Abstract
Background
Remote ischaemic preconditioning (RIPC) has been shown to have a protective role on vital organs exposed to reperfusion injury. The aim of this systematic review was to evaluate the effects of non-invasive RIPC on clinical and biochemical outcomes in patients undergoing non-cardiac surgery
Methods
A systematic literature search of PubMed, EMBASE, Scopus, and Cochrane databases was carried out in February 2020. RCTs investigating the effect of non-invasive RIPC in adults undergoing non-cardiac surgery were included. Meta-analyses and trial sequential analyses (TSAs) were performed on cardiovascular events, acute kidney injury, and short- and long-term mortality.
Results
Some 43 RCTs including 3660 patients were included. The surgical areas comprised orthopaedic, vascular, abdominal, pulmonary, neurological, and urological surgery. Meta-analysis showed RIPC to be associated with fewer cardiovascular events in non-cardiac surgery (13 trials, 1968 patients, 421 events; odds ratio (OR) 0.68, 95 per cent c.i. 0.47 to 0.96; P = 0.03). Meta-analyses of the effect of RIPC on acute kidney injury (12 trials, 1208 patients, 211 events; OR 1.14, 0.78 to 1.69; P = 0.50; I2 = 9 per cent), short-term mortality (7 trials, 1239 patients, 65 events; OR 0.65, 0.37 to 1.12; P = 0.12; I2 = 0 per cent), and long-term mortality (4 trials, 1167 patients, 9 events; OR 0.67, 0.18 to 2.55; P = 0.56; I2 = 0 per cent) showed no significant differences for RIPC compared with standard perioperative care in non-cardiac surgery. However, TSAs showed that the required information sizes have not yet been reached.
Conclusion
Application of RIPC to non-cardiac surgery might reduce cardiovascular events, but not acute kidney injury or all-cause mortality, but currently available data are inadequate to confirm or reject an assumed intervention effect.
This systematic review including meta-analyses and trial sequential analyses evaluated the orthopaedic effects of non-invasive remote ischaemic preconditioning on clinical and biochemical outcomes in patients undergoing non-cardiac surgery. Only RCTs were included.
potential benefits remain unclear
Resumen
Antecedentes
Se ha demostrado que el acondicionamiento isquémico remoto (remote ischaemic preconditioning, RIPC) tiene un papel protector de los órganos vitales frente a la lesión por reperfusión. El objetivo de esta revisión sistemática fue evaluar los resultados clínicos y bioquímicos del RIPC no invasivo en pacientes sometidos a cirugía no cardíaca (Prospero CRD42019123171).
Métodos
En febrero de 2020 se realizó una búsqueda bibliográfica sistemática en las bases de datos PubMed, EMBASE, Scopus y Cochrane. Se incluyeron los ensayos clínicos aleatorizados y controlados (randomised controlled trials, RCTs) que investigaron el efecto del RIPC no invasivo en adultos sometidos a cirugía no cardíaca. Se realizaron metaanálisis y análisis secuenciales de ensayos (trial sequential analyses, TSA) para los eventos cardiovasculares, la lesión renal aguda y la mortalidad a corto y a largo plazo.
Resultados
Se incluyeron 43 RCTs con 3.660 pacientes sometidos a cirugía ortopédica, vascular, abdominal, pulmonar, neurológica y urológica. El metaanálisis mostró que el RIPC se asociaba con una reducción de los eventos cardiovasculares en la cirugía no cardíaca (13 ensayos; 1.968 pacientes; 421 eventos; razón de oportunidades, odds ratio, OR 0,68; i.c. del 95% (0,47-0,96); P = 0,03)). Los metaanálisis del efecto del RIPC sobre la lesión renal aguda (12 ensayos; 1.208 pacientes; 211 eventos; OR 1,14, i.c. del 95% (0,78-1,69); P = 0,50, I2 = 9%)), la mortalidad a corto plazo (7 ensayos; 1.239 pacientes; 65 eventos; OR 0,65, i.c. del 95% (0,37-1,12); P = 0,12, I2 = 0%)) y la mortalidad a largo plazo (4 ensayos; 1.167 pacientes; 9 eventos; OR 0,67, i.c. del 95% (0,18- 2,55); P = 0,56, I2 = 0%)) no mostraron diferencias significativas al comparar el RIPC con la atención perioperatoria estándar en cirugía no cardíaca. Sin embargo, los TSAs pusieron en evidencia que el volumen necesario de información no se había alcanzado.
Conclusión
La aplicación del RIPC antes de la cirugía no cardíaca podría reducir los eventos cardiovasculares, pero no la lesión renal aguda o la mortalidad por cualquier causa, pero los datos actualmente disponibles son insuficientes para confirmar o rechazar un supuesto efecto una intervención basada en el RIPC.
Introduction
Ischaemic preconditioning involves exposure of tissues or organs to brief episodes of ischaemia and reperfusion in order to initiate a systemic response that protects tissue and organs from reperfusion injury1,2. Remote ischaemic preconditioning (RIPC) most often refers to ischaemic preconditioning where a remote tissue or organ, such as the upper or lower extremity, is exposed to short cycles of ischaemia and reperfusion by repetitive inflation and deflation of a BP cuff1,2.
Recent meta-analyses of the effect of RIPC have either included non-surgical studies3, cardiovascular surgical studies4,5, or all invasive procedures6. Considering that patients undergoing surgery are exposed to a great burden of surgical stress, which increases activity and oxygen demand7, it is likely that both the mechanism and effect of RIPC differ considerably between a surgical and non-surgical setting. Moreover, cardiac surgery interferes with the natural pathophysiological response of the heart and vascular structures, for example when using extracorporeal circulation. This might affect the mechanisms and effects of RIPC in ways that non-cardiac surgery does not8.
The surgical stress response can cause hypercoagulability, endothelial dysfunction, immunological dysfunction, and activation of the sympathetic nervous system9–11. All of these are possible contributors to the pathophysiology of a variety of postoperative complications9–11. Experimental and clinical studies12–14 have suggested that the local tissue damage caused by RIPC leads to activation of systemic anti-inflammatory and antithrombotic mechanisms, and induces a cytoprotective state through activation of humoral mediators and neuronal signal transfer. Therefore, RIPC might have the potential to reduce the surgical stress response and the occurrence of postoperative complications.
The aim of the present study was to conduct a systematic review of RCTs of the effect of RIPC on biomarkers, clinical outcomes, and mortality in adult patients undergoing acute or elective non-cardiac surgery compared with standard preoperative and perioperative care.
Methods
Before initiation of the systematic review and meta-analysis, a written study protocol was registered with Prospero (CRD42019123171). The study is reported in accordance with the PRISMA statement15.
Study eligibility criteria
RCTs with a RIPC protocol were included. Both elective and acute surgery was included, and controls were defined as adult patients (aged at least 18 years of age) undergoing non-cardiac surgery without RIPC (standard preoperative and perioperative care or sham procedure) in hospital. Only original articles in English were included. Trials investigating exclusively intracorporal RIPC and trials including deceased or brain-dead donors were excluded.
Literature search strategy
A systematic literature search was conducted by one investigator under the guidance of an information specialist from the reference library at the University of Copenhagen in the MEDLINE, Embase, Cochrane, and SCOPUS databases. The full electronic search strategy is shown in Appendix S1. The searches were carried out from the inception date of each database to 3 February 2020.
Data collection and extraction
Duplicates were resolved in Mendeley before uploading references to Covidence (Covidence systematic review software; Veritas Health Innovation, Melbourne, Victoria, Australia). Records were screened by title and abstract by two assessors independently. Full reports were obtained for all titles that met the inclusion criteria and in cases of uncertainty. Any disagreement between the two assessors was settled by discussion with a third evaluator. To ensure literature saturation, the reference lists of included studies or relevant reviews identified through the search were examined.
Data assessment
The following data were extracted from the trials: title; design; inclusion and exclusion criteria; number of patients included and distribution into intervention groups, sham or control groups; surgical setting (acute, emergency or elective); surgical and anaesthetic details; sham or control specifications; timing of intervention in relation to anaesthesia and surgery; anatomical site of RIPC application; number of RIPC cycles; duration of ischaemia and reperfusion; inflation pressure of the tourniquet; clinical outcomes including mortality, biomarker outcomes, and duration of follow-up; and limitations. If there was any uncertainty regarding these data, the authors of the study in question were contacted.
The Cochrane risk-of-bias tool16 was used to assess the risk of methodological bias in all included trials. Quantitative analyses were planned to examine the association between RIPC and clinical outcomes where more than three trials had homogeneous clinical outcomes. Statistical analyses were performed with Review Manager version 5.3 (Nordic Cochrane Centre, Copenhagen, Denmark)17. Unadjusted odds ratios (ORs) with 95 per cent confidence intervals were reported for the meta-analyses. Heterogeneity was explored using the I2 statistic; if I2 exceeded 0 per cent, a random-effects model was used. Funnel plots were designed to evaluate the risk of publication bias. Planned sensitivity analyses were performed for each meta-analysis: excluding small trials (fewer than 40 patients in 1 arm); or excluding trials with a high risk of bias. Subgroup analyses for each surgical specialty were also undertaken.
