Abstract
The global obesity epidemic is a major contributor to chronic disease and disability in the world today. Since the discovery of leptin in 1994, a multitude of studies have characterized the pathological changes that occur within adipose tissue in the obese state. One significant change is the dysregulation of adipokine production. Adipokines are an indispensable link between metabolism and optimal immune system function; however, their dysregulation in obesity contributes to chronic low-grade inflammation and disease pathology. Herein, I will highlight current knowledge on adipokine structure and physiological function, and focus on the known roles of these factors in the modulation of the immune response. I will also discuss adipokines in rheumatic and autoimmune diseases.
Keywords: adipokines, autoimmunity, immunity, inflammation, obesity
Introduction
The essential link between the adipose tissue, metabolism, and the immune system is perhaps best demonstrated in Drosophila, in which adipose tissue, the liver, and the immune system are all situated together in the same organ, the fat body. Drosophila responds to different microbes by producing antimicrobial peptides within the fat body [1–3], and the fat body also senses sugar and amino acids in the hemolymph to control the release of insulin-like peptides [4]. When Drosophila is infected with pathogens, they develop hyperglycemia and deplete fat reserves [5]. The metabolic changes that occur concomitantly with infection are evolutionarily conserved; as early as the late 19th century, physicians noted transient hyperglycemia in cases of meningitis [6]. It has been hypothesized that the insulin resistance and hyperglycemia associated with some bacterial infections are beneficial, allowing nutrients to become available for driving the immune response rather than performing non-essential functions [7]. An enormous amount of energy (∼25% of the basal metabolic rate) is needed to maintain the immune system in an activated state to combat a pathogen [8]. Thus, while modern mammals have a delineation of immune and metabolic organs, the two processes remain necessarily linked. However, in pathological conditions of nutrient excess or obesity, the link between metabolism and the immune system may be dysfunctional. It was reported in the 1960s that adipose tissue had infiltration of immune cells such as macrophages and mast cells in animal models of obesity [9,10]. In the past 50 years, the prevalence of obesity worldwide has increased rapidly. At the same time, major advances have been made in our understanding of adipose tissue as an endocrine organ that impacts physiological function, including the immune response. In this review, I will discuss adipokines and their dysregulation in obesity as a link between inflammation, immune system dysfunction, and autoimmune disease.
Adipose tissue and its changes in obesity
Adipose is the primary energy storage site in the body, in the form of neutral triglycerides. It is also an endocrine organ that secretes various cytokines, chemokines, and hormonal factors, or adipokines, that regulate diverse processes including feeding behavior and immunity. To date, more than 600 adipokines have been identified, not including fatty acids and other metabolites [11]. The notion that adipose tissue secreted hormonal factors was suggested in the 1950s by Kennedy who noted that there was a ‘lipostatic’ factor acting in the brain to control food intake in rats [12]. This idea was further strengthened by a series of studies by Coleman at Jackson Laboratories in the 1970s, using two obese animal models: the ob/ob mouse and the db/db mouse. Coleman discovered through parabiosis experiments of an ob/ob mouse with a wild type mouse improved glucose and insulin metabolism and decreased food intake in the ob/ob mouse. Parabiosis of a db/db mouse with a wild type mouse resulted in increased adiposity and body weight. Based on these experiments, Coleman concluded that ob/ob mice do not produce the factor required to regulate body weight and food intake, while db/db mice lack the receptor needed to respond to the factor [13,14]. Twenty years later, this factor was identified as leptin [15].
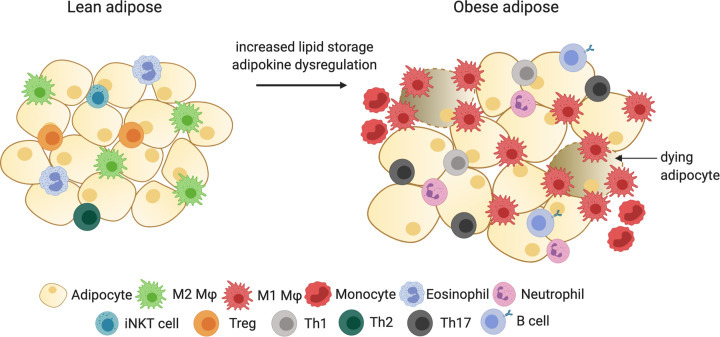
White adipose tissue (WAT) is organized into several depots in the body, including under the skin (subcutaneous), within the abdominal cavity (visceral), and in other small depots within most organs. Up to 10–20% of adipose is visceral in men and 5–8% in women [16]. Multiple physiological differences exist between subcutaneous and visceral WAT; adipocytes from visceral WAT are more insulin resistant, metabolically active, and have greater lipolytic activity [17]. Furthermore, the accumulation of visceral fat is associated with increased risk for the development of type 2 diabetes and metabolic syndrome [18–20]. While WAT is primarily composed of adipocytes, it also contains pre-adipocytes, immune cells, fibroblasts, and vascular cells, which are collectively known as the stromal vascular fraction. The number and phenotype of these cells varies depending on the adipose tissue depot, and is also different between obese and lean individuals [21]. Lean WAT is commonly composed of immune cells that are predominately regulatory and immunosuppressive in nature, including M2-like adipose tissue macrophages (ATMs), regulatory T cells (Tregs), T helper (Th) type 2 cells, iNKT cells, and eosinophils. In lean humans and mice, ATMs are the predominant immune cell present in the WAT and comprise ∼5–15% of total cells within the tissue [22]. The M2 ATMs are uniformly distributed within the adipose and perform diverse physiological functions, including promoting dead adipocyte clearance, inhibiting proliferation of adipocyte progenitors, and secreting anti-inflammatory cytokines such as IL-10, IL-4, IL-13, and IL-1Rα [23,24]. Recent studies revealed that in healthy murine adipose, most ATMs are derived from embryonic yolk-sac precursors and self-renew within the adipose [25,26].
The changes that occur in the WAT as a result of obesity are complex. Increased lipid storage results in adipocyte hypertrophy, hypoxia, and increased cell death. This adipose tissue dysfunction promotes a microenvironment in which the adipocytes begin to secrete proinflammatory cytokines, including TNF-α, IL-6, IL-8, and MCP-1. MCP-1 and other chemokines produced by adipocytes and immune cells promote increased infiltration of circulating monocytes and other innate and adaptive immune cells into the adipose tissue [27–29]. Increased monocyte infiltration [22], self-renewal of tissue-resident ATMs [30,31], and tissue retention of macrophages [32] all contribute to profound increases in the number of macrophages within obese adipose. While macrophages in lean WAT are uniformly distributed, most cluster around apoptotic adipocytes in ‘crown-like structures’ in obese adipose [22,24]. In addition to increased numbers of macrophages, the proinflammatory milieu within obese WAT promotes changes to ATM phenotypes [24]. While initially it was thought that obesity led to an increase in proinflammatory M1 macrophages in the WAT, it was recently discovered that unique ‘metabolically active macrophages’ with a distinct proinflammatory profile are present in obese WAT [33]. In addition, increased numbers of mast cells, dendritic cells (DCs), CD4+ Th1 and Th17 cells, and CD8+ cytotoxic T lymphocytes [34–37] are present in obese WAT. A summary of the immunological changes that occur in adipose tissue with the onset of obesity is discussed in Figure 1.
Figure 1. Immune cell changes in response to obesity in adipose tissue.
Lean adipose is dominated by alternatively activated M2 macrophages, Th2 cells, Tregs, iNKT cells, and eosinophils, while obese adipose has increased influx of monocytes, proinflammatory M1 macrophages, Th1 cells, Th17 cells, neutrophils, and B cells. Created with Biorender.com.
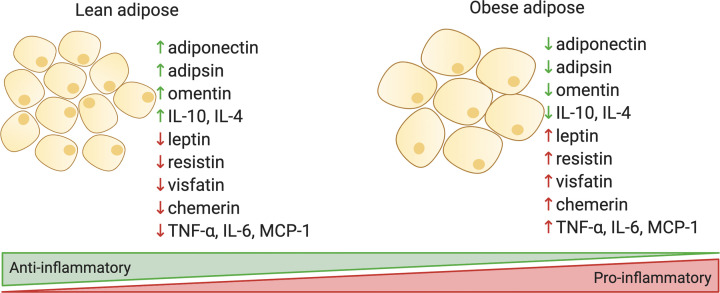
Since it was discovered in the early 1990s that the WAT of obese animals exhibits increased expression of TNF-α [38], studies into the link between obesity and inflammation have increased exponentially. In the next sections, the adipokines that are predominantly produced within WAT and also pro- and anti-inflammatory cytokines that are highly produced in adipose will be discussed. A summary of these adipokines and their changes in obesity are illustrated in Figure 2. Emphasis will be placed on the immunomodulatory effects of the adipokines, including the role of dysregulated adipokines in autoimmune diseases. Clinical data provide evidence that the rates of autoimmunity are increasing, in parallel with the rise in obesity and metabolic syndrome [39]. In support of this concept, diet-induced obesity exacerbates autoimmune disease manifestations in several animal models, including inflammatory bowel disease [40], collagen-induced arthritis [41], experimental autoimmune encephalomyelitis (EAE, a model of multiple sclerosis) [42,43], and systemic lupus erythematosus (SLE) [44]. Thus, the levels of adipokines in autoimmunity and any functional data on their role in autoimmune disease pathogenesis will be explored.
Figure 2. Adipokines in lean and obese states.
Adipokines that are discussed in this review are summarized. Created with Biorender.com.
Leptin
Structure and function
Perhaps the most well-studied adipokine is leptin, which was identified 1994 by Jeffrey Friedman’s group as the product of the obese (ob) gene in mice and the lep gene in humans [15]. Mice that have a loss of function of the ob gene have hyperphagia, weight gain, and insulin resistance, conditions which are improved upon administration of exogenous leptin [45]. Leptin is a 16-kDa nonglycosylated protein that is highly produced by subcutaneous WAT [46–48]. The structure of leptin consists of a bundle of four α-helices that are stabilized by cysteine disulfide bonds, in a structure similar to granulocyte-colony stimulating factor (G-CSF) and IL-6. Leptin signals through the leptin receptor (LEPR in humans, Ob-R in mice), which is a single membrane-spanning receptor that belongs to the class I cytokine receptor family [49]. There are six known isoforms of the leptin receptor: the long form (ObRb), four isoforms with short cytoplasmic tails (ObRa, ObRc, ObRd, and ObRe) and a soluble form (ObRf). ObRb has a full-length cytoplasmic tail containing three conserved tyrosine residues that can mediate downstream signaling [50].
In the brain, leptin acts on cells in specific hypothalamic nuclei that express the long form of the leptin receptor and mediates downstream signaling pathways to control appetite and energy expenditure [51]. While obesity is associated with elevated circulating leptin in proportion to increased fat mass, obese patients are generally resistant to leptin’s effects. Multiple mechanisms likely contribute to the development of leptin resistance in obesity, including negative regulation of ObRb signaling in the hypothalamus [52,53], increased proinflammatory cytokine signaling in the brain [54], cleavage of hypothalamic ObR by increased matrix metalloproteinase (MMP)-2 activity [55], and reduced transport of leptin across the blood–brain barrier [56].
Leptin and immune function
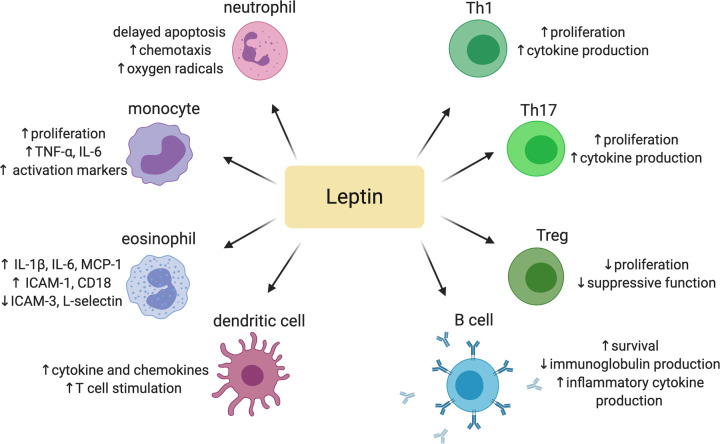
In addition to leptin’s role in controlling energy balance, it also affects many aspects of immune function (Figure 3). During fasting or periods of starvation, leptin levels decrease [57], leading to impairments in cell-mediated immunity, including delayed-type hypersensitivity reactions and T cell mitogen responses [58]. Gainsford et al. determined that the long (ObRb) and short form (ObRa) of the leptin receptor are expressed by immune cells. The same study also showed that the binding of leptin to ObRb stimulated proliferation of clonal immune cell lines [59]. In addition, the effects of leptin on cells of both the innate and adaptive immune systems have been investigated. Monocytes and macrophages express ObRa and ObRb. Leptin increases the proliferation of monocytes and induces the expression of inflammatory cytokines (TNF-α and IL-6) and surface activation markers [60]. Neutrophils only have a short form of the receptor (ObRa) and thus lack some downstream signaling capability [61]. Neutrophils incubated with leptin in vitro have delayed apoptosis, and MAPK signaling downstream of ObRa was responsible for the anti-apoptotic activity [62]. However, the biological significance of this and other studies remain unclear given that Kamp et al. determined that supraphysiological concentrations of leptin were needed to elicit these effects in neutrophils [63]. Leptin also promotes neutrophil chemotaxis. Intraperitoneal administration of leptin in mice results in the migration of neutrophils to the peritoneum, although the mechanism is likely an indirect effect of leptin inducing TNF-α and chemokine production by monocytes and macrophages [64]. In eosinophils, leptin promotes the surface expression of the adhesion molecules ICAM-1 and CD18, but down-regulates surface expression of ICAM-3 and L-selectin. Leptin also induces the production of inflammatory cytokines, including IL-1β, IL-6, and MCP-1 in eosinophils [65]. Both immature and mature DCs express ObRb. When treated in vitro with exogenous leptin, ObRb is up-regulated on the surface, but there is no modulation of surface activation marker expression. Leptin treatment does, however, induce cytokine and chemokine production by DCs. In addition, leptin-treated DCs can more efficiently stimulate heterologous T cells and induce naïve T cells to differentiate into Th1 cells [66].
Figure 3. Immunomodulatory effects of leptin.
Created with Biorender.com.
Both naïve and activated T cells express the leptin receptor, but T cells further up-regulate the expression of the leptin receptor upon stimulation or activation [67]. Howard et al. showed that proliferation of CD4+ T cells in response to allogeneic target cells is enhanced in the presence of leptin. The same study also reported that leptin had a more pronounced proliferative effect on naïve T cells compared to memory T cells [68]. Leptin has a differential effect on Th subsets by promoting Th1 cytokine production and suppressing Th2 cytokine production [69], and it also enhances de novo differentiation of cultured Th17 cells [70]. Conversely, leptin acts as a negative signal for proliferation of thymically derived Tregs. CD4+FoxP3+ Tregs express higher levels of both leptin and ObR as compared with other effector T cells, and neutralization of leptin results in Treg proliferation. Similarly, when wild type mice are treated with a leptin neutralizing antibody, there is an increase in Treg proliferation. Importantly, these cells retain their suppressive phenotype [71]. In an important study linking nutrition, leptin, and T cell function, Saucillo et al. demonstrated that leptin is needed for activated T cells to up-regulate both glucose uptake through the GLUT1 transporter as well as glucose metabolism. The glycolytic metabolism is needed for optimal proliferation and cytokine production [72]. The requirement for leptin is restricted to Th1 and Th17 effector cells [73], while Tregs do not require leptin and instead utilize oxidative metabolism to fuel their suppressive function [74].
