Abstract
In the backdrop of an abundance of lignin in jute, the main focus of the present study was to conduct a quality assessment of four delignified jute lines (in which four lignin biosynthetic genes were individually downregulated) across advanced generations for industrial applications. To this end, the transgenic lines were advanced to 7th (COMT and C4H lines) and 5th (C3H and F5H lines) transformed generations. The results exhibit approximately 16–25% reduction in acid-insoluble lignin for the whole stem and 13–14% reduction in fiber lignin content for all four transgenic lines compared to the control. The altered lignin composition led to a 3–6% increase in the cellulose content and a small improvement in the enzymatic release of glucose. Lignin reduction led to an exposure of the underlying fibrils in transgenic lines as observed through a scanning electron microscope whereas, it was undiscernible in the control fiber. Furthermore, an analysis of the mechanical properties appears almost similar to that of the control with no morphological deformities. Jute fibers from the transgenic lines offer tremendous cost-effective implications from an economic perspective.
Keywords: Jute, Lignocellulose, Lignin biosynthetic genes, Tensile strength, Jute fiber
Jute, lignocellulose, lignin biosynthetic genes, tensile strength, jute fiber.
1. Introduction
In recent years, an increasing environmental and sustainability awareness has led to a growing interest in promoting the use of biodegradable fiber and pulp for industrial purposes as their use has a favorable impact on both the economy and the environment (Ozgen, 2012; Monteiro et al., 2018; Pereira et al., 2019a, 2019b).
Jute fibers are mainly composed of lignocellulosic biomass like cellulose, hemicellulose and lignin with a cellulose content of 45%–71.5% and lignin 12%–26% (Selver et al., 2018). Researchers have found diverse mechanical properties of this fiber. Its tensile properties and specific strength increase its potential use in different applications for the production of bio-composites and bio-plastics (Mishra and Biswas, 2013). Due to their mechanical properties, jute fibers are considered analogous to glass mainly for their specific strength and modulus (Li et al., 2007). Natural jute fibers bestow several advantages. These include biodegradability, renewability, low cost, high abundance and a low requirement of pesticides and fertilizers for its production (Pickering et al., 2016). Jute is thus considered the “Fiber of the Future”, remarkably attractive to several industries as a raw material (Saha et al., 2010).
Automotive industry is another potential area for its usage as bio-fiber (Alves et al., 2010). Natural fiber compounds in automobiles provide improved thermal and acoustic insulation compared to fiberglass and lessen the irritation of the skin and respiratory system (Bergfjord and Holst, 2010). Jute has thus penetrated various diversified sectors where natural fibers are progressively becoming better substitutes (Asadullah et al., 2008). Among many, significant industries are celluloid products (films), nonwoven composites (psydo-wood), nonwoven textiles (for car interiors and other uses), etc. (Oboh et al., 2009).
In addition, bio-fibers can substitute petroleum-based synthetic polymers on account of their low production costs, biodegradable nature, physical properties and environmental friendliness. Plant fiber or biomass can be used as raw materials to produce biofuel. Various microorganisms and enzymes are available for degrading cellulosic biomass and converting them into biofuels including bioethanol and biogas (Chirat, 2017). Regarding deforestation, jute fibers may also serve as an alternative source for paper pulp (Rastogi and Dwivedi, 2014).
Although there is a strong demand for jute in both local and international markets, limitations exist in terms of fiber quality, limited productivity and profitability. This is because the presence of lignin hinders the processing of plants for various industrial purposes (Kumar et al., 2009) and confers poor elastic properties to fibers as compared to non-lignocellulosic fibers, for example, cotton (Kwiatkowska et al., 2007). Also, the presence of lignin gives resistance in downstream processing of jute as a raw material for pulping and biofuel production (Wang et al., 2012) and a major barrier for its use as a textile fiber (Maity et al., 2012). Moreover, in lignocellulosic biomass, the strong association of lignin with cellulose and hemicellulose, prevents enzymes from breaking down the cellulosic matter to produce energy and thus lignin limits digestibility of the same by micro-organisms and subsequently decreases energy production (Shadle et al., 2007).
In consideration of these issues, studies have shown that developing jute variations having low lignin content would result in superior fiber quality and consequently better profitability (Bogoeva et al., 2007; Del-Río et al., 2009). Inspired by this, several studies have been carried out on the selective removal of lignin using chemical methods (Zhang et al., 2013; Bharathiraja et al., 2014). Nevertheless, chemical delignification methods are expensive and detrimental to the environment (Chanoca et al., 2019). The conventional delignification process employs extensive chemicals like a bleaching agent and sodium hypochlorite which triggers damage to the cellulosic matter and imposes severe impairment to the environment by releasing toxic pollutants.
For quite some time, RNAi technology, especially the utilization of miRNA-based genetic engineering techniques has offered new approaches to modify lignins and lignocellulosic materials (Bewg et al., 2016). Genetic engineering approaches have advantages of specificity, efficacy, eco-friendliness, as compared to conventional chemical methods. The effectiveness of such approaches is in the down-regulation or silencing of the expression of enzymes involved in the lignin biosynthetic pathway (J.P. Wang et al., 2018; Rastogi and Dwivedi, 2014).
One study showed the lignin content in delignified Zea mays to be reduced by 7–8.7% through the Klason (acid-insoluble) lignin method which is recognized to be more precise than the acid detergent lignin estimation method (Park et al., 2014). Lignin reduction has also been visualized via scanning electron microscopy (SEM) which is appropriate for examining the topography and composition of samples. In one such study, efficient lignin reduction after pretreatment of the lignocellulosic plant was observed through SEM analysis (Bharathiraja et al., 2014). Moreover, the reduction in lignin content of the cell wall was found to be compensated with a high level of cellulose and arabinoxylan accumulation in maize (Fornalé et al., 2012). Likewise, the repression of a single lignin biosynthetic pathway gene, O-methyltransferase (OMT) has shown a concomitant increase in the cellulose content as a result of the reduction in the amount of lignin in maize (Piquemal et al., 2002). Many research works have revealed a correlation between lignin reduction and the improvement of fiber tensile parameters, such as density, tensile strength and elongation (Q. Wang et al., 2018; Zhang et al., 2013). Similarly, it has been demonstrated that delignified plants (using RNAi technology) result in improved fiber quality and consequently better profitability (Verma and Dwivedi, 2014; Bogoeva et al., 2007).
Previous work to reduce the lignin content in jute using RNAi techniques showed stable incorporation of fine-tuned alteration of four different lignin biosynthetic pathway genes namely, caffeic acid O-methyltransferase (COMT), cinnamate 4-hydroxylase (C4H), ferulic acid 5-hydroxylase (F5H) and coumarate 3-hydroxylase (C3H) (Shafrin et al., 2015, 2017).
However, considering the perspective of commercial deployment of genetically manipulated plants, it is of interest to learn how the advancement in transgenic lines i.e. lignin reduction affects the physical and biochemical properties in advanced generations of delignified jute as well as the quality of its fibers. This is crucial from a practical application viewpoint as it is obligatory to carry out field trials and obtain government consent before commercial implementation.
In light of the above, this study aimed at conducting the advancement of the transgenic jute lines to facilitate potential industrial applications of jute. To this aim, the effectiveness of lignin downregulation was evaluated across all transgenic generations of the four delignified jute through morphometric, histochemical, mechanical, chemical and SEM analyses. The advancement in COMT and C4H were carried out up to 7th and F5H and C3H were transformed up to 5th generations under uniform environmental conditions for analyzing stable transgenesis. The study intended to observe the transformations in physical and chemical properties such as changes in lignin and cellulose content, recalcitrance to glucose release and phenotypic changes if any due to lignin reduction. Moreover, to check the quality of the delignified jute fibers, several parameters i.e. tensile strength, elongation at break were used as performance indicators to assess the fiber tensile properties compared to the control. Furthermore, variation in growth compensation was also analyzed in the transgenic lines.
