Abstract
Exopolysaccharides (EPS) isolated from microalgae are promising immune cell proliferation agents, that could be potentially used as immunostimulants. In the current study, Scenedesmus acutus (S. acutus) was grown under varying nutrient (sulphur and phosphorus) concentrations to enhance the EPS production, and the isolated EPS were assessed for their effect on cell proliferation using peripheral blood mononuclear cells (PBMC). Five different concentrations of MgSO4 (0, 0.25, 0.5, 1.0 and 1.25 g/L) and K2HPO4 (0, 0.2, 0.6, 0.8 and 1.0 g/L) were taken as compared to the normal culture conditions (0.75 g/L MgSO4 and 0.4 g/L K2HPO4) with the intention to enrich EPS secretion. LC–MS, FTIR and NMR analysis revealed that isolated EPS have the characteristic spectrum of hetero-polysaccharides (octa-saccharides). Immunostimulatory property of EPS was demonstrated by their ability to augment PBMC proliferation as measured by MTT assay. Further, increase in the glucose content and proliferative index was observed for EPS obtained under higher concentrations of MgSO4 (1 and 1.25 g/L) and K2HPO4 (0.6 and 0.8 g/L) relative to normal culture conditions. Effects of the generated EPS under varying concentration of MgSO4 (r = 0.84–0.99) and K2HPO4 (r = 0.76–0.97) remained strongly correlated with cell count, chlorophyll content, total biomass, glucose, proliferative index and its scavenging activity. Collectively, our data not only showed that EPS generated by S. acutus under higher concentration of K2HPO4 and MgSO4 possess improved immunostimulatory properties, but also provides convincing evidence towards nutritional optimization of alga for enhanced EPS production with better bioactivities.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-021-02720-z.
Keywords: Cell growth, Peripheral blood mononuclear cells, Extracellular polysaccharides, MTT assay, Modified culture conditions
Introduction
Microalgae secrete different extracellular polymeric substances which include nucleic acids, polysaccharides, proteins and lipids. Micro-algal extracellular polysaccharides (EPS) have drawn attention to the researchers owing to their wide applications in pharmaceuticals, therapeutics, mechanical and food industry. Currently, EPS are greatly used in the food industry as gelling agents and thickeners which are being used to improve the texture and quality of the food products (Xiao and Zheng 2016). EPS are also reported to exhibit different bioactive properties such as antiviral, anticancer, antioxidant, antihyperlipidaemic and immunomodulatory activities (Xiao and Zheng 2016). EPS produced by different microalgae such as Chlorella pyrenoidosa, Scenedesmus sp. and Chlorococcum sp. were recently reported to exhibit antitumor effects against human colon cancer cell lines HCT116 and HCT8, and also showed antioxidant potential (Zhang et al. 2019).
In the last decade, various microalgae were explored in the search of novel bioactive leads with immunomodulatory properties (Riccio and Lauritano 2020). Such immunomodulatory compounds may either reduce inflammation associated with chronic conditions or increase immune response against invading pathogens and cancer. For instance, EPS isolated from Chlorella stigmatophora and Phaeodactylum tricornutum reduced inflammatory edema in rats (Guzmán et al. 2003). Hetero-polysaccharides obtained from Dunaliella salina increased the production of IFN-γ, TNF-α and TGF-β in peripheral blood mononuclear cells (PBMC) (Goyal et al. 2019). Similarly, EPS isolated from microalgae Thraustochytriidae sp. showed immunostimulatory effects by inducing B cell proliferation (Park et al. 2017).
For the present study, we have selected Scenedesmus acutus (S. acutus) which is a green, unicellular, non-mobile freshwater photosynthetic microalgae that exist in colonies of 4–8 cells, often displaying filamentous form (Trainor 1996). The literature survey revealed that Scenedesmus sp. has been used to isolate various bioactive compounds which have wide range of applications in the fields of bio-remediation, bio-fuel production (bio-hydrogen, bio-diesel, bio-ethanol), waste water treatment and energy production (Martínez et al. 2000; McGinn et al. 2012; Kondaveeti et al. 2014). In addition, Scenedesmus sp. has shown different bioactive properties such as antibacterial, antiinflammatory, antioxidant and antidiabetic (Dantas et al. 2019; Patil and Kaliwal 2019). In the current study, EPS were isolated from S. acutus and their effect was evaluated on human PBMC consisting of lymphocytes and monocytes.
Earlier studies were intended to optimize the metabolite production in microalgae trend by tweaking the culture parameters including pH, light, aeration, temperature and medium compositions. Data showed that increased production of β-carotene and glycerols in Dunaliella could be achieved by alerting the concentration of NaCl and nitrites in culture medium (Ben-Amotz et al. 1982; Ben-Amotz 1995). Further, Dunaliella salina grown under stress condition using nitrogen, salinity and temperature increased the carotene yield with improved cytotoxic activities (Singh et al. 2016). In general, the trend in better EPS production was observed to be greatly influenced by modifying the culture medium or under stress conditions (Markou and Nerantzis 2013). Evidently, Scenedesmus sp. produced 1.6-fold more EPS under increased acidic conditions (pH 6) relative to normal culture conditions (pH 6.8) (Angelaalincy et al. 2017). Based on these previous reports, we have attempted to enrich the EPS secretion from S. acutus by inducing stress conditions using varying concentrations of sulphur and phosphorus in culture medium. Further, EPS secreted under these nutritional stress conditions was assessed for its effect on cell proliferation and antioxidant activity.
