Abstract
Objective
The study aims were to determine whether BST-CarGel, a chitosan scaffold for cartilage repair, can be mixed with bone marrow aspirate concentrate (BMAC) to create a cell seeded implant with comparative properties to standard BST-CarGel mixed with blood.
Design
Whole blood and bone marrow were harvested from 12 patients who underwent cartilage repair surgery using BMAC after informed consent. A validated in vitro testing model was used to assess the following 6 conditions: (1) BST-CarGel mixed with whole blood (CG-WB), (2) BST-CarGel mixed with bone marrow (CG-BM), (3) BST-CarGel mixed with bone marrow concentrate (CG-BMAC), (4) whole blood (WB), (5) bone marrow (BM), and (6) bone marrow concentrate and batroxobin (BMAC-BTX). Cell retention and viability within the BST-CarGel/BMAC clots were investigated.
Results
In our study, BM and BMAC (processed using the Harvest, SmartPrep2 system and reactivated with batroxibin) when combined with BST-CarGel produced a product that had similar clot contraction, macroscopic properties, and histological appearance to standard BSTCarGel mixed with blood. Mononucleated cells from the BMAC were retained within the scaffold and remained viable until clot dissolution in vitro.
Conclusions
By combining BST-CarGel with BMAC in the manner described, bone marrow–derived mononucleated cells can be retained within the chondral defect potentially negating the need for microfracture. Further in vivo work is required to confirm these potential benefits and determine if this combination will result in more durable cartilage repair and improved clinical outcomes.
Keywords: cartilage, bone marrow concentrate, scaffold, chitosan
Introduction
The microfracture (MF) technique represents a first-line treatment option for small- to medium-size full-thickness cartilage defects in the knee, showing a decrease in pain and improved knee function after 1- or 2-year follow-up.1-3 In marrow-stimulating procedures, perforations to the subchondral bone allow blood marrow–derived cells to migrate into the holes and defect forming a blood clot. The subsequent wound repair cascade comprised of an acute inflammatory response and cell chemotaxis leads to the generation of a vascularized granulation tissue, and the potential proliferation of pluripotent mesenchymal progenitor cells with a capacity to differentiate into multiple mesenchymal cell types.4 Bone remodeling can proceed along with the induction of chondrogenesis, that resembles endochondral ossification, to form new bone in deeper zones and fibrous or fibrocartilaginous tissues in the more superficial chondral region.1,5,6
However, longer term follow-up has demonstrated increasing failure over time with the MF technique7,8 particularly in lesions >4 cm.2,9 As a consequence, clinicians and researchers have tried to improve MF results by augmenting the blood clot with various scaffolds, a treatment termed autologous matrix induced chondrogenesis (AMIC). The quality of the data regarding the results for AMIC is currently insufficient to recommend one scaffold over another with few prospective studies comparing the results of AMIC to MF or autologous chondrocyte implantation.10
BST-CarGel (Smith & Nephew, Andover, MA) was developed by Hoemann et al. to stabilize the blood clot in the cartilage lesion following MF by dispersing a soluble polymer scaffold containing chitosan throughout the blood.11,12 Chitosan is an abundant glucosamine polysaccharide derived from the exoskeleton of crustaceans with low toxicity along with good biocompatibility, biodegradability, and tissue adhesive properties.13 By dissolving chitosan in an aqueous glycerophosphate buffer,11 BST-CarGel is obtained as a liquid chitosan solution having physiological pH. When mixed with fresh, autologous whole blood, BST-CarGel does not interfere with normal coagulation, but reinforces the resulting clot by impeding its retraction.14 The increased clot adhesivity within the lesion as a consequence of chitosan’s cationic nature ensures prolonged activation of tissue repair processes by maintaining critical blood components above the marrow access holes.12,14,15
An 80-patient prospective randomized controlled trial of BST-CarGel versus MF for the treatment of isolated full-thickness lesions in the knee resulted in more voluminous, higher quality cartilage repair at 1 year.13 The benefit was sustained over 5 years, supporting the hypothesis that BST-CarGel increases the consistency of cartilage repair and enhances long-term structural superiority compared with MF.16 The study was not powered for clinical outcome and no significant difference in clinical scores was seen between groups.
The potential flaw in the AMIC process is the continued need for the MF to provide a source of pluripotent cells in order to induce repair. The bone changes induced by the MF potentially will compromise the long-term results. Bone overgrowth following MF has been reported in up to 80% of cases,17 with its presence associated with a 10 times increased rate of failure.18 In an attempt to avoid microfracture researchers have explored the potential of using bone marrow aspirate concentrate (BMAC) as an easily accessible source of pluripotent cells, with promising results reported in the literature.19,20 There exist little data within the literature regarding the most effective scaffold/BMAC combination with previous work suggesting cell retention as a potential issue with textile-based scaffolds.21
This study aimed to determine whether BST-CarGel can be mixed with BMAC to create a cell seeded implant with comparative properties to standard BST-CarGel mixed with blood. We also aimed to confirm cell retention and viability within the BST-CarGel/BMAC clots.
Methods
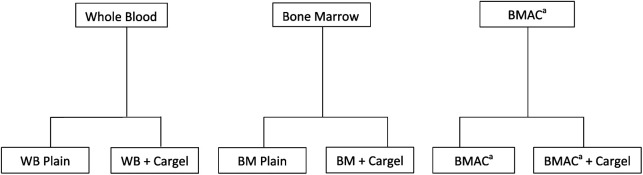
Ethical approval was obtained to perform the study from the regional ethical committee, reference 12/EE/0136. Twelve patients undergoing cartilage repair surgery using BMAC consented to take part. The in vitro testing model used was a validated model developed by the product developing team and one used to undertake quality assurance testing of the chitosan product (data on file). Six conditions were tested: (1) BST-CarGel mixed with whole blood (CG-WB), (2) BST-CarGel mixed with bone marrow (CG-BM), (3) BST-CarGel mixed with bone marrow concentrate (CG-BMAC), (4) whole blood (WB), (5) bone marrow (BM), and (6) bone marrow concentrate and batroxobin (Plateltex, Prague, Czech Republic) (BMAC-BTX). See Figure 1 .
