Abstract
Saving lives and flattening the curve are the foremost priorities during the ongoing pandemic spread of SARS-CoV-2. Developing cutting-edge technology and collating available evidence would support frontline health teams. Nutritional adequacy improves general health and immunity to prevent and assuage infections. This review aims to outline the potential role of probiotics in fighting the COVID-19 by covering recent evidence on the association between microbiota, probiotics, and COVID-19, the role of probiotics as an immune-modulator and antiviral agent. The high basic reproduction number (R0) of SARS-CoV-2, absence of conclusive remedies, and the pleiotropic effect of probiotics in fighting influenza and other coronaviruses together favour probiotics supplements. However, further support from preclinical and clinical studies and reviews outlining the role of probiotics in COVID-19 are critical. Results are awaited from many ongoing clinical trials investigating the benefits of probiotics in COVID-19.
Key Words: Coronavirus, COVID-19, Immunity, Microbiota, Probiotics, SARS-CoV-2
Introduction
The novel coronavirus pandemic of 2019 (COVID-19) (1), an emerging infectious disease (EID) caused by multiple strains of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) has spread to 216 countries and territories, with catastrophic impact on global health and economy. As of 12th August 2020, there are 20.4 million cumulative confirmed cases of COVID-19, including 0.74 million deaths, with a case-fatality rate (CFR) of 3.6% (2). Among WHO regions, the Americas and Europe have the highest confirmed cases. Ten most affected countries are the United States of America (USA), Brazil, India, Russia, South Africa, Mexico, Peru, Colombia, Chile, and Iran (3).
COVID-19 mortality rates vary significantly across different countries. Currently, CFR has been higher in the United Kingdom (14.8%), Italy (14.0%), France (12.4%), Mexico (10.9%), Spain (8.7%) than in Brazil (3.3%), USA (3.2%), India (2.0%), South Africa (1.9%) and Russia (1.7%) (2), but the reasons for this variation remain ambiguous. Differences in virus strains, rate of COVID-19 testing, quality, and access to the healthcare system, and preventive strategies are among the listed reasons (4,5). Demographic characteristics such as the proportion of elderly, dietary and lifestyle patterns, comorbidities, and socioeconomic status, also influence the susceptibility, severity, and fatality of COVID-19 (5).
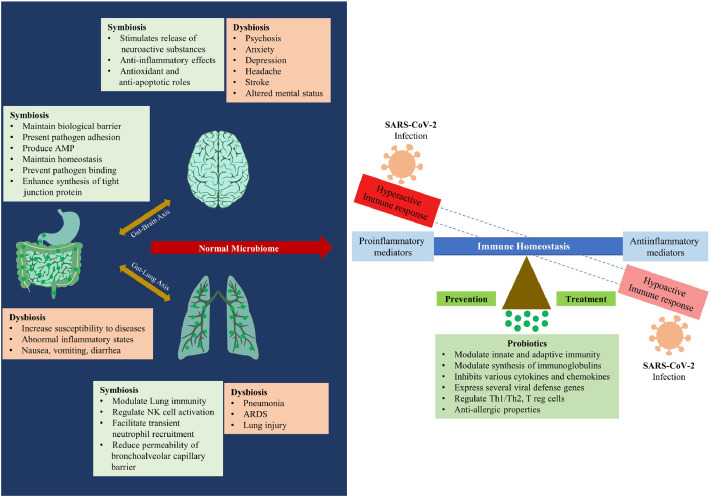
Most COVID-19 cases are mild to moderate with self-limiting respiratory illness. Geriatrics and individuals with hypertension, diabetes, cardiac diseases, pulmonary diseases, and cancer are extremely vulnerable to severe COVID-19 (1). A meta-analysis with more than 50000 COVID-19 cases found a pooled incidence of 20.2% severity and 3.1% mortality (6). Inflammatory markers like c-reactive protein (CRP) and lymphocytopenia are significantly correlated with severity. The immune status of certain individuals seems to fight COVID-19 better than others (4). Most trials repurposing antivirals have not proven effective so far. Moreover, a subset of patients develops a potentially life-threatening hyperinflammatory state called cytokine storm, accompanied by multi-organ dysfunction, respiratory failure, and a clinically distinct hypercoagulable state of the pulmonary vasculature. COVID-19 requires a multidimensional therapeutic approach, ranging from virus-targeted interventions in the early stages to immunomodulation in late stages (7). While dexamethasone reduces the mortality rate in severe COVID-19 patients, it has limited value in mild disease (8). Besides, WHO and many countries have advised caution in implementing steroid therapy in COVID-19 patients with co-morbidities such as diabetes and hypertension (9). Suppressing the exaggerated immune response can protect the lungs, but containing the infection needs a fully functional immune system. Therefore, fine-tuning the host-microbiota balance could be useful in COVID-19, especially with co-morbidities. Considering the immunomodulatory (10), anti-inflammatory (11), antioxidant (12), and antiviral (13) actions of probiotics, we hypothesize that a pleiotropic mechanism could be a preventive /curative option for COVID-19 (Figure 1 ).
