in keloids (80% were earlobe keloids) resulted in a lower recurrence rate (18.7%) compared to adjuvant post-operative TAC injections (58.4%).106 When combined with intralesional TAC, INF-α2b caused a significant decrease in keloid volume (86.6%; P = 0.002) and depth (81.6%; P = 0.005).107 The majority of these scars were located on the chest, shoulders, upper arms and back. No keloid recurrence was reported.107
Tamoxifen
Tamoxifen is a synthetic non-steroidal anti-estrogen which has been exploited extensively in both chemoprevention and breast cancer treatment.108 Tamoxifen modifies RNA transcription, hinders keloidal fibroblast proliferation, influences the cell cycle in the G-phase and suppresses insulin-like growth factor production.109,110 One study showed that tamoxifen dose-dependently waned the expression of TGF-β1 in keloid fibroblast cultures.111 Moreover, it was capable of impeding lattice contraction in a dose-and time-dependent manner in vitro.110 Additionally, topical application of a 2% tamoxifen ointment on third-degree burns in rats augmented angiogenesis and decreased fibrotic tissue thickness.112 In Pasquetti et al.’s study, topical application of 0.1% tamoxifen citrate on keloids or hypertrophic scars located on the sternum, shoulders, abdomen and upper limbs had satisfactory results with a substantial decrement in lesion height, width and length.113 Soares-Lopes et al. found that the intralesional administration of tamoxifen in 13 patients with keloids resulted in a significant reduction in collagen fibres and fibroblasts (P <0.0001).114
RNA-based therapies
RNA interference (RNAi) is a biological process for gene-specific RNA degradation. It is mediated by small interfering RNAs (siRNAs).115 During the past few years, several attempts have been made to inhibit gene expression by siRNAs in keloid fibroblasts. For example, siRNA targeting TIMP-1/-2 resulted in degradation of collagen type I in keloid fibroblasts.116 In Shin et al.’s study, heat shock protein 70 knockdown using siRNAs caused a marked decrement in collagen production in keloid fibroblasts compared to controls.117 Furthermore, transfection of keloid fibroblasts with siRNA targeting the human wingless-related mouse mammary tumour virus integration site 2 led to considerably slower growth and a substantial delay in cell doubling time.118 These studies show that RNA-based therapies hold potential for treating keloids.
Conclusion
Although a plethora of therapeutic options are now available for keloids, they still remain an ongoing clinical challenge for both patients and clinicians. Given the complex process of keloid formation, a deeper understanding of the molecular mechanisms that drive development and recurrence of keloids would open further avenues for developing innovative treatments. Several promising therapeutic approaches, such as the use of mesenchymal stem cells, autologous fat grafting, microneedle physical contact and RNA-based therapies, are currently underway. Nevertheless, there is still a great need for high-quality RCTs with sufficient sample sizes.
Footnotes
AUTHORS’ CONTRIBUTIONS
All authors contributed equally for writing and revision of this manuscript. The authors reviewed and approved the final submitted manuscript.
References
- 1.Mari W, Alsabri SG, Tabal N, Younes S, Sherif A, Simman R. Novel insights on understanding of keloid scar: Article review. J Am Coll Clin Wound Spec. 2016;7:1–7. doi: 10.1016/j.jccw.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chodon T, Sugihara T, Igawa HH, Funayama E, Furukawa H. Keloid-derived fibroblasts are refractory to Fas-mediated apoptosis and neutralization of autocrine transforming growth factor-beta1 can abrogate this resistance. Am J Pathol. 2000;157:1661–9. doi: 10.1016/s0002-9440(10)64803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porter JP. Treatment of the keloid: What is new? Otolaryngol Clin North Am. 2002;35:207–20. doi: 10.1016/s0030-6665(03)00103-8. [DOI] [PubMed] [Google Scholar]