Figure 1.
Schematic representation of the clot variants studied. BMAC, bone marrow aspirate concentrate.
aActivated using batroxobin.
For each patient, 10 mL of blood was taken at the time of anesthesia induction. Bone marrow collection was then undertaken from the anterior iliac crest by the senior author (MS) using a Jamshidi needle prerinsed with 1:1000 heparin. Sixty milliliters of bone marrow were then withdrawn into 6 × 10-mL syringes prefilled with 1 mL of 1:1000 heparin solution. Finally, 10 mL of bone marrow was withdrawn into an empty 10 mL syringe. The Harvest, SmartPrep2 system (Smart PRePVR, Harvest Technologies, Plymouth, MA) was used to prepare the BMAC as this was found to provide the optimum BM to plasma ratio on preliminary testing. The 60 mL of anticoagulated bone marrow was first transferred into the MarrowPREP filter bag (to remove bone spicules) and then the filtered marrow was transferred into the SmartPrep2 container, for centrifugation as per device instructions. After centrifugation, approximately 6 mL of BMAC was obtained on average. BST-CarGel reconstituted product was prepared as per the instructions of use prior to mixing with blood, bone marrow, and bone marrow concentrate (BMAC). Batroxobin enzyme (BTX, Plateltex, Prague, Czech Republic) was used as external clotting agent to reactivate the BMAC (which contained anticoagulant) specimen. Lyophilized BTX (5 U) was resuspended with 0.5 mL of 10% calcium gluconate (CaG). Once BST-CarGel and BMAC were mixed, BTX was added immediately before dispensing these mixtures into glass tubes.
For each of the test conditions, approximately 350 μL of mixture was placed in a glass test tube seated in a water bath at 37°C for 60 minutes. The time taken for a half sphere clot to form at the base of the test tube was recorded. The percentage clot retraction and macroscopic structure of each generated clot was evaluated at 60 minutes. Three test tubes were used for each test condition. The BST-CarGel/specimen clot samples should be “clotted” or “clotted on the edges, with a fragile or liquid” center within 15 minutes to be considered acceptable and comparable to BST-CarGel/blood clots.
Percentage of clot retraction was evaluated based on the volume of serum extruded from the clot in the test tubes during contraction. After the 60-minute clotting period at 37°C, the glass tubes were removed from the water bath and their weight individually (including the clot and serum) recorded. All liquid (serum or unclotted blood) extruded from the clot was carefully removed using a 1-mL syringe with an 18G 11⁄2 needle without disrupting the clot. The tube with only the clot inside was then weighed. The percentage of clot retraction was calculated as the percentage ratio of difference in weights between the clot samples with and without serum using the following formula (Equation 1):
| (1) |
The clots without serum from the % clot retraction test were used to assess macroscopic appearance in all groups. The clot color, firmness, and shape along with the presence of fragmentation were documented.
Histological Analysis
Toluidine blue staining method was chosen to evaluate clot homogeneity as it specifically stains each component throughout the clot: erythrocytes—blue (blue-green); white blood cells—dark purple; chitosan—light blue. Briefly, blood clots were fixed in 10% neutral buffered formalin and then cut into 2 halves through the middle and the cut face of the clot was placed face down into the embedding block. The paraffin blocks were then sectioned into 5-μm slices and mounted on glass slides. Two slides were prepared for each sample. These slides were then stained following the toluidine blue staining method and digitally scanned and viewed at magnifications up to 40× to evaluate the clot mixing homogeneity. The criteria for acceptable histology results are defined in Table 1 .
Table 1.
Criteria for Histological Grading.
| Histology Grade | Clot Characteristics |
|---|---|
| Pass | Chitosan is evenly distributed throughout the clot/between the blood cells. Small and/or larger chitosan aggregates may be present, but overall staining intensity is uniform. In addition, red blood cells have a normal/typical biconcave discoid shape. |
| Fail | Chitosan did not mix with whole blood cells (presence of regions of complete chitosan exclusion) and/or 2 separate phases are apparent. Red blood cells appear lysed or distorted (not normal/typical discoid shape). |
Cell Culture
Clots were cultured in the glass tubes in 2 mL of chondrogenic or nonchondrogenic media that was refreshed every 2 days. All clots were stored in a cell culture incubator at 37°C and 5% CO2.
Chondrogenic Media
DMEM/F-12 (50%/50% vol. mix, Gibco 21331-046, Life Technologies, Warrington, UK) with 1% vol., ITS-X (Gibco 51500-056, Life Technologies), 2.4% vol. HEPES buffer (H0887, Sigma-Aldrich, UK), 2.4%vol l-glutamine (G7513, Sigma-Aldrich, Gillingham, UK), 1% vol. penicillin/streptomycin (P4333-20ML Sigma-Aldrich), 100 nM dexamethasone (Sigma-Aldrich), 27.5 µg/mL ascorbic acid (2-phospho-l-ascorbic acid [49752-10G, Sigma-Aldrich]), 1.25 µg/mL bovine serum albumin (A8806-1G, Sigma-Aldrich), and 10 ng/mL TGF-β1 (PHG9214, Life Technologies).