2. Materials and methods
2.1. Plant materials
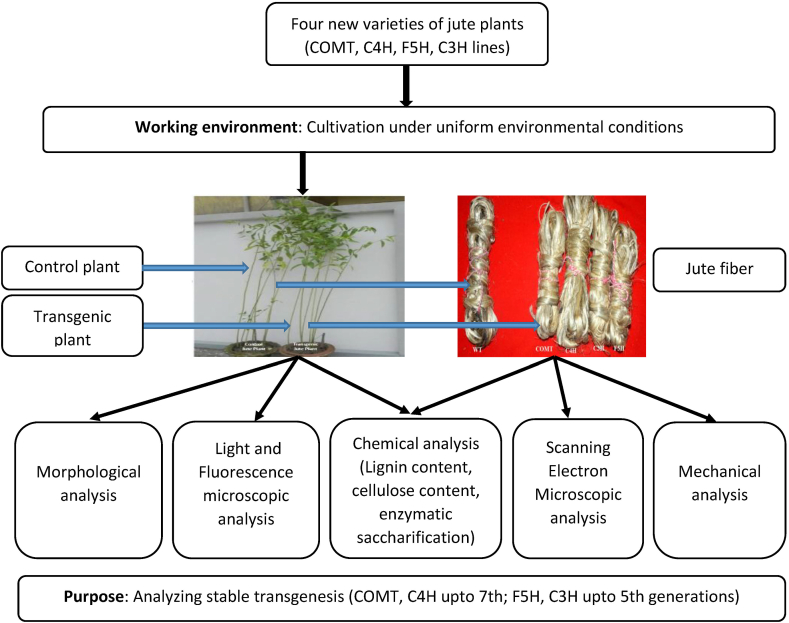
Among several lignin biosynthetic pathway genes, the following four i.e. COMT, F5H, C3H and C4H genes were downregulated in jute (Corchorus olitorius var. 0–9897) (Shafrin et al., 2015, 2017). After successful transformation (T), each of the four transgenic lines was advanced to several generations. The complete process is outlined in Figure 1.
Figure 1.
Flowchart of the complete study.
To advance the transgenic lines, seeds from the first transformed generation of all four transgenic lines were grown repeatedly. COMT and C4H transgenic lines were advanced up to the seventh generation whereas C3H and F5H transgenic lines were advanced to the fifth transformed generation. For comparative performance analysis, all four transgenic and control jute plants were cultivated in a controlled environment maintaining the same conservational conditions. After four months, matured jute plants were harvested at the end of the vegetation period. For experimental analysis, only the middle portion of the stem was collected. In order to collect the jute fiber, retting was done after harvesting. Jute stems were tied together and soaked in water. After 20 days of retting, the fibers were stripped from the jute stalk (Ali et al., 2015). The fibers were washed and dried for further chemical analysis.
2.2. Processes of chemical analysis
In this study, three different chemical analysis methods were studied to identify and understand the successful downregulation of the lignin pathway genes in all four transgenic lines compared to the control jute plant.
2.2.1. Lignin content
The lignin content of the whole stem and fiber was measured to estimate the acid-insoluble lignin using the Klason lignin estimation method with slight modifications (Tanmoy et al., 2015) for both transgenic and control jute plants. Plant stems and fiber were dried overnight at 105 °C to obtain the dry weight. For the experiment, 0.5gm of dry and ground samples were taken and denoted as W1. The samples were then hydrolyzed with 72% H2SO4 at room temperature, followed by 4% H2SO4 hydrolysis at boiling temperature. The hydrolyzed samples were vacuum filtered in glass crucibles with a silica filter to collect the sample residue. After purification, the crucibles with the residual content were heated at 105 °C in an oven. Next, the weight of the dried residual samples in the crucibles was taken and represented as W2. The residual samples in the crucibles were next heated at 575 ± 25 °C in a muffle furnace. At that point, the crucibles were cooled in a desiccator, the weight measured and designated as W3. With all these measured weights, the percentage of acid-insoluble lignin was calculated using the formula given as follows (Templeton and Ehrman, 1995):
| Acid Insoluble Lignin (%) = {(W3– W2)/W1} ×100 |
2.2.2. Cellulose content
The cellulose content was determined for both transgenic and control plants using the colorimetric method as described by Updegraff (1969). At first, samples from the whole stem were digested with a mixture of acetic and nitric acid (8:1 v/v) solution for 1 h at 100 °C and then centrifuged (14000 rpm, 5min). The precipitates were washed with water and digested again with 72% H2SO4 (v/v). Then the addition of cold anthrone reagent to the samples resulted in a green color which was measured spectrophotometrically at 620 nm. Finally, commercially available cellulose was used as a basis to generate the calibration curve.
2.2.3. Enzymatic saccharification
Neutral Detergent Fiber (NDF) was prepared for enzymatic saccharification (sugar release per unit of biomass) using an analytical technique described by Soest et al. (1991). The fiber was then incubated with commercial cellulase (Sigma, A. niger) for 24 h at 37 °C at 80 FPU (filter paper unit)/g NDF and reduced sugars were measured using dinitrosalicylic (DNS) acid at 540 nm (Miller, 1959).
2.3. Microscopic analysis
In the microscopic analysis, various observational approaches were considered using three types of microscopes to visualize and realize the successful downregulation of the lignin pathway genes.
2.3.1. Light microscopic analysis
The histochemical analysis was performed using phloroglucinol to analyze the lignin content of all four transgenic and control jute lines. The stem samples were collected after 45 days of growth and cut 10 cm above the root. Cross-sectioning (15 μm) of the jute stems was performed via a microtome (Thermo Scientific MICROM HM 430). Then, mounted segments of plant stems were stained with the phloroglucinol-HCl solution (a mixture of one volume of concentrated HCl and two volumes of 3 % phloroglucinol solution in absolute alcohol) (Liljegren, 2010) and sections were observed under a light microscope (Nikon ECLIPSE Ci) where lignified tissues appeared as red-violet in color.
2.3.2. Fluorescence microscope
The further histochemical analysis was carried out using calcofluor to analyze the cellulose content of all four transgenic and control jute plants. The sample collection and cross-sectioning procedures were the same as described for the light microscopic analysis section. The samples were first mounted in a 0.01% calcofluor white solution with a drop of 1N NaOH. After 5 min, samples were washed with distilled water to rinse out the calcofluor white solution and observed under a fluorescence microscope (EVOS® FL Imaging System).
2.3.3. Scanning electron microscope (SEM)
Surface morphological features of the transgenic and control jute fibers were observed by SEM (Zeiss sigma VP FE-SEM) and compared to analyze lignin reduction in all transgenic lines. Five elementary jute fiber samples from each transgenic and control jute plants were carefully cut into small pieces of similar size and mounted with adhesive tape on stubs, sputter-coated with gold and then examined under SEM operated at 10kV. Repeated images were taken for each transgenic and control sample to confirm the reproducibility of the result.
2.3.4. Mechanical analysis
For the analysis of tensile properties of all transgenic and control jute fibers, individual elementary fibers were separated manually from the fiber bundles. Jaw separation of 50 mm and a maximum load cell capacity of 5000 N were used for assessment. The tests were carried out with a speed of 5 mm/min. Tensile testing was performed at room temperature. For each sample, 20 individual fibers were studied and the data represented as average ±standard error (SE).