Figure 1.
represents the proposed role of probiotics in the management of COVID-19. Dysbiosis (an altered gut microbial flora) predisposes the individual to abnormal inflammatory status and increases the susceptibility to the disease. Probiotic supplementation helps to maintain symbiosis in the GIT and thereby modulate the immune system. The symbiotic state also helps to control the severity of the disease via the gut-lung and gut-brain axes.
Microbiota and Immunity
Microbiotas constitute the entire spectrum of microbial communities residing in the host, such as bacteria, viruses, fungi, and protozoans. The human body has more microbes than human cells, occupying mucosal membranes, and the skin. The human microbiota's role in shaping the immunity and preserving homeostasis has been extensively studied, particularly in the gut, where microbes are most abundant (14). Germ-free mice have demonstrated the protective effects of the microbiota in pathological conditions, including infections, inflammatory and metabolic disorders of multiple body parts, including lungs (15,16). The host-microbiota symbiosis is sensitive to genetic makeup, antibiotic use, dietary pattern, allergens, and infective agents, all of which can alter the microbiota composition. ‘Dysbiosis’, resulting from the host-microbiome maladjustment, can increase susceptibility or severity of diseases and multiple health issues concerning the gastrointestinal (GI) and distal sites such as lungs, brain, vagina, liver, etc. Alteration of the microbiota in gut and lung has been observed in metabolic and respiratory illnesses (14), such as inflammatory bowel diseases (IBD), obesity, type 2 diabetes, cardiovascular disease, Alzheimer's disease, and depression (17., 18., 19., 20., 21., 22.). Disturbance in lung microbiota in chronic obstructive airway disease, asthma, tuberculosis, cystic fibrosis, etc. implies the influence of the lung microbiota in pulmonary health and illnesses (23., 24., 25., 26.). Dysbiosis also creates an imbalance in the degree of activation of leucocytes, leading to lung injury. Gut microbiota modulates the immunity of the lung via the gut-lung axis, implying crosstalk between different mucosal sites of the human body (27). Likewise, the gut-brain axis presents a bidirectional exchange through neural, humoral, endocrine, and immune connections, presumed to be mediated by bacterial metabolites such as short-chain fatty acids (SCFAs) (28). SCFAs modify neuronal excitability, like the many microbiota-derived neuroactive substances (histamine, acetylcholine, γ-aminobutyric acid, dopamine, and tryptophan, a precursor in serotonin biosynthesis). Thus, gut microbiota intervenes in the crosstalk between the central nervous system (CNS) and the enteric nervous system (ENS), thereby linking brain centers with peripheral intestinal functions for emotional as well as cognitive functions (28). The role of the gut-brain axis is exemplified by the dysbiosis associated with CNS diseases and functional GI disorders.
Gut microbiota is also involved in the pathogenesis of sepsis and acute respiratory distress syndrome (ARDS) (29). Depletion of gut microbial diversity can cause dysbiosis, which is therefore attributed to several pathologies. Geriatrics have reduced the diversity of gut microbiota, including beneficial microbes such as Bifidobacteria species (30) suggesting microbiotal interaction with the gut-lung axis. Thus, modulating the microbiome could demonstrate antiviral effects.
Host-microbiota interactions are bidirectional, complex, and potentially modulates the development and function of innate and adaptive immune systems (31,32). Commensals maintain homeostasis by releasing antimicrobial peptides (AMPs) and compete with pathogens for nutrition and space at the site of infection (33), demonstrating a mutual relationship between gut microbiota and immune homeostasis, to be exploited in the current pandemic. Signals by gut microbiota can tune the immune-mediated cells for proinflammatory (helper T cells type 17; Th17) and anti-inflammatory (regulatory T cells; Tregs) responses, determining susceptibility to different illnesses (34). Coronavirus infections can be countered by healthy gut microbiota that protects the lungs and vital organs from an exaggerated immunological response.