Live-Dead Staining
Clots were transferred from the glass test tubes to 35-mm live cell imaging dishes (CELLview 627860, Greiner Bio-One, Stonehouse, UK). Calcein AM stock solution of 4 μM was prepared in anhydrous dimethyl sulfoxide (DMSO), while a propidium iodide stock solution of 2 mM was prepared with a 1:4 v/v mix of DMSO/ultrapure water. A working live/dead staining solution was then prepared by adding 0.5 μL of stock calcein AM and 2 μL of stock propidium to every 1 mL DMEM/F-12 culture media (2 mL of live/dead working solution is required per clot). Samples were stained by adding 2 mL of the live/dead working solution to the culture dish containing the clot and incubating for 15 minutes in a cell culture incubator at 37°C and 5% CO2.
Confocal Fluorescence Imaging
Zeiss LSM 710 confocal fluorescence unit attached to a Zeiss Observer.Z1 microscope with a heated stage (Carl Zeiss Ltd., Cambridge, UK). Live and dead dyes were excited, and the fluorescence collected independently as follows: calcein AM—excited with 488-nm laser, ~3% transmission, spectral detection range 500 to 592 nm; propidium iodide—excited with 543-nm laser, ~7% transmission, spectral detection range 595 to 704 nm. Four frames were collected per channel and averaged to form a final image slice for each channel. Images were acquired at 1024 × 1024 pixels and 16-bit color depth.
A global view of the clot was obtained by acquiring a 2-dimensional single image slice with 12-μm slice thickness using a ×10 (EC Plan-Neofluar 10×/0.30 M27) objective lens. Higher magnification 3-dimensional z-stacks were acquired using a ×20 (EC PlanN NA = 0.5 DIC) objective lens.
Results
The results of the blood, bone marrow, and BMAC analysis pre- and postcentrifugation are shown in Table 2 . The white cell concentration on average increased by a factor of 6.9 following centrifugation of the bone marrow.
Table 2.
Cell Numbers Contained Within Blood and Bone Marrow Pre- and Postcentrifugation.
| Whole Blood | Bone Marrow | BMAC | × Fold Increase | |
|---|---|---|---|---|
| WBC (109/L) range: 4-11 | 6.1 STDV 2.0 |
11.7 STDV 5.8 |
82.9 STDV 51.0 |
6.9 STDV 1.8 |
| LYMP (109/L) range: 1-4 | 1.8 STDV 0.4 |
2.3 STDV 0.4 |
14.8 STDV 3.5 |
6.7 STDV 2.7 |
| MONO (109/L) range: 0.2-0.8 | 0.5 STDV 0.2 |
0.6 STDV 0.4 |
3.6 STDV 2.7 |
6.7 STDV 2.6 |
| PLT (109/L) range: 150-450 | 181.7 STDV 39.6 |
24.2a
STDV 22.6 |
136.8 STDV 83.1 |
5.3 STDV 1.7 |
WBC = white blood cells; LYMP = lymphocytes; MONO = monocytes; PLT = platelets; STDV = standard deviation.
Platelets come from megakaryocyte cells and the low number found in bone marrow versus whole blood is normal, as it is only when megakaryocyte reach maturity that platelets are released in blood circulation.
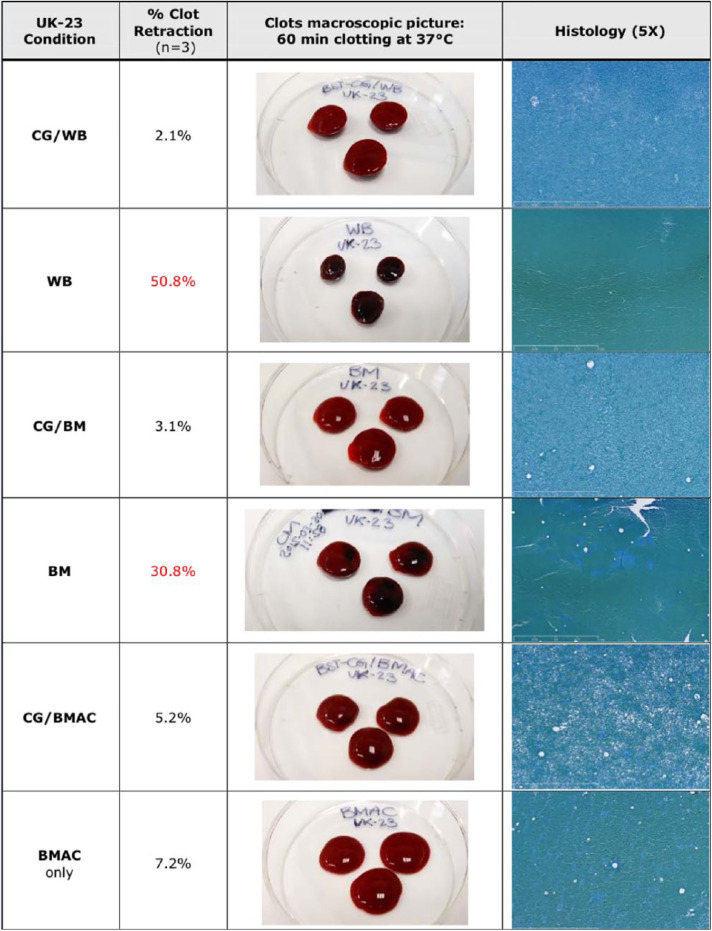
The macroscopic appearance of the BST-CarGel clots were all very similar. The clots in all BST-CG groups were classified as a pass at the 15-minute observation time point (or less). However, 2 out of 12 bone marrow specimens were not fully clotted within 15 minutes. This may be attributable to the fact that these particular samples might have been contaminated with whole blood, which would have increased the coagulation time by dilution. Indeed, it is usual to observe that the whole blood condition is not entirely clotted after 15 minutes. On the other hand, the use of batroxobin accelerated the clotting of CG-BMAC samples and reduced the clotting time to approximately 5 minutes on average. The CG-BMAC clots were softer on manipulation and handling compared to the CG-WB and CG-BM groups.