- 4.Bran GM, Goessler UR, Hormann K, Riedel F, Sadick H. Keloids: Current concepts of pathogenesis (review) Int J Mol Med. 2009;24:283–93. doi: 10.3892/ijmm_00000231. [DOI] [PubMed] [Google Scholar]
- 5.Marttala J, Andrews JP, Rosenbloom J, Uitto J. Keloids: Animal models and pathologic equivalents to study tissue fibrosis. Matrix Biol. 2016;51:47–54. doi: 10.1016/j.matbio.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berman B, Bieley HC. Adjunct therapies to surgical management of keloids. Dermatol Surg. 1996;22:126–30. doi: 10.1111/j.1524-4725.1996.tb00493.x. [DOI] [PubMed] [Google Scholar]
- 7.Cheng ET, Nowak KC, Koch RJ. Effect of blended carbon dioxide and erbium: YAG laser energy on preauricular and ear lobule keloid fibroblast secretion of growth factors: A serum-free study. Arch Facial Plast Surg. 2001;3:252–7. doi: 10.1001/archfaci.3.4.252. [DOI] [PubMed] [Google Scholar]
- 8.Murray JC, Pollack SV, Pinnell SR. Keloids: A review. J Am Acad Dermatol. 1981;4:461–70. doi: 10.1016/s0190-9622(81)70048-3. [DOI] [PubMed] [Google Scholar]
- 9.Ghazawi FM, Zargham R, Gilardino MS, Sasseville D, Jafarian F. Insights into the pathophysiology of hypertrophic scars and keloids: How do they differ? Adv Skin Wound Care. 2018;31:582–95. doi: 10.1097/01.ASW.0000527576.27489.0f. [DOI] [PubMed] [Google Scholar]
- 10.Glass DA., 2nd Current understanding of the genetic causes of keloid formation. J Investig Dermatol Symp Proc. 2017;18:S50–3. doi: 10.1016/j.jisp.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 11.Child FJ, Fuller LC, Higgins EM, Du Vivier AW. A study of the spectrum of skin disease occurring in a black population in south-east London. Br J Dermatol. 1999;141:512–17. doi: 10.1046/j.1365-2133.1999.03047.x. [DOI] [PubMed] [Google Scholar]
- 12.Jones CD, Guiot L, Samy M, Gorman M, Tehrani H. The use of chemotherapeutics for the treatment of keloid scars. Dermatol Reports. 2015;7:5880. doi: 10.4081/dr.2015.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee YI, Kim J, Yang CE, Hong JW, Lee WJ, Lee JH. Combined therapeutic strategies for keloid treatment. Dermatol Surg. 2019;45:802–10. doi: 10.1097/DSS.0000000000001695. [DOI] [PubMed] [Google Scholar]
- 14.Moravvej H, Memariani M, Memariani H. Quercetin: A potential treatment for keloids. Sultan Qaboos Univ Med J. 2019;19:e372–3. doi: 10.18295/squmj.2019.19.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mafong EA, Ashinoff R. Treatment of hypertrophic scars and keloids: A review. Aesthetic Surg J. 2000;20:114–21. doi: 10.1067/maj.2000.106649. [DOI] [Google Scholar]
- 16.Betarbet U, Blalock TW. Keloids: A review of etiology, prevention, and treatment. J Clin Aesthet Dermatol. 2020;13:33–43. [PMC free article] [PubMed] [Google Scholar]
- 17.National Institute for Health and Clinical Excellence (NICE) [Accessed: Jul 2020]. From http://www.nice.org.uk/
- 18.Stewart J, Glass DA., 2nd Plasma angiotensin-converting enzyme levels in patients with keloids and/or hypertension. Wounds. 2018;30:E71–2. [PMC free article] [PubMed] [Google Scholar]
- 19.Morihara K, Takai S, Takenaka H, Sakaguchi M, Okamoto Y, Morihara T, et al. Cutaneous tissue angiotensin-converting enzyme may participate in pathologic scar formation in human skin. J Am Acad Dermatol. 2006;54:251–7. doi: 10.1016/j.jaad.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 20.Ogawa R, Arima J, Ono S, Hyakusoku H. CASE REP ORT Total management of a severe case of systemic keloids associated with high blood pressure (hypertension): Clinical symptoms of keloids may be aggravated by hypertension. Eplasty. 2013;13:e25. [PMC free article] [PubMed] [Google Scholar]
- 21.Niazi F, Hooshyar SH, Hedayatyanfard K, Ziai SA, Doroodgar F, Niazi S, et al. Detection of angiotensin II and AT1 receptor concentrations in keloid and hypertrophic scar. J Clin Aesthet Dermatol. 2018;11:36–9. [PMC free article] [PubMed] [Google Scholar]
