Abstract
Study Objectives:
The aim of this study was to determine the impact of serious parental injury on adolescent sleep disorder diagnoses, outpatient care, and medication use.
Methods:
U.S. military personnel who sustained a serious injury and were parents of adolescents aged 10–18 years were identified. Included adolescents were enrolled in the Military Health System for 2 years before their parent’s injury and 2 years after the injury. We used logistic regression clustered by adolescents to compare the odds of having a sleep diagnosis and negative binomial regression analysis clustered by adolescents to compare outpatient sleep disorder visits and sleep medication days before and after parental injury.
Results:
There were 96,318 parents seriously injured during 2004–2014 who had 117,577 children aged 10–18 years in 2002–2016. Approximately 2% of adolescents had a sleep disorder diagnosis, both before and after their parent’s injury or illness. Outpatient sleep disorder visits increased 36% after a parent’s injury (incidence rate ratio 1.36 [1.24–1.50]), with a twofold increase in insomnia visits (incidence rate ratio 2.35 [2.08–2.64]). Increases in sleep visits were most pronounced in adolescents of parents with traumatic brain injury, comorbid traumatic brain injury and posttraumatic stress disorder, battle injury, and those who were medically discharged from the military. The number of adolescents using sleep medications increased, but sleep medication days did not increase.
Conclusions:
Adolescents in our study used more outpatient medical care for sleep disorders; sleep medication use increased after parental injury. Sleep disorders should be considered in the care of adolescents with injured parents.
Citation:
Ahmed S, Gorman GH, Susi A, Robertson BD, Collen JF, Hisle-Gorman EJ. Impact of parental injury on adolescent sleep. J Clin Sleep Med. 2020;16(9):1437–1444.
Keywords: adolescent, sleep, parents, posttraumatic stress disorders, brain injuries, wounds and injuries, military family, family health
BRIEF SUMMARY
Current Knowledge/Study Rationale: Stress within a family unit increases after a parent is injured and likely impacts the sleep of the entire household, which may include adolescents, who are already uniquely vulnerable to sleep disturbances. The full impact of parental injury on adolescent sleep has yet to be fully explored.
Study Impact: Adolescents are at an increased risk of outpatient care utilization and medication use to treat sleep disorders after a parent is injured. Early screening and detection of sleep disorders in these adolescents are crucial to prevent adverse consequences of poor sleep.
INTRODUCTION
Parental physical or mental illnesses and injuries can significantly impact a family’s ability to function, increase family stress, and affect the well-being of their children.1,2 Adolescents are particularly vulnerable to difficulties with sleep.3,4 For children, poor sleep can be a serious consequence of stress, and quality sleep is a vital element in maintaining child health and well-being.5,6
The military represents a vulnerable population with regard to sleep. Sleep disorders are more common in the military, where significantly more active-duty service members (ADSMs) habitually obtain 6 or fewer hours of sleep per night and have higher rates of obstructive sleep apnea and insomnia than the general population.7–10 ADSMs recovering from injury and illness often struggle with poor sleep. Many consider posttraumatic stress disorder (PTSD) and traumatic brain injury (TBI) to be the signature invisible injuries of the wars in Iraq and Afghanistan, and these are often accompanied by a high rate of sleep problems, including excessive daytime sleepiness, sleep-disordered breathing (SDB), insomnia, and hypersomnia.11,12 Disturbed sleep is a cardinal feature of mood disorders (such as PTSD, depression, and anxiety), and these conditions can perpetuate one another.13,14 What has not been established is whether or how sleep and health in ADSM impact family members, especially over the past two decades of war.
Chronic parental illness has been linked with increased behavioral and mental health problems in children.15–18 A large retrospective Finnish cohort study found that children of parents with cancer utilized more specialized inpatient and outpatient psychiatric care compared with their peers, more so if one of their parents had a comorbid psychiatric disorder.15 Severe parental mental illness has been associated with increased risk for attention deficit hyperactivity disorder and autism spectrum disorder in their offspring.16 In smaller cohorts, parental multiple sclerosis and stroke have been linked to childhood interpersonal and behavioral problems.17,18
Beyond effects on behavioral and mental health, parental injury has been found to increase the use of psychoactive medication use by children.1,19–21 Children of parents treated for unintentional injury were more likely to report posttraumatic stress and depressive symptoms postinjury.1 Parental TBI has been associated with emotional and behavioral difficulties in children, including depressive symptoms.19,20
Sleep disturbances are prevalent in the pediatric population but may be underdiagnosed.22 Rates of specific sleep disorders range from 0.004% to 1.2% for parasomnias, 0.005% to 0.2% for circadian-rhythm sleep disorder, 1% for SDB, and 4% to 11% for insomnia.22–24 Many adolescents, however, are symptomatic and experience poor sleep without an actual diagnosis. Adolescents are inherently at risk for sleep issues, which can have significant adverse implications on development, even without a known stressor.3,4,25 During puberty, adolescents are at risk of experiencing sleep-phase delay, with a predilection to fall asleep later in the evening26; this tendency, together with the high demands of schoolwork and extracurricular activities, may lead to sleep deprivation and poor sleep hygiene. This risk for poor sleep is at odds with adolescents’ need for sleep, as consistently obtaining sufficient sleep is vital for adequate development of the white matter of adolescent brains.25 Animal models and short-term experiments in humans have shown that sleep deprivation can lead to deficits in cognition, vigilance, memory, mood, behavior, immune function, and the ability to learn.6,27 Expected developmental changes in adolescent sleep patterns, including delayed sleep phase and psychosocial stressors from school, are likely tolerable in isolation but may prove unbearable when compounded by stressors such as parental injury, which may then lead to an increase in sleep disturbances from these stressors.
Whereas research has linked parental injuries with negative health outcomes in younger children, the impact of parental injury on adolescents has not been well explored, nor has the impact of parental injury on the sleep of children of any age.28 Children in military families may be at increased risk for sleep disturbances owing to their increased exposure to stressors such as parental deployment and battle injury. This study sought to explore any relationship between parental injury and sleep-related medical care and the use of sleep medication in military-connected adolescents.