The clot retraction data for all test conditions is shown in Figure 2 . The CG-WB samples demonstrated 2.6% retraction, which is standard retraction for the BST-CG product. CG-BM demonstrated very similar contraction; however, the CG-BMAC samples had a mean contraction of 10.3%.
Figure 2.
Example of contraction data, macroscopic appearance and histology for all test conditions. Erythrocytes—blue (blue-green); white blood cells—dark purple; chitosan—light blue. CG, CarGel; BM, bone marrow; BMAC, bone marrow aspirate concentrate; WB, whole blood.
A summary of the data on clot morphology and composition for a single patient is shown in Figure 2 . CG-WB clots exhibited the typical uniform distribution of chitosan without any chitosan aggregates. Most Red blood cells had the typical biconcave morphology with small areas of agglutination. The CG-BM clots showed similar appearances with all clots classified as a pass.
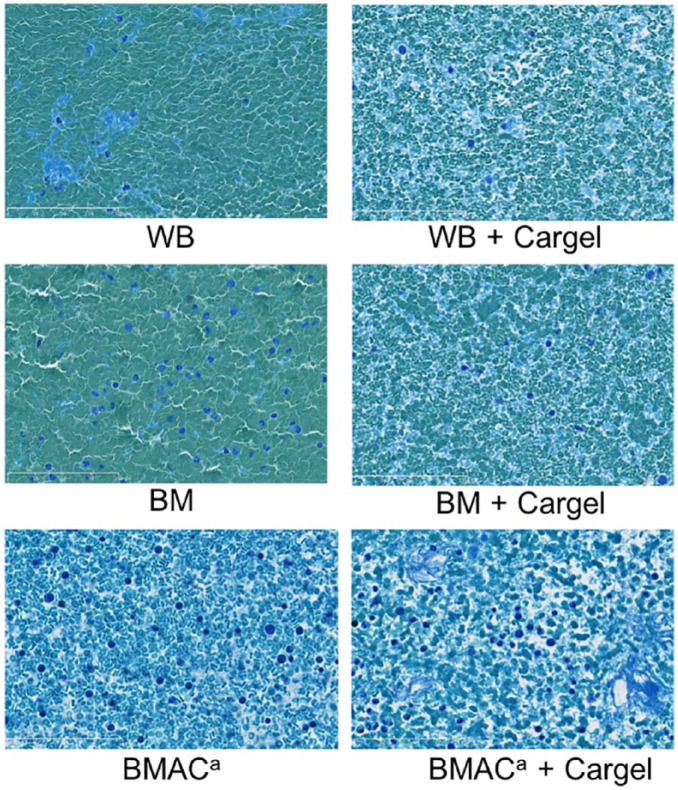
The CG-BMAC clots demonstrated mostly homogenous distribution of chitosan throughout the clot and the red blood cells (RBCs) were biconcave in shape with small areas of agglutination. However, there was variation observed in this group. Eight of 24 clots examined demonstrated roundish/swollen RBCs without the typical biconcave morphology. The space around the RBCs was filled up with a light blue “film” of uncertain origin. A high number of other cell types were visible as well as adipocytes ( Fig. 3 ).
Figure 3.
×40 Magnification toluidine blue stain of all studied clot variants. WB = whole blood; BM = bone marrow; BMAC = bone marrow aspirate concentrate. Erythrocytes—blue (blue-green); white blood cells—dark purple; chitosan—light blue.
aActivated using batroxobin.
Cell Viability
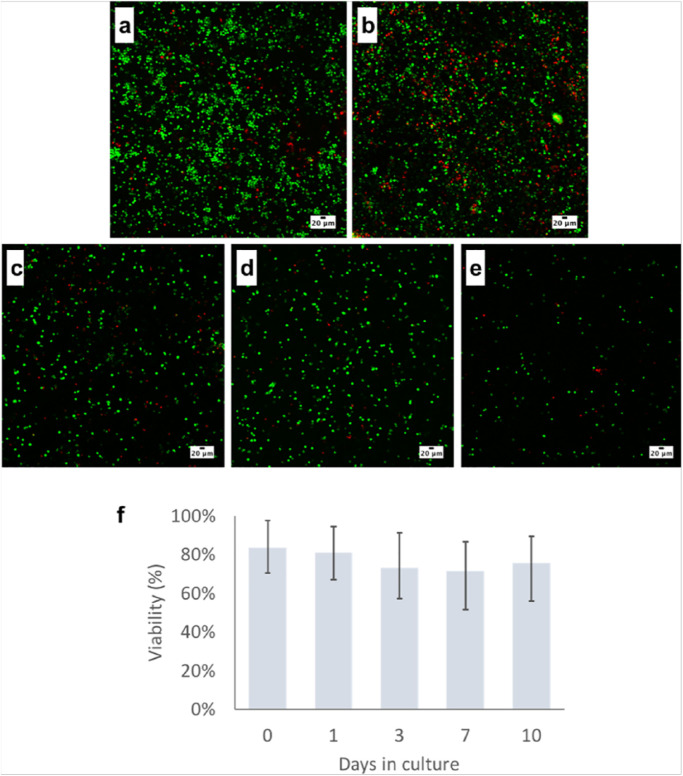
The clots started to fragment in culture between day 3 and day 5, leading to loss of mostly live cells adhered to the gel material during media changes. As can be seen over the image timepoints in Figure 4c-e , the cell density decreased across the clot fragments, which reduced the total cell count and increased the range in estimated cell viability over time. The average viability dropped to 70% to 73% at days 3 to 7 due to the loss of cells attached to broken clot fragments, but homogenization of the remaining clot and clot fragments to recover the entire cell population showed that the overall viability was still >86%.
