Abstract
This review compares the effects of peripheral dexamethasone and dexmedetomidine on postoperative analgesia. We included six randomized controlled trials (354 patients) through a systematic literature search. We found that analgesia duration was comparable between dexamethasone and dexmedetomidine (58.59 min, 95% CI (confidence interval), − 66.13, 183.31 min) with extreme heterogeneity. Secondary outcome was also compared and no significant difference was observed in sensory block onset and duration and motor block duration and also for postoperative nausea and vomiting. It is noteworthy that dexamethasone reduced analgesic consumption (fentanyl) by 29.12 mcg compared with dexmedetomidine. We performed subgroup analyses and found no significant difference between the following: (1) lidocaine vs ropivacaine (P = 0.28), (2) nerve block vs nerve block + general anesthesia (P = 0.47), and (3) upper limb surgery vs thoracoscopic pneumonectomy (P = 0.27). We applied trial sequential analysis to assess the risks of type I and II errors and concluded that the meta-analysis was insufficiently powered to answer the clinical question, and further analysis is needed to establish which adjuvant is better. In conclusion, we believe that existing research indicates that dexamethasone and dexmedetomidine have equivalent analgesic effects in peripheral nerve blocks.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00540-021-02895-y.
Keywords: Dexamethasone, Dexmedetomidine, Analgesia, Adjuvants, Nerve block
Introduction
Nerve blocks have been widely used for postoperative pain control in recent years, but the analgesic duration of local anesthetics is time-limited. With the development of multimodal analgesia, there are on-going studies to prolong the time of analgesic. One area of focus has been the addition of adjuvant medications to local anesthetics. Medications that have been previously investigated include opioids, clonidine, buprenorphine, dexmedetomidine, and dexamethasone [1].
Dexamethasone has been evaluated as an adjuvant either peripherally or intravenously [2]. A meta-analysis has confirmed that peripheral dexamethasone with local anesthetics prolongs the analgesic duration of the brachial plexus block [3]. The mechanism of action may involve suppressing transmission in thin unmyelinated C-fibers [4], a local vasoconstrictive effect [5], and anti-inflammatory actions [6]. Dexmedetomidine, an α2 adrenoreceptor, has also been found to prolong loco-regional analgesia in studies in vivo and in vitro [7, 8]. An in vivo study of a peripheral nerve block in rats found that the analgesic effect of dexmedetomidine is related to the block of hyperpolarization-activated cations [9]. Most published studies compared dexmedetomidine or dexamethasone as local anesthetic adjuvants with placebo [10, 11], and concluded that both prolong analgesia time. However, they also added their own corresponding adverse reactions: bradycardia, hypotension, and excessive sedation caused by dexmedetomidine [7, 12], whereas dexamethasone increased glucose concentration [13, 14]. Therefore, intuitive evidence is needed to compare the benefit-to-risk ratio of the two adjuvants. The objective of this systematic review and meta-analysis is to assess the effect of dexmedetomidine compared with dexamethasone peripherally on postoperative pain outcomes in patients undergoing surgery under regional or combined regional and general anesthesia.
Materials and methods
Search strategy and selection criteria
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines [15] for the preparation of this review. Randomized controlled trials examining the effect of dexmedetomidine and dexamethasone on the duration of the block after a single-shot nerve block were evaluated using a predefined protocol (Supplemental Digital Content 1. PRISMA NMA Checklist). The review was registered on PROSPERO with the registration number CRD42020202582.
We performed a systematic electronic literature search in the databases PubMed (https://pubmed.ncbi.nlm.nih.gov/), Embase (http://www.embase.com/), the Cochrane library (https://www.cochranelibrary.com/), and Web of Science (http://apps.webofknowledge.com/) without time limits. Two authors independently screened articles to determine their qualifications. EndNote was used to manage eligible studies. We mainly used the combination of subject words and free words in the search. The exact search strategies for different databases are described in Table 1. Our search was limited to randomized trials published in the English language. Trials that are unpublished or in progress were not included.
Table 1.
Search strategy
| Search strategy for PUBMED (38) |
|---|
| #1. ((((((((((((((((((((regional anaesthesia) OR (Conduction Anesthesia)) OR (Anesthesia, Regional)) OR (Regional Anesthesia)) OR (nerve block)) OR (Block, Nerve)) OR (Blocks, Nerve)) OR (Nerve Blocks)) OR (Nerve Blockade)) OR (Blockade, Nerve)) OR (Blockades, Nerve)) OR (Nerve Blockades)) OR (Chemical Neurolysis)) OR (Chemical Neurolyses)) OR (Neurolyses, Chemical)) OR (Neurolysis, Chemical)) OR (Chemodenervation)) OR (Chemodenervations)) OR (peripheral block)) |
| #2. (((((((((((Dexamethasone) OR (Methylfluorprednisolone)) OR (Hexadecadrol)) OR (Decameth)) OR (Decaspray)) OR (Dexasone)) OR (Hexadrol)) OR (Oradexon)) OR (Glucocorticoid)) OR (cortison)) OR (corticosteroid))) |
| #3. ((((((((Medetomidine) OR (Levomedetomidine)) OR (Medetomidine Hydrochloride)) OR (Hydrochloride, Medetomidine)) OR (Dexmedetomidine)) OR (Precedex)) OR (Dexmedetomidine Hydrochloride)) OR (Hydrochloride, Dexmedetomidine)) |
| #4. #1 AND #2 AND #3 |
| Search strategy for The Cochrane Library (10) |
| #1 MeSH descriptor: [Nerve Block] explode all trees |
| #2 (“Block, Nerve” or “Blocks, Nerve” or “Nerve Blocks” or “Nerve Blockade” or “Blockade, Nerve” or” Blockades, Nerve” or “Nerve Blockades” or “Chemical Neurolysis” or “Chemical Neurolyses” or “Neurolyses, Chemical” or “Neurolysis, Chemical”):ti,ab,kw |
| #3 #1 or #2 |
| #4 MeSH descriptor: [dexamethasone] explode all trees |
| #5 (Methylfluorprednisolone or Hexadecadrol or Decameth or Decaspray or Dexasone or Dexpak or Maxidex or Millicorten or Oradexon or Decaject or Hexadrol):ti,ab,kw |
| #6 #4 or #5 |
| #7 MeSH descriptor: [dexmedetomidine] explode all trees |
| #8 (Levomedetomidine or “Hydrochloride, Medetomidine” or “Medetomidine Hydrochloride” or Medetomidine or Precedex or “Dexmedetomidine Hydrochloride” or “Hydrochloride, Dexmedetomidine”):ti,ab,kw |
| #9 #7 or #8 |
| #10 #3 and #6 and #9 |
| Search strategy for Web of Science (46) |