Pattern recognition receptors (PRRs) on host cells can recognize microbe-associated molecular patterns (MAMPs) and pathogen-associated molecular patterns (PAMPs) (34,35), possibly generating memory response at primary exposure. PRRs primarily comprises of the families of toll-like receptors (TLRs), C-type lectin receptors (CLRs), nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), and RIG-I–like receptors (RLRs). TLRs recognize the MAMPS and PAMPs and induce immunological reactions based on cell type, ligand, or receptor (35). TLR stimulation by cell wall components and flagellin of gut microbiota is necessary for mounting the immune responses towards influenza. In contrast, oral SCFA-induced anti-inflammatory actions are attributed to decreased pulmonary pathology following bacterial and viral infections in mice (36). Effectively engaging PRRs expressing innate cells with gut- or non-microbial ligands is essential for the protective mechanism, independent of adaptive immunity during exposure to a pathogen or secondary infection. The gut microbiota secretes SCFA metabolites (e.g., acetate, butyrate, and propionate and secondary bile acids) that generate immunomodulatory signals. Commensals such as Lactobacillus, Bacteroides, and Bifidobacteria bind receptors in dendritic cells (DCs) and macrophages, subsequently regulating their metabolism and immune response functions (37). More importantly, probiotics like Bifidobacterium lactis increased mononuclear leukocytes and antitumour action of natural killer (NK) cells in healthy geriatric volunteers (38). Balanced gut microbiota composition significantly impacts the efficiency of the lung immunity of the host (14). Moreover, germ-free (GF) mice have been found to suffer from impaired pathogen clearance in their lungs (15). Interestingly, lung infection with influenza virus (IFV) in mice enriches Enterobacteriaceae and depletes Lactobacilli and Lactococci in the gut microbiota (39).
Microbiota and COVID-19
A pilot study with sequencing faecal samples of 15 patients showed that COVID-19 infections significantly altered faecal microbiomes characterized by a decline in beneficial commensals and enrichment of opportunistic pathogens (40). The baseline abundance of Coprobacillus and few Clostridium species have been correlated with the severity of COVID-19. Similarly, the abundance of Faecalibacterium prausnitzii, an anti-inflammatory bacterium, was inversely correlated to COVID-19 severity, demonstrating the influence of SARS-CoV-2 on gut microbiome (40). Similarly, Liu F, et al. and Yeoh YK, et al. reported significant alterations in the gut microbiome and dysbiosis in COVID 19 patients, associated with disease severity and inflammatory markers (41,42).
Many COVID-19 patients present with GI symptoms and have sepsis that may originate in the gut (43). GI symptoms correlate with profound disease severity. Moreover, angiotensin-converting enzyme 2 (ACE 2) and virus nucleocapsid protein have been detected in GI epithelial cells, and virus particles have been isolated from faeces. Once the virus enters the epithelial cells of GIT and faeces, COVID-19 patients become highly infectious (44). Since SARS-CoV-2 binds ACE 2 receptor for its entry into human cells (45), the high expression of angiotensin II receptor type 2 (AT2) cells of the lung, stratified epithelial cells of the esophagus, and absorptive enterocytes of ileum and colon are critical for infectivity. Bioinformatics data indicate that, like the respiratory system, the GI system is an important route for SARS-CoV-2 infection (46). ACE 2 is an essential regulator of intestinal inflammation (46) and significantly influences the composition of gut microbiota, thereby affecting cardiopulmonary diseases (47). Pneumonia and subsequent ARDS are critical clinical manifestations of COVID-19, particularly in geriatric and immuno-compromised patients (48).
Gou W, et al. suggest that the gut microbiome of healthy non-infected individuals is highly predictive of the blood proteomic biomarkers of COVID-19 severity (49). Disruption of the healthy gut microbiome potentially predisposes healthy individuals to abnormal inflammatory states, possibly accounting for COVID-19 susceptibility and severity. Restoration of commensal probiotic strains may enhance the recovery of the lung and the gut via host-derived cytokines and chemokines in addition to microbiota-derived SCFAs (14). Since human microbiota plays a vital role in immunomodulation, it can impact SARS-CoV-2 infection. Current evidence supports the potential role of microbiota in the susceptibility, progression, and severity of COVID-19. Five studies directly connect microbiota with COVID-19, supporting the presumptive role of probiotics in both prevention and treatment of COVID-19 (40., 41., 42.,49,50) (Table 1 ).
Table 1.