METHODS
Inclusion criteria
A self-controlled case series was conducted using the Military Health System Medical Data Repository, which is a comprehensive record inclusive of all inpatient and outpatient care provided at military and civilian medical treatment facilities, as well as outpatient prescriptions. All children were covered under TRICARE, the low-cost/no-cost health care program for uniformed service members, retirees, and their dependents in the United States and abroad. All parents who incurred an injury between October 1, 2004, and September 30, 2014, were identified by their inclusion in the Ill, Injured, and Wounded Dataset of the Medical Data Repository, which records injuries in the ADSM. This database was initially created to determine the cost of care for ADSM injured in battle but now includes anyone with a serious injury or illness of a type or severity similar to those that could be incurred in battle. Children of injured ADSM enrolled in the Military Health System and covered under TRICARE for the entire 2 years before and 2 years after their parents were injured were identified using the Defense Eligibility and Enrollment Reporting System and were included in the study. Families who moved during the study period and received care at multiple military hospitals or civilian providers were still included so long as they were covered under TRICARE for the entire 4-year period. Children who did not have 2 full years of care before and after the injury were not included. We do not expect to have lost many children to follow-up as care in the Warrior Care and Transition program and the time to medically related retirement is not a quick process. Child age was determined at the time of their parent’s injury.
For each adolescent, the 2 years after the parent’s injury was compared with the 2 years immediately preceding their parent’s injury, which was considered the control period. Children were aged 12 to 16 years at the time of their parent’s injury and were aged 10 to 18 years over the full course of the study.
Parents’ serious injuries were identified as occurring in battle or in daily life and included physical injuries, such as burns, amputation, shrapnel injury, fracture, spinal cord injury, blindness, as well as PTSD and TBI. PTSD and TBI are more likely to impact sleep health, and parental sleep issues have been shown to impact the sleep of others in the household and may adversely impact an adolescent’s sleep.29–31 Parents injured during combat were identified in the Ill, Injured, and Wounded dataset by a “flag” denoting an injury sustained during hostilities. It is likely that this identification method missed some parents whose injuries were not immediately documented related to battle, for example, those with conditions such as PTSD or TBI that were rooted in deployment, but not documented in the Ill, Injured, and Wounded database owing to delayed presentation (ie, after return from combat). The dataset also included an indicator of those who were medically retired from the military as a result of their injuries, which acted as a surrogate for the most severe injuries.
Sleep disorder diagnoses
The International Classification of Diseases 9th edition (ICD-9) diagnostic codes identifies outpatient visits for sleep disorders. The Clinical Classification System of the Agency for Healthcare Research and Quality multilevel diagnosis categories for sleep disorders are used to subgroup sleep disorder diagnoses. Sleep subcategories include circadian-rhythm sleep disorder, hypersomnia, insomnia, narcolepsy, parasomnias, restless legs syndrome (RLS), SDB, and sleep disorder–not otherwise specified (NOS).
Sleep medication use
Outpatient pharmacy records identify prescriptions for sleep medications and number of days on sleep medications both before and after the date of injury. Sleep medications were only counted for the time period (before or after a parent’s injury) if the child had a sleep disorder diagnosis in that same time period, as many medications used for sleep can be used for other conditions as well. Classes of medications studied included benzodiazepines, hypnotics, tricyclic antidepressants, antihistamines, melatonin receptor agonists, orexin receptor antagonists, alpha2-adrenergic receptor agonists, the serotonin antagonist and reuptake inhibitor trazodone, and the tetracyclic antidepressant mirtazapine (Supplemental Table S1).
Statistical analysis
The prevalence of sleep diagnoses overall and by type and sleep medication use overall and by class were determined before and after a parent’s injury and compared using the χ2 statistic. Unadjusted and adjusted logistic regression clustered by adolescent compared the odds of having a sleep diagnosis. Unadjusted and adjusted negative binomial regression analysis clustered by adolescent calculated the incidence rate ratios (IRR) comparing outpatient visits for sleep diagnoses and prescribed days of sleep medication use before and after the parent’s injury. Negative binomial regression can determine the relative rate of counted events, such as outpatient visits or days of medication use. Adjusted models controlled for child’s sex, adolescent age at time of parental injury, years of parental deployment, and comorbid conditions (eg, attention deficit hyperactivity disorder, autism spectrum disorder, and mood disorders) associated with sleep issues in children and adolescents.13,32,33 Adolescents were categorized as having the comorbid condition if they carried the diagnosis at any point during the study period. Secondary analysis examined the impact of parental injury stratified by parental injury type, severe parental injury as operationalized by medical retirement, and parental injury setting (battle vs nonbattle). Parents with PTSD and/or TBI with or without a co-occurring physical injury were classified as having PTSD, TBI, or comorbid PTSD-TBI for secondary analysis, as these were the signature injuries of these wars and can be particularly disruptive to sleep.29,30
P values were considered statistically significant at P < .05. Analyses were conducted using Stata Intercooled 13 (Stata Corp, College Station, Texas). This study was reviewed and approved by the local Institutional Review Board.