| #1 TS = (regional anaesthesia OR Conduction Anesthesia OR Anesthesia, Regional OR Regional Anesthesia OR nerve block OR Block, Nerve OR Blocks, Nerve OR Nerve Blocks OR Nerve Blockade OR Blockade, Nerve OR Blockades, Nerve OR Nerve Blockades OR Chemical Neurolysis OR Chemical Neurolyses OR Neurolyses, Chemical OR Neurolysis, Chemical OR Chemodenervation OR Chemodenervations OR peripheral block) |
| #2 TS = (Dexamethasone OR Methylfluorprednisolone OR Hexadecadrol OR Decameth OR Decaspray OR Dexasone OR Hexadrol OR Oradexon OR Glucocorticoid OR cortison OR corticosteroid) |
| #3 TS = (Medetomidine OR Levomedetomidine OR Medetomidine Hydrochloride OR Hydrochloride, Medetomidine OR Dexmedetomidine OR Precedex OR Dexmedetomidine Hydrochloride OR Hydrochloride, Dexmedetomidine) |
| #4 #1 and #2 and #3 |
| Search strategy for EMBASE (114) |
| 1. (‘dexmedetomidine’/exp OR ‘precedex’:ab,ti OR ‘dexmedetomidine hydrochloride’:ab,ti OR ‘hydrochloride, dexmedetomidine’:ab,ti OR ‘medetomidine’:ab,ti OR ‘levomedetomidine’:ab,ti OR ‘medetomidine hydrochloride’:ab,ti OR ‘hydrochloride, medetomidine’:ab,ti) |
| 2. (‘dexamethasone’/exp OR ‘methylfluorprednisolone’:ab,ti OR ‘hexadecadrol’:ab,ti OR ‘decameth’:ab,ti OR ‘decaspray’:ab,ti OR ‘dexasone’:ab,ti OR ‘dexpak’:ab,ti OR ‘oradexon’:ab,ti OR ‘decaject’:ab,ti OR ‘hexadrol’:ab,ti OR ‘glucocorticoids’:ab,ti OR ‘glucocorticoid’:ab,ti OR‘ cortison’:ab,ti OR ‘corticosteroids’:ab,ti OR ‘corticoids’:ab,ti) |
| 3. (‘nerve block’/exp OR ‘block, nerve’:ab,ti OR ‘blocks, nerve’:ab,ti OR ‘nerve blocks’:ab,ti OR ‘nerve blockade’:ab,ti OR ‘blockade, nerve’:ab,ti OR ‘blockades, nerve’:ab,ti OR ‘nerve blockades’:ab,ti OR ‘chemical neurolysis’:ab,ti OR ‘chemical neurolyses’:ab,ti OR ‘anesthesia, regional’:ab,ti OR ‘peripheral block’:ab,ti OR ‘regional anesthesia’:ab,ti) |
| 4. 1 and 2 and 3 |
Inclusion and exclusion criteria
We included randomized controlled trials assessing the duration of analgesia after adding peripheral dexmedetomidine or dexamethasone as an adjuvant to local anesthetics. We performed inclusion criteria according to PICO [16].
Patients: adults undergoing surgery with peripheral nerve block alone or combined with general anesthesia.
Intervention: addition of dexamethasone to local anesthetic for perioperative analgesia.
Comparison: addition of dexmedetomidine to local anesthetic for perioperative analgesia.
Outcome: duration of analgesia, sensory block onset and duration time, motor block onset and duration time, analgesic consumption, and adverse effects.
Patients aged under 18 years and animal studies were excluded. Similarly, we also excluded observational cohort studies, case–control studies, and reviews.
Data collection and presentation
Two authors extracted data independently. Disagreements were resolved by discussion until a consensus was reached or by consulting a third author. We selected the duration of analgesia as the primary outcome, while sensory block onset and duration time, motor block onset and duration time, analgesic consumption, and adverse effects were secondary outcomes. The duration of analgesia was defined as the time from onset of adequate sensory block to the time that the patient first requested analgesic medication. We also defined sensory and motor block onset as the time interval between the end of local anesthetic injection and the loss of pinprick sensation or motor function. Sensory and motor block duration were considered as the time interval between a successful block and the complete reappearance of all the senses and recovery of motor function. Analgesic consumption was defined as postoperative fentanyl consumption. We also retrieved perioperative adverse effects such as bradycardia, hypotension, dizziness, postoperative nausea and vomiting, Horner’s syndrome, hoarseness of voice, and hyperglycemia.
Assessment of bias risks
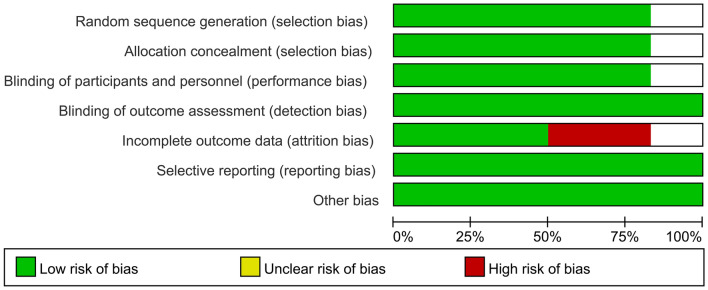
Two reviews independently assessed the quality of the selected studies according to the Cochrane collaboration's tool [17] for randomized controlled trials. We used the Review Manager 5.3 Risk of Bias tool to analyze the methodological quality of the studies. This tool allows for an assessment of the risks of selection bias (random sequence generation, allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective reporting), and other bias.
Meta-analyses
We decided to perform meta-analyses when at least two studies were identified. Review Manager (RevMan, version 5.3) Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014 was used for the meta-analysis. Dichotomous and continuous outcomes were analyzed using random-effects modeling. The risk ratios and 95% confidence intervals (CIs) are reported for dichotomous outcomes, while the mean difference and 95% CI are reported for continuous outcomes. The heterogeneity of the eligible studies was measured using the I2 test [18], we explored the sources of heterogeneity of the primary outcome by subgroup analysis or sensitivity.
Subgroup analysis
We grouped the included studies and performed subgroup analysis three times according to the types of local anesthetics, methods of anesthesia, and type of surgery. The specific classification is as follows: (1) lidocaine vs ropivacaine, (2) nerve block vs nerve block + general anesthesia, and (3) upper limb surgery vs thoracoscopic pneumonectomy.
Sensitivity analysis
Some studies have certain characteristics, for example, the methodological quality of several studies is low or the sample is small, we can judge whether these characteristics have affected the conclusion through sensitivity analysis, that is, by adding or removing these studies and observing the consistency of the meta-analysis.
Trial sequential analysis
When the number of trials included in a meta-analysis is small with an insufficient sample size, random errors may lead to erroneous results [19, 20]. Trial sequential analysis is a statistical approach that combines multiple techniques, it quantifies the required evidence and provides specific values for the required information size. Results are presented as a graph that contains the cumulative Z-curve (the Z test value at each meta-analysis update), conventional level of significance, number of patients in the meta-analysis, estimated required information size, and trial sequential significance boundaries. The trial sequential significance boundaries are constructed by adjusting the thresholds for significance so that the overall risk of type 1 error is less than the desired level (usually 5%). A cumulative Z-curve that is greater than the trial sequential boundary is considered a statistically significant effect.