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach was used to assess the quality of the evidence associated with each of the clinical outcomes using GRADEpro software18. The GRADE tool uses factors to upgrade or downgrade the quality assessment of an outcome, and then rates each variable as a very low-, low-, moderate-, or high-quality outcome.
Trial sequential analysis (TSA) was performed on all meta-analysis outcomes in order to adjust for the risk of drawing a conclusion on the basis of random error, type I errors or underestimations (type II errors)19,20. TSA provides a detailed imprecision assessment in the GRADE system as well as the means of calculating the heterogeneity-adjusted required information size (RIS), defined as the required number of participants or events necessary in a meta-analysis to detect or reject an assumed intervention effect20,21. The analysis applied the proportion of patients with each outcome in the standard-care group, the heterogeneity (I2) estimate from each meta-analysis, the assumption of a relative risk reduction of the RIPC intervention effect of 20 per cent22, and the assumptions of an overall type I error of 5 per cent and power of 80 per cent. Each trial was added sequentially in the TSA by publication year, which provided a timewise series of points that formed the basis of the cumulative analysis. TSA was performed using TSA software v0.9.5.10 Beta (Copenhagen Trial Unit, Copenhagen, Denmark)22.
Results
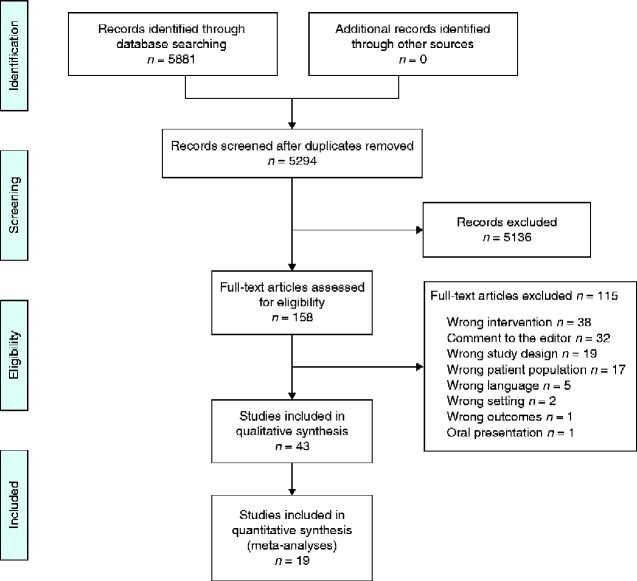
From 5881 citations, a total of 43 RCTs investigating the effect of RIPC on clinical or biomarker outcomes in non-cardiac surgery were included in the review (3660 patients) (Fig. 1). All studies were published in peer-reviewed journals between 2006 and 2020. Study characteristics are listed in Table S1. The overall risk of bias was low (Fig. S1).
Fig. 1.
PRISMA showing selection of articles for review
The surgical areas investigated comprised vascular surgery (13 trials)23–36, orthopaedic surgery (13 trials)37–49, urological surgery (7 trials)50–57, abdominal surgery (7 trials)58–64, pulmonary surgery (2 trials)65,66, and neurosurgery (1 trial)67. Across the trials, there were 104 different outcome measures, of which 58 were solely examined in one study. Outcomes investigated in only one study and as a secondary outcome were not further described in this review.
Cardiovascular events
Trials and outcomes
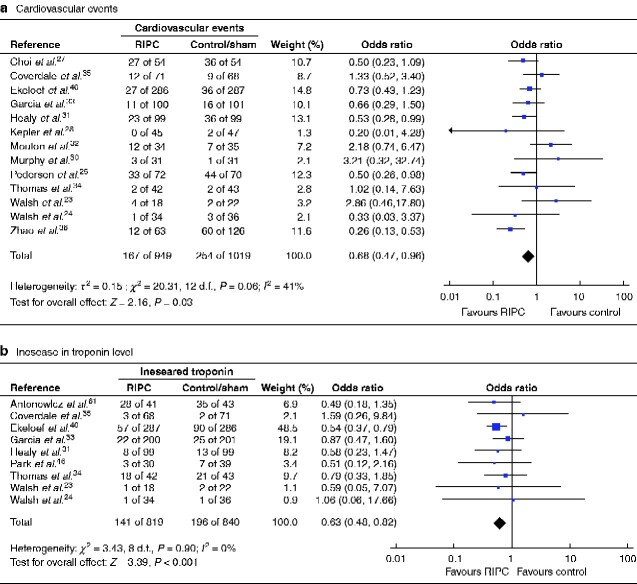
Thirteen trials23–25,27,28,30–36,40 reported on cardiovascular events and were included in the meta-analysis. They reported on cardiovascular death, myocardial infarction, myocardial injury, new arrhythmia, cardiac arrest, cardiac failure, low cardiac output syndrome, ischaemic ECG changes, stroke, hypoperfusion syndrome, transient ischaemic attack and newly ischaemic brain lesions on MRI. One trial included emergent orthopaedic surgery on patients with known cardiovascular risk factors and twelve trials included patients undergoing vascular surgery.
RIPC was associated with reduced cardiovascular events in non-cardiac surgery (13 trials, 1968 patients, 421 events; OR 0.68, 95 per cent c.i. 0.47 to 0.96; P = 0.03) (Fig. 2a). Heterogeneity between trials was considerable (I2 = 41 per cent) and the funnel plot was asymmetrical, showing risk of publication bias (missing small negative trials) (Fig. S2a). The risk-of-bias assessment showed little evidence of bias within any of the trials. Sensitivity analysis excluding small trials reduced the heterogeneity (I2 = 18 per cent) and the analysis still favoured application of RIPC compared with control (9 trials, 1727 patients, 388 events; OR 0.56, 0.42 to 0.76; P < 0.001). Sensitivity analysis excluding the trial in emergency hip surgery in which the patients had known cardiovascular risk factors, leaving only trials in vascular surgery, changed the result, showing no effect of RIPC (12 trials, 1395 patients, 358 events; OR 0.68, 0.45 to 1.03; P = 0.07). According to the GRADE assessment, the quality of evidence was low (Table 1) and TSA showed a RIS of 3150 participants (Fig. 3).
Fig. 2.
Forest plot of RCTs comparing rates of cardiovascular events and increased postoperative troponin level in remote ischaemic preconditioning and control/sham groups
a Cardiovascular events and b increased postoperative troponin level. Inverse-variance random-effects (a) and inverse-variance fixed-effect (b) models were used for meta-analysis. Odds ratios are shown with 95 per cent confidence intervals.
Table 1.
Assessment of the quality of evidence of RCTs included in the meta-analyses, using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach
| Studies | Certainty assessment |
Proportion with event
*
|
Effect
†
|
Certainty | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Risk of bias | Inconsistency | Indirectness | Imprecision | Other | RIPC | Control/sham | Relative | Absolute | ||
| Cardiovascular events | ||||||||||
| 13 RCTs | Not serious | Serious‡ | Not serious | Not serious | Publication bias strongly suspected§ | 167 of 949 (17.6) | 254 of 1019 (24.9) | OR 0.68 (0.47, 0.96) | 65 fewer (from 114 to 8 fewer) per 1000 | Low |
| Acute kidney injury | ||||||||||
| 12 RCTs | Not serious | Not serious¶ | Not serious | Not serious | None | 110 of 603 (18.2) | 101 of 605 (16.7) | OR 1.14 (0.78, 1.69) | 19 more (from 32 fewer to 86 more) per 1000 | High |
| Mortality ≤ 90 days | ||||||||||
| 7 RCTs | Not serious | Not serious | Not serious | Very serious# | None | 27 of 619 (4.4) | 38 of 620 (6.1) | OR 0.65 (0.37, 1.12) | 21 fewer (from 38 fewer to 7 more) per 1000 | Low |
| Mortality > 90 days | ||||||||||
| 4 RCTs | Not serious | Not serious | Not serious | Very serious# | None | 3 of 585 (0.5) | 6 of 582 (1.0) | OR 0.67 (0.18, 2.55) | 3 fewer (from 8 fewer to 16 more) per 1000 | Low |
Values in parentheses are
percentages and
95 per cent confidence intervals.
I 2 = 41 per cent.
Funnel plot asymmetrical.
I 2 = 9 per cent.
Small number of events, wide confidence interval. RIPC, remote ischaemic preconditioning; OR, odds ratio.
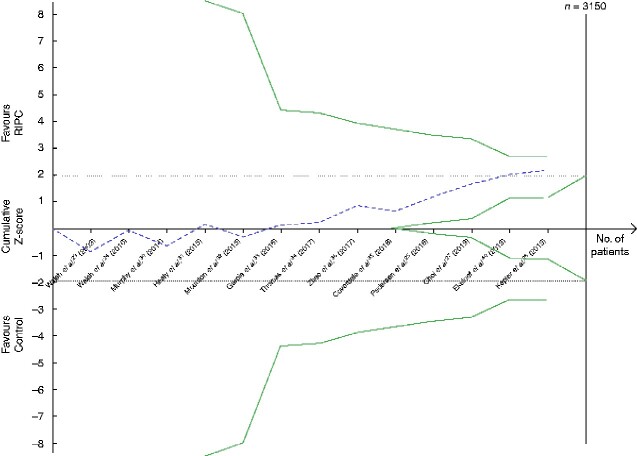
Fig. 3.