Material and methods
Growth conditions and cell count
Scenedesmus acutus (S. acutus) culture was procured from the Council of Scientific and Industrial Research, National Chemical Laboratory, Pune, India, and maintained on modified BG-11 medium (Rippka et al. 1979). As compared to normal condition (0.75 g/L MgSO4 and 0.4 g/L K2HPO4), five different concentrations of MgSO4 (0, 0.25, 0.5, 1.0 and 1.25 g/L) and K2HPO4 (0, 0.2, 0.6, 0.8 and 1.0 g/L) were chosen to culture S. acutus with the intention to enhance EPS production. The culture was maintained at 28 ± 2 °C with 12/12 h of photoperiodism (light/dark) for 20 days to extract EPS.
Cell growth was monitored by measuring the cell count of S. acutus with a Neubauer haemocytometer after every 3–4 days over a 20-day period. The cells of S. acutus were either fixed by Lugol’s iodine solution or directly mixed with Trypan blue dye before counting (Auinger et al. 2008).
Dry weight biomass
To determine the total dry weight biomass of algae (mg/L), the grown culture was centrifuged (10,000 rpm; 30 min). Cells were collected in pre-weighed tubes after washing thrice with distilled water (to remove extra chemicals, debris and salts), and were put in the oven for drying at 80 °C until a constant weight was obtained.
Estimation of chlorophyll content
Chlorophyll content was estimated as mentioned earlier with slight modification (Sarker et al.2020a, b; Sarker and Oba 2020a; Singh et al. 2016). The cells in their log phase were taken in micro-centrifuge tubes and centrifuged (8000 rpm; 15 min). Supernatant was discarded and pellet was mixed with 80% acetone diluted with autoclaved distilled water. The mixture was vortexed for 30–40 s and the tubes were kept for 24 h (dark conditions). Following incubation, the tubes were centrifuged (3000 rpm; 10 min) again. The resultant supernatant was collected and absorbance was measured by spectrophotometer at 645 nm and 661.5 nm. Chlorophyll a, b and total chlorophyll concentration was measured by using the following equations (Singh et al. 2016):
Extraction of extracellular polysaccharides
The collected culture was centrifuged (10,000 rpm; 30 min) to separate the cells from debris. Supernatant was then filtered using Whatman filter paper and heated at 50–70 °C on a hot plate till the final volume reduced to 1/6th of the original volume. The concentrated supernatant was then subjected to alcoholic precipitation, where an equal volume of chilled ethanol was added to precipitate extracellular polymeric substances (Goyal et al. 2019). The mixture was kept in chilled conditions (4 °C) for 16–18 h before centrifugation (10,000 rpm; 10 min) was done. The supernatant ethanol was discarded while the obtained pellet was dissolved in Milli-Q water after washing it thrice with absolute ethanol.
The dissolved pellet was dialysed against double distilled water (48 h) to remove the salts and impurities. Trichloroacetic acid (TCA) precipitation was carried out to remove proteins by incubating the dialysed solution with 20% TCA for 1 h (Goyal et al. 2019). The obtained solution was centrifuged (14,000 rpm; 10 min) and the protein pellet was discarded. The supernatant was dialysed again to remove the remaining TCA and subjected to lyophilization to obtain crude EPS powder. Sugar and protein content were estimated by phenol–sulphuric and Bradford assay by taking glucose and bovine serum albumin (BSA) as standard, respectively (Bradford 1976; Masuko et al. 2005).
LC–MS, FTIR and NMR spectroscopy
LC–MS was carried out for recording MS of EPS using Waters, Micromass Q-TOF micro instrument (Panjab University, SAIF lab) with electron spray ionization and collision energy of 4ev.
FTIR absorption of isolated EPS was recorded using Perkin Elmer Spectrum RX-IFTIR (Panjab University, SAIF lab) with resolution of 1 cm−1 and scanned frequency range of 4000–600 cm−1 to get the imprinted pattern of absorption and transmission. The processed spectra were converted into.csv format and graphs were prepared using origin 6.0 software (MicroGal Origin, USA).
The 1H spectra of EPS were recorded on Bruker Advance II 400 MHz in DMSO (Panjab University, SAIF lab). The working 1H frequency was 400 MHz. Different spectral peaks were recorded to determine the number of protons and their positions as chemical shift.
Cell proliferation assay
Ficoll density gradient method was used to isolate the peripheral blood mononuclear cells (PBMC) as mentioned earlier (Lohia and Baranwal 2017). Informed consent was taken from healthy volunteers prior to the collection of blood (5 mL). Approval was obtained from the institutional ethical committee prior to the study.
The growth effect of EPS on freshly isolated PBMC was assessed by MTT (3-(4,5-dimethylthiazol-2-yl)-2-5-diphenyl tetrazolium bromide) assay (Lohia and Baranwal 2017). Crude EPS dissolved in sterilized water were added in 96-well microtiter plate containing PBMC (2 × 105 cells/well). Concanavalin A (ConA; 10 µg/mL, Sigma-Aldrich) was added in PBMC containing wells which served as the positive control. The final volume of RPMI (Sigma-Aldrich), containing 10% (v/v) fetal bovine serum (FBS) (Gibco, Waltham, MA), 100 IU/mL penicillin, 100 µg/mL streptomycin and 2.5 µg/mL amphotericin (Sigma-Aldrich), was kept as 200 µL. After 48 h of incubation at 37 °C with 5% CO2, 20 μL MTT (5 mg/mL in PBS) was added in each well and the plate was again incubated for 4 h. Next, 170 μL of media was discarded and 100 μL of DMSO was added in each well. The absorbance was recorded at 570 nm (reference wavelength 620 nm) on ELISA plate reader (iTecan Infinite Pro ELISA reader). The cell growth was calculated as proliferative index by taking ratio of absorbance of sample (cells treated with ConA/EPS) and absorbance of untreated cells (cells only).