Both ob/ob and db/db mice have lower numbers of circulating B cells and fewer bone marrow B cells [75,76]. Specifically, the bone marrow B-cell compartment of ob/ob or fasted mice have lower levels of pro-B, pre-B, and immature B cells and increased levels of mature B cells as compared with wild type mice [76,77]. Interestingly, these bone marrow changes may be the result of central leptin signaling. For example, Tanaka et al. showed that intracerebroventricular (ICV) leptin administration to ob/ob mice results in the normalization of the bone marrow B cell compartment [77]. In vitro studies on B cells revealed that leptin induces the production of TNF-α, IL-6, and IL-10 in freshly isolated B cells from human subjects [78]. This expression of TNF-α by B cells is actually a negative regulator of immunoglobulin production [79]. In a recent study by Frasca et al., the culture of B cells isolated from young lean individuals in the presence of leptin resulted in reduced class switch recombination and vaccine-specific IgG production [80]. The effect of leptin on B cells may be responsible for decreased production of protective antibodies in response to vaccination or infection in individuals with obesity and elevated circulating leptin [81].
Leptin in autoimmunity
Mice deficient in leptin signaling are protected from various models of autoimmune disease including SLE [82], multiple sclerosis (MS) [83], rheumatoid arthritis (RA) [84], and type 1 diabetes [85]. The pro-inflammatory effect of leptin, including promoting Th1 and Th17 responses and decreasing Treg responses, is echoed in the CD4+ T cell subset dysregulation in many autoimmune diseases. When SLE is induced in ob/ob mice using the hydrocarbon oil pristane, the mice do not develop autoantibodies and have elevated Treg as compared with C57BL/6 mice [82]. A spontaneous model of SLE, the NZBWF1 mouse, has elevated levels of leptin as compared with control mice, and leptin levels correlate with increased disease activity. Antagonism of leptin in NZBWF1 mice delays disease progression [82]. The majority of clinical studies have found that leptin levels are increased in SLE patients [86]. Wang et al. showed that leptin levels in patients inversely correlated with Treg frequency, and that patients with higher leptin levels had higher disease activity [87]. When the EAE model of MS is induced in wild type mice, there is a surge of leptin in the circulation that precedes acute onset of symptoms. Pathogenic Th1 cells were found to be a source of leptin in these experiments [83]. Patients with the relapsing-remitting form of MS have been reported as having similar leptin levels as healthy subjects [88], or as having higher leptin levels [89]. However, a recent study did determine that increased leptin was associated with an elevated risk for later MS development in adults younger than 20 [90].
In general, circulating leptin levels are increased in patients with RA [91]; however, there have been some discrepancies in the literature [92,93]. In an animal model, leptin-deficient mice are protected from the development of antigen-induced experimental arthritis. The mice developed less severe inflammation in the synovium of the arthritic knee and had lower serum levels of anti-methylated BSA [84]. In a collagen-induced model of RA, leptin enhanced Th17 proliferation in inflamed joints and exacerbated inflammation [94]. Bokarewa et al. measured leptin in both plasma and synovial fluid and determined that patients with non-erosive joint disease had lower levels of leptin in the synovial fluid as compared with those with an erosive phenotype, suggesting that local leptin consumption in the inflamed joints is protective [95]. Conversely, leptin synergizes with IL-1 to induce nitric oxide synthase type II activity in chondrocytes, promoting inflammation [96]. Many unanswered questions remain regarding the role of leptin in the pathogenesis of RA and the other autoimmune diseases discussed above. For example, the role of leptin signaling on pathogenic autoreactive B and T lymphocytes is unexplored. In addition, in light of the central effects of leptin on immune function, the development of central leptin resistance in obesity in may also play a pathogenic role in autoimmune disease progression.
Adiponectin
Structure and function
Adiponectin was identified by several research groups in the mid 1990s as a 30-kDa protein secreted by adipose tissue [97–100]. Adiponectin is structurally similar to the complement component C1q and is comprised of an N terminal signal sequence, a cysteine-rich variable region needed for multimer formation, a collagen-like domain, and a C-terminal globular domain. Adiponectin forms a trimer via hydrophobic interactions between the globular domains and triple helix formation by the collagen domains. It further oligomerizes via interchain disulfide bonds into multimers consisting of four to six trimers [101,102]. Trimers, medium molecular weight hexamers, and high molecular weight multimers are present in the bloodstream [101,103,104]. Two adiponectin receptors were initially identified (AdipoR1 and AdipoR2) [105], and more recently, a third non-signaling receptor for adiponectin was identified, T-cadherin [106]. The different forms of adiponectin bind to the different receptors: trimers bind to AdipoR1, hexamers bind to AdipoR2, and hexamers and high molecular weight multimers bind to T-cadherin [105,106]. Adiponectin is the most abundant adipokine in the circulation; however, adiponectin levels are inversely correlated to BMI, triglyceride levels, and insulin resistance [107,108]. Adiponectin is an endogenous insulin sensitizer of target organs including the skeletal muscle and liver. Injection of mice with recombinant adiponectin results in decreased blood glucose [109], and it can reverse insulin resistance in mouse models of obesity [110]. Adiponectin decreases insulin resistance by increasing fatty acid oxidation and stimulating glucose utilization via the activation of AMPK [110–112].
Adiponectin and immune function
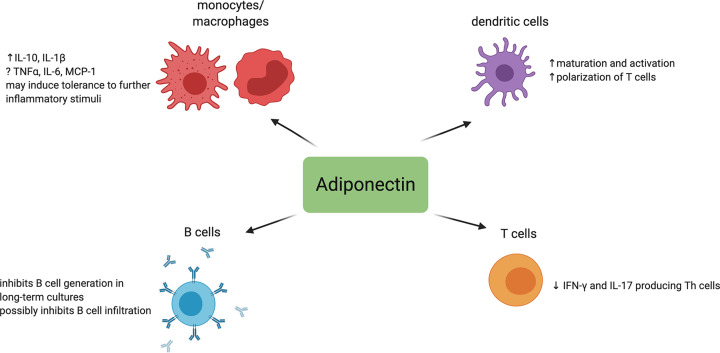
AdipoR1, AdipoR2, and T-cadherin are present on the surface of immune cells, and the differential effects of adiponectin on immune function may arise from which receptor type is stimulated on the cell. This review will focus on some of the more frequently observed effects of adiponectin on immune cells, which are summarized in Figure 4. In monocytes/macrophages, adiponectin suppresses the production of TNF-α and IL-6 [113,114] and induces the production of the anti-inflammatory mediators IL-10 and IL-1 receptor antagonist [114,115]. Mice deficient in adiponectin display increased numbers of classically activated M1 macrophages in their adipose tissue. These M1 macrophages have increased production of the cytokines TNF-α, IL-6, and MCP-1 [116]. In elegant studies by Tsatsanis et al., it was demonstrated that the globular form of adiponectin (only containing the C-terminal globular domain) actually induced TNF-α and IL-6 production in macrophages. However, the cells were then tolerant to further exposure to adiponectin or an additional pro-inflammatory stimulus, thus the net effect of adiponectin was anti-inflammatory [117]. In bone marrow-derived DCs, adiponectin has a more proinflammatory effect by inducing DC maturation and activation, resulting in the polarization of naïve T cells into Th1 and Th17 cells [118]. A contrasting report shows that adiponectin has the opposite effect on DCs and instead up-regulates Tregs [119]. Reasons for the discrepancies between these studies could be differences in the methods used to generate DCs, when the adiponectin is added to the media, and differential expression of adiponectin receptors on the DCs.
Figure 4. Immunomodulatory effects of adiponectin.
Created with Biorender.com.
Several recent studies have highlighted the effect of adiponectin on T cells. When naïve CD4+ T cells are cultured in the presence of adipocytes from high fat diet-fed mice, the frequency of IFN-γ+ and IL-17+ T cells are increased, but they are not when cultured in the presence of adipocytes from mice fed normal chow. Further experiments indicated that adipocyte-derived adiponectin was responsible for the reduction in the IFN-γ and IL-17-producing T lymphocytes in mice fed a normal diet [120]. More recently it was demonstrated in murine cardiac transplant studies that CD4+ T cells are also an important local source of adiponectin in the transplanted heart [121]. Comparatively, less is known about the effect of adiponectin on B cells. Adiponectin inhibits B-cell generation in long-term bone marrow cultures, likely by induction of prostaglandin synthesis [122]. It is possible that the different circulating forms of adiponectin have differential effects on immune cells, yielding either pro- or anti-inflammatory effects, a concept that has not been fully explored. As an example, an excellent review by Fantuzzi highlighted the apparently contradictory effects of adiponectin on NFkB activation in different cell types using different forms of the protein [123]. Another study demonstrated that the low molecular weight form of adiponectin induces IL-10 production, but the hexamer and multimer forms stimulate the production of MCP-1 and IL-8 [124].
Adiponectin in autoimmunity
Unlike conditions of obesity, in which circulating adiponectin levels are lower than healthy controls, adiponectin is elevated in many autoimmune diseases including SLE [125], RA [126], and type 1 diabetes [127], but it is lower in MS [128]. It is likely that the increase in adiponectin in some autoimmune conditions is a response to the chronic elevations in inflammatory cytokines that are present in these diseases. It is also possible that the proinflammatory effects of adiponectin discussed above may play a role in disease pathogenesis. To ascertain the role of adiponectin in SLE, adiponectin-deficient mice were generated in the MRL/lpr model of SLE. The mice had an exacerbated inflammatory phenotype with lymphadenopathy, splenomegaly, and increased autoantibodies compared with MRL/lpr mice that had adiponectin [129]. In RA, adiponectin is up-regulated in the inflamed joints of patients and is positively correlated with IL-6 levels, suggesting that adiponectin may be involved in the pathogenesis of local inflammation in the joints [130]. In a mouse model of collagen-induced arthritis, injection of adiponectin into the joint exacerbated the arthritic phenotype and increased the percentage of Th17 cells [131]. In type 1 diabetes, adipokine receptor expression is decreased in monocytes and other antigen-presenting cells. Reduced adiponectin receptor expression results in increased T-cell proliferation [132]. Because of the aforementioned differential effects of circulating forms of adiponectin, it may also be important to assess circulating ratios of each form in autoimmune diseases to gain a better understanding of adiponectin in autoimmune disease pathogenesis.
Resistin
Structure and function
Resistin was first discovered in obese mice as an adipocyte-derived protein involved in obesity-mediated insulin resistance [133,134]. Resistin is a 12.5-kDa cysteine-rich protein that consists of a signal peptide, a variable region, and a conserved C terminus. The cysteines form disulfide bonds that facilitate its association into a trimer and then interchain disulfide bond formation leads to the formation of a high molecular weight hexamer. In mice, resistin circulates as a low molecular weight trimer and a high molecular weight hexamer. In humans, it exists in the circulation as a trimer and as a high molecular weight oligomer that is 660 kDa [135]. Interestingly, resistin is highly expressed in PBMCs, bone marrow, and macrophages in humans and is only minimally produced by adipocytes, unlike in mice [136,137]. Decorin [138] and ROR1 [139] are putative receptors for murine resistin, while adenylyl cyclase-associated protein 1 (CAP1) is a receptor for human resistin [140]. Resistin was also shown to compete for binding with LPS to TLR4, although direct binding of resistin to TLR4 was not demonstrated [141]. Resistin plays a role in the suppression of insulin-mediated signaling in adipocytes by activating SOCS3 [142]. Ob/ob mice that lack resistin have improved glucose tolerance and insulin sensitivity [143]. Clinical studies indicate circulating levels of resistin only weakly correlate with body fat or BMI [144], and do not correlate with insulin resistance or metabolic syndrome [145,146], which suggests divergent functions of resistin in rodents and humans.
Resistin and immune function
Resistin seems to interact more directly with immune processes in humans as compared with mice [147]. The binding of resistin to CAP1 on human monocytes stimulates NFkB-dependent transcription of inflammatory cytokines in vitro. Another study similarly showed that resistin stimulates IL-12 and TNF-α production in macrophages in a NFkB-dependent manner [148]. Although it has not been formally proven that resistin acts through TLR4 as discussed above, the induction of proinflammatory signals may be via resistin’s binding to TLR4, in addition to CAP1. Proinflammatory cytokines including CRP, TNF-α, IL-1β, IL-6 can induce resistin release by PBMCs and infiltrating monocytes [149,150]. While the majority of studies indicate that resistin functions in a proinflammatory manner, others have shown a different role. Resistin decreases antigen uptake and cytokine production by monocyte-derived human DCs [151], leading to the expansion of Tregs [152]. Currently, little is known about the role of resistin in modulating other immune cells, including B lymphocytes, NK cells, and neutrophils, among others.
Resistin in autoimmunity
Senolt et al. determined that resistin is expressed in the inflamed synovial fluid in RA and osteoarthritis, and that resistin production colocalized with macrophages, B cells, and plasma cells [147]. In RA patients who were given the anti-TNF-α antibody infliximab, there was a rapid decrease in circulating resistin levels, suggesting that resistin may play a pathogenic role in inflammatory processes [153]. Studies in murine arthritis showed that when mouse resistin was injected into the knee joint of normal mice, immune cells infiltrated the synovial tissue and led to arthritis [154]. Resistin in SLE has also been investigated, and serum resistin correlated with markers of inflammation in SLE including proinflammatory cytokines, C reactive protein, and total IgG [153]. A similar study also linked resistin levels with inflammatory markers in SLE and increased SLE disease activity scores, but resistin did not correlate with adiposity or insulin resistance [155]. A clinical study examining adipokines in MS found elevated levels of resistin in MS patients compared with age-matched controls [156].
Adipsin
Structure and function
The serine protease adipsin was identified in adipose tissue in the late 1980s by Spiegelman’s group [157]. In later studies, it was determined that adipsin is actually complement factor D, which plays a role in the alternative pathway of complement activation [158]. The complement system is one of the main mechanisms by which the body recognizes foreign antigens and pathogens, and it also plays an important role in the regulation of the innate and adaptive immune systems [159]. Adipsin is primarily produced in adipose tissue, while other soluble complement components are mostly made in the liver [160]. Other components of the alternative complement pathway, including C3 and Factor B, are also produced within adipose tissue to a lesser extent [161,162]. C3, Factor B, and adipsin are required for the production of C3a. C3a is an anaphylatoxin that promotes inflammation, chemotaxis, vascular permeability, and leukocyte activation [163]. The receptor for C3a, C3aR, is expressed by adipocytes and macrophages in the adipose tissue and is increased in mice administered a high-fat diet. Mice that lack C3aR are protected from high-fat diet induced metabolic dysfunction [164]. C3adesArg, which is a C3a cleavage product produced by exopeptidase activity, regulates triglyceride synthesis and glucose uptake in cultured adipocytes [165,166]. Adipsin also promotes adipocyte differentiation through the C3a/C3aR axis [167]. Adipsin is decreased in models of obesity and diabetes [168]. Adipsin−/− mice, when administered a high-fat diet, gain less weight compared with WT mice and have less adipose tissue inflammation, fewer macrophage crown-like structures, and diminished numbers of mast cells. Despite lower levels of adiposity, however, adipsin−/− mice have impaired glucose tolerance after 16 weeks of high-fat feeding, but no changes in insulin sensitivity. Further interrogation of adipsin−/− mice showed that these mice had insulinopenia due to a defect in pancreatic β-cell function. Furthermore, db/db mice, which have low adipsin, when given recombinant adipsin via an adenoviral vector, have improved fasting blood glucose and an improvement in glucose tolerance, which is mediated by C3a [169]. These studies reveal a complicated role of adipsin in inflammation and glucose homeostasis.