Studies on association of microbiota with COVID-19 patients
| Sl. no. | Author, Year, place | Study title | Objectives | Method | Key Findings |
|---|---|---|---|---|---|
| 1. | Gu S, et al., (2020), China (50) | Alterations of the gut microbiota in patients with COVID-19 or H1N1 influenza | To study the gut microbial flora in COVID-19, H1N1 patients and healthy individuals | Prospective cohort study (n = 84) | Gut microbiome of COVID-19 and H1N1 patients were significantly different from healthy individuals. An abundance of opportunistic pathogens and relatively lower beneficial symbionts were observed in COVID-19 patients. H1N1 patients had a lower diversity and overall different microbial composition. |
| 2. | Zuo T, et al., (2020), Hong Kong (40) | Alterations in gut microbiota of patients with COVID-19 during time of hospitalization | To investigate faecal microbiome in hospitalised COVID-19 patients | Prospective cohort study (n = 15) | Significant alteration in gut microbiome was seen in COVID-19 patients. Baseline microbial composition correlated with COVID-19 severity. This dysbiosis persisted even after the symptoms resolved and virus had cleared. |
| 3. | Gou W, et al., (2020), China (49) | Gut microbiota may underlie the predisposition of healthy individuals to COVID-19 | To identify proteomic biomarkers that may indicate predisposition to COVID-19 in healthy individuals To assess influence of gut microbiota on these biomarkers in healthy individuals | Multi-methodological study | Identified 20 blood proteomic biomarkers and drafted a proteomic risk score that could predict severity of COVID-19 in patients Gut microbial features were predictive of blood proteomic markers indicating a potential biological mechanism on disease predisposition. |
| 4. | Liu F, et al., (2021), China (41) | Gastrointestinal disturbance and effect of fecal microbiota transplantation in discharged COVID-19 patients | To study the effect of fecal microbiota transplantation in GI disturbances, gut microbiota and immune system after SARS-CoV-2 infection | Prospective interventional study (n = 11) | Gut dysbiosis was observed in patients after SARS-CoV-2 infection. Fecal microbiota transplantation attenuated GI symptoms, partially restored gut microbiome, and significantly altered B lymphocytes. |
| 5. | Yeoh YK, et al., (2021), Hong Kong (42) | Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19 | To assess the association between gut microbiota and COVID-19 patients | Prospective cohort study (n = 165) | Significant alteration in the gut microbiome was observed in COVID 19 patients, associated with disease severity and plasma concentrations of several chemokines, cytokines, and inflammatory markers Significant decrease in gut microbes with immunomodulatory properties were seen in COVID 19 patients. |
Probiotics and Immunity
Food and Agriculture Organization (FAO) under the United Nations and the World Health Organization (WHO) define probiotics as “live microorganisms, when administered in adequate amounts, confer a health benefit on the host” (51). Probiotic formulations are microecological products that improve the intestinal flora's architecture, diminish the growth of harmful microbes, and improve the immune response (51,52). Currently, most extensively researched probiotics include Lactobacillus, Bifidobacteria, Escherichia coli, Enterococcus, etc. Although probiotics' mechanism profoundly focuses on the GIT, the effect of probiotics is not confined to the initial infection site. Probiotics can act on the entire body via immune modulation. Probiotics and their antigenic metabolites are phagocytosed by microfold cells to form endosomes in gut-associated lymphoid tissues. These antigens are suddenly released and taken in by DCs, which can transport them to local lymph nodes and consequently activate naive T and B cells to differentiate into different effector subpopulations. This initiates the release of the relevant cytokines and various immune responses (52).
Pleiotropism of probiotics comprises of strengthening biological barriers in the intestinal tract and modulating the equilibrium of intestinal flora. Probiotics provide antimicrobial action, restore intestinal epithelial cell function, inhibit adhesion and growth of pathogens via a space-occupying effect, augment competitive antagonism, secrete antimicrobial substance like bacteriocins, increase activity of digestive enzymes, and synthesize organic acids, etc (53). Probiotics increase the expression and release of mucous glycoproteins via enhanced tight junction proteins synthesis between epithelial cells, thereby improving epithelial integrity and mechanical barrier function, and preventing the displacement of the intestinal microbes and endotoxins. Probiotics also initiate repair of damaged barrier function mediated by the tight junction complex reconstruction via enhanced expression and redistribution of zonula occludens (ZO-2) proteins of the tight junction and protein kinase C (PKC) (53,54). The introduction of probiotics prevents cytokine-induced epithelial damage, which is also attributed to mucosal barrier reinforcement. Besides, probiotics enhance mucous secretion, improve barrier function, and pathogen exclusion. Probiotics also prevent pathogen binding by promoting qualitative alterations in intestinal mucins (53,54). Interestingly, the bacterial component is also degraded into an AMP, which lends anti-pathogenic properties to the host. This constitutes an example for an evolutionarily beneficial pleiotropic effect of large surface proteins. Besides, probiotics also induce the release of defensins from epithelia, the small peptides active against bacteria, fungi, and viruses. Additionally, these peptides steady the barrier function of the gut. Probiotics inhibit the binding of the pathogen via steric hindrance at pathogen receptors of the enterocyte. Specific metabolites of probiotics modulate signalling and metabolic pathways in different cells. Diverse components of the probiotic metabolome (e.g., hydrogen peroxide, amines, organic acids, and bacteriocins) interact with multiple targets in host metabolic pathways that regulate inflammation, angiogenesis, metastasis and cellular proliferation, differentiation, and apoptosis (54).