Figure 4.
Live cell confocal fluorescence images (a-e) (z-projections) showing the live (green) and dead (red) cell populations within a single clot from the study. Images a and b were captured at day 1 in 2 locations in the clot and demonstrate the large number of viable cells spread through lateral cross-section of a clot. Images c, d, and e represent clots at days 3, 7, and 10, respectively, where good cell viability was maintained. Cell density appears to decrease gradually as the clots gradually break up in culture. The viability plot (f) presents the average cell viability across all samples grouped by timepoint with error bars representing range of viability values.
Discussion
The main findings of this study were that BM and BMAC (processed using the Harvest system and reactivated with batroxobin) when combined with BST-CarGel produced a product that had similar clot retraction, macroscopic properties and histological appearance to standard BST-CarGel mixed with blood. Mononucleated cells from the BMAC were retained within the scaffold and remained viable until clot dissolution in vitro.
MF results in bone overgrowth which reduces the volume of the cartilage regenerate.18 The subchondral bone is also postulated to become stiffer which results in increased stress on the fibrocartilage regenerate. These are thought to be some of the reasons in association with poor clot stability14 why microfracture results appear to be less durable than those for ACI—a technique that does not disrupt the subchondral bone. The application of BMAC to chondral defects has shown promising results in both animal models22 and in humans.20,23,24 The aim is to provide a source of mononucleated cells that could augment and drive host repair and consequently this would negate the need to penetrate the subchondral bone. However, there is very little literature to guide the decision as to which is the ideal scaffold to deliver and retain the cells within a chondral defect.
Bone marrow from the iliac crest has been shown by De Girolamo et al.25 to contain more cells with a mesenchymal stem cell phenotype (CD34−/CD45 low/CD271 high) (0.04%) compared with the subchondral location of the defect (0.02%) and so potentially its application in a chondral defect would result in increased mesenchymal stem cell concentration compared with that provided by MF. By centrifuging the BM to create BMAC the mononucleated cell concentration can be increased further by a factor of 6. However, the process of centrifugation requires anticoagulation which affects the normal clotting process that BST-CarGel requires to gelate. Batroxabin is a thrombin-like enzyme of Bothrops atrox moojeni venom, and it specifically cleaves fibrinogen alpha chain, resulting in the formation of non-crosslinked fibrin clots. In preliminary testing this enzyme was found to be the most effective method of reactivating the BMAC without causing excessive contraction. This is believed to be due to its mechanism of coagulation via fibrin and not platelet activation.26,27 Batroxobin has been used in clinical trials aiming to reduce perioperative blood loss in spinal surgery28 and to prevent restenosis/reocclusion following angioplasty29.
The main aim of the study was to replicate the macroscopic and histological appearance of the standard BST-CarGel using BMAC instead of whole blood, given the scaffolds proven effectiveness to improve the quality of the cartilage regenerate in both an animal model and in humans. Hoemann et al.14 demonstrated in a sheep model that the application of a chitosan-glycerol phosphate/blood clot to condylar defects resulted in a net increase in fill (52%), a modest but significant increase in hyaline character (86%) with more columnar organization and a complete restoration of normal glycosaminoglycan levels compared with MF alone.14 Stanish et al.13 retrieved 38 repair tissue biopsies at 1 year posttreatment as part of a randomized controlled trial of BST-CarGel versus MF alone in humans. They found significantly better zonal organization and collagen characteristics for the BST-CarGel biopsies over the MF biopsies. While there was no clinical difference detected between the 2 groups at 12 months, it is recognized that improved collagen content and zonal organization are necessary components for long-term durability of repair cartilage given collagen breakdown is considered to be a critical step in the progression of osteoarthritis.
The aim of BST-CarGel is to stabilize the clot produced by MF and prevent clot contraction within the defect. Bone marrow contains less platelets (as platelets are only released into the circulation through the cytoplasmic fragmentation of megakaryocytes cells) and less fibrinogen (as this protein is secreted by hepatocyte cells into the circulation) than blood. However, the percentage clot retraction of BST-CarGel/BM in this study was within the normal range of percentage clot retraction observed for BST-CarGel/blood clots. These results are likely attributable to the fact that during aspiration of bone marrow, venous blood was also aspirated. Therefore, bone marrow is never 100% composed of bone marrow elements, but it is also “contaminated” with venous blood. In this experiment, bone marrow elements were observed on histology (i.e., adipocytes cells and precursor’s cells surrounded with collagen structures) and therefore the BM specimen collected was effectively composed of bone marrow and not only of blood. CG/BMAC demonstrated contraction on average of 10%, which we feel is within an acceptable clinical range. This increased contraction is likely due to the increased platelet activation and augmented clotting process. This would be in agreement with the reduced clotting time of less than 5 minutes in this group compared with the 15 minutes in whole blood control.
Macroscopically, the CG-BMAC clots were softer compared with the CG clots generated using blood and bone marrow which is likely due to the increased cellularity of the clots. The structural properties of the CarGel clots were not objectively compared by undertaking mechanical testing and therefore were not objectively quantified during this study. The senior author (MS) has implanted CG/BMAC in vivo and the physical properties were comparable to CG-WB and appear to be sufficient to allow stable implantation.
