Abstract
Objective
To evaluate the effects of TRB-N0224, a chemically modified curcumin (CMC) with zinc binding properties and improved pharmacokinetics, in a rabbit anterior cruciate ligament (ACL) transection injury–induced model of osteoarthritis (OA).
Design
Thirty-eight skeletally mature New Zealand white rabbits were studied in 4 groups: a sham with arthrotomy (n = 6), control with ACL transection (n = 6), and 2 treatment groups with ACL transection and administration of TRB-N0224 at low (25 mg/kg/day) (n = 13) and high (50 mg/kg/day) (n = 13) doses. After euthanization at 12 weeks, outcomes were measured by post-necropsy gross morphology, biomechanics, and cartilage and synovium histology. Rabbit blood ELISA quantified cytokine and matrix metalloproteinase (MMP) concentrations at 0, 4, 8, and 12 weeks.
Results
Both treatment doses had fewer distal femoral condyle erosive defects than the control; the low dose demonstrated a mean 78% decrease (P < 0.01). Histologically, the low- and high-dose treatment groups had fewer cartilage pathologic changes and less severe synovitis than the control. CMC alone did not have a major effect on the biomechanics of healthy cartilage or cartilage in the ACL transection model, as demonstrated in 5 of the 6 measured properties/regions (P < 0.05). ELISA results suggested that the key mediators of OA, (interleukin) IL-1β, IL-6, TNFα (tumor necrosis factor-α), MMP-9, and MMP-13, had decreased concentrations with TRB-N0224 treatment at different time points between weeks 4 to 12 (P < 0.05).
Conclusions
In the pathogenesis of OA, an imbalance exists between catabolic and anabolic mediators. These results suggest the potential of TRB-N0224 to modulate MMP and cytokine levels, slowing the macroscopic and histopathological progression of OA.
Keywords: osteoarthritis, chemically modified curcumin, MMP inhibitors, inflammation, cytokines, DMOAD
Introduction
The pathophysiology of osteoarthritis (OA) involves the dysregulated production of inflammatory mediators IL-1β (interleukin), IL-6, and TNFα (tumor necrosis factor-α) by activated chondrocytes, synoviocytes, and mononuclear cells.1,2 IL-1β stimulates the activation of matrix metalloproteinases (MMPs), suppresses synthesis of type II collagen and aggrecan (aggregating proteoglycan), and induces chondrocyte apoptosis.3,4 IL-6 has a similar role, activating MMPs and reducing type II collagen expression.5 TNFα plays an additive part by inducing production of IL-6, while also stimulating the activation of MMPs and suppressing type II collagen and aggrecan synthesis.4,6 MMPs are zinc-dependent enzymes that degrade aggrecan and collagen, the principle components of articular cartilage, by enzymatic cleavage.1 Inducible, unbalanced MMPs can reach pathological levels leading to chondrocyte apoptosis and plays a central role in OA.7,8
TRB-N0224 is a chemically modified curcumin (CMC) that combined the known effects of tetracyclines and curcumin, a component of turmeric, on inflammatory mediators in OA. Tetracyclines inhibit MMPs by binding zinc in the enzyme’s catalytic site.9 Periostat and Oracea are non-antimicrobial doses of doxycycline, approved for the treatment of periodontal disease and rosacea by inhibiting MMPs. However, the dosage of these drugs is limited by their side effects and the potential development of antibiotic resistant bacteria.10-12 Curcumin has been shown to have anti-inflammatory properties through suppression of the NFκB pathway.13 However, unmodified curcumin has poor solubility, rapid metabolism, and low biological activity resulting from the low plasma and tissue levels.14,15 To address these limitations, a 20% curcuminoid, compared to the natural 5% of turmeric, curcumin-phosphatidyl choline complex called Meriva, was developed.14,16 A study of 50 OA patients found Meriva improved patient reported outcomes (WOMAC scores) by 58%, decreased joint pain and improved function at 3 months and 8 months, and decreased IL-6 and IL-1β plasma levels.17
TRB-N0224 (CMC) is a novel and proprietary phenyl amino carbonyl analog of curcumin with the zinc-binding features of tetracyclines. Curcumin was selected as a parent structure for 2 reasons: it has a 1,3-diketone moiety akin to tetracyclines and the inherent anti-inflammatory properties of curcumin itself. The pharmacokinetic properties of curcumin were improved by addition of an electron-withdrawing group that increased the acidic character of the enolic system. This significantly improved the solubility and decreased the metabolism rate of CMC with no observed toxicity to date. In our preliminary studies, CMC was shown to have pleiotropic anti-inflammatory effects, most likely as a MMP modulator and cytokine inhibitor. CMC was studied in vitro for chondroprotective effects against IL-1β and Onctostatin M–induced chondrolysis in a bovine femoral condyle articular cartilage model by measuring 35S sulfate release at 24, 48, and 72 hours.18 CMC demonstrated a chondroprotective effect, as evidenced by a lowered release of 35S at all time points and improved histological outcomes.18
Hypothesis
Our aim was to evaluate the efficacy of CMC in an in vivo rabbit injury–induced model that closely resembled the human OA disease progression. We hypothesized that administration of CMC will lower tissue and serum levels of pro-inflammatory cytokines and MMPs, and that decreased levels of these inflammatory mediators will prevent OA progression macroscopically, histologically, and biomechanically.
Methods
Animals and Surgical Model
The Institutional Animal Care and Use Committee (IACUC) (Protocol #2014-013) approved this study. Thirty-eight skeletally mature male New Zealand white rabbits (4.5 kg average weight) were randomized into 4 groups. These included one sham group with arthrotomy to anterior cruciate ligament (ACL) exposure without transection (n = 6) and 3 groups with ACL transection: a control without additional treatment (n = 6), a low-dose (25 mg/kg/day) CMC (n = 13) treatment group, and a high-dose (50 mg/kg/day) CMC (n = 13) treatment group ( Table 1 ).
Table 1.
