Abstract
Vagus nerve stimulation (VNS) paired with motor rehabilitation enhances recovery of function after neurological injury in rats and humans. This effect is ascribed to VNS-dependent facilitation of plasticity in motor networks. Previous studies document an inverted-U relationship between VNS intensity and cortical plasticity, such that moderate intensities increase plasticity, while low or high intensity VNS does not. We tested the interaction of moderate and high intensity VNS trains to probe the mechanisms that may underlie VNS-dependent plasticity. Rats performed a behavioral task where VNS was paired with jaw movement during chewing. For five days, subjects received 100 pairings of moderate intensity VNS (Standard VNS), 100 pairings alternating between moderate and high intensity VNS (Interleaved VNS), or 50 pairings of moderate intensity VNS (Short VNS) approximately every 8 seconds. After the final behavioral session, intracortical microstimulation (ICMS) was used to evaluate movement representations in motor cortex. 100 pairings of moderate intensity VNS enhanced motor cortex plasticity. Replacing half of moderate intensity stimulation with high intensity VNS blocked this enhancement of plasticity. Removing high intensity stimulation, leaving only 50 pairings of moderate intensity VNS, reinstated plasticity. These results demonstrate that there is a period for at least 8 seconds after high intensity stimulation in which moderate intensity VNS is not able to engage mechanisms required for synaptic reorganization. More importantly, this study demonstrates that changes in stimulation parameters are a critical determinant of the magnitude of plasticity and likely the efficacy of VNS-enhanced recovery.
Keywords: Vagus nerve stimulation, intracortical microstimulation, cortical reorganization, motor cortex, synaptic plasticity
1. Introduction
Vagus nerve stimulation (VNS) paired with rehabilitation has recently emerged as a strategy to enhance recovery of motor function after a range of neurological injuries including stroke, traumatic brain injury, neuropathy, and spinal cord injury [1–11]. Greater recovery is attributed to VNS-dependent enhancement of synaptic plasticity in motor and sensory networks within the central nervous system during rehabilitation [12,13]. Thus, defining stimulation strategies that maximize plasticity may provide a means to optimize the efficacy of VNS therapy.
Activation of several neuromodulatory nuclei are required for VNS-mediated synaptic plasticity. VNS rapidly engages the nucleus basalis (NB) and locus coeruleus (LC), which in turn release the neuromodulators acetylcholine and norepinephrine, respectively, throughout the brain [14–18]. Acetylcholine and norepinephrine, as well as serotonin, bind to and activate G-protein coupled receptors (GPCRs) [19–22]. Simultaneous activation of these GPCRs paired with the neural activity associated with rehabilitation transiently increases synaptic plasticity, resulting in VNS potentiating neural circuits that contribute to recovery and enhancing the therapeutic effects of rehabilitation [23].
Skilled motor learning causes the area of motor cortex that elicits movement of the trained muscles to transiently expand. This expansion is driven by synaptic long-term potentiation in excitatory and inhibitory cortical networks that control movement [24–26]. Neuromodulators can influence synaptic plasticity, thus, timed engagement of neuromodulatory networks during motor training can shape training-dependent cortical plasticity. As a result, repeatedly pairing VNS with motor training drives specific expansion of motor representations via activation of neuromodulatory networks [18,27–31].
The stimulation parameters of VNS, including intensity, frequency, and duration, can influence the level of activity in neuromodulatory nuclei [18]. For example, VNS intensity scales linearly with LC activation, such that 1.6 mA VNS promotes twice the neuromodulator release of 0.8 mA VNS. Paradoxically, this increase in neuromodulatory activity does not translate to increases in the magnitude of cortical plasticity. Instead, VNS-directed plasticity exhibits an inverted-U relationship with increases in stimulation intensity, such that 0.8 mA VNS significantly increases cortical plasticity while 0.4 and 1.6 mA VNS do not [28,31,32]. Highlighting the clinical importance of these findings, an equivalent inverted-U effect is observed between VNS intensity and recovery after stroke [8].
While the absence of VNS-mediated plasticity at 0.4 mA can be ascribed to insufficient activation of neuromodulatory nuclei and minimal activation of GPCRs required for synaptic reorganization, the neural mechanisms underlying an absence of plasticity at higher intensities such as 1.6 mA are unclear. Overactivation of neuromodulatory systems at high stimulation intensities could lead to desensitization of mechanisms promoting VNS-mediated plasticity. If so, high intensity VNS should interfere with moderate intensity VNS and suppress plasticity-enhancing effects for the duration of this desensitization. Alternatively, if high intensity VNS does not desensitize neuromodulatory pathways critical for VNS-mediated plasticity, there should be no temporal interaction between the two, and moderate intensity VNS should still enhance plasticity.
Here, we tested the temporal interaction of moderate and high intensity VNS trains to probe the potential mechanisms that may underlie VNS-dependent plasticity. To do so, we conducted an experiment in rats pairing both 0.8 mA and 1.6 mA VNS during motor training, and using intracortical microstimulation (ICMS) we observed the magnitude of subsequent motor cortex plasticity (Fig. 1).
Figure 1.