RESULTS
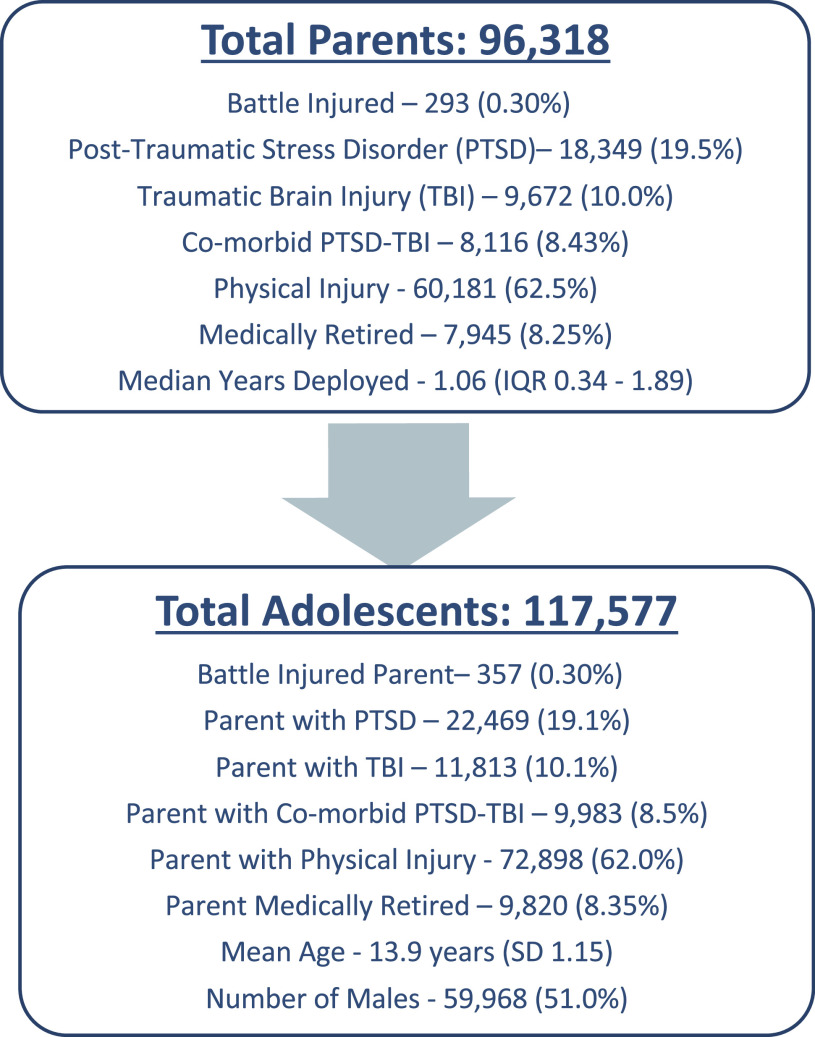
This study comprised 117,577 adolescents of 96,318 injured parents (Figure 1). At the time of the parent’s injury, the children were a mean age of 13.9 years (standard deviation [SD] 1.15), and 51% were male. Median age for adolescents increased from 15.4 to 17.1 years after parental injury (P < .001) for all sleep disorders, as well as specifically for each sleep disorder except hypersomnia and narcolepsy. Approximately 38% of adolescents had a parent with PTSD and/or TBI, which included 22,469 (19.1%) who had a parent with PTSD, 11,813 (10.1%) with a parent with a TBI, and 9,983 (8.5%) had a parent with comorbid PTSD-TBI. There were 357 (0.3%) adolescents whose parents sustained a battle injury, and 9,820 (8.35%) had parents who were medically retired from the military after their injury (Figure 1).
Figure 1. Demographics of included parents and adolescents.
Sleep disorder prevalence
The number of adolescents with a sleep disorder diagnosis after their parent’s injury, 2,249 (1.9%), did not differ significantly from the preinjury number, 2,342 (2.0%; P = .17) using χ2 analysis. The most common sleep disorder diagnosis was insomnia, followed by sleep disorder–NOS. After parental injury, the number of adolescents with insomnia increased significantly, and those with diagnosed parasomnias, RLS, SDB, and sleep disorder–NOS decreased (Table 1). In logistic regression analysis, the odds of a sleep diagnosis after parental injury was unchanged in unadjusted (odds ratio 0.96 [0.91–1.01]) and slightly decreased in adjusted analysis (odds ratio 0.93 [0.88–0.99] Table 2).
Table 1.
Adolescents with sleep disorder diagnoses and sleep medication use before and after parental injury.
| No. and Percentage (%) of Adolescents before Parental Injury | No. and Percentage (%) of Adolescents after Parental Injury | P Value | |
|---|---|---|---|
| All diagnoses | 2,342 (1.99) | 2,249 (1.91) | .166 |
| CRSD | 47 (.04) | 59 (.04) | .244 |
| Hypersomnia | 4 (0) | 3 (0) | .705 |
| Insomnia | 961 (.82) | 1166 (.99) | < .001 |
| Narcolepsy | 35 (.03) | 39 (.03) | .642 |
| Parasomnias | 68 (0.06) | 26 (.02) | < .001 |
| RLS | 69 (0.06) | 42 (.04) | .010 |
| SDB | 739 (.63) | 526 (.45) | < .001 |
| Sleep disorder–NOS | 793 (.57) | 698 (.59) | .014 |
| Any sleep medication | 590 (.50) | 692 (.59) | .004 |
| Benzodiazepines | 16 (.01) | 25 (.02) | .160 |
| Hypnotics | 75 (.06) | 137 (.12) | < .001 |
| Tricyclic antidepressants | 6 (.01) | 8 (.01) | .593 |
| Antihistamines | 176 (.15) | 171 (.15) | .788 |
| Melatonin receptor agonists | 9 (.01) | 10 (.01) | .819 |
| Orexin receptor antagonists | 0 (0) | 1 (0) | .267 |
| α2-adrenergic receptor agonists | 140 (.12) | 108 (.09) | .042 |
| Serotonin antagonist and reuptake inhibitors | 149 (.13) | 241 (.20) | < .001 |
| Tetracyclic antidepressants | 154 (.13) | 175 (.15) | .247 |
CRSD = circadian rhythm sleep disorder, NOS = not otherwise specified, RLS = restless leg syndrome, SDB = sleep-disordered breathing.
Table 2.
Unadjusted and adjusted rates of outpatient visits for sleep disorders and sleep medication days.
| Sleep Diagnoses OR (95% CI) | Sleep Outpatient Visits IRR (95% CI) | Sleep Medication Days IRR (95% CI) | |
|---|---|---|---|
| Unadjusted impact of parental injury | .96 (.91–1.01) | 1.33 (1.22–1.46) | 1.01 (.83–1.23) |
| Adolescent sex | .96 (.90–1.03) | 1.01 (.89–1.14) | 0.75 (.46–1.22) |
| Adolescent age | 1.03 (1.00–1.06) | 1.04 (.99–1.09) | 1.37 (1.15–1.62) |
| Parental years deployed | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) |
| Adolescent ADHD | 2.39 (2.20–2.60) | 3.39 (2.93–3.93) | 7.72 (4.96–12.0) |
| Adolescent ASD | 1.46 (1.25–1.71) | 4.67 (2.79–7.81) | 20.6 (11.4–37.0) |
| Adolescent mood disorders | 2.36 (2.16–2.58) | 3.64 (3.23–4.10) | 6.64 (4.96–8.89) |
| Adjusted impact of parental injury | .93 (.88–.99) | 1.36 (1.24–1.50) | 1.12 (.80–1.57) |
ADHD = attention deficit hyperactivity disorder, ASD = autism spectrum disorder, CI = confidence interval, IRR = incidence rate ratio, OR = odds ratio.