We used trial sequential analysis on the duration of analgesia. We calculated the required information size (RIS) allowing for type 1 error of 0.05, and type 2 error of 0.20, mean difference from the effect estimate from the random-effects model, and estimated variance and heterogeneity from that present in the included trials. We constructed trial sequential analysis boundaries based on the O’Brien–Fleming alpha-spending function. Trial sequential analysis software (version 0.9 Copenhagen Trial Unit, Copenhagen, Denmark) was used to perform the analysis.
Grading of recommendations assessment, development, and evaluation (GRADE) system
We used GRADE [21] to rate the quality of evidence and the strength of recommendation of our outcome. Based on key elements including the risk of bias, inconsistency, indirectness, imprecision, and publication bias, the GRADE tool classifies the strength of synthesized evidence into four categories:
High quality: further research is very unlikely to change our confidence in the estimate of effects.
Moderate quality: further research is likely to alter the confidence in the estimate of the effect.
Low quality: further research is very likely to alter the confidence in the estimate of the effect.
Very low quality: we are very uncertain about the estimate.
Results
Our database search strategy retrieved 209 potentially relevant records published. Of these, a total of six full-text randomized trials were included in the final analysis. Figure 1 represents a flow diagram following the PRISMA template.
Figure 1.
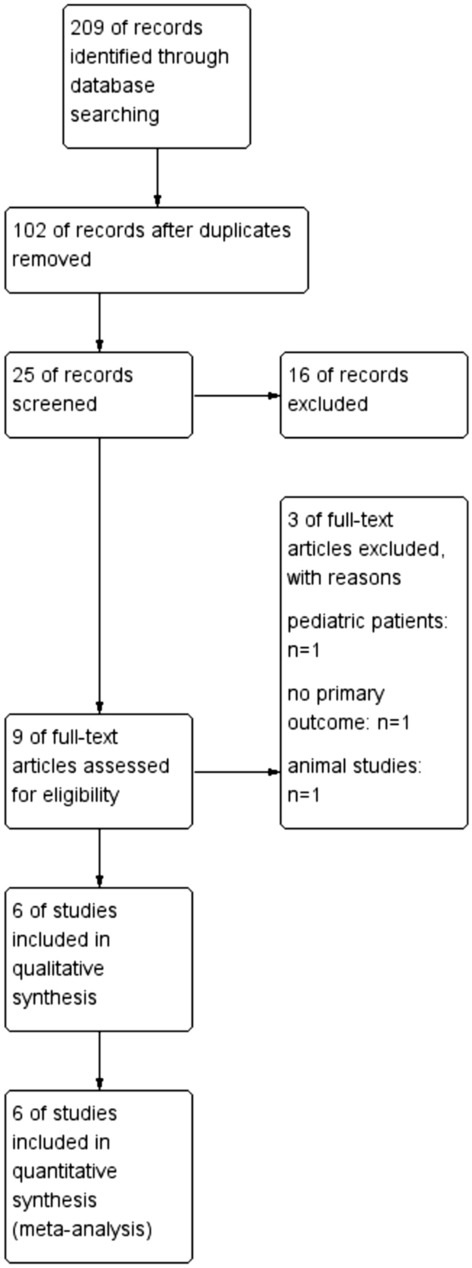
Study flow diagram (PRISMA template)
Trial characteristics
Table 2 contains the details of the included studies. Table 3 provides quantitative results about secondary outcomes.
Table 2.
Details of the included trials
| Study | Number of patient | Type of surgery | Nerve block | Dose | Primary outcome | Other anaesthesia techniques | Postoperative analgesia | ||
|---|---|---|---|---|---|---|---|---|---|
| DeM | DeA | DeM | DeA | ||||||
| Sandeep Kataria | 30 | 30 | Arthroscopic shoulder surgery | Ultrasound guided interscalene block 0.5% ropivacaine 20 mL | 0.5 mcg/kg | 8 mg | Duration of analgesia | General anaesthesia with endotracheal intubation:fentanyl 2 mcg/kg, propofol 2 mg/kg and vecuronium 0.1 mg/kg. supplement fentanyl intra-operatively in the dose of 1 mcg/kg if there was 20% increase from the baseline parameters | PCIA (The pump was set to deliver patient-controlled boluses of 10 mcg of fentanyl, with lock-out interval of 6 min, maximum 4 h dose of fentanyl being 400 mcg) |
| Siamak Yaghoobi | 26 | 25 | Forearm fracture surgery | Ultrasound-guided infraclavicular brachial plexus block 28 mL lidocaine 2% | 1 mcg/kg | 8 mg | the time to the first requirement of analgesic supplement and the total analgesic consumption in the first 6 h postoperatively | Premedication: administration of 0.02 mg/kg midazolam and 2 µg/kg fentanyl | I.V. pethidine 25 mg,when VAS ≥ 4 |
| Panpan zhang | 20 | 20 | Thoracoscopic pneumonectomy | Intercostal nerve block 28 mL 0.5% ropivacaine | 1 mcg/kg | 10 mg | Duration of analgesia | General anesthesia was induced with 0.08–0.10 mg/kg of midazolam, 0.15–0.30 mg/kg of etomidate, 2–4 µg/kg of fentanyl and 0.12 mg/kg of cisatracurium. Maintenance of anesthesia was achieved with propofol, sevoflurane, remifentanil and atracurium | received PCIA for postoperative analgesia. PCIA was administered for VAS ≥ 4 or on patient request |
| Julián Aliste | 53 | 56 | Upper limb surgery | Ultrasound-guided infraclavicular brachial plexus block 35 mL of lidocaine 1%–bupivacaine 0.25% with epinephrine 5 µg/mL | 100 µg | 5 mg | Duration of motor block | Premedication: (0.015–0.03 mg/kg of midazolam and 0.6 µg/kg of fentanyl | Not described |
| Myeong Jong Lee | 17 | 17 | Elective forearm and hand surgery | Ultrasound-guided axillary brachial plexus blocks with nerve stimulation 20 ml of 0.5% ropivacaine | 100 µg | 10 mg | The duration of the sensory block | When VAS ≥ 4 or an uncomfortable sensation developed during surgery, a 50 g bolus of fentanyl was administered intravenously. If pain persisted 5 min after administration of fentanyl, an additional 50 g fentanyl was given | Not described |
| Zhixin Gao | 30 | 30 | Video-assisted thoracoscopic lobectomy surgery | Ultrasound-guided erector spinae plane block 0.5% ropivacaine 30 mL | 1 mcg/kg | 10 mg | Postoperative PCA use during the first 72 h | General anesthesia was induced with 0.03 mg/kg midazolam, 0.5 μg/kg of sufentanil, 0.9 mg/kg Rocuronium bromide and Propofol. Maintenance of anesthesia was achieved with propofol, sevoflurane, remifentanil and cisatracurium | Sufentanil (0.1–0.2 μg/kg) and flurbiprofen (50 mg) were intravenously administered, followed by PCA pump use before the end of the surgery. PCA capacity was 250 mL and contained 7.5 μg/kg sufentanil and 250 mg flurbiprofen |
DeM dexmedetomidine, DeA dexamethasone, PCIA patient-controlled intravenous analgesia, VAS visual analogue scale, PCA patient controlled analgesia
Table 3.