Adipsin in autoimmunity
Complement has been extensively studied in autoimmune disease, and deficiencies in early complement components often result in autoimmune disease [170]. Similar to adiponectin, adipsin levels are elevated in several autoimmune diseases. In the MRL/lpr model of SLE, mice that lack Factor D or adipsin have a decrease in SLE disease activity and lessened renal injury [171]. Adipsin levels are reported to be higher in SLE patients as compared with healthy controls, and were also higher in SLE patients with renal manifestations [172]. In an immune complex-mediated model of RA, synovial adipose tissue was shown to be a site of local adipsin production [173]. Also, in a serum transfer model of inflammatory arthritis, adipsin was found to be central to the pathogenesis of the disease. Fat-free mice display no evidence of arthritis, as evidenced by a lack of paw or ankle swelling, and transfer of adipose tissue into fat-free mice restores the arthritic phenotype. However, recipients of adipsin-deficient, but not leptin or adiponectin-deficient adipose remain resistant to arthritis development [174]. A clinical study in MS patients found a strong association with adipsin levels and neurological disability [175]. It will be important to further analyze the role of adipsin in animal models of autoimmune disease to fully understand whether adipsin is pro- or anti-inflammatory.
Chemerin
Structure and function
Chemerin, also known as tazarotene-induced gene 2 (TIG2) or retinoic acid receptor responder 2 (Rarres2), is a chemoattractant protein that is secreted from adipose tissue, primarily by adipocytes. It is also expressed at high levels in placenta, liver, and lung [176,177] and was initially identified as a retinoic acid responsive gene in the skin [178]. The majority of circulating chemerin is the inactive prochemerin form, which can be cleaved at the C-terminus by extracellular proteases including plasmin, elastase, and cathepsin G to yield various forms that differ in their biological activities and receptor affinities [179]. Chemerin can bind to several receptors, including the G-protein coupled receptor chemokine-like receptor 1 (CMKLR1) [176,177] and G protein-coupled receptor 1 (GPR1), which have a high degree of sequence identity to each other [180]. It can also bind to C-C chemokine receptor-like 2 (CCRL2), but with a lower affinity than it does to CMKLR1 or GPR1 [181]. Further, unlike CMKLR1 and GPR1, binding of chemerin to CCRL2 does not trigger downstream signaling events [181]. It has been suggested that binding to CCRL2 may serve to increase concentrations of chemerin locally so that the protein can bind cells with CMKLR1 on their surface [182]. Chemerin plays a role in adipocyte differentiation [176], and regulates the function of adipocytes by controlling the expression of the glucose transporter GLUT4, the synthesis of triglycerides, and the expression of leptin and adiponectin. The first analysis of chemerin in obese humans indicated that circulating levels positively correlated with BMI and markers of obesity [183,184]. Chemerin also increases in mice administered a high-fat diet in some strains (C57BL/6 and SVB) [185,186], but not in NMRI mice [187]. Other studies show a potentially bimodal response to chemerin; acute ICV administration of chemerin leads to decreased body weight, but chronic infusion increases body weight [188]. Inconsistent phenotypes have been reported for CMKLR1−/− mice. Ernst et al. showed that CMKLR1−/− mice have decreased food intake and body weight on either low- or high-fat diet [189], but several other studies found no effect on body composition [190,191].
Chemerin and immune function
Several immune cell subsets express one or more forms of the chemerin receptor. Myeloid, plasmacytoid, and immature DCs all express CMKLR1 [192,193]. Chemerin induces the migration of blood-derived DCs through an endothelial cell monolayer. Chemerin is also expressed by high endothelial venules, indicating an important role for chemerin in cell trafficking [192]. In addition, chemerin recruits circulating plasmacytoid DCs to visceral WAT by interacting with CMKLR1 on the DCs. The DCs then produce type I interferons, leading to proinflammatory polarization of adipose tissue-resident macrophages [194]. Monocyte-derived macrophages also express CMKLR1, and chemerin similarly promotes macrophage migration [195,196]. Herova et al. determined that CMKLR1 is only expressed on M1 macrophages, but not M2 macrophages, indicating that the receptor is involved in recruitment of M1 macrophages to inflamed tissues [197]. Chemerin is also a potent chemoattractant for NK cells and functions to traffic CD56lowCD16+ NK cells into sites of inflammation [198]. The non-signaling receptor for chemerin, CCRL2, is expressed by macrophages, DCs, mast cells, neutrophils, T cells, and NK cells in humans [199]. This receptor is also engaged in the regulation of immune cell recruitment, to dampen an acute inflammatory response. Mice that lack CCRL2 have an exacerbated acute inflammatory response to peritonitis and have increased neutrophils and monocytes in the peritoneal cavity [200].
Chemerin in autoimmunity
Because of its role in inflammatory cell recruitment, chemerin has been examined in various autoimmune diseases. Chemerin is important in the pathogenesis of psoriasis. In psoriatic lesions, chemerin expressed by fibroblasts, mast cells, and endothelial cells promotes migration of plasmacytoid DCs to the site of inflammation [201]. Circulating chemerin levels are also elevated in psoriasis [202]. Similarly, chemerin is detected in SLE skin lesions, in the endothelium of dermal blood vessels, and in plasmacytoid DCs within the lesions [192]. In addition, local production of chemerin in the kidneys in SLE patients with lupus nephritis leads to the recruitment of CMKLR1+ plasmacytoid DCs to the kidneys [203]. However, one clinical study measured circulating chemerin levels in SLE patients and found no difference compared with healthy control subjects [172]. In RA patients, elevated chemerin is associated with disease activity but not obesity [204,205]. In two mouse models of arthritis, CCRL2−/− mice are protected from disease [206]. In the EAE model of MS, CMKLR1−/− mice had lessened CNS inflammation and disease pathology, suggesting that chemerin-mediated recruitment of immune cells is important for the pathogenesis of experimental MS [207]. Unlike the arthritis study, mice that lack CCRL2 have exacerbated disease in the EAE model [208]. Taken together, these studies indicate that the disease model and the chemerin receptor targeted are important factors to consider when evaluating the role of chemerin in autoimmune disease pathogenesis. Also, many of these studies have examined the local effects of chemerin in damaged tissues, and thus it is unknown whether chemerin from the adipose itself plays a pathogenic role in immune cell recruitment in autoimmune disease.
Cytokines produced by adipose tissue
TNF-α
One of the first cytokines that was found to be produced by adipose tissue was TNF-α. Spiegelman’s group determined that the primary endogenous sources of TNF-α were the spleen and visceral WAT in both db/db mice and lean littermates, but that the expression of TNF-α mRNA was five- to ten-fold higher in the adipose of db/db mice. These results were echoed in other models of obesity and diabetes including the obese Zucker rat and the ob/ob mouse [38]. Studies in humans similarly showed that obese adipose tissue produces more TNF-α [209], and that circulating TNF-α correlates with BMI [210,211]. TNF-α is produced by both adipocytes and ATMs within adipose tissue, but it is likely like that majority of adipose TNF-α is produced by ATMs [22]. TNF-α is a 26-kDa protein that is synthesized as a transmembrane monomer, which can then be cleaved by TNF-α converting enzyme to yield the 17-kDa soluble form. Both forms exist as trimers that have biological activity [212]. TNF-α has two distinct surface receptors, TNFR1 and TNFR2, that are similar in their extracellular ligand-binding domains but differ markedly in their intracellular signaling domains [213]. Most studies indicate that the majority of signaling in adipose is downstream of the TNFR1 [214]. The myriad effects of TNF-α signaling within the adipose tissue have been expertly reviewed previously (please see [215,216]), but some downstream pathways that are activated in adipose include apoptosis, ceramide production, MAPK activation, and NFkB activation.
The initial trigger that leads to TNF-α production in adipose tissue is not completely understood. One likely mechanism is that the increase in adipocyte death that occurs in adipose tissue expansion serves as a chemoattractant signal for circulating monocytes. These infiltrating cells are an important source of TNF-α within the adipose. In addition, excess dietary fatty acids as well as increases in circulating fatty acids from increased lipolysis can affect cytokine production, leading to increases in TNF-α [215,217]. TNF-α actions in the adipose also cause changes in the production of other adipokines, including adiponectin [218], leptin [219], and visfatin [220], and promote the release of other proinflammatory cytokines. It is unclear whether the elevated circulating levels of TNF-α that are present in obesity are a direct result of the increased TNF-α production within the adipose. Nonetheless, it is important to recognize the effect of increased systemic TNF-α on various cells of the innate and adaptive immune systems. Under normal conditions, acute increases in TNF-α result in activation of effector Th cells, which is important in the defense against pathogens. This activation is then dampened due to the effect of TNF-α on Treg. On the other hand, chronic exposure to TNF-α as in obesity may lead to immunosuppression and increased incidence of autoimmune disease.
IL-6
IL-6 is produced by adipocytes, macrophages, skeletal muscle, endothelial cells, and fibroblasts [221]. Structurally, IL-6 is a single glycosylated protein that consists of four α-helices [222]. To mediate its effects, IL-6 binds to the α chain of the IL-6 receptor which then associates with the gp130 membrane glycoprotein for signal transduction [223]. Within WAT, ATMs and adipocytes are the main source of IL-6 [22]. Adipose-derived IL-6 is an important source of circulating IL-6; it has been estimated that approximately one-third of the circulating IL-6 is derived from adipose tissue [224]. Similar to TNF-α, increased IL-6 levels correlate with BMI and waist circumference in clinical data [225]. IL-6, like many other adipokines discussed in this review, has both pro- and anti-inflammatory effects, with a central role in host defense, inflammation, lipid metabolism, and insulin resistance. Indeed, it is a still a matter of debate as to whether IL-6 is helpful or harmful in the context of insulin resistance and obesity. Acute treatment with IL-6 in mice abrogates insulin’s ability to suppress hepatic glucose production, reduces insulin-stimulated glucose uptake in skeletal muscle, and increases circulating triglycerides [226,227]. On the other hand, IL-6−/− mice become obese as they age and develop decreased glucose tolerance, and treatment with IL-6 decreases body weight in IL-6−/− mice, while having no effect on wild type animals [228]. Recently, studies by Han et al. showed that IL-6 produced by adipocytes promotes ATM accumulation, but does not affect glucose or insulin tolerance. On the other hand, IL-6 produced by myeloid cells in the adipose inhibits ATM accumulation, suppresses M1 polarization, and improves glucose tolerance [229].
MCP-1
MCP-1 or CCL2 is a potent chemotactic protein that is mostly made by macrophages and endothelial cells. Human MCP-1 is 13-kDa protein that is a member of the MCP family consisting of at least four members (MCP-1,-2,-3,-4), and it exerts its action by binding to its receptor CCR2 [230]. The CCR2A isoform is expressed by mononuclear cells and vascular smooth muscle cells, while CCR2B is expressed by monocytes and NK cells. MCP-1 was discovered to be secreted by adipose tissue in an exploratory study using 3T3-L1 adipocytes searching for factors that were responsible for insulin resistance. MCP-1 was highly up-regulated in 3T3-L1 cells that were deprived of glucose [231]. Circulating levels of MCP-1 are elevated in obese mice and humans [232]. MCP-1 is important in the recruitment of circulating macrophages to obese adipose tissue [233]. Furthermore, local proliferation of macrophages in adipose is also dependent on MCP-1, as mice with depleted blood monocytes still have MCP-1-dependent proliferation of macrophages in their adipose [30]. Kanda et al. showed that high-fat diet-induced insulin resistance and hepatic steatosis were reduced in mice MCP-1 knockout mice [231]. These results were not echoed in a subsequent study, where it was determined that high-fat diet-fed mice that lacked MCP-1 had no changes in insulin sensitivity or macrophage adipose accumulation [234]. It is likely that in this study other chemokines were significant in monocyte recruitment to adipose tissue.
IL-10
IL-10 is a cytokine that is produced by a wide range of cell types, including Th2 cells, macrophages, and B cells. It can have numerous effects on various cell types, but its principal function is to limit the inflammatory response. IL-10 stimulates the release of soluble TNF-α receptor and IL-1 receptor antagonist, which serves to decrease the activity of inflammatory cytokines [235]. It also inhibits the action of Th1 cells by reducing levels of the cytokines IL-6, IFN-γ, and TNF-α [236]. In the adipose tissue, IL-10, the regulation of its secretion, and its role in WAT metabolism and insulin sensitivity is unclear. In nonobese diabetic mice, IL-10 treatment prevents diabetes onset [237], and IL-10 treatment can prevent lipid-mediated insulin resistance in skeletal muscle. In obese humans and in obese animal models, WAT produces more IL-10 [238]. The elevated levels of IL-10 are likely an attempt to inhibit continued inflammatory cytokine production in the adipose. There are many discrepancies in the literature on circulating IL-10 in obesity. Several studies find that IL-10 is higher in obese subjects [237–239], while others report lower circulating levels [240,241]. Because IL-10 can have pro-inflammatory effects in certain conditions, further studies are needed to fully understand the dynamics of IL-10 production in adipose tissue and obesity.
As a reference, some additional adipokines, their change in obesity, immune function, and levels in autoimmune disease are summarized in Table 1. Overall, data are limited on this diverse group of molecules and any pathogenic role they may have in obesity or autoimmunity. For example, angiopoietin-like protein 2 is increased in obesity and promotes accumulation of immune cells in adipose tissue [242,243], but its levels in autoimmune diseases have not been assessed. Omentin, which is decreased in obesity, is associated with increased levels of IL-4 and IL-13 and promotes Th2-type responses [244]. Omentin is decreased in psoriasis [245], but increased in patients with lupus nephritis [246]. It is unknown whether omentin functions in a pro- or anti-inflammatory manner in autoimmunity.
Table 1. Additional adipokines and their role in immunity and autoimmunity.
| Adipokine | Change in obesity | Immune function | Role in autoimmune disease (if known) |
|---|---|---|---|
| Visfatin/PBEF | Increased | Stimulates early B-cell formation [247] Stimulates inflammatory cytokine production [248] |
Increased in SLE [249] and RA [250] |
| RBP-4 | Increased | Induces antigen-presenting cell activation leading to Th1 polarization in adipose [251] Stimulates inflammatory cytokines in macrophages [252] |
Increased in the urine of SLE patients with lupus nephritis [253] |
| Lipocalin-2/NGAL | Increased | Expressed in neutrophil granules [254] Increases Treg and tolerogenic molecule expression [255] Functions in neutrophil recruitment and adhesion in adipose |
Increased in urine of SLE patients with renal involvement [256] Increased in synovial fluid in RA [257] Promotes EAE [258] |
| Angiopoietin-like protein 2 | Increased | Promotes chemotaxis of monocytes/macrophages [242] Increased macrophage accumulation in adipose and increases in M1 macrophages [243] Increases CD8+ T-cell accumulation in adipose [243] |
Produced in the synovium in RA [259] in circulation in autoimmune disease |
| Progranulin | Increased | Binds to TNF receptors and counteracts TNF proinflammatory signaling [260] Induces Treg [261] Inhibits CXCL9 and CXCL10 release [262] |
Increased in RA [263], psoriasis [264], SLE [265] |
| Omentin | Decreased | Associated with higher levels of IL-13, IL-4, and IL-1β [244] Inhibits NFkB activation [266] |
Decreased in psoriasis [245] Higher in SLE patients with lupus nephritis [246] |
Concluding remarks
The past three decades of research have yielded a tremendous influx of data on adipokines and their role in immune function, and not surprisingly, have sparked many new questions. A common finding is that most of the adipokines have either pro- or anti-inflammatory properties depending on the animal model, in vitro culture conditions, or, in the case of human studies, characteristics of the study group. These discrepancies highlight the importance of furthering our understanding of adipokines as part of a large network in which immune cells respond to a complex milieu of factors. In addition, there are many other adipokines not extensively discussed in this review that affect immune function and warrant further study. Finally, as rates of obesity have skyrocketed, so have the diagnoses of autoimmune diseases. This leads to the question, are the dysregulated adipokines in obesity promoting an environment for the development of autoimmunity, or does autoimmune disease promote an inflammatory environment in the adipose and the later development of obesity?