Probiotics modulate the innate and adaptive immune responses, facilitating the immune system's development and maturation. Probiotics regulate host-pathogen interactions by initiating the innate immune responses that comprise of TLR, nuclear factor kappa B (NF-κB), mitogen-activated protein kinase (MAPK), and c-Jun NH2-terminal kinase (JNK) pathways. Probiotics such as Lactobacillus and Bifidobacterium can restore host-health by eliminating pathogens and regulating immune responses in intestinal epithelial cells. Moreover, probiotics enhance the viability of NK cells and macrophages and stimulate the release of secretory immunoglobulin A (IgA). As probiotics activate relevant immune reactions mediated by TLR and NLR, regulating Th1/Th2 immune responses increase Treg's counts that secrete IL-10 and transforming growth factor (TGF)-β, strengthening their function, thereby decreasing the antigen-specific IgE levels (53., 54., 55.). Clinical trials have demonstrated that certain Bifidobacterium species could promote the signalling of TGF-β and increase peripheral Treg cells (56,57). Lactobacillus plantarum NCU116-induced expression of Th17 and Treg immune response expresses specific transcription factors like retinoic acid receptor-related-orphan-receptor-gamma t (RORγt) and forkhead box protein P3 (FOXP3), in mice. This strain enhances intestinal mucosal immunity and modulates Th17/Treg balance, which is attributed to the TLR pathway in DCs (58).
Probiotics stimulate the immune system, thereby increasing Igs generation, enhancing macrophages and lymphocytes activity, and interferon (IFN)-ɤ stimulation (10). Probiotics may also inhibit the immune system, primarily embodied in their anti-inflammatory response. Thus, probiotics have both positive and negative effects on the immune system. Many immunomodulatory signalling molecules of the probiotics are MAMPs that interact with the host cell's transmembrane PRRs. Immunomodulation with probiotics attenuate the synthesis of several cytokines and chemokines and induce anti-inflammatory molecules, predominantly via TLRs, Th17/Treg, or NF-κB signalling pathways. Upon activation by commensals or probiotics, DCs initiate a suitable response like Th0 to Treg differentiation, which can inhibit Th1, Th2, and Th17 inflammatory responses. Probiotics can suppress intestinal inflammation by downregulating TLR expression and the release of metabolites (which inhibit TNF-α from entering blood mononuclear cells), thereby inhibiting the signalling of NF-κB in enterocytes (53,54). Furthermore, both intracellular NLRs, and extracellular CLRs, can transmit signals through interaction with microbes (52., 53., 54., 55.). Lactobacillus rhamnosus GR-1 overcomes Escherichia coli-induced cellular structure destruction and inflammation, partially by endorsing synergism of TLR2 and nucleotide-binding oligomerization domain-containing protein 1 (NOD1) and decreasing NLRP3 inflammasome activation (59). Anti-allergy properties include polarizing the balance of Th1/Th2 toward a Th1 response, thereby reducing Th2 cytokines (like IL-4, IL-5, and IL-13) secretion, along with reduced IgE levels and elevated synthesis of CRP and IgA (60).