Trial sequential analysis of the effect of remote ischaemic preconditioning on cardiovascular events
Relative risk reduction of cardiovascular events is 20 per cent, acceptable risk of type I error is 5 per cent, and power is 20 per cent on a two-sided graph. The Z-curve did not reach the diversity-adjusted required information size for a 20 per cent relative risk reduction of a cardiovascular event, of 3150 patients. Neither did the Z-curve cross the monitoring efficacy boundary (upper curve) or the futility boundary (lower curve). RIPC, remote ischaemic preconditioning.
The black dotted lines are the conventional efficacy boundaries (nominal statistical significance). The uppermost green line and the lowermost green line are both the monitoring efficacy boundaries on a two-sided test. The two shorter green line (within the dotted lines representing the conventional efficacy boundaries) are the futility boundaries on a two-sided test.
Biochemical markers
Twelve trials 23–25,30–35,40,46,61 measured troponin levels (TnI or TnT) as an individual outcome. Nine of these quantified troponin measurements in comparable units and were included in a meta-analysis This showed that RIPC was associated with a reduced occurrence of increased troponin level (9 trials, 1659 patients, 337 events; OR 0.63, 95 per cent c.i. 0.48 to 0.82; P < 0.001) (Fig. 2b). Trials were homogeneous (I2 = 0 per cent) but the funnel plot were asymmetrical (Fig. S2b), indicating publication bias with small trials (both positive and negative) lacking. Sensitivity analyses excluding small trials or trials with a high risk of bias did not change the results, but subgroup analysis of patients undergoing vascular surgery showed no benefit of RIPC (6 trials, 933 patients, 117 events; OR 0.80, 0.53 to 1.22; P = 0.30).
Acute kidney injury
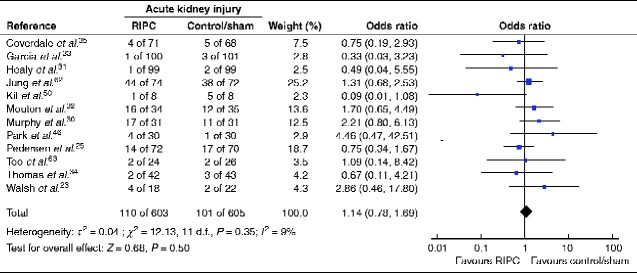
Twelve trials reported on acute kidney injury either in accordance with Acute Kidney Injury Network (AKIN) criteria30,32,33,46,63, using measurements similar to AKIN criteria23,25,31,35,50,62, or the Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease (RIFLE) criteria34. Eight trials included patients undergoing major vascular surgery, one46 was a trial in orthopaedic surgery, one50 included patients having open partial nephrectomy, one62 involved liver transplantation, and one63 included patients undergoing liver resection.
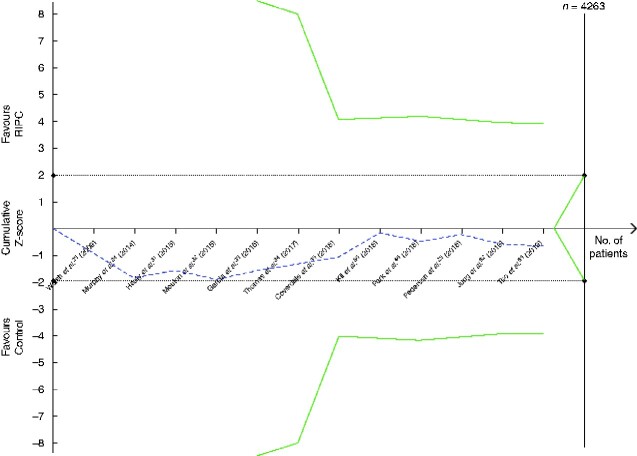
RIPC did not reduce the occurrence of acute kidney injury (12 trials, 1208 patients, 211 events; OR 1.14, 95 per cent c.i. 0.78 to 1.69; P = 0.50) (Fig. 4). Heterogeneity between trials was acceptable (I2 = 9 per cent) and the funnel plot was nearly symmetrical (Fig. S3). Sensitivity analysis showed that exclusion of one small trial50 with only 16 patients reduced I2 to 0 per cent for the remaining 11 trials (1192 patients, 205 events; OR 1.22, 0.85 to 1.74; P = 0.28). There was no gain in heterogeneity or significance in sensitivity analysis by excluding trials with a larger risk of bias; however, in a subgroup analysis investigating only patients having vascular surgery, heterogeneity was also reduced to I2 = 0 per cent (8 trials, 936 patients, 114 events; OR 1.12, 0.72 to 1.75; P = 0.61). The quality of evidence according to GRADE assessment was high (Table 1), but TSA showed a required enrolment of 4263 patients (Fig. 5).
Fig. 4.
Forest plot of RCTs comparing rates of acute kidney injury in remote ischaemic preconditioning and control/sham groups
An inverse-variance random-effects model was used for meta-analysis. Odds ratios are shown with 95 per cent confidence intervals.
Fig. 5.
Trial sequential analysis of the effect of remote ischaemic preconditioning on acute kidney injury
Relative risk reduction of acute kidney injury is 20 per cent, acceptable risk of type I error is 5 per cent, and power is 20 per cent on a two-sided graph. The Z-curve did not reach the diversity-adjusted required information size of 4263 patients. Neither did the Z-curve cross the monitoring efficacy boundary (upper curve) or the futility boundary (lower curve). RIPC, remote ischaemic preconditioning.
The black dotted lines are the conventional efficacy boundaries (nominal statistical significance). The uppermost green line and the lowermost green line are both the monitoring efficacy boundaries on a two-sided test. The two shorter green line (within the dotted lines representing the conventional efficacy boundaries) are the futility boundaries on a two-sided test.
Short- and long-term all-cause mortality
Seven trials23,25,30,31,34,35,40 reported short-term all-cause mortality (within 90 days of operation). All but one was in vascular surgery. The other trial40 included patients with at least one cardiovascular risk factor having hip surgery. One trial25 of patients undergoing open repair of a ruptured abdominal aortic aneurysm (AAA) had a distinctly higher mortality rate than the remaining trials. Three32–34 of the four trials reporting on long-term all-cause mortality (after postoperative day 90) were in vascular surgery. The other trial51 involved patients undergoing live-donor renal transplantation and reported 1-year mortality. This trial has also recently reported 5-year mortality57, but these results are not included in the present meta-analysis.
A further five trials29,53,59,64,65 registered short-term all-cause mortality and two36,54 recorded long-term all-cause mortality, but there were no events in any of these trials and they were not included in the meta-analyses of mortality.
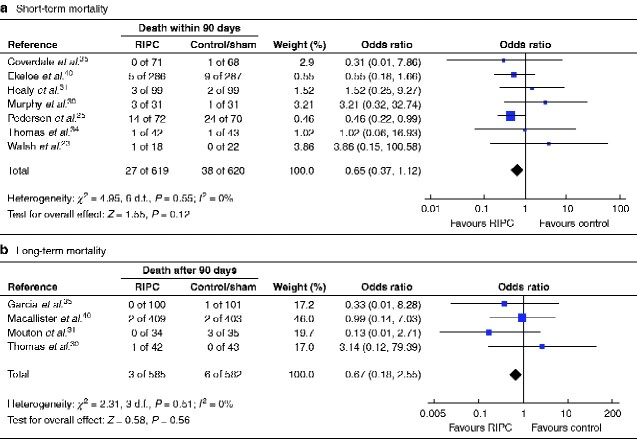
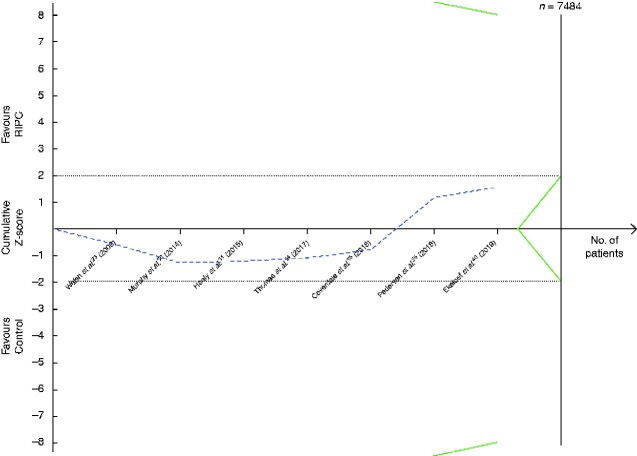
Neither short-term (7 trials, 1239 patients, 65 events; OR 0.65, 95 per cent c.i. 0.37 to 1.12; P = 0.12) (Fig. 6a) or long-term mortality (4 trials, 1167 patients, 9 events; OR 0.67, 0.18 to 2.55; P = 0.56) (Fig. 6b) were affected by RIPC in the meta-analyses. Heterogeneity between trials was minimal (I2 = 0 per cent) in both meta-analyses. The funnel plot for long-term mortality was symmetrical, but that for short-term mortality indicated publication bias with a lack of negative trials (Fig. S4). Exclusion of small trials changed the results towards there being an effect of RIPC on short-term mortality (5 trials, 1137 patients, 60 events; OR 0.56, 0.31 to 0.99; P = 0.04; I2 = 0 per cent). There was no change after exclusion of trials with a higher risk of bias. The body of evidence was of low quality according to GRADE assessment (Table 1). To confirm or reject whether RIPC has an effect on short- or long-term mortality, TSA indicated that a RIS of at least 7484 and 33 003 patients respectively would be necessary (Fig. 7).