Representation of the 4 Groups Studied.
| Groups | n | ACL Transection | TRBN0224 (CMC) |
|---|---|---|---|
| Control | 6 | Yes | No |
| Sham | 6 | No | No |
| High dose | 13 | Yes | 50 kg/mg/day |
| Low dose | 13 | Yes | 25 kg/mg/day |
ACL = anterior cruciate ligament; CMC = curcumin.
Rabbits were given a pre-anesthetic injection of ketamine (26 mg/kg), xylazine (0.78 mg/kg), and maintained on isoflourane (1.5% to 3%). Cephalotin (13 mg/kg) was given pre-surgery and post-surgery for 2 days at 12-hour intervals with butorphanol (0.1 to 0.5 mg/kg) at 4-hour intervals. The ACL was exposed through an aseptic, medial para-patellar arthrotomy with lateral patellar dislocation19,20 and transected approximately mid-ACL with a number-11 scalpel blade. The sham operation exposed the ACL identically to transection groups, but without ligament transection. The joint capsule and skin was closed in layers with 4-0 vicryl resorbable sutures. No significant complications occurred during surgery or post-surgery. CMC was administered systemically by daily oral gavage at 2 doses (25 or 50 mg/kg); the control and sham rabbits did not receive daily oral gavage. Rabbits were euthanized 12 weeks post-surgery.
Macroscopic Examination
The rabbit distal femoral condyles and tibial plateaus were exposed and photographed post necropsy. The articular surfaces were analyzed for osteophyte formation, erosions, and other abnormalities of the total condyle surface area. The program Image J from the National Institutes of Health was used to quantify the percentage of surface area containing erosions.
Biomechanical Testing
For biomechanics, rabbit condyles were thawed at room temperature and were kept hydrated in a phosphate-buffered saline bath during testing. The femur epiphyses were cut flat with a band saw, and the cut surface was mounted to a custom sample holder using cyanoacrylate glue. Positioning of the cartilage surface was adjusted to achieve perpendicular contact between indenter and the cartilage surface using a ball and socket tilt stage mounted on top of an x,y-micromanipulator linear stage (Newport). Indentation testing was performed at the central part of both the lateral and medial femoral condyles on regions of intact cartilage, to ensure full contact with the indenter. Indentation tests were performed using a spherical stainless steel indenter (1.5 mm radius) attached to a material test frame (Instron 5566) through a custom holder, and loads were measured with a 10 N load cell. After establishing contact with the articular surface, an instantaneous step indentation of 100 µm was applied, and the resulting load was measured until equilibrium. Force and displacement data were analyzed using Hertzian biphasic theory (HBT), adapted from approach by Moore et al., to simultaneously quantify cartilage contact (compressive) modulus (EY−), tensile modulus (EY+), and permeability (k).21 Tissue thickness was then measured at the site of indentation using a needle indenter. Tissue thickness was determined from the force versus needle distance curve, which has sharp peak corresponding to the subchondral bone as the needle indenter is pushed through the cartilage layer. The indentation and thickness measurement was performed on the medial and lateral femoral condyles, while randomizing the order of testing. Analysis of the contralateral knees was also performed to determine the effects of the systemic oral CMC administration (labeled “drug”) on the noninjured joints.
Histologic Analysis
Histological preparation followed ORS international guidelines.22 After the necropsy at 12 weeks, 2 samples from the control and sham groups as well as 5 samples from the high- and low-dose groups were used for cartilage histology. The whole knee specimen was fixed in 10% formalin and subsequently decalcified in 5% formic acid. Five-micrometer-thick sections of the tibial femoral condyle were stained with safranin-O/fast green. Magnification digital images (10×) were captured on an Olympus BX-60 microscope. The histological progression was graded in a blinded fashion according to the Mankin scale for OA, a 14-point score involving cellular changes, architectural changes, and presence of safranin-O matrix staining.22-24 One sample of synovium was taken from the control sham group, and 2 samples from the high- and low-dose groups for synovium histology. Each sample was also fixed and stained with hematoxylin-eosin. The synovium was graded in a blinded fashion for severity of synovitis, taking into account lining cell layer hyperplasia, resident cellular density, and leukocyte infiltrate.25,26
ELISA
Rabbit blood was collected and analyzed by the RayBio Rabbit ELISA Kit for Cell Culture Supernatants, Plasma, and Serum samples. The analytes quantified using ELISA were IL-1β, IL-6, TNFα, MMP-9, and MMP-13. Blood was drawn at 0, 4, 8, and 12 weeks; week 0 was immediately prior to surgery. Whole rabbit blood was collected from each animal into a BD vacutainer without additives, kept at room temperature for approximately 20 minutes, and then centrifuged for 10 minutes at 3,000 rpm. Aliquots of the serum were either used immediately or stored at −80°C and brought to room temperature (18-25°C) along with the reagents before use. The serum samples were diluted 2-fold and 5-fold, and were run in duplicate. All reagents, samples, and standards were prepared as per manufacturer’s instructions. Briefly, 100 µL standard or sample was added to each well and incubated for 2.5 hours at room temperature. One hundred microliters of prepared biotin antibody was then added and the samples incubated an additional hour at room temperature. Next, 100 µL of prepared streptavidin solution was added and the samples incubated for another 45 minutes at room temperature. Last, 100 µL TMB One-Step Substrate Reagent was added and the samples incubated for 30 minutes at room temperature. Fifty microliters of Stop Solution was then added to each well and absorbance measured at 450 nm immediately by spectrophotometry.
Statistical Analysis
For the macroscopic results, a one-way ANOVA was used to test for differences in osteophytes and erosive lesions between the 4 groups followed by the Bonferroni post hoc test. Significance was set at P < 0.05, and statistical analysis was performed in SPSS.
For biomechanics, we did not observe a dose response of CMC on measured properties; therefore, the low- and high-dose groups were pooled together. A 2-way ANOVA was performed to determine effect of injury (ACL transection) and effect of treatment (CMC) on mechanical properties and tissue thickness. Group comparisons were performed with using LSD post hoc test, with P < 0.05 considered significant. Statistical analysis was performed in Statistica.