2.4. Statistical analysis
Morphological studies were carried out using five randomly selected plants of each generation. For chemical analysis such as lignin, cellulose content and enzymatic glucose release, independently selected two biological replicates and technical triplicates were used for each generation of jute lines. For mechanical analysis, 20 samples were taken into account and average data were presented for all transgenic and control fiber. Analyzed data were presented as mean ± standard error.
To evaluate the statistical significance of obtained data, one-way ANOVA and Tukey's tests were carried out through R program. For experimental data analysis, ‘∗∗∗’ indicates P value < 0.001, ‘∗∗’ indicates P value < 0.01, ‘∗’ indicates P value < 0.05; are considered as statistically significant.
3. Results
3.1. Morphological characteristics of transgenic plants across different generations
Jute, one of the best natural bast fibers, is losing its diversification because of an abundance of lignin polymer that renders the plant material nearly inaccessible for downstream processes. In this regard, a previous study had successfully carried out the downregulation of four lignin biosynthetic genes of jute (Shafrin et al., 2015, 2017). In continuation, the current study was designed to assess if the lowered lignin content in the same transgenic plants had been effective in enhancing the quality of the fiber by increasing the cellulose content as well as decreasing the recalcitrance to glucose release. Such changes would allow jute to be suitable for biofuel production, paper and pulp industries as well as other industrial purposes. The analyses were performed to visualize the effect of individual down-regulation of four different lignin biosynthetic genes across all the advanced generations (2nd, 3rd, 4th, 5th, 6th and 7th) of transgenic plants in terms of lignin and cellulose content, enzymatic glucose release and fiber strength.
To determine phenotypic variations if any, the delignified transgenic generations were analyzed all through the growth period. All transgenic and control plants were grown under the same environmental field conditions for a comparable appraisal. Besides, morphological evaluations were measured and compared to conclude if any changes in transgenic generations had resulted from lignin reduction. The analysis as reflected in Table 1 shows that plant height and width, pod length and number remained almost the same in each transgenic generation as in the control plants. This evidently indicates that there was no distinct variation in vegetative and reproductive growth characteristics due to lignin downregulation. For statistical analysis, plant samples were arbitrarily chosen ensuring two biological duplicates with three technical triplicates from individual generations.
Table 1.
Measurement of growth and yield parameters of control and transgenic lines of jute plant.
| Parameters | Plant height (cm) | Plant width (cm) | Pod number | Pod length (cm) | |
|---|---|---|---|---|---|
| Control plant | 350.72 ± 1.66a | 1.20 ± 0.006a | 60.67 ± 1.20a | 5.00 ± 0.06a | |
| COMT lines | T2 | 350.74 ± 1.47a | 1.21 ± 0.01a | 62.33 ± 0.88a | 4.97 ± 0.12a |
| T3 | 351.97 ± 0.51a | 1.20 ± 0.003a | 61.00 ± 1.15a | 4.93 ± 0.12a | |
| T4 | 350.01 ± 1.28a | 1.19 ± 0.008a | 60.67 ± 0.88a | 5.03 ± 0.09a | |
| T5 | 349.56 ± 0.46a | 1.20 ± 0.005a | 60.00 ± 1.15a | 5.00 ± 0.06a | |
| T6 | 349.81 ± 1.48a | 1.18 ± 0.003a | 58.67 ± 1.20a | 4.97 ± 0.12a | |
| T7 | 351.64 ± 0.67a | 1.20 ± 0.005a | 60.33 ± 1.86a | 5.07 ± 0.15a | |
| C4H lines | T2 | 351.63 ± 0.63a | 1.20 ± 0.005a | 60.33 ± 1.20a | 5.00 ± 0.06a |
| T3 | 350.90 ± 0.82a | 1.18 ± 0.003a | 60.00 ± 1.15a | 5.00 ± 0.06a | |
| T4 | 351.40 ± 0.84a | 1.21 ± 0.008a | 59.00 ± 1.15a | 5.07 ± 0.12a | |
| T5 | 350.76 ± 1.24a | 1.19 ± 0.005a | 60.67 ± 1.86a | 5.07 ± 0.09a | |
| T6 | 349.99 ± 0.99a | 1.20 ± 0.005a | 60.67 ± 1.76a | 5.00 ± 0.12a | |
| T7 | 351.10 ± 0.65a | 1.19 ± 0.005a | 60.33 ± 1.76a | 5.00 ± 0.12a | |
| C3H lines | T2 | 351.71 ± 0.46a | 1.19 ± 0.005a | 59.67 ± 0.88a | 5.07 ± 0.12a |
| T3 | 350.84 ± 0.87a | 1.20 ± 0.005a | 61.00 ± 1.53a | 4.90 ± 0.12a | |
| T4 | 351.04 ± 0.95a | 1.18 ± 0.008a | 60.00 ± 1.53a | 4.87 ± 0.09a | |
| T5 | 351.40 ± 0.79a | 1.20 ± 0.005a | 59.33 ± 0.88a | 5.07 ± 0.09a | |
| F5H lines | T2 | 350.28 ± 0.31a | 1.20 ± 0.003a | 59.67 ± 1.76a | 4.93 ± 0.09a |
| T3 | 350.34 ± 0.56a | 1.19 ± 0.005a | 59.00 ± 1.73a | 5.00 ± 0.12a | |
| T4 | 349.66 ± 0.87a | 1.21 ± 0.003a | 60.33 ± 0.88a | 5.00 ± 0.06a | |
| T5 | 350.62 ± 0.76a | 1.19 ± 0.008a | 60.67 ± 0.88a | 5.00 ± 0.06a | |
Results are shown as means ± standard error. Statistical analyses were done by one-way ANOVA and Tukey's test, For every single parameter, averages that do not share a letter are significantly different. (cm = centimeter; Control = Wild type plant).
3.2. Observations from chemical analysis
3.2.1. Analysis of lignin content
Lignin reduction was analyzed across all transformed generations of the four transgenic lines as an effect of the downregulation of lignin genes. To measure lignin reduction, up to T7 of two lines (COMT and C4H) and T5 of the other two lines (F5H and C3H) were taken into consideration and compared to the control. A significant decrease in the lignin content was attained for all transgenic types in contrast to the control. As Table 2 illustrates, on average, 21.45%, 22.08%, 22.72% and 21.66% decrease in the lignin content was observed for the whole stem of the COMT, C4H, F5H and C3H lines respectively. On the contrary, approximately 15.47%, 16.11%, 12.11% and 14.72% reductions in fiber lignin were obtained for COMT, C4H, F5H and C3H lines respectively when compared with control data as shown in Table 2. Klason lignin value of all transgenic generations of jute was statistically analyzed using ANOVA which showed a significant reduction compared to the control.
Table 2.
Summary of lignin content, cellulose content and enzymatic glucose release of control and transgenic jute plants.