Fig. 6.
Forest plot of RCTs comparing rates of death within 90 days or more than 90 days after surgery in remote ischaemic preconditioning and control/sham groups
a Short-term and b long-term mortality. An inverse-variance fixed-effect model was used for meta-analysis. Odds ratios are shown with 95 per cent confidence intervals.
Fig. 7.
Trial sequential analysis of the effect of remote ischaemic preconditioning on short-term mortality
Relative risk reduction of short-term mortality is 20 per cent, acceptable risk of type I error is 5 per cent, and power is 20 per cent on a two-sided graph. The Z-curve did not reach the diversity-adjusted required information size of 7484 patients. Neither did the Z-curve cross the monitoring efficacy boundary (upper curve) or the futility boundary (lower curve). Too few trials and numbers of patients were available for a trial sequential analysis graph to be produced for the effect of remote ischaemic preconditioning (RIPC) on long-term mortality.
The black dotted lines are the conventional efficacy boundaries (nominal statistical significance). The uppermost green line and the lowermost green line are both the monitoring efficacy boundaries on a two-sided test. The two shorter green line (within the dotted lines representing the conventional efficacy boundaries) are the futility boundaries on a two-sided test.
Adaptive immune response
One trial43 investigated the effect of RIPC on the adaptive immune response in patients undergoing cruciate ligament reconstruction. It found that RIPC modulated T cell responses through reduced activation and proinflammatory cytokine production by CD4 cells, while preventing CD4/CD8 derangement.
Inflammatory markers
Ten trials29,37,38,41–44,51,58,65 studied the effect of RIPC on circulating levels of inflammatory markers. Of these, five trials29,43,44,58,65 showed a significant difference in favour of RIPC lowering the levels of inflammatory markers such as interleukin (IL) 6, IL-1β, interferon γ, and tumour necrosis factor (TNF) α, whereas the remaining five trials37,38,41,42,51 did not. The trials that did not show significant results were studies of total knee arthroplasty37,38,42, shoulder surgery in patients in the beach chair position41, and RIPC in live-donor renal transplantation where patients were immunosuppressed51. However, among the RIPC-positive trials were also two studies43,44 of RIPC in orthopaedic surgery, and one each in colonic surgery58, pulmonary resection65, major vascular surgery29.
One orthopaedic trial45 measured levels of inflammatory markers in the periarticular drainage fluid (IL-6 and TNF-α). No differences were seen between controls and the RIPC group.
Oxidative stress markers
Six trials29,44,47,48,52,65 investigated the effect of RIPC on the levels of circulating oxidative stress markers. All measured malondialdehyde, three measured superoxide dismutase as well, and one trial also measured glutathione peroxidase, total antioxidant capacity, and total oxidant status. Five29,44,47,48,65 of these six trials showed that RIPC reduced oxidative stress. These trials comprised both major29,65 and minor surgery44,47,48.
Neurological injury
Seven trials investigated whether RIPC had any neuroprotective effect on ischaemic lesions, markers of neurological injury, and cognitive assessment scales. The outcomes included levels of neurone-specific enolase (NSE)36,39 and S100 calcium-binding protein B (S-100B)36,39,58, regional cerebral oxygenation38,41, the Montreal Cognitive Assessment (MoCA) score58, median nerve somatosensory-evoked potentials (SEPS)39, Japanese Orthopaedic Association criteria for the evaluation of operative results in patients with cervical myelopathy (JOA scale)39 or saccadic latency24. One trial36 in vascular surgery (carotid artery stenting) also investigated the incidence of new ischaemic lesions, and another trial67 in brain surgery investigated both the incidence of new ischaemic lesions and infarct volumes. Trials investigating neurological injury comprised both minor24,36,39 and moderate–major surgery38,58,67.
Six of the seven trials showed significant neuroprotective effects of RIPC on NSE36,39, S-100B39,58, MoCA score58, JOA scale39, regional cerebral oxygenation38,41, incidence of new ischaemic lesions36,67, and infarct volumes67. Only median nerve SEPS and saccadic latency did not show any response to RIPC24,39.
Pulmonary injury and function
Six trials studied the effect of RIPC on pulmonary injury and function. The outcomes investigated were: arterial–alveolar oxygen tension ratio and alveolar–arterial oxygen tension difference29,44,65,66, ratio of arterial oxygen partial pressure and fraction of inspired oxygen and respiratory index38,44,65,66, urinary desmosine level37, static and dynamic lung compliance, as well as acute lung injury65. One trial66 measured levels of 8-isoprostane, nitrite + nitrate, and hydrogen peroxide, and pH in both blood and exhaled breath condensate.
Five29,38,44,65,66 of the six trials investigating the effects of RIPC on pulmonary injury showed a beneficial effect of RIPC on the broad variety of markers of lung injury described above. Only one study37, which measuring urinary desmosine levels, did not find a significant difference between the control and RIPC groups. Trials investigating lung injury comprised primarily major procedures29,37,38,65,66 in orthopaedic, vascular, and pulmonary surgery, and only one trial44 in minor surgery (orthopaedic).
Renal transplantation and renal injury
Renal transplantation
Live-donor renal transplantation and the effects of RIPC were investigated in four trials51–53,56 that measured estimated glomerular filtration rate (eGFR), creatinine concentration, urinary volume, levels of serum cystatin C, plasma neutrophil gelatinase-associated lipocalin (NGAL), urinary neutrophil gelatinase-associated lipocalin, urinary retinol-binding protein (RBP), urinary N-acetyl-d-glucosaminidase, and urinary liver-type fatty acid-binding protein, and outcomes in relation to graft function and rejection. One trial56 also investigated the incidence of chronic kidney disease 1 year after surgery in donors, according to the Kidney Disease Improving Global Outcomes criteria. Another trial51 assessed changes in tissue pathology. Overall, the results were negative, with the exception of two trials. In one multicentre, international trial51, RIPC decreased the incidence of delayed graft function (secondary outcome), and at 5-year follow-up there was a sustained improvement in eGFR after RIPC immediately before surgery compared with that in controls (P = 0.004) (primary outcome)57. Another trial56 reported that creatinine levels were increased significantly in the donor control group at discharge (P = 0.003), and donors with high creatinine levels at discharge had a higher prevalence of chronic kidney disease after 1 year (P = 0.003) (both secondary outcomes).
Renal injury in nephrectomy
Three trials in partial nephrectomy, both open50 and laparoscopic54,55, showed a significant advantageous effect of RIPC. One trial54 assessed absolute change in GFR of the affected kidney by renal scintigraphy and urinary RBP measurement, and another50 assessed eGFR, serum creatinine, fraction of excreted sodium, and acute kidney injury in accordance with AKIN criteria. The final trial55 exposed one group of patients to RIPC 24 h before surgery and another group immediately before surgery. Plasma NGAL and serum cystatin C levels were decreased significantly in intervention groups compared with controls, and late-phase protection was more prominent. Furthermore, GFR assessed by renal scintigraphy was lower in both intervention groups at 3-month follow-up.
Renal injury in vascular surgery
Four trials in vascular surgery investigated the renoprotective effect of RIPC. Three30,33,34 reported no effect of RIPC. One trial23, which included patients undergoing elective endovascular aneurysm repair, found that RIPC was associated with improved tubular function indicated by a significantly higher urine albumin/creatinine ratio and lower urinary RBP levels, implying less distal tubule damage.
Liver injury
Five trials59,60,62–64 investigated the hepatoprotective effect of RIPC. All trials included liver surgery only. Four trials investigated RIPC in patients undergoing major hepatectomy59,63,64 or liver resections60 for hepatocellular carcinoma or colorectal liver metastasis. The trial on liver resections compared the hepatoprotective effects of RIPC versus local ischaemic preconditioning. The four trials had different results. One59 showed a reduction in postoperative serum aminotransferase levels and increased indocyanine green (ICG) clearance, but no effect on serum bilirubin or histological examination of the resected liver specimen for signs of ischaemia–reperfusion injury, steatosis, and fibrosis. Another trial60, however, found ischaemic preconditioning to be beneficial in terms of postoperative serum cholinesterase and serum bilirubin levels, as well as a higher Doppler ultrasound flow through the hepatic artery; there were significant differences between groups in histopathological evaluation of hepatocyte necrosis. Prothrombin time (PT), activated partial thromboplastin time, and alkaline phosphatase, albumin, total protein, and carbamide levels did not differ between the groups. The third trial63 assessed postoperative liver function by measurement of alanine (ALT) and aspartate (AST) aminotransferase, and ICG levels; and the fourth trial64 by total bilirubin, ALT and AST levels, PT, international normalized ratio, and serum albumin level. Neither trial found differences between the RIPC and control groups.