Free radical scavenging (antioxidant) activity
Antioxidant ability of extracted EPS was measured using DPPH (2,2-diphenyl-1-picrylhydrazyl) assay as mentioned earlier with slight modification (Sarker and Oba 2019a, b, 2020b; Singh et al. 2016). EPS dissolved in water was added in varying concentrations (100–1000 μg/mL) to each well in the 96-well plate. 30 μL of prepared ascorbic acid (100 μg/mL) was added in the well which served as a positive control. 150 μL of prepared DPPH (100 μM) dissolved in methanol was added to each well and the final volume of each well was made up to 200 μL using methanol. The plate was kept for incubation in dark for 45 min and then the absorbance was measured at 517 nm on ELISA plate reader (iTecan Infinite Pro ELISA reader). Radical scavenging activity was calculated using the following formula:
Statistical analysis
The data were expressed as the mean ± standard error of mean. All the experiments were carried out in triplicates. Data were analyzed using the analysis of variance (ANOVA) and the means were compared using Tukey’s test at p < 0.05. Correlation analysis and heat map was done in python software.
Result
Estimation of glucose content in EPS
Sugar content in isolated EPS was estimated by taking glucose as standard using phenol–sulphuric acid method. The glucose content was found to be 50.76 ± 6.28 μg/mg of EPS. The Bradford assay confirmed the absence of protein content in isolated EPS.
FTIR and 1H NMR spectroscopy
The positive ion reflector mode represents the spectrum of 1356 (intensity of 964), which appears to be octa-saccharides (ESM_1). The octa-saccharides were hetero-oligosaccharides consisting of hexose and pentose sugar and may be associated with some ions. A series of m/z representing monosaccharide units such as pentose (149.03) and methyl pentose (163.05) were also present in the MS spectrum (ESM_1). Presence of m/z peak of di-saccharides, tri-saccharides and tetra-saccharides showed that these peaks were due to fragmentation of octa-saccharides.
Characterization principles of FTIR and 1H NMR spectroscopy were used to confirm and determine the nature of sugars present in the extract. The FTIR spectra of the isolated EPS showed characteristic absorption related to polysaccharides (ESM_2) such as peaks with multiple splits between 2800 and 3000 cm−1 associated with C–H stretching while O–H stretching band at 3339 cm−1 (Goyal et al. 2019). FTIR spectra also showed vibrations of C=O (1650 cm−1) and C–O–C (1050 cm−1) which confirmed the presence of polysaccharides (Guo et al. 2016). Halo stretching and sulphate groups noticed at 630 and 1341 cm−1, respectively, showed the presence of halo and sulphate groups along with the polysaccharides (Goyal et al. 2019).
The NMR spectroscopy was performed to determine the structure (protons) of the compound. The chemical shift obtained between 5.1–5.4 and 4.8–4.9 ppm attributed towards the presence of α- and β-anomeric carbon of pentose and hexose sugars, respectively (ESM_3a) (Mishra et al. 2011). Ppm peaks from 3.2 to 3.4 showed the presence of hydroxyl group while 3.1 ppm represented alkyl halide (ESM_3b).
Cell proliferative and antioxidant effect of extracted EPS
Extracted EPS were assessed for cell proliferative effect on PBMC using MTT assay. It was observed that the proliferative index of PBMC relatively increased with the increase in EPS concentration from 100 to 1000 μg/mL (Fig. 1). Proliferative index of more than one at all concentrations indicated the EPS-induced PBMC proliferation (Fig. 1). Interestingly, the proliferative index at 750 and 1000 μg/mL was found to be significantly higher than that of concanavalin A (positive control).
Fig. 1.
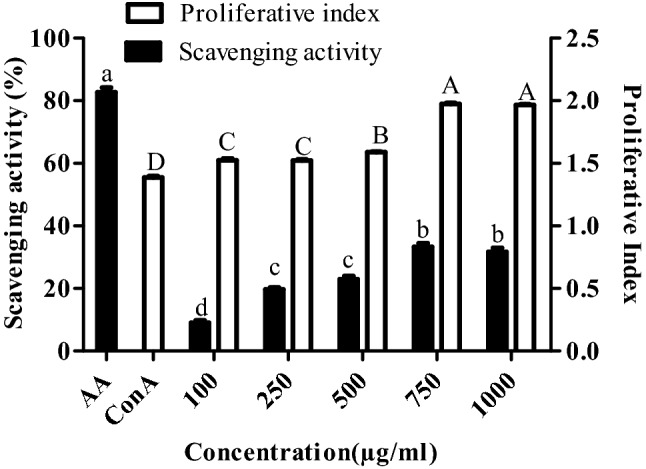
Cell proliferative and scavenging activity of exopolysaccharides. Cell proliferation was carried out in peripheral blood mononuclear cells by MTT assay while scavenging activity by DPPH assay. Proliferative index is the ratio of absorbance of the concanavalin A/exopolysaccharides treated and untreated cells. Bars which share the common (upper and lower case) letter among the treatments are not significantly different at p < 0.05
The EPS antioxidant activity was analyzed via DPPH assay in which ascorbic acid served as the positive control. The percentage free radical scavenging activity increased with increased EPS concentrations and maximum activity was found at concentrations of 750 and 1000 µg/ml (Fig. 1). Overall, from the data it appeared that isolated EPS possess less antioxidant activity.
