Abstract
Many general anesthetics potentiate GABAA receptors but their neuroanatomic sites of action are less clear. GABAergic neurons in the rostromedial tegmental nucleus (RMTg) send inhibitory projections to multiple arousal-promoting nuclei, but the role of these neurons in modulating consciousness is unknown. In this study, designer receptors were targeted to RMTg GABAergic neurons of Vgat-ires-Cre mice. DREADDs expression was found in the RMTg and other brainstem regions. Activation of these neurons decreased movement and exploratory behavior, impaired motor coordination, induced EEG oscillations resembling NREM sleep without loss of righting, and reduced the dose requirement for sevoflurane-induced unconsciousness. These results suggest that GABAergic neurons in the RMTg and other brainstem regions promote sedation and facilitate sevoflurane-induced unconsciousness.
INTRODUCTION
Many general anesthetics potentiate GABAA receptors,1 however the mechanisms underlying anesthetic-induced unconsciousness at the level of neural circuits and systems are unclear.2 Activation of arousal systems can restore consciousness in anesthetized animals,3,4 and neurons in the ventral tegmental area (VTA) promote wakefulness from natural sleep5,6 and general anesthesia.7 The rostromedial tegmental nucleus (RMTg) is a key hub for relaying information to dopaminergic systems,8 receiving excitatory glutamatergic inputs from the lateral habenula (LHb)9 and sending inhibitory projections to the VTA and other subcortical arousal nuclei.10 Propofol activates excitatory glutamatergic projections from the LHb to the RMTg,11 suggesting that it may produce unconsciousness in part by activating inhibitory RMTg GABAergic neurons downstream from the LHb. In this study, we targeted Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) to GABAergic neurons in the RMTg in adult mice, to test the hypothesis that activating these neurons induces sedation and increases sensitivity to sevoflurane-induced unconsciousness.
METHODS
Mice
All animal procedures were reviewed and approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee. Adult male and female Vgat-ires-Cre mice (Jackson Laboratory, stock number 016962) were kept on a 12:12 hr light/dark cycle (lights on at 7:00AM and off at 7:00PM) with food and water provided ad libitum. Mice had at least three weeks of recovery after surgery and three days of rest between experiments.
Stereotaxic Virus Injections
DREADDs were used to modulate the activity of RMTg GABAergic neurons. Vgat-ires-Cre mice (n=9, 4 males, 5 females) were anesthetized with isoflurane and the RMTg (-3.9 mm anterior/posterior, ±0.4 mm lateral, and −4.8 mm dorsal/ventral to bregma) was targeted using previously published coordinates.12 Mice underwent bilateral injections with a micropipette of a viral construct (AAV5-hSyn-DIO-hM3Dq-mCherry, University of North Carolina Vector Core, UNC Chapel Hill, NC) that elicits Cre-dependent expression of designer excitatory (hM3Dq) receptors in GABAergic neurons. Control animals (n=9, 4 males, 5 females) were Vgat-ires-Cre mice injected with a viral construct containing only the mCherry reporter (AAV8-hSyn-DIO-mCherry, UNC Vector Core). The titer was 3.8×1012 and the injection volume was 400 nL on each side.
Behavioral Testing
Approximately 5 weeks after virus injection, all mice underwent habituation on the accelerating rotarod over 2 days. On the first day they were placed on the rotarod at a constant speed (4 RPM) and had to run for at least 60 seconds (5 minutes max) and on the second day they underwent two trials during which the rotarod started at 4 RPM and accelerated to 40 RPM over a period of 5 minutes (up 2 RPM every 15 seconds). At least 30 minutes of rest was given between trials. After habituation, four baseline measurements (two per day) were taken for each animal, with the rotarod starting at 4 RPM and accelerating to 50 RPM over 5 minutes (increase by 1 RPM every 5 seconds). Baseline measurements were averaged for each animal.
On experimental days, mice received clozapine-N-oxide (CNO, 1 mg/kg i.p.) or saline (vehicle) and 30 minutes later they were placed in an open field (40 cm × 40 cm). After recording open field activity for 5 minutes using a video tracking system (Any-Maze, Stoetling Co., USA) the total distance traveled was recorded. After the open field test, the mice were placed on the rotarod under the same conditions as the baseline measurements. After a 30-minute rest period, the mice were placed on the rotarod again for a second trial, and the results from both trials were averaged. At least 3 days of rest were provided between experimental days. Experimenters were blinded to the intervention (saline vs. CNO) as well as the animal group (hM3Dq vs. mCherry control). The mice were numbered by a second person and the assignments were revealed only after the experiments.
