Abstract
Chronic renal replacement therapy by either a kidney transplant (KTX) or hemodialysis (HD) predisposes patients to an increased risk for adverse outcomes of COVID-19. However, details on this interaction remain incomplete. To provide further characterization, we undertook a retrospective observational cohort analysis of the majority of the hemodialysis and renal transplant population affected by the first regional outbreak of severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2) in Germany. In a region of 250,000 inhabitants we identified a total of 21 cases with SARS-CoV-2 among 100 KTX and 260 HD patients, that is, 7 KTX with COVID-19, 14 HD with COVID-19, and 3 HD with asymptomatic carrier status. As a first observation, KTX recipients exhibited trends for a higher mortality (43 vs 18%) and a higher proportion of acute respiratory distress syndrome (ARDS) (57 vs 27%) when compared to their HD counterparts. As a novel finding, development of ARDS was significantly associated with the time spent on previous renal replacement therapy (RRT), defined as the composite of dialysis time and time on the transplant (non-ARDS 4.3 vs ARDS 10.6 years, P = .016). Multivariate logistic regression analysis showed an OR of 1.7 per year of RRT. The association remained robust when analysis was confined to KTX patients (5.1 vs 13.2 years, P = .002) or when correlating the time spent on a renal transplant alone (P = .038). Similarly, longer RRT correlated with death vs survival (P = .0002). In conclusion our data suggest renal replacement vintage as a novel risk factor for COVID-19-associated ARDS and death. The findings should be validated by larger cohorts.
Keywords: acute respiratory distress syndrome, Charlson comorbidity index, end stage kidney disease, hospital frailty risk score, immunocompromised, severe acute respiratory distress syndrome coronavirus 2, viral shedding
Key Points
Question: What predisposes dialysis and kidney transplant patients to an unfavorable outcome of COVID-19?
Findings: Patients developing ARDS exhibited a significantly longer history of previous RRT than those without ARDS (10.6 vs 4.3 years) with an OR for ARDS of 1.7 per year of RRT. No other characteristic including CCI, HFRS or counting diagnoses yielded an interaction of comparable strength.
Meaning: These data from 21 patients of the first regional German SARS-CoV-2 outbreak suggest time on previous RRT as a novel risk factor for patients with end stage renal disease, especially transplant carriers. Validation by larger cohorts is warranted.
1. Introduction
The coronavirus SARS-CoV-2 (CoV-2)-associated disease 2019 (COVID-19) is primarily characterized by a hypoxia-inducing prolonged pneumonia, potentially developing into an acute respiratory distress syndrome (ARDS), that is often associated with acute kidney injury (AKI) and endovascular dysfunction. Conversely pre-existent renal disease and especially end-stage renal disease itself represents a putative risk factor for worse outcomes in COVID-19.[1] The background situation is that for a proper allocation of resources and the best individual care, a better understanding of these interactions is urgently needed.[2] Specifically, for practicing nephrologists this concerns the impact of carrying a renal transplant vs being on chronic hemodialysis and potential additional risk factors within these 2 patient groups. The objective of this study was to gain additional information on these points by comparing the characteristics of KTX and HD patients with COVID-19 and to inform this by a very high degree of detail in the recording of patients’ histories and clinical courses. According to the planning, the hypothesis of additional identifiable risk factors was intended to be tested. In order to obtain such insight, we identified and analyzed a group of 21 patients on RRT who developed COVID-19 or were CoV-2 positive from the epicenter of the first German outbreak in a region of 250,000 inhabitants. The patients were treated in homogeneous distribution by a small and defined set of care providers both prior to and during COVID-19. Importantly, the region of the outbreak was also well characterized in terms of the epidemiological virology of its general population allowing for a contextualization of our results.[3]
The study flow diagram in Figure 1 summarizes the population and patient background numbers and subsequently enrolled patients.
Figure 1.
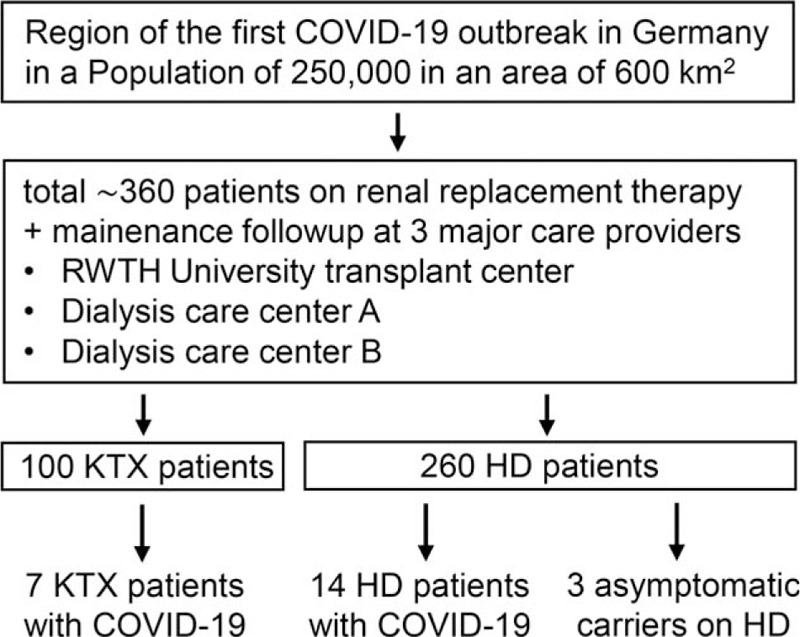
Study flow chart. Within the given region and its renal replacement therapy patient population of ∼360 individuals, a total of 21 individuals with SARS-CoV-2 was identified using vigilant maintenance follow-up and inter-institutional communication by the 3 care providers serving the vast majority of renal patients in the area.