Measurement of count and chlorophyll content of S. acutus cells grown under K2HPO4 and MgSO4 stress conditions
Scenedesmus acutus was grown in different mediums by changing the concentration of sulphur (MgSO4) and phosphorus (K2HPO4) so as to induce nutritional stress. Five different concentrations of MgSO4 (0, 0.25, 0.5, 1.0 and 1.25 g/L) and K2HPO4 (0, 0.2, 0.6, 0.8 and 1.0 g/L) were considered for the study. As compared to the normal nutritional concentrations (0.4 g/L K2HPO4 and 0.75 g/L MgSO4), the cell growth was observed to be significantly increased at 0.6 g/L K2HPO4 and 1.25 g/L MgSO4 (Fig. 2). The chlorophyll content was found to be significantly higher at 0.6, 0.8 and 1.0 g/L K2HPO4 and 0.5, 1.0 and 1.25 g/L MgSO4 as compared to normal conditions (Fig. 2).
Fig. 2.
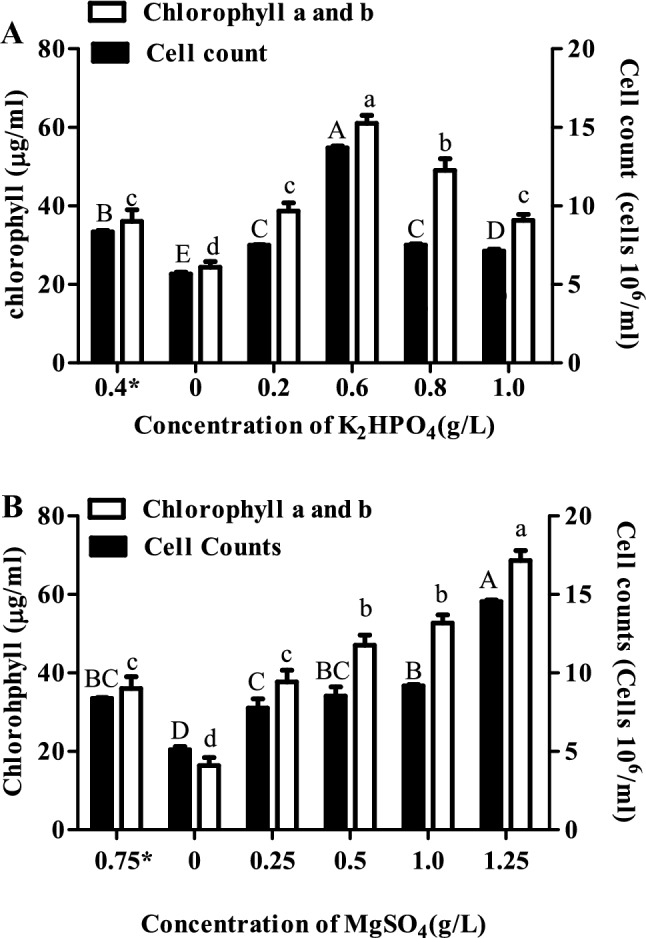
Cell count and chlorophyll content of Scenedesmus acutus grown under a K2HPO4 and b MgSO4 stress conditions. Bars which share the common (upper and lower case) letter among the treatments are not significantly different at p < 0.05
Dry weight biomass and sugar content in extracted EPS from S. acutus cells grown under stress conditions
The dry weight biomass of S. acutus cells was found to be significantly more at K2HPO4 (0.6 g/L) and MgSO4 (1 and 1.25 g/L) in comparison with normal conditions. As compared to normal conditions, glucose content was observed significantly higher at 0.6 and 0.8 g/L K2HPO4 as well as at 1 and 1.25 g/L MgSO4 stress (Fig. 3). At some concentrations, glucose content could not be detected, hence not shown in Fig. 3.
Fig. 3.
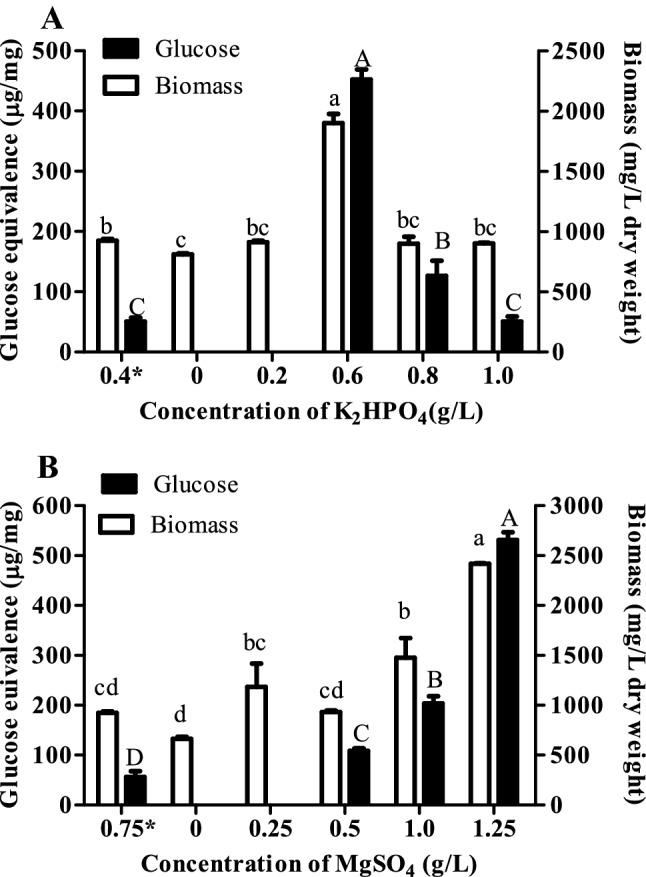
Biomass and glucose content in extracted exopolysaccharides from Scenedesmus acutus grown under a K2HPO4 and b MgSO4 stress conditions. Bars which share the common (upper and lower case) letter among the treatments are not significantly different at p < 0.05
Cell proliferative and antioxidant effect of extracted EPS from S. acutus cells grown under stress conditions
Cell proliferation and antioxidant effect was assessed at EPS concentration of 1 mg/ml. Proliferative index of PBMC significantly increased at 0.6 and 0.8 g/L K2HPO4 as well as at 1 and 1.25 g/L MgSO4 stress conditions as compared to normal conditions (Fig. 4). Proliferative index was observed to be associated with glucose content as glucose concentration was also higher for both K2HPO4 and MgSO4 at same concentrations. Antioxidant effect was also found to be higher at 0.6 g/L K2HPO4 and 1.25 g/L MgSO4 (Fig. 4). Interestingly, proliferative index and antioxidant effect was found to be significantly lower at same concentrations of K2HPO4 and MgSO4 at which glucose content could not be detected earlier (Fig. 4).