Quantitative results
| Time-to-event outcomes | Studies included | DeA | DeM | Risk ratios or weighed mean (95% CI) | P value for statistical significance | P value for heterogeneity | I2 test for heterogeneity | ||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean or n/N | N | Mean or n/N | ||||||
| Sensory block onset (min) | [14–17] | 132 | 12.8 | 133 | 12.66 | 0.4 (− 1.24, 2.04) | 0.64 | 0.04 | 64% |
| Sensory block duration (min) | [14, 15, 17, 18] | 128 | 656.15 | 126 | 707.52 | − 9.55 (− 186.07, 166.98) | 0.92 | < 0.01 | 91% |
| Motor block onset (min) | [14, 16] | 55 | 9.47 | 56 | 8.78 | 0.67 (0.03, 1.32) | 0.04 | 0.97 | 0 |
| Motor block duration (min) | [14, 17] | 81 | 634.49 | 79 | 570.96 | 61.85 (− 178.16, 301.86) | 0.61 | < 0.01 | 96% |
|
Fentanyl consumption (mcg) |
[16, 19] | 50 | 126.5 | 50 | 138.665 | − 29.12 (− 45.18, − 13.06) | < 0.01 | 0.43 | 0 |
| Postoperative nausea | [14–16, 18, 19] | 122 | 7/122 | 123 | 4/123 | 1.6 (0.24, 10.83) | 0.62 | 0.21 | 38% |
| Postoperative vomiting | [14–16, 18, 19] | 122 | 8/122 | 123 | 2/123 | 3.89 (0.88, 17.16) | 0.08 | 0.76 | 0 |
DeA dexamethasone, min minute, DeM dexmedetomidine, CI confidence interval
Risk of bias assessment
The methodological quality of the studies is given in Figs. 2, 3, and Table 4. We assessed five [22–26] out of six trials as low risk of bias. One trial [27] was an unclear risk due to the selection bias, performance bias, and attrition bias.
Figure 2.
Risk of bias graph
Figure 3.
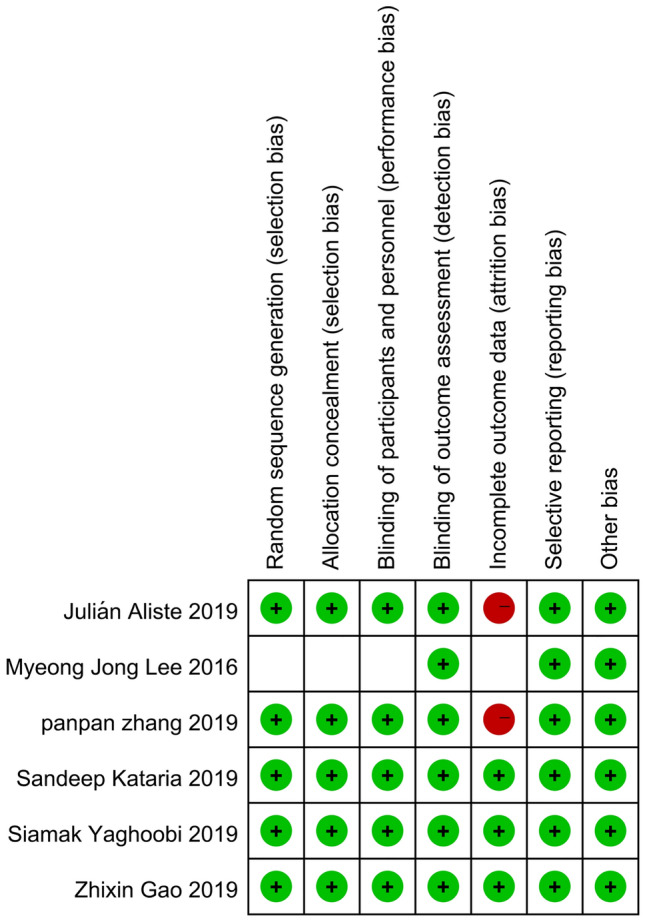
Risk of bias assessment
Table 4.
The assessment judgment outcomes of RCTs
| Author name (Year) | Sequence generation (Selection bias) | Allocation concealment (Selection bias) | Blinding of Participants and personnel (Performance bias) | Blinding of outcome assessors (Detection bias) | Incomplete outcome data (Attrition bias) | Selective outcome reporting (Reporting bias) | Other bias | Overall |
|---|---|---|---|---|---|---|---|---|
| Julián Aliste (2019) | Low risk | Low risk | Low risk | Low risk | High risk | Low risk | Low risk | Low risk |
| Double blinding | Double blinding | Double blinding | Some patients lost to follow-up | The study protocol is available and all of the study’s prespecified outcomes of interest have been reported in the prespecified way | The study appears to be free of other sources of bias | |||
| Myeong Jong Lee (2016) | Unclear risk | Unclear risk | Unclear risk | Low risk | Unclear risk | Low risk | Low risk | Unclear risk |
| didn’t mention Sequence generation | didn’t mention weather about blinding | didn’t mention weather about blinding | Double blinding | Not mentioned | The study protocol is available and all of the study’s prespecified outcomes of interest have been reported in the prespecified way | The study appears to be free of other sources of bias | ||
| Panpan Zhang (2019) | Low risk | Low risk | Low risk | Low risk | High risk | Low risk | Low risk | Low risk |
| Double blinding | Double blinding | Double blinding | Some patients lost to follow-up | The study protocol is available and all of the study’s prespecified outcomes of interest have been reported in the prespecified way | The study appears to be free of other sources of bias | |||
| Sandeep Kataria (2019) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Double blinding | Double blinding | Double blinding | No missing outcome data or loss to follow-up | The study protocol is available and all of the study’s prespecified outcomes of interest have been reported in the prespecified way | The study appears to be free of other sources of bias | |||
| Siamak Yaghoobi (2019) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Double blinding | Double blinding | Double blinding | Some patients lost to follow-up | The study protocol is available and all of the study’s prespecified outcomes of interest have been reported in the prespecified way | The study appears to be free of other sources of bias | |||
| Zhixin Gao (2019) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Double blinding | Double blinding | Double blinding | No missing outcome data or loss to follow-up | The study protocol is available and all of the study’s prespecified outcomes of interest have been reported in the prespecified way | The study appears to be free of other sources of bias |
Synthesis of results
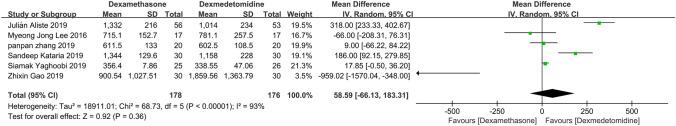
Primary outcome: duration of analgesia
Figure 4 shows the meta-analysis for the primary outcome including six trials [22–27] that had data for this outcome. When comparing peripheral dexamethasone with dexmedetomidine, the estimated duration of analgesia was 58.59 min (95%CI: − 66.13, 183.31; P = 0.36) longer in the peripheral dexamethasone group. But this difference did not reach statistical significance. The heterogeneity among the pooled studies was significant (I2 = 93%; P < 0.00001).