2. Materials and methods
2.1. Patients
The county of Heinsberg in the western part of Germany comprises ∼250,000 inhabitants at a density of 405 per square kilometer,[3] ∼260 patients on chronic dialysis, and ∼100 patients who are renal transplant carriers, indicating a 0.15% prevalence of renal replacement therapy (RRT), which is in line with the national average (Fig. 1).[4] In mid-February 2020 the region became the epicenter of the first nationally reported outbreak of SARS-CoV-2/COVID-19, emerging from a traditional carnival session attended by several hundred people in the town of Gangelt.[3] In response to this event, we established a registry enrolling all regional patients on chronic renal replacement therapy developing COVID-19 or positivity for SARS-CoV-2 as the local institutions covering and treating the vast majority of renal patients in the area. This was done in a joint effort among the 2 ambulatory dialysis care providers (termed provider A and B) serving the area and the reference renal transplant center at RWTH Aachen University. RWTH Aachen University Hospital was the tertiary care center located closest to the epicenter (40 km) treating the highest number of more severely affected inpatients in the course of the COVID-19 outbreak (total of 133 individuals between February 24 and June 17, 2020; general hospital demographics: 1400 beds; 50,000 inpatients and 200,000 clinic patients per year). During the crisis, the maximum capacities of ventilatory ICU beds and renal replacement therapy were increased from ∼120 to 200 patients and 25 to 55 treatments per day, respectively, thus securing without exception the availability of optimal care and the capacity to accept referrals from community centers. The geography and the regional subdistribution of SARS-CoV-2/COVID-19 cases in our patients on renal replacement therapy (both KTX and HD) is summarized in Supplementary Figure 1. The center-specific allocation of these patients prior and during their SARS-CoV-2-associated illness is summarized in Supplementary Figure 2.
2.2. Data collection
This study was approved by the University's institutional review board (IRB) under the protocol number EK 233/20. Patients were identified during clinical routine, for example, thrice weekly dialysis, ambulatory visits, telephone investigations (similar to published practice),[5] communication of the nephrology consult service with ambulatory providers, and the intensive care service. Review of the University hospital's electronic virology database and of the COVID-19 Aachen Study Registry (COVAS) enlisting all patients treated for COVID-19[6] revealed no additional participants, confirming a comprehensive inclusion of the target population. KTX patients had been instructed to only seek medical treatment when symptomatic for COVID-19, then resulting in their diagnosis, in line with recommendations.[7,8] The dialysis population was subjected to a more proactive screening by throat swabs for those deemed at increased risk for example, by contact to a dialysis roommate or a family member, thereby yielding additional detection of SARS-CoV-2 RNA in 3 asymptomatic HD patients. Clinical data of enrollees were systematically recorded and observation of each patient was performed at least until a definitive outcome was reached, that is, recovery with discharge or death. Patients experienced the onset of COVID-19 symptoms or CoV-2 viral RNA detection between February 18 and April 23, 2020. Data collection was closed by May 27, 2020, when the last hospitalized patient achieved a final outcome. None of the 21 patients had a loss to follow-up.
2.3. Virologic studies
Acute SARS-CoV-2 infection was detected using real time PCR (SARS-CoV-2 Virus RT PCR Kit 1.0, Altona, Hamburg, Germany)[9] with a sensitivity and specificity of 93.1% and 100%, respectively.[10] SARS-CoV-2-speific Immunoglobulin G seroconversion was detected by the Anti-SARS-CoV-2 ELISA (IgG) (Euroimmun, Lübeck, Germany) (sensitivity 86.4%, specificity 96.2%).[11]
2.4. Radiologic studies
Patient-specific radiologic data are provided when available to support the clinical description of single patients (Supplementary Table 2). Classification of radiologic images was performed according to the Corona Virus imaging Reporting and Data System (CoV-RADS), grades 1 to 5 as recently published[10] and as detailed in legends to Supplementary Table 2.
2.5. Score calculation
The number of diagnoses was calculated by counting each patient's historically accumulated ICD (International statistical classification of diseases and related health problems) diagnoses. The Charlson cormorbidity index, CCI[12] was calculated using Microsoft Excel informed by chart review. The geriatric index, Hospital frailty risk score, HFRS[13] was computed using a self-designed Python script for Microsoft Excel based on ICD history.
2.6. Statistical analysis
Basic statistical analysis was performed using GraphPad Prism 6 with the test methods as indicated in legends to Tables and Figures. These comprised log-rank-test, student t test, if appropriate with Welch correction, analysis of variance (ANOVA) with post-hoc Tuckey test, Mann–Whitney test, and Fisher exact test. Missing data are indicated in tables throughout by empty spaces, a dash or a comment, as appropriate. In Table 1, visual analysis revealed that significance testing between groups or their combinations lacked statistical power and was thus omitted. In Table 2, visual analysis for each parameter led to the conclusion that application of one-sided t test (with Welch correction if needed) in a univariate manner was appropriate. This was confirmed by similar significance results upon non-parameteric Mann–Whitney testing (data not shown). To identify risk factors for ARDS, binomial logistic regression was performed using PROC logistic in SAS (Version 9.4, SAS Institute Inc, Cary, NC). Variables with P values <.25 in the univariate logistic regression analysis were potentially eligible for testing in the multivariate model, that is, HFRS, diagnoses, RRT time (years) and HD vs KTX. The variable baseline GFR was excluded for multivariate testing since it was only measurable for KTX patients. The variables HFRS and duration of RRT (RRT, years) showed a strong correlation with the number of diagnoses (r = 0.8 and r = 0.6, respectively, PROC corr in SAS). These interactions were expected, given the calculation of HFRS from ICD-codes and the probability to accumulate more diagnoses with longer RRT time. Given the universal character of age and the relevance of RRT time and HD vs KTX, these 3 parameters were included in the multivariate analysis. Choice of a larger group of characteristics was precluded by the limited patient number. Interestingly, body-mass-index (BMI), as an established risk factor for a severe COVID-19 course[1] when >30 kg/m2 was actually lower in patients with ARDS in our cohort and showed a P value >.25 in univariate logistic regression for ARDS; hence it was also omitted.