Fig. 4.
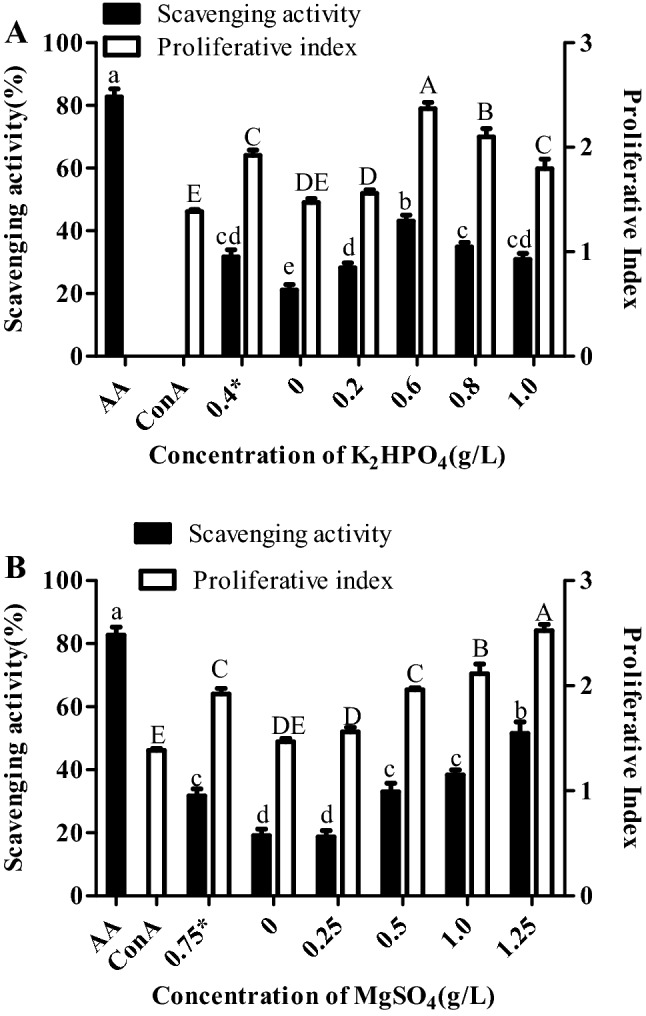
Cell proliferative and scavenging activity of extracted exopolysaccharides from Scenedesmus acutus grown under a K2HPO4 and b MgSO4 stress conditions. Cell proliferation was carried out in peripheral blood mononuclear cells by MTT assay while scavenging activity by DPPH assay. Proliferative index is ratio of absorbance of the concanavalin A/exopolysaccharides treated and untreated cells. Bars which share the common (upper and lower case) letter among the treatments are not significantly different at p < 0.05. ConA: concanavalin A: AA: ascorbic acid
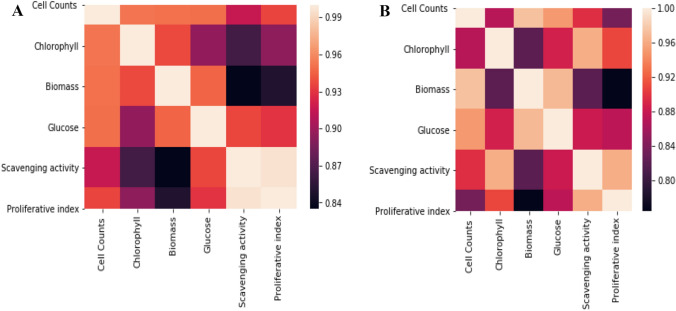
Correlation analysis of different parameters considered in stress conditions
All the parameters considered were found to be strongly correlated with each other. The range of correlation was observed to be 0.84–0.99 (MgSO4) and 0.76–0.97 (K2HPO4) as represented in heat map (Fig. 5). The correlation value was 0.94 (glucose content vs scavenging activity) and 0.93 (glucose content vs proliferative index) for MgSO4. In case of K2HPO4, the observed correlation value was 0.88 (glucose content vs scavenging activity) and 0.87 (glucose content vs proliferative index).
Fig. 5.