- 22.Hedayatyanfard K, Ziai SA, Niazi F, Habibi I, Habibi B, Moravvej H. Losartan ointment relieves hypertrophic scars and keloid: A pilot study. Wound Repair Regen. 2018;26:340–3. doi: 10.1111/wrr.12648. [DOI] [PubMed] [Google Scholar]
- 23.Ardekani GS, Aghaei S, Nemati MH, Handjani F, Kasraee B. Treatment of a post-burn keloid scar with topical captopril: Report of the first case. Plast Reconstr Surg. 2009;123:112e–3. doi: 10.1097/PRS.0b013e31819a34db. [DOI] [PubMed] [Google Scholar]
- 24.Fang QQ, Wang XF, Zhao WY, Ding SL, Shi BH, Xia Y, et al. Angiotensin-converting enzyme inhibitor reduces scar formation by inhibiting both canonical and noncanonical TGF-β1 pathways. Sci Rep. 2018;8:3332. doi: 10.1038/s41598-018-21600-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iannello S, Milazzo P, Bordonaro F, Belfiore F. Low-dose enalapril in the treatment of surgical cutaneous hypertrophic scar and keloid--Two case reports and literature review. MedGenMed. 2006;8:60. [PMC free article] [PubMed] [Google Scholar]
- 26.Uzun H, Bitik O, Hekimoğlu R, Atilla P, Kayikçioğlu AU. Angiotensin-converting enzyme inhibitor enalapril reduces formation of hypertrophic scars in a rabbit ear wounding model. Plast Reconstr Surg. 2013;132:e361–71. doi: 10.1097/PRS.0b013e31829acf0a. [DOI] [PubMed] [Google Scholar]
- 27.Bergson P, Lipkind G, Lee SP, Duban ME, Hanck DA. Verapamil block of T-type calcium channels. Mol Pharmacol. 2011;79:411–19. doi: 10.1124/mol.110.069492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Margaret Shanthi FX, Ernest K, Dhanraj P. Comparison of intralesional verapamil with intralesional triamcinolone in the treatment of hypertrophic scars and keloids. Indian J Dermatol Venereol Leprol. 2008;74:343–8. doi: 10.4103/0378-6323.42899. [DOI] [PubMed] [Google Scholar]
- 29.Danielsen PL, Rea SM, Wood FM, Fear MW, Viola HM, Hool LC, et al. Verapamil is less effective than triamcinolone for prevention of keloid scar recurrence after excision in a randomized controlled trial. Acta Derm Venereol. 2016;96:774–8. doi: 10.2340/00015555-2384. [DOI] [PubMed] [Google Scholar]
- 30.Kant SB, van den Kerckhove E, Colla C, Tuinder S, van der Hulst RRWJ, Piatkowski de Grzymala AA. A new treatment of hypertrophic and keloid scars with combined triamcinolone and verapamil: A retrospective study. Eur J Plast Surg. 2018;41:69–80. doi: 10.1007/s00238-017-1322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawrence WT. Treatment of earlobe keloids with surgery plus adjuvant intralesional verapamil and pressure earrings. Ann Plast Surg. 1996;37:167–9. doi: 10.1097/00000637-199608000-00008. [DOI] [PubMed] [Google Scholar]
- 32.El-Kamel MF, Selim MK, Alghobary MF. Keloidectomy with core fillet flap and intralesional verapamil injection for recurrent earlobe keloids. Indian J Dermatol Venereol Leprol. 2016;82:659–65. doi: 10.4103/0378-6323.187084. [DOI] [PubMed] [Google Scholar]
- 33.Chen S. Clinical uses of botulinum neurotoxins: current indications, limitations and future developments. Toxins (Basel) 2012;4:913–39. doi: 10.3390/toxins4100913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Austin E, Koo E, Jagdeo J. The cellular response of keloids and hypertrophic scars to botulinum toxin A: A comprehensive literature review. Dermatol Surg. 2018;44:149–57. doi: 10.1097/DSS.0000000000001360. [DOI] [PubMed] [Google Scholar]
- 35.Gauglitz GG, Bureik D, Dombrowski Y, Pavicic T, Ruzicka T, Schauber J. Botulinum toxin A for the treatment of keloids. Skin Pharmacol Physiol. 2012;25:313–18. doi: 10.1159/000342125. [DOI] [PubMed] [Google Scholar]
- 36.Lee HJ, Jang YJ. Recent understandings of biology, prophylaxis and treatment strategies for hypertrophic scars and keloids. Int J Mol Sci. 2018;19:711. doi: 10.3390/ijms19030711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Viera MH, Amini S, Valins W, Berman B. Innovative therapies in the treatment of keloids and hypertrophic scars. J Clin Aesthet Dermatol. 2010;3:20–6. [PMC free article] [PubMed] [Google Scholar]