Table 1.
Cohort baseline characteristics.
| Kidney transplant with COVID-19 | Hemodialysis with COVID-19 | Hemodialysis with asymptomatic CoV-2 | |
| Characteristic | (N = 7) | (N = 11) | (N = 3) |
| Baseline | |||
| Age, median y (IQR) | 62 (55–68) | 69 (65–73) | 79 (65–87) |
| Sex, female N (%) | 3 (43) | 6 (55) | 2 (67) |
| ARDS, N (%) | 4 (57) | 3 (27) | 0 (0) |
| AKI, N (%) | 4 (57) | n/a | n/a |
| Outcome | |||
| Median follow-up, d (IQR) | 69 (22–70) | 64 (45–69) | 67 (35–78) |
| Still hospitalized, N (%) | 0 (0) | 0 (0) | n/a |
| Deceased, N (%) | 3 (43) | 2 (18) | 0 (0) |
| Recovered, N (%) | 4 (57) | 9 (82) | 3 (100) |
| Multimorbidity indicators | |||
| Diagnoses, median N (IQR) | 34 (31–57) | 26 (21–34) | 22 (19–23) |
| CCI, median points (IQR) | 6 (4–8) | 6 (6–8) | 4 (7–10) |
| HFRS, median points (IQR) | 13.0 (4.6–22.4) | 3.5 (2.4–6.9) | 3.8 (2.4–10.0) |
| Time on RRT, median y (IQR) | 9.6 (5.1–14.9) | 4.1 (0.9–4.7) | 4.5 (3.3–6.5) |
| Time on KTX, median y (IQR) | 5.4 (3.8–8.5) | n/a | n/a |
| Risk factors | |||
| BMI 25–29.9, N (%) | 1 (14) | 5 (45) | 2 (67) |
| BMI ≧30.0, N (%) | 0 (0) | 4 (36) | 1 (33) |
| Hypertension, N (%) | 5 (71) | 9 (82) | 3 (100) |
| Diabetes mellitus, N (%) | 4 (57) | 4 (36) | 3 (100) |
| RAAS blockade, N (%) | 4 (57) | 6 (55) | 3 (100) |
| ABO-type A, N (%) | 6 (86) | 5 (45) | 1 (33) |
| Management and highest level of care | |||
| Outpatient only, N (%) | 3 (43) | 5 (45) | 3 (100) |
| Hospitalized, N (%) | 4 (57) | 6 (55) | 0 (0) |
| No oxygen, N (%) | 3 (43) | 6 (55) | 3 (100) |
| Oxygen, N (%) | 1 (14) | 2 (18) | 0 (0) |
| MEV or ECMO, N (%) | 3 (43) | 3 (27) | 0 (0) |
| Duration of | |||
| Illness, median d (IQR) | 21 (15–26) | 14 (14–21) | 0 (0) |
| Hospitalization, median d (IQR) | 36 (14–62) | 22 (5–37) | n/a |
| CoV-2 shedding, median d (IQR) | 18 (15–34) | 18 (14–21) | 21 (13–33) |
AKI = acute kidney injury according to KDIGO (kidney disease improving global outcomes), ARDS = acute respiratory distress syndrome, BMI = body mass index in kg/m2, CCI = Charlson comorbidity index, CoV-2 = SARS-CoV-2 RNA detected by polymerase-chain-reaction, ECMO = extracorporeal membrane oxygenation, HD = hemodialysis, HFRS = hospital frailty risk score, IQR = interquartile range, MEV = mechanical ventilation, n/a = not assessed or not applicable, N = number, RAAS = renin angiotensin aldosterone, RRT = renal replacement therapy.
Table 2.
Univariate analysis for quantitative and qualitative differences in characteristics of non-ARDS vs ARDS patients.
| Non-ARDS | ARDS | Onetailed t test | |
| Characteristic | (N = 14∗) | (N = 7∗) | P value |
| Parametric variables | |||
| Age, median years (IQR) | 67.0 (59.5–79.0) | 67.0 (62.0–69.0) | .447 |
| BMI, median (IQR) | 28.3 (24.3–29.8) | 23.8 (23.5–36.2) | .433 |
| CCI, median points (IQR) | 6.5 (5.8–7.3) | 8.0 (6.0–8.0) | .202 |
| HFRS, median points (IQR) | 3.7 (2.4–10.1) | 8.5 (4.6–22.4) | .111 |
| Diagnoses, N (IQR) | 24.5 (19.8–34.0) | 31.0 (23.0–57.0) | .044 |
| RRT, median years (IQR) | 4.3 (3.0–5.3) | 10.6 (4.5–14.9) | .016 |
| RRT in KTX, median years (IQR) | 5.1 (3.5–5.9)† | 13.2 (10.0–14.9)‡ | .002 |
| KTT in KTX, median years (IQR) | 3.8 (2.8–5.1)† | 8.2 (6.0–13.3)‡ | .038 |
| HDT in KTX, median years (IQR) | 0.7 (0.0–2.1)† | 3.6 (0.3–6.9)‡ | .138 |
| Baseline GFR in KTX, median (IQR) | 65 (40–68)† | 22 (16–51)‡ | .047 |
| Binomial variables | |||
| RAAS blockade, N (%) | 9 (64) | 4 (57) | 1.000 |
| Hypertension, N (%) | 12 (86) | 6 (86) | .753 |
| Diabetes mellitus, N (%) | 7 (50) | 3 (43) | .562 |
| ABO type A, N (%) | 7 (58) | 5 (71) | .474 |
| HD vs KTX, N (%) | 3 (21) | 4 (57) | .127 |
ARDS = acute respiratory distress syndrome, BMI = body mass index in kg/m2, CCI = Charlson comorbidity index, GFR = glomerular filtration rate in ml/minutes/1.73m2, HD = hemodialysis, HDT = hemodialysis time in transplant recipients, HFRS = hospital frailty risk score, KTT = kidney transplant time, KTX = kidney transplant recipients.