Heatmap showing the correlation between different parameters analyzed for Scenedesmus acutus grown under a K2HPO4 and b MgSO4 stress conditions
Discussion
In the current situation, when world is facing challenges with different infectious diseases (COVID-19, influenza and tuberculosis) and cancer, there is need of molecules having potentiality to enhance the proliferation of immune cells. People having weakened immune system are more prone to different health disorders; hence, they are advised to take immunostimulators. Considering the need of immunostimulants, exopolysaccharides (EPS) produced by various microorganisms (algae, fungi and yeasts) have been explored for immune cell stimulating potential. EPS have been reported to interact with immune system and play diverse role in modulating both innate and adaptive immune system (Ferreira et al. 2015). EPS produced by several species of microalgae such as Chlorella, Dunaliella, Spirulina, Nostoc, Rhodella, Ulva have been known to produce immunomodulatory polysaccharides (De Jesus Raposo et al. 2015). The chemical composition of microalgal culture media has been altered to induce enhanced production of bioactive compounds (Markou and Nerantzis 2013; Minhas et al. 2016; Vaz et al. 2016). In the present study, S. acutus grown in medium with increased concentration of MgSO4 and K2HPO4 exhibited enhanced EPS production. Further, the increased proliferative index of PBMC and antioxidant activity was found correlated with EPS produced under these high concentrations of MgSO4 and K2HPO4 in culture medium.
LC–MS analysis indicated that EPS are hetero-polysaccharides (octa-saccharides) consisting of pentose and hexose sugars and associated with ions which is in accordance with m/z peak obtained in previous studies (Mishra and Jha 2009; Goyal et al. 2019). FTIR and NMR analysis results of isolated EPS from S. acutus were compared to those of earlier studies (Mishra et al. 2011; Guo et al. 2016) and the peaks obtained were found to be associated with sugar. Evaluations of the effect of EPS on PBMC growth revealed the dose-dependent increase in cell proliferation. In one of our earlier studies with EPS isolated from Dunaliella salina, similar effect was observed at higher concentration and growth inhibition was reported at lower concentrations (Goyal et al. 2019). In the current study, an increase in PBMC proliferation is observed at tested concentrations (100–1000 μg/mL) indicating that these EPS are more potent in enhancing the growth of immune cells. Paramylon (β-glucans) obtained from Euglena gracilis also showed stimulation of PBMC by inducing the production of pro-inflammatory factors (NO, TNF-α, IL-6, and COX-2) (Russo et al. 2017). Sulfated polysaccharides from Cystoseira indica exhibited immunostimulatory activity by inducing the release of inflammatory cytokines (TNF-α, IL-1β, IL-6 and IL-10) in RAW264.7 murine macrophage cells (Bahramzadeh et al. 2020). Immunomodulatory activity against PBMC and macrophages was also reported in EPS obtained from Porphyridium tricornutum and Chlorella stigmatophora (Guzmán et al. 2003). In different studies, polysaccharides isolated from different microalgae such as Porphyridium, Rhodella, Arthrospira have exhibited antioxidant activity (De Jesus Raposo et al. 2015). In accordance with these reports, EPS isolated from S. acutus have shown the antioxidant activity, but the activity appeared to be less pronounced at the tested concentration.
In our earlier studies on Dunaliella Salina, different stress conditions such as salinity, nitrogen and temperature were used and the cytotoxic (MCF-7 breast cancer line) and antioxidant effect was found to be increased due to enhancement in carotenes production (Singh et al. 2016). Apart from nitrogen, sulphur and phosphorus are also essential component in the medium for the microbial growth (Minhas et al. 2016). As compared to normal conditions (0.4 g/L K2HPO4 and 0.75 g/L MgSO4), a concentration of 0.6 g/L K2HPO4 and 1.25 g/L MgSO4 exhibited highest cell count, chlorophyll content, biomass, glucose, proliferative index and scavenging activity. No glucose content could be detected at either low concentration (0.2 g/L K2HPO4 and 0.25 g/L MgSO4) or medium deficient in K2HPO4 and MgSO4, which indicated that stress conditions with low or no sulphur and phosphorus concentration might not be suitable for S. acutus to produce EPS. A strong correlation was observed between glucose content and cell growth, chlorophyll content and biomass indicating that the production of EPS is dependent on these factors. Further, the significantly increased glucose content and proliferative index at K2HPO4 (0.6 and 0.8 g/L) and MgSO4 (1 and 1.25 g/L) as compared to normal conditions, indicated that increased PBMC proliferation was due to the EPS production. Nitrates, phosphorus and sulfates have been used as stress condition on different microalgae to enhance the EPS production (Markou and Nerantzis 2013). In one of the recent reports which was done with culture of Porphyridium purpureum, it was shown that there was accumulation of EPS with the increase in carbon/nitrogen ratio (Li et al. 2020). Enhanced EPS production was reported in nitrogen and sulphate deficiency in Rhodella reticulate (Arad et al. 1992; Sutherland 1996). In contrast to this report, we could not detect EPS production under phosphorus and sulphur-deficient conditions while enhanced EPS production was observed upon increasing the concentration of phosphorus and sulphur. In another study, nitrogen starvation did not show any effect on EPS production in Synechocystis and Phormidium (De Philippis and Vincenzini 1998). Medina-Cabrera et al. (2020) evaluated the effect of medium composition such as CaCl2, NaNO3, NaCl, MgSO4 and KH2PO4 on EPS generation from Porphyridium sordidum and Porphyridium purpureum. It was observed that presence of CaCl2 and NaNO3 and deficiency of NaCl, MgSO4 and KH2PO4 enhanced the EPS production in Porphyridium sordidum. The results were contrasting for Porphyridium purpureum where KH2PO4 and MgSO4 presence and CaCl2, NaNO3, NaCl deficiency favors the EPS enrichment. Hence, these studies showed that the effect of altered culture conditions and other parameters on EPS production was purely dependent on the strain and species of microalgae taken.