Probiotics and Vitamin D
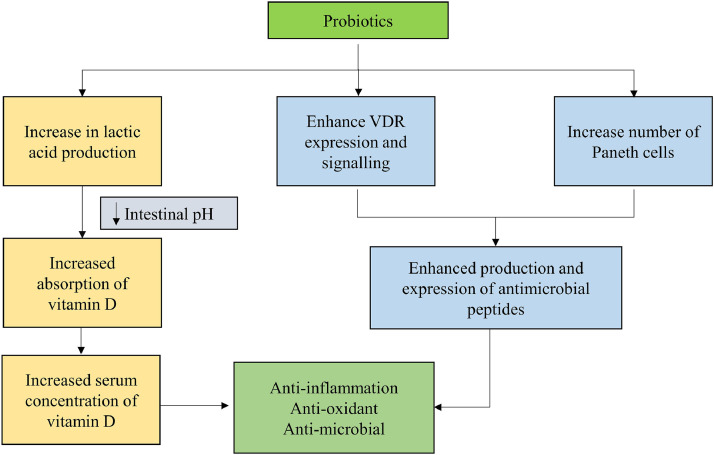
Vitamin D deficiency (VDD) partly explains geographical differences in COVID-19 susceptibility, severity, and mortality. The immunomodulatory effects of vitamin D suggest a definitive role of vitamin D in viral infections (55,61). Vitamin D can also alleviate severe complications and mortality related to COVID-19 and inhibit cytokine storm by simultaneously boosting the innate immunity and evading the exaggeration of the adaptive immunity (4). Interestingly, current findings also associate VDD to common comorbidities that increase the severity of COVID-19 such as certain cancers, autoimmune disorders, diabetes, and cardiovascular diseases. A well-designed multicentric randomized controlled trial (RCT) with Lactobacillus reuteri NCIMB 30242 probiotics strain (for nine weeks) increased serum vitamin D level by 25% (62). Increased vitamin D levels may be attributed to the probiotic secreting lactic acid and lowering intestinal pH (Figure 2 ). When a probiotic was combined with a vitamin D supplement, vitamin D absorption improved further. Probiotics Lactobacillus plantarum and Lactobacillus rhamnosus enhanced the expression of vitamin D receptor (VDR) protein and its transcriptional activity, increasing the expression of an AMP, viz. cathelicidin (63). Probiotics-induced signalling modulation has been demonstrated in VDR knock-out mice in a salmonella-induced colitis model (64). Probiotics confer both physiological as well as histological protection in VDR+/+ mice, but not in VDR–/– mice, suggesting that the probiotic protection in colitis depends on the VDR pathway. Probiotic supplementation also increases Paneth cells, thereby enhancing host defense by secreting AMPs. Elucidating the probiotics' mechanism in enhancing VDR signalling and inhibiting inflammation, makes them an attractive agent for preventing and treating COVID-19 related chronic inflammation (55,61).
Figure 2.
Explicit the probable combined/ synergistic effect of probiotics with vitamin D in COVID-19. Probiotic secreting lactic acid will lower the intestinal pH, thereby increasing the absorption of vitamin D and consequently improves its levels. Certain probiotics enhances the expression of vitamin D receptor (VDR) protein and its transcriptional activity, subsequently increasing the expression of cathelicidin. Moreover, probiotics also increases the number of paneth cells, thereby enhancing the levels and expression of defensins.
Probiotics and Viral Infections
As discussed earlier, protection by probiotics includes synthesis of antimicrobial agents, immune-modulatory responses, and enhancement of innate host defense. Certain probiotics also have anti-viral effects, including against coronavirus (65). Enterococcus faecium inhibits replication of enteropathogenic coronavirus transmissible gastroenteritis virus (TGEV) in swine testicular cells. This involves direct interference of virus attachments, adsorptive trapping or virus particle inactivation through surface components of the probiotics and stimulating the synthesis of pro-inflammatory cytokines IL-6 and IL-8 and nitric oxide (NO). Probiotics can also interfere with ACE 2, the primary host receptor of the SARS-CoV-2. For instance, during milk fermentation, Lactobacillus helveticus and Lactobacillus casei release peptides with high affinity for ACE. Bovine milk fermented with Lactobacillus species yields fermented products enriched with ACE-inhibitory peptides (66), of which many are resistant to GI digestion and inhibit ACE in the renin-angiotensin system (RAS). In vitro and in vivo experiments have demonstrated antihypertensive effects of fermented milk products (67,68).