Statistical analysis using standard or Welch corrected t test for parametric and Fisher exact test for binomial variables, respectively.
unless otherwise specified.
N = 3.
N = 4.
3. Results
3.1. Major findings
The study population comprised 21 subjects on RRT identified as described above. As detailed in Table 1, the cohort's composition consisted of 7 KTX plus 11 HD patients suffering from COVID-19 and an additionally 3 asymptomatic HD patients identified to be CoV-2-positive by throat PCR screening. According to visual analysis, baseline demographics, median follow-up time, risk factors (except for obesity), rates of ambulatory and in-hospital treatment, and the duration of CoV-2 RNA shedding were similar between KTX and HD COVID-19 patients. However, in comparison to HD COVID-19 patients, in KTX recipients with COVID-19 the rates of ARDS and death were almost doubled and the lengths of clinical illness and hospitalization increased by 50%, yet without statistical significance. This case fatality is further illustrated by a Kaplan Meier plot (Fig. 2). Due to low patient numbers, univariate or multiple variable analysis of Table 1 or a meaningful log-rank-test for Figure 2 could not be performed. Four out of 7 KTX recipients developed AKI. Except for the Charlson comorbidity index, CCI,[12] morbidity indicators were more pronounced in the KTX group, such as the number of diagnoses, the geriatric hospital frailty risk score, HFRS (based on ICD codes of previous hospitalizations)[13] and the time spent on previous RRT (Table 1). In summary, our KTX patients developing COVID-19 exhibited trends for a higher degree of baseline multimorbidity and also showed higher rates of ARDS, death, longer morbidity and longer hospitalization.
Figure 2.
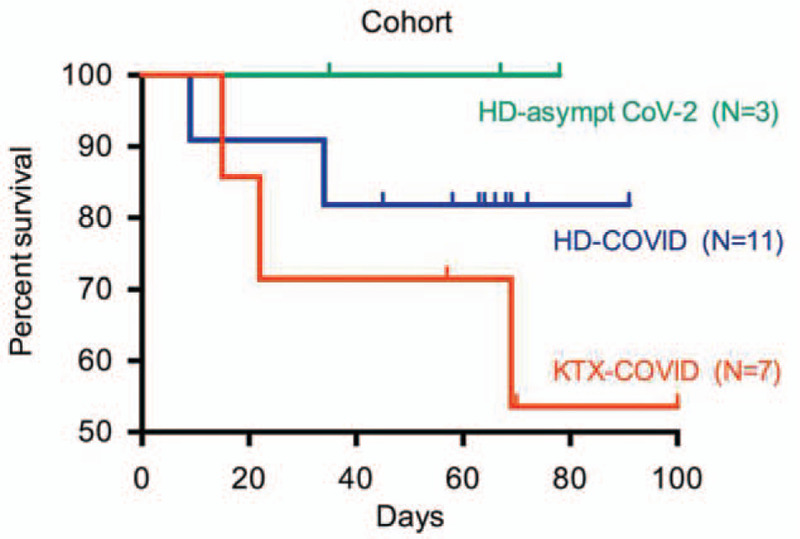
Patient survival within the cohort. KTX-COVID = kidney transplant with COVID-19, HD-COVID = hemodialysis with COVID-19, HD-asympt CoV-2 = hemodialysis with asymptomatic detection of SARS-CoV-2-RNA, N = number of patients.
To further explore the interaction between pre-existing morbidity and unfavorable outcomes, we performed a univariate analysis for the development of ARDS (Table 2). The entire cohort of 21 patients was dissected into N = 14 who did not and N = 7 who did develop an ARDS. To increase sensitivity the non-ARDS group also comprised the 3 individuals on HD who did not develop COVID-19 but were asymptomatic carriers of SARS-CoV-2 detected by screening procedures at the dialysis centers. The ARDS group was composed of 4 KTX and 3 HD cases. In this univariate one-sided t test analysis, the absolute number of diagnoses and the time on previous RRT were significantly higher in the ARDS group, that is, by 27 and 147% respectively (median years on RRT 4.3 non-ARDS vs 10.6 ARDS) (Table 2). Following this result and to understand if this effect was conserved in the renal transplant population, we probed the KTX cohort alone for both previous time on RRT and also for previous time on renal transplant (kidney transplant time, KTT). Again, there were robustly significant results, suggesting, that especially in the renal transplant population, the length of previous renal replacement therapy and of transplantation dwelling are associated with the development of COVID-19-related ARDS and/or death (Table 2). Similar results were obtained for the alternative outcome of survival vs non-survival, yielding signals of significance again for the time spent on previous RRT over all 3 patient groups (P = .0002), and within the KTX group for RRT (P = .024) and KTT (P = .014), respectively.
To verify these findings by a more conservative approach we also performed a separate analysis of the ARDS and non-ARDS groups without the 3 asymptomatic SARS-CoV-2 positive individuals on HD by a comprehensive ANOVA analysis with posthoc testing, as shown graphically in Figure 3. Again, there was a statistically significant signal concerning the association of previous renal replacement duration and development of fatal ARDS.