Conclusion
In the present investigation, hetero-polysaccharides obtained from S. acutus have shown increase in cell proliferation in a dose-dependent manner which may indicate their immunostimulating properties. However, further estimation is required in different experimental models to understand the mechanism behind their immunostimulating activity. We also observed that, at the higher concentrations of K2HPO4 (0.6 and 0.8 g/L) and MgSO4 (1 and 1.25 g/L), both glucose content and proliferative index were high which represented that the enhanced EPS production with improved bioactive properties can be achieved by increasing the concentration of phosphorus and sulphur.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The author would like to acknowledge to Dr. Akshey Jain from Nitin Nursing Home Patiala for providing us the blood needed for carrying out the experiments. The authors also would like to acknowledge SAIF, Punjab University, Chandigarh, for FTIR and NMR analysis.
Author contributions
TP: conduct of experiments, compilation of results and figures. MB: formulation of idea, manuscript writing.
Declarations
Conflict of interest
No competing financial interests exist.
References
- Angelaalincy M, Senthilkumar N, Karpagam R, et al. Enhanced extracellular polysaccharide production and self-sustainable electricity generation for PAMFCs by Scenedesmus sp. SB1. ACS Omega. 2017;2:3754–3765. doi: 10.1021/acsomega.7b00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arad SM, Lerental YB, Dubinsky O. Effect of nitrate and sulfate starvation on polysaccharide formation in Rhodella reticulata. Bioresour Technol. 1992;42:141–148. doi: 10.1016/0960-8524(92)90073-7. [DOI] [Google Scholar]
- Auinger BM, Pfandl K, Boenigk J. Improved methodology for identification of protists and microalgae from plankton samples preserved in Lugol’s iodine solution: combining microscopic analysis with single-cell PCR. Appl Environ Microbiol. 2008;74:2505–2510. doi: 10.1128/AEM.01803-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahramzadeh S, Tabarsa M, You S, et al. Purification, structural analysis and mechanism of murine macrophage cell activation by sulfated polysaccharides from Cystoseira indica. Carbohydr Polym. 2020;205:261–270. doi: 10.1016/j.carbpol.2018.10.022. [DOI] [PubMed] [Google Scholar]
- Ben-Amotz A. New mode of Dunaliella biotechnology: two-phase growth for β-carotene production. J Appl Phycol. 1995;7:65–68. doi: 10.1007/BF00003552. [DOI] [Google Scholar]
- Ben-Amotz A, Sussman I, Avron M. Glycerol production by Dunaliella. In: Mislin H, Bachofen R, editors. New trends in research and utilization of solar energy through biological systems. Birkhäuser: Basel; 1982. pp. 55–58. [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- de Dantas DMM, de Oliveira CYB, Costa RMPB, et al. Evaluation of antioxidant and antibacterial capacity of green microalgae Scenedesmus subspicatus. Food Sci Technol Int. 2019;25:318–326. doi: 10.1177/1082013218825024. [DOI] [PubMed] [Google Scholar]
- De Philippis R, Vincenzini M. Exocellular polysaccharides from cyanobacteria and their possible applications. FEMS Microbiol Rev. 1998;22:151–175. doi: 10.1016/S0168-6445(98)00012-6. [DOI] [Google Scholar]
- De Jesus Raposo MF, De Morais AMB, De Morais RMSC. Marine polysaccharides from algae with potential biomedical applications. Mar Drugs. 2015;13:2967–3028. doi: 10.3390/md13052967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira SS, Passos CP, Madureira P, et al. Structure-function relationships of immunostimulatory polysaccharides: a review. Carbohydr Polym. 2015;132:378–396. doi: 10.1016/j.carbpol.2015.05.079. [DOI] [PubMed] [Google Scholar]
- Goyal M, Baranwal M, Pandey SK, Reddy MS. Hetero-polysaccharides secreted from Dunaliella salina exhibit immunomodulatory activity against peripheral blood mononuclear cells and RAW 264.7 macrophages. Indian J Microbiol. 2019;59:428–435. doi: 10.1007/s12088-019-00818-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Wang X, Liu J. Composition analysis of fractions of extracellular polymeric substances from an activated sludge culture and identification of dominant forces affecting microbial aggregation. Sci Rep. 2016;6:1–9. doi: 10.1038/srep28391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán S, Gato A, Lamela M, et al. Anti-inflammatory and immunomodulatory activities of polysaccharide from Chlorella stigmatophora and Phaeodactylum tricornutum. Phyther Res. 2003;17:665–670. doi: 10.1002/ptr.1227. [DOI] [PubMed] [Google Scholar]
- Kondaveeti S, Choi KS, Kakarla R, Min B. Microalgae Scenedesmus obliquus as renewable biomass feedstock for electricity generation in microbial fuel cells (MFCs) Front Environ Sci Eng. 2014;8:784–791. doi: 10.1007/s11783-013-0590-4. [DOI] [Google Scholar]
- Li S, Ji L, Chen C, et al. Efficient accumulation of high-value bioactive substances by carbon to nitrogen ratio regulation in marine microalgae Porphyridium purpureum. Bioresour Technol. 2020;309:123362. doi: 10.1016/j.biortech.2020.123362. [DOI] [PubMed] [Google Scholar]
- Lohia N, Baranwal M. Immune responses to highly conserved influenza A virus matrix 1 peptides. Microbiol Immunol. 2017;61:225–231. doi: 10.1111/1348-0421.12485. [DOI] [PubMed] [Google Scholar]