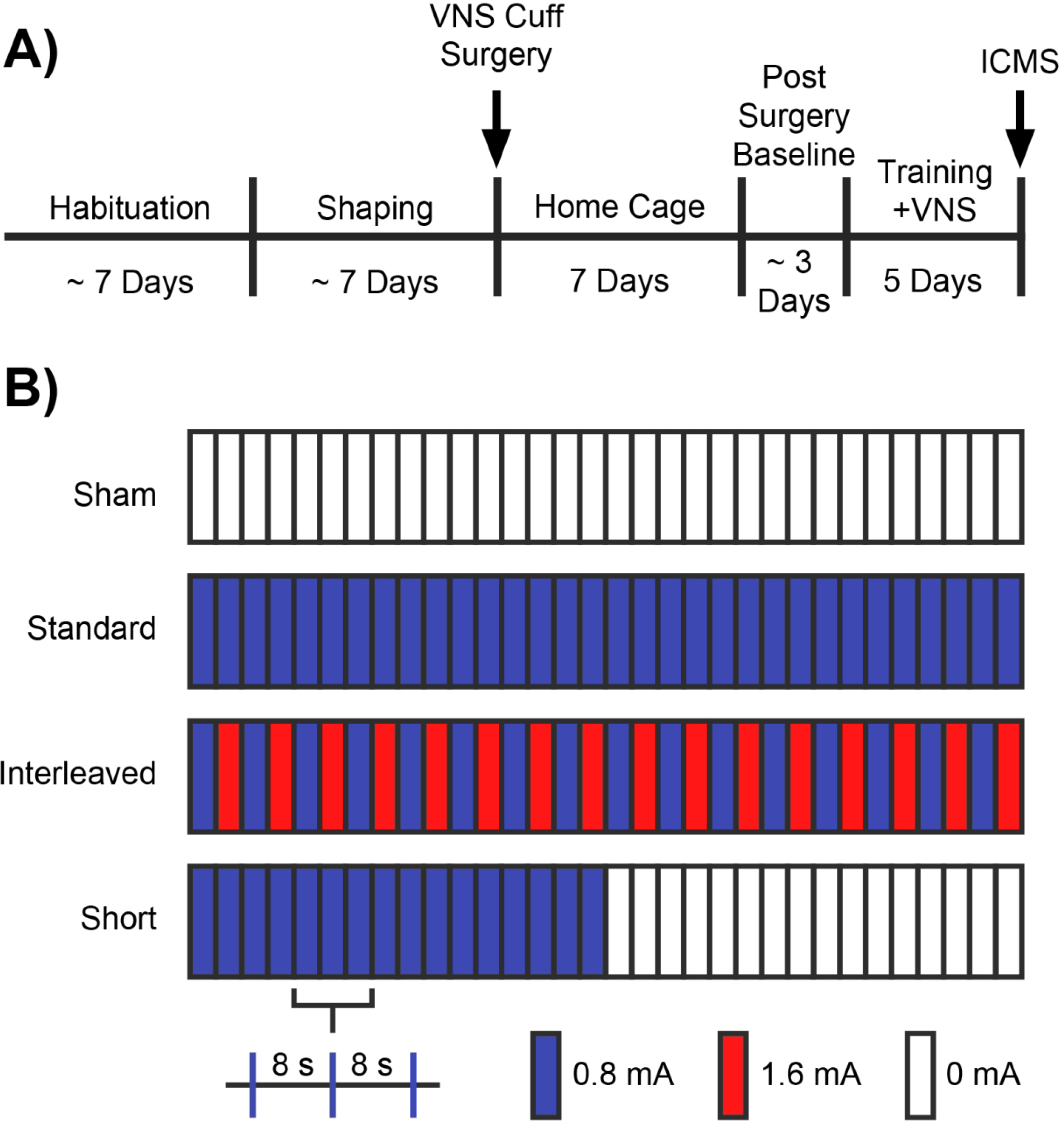
Behavioral task and experimental design. (A) Experimental timeline. (B) Overview of experimental groups. Groups received different paradigms of VNS paired with behavioral training: 100 pairings at a moderate intensity (Standard VNS), 100 pairings of interleaved moderate and high intensity stimulation (Interleaved VNS), 50 parings at a moderate intensity (Short VNS), or equivalent behavioral training with without VNS (Sham). Stimulation occurred at 8 second intervals.
2. Results
Pairing trains of VNS with motor training drives robust, specific plasticity in motor cortex [13,27–30]. The magnitude of plasticity displays an inverted-U relationship with stimulation intensity, such that moderate intensity VNS enhances plasticity while high intensity VNS fails to enhance plasticity [28,32]. Here, we tested the temporal interaction of moderate and high intensity VNS trains to probe the potential mechanisms that may underlie VNS-dependent plasticity. To do so, rats performed a simple behavioral task for five days during which VNS at either moderate or both moderate and high intensities was paired with jaw movement during chewing (Fig. 1). Within 24 hours of the final behavioral training session, motor cortex movement representations were documented using intracortical microstimulation (ICMS) with area of jaw representation as the primary experimental outcome.
2.1. Moderate intensity VNS at 0.8 mA paired with motor training enhances motor cortex plasticity
We first sought to confirm that pairing standard, moderate intensity VNS with motor training could enhance motor cortex plasticity. Group analysis of motor cortex area eliciting jaw movement revealed significant differences between groups (One-way ANOVA, F[3,19] = 8.170, p = 0.004). Standard VNS paired with chewing significantly increased jaw representation compared to equivalent training on the task without VNS (Standard VNS: 2.69 ± 0.27 mm2; Sham: 1.25 ± 0.24 mm2, Unpaired t-test, p = 0.004) (Fig. 2A). These findings confirm that VNS at moderate intensity 0.8 mA paired with training enhances motor cortex plasticity, as previously reported [27–30].
Figure 2.