Acknowledgements
I would like to thank Dr. Michael Ryan for his helpful discussions and critical reading of this review.
Abbreviations
- ATM
adipose tissue macrophage
- BMI
body mass index
- CAP1
adenylyl cyclase-associated protein 1
- CCR2
chemokine receptor type 2
- CCRL2
C–C chemokine receptor-like 2
- CMKLR1
chemokine-like receptor 1
- DC
dendritic cell
- EAE
experimental autoimmune encephalomyelitis
- G-CSF
granulocyte-colony stimulating factor
- GLUT
glucose transporter
- GPR1
G protein-coupled receptor 1
- IFN
interferon
- LPS
lipopolysaccharide
- MCP
monocyte chemoattractant protein
- MMP
matrix metalloproteinase
- MS
multiple sclerosis
- PBMC
peripheral blood mononuclear cell
- RA
rheumatoid arthritis
- Rarres2
retinoic acid receptor responder 2
- SLE
systemic lupus erythematosus
- Th
T helper
- TIG2
tazarotene-induced gene 2
- TLR
toll-like receptor
- Treg
regulatory T cell
- WAT
white adipose tissue
Competing Interests
The author declares that there are no competing interests associated with the manuscript.
References
- 1.Lemaitre B., Kromer-Metzger E., Michaut L., Nicolas E., Meister M., Georgel P.et al. (1995) A recessive mutation, immune deficiency (imd), defines two distinct control pathways in the Drosophila host defense. Proc. Natl. Acad. Sci. U.S.A. 92, 9465–9469 10.1073/pnas.92.21.9465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lemaitre B., Nicolas E., Michaut L., Reichhart J.M. and Hoffmann J.A. (1996) The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86, 973–983 10.1016/S0092-8674(00)80172-5 [DOI] [PubMed] [Google Scholar]
- 3.Lemaitre B., Reichhart J.M. and Hoffmann J.A. (1997) Drosophila host defense: differential induction of antimicrobial peptide genes after infection by various classes of microorganisms. Proc. Natl. Acad. Sci. U.S.A. 94, 14614–14619 10.1073/pnas.94.26.14614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boulan L., Milan M. and Leopold P. (2015) The systemic control of growth. Cold Spring Harb. Perspect. Biol. 7, 10.1101/cshperspect.a019117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee K.A. and Lee W.J. (2018) Immune-metabolic interactions during systemic and enteric infection in Drosophila. Curr. Opin. Insect. Sci. 29, 21–26 10.1016/j.cois.2018.05.014 [DOI] [PubMed] [Google Scholar]
- 6.Fox M.J., Kuzma J.F. and Washam W.T. (1947) Transitory diabetic syndrome associated with meningococcic meningitis. Arch. Intern. Med. 79, 614–621 10.1001/archinte.1947.00220120044003 [DOI] [PubMed] [Google Scholar]
- 7.Chawla A., Nguyen K.D. and Goh Y.P. (2011) Macrophage-mediated inflammation in metabolic disease. Nat. Rev. Immunol. 11, 738–749 10.1038/nri3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Straub R.H., Cutolo M., Buttgereit F. and Pongratz G. (2010) Energy regulation and neuroendocrine-immune control in chronic inflammatory diseases. J. Intern. Med. 267, 543–560 10.1111/j.1365-2796.2010.02218.x [DOI] [PubMed] [Google Scholar]
- 9.Hellman B. (1965) Studies in obese-hyperglycemic mice. Ann. N.Y. Acad. Sci. 131, 541–558 10.1111/j.1749-6632.1965.tb34819.x [DOI] [PubMed] [Google Scholar]
- 10.Hausberger F.X. (1966) Pathological changes in adipose tissue of obese mice. Anat. Rec. 154, 651–660 10.1002/ar.1091540311 [DOI] [PubMed] [Google Scholar]
- 11.Lehr S., Hartwig S. and Sell H. (2012) Adipokines: a treasure trove for the discovery of biomarkers for metabolic disorders. Proteomics Clin. Appl. 6, 91–101 10.1002/prca.201100052 [DOI] [PubMed] [Google Scholar]
- 12.Kennedy G.C. (1953) The role of depot fat in the hypothalamic control of food intake in the rat. Proc. R. Soc. Lond. B Biol. Sci. 140, 578–596 10.1098/rspb.1953.0009 [DOI] [PubMed] [Google Scholar]
- 13.Coleman D.L. (1973) Effects of parabiosis of obese with diabetes and normal mice. Diabetologia 9, 294–298 10.1007/BF01221857 [DOI] [PubMed] [Google Scholar]
- 14.Coleman D.L. and Hummel K.P. (1973) The influence of genetic background on the expression of the obese (Ob) gene in the mouse. Diabetologia 9, 287–293 10.1007/BF01221856 [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y., Proenca R., Maffei M., Barone M., Leopold L. and Friedman J.M. (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 10.1038/372425a0 [DOI] [PubMed] [Google Scholar]
- 16.Wajchenberg B.L. (2000) Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr. Rev. 21, 697–738 10.1210/edrv.21.6.0415 [DOI] [PubMed] [Google Scholar]
- 17.Arner P. (1995) Differences in lipolysis between human subcutaneous and omental adipose tissues. Ann. Med. 27, 435–438 10.3109/07853899709002451 [DOI] [PubMed] [Google Scholar]
- 18.Kissebah A.H., Vydelingum N., Murray R., Evans D.J., Hartz A.J., Kalkhoff R.K.et al. (1982) Relation of body fat distribution to metabolic complications of obesity. J. Clin. Endocrinol. Metab. 54, 254–260 10.1210/jcem-54-2-254 [DOI] [PubMed] [Google Scholar]
- 19.Pouliot M.C., Despres J.P., Nadeau A., Moorjani S., Prud’Homme D., Lupien P.J.et al. (1992) Visceral obesity in men. Associations with glucose tolerance, plasma insulin, and lipoprotein levels. Diabetes 41, 826–834 10.2337/diab.41.7.826 [DOI] [PubMed] [Google Scholar]
- 20.Gastaldelli A., Miyazaki Y., Pettiti M., Matsuda M., Mahankali S., Santini E.et al. (2002) Metabolic effects of visceral fat accumulation in type 2 diabetes. J. Clin. Endocrinol. Metab. 87, 5098–5103 10.1210/jc.2002-020696 [DOI] [PubMed] [Google Scholar]
- 21.Ouchi N., Parker J.L., Lugus J.J. and Walsh K. (2011) Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 11, 85–97 10.1038/nri2921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weisberg S.P., McCann D., Desai M., Rosenbaum M., Leibel R.L. and Ferrante A.W. Jr (2003) Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 10.1172/JCI200319246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nawaz A., Aminuddin A., Kado T., Takikawa A., Yamamoto S., Tsuneyama K.et al. (2017) CD206(+) M2-like macrophages regulate systemic glucose metabolism by inhibiting proliferation of adipocyte progenitors. Nat. Commun. 8, 286 10.1038/s41467-017-00231-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lumeng C.N., Bodzin J.L. and Saltiel A.R. (2007) Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184 10.1172/JCI29881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hassnain Waqas S.F., Noble A., Hoang A.C., Ampem G., Popp M., Strauss S.et al. (2017) Adipose tissue macrophages develop from bone marrow-independent progenitors in Xenopus laevis and mouse. J. Leukoc. Biol. 102, 845–855 10.1189/jlb.1A0317-082RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waqas S.F.H., Hoang A.C., Lin Y.T., Ampem G., Azegrouz H., Balogh L.et al. (2017) Neuropeptide FF increases M2 activation and self-renewal of adipose tissue macrophages. J. Clin. Invest. 127, 3559 10.1172/JCI95841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nara N., Nakayama Y., Okamoto S., Tamura H., Kiyono M., Muraoka M.et al. (2007) Disruption of CXC motif chemokine ligand-14 in mice ameliorates obesity-induced insulin resistance. J. Biol. Chem. 282, 30794–30803 10.1074/jbc.M700412200 [DOI] [PubMed] [Google Scholar]
- 28.Huber J., Kiefer F.W., Zeyda M., Ludvik B., Silberhumer G.R., Prager G.et al. (2008) CC chemokine and CC chemokine receptor profiles in visceral and subcutaneous adipose tissue are altered in human obesity. J. Clin. Endocrinol. Metab. 93, 3215–3221 10.1210/jc.2007-2630 [DOI] [PubMed] [Google Scholar]
- 29.Duffaut C., Zakaroff-Girard A., Bourlier V., Decaunes P., Maumus M., Chiotasso P.et al. (2009) Interplay between human adipocytes and T lymphocytes in obesity: CCL20 as an adipochemokine and T lymphocytes as lipogenic modulators. Arterioscler. Thromb. Vasc. Biol. 29, 1608–1614 10.1161/ATVBAHA.109.192583 [DOI] [PubMed] [Google Scholar]
- 30.Amano S.U., Cohen J.L., Vangala P., Tencerova M., Nicoloro S.M., Yawe J.C.et al. (2014) Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. 19, 162–171 10.1016/j.cmet.2013.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng C., Yang Q., Cao J., Xie N., Liu K., Shou P.et al. (2016) Local proliferation initiates macrophage accumulation in adipose tissue during obesity. Cell Death Dis. 7, e2167 10.1038/cddis.2016.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramkhelawon B., Hennessy E.J., Menager M., Ray T.D., Sheedy F.J., Hutchison S.et al. (2014) Netrin-1 promotes adipose tissue macrophage retention and insulin resistance in obesity. Nat. Med. 20, 377–384 10.1038/nm.3467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kratz M., Coats B.R., Hisert K.B., Hagman D., Mutskov V., Peris E.et al. (2014) Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 20, 614–625 10.1016/j.cmet.2014.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bertola A., Ciucci T., Rousseau D., Bourlier V., Duffaut C., Bonnafous S.et al. (2012) Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients. Diabetes 61, 2238–2247 10.2337/db11-1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stefanovic-Racic M., Yang X., Turner M.S., Mantell B.S., Stolz D.B., Sumpter T.L.et al. (2012) Dendritic cells promote macrophage infiltration and comprise a substantial proportion of obesity-associated increases in CD11c+ cells in adipose tissue and liver. Diabetes 61, 2330–2339 10.2337/db11-1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J., Divoux A., Sun J., Zhang J., Clement K., Glickman J.N.et al. (2009) Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat. Med. 15, 940–945 10.1038/nm.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishimura S., Manabe I., Nagasaki M., Eto K., Yamashita H., Ohsugi M.et al. (2009) CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 15, 914–920 10.1038/nm.1964 [DOI] [PubMed] [Google Scholar]
- 38.Hotamisligil G.S., Shargill N.S. and Spiegelman B.M. (1993) Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259, 87–91 10.1126/science.7678183 [DOI] [PubMed] [Google Scholar]
- 39.Manzel A., Muller D.N., Hafler D.A., Erdman S.E., Linker R.A. and Kleinewietfeld M. (2014) Role of “Western diet” in inflammatory autoimmune diseases. Curr. Allergy Asthma Rep. 14, 404 10.1007/s11882-013-0404-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paik J., Fierce Y., Treuting P.M., Brabb T. and Maggio-Price L. (2013) High-fat diet-induced obesity exacerbates inflammatory bowel disease in genetically susceptible Mdr1a-/- male mice. J. Nutr. 143, 1240–1247 10.3945/jn.113.174615 [DOI] [PubMed] [Google Scholar]
- 41.Jhun J.Y., Yoon B.Y., Park M.K., Oh H.J., Byun J.K., Lee S.Y.et al. (2012) Obesity aggravates the joint inflammation in a collagen-induced arthritis model through deviation to Th17 differentiation. Exp. Mol. Med. 44, 424–431 10.3858/emm.2012.44.7.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Winer S., Paltser G., Chan Y., Tsui H., Engleman E., Winer D.et al. (2009) Obesity predisposes to Th17 bias. Eur. J. Immunol. 39, 2629–2635 10.1002/eji.200838893 [DOI] [PubMed] [Google Scholar]
- 43.Timmermans S., Bogie J.F., Vanmierlo T., Lutjohann D., Stinissen P., Hellings N.et al. (2014) High fat diet exacerbates neuroinflammation in an animal model of multiple sclerosis by activation of the Renin Angiotensin system. J. Neuroimmune Pharmacol. 9, 209–217 10.1007/s11481-013-9502-4 [DOI] [PubMed] [Google Scholar]
- 44.Hanna Kazazian N., Wang Y., Roussel-Queval A., Marcadet L., Chasson L., Laprie C.et al. (2019) Lupus autoimmunity and metabolic parameters are exacerbated upon high fat diet-induced obesity due to TLR7 signaling. Front. Immunol. 10, 2015 10.3389/fimmu.2019.02015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Friedman J.M. and Halaas J.L. (1998) Leptin and the regulation of body weight in mammals. Nature 395, 763–770 10.1038/27376 [DOI] [PubMed] [Google Scholar]
- 46.Senaris R., Garcia-Caballero T., Casabiell X., Gallego R., Castro R., Considine R.V.et al. (1997) Synthesis of leptin in human placenta. Endocrinology 138, 4501–4504 10.1210/endo.138.10.5573 [DOI] [PubMed] [Google Scholar]
- 47.Bado A., Levasseur S., Attoub S., Kermorgant S., Laigneau J.P., Bortoluzzi M.N.et al. (1998) The stomach is a source of leptin. Nature 394, 790–793 10.1038/29547 [DOI] [PubMed] [Google Scholar]
- 48.Wang J., Liu R., Hawkins M., Barzilai N. and Rossetti L. (1998) A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature 393, 684–688 10.1038/31474 [DOI] [PubMed] [Google Scholar]
- 49.Tartaglia L.A., Dembski M., Weng X., Deng N., Culpepper J., Devos R.et al. (1995) Identification and expression cloning of a leptin receptor, OB-R. Cell 83, 1263–1271 10.1016/0092-8674(95)90151-5 [DOI] [PubMed] [Google Scholar]
- 50.Baumann H., Morella K.K., White D.W., Dembski M., Bailon P.S., Kim H.et al. (1996) The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc. Natl. Acad. Sci. U.S.A. 93, 8374–8378 10.1073/pnas.93.16.8374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kwon O., Kim K.W. and Kim M.S. (2016) Leptin signalling pathways in hypothalamic neurons. Cell. Mol. Life Sci. 73, 1457–1477 10.1007/s00018-016-2133-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bjorbaek C., Elmquist J.K., Frantz J.D., Shoelson S.E. and Flier J.S. (1998) Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol. Cell 1, 619–625 10.1016/S1097-2765(00)80062-3 [DOI] [PubMed] [Google Scholar]
- 53.Cheng A., Uetani N., Simoncic P.D., Chaubey V.P., Lee-Loy A., McGlade C.J.et al. (2002) Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Dev. Cell 2, 497–503 10.1016/S1534-5807(02)00149-1 [DOI] [PubMed] [Google Scholar]