Outpatient care for sleep disorders
In unadjusted analysis, there was an overall 33% increase in outpatient visits for sleep disorders (IRR 1.33 [1.22–1.46]) after parental injury. Increased visits were associated with attention deficit hyperactivity disorder, autism spectrum disorder, and mood disorders (Table 2), and male sex, adolescent age, and years of parental deployment were not significantly associated with sleep visits in unadjusted analysis. The median age of all sleep visits, however, before injury was 15.4 (95% confidence interval [13.1–17.4]) years, which is significantly different from the median age of sleep visits after parental injury (17.1 [15.1–19.1]) in rank sum. After adjustment, there was a 36% increase in visits for any sleep disorder diagnosis (IRR 1.36 [1.24–1.50]). The rate of visits for circadian-rhythm sleep disorder, narcolepsy, insomnia, and sleep disorder–NOS were increased after injury in unadjusted and adjusted models, yet visits for RLS, SDB, hypersomnia, and parasomnias were not significantly different (Table 3).
Table 3.
Unadjusted and adjusted rates of outpatient visits for sleep disorders by specific diagnosis.
| Outpatient Visits: Unadjusted IRR (95% CI) | Outpatient Visits: Adjusted IRR (95% CI)a | |
|---|---|---|
| Any sleep disorder diagnosis | 1.33 (1.22–1.46) | 1.36 (1.24–1.50) |
| Circadian rhythm sleep disorder | 1.52 (.92–2.54) | 1.57 (1.01–2.45) |
| Hypersomnia | 1.75 (.38–7.96) | * |
| Insomnia | 2.05 (1.82–2.30) | 2.35 (2.08–2.64) |
| Narcolepsy | 2.79 (.97–7.98) | 1.91 (1.01–3.63) |
| Parasomnias | 0.42 (.20–0.86) | * |
| Restless legs syndrome | 0.78 (.49–1.26) | 0.95 (.54–1.66) |
| Sleep-disordered breathing | 0.92 (.79–1.06) | 0.93 (.79–1.10) |
| Sleep disorder–NOS | 1.12 (.96–1.30) | 1.27 (1.10–1.46) |
Insufficient numbers of cases to run adjusted analysis. aAdjusted for adolescent sex, age, parental years deployed, adolescent attention deficit hyperactivity disorder, autism spectrum disorder, and mood disorders. CI = confidence interval, IRR = incidence rate ratio, NOS = not otherwise specified.
Sleep medication prevalence and medication days
After a parent’s injury, the number of adolescents using sleep medications increased significantly from 590 to 692 adolescents (P = .004), driven by increased use of hypnotic and serotonin antagonist and reuptake inhibitor use (Table 1); however, in unadjusted and adjusted analyses, the number of sleep medication days did not significantly differ in the postinjury period compared with the time preceding parental injury (Table 2).
Secondary analysis by type of parent injury
In unadjusted and adjusted analyses outpatient visits for sleep disorders increased in adolescents of parents with all types of injuries (Table 4). Increases were most pronounced in children of parents with TBI (IRR 1.67 [1.25–2.22]) and parents with comorbid PTSD-TBI (IRR 1.64 [1.34–2.01]). In unadjusted analysis, sleep medication days increased twofold in adolescents whose parents had comorbid PTSD-TBI (IRR 2.09 [1.15–3.81]); however, after adjustment, none of the categories of parental injury was associated with increased medication use (Table 4).
Table 4.
Adolescents’ outpatient visits for sleep disorders and medication days by type and severity of parental injury: unadjusted and adjusted rates of outpatient visits for sleep disorders and sleep medication days.
| Sleep Outpatient Visits IRR (95% CI) | Sleep Medication Days IRR (95% CI) | |||
|---|---|---|---|---|
| Unadjusted | Adjusteda | Unadjusted | Adjusteda | |
| Physical injury | 1.22 (1.11–1.35) | 1.28 (1.14–1.43) | .87 (.70–1.07) | .99 (.68–1.44) |
| PTSD | 1.27 (1.10–1.46) | 1.36 (1.15–1.60) | .93 (.65–1.34) | 1.09 (.57–2.09) |
| TBI | 1.66 (1.17–2.35) | 1.67 (1.25–2.22) | 1.11 (.72–1.72) | 1.02 (.55–1.89) |
| PTSD and TBI | 1.91 (1.52–2.41) | 1.64 (1.34–2.01) | 2.09 (1.15–3.81) | 2.14 (.95–4.88) |
| Nonbattle injury | 1.33 (1.22–1.46) | 1.36 (1.23–1.50) | 1.00 (.82–1.22) | 1.05 (.74–1.50) |
| Battle injury | 1.52 (.62–3.74) | 2.48 (.89–6.88) | 2.65 (.40–17.5) | 19.1 (3.10–119) |
| Not medically retired | 1.29 (1.17–1.41) | 1.31 (1.18–1.45) | .94 (.76–1.16) | 1.05 (.73–1.51) |
| Medically retired | 1.85 (1.54–2.23) | 1.98 (1.61–2.45) | 1.74 (1.10–2.73) | 1.79 (.83–3.88) |
Adjusted for adolescent sex, age, parental years deployed, adolescent attention deficit hyperactivity disorder, autism spectrum disorder, and mood disorders. CI = confidence interval, IRR = incidence rate ratio, PTSD = posttraumatic stress disorder, TBI = traumatic brain injury.