EEG/EMG Recordings and Analysis
Approximately 10–11 weeks after virus injection, mice underwent surgery to place EEG and EMG electrodes as previously described.7,13 Extradural EEG screws were placed over the prefrontal cortex and parietal cortex, and two EMG electrodes were placed in the nuchal muscles. Mice received post-operative analgesia during recovery.
Approximately 4 weeks after electrode implantation, EEG and EMG recordings were conducted with a sampling frequency of 1000 Hz. Although all 9 hM3Dq mice were implanted with electrodes, 4 had high-voltage EEG artifacts suggesting that the electrodes or ground screws had become loose during the recovery period after surgery. Because these mice did not produce interpretable data, their recordings were excluded. The 5 remaining mice were used for analysis.
To record activity during natural sleep, EEG and EMG were continuously recorded between 10am and 2pm, when mice were likely to sleep. To test whether CNO induced sedation, recordings were conducted 3:30pm-6:30pm, when mice were likely to be awake. After 10–30 minutes of recording baseline awake activity, mice received CNO and were returned to their cages for two hours of continuous recording.
Data and EEG/EMG analysis were adapted from previous methods.7,13 Power spectral density estimates were calculated from EEG data during periods of NREM sleep, wakefulness, and after CNO. Estimates were used to construct bootstrapped 95% confidence intervals around the mean power spectral density for each state (see “Statistical Analysis” below for details).
Anesthetic Sensitivity Testing
After completing the EEG/EMG recordings, a final set of experiments was conducted in hM3Dq mice to test sensitivity to sevoflurane-induced loss of righting after saline vs. CNO. These experiments were carried out approximately 8 weeks after electrode implantation surgery. Mice received saline or CNO and were anesthetized 20 minutes later with sevoflurane in an acrylic chamber with a heating pad. Gas concentrations in the chamber were monitored, and the dose of inhaled sevoflurane was increased by 0.2% increments. After the sevoflurane concentration in the chamber reached each dose, it was held for 10 minutes to allow for equilibration with the brain concentration prior to testing for LORR. Precise concentrations were measured with a Riken FI-21 (RKI Instruments, Union City, CA) after each equilibration period until loss of righting occurred.
Histology
Designer receptor expression was confirmed by the presence of mCherry on histological analysis. For this analysis, the brains were sliced at 40–60 μm and treated following previous methods.13 Designer receptor expression in GABAergic neurons was confirmed by co-localization of mCherry with fluorescent in situ hybridization (FISH) for the vesicular GABA transporter (VGAT). For FISH, two experimental mice were used (one control and one hM3Dq) to confirm co-localization of mCherry with VGAT. These brains were serially sectioned coronally at 10–15 μm, and triple-label FISH was performed. Specimens were incubated with target probes for mouse VGAT (Mm-Slc32a1, target region 894–2037, Catalog no. 319191, Advanced Cell Diagnostics) and mCherry (target region 23–681, Catalog no. 431201, Advanced Cell Diagnostics). The slices underwent four serial amplification incubations, the last of which contained fluorescent probes (Atto 550 and Atto 647, Catalog no. 320850, Advanced Cell Diagnostics) individually targeted to the Slc32a1 and mCherry probes.
Statistical Analysis
To accommodate the repeated measures for treatment (CNO, Saline), a linear mixed effects model was conducted that specified a random intercept for animal and fixed effects for DREADD group (hM3Dq, control), treatment (CNO, saline), and group × treatment. Separate models were conducted for the rotarod, LORR, and the open field test. Sensitivity analyses were also conducted examining the impact of sex on the experimental effects. Statistical analysis was performed with GraphPad Prism 7 (GraphPad Software, La Jolla, CA) and R 3.4. All group data is reported as mean ± SD. Where appropriate, p < 0.05 was used to assess statistical significance.