Figure 4.
Meta-analysis: duration of analgesia (min), min, minute
Secondary outcomes
The meta-analysis is shown on the following outcomes.
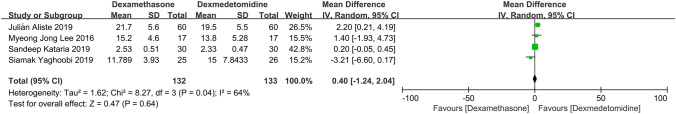
Sensory block onset
This outcome was reported in four studies (Fig. 5) [23–25, 27]. When comparing peripheral dexamethasone with dexmedetomidine, the estimated onset of sensory block was 0.40 min (95% CI: − 1.24, 2.04; P = 0.64) longer in the peripheral dexmedetomidine group. This difference was not statistically significant. The heterogeneity among the pooled studies was significant (I2 = 64%; P = 0.04).
Figure 5.
Meta-analysis: sensory block onset (min), min, minute
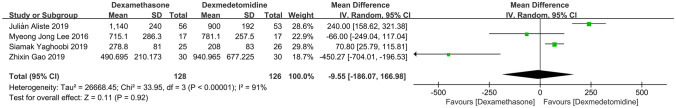
Sensory block duration
Four studies reported this variable (Fig. 6) [23, 24, 26, 27]. When comparing peripheral dexamethasone with dexmedetomidine, the estimated duration of the sensory block was 9.55 min (95% CI: − 186.07, 166.98; P = 0.92) longer in the peripheral dexmedetomidine group. This difference was not statistically significant. The heterogeneity among the pooled studies was also high (I2 = 91%; P ≤ 0.00001).
Figure 6.
Meta-analysis: sensory block duration (min), min, minute
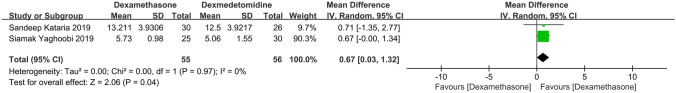
Motor block onset
This outcome was reported in two studies (Fig. 7) [23, 25]. When comparing peripheral dexamethasone with dexmedetomidine, the estimated onset of the motor block was 0.67 min (95% CI: 0.03, 1.32; P = 0.04) longer in the peripheral dexamethasone group. The heterogeneity among trials was insignificant (I2 = 0; P = 0.97).
Figure 7.
Meta-analysis: motor block onset (min), min, minute
Motor block duration
Four studies reported this variable (Fig. 8) [23, 24]. When comparing peripheral dexamethasone with dexmedetomidine, the estimated duration of the motor block was 61.85 min (95% CI: − 178.16, 301.86; P = 0.61) longer in the peripheral dexamethasone group. This difference was not statistically significant. The heterogeneity among the pooled studies was significant (I2 = 96%; P < 0.00001).
Figure 8.
Meta-analysis: motor block duration (min), min, minute
Analgesic consumption (fentanyl)
This outcome was reported in two studies (Fig. 9) [22, 25]. When comparing peripheral dexamethasone with dexmedetomidine, the estimated analgesic consumption was 29.12 mcg (95% CI: − 45.18, − 13.06; P < 0.0004) more in the peripheral dexmedetomidine group. There was no heterogeneity among the pooled studies (I2 = 0; P = 0.43).
Figure 9.
Meta-analysis: analgesic consumption (fentanyl)
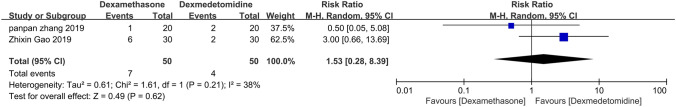
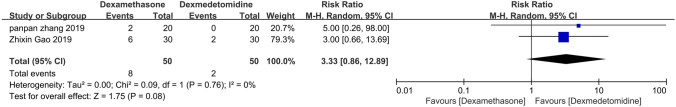
Adverse outcomes
There was no significant difference in postoperative nausea and vomiting assessed in five studies (Figs. 10, 11) [22, 23, 25–27]. Only one trial reported bradycardia [27], dizziness [22], Horner’s syndrome [25], and hoarseness of voice [25]. There were no reports of perioperative and postoperative hyperglycemia caused by dexamethasone.
Figure 10.
Meta-analysis: postoperative nausea
Figure 11.
Meta-analysis: postoperative vomiting
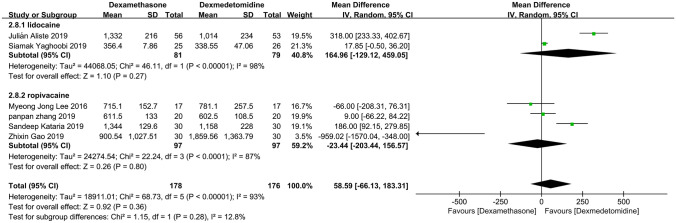
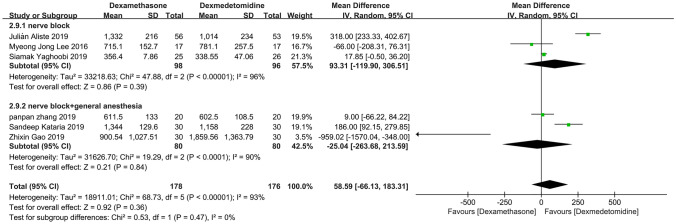
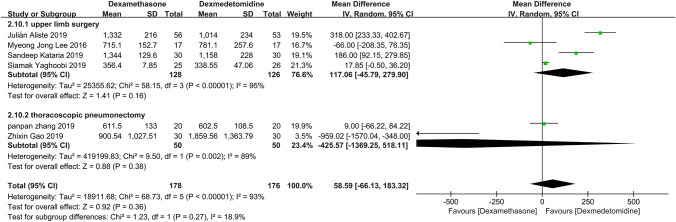
Subgroup analysis
Through three times subgroup analysis (Figs. 12, 13, 14), we believed there was no significant difference between the subgroups: (1) lidocaine vs ropivacaine (P = 0.28), (2) nerve block vs nerve block + general anesthesia (P = 0.47), and (3) upper limb surgery vs thoracoscopic pneumonectomy (P = 0.27), and the heterogeneity remained substantial.
Figure 12.
Meta-analysis: duration of analgesia (min): lidocaine versus ropivacaine subgroups, min, minute
Figure 13.
Meta-analysis: duration of analgesia (min): nerve block versus nerve block + general anesthesia subgroups, min, minute
Figure 14.