| Parameters | % Klason lignin (whole stem) | % Lignin reduction (whole stem) | % Klason lignin (fiber) | % Lignin reduction (fiber) | % Cellulose (whole stem) | % Increase in cellulose content (whole stem) | Amount of glucose released (unit/hour of sample) | |
|---|---|---|---|---|---|---|---|---|
| Control plant | 30.56 ± 0.51a | 14.09 ± 0.14a | 31.28 ± 0.31b | 2.04 ± 0.35ab | ||||
| COMT lines | T2 | 23.74 ± 0.54b∗∗∗ | 22.31 | 11.87 ± 0.16def∗∗∗ | 15.76 | 32.88 ± 0.47a∗ | 5.11 | 2.41 ± 0.14ab |
| T3 | 24.63 ± 0.34b∗∗∗ | 19.40 | 12.36 ± 0.26bcd∗∗∗ | 12.28 | 32.84 ± 0.36a∗ | 5.01 | 2.77 ± 0.18ab | |
| T4 | 23.86 ± 0.67b∗∗∗ | 21.93 | 11.65 ± 0.15ef∗∗∗ | 17.32 | 33.03 ± 0.22a∗∗ | 5.59 | 2.96 ± 0.14a | |
| T5 | 23.59 ± 0.44b∗∗∗ | 22.80 | 12.07 ± 0.11cde∗∗∗ | 14.34 | 32.56 ± 0.36a | 4.09 | 2.40 ± 0.46ab | |
| T6 | 24.09 ± 0.60b∗∗∗ | 21.15 | 11.66 ± 0.21ef∗∗∗ | 17.25 | 33.32 ± 0.11a∗∗∗ | 6.52 | 2.32 ± 0.09ab | |
| T7 | 24.11 ± 0.55b∗∗∗ | 21.09 | 11.82 ± 0.22def∗∗∗ | 16.11 | 33.29 ± 0.18a∗∗ | 6.44 | 2.75 ± 0.12ab | |
| C4H lines | T2 | 23.59 ± 0.44b∗∗∗ | 22.80 | 11.43 ± 0.10f∗∗∗ | 18.88 | 32.74 ± 0.32a | 4.69 | 2.37 ± 0.19ab |
| T3 | 24.29 ± 0.35b∗∗∗ | 20.52 | 12.25 ± 0.24bcde∗∗∗ | 13.06 | 32.83 ± 0.36a∗ | 4.98 | 2.42 ± 0.29ab | |
| T4 | 24.18 ± 0.40b∗∗∗ | 20.86 | 11.70 ± 0.05ef∗∗∗ | 16.96 | 32.81 ± 0.28a∗ | 4.91 | 2.78 ± 0.19ab | |
| T5 | 23.85 ± 0.69b∗∗∗ | 21.96 | 11.99 ± 0.22def∗∗∗ | 14.90 | 32.56 ± 0.27a | 4.09 | 2.80 ± 0.24ab | |
| T6 | 23.26 ± 0.43b∗∗∗ | 23.87 | 11.81 ± 0.13def∗∗∗ | 16.18 | 33.18 ± 0.28a∗∗ | 6.09 | 2.28 ± 0.19ab | |
| T7 | 23.70 ± 0.21b∗∗∗ | 22.44 | 11.73 ± 0.34ef∗∗∗ | 16.75 | 33.22 ± 0.18a∗∗ | 6.18 | 2.73 ± 0.12ab | |
| C3H lines | T2 | 23.97 ± 0.62b∗∗∗ | 21.56 | 12.19 ± 0.08bcde∗∗∗ | 13.48 | 32.69 ± 0.43a. | 4.51 | 2.39 ± 0.47ab |
| T3 | 23.77 ± 0.32b∗∗∗ | 22.22 | 11.73 ± 0.06ef∗∗∗ | 16.75 | 32.94 ± 0.29a∗ | 5.30 | 2.18 ± 0.33ab | |
| T4 | 23.91 ± 0.50b∗∗∗ | 21.74 | 12.03 ± 0.23cde∗∗∗ | 14.62 | 33.38 ± 0.06a∗∗∗ | 6.72 | 1.95 ± 0.30b | |
| T5 | 24.10 ± 0.32b∗∗∗ | 21.13 | 12.10 ± 0.14cde∗∗∗ | 14.12 | 33.27 ± 0.08a∗∗ | 6.37 | 2.53 ± 0.14ab | |
| F5H lines | T2 | 23.15 ± 0.08b∗∗∗ | 24.25 | 12.14 ± 0.25cde∗∗∗ | 13.84 | 31.53 ± 0.30b | 0.81 | 2.58 ± 0.16ab |
| T3 | 24.17 ± 0.19b∗∗∗ | 20.91 | 12.73 ± 0.06b∗∗∗ | 9.65 | 32.74 ± 0.31a | 4.68 | 2.33 ± 0.28ab | |
| T4 | 23.70 ± 0.18b∗∗∗ | 22.43 | 12.59 ± 0.04bc∗∗∗ | 10.65 | 33.38 ± 0.01a∗∗∗ | 6.72 | 2.12 ± 0.25ab | |
| T5 | 23.44 ± 0.50b∗∗∗ | 23.29 | 12.07 ± 0.15cde∗∗∗ | 14.34 | 33.45 ± 0.08a∗∗∗ | 6.93 | 2.60 ± 0.14ab | |
Results are given as means ± standard error. Statistical analyses were done by one-way ANOVA and Tukey's test, P value <0.001 considered as highly significant where ‘∗∗∗’ indicates P < 0.001, ‘∗∗’ indicates P < 0.01, ‘∗’ indicates P < 0.05. For every single parameter, averages that do not share a letter are significantly different. (Control = Wild type plant).
3.2.2. Effect on cellulose content
It has been shown by others that the total lignin-cellulose mass remains unchanged after lignin reduction due to compensatory measures which may contribute to metabolic flexibility and growth benefit to withstand the structural integrity of a plant (Wei et al., 2005; Hu et al., 1999). In this study, all generations of four transgenic lines exhibited substantial growth in cellulose content compared to the control. For COMT, C4H, F5H and C3H transgenic lines, cellulose content on an average augmented 5.28%, 4.95%, 4.06% and 5.50 % respectively as represented in Table 2.
3.2.3. Efficiency of glucose release
Usually, a decrease in recalcitrance by lignin reduction is signified as an increase in enzymatic saccharification rate. The rise in sugar release due to an increased saccharification rate was found in delignified sugarcane under a demarcated pre-treatment condition (Bewg et al., 2016). Likewise, in our case, a slight improvement was observed in the amount of glucose released for all transgenic lines than the control jute plants as illustrated in Table 2.
3.3. Observations from microscopic analysis
3.3.1. Phloroglucinol staining
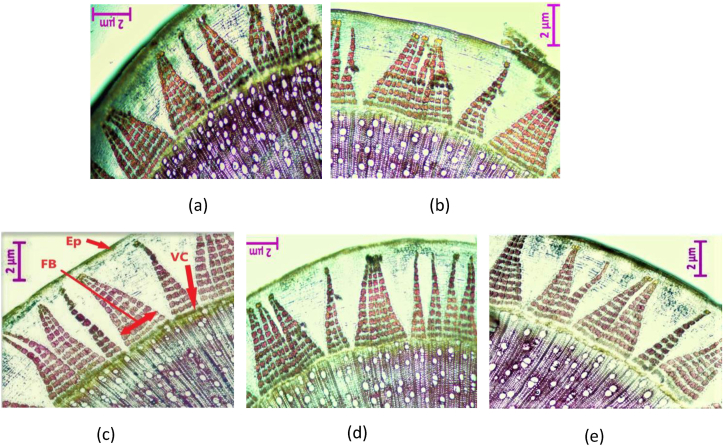
The presence of lignin in stem cross-sections of control and transgenic generations were observed under a light microscope with 20X magnification using phloroglucinol-HCl. Phloroglucinol, which specifically binds with hydroxy-cinnamaldehyde end-groups of lignin develops a purple or reddish chromophore with the color intensity reflecting the amount of lignin present (Blaschek et al., 2020). Comparatively, the intensities of the purple coloration detected in fiber and xylem regions of the transgenic stems were lower as displayed in Figure 2 (b-e). This indicates a significantly lower deposition of lignin in these locations as compared to control (Figure 2 a). The epidermis, cortex and vascular cambium portions of all stem sections did no stain due to an absence of lignin. For experimental analysis, 90 days old jute plants were used in this study.
Figure 2.
Histochemical assay of lignin. Lignin deposition observed in jute stems of control plant (a), transgenic lines COMT-T6 (b), C4H-T6 (c), F5H-T4 (d) and C3H-T4 (e). (Ep: epidermis; FB: fiber bundle VC: vascular cambium).