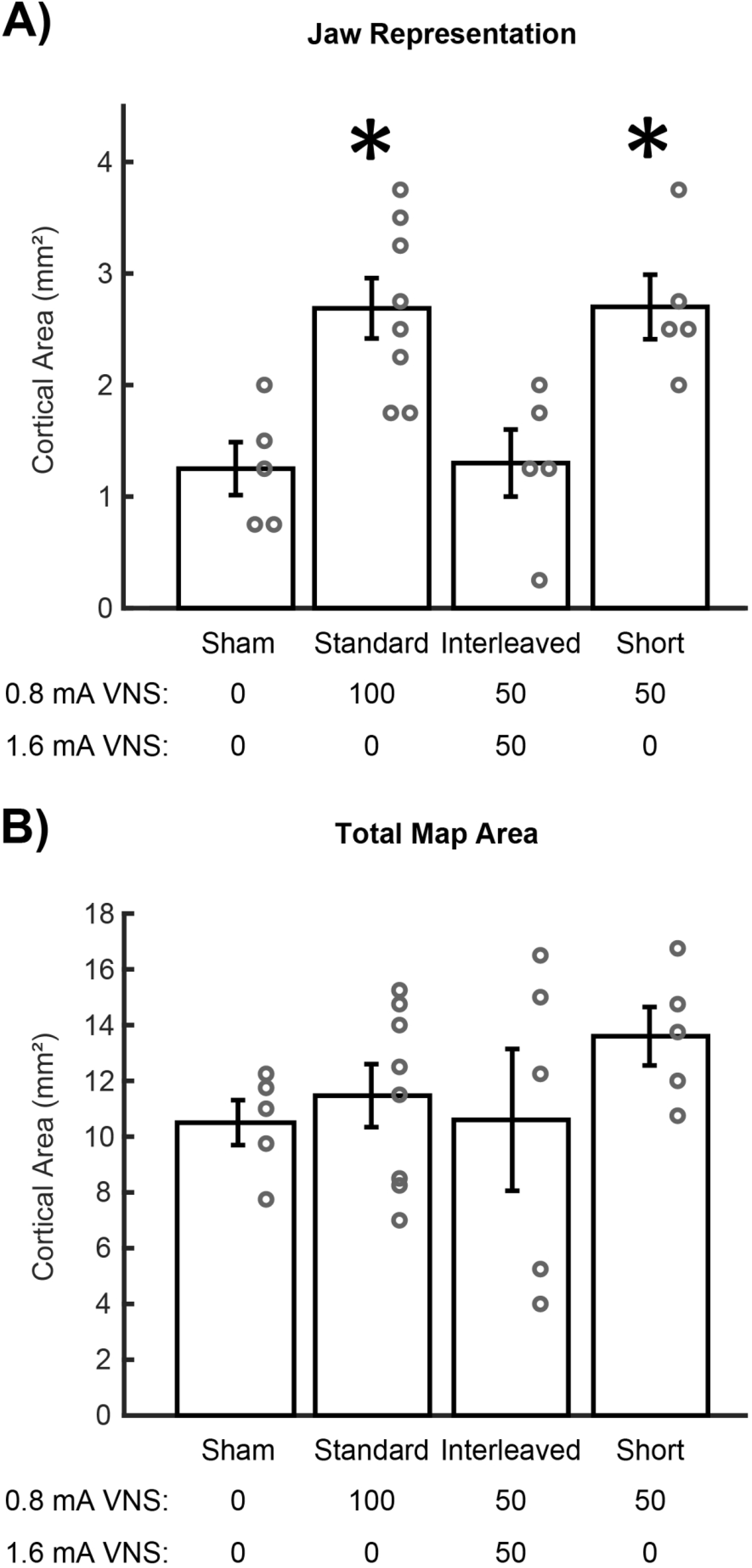
High intensity VNS disrupts cortical plasticity. (A) Standard VNS and Short VNS paired with chewing significantly increases jaw movement representation area in motor cortex compared to equivalent behavioral experience without VNS. Interleaved VNS fails to enhance plasticity. (B) No change in total motor cortex area was observed between groups. Bars represent mean ± SEM. “*” indicates p < 0.008.
2.2. Adding high intensity 1.6 mA VNS interleaved with 0.8 mA VNS disrupts motor cortex plasticity
Next, we interleaved high intensity 1.6 mA VNS, which does not enhance plasticity [28,31,32], alongside pro-plasticity moderate intensity 0.8 mA VNS to clarify the action of high intensity VNS. Interleaved VNS resulted in significantly less jaw representation in motor cortex compared to Standard VNS (Interleaved VNS: 1.30 ± 0.30 mm2; Standard VNS: 2.69 ± 0.27 mm2, Unpaired t-test, p = 0.007). Interleaved VNS paired with chewing also failed to enhance jaw representation in motor cortex compared to Sham animals (Interleaved VNS: 1.30 ± 0.30 mm2; Sham: 1.25 ± 0.24 mm2, Unpaired t-test, p = 0.899) (Fig. 2A). This demonstrates that VNS at high intensities interferes with moderate 0.8 mA VNS-mediated plasticity.
2.3. 0.8 mA VNS alone enhances VNS-mediated motor cortex plasticity
To confirm that high intensity stimulation was disrupting VNS-mediated plasticity, we removed the 1.6 mA stimulation, leaving only 50 moderate intensity 0.8 mA stimulations per session (Short VNS). Removal of high intensity stimulation restored motor cortex plasticity. Short VNS significantly increased jaw representation compared to Sham animals (Short VNS: 2.70 ± 0.29 mm2; Sham: 1.25 ± 0.24 mm2, Unpaired t-test, p = 0.005) and comparable to Standard VNS (Short VNS: 2.70 ± 0.29 mm2; Standard VNS: 2.69 ± 0.27 mm2, Unpaired t-test, p = 0.976) (Fig. 2A). These findings additionally confirm that moderate intensity stimulation enhances cortical plasticity, and high intensity stimulation prevents VNS-dependent plasticity.
2.4. VNS does not affect total motor cortex representation or movement thresholds
Consistent with previous studies [27–30], no differences in other cortical movement representations were observed between groups, indicating that VNS-dependent synaptic plasticity is specific to the paired movement (One-way ANOVA, forelimb: F[3,19] = 0.426, p = 0.661; vibrissa: F[3,19] = 0.376, p = 0.693; neck: F[3,19] = 0.268, p = 0.769; hindlimb: F[3,19] = 2.097, p = 0.157). Additionally, total motor cortex area was also not affected by VNS (One-way ANOVA, F[3,19] = 1.796, p = 0.200) (Fig. 2B). As expected, group analysis revealed no differences across groups in average stimulation threshold required to elicit movement (One-way ANOVA, F[3,19] = 2.049, p = 0.141).
3. Discussion
VNS has repeatedly proven effective at enhancing cortical plasticity using a stimulation paradigm of 0.8 mA, 100 μs pulse width, 30 Hz frequency with a pulse train of 0.5 s. Variation of these stimulation parameters can significantly influence the degree of subsequent cortical plasticity. There is an inverted-U relationship between degree of synaptic plasticity and pulse duration [33], frequency [33], and intensity of VNS [28,31,32,34–36]. Intensity has been the most studied of these parameters, with high intensity VNS consistently failing to enhance synaptic plasticity compared to moderate intensities. Although high intensity VNS fails to enhance plasticity when delivered alone, it is unclear whether the mechanisms engaged by high intensity VNS interact with and disrupt subsequent moderate intensity VNS.
In this study, we tested the temporal interaction of moderate and high intensity VNS to probe the potential mechanisms that may underlie VNS-dependent plasticity. To do so, we conducted an experiment in rats pairing both 0.8 mA and 1.6 mA approximately every 8 seconds VNS during motor training, and using ICMS we observed the magnitude of subsequent motor cortex plasticity. We confirm moderate intensity 0.8 mA VNS enhances plasticity. Replacing half of moderate intensity stimulation with high intensity 1.6 mA VNS blocks this VNS-dependent enhancement of plasticity. Removing the high intensity stimulation reinstates plasticity. These results demonstrate that there is a period of time lasting at least 8 seconds after high intensity stimulation in which moderate intensity VNS is not able to enhance synaptic plasticity.