Impact of battle injury
In adolescents of nonbattle-injured parents, outpatient visits for sleep disorders increased by 33% in unadjusted (IRR 1.33 [1.22–1.46]) and 36% in adjusted analysis (IRR 1.36 [1.23–1.50]). Sleep medication use did not increase in adolescents of parents not injured in battle, in unadjusted or adjusted analysis. In adolescents whose parents sustained a battle injury, sleep visits did not significantly change after parental injury in unadjusted or adjusted analysis; however, in adjusted analysis, sleep medication days were significantly increased in adolescent of battle-injured parents (IRR 19.1 [3.10–119], Table 4).
Secondary analysis by injury severity
In adolescents of nonmedically retired parents, outpatient visits for sleep disorders increased in unadjusted (IRR 1.29 [1.17–1.41]) and adjusted analysis (IRR 1.31 [1.18–1.45]; Table 4). Sleep medication days did not significantly change for children of nonmedically retired parents. In adolescents of medically retired parents, visits also increased after their parent’s injury in unadjusted (IRR 1.85 [1.54–2.23]) and adjusted analysis (IRR 1.98 [1.61–2.45]). Sleep medication days increased 74% (IRR 1.74 [1.10–2.73]) in unadjusted analysis of adolescents of medically retired parents, and there was no significant change in adjusted analysis (Table 4).
DISCUSSION
Adolescents used more outpatient care for sleep disorders after a parent’s injury. Care increases were especially pronounced in teens whose parents experienced TBI, had comorbid TBI-PTSD, or endured injuries sufficiently severe to lead to medical retirement. The increase in outpatient care for sleep disorders after parental injury without an increase in the number of adolescents with sleep disorder diagnoses suggests increased severity after parental injury in those with existing sleep issues. After a parent is injured, the focus of family medical and emotional needs may shift toward parental rather than child needs.34 This shift in focus away from the child may lead to significant disruptions in teenage daily routines and activities, altering their sleep schedule and habits and resulting in sleep difficulties.
Despite previous survey reports that up to 60% of adolescents report difficulties with sleep and inadequate sleep duration, this study’s finding that 2% of included teens had formally diagnosed sleep disorders both before and after their parent’s injury is consistent with a previous estimate of 3.7% of sleep disorder diagnoses in 0- to 18-year olds.22,35–37 The discrepancy between survey response and medical diagnoses likely relates to adolescents underreporting sleep difficulties in the primary-care setting, teens not viewing sleep as a medical issue, providers not asking about sleep, or providers not coding accurately (or at all) for sleep issues. Comorbid mood disorders in adolescents that were treated after a parent’s injury with medication may have also led to improvement in sleep disorders.
Insomnia was the most common sleep disorder diagnosis experienced by adolescents. The prevalence of insomnia in this study (0.82%–0.99%) was lower than previously reported rates based on random sample surveying of teens (4%–11%) who were asked about Diagnostic and Statistical Manual of Mental Disorders (4th ed.) criteria for insomnia.23,24 Rates, however, are considerably higher than reported rates of insomnia identified by ICD-9 codes in a medical setting (0.05%).22 After parental injury, both the proportion of adolescents with insomnia and the rate of outpatient visits for insomnia increased, which indicates increased insomnia prevalence and severity. Adolescents are particularly vulnerable to insomnia, as teens experience changes in their circadian rhythm as they progress through puberty.26 Parental injury may exacerbate these vulnerabilities if it leads to increased caregiving responsibilities for the adolescent, more family conflict, or increases parental injury-related sleep disturbances, all of which can impact adolescents’ sleep practices.29,30,34,38
Outpatient visits for narcolepsy, circadian-rhythm sleep disorder, and sleep disorder–NOS also increased after parental injury, despite small decreases or no change in the prevalence of these diagnoses, which again suggests increased severity after parental injury in teens with these conditions. The effect of puberty on adolescent circadian rhythm may negatively impact teenagers’ sleep.26 In addition, symptoms resembling narcolepsy can be seen in those with inadequate sleep overall.39 Like insomnia, these normal adolescent vulnerabilities to sleep issues are likely exacerbated by family stress and poor parental sleep related to parent’s injury.
A lower proportion of adolescents had diagnoses for parasomnias, RLS, and SDB after parental injury; and visit rates for these diagnoses did not significantly change after parental injury. The prevalence of parasomnia (0.004%–1.2%), RLS (0.02%), and SDB (1.0%) diagnoses was consistent with previously reported ICD-9 code-based prevalence estimates.22 These diagnoses are less likely to be associated with an adolescent-aged specific vulnerability. Decreased care for these diagnoses likely relates to a family shift in focus to the injured parent or increased resolution of the sleep disorder owing to care or age.34,40 It cannot be assumed that these disorders resolved after the parent’s injuries; however, a diagnosis of SDB may resolve over time because of the expected airway maturation of older children or if the adolescent received an adenotonsillectomy or initiated continuous positive airway pressure treatment at home.41,42 Many children with SDB also have difficulties with bedwetting, a type of parasomnia, and after proper treatment of their SDB, their bedwetting may resolve as well.43 Hypersomnia in adolescents may improve over time if medication treatment was initiated.
The proportion of adolescents using medications for sleep disorders increased in the postinjury period, yet there was no significant change in the number of medication days after a parent’s injury. Results may suggest increased short-term trialing of medication therapy for sleep disorders.
The percentage of adolescents who used a medication for a sleep disorder in this study (0.5%–0.6%) was consistent with a reported rate of 0.3% in a study that also used pharmaceutical data linked with diagnoses.44 This estimate was lower, however, than the rate of 5.1% reported from a study that looked at prescriptions but did not link medications to known sleep conditions.22 Trazodone, mirtazapine, and antihistamines were the most commonly prescribed medications for sleep in military-connected adolescents, which is consistent with reported sleep prescribing patterns in older children.22,45 Mirtazapine and trazodone have sedative effects that make them more commonly used in patients with both depressive symptoms and sleep difficulties, and some studies have found them to be effective in treating adult sleep difficulties as well.46,47 Providers may feel more comfortable prescribing these medications in older children because of their larger size and ability to tolerate potential side effects. Use of antihistamines was likely undercounted as these medications can be purchased over the counter.