- 54.Thaler J.P., Yi C.X., Schur E.A., Guyenet S.J., Hwang B.H., Dietrich M.O.et al. (2012) Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Invest. 122, 153–162 10.1172/JCI59660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mazor R., Friedmann-Morvinski D., Alsaigh T., Kleifeld O., Kistler E.B., Rousso-Noori L.et al. (2018) Cleavage of the leptin receptor by matrix metalloproteinase-2 promotes leptin resistance and obesity in mice. Sci. Transl. Med. 10(455), eaah6324 10.1126/scitranslmed.aah6324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Banks W.A., DiPalma C.R. and Farrell C.L. (1999) Impaired transport of leptin across the blood-brain barrier in obesity. Peptides 20, 1341–1345 10.1016/S0196-9781(99)00139-4 [DOI] [PubMed] [Google Scholar]
- 57.Grinspoon S., Gulick T., Askari H., Landt M., Lee K., Anderson E.et al. (1996) Serum leptin levels in women with anorexia nervosa. J. Clin. Endocrinol. Metab. 81, 3861–3863 [DOI] [PubMed] [Google Scholar]
- 58.Cason J., Ainley C.C., Wolstencroft R.A., Norton K.R. and Thompson R.P. (1986) Cell-mediated immunity in anorexia nervosa. Clin. Exp. Immunol. 64, 370–375 [PMC free article] [PubMed] [Google Scholar]
- 59.Gainsford T., Willson T.A., Metcalf D., Handman E., McFarlane C., Ng A.et al. (1996) Leptin can induce proliferation, differentiation, and functional activation of hemopoietic cells. Proc. Natl. Acad. Sci. U.S.A. 93, 14564–14568 10.1073/pnas.93.25.14564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Santos-Alvarez J., Goberna R. and Sanchez-Margalet V. (1999) Human leptin stimulates proliferation and activation of human circulating monocytes. Cell. Immunol. 194, 6–11 10.1006/cimm.1999.1490 [DOI] [PubMed] [Google Scholar]
- 61.Zarkesh-Esfahani H., Pockley A.G., Wu Z., Hellewell P.G., Weetman A.P. and Ross R.J. (2004) Leptin indirectly activates human neutrophils via induction of TNF-alpha. J. Immunol. 172, 1809–1814 10.4049/jimmunol.172.3.1809 [DOI] [PubMed] [Google Scholar]
- 62.Bruno A., Conus S., Schmid I. and Simon H.U. (2005) Apoptotic pathways are inhibited by leptin receptor activation in neutrophils. J. Immunol. 174, 8090–8096 10.4049/jimmunol.174.12.8090 [DOI] [PubMed] [Google Scholar]
- 63.Kamp V.M., Langereis J.D., van Aalst C.W., van der Linden J.A., Ulfman L.H. and Koenderman L. (2013) Physiological concentrations of leptin do not affect human neutrophils. PLoS ONE 8, e73170 10.1371/journal.pone.0073170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Souza-Almeida G., D’Avila H., Almeida P.E., Luna-Gomes T., Liechocki S., Walzog B.et al. (2018) Leptin mediates in vivo neutrophil migration: involvement of tumor necrosis factor-alpha and CXCL1. Front. Immunol. 9, 111 10.3389/fimmu.2018.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wong C.K., Cheung P.F. and Lam C.W. (2007) Leptin-mediated cytokine release and migration of eosinophils: implications for immunopathophysiology of allergic inflammation. Eur. J. Immunol. 37, 2337–2348 10.1002/eji.200636866 [DOI] [PubMed] [Google Scholar]
- 66.Mattioli B., Straface E., Quaranta M.G., Giordani L. and Viora M. (2005) Leptin promotes differentiation and survival of human dendritic cells and licenses them for Th1 priming. J. Immunol. 174, 6820–6828 10.4049/jimmunol.174.11.6820 [DOI] [PubMed] [Google Scholar]
- 67.Papathanassoglou E., El-Haschimi K., Li X.C., Matarese G., Strom T. and Mantzoros C. (2006) Leptin receptor expression and signaling in lymphocytes: kinetics during lymphocyte activation, role in lymphocyte survival, and response to high fat diet in mice. J. Immunol. 176, 7745–7752 10.4049/jimmunol.176.12.7745 [DOI] [PubMed] [Google Scholar]
- 68.Howard J.K., Lord G.M., Matarese G., Vendetti S., Ghatei M.A., Ritter M.A.et al. (1999) Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J. Clin. Invest. 104, 1051–1059 10.1172/JCI6762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lord G.M., Matarese G., Howard J.K., Baker R.J., Bloom S.R. and Lechler R.I. (1998) Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 394, 897–901 10.1038/29795 [DOI] [PubMed] [Google Scholar]
- 70.Yu Y., Liu Y., Shi F.D., Zou H., Matarese G. and La Cava A. (2013) Cutting edge: Leptin-induced RORgammat expression in CD4+ T cells promotes Th17 responses in systemic lupus erythematosus. J. Immunol. 190, 3054–3058 10.4049/jimmunol.1203275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Rosa V., Procaccini C., Cali G., Pirozzi G., Fontana S., Zappacosta S.et al. (2007) A key role of leptin in the control of regulatory T cell proliferation. Immunity 26, 241–255 10.1016/j.immuni.2007.01.011 [DOI] [PubMed] [Google Scholar]
- 72.Saucillo D.C., Gerriets V.A., Sheng J., Rathmell J.C. and Maciver N.J. (2014) Leptin metabolically licenses T cells for activation to link nutrition and immunity. J. Immunol. 192, 136–144 10.4049/jimmunol.1301158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gerriets V.A., Danzaki K., Kishton R.J., Eisner W., Nichols A.G., Saucillo D.C.et al. (2016) Leptin directly promotes T-cell glycolytic metabolism to drive effector T-cell differentiation in a mouse model of autoimmunity. Eur. J. Immunol. 46, 1970–1983 10.1002/eji.201545861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Michalek R.D., Gerriets V.A., Jacobs S.R., Macintyre A.N., MacIver N.J., Mason E.F.et al. (2011) Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 186, 3299–3303 10.4049/jimmunol.1003613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bennett B.D., Solar G.P., Yuan J.Q., Mathias J., Thomas G.R. and Matthews W. (1996) A role for leptin and its cognate receptor in hematopoiesis. Curr. Biol. 6, 1170–1180 10.1016/S0960-9822(02)70684-2 [DOI] [PubMed] [Google Scholar]
- 76.Claycombe K., King L.E. and Fraker P.J. (2008) A role for leptin in sustaining lymphopoiesis and myelopoiesis. Proc. Natl. Acad. Sci. U.S.A. 105, 2017–2021 10.1073/pnas.0712053105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tanaka M., Suganami T., Kim-Saijo M., Toda C., Tsuiji M., Ochi K.et al. (2011) Role of central leptin signaling in the starvation-induced alteration of B-cell development. J. Neurosci. 31, 8373–8380 10.1523/JNEUROSCI.6562-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Agrawal S., Gollapudi S., Su H. and Gupta S. (2011) Leptin activates human B cells to secrete TNF-alpha, IL-6, and IL-10 via JAK2/STAT3 and p38MAPK/ERK1/2 signaling pathway. J. Clin. Immunol. 31, 472–478 10.1007/s10875-010-9507-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Frasca D., Diaz A., Romero M., Landin A.M. and Blomberg B.B. (2014) High TNF-alpha levels in resting B cells negatively correlate with their response. Exp. Gerontol. 54, 116–122 10.1016/j.exger.2014.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Frasca D., Diaz A., Romero M. and Blomberg B.B. (2020) Leptin induces immunosenescence in human B cells. Cell. Immunol. 348, 103994 10.1016/j.cellimm.2019.103994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Frasca D., Ferracci F., Diaz A., Romero M., Lechner S. and Blomberg B.B. (2016) Obesity decreases B cell responses in young and elderly individuals. Obesity (Silver Spring) 24, 615–625 10.1002/oby.21383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lourenco E.V., Liu A., Matarese G. and La Cava A. (2016) Leptin promotes systemic lupus erythematosus by increasing autoantibody production and inhibiting immune regulation. Proc. Natl. Acad. Sci. U.S.A. 113, 10637–10642 10.1073/pnas.1607101113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sanna V., Di Giacomo A., La Cava A., Lechler R.I., Fontana S., Zappacosta S.et al. (2003) Leptin surge precedes onset of autoimmune encephalomyelitis and correlates with development of pathogenic T cell responses. J. Clin. Invest. 111, 241–250 10.1172/JCI200316721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Busso N., So A., Chobaz-Peclat V., Morard C., Martinez-Soria E., Talabot-Ayer D.et al. (2002) Leptin signaling deficiency impairs humoral and cellular immune responses and attenuates experimental arthritis. J. Immunol. 168, 875–882 10.4049/jimmunol.168.2.875 [DOI] [PubMed] [Google Scholar]
- 85.Matarese G., Sanna V., Lechler R.I., Sarvetnick N., Fontana S., Zappacosta S.et al. (2002) Leptin accelerates autoimmune diabetes in female NOD mice. Diabetes 51, 1356–1361 10.2337/diabetes.51.5.1356 [DOI] [PubMed] [Google Scholar]
- 86.Diaz-Rizo V., Bonilla-Lara D., Gonzalez-Lopez L., Sanchez-Mosco D., Fajardo-Robledo N.S., Perez-Guerrero E.E.et al. (2017) Serum levels of adiponectin and leptin as biomarkers of proteinuria in lupus nephritis. PLoS ONE 12, e0184056 10.1371/journal.pone.0184056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang X., Qiao Y., Yang L., Song S., Han Y., Tian Y.et al. (2017) Leptin levels in patients with systemic lupus erythematosus inversely correlate with regulatory T cell frequency. Lupus 26, 1401–1406 10.1177/0961203317703497 [DOI] [PubMed] [Google Scholar]
- 88.Batocchi A.P., Rotondi M., Caggiula M., Frisullo G., Odoardi F., Nociti V.et al. (2003) Leptin as a marker of multiple sclerosis activity in patients treated with interferon-beta. J. Neuroimmunol. 139, 150–154 10.1016/S0165-5728(03)00154-1 [DOI] [PubMed] [Google Scholar]
- 89.Matarese G., Carrieri P.B., La Cava A., Perna F., Sanna V., De Rosa V.et al. (2005) Leptin increase in multiple sclerosis associates with reduced number of CD4(+)CD25+ regulatory T cells. Proc. Natl. Acad. Sci. U.S.A. 102, 5150–5155 10.1073/pnas.0408995102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bistrom M., Hultdin J., Andersen O., Alonso-Magdalena L., Jons D., Gunnarsson M.et al. (2021) Leptin levels are associated with multiple sclerosis risk. Mult. Scler., 1352458520905033 10.1177/1352458520905033 [DOI] [PubMed] [Google Scholar]
- 91.Targonska-Stepniak B., Majdan M. and Dryglewska M. (2008) Leptin serum levels in rheumatoid arthritis patients: relation to disease duration and activity. Rheumatol. Int. 28, 585–591 10.1007/s00296-007-0480-9 [DOI] [PubMed] [Google Scholar]
- 92.Tian G., Liang J.N., Wang Z.Y. and Zhou D. (2014) Emerging role of leptin in rheumatoid arthritis. Clin. Exp. Immunol. 177, 557–570 10.1111/cei.12372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Anders H.J., Rihl M., Heufelder A., Loch O. and Schattenkirchner M. (1999) Leptin serum levels are not correlated with disease activity in patients with rheumatoid arthritis. Metabolism 48, 745–748 10.1016/S0026-0495(99)90174-9 [DOI] [PubMed] [Google Scholar]
- 94.Deng J., Liu Y., Yang M., Wang S., Zhang M., Wang X.et al. (2012) Leptin exacerbates collagen-induced arthritis via enhancement of Th17 cell response. Arthritis Rheum. 64, 3564–3573 10.1002/art.34637 [DOI] [PubMed] [Google Scholar]
- 95.Bokarewa M., Bokarew D., Hultgren O. and Tarkowski A. (2003) Leptin consumption in the inflamed joints of patients with rheumatoid arthritis. Ann. Rheum. Dis. 62, 952–956 10.1136/ard.62.10.952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Otero M., Lago R., Lago F., Reino J.J. and Gualillo O. (2005) Signalling pathway involved in nitric oxide synthase type II activation in chondrocytes: synergistic effect of leptin with interleukin-1. Arthritis Res. Ther. 7, R581–R591 10.1186/ar1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Scherer P.E., Williams S., Fogliano M., Baldini G. and Lodish H.F. (1995) A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 270, 26746–26749 10.1074/jbc.270.45.26746 [DOI] [PubMed] [Google Scholar]
- 98.Hu E., Liang P. and Spiegelman B.M. (1996) AdipoQ is a novel adipose-specific gene dysregulated in obesity. J. Biol. Chem. 271, 10697–10703 10.1074/jbc.271.18.10697 [DOI] [PubMed] [Google Scholar]
- 99.Maeda K., Okubo K., Shimomura I., Funahashi T., Matsuzawa Y. and Matsubara K. (1996) cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1). Biochem. Biophys. Res. Commun. 221, 286–289 10.1006/bbrc.1996.0587 [DOI] [PubMed] [Google Scholar]
- 100.Nakano Y., Tobe T., Choi-Miura N.H., Mazda T. and Tomita M. (1996) Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J. Biochem. 120, 803–812 10.1093/oxfordjournals.jbchem.a021483 [DOI] [PubMed] [Google Scholar]
- 101.Waki H., Yamauchi T., Kamon J., Ito Y., Uchida S., Kita S.et al. (2003) Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J. Biol. Chem. 278, 40352–40363 10.1074/jbc.M300365200 [DOI] [PubMed] [Google Scholar]
- 102.Tsao T.S., Tomas E., Murrey H.E., Hug C., Lee D.H., Ruderman N.B.et al. (2003) Role of disulfide bonds in Acrp30/adiponectin structure and signaling specificity. Different oligomers activate different signal transduction pathways. J. Biol. Chem. 278, 50810–50817 10.1074/jbc.M309469200 [DOI] [PubMed] [Google Scholar]
- 103.Pajvani U.B., Du X., Combs T.P., Berg A.H., Rajala M.W., Schulthess T.et al. (2003) Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications fpr metabolic regulation and bioactivity. J. Biol. Chem. 278, 9073–9085 10.1074/jbc.M207198200 [DOI] [PubMed] [Google Scholar]
- 104.Tsao T.S., Murrey H.E., Hug C., Lee D.H. and Lodish H.F. (2002) Oligomerization state-dependent activation of NF-kappa B signaling pathway by adipocyte complement-related protein of 30 kDa (Acrp30). J. Biol. Chem. 277, 29359–29362 10.1074/jbc.C200312200 [DOI] [PubMed] [Google Scholar]
- 105.Yamauchi T., Kamon J., Ito Y., Tsuchida A., Yokomizo T., Kita S.et al. (2003) Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423, 762–769 10.1038/nature01705 [DOI] [PubMed] [Google Scholar]
- 106.Hug C., Wang J., Ahmad N.S., Bogan J.S., Tsao T.S. and Lodish H.F. (2004) T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc. Natl. Acad. Sci. U.S.A. 101, 10308–10313 10.1073/pnas.0403382101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Arita Y., Kihara S., Ouchi N., Takahashi M., Maeda K., Miyagawa J.et al. (1999) Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 257, 79–83 10.1006/bbrc.1999.0255 [DOI] [PubMed] [Google Scholar]
- 108.Kadowaki T., Yamauchi T., Kubota N., Hara K., Ueki K. and Tobe K. (2006) Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Invest. 116, 1784–1792 10.1172/JCI29126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Berg A.H., Combs T.P., Du X., Brownlee M. and Scherer P.E. (2001) The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat. Med. 7, 947–953 10.1038/90992 [DOI] [PubMed] [Google Scholar]