Data was analyzed using MATLAB R2020b (MathWorks, Natick, MA). Time-frequency multitaper spectral density estimates (spectrograms) were constructed using the Chronux toolbox (Cold Spring Harbor, NY). All EEG and EMG analyses were adapted from methods described previously.7,13 EEG recordings were bandpass filtered between 1 and 50 Hz using a minimum-order FIR filter constructed using the Parks-McClellan algorithm. EMG recordings were highpass filtered at 100 Hz using a minimum-order FIR filter constructed using the Parks-McClellan algorithm. Zero-phase filtering was accomplished using the MATLAB “filtfilt” function. EEG and EMG recordings were then used to manually sleep score the recording sessions. Power spectral density estimates of EEG recordings were constructed using the multitaper method. The Chronux function “mtspecgramc” was used with a non-overlapping 2-s window, a frequency resolution of 2 Hz, a half-bandwidth of 2, and 3 tapers. Power values of the spectral estimates were converted to decibels and normalized per recording session using z-scoring. The mean power spectral density estimate was calculated from bouts of NREM sleep during the baseline recordings (9.0 hours), bouts of awake activity during the baseline recordings (9.7 hours), and bouts of CNO-induced slow oscillations after the CNO injection (3.7 hours).
In order to assess whether the power spectral density estimates from these distinct states (NREM sleep, awake, or CNO-induced oscillations) were significantly different across frequency range of interest, 95% confidence intervals around the mean power spectral density were constructed using a percentile block bootstrap procedure (see Supplemental Fig. 1). The bootstrap is a distribution-independent way to estimate confidence intervals. In order to account for short-term dependencies in the time-series power spectral density estimates, individual estimates were not resampled. Instead, individual bouts were identified for each state (NREM sleep baseline: 31 bouts, awake baseline: 37 bouts, CNO-induced slow oscillations: 11 bouts), and those bouts were then sampled with replacement and a bootstrap distribution was formed by taking the mean across all of those resampled bouts. The total number of bootstrap samples calculated for each state was 10,000. Confidence intervals were then found at each frequency point using the bootstrap distribution, where the lower and upper bounds were the 2.5th percentile and the 97.5th percentile values at each frequency point.
Animal numbers (n=9 per group/condition) were chosen based on our previous experience with similar experiments and behavioral assays.7 This sample size allows for 80% power to detect a difference as small as d = 1.4, assuming an alpha = 0.05 for a two-sided hypothesis test and an independent t-test with n = 9 per group. For example, this equates to having sufficient power to detect between-group differences of >= 7.1 m on the open field test (within-group comparisons will have more power).
RESULTS
In all mice, there was robust expression of hM3Dq in the RMTg12 (Fig. A, top) that was limited to GABAergic neurons (Fig. A, bottom). We also found variable expression of hM3Dq in GABAergic neurons in the pons and medulla, likely due to virus spread outside the RMTg. The extent of mCherry reporter expression for each hM3Dq mouse is shown in Supplemental Fig 2. Group data for the open field test (Fig. B) shows that in control mice, the total distance traveled was similar after saline (12.7 ± 4.1 m) vs. CNO (11.0 ± 4.5 m). However, in hM3Dq mice the total distance was 18.1 ± 7.1 m after saline and 3.7 ± 4.5 m after CNO (Group × Treatment interaction, F[1, 16] = 25.0, p = 0.0001). As shown in Fig. C, in control mice no meaningful difference was observed for time on the accelerating rotarod after saline (124 ± 34 sec) vs. CNO (128 ± 27 sec). However, in hM3Dq mice the time was 139 ± 42 sec after saline and 71 ± 55 sec after CNO (Group × Treatment interaction, F[1, 16] = 29.2, p < 0.0001).
Fig.