The histology of the BST-CarGel/BMAC clots were all satisfactory and on the whole, showed similar structure to the BST/WB clots. There was even distribution of chitosan with biconcave structure of the RBCs in the majority of clots. There was variation in the CG-BMAC clots, with 36% of clots demonstrating a number of blue patches. We believe these represent de-granulated platelet aggregates, fibrin, and chitosan with no red blood cells. This was likely secondary to the heparinized blood, as calcium is not chelated, it permits platelet aggregation and partial platelet activation following the addition of batroxobin. Whether the presence of this variation would affect the scaffold performance and the clinical outcome is unknown.
The viability data demonstrated that the cells remained viable until clot disruption. The polycationic nature of chitosan facilitates adhesion to negatively charged surfaces of tissues and cells,15 including cartilage. This results in increased clot adhesivity within the lesion and contributes to cell retention. This will potentially result in prolonged activation of the tissue repair processes by maintaining greater numbers of mononucleated cells within the lesion. The importance of cell retention at the defect site has been demonstrated by Kim et al.,30 who demonstrated that implantation of mesenchymal stem cells in a chondral defect was superior compared with injection, probably secondary to more effective delivery of paracrine factors and less cell death.
While a number of investigators have assessed the growth and chondrogenic potential of bone marrow derived mesenchymal stem cells on different scaffolds,31-35 there are very few studies which have undertaken the same using BMAC. Grigolo et al.36 seeded BMAC on a nanostructured biomimetic 3-layer gradient scaffold composed of type I collagen and magnesium enriched hydroxyapatite. They were able to demonstrate that BMAC could differentiate along chondrogenic and osteogenic pathways as evaluated by the expression and production of specific matrix molecules. Cavallo et al.37 seeded 0.1 mL of BMAC on a 0.5 cm × 0.5 cm pieces of hyaluronic scaffold (Hyaff-11) and cultured them for 52 days. They demonstrated that cells within BMAC seeded onto the Hyaff-11 scaffold and were able to differentiate down a chondrogenic pathway, expressing and producing sulfated glycosaminoglycans, SOX-9, aggrecan, and collagen type II. However, this is in contradiction to the findings of Kohli et al.21 who seeded 30 μL of BMAC on Chondro-Gide, Alpha Chondro Shield, and Hyalofast. All cell-seeded scaffolds were fed with standard culture media, 3 times a week for a period of 4 weeks. Alpha Chondro Shield and Hyalofast demonstrated the best initial cell seeding with BMAC on all 3 scaffolds remaining viable after 1 day and 1 week. Very few viable cells were visualized after 7 days and no cells were seen to be incorporated in either of the 3 scaffolds after 4 weeks in culture with cell retention postulated as the main cause.
The viability tests in this study were only carried out to 7 days. This was secondary to technical difficulties due to rapid clot degradation in vitro. BST-CarGel degrades in vivo over a period of 5 weeks; however, the clots in all test groups began to lose structural integrity after 7 to 10 days in media. We hypothesized that the fragmentation occurred due to a combination regular media replacement gradually breaking its adherence to culture surfaces (despite best efforts to be careful) and loss of 3-dimensional structural support when the clots had to be transferred to imaging dishes to be examined. Culturing the clots in imaging dishes reduced the impact of damage from moving the clots but came at the expense of structural support compared with the test tube. We also believe that the decreased viscosity of the media compared with synovial fluid penetrated the clots to a greater extent resulting in their early disruption. The increased fragility over time meant it became technically difficult to avoid scaffold and cell loss and consequently a decision was made not to image beyond the 7-day period.
The results of this study confirm that BMAC can be combined with BST-CarGel when prepared in a specific manner to produce a scaffold that has similar properties to standard BST-CarGel mixed with blood. BST-CarGel has previously been shown to be a biologically active scaffold, improving histological repair following microfracture. By combining BST-CarGel with BMAC in the manner described, bone marrow–derived mononucleated cells can be retained within the chondral defect potentially negating the need for microfracture. Further in vivo work is required to confirm these potential benefits and determine if this combination will result in more durable cartilage repair and improved clinical outcomes.
Footnotes
Authors’ Note: The study was conducted at both the Royal Orthopaedic Hospital NHS Foundation Trust and the School of Chemical Engineering at the University of Birmingham.
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The funding for this study was provided through a research grant by Piramal and Smith & Nephew.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Martyn Snow is a consultant for Smith & Nephew.
Ethical Approval: Ethical approval was obtained to perform the study from the regional ethical committee (reference 12/EE/0136).
Informed Consent: Written informed consent was obtained from all subjects before the study.
Trial Registration: Not applicable.