3.3.2. Calcofluor staining
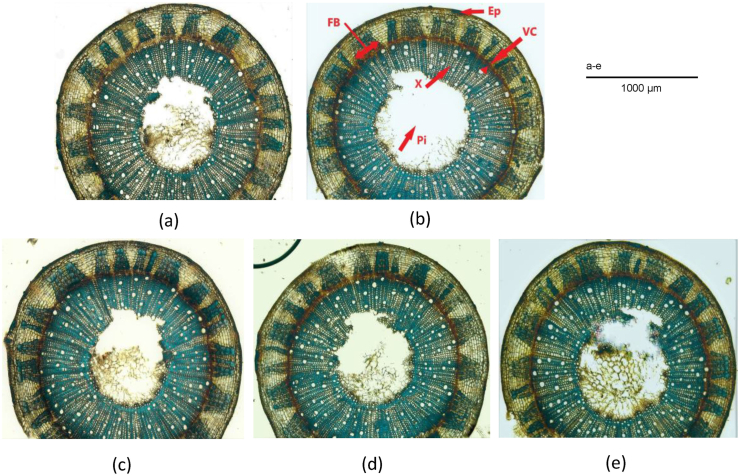
To look for variations in the cellulose content in transgenic and control lines, a further histochemical analysis was performed with stem cross-sections of 45 days old plants using calcofluor white. These cross-sections were analyzed at 10X magnification under a fluorescence microscope. Calcofluor white stains cellulose and yields cyan-green color under fluorescence light (Coen et al., 2019).
The epidermis, cortex, fiber cell and the xylem contain cellulose as the major polysaccharide polymer in their cell walls. Histochemical analysis revealed an intense color all over the stem section of transgenic lines as presented in Figure 3 (b-e). The bright staining reflects an increase in the accessibility of cellulose at these locations for all the transgenic lines than the control.
Figure 3.
Histochemical assay of cellulose. Cellulose deposition observed in jute stems of control plant (a), transgenic lines COMT-T6 (b), C4H-T6 (c), F5H-T4 (d) and C3H-T4 (e). (Ep: epidermis; FB: fiber bundle; VC: vascular cambium; X: xylem; Pi: pith).
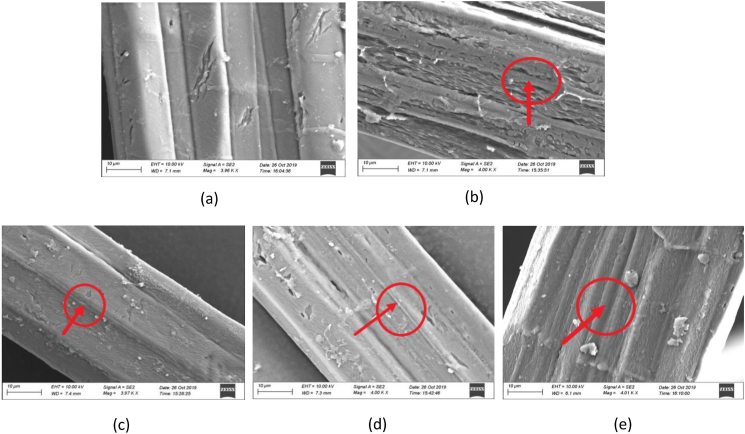
3.3.3. Scanning electron microscope analysis of jute fiber
SEM was used for understanding the influence of lignin reduction on the stem surfaces of transgenic lines. The surface morphological features of wild type and transgenic elementary fibers were observed through SEM for identifying variations among the fibers. Figure 4 (a) demonstrates the control and Figure 4 (b-e) portray all the transgenic specimens as highlighted in red circles. Generally, the bundles of bast fibers comprise 10–25 elementary fibers which are mainly composed of elementary fibrils covered by lignin and pectin (Mohanty et al., 2005). It can be seen that the presence of lignin on the control is much higher than all the transgenic samples. More specifically, if we consider two regions on the control specimen and the transgenic samples (marked with red circles), no small fibrils are witnessed on the control specimen as they are hidden underneath the lignin layer (Figure 4a). On the other hand, COMT transgenic line marked in red circle (Figure 4b) indicates the exposure of small fibrils on the fiber surface as a result of delignification. Similarly, Figure 4 (c-e) for C4H, F5H and C3H transgenic lines exemplify comparable features as detected for COMT transgenic line and a reduction of lignin exposed the underlying fibrils. Consequently, SEM steered figure analyses confirm all transgenic fibers to have less lignin compared to the control.
Figure 4.
Scanning Electron Microscope (SEM) images of the surface of control (wild type) (a) and transgenic lines COMT-T6 (b), C4H-T6 (c), F5H-T4 (d) and C3H-T4 (e). Red circles indicate microfibril exposure on transgenic fiber surface.
3.4. Effect of lignin reduction on mechanical properties of jute fiber
Different mechanical properties of delignified transgenic and control jute fibers are outlined in Table 3. It is noticed that control jute fiber has an average tensile strength of 493.80 MPa and a breaking elongation of 3.25%.
Table 3.
Measurement of mechanical properties of control (wild type) and transgenic lines of jute fiber.
| Parameters | Fiber tensile strength (MPa) | Breaking elongation (%) |
|---|---|---|
| Control plant | 493.80 ± 32.51a | 3.25 ± 0.13a |
| COMT-T6 lines | 512.50 ± 35.54a | 3.56 ± 0.11a |
| C4H-T6 lines | 548.77 ± 32.04a | 3.49 ± 0.17a |
| C3H-T4 lines | 576.13 ± 30.89a | 3.42 ± 0.09a |
| F5H-T4 lines | 570.97 ± 27.22a | 3.54 ± 0.12a |
Results are shown as means ± standard error. Statistical analyses were done by one-way ANOVA and Tukey's test (20 samples). For every single parameter, averages that do not share a letter are significantly different. In this case, data were not significant. (MPa = Megapascal; Control = wild type plant).
Comparative analyses indicate that all transgenic jute fibers exhibit an increased tensile strength ranging between 512.50 to 576.13 MPa. The average breaking elongation of individual fibers was found to increase modestly ranging between 3.42% - 3.56%. A reduction of lignin possibly leads to the slippage of cellulosic microfibrils allowing for a rearrangement of the microfibrillar assembly. Therefore, the average tensile strength and breaking elongation of transgenic fibers showed a minute increase with associated lignin reduction. It is worth noting that the tensile strength and breaking elongation of jute fiber exhibited a moderately high standard error. A possible reason could be attributed to the difficulty of extracting uniform fiber (elementary) samples because of their very thin nature (Sengupta and Palit, 2004).
4. Discussion
With an understanding that transgenic jute with reduced lignin has immense contribution in value-added utilization of bio-based material in the textile industry, forage and bioenergy production, paper and pulp industry, the current study explored the benefits of lignin reduction for diversified applications of this cash crop.
We performed experiments across different generations of each transgenic line along with the control jute plant. Among the four lines available, two transgenic plants (COMT and C4H) were advanced up to the T7 generation whereas for C3H and F5H the lines were up to T5. Klason lignin estimation method was used to measure the acid-insoluble lignin content. As illustrated in Table 2, lignin was found to be significantly reduced in all four transgenic jute lines compared to the control. Reduction in lignin content was persistent across the different generations of each line (T2-T7 in COMT and C4H and T2-T5 in C3H and F5H) proving the stability of the lignin gene manipulation. Findings of this experiment comply with similar downregulation of COMT gene expression that had reduced the lignin content in switchgrass (Fu et al., 2011) and downregulation of C4H, C3H or F5H genes in alfalfa plants which greatly reduced lignin without a significant impact on its composition (Chen et al., 2006).