The fifth trial62 investigated the effect of RIPC on donors and recipients after liver transplantation. The donors underwent RIPC before surgery. The postoperative AST concentration was lower in recipients who received a preconditioned graft (P = 0.029), but there were no differences in postoperative AST or ALT levels in donors. RIPC did not reduce the incidence of delayed graft hepatic function, early allograft dysfunction or graft failure.
Intestinal injury
Three trials25,29,31, all in vascular surgery, assessed the effect of RIPC on intestinal injury. One trial29 investigated the impact of RIPC in patients undergoing elective open infrarenal AAA repair. Intestinal injury was assessed by measuring the serum concentrations of intestinal fatty acid-binding protein and endotoxin, and the activity of diamine oxidase. To evaluate intestinal function, a modified intestinal dysfunction score was recorded from 72 h after operation. All biomarkers reflecting intestinal injury were reduced in favour of RIPC (P < 0.001).
Two trials25,31 investigated the effect of RIPC on intestinal injury as a clinical outcome of intestinal ischaemia. One trial25, in open surgery for ruptured AAA, defined intestinal ischaemia in terms of surgical removal of ischaemic bowel. Ischaemic bowel developed in five patients in the RIPC group and 12 in the control group (P = 0.052). The other trial31, comprising a composite of elective major vascular surgery, defined intestinal ischaemia as small or large bowel ischaemia requiring laparotomy, found at autopsy or proven on colonic biopsy; no significant difference between the control and RIPC groups was found.
Muscular injury and pain
Four trials in orthopaedic surgery (total knee replacement and knee ligamentoplasty) examined the effects of RIPC on postoperative pain37,45,49, analgesic consumption45,49, muscle oxygenation45, muscular injury49, and gene expression profile of muscle biopsies42.
The three trials investigating pain scores and analgesic consumption showed heterogenous results. One49 reported no difference in pain scores, but a reduction in analgesic consumption in the RIPC group. The other two37,45 reported less pain at rest and during exercise, but found no differences in analgesic consumption. All three trials performed RIPC on the operative limb just before surgery.
RIPC did not have any effect on muscular oxygenation45 or injury measured as the level of myoglobin and creatinine phosphokinase in plasma49. However, muscle gene expression profiles showed a statistically significant increase in the expression of oxidative stress defence genes, immediate early response genes, and mitochondrial genes. Upregulation of prosurvival genes was also observed and correlated with a downregulation of proapoptotic gene expression42.
Arterial stiffness
One trial26 investigated the effect of RIPC on arterial stiffness parameters (augmentation index and pulse wave velocity) as a primary outcome in patients undergoing vascular surgery. There were no significant differences between the RIPC and control groups.
Discussion
In this systematic review of the effect of RIPC on clinical or biomarker outcomes in non-cardiac surgery, meta-analysis showed a positive association between RIPC and improved cardiovascular outcomes. There was, however, substantial heterogeneity, possibly as a consequence of several different cardiovascular outcomes being included. The majority of included patients were undergoing vascular surgery, but the timing of RIPC in relation to anaesthesia and surgery differed between studies, as did the number and duration of cycles, and the anatomical site of RIPC. The positive effects of RPIC remained in a sensitivity analysis excluding small trials.
The meta-analysis of acute kidney injury did not show an effect of RIPC. The quality of evidence according to GRADE assessment was high, but the TSA showed that the required information size had not yet been reached. Finally, RIPC had no effect on all-cause short- or long-term postoperative mortality. The body of evidence was of low quality according to GRADE assessment, and the TSA indicated that the RIS had not been reached.
The finding of a reduction in cardiovascular events with use of RIPC in non-cardiac surgery contradicts the findings of a meta-analysis6 that included both ischaemic preconditioning and postconditioning, central and remote conditioning, in both children and adults undergoing invasive procedures, including cardiac surgery. It showed no effect of ischaemic conditioning (both central and remote) on the overall risk of death or cardiovascular events. Furthermore, effects on stroke and acute kidney injury were uncertain given methodological concerns and low event rates. Furthermore, meta-analyses investigating the clinical effect of RIPC in only one surgical area, such as AAA repair4,68 and cardiac surgery5,69, did not consistently show cardiac or renal protection, and there was no effect on mortality. Some of the meta-analyses did, however, show a reduced incidence of acute kidney injury5,69 and mortality5 in subgroup analyses of patients receiving volatile anaesthetics in cardiac surgery.
Despite investigation of the use of RIPC in a number of clinical settings over several decades70, the underlying mechanism is still not fully understood. Several experimental and clinical studies71–75 have suggested that the stimulus is transmitted from the preconditioned tissue to other organs by humoral, neural, and systemic anti-inflammatory mediators. Considering the complex interaction of pathways in which RIPC might exert its effect, it is tempting to establish the hypothesis that the effect of RIPC also depends on the level of surgical stress the patients are exposed to. This hypothesis remains untested. Interestingly, there seems to be a pattern in the organs protected by RIPC. Focusing on the target organ, twice as many trials showed significant results in the form of protection of the operated organ28,36,39,50,51,54,55,59,60,65–67, than in trials investigating a protective effect on target organs other than the operated organ18,21,28,30,44. This gives further reason to believe that the amount of surgical stress is crucial for demonstrating an important effect of RIPC. Trials that did not show significant protective results in the operated organ either referred to renal protection in renal transplantation52,53, liver protection in hepatectomy63,64, or cardiovascular protection in vascular surgery24,31–34.
Many trials have shown a significant effect of RIPC on inflammatory markers29,43,44,58,65 and oxidative stress markers29,44,47,48,65, supporting the hypothesis that RIPC has an opposing effect on surgical stress. Cardiovascular complications account for a substantial proportion of both postoperative complications and mortality in non-cardiac surgery76–78. The pathophysiology of perioperative cardiac events has not been fully clarified, even though efforts to identify the underlying pathophysiology have been made79–85. The systemic stress response leads to a myocardial oxygen supply–demand mismatch, which, in the presence of endothelial dysfunction, stress-induced rupture of arteriosclerotic plaques, and hypercoagulability, may cause myocardial injury77. A reduction in the surgical stress response resulting from RIPC could partially explain the reduced risk of cardiovascular events after non-cardiac surgery using such preconditioning.
Methods of performing RIPC, timing, anaesthesia, surgical procedures, and patient populations differed, and this was of concern when comparing the trials in this meta-analysis. Furthermore, low event rates for clinical outcomes, especially death, prevented meaningful comparison between trials and statistical power was generally low (only 5 RCTs in this review included more than 200 patients). RIS values determined by TSA were 3150, 4263, 7484, and 33 003 to confirm or reject any effect of RIPC on serious cardiovascular events, acute kidney injury, short-term and long-term mortality respectively. These numbers are similar to those of TSA in another meta-analysis6.
Small proof-of-concept studies reported an effect of RIPC on several biomarkers, although not enough to demonstrate a clinical effect. Trials measuring oxidative stress markers (5 of 6 trials29,44,47,48,65), markers of neurological injury (6 of 7 trials36,38,39,41,58,67), markers of lung injury (5 of 6 trials29,38,44,65,66), and markers of renal injury in nephrectomy (3 of 3 trials50,54,55) reported positive results of RIPC. Only one trial29 investigated markers of intestinal injury, with positive results.
Several trials lacked detailed information on the choice of anaesthesia, which is believed to have an impact on the effect on RIPC. Propofol may inhibit, whereas sevoflurane might preserve, myocardial protection afforded by RIPC86,87. However, a recent study88 investigated the effect of RIPC on humans in settings of anaesthesia with propofol, sevoflurane or carvedilol before RIPC (no anaesthesia) and a control group. Plasma was perfused through an isolated rat heart subjected to 30 min of ischaemia and 60 min of reperfusion; thereafter, myocardial infarct size was determined. Controls not exposed to either propofol, sevoflurane or carvedilol had significantly reduced myocardial infarct sizes after RIPC, suggesting that all three agents blocked the effect of RIPC88.
RIPC is believed to exert both early and late protection; the early phase of protection lasts only a few hours after the RIPC stimulus, and is followed 24 h later by a second window lasting for up to 48 h89. Most trials in this review applied RIPC after induction of anaesthesia; nevertheless one trial55 also applied RIPC to investigate the late-phase protection, and reported significant results and a more prominent protection than that seen in the early phase. One trial36 exposed subjects to RIPC twice daily for 2 weeks before carotid artery stenting, with positive results in terms of the incidence and volume of new ischaemic lesions in the brain after stenting. Another trial60 also reported a significant effect of RIPC applied after the laparotomy incision. This trial involved patients undergoing liver resections, and in all patients the Pringle manoeuvre was used to avoid blood loss, which itself might work as a preconditioning stimulus. This consideration is also relevant in vascular surgery, where the cross-clamping techniques might exert a preconditioning stimulus, leading to underestimation of the effect in the intervention groups compared with controls31,33–35.