Descriptive statistics alone, the mean and standard deviation, were compared for histological outcomes, as a result of the small sample size.
A 2-way repeated-measures ANOVA with estimated marginal means was employed to evaluate the effect of CMC and time on cytokine and MMP inhibitor concentrations between the treatment groups. A Bonferroni multiple comparisons post hoc analysis was subsequently used to determine differences between groups with significance set at P < 0.05. Statistical analysis was performed in SPSS.
Results
Macroscopic Features of Osteoarthritis
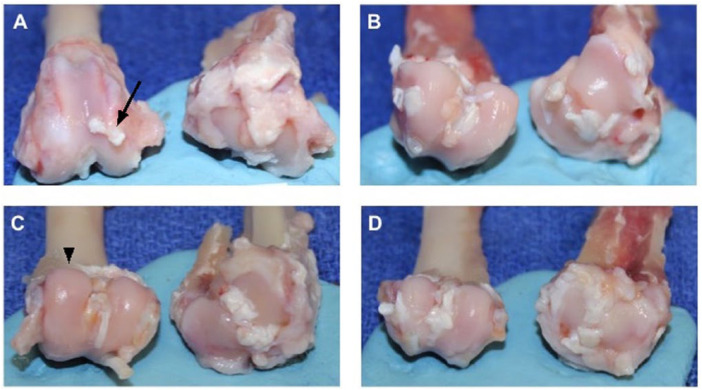
Macroscopic measurements of OA progression included gross fibrillation, osteophyte formation, and bone eburnation ( Fig. 1 ). The control group exhibited a significantly higher percentage of surface erosions on the distal femoral condyle as compared to the sham and low-dose groups ( Fig. 1A vs. B , C , respectively). The high-dose group exhibited a similar trend to the low-dose group ( Fig. 1D vs. C ). After 12 weeks, the low-dose group had a mean of 77.8% fewer articular cartilage erosive lesions on both the femoral condyle and tibial plateau than the control. The articular surfaces in the high- and low-dose treatment groups specifically exhibited fewer osteophytes than the control group, but this finding did not reach statistical significance ( Fig. 2 ).
Figure 1.
Exposed distal femoral condyles (left) and tibial plateau (right). (A) Control demonstrates cartilage damage, osteophytes (arrow), and eburnated bone on medial tibial plateau. (B) The sham cartilage appears smooth and glistening (arrow head) with no fibrillations on both articular surfaces compared to control. (C) The low-dose and (D) high-dose treatment groups exhibit fewer focal areas of fibrillation and osteophytes than control.
Figure 2.
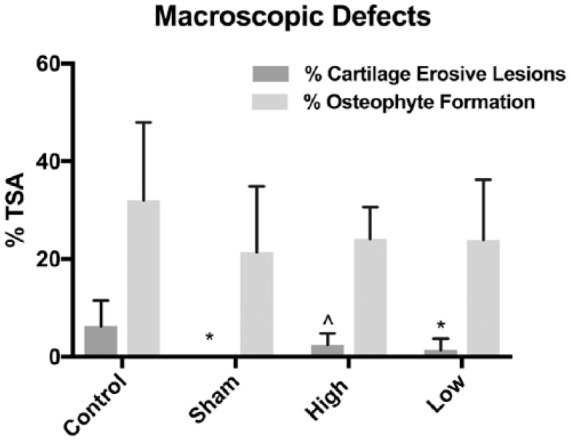
Differences in cartilage erosive lesions and osteophyte formation between groups. Each data set is presented normalized (percentage) relative to the total surface area (TSA) of the distal femoral condyle. *Signifies P < 0.01 versus control group, and ^indicates P = 0.0595 (trend).
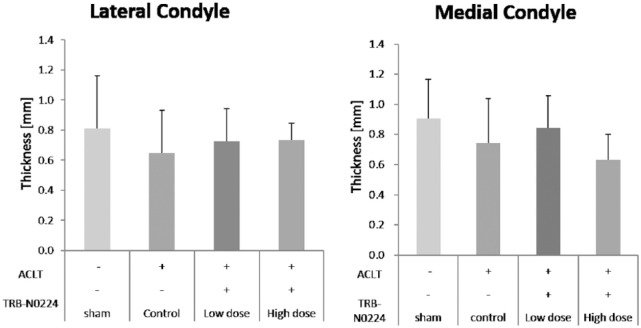
Altered Cartilage Biomechanics
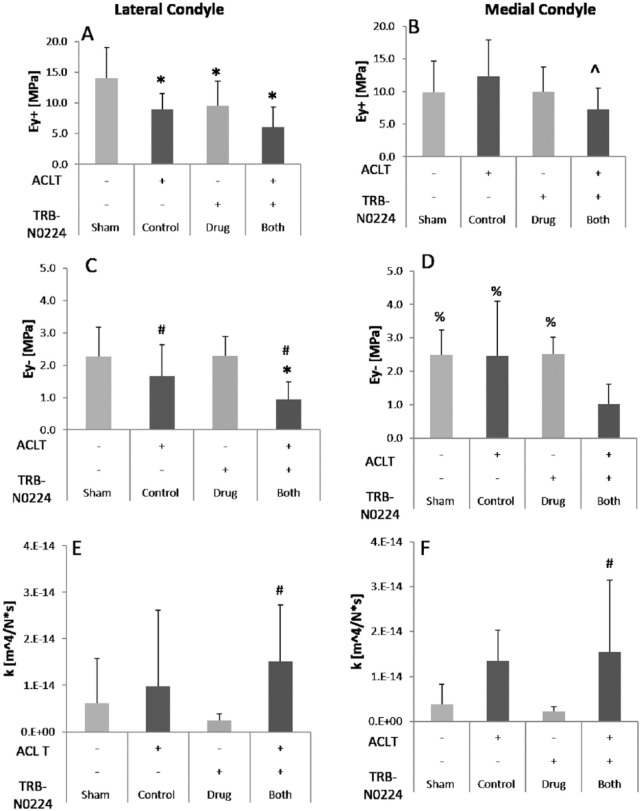
The HBT analysis yielded high-quality fits of the measured biomechanical data (R2 = 0.85 to 0.9). The cartilage thickness was found to be similar across all groups in both the lateral and media femoral condyles, with a mean thickness of 0.76 ± 0.33 mm and 0.91 ± 0.26 mm in sham groups, respectively ( Fig. 3 ). In evaluating Ey+, a significant effect of ACL injury (P = 0.007) and treatment (P = 0.017) was observed in the lateral compartment, but not the medial femoral condyle. Ey+ was significantly lower in the control lateral condyle (of the joints that received ACL transection) versus sham group. Oral administration of CMC reduced lateral Ey+ compared to sham, even in the presence of ACL transection ( Fig. 4A ). In the medial compartment, Ey+ in ACLT+CMC (TRB-N0224) treated groups was lower than control (ACLT only) group ( Fig. 4B ). In evaluating EY−, a significant effect of ACL injury was observed in the lateral (P = 0.0016) and medial (P = 0.038) condyles. In the lateral condyle, administration of CMC resulted in significantly higher E-− compared to control ( Fig. 4c ). In evaluating permeability (k), group comparisons indicated that ACLT + CMC groups had higher permeability than drug administration alone ( Fig. 4E and F ).