In adolescents with a sleep diagnosis, the proportion of adolescents prescribed sleep medications increased, although the mean number of medication days did not change after parental injury. The increase in the number of adolescents prescribed a medication after parental injury is consistent with increased sleep visits during this same period. If increased visits indicate increased severity, it would likely follow that increased severity led to medication use, at least as a trial treatment. Physicians may be more likely to trial sleep medications in adolescents with known acute family stressors if effectively implementing behavioral interventions is not thought to be feasible (because of limited resources or family conditions) in already stressed families.48
In the aggregate, there may not be increases in medication days as some adolescents may be taken off long-term sleep medication prescriptions after parental injury. In many cases, injured ADSM and their families are moved to large medical treatment facilities to better serve the needs of the injured ADSM. These larger medical facilities are likely to have specialized sleep medicine services. In cases where sleep care is moved to specialized sleep clinics, the first step is often cessation of all long-term sleep medications, as these specialized practitioners can better diagnose the root causes of sleep issues when patients are not taking medication; however, a limitation of our dataset is that we are unable to elucidate reasons for changes in sleep medication prescriptions owing to variability in the location of care and provider prescribing practices across our population.
Secondary analysis showed more pronounced increases in sleep visits from the preinjury to postinjury periods in adolescents of parents with TBI and comorbid PTSD-TBI and children of medically retired parents. Adolescent sleep habits have been linked to parental sleep habits; that is, when a parent is experiencing PTSD-related nightmares or delayed-phase circadian rhythm sleep disturbance from TBI, the adolescent’s sleep may be disrupted as a result.31 The more pronounced increases in care for these teens may suggest that adolescent sleep is more impacted in cases of more severe injury and in injury types that are likely to directly impact the sleep of injured parents. These findings are consistent with research suggesting that poor parental emotional health and parental anger, which can increase with PTSD and/or TBI, are associated with sleep difficulties in their children.49 In addition, parental TBI has been associated with depressive symptoms in children, which may manifest as poor or inadequate sleep.19,20 Findings are consistent with research that suggests the severity of parental emotional problems is predictive of the severity of their children’s sleep issues.49,50
In adolescents of battle-injured parents specifically, the visit rate for sleep disorders after parental injury did not significantly differ from the preinjury period. The military has implemented a robust warrior care and transition program to care for battle-injured ADSM and their families that provides intensive care to the individual and their family as well. Results may suggest that adolescent sleep is not so negatively impacted when families are provided comprehensive care.
Our study was limited by reliance on ICD-9 codes and prescription billing data. ICD-9 coding may not provide adequate information about the severity of adolescent sleep disorders or parental injuries. Less severe sleep problems may have been underidentified, and over-the-counter sleep aids, such as melatonin or antihistamines, are often the first-line treatment for sleep issues and may not have been captured in this study. Whereas medication analyses were limited to those with sleep disorder diagnoses, we were unable to link prescriptions to a diagnosis, so it is possible that some medications were prescribed for nonsleep issues.
Preinjury periods were also likely periods of parental deployment, especially in the case of battle-injured parents, as battle injures by definition occur during combat deployment. ADSMs are frequently sent on deployment in close succession. Deployments in the preinjury period may have increased family stress and resulted in less pronounced differences in preinjury and postinjury sleep care and medication use. The recorded prevalence of battle-injured parents (0.3%) in this study was low compared with recent statistics of war-related injuries in ADSMs.51 Discrepancies likely relate to miscoding of injuries if diagnoses were made after the acute injury period, such as PTSD and TBI, which may take time to develop, or challenges related to completing accurate documentation in combat settings.
Finally, because of the nature of this study’s design, we are not able to establish causation between parental injury and sleep disorder diagnoses and medication use. Sleep disorder prevalence rates may be affected by a host of family-level factors and stressors that we were unable to control for, such as family structure and income, parental mental illness, frequent military household moves, parental substance abuse, domestic violence, and divorce or separation. In addition, later school start times, expected delayed sleep-phase changes as children age, or other comorbid medical conditions also may have affected sleep disorder prevalence. All these confounders could affect the ability of parents to care for their children, especially in the setting of a stressor such as serious parental injury. Diagnosis and treatment of sleep disorders may vary as an adolescent ages, and although adolescent age was controlled for in this study, it may not fully account for this age-related variation in care.
The strengths of this study included a large sample of parents and adolescents followed up over a long period. A wide variety of parental injuries were studied, including physical injuries, TBI, and PTSD sustained both in and out of the battle setting. Use of an open-access health care system with low- to no-cost health care decreased access-to-care bias. Because some indications for medications commonly used for sleep disorders included nonsleep conditions, medication analyses were limited to adolescents with a sleep diagnosis. The results of this study are generalizable to a nonmilitary population as most of the injured parents were injured in nonbattle-related activities. Finally, the use of an extended preinjury and postinjury period allowed for examination of the ongoing impact of injury on children and families.
CONCLUSIONS
Sleep disorders can affect the entire household. When providing care to adolescents with known family stressors, including parental injury, providers should inquire about adolescents’ sleep as a potential area for intervention. Existing sleep conditions are exacerbated by family stress, and sleep should be included in long-term care and treatment planning for adolescents in families where one or more parents has experienced injury.
DISCLOSURE STATEMENT
All authors have met requirements for authorship, have read and approved the manuscript, and agree with its submission to the Journal of Clinical Sleep Medicine. This work was performed at National Capital Consortium–Pediatrics Residency, Walter Reed National Military Medical Center, Bethesda, Maryland. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors have no financial relationships or conflicts of interest to disclose. The views expressed in this manuscript are those of the authors and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or U.S. Government.
SUPPLEMENTARY MATERIAL
ACKNOWLEDGMENTS
This study was performed with the assistance of the Department of Pediatric Military Research at the Uniformed Services University in Bethesda, Maryland, and the Department of Pediatrics at the Walter Reed National Military Medical Center in Bethesda, Maryland.