It has been reported that even though lignin deposition in the cell wall is a critical phase in adaptation, plants can endure up to 40% decrease in lignin without major adversarial effects on normal plant growth, physiology and susceptibility to diseases in greenhouse conditions (Zhong et al., 2000). A summary of our findings (Table 1) disclose that lignin reduction in the transgenic whole stem is below 25% and for fiber, it is 15%. Lignin reduction is therefore not expected to have any significant growth deformities in transgenic jute plants compared to the control. A study carried out by Shafrin et al. (2015) on earlier generations of these transgenic lines attests to the same.
In principle, lignin and cellulose depositions are regulated in a compensatory fashion and the total lignin–cellulose mass remains unchanged (Hu et al., 1999). Comparing the results of lignin and cellulose content as summarized in Table 2, it is conceivable that lignin reduction is compensated with a higher level of cellulose accumulation in all transgenic jute plants. Lignin reduction is found to be linked to an associated increase in cellulose content leading to an improvement in saccharification efficiency (Sykes et al., 2015). In this essence, our analysis has also pointed at an increase in glucose release after enzymatic saccharification in different transgenic jute lines compared to the control as summed up in Table 2.
In a further study, the results of SEM analysis on transgenic and control jute fiber surfaces (presented in Figure 4 a-e) demonstrate the effects of lignin gene downregulation. The investigation reveals exposed microfibrils (in red circles) on the surface of transgenic fibers whereas these microfibrils are hidden below the lignin layer in control fiber. This comparative analysis of the presence of microfibrils on fiber surface asserts a lower lignin content in all transgenic fibers which is comparable to a similar observation made by Abraham et al. (2011). Another analysis made by Wang et al. (2018) identified that untreated poplar fiber pulp had a lower extent of fibrillation than pulp treated for lignin reduction.
The effect of lignin content on the mechanical properties of control and transgenic jute fibers were investigated in terms of their tensile strength and percentage of elongation at break. Most of the existing studies for improving mechanical properties in jute fiber were done through surface modification of the fiber where the treatment involved a chemical process for improving the adhesion at the interface (Wang et al., 2019; Roy et al., 2012; Saha et al., 2010). These findings indicate that alkali concentration and treatment duration are dominant factors in achieving a better tensile strength performance. For the same alkali concentration and treatment duration, some studies (Saha et al., 2010; Roy et al., 2012; Zafar et al., 2016; Maharana et al., 2020) have revealed a very different outcome. Additionally, studies by Zafar et al. (2016) and Wang et al. (2019) show an insignificant increase (below 5%) in tensile strength. Therefore, based on these studies, it can be concluded that a linear relationship between non-cellulosic materials and mechanical properties does not exist. In these studies, the main reason for enhancement in strength and elongation at break is identified as the partial removal of the amorphous materials (hemicellulose, lignin or pectin) from the interfibrillar region. However, none of these studies have established or presented any explicit relationship between lignin removal and its effect on mechanical properties. Therefore, the contribution of lignin in mechanical properties can not be evidently obtained from the existing studies. Also, chemical treatment requires extensive chemicals that release toxic pollutants into the environment (Burrola-Núñez et al., 2019).
On the contrary, a study by (Mandal and Datta, 2014) on jute stem has shown that with a 62% lignin reduction, the tensile strength of the mutant had increased by 23%. However, the study used a mutational approach which is considered to result in random mutations in the genome, leading to a decrease in fiber yield and other abnormalities in the mutant (Sengupta and Palit, 2004; Majumder et al., 2020).
At this point, our study stands out from the existing studssies in two ways. Firstly, comparative results indicate that the transgenic jute fibers exhibit little variation in tensile strength with regard to the control fiber as illustrated in Table 3. Secondly, this study shows approximately 17% tensile strength and 10% breaking elongation rise for only a 23% reduction of lignin possibly due to slippage and displacement of cellulose microfibrils resulting from reduced lignin (Jo et al. 2016; Zafar et al., 2016). A similar investigation has been reported for palm fiber (Oushabi et al., 2017) in which enhanced tensile strength is observed as a result of effective non-cellulosic lignin removal.
Moreover, according to the results obtained (Table 3), increased values of breaking elongation were observed for the delignified transgenic jute lines than the control fiber. Similar analyses of mechanical properties were made by Zhang et al. (2013) and Saha et al. (2010) with both reporting that the removal of non-cellulosic materials like lignin from the interfibrillar space leads to fiber elongation.
In textile industries, there is a revived interest in the use of plant fibers because of the non-renewable properties of the synthetic ones. The presence of lignin in plant fiber confers poor elastic properties. In paper pulp industries, the use of a chemical delignification process to produce high-quality paper damage the polysaccharide components of the fiber and release toxic pollutants into the environment. A soluton to these problems can be found inthe four different genetically engineered lines of jute plant. These lines with reduced lignin show a concomitant growth in cellulose content avoiding chemical pretreatment, asserting its profitability and environment friendliness. Furthermore, eco-friendly production of biofuel from its increased cellulosic biomass will lead to a cost-effective use of these new jute lines. Overall, such high-quality attributes of transgenic jute lines have the potential to enforce jute application as a sustainable source of bio-based material for commercial resolutions viz. textile, paper and pulping, biofuel with far-reaching economic acceleration for jute producing countries.
5. Conclusion
In this study, the influence of lignin gene downregulation on different generations of four transgenic lines (COMT, C4H, C3H and F5H) was investigated. The findings indicate growth in cellulose content, a slight enhancement in saccharification and confirmed the exposure of underlying microfibrils as a result of significant lignin reduction. Microscopic studies of jute stem sections are seen to fully comply with biochemical analysis in terms of lignin and cellulose content. Furthermore, a minute increase in tensile strength and breaking elongation of transgenic jute fiber was observed. However, no alterations in the morphology and defense mechanisms in transgenic jute traits were observed. Overall, these assert the great potential of down-regulated transgenic jute lines for industrial applications. From the perspective of practical application, it is mandatory to proceed with field trial and government approval to implement the new jute transgenic lines. This will be the future focus of this research.
Declarations
Author contribution statement
Mousumi Nath: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Farhana Tasnim Chowdhury: Analyzed and interpreted the data.
Shabbir Ahmed, Avizit Das: Performed the experiments, Analyzed and interpreted the data.
Mohammad Riazul Islam, Haseena Khan: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
All the authors greatly acknowledge the support rendered by the Bangladesh Council of Scientific and Industrial Research (BCSIR), Dhaka, Bangladesh.