Figure 3.
Cartilage tissue thickness in lateral and medial femoral condyles at site of indentation. No significant effect of surgery or treatment administration was observed on cartilage thickness.
Figure 4.
Indentation testing of the lateral (left) and medial (right) femoral condyle cartilage. (A, B) Tensile modulus (Ey+); (C, D) Compressive modulus (EY−); (E, F) Permeability (k). Lateral condyle had more sensitivity to biomechanical changes compared to medial condyle. A significant effect of surgery was observed in lateral Ey+ (A: P < 0.006), as well as lateral and medial EY− (C: P < 0.002; D: P < 0.05). A significant effect of treatment was observed in lateral Ey+ (A: P < 0.02) and medial EY− (D: P = 0.053). *Denotes P < 0.05 when compared to “Sham”; # signifies P < 0.05 compared to “Drug”; ^ signifies P < 0.05 compared to “Control”; % signifies P < 0.05 compared to “Both.”
Histopathologic Features
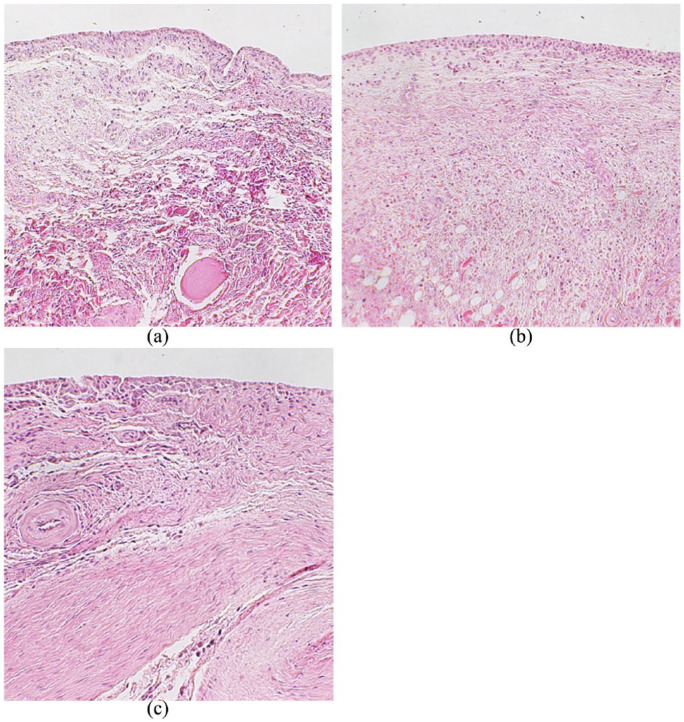
Control synovium samples had a higher-grade synovitis than the high- or low-dose treatment groups. The control group exhibited a mean score of 3, while the high- and low-dose groups resulted in mean scores of 2 and 1.50, respectively. The histopathological changes seen in the control specimens included moderate reactive synovial hyperplasia of the cell lining layer and an increase in density of the resident cells ( Fig. 5A ), as compared to a mild increase in cell lining layer and local inflammatory cell density in the low- and high-dose groups ( Fig. 5B and C ).
Figure 5.
Synovium histopathological samples at 10× magnification. The control group (A) had enlargement of the synovial lining cell layer and increased density of resident cells. The low-dose group (B) demonstrated mild reactive synovial hyperplasia. The high-dose group exhibited the lowest grade synovitis, with a single cell lining layer to the right (C).
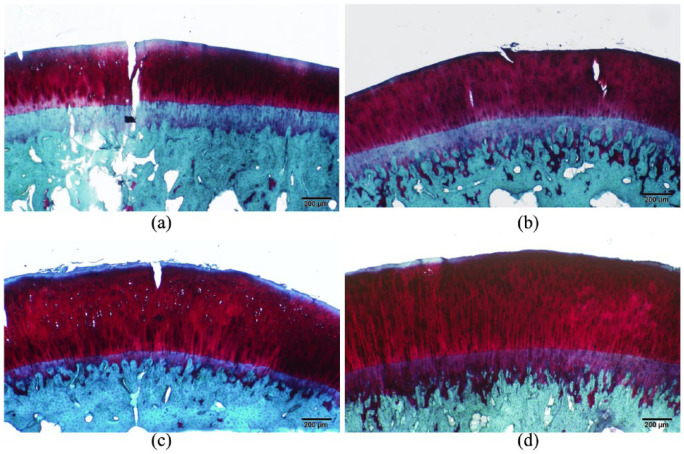
The articular cartilage histopathology showed similar results. The Mankin scale for histopathological grading found lower mean scores in the low (2.60 ± 0.548) and high (1.25 ± 1.258) dose treatment groups as compared to the control (3.5 ± 0.707). The control group exhibited a greater number of features of OA progression, including fissuring at the articular surface extending down to the subchondral plate, decreased safranin-O staining indicative of decreased aggrecan concentration, and other structural abnormalities ( Fig. 6 ). The sham group did not exhibit these histopathological features of OA (0 ± 0.0).