- Markou G, Nerantzis E. Microalgae for high-value compounds and biofuels production: a review with focus on cultivation under stress conditions. Biotechnol Adv. 2013;31:1532–1542. doi: 10.1016/j.biotechadv.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Martínez ME, Sánchez S, Jiménez JM, et al. Nitrogen and phosphorus removal from urban wastewater by the microalga Scenedesmus obliquus. Bioresour Technol. 2000;73:263–272. doi: 10.1016/S0960-8524(99)00121-2. [DOI] [Google Scholar]
- Masuko T, Minami A, Iwasaki N, et al. Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Anal Biochem. 2005;339:69–72. doi: 10.1016/j.ab.2004.12.001. [DOI] [PubMed] [Google Scholar]
- McGinn PJ, Dickinson KE, Park KC, et al. Assessment of the bioenergy and bioremediation potentials of the microalga Scenedesmus sp. AMDD cultivated in municipal wastewater effluent in batch and continuous mode. Algal Res. 2012;1:155–165. doi: 10.1016/j.algal.2012.05.001. [DOI] [Google Scholar]
- Medina-Cabrera EV, Rühmann B, Schmid J, Sieber V. Optimization of growth and EPS production in two Porphyridum strains. Bioresour Technol Rep. 2020;11:100486. doi: 10.1016/j.biteb.2020.100486. [DOI] [Google Scholar]
- Minhas AK, Hodgson P, Barrow CJ, Adholeya A. A review on the assessment of stress conditions for simultaneous production of microalgal lipids and carotenoids. Front Microbiol. 2016;7:1–19. doi: 10.3389/fmicb.2016.00546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A, Jha B. Isolation and characterization of extracellular polymeric substances from micro-algae Dunaliella salina under salt stress. Bioresour Technol. 2009;100:3382–3386. doi: 10.1016/j.biortech.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Mishra A, Kavita K, Jha B. Characterization of extracellular polymeric substances produced by micro-algae Dunaliella salina. Carbohydr Polym. 2011;83:852–857. doi: 10.1016/j.carbpol.2010.08.067. [DOI] [Google Scholar]
- Park GT, Go RE, Lee HM, et al. Potential anti-proliferative and immunomodulatory effects of marine microalgal exopolysaccharide on various human cancer cells and lymphocytes in vitro. Mar Biotechnol. 2017;19:136–46. doi: 10.1007/s10126-017-9735-y. [DOI] [PubMed] [Google Scholar]
- Patil L, Kaliwal BB. Microalga Scenedesmus bajacalifornicus BBKLP-07, a new source of bioactive compounds with in vitro pharmacological applications. Bioprocess Biosyst Eng. 2019;42:979–994. doi: 10.1007/s00449-019-02099-5. [DOI] [PubMed] [Google Scholar]
- Riccio G, Lauritano C. Microalgae with immunomodulatory activities. Mar Drugs. 2020;18:2. doi: 10.3390/md18010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippka R, Deruelles J, Waterbury JB. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology. 1979;111:1–61. doi: 10.1099/00221287-111-1-1. [DOI] [Google Scholar]
- Russo R, Barsanti L, Evangelista V, et al. Euglena gracilis paramylon activates human lymphocytes by upregulating pro-inflammatory factors. Food SciNutr. 2017;5:205–214. doi: 10.1002/fsn3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker U, Oba S. Antioxidant constituents of three selected red and green color Amaranthus leafy vegetable. Sci Rep. 2019;9:1–11. doi: 10.1038/s41598-019-52033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker U, Oba S. Nutraceuticals, antioxidant pigments, and phytochemicals in the leaves of Amaranthus spinosus and Amaranthus viridis weedy species. Sci Rep. 2019;9:1–10. doi: 10.1038/s41598-019-50977-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker U, Oba S. Nutrients, minerals, pigments, phytochemicals, and radical scavenging activity in Amaranthus blitum leafy vegetables. Sci Rep. 2020;10:1–9. doi: 10.1038/s41598-020-59848-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker U, Oba S. The response of salinity stress-induced A. tricolor to growth, anatomy, physiology, non-enzymatic and enzymatic antioxidants. Front Plant Sci. 2020;11:559876. doi: 10.3389/fpls.2020.559876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker U, Hossain MM, Oba S. Nutritional and antioxidant components and antioxidant capacity in green morph Amaranthus leafy vegetable. Sci Rep. 2020;10:1–10. doi: 10.1038/s41598-020-57687-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker U, Oba S, Daramy MA. Nutrients, minerals, antioxidant pigments and phytochemicals, and antioxidant capacity of the leaves of stem amaranth. Sci Rep. 2020;10:1–9. doi: 10.1038/s41598-020-60252-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Baranwal M, Reddy SM. Antioxidant and cytotoxic activity of carotenes produced by Dunaliella salina under stress. Pharm Biol. 2016 doi: 10.3109/13880209.2016.1153660. [DOI] [PubMed] [Google Scholar]
- Sutherland IW. Extracellular polysaccharides. In: Rehm HJ, Reed G, editors. Biotechnology: products of primary metabolism. Weinheim: Wiley; 1996. pp. 613–657. [Google Scholar]
- Trainor FR. Reproduction in Scenedesmus. Korean J Phycol. 1996;11:183–201. [Google Scholar]
- Vaz S, Moreira JB, De MMG, et al. ScienceDirect Microalgae as a new source of bioactive compounds in food supplements. Curr Opin Food Sci. 2016;7:73–77. doi: 10.1016/j.cofs.2015.12.006. [DOI] [Google Scholar]
- Xiao R, Zheng Y. Overview of microalgal extracellular polymeric substances (EPS) and their applications. Biotechnol Adv. 2016;34:1225–1244. doi: 10.1016/j.biotechadv.2016.08.004. [DOI] [PubMed] [Google Scholar]
- Zhang J, Liu L, Ren Y, Chen F. Characterization of exopolysaccharides produced by microalgae with antitumor activity on human colon cancer cells. Int J Biol Macromol. 2019;128:761–767. doi: 10.1016/j.ijbiomac.2019.02.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.