Figure 3.
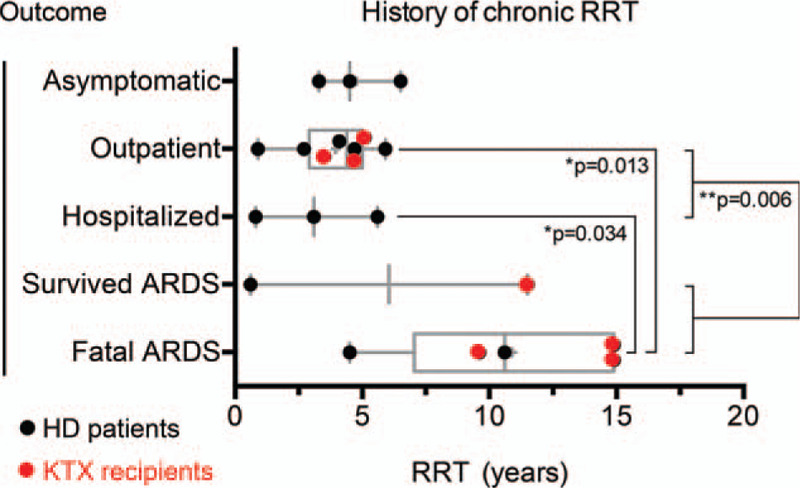
Time on previous chronic renal replacement therapy, RRT in years stratified by outcome. Asymptomatic, patients on hemodialysis with asymptomatic shedding of SARS-CoV-2 RNA; outpatient, patients with COVID-19 treated as outpatients only; hospitalized, patients who were hospitalized but did not develop an acute respiratory distress syndrome (ARDS); survived ARDS, patients with ARDS followed by recovery and discharge; fatal ARDS, patients with ARDS followed by death. Each data point represents 1 single patient. Horizontal bars: median. Boxes: 25% to 75% interquartile range, IQR. Whiskers: 10% to 90% IQR. Statistical testing using ANOVA with Tuckey post-hoc analysis. Of note, patient HD 9 who developed nonfatal ARDS despite a very short duration of previous RRT of 0.6 years (left black dot under “Survived ARDS”) suffered from chronic asthma as a known non-renal risk factor for developing ARDS.
Despite the caution that must be advised in the interpretation of such results given the overall limited patient number of our study, we performed logistic regression analyses for risk factors of ARDS as detailed in the methods section. The results are provided in Table 3, confirming the signal for time on previous RRT as a risk factor for development of ARDS.
Table 3.
Logistic regression analyses for the outcome variable of Non-ARDS (N = 14)vs ARDS (N = 7).
| Univariate logistic regression | Multivariate logistic regression | |||||
| Characteristic | OR | CI | P value | OR | CI | P value |
| Parametric variables | ||||||
| Age, years | 0.995 | 0.912–1.086 | .917 | 0.969 | 0.858–1.095 | .617 |
| BMI | 1.016 | 0.881–1.171 | .831 | |||
| CCI, points | 1.249 | 0.742–2.102 | .403 | |||
| HFRS, points | 1.096 | 0.969–1.239 | .145 | |||
| Diagnoses, N | 1.102 | 0.993–1.222 | .067 | |||
| RRT, years | 1.583 | 1.057–2.370 | .026 | 1.701 | 1.001–2.919 | .049 |
| Baseline GFR | 0.921 | 0.827–1.025 | .133 | |||
| Binomial variables | ||||||
| RAAS blockade | 1.350 | 0.212–8.617 | .751 | |||
| Hypertension | 1.000 | 0.075–13.367 | 1.000 | |||
| Diabetes mellitus | 1.333 | 0.214–8.288 | .758 | |||
| ABO type A | 0.640 | 0.088–4.656 | .659 | |||
| HD vs KTX | 0.205 | 0.029–1.463 | .114 | 0.429 | 0.012–15.510 | .644 |
CI = confidence interval, OR = odds ratio. See Table 2 for further corresponding values and abbreviations.
None of the fatal or adverse outcomes was due to a limitation in the availability of medical resources (see methods for details). As an indicator, extracorporeal membrane oxygenation (ECMO) was administered (e.g., KTX-COVID #6) and 4 out of 5 deaths occurred at the University hospital itself, with the remaining fatal case being adequately intubated and managed at local hospital level but developing rapidly progressive multiorgan failure (HD-COVID #10).
3.2. Individual patients
Individual patient's details are provided as Supplementary information summarizing baseline characteristics (Supplementary Table 1), risk factors (Supplementary Table 2), laboratory and radiology investigations (Supplementary Table 3) and the presenting symptoms (Supplementary Table 4). All 5 fatal outcomes occurred in patients with ARDS and were due to fulminant vasomotor paresis either around the time of requirement for invasive respiratory support (KTX-COVID #6; HD-COVID #10), or following very long intensive care courses with development of nosocomial sepsis (KTX-COVID #5, #7; HD-COVID #11). Serious complications were rhabdomyolysis, reactivation of herpes viruses, need for red blood cell transfusions, hemorrhage and importantly, micro-thrombosis, and myocarditis. One multimorbid senior patient, KTX-COVID #4, was managed by comfort care based on his multimorbidity, but recovered from mild ARDS after a week-long course. Across the cohort, the main laboratory abnormalities on admission were
-
1.
lymphopenia
-
2.
elevated D-dimers
-
3.
elevated lactate-dehydrogenase (LDH)
-
4.
elevated C-reactive protein (CRP)Of those, CRP and LDH proved significantly (Supplementary Table 5).