Figure 6.
Histopathological images of the tibial femoral condyle cartilage: The control (A) demonstrated fissuring and reduction of safranin-O staining. The sham (B) and high-dose (D) groups were nearly indistinguishable, representing normal articular cartilage. The low-dose group (C) exhibited mild fissuring.
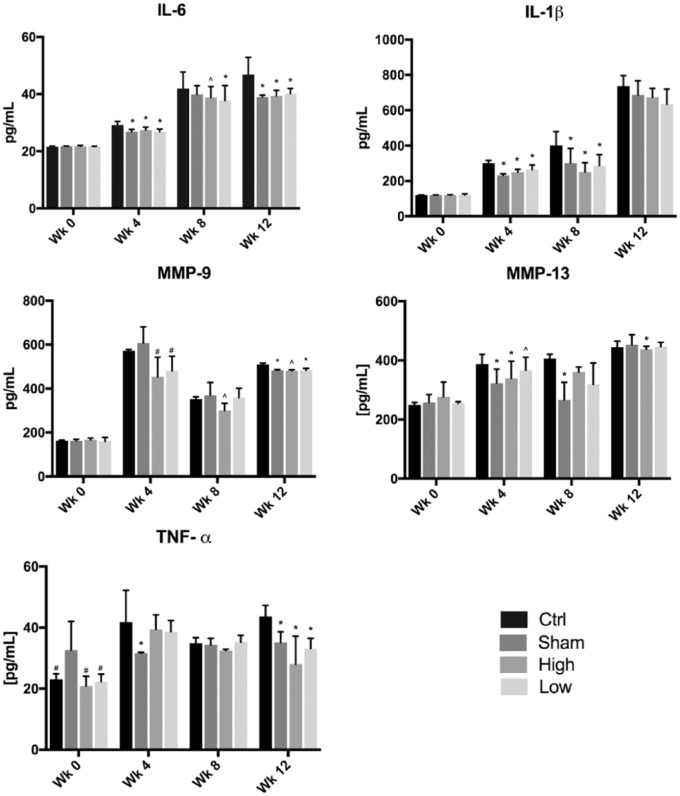
The Effect of CMC on Cytokines and Matrix Metalloproteinases
At week 0, no significant inflammatory mediator concentration differences existed between groups. At week 4, MMP-13, IL-6, and IL-1β showed significantly decreased and trends of decreased concentrations in the sham, high-, and low-dose groups as compared to the control ( Fig. 7 ). The concentration of IL-6 from weeks 4 to 12 was lower in treatment groups than the control, with levels similar to the sham, although no difference existed between dosages. No statistically significant difference in any inflammatory mediator existed between the low- and high-dose groups at a single time point. In addition, both treatment groups and the sham group did not have significant differences in concentrations of any inflammatory mediators, except for increased levels of TNFα in the sham as compared to the treatment groups at week 4.
Figure 7.
Concentration results from pro-inflammatory cytokine and MMP ELISA. *Indicates P < 0.05 compared to control group; ^denotes a trend, or P = ~0.06, compared to the control; #signifies P < 0.05 compared to sham group.
IL-1β concentrations were decreased in treatment groups at weeks 4 and 8, but this finding lost significance at week 12. TNFα levels in the sham hardly varied between weeks. The control group also exhibited similar concentrations in week 4 as in week 12. At week 12, TNFα concentration in the treatment groups and sham was significantly less than the control.
MMP-9 had less consistent sham concentrations at week 4, but this resulted in significantly decreased MMP-9 in the sham and low-dose groups compared to the control at week 12. The MMP-9 concentration in the high-dose group was not significantly decreased from the control. The opposite occurred in MMP-13, where only the high-dose group had significantly decreased concentrations at weeks 4 and 12.
Discussion
Current treatment options for OA manage daily pain and inflammation without prevention of OA progression. Innovative therapeutic options are needed to decrease symptom severity and protect articular cartilage from further damage.14 The results of this study suggest that CMC modulated pro-inflammatory cytokine and MMP concentrations resulting in decreased articular cartilage erosive lesions and histopathological OA progression. The outcomes of this study are impactful because they demonstrate that CMC combined the positive effects of curcumin and tetracyclines, 2 innovative potential treatments for OA, to suppress the systemic expression of 5 inflammatory mediators of OA. Additionally, CMC was successful as an oral, systemic medication rather than local injection treatment. These findings support the notion that CMC offers a step toward a DMOAD that alters the natural progression of OA.
Curcumin has been shown to lower levels of IL-1β,27,28 IL-6,29 TNFα,28 and MMP-9,30 and MMP-1328 likely through suppression of the NFκB pathway.30 For CMC, this quality was enhanced by its zinc-binding capabilities. CMC decreased the concentrations of all 5 studied inflammatory mediators at variable time points between weeks 4, 8, or 12, and at no point did the concentration increase relative to the control. This result is highly indicative of CMC exhibiting an inhibitory effect on inflammation. The lack of significant differences between the treatment and sham groups suggests CMC lowered concentrations to levels seen in uninjured rabbits. The close relationship of the high- and low-dose groups implies that increasing, or nearly doubling, the dosage had little effect on CMC’s modulation capabilities. We believe this is due to the increased bioavailability offered by the modified drug. It is of interest that the cytokine concentrations in the sham group also increased post arthrotomy without ACL transection. This could be attributed to increase in inflammatory mediators from arthrotomy alone. However, this increase in cytokines did not translate to significant macroscopic or histologic fractures of OA, mostly likely because the cytokines were at significantly lower levels than the other 3 groups. Perhaps more global histopathology would reveal pathologic features. High doses of curcumin have been shown to induce generation of reactive oxygen species, which can lead to myocardial tissue damage.31 This is concerning for having a counterproductive effect, and emphasizes the importance of utilizing the lowest possible effective dose. Although neither dose was associated with toxicities, the lowest effective dose of CMC is preferable to prevent potential adverse effects.