ABBREVIATIONS
- ADSM
active-duty service member
- ICD
International Classification of Diseases
- IRR
incidence rate ratio
- NOS
not otherwise specified
- PTSD
posttraumatic stress disorder
- RLS
restless legs syndrome
- SDB
sleep-disordered breathing
- TBI
traumatic brain injury
REFERENCES
- 1.Rivara FP, McCarty CA, Shandro J, Wang J, Zatzick D. Parental injury and psychological health of children. Pediatrics. 2014;134(1):e88–e97. 10.1542/peds.2013-3273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mc Elroy S, Hevey D. Relationship between adverse early experiences, stressors, psychosocial resources and wellbeing. Child Abuse Negl. 2014;38(1):65–75. 10.1016/j.chiabu.2013.07.017 [DOI] [PubMed] [Google Scholar]
- 3.National Sleep Foundation . Adolescent Sleep Needs and Patterns: Research Report and Resource Guide. Arlington, VA: National Sleep Foundation; 2000. [Google Scholar]
- 4.Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Dev. 1998;69(4):875–887. 10.1111/j.1467-8624.1998.tb06149.x [DOI] [PubMed] [Google Scholar]
- 5.Duke NN, Borowsky IW. Health status of adolescents reporting experiences of adversity. Glob Pediatr Health. 2018;52333794X18769555. 10.1177/2333794X18769555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukherjee S, Patel SR, Kales SN, et al. An official American Thoracic Society Statement: the importance of healthy sleep: recommendations and future priorities. Am J Respir Crit Care Med. 2015;191(12):1450–1458. 10.1164/rccm.201504-0767ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caldwell JA, Knapik JJ, Shing TL, Kardouni JR, Lieberman HR. The association of insomnia and sleep apnea with deployment and combat exposure in the entire population of US army soldiers from 1997 to 2011: a retrospective cohort investigation. Sleep. 2019;42(8):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caldwell JA, Knapik JJ, Lieberman HR. Trends and factors associated with insomnia and sleep apnea in all United States military service members from 2005 to 2014. J Sleep Res. 2017;26(5):665–670. 10.1111/jsr.12543 [DOI] [PubMed] [Google Scholar]
- 9. Armed Forces Health Surveillance Center. Obstructive sleep apnea, active component, US Armed Forces, January 2000-December 2009. MSMR. [Google Scholar]
- 10. Armed Forces Health Surveillance Center. Insomnia, active component, US Armed Forces, January 2000-December 2009. MSMR. [Google Scholar]
- 11.Tanielian T, Jaycox LH. Invisible Wounds of War: Psychological and Cognitive Injuries: Their Consequences, and Services to Assist Recovery. Santa Monica, CA: Rand Corporation; 2008. [Google Scholar]
- 12.National Council on Disability . Invisible Wounds: Serving Service Members and Veterans with PTSD and TBI. Washington, DC: National Council on Disability; 2009.
- 13.Rumble ME, White KH, Benca RM. Sleep disturbances in mood disorders. Psychiatr Clin North Am. 2015;38(4):743–759. 10.1016/j.psc.2015.07.006 [DOI] [PubMed] [Google Scholar]
- 14.Germain A. Sleep disturbances as the hallmark of PTSD: where are we now?. Am J Psychiatry. 2013;170(4):372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niemelā M, Paananen R, Hakko H, Merikukka M, Gissler M, Rasanen S. The prevalence of children affected by parental cancer and their use of specialized psychiatric services: the 1987 Finnish Birth Cohort study. Int J Cancer. 2012;131(9):2117–2125. 10.1002/ijc.27466 [DOI] [PubMed] [Google Scholar]
- 16.McCoy BM, Rickert ME, Class QA, Larsson H, Lichtenstein P, D’Onofrio BM. Mediators of the association between parental severe mental illness and offspring neurodevelopmental problems. Ann Epidemiol. 2014;24(9):629–634. 10.1016/j.annepidem.2014.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Judicibus MA, McCabe MP. The impact of parental multiple sclerosis on the adjustment of children and adolescents. Adolescence. 2004;39(155):551–569. [PubMed] [Google Scholar]
- 18.van de Port IG, Visser-Meily AM, Post MW, Lindeman E. Long-term outcome in children of patients after stroke. J Rehabil Med. 2007;39(9):703–707. [DOI] [PubMed] [Google Scholar]
- 19.Niemela M, Kinnunen L, Paananen R, et al. Parents’ traumatic brain injury increases their children’s risk for use of psychiatric care: the 1987 Finnish Birth Cohort study. Gen Hosp Psychiatry. 2014;36(3):337–341. [DOI] [PubMed] [Google Scholar]
- 20.Tiar AM, Dumas JE. Impact of parental acquired brain injury on children: review of the literature and conceptual model. Brain Inj. 2015;29(9):1005–1017. 10.3109/02699052.2014.976272 [DOI] [PubMed] [Google Scholar]
- 21.Hisle-Gorman E, Susi A, Gorman GH. The impact of military parents’ injuries on the health and well-being of their children. Health Aff. (Millwood). 2019;38(8):1358–1365. 10.1377/hlthaff.2019.00276 [DOI] [PubMed] [Google Scholar]
- 22.Meltzer LJ, Johnson C, Crosette J, Ramos M, Mindell JA. Prevalence of diagnosed sleep disorders in pediatric primary care practices. Pediatrics. 2010;125(6):e1410–e1418. 10.1542/peds.2009-2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson EO, Roth T, Schultz L, Breslau N. Epidemiology of DSM-IV insomnia in adolescence: lifetime prevalence, chronicity, and an emergent gender difference. Pediatrics. 2006;117(2):e247–e256. 10.1542/peds.2004-2629 [DOI] [PubMed] [Google Scholar]
- 24.Ohayon MM, Roberts RE, Zulley J, Smirne S, Priest RG. Prevalence and patterns of problematic sleep among older adolescents. J Am Acad Child Adolesc Psychiatry. 2000;39(12):1549–1556. 10.1097/00004583-200012000-00019 [DOI] [PubMed] [Google Scholar]