References
- Abraham E., Deepa B., Pothan L.A., Jacob M., Thomas S., Cvelbar U., Anandjiwala R. Extraction of nanocellulose fibrils from lignocellulosic fibres: a novel approach. Carbohydr. Polym. 2011;86(4):1468–1475. [Google Scholar]
- Ali M., Kozan O., Rahman A., Islam K., Hossain M. Jute retting process: present practice and problems in Bangladesh. Agric. Eng. Int. CIGR J. 2015;17:243–247. [Google Scholar]
- Alves C., Ferrão P., Silva A., Reis L., Freitas M.J., Rodrigues L.B., Alves D.E. Ecodesign of automotive components making use of natural jute fiber composites. J. Clean. Prod. 2010;18(4):313–327. [Google Scholar]
- Asadullah M., Rahman M.A., Ali M.M., Motin M.A., Sultan M.B., Alam M.R., Rahman M.S. Jute stick pyrolysis for bio-oil production in fluidized bed reactor. Bioresour. Technol. 2008;99(1):44–50. doi: 10.1016/j.biortech.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Bergfjord C., Holst B. A procedure for identifying textile bast fibres using microscopy: flax, nettle/ramie, hemp and jute. Ultramicroscopy. 2010;110(9):1192–1197. doi: 10.1016/j.ultramic.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Bewg W.P., Poovaiah C., Lan W., Ralph J., Coleman H.D. RNAi downregulation of three key lignin genes in sugarcane improves glucose release without reduction in sugar production. Biotechnol. Biofuels. 2016;9:270. doi: 10.1186/s13068-016-0683-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharathiraja B., Sudharsanaa T., Bharghavi A., Sowmeya G.S., Balaram G. Insights on lignocellulosic pretreatments for biofuel production- SEM and reduction of lignin analysis. Int. J. ChemTech. Res. 2014;6:4334–4444. [Google Scholar]
- Blaschek L., Champagne A., Dimotakis C., Nuoendagula, Decou R., Hishiyama S., Kratzer S., Kajita S., Pesquet E. Cellular and genetic regulation of coniferaldehyde incorporation in lignin of herbaceous and woody plants by quantitative wiesner staining. Front. Plant Sci. 2020;11:109. doi: 10.3389/fpls.2020.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogoeva G.G., Avella M., Malinconico M., Buzarovska A., Grozdanov A., Gentile G., Errico M.E. Natural fiber eco-composites. Polym. Compos. 2007;28(1):98–107. [Google Scholar]
- Burrola-Núñez H., Herrera-Franco P.J., Rodríguez-Félix D.E., Soto-Valdez H., Madera-Santana T.J. Surface modification and performance of jute fibers as reinforcement on polymer matrix: an overview. J. Nat. Fibers. 2019;16(7):944–960. [Google Scholar]
- Chanoca A., de Vries L., Boerjan W. Lignin engineering in forest trees. Front. Plant Sci. 2019;10:912. doi: 10.3389/fpls.2019.00912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Reddy M.S.S., Temple S., Jackson L., Shadle G., Dixon R.A. Multi-site genetic modulation of monolignol biosynthesis suggests new routes for formation of syringyl lignin and wall-bound ferulic acid in alfalfa (Medicago sativa L.) Plant J.: Cell. Mol. Biol. (Noisy-Le-Grand) 2006;48:113–124. doi: 10.1111/j.1365-313X.2006.02857.x. [DOI] [PubMed] [Google Scholar]
- Chirat C. Use of vegetal biomass for biofuels and bioenergy. Competition with the production of bioproducts and materials. Compt. Rendus Phys. 2017;18:462–468. [Google Scholar]
- Coen O., Lu J., Xu W., Vos D.D., Péchoux C., Domergue F., Grain D., Lepiniec L., Magnani E. Deposition of a cutin apoplastic barrier separating seed maternal and zygotic tissues. BMC Plant Biol. 2019;19:304. doi: 10.1186/s12870-019-1877-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del-Río J.C., Marques G., Rodríguez I.M., Gutiérrez A. Chemical composition of lipophilic extractives from jute (Corchorus capsularis) fibers used for manufacturing of high-quality paper pulps. Ind. Crop. Prod. 2009;30(2):241–249. [Google Scholar]
- Fornalé S., Capellades M., Encina A., Wang K., Irar S., Lapierre C., Ruel K., Joseleau J.P., Berenguer J., Puigdomènech P., Rigau J., Caparrós-Ruiz D. Altered lignin biosynthesis improves cellulosic bioethanol production in transgenic maize plants down-regulated for cinnamyl alcohol dehydrogenase. Mol. Plant. 2012;5(4):817–830. doi: 10.1093/mp/ssr097. [DOI] [PubMed] [Google Scholar]
- Fu C., Mielenz J.R., Xiao X., Ge Y., Hamilton C.Y., Rodriguez M., Jr., Chen F., Foston M., Ragauskas A., Bouton J., Dixon R.A., Wang Z.Y. Genetic manipulation of lignin reduces recalcitrance and improves ethanol production from switchgrass. Proc. Natl. Acad. Sci. U.S.A. 2011;108(9):3803–3808. doi: 10.1073/pnas.1100310108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W.J., Harding S.A., Lung J., Popko J.L., Ralph J., Stokke D.D., Tsai C.J., Chiang V.L. Repression of lignin biosynthesis promotes cellulose accumulation and growth in transgenic trees. Nat. Biotechnol. 1999;17(8):808–812. doi: 10.1038/11758. [DOI] [PubMed] [Google Scholar]
- Jo B.W., Chakraborty S., Kim H. Efficacy of alkali-treated jute as Fibre reinforcement in enhancing the mechanical properties of cement mortar. Mater. Struct. 2016;49(3):1093–1104. [Google Scholar]
- Kumar P., Barrett D., Delwiche M.J., Stroeve P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res. 2009;48(8):3713–3729. [Google Scholar]
- Kwiatkowska M.W., Starzycki M., Zebrowski J., Oszmiański J., Szopa J. Lignin deficiency in transgenic flax resulted in plants with improved mechanical properties. J. Biotechnol. 2007;128(4):919–934. doi: 10.1016/j.jbiotec.2006.12.030. [DOI] [PubMed] [Google Scholar]
- Li X., Tabil L.G., Panigrahi S. Chemical treatments of natural fiber for use in natural fiber-reinforced composites: a review. J. Polym. Environ. 2007;15:25–33. [Google Scholar]
- Liljegren S. Phloroglucinol stain for lignin. Cold Spring Harb. Protoc. 2010;2010(1) doi: 10.1101/pdb.prot4954. pdb.prot4954. [DOI] [PubMed] [Google Scholar]
- Maharana S.M., Pandit M.K., Pradhan A.K. Effect of chemical treatment and fumed silica coating on tensile and thermogravimetric properties of jute yarn. Mater. Today: Proceedings. 2020;27(3):2693–2698. [Google Scholar]
- Maity S., Chowdhury S., Datta A.K. Jute biology, diversity, cultivation, pest control, fiber production and genetics. In: Lichtfouse E., editor. Vol. 9. 2012. pp. 227–262. (Organic Fertilisation, Soil Quality and Human Health). [Google Scholar]
- Majumder S., Saha P., Datta K., Datta S.K. Advancement in Crop Improvement Techniques. Woodhead Publishing; 2020. Fiber crop, jute improvement by using genomics and genetic engineering; pp. 363–383. Chapter 22. 2020. [Google Scholar]
- Mandal A., Datta A.K. Stability analysis of a high fibre yield and low lignin content "thick stem" mutant in tossa jute (Corchorus olitorius L.) BioMed Res. Int. 2014:539869. doi: 10.1155/2014/539869. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959;31:426–428. [Google Scholar]
- Mishra V., Biswas S. Physical and mechanical properties of Bi-directional jute fiber epoxy composites. Procedia Eng. 2013;51:561–566. [Google Scholar]
- Mohanty A.K., Manjusri M., Drzal L.T. CRC Press, Taylor & Francis; Boca Raton: 2005. Natural Fibres, Biopolymers and Biocompo- Sites. [Google Scholar]
- Monteiro S.N., Pereira A.C., Ferreira C.L., Pereira J.É., Weber R.P., Assis F.S.D. Performance of plain woven jute fabric-reinforced polyester matrix composite in multilayered ballistic system. Polymers. 2018;10(3):230. doi: 10.3390/polym10030230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oboh G., Raddatz H., Henle T. Characterization of the antioxidant properties of hydrophilic and lipophilic extracts of Jute (Corchorus olitorius) leaf. Int. J. Food Sci. Nutr. 2009;60(2):124–134. doi: 10.1080/09637480902824131. [DOI] [PubMed] [Google Scholar]