The results of the biomechanical testing did not correlate with the other measured parameters. This could be attributed to the constraints of testing at a single time point and from one location of the femoral condyle that was suitably covered with intact articular cartilage required for testing. The presence of surface erosion is an obstacle for indentation testing, because full contact between the indenter and the cartilage surface is required, and therefore testing could not be performed in eroded tissue regions. Nevertheless, the ACL transection was found to diminish the tensile modulus of cartilage, and induced a higher cartilage permeability. However, the compressive modulus was less sensitive to the transection injury at the 12-week time point. Analysis of the contralateral limbs indicates that administration of CMC alone did not have a major effect on the biomechanics of healthy cartilage or the ACL transected joints, as demonstrated in 5 of the 6 measured properties/regions. Results show that tensile modulus was most sensitive to injury and treatment, which is consistent with the qualitative histological changes in collagen deposition observed in the study ( Fig. 6 ). This is consistent with quantitative results of several other studies have found that administering curcumin increased collagen deposition.32,33 Previous studies also show that greater alterations in the collagen orientation angle occurs with ACLT and such alterations extend deeper into the cartilage layer, which may be contributing to the measured altered biomechanics.34 Interestingly, the HBT indentation approach showed more sensitivity to detecting differences in cartilage permeability than previously reported.35
An important novelty of our study is that as an oral agent CMC had systemic effects and modulated the disease progression in the articular cartilage and synovium. While the sensitivity of blood cytokine measurement for detection of joint injury remains to be fully evaluated, several recent studies have shown that both systemic and local inflammatory mediators are involved in the animal ACL transection model of OA, predominantly in rats.36-38 The significant differences in pro-inflammatory cytokine and MMP levels between the sham and ACL transection control groups in our study further demonstrate that systemic changes in serum cytokine concentrations result from rabbit ACL injury and associated OA progression. In addition, other studies have also found that systemic agents, delivered orally or by intramuscular injection, modulated both serum and joint tissue inflammatory cytokine concentrations in the rat ACL transection model indicating that a local therapeutic effect occurs with systemic drug agent.39-45 Fewer such studies exist in rabbits; previous studies of oral agents in the rabbit ACL transection model primarily quantified cartilage cytokine levels.46-48 To our knowledge, only one other study correlating the effects of oral treatment and serum cytokine levels in rabbits exists, making our study of serum cytokine concentrations unique.49 In the current study, we chose to measure blood cytokine concentrations to investigate if oral drug administration led to systemic immunomodulatory effects, seeing that the therapy was an oral agent rather than local intra-articular injection, as most past studies of DMOADs have been. Quantifying the immunomodulatory effects of CMC on synovial fluid is an important future direction for our study.
One limitation of the study is that joint analysis was performed at 12 weeks post-surgery. Other prior studies have shown changes in response to rabbit ACLT from 4- to 9-week time frame. Moreover, the temporal analysis of cytokine levels in the blood of IL-1β, IL-6, and MMP-9 were found to be elevated in injured animals at 4 and 8 weeks, but that resolution of cytokine levels occurred by 12 weeks. Another limitation is the small sample size, in particular for the histopathology. Future studies would require the use of more rabbits, so that additional cartilage and synovium could be used for analysis.
Nevertheless, in the pathogenesis of OA, an imbalance exists between catabolic and anabolic mediators. CMC seems to offset this imbalance by reducing pro-inflammatory and catabolic mediators. Studies have shown that decreasing IL-1β concentration inhibited the destructive articular cartilage process and allowed for collagen deposition to occur.3 Despite the small sample size and power, this shift from catabolism to anabolism resulted in fewer erosive lesions and preserved cartilage and synovial histology in our samples. The treatment groups both exhibited fewer features of OA macroscopically and histologically, with no significant differences between dosages.
In summary, this study built upon the previous knowledge of CMC’s chondroprotective effects in vitro18 and further demonstrated these chondroprotective effects in vivo in a rabbit injury–induced model of OA. These results indicate the potential of CMC to modulate concentrations of several pro-inflammatory cytokines and MMPs leading to reduced inflammation, preserved joint cartilage, and slowed OA progression. CMC should continue to be studied as a potential therapeutic oral DMOAD in OA.
Footnotes
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by NIH Grant 1R41AG050021-01.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics Approval: Ethical approval for this study was obtained from the Institutional Animal Care and Use Committee (IACUC) at The Feinstein Institute (protocol #2014-013).
Animal Welfare: The present study followed international, national, and/or institutional guidelines for humane animal treatment and complied with relevant legislation.