Heterogeneity in RIPC procedures across trials (timing, duration, and number of cycles) was notable, ranging from one cycle of 5 min to two cycles of 10 min on one leg after another, to four cycles of 5 min of ischaemia and reperfusion24,37,51. An experimental study90 has shown that the effects of RIPC differ depending on the number and duration of cycles. It even seems that prolonged cycles lasting 10 min can abrogate the protective effect90. Furthermore, differences in eligibility criteria are of concern when comparing trials. For instance, co-morbidities such as hypercholesterolaemia and diabetes have been shown to interfere with the efficacy of RIPC91–94, as has advanced age95.
In general, the trials in this review had a low overall risk of bias, but most had small sample sizes and funnel plots revealed signs of publication bias, particularly among trials reporting on cardiovascular events. Furthermore, many of the trials were underpowered in terms of exploring any impact RIPC might have on postoperative clinical outcomes, and several studies did not report anaesthetic regimens in detail.
The evidence remains insufficient to reach a firm conclusion on the effects of RIPC in non-cardiac surgery. Further understanding of the mechanisms underlying RIPC is required to design a trial with sufficient statistical power, using an optimal RIPC process, with relevant eligibility criteria and using optimal anaesthesia.
Funding
No funding.
Disclosure. The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS Open online
Supplementary Material
References
- 1. Hausenloy DJ, Yellon DM.. Preconditioning and postconditioning: underlying mechanisms and clinical application. Atherosclerosis 2009;204:334–341 [DOI] [PubMed] [Google Scholar]
- 2. Kharbanda RK, Nielsen TT, Redington AN.. Translation of remote ischaemic preconditioning into clinical practice. Lancet 2009;374:1557–1565 [DOI] [PubMed] [Google Scholar]
- 3. Man C, Gong D, Zhou Y, Fan Y.. Meta-analysis of remote ischemic conditioning in patients with acute myocardial infarction. Sci Rep 2017;7:43529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang MH, Du X, Guo W, Liu XP, Jia X, Wu Y.. Effect of remote ischemic preconditioning on complications after elective abdominal aortic aneurysm repair: a meta-analysis with randomized control trials. Vasc Endovascular Surg 2019;53:387–394 [DOI] [PubMed] [Google Scholar]
- 5. Xie J, Zhang X, Xu J, Zhang Z, Klingensmith NJ, Liu S. et al. Effect of remote ischemic preconditioning on outcomes in adult cardiac surgery: a systematic review and meta-analysis of randomized controlled studies. Anesth Analg 2018;127:30–38 [DOI] [PubMed] [Google Scholar]
- 6. Sukkar L, Hong D, Wong MG, Badve SV, Rogers K, Perkovic V. et al. Effects of ischaemic conditioning on major clinical outcomes in people undergoing invasive procedures: systematic review and meta-analysis. BMJ 2016;355:i5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Desborough JP. The stress response to trauma and surgery. Br J Anaesth 2000;85:109–117 [DOI] [PubMed] [Google Scholar]
- 8. Hudson W. The physiological ascpects of extracorporeal circulation. Br J Anaesth 1959;31:378–392 [DOI] [PubMed] [Google Scholar]
- 9. Górka J, Polok K, Iwaniec T, Górka K, Włudarczyk A, Fronczek J. et al. Altered preoperative coagulation and fibrinolysis are associated with myocardial injury after non-cardiac surgery. Br J Anaesth 2017;118:713–719 [DOI] [PubMed] [Google Scholar]
- 10. Ekeloef S, Larsen MHH, Schou-Pedersen AMV, Lykkesfeldt J, Rosenberg J, Gögenür I.. Endothelial dysfunction in the early postoperative period after major colon cancer surgery. Br J Anaesth 2017;118:200–206 [DOI] [PubMed] [Google Scholar]
- 11. Kimura F, Shimizu H, Yoshidome H, Ohtsuka M, Miyazaki M.. Immunosuppression following surgical and traumatic injury. Surg Today 2010;40:793–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heusch G, Bøtker HE, Przyklenk K, Redington A, Yellon D.. Remote ischemic conditioning. J Am Coll Cardiol 2015;65:177–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kharbanda RK, Peters M, Walton B, Kattenhorn M, Mullen M, Klein N. et al. Ischemic preconditioning prevents endothelial injury and systemic neutrophil activation during ischemia–reperfusion in humans in vivo. Circulation 2001;103:1624–1630 [DOI] [PubMed] [Google Scholar]
- 14. Pedersen CM, Cruden NL, Schmidt MR, Lau C, Bøtker HE, Kharbanda RK. et al. Remote ischemic preconditioning prevents systemic platelet activation associated with ischemia–reperfusion injury in humans. J Thromb Haemost 2011;9:404–407 [DOI] [PubMed] [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP.. preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:1–6 [PMC free article] [PubMed] [Google Scholar]
- 16. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD. et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cochrane Training. RevMan 5.https://community.cochrane.org/help/tools-and-software/revman-5 (accessed 13 May 2019)
- 18. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P. et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Simmonds M, Salanti G, McKenzie Joanne, Elliott J.. Living systematic reviews: 3. Statistical methods for updating meta-analyses. J Clin Epidemiol 2017;91:38–46 [DOI] [PubMed] [Google Scholar]
- 20. Wetterslev J, Jakobsen JC, Gluud C.. Trial sequential analysis in systematic reviews with meta-analysis. BMC Med Res Methodol 2017;17:1–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jakobsen JC, Gluud C, Winkel P, Lange T, Wetterslev J.. The thresholds for statistical and clinical significance—a five-step procedure for evaluation of intervention effects in randomised clinical trials. BMC Med Res Methodol 2014;14:1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wetterslev J, Thorlund K, Brok J, Gluud C.. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol 2008;61:64–75 [DOI] [PubMed] [Google Scholar]
- 23. Walsh SR, Boyle JR, Tang TY, Sadat U, Cooper DG, Lapsley M. et al. Remote ischemic preconditioning for renal and cardiac protection during endovascular aneurysm repair: a randomized controlled trial. J Endovasc Ther 2009;16:680–689 [DOI] [PubMed] [Google Scholar]
- 24. Walsh SR, Nouraei SA, Tang TY, Sadat U, Carpenter RH, Gaunt ME. et al. Remote ischemic preconditioning for cerebral and cardiac protection during carotid endarterectomy: results from a pilot randomized clinical trial. Vasc Endovascular Surg 2010;44:434–439 [DOI] [PubMed] [Google Scholar]
- 25. Pedersen TF, Budtz-Lilly J, Petersen CN, Hyldgaard J, Schmidt JO, Kroijer R. et al. Randomized clinical trial of remote ischaemic preconditioning versus no preconditioning in the prevention of perioperative myocardial infarction during open surgery for ruptured abdominal aortic aneurysm. Br J Surg Open 2018;2:112–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kepler T, Kuusik K, Lepner U, Starkopf J, Zilmer M, Eha J. et al. The effect of remote ischaemic preconditioning on arterial stiffness in patients undergoing vascular surgery: a randomised clinical trial. Eur J Vasc Endovasc Surg 2019;57:868–875 [DOI] [PubMed] [Google Scholar]
- 27. Choi ES, Lee YS, Park BS, Kim BG, Sohn HM, Jeon YT.. Effects of combined remote ischemic pre-and post-conditioning on neurologic complications in moyamoya disease patients undergoing superficial temporal artery–middle cerebral artery anastomosis. J Clin Med 2019;8:638–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kepler T, Kuusik K, Lepner U, Starkopf J, Zilmer M, Eha J. et al. Remote ischaemic preconditioning attenuates cardiac biomarkers during vascular surgery: a randomised clinical trial. Eur J Vasc Endovasc Surg 2020;59:301–308 [DOI] [PubMed] [Google Scholar]
- 29. Li C, Li YSS, Xu M, Wen SHH, Yao X, Wu Y. et al. Limb remote ischemic preconditioning for intestinal and pulmonary protection during elective open infrarenal abdominal aortic aneurysm repair: a randomized controlled trial. Anesthesiology 2013;118:842–852 [DOI] [PubMed] [Google Scholar]
- 30. Murphy N, Vijayan A, Frohlich S, O’Farrell F, Barry M, Sheehan S. et al. Remote ischemic preconditioning does not affect the incidence of acute kidney injury after elective abdominal aortic aneurysm repair. J Cardiothorac Vasc Anesth 2014;28:1285–1292 [DOI] [PubMed] [Google Scholar]
- 31. Healy DA, Boyle E, McCartan D, Bourke M, Medani M, Ferguson J. et al. A multicenter pilot randomized controlled trial of remote ischemic preconditioning in major vascular surgery. Vasc Endovascular Surg 2015;49:220–227 [DOI] [PubMed] [Google Scholar]