- 38.Zhibo X, Miaobo Z. Intralesional botulinum toxin type A injection as a new treatment measure for keloids. Plast Reconstr Surg. 2009;124:e275–7. doi: 10.1097/PRS.0b013e3181b98ee7. [DOI] [PubMed] [Google Scholar]
- 39.Shaarawy E, Hegazy RA, Abdel Hay RM. Intralesional botulinum toxin type A equally effective and better tolerated than intra-lesional steroid in the treatment of keloids: A randomized controlled trial. J Cosmet Dermatol. 2015;14:161–6. doi: 10.1111/jocd.12134. [DOI] [PubMed] [Google Scholar]
- 40.Pruksapong C, Yingtaweesittikul S, Burusapat C. Efficacy of botulinum toxin A in preventing recurrence keloids: Double blinded randomized controlled trial study: Intraindividual subject. J Med Assoc Thai. 2017;100:280–6. [PubMed] [Google Scholar]
- 41.Rasaii S, Sohrabian N, Gianfaldoni S, Hadibarhaghtalab M, Pazyar N, Bakhshaeekia A, et al. Intralesional triamcinolone alone or in combination with botulinium toxin A is ineffective for the treatment of formed keloid scar: A double blind controlled pilot study. Dermatol Ther. 2019;32:e12781. doi: 10.1111/dth.12781. [DOI] [PubMed] [Google Scholar]
- 42.Batifol D, de Boutray M, Galmiche S, Finiels PJ, Jammet P. Treatment of keloid scars by botulinum toxin. J Ear Nose Throat Disord. 2017;2:1024. [Google Scholar]
- 43.Bi M, Sun P, Li D, Dong Z, Chen Z. Intralesional injection of botulinum toxin type A compared with intralesional injection of corticosteroid for the treatment of hypertrophic scar and keloid: A systematic review and meta-analysis. Med Sci Monit. 2019;25:2950–8. doi: 10.12659/MSM.916305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sohrabi C, Goutos I. The use of botulinum toxin in keloid scar management: A literature review. Scars Burn Heal. 2020;6:2059513120926628. doi: 10.1177/2059513120926628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thell K, Hellinger R, Schabbauer G, Gruber CW. Immuno-suppressive peptides and their therapeutic applications. Drug Discov Today. 2014;19:645–53. doi: 10.1016/j.drudis.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Viera MH, Vivas AC, Berman B. Update on keloid management: Clinical and basic science advances. Adv Wound Care (New Rochelle) 2012;1:200–6. doi: 10.1089/wound.2011.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelly AP. Medical and surgical therapies for keloids. Dermatol Ther. 2004;17:212–18. doi: 10.1111/j.1396-0296.2004.04022.x. [DOI] [PubMed] [Google Scholar]
- 48.Nasr IS. Topical tacrolimus in dermatology. Clin Exp Dermatol. 2000;25:250–4. doi: 10.1046/j.1365-2230.2000.00628.x. [DOI] [PubMed] [Google Scholar]
- 49.Wu CS, Wu PH, Fang AH, Lan CC. FK506 inhibits the enhancing effects of transforming growth factor (TGF)-β1 on collagen expression and TGF-β/Smad signalling in keloid fibroblasts: Implication for new therapeutic approach. Br J Dermatol. 2012;167:532–41. doi: 10.1111/j.1365-2133.2012.11023.x. [DOI] [PubMed] [Google Scholar]
- 50.Kim A, DiCarlo J, Cohen C, McCall C, Johnson D, McAlpine B, et al. Are keloids really "gli-loids"?: High-level expression of gli-1 oncogene in keloids. J Am Acad Dermatol. 2001;45:707–11. doi: 10.1067/mjd.2001.117736. [DOI] [PubMed] [Google Scholar]
- 51.Menezes MCS, Vasconcellos LS, Nunes CB, Alberti LR. Evaluation of the use of tacrolimus ointment for the prevention of hypertrophic scars in experimental model. An Bras Dermatol. 2019;94:164–71. doi: 10.1590/abd1806-4841.20197490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berman B, Poochareon V, Villa AM. An open-label pilot study to evaluate the safety and tolerability of tacrolimus ointment 0.1% for the treatment of keloids. Cos Derm. 2005;18:399–404. [Google Scholar]
- 53.Borzykh YA. The efficacy of topical tacrolimus in complex treatment of keloids. 2014;15:50–2. doi: 10.22141/1608-1706.1.15.2014.81264. [DOI] [Google Scholar]
- 54.Sehgal SN. Sirolimus: Its discovery, biological properties, and mechanism of action. Transplant Proc. 2003;35:S7–14. doi: 10.1016/s0041-1345(03)00211-2. [DOI] [PubMed] [Google Scholar]