ORCID iD: Josephine R. Coury  https://orcid.org/0000-0002-4511-1172
https://orcid.org/0000-0002-4511-1172
References
- 1. Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39:237-46. [PubMed] [Google Scholar]
- 2. Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33-42. [DOI] [PubMed] [Google Scholar]
- 3. Shakibaei M, Schulze-Tanzil G, John T, Mobasheri A. Curcumin protects human chondrocytes from IL-l1beta-induced inhibition of collagen type II and beta1-integrin expression and activation of caspase-3: an immunomorphological study. Ann Anat. 2005;187:487-97. [DOI] [PubMed] [Google Scholar]
- 4. Lefebvre V, Peeters-Joris C, Vaes G. Modulation by interleukin 1 and tumor necrosis factor alpha of production of collagenase, tissue inhibitor of metalloproteinases and collagen types in differentiated and dedifferentiated articular chondrocytes. Biochim Biophys Acta. 1990;1052:366-78. [DOI] [PubMed] [Google Scholar]
- 5. Alonzi T, Fattori E, Lazzaro D, Costa P, Probert L, Kollias G. et al. Interleukin 6 is required for the development of collagen-induced arthritis. J Exp Med. 1998;187:461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saklatvala J. Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature. 1986;322:547-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davidson RK, Waters JG, Kevorkian L, Darrah C, Cooper A, Donell ST. et al. Expression profiling of metalloproteinases and their inhibitors in synovium and cartilage. Arthritis Res Ther. 2006;8:R124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zeng GQ, Chen AB, Li W, Song JH, Gao CY. High MMP-1, MMP-2, and MMP-9 protein levels in osteoarthritis. Genet Mol Res. 2015;14:14811-22. [DOI] [PubMed] [Google Scholar]
- 9. Pasternak B, Aspenberg P. Metalloproteinases and their inhibitors-diagnostic and therapeutic opportunities in orthopedics. Acta Orthop. 2009;80:693-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Emingil G, Atilla G, Sorsa T, Luoto H, Kirilmaz L, Baylas H. The effect of adjunctive low-dose doxycycline therapy on clinical parameters and gingival crevicular fluid matrix metalloproteinase-8 levels in chronic periodontitis. J Periodontol. 2004;75:106-15. [DOI] [PubMed] [Google Scholar]
- 11. Caton J, Ryan ME. Clinical studies on the management of periodontal diseases utilizing subantimicrobial dose doxycycline (SDD). Pharmacol Res. 2011;63:114-20. [DOI] [PubMed] [Google Scholar]
- 12. Park JM, Mun JH, Song M, Kim HS, Kim BS, Kim MB. et al. Propranolol, doxycycline and combination therapy for the treatment of rosacea. J Dermatol. 2015;42:64-9. [DOI] [PubMed] [Google Scholar]
- 13. Csaki C, Mobasheri A, Shakibaei M. Synergistic chondroprotective effects of curcumin and resveratrol in human articular chondrocytes: inhibition of IL-1 beta-induced NF-kappa B-mediated inflammation and apoptosis. Arthritis Res Ther. 2009;11:R165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Henrotin Y, Priem F, Mobasheri A. Curcumin: a new paradigm and therapeutic opportunity for the treatment of osteoarthritis: curcumin for osteoarthritis management. Springerplus. 2013;2:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807-18. [DOI] [PubMed] [Google Scholar]
- 16. Belcaro G, Cesarone MR, Dugall M, Pellegrini L, Ledda A, Grossi MG. et al. Product-evaluation registry of Meriva®, a curcumin-phosphatidylcholine complex, for the complementary management of osteoarthritis. Panminerva Med. 2010;52:55-62. [PubMed] [Google Scholar]
- 17. Belcaro G, Cesarone MR, Dugall M, Pellegrini L, Ledda A, Grossi MG. et al. Efficacy and safety of Meriva®, a curcumin-phosphatidylcholine complex, during extended administration in osteoarthritis patients. Altern Med Rev. 2010;15:337-44. [PubMed] [Google Scholar]
- 18. Katzap E, Goldstein MJ, Shah NV, Schwartz J, Razzano P, Golub L. et al. The chondroprotective properties of curcumin (Curcuma longa) and curcumin derived polyenolic zinc binding inhibitors against IL-1β and OsM-induced chondrolysis. Poster presented at: Orthopaedic Society Research Annual Meeting; February 4-7, 2012; San Francisco, CA. [Google Scholar]
- 19. Tochigi Y, Vaseenon T, Heiner AD, Fredericks DC, Martin JA, Rudert MJ. et al. Instability dependency of osteoarthritis development in a rabbit model of graded anterior cruciate ligament transection. J Bone Joint Surg Am. 2011;93:640-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yoshioka M, Coutts RD, Amiel D, Hacker SA. Characterization of a model of osteoarthritis in the rabbit knee. Osteoarthritis Cartilage. 1996;4:87-98. [DOI] [PubMed] [Google Scholar]
- 21. Moore AC, DeLucca JF, Elliott DM, Burris DL. Quantifying cartilage contact modulus, tension modulus, and permeability with Hertzian biphasic creep. J Tribol. 2016;138:0414051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pritzker KPH, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA. et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14:13-29. [DOI] [PubMed] [Google Scholar]
- 23. Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971;53:523-37. [PubMed] [Google Scholar]
- 24. Ostergaard K, Andersen CB, Petersen J, Bendtzen K, Salter DM. Validity of histopathological grading of articular cartilage from osteoarthritic knee joints. Ann Rheum Dis. 1999;58:208-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Krenn V, Morawietz L, Burmester GR, Kinne RW, Mueller-Ladner U, Muller B. et al. Synovitis score: discrimination between chronic low-grade and high-grade synovitis. Histopathology. 2006;49:358-64. [DOI] [PubMed] [Google Scholar]
- 26. Krenn V, Morawietz L, Haüpl T, Neidel J, Petersen I, König A. Grading of chronic synovitis—a histopathological grading system for molecular and diagnostic pathology. Pathol Res Pract. 2002;198:317-25. [DOI] [PubMed] [Google Scholar]