Meta-analysis: duration of analgesia (min): upper limb surgery versus thoracoscopic pneumonectomy subgroups, min, minute
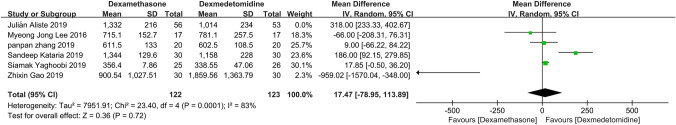
Sensitivity analysis
In Fig. 15, the meta-analysis is shown after sensitivity analysis. The heterogeneity was high in our primary outcome. After removing one study [24], the I2 was lower from 93 to 83%, but there was still no statistical difference in the duration of analgesia.
Figure 15.
Sensitivity analysis: duration of analgesia (min), min, minute
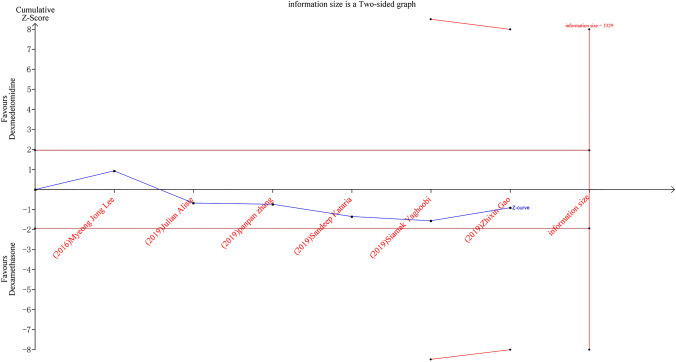
Trial sequential analysis
In Fig. 16, we demonstrate that the trial sequential analysis (TSA) curve neither crosses the traditional boundary value nor the TSA boundary value, and the cumulative information size does not reach the required information size, indicating that the meta-analysis was insufficiently powered to answer the clinical question defined by the assumptions used, and more data are needed to establish this.
Figure 16.
Trial-sequential analysis of six trials comparing perineural dexamethasone with dexmedetomidine for the duration of analgesia
Grade
We assigned the GRADE level of “low quality” to our primary outcome “duration of analgesia” (Table 5). This assessment was based on the risk of bias, demonstrated by insufficient details regarding blinding and concealment of sequence allocation and some outcomes were incomplete. Regarding the inconsistency, the I2 is high and we did not assess the risk of publication bias because of the few studies included. As a result of our assessment of the risk of bias, inconsistency, and publication bias, we down-graded the level of evidence three times, resulting in our assessment of the primary outcome being “low quality”.
Table 5.
The grading of recommendations assessment, development and evaluation (GRADE) approach
| No of studies | Design | Quality assessment | No of patients | Effects | Quality | Importance | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Analgesia | Control | Relative (95% CI) | Absolute | ||||
| Duration of analgesia (Better indicated by lower values) | ||||||||||||
| 6 | Randomised trials | Seriousa | Seriousb | No serious indirectness | No serious imprecision | None | 178 | 176 | – | MD 58.59 higher (66.13 lower to 183.31 higher) |
⊕⊕ΟΟ Low |
Critical |
| Sensory block onset (Better indicated by lower values) | ||||||||||||
| 4 | Randomised trials | Seriousa | Very seriousc | No serious indirectness | No serious imprecision | None | 132 | 133 | – | MD 0.4 higher (1.24 lower to 2.04 higher) |
⊕ΟΟΟ Very Low |
Important |
| Sensory block duration (Better indicated by lower values) | ||||||||||||
| 4 | Randomised trials | Seriousa | Very seriousc | No serious indirectness | No serious imprecision | None | 128 | 126 | – | MD 9.55 lower (186.07 lower to 166.98 higher) |
⊕ΟΟΟ Very Low |
Important |
| Motor block onset (Better indicated by lower values) | ||||||||||||
| 2 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | No serious imprecision | None | 55 | 56 | – | MD 0.67 higher (0.03 to 1.32 higher) |
⊕⊕⊕⊕ High |
Important |
| Motor block duration (Better indicated by lower values) | ||||||||||||
| 2 | Randomised trials | Seriousa | Very seriousc | No serious indirectness | No serious imprecision | None | 81 | 79 | – | MD 61.85 higher (178.16 lower to 301.86 higher) |
⊕ΟΟΟ Very Low |
Important |
| Analgesic consumption (Better indicated by lower values) | ||||||||||||
| 2 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | None | 50 | 50 | – | MD 29.12 lower (45.18 to 13.06 lower) |
⊕⊕⊕Ο Moderate |
Critical |
| No of studies | Design | Quality assessment | No of patients | Effect | Quality | Importance | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Adverse reaction | Control | Relative (95% CI) | Absolute | ||||
| Postoperative nausea | ||||||||||||
| 2 | Randomised trials | Seriousd | No serious inconsistency | No serious indirectness | No serious imprecision | None | 7/50 (14%) | 4/50 (8%) | RR 1.53 (0.28 to 8.39) | 42 more per 1000 (from 58 fewer to 591 more) |
⊕⊕⊕Ο Moderate |
Important |
| – | 8.3% | 44 more per 1000 (from 60 fewer to 613 more) | ||||||||||
| Postoperative vomiting | ||||||||||||
| 2 | Randomised trials | Seriousd | No serious inconsistency | No serious indirectness | No serious imprecision | None | 8/50 (16%) | 2/50 (4%) | RR 3.33 (0.86 to 12.89) | 93 more per 1000 (from 6 fewer to 476 more) |
⊕⊕⊕Ο Moderate |
Important |
| – | 3.3% | 77 more per 1000 (from 5 fewer to 392 more) | ||||||||||
aSome authors did not provide sufficient details regarding blinding and concealment of sequence allocation and some outcome is incomplete.
bI2 statistic was high without satisfactory explanation by subgroup analysis, but it can be reduced by sensitivity analysis
cI2 statistic was high without satisfactory explanation by subgroup analysis
dSome outcome is incomplete
Discussion
This is the first review to assess the direct effects of adjuvants, such as dexamethasone and dexmedetomidine, when applied to a regional block. Previously, Albrecht [28] conducted an indirect meta-analysis to identify the superior adjuvant by comparing dexamethasone and dexmedetomidine, and believed that dexamethasone was superior. In our meta-analysis, however, dexmedetomidine appears to have a comparable duration of analgesia with dexamethasone. A GRADE level of “low quality” was assigned to this primary outcome.
We also observed a longer duration of sensory block onset (0.40 min) and duration (9.55 min) with peripheral dexmedetomidine, and the difference was not statistically significant. The motor block was longer in the peripheral dexamethasone group, the time of onset and duration was 0.67 and 61.85 min, respectively, but the difference in motor block duration was insignificant. There was no significant difference in postoperative nausea and vomiting. It is noteworthy that dexamethasone reduced analgesic consumption (fentanyl) by 29.12 mcg compared with dexmedetomidine.