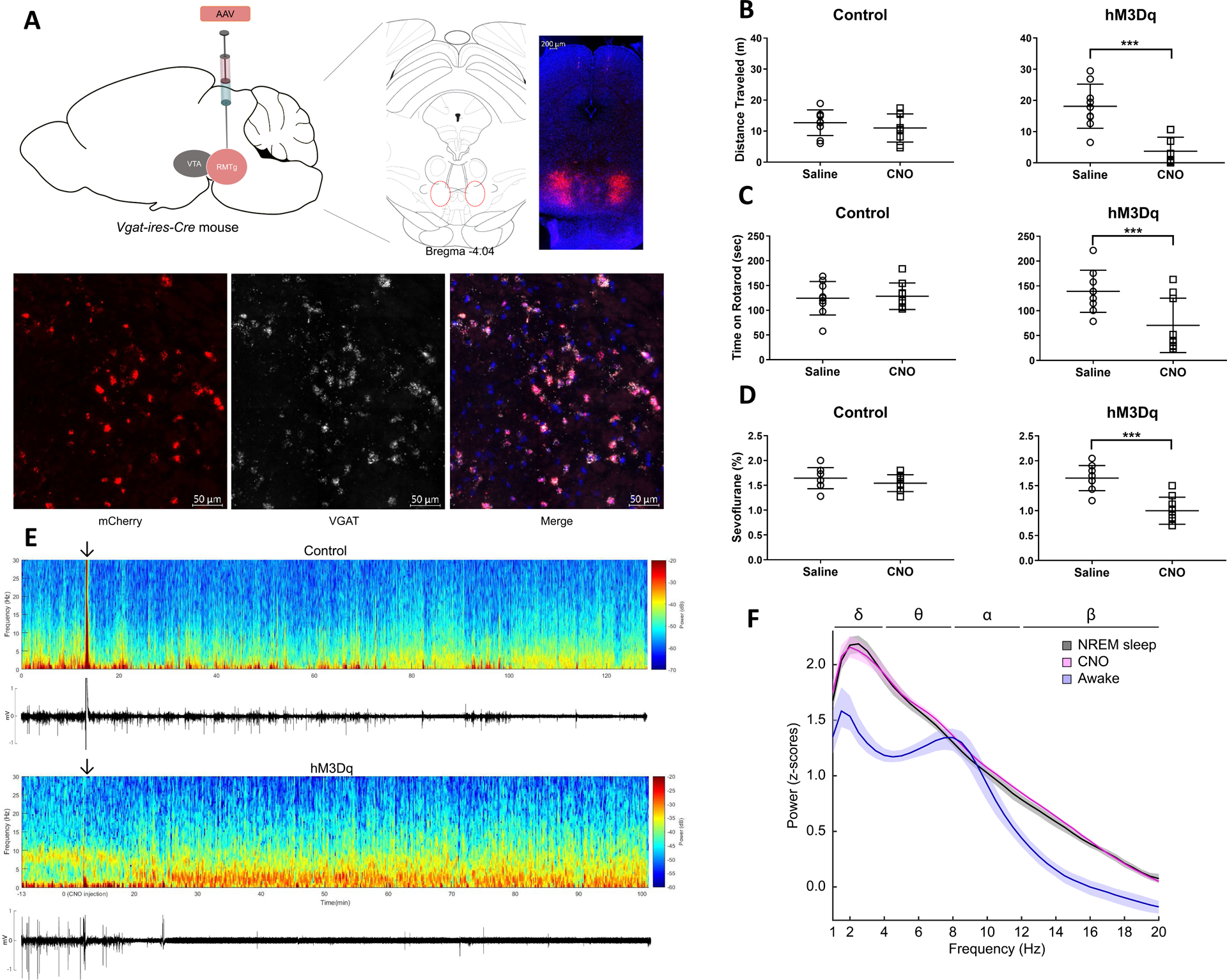
A. Bilateral expression of hM3Dq-mCherry in RMTg GABAergic neurons of Vgat-ires-Cre mice. Representative expression pattern of hM3Dq-mCherry in the RMTg (top). Co-localization of mCherry and VGAT (visualized with fluorescent in situ hybridization) demonstrates selective expression of hM3Dq in GABAergic neurons (bottom).
B. Activation of GABAergic neurons decreases the total distance traveled in the open field test. Scatter plots showing total distance traveled (meters) in the 5-minute open field test after i.p. administration of saline (vehicle) or CNO (1 mg/kg) for control and hM3Dq mice (lines represent mean and SD). Although there was no significant change in control mice after CNO, total distance traveled was significantly decreased in hM3Dq mice.
C. Activation of GABAergic neurons decreases the total time on an accelerating rotarod. Scatter plots showing total time (seconds) on the accelerating rotarod (lines represent mean and SD). CNO (1 mg/kg) significantly decreased total rotarod time in hM3Dq mice compared to saline, but there was no significant difference in control mice.
D. Activation of GABAergic neurons decreases the dose requirement for sevoflurane-induced unconsciousness. Scatter plots show that in control mice, CNO (1 mg/kg) had no significant effect on sensitivity to sevoflurane-induced loss of righting reflex (LORR) compared to saline (lines represent mean and SD). In hM3Dq mice, however, CNO significantly reduced the dose of sevoflurane required for LORR.
E. Activation of GABAergic neurons increases EEG spectral power at low frequencies and decreases EMG activity. Spectrogram of a continuous EEG recording from a representative control mouse before and after CNO injection (top). The arrow indicates the time of CNO administration. The animal remained awake and active, as demonstrated by low spectral power in the delta range (1–4 Hz), and high EMG activity. However, in a representative hM3Dq mouse, approximately 20 minutes after CNO, spectral power increased at low frequencies (1–4 Hz) and persisted for the duration of the recording. The corresponding EMG activity below the spectrogram shows that the increase in EEG delta power coincided with decreased movement.