This study leveraged the inverted-U relationship between VNS intensity and was designed to differentiate between two possible outcomes (Fig. 3). In the first option, high intensity stimulation engages a signaling pathway with a rapid decay, and thus does not interfere with moderate intensity (Fig. 3B). Here, high intensity stimulation trains result in activation that exceed the effective range, indicated in green, but the signal engagement rapidly decays to baseline levels. Subsequent trains of moderate intensity stimulation then produce activation that peaks in the effective range, and thus cortical plasticity is enhanced. In the second option, high intensity stimulation engages a signaling pathway that slowly decays, and as a result, interacts with the plasticity-enhancing action of moderate intensity stimulation trains (Fig. 3C). In this scenario, high intensity VNS results in engagement of a signaling pathway that exceeds the effective range and slowly decays. Subsequent moderate intensity trains build on this overactivation and push the signal engagement further out of the effect range, resulting in no enhancement of plasticity. Our findings are consistent with the second option (Fig. 3C). This indicates that the action of trains of VNS delivered within the order of ten seconds may interact, which raises the need to consider this interaction when selecting stimulation paradigms.
Figure 3.
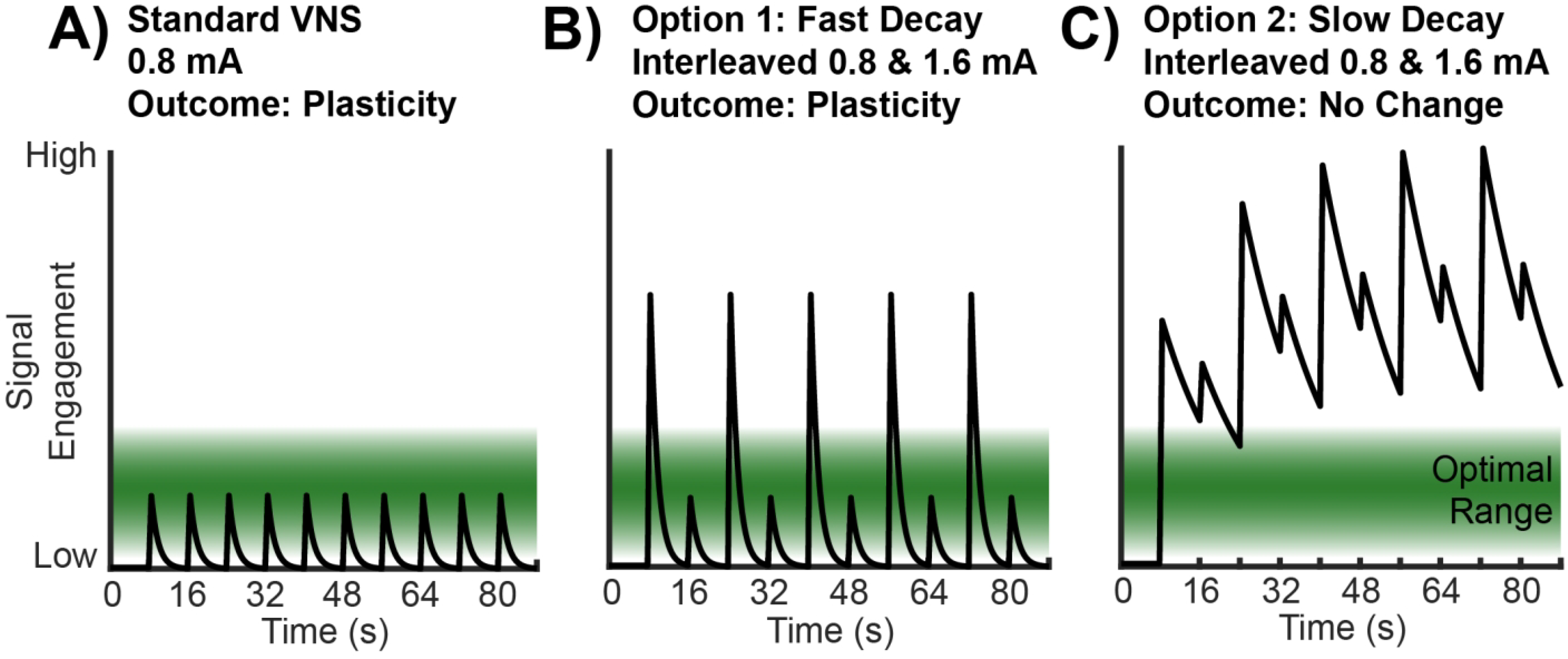
Conceptual models of the temporal engagement of plasticity enhancement pathways by VNS. (A) The green band indicates the level of engagement of signaling pathways that promotes plasticity. Moderate intensity trains of VNS produce signal engagement that peaks within the effective range, resulting in enhanced synaptic plasticity observed in the Standard VNS group. (B) In this model, signal levels decay rapidly. High intensity VNS produces activation that peaks above the effective range and thus does not facilitate plasticity. However, interleaved moderate intensity trains produce signal engagement within the effective range, which results in enhanced plasticity. In this model, the Interleaved VNS group would exhibit enhanced plasticity. Our results are inconsistent with this model. (C) In this model, signal levels decay slowly. High intensity VNS produces activation that peaks above the effective range. Interleaved moderate intensity trains build on this overactivation and also peak outside the optimal range, thus no enhancement of plasticity is observed. In this model, the Interleaved VNS group would not exhibit enhanced plasticity, which is accordant with the results reported in this study.
The mechanisms engaged by VNS may provide insight into why high intensity VNS fails to promote plasticity, and why it continues to disrupt the plasticity-enhancing effects of subsequent moderate intensity stimulation. VNS rapidly engages the NB and LC, which release the neuromodulators acetylcholine and norepinephrine. Increasing the current intensity of VNS proportionally increases the release of these neuromodulators [18]. Acetylcholine and norepinephrine then activate GPCRs, which in turn signal for the production of new proteins and other cellular machinery needed for synaptic reorganization. High intensity VNS may disrupt this process due to an over-release of neuromodulators and overactivation and desensitization of these GPCRs, which are known to exhibit notable short-term and long-term desensitization [19,20,29,30]. This desensitization could be due to overstimulation and subsequent inactivation of relevant G-proteins via β-arrestin, which can occur on a timescale of minutes [19,20], or due to GPCR internalization or even lysosomal degradation, which may have long-lasting effects on a timescale of hours or days [20]. The timescales of these forms of desensitization could account for the ability of high intensity VNS to disrupt the effects of subsequent moderate intensity stimulation.