In our meta-analysis, we performed a subgroup analysis of three aspects. We found there was no significant difference between the subgroups, which indicates that the type of local anesthetic, methods of anesthesia, and type of surgery were not the reason for the high heterogeneity. Therefore, we suspect that it may be related to the dose of adjuvants and the concentration and volume of local anesthetics. However, we could not conduct a meta-regression to assess a dose–response effect because of the few studies included. We concluded from other studies that the two may be powerful influencing factors. Woo et al. carried out a randomized controlled trial and evaluated the effect of different doses of dexamethasone on the duration of single-shot interscalene brachial plexus block using ropivacaine 0.5% [29]. They concluded that dexamethasone demonstrated a dose-dependent effect on the duration of analgesia. However, Kirkham et al. conducted a meta-regression and believed 4 mg of peripheral dexamethasone represents a ceiling dose in terms of prolonging analgesia duration with very low-quality evidence [14]. The latest randomized controlled trial comparing the analgesic time of different doses of peripheral dexamethasone found 2, 5, and 8 mg of dexamethasone provide clinically equivalent sensorimotor and analgesic durations for ultrasound-guided infraclavicular block although 5 mg provided a longer analgesic duration (2.7 h) than 2 mg [30]. Therefore, the dose–effect relationship of dexamethasone is still unclear. Fredrickson et al. found that block duration is influenced by both local anesthetic volume and concentration [31].
However, we lowered the I2 of the primary outcome through sensitivity analysis. Using this, we removed one study [24] that we believed was the main source of heterogeneity. There are several explanations for the high inconsistency. First, the local anesthetic–epinephrine mixture may affect the outcome. Epinephrine itself acts as a vasoconstrictor and can prolong the duration of analgesia [32]. While Saied et al. [33] conducted an observational study and deem that epinephrine does not affect the duration of analgesia of brachial plexus block when added to ropivacaine with or without other adjuvants. Second, the number of patients in the included studies might not be adequate, although they all had considered the sample size. In addition, the included studies selected different optimal doses of the adjunct according to the different original trials.
For heterogeneity that cannot be explained by subgroup analysis and sensitivity analysis, we believed that analgesics used during peri-operation may be a critical factor. In one trial [26], patients were administered analgesia intravenously before the end of surgery, and this resulted in greater heterogeneity.
The results of our review are subject to several limitations. First, the trials included herein were small without enough power confirmed by trial sequential analysis and characterized by high levels of heterogeneity, factors that limit the clinical combinability of the source trials, and the generalizability of our results. Second, the GRADE level that we assigned to our study was only low quality for the conclusions and for the different dosages of adjuvants, we did not conduct a meta-regression to assess a dose–response effect. Also, contour-enhanced funnel plots for publication bias was limited, because the included trials were small. Finally, we did not consider the neurotoxicity of these two adjuncts, because there were no reports in our included studies. A study has demonstrated the safety of dexmedetomidine sciatic nerve block in rats [8]. Ferré et al. [34] showed that peripheral dexamethasone had a protective effect against the neural inflammation induced by bupivacaine and attenuated neural inflammation in the animal experiments. However, dexamethasone [35] and dexmedetomidine are used off-label. Even though it is widely used on an international level and has been investigated in many scientific trials, the US Food and Drug Administration does not approve dexamethasone or dexmedetomidine for peripheral administration.
In summary, dexamethasone was comparable with dexmedetomidine in terms of analgesia. However, because of the number of studies included, further comparisons are encouraged. The optimal dosages remain uncertain. Future dose-finding studies are required to elucidate the optimal dose of dexamethasone and dexmedetomidine.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Zhenguo Song and Shenyue Pang contributed equally to this work. ZGS and SYP take responsibility for the content of the manuscript. SYP was involved in the conception, hypothesis delineation, and design of the study and in the acquisition and analysis of the data and editing the manuscript. ZGS was involved in designing the study, analyzing the data, and writing and editing the manuscript. GYW was involved in conceiving the idea for the study and editing the manuscript. ZZ was involved in conceiving the idea for the study, generating the hypotheses and editing the manuscript. All authors read and approved the final manuscript. ZGS is the guarantor of the article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article this work was supported by The Science and Technology Development Fund of Tianjin Education Commission for Higher Education (Tianjin, China; No. 2018KJ066).
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest in preparing this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bailard NS, Ortiz J, Flores RA. Additives to local anesthetics for peripheral nerve blocks: evidence, limitations, and recommendations. Am J Health Syst Pharm. 2014;71(5):373–385. doi: 10.2146/ajhp130336. [DOI] [PubMed] [Google Scholar]
- 2.Heesen M, Klimek M, Imberger G, Hoeks SE, Rossaint R, Straube S. Co-administration of dexamethasone with peripheral nerve block: intravenous vs perineural application: systematic review, meta-analysis, meta-regression and trial-sequential analysis. Br J Anaesth. 2018;120(2):212–227. doi: 10.1016/j.bja.2017.11.062. [DOI] [PubMed] [Google Scholar]
- 3.Choi S, Rodseth R, McCartney CJ. Effects of dexamethasone as a local anaesthetic adjuvant for brachial plexus block: a systematic review and meta-analysis of randomized trials. Br J Anaesth. 2014;112(3):427–439. doi: 10.1093/bja/aet417. [DOI] [PubMed] [Google Scholar]
- 4.Johansson A, Hao J, Sjölund B. Local corticosteroid application blocks transmission in normal nociceptive C-fibres. Acta Anaesthesiol Scand. 1990;34(5):335–338. doi: 10.1111/j.1399-6576.1990.tb03097.x. [DOI] [PubMed] [Google Scholar]
- 5.Shishido H, Kikuchi S, Heckman H, Myers RR. Dexamethasone decreases blood flow in normal nerves and dorsal root ganglia. Spine (Phila Pa 1976) 2002;27(6):581–586. doi: 10.1097/00007632-200203150-00005. [DOI] [PubMed] [Google Scholar]
- 6.Barnes PJ. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci (Lond). 1998;94(6):557–572. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- 7.Abdallah FW, Brull R. Facilitatory effects of perineural dexmedetomidine on neuraxial and peripheral nerve block: a systematic review and meta-analysis. Br J Anaesth. 2013;110(6):915–925. doi: 10.1093/bja/aet066. [DOI] [PubMed] [Google Scholar]