ORCID iD: Joseph Pagkalos  https://orcid.org/0000-0002-9890-9831
https://orcid.org/0000-0002-9890-9831
References
- 1. Gill TJ, Asnis PD, Berkson EM. The treatment of articular cartilage defects using the microfracture technique. J Orthop Sports Phys Ther. 2006;36(10):728-38. doi: 10.2519/jospt.2006.2444 [DOI] [PubMed] [Google Scholar]
- 2. Knutsen G, Drogset JO, Engebretsen L, Grøntvedt T, Ludvigsen TC, Løken S. et al. A randomized multicenter trial comparing autologous chondrocyte implantation with microfracture: long-term follow-up at 14 to 15 years. J Bone Joint Surg Am. 2016;98(16):1332-9. doi: 10.2106/JBJS.15.01208 [DOI] [PubMed] [Google Scholar]
- 3. Mithoefer K, Williams RJ, 3rd, Warren RF, Potter HG, Spock CR, Jones EC. et al. The microfracture technique for the treatment of articular cartilage lesions in the knee. A prospective cohort study. J Bone Joint Surg Am. 2005;87(9):1911-20. doi: 10.2106/JBJS.D.02846 [DOI] [PubMed] [Google Scholar]
- 4. Compston JE. Bone marrow and bone: a functional unit. J Endocrinol. 2002;173(3):387-94. [DOI] [PubMed] [Google Scholar]
- 5. Shapiro F, Koide S, Glimcher MJ. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1993;75(4):532-53. [DOI] [PubMed] [Google Scholar]
- 6. Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;(391 Suppl):S362-S369. doi: 10.1097/00003086-200110001-00033 [DOI] [PubMed] [Google Scholar]
- 7. Solheim E, Hegna J, Inderhaug E, Øyen J, Harlem T, Strand T. Results at 10-14 years after microfracture treatment of articular cartilage defects in the knee. Knee Surg Sports Trau-matol Arthrosc. 2016;24(5):1587-93. doi: 10.1007/s00167-014-3443-1 [DOI] [PubMed] [Google Scholar]
- 8. Mithoefer K, Gill TJ, Cole BJ, Williams RJ, Mandelbaum BR. Clinical outcome and return to competition after microfracture in the athlete’s knee. Cartilage. 2010;1(2):113-20. doi: 10.1177/1947603510366576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goyal D, Keyhani S, Lee EH, Hui JHP. Evidence-based status of microfracture technique: a systematic review of level I and II studies. Arthroscopy. 2013;29(9):1579-88. doi: 10.1016/j.arthro.2013.05.027 [DOI] [PubMed] [Google Scholar]
- 10. Gao L, Orth P, Cucchiarini M, Madry H. Autologous matrix-induced chondrogenesis: a systematic review of the clinical evidence. Am J Sports Med. Epub 2017 November 1. doi: 10.1177/0363546517740575 [DOI] [PubMed] [Google Scholar]
- 11. Chenite A, Chaput C, Wang D, Combes C, Buschmann MD, Hoemann CD. et al. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials. 2000;21(21):2155-61. [DOI] [PubMed] [Google Scholar]
- 12. Hoemann CD, Hurtig M, Rossomacha E, Sun J, Chevrier A, Shive MS. et al. Chitosan-glycerol phosphate/blood implants improve hyaline cartilage repair in ovine microfracture defects. J Bone Joint Surg Am. 2005;87(12):2671-86. doi: 10.2106/JBJS.D.02536 [DOI] [PubMed] [Google Scholar]
- 13. Stanish WD, McCormack R, Forriol F, Mohtadi N, Pelet S, Desnoyers J. et al. Novel scaffold-based BST-CarGel treatment results in superior cartilage repair compared with microfracture in a randomized controlled trial. J Bone Joint Surg Am. 2013;95(18):1640-50. doi: 10.2106/JBJS.L.01345 [DOI] [PubMed] [Google Scholar]
- 14. Hoemann CD, Sun J, McKee MD, Chevrier A, Rossomacha E, Rivard GE. et al. Chitosan-glycerol phosphate/blood implants elicit hyaline cartilage repair integrated with porous subchondral bone in microdrilled rabbit defects. Osteoarthritis Cartilage. 2007;15(1):78-89. doi: 10.1016/j.joca.2006.06.015 [DOI] [PubMed] [Google Scholar]
- 15. Chevrier A, Hoemann CD, Sun J, Buschmann MD. Chitosan-glycerol phosphate/blood implants increase cell recruitment, transient vascularization and subchondral bone remodeling in drilled cartilage defects. Osteoarthritis Cartilage. 2007;15(3):316-27. doi: 10.1016/j.joca.2006.08.007 [DOI] [PubMed] [Google Scholar]
- 16. Shive MS, Stanish WD, McCormack R, Forriol F, Mohtadi N, Pelet S. et al. BST-CarGel® treatment maintains cartilage repair superiority over microfracture at 5 years in a multicenter randomized controlled trial. Cartilage. 2015;6(2):62-72. doi: 10.1177/1947603514562064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shive MS, Restrepo A, Totterman S, Tamez-Peña J, Schreyer E, Steinwachs M. et al. Quantitative 3D MRI reveals limited intra-lesional bony overgrowth at 1 year after microfracture-based cartilage repair. Osteoarthritis Cartilage. 2014;22(6):800-4. doi: 10.1016/j.joca.2014.03.020 [DOI] [PubMed] [Google Scholar]
- 18. Mithoefer K, Venugopal V, Manaqibwala M. Incidence, degree, and clinical effect of subchondral bone overgrowth after microfracture in the knee. Am J Sports Med. 2016;44(8):2057-63. doi: 10.1177/0363546516645514 [DOI] [PubMed] [Google Scholar]
- 19. Holton J, Imam MA, Snow M. Bone marrow aspirate in the treatment of chondral injuries. Front Surg. 2016;3:33. doi: 10.3389/fsurg.2016.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gobbi A, Karnatzikos G, Sankineani SR. One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee. Am J Sports Med. 2014;42(3):648-57. doi: 10.1177/0363546513518007 [DOI] [PubMed] [Google Scholar]