Alternatively, mechanisms other than GPCR desensitization may be at work. Activation of opposing families of adrenergic receptors could account for the disruption of VNS-mediated plasticity after high intensity stimulation. Increasing VNS intensity drives proportionate increases in LC activity, which in turn increases levels of norepinephrine release in motor cortex [18]. Activation of high affinity α-receptors and low affinity β-receptors differentially modulate the outcome of spike-timing-dependent plasticity depending on norepinephrine concentration, leading to either long-term potentiation or depression of synapses [37]. Under this model, moderate intensities of VNS would produce maximal activation of pro-plasticity α-receptors and minimize activation of pro-stability β-receptors, while high intensity VNS would activate β-receptors, leading to a robust, overriding stability of circuits. Indeed, similar activation of noradrenergic receptors and their effects on plasticity has been previously described at timescales that could account for the disruptive effects of high intensity VNS reported here [28,37–39].
Another possibility for high intensity stimulation’s disruption of VNS-mediated plasticity is recruitment of inhibitory interneurons, resulting in increased stability of activated networks. Cholinergic modulation of sensory stimuli via GABAergic interneurons in cortico-cortical networks can influence learning and memory retrieval and may play a role in the selective potentiation of circuits paired with VNS during training [40]. Varying the concentration of acetylcholine can differentially activate feedback inhibition mechanisms via muscarinic receptors, which can lead to either suppression or amplification of sensory information in cortical networks. Under this model, high intensity VNS raises cholinergic levels in motor cortex to the point that inhibitory mechanisms dominate, causing the nervous system to promote circuit stability and overriding any signal from moderate intensity VNS for synapses to reorganize.
VNS can selectively enhance synaptic reorganization when paired with an array of training and rehabilitative paradigms [2,12,27–31]. Furthermore, VNS-paired rehabilitation enhances recovery compared to rehabilitation alone in a number of injuries including stroke, traumatic brain injury, neuropathy, and spinal cord injury [1,2,4–6,9,11,12]. VNS parameters that enhance plasticity yield recovery, while those that fail to enhance plasticity do not provide any functional benefits [8]. This suggests that synaptic reorganization is the driving force behind VNS-mediated recovery after injury.
The range of VNS intensities that enhance synaptic plasticity is narrow [31], and therefore it is of the upmost importance to accurately deliver stimulation, as small deviations from optimal stimulation parameters could result in a lack of treatment efficacy. Variations in nerve cuff implantation, scarring, and natural variations between patients could alter vagal fiber activation [41], which could conceivably alter neuromodulatory response and the resulting magnitude of synaptic reorganization. In order to counteract these variations it would seem reasonable to employ a VNS-rehabilitation paradigm in which the intensities of VNS ramp from slightly below to slightly above 0.8 mA throughout treatment to ensure patients receive optimal vagal fiber activation. However, we demonstrate that high intensity stimulation can disrupt VNS-mediated plasticity, meaning such a paradigm could be counterproductive to patient recovery. A better understanding of the neural basis for VNS-mediated synaptic plasticity at moderate intensities, and disruption of this synaptic plasticity at higher intensities, could open the door for greater optimization and increased treatment efficacy in the clinic.
4. Methods
4.1. Subjects
Thirty-six female Sprague Dawley rats weighing approximately 250 grams were used in this study (Charles River Labs). All rats were housed in a reversed 12:12 hour light-dark cycle. Rats that underwent behavioral training were food restricted on weekdays during shaping and training with ad libitum access to food on weekends. All rats were maintained at or above 85% body weight relative to the beginning of shaping. All handling, housing, stimulation, and surgical procedures were approved by The University of Texas at Dallas Institutional Animal Care and Use Committee. Data from a subset of subjects used in this study has been previously published [31]. All subjects were run concurrently.
4.2. Behavioral training
Rats were trained on a simple automated behavioral task that allowed triggering of VNS during chewing [31]. The behavioral training apparatus consisted of an acrylic cage with a nosepoke food dispenser at one end. A food pellet (45 mg dustless precision pellet, BioServ, Frenchtown, NJ) was delivered to the food dispenser. An infrared beam sensor positioned in the food dispenser was used to determine when the rat entered the nosepoke to retrieve the food pellet. Upon breaking the infrared beam, another pellet was dispensed after an eight second delay. Additionally, in the appropriate groups, VNS was triggered 3 seconds after beam break. This stimulation timing results in reliable delivery of VNS during chewing [31]. Each behavioral session continued until either 100 pellets had been dispensed, or until 1 hour had elapsed. Rats received a supplement of approximately 100 food pellets if they did not receive at least 100 pellets in a day to maintain weight.
Rats performed the task twice per day, 5 days per week, with daily sessions separated by at least 2 hours. Rats were shaped on the task until they reliably consumed 100 pellets within 1 hour each session (Fig. 1A). Rats were then implanted with a VNS cuff and recovered for 7 days in their home cage with ad libitum access to food and water. Seven days after surgery, rats were allocated to one of four groups to receive 10 additional training sessions over 5 days. Groups received either VNS at 0.8 mA paired with 100 trials per session (Standard VNS), VNS at 0.8 mA paired with 50 trials per session (Short VNS), VNS alternating between 0.8 and 1.6 mA on each successive trial for 100 trials per session (Interleaved VNS), or sham stimulation (Sham) (Fig. 1B). Each train of VNS consisted of a 500 ms burst triggered 3 seconds after nosepoke beam break, which reliably results in delivery of VNS during chewing [31]. Twenty-four hours after the conclusion of behavioral training, all rats underwent ICMS motor cortex mapping.
4.3. Surgical implantation
All surgeries were performed using aseptic technique under general anesthesia. Rats were implanted with a stimulating cuff on the left cervical vagus nerve as described in previous studies [4–6,28,42]. Rats were anesthetized with ketamine hydrochloride (50 mg/kg, i.p.), xylazine (20 mg/kg, i.p.), and acepromazine (5 mg/kg, i.p.), and were placed in a stereotactic apparatus. An incision was made down the midline of the head to expose the skull. Bone screws were inserted into the skull at points surrounding the lamboid suture and over the cerebellum. A two-channel connector was mounted to the screws using acrylic. The rat was then removed from the stereotactic apparatus and placed in a supine position.