Garcia-Crespo KE, et al. found that intranasal inoculation of Lactobacillus reuteri F275 protects mice from lethal infection of a pneumonia virus of mice (PVM) (69). Intranasal Lactobacillus reuteri inoculation prompted immediate and transient neutrophil recruitment along with pro-inflammatory mediators including chemokine (C-X-C motif) ligand (CXCL) 1, CXCL10, chemokine (C-C motif) ligand (CCL) 2, CCL3, tumour necrosis factor (TNF)-α and interleukin (IL)-17A, but not Th1 cytokines. Lactobacillus-mediated heterologous immune response warrants investigations into novel antiviral strategies (69). Pre-treatment with Lactobacillus rhamnosus GG (LGG) in transgenic mice enhanced immune-gene transcription during early IFV infection with upregulation of type 1 IFN pathways (70). LGG's protective effect is mediated via a myeloid differentiation factor (MyD)88-dependent mechanism, specifically via TLR4. LGG can provide early control of IFV and improve transcriptional responsiveness, acting as a simple and safe strategy to protect neonates. Intranasal LGG also protected mice from infection with IFV by stimulating respiratory cell-mediated immunity following up-regulation of lung NK cell activation (71). In the LGG-treated mice, lung IL-1β, TNF, and monocyte chemoattractant protein (MCP)-1 mRNA expression were potentially higher than in control mice. IL-1β stimulates the synthesis of IL-2 and affect proliferation and differentiation of NK cells. Along with IL-12, TNF works as a co-stimulator for the activation of NK cells. MCP-1 induces the migration and accumulation of NK cells in the infected tissue and activates NK. Thus, in LGG-treated mice, up-regulation of lung IL-1β, TNF, and MCP-1 mRNA expression may also be attributed to the stimulation of the lung NK cells. These cells are the major components of the host-nonspecific cell-mediated immune system and take part in recognizing and controlling a broad range of pathogens, including viruses. Previous reports have also proposed that oral administration of LGG might protect host animals from IFV infection via interaction with gut-associated immune cells like pancreatic polypeptide (PP) cells, and by direct up-regulation of respiratory immunity. Intranasal administration of Lactobacillus casei DK128 protected mice from various IFV subtypes by lowering weight loss and reducing viral loads (72). Mice protected against primary viral infection with pretreatment of a probiotic strain developed heterosubtypic immunity towards secondary virus infection.
Antiviral efficacy of Lactobacillus plantarum DK119 was dose and route dependent (73) and raised the concentration of cytokines IL-12 and IFN-γ in bronchoalveolar lavage fluids of IFV infected mice and was accompanied by diminished inflammation. Modulating innate host immunity of DC and macrophages, and production of cytokine release patterns could be likely antiviral mechanisms on IFV. Probiotic treatment enhanced the levels of L-12, IFN-γ, and reduced inflammatory cytokines such as IL-4, IL-6, and TNFα in mice. The study concluded that the use of Lactobacillus plantarum DK119 protected mice from H1N1 and H3N2 IFV infections, possibly by enhancing the innate immune response of CD11c+ DC and macrophages and antiviral cytokines, resulting in the fall of viral loads in the lungs. Lactobacillus induce human DCs to release Th1/Th17 cytokines such as TNF-α, IL-12P70, IL-12P40 and IL-23 (74). Moreover, Lactobacillus was reported to stimulate the synthesis of Th1 polarizing cytokine IL-12 by inducing murine DCs via TLR 2-dependent mechanisms (75). The results revealed that specific Lactobacilli trigger viral defense genes expression in DCs in a TLR 2 dependent manner on IFN-β. Waki N, et al. have shown that regular consumption of a probiotic drink containing Lactobacillus brevis KB290a decreases incidence of influenza in 1089 school children, especially in unvaccinated individuals (76).
Lee DK, et al. demonstrated antiviral effect of Bifidobacterium adolescentis SPM 0212 isolated from healthy Koreans against the hepatitis B virus (HBV) (77). Its antiviral activity is associated with the Mx-GTPase pathway. The cell extract of the probiotic strain significantly upregulates gene expression of IFN-signalling components such as signal transducer and activator of transcription 1 (STAT1) and interferon-induced protein 6–16 gene. Consequently, myxovirus resistance gene A (MxA) expression improved significantly. MxA protein binds viral nucleocapsids or other viral components and degrade them. Viruses susceptible to MxA activation are coxsackievirus, orthobunyavirus, hantavirus, phlebovirus, dugbe virus, and HBV. Another study demonstrated that Lactobacillus ruminis SPM0211, Bifidobacterium longum SPM1205, and SPM1206 effectively inhibited rotavirus replication in vitro and in vivo (78). Anti-rotavirus properties of these three probiotic strains may be attributed to immunomodulatory response via enhancing type 1 IFNs, key regulators in the IFN signalling pathway. Selected microbiota strains as probiotics favour host immunity against viral pathogens as demonstrated in the treatment of intestinal disorders, with potential promise in respiratory diseases including COVID-19.