F. Activation of GABAergic neurons produces an EEG power spectrum similar to NREM sleep. Group power spectral density estimates comparing EEG recordings taken during wakefulness, NREM sleep, and after CNO. The 95% bootstrapped confidence intervals are shown in the shaded areas. CNO (pink) induced a power spectrum very similar to NREM sleep (black) and distinct from the awake state (blue).
Activation of RMTg and brainstem GABAergic neurons produced evidence of sedation, but not unconsciousness as defined by loss of the righting reflex (LORR). Therefore, we tested whether activation of these GABAergic neurons alters sensitivity to sevoflurane-induced LORR (Fig. D). In control mice, no practical difference was observed after saline (1.65 ± 0.21%) vs. CNO (1.54 ± 0.17%). In hM3Dq mice, however, the inhaled sevoflurane concentration required to induce LORR was 1.65 ± 0.25% after saline and 1.00 ± 0.27% after CNO (Group × Treatment interaction, F[1, 16] = 18.4, p = 0.0006).
The effect of sex on the experimental effects was also evaluated using the linear mixed effects model with gender specified as a fixed effect for main effects and interactions. There were no three-way interactions (i.e., group × treatment × sex) for any of the variables (p > 0.238). However, there was evidence for a group × sex interaction only for the open field test (F[1, 14] = 16.98, p = 0.001). This appeared to be driven, at least in part, by a reduced degree of within-group variation.
Finally, the effects of CNO on EEG/EMG activity were assessed in hM3Dq mice and compared to natural sleep. As shown in Fig. E, a decrease in EMG activity was observed 20–40 minutes after CNO administration, which coincided with the appearance of EEG delta oscillations (1–4 Hz). The mean power spectral density estimates of the EEG after CNO was very similar to NREM sleep, and the bootstrapped 95% confidence intervals around the mean power spectral density estimates overlapped from 1 to 20 Hz (Fig. F). In contrast, the awake power spectral density estimates were characterized by lower power at most frequencies, indicated by non-overlapping 95% confidence intervals.
DISCUSSION
In this study, hM3Dq expression was targeted to RMTg GABAergic neurons in Vgat-ires-Cre mice to test the hypothesis that these neurons modulate arousal and anesthetic sensitivity. Robust expression of hM3Dq was observed in RMTg GABAergic neurons in all mice, but we also found variable expression in the pons and medulla, likely due to virus spread outside the RMTg. Therefore, the observed changes in behavior and neurophysiology were likely due to the combined effects of activating GABAergic neurons in the RMTg and other brainstem regions.
In hM3Dq mice, CNO produced large decreases in exploratory behavior and distance traveled in the open field test, and diminished motor coordination on the accelerating rotarod. Although it can be difficult to distinguish whether decreased motor activity is due to decreased arousal or direct inhibition of motor systems, EEG and EMG recordings demonstrated the appearance of oscillations that resembled NREM sleep and coincided with loss of muscle tone, suggesting decreased arousal as the underlying cause. Additionally, CNO decreased the dose requirement for sevoflurane-induced loss of righting by approximately 40% in hM3Dq mice, suggesting that activation of GABAergic neurons in the RMTg and other brainstem regions facilitates the transition to sevoflurane-induced unconsciousness.
It was previously reported that nonselective chemogenetic stimulation of the RMTg region increases NREM sleep and decreases rapid eye movement (REM) sleep in rats.14 In this study, we elicited DREADDs expression only in GABAergic neurons using Vgat-ires-Cre mice, and found that selective activation of these neurons in the RMTg and brainstem produces a NREM sleep-like state with increased sensitivity to sevoflurane-induced unconsciousness. High-dose CNO by itself may decrease motor activity and promote sedation,15 however the control mice in our study did not exhibit significant behavioral changes after receiving the same dose of CNO that induced profound sedation in hM3Dq mice, indicating that our results were not due to any off-target sedating effects of the designer ligand. However, activation of GABAergic neurons in the RMTg and other brainstem regions was insufficient to induce unconsciousness as defined by LORR. This suggests that while these neurons modulate levels of consciousness and facilitate the hypnotic actions of inhaled anesthetics, other inhibitory pathways are also involved in sevoflurane-induced unconsciousness. Because the goal of this study was to explore neural circuit mechanisms of decreased arousal and loss of consciousness, nociception was not tested.