Radiologic pulmonary findings were graded as described and were consistent with the published literature;[10] an example for the development of a severe course of pulmonary infiltrations (from patient KTX-COVID #5) is shown in Supplementary Figure 4. The most frequent symptoms (based on the entire cohort of N = 21) were fever (67%), cough, weakness (52% each), and the composite of diarrhea and nausea (33%) (Supplementary Table 4 for details). Anosmia[14] was reported by 2 KTX recipients with a near-normal eGFR of 40 to 60 ml/minutes, but by none of the other KTX or HD patients who all had a substantially lower renal clearance. This underreporting might be due to a high prevalence of pre-existing uremic olfactory dysfunction in the second group.[15]
3.3. Immunosuppression, therapeutics, and immune responses
The handling of immunosuppression in KTX recipients is detailed in Supplementary Table 1. In brief, mycophenolic acid (MPA) but not calcineurin inhibitors (CNI) were removed immediately in 5 out of 7 cases, in line with current practice and recommendations.[16–18] When ARDS developed (KTX-COVID #4–7), CNIs were also withdrawn (days 11–14). Following such reductions, maintenance prednisolone was escalated to 10 to 20 mg/d but not further. In retrospect, all hospitalized KTX patients presented with CNI-level elevation (average 10.0 ng/ml; target 4–7 ng/ml) that proved difficult to handle (Supplementary Fig. 3). In conclusion, earlier discontinuation might have simplified management.
Neither KTX nor HD cases received drugs or steroids with intent to target COVID-19 or SARS-CoV-2. This stands in contrast to most early practice published by nephrologists worldwide, reporting the preponderant and often combined engagement of for example, hydroxychloroquine, azithromycin, antivirals, interleukin-6 blockers, and chemokine receptor 5 blockers.[17,19–25]
While the median shedding time of SARS-CoV-2 was equal among the 3 patient subgroups (Table 1), transplant recipient (KTX-COVID #4) and 1 hemodialysis patient under CNI-treatment for myasthenia gravis (HD-COVID #8) exhibited remarkably long positivity of SARS-CoV-2 viral load of 47 and 32 days despite earlier clinical recovery, respectively. Such courses have been observed by others.[26,27] Serologic IgG responses were documented as detailed in Supplementary Table 1. Of interest, patient KTX-COVID #3, who fulfilled bona fide criteria of SARS-CoV-2 infection including anosmia and a positive PCR result, was tested negative on serology, suggesting a non-response. In general, SARS-CoV-2-specific IgG titers of immunosuppressed (i.e., KTX-COVID #2–6 plus HD-COVID #8) vs non-immunosuppressed individuals (HD-COVID #1–2, 4–8, 11) were very similar: 4.1 (IQR 1.8–7.9) vs 4.5 (IQR 2.0–10.8), respectively, lacking significance (one-tailed t test, P = .298).
4. Discussion
The major and novel observation of this work is that the duration of renal replacement therapy prior to developing COVID-19 as well as the baseline glomerular filtration rate (GFR) was significantly associated with an ensuing ARDS or death in renal transplant carriers (Table 2, Fig. 2). Thereby, our data extend the findings of a previous report from a Spanish hemodialysis cohort[22] to the renal transplant community. Therein, hemodialysis patients were found to have an adjusted Hazard Ratio for in-hospital death of 1.008 (P = .019) per month of dialysis history (50% of a total of 36 patients achieved definite outcomes at the time of data analysis: 11 deceased, 7 recovered, 18 still hospitalized). In support of this interaction, the Wuhan dialysis cohort also observed a substantially higher median dialysis age in the 2 individuals that died until analysis closure,[28] while 2 additional early-outcome cohorts with homogeneously brief dialysis histories were inherently underpowered to detect such an association.[19,29] Owing to their different focus (death rather than ARDS) and lack of completed outcomes, there are currently no further studies directly addressing the issue, neither in KTX (cohort sizes 15–144),[17,20,23,30–32] nor in dialysis patients (cohort sizes 32–154).[33–37] The recent elegant multicentric search for death-associated risk factors in 144 KTX patients by Cravedi, Riella and colleagues deserves special mentioning, since it detected a signal of significance for a lower baseline GFR.[32] The potential mechanism for the association of duration of end stage renal disease including time on transplant with the susceptibility for ARDS could be chronic cellular substrate modifications facilitating either the entry or the pathogenicity of SARS-CoV-2. Iatrogenic or uremic immune paresis and endothelial strain could all lie at the root of such modifications.[38,39] The complement system should also be taken into account as a potential culprit.[40] In sum, renal replacement age might be a particularly strong indicator of multimorbidity and biologic age, more powerful than counting diagnoses, calculating the CCI or computing the geriatric frailty-associated HFRS (see above). Once validated by the analysis of larger cohorts, it would be intriguing to study the mechanistic biology of such an interaction.
Several epidemiological key metrics can be inferred from our study. First, in HD patients the risk of being SARS-CoV-2-positive and of developing COVID-19 was 5.3% and 4.1%, respectively (calculated as 14 or 11 cases/260 HD patients). This is identical to the findings of a similar Italian dialysis cohort[29] and also supported by data from Spain.[41] This incidence lies somewhat above the officially reported case-rate of 3.1% in the Heinsberg area's general population but below the 15.5% infection-rate established in the area's hotspot by proactive combinatorial PCR and serologic screening of ∼1000 persons in ∼400 households.[3] Given the lower number of KTX patients enrolled, we are cautious to derive any incidence, though the resulting 7% (7/∼100 KTX recipients) would be in line with the current literature (see above).