- 8.Brummett CM, Norat MA, Palmisano JM, Lydic R. Perineural administration of dexmedetomidine in combination with bupivacaine enhances sensory and motor blockade in sciatic nerve block without inducing neurotoxicity in rat. Anesthesiology. 2008;109(3):502–511. doi: 10.1097/ALN.0b013e318182c26b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brummett CM, Hong EK, Janda AM, Amodeo FS, Lydic R. Perineural dexmedetomidine added to ropivacaine for sciatic nerve block in rats prolongs the duration of analgesia by blocking the hyperpolarization-activated cation current. Anesthesiology. 2011;115(4):836–843. doi: 10.1097/ALN.0b013e318221fcc9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cummings KC, Napierkowski DE, Parra-Sanchez I, Kurz A, Dalton JE, Brems JJ, et al. Effect of dexamethasone on the duration of interscalene nerve blocks with ropivacaine or bupivacaine. Br J Anaesth. 2011;107(3):446–453. doi: 10.1093/bja/aer159. [DOI] [PubMed] [Google Scholar]
- 11.Bjorn S, Linde F, Nielsen KK, Borglum J, Hauritz RW, Bendtsen TF. Effect of perineural dexamethasone on the duration of single injection saphenous nerve block for analgesia after major ankle surgery: a randomized controlled study. Reg Anesth Pain Med. 2017;42(2):210–216. doi: 10.1097/AAP.0000000000000538. [DOI] [PubMed] [Google Scholar]
- 12.Vorobeichik L, Brull R, Abdallah FW. Evidence basis for using perineural dexmedetomidine to enhance the quality of brachial plexus nerve blocks: a systematic review and meta-analysis of randomized controlled trials. Br J Anaesth. 2017;118(2):167–181. doi: 10.1093/bja/aew411. [DOI] [PubMed] [Google Scholar]
- 13.Albrecht E, Kern C, Kirkham KR. A systematic review and meta-analysis of perineural dexamethasone for peripheral nerve blocks. Anaesthesia. 2015;70(1):71–83. doi: 10.1111/anae.12823. [DOI] [PubMed] [Google Scholar]
- 14.Kirkham KR, Jacot-Guillarmod A, Albrecht E. Optimal dose of perineural dexamethasone to prolong analgesia after brachial plexus blockade: a systematic review and meta-analysis. Anesth Analg. 2018;126(1):270–279. doi: 10.1213/ANE.0000000000002488. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Loveren C, Aartman IH. The PICO (patient-intervention-comparison-outcome) question. Ned Tijdschr Tandheelkd. 2007;114(4):172–178. [PubMed] [Google Scholar]
- 17.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 19.Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta-analyses? Int J Epidemiol. 2009;38(1):276–286. doi: 10.1093/ije/dyn179. [DOI] [PubMed] [Google Scholar]
- 20.Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol. 2008;61(1):64–75. doi: 10.1016/j.jclinepi.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 21.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang P, Liu S, Zhu J, Rao Z, Liu C (2019) Dexamethasone and dexmedetomidine as adjuvants to local anesthetic mixture in intercostal nerve block for thoracoscopic pneumonectomy: a prospective randomized study. Reg Anesth Pain Med. [DOI] [PubMed]
- 23.Yaghoobi S, Shahamat H, Alizadeh A, Khezri MB. Comparing postoperative analgesic effect of dexmedetomidine or dexamethasone added to lidocaine through infraclavicular block in forearm surgery. Clin J Pain. 2019;35(9):766–771. doi: 10.1097/AJP.0000000000000736. [DOI] [PubMed] [Google Scholar]
- 24.Aliste J, Layera S, Bravo D, Fernández D, Jara Á, García A, et al (2019) Randomized comparison between perineural dexamethasone and dexmedetomidine for ultrasound-guided infraclavicular block. Reg Anesth Pain Med. [DOI] [PubMed]
- 25.Kataria S, Mitra S, Saroa R, Jindal S, Gupta R. A Randomized double blinded trial comparing dexmedetomidine with dexamethasone as an adjunct to Ropivacaine in ultrasound guided interscalene block for arthroscopic shoulder surgery. Asian J Anesthesiol. 2019;57(1):10–18. doi: 10.6859/aja.201903_57(1).0003. [DOI] [PubMed] [Google Scholar]
- 26.Gao Z, Xiao Y, Wang Q, Li Y. Comparison of dexmedetomidine and dexamethasone as adjuvant for ropivacaine in ultrasound-guided erector spinae plane block for video-assisted thoracoscopic lobectomy surgery: a randomized, double-blind, placebo-controlled trial. Ann Transl Med. 2019;7(22):668. doi: 10.21037/atm.2019.10.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee MJ, Koo DJ, Choi YS, Lee KC, Kim HY. Dexamethasone or dexmedetomidine as local anesthetic adjuvants for ultrasound-guided axillary brachial plexus blocks with nerve stimulation. Korean J Pain. 2016;29(1):29–33. doi: 10.3344/kjp.2016.29.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albrecht E, Vorobeichik L, Jacot-Guillarmod A, Fournier N, Abdallah FW. Dexamethasone is superior to dexmedetomidine as a perineural adjunct for supraclavicular brachial plexus block: systematic review and indirect meta-analysis. Anesth Analg. 2019;128(3):543–554. doi: 10.1213/ANE.0000000000003860. [DOI] [PubMed] [Google Scholar]
- 29.Woo JH, Kim YJ, Kim DY, Cho S. Dose-dependency of dexamethasone on the analgesic effect of interscalene block for arthroscopic shoulder surgery using ropivacaine 0.5%: a randomised controlled trial. Eur J Anaesthesiol. 2015;32(9):650–655. doi: 10.1097/EJA.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 30.Bravo D, Aliste J, Layera S, Fernández D, Leurcharusmee P, Samerchua A, et al. A multicenter, randomized comparison between 2, 5, and 8 mg of perineural dexamethasone for ultrasound-guided infraclavicular block. Reg Anesth Pain Med. 2019;44(1):46–51. doi: 10.1136/rapm-2018-000032. [DOI] [PubMed] [Google Scholar]
- 31.Fredrickson MJ, Abeysekera A, White R. Randomized study of the effect of local anesthetic volume and concentration on the duration of peripheral nerve blockade. Reg Anesth Pain Med. 2012;37(5):495–501. doi: 10.1097/AAP.0b013e3182580fd0. [DOI] [PubMed] [Google Scholar]
- 32.Tschopp C, Tramèr MR, Schneider A, Zaarour M, Elia N. Benefit and harm of adding epinephrine to a local anesthetic for neuraxial and locoregional anesthesia: a meta-analysis of randomized controlled trials with trial sequential analyses. Anesth Analg. 2018;127(1):228–239. doi: 10.1213/ANE.0000000000003417. [DOI] [PubMed] [Google Scholar]
- 33.Saied NN, Gupta RK, Saffour L, Helwani MA. Dexamethasone and clonidine, but not epinephrine, prolong duration of ropivacaine brachial plexus blocks, cross-sectional analysis in outpatient surgery setting. Pain Med. 2017;18(10):2013–2026. doi: 10.1093/pm/pnw198. [DOI] [PubMed] [Google Scholar]
- 34.Ferré F, Krin A, Sanchez M, Ancelin D, Cavaignac E, Charre A, et al. Perineural dexamethasone attenuates liposomal bupivacaine-induced delayed neural inflammation in mice in vivo. Br J Anaesth. 2020;125(2):175–183. doi: 10.1016/j.bja.2020.04.091. [DOI] [PubMed] [Google Scholar]
- 35.Neal JM, Rathmell JP, Rowlingson JC. Publishing studies that involve “off-label” use of drugs: formalizing Regional Anesthesia and Pain Medicine's policy. Reg Anesth Pain Med. 2009;34(5):391–392. doi: 10.1097/AAP.0b013e3181b87066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.