- 55.Ong CT, Khoo YT, Mukhopadhyay A, Do DV, Lim IJ, Aalami O, et al. mTOR as a potential therapeutic target for treatment of keloids and excessive scars. Exp Dermatol. 2007;16:394–404. doi: 10.1111/j.1600-0625.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- 56.Shegogue D, Trojanowska M. Mammalian target of rapamycin positively regulates collagen type I production via a phosphatidylinositol 3-kinase-independent pathway. J Biol Chem. 2004;279:23166–75. doi: 10.1074/jbc.M401238200. [DOI] [PubMed] [Google Scholar]
- 57.Park J, Ha H, Ahn HJ, Kang SW, Kim YS, Seo JY, et al. Sirolimus inhibits platelet-derived growth factor-induced collagen synthesis in rat vascular smooth muscle cells. Transplant Proc. 2005;37:3459–62. doi: 10.1016/j.transproceed.2005.09.066. [DOI] [PubMed] [Google Scholar]
- 58.Thorn CF, Oshiro C, Marsh S, Hernandez-Boussard T, McLeod H, Klein TE, et al. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharmacogenet Genomics. 2011;21:440–6. doi: 10.1097/FPC.0b013e32833ffb56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tacar O, Sriamornsak P, Dass CR. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol. 2013;65:157–70. doi: 10.1111/j.2042-7158.2012.01567.x. [DOI] [PubMed] [Google Scholar]
- 60.Muszyńska A, Pałka J, Wołczyński S. Doxorubicin-induced inhibition of prolidase activity in human skin fibroblasts and its implication to impaired collagen biosynthesis. Pol J Pharmacol. 1998;50:151–7. [PubMed] [Google Scholar]
- 61.Sasaki T, Holeyfield KC, Uitto J. Doxorubicin-induced inhibition of prolyl hydroxylation during collagen biosynthesis in human skin fibroblast cultures. Relevance to impaired wound healing. J Clin Invest. 1987;80:1735–41. doi: 10.1172/JCI113265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berman B, Maderal A, Raphael B. Keloids and hypertrophic scars: Pathophysiology, classification, and treatment. Dermatol Surg. 2017;43:S3–18. doi: 10.1097/DSS.0000000000000819. [DOI] [PubMed] [Google Scholar]
- 63.Perry D, Colthurst J, Giddings P, McGrouther DA, Morris J, Bayat A. Treatment of symptomatic abnormal skin scars with electrical stimulation. J Wound Care. 2010;19:447–53. doi: 10.12968/jowc.2010.19.10.79092. [DOI] [PubMed] [Google Scholar]
- 64.Ud-Din S, Bayat A. Electrical stimulation and cutaneous wound healing: A review of clinical evidence. Healthcare (Basel) 2014;2:445–67. doi: 10.3390/healthcare2040445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ud-Din S, Giddings Dip P, Colthurst J, Whiteside S, Morris J, Bayat A. Significant reduction of symptoms of scarring with electrical stimulation: Evaluated with subjective and objective assessment tools in a prospective noncontrolled case series. Wounds. 2013;25:212–24. [PubMed] [Google Scholar]
- 66.Sebastian A, Allan E, Allan D, Colthurst J, Bayat A. Addition of novel degenerate electrical waveform stimulation with photodynamic therapy significantly enhances its cytotoxic effect in keloid fibroblasts: First report of a potential combination therapy. J Dermatol Sci. 2011;64:174–84. doi: 10.1016/j.jdermsci.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 67.Nicoletti G, Perugini P, Bellino S, Capra P, Malovini A, Jaber O, et al. Scar remodeling with the association of monopolar capacitive radiofrequency, electric stimulation, and negative pressure. Photomed Laser Surg. 2017;35:246–58. doi: 10.1089/pho.2016.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Waghule T, Singhvi G, Dubey SK, Pandey MM, Gupta G, Singh M, et al. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed Pharmacother. 2019;109:1249–58. doi: 10.1016/j.biopha.2018.10.078. [DOI] [PubMed] [Google Scholar]