Casualty rates in the comparable publication[22] and in ERA/EDTA's pan-European ERACODA database[42] were recently reported to be around 18 to 25% for both HD and KTX patients. This was similar in our HD subcohort (18%) but not in our KTX stratum which displayed an excessively high mortality of 43%. Most published KTX cohorts are placed somewhat in the middle of this (see above). Part of our figures might relate to a higher degree of morbidity in our KTX subcohort for example, as suggested by a Charlson comorbidity index of 6 vs 1 of 4 points when compared to Goicoechea et al.[22] In addition, blood type A, which has been reported as a potential risk factor for both infection with[43] and the severity of CoV-2/COVID-19[44] was also overrepresented in our KTX subcohort. Finally, as rule, much of the currently available published data is not yet based on 100% achieved endpoints. For example, in Alberci et al[20] a death-toll of 25% in KTX recipients was calculated for KTX recipients, while a remaining 60% of patients was still hospitalized awaiting their outcome.
There are several limitations and strengths to our study. Owing to the restriction by its local design, the main limitation is low patient number, precluding the statistical evaluation of baseline characteristics and harboring a certain risk for skew. One strength lies in the consistent inclusion of the renal replacement population of an entire epicenter, allowing for epidemiologic contextualization. In addition, all study subjects had reached a definitive outcome, thus contrasting many of the pandemic's early reports (see above). Finally, our patients were treated by a well-defined set of caregivers with no issues of bias from patient capacity-overload.
In conclusion, we present time on RRT as a novel risk factor for ARDS and death in patients on renal transplantation. We suggest that this correlation be validated by ongoing large cohort analyses and interrogated in more depth.
Suppl Figure 1.
Suppl Figure 2.
Author contributions
L.V., T.K., C.S., A.S.M., U.K., C.W., A.K., T.R., and G.S.B. were involved in patient care, data contribution, and manuscript writing. M.D. and N.M. represent the COVAS registry. M.K. and M.S.H. performed virological and radiological analyses, respectively. G.S.B., C.S., and C.W. performed data analysis. J.F., T.R., and G.S.B. had the idea and planned the study. G.S.B. drafted the manuscript. L.V., T.K., C.S., T.R. and G.S.B. contributed equally and share first and senior authorships, respectively. The authors thank Stephanie Wied, MSc, Institute for Medical Statistics, RWTH Aachen University for valuable discussions on statistical planning.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: AKI = acute kidney injury, ARDS = acute respiratory distress syndrome, CCI = Charlson comorbidity index, COVID-19 = coronavirus disease-2019, HD = hemodialysis, HFRS = hospital frailty risk score, KTX = kidney transplant, RRT = renal replacement therapy (by either HD or as a composite of HD and KTX in transplant patients), SARS-CoV-2 = severe acute respiratory distress syndrome coronavirus 2.
How to cite this article: Villa L, Krüger T, Seikrit C, Mühlfeld AS, Kunter U, Werner C, Kleines M, Schulze-Hagen M, Dreher M, Kersten A, Marx N, Floege J, Rauen T, Braun GS. Time on previous renal replacement therapy is associated with worse outcomes of COVID-19 in a regional cohort of kidney transplant and dialysis patients. Medicine. 2021;100:10(e24893).
LV, TK, CS, TR, and GSB contributed equally to this work.
The authors have no funding and conflicts of interests to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental digital content is available for this article.
References
- [1].Gandhi RT, Lynch JB, del Rio C. Mild or Moderate Covid-19. N Engl J Med 2020;383:1757–66. [DOI] [PubMed] [Google Scholar]
- [2].Lai Q, Spoletini G, Bianco G, et al. SARS-CoV2 and immunosuppression: A double-edged sword. Transpl Infect Dis 2020;22:e13404.doi: 10.1111/tid.13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Streeck H, Schulte B, Kuemmerer B, et al. Infection fatality rate of SARS-CoV-2 infection in a German community with a super-spreading event. Med Rxiv 2020;2020.2005.2004.20090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Caskey FJ, Schober-Halstenberg HJ, Roderick PJ, et al. Exploring the differences in epidemiology of treated ESRD between Germany and England and Wales. Am J Kidney Dis 2006;47:445–54. [DOI] [PubMed] [Google Scholar]
- [5].Angeletti A, Trivelli A, Magnasco A, et al. Risk of COVID-19 in young kidney transplant recipients. Results from a single-center observational study. Clin Transplant 2020;e13889. [DOI] [PubMed] [Google Scholar]
- [6].Dreher M, Kersten A, Bickenbach J, et al. The Characteristics of 50 hospitalized COVID-19 patients with and without ARDS. Dtsch Arztebl Int 2020;117:271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Santos-Parker KS, Santos-Parker JR, Highet A, et al. Practice change amidst the COVID-19 pandemic: harnessing the momentum for expanding telehealth in transplant. Clin Transplant 2020;e13897. [DOI] [PubMed] [Google Scholar]
- [8].Mehta SA, Leonard J, Labella P, et al. Outpatient management of kidney transplant recipients with suspected COVID-19-Single-center experience during the New York City surge. Transpl Infect Dis 2020;22:e13383.doi: 10.1111/tid.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill 2020;25:2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schulze-Hagen M, Hübel C, Meier-Schroers M, et al. Low-dose chest CT for the diagnosis of COVID-19. Dtsch Arztebl Int 2020;117:389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Krüttgen A, Cornelissen CG, Dreher M, et al. Comparison of four new commercial serologic assays for determination of SARS-CoV-2 IgG. J Clin Virol 2020;128:104394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- [13].Gilbert T, Neuburger J, Kraindler J, et al. Development and validation of a hospital frailty risk score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet 2018;391:1775–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Spinato G, Fabbris C, Polesel J, et al. Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA 2020;323:2089–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nigwekar SU, Weiser JM, Kalim S, et al. Characterization and correction of olfactory deficits in kidney disease. J Am Soc Nephrol 2017;28:3395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Maggiore U, Abramowicz D, Crespo M, et al. How should I manage immunosuppression in a kidney transplant patient with COVID-19? An ERA-EDTA DESCARTES expert opinion. Nephrol Dial Transplant 2020;35:899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Akalin E, Azzi Y, Bartash R, et al. Covid-19 and kidney transplantation. N Engl J Med 2020;382:2475–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bosch F, Borner N, Kemmner S, et al. Attenuated early inflammatory response in solid organ recipients with COVID-19. Clin Transplant 2020;e14027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Trujillo H, Caravaca-Fontán F, Sevillano Á, et al. SARS-CoV-2 infection in hospitalized patients with kidney disease. Kidney Int Rep 2020;5:905–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Alberici F, Delbarba E, Manenti C, et al. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int 2020;97:1083–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Alberici F, Delbarba E, Manenti C, et al. Management of patients on dialysis and with kidney transplant during SARS-COV-2 (COVID-19) pandemic in Brescia, Italy. Kidney Int Rep 2020;5:580–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Goicoechea M, Sánchez Cámara LA, Macías N, et al. COVID-19: clinical course and outcomes of 36 maintenance hemodialysis patients from a single center in Spain. Kidney Int 2020;98:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Columbia University Kidney Transplant Program. Early description of coronavirus 2019 disease in kidney transplant recipients in New York. J Am Soc Nephrol 2020;31:1150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang R, He H, Liao C, et al. Clinical outcomes of hemodialysis patients infected with severe acute respiratory syndrome coronavirus 2 and impact of proactive chest computed tomography scans. Clin Kidney J 2020;13:328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mella A, Mingozzi S, Gallo E, et al. Case series of six kidney transplanted patients with COVID-19 pneumonia treated with tocilizumab. Transpl Infect Dis 2020;e13348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gajurel K. Persistently positive severe acute respiratory syndrome coronavirus 2 (SARS-COV2) nasopharyngeal PCR in a kidney transplant recipient. Transpl Infect Dis. e13408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Man Z, Jing Z, Huibo S, et al. Viral shedding prolongation in a kidney transplant patient with COVID-19 pneumonia. Am J Transplant 2020;20:2626–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ma Y, Diao B, Lv X, et al. Epidemiological, clinical, and immunological features of a cluster of COVID-19 contracted hemodialysis patients. Kidney Int Rep 2020;5:1333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fontana F, Giaroni F, Frisina M, et al. Severe acute respiratory SARS-CoV-2 infection in dialysis patients in northern Italy: a single-centre experience. Clin Kidney J 2020;13:334–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Husain SA, Dube G, Morris H, et al. Early Outcomes of Outpatient Management of Kidney Transplant Recipients with Coronavirus Disease 2019. Clin JAm Soc Nephrol 2020;15:1174–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Roberts MB, Izzy S, Tahir Z, Al Jarrah A, Fishman JA, El Khoury J. COVID-19 in solid organ transplant recipients: dynamics of disease progression and inflammatory markers in ICU and non-ICU admitted patients. Transpl Infect Dis. e13407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cravedi P, Suraj SM, Azzi Y, et al. COVID-19 and kidney transplantation: results from the TANGO International Transplant Consortium. Am J Transplant. 2020;20:3140–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Li CMYM, Can T, Dongdong M, Sheng W, Haifeng L, Fei F. An analysis on the clinical features of MHD patients with coronavirus disease 2019: a single center study. https://wwwresearchsquarecom/article/rs-18043/v1. 2020. [Google Scholar]
- [34].Xiong F, Tang H, Liu L, et al. Clinical characteristics of medical interventions for COVID-19 in hemodialysis patients in Wuhan, China. J Am Soc Nephrol 2020;31:1387–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Valeri AM, Robbins-Juarez SY, Stevens JS, et al. Presentation and outcomes of patients with ESKD and COVID-19. J Am Soc Nephrol 2020;31:1409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Alberici F, Delbarba E, Manenti C, et al. A report from the Brescia Renal COVID task force on the clinical characteristics and short-term outcome of hemodialysis patients with SARS-CoV-2 infection. Kidney Int 2020;98:20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Du X, Li H, Dong L, et al. Clinical features of hemodialysis patients with COVID-19: a single-center retrospective study on 32 patients. Clin Exp Nephrol 2020;1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Puelles VG, Lütgehetmann M, Lindenmeyer MT, et al. Multiorgan and Renal Tropism of SARS-CoV-2. N Engl J Med 2020;383:590–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020;395:1417–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Franzin R, Stasi A, Fiorentino M, et al. Inflammaging and complement system: a link between acute kidney injury and chronic graft damage. Front Immunol 2020;11:734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Rodrigo E, Piñera VC, Setién MA, et al. Silent COVID-19 in haemodialysis facilities in Cantabria, Spain: an ecological study. Clin Kidney J 2020;13:475–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42]. Group EW. ERACODA - The ERA-EDTA COVID-19 Database for Patients on Kidney Replacement Therapy. Available at https://wwwera-edtaorg/en/wp-content/uploads/2020/04/ERACODA-Study-Report-2020-04-22pdf. [Google Scholar]
- [43].Zhao J, Yang Y, Huang H, et al. Relationship between the ABO blood group and the COVID-19 susceptibility. Med Rxiv 2020;2020.2003.2011.20031096. [Google Scholar]
- [44].Ellinghaus D, Degenhardt F, Bujanda L, et al. The ABO blood group locus and a chromosome 3 gene cluster associate with SARS-CoV-2 respiratory failure in an Italian-Spanish genome-wide association analysis. medRxiv 2020;2020.2005.2031.20114991. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