Probiotics and COVID-19: Current Perspectives
A few reports cite indirect evidence for the association between probiotics and COVID-19, mostly based on previous coronavirus and other viral infections. A systematic review by Pourhossein M, et al. described the probiotics’ efficacy against viral diseases, where more than 20 strains improved the anti-inflammatory ILs and antibody production against viruses (79). Moreover, viral loads were lowered upon probiotic supplementation, possibly via immune modulation by microbiota that fights COVID-19 directly and decreases the risk of secondary infections due to prolonged antibiotic exposure from multiple experimental COVID-19 treatments (80). China's National Health Commission recommended probiotics for severe COVID-19 patients to restore the intestinal balance and protect from secondary infections. Moreover, gut microbiota's role in modulating lung diseases has been amply demonstrated. Diminished diversity of gut microbiota in the elderly might be causally related to higher COVID-19 fatality. Taxonomic changes and ageing have been correlated with decreased metabolic potential of microbiome, including diminished generation of SCFAs (30). Improving gut microbiota profile by probiotic supplements could, therefore, enhance immunity, suggesting a possible prophylactic strategy in geriatrics and immunocompromised people.
SARS-CoV-2 compromises lung function via binding of ACE 2 receptors on the alveolar epithelial cells. However, viral RNA was recently detected in the faeces of COVID-19 patients (43,44). Indeed, the intestinal epithelial cells, predominantly the small intestinal enterocytes, also express ACE 2 receptors that are critically involved in cardiovascular physiology, pathology, and acute lung injury, including ARDS. ACE 2 is an important regulator of intestinal inflammation. Moreover, there is a wide disparity in COVID susceptibility and disease progression. Gou W, et al. suggest that susceptibility among diverse cohorts might be related to composition of microbiota (49). The faecal metabolites suggest that an amino acid-related pathway may provide the key link between the core gut microbiota, inflammation, and COVID-19 susceptibility. The core gut microbial features and related metabolites may serve as potential preventive/ treatment targets for intervention, especially among susceptible populations (34).
A report from China suggests that COVID-19 patients suffer microbial dysbiosis with depleted Lactobacillus and Bifidobacterium, with over 60% of patients expressing GI symptoms such as diarrhoea, nausea, and vomiting (81). GI symptoms also imply greater severity. Around 58–71% of COVID-19 patients were administered antibiotics in China, and diarrhoea was reported in 2–36% of them. Reinforcement of colonic microflora using probiotics diminishes secondary infection and diarrhoea in patients receiving antibiotics. A meta-analysis by Kang EJ, et al. revealed that probiotics such as Lactobacillus and Bifidobacterium produce a modest decrease in common cold, a symptom of SARS-CoV-2 infection (82). In these studies, probiotic supplementation either decreased the severity or shortened the duration of disease 2–47% of COVID-19 patients need invasive mechanical ventilation due to development ARDS. A meta-analysis of RCTs suggests that probiotics decreased ventilator-associated pneumonia (VAP) (83) and reduced the duration of antibiotic use for VAP.
COVID-19 is a multi-organ phenomenon, requiring appropriate systemic inflammatory control for overall survival. Besides, COVID-19 infection expresses new presentations, such as pediatric multisystem inflammatory syndrome, which includes features like Kawasaki disease (84). ‘Cytokine storm’ or the excessive release of inflammatory cytokines is the reason for severity and death of COVID-19 patients (4). Therefore, anti-cytokine therapy for the suppression of the hyperinflammatory states is a recommended strategy to treat severe COVID-19. So far, many preclinical studies with probiotics have focused on influenza and pneumonia, demonstrating benefits from oral or nasal administration of probiotics, which prolonged survival, reduced weight loss, diminished viral loads in the lung, and minimized bronchial epithelial damage (70., 71., 72., 73.). Protection was mediated by immune regulation, distinguished by potent viricidal properties by early recruitment of innate immune system through alveolar macrophages, NK lymphocytes and heightened proinflammatory cytokines such as TNF-α and IL-6, etc. This inflammatory boost is followed by a rapid decline, attributed to enhanced anti-inflammatory mediators like Treg cells and IL-10 in the lungs, diminishing lung injuries (14). Moreover, probiotics’ ability to modulate vitamin D/VDR and balancing the composition and growth of gut microbiota (34), together suggest the immunomodulatory potential in ameliorating the cytokine storm. Therefore, the use of probiotics with anti-inflammatory effects could maintain the equilibrium of intestinal microecology and prevent secondary infection in COVID-