- 69.Tan CWX, Tan WD, Srivastava R, Yow AP, Wong DWK, Tey HL. Dissolving triamcinolone-embedded microneedles for the treatment of keloids: A single-blinded intra-individual controlled clinical trial. Dermatol Ther (Heidelb) 2019;9:601–11. doi: 10.1007/s13555-019-00316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yeo DC, Balmayor ER, Schantz JT, Xu CJ. Microneedle physical contact as a therapeutic for abnormal scars. Eur J Med Res. 2017;22:28. doi: 10.1186/s40001-017-0269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xue P, Yeo DCL, Chuah YJ, Tey HL, Kang Y, Xu CJ. Drug-eluting microneedles for self-administered treatment of keloids. Technology. 2014;2:144–52. doi: 10.1142/S2339547814500137. [DOI] [Google Scholar]
- 72.Marasca C, Ruggiero A, Marasca D, Megna M, Fabbrocini G. Skin needling in combination with antimicrobial peptides for the treatment of keloids: A novel perspective proposal and approach for skin scars. Dermatology. 2020;236:601–2. doi: 10.1159/000507927. [DOI] [PubMed] [Google Scholar]
- 73.Moravvej H, Memariani M, Memariani H, Robati RM, Gheisari M. Can antimicrobial peptides be repurposed as a novel therapy for keloids? Dermatology. 2020;20:1–3. doi: 10.1159/000506831. [DOI] [PubMed] [Google Scholar]
- 74.Reilly JM, Bluman E, Tenforde AS. Effect of shockwave treatment for management of upper and lower extremity musculoskeletal conditions: A narrative review. PM&R. 2018;10:1385–403. doi: 10.1016/j.pmrj.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 75.Romeo P, Lavanga V, Pagani D, Sansone V. Extracorporeal shock wave therapy in musculoskeletal disorders: A review. Med Princ Pract. 2014;23:7–13. doi: 10.1159/000355472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang L, Weng C, Zhao Z, Fu X. Extracorporeal shock wave therapy for chronic wounds: A systematic review and meta-analysis of randomized controlled trials. Wound Repair Regen. 2017;25:697–706. doi: 10.1111/wrr.12566. [DOI] [PubMed] [Google Scholar]
- 77.Hitchman LH, Totty JP, Raza A, Cai P, Smith GE, Carradice D, et al. Extracorporeal shockwave therapy for diabetic foot ulcers: A systematic review and meta-analysis. Ann Vasc Surg. 2019;56:330–9. doi: 10.1016/j.avsg.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 78.Taheri P, Khosrawi S, Mazaheri M, Parsa MA, Mokhtarian A. Effect of extracorporeal shock wave therapy on improving burn scar in patients with burnt extremities in Isfahan, Iran. J Res Med Sci. 2018;23:81. doi: 10.4103/jrms.JRMS_681_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fioramonti P, Cigna E, Onesti MG, Fino P, Fallico N, Scuderi N. Extracorporeal shock wave therapy for the management of burn scars. Dermatol Surg. 2012;38:778–82. doi: 10.1111/j.1524-4725.2012.02355.x. [DOI] [PubMed] [Google Scholar]
- 80.Wang CJ, Ko JY, Chou WY, Cheng JH, Kuo YR. Extracorporeal shockwave therapy for treatment of keloid scars. Wound Repair Regen. 2018;26:69–76. doi: 10.1111/wrr.12610. [DOI] [PubMed] [Google Scholar]
- 81.Cui HS, Hong AR, Kim JB, Yu JH, Cho YS, Joo SY, et al. Extra-corporeal shock wave therapy alters the expression of fibrosis-related molecules in fibroblast derived from human hypertrophic scar. Int J Mol Sci. 2018;19:124. doi: 10.3390/ijms19010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Klinger M, Marazzi M, Vigo D, Torre M. Fat injection for cases of severe burn outcomes: A new perspective of scar remodeling and reduction. Aesthetic Plast Surg. 2008;32:465–9. doi: 10.1007/s00266-008-9122-1. [DOI] [PubMed] [Google Scholar]
- 83.Brongo S, Nicoletti GF, La Padula S, Mele CM, D'Andrea F. Use of lipofilling for the treatment of severe burn outcomes. Plast Reconstr Surg. 2012;130:e374–6. doi: 10.1097/PRS.0b013e3182590387. [DOI] [PubMed] [Google Scholar]
- 84.Silva VZ, Neto Albacete A, Horácio GS, Andrade GA, Procópio LD, Coltro PS, et al. Evidences of autologous fat grafting for the treatment of keloids and hypertrophic scars. Rev Assoc Med Bras 1992. 2016;62:862–6. doi: 10.1590/1806-9282.62.09.862. [DOI] [PubMed] [Google Scholar]