- 110.Yamauchi T., Kamon J., Waki H., Terauchi Y., Kubota N., Hara K.et al. (2001) The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 7, 941–946 10.1038/90984 [DOI] [PubMed] [Google Scholar]
- 111.Fruebis J., Tsao T.S., Javorschi S., Ebbets-Reed D., Erickson M.R., Yen F.T.et al. (2001) Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc. Natl. Acad. Sci. U.S.A. 98, 2005–2010 10.1073/pnas.98.4.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yamauchi T., Kamon J., Minokoshi Y., Ito Y., Waki H., Uchida S.et al. (2002) Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 8, 1288–1295 10.1038/nm788 [DOI] [PubMed] [Google Scholar]
- 113.Yokota T., Oritani K., Takahashi I., Ishikawa J., Matsuyama A., Ouchi N.et al. (2000) Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood 96, 1723–1732 10.1182/blood.V96.5.1723 [DOI] [PubMed] [Google Scholar]
- 114.Wulster-Radcliffe M.C., Ajuwon K.M., Wang J., Christian J.A. and Spurlock M.E. (2004) Adiponectin differentially regulates cytokines in porcine macrophages. Biochem. Biophys. Res. Commun. 316, 924–929 10.1016/j.bbrc.2004.02.130 [DOI] [PubMed] [Google Scholar]
- 115.Wolf A.M., Wolf D., Rumpold H., Enrich B. and Tilg H. (2004) Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem. Biophys. Res. Commun. 323, 630–635 10.1016/j.bbrc.2004.08.145 [DOI] [PubMed] [Google Scholar]
- 116.Ohashi K., Parker J.L., Ouchi N., Higuchi A., Vita J.A., Gokce N.et al. (2010) Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J. Biol. Chem. 285, 6153–6160 10.1074/jbc.M109.088708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tsatsanis C., Zacharioudaki V., Androulidaki A., Dermitzaki E., Charalampopoulos I., Minas V.et al. (2005) Adiponectin induces TNF-alpha and IL-6 in macrophages and promotes tolerance to itself and other pro-inflammatory stimuli. Biochem. Biophys. Res. Commun. 335, 1254–1263 10.1016/j.bbrc.2005.07.197 [DOI] [PubMed] [Google Scholar]
- 118.Jung M.Y., Kim H.S., Hong H.J., Youn B.S. and Kim T.S. (2012) Adiponectin induces dendritic cell activation via PLCgamma/JNK/NF-kappaB pathways, leading to Th1 and Th17 polarization. J. Immunol. 188, 2592–2601 10.4049/jimmunol.1102588 [DOI] [PubMed] [Google Scholar]
- 119.Tsang J.Y., Li D., Ho D., Peng J., Xu A., Lamb J.et al. (2011) Novel immunomodulatory effects of adiponectin on dendritic cell functions. Int. Immunopharmacol. 11, 604–609 10.1016/j.intimp.2010.11.009 [DOI] [PubMed] [Google Scholar]
- 120.Surendar J., Frohberger S.J., Karunakaran I., Schmitt V., Stamminger W., Neumann A.L.et al. (2019) Adiponectin limits IFN-gamma and IL-17 producing CD4 T cells in obesity by restraining cell intrinsic glycolysis. Front. Immunol. 10, 2555 10.3389/fimmu.2019.02555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Danturti S., Keslar K.S., Steinhoff L.R., Fan R., Dvorina N., Valujskikh A.et al. (2017) CD4+ T lymphocytes produce adiponectin in response to transplants. JCI Insight 2, 10.1172/jci.insight.89641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yokota T., Meka C.S., Kouro T., Medina K.L., Igarashi H., Takahashi M.et al. (2003) Adiponectin, a fat cell product, influences the earliest lymphocyte precursors in bone marrow cultures by activation of the cyclooxygenase-prostaglandin pathway in stromal cells. J. Immunol. 171, 5091–5099 10.4049/jimmunol.171.10.5091 [DOI] [PubMed] [Google Scholar]
- 123.Fantuzzi G. (2013) Adiponectin in inflammatory and immune-mediated diseases. Cytokine 64, 1–10 10.1016/j.cyto.2013.06.317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Song H., Chan J. and Rovin B.H. (2009) Induction of chemokine expression by adiponectin in vitro is isoform dependent. Transl. Res. 154, 18–26 10.1016/j.trsl.2009.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dini A.A., Wang P. and Ye D.Q. (2017) Serum adiponectin levels in patients with systemic lupus erythematosus: a meta-analysis. J. Clin. Rheumatol. 23, 361–367 10.1097/RHU.0000000000000580 [DOI] [PubMed] [Google Scholar]
- 126.Fatel E.C.S., Rosa F.T., Simao A.N.C. and Dichi I. (2018) Adipokines in rheumatoid arthritis. Adv. Rheumatol. 58, 25 10.1186/s42358-018-0026-8 [DOI] [PubMed] [Google Scholar]
- 127.Imagawa A., Funahashi T., Nakamura T., Moriwaki M., Tanaka S., Nishizawa H.et al. (2002) Elevated serum concentration of adipose-derived factor, adiponectin, in patients with type 1 diabetes. Diabetes Care 25, 1665–1666 10.2337/diacare.25.9.1665 [DOI] [PubMed] [Google Scholar]
- 128.Musabak U., Demirkaya S., Genc G., Ilikci R.S. and Odabasi Z. (2011) Serum adiponectin, TNF-alpha, IL-12p70, and IL-13 levels in multiple sclerosis and the effects of different therapy regimens. NeuroImmunoModulation 18, 57–66 10.1159/000317393 [DOI] [PubMed] [Google Scholar]
- 129.Parker J., Menn-Josephy H., Laskow B., Takemura Y. and Aprahamian T. (2011) Modulation of lupus phenotype by adiponectin deficiency in autoimmune mouse models. J. Clin. Immunol. 31, 167–173 10.1007/s10875-010-9486-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schaffler A., Ehling A., Neumann E., Herfarth H., Tarner I., Scholmerich J.et al. (2003) Adipocytokines in synovial fluid. JAMA 290, 1709–1710 [DOI] [PubMed] [Google Scholar]
- 131.Sun X., Feng X., Tan W., Lin N., Hua M., Wei Y.et al. (2015) Adiponectin exacerbates collagen-induced arthritis via enhancing Th17 response and prompting RANKL expression. Sci. Rep. 5, 11296 10.1038/srep11296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pang T.T., Chimen M., Goble E., Dixon N., Benbow A., Eldershaw S.E.et al. (2013) Inhibition of islet immunoreactivity by adiponectin is attenuated in human type 1 diabetes. J. Clin. Endocrinol. Metab. 98, E418–E428 10.1210/jc.2012-3516 [DOI] [PubMed] [Google Scholar]
- 133.Steppan C.M., Bailey S.T., Bhat S., Brown E.J., Banerjee R.R., Wright C.M.et al. (2001) The hormone resistin links obesity to diabetes. Nature 409, 307–312 10.1038/35053000 [DOI] [PubMed] [Google Scholar]
- 134.Li F.P., He J., Li Z.Z., Luo Z.F., Yan L. and Li Y. (2009) Effects of resistin expression on glucose metabolism and hepatic insulin resistance. Endocrine 35, 243–251 10.1007/s12020-009-9148-4 [DOI] [PubMed] [Google Scholar]
- 135.Gerber M., Boettner A., Seidel B., Lammert A., Bar J., Schuster E.et al. (2005) Serum resistin levels of obese and lean children and adolescents: biochemical analysis and clinical relevance. J. Clin. Endocrinol. Metab. 90, 4503–4509 10.1210/jc.2005-0437 [DOI] [PubMed] [Google Scholar]
- 136.Patel L., Buckels A.C., Kinghorn I.J., Murdock P.R., Holbrook J.D., Plumpton C.et al. (2003) Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem. Biophys. Res. Commun. 300, 472–476 10.1016/S0006-291X(02)02841-3 [DOI] [PubMed] [Google Scholar]
- 137.Codoner-Franch P. and Alonso-Iglesias E. (2015) Resistin: insulin resistance to malignancy. Clin. Chim. Acta 438, 46–54 10.1016/j.cca.2014.07.043 [DOI] [PubMed] [Google Scholar]
- 138.Daquinag A.C., Zhang Y., Amaya-Manzanares F., Simmons P.J. and Kolonin M.G. (2011) An isoform of decorin is a resistin receptor on the surface of adipose progenitor cells. Cell Stem Cell 9, 74–86 10.1016/j.stem.2011.05.017 [DOI] [PubMed] [Google Scholar]
- 139.Sanchez-Solana B., Laborda J. and Baladron V. (2012) Mouse resistin modulates adipogenesis and glucose uptake in 3T3-L1 preadipocytes through the ROR1 receptor. Mol. Endocrinol. 26, 110–127 10.1210/me.2011-1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lee S., Lee H.C., Kwon Y.W., Lee S.E., Cho Y., Kim J.et al. (2014) Adenylyl cyclase-associated protein 1 is a receptor for human resistin and mediates inflammatory actions of human monocytes. Cell Metab. 19, 484–497 10.1016/j.cmet.2014.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tarkowski A., Bjersing J., Shestakov A. and Bokarewa M.I. (2010) Resistin competes with lipopolysaccharide for binding to toll-like receptor 4. J. Cell. Mol. Med. 14, 1419–1431 10.1111/j.1582-4934.2009.00899.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Steppan C.M., Wang J., Whiteman E.L., Birnbaum M.J. and Lazar M.A. (2005) Activation of SOCS-3 by resistin. Mol. Cell. Biol. 25, 1569–1575 10.1128/MCB.25.4.1569-1575.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Qi Y., Nie Z., Lee Y.S., Singhal N.S., Scherer P.E., Lazar M.A.et al. (2006) Loss of resistin improves glucose homeostasis in leptin deficiency. Diabetes 55, 3083–3090 10.2337/db05-0615 [DOI] [PubMed] [Google Scholar]
- 144.Utzschneider K.M., Carr D.B., Tong J., Wallace T.M., Hull R.L., Zraika S.et al. (2005) Resistin is not associated with insulin sensitivity or the metabolic syndrome in humans. Diabetologia 48, 2330–2333 10.1007/s00125-005-1932-y [DOI] [PubMed] [Google Scholar]
- 145.Vozarova de Courten B., Degawa-Yamauchi M., Considine R.V. and Tataranni P.A. (2004) High serum resistin is associated with an increase in adiposity but not a worsening of insulin resistance in Pima Indians. Diabetes 53, 1279–1284 10.2337/diabetes.53.5.1279 [DOI] [PubMed] [Google Scholar]
- 146.Heilbronn L.K., Rood J., Janderova L., Albu J.B., Kelley D.E., Ravussin E.et al. (2004) Relationship between serum resistin concentrations and insulin resistance in nonobese, obese, and obese diabetic subjects. J. Clin. Endocrinol. Metab. 89, 1844–1848 10.1210/jc.2003-031410 [DOI] [PubMed] [Google Scholar]
- 147.Senolt L., Housa D., Vernerova Z., Jirasek T., Svobodova R., Veigl D.et al. (2007) Resistin in rheumatoid arthritis synovial tissue, synovial fluid and serum. Ann. Rheum. Dis. 66, 458–463 10.1136/ard.2006.054734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Silswal N., Singh A.K., Aruna B., Mukhopadhyay S., Ghosh S. and Ehtesham N.Z. (2005) Human resistin stimulates the pro-inflammatory cytokines TNF-alpha and IL-12 in macrophages by NF-kappaB-dependent pathway. Biochem. Biophys. Res. Commun. 334, 1092–1101 10.1016/j.bbrc.2005.06.202 [DOI] [PubMed] [Google Scholar]
- 149.Kaser S., Kaser A., Sandhofer A., Ebenbichler C.F., Tilg H. and Patsch J.R. (2003) Resistin messenger-RNA expression is increased by proinflammatory cytokines in vitro. Biochem. Biophys. Res. Commun. 309, 286–290 10.1016/j.bbrc.2003.07.003 [DOI] [PubMed] [Google Scholar]
- 150.Filkova M., Haluzik M., Gay S. and Senolt L. (2009) The role of resistin as a regulator of inflammation: Implications for various human pathologies. Clin. Immunol. 133, 157–170 10.1016/j.clim.2009.07.013 [DOI] [PubMed] [Google Scholar]
- 151.Son Y.M., Ahn S.M., Jang M.S., Moon Y.S., Kim S.H., Cho K.K.et al. (2008) Immunomodulatory effect of resistin in human dendritic cells stimulated with lipoteichoic acid from Staphylococcus aureus. Biochem. Biophys. Res. Commun. 376, 599–604 10.1016/j.bbrc.2008.09.037 [DOI] [PubMed] [Google Scholar]
- 152.Son Y.M., Ahn S.M., Kim G.R., Moon Y.S., Kim S.H., Park Y.M.et al. (2010) Resistin enhances the expansion of regulatory T cells through modulation of dendritic cells. BMC Immunol. 11, 33 10.1186/1471-2172-11-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gonzalez-Gay M.A., Garcia-Unzueta M.T., Gonzalez-Juanatey C., Miranda-Filloy J.A., Vazquez-Rodriguez T.R., De Matias J.M.et al. (2008) Anti-TNF-alpha therapy modulates resistin in patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 26, 311–316 [PubMed] [Google Scholar]
- 154.Bokarewa M., Nagaev I., Dahlberg L., Smith U. and Tarkowski A. (2005) Resistin, an adipokine with potent proinflammatory properties. J. Immunol. 174, 5789–5795 10.4049/jimmunol.174.9.5789 [DOI] [PubMed] [Google Scholar]
- 155.Baker J.F., Morales M., Qatanani M., Cucchiara A., Nackos E., Lazar M.A.et al. (2011) Resistin levels in lupus and associations with disease-specific measures, insulin resistance, and coronary calcification. J. Rheumatol. 38, 2369–2375 10.3899/jrheum.110237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Emamgholipour S., Eshaghi S.M., Hossein-nezhad A., Mirzaei K., Maghbooli Z. and Sahraian M.A. (2013) Adipocytokine profile, cytokine levels and foxp3 expression in multiple sclerosis: a possible link to susceptibility and clinical course of disease. PLoS ONE 8, e76555 10.1371/journal.pone.0076555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cook K.S., Min H.Y., Johnson D., Chaplinsky R.J., Flier J.S., Hunt C.R.et al. (1987) Adipsin: a circulating serine protease homolog secreted by adipose tissue and sciatic nerve. Science 237, 402–405 10.1126/science.3299705 [DOI] [PubMed] [Google Scholar]
- 158.Rosen B.S., Cook K.S., Yaglom J., Groves D.L., Volanakis J.E., Damm D.et al. (1989) Adipsin and complement factor D activity: an immune-related defect in obesity. Science 244, 1483–1487 10.1126/science.2734615 [DOI] [PubMed] [Google Scholar]
- 159.Ricklin D., Hajishengallis G., Yang K. and Lambris J.D. (2010) Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 11, 785–797 10.1038/ni.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Qin X. and Gao B. (2006) The complement system in liver diseases. Cell Mol. Immunol. 3, 333–340 [PubMed] [Google Scholar]
- 161.Choy L.N., Rosen B.S. and Spiegelman B.M. (1992) Adipsin and an endogenous pathway of complement from adipose cells. J. Biol. Chem. 267, 12736–12741 10.1016/S0021-9258(18)42338-1 [DOI] [PubMed] [Google Scholar]