An incision was made on the left side of the neck and the overlying musculature was blunt dissected to reveal the left cervical vagus nerve. The nerve was gently dissected away from the carotid artery. A cuff electrode was implanted surrounding the vagus nerve, and the leads were tunneled subcutaneously to connect with the two-channel connector mounted on the skull. Nerve activation was confirmed by observation of a ≥ 5% drop in blood oxygen saturation in response to a 10 s stimulation train of VNS, as in previous studies [28]. The head and neck incisions were then sutured, and rats received subcutaneous injections of 4 mL 50:50 0.9% saline 5% dextrose solution. A seven day recovery period followed surgery during which animals did not perform behavioral training. All rats underwent implantation procedures.
4.4. Vagus nerve stimulation
Upon return to behavioral testing after surgery, rats were randomly assigned to groups to receive either 100 stimulations of VNS at 0.8 mA per session (Standard VNS, n = 8), 50 stimulations of 0.8 mA VNS per session (Short VNS, n = 5), 100 stimulations of VNS alternating between 0.8 and 1.6 mA per session (Interleaved VNS, n = 5), or equivalent behavioral training without stimulation (Sham, n = 5) (Fig. 1B). In the initial sessions after implantation, no stimulation was delivered in any group while rats were allowed to acclimate to being attached to stimulating cables until they reliably consumed 100 pellets in a one hour session. Once acclimated, rats then underwent five days of training and received VNS stimulation according to their group. VNS was triggered 3 seconds after nosepoke beam break once a pellet had been dispensed during behavioral training, resulting in stimulation that was consistently delivered during chewing of the pellet [31]. Each 0.5 s stimulation train consisted of 100 μsec biphasic pulses delivered at 30 Hz at an intensity of either 0.8 mA or 1.6 mA, as appropriate for each experimental group. A digital oscilloscope (PicoScope 2204A, PP906, Pico Technology) was used to monitor voltage across the electrodes during each stimulation to ensure cuff functionality.
4.5. Intracortical microstimulation mapping
Approximately 24 hours after their last behavioral session, rats underwent ICMS to derive cortical movement representation maps according to standard procedures [27,28,43–46]. Rats were anesthetized with intraperitoneal injections of ketamine hydrochloride (80 mg/kg) and xylazine (10 mg/kg). Rats received supplemental doses of ketamine as necessary throughout the procedure in order to maintain a consistent level of anesthesia as indicated by breathing rate, vibrissae whisking, and toe pinch reflex. Rats were placed in a stereotactic apparatus and a craniotomy and durotomy were performed to expose the left motor cortex (4 mm to −3 mm AP and 0.25 mm to 5 mm ML). To prevent cortical swelling, a small incision was made in the cisterna magna.
Connected to a pulse stimulator (Model 2100, A-M Systems, Sequim, WA, ±100 V), a tungsten electrode (0.65 ± 0.8 MΩ) (UEWMEGSEBN3M, FHC, Bowdoin, ME) was lowered into the brain to a depth of 1.8 mm. Stimulation sites were chosen at random on a grid with sites set 500 μm apart from each other. The next stimulation site was placed at least 1 mm away from the previous site whenever possible. Stimulation consisted of a 40 ms pulse train of 10 monophasic 200 μs cathodal pulses. Stimulation was increased from 10 μA until a movement was observed or until a maximum of 250 μA was reached. ICMS was conducted blinded with two experimenters, as previously described [27–29]. The first experimenter placed the electrode and recorded the data for each site. The second experimenter, blinded to group and electrode location, delivered stimulations and classified movements. Movements were classified into the following categories: jaw, neck, vibrissa, forelimb, and hindlimb. After the completion of ICMS, VNS cuff functionality was confirmed by a stimulation-evoked decrease in blood oxygen saturation in response to a 10 s VNS train, as previously described [28,47]. All maps from ICMS are included in the supplemental materials.
4.6. Subject exclusion
Twenty-three subjects were analyzed in the final results of the study out of a total of 36 subjects. Of the 13 subjects not used in final analysis, 5 subjects were excluded due to non-functional stimulating cuffs (indicated by digital oscilloscope readings exceeding 40 V peak-to-peak), 3 subjects were excluded due to headcap failure, 3 subjects were excluded due to a lack of drop in blood oxygen saturation in response to VNS during ICMS, 1 subject died during VNS surgery, and 1 subject died during ICMS surgery. All exclusion criteria were predefined before beginning data collection and are consistent with previous studies [27–31].
4.7. Statistics
The primary outcome of this study was area of motor cortex generating jaw movements. All other movement representations were analyzed as secondary outcome measures. A one-way ANOVA was used to identify differences across groups. Post hoc unpaired two-tailed t-tests using a Bonferroni-corrected alpha of 0.008 were used to determine statistically significant differences between individual groups, as appropriate. Statistical tests for each comparison are noted in the text. All data are reported as mean ± SEM.
Supplementary Material
Research Highlights.
Moderate intensity VNS paired with training enhances synaptic plasticity
High intensity VNS fails to enhance synaptic plasticity
Interleaving moderate and high intensity VNS fails to enhance synaptic plasticity
High intensity VNS disrupts mechanisms required for VNS-mediated plasticity
High intensity stimulation blocks VNS-mediated plasticity for least 8 seconds
Acknowledgements
We would like to thank Sarah Jacob, Anjana Swami, Kamal Safadi, Anusheh Kashif, Armaan Somaney, Amina Aslam, Yabsera Mesfin, Vishal Kumar, and Zainab Haider for help with behavioral training. We would also like to thank David Pruitt and Jesse Bucksot for help with programming and troubleshooting.
Sources of funding
This work was supported by the National Institutes of Health R01NS094384 and R01NS103803 and by the DARPA BTO Targeted Neuroplasticity Training (TNT) program under the auspices of Dr. Tristan McClure-Begley through the Space and Naval Warfare Systems Center, Pacific Grant/Contract No. N66001–17-2–4011.