- Oushabi A., Sair S., Hassani F.O., Abboud Y., Tanane O., Abdeslam E.B. The effect of alkali treatment on mechanical, morphological and thermal properties of date palm fibers (DPFs): study of the interface of DPF–Polyurethane composite. S. Afr. J. Chem. Eng. 2017;23:116–123. [Google Scholar]
- Ozgen B. New biodegradable fibres, yarn properties and their applications in textiles: a review. Industria Textila. 2012;63:3–7. [Google Scholar]
- Park S.H., Ong R.G., Mei C., Sticklen M. Lignin down-regulation of Zea mays via dsRNAi and Klason lignin analysis. JoVE: JoVE. 2014;89:51340. doi: 10.3791/51340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira A.C., Assis F.S.D., Filho F.D.C.G., Oliveira M.S., Demosthenes L.C.D.C., Lopera H.A.C., Monteiro S.N. Ballistic performance of multilayered armor with intermediate polyester composite reinforced with fique natural fabric and fibers. J. Mat. Res. Tech. 2019;8(5):4221–4226. [Google Scholar]
- Pereira A.C., Assis F.S.D., Filho F.D.C.G., Costa F.D., Oliveira M.S., Lima E.S., Lopera H.A.C., Monteiro S.N. Evaluation of the projectile's loss of energy in polyester composite reinforced with fique fiber and fabric. Mater. Res. 2019;22(Suppl. 1) [Google Scholar]
- Pickering K., Efendy M.A., Le T. A review of recent developments in natural fibre composites and their mechanical performance. Compos. Part A Appl. Sci. Manuf. 2016;83:98–112. [Google Scholar]
- Piquemal J., Chamayou S., Nadaud I., Beckert M., Barrière Y., Mila I., Lapierre C., Rigau J., Puigdomenech P., Jauneau A., Digonnet C., Boudet A.M., Goffner D., Pichon M. Downregulation of caffeic acid O-methyltransferase in maize revisited using a transgenic approach. Plant Physiol. 2002;130:1675–1685. doi: 10.1104/pp.012237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi S., Dwivedi U. Lignin genetic engineering for improvement of wood quality: applications in paper and textile industries, fodder and bioenergy production. South Afr. J. Bot. 2014;91:107–125. [Google Scholar]
- Roy A., Chakraborty S., Kundu S.P., Basak R.K., Majumder S.B., Adhikari B. Improvement in mechanical properties of jute fibres through mild alkali treatment as demonstrated by utilisation of the Weibull distribution model. Bioresour. Technol. 2012;107:222–228. doi: 10.1016/j.biortech.2011.11.073. [DOI] [PubMed] [Google Scholar]
- Saha P., Manna S., Chowdhury S.R., Sen R., Roy D., Adhikari B. Enhancement of tensile strength of lignocellulosic jute fibers by alkali-steam treatment. Bioresour. Technol. 2010;101(9):3182–3187. doi: 10.1016/j.biortech.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Selver E., Ucar N., Gulmez T. Effect of stacking sequence on tensile, flexural and thermomechanical properties of hybrid flax/glass and jute/glass thermoset composites. J. Ind. Textil. 2018;48:494–520. [Google Scholar]
- Sengupta G., Palit P. Characterization of a lignified secondary phloem fibre-deficient mutant of jute (corchorus capsularis) Ann. Bot. 2004;93:211–220. doi: 10.1093/aob/mch029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadle G., Chen F., Reddy M.S.S., Jackson L., Nakashima J., Dixon R.A. Down-regulation of hydroxycinnamoyl CoA: Shikimate hydroxycinnamoyl transferase in transgenic alfalfa affects lignification, development and forage quality. Phytochemistry. 2007;68(11):1521–1529. doi: 10.1016/j.phytochem.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Shafrin F., Ferdous A.S., Sarkar S.K., Ahmed R., Amin A., Hossain K., Sarker M., Rencoret J., Gutiérrez A., Rio J.C., Sanan-Mishra N., Khan H. Modification of monolignol biosynthetic pathway in jute: different gene, different consequence. Sci. Rep. 2017;7:39984. doi: 10.1038/srep39984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafrin F., Das S.S., Sanan-Mishra N., Khan H. Artificial miRNA-mediated down-regulation of two monolignoid biosynthetic genes (C3H and F5H) cause reduction in lignin content in jute. Plant Mol. Biol. 2015;89(4-5):511–527. doi: 10.1007/s11103-015-0385-z. [DOI] [PubMed] [Google Scholar]
- Soest P.J.V., Robertson J.B., Lewis B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991;74(10):3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- Sykes R.W., Gjersing E.L., Foutz K., Rottmann W.H., Kuhn S.A., Foster C.E., Ziebell A., Turner G.B., Decker S.R., Hinchee M.A., Davis M.F. Down-regulation of p-coumaroyl quinate/shikimate 3'-hydroxylase (C3'H) and cinnamate 4-hydroxylase (C4H) genes in the lignin biosynthetic pathway of Eucalyptus urophylla × E. grandis leads to improved sugar release. Biotechnol. Biofuels. 2015;8:128. doi: 10.1186/s13068-015-0316-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanmoy A., Alum M., Islam M., Farzana T., Khan H. Jute (Corchorus olitorius var. O-72) stem lignin: variation in content with age. Bangladesh J. Bot. 2015;43(3):309–314. [Google Scholar]
- Templeton D., Ehrman T. National Renewable Energy Laboratory (NREL), Technical Report NREL LAP-003; Golden, CO: 1995. Determination of Acid-Insoluble Lignin in Biomass. [Google Scholar]
- Updegraff D.M. Semimicro determination of cellulose in biological materials. Anal. Biochem. 1969;32(3):420–424. doi: 10.1016/s0003-2697(69)80009-6. [DOI] [PubMed] [Google Scholar]
- Verma S.R., Dwivedi U.N. Lignin genetic engineering for improvement of wood quality: applications in paper and textile industries, fodder and bioenergy production. South Afr. J. Bot. 2014;91:107–125. [Google Scholar]
- Wang H., Memon H., Am Hassan E., Miah M.S., Ali M.A. Effect of jute fiber modification on mechanical properties of jute fiber composite. Materials. 2019;12(8):1226. doi: 10.3390/ma12081226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Xue Y., Chen Y., Li R., Wei J. Lignin modification improves the biofuel production potential in transgenic Populus tomentosa. Ind. Crop. Prod. 2012;37(1):170–177. [Google Scholar]
- Wang J.P., Matthews M.L., Williams C.M., Shi R., Yang C.M., Tunlaya-Anukit S., Chen H.C., Li Q.Z., Liu J., Lin C.Y. Improving wood properties for wood utilization through multi-omics integration in lignin biosynthesis. Nat. Commun. 2018;9:1579. doi: 10.1038/s41467-018-03863-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Xiao S., Shi S.Q., Cai L. Effect of light-delignification on mechanical, hydrophobic, and thermal properties of high-strength molded fiber materials. Sci. Rep. 2018;8:955. doi: 10.1038/s41598-018-19623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei T., Nelson A., Johnson E. Increasing cellulose production and transgenic plant growth in forest tree species. J. For. Res. 2005;16:67. [Google Scholar]
- Zafar M.T., Maiti S.N., Ghosh A.K. Effect of surface treatment of jute fibers on the interfacial adhesion in poly(lactic acid)/jute fiber biocomposites. Fibers Polym. 2016;17:266–274. [Google Scholar]
- Zhang S.Y., Fei B.H., Yu Y., Cheng H.T., Wang C.G. Effect of the amount of lignin on tensile properties of single wood fibers. Forest Science and Practice. 2013;15:56–60. [Google Scholar]
- Zhong R., Morrison W.H., Himmelsbach D.S., Poole F.L., Ye Z.H. Essential role of caffeoyl coenzyme A O-methyltransferase in lignin biosynthesis in woody poplar plants. Plant Physiol. 2000;124:563–578. doi: 10.1104/pp.124.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.