- 32. Mouton R, Pollock J, Soar J, Mitchell DC, Rogers CA.. Remote ischaemic preconditioning versus sham procedure for abdominal aortic aneurysm repair: an external feasibility randomized controlled trial. Trials 2015;16:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Garcia S, Rector TS, Zakharova M, Herrmann RR, Adabag S, Bertog S. et al. Cardiac Remote Ischemic Preconditioning Prior to Elective Vascular Surgery (CRIPES): a prospective, randomized, sham-controlled phase II clinical trial. J Am Heart Assoc 2016;5:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thomas KN, Cotter JD, Williams MJA, van Rij AM.. Repeated episodes of remote ischemic preconditioning for the prevention of myocardial injury in vascular surgery. Vasc Endovascular Surg 2016;50:140–146 [DOI] [PubMed] [Google Scholar]
- 35. Coverdale NS, Hamilton A, Petsikas D, McClure RS, Malik P, Milne B. et al. Remote ischemic preconditioning in high-risk cardiovascular surgery patients: a randomized-controlled trial. Semin Thorac Cardiovasc Surg 2018;30:26–33 [DOI] [PubMed] [Google Scholar]
- 36. Zhao W, Meng R, Ma C, Hou B, Jiao L, Zhu F. et al. Safety and efficacy of remote ischemic preconditioning in patients with severe carotid artery stenosis before carotid artery stenting: a proof-of-concept, randomized controlled trial. Circulation 2017;135:1325–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Memtsoudis SG, Valle AG, Jules-Elysse K, Poultsides L, Reid S, Starcher B. et al. Perioperative inflammatory response in total knee arthroplasty patients: impact of limb preconditioning. Reg Anesth Pain Med 2010;35:412–416 [DOI] [PubMed] [Google Scholar]
- 38. Oh CSS, Kim SHH, Lee J, Rhee KY.. Impact of remote ischaemic preconditioning on cerebral oxygenation during total knee arthroplasty. Int J Med Sci 2017;14:115–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hu S, Dong HL, Li YZ, Luo ZJ, Sun L, Yang QZ. et al. Effects of remote ischemic preconditioning on biochemical markers and neurologic outcomes in patients undergoing elective cervical decompression surgery: a prospective randomized controlled trial. J Neurosurg Anesthesiol 2010;22:46–52 [DOI] [PubMed] [Google Scholar]
- 40. Ekeloef S, Homilius M, Stilling M, Ekeloef P, Koyuncu S, Münster AMB. et al. The effect of remote ischaemic preconditioning on myocardial injury in emergency hip fracture surgery (PIXIE trial): phase II randomised clinical trial. BMJ 2019;367:16385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Oh CS, Sa M, Park HJ, Piao L, Oh KS, Kim SH.. Effects of remote ischemic preconditioning on regional cerebral oxygen saturation in patients in the beach chair position during shoulder surgery: a double-blind randomized controlled trial. J Clin Anesth 2020;61:109661. [DOI] [PubMed] [Google Scholar]
- 42. Murphy T, Walsh PM, Doran PP, Mulhall KJ.. Transcriptional responses in the adaptation to ischaemia–reperfusion injury: a study of the effect of ischaemic preconditioning in total knee arthroplasty patients. J Transl Med 2010;8:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sullivan PJ, Sweeney KJ, Hirpara KM, Malone CB, Curtin W, Kerin MJ.. Cyclical ischaemic preconditioning modulates the adaptive immune response in human limb ischaemia–reperfusion injury. Br J Surg 2009;96:381–390 [DOI] [PubMed] [Google Scholar]
- 44. Lin L, Wang L, Wang W, Jin L, Zhao X, Zheng L. et al. Ischemic preconditioning attenuates pulmonary dysfunction after unilateral thigh tourniquet-induced ischemia–reperfusion. Anesth Analg 2010;111:539–543 [DOI] [PubMed] [Google Scholar]
- 45. Memtsoudis SG, Stundner O, Yoo D, Gonzalez Della Valle A, Boettner F, Bombardieri AM. et al. Does limb preconditioning reduce pain after total knee arthroplasty? A randomized, double-blind study. Clin Orthop Relat Res 2014;472:1467–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Park SK, Hur M, Yoo S, Choi JY, Kim WH, Kim JT. et al. Effect of remote ischaemic preconditioning in patients with ischaemic heart disease undergoing orthopaedic surgery: a randomized controlled trial. Br J Anaesth 2018;120:198–200 [DOI] [PubMed] [Google Scholar]
- 47. Koca K, Yurttas Y, Cayci T, Bilgic S, Kaldirim U, Durusu M. et al. The role of preconditioning and N-acetylcysteine on oxidative stress resulting from tourniquet-induced ischemia–reperfusion in arthroscopic knee surgery. J Trauma 2011;70:717–723 [DOI] [PubMed] [Google Scholar]
- 48. Van M, Olguner C, Koca U, Sisman AR, Muratli K, Karci A. et al. Ischaemic preconditioning attenuates haemodynamic response and lipid peroxidation in lower-extremity surgery with unilateral pneumatic tourniquet application: a clinical pilot study. Adv Ther 2008;25:355–366 [DOI] [PubMed] [Google Scholar]
- 49. Orban JC, Levraut J, Gindre S, Deroche D, Schlatterer B, Ichai C. et al. Effects of acetylcysteine and ischaemic preconditioning on muscular function and postoperative pain after orthopaedic surgery using a pneumatic tourniquet. Eur J Anaesthesiol 2006;23:1025–1030 [DOI] [PubMed] [Google Scholar]
- 50. Kil H, Kim J, Choi Y, Lee H, Kim T, Kim J. et al. Effect of combined treatment of ketorolac and remote ischemic preconditioning on renal ischemia–reperfusion injury in patients undergoing partial nephrectomy: pilot study. J Clin Med 2018;7:470–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. MacAllister R, Clayton T, Knight R, Robertson S, Nicholas J, Motwani M. et al. REmote preconditioning for Protection Against Ischaemia–Reperfusion in renal transplantation (REPAIR): a multicentre, multinational, double-blind, factorial designed randomised controlled trial. Effic Mech Eval 2015;2:1–60 [PubMed] [Google Scholar]
- 52. Chen Y, Zheng H, Wang X, Zhou Z, Luo A, Tian Y.. Remote ischemic preconditioning fails to improve early renal function of patients undergoing living-donor renal transplantation: a randomized controlled trial. Transplantation 2013;95:4–6 [DOI] [PubMed] [Google Scholar]
- 53. Nicholson ML, Pattenden CJ, Barlow AD, Hunter JP, Lee G, Hosgood SA. et al. A double blind randomized clinical trial of remote ischemic conditioning in live donor renal transplantation. Medicine (Baltimore) 2015;94:e1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Huang J, Chen Y, Dong B, Kong W, Zhang J, Xue W. et al. Effect of remote ischaemic preconditioning on renal protection in patients undergoing laparoscopic partial nephrectomy: a ‘blinded’ randomised controlled trial. BJU Int 2013;112:74–80 [DOI] [PubMed] [Google Scholar]
- 55. Y Hou Y, Li Y, F He S, Song J, X Yu D, Wong GTC. et al. Effects of differential-phase remote ischemic preconditioning intervention in laparoscopic partial nephrectomy: a single blinded, randomized controlled trial in a parallel group design. J Clin Anesth 2017;41:21–28 [DOI] [PubMed] [Google Scholar]
- 56. Bang JY, Kim SG, Oh J, Kim SO, Go YJ, Hwang GS. et al. Impact of remote ischemic preconditioning conducted in living kidney donors on renal function in donors and recipients following living donor kidney transplantation: a randomized clinical trial. J Clin Med 2019;8:713–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Veighey KV, Nicholas JM, Clayton T, Knight R, Robertson S, Dalton N. et al. Early remote ischaemic preconditioning leads to sustained improvement in allograft function after live donor kidney transplantation: long-term outcomes in the REnal Protection Against Ischaemia–Reperfusion in transplantation (REPAIR) randomised trial. Br J Anaesth 2019;123:584–591 [DOI] [PubMed] [Google Scholar]
- 58. He Z, Xu N, Qi S.. Remote ischemic preconditioning improves the cognitive function of elderly patients following colon surgery. Medicine (Baltimore) 2017;96:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kanoria S, Robertson FP, Metha NN, Fusai G, Sharma D, Davidson BR.. Effect of remote ischaemic preconditioning on liver injury in patients undergoing major hepatectomy for colorectal liver metastasis: a pilot randomised controlled feasibility trial. World J Surg 2017;41:1322–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rakic M, Patrlj L, Amic F, Aralica G, Grgurević I.. Comparison of hepatoprotective effect from ischemia–reperfusion injury of remote ischemic preconditioning of the liver vs local ischemic preconditioning of the liver during human liver resections. Int J Surg 2018;54:248–253 [DOI] [PubMed] [Google Scholar]
- 61. Antonowicz SS, Cavallaro D, Jacques N, Brown A, Wiggins T, Haddow JB. et al. Remote ischemic preconditioning for cardioprotection in elective inpatient abdominal surgery—a randomized controlled trial. BMC Anesthesiol 2018;18:76–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jung KW, Kang J, Kwon HM, Moon YJ, Jun IG, Song JG. et al. Effect of remote ischemic preconditioning conducted in living liver donors on postoperative liver function in donors and recipients following liver transplantation. Ann Surg 2020;271:646–653 [DOI] [PubMed] [Google Scholar]