Footnotes
Conflict of interest
MPK has a financial interest in MicroTransponder, Inc., which is developing VNS for stroke. RLR has a financial interest in Teliatry, Inc., and X-Nerve, Inc., which may commercialize targeted plasticity therapy. All other authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hays SA, Ruiz A, Bethea T, Khodaparast N, Carmel JB, Rennaker RL, et al. Vagus nerve stimulation during rehabilitative training enhances recovery of forelimb function after ischemic stroke in aged rats. Neurobiol Aging 2016;43:111–8. doi: 10.1016/j.neurobiolaging.2016.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Meyers EC, Solorzano BR, James J, Ganzer PD, Lai ES, Rennaker RL, et al. Vagus Nerve Stimulation Enhances Stable Plasticity and Generalization of Stroke Recovery. Stroke 2018;49:710–7. doi: 10.1161/STROKEAHA.117.019202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Darrow MJ, Mian TM, Torres M, Haider Z, Danaphongse T, Rennaker RL, et al. Restoration of Somatosensory Function by Pairing Vagus Nerve Stimulation with Tactile Rehabilitation. Ann Neurol 2020;87:194–205. doi: 10.1002/ana.25664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Khodaparast N, Hays SA, Sloan AM, Hulsey DR, Ruiz A, Pantoja M, et al. Vagus nerve stimulation during rehabilitative training improves forelimb strength following ischemic stroke. Neurobiol Dis 2013;60:80–8. doi: 10.1016/j.nbd.2013.08.002. [DOI] [PubMed] [Google Scholar]
- [5].Khodaparast N, Hays SA, Sloan AM, Fayyaz T, Hulsey DR, Rennaker RL, et al. Vagus Nerve Stimulation Delivered During Motor Rehabilitation Improves Recovery in a Rat Model of Stroke. Neurorehabil Neural Repair 2014;28:698–706. doi: 10.1177/1545968314521006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hays SA, Khodaparast N, Ruiz A, Sloan AM, Hulsey DR, Rennaker RL, et al. The timing and amount of vagus nerve stimulation during rehabilitative training affect poststroke recovery of forelimb strength. Neuroreport 2014;25:682–8. doi: 10.1097/WNR.0000000000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Darrow MJ, Torres M, Sosa MJ, Danaphongse TT, Haider Z, Rennaker RL, et al. Vagus Nerve Stimulation Paired With Rehabilitative Training Enhances Motor Recovery After Bilateral Spinal Cord Injury to Cervical Forelimb Motor Pools. Neurorehabil Neural Repair 2020;34:200–9. doi: 10.1177/1545968319895480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pruitt DT, Danaphongse TT, Lutchman M, Patel N, Reddy P, Wang V, et al. Optimizing Dosing of Vagus Nerve Stimulation for Stroke Recovery. Transl Stroke Res 2020. doi: 10.1007/s12975-020-00829-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pruitt DT, Schmid AN, Kim LJ, Abe CM, Trieu JL, Choua C, et al. Vagus Nerve Stimulation Delivered with Motor Training Enhances Recovery of Function after Traumatic Brain Injury. J Neurotrauma 2016;33:871–9. doi: 10.1089/neu.2015.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kimberley TJ, Pierce D, Prudente CN, Francisco GE, Yozbatiran N, Smith P, et al. Vagus nerve stimulation paired with upper limb rehabilitation after chronic stroke: A blinded randomized pilot study. Stroke 2018. doi: 10.1161/STROKEAHA.118.022279. [DOI] [PubMed] [Google Scholar]
- [11].Dawson J, Pierce D, Dixit A, Kimberley TJ, Robertson M, Tarver B, et al. Safety, Feasibility, and Efficacy of Vagus Nerve Stimulation Paired With Upper-Limb Rehabilitation After Ischemic Stroke. Stroke 2016;47:143–50. doi: 10.1161/STROKEAHA.115.010477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ganzer PD, Darrow MJ, Meyers EC, Solorzano BR, Ruiz AD, Robertson NM, et al. Closed-loop neuromodulation restores network connectivity and motor control after spinal cord injury. Elife 2018;7:1–19. doi: 10.7554/eLife.32058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Meyers EC, Kasliwal N, Solorzano BR, Lai E, Bendale G, Berry A, et al. Enhancing plasticity in central networks improves motor and sensory recovery after nerve damage. Nat Commun 2019;10:5782. doi: 10.1038/s41467-019-13695-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Roosevelt RW, Smith DC, Clough RW, Jensen RA, Browning RA. Increased extracellular concentrations of norepinephrine in cortex and hippocampus following vagus nerve stimulation in the rat. Brain Res 2006;1119:124–32. doi: 10.1016/j.brainres.2006.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dorr AE. Effect of Vagus Nerve Stimulation on Serotonergic and Noradrenergic Transmission. J Pharmacol Exp Ther 2006;318:890–8. doi: 10.1124/jpet.106.104166. [DOI] [PubMed] [Google Scholar]
- [16].Follesa P, Biggio F, Gorini G, Caria S, Talani G, Dazzi L, et al. Vagus nerve stimulation increases norepinephrine concentration and the gene expression of BDNF and bFGF in the rat brain. Brain Res 2007;1179:28–34. doi: 10.1016/j.brainres.2007.08.045. [DOI] [PubMed] [Google Scholar]
- [17].Seol GH, Ziburkus J, Huang S, Song L, Kim IT, Takamiya K, et al. Neuromodulators Control the Polarity of Spike-Timing-Dependent Synaptic Plasticity. Neuron 2007;55:919–29. doi: 10.1016/j.neuron.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hulsey DR, Riley JR, Loerwald KW, Rennaker RL, Kilgard MP, Hays SA. Parametric characterization of neural activity in the locus coeruleus in response to vagus nerve stimulation. Exp Neurol 2017;289:21–30. doi: 10.1016/j.expneurol.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci 2004;27:107–44. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- [20].Rajagopal S, Shenoy SK. GPCR desensitization: Acute and prolonged phases. Cell Signal 2018. doi: 10.1016/j.cellsig.2017.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lovinger DM. Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology 2010;58:951–61. doi: 10.1016/j.neuropharm.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jong Y-JI, Harmon SK, O’Malley KL. Intracellular GPCRs Play Key Roles in Synaptic Plasticity. ACS Chem Neurosci 2018;9:2162–72. doi: 10.1021/acschemneuro.7b00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Engineer ND, Kimberley TJ, Prudente CN, Dawson J, Tarver WB, Hays SA. Targeted Vagus Nerve Stimulation for Rehabilitation After Stroke. Front Neurosci 2019;13. doi: 10.3389/fnins.2019.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Buonomano DV, Merzenich MM. CORTICAL PLASTICITY: From Synapses to Maps. Annu Rev Neurosci 1998;21:149–86. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- [25].Rioult-Pedotti MS, Friedman D, Hess G, Donoghue JP. Strengthening of horizontal cortical connections following skill learning. Nat Neurosci 1998;1:230–4. doi: 10.1038/678. [DOI] [PubMed] [Google Scholar]