- 85.Seo BF, Jung SN. The immunomodulatory effects of mesenchymal stem cells in prevention or treatment of excessive scars. Stem Cells Int. 2016;2016 doi: 10.1155/2016/6937976. 6937976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang Q, Liu LN, Yong Q, Deng JC, Cao WG. Intralesional injection of adipose-derived stem cells reduces hypertrophic scarring in a rabbit ear model. Stem Cell Res Ther. 2015;6:145. doi: 10.1186/s13287-015-0133-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li Y, Zhang W, Gao J, Liu J, Wang H, Li J, et al. Adipose tissue-derived stem cells suppress hypertrophic scar fibrosis via the p38/MAPK signaling pathway. Stem Cell Res Ther. 2016;7:102. doi: 10.1186/s13287-016-0356-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang X, Ma Y, Gao Z, Yang J. Human adipose-derived stem cells inhibit bioactivity of keloid fibroblasts. Stem Cell Res Ther. 2018;9:40. doi: 10.1186/s13287-018-0786-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fang F, Huang RL, Zheng Y, Liu M, Huo R. Bone marrow derived mesenchymal stem cells inhibit the proliferative and profibrotic phenotype of hypertrophic scar fibroblasts and keloid fibroblasts through paracrine signaling. J Dermatol Sci. 2016;83:95–105. doi: 10.1016/j.jdermsci.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 90.Craft N, Birnbaum R, Quanquin N, Erfe MCB, Quant C, Haskell J, et al. Topical resiquimod protects against visceral infection with Leishmania infantum chagasi in mice. Clin Vaccine Immunol. 2014;21:1314–22. doi: 10.1128/CVI.00338-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Del Rosso JQ. The use of topical imiquimod for the treatment of actinic keratosis: a status report. Cutis. 2005;76:241–8. [PubMed] [Google Scholar]
- 92.Berman B, Kaufman J. Pilot study of the effect of postoperative imiquimod 5% cream on the recurrence rate of excised keloids. J Am Acad Dermatol. 2002;47:S209–11. doi: 10.1067/mjd.2002.126585. [DOI] [PubMed] [Google Scholar]
- 93.Chuangsuwanich A, Gunjittisomram S. The efficacy of 5% imiquimod cream in the prevention of recurrence of excised keloids. J Med Assoc Thai. 2007;90:1363–7. [PubMed] [Google Scholar]
- 94.Martín-García RF. Imiquimod: An effective alternative for the treatment of invasive cutaneous squamous cell carcinoma. Dermatol Surg. 2005;31:371–4. doi: 10.1111/j.1524-4725.2005.31093. [DOI] [PubMed] [Google Scholar]
- 95.Stashower ME. Successful treatment of earlobe keloids with imiquimod after tangential shave excision. Dermatol Surg. 2006;32:380–6. doi: 10.1111/j.1524-4725.2006.32077.x. [DOI] [PubMed] [Google Scholar]
- 96.Berman B, Harrison-Balestra C, Perez OA, Viera M, Villa A, Zell D, et al. Treatment of keloid scars post-shave excision with imiquimod 5% cream: A prospective, double-blind, placebo-controlled pilot study. J Drugs Dermatol. 2009;8:455–8. [PubMed] [Google Scholar]
- 97.Cação FM, Tanaka V, Messina MC. Failure of imiquimod 5% cream to prevent recurrence of surgically excised trunk keloids. Dermatol Surg. 2009;35:629–33. doi: 10.1111/j.1524-4725.2009.01101.x. [DOI] [PubMed] [Google Scholar]
- 98.Lin WC, Liou SH, Kotsuchibashi Y. Development and characterisation of the imiquimod poly(2-(2-methoxyethoxy)ethyl methacrylate) hydrogel dressing for keloid therapy. Polymers (Basel) 2017;9:579. doi: 10.3390/polym9110579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wolfram D, Tzankov A, Pülzl P, Piza-Katzer H. Hypertrophic scars and keloids--A review of their pathophysiology, risk factors, and therapeutic management. Dermatol Surg. 2009;35:171–81. doi: 10.1111/j.1524-4725.2008.34406.x. [DOI] [PubMed] [Google Scholar]
- 100.Shridharani SM, Magarakis M, Manson PN, Singh NK, Basdag B, Rosson GD. The emerging role of antineoplastic agents in the treatment of keloids and hypertrophic scars: A review. Ann Plast Surg. 2010;64:355–61. doi: 10.1097/SAP.0b013e3181afaab0. [DOI] [PubMed] [Google Scholar]
- 101.Yokozeki M, Baba Y, Shimokawa H, Moriyama K, Kuroda T. Interferon-gamma inhibits the myofibroblastic phenotype of rat palatal fibroblasts induced by transforming growth factor-beta1 in vitro. FEBS Lett. 1999;442:61–4. doi: 10.1016/s0014-5793(98)01626-3. [DOI] [PubMed] [Google Scholar]