- 25.Telzer EH, Goldenberg D, Fuligni AJ, Lieberman MD, Galvan A. Sleep variability in adolescence is associated with altered brain development. Dev Cogn Neurosci. 2015;1416–22. 10.1016/j.dcn.2015.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagenauer MH, Perryman JI, Lee TM, Carskadon MA. Adolescent changes in the homeostatic and circadian regulation of sleep. Dev Neurosci. 2009;31(4):276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown LK. Can sleep deprivation studies explain why human adults sleep?. Curr Opin Pulm Med. 2012;18(6):541–545. [DOI] [PubMed] [Google Scholar]
- 28.Hisle-Gorman E, Harrington D, Nylund CM, Tercyak KP, Anthony BJ, Gorman GH. Impact of parents’ wartime military deployment and injury on young children’s safety and mental health. J Am Acad Child Adolesc Psychiatry. 2015;54(4):294–301. 10.1016/j.jaac.2014.12.017 [DOI] [PubMed] [Google Scholar]
- 29.Castriotta RJ, Wilde MC, Lai JM, Atanasov S, Masel BE, Kuna ST. Prevalence and consequences of sleep disorders in traumatic brain injury. J Clin Sleep Med. 2007;3(4):349–356. 10.5664/jcsm.26855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams SG, Collen J, Orr N, Holley AB, Lettieri CJ. Sleep disorders in combat-related PTSD. Sleep Breath. 2015;19(1):175–182. 10.1007/s11325-014-0984-y [DOI] [PubMed] [Google Scholar]
- 31.Fuligni AJ, Tsai KM, Krull JL, Gonzales NA. Daily concordance between parent and adolescent sleep habits. J Adolesc Health. 2015;56(2):244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malow BA, Katz T, Reynolds AM, et al. Sleep difficulties and medications in children with autism spectrum disorders: a registry study. Pediatrics. 2016;137(Suppl 2)S98–S104. [DOI] [PubMed] [Google Scholar]
- 33.Owens JA. The ADHD and sleep conundrum: a review. J Dev Behav Pediatr. 2005;26(4):312–322. 10.1097/00004703-200508000-00011 [DOI] [PubMed] [Google Scholar]
- 34.Rolland J. Parental illness and disability: a family systems framework. J Fam Ther. 1999;21(3):242–266. 10.1111/1467-6427.00118 [DOI] [Google Scholar]
- 35.National Sleep Foundation . 2006 Sleep in America Poll Highlights and Key Findings. Washington, DC: National Sleep Foundation; 2006.
- 36.Pagel JF, Forister N, Kwiatkowki C. Adolescent sleep disturbance and school performance: the confounding variable of socioeconomics. J Clin Sleep Med. 2007;3(1):19–23. [PubMed] [Google Scholar]
- 37.Vignau J, Bailly D, Duhamel A, Vervaecke P, Beuscart R, Collinet C. Epidemiologic study of sleep quality and troubles in French secondary school adolescents. J Adolesc Health. 1997;21(5):343–350. 10.1016/S1054-139X(97)00109-2 [DOI] [PubMed] [Google Scholar]
- 38.Gregory AM, Caspi A, Moffitt TE, Poulton R. Family conflict in childhood: a predictor of later insomnia. Sleep. 2006;29(8):1063–1067. 10.1093/sleep/29.8.1063 [DOI] [PubMed] [Google Scholar]
- 39.Dunne L, Patel P, Maschauer EL, Morrison I, Riha RL. Misdiagnosis of narcolepsy. Sleep Breath. 2016;20(4):1277–1284. 10.1007/s11325-016-1365-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gorman LA, Fitzgerald HE, Blow AJ. Parental combat injury and early child development: a conceptual model for differentiating effects of visible and invisible injuries. Psychiatr Q. 2010;81(1):1–21. 10.1007/s11126-009-9116-4 [DOI] [PubMed] [Google Scholar]
- 41.Kass LJ. Sleep problems. Pediatr Rev. 2006;27(12):455–462. 10.1542/pir.27-12-455 [DOI] [PubMed] [Google Scholar]
- 42.Nelson W. Nelson Textbook of Pediatrics. 21st ed. Philadelphia, PA: Elsevier; 2019. [Google Scholar]
- 43.Park S, Lee JM, Sim CS, et al. Impact of adenotonsillectomy on nocturnal enuresis in children with sleep-disordered breathing: a prospective study. Laryngoscope. 2016;126(5):1241–1245. 10.1002/lary.25934 [DOI] [PubMed] [Google Scholar]
- 44.Harris G. Sleeping pill use by youths soars, study says. The New York Times. February 10, 2019, 2005; October 19, 2005.
- 45.Stojanovski SD, Rasu RS, Balkrishnan R, Nahata MC. Trends in medication prescribing for pediatric sleep difficulties in US outpatient settings. Sleep. 2007;30(8):1013–1017. 10.1093/sleep/30.8.1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aslan S, Isik E, Cosar B. The effects of mirtazapine on sleep: a placebo controlled, double-blind study in young healthy volunteers. Sleep. 2002;25(6):677–679. 10.1093/sleep/25.6.666 [DOI] [PubMed] [Google Scholar]
- 47.Owens JA, Moturi S. Pharmacologic treatment of pediatric insomnia. Child Adolesc Psychiatr Clin N Am. 2009;18(4):1001–1016. 10.1016/j.chc.2009.04.009 [DOI] [PubMed] [Google Scholar]
- 48.Owens JA, Babcock D, Blumer J, et al. The use of pharmacotherapy in the treatment of pediatric insomnia in primary care: rational approaches. A consensus meeting summary. J Clin Sleep Med. 2005;1(1):49–59. 10.5664/jcsm.26297 [DOI] [PubMed] [Google Scholar]
- 49.Smaldone A, Honig JC, Byrne MW. Sleepless in America: inadequate sleep and relationships to health and well-being of our nation’s children. Pediatrics. 2007;119(Suppl 1):S29–S37. 10.1542/peds.2006-2089F [DOI] [PubMed] [Google Scholar]
- 50.El-Sheikh M, Kelly RJ, Bagley EJ, Wetter EK. Parental depressive symptoms and children’s sleep: the role of family conflict. J Child Psychol Psychiatry. 2012;53(7):806–814. 10.1111/j.1469-7610.2012.02530.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Le TD, Gurney JM, Nnamani NS, et al. A 12-year analysis of nonbattle injury among US service members deployed to Iraq and Afghanistan. JAMA Surg. 2018;153(9):800–807. 10.1001/jamasurg.2018.1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.