- [26].Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science 2000;290:533–6. [DOI] [PubMed] [Google Scholar]
- [27].Porter BA, Khodaparast N, Fayyaz T, Cheung RJ, Ahmed SS, Vrana WA, et al. Repeatedly Pairing Vagus Nerve Stimulation with a Movement Reorganizes Primary Motor Cortex. Cereb Cortex 2012;22:2365–74. doi: 10.1093/cercor/bhr316. [DOI] [PubMed] [Google Scholar]
- [28].Morrison RA, Hulsey DR, Adcock KS, Rennaker RL, Kilgard MP, Hays SA. Vagus nerve stimulation intensity influences motor cortex plasticity. Brain Stimul 2019;12:256–62. doi: 10.1016/j.brs.2018.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hulsey DR, Hays SA, Khodaparast N, Ruiz A, Das P, Rennaker RL, et al. Reorganization of Motor Cortex by Vagus Nerve Stimulation Requires Cholinergic Innervation. Brain Stimul 2016;9:174–81. doi: 10.1016/j.brs.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hulsey DR, Shedd CM, Sarker SF, Kilgard MP, Hays SA. Norepinephrine and serotonin are required for vagus nerve stimulation directed cortical plasticity. Exp Neurol 2019. doi: 10.1016/j.expneurol.2019.112975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Morrison RA, Danaphongse TT, Pruitt DT, Adcock KS, Mathew JK, Abe ST, et al. A limited range of vagus nerve stimulation intensities produce motor cortex reorganization when delivered during training. Behav Brain Res 2020;391:112705. doi: 10.1016/j.bbr.2020.112705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Borland MS, Vrana WA, Moreno NA, Fogarty EA, Buell EP, Sharma P, et al. Cortical Map Plasticity as a Function of Vagus Nerve Stimulation Intensity. Brain Stimul 2016;9:117–23. doi: 10.1016/j.brs.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Buell EP, Borland MS, Loerwald KW, Chandler C, Hays SA, Engineer CT, et al. Vagus Nerve Stimulation Rate and Duration Determine whether Sensory Pairing Produces Neural Plasticity. Neuroscience 2019. doi: 10.1016/j.neuroscience.2019.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Clark KB, Krahl SE, Smith DC, Jensen RA. Post-training Unilateral Vagal Stimulation Enhances Retention Performance in the Rat. Neurobiol Learn Mem 1995;63:213–6. doi: 10.1006/nlme.1995.1024. [DOI] [PubMed] [Google Scholar]
- [35].Clark KB, Smith DC, Hassert DL, Browning RA, Naritoku DK, Jensen RA. Posttraining Electrical Stimulation of Vagal Afferents with Concomitant Vagal Efferent Inactivation Enhances Memory Storage Processes in the Rat. Neurobiol Learn Mem 1998;70:364–73. doi: 10.1006/nlme.1998.3863. [DOI] [PubMed] [Google Scholar]
- [36].Clark KB, Naritoku DK, Smith DC, Browning RA, Jensen RA. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat Neurosci 1999;2:94–8. doi: 10.1038/4600. [DOI] [PubMed] [Google Scholar]
- [37].Salgado H, Köhr G, Treviño M. Noradrenergic ‘Tone’ Determines Dichotomous Control of Cortical Spike-Timing-Dependent Plasticity. Sci Rep 2012;2:417. doi: 10.1038/srep00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci 2005;28:403–50. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- [39].Hansen N, Manahan-Vaughan D. Locus Coeruleus Stimulation Facilitates Long-Term Depression in the Dentate Gyrus That Requires Activation of β-Adrenergic Receptors. Cereb Cortex 2015. doi: 10.1093/cercor/bht429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hasselmo ME. The role of acetylcholine in learning and memory. Curr Opin Neurobiol 2006;16:710–5. doi: 10.1016/j.conb.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bucksot JE, Wells AJ, Rahebi KC, Sivaji V, Romero-Ortega M, Kilgard MP, et al. Flat electrode contacts for vagus nerve stimulation. PLoS One 2019;14:e0215191. doi: 10.1371/journal.pone.0215191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hays SA, Khodaparast N, Hulsey DR, Ruiz A, Sloan AM, Rennaker RL, et al. Vagus Nerve Stimulation During Rehabilitative Training Improves Functional Recovery After Intracerebral Hemorrhage. Stroke 2014;45:3097–100. doi: 10.1161/STROKEAHA.114.006654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kleim JA, Bruneau R, Calder K, Pocock D, VandenBerg PM, MacDonald E, et al. Functional Organization of Adult Motor Cortex Is Dependent upon Continued Protein Synthesis. Neuron 2003;40:167–76. doi: 10.1016/S0896-6273(03)00592-0. [DOI] [PubMed] [Google Scholar]
- [44].Neafsey EJ, Sievert C. A second forelimb motor area exists in rat frontal cortex. Brain Res 1982;232:151–6. doi: 10.1016/0006-8993(82)90617-5. [DOI] [PubMed] [Google Scholar]
- [45].Neafsey EJ, Bold EL, Haas G, Hurley-Gius KM, Quirk G, Sievert CF, et al. The organization of the rat motor cortex: a microstimulation mapping study. Brain Res 1986;396:77–96. [DOI] [PubMed] [Google Scholar]
- [46].Pruitt DT, Schmid AN, Danaphongse TT, Flanagan KE, Morrison RA, Kilgard MP, et al. Forelimb training drives transient map reorganization in ipsilateral motor cortex. Behav Brain Res 2016;313:10–6. doi: 10.1016/j.bbr.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Rios M, Bucksot J, Rahebi K, Engineer C, Kilgard M, Hays S. Protocol for Construction of Rat Nerve Stimulation Cuff Electrodes. Methods Protoc 2019. doi: 10.3390/mps2010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