- 27. Das L, Vinayak M. Curcumin attenuates carcinogenesis by down regulating proinflammatory cytokine interleukin-1 (IL-1α and IL-1β) via modulation of AP-1 and NF-1L6 in lymphoma bearing mice. Int Immunopharmacol. 2014;20:141-7. [DOI] [PubMed] [Google Scholar]
- 28. Zhang Z, Leong DJ, Xu L, He Z, Wang A, Navati M. et al. Curcumin slows osteoarthritis progression and relieves osteoarthritis-associated pain symptoms in a post-traumatic osteoarthritis mouse model. Arthritis Res Ther. 2016;18:128. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 29. Rahimnia AR, Panahi Y, Alishiri G, Sharafi M, Sahebkar A. Impact of supplementation with curcuminoids on systemic inflammation in patients with knee osteoarthritis: findings from a randomized double-blind placebo-controlled trial. Drug Res (Stuttg). 2015;65:521-5. [DOI] [PubMed] [Google Scholar]
- 30. Shakibaei M, John T, Schulze-Tanzil G, Lehmann I, Mobasheri A. Suppression of NF-kappa B activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: implications for the treatment of osteoarthritis. Biochem Pharmacol. 2007;73:1434-45. [DOI] [PubMed] [Google Scholar]
- 31. Tanwar V, Sachdeva J, Kishore K, Mittal R, Nag TC, Ray R. et al. Dose-dependent actions of curcumin in experimentally induced myocardial necrosis: a biochemical, histopathological, and electron microscopic evidence. Cell Biochem Funct. 2010;28:74-82. [DOI] [PubMed] [Google Scholar]
- 32. Guimarães MR, Coimbra LS, de Aquino SG, Spolidorio LC, Kirkwood KL, Rossa C., Jr. Potent anti-inflammatory effects of systemically administered curcumin modulate periodontal disease in vivo. J Periodontal Res. 2011;46:269-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jagetia GC, Rajanikant GK. Curcumin treatment enhances the repair and regeneration of wounds in mice exposed to hemibody gamma-irradiation. Plast Reconstr Surg. 2005;115:515-28. [DOI] [PubMed] [Google Scholar]
- 34. Florea C, Malo MK, Rautiainen J, Mäkelä JT, Fick JM, Nieminen MT. et al. Alterations in subchondral bone plate, trabecular bone and articular cartilage properties of rabbit femoral condyles at 4 weeks after anterior cruciate ligament transection. Osteoarthritis Cartilage. 2015;23:414-22. [DOI] [PubMed] [Google Scholar]
- 35. Sah RL, Yang AS, Chen AC, Hant JJ, Halili RB, Yoshioka M. et al. Physical properties of rabbit articular cartilage after transection of the anterior cruciate ligament. J Orthop Res. 1997;15:197-203. [DOI] [PubMed] [Google Scholar]
- 36. Wang XJ, Zhang H, Zhan HS, Ding DF. Establishment of chondrocyte degeneration model in vitro by rat serum [in Chinese]. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2015;44:308-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Braza-Boils A, Alcaraz MJ, Ferrandiz ML. Regulation of the inflammatory response by tin protoporphyrin IX in the rat anterior cruciate ligament transection model of osteoarthritis. J Orthop Res. 2011;29:1375-82. [DOI] [PubMed] [Google Scholar]
- 38. Finn A, Angeby Moller K, Gustafsson C, Abdelmoaty S, Nordahl G, Ferm M. et al. Influence of model and matrix on cytokine profile in rat and human. Rheumatology (Oxford). 2014;53:2297-305. [DOI] [PubMed] [Google Scholar]
- 39. Xu Y, Liu Q, Liu ZL, Lim L, Chen WH, Lin N. Treatment with SiMiaoFang, an anti-arthritis Chinese herbal formula, inhibits cartilage matrix degradation in osteoarthritis rat model. Rejuvenation Res. 2013;16:364-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jia PT, Zhang XL, Zuo HN, Lu X, Li L. Articular cartilage degradation is prevented by tanshinone IIA through inhibiting apoptosis and the expression of inflammatory cytokines. Mol Med Rep. 2017;16:6285-9. [DOI] [PubMed] [Google Scholar]
- 41. Xu Y, Dai GJ, Liu Q, Liu ZL, Song ZQ, Li L. et al. Sanmiao formula inhibits chondrocyte apoptosis and cartilage matrix degradation in a rat model of osteoarthritis. Exp Ther Med. 2014;8:1065-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Balaganur V, Pathak NN, Lingaraju MC, More AS, Latief N, Kumari RR. et al. Effect of S-methylisothiourea, an inducible nitric oxide synthase inhibitor, in joint pain and pathology in surgically induced model of osteoarthritis. Connect Tissue Res. 2014;55:367-77. [DOI] [PubMed] [Google Scholar]
- 43. Ferrándiz ML, Terencio MC, Carceller MC, Ruhí R, Dalmau P, Vergés J. et al. Effects of BIS076 in a model of osteoarthritis induced by anterior cruciate ligament transection in ovariectomised rats. BMC Musculoskelet Disord. 2015;16:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Francischi JN, Yokoro CM, Poole S, Tafuri WL, Cunha FQ, Teixeira MM. Anti-inflammatory and analgesic effects of the phosphodiesterase 4 inhibitor rolipram in a rat model of arthritis. Eur J Pharmacol. 2000;399:243-9. [DOI] [PubMed] [Google Scholar]
- 45. Rocha FA, Silva FS, Jr, Leite AC, Leite AK, Girão VC, Castro RR. et al. Tadalafil analgesia in experimental arthritis involves suppression of intra-articular TNF release. Br J Pharmacol. 2011;164:828-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen D, Zhang Z, Cao J, Zhang D, Jia B. Effect of glucosamine hydrochloride capsules on articular cartilage of rabbit knee joint in osteoarthritis [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2010;24:287-91. [PubMed] [Google Scholar]
- 47. Lu HT, Hsieh MS, Cheng CW, Yao LF, Hsu TY, Lan J. et al. Alterative effects of an oral alginate extract on experimental rabbit osteoarthritis. J Biomed Sci. 2015;22:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu XX, Li XH, Zhou JT. Research on mechanism of Tougu Xiaotong Granule in preventing and treating knee osteoarthritis [in Chinese]. Zhongguo Zhong Xi Yi Jie He Za Zhi 2007;27:50-4. [PubMed] [Google Scholar]
- 49. Zhu FX, Zhou RH, Shi YH, Qin Y. Effect of parthenolide on serum expressions of interleukin-1beta and tumor necrosis factor-alpha in rabbits with knee osteoarthritis [in Chinese]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2013;33:1382-4. [PubMed] [Google Scholar]