- 162.Shim K., Begum R., Yang C. and Wang H. (2020) Complement activation in obesity, insulin resistance, and type 2 diabetes mellitus. World J. Diabetes 11, 1–12 10.4239/wjd.v11.i1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Klos A., Tenner A.J., Johswich K.O., Ager R.R., Reis E.S. and Kohl J. (2009) The role of the anaphylatoxins in health and disease. Mol. Immunol. 46, 2753–2766 10.1016/j.molimm.2009.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mamane Y., Chung Chan C., Lavallee G., Morin N., Xu L.J., Huang J.et al. (2009) The C3a anaphylatoxin receptor is a key mediator of insulin resistance and functions by modulating adipose tissue macrophage infiltration and activation. Diabetes 58, 2006–2017 10.2337/db09-0323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Baldo A., Sniderman A.D., St-Luce S., Avramoglu R.K., Maslowska M., Hoang B.et al. (1993) The adipsin-acylation stimulating protein system and regulation of intracellular triglyceride synthesis. J. Clin. Invest. 92, 1543–1547 10.1172/JCI116733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cianflone K., Xia Z. and Chen L.Y. (2003) Critical review of acylation-stimulating protein physiology in humans and rodents. Biochim. Biophys. Acta 1609, 127–143 10.1016/S0005-2736(02)00686-7 [DOI] [PubMed] [Google Scholar]
- 167.Song N.J., Kim S., Jang B.H., Chang S.H., Yun U.J., Park K.M.et al. (2016) Small molecule-induced complement factor D (Adipsin) promotes lipid accumulation and adipocyte differentiation. PLoS ONE 11, e0162228 10.1371/journal.pone.0162228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Flier J.S., Cook K.S., Usher P. and Spiegelman B.M. (1987) Severely impaired adipsin expression in genetic and acquired obesity. Science 237, 405–408 10.1126/science.3299706 [DOI] [PubMed] [Google Scholar]
- 169.Lo J.C., Ljubicic S., Leibiger B., Kern M., Leibiger I.B., Moede T.et al. (2014) Adipsin is an adipokine that improves beta cell function in diabetes. Cell 158, 41–53 10.1016/j.cell.2014.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Macedo A.C. and Isaac L. (2016) Systemic lupus erythematosus and deficiencies of early components of the complement classical pathway. Front. Immunol. 7, 55 10.3389/fimmu.2016.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Elliott M.K., Jarmi T., Ruiz P., Xu Y., Holers V.M. and Gilkeson G.S. (2004) Effects of complement factor D deficiency on the renal disease of MRL/lpr mice. Kidney Int. 65, 129–138 10.1111/j.1523-1755.2004.00371.x [DOI] [PubMed] [Google Scholar]
- 172.Chougule D., Nadkar M., Venkataraman K., Rajadhyaksha A., Hase N., Jamale T.et al. (2018) Adipokine interactions promote the pathogenesis of systemic lupus erythematosus. Cytokine 111, 20–27 10.1016/j.cyto.2018.08.002 [DOI] [PubMed] [Google Scholar]
- 173.Arend W.P., Mehta G., Antonioli A.H., Takahashi M., Takahashi K., Stahl G.L.et al. (2013) Roles of adipocytes and fibroblasts in activation of the alternative pathway of complement in inflammatory arthritis in mice. J. Immunol. 190, 6423–6433 10.4049/jimmunol.1300580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Li Y., Zou W., Brestoff J.R., Rohatgi N., Wu X., Atkinson J.P.et al. (2019) Fat-produced adipsin regulates inflammatory arthritis. Cell Rep. 27, 2809.e3–2816.e3 10.1016/j.celrep.2019.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Natarajan R., Hagman S., Hamalainen M., Leppanen T., Dastidar P., Moilanen E.et al. (2015) Adipsin is associated with multiple sclerosis: a follow-up study of adipokines. Mult. Scler. Int. 2015, 371734 10.1155/2015/371734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Goralski K.B., McCarthy T.C., Hanniman E.A., Zabel B.A., Butcher E.C., Parlee S.D.et al. (2007) Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J. Biol. Chem. 282, 28175–28188 10.1074/jbc.M700793200 [DOI] [PubMed] [Google Scholar]
- 177.Bozaoglu K., Bolton K., McMillan J., Zimmet P., Jowett J., Collier G.et al. (2007) Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology 148, 4687–4694 10.1210/en.2007-0175 [DOI] [PubMed] [Google Scholar]
- 178.Nagpal S., Patel S., Jacobe H., DiSepio D., Ghosn C., Malhotra M.et al. (1997) Tazarotene-induced gene 2 (TIG2), a novel retinoid-responsive gene in skin. J. Invest. Dermatol. 109, 91–95 10.1111/1523-1747.ep12276660 [DOI] [PubMed] [Google Scholar]
- 179.Mattern A., Zellmann T. and Beck-Sickinger A.G. (2014) Processing, signaling, and physiological function of chemerin. IUBMB Life 66, 19–26 10.1002/iub.1242 [DOI] [PubMed] [Google Scholar]
- 180.Rourke J.L., Muruganandan S., Dranse H.J., McMullen N.M. and Sinal C.J. (2014) Gpr1 is an active chemerin receptor influencing glucose homeostasis in obese mice. J. Endocrinol. 222, 201–215 10.1530/JOE-14-0069 [DOI] [PubMed] [Google Scholar]
- 181.De Henau O., Degroot G.N., Imbault V., Robert V., De Poorter C., McHeik S.et al. (2016) Signaling properties of chemerin receptors CMKLR1, GPR1 and CCRL2. PLoS ONE 11, e0164179 10.1371/journal.pone.0164179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zabel B.A., Nakae S., Zuniga L., Kim J.Y., Ohyama T., Alt C.et al. (2008) Mast cell-expressed orphan receptor CCRL2 binds chemerin and is required for optimal induction of IgE-mediated passive cutaneous anaphylaxis. J. Exp. Med. 205, 2207–2220 10.1084/jem.20080300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bozaoglu K., Segal D., Shields K.A., Cummings N., Curran J.E., Comuzzie A.G.et al. (2009) Chemerin is associated with metabolic syndrome phenotypes in a Mexican-American population. J. Clin. Endocrinol. Metab. 94, 3085–3088 10.1210/jc.2008-1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chakaroun R., Raschpichler M., Kloting N., Oberbach A., Flehmig G., Kern M.et al. (2012) Effects of weight loss and exercise on chemerin serum concentrations and adipose tissue expression in human obesity. Metabolism 61, 706–714 10.1016/j.metabol.2011.10.008 [DOI] [PubMed] [Google Scholar]
- 185.Wargent E.T., Zaibi M.S., O’Dowd J.F., Cawthorne M.A., Wang S.J., Arch J.R.et al. (2015) Evidence from studies in rodents and in isolated adipocytes that agonists of the chemerin receptor CMKLR1 may be beneficial in the treatment of type 2 diabetes. PeerJ 3, e753 10.7717/peerj.753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lloyd J.W., Zerfass K.M., Heckstall E.M. and Evans K.A. (2015) Diet-induced increases in chemerin are attenuated by exercise and mediate the effect of diet on insulin and HOMA-IR. Ther. Adv. Endocrinol. Metab. 6, 189–198 10.1177/2042018815589088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hansen I.R., Jansson K.M., Cannon B. and Nedergaard J. (2014) Contrasting effects of cold acclimation versus obesogenic diets on chemerin gene expression in brown and brite adipose tissues. Biochim. Biophys. Acta 1841, 1691–1699 10.1016/j.bbalip.2014.09.003 [DOI] [PubMed] [Google Scholar]
- 188.Helfer G., Ross A.W., Thomson L.M., Mayer C.D., Stoney P.N., McCaffery P.J.et al. (2016) A neuroendocrine role for chemerin in hypothalamic remodelling and photoperiodic control of energy balance. Sci. Rep. 6, 26830 10.1038/srep26830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ernst M.C., Haidl I.D., Zuniga L.A., Dranse H.J., Rourke J.L., Zabel B.A.et al. (2012) Disruption of the chemokine-like receptor-1 (CMKLR1) gene is associated with reduced adiposity and glucose intolerance. Endocrinology 153, 672–682 10.1210/en.2011-1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rouger L., Denis G.R., Luangsay S. and Parmentier M. (2013) ChemR23 knockout mice display mild obesity but no deficit in adipocyte differentiation. J. Endocrinol. 219, 279–289 10.1530/JOE-13-0106 [DOI] [PubMed] [Google Scholar]
- 191.Gruben N., Aparicio Vergara M., Kloosterhuis N.J., van der Molen H., Stoelwinder S., Youssef S.et al. (2014) Chemokine-like receptor 1 deficiency does not affect the development of insulin resistance and nonalcoholic fatty liver disease in mice. PLoS ONE 9, e96345 10.1371/journal.pone.0096345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Vermi W., Riboldi E., Wittamer V., Gentili F., Luini W., Marrelli S.et al. (2005) Role of ChemR23 in directing the migration of myeloid and plasmacytoid dendritic cells to lymphoid organs and inflamed skin. J. Exp. Med. 201, 509–515 10.1084/jem.20041310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Li S., Wang X., Li Y., Kost C.K. Jr and Martin D.S. (2013) Bortezomib, a proteasome inhibitor, attenuates angiotensin II-induced hypertension and aortic remodeling in rats. PLoS ONE 8, e78564 10.1371/journal.pone.0078564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ghosh A.R., Bhattacharya R., Bhattacharya S., Nargis T., Rahaman O., Duttagupta P.et al. (2016) Adipose recruitment and activation of plasmacytoid dendritic cells fuel metaflammation. Diabetes 65, 3440–3452 10.2337/db16-0331 [DOI] [PubMed] [Google Scholar]
- 195.Samson M., Edinger A.L., Stordeur P., Rucker J., Verhasselt V., Sharron M.et al. (1998) ChemR23, a putative chemoattractant receptor, is expressed in monocyte-derived dendritic cells and macrophages and is a coreceptor for SIV and some primary HIV-1 strains. Eur. J. Immunol. 28, 1689–1700 [DOI] [PubMed] [Google Scholar]
- 196.Zhang R.L., Wang Q.Q. and Yang L.J. (2019) Chemerin induced by Treponema pallidum predicted membrane protein Tp0965 mediates the activation of endothelial cell via MAPK signaling pathway. J. Cell. Biochem. 120, 19621–19634 10.1002/jcb.29269 [DOI] [PubMed] [Google Scholar]
- 197.Herova M., Schmid M., Gemperle C. and Hersberger M. (2015) ChemR23, the receptor for chemerin and resolvin E1, is expressed and functional on M1 but not on M2 macrophages. J. Immunol. 194, 2330–2337 10.4049/jimmunol.1402166 [DOI] [PubMed] [Google Scholar]
- 198.Parolini S., Santoro A., Marcenaro E., Luini W., Massardi L., Facchetti F.et al. (2007) The role of chemerin in the colocalization of NK and dendritic cell subsets into inflamed tissues. Blood 109, 3625–3632 10.1182/blood-2006-08-038844 [DOI] [PubMed] [Google Scholar]
- 199.Bondue B., Wittamer V. and Parmentier M. (2011) Chemerin and its receptors in leukocyte trafficking, inflammation and metabolism. Cytokine Growth Factor Rev. 22, 331–338 10.1016/j.cytogfr.2011.11.004 [DOI] [PubMed] [Google Scholar]
- 200.Regan-Komito D., Valaris S., Kapellos T.S., Recio C., Taylor L., Greaves D.R.et al. (2017) Absence of the non-signalling chemerin receptor CCRL2 exacerbates acute inflammatory responses in vivo. Front. Immunol. 8, 1621 10.3389/fimmu.2017.01621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Albanesi C., Scarponi C., Pallotta S., Daniele R., Bosisio D., Madonna S.et al. (2009) Chemerin expression marks early psoriatic skin lesions and correlates with plasmacytoid dendritic cell recruitment. J. Exp. Med. 206, 249–258 10.1084/jem.20080129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nakajima H., Nakajima K., Nagano Y., Yamamoto M., Tarutani M., Takahashi M.et al. (2010) Circulating level of chemerin is upregulated in psoriasis. J. Dermatol. Sci. 60, 45–47 10.1016/j.jdermsci.2010.07.013 [DOI] [PubMed] [Google Scholar]
- 203.De Palma G., Castellano G., Del Prete A., Sozzani S., Fiore N., Loverre A.et al. (2011) The possible role of ChemR23/Chemerin axis in the recruitment of dendritic cells in lupus nephritis. Kidney Int. 79, 1228–1235 10.1038/ki.2011.32 [DOI] [PubMed] [Google Scholar]
- 204.Ha Y.J., Kang E.J., Song J.S., Park Y.B., Lee S.K. and Choi S.T. (2014) Plasma chemerin levels in rheumatoid arthritis are correlated with disease activity rather than obesity. Joint Bone Spine 81, 189–190 10.1016/j.jbspin.2013.07.013 [DOI] [PubMed] [Google Scholar]
- 205.Tolusso B., Gigante M.R., Alivernini S., Petricca L., Fedele A.L., Di Mario C.et al. (2018) Chemerin and PEDF are metaflammation-related biomarkers of disease activity and obesity in rheumatoid arthritis. Front. Med. (Lausanne) 5, 207 10.3389/fmed.2018.00207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Del Prete A., Martinez-Munoz L., Mazzon C., Toffali L., Sozio F., Za L.et al. (2017) The atypical receptor CCRL2 is required for CXCR2-dependent neutrophil recruitment and tissue damage. Blood 130, 1223–1234 10.1182/blood-2017-04-777680 [DOI] [PubMed] [Google Scholar]
- 207.Graham K.L., Zabel B.A., Loghavi S., Zuniga L.A., Ho P.P., Sobel R.A.et al. (2009) Chemokine-like receptor-1 expression by central nervous system-infiltrating leukocytes and involvement in a model of autoimmune demyelinating disease. J. Immunol. 183, 6717–6723 10.4049/jimmunol.0803435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Mazzon C., Zanotti L., Wang L., Del Prete A., Fontana E., Salvi V.et al. (2016) CCRL2 regulates M1/M2 polarization during EAE recovery phase. J. Leukoc. Biol. 99, 1027–1033 10.1189/jlb.3MA0915-444RR [DOI] [PubMed] [Google Scholar]
- 209.Hotamisligil G.S., Arner P., Caro J.F., Atkinson R.L. and Spiegelman B.M. (1995) Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J. Clin. Invest. 95, 2409–2415 10.1172/JCI117936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Olszanecka-Glinianowicz M., Zahorska-Markiewicz B., Janowska J. and Zurakowski A. (2004) Serum concentrations of nitric oxide, tumor necrosis factor (TNF)-alpha and TNF soluble receptors in women with overweight and obesity. Metabolism 53, 1268–1273 10.1016/j.metabol.2004.07.001 [DOI] [PubMed] [Google Scholar]
- 211.Zahorska-Markiewicz B., Janowska J., Olszanecka-Glinianowicz M. and Zurakowski A. (2000) Serum concentrations of TNF-alpha and soluble TNF-alpha receptors in obesity. Int. J. Obes. Relat. Metab. Disord. 24, 1392–1395 10.1038/sj.ijo.0801398 [DOI] [PubMed] [Google Scholar]
- 212.Black R.A., Rauch C.T., Kozlosky C.J., Peschon J.J., Slack J.L., Wolfson M.F.et al. (1997) A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature 385, 729–733 10.1038/385729a0 [DOI] [PubMed] [Google Scholar]
- 213.MacEwan D.J. (2002) TNF receptor subtype signalling: differences and cellular consequences. Cell. Signal. 14, 477–492 10.1016/S0898-6568(01)00262-5 [DOI] [PubMed] [Google Scholar]
- 214.Uysal K.T., Wiesbrock S.M. and Hotamisligil G.S. (1998) Functional analysis of tumor necrosis factor (TNF) receptors in TNF-alpha-mediated insulin resistance in genetic obesity. Endocrinology 139, 4832–4838 10.1210/endo.139.12.6337 [DOI] [PubMed] [Google Scholar]