- 102.Nedelec B, Shen YJ, Ghahary A, Scott PG, Tredget EE. The effect of interferon alpha 2b on the expression of cytoskeletal proteins in an in vitro model of wound contraction. J Lab Clin Med. 1995;126:474–84. [PubMed] [Google Scholar]
- 103.Espinassouze F, Heid E, Grosshans E. [Treatment of keloid by intralesional injections of interferon alfa-2B]. Ann Dermatol Venereol. 1993;120:629–30. [PubMed] [Google Scholar]
- 104.Wong TW, Chiu HC, Yip KM. Intralesional interferon alpha-2b has no effect in the treatment of keloids. Br J Dermatol. 1994;130:683–5. doi: 10.1111/j.1365-2133.1994.tb13125.x. [DOI] [PubMed] [Google Scholar]
- 105.al-Khawajah MM. Failure of interferon-alpha 2b in the treatment of mature keloids. Int J Dermatol. 1996;35:515–17. doi: 10.1111/j.1365-4362.1996.tb01671.x. [DOI] [PubMed] [Google Scholar]
- 106.Berman B, Flores F. Recurrence rates of excised keloids treated with postoperative triamcinolone acetonide injections or interferon alfa-2b injections. J Am Acad Dermatol. 1997;37:755–7. doi: 10.1016/s0190-9622(97)70113-0. [DOI] [PubMed] [Google Scholar]
- 107.Lee JH, Kim SE, Lee AY. Effects of interferon-alpha2b on keloid treatment with triamcinolone acetonide intralesional injection. Int J Dermatol. 2008;47:183–6. doi: 10.1111/j.1365-4632.2008.03426.x. [DOI] [PubMed] [Google Scholar]
- 108.Shagufta, Ahmad I. Tamoxifen a pioneering drug: An update on the therapeutic potential of tamoxifen derivatives. Eur J Med Chem. 2018;143:515–31. doi: 10.1016/j.ejmech.2017.11.056. [DOI] [PubMed] [Google Scholar]
- 109.Mancoll JS, Zhao J, McCauley RL, Phillips LG. The inhibitory effect of tamoxifen on keloid fibroblasts. Surg Forum. 1996;47:718–20. [Google Scholar]
- 110.Hu D, Hughes MA, Cherry GW. Topical tamoxifen--A potential therapeutic regime in treating excessive dermal scarring? Br J Plast Surg. 1998;51:462–9. doi: 10.1054/bjps.1997.0100. [DOI] [PubMed] [Google Scholar]
- 111.Mikulec AA, Hanasono MM, Lum J, Kadleck JM, Kita M, Koch RJ. Effect of tamoxifen on transforming growth factor beta1 production by keloid and fetal fibroblasts. Arch Facial Plast Surg. 2001;3:111–14. doi: 10.1001/archfaci.3.2.111. [DOI] [PubMed] [Google Scholar]
- 112.Mehrvarz S, Ebrahimi A, Sahraei H, Bagheri MH, Fazili S, Manoochehry S, et al. Effects of topical tamoxifen on wound healing of burned skin in rats. Arch Plast Surg. 2017;44:378–83. doi: 10.5999/aps.2017.44.5.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pasquetti AF, Mota WN, dos Santos MR, de Farias Brito HL. Uso do tamoxifeno no tratamento de queloides e cicatrizes hipertroficas. BJPS. 2010;25:372–8. [Google Scholar]
- 114.Soares-Lopes LR, Soares-Lopes IM, Filho LL, Alencar AP, da Silva BB. Morphological and morphometric analysis of the effects of intralesional tamoxifen on keloids. Exp Biol Med (Maywood) 2017;242:926–9. doi: 10.1177/1535370217700524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang Z, Gao Z, Shi Y, Sun Y, Lin Z, Jiang H, et al. Inhibition of Smad3 expression decreases collagen synthesis in keloid disease fibroblasts. J Plast Reconstr Aesthet Surg. 2007;60:1193–9. doi: 10.1016/j.bjps.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 116.Aoki M, Miyake K, Ogawa R, Dohi T, Akaishi S, Hyakusoku H, et al. siRNA knockdown of tissue inhibitor of metalloproteinase-1 in keloid fibroblasts leads to degradation of collagen type I. J Invest Dermatol. 2014;134:818–26. doi: 10.1038/jid.2013.396. [DOI] [PubMed] [Google Scholar]
- 117.Shin JU, Lee WJ, Tran TN, Jung I, Lee JH. Hsp70 knockdown by siRNA decreased collagen production in keloid fibroblasts. Yonsei Med J. 2015;56:1619–26. doi: 10.3349/ymj.2015.56.6.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cai Y, Yang W, Pan M, Wang C, Wu W, Zhu S. Wnt2 knock down by RNAi inhibits the proliferation of in vitro-cultured human keloid fibroblasts. Medicine (Baltimore) 2018;97:e12167. doi: 10.1097/MD.0000000000012167. [DOI] [PMC free article] [PubMed] [Google Scholar]