It has been reported that microinjections of GABAergic anesthetics such as pentobarbital and propofol in the mesopontine tegmental anesthesia area (MPTA) produces a state of unconsciousness akin to general anesthesia in rodents,16 and lesioning MPTA neurons produces resistance to these anesthetics.17 It is possible that the sedation and increased sensitivity to sevoflurane observed in our study was mediated, at least in part, by increased levels of GABA in the MPTA.
In conclusion, GABAergic neurons in the RMTg and other brainstem regions are likely to play important roles in sleep and the sedative actions of GABAergic anesthetics. Our results encourage further work to characterize how brainstem GABAergic neurons are involved in consciousness, sleep, and anesthesia.
Supplementary Material
Funding:
This work was supported by grants R01-GM126155 and P01-GM118269 from the National Institutes of Health, and grant 220020406 from the James S. McDonnell Foundation.
GLOSSARY OF TERMS
- GABA
gamma-aminobutyric acid
- RMTg
rostromedial tegmental nucleus
- VTA
ventral tegmental area
- LHb
lateral habenula
- NREM
non-rapid eye movement
- REM
rapid eye movement
- DREADDs
Designer Receptors Exclusively Activated by Designer Drugs
- CNO
clozapine-N-oxide
- EEG
electroencephalogram
- EMG
electromyogram
- FISH
fluorescent in situ hybridization
- VGAT
vesicular GABA transporter
- LORR
loss of the righting reflex
Footnotes
Conflicts of Interest: Ken Solt is a consultant for Takeda Pharmaceuticals.
REFERENCES
- 1.Hemmings HC Jr, Riegelhaupt PM, Kelz MB, et al. Towards a Comprehensive Understanding of Anesthetic Mechanisms of Action: A Decade of Discovery. Trends Pharmacol Sci. 2019;40(7):464–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9(5):370–386. [DOI] [PubMed] [Google Scholar]
- 3.Pal D, Dean JG, Liu T, et al. Differential Role of Prefrontal and Parietal Cortices in Controlling Level of Consciousness. Curr Biol. 2018;28(13):2145–2152 e2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelz MB, Garcia PS, Mashour GA, Solt K. Escape From Oblivion: Neural Mechanisms of Emergence From General Anesthesia. Anesth Analg. 2019;128(4):726–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eban-Rothschild A, Rothschild G, Giardino WJ, Jones JR, de Lecea L. VTA dopaminergic neurons regulate ethologically relevant sleep-wake behaviors. Nat Neurosci. 2016;19(10):1356–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu X, Li W, Ma Y, et al. GABA and glutamate neurons in the VTA regulate sleep and wakefulness. Nat Neurosci. 2019;22(1):106–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor NE, Van Dort CJ, Kenny JD, et al. Optogenetic activation of dopamine neurons in the ventral tegmental area induces reanimation from general anesthesia. Proc Natl Acad Sci U S A. 2016;113(45):12826–12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrot M, Sesack SR, Georges F, Pistis M, Hong S, Jhou TC. Braking dopamine systems: a new GABA master structure for mesolimbic and nigrostriatal functions. J Neurosci. 2012;32(41):14094–14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: A structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol. 2009;513(6):566–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lavezzi HN, Zahm DS. The mesopontine rostromedial tegmental nucleus: an integrative modulator of the reward system. Basal Ganglia. 2011;1(4):191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gelegen C, Miracca G, Ran MZ, et al. Excitatory Pathways from the Lateral Habenula Enable Propofol-Induced Sedation. Curr Biol. 2018;28(4):580–587 e585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stamatakis AM, Stuber GD. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat Neurosci. 2012;15(8):1105–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guidera JA, Taylor NE, Lee JT, et al. Sevoflurane Induces Coherent Slow-Delta Oscillations in Rats. Front Neural Circuits. 2017;11:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang SR, Hu ZZ, Luo YJ, et al. The rostromedial tegmental nucleus is essential for non-rapid eye movement sleep. PLoS Biol. 2018;16(4):e2002909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomez JL, Bonaventura J, Lesniak W, et al. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science. 2017;357(6350):503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minert A, Yatziv SL, Devor M. Location of the Mesopontine Neurons Responsible for Maintenance of Anesthetic Loss of Consciousness. J Neurosci. 2017;37(38):9320–9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minert A, Baron M, Devor M. Reduced Sensitivity to Anesthetic Agents upon Lesioning the Mesopontine Tegmental Anesthesia Area in Rats Depends on Anesthetic Type. Anesthesiology. 2020;132(3):535–550. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