- 63. Teo JY, Ho AFW, Bulluck H, Gao F, Chong J, Koh YX. et al. Effect of remote ischemic preconditioning on liver injury in patients undergoing liver resection: the ERIC-LIVER trial. HPB (Oxford) 2020;22:1250–1257 [DOI] [PubMed] [Google Scholar]
- 64. Liu X, Cao L, Zhang T, Guo R, Lin W.. Effect of remote ischemic preconditioning in patients undergoing hepatectomy with portal triad clamping: a randomized controlled trial. Anesth Analg 2019;129:1742–1748 [DOI] [PubMed] [Google Scholar]
- 65. Li C, Xu M, Wu Y, Li YS, Huang WQ, Liu KX.. Limb remote ischemic preconditioning attenuates lung injury after pulmonary resection under propofol–remifentanil anesthesia: a randomized controlled study. Anesthesiology 2014;121:249–259 [DOI] [PubMed] [Google Scholar]
- 66. Garcia-de-la-Asuncion J, Bruno L, Perez-Griera J, Galan G, Morcillo A, Wins R. et al. Remote ischemic preconditioning decreases oxidative lung damage after pulmonary lobectomy: a single-center randomized, double-blind, controlled trial. Anesth Analg 2017;125:499–506 [DOI] [PubMed] [Google Scholar]
- 67. Sales AHA, Barz M, Bette S, Wiestler B, Ryang YM, Meyer B. et al. Impact of ischemic preconditioning on surgical treatment of brain tumors: a single-center, randomized, double-blind, controlled trial. BMC Med 2017;15:137–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. De Freitas S, Hicks CW, Mouton R, Garcia S, Healy D, Connolly C. et al. Effects of ischemic preconditioning on abdominal aortic aneurysm repair: a systematic review and meta-analysis. J Surg Res 2019;235:340–349 [DOI] [PubMed] [Google Scholar]
- 69. Deferrari G, Bonanni A, Bruschi M, Alicino C, Signori A.. Remote ischaemic preconditioning for renal and cardiac protection in adult patients undergoing cardiac surgery with cardiopulmonary bypass: systematic review and meta-analysis of randomized controlled trials. Nephrol Dial Transplant 2018;33:813–824 [DOI] [PubMed] [Google Scholar]
- 70. Hu J, Liu S, Jia P, Xu X, Song N, Zhang T. et al. Protection of remote ischemic preconditioning against acute kidney injury: a systematic review and meta-analysis. Crit Care 2016;20:111–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lang SC, Elsässer A, Scheler C, Vetter S, Tiefenbacher CP, Kübler W. et al. Myocardial preconditioning and remote renal preconditioning. Identifying a protective factor using proteomic methods. Basic Res Cardiol 2006;101:149–158 [DOI] [PubMed] [Google Scholar]
- 72. Dickson EW, Reinhardt CP, Renzi FP, Becker RC, Porcaro WA, Heard SO.. Ischemic preconditioning may be transferable via whole blood transfusion: preliminary evidence. J Thromb Thrombolysis 1999;8:123–129 [DOI] [PubMed] [Google Scholar]
- 73. Gho BCG, Schoemaker RG, van den Doel MA, Duncker DJ, Verdouw PD.. Myocardial protection by brief ischemia in noncardiac tissue. Circulation 1996;94:2193–2200 [DOI] [PubMed] [Google Scholar]
- 74. Konstantinov IE, Arab S, Kharbanda RK, Li J, Cheung MMH, Cherepanov V. et al. The remote ischemic preconditioning stimulus modifies inflammatory gene expression in humans. Physiol Genomics 2004;19:143–150 [DOI] [PubMed] [Google Scholar]
- 75. Shimizu M, Saxena P, Konstantinov IE, Cherepanov V, Cheung MMH, Wearden P. et al. Remote ischemic preconditioning decreases adhesion and selectively modifies functional responses of human neutrophils. J Surg Res 2010;158:155–161 [DOI] [PubMed] [Google Scholar]
- 76. Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) Study Investigators; Devereaux PJ, Chan MT, Alonso-Coello P, Walsh M, Berwanger O, Villar J. et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012;307:2295–2304 [DOI] [PubMed] [Google Scholar]
- 77. Kristensen S, Knuuti J, Saraste A, Anker S, Bøtker H, De Hert S. et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management. Eur J Anaesthesiol 2014;31:517–573 [DOI] [PubMed] [Google Scholar]
- 78. Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH.. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. CMAJ 2005;173:627–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mangano DT, Browner WS, Hollenberg M, London MJ, Tubau JF, Tateo IM.. Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. N Engl J Med 1990;323:1781–1788 [DOI] [PubMed] [Google Scholar]
- 80. Ashton CM, Petersen NJ, Wray NP, Kiefe CI, Dunn JK, Wu L. et al. The incidence of perioperative myocardial infarction in men undergoing noncardiac surgery. Ann Intern Med 1993;118:504–510 [DOI] [PubMed] [Google Scholar]
- 81. Badner NH, Knill RL, Brown JE, Novick TV, Gelb AW.. Myocardial infarction after noncardiac surgery. Anesthesiology 1998;88:572–578 [DOI] [PubMed] [Google Scholar]
- 82. Sprung J, Warner ME, Contreras MG, Schroeder DR, Beighley CM, Wilson GA. et al. Predictors of survival following cardiac arrest in patients undergoing noncardiac surgery: a study of 518 294 patients at a tertiary referral center. Anesthesiology 2003;99:259–269 [DOI] [PubMed] [Google Scholar]
- 83. Dawood MM, Gutpa DK, Southern J, Walia A, Atkinson JB, Eagle KA.. Pathology of fatal perioperative myocardial infarction: implications regarding pathophysiology and prevention. Int J Cardiol 1996;57:37–44 [DOI] [PubMed] [Google Scholar]
- 84. Cohen MC, Aretz TH.. Histological analysis of coronary artery lesions in fatal postoperative myocardial infarction. Cardiovasc Pathol 1999;8:133–139 [DOI] [PubMed] [Google Scholar]
- 85. Hanson I, Kahn J, Dixon S, Goldstein J.. Angiographic and clinical characteristics of type 1 versus type 2 perioperative myocardial infarction. Catheter Cardiovasc Interv 2013;82:622–628 [DOI] [PubMed] [Google Scholar]
- 86. Kottenberg E, Thielmann M, Bergmann L, Heine T, Jakob H, Heusch G. et al. Protection by remote ischemic preconditioning during coronary artery bypass graft surgery with isoflurane but not propofol—a clinical trial. Acta Anaesthesiol Scand 2012;56:30–38 [DOI] [PubMed] [Google Scholar]
- 87. Pagel PS, Crystal GJ.. The discovery of myocardial preconditioning using volatile anesthetics: a history and contemporary clinical perspective. J Cardiothorac Vasc Anesth 2018;32:1112–1134 [DOI] [PubMed] [Google Scholar]
- 88. Cho YJ, Nam K, Kim TK, Choi SW, Kim SJ, Hausenloy DJ. et al. Sevoflurane, propofol and carvedilol block myocardial protection by limb remote ischemic preconditioning. Int J Mol Sci 2019;20:269–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Loukogeorgakis SP, Panagiotidou AT, Broadhead MW, Donald A, Deanfield JE, MacAllister RJ.. Remote ischemic preconditioning provides early and late protection against endothelial ischemia–reperfusion injury in humans: role of the autonomic nervous system. J Am Coll Cardiol 2005;46:450–456 [DOI] [PubMed] [Google Scholar]
- 90. Johnsen J, Pryds K, Salman R, Løfgren B, Kristiansen SB, Bøtker HE. et al. The remote ischemic preconditioning algorithm: effect of number of cycles, cycle duration and effector organ mass on efficacy of protection. Basic Res Cardiol 2016;111:1–10 [DOI] [PubMed] [Google Scholar]
- 91. Kocić I, Konstański Z, Kaminski M, Dworakowska D, Dworakowski R.. Experimental hyperlipidemia prevents the protective effect of ischemic preconditioning on the contractility and responsiveness to phenylephrine of rat-isolated stunned papillary muscle. Gen Pharmacol 1999;33:213–219 [DOI] [PubMed] [Google Scholar]
- 92. Ueda Y, Kitakaze M, Komamura K, Minamino T, Asanuma H, Sato H. et al. Pravastatin restored the infarct size-limiting effect of ischemic preconditioning blunted by hypercholesterolemia in the rabbit model of myocardial infarction. J Am Coll Cardiol 1999;34:2120–2125 [DOI] [PubMed] [Google Scholar]
- 93. Kristiansen SB, Løfgren B, Støttrup NB, Khatir D, Nielsen-Kudsk JE, Nielsen TT. et al. Ischaemic preconditioning does not protect the heart in obese and lean animal models of type 2 diabetes. Diabetologia 2004;47:1716–1721 [DOI] [PubMed] [Google Scholar]
- 94. Whittington HJ, Harding I, Stephenson CIM, Bell R, Hausenloy DJ, Mocanu MM. et al. Cardioprotection in the aging, diabetic heart: the loss of protective Akt signalling. Cardiovasc Res 2013;99:694–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Behmenburg F, Heinen A, Bruch LV, Hollmann MW, Huhn R.. Cardioprotection by remote ischemic preconditioning is blocked in the aged rat heart in vivo. J Cardiothorac Vasc Anesth 2017;31:1223–1226 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.