- 215.Cawthorn W.P. and Sethi J.K. (2008) TNF-alpha and adipocyte biology. FEBS Lett. 582, 117–131 10.1016/j.febslet.2007.11.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sethi J.K. and Hotamisligil G.S. (1999) The role of TNF alpha in adipocyte metabolism. Semin. Cell Dev. Biol. 10, 19–29 10.1006/scdb.1998.0273 [DOI] [PubMed] [Google Scholar]
- 217.Kelley D.S. (2001) Modulation of human immune and inflammatory responses by dietary fatty acids. Nutrition 17, 669–673 10.1016/S0899-9007(01)00576-7 [DOI] [PubMed] [Google Scholar]
- 218.Ruan H., Hacohen N., Golub T.R., Van Parijs L. and Lodish H.F. (2002) Tumor necrosis factor-alpha suppresses adipocyte-specific genes and activates expression of preadipocyte genes in 3T3-L1 adipocytes: nuclear factor-kappaB activation by TNF-alpha is obligatory. Diabetes 51, 1319–1336 10.2337/diabetes.51.5.1319 [DOI] [PubMed] [Google Scholar]
- 219.Kirchgessner T.G., Uysal K.T., Wiesbrock S.M., Marino M.W. and Hotamisligil G.S. (1997) Tumor necrosis factor-alpha contributes to obesity-related hyperleptinemia by regulating leptin release from adipocytes. J. Clin. Invest. 100, 2777–2782 10.1172/JCI119824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hector J., Schwarzloh B., Goehring J., Strate T.G., Hess U.F., Deuretzbacher G.et al. (2007) TNF-alpha alters visfatin and adiponectin levels in human fat. Horm. Metab. Res. 39, 250–255 10.1055/s-2007-973075 [DOI] [PubMed] [Google Scholar]
- 221.Tanaka T., Narazaki M. and Kishimoto T. (2014) IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 6, a016295 10.1101/cshperspect.a016295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kaur S., Bansal Y., Kumar R. and Bansal G. (2020) A panoramic review of IL-6: Structure, pathophysiological roles and inhibitors. Bioorg. Med. Chem. 28, 115327 10.1016/j.bmc.2020.115327 [DOI] [PubMed] [Google Scholar]
- 223.Garbers C., Hermanns H.M., Schaper F., Muller-Newen G., Grotzinger J., Rose-John S.et al. (2012) Plasticity and cross-talk of interleukin 6-type cytokines. Cytokine Growth Factor Rev. 23, 85–97 10.1016/j.cytogfr.2012.04.001 [DOI] [PubMed] [Google Scholar]
- 224.Makki K., Froguel P. and Wolowczuk I. (2013) Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm. 2013, 139239 10.1155/2013/139239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Pou K.M., Massaro J.M., Hoffmann U., Vasan R.S., Maurovich-Horvat P., Larson M.G.et al. (2007) Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation 116, 1234–1241 10.1161/CIRCULATIONAHA.107.710509 [DOI] [PubMed] [Google Scholar]
- 226.Kim H.J., Higashimori T., Park S.Y., Choi H., Dong J., Kim Y.J.et al. (2004) Differential effects of interleukin-6 and -10 on skeletal muscle and liver insulin action in vivo. Diabetes 53, 1060–1067 10.2337/diabetes.53.4.1060 [DOI] [PubMed] [Google Scholar]
- 227.Nonogaki K., Fuller G.M., Fuentes N.L., Moser A.H., Staprans I., Grunfeld C.et al. (1995) Interleukin-6 stimulates hepatic triglyceride secretion in rats. Endocrinology 136, 2143–2149 10.1210/endo.136.5.7720663 [DOI] [PubMed] [Google Scholar]
- 228.Wallenius V., Wallenius K., Ahren B., Rudling M., Carlsten H., Dickson S.L.et al. (2002) Interleukin-6-deficient mice develop mature-onset obesity. Nat. Med. 8, 75–79 10.1038/nm0102-75 [DOI] [PubMed] [Google Scholar]
- 229.Han M.S., White A., Perry R.J., Camporez J.P., Hidalgo J., Shulman G.I.et al. (2020) Regulation of adipose tissue inflammation by interleukin 6. Proc. Natl. Acad. Sci. U.S.A. 117, 2751–2760 10.1073/pnas.1920004117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Deshmane S.L., Kremlev S., Amini S. and Sawaya B.E. (2009) Monocyte chemoattractant protein-1 (MCP-1): an overview. J. Interferon Cytokine Res. 29, 313–326 10.1089/jir.2008.0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kanda H., Tateya S., Tamori Y., Kotani K., Hiasa K., Kitazawa R.et al. (2006) MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Invest. 116, 1494–1505 10.1172/JCI26498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kim C.S., Park H.S., Kawada T., Kim J.H., Lim D., Hubbard N.E.et al. (2006) Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int. J. Obes. (Lond.) 30, 1347–1355 10.1038/sj.ijo.0803259 [DOI] [PubMed] [Google Scholar]
- 233.Bremer A.A., Devaraj S., Afify A. and Jialal I. (2011) Adipose tissue dysregulation in patients with metabolic syndrome. J. Clin. Endocrinol. Metab. 96, E1782–E1788 10.1210/jc.2011-1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Inouye K.E., Shi H., Howard J.K., Daly C.H., Lord G.M., Rollins B.J.et al. (2007) Absence of CC chemokine ligand 2 does not limit obesity-associated infiltration of macrophages into adipose tissue. Diabetes 56, 2242–2250 10.2337/db07-0425 [DOI] [PubMed] [Google Scholar]
- 235.Joyce D.A., Gibbons D.P., Green P., Steer J.H., Feldmann M. and Brennan F.M. (1994) Two inhibitors of pro-inflammatory cytokine release, interleukin-10 and interleukin-4, have contrasting effects on release of soluble p75 tumor necrosis factor receptor by cultured monocytes. Eur. J. Immunol. 24, 2699–2705 10.1002/eji.1830241119 [DOI] [PubMed] [Google Scholar]
- 236.MacKenzie K.F., Pattison M.J. and Arthur J.S. (2014) Transcriptional regulation of IL-10 and its cell-specific role in vivo. Crit. Rev. Immunol. 34, 315–345 10.1615/CritRevImmunol.2014010694 [DOI] [PubMed] [Google Scholar]
- 237.Pennline K.J., Roque-Gaffney E. and Monahan M. (1994) Recombinant human IL-10 prevents the onset of diabetes in the nonobese diabetic mouse. Clin. Immunol. Immunopathol. 71, 169–175 10.1006/clin.1994.1068 [DOI] [PubMed] [Google Scholar]
- 238.Juge-Aubry C.E., Somm E., Pernin A., Alizadeh N., Giusti V., Dayer J.M.et al. (2005) Adipose tissue is a regulated source of interleukin-10. Cytokine 29, 270–274 [DOI] [PubMed] [Google Scholar]
- 239.Manigrasso M.R., Ferroni P., Santilli F., Taraborelli T., Guagnano M.T., Michetti N.et al. (2005) Association between circulating adiponectin and interleukin-10 levels in android obesity: effects of weight loss. J. Clin. Endocrinol. Metab. 90, 5876–5879 10.1210/jc.2005-0281 [DOI] [PubMed] [Google Scholar]
- 240.Esposito K., Pontillo A., Giugliano F., Giugliano G., Marfella R., Nicoletti G.et al. (2003) Association of low interleukin-10 levels with the metabolic syndrome in obese women. J. Clin. Endocrinol. Metab. 88, 1055–1058 10.1210/jc.2002-021437 [DOI] [PubMed] [Google Scholar]
- 241.Bluher M., Fasshauer M., Tonjes A., Kratzsch J., Schon M.R. and Paschke R. (2005) Association of interleukin-6, C-reactive protein, interleukin-10 and adiponectin plasma concentrations with measures of obesity, insulin sensitivity and glucose metabolism. Exp. Clin. Endocrinol. Diabetes 113, 534–537 10.1055/s-2005-872851 [DOI] [PubMed] [Google Scholar]
- 242.Tabata M., Kadomatsu T., Fukuhara S., Miyata K., Ito Y., Endo M.et al. (2009) Angiopoietin-like protein 2 promotes chronic adipose tissue inflammation and obesity-related systemic insulin resistance. Cell Metab. 10, 178–188 10.1016/j.cmet.2009.08.003 [DOI] [PubMed] [Google Scholar]
- 243.Sasaki Y., Ohta M., Desai D., Figueiredo J.L., Whelan M.C., Sugano T.et al. (2015) Angiopoietin like protein 2 (ANGPTL2) promotes adipose tissue macrophage and T lymphocyte accumulation and leads to insulin resistance. PLoS ONE 10, e0131176 10.1371/journal.pone.0131176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Zabetian-Targhi F., Mirzaei K., Keshavarz S.A. and Hossein-Nezhad A. (2016) Modulatory role of Omentin-1 in inflammation: cytokines and dietary intake. J. Am. Coll. Nutr. 35, 670–678 10.1080/07315724.2015.1126207 [DOI] [PubMed] [Google Scholar]
- 245.Ismail S.A. and Mohamed S.A. (2012) Serum levels of visfatin and omentin-1 in patients with psoriasis and their relation to disease severity. Br. J. Dermatol. 167, 436–439 10.1111/j.1365-2133.2012.10980.x [DOI] [PubMed] [Google Scholar]
- 246.Zhang T.P., Li H.M., Leng R.X., Li X.P., Li X.M., Pan H.F.et al. (2016) Plasma levels of adipokines in systemic lupus erythematosus patients. Cytokine 86, 15–20 10.1016/j.cyto.2016.07.008 [DOI] [PubMed] [Google Scholar]
- 247.Samal B., Sun Y., Stearns G., Xie C., Suggs S. and McNiece I. (1994) Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol. Cell. Biol. 14, 1431–1437 10.1128/MCB.14.2.1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Luk T., Malam Z. and Marshall J.C. (2008) Pre-B cell colony-enhancing factor (PBEF)/visfatin: a novel mediator of innate immunity. J. Leukoc. Biol. 83, 804–816 10.1189/jlb.0807581 [DOI] [PubMed] [Google Scholar]
- 249.Chung C.P., Long A.G., Solus J.F., Rho Y.H., Oeser A., Raggi P.et al. (2009) Adipocytokines in systemic lupus erythematosus: relationship to inflammation, insulin resistance and coronary atherosclerosis. Lupus 18, 799–806 10.1177/0961203309103582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Otero M., Lago R., Gomez R., Lago F., Dieguez C., Gomez-Reino J.J.et al. (2006) Changes in plasma levels of fat-derived hormones adiponectin, leptin, resistin and visfatin in patients with rheumatoid arthritis. Ann. Rheum. Dis. 65, 1198–1201 10.1136/ard.2005.046540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Moraes-Vieira P.M., Yore M.M., Dwyer P.M., Syed I., Aryal P. and Kahn B.B. (2014) RBP4 activates antigen-presenting cells, leading to adipose tissue inflammation and systemic insulin resistance. Cell Metab. 19, 512–526 10.1016/j.cmet.2014.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Norseen J., Hosooka T., Hammarstedt A., Yore M.M., Kant S., Aryal P.et al. (2012) Retinol-binding protein 4 inhibits insulin signaling in adipocytes by inducing proinflammatory cytokines in macrophages through a c-Jun N-terminal kinase- and toll-like receptor 4-dependent and retinol-independent mechanism. Mol. Cell. Biol. 32, 2010–2019 10.1128/MCB.06193-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Go D.J., Lee J.Y., Kang M.J., Lee E.Y., Lee E.B., Yi E.C.et al. (2018) Urinary vitamin D-binding protein, a novel biomarker for lupus nephritis, predicts the development of proteinuric flare. Lupus 27, 1600–1615 10.1177/0961203318778774 [DOI] [PubMed] [Google Scholar]
- 254.Kjeldsen L., Bainton D.F., Sengelov H. and Borregaard N. (1994) Identification of neutrophil gelatinase-associated lipocalin as a novel matrix protein of specific granules in human neutrophils. Blood 83, 799–807 10.1182/blood.V83.3.799.799 [DOI] [PubMed] [Google Scholar]
- 255.La Manna G., Ghinatti G., Tazzari P.L., Alviano F., Ricci F., Capelli I.et al. (2014) Neutrophil gelatinase-associated lipocalin increases HLA-G(+)/FoxP3(+) T-regulatory cell population in an in vitro model of PBMC. PLoS ONE 9, e89497 10.1371/journal.pone.0089497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Pitashny M., Schwartz N., Qing X., Hojaili B., Aranow C., Mackay M.et al. (2007) Urinary lipocalin-2 is associated with renal disease activity in human lupus nephritis. Arthritis Rheum. 56, 1894–1903 10.1002/art.22594 [DOI] [PubMed] [Google Scholar]
- 257.Katano M., Okamoto K., Arito M., Kawakami Y., Kurokawa M.S., Suematsu N.et al. (2009) Implication of granulocyte-macrophage colony-stimulating factor induced neutrophil gelatinase-associated lipocalin in pathogenesis of rheumatoid arthritis revealed by proteome analysis. Arthritis Res. Ther. 11, R3 10.1186/ar2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Nam Y., Kim J.H., Seo M., Kim J.H., Jin M., Jeon S.et al. (2014) Lipocalin-2 protein deficiency ameliorates experimental autoimmune encephalomyelitis: the pathogenic role of lipocalin-2 in the central nervous system and peripheral lymphoid tissues. J. Biol. Chem. 289, 16773–16789 10.1074/jbc.M113.542282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Okada T., Tsukano H., Endo M., Tabata M., Miyata K., Kadomatsu T.et al. (2010) Synoviocyte-derived angiopoietin-like protein 2 contributes to synovial chronic inflammation in rheumatoid arthritis. Am. J. Pathol. 176, 2309–2319 10.2353/ajpath.2010.090865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Tang W., Lu Y., Tian Q.Y., Zhang Y., Guo F.J., Liu G.Y.et al. (2011) The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science 332, 478–484 10.1126/science.1199214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Wei F., Zhang Y., Zhao W., Yu X. and Liu C.J. (2014) Progranulin facilitates conversion and function of regulatory T cells under inflammatory conditions. PLoS ONE 9, e112110 10.1371/journal.pone.0112110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Mundra J.J., Jian J., Bhagat P. and Liu C.J. (2016) Progranulin inhibits expression and release of chemokines CXCL9 and CXCL10 in a TNFR1 dependent manner. Sci. Rep. 6, 21115 10.1038/srep21115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Yamamoto Y., Takemura M., Serrero G., Hayashi J., Yue B., Tsuboi A.et al. (2014) Increased serum GP88 (Progranulin) concentrations in rheumatoid arthritis. Inflammation 37, 1806–1813 10.1007/s10753-014-9911-4 [DOI] [PubMed] [Google Scholar]
- 264.Huang K., Chen A., Zhang X., Song Z., Xu H., Cao J.et al. (2015) Progranulin is preferentially expressed in patients with psoriasis vulgaris and protects mice from psoriasis-like skin inflammation. Immunology 145, 279–287 10.1111/imm.12446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Tanaka A., Tsukamoto H., Mitoma H., Kiyohara C., Ueda N., Ayano M.et al. (2012) Serum progranulin levels are elevated in patients with systemic lupus erythematosus, reflecting disease activity. Arthritis Res. Ther. 14, R244 10.1186/ar4087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Tan B.K., Adya R., Farhatullah S., Chen J., Lehnert H. and Randeva H.S. (2010) Metformin treatment may increase omentin-1 levels in women with polycystic ovary syndrome. Diabetes 59, 3023–3031 10.2337/db10-0124 [DOI] [PMC free article] [PubMed] [Google Scholar]