- 21. Kohli N, Snow M, Johnson E. A comparison of culture expanded mesenchymal stem cells versus minimally manipulated bone marrow cells for their growth and chondrogenic differentiation in clinically relevant cell scaffolds with potential for cartilage repair. Orthop Proc. 2014;96-B(Suppl 11):239. doi: 10.1302/1358-992X.96BSUPP_11.CORS2013-239 [DOI] [Google Scholar]
- 22. Zhang Y, Wang F, Chen J, Ning Z, Yang L. Bone marrow-derived mesenchymal stem cells versus bone marrow nucleated cells in the treatment of chondral defects. Int Orthop. 2012;36(5):1079-86. doi: 10.1007/s00264-011-1362-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chahla J, Dean CS, Moatshe G, Pascual-Garrido C, Serra Cruz R, LaPrade RF. Concentrated bone marrow aspirate for the treatment of chondral injuries and osteoarthritis of the knee: a systematic review of outcomes. Orthop J Sports Med. 2016;4(1):2325967115625481. doi: 10.1177/2325967115625481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chang F, Ishii T, Yanai T, Mishima H, Akaogi H, Ogawa T. et al. Repair of large full-thickness articular cartilage defects by transplantation of autologous uncultured bone-marrow-derived mononuclear cells. J Orthop Res. 2008;26(1):18-26. doi: 10.1002/jor.20470 [DOI] [PubMed] [Google Scholar]
- 25. de Girolamo L, Bertolini G, Cervellin M, Sozzi G, Volpi P. Treatment of chondral defects of the knee with one step matrix-assisted technique enhanced by autologous concentrated bone marrow: in vitro characterisation of mesenchymal stem cells from iliac crest and subchondral bone. Injury. 2010;41(11):1172-77. doi: 10.1016/j.injury.2010.09.027 [DOI] [PubMed] [Google Scholar]
- 26. Carr ME, Jr, Carr SL, Tildon T, Fisher LM, Martin EJ. Batroxobin-induced clots exhibit delayed and reduced platelet contractile force in some patients with clotting factor deficiencies. J Thromb Haemost. 2003;1(2):243-9. doi: 10.1046/j.1538-7836.2003.00021.x [DOI] [PubMed] [Google Scholar]
- 27. Carr ME, Jr, Carr SL, Greilich PE. Heparin ablates force development during platelet mediated clot retraction. Thromb Haemost. 1996;75(4):674-8. [PubMed] [Google Scholar]
- 28. Nagabhushan RM, Shetty AP, Dumpa SR, Subramanian B, Kanna RM, Shanmuganathan R. Effectiveness and safety of batroxobin, tranexamic acid and a combination in reduction of blood loss in lumbar spinal fusion surgery. Spine (Phila Pa 1976). 2018;43(5):E267-E273. doi: 10.1097/BRS.0000000000002315 [DOI] [PubMed] [Google Scholar]
- 29. Wang J, Zhu YQ, Li MH, Zhao JG, Tan HQ, Wang JB. et al. Batroxobin plus aspirin reduces restenosis after angioplasty for arterial occlusive disease in diabetic patients with lower-limb ischemia. J Vasc Interv Radiol. 2011;22(7):987-94. doi: 10.1016/j.jvir.2011.03.015 [DOI] [PubMed] [Google Scholar]
- 30. Kim YS, Kwon OR, Choi YJ, Suh DS, Heo DB, Koh YG. Comparative Matched-pair analysis of the injection versus implantation of mesenchymal stem cells for knee osteoarthritis. Am J Sports Med. 2015;43(11):2738-46. doi: 10.1177/0363546515599632 [DOI] [PubMed] [Google Scholar]
- 31. Bosnakovski D, Mizuno M, Kim G, Takagi S, Okumura M, Fujinaga T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol Bioeng. 2006;93(6):1152-63. doi: 10.1002/bit.20828 [DOI] [PubMed] [Google Scholar]
- 32. Gigante A, Manzotti S, Bevilacqua C, Orciani M, Di Primio R, Mattioli-Belmonte M. Adult mesenchymal stem cells for bone and cartilage engineering: effect of scaffold materials. Eur J Histochem. 2008;52(3):169-74. [DOI] [PubMed] [Google Scholar]
- 33. Kohli N, Wright KT, Sammons RL, Jeys L, Snow M, Johnson WE. An in vitro comparison of the incorporation, growth, and chondrogenic potential of human bone marrow versus adipose tissue mesenchymal stem cells in clinically relevant cell scaffolds used for cartilage repair. Cartilage. 2015;6(4):252-63. doi: 10.1177/1947603515589650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jakobsen RB, Shahdadfar A, Reinholt FP, Brinchmann JE. Chondrogenesis in a hyaluronic acid scaffold: comparison between chondrocytes and MSC from bone marrow and adipose tissue. Knee Surg Sports Traumatol Arthrosc. 2010;18(10):1407-16. doi: 10.1007/s00167-009-1017-4 [DOI] [PubMed] [Google Scholar]
- 35. You M, Peng G, Li J, Ma P, Wang Z, Shu W. et al. Chondrogenic differentiation of human bone marrow mesenchymal stem cells on polyhydroxyalkanoate (PHA) scaffolds coated with PHA granule binding protein PhaP fused with RGD peptide. Biomaterials. 2011;32(9):2305-13. doi: 10.1016/j.biomaterials.2010.12.009 [DOI] [PubMed] [Google Scholar]
- 36. Grigolo B, Cavallo C, Desando G, Manferdini C, Lisignoli G, Ferrari A. et al. Novel nano-composite biomimetic biomaterial allows chondrogenic and osteogenic differentiation of bone marrow concentrate derived cells. J Mater Sci Mater Med. 2015;26(4):173. doi: 10.1007/s10856-015-5500-9 [DOI] [PubMed] [Google Scholar]
- 37. Cavallo C, Desando G, Columbaro M, Ferrari A, Zini N, Facchini A. et al. Chondrogenic differentiation of bone marrow concentrate grown onto a hylauronan scaffold: Rationale for its use in the treatment of cartilage lesions. J Biomed Mater Res A. 2013;101(6):1559-70. doi: 10.1002/jbm.a.34460 [DOI] [PubMed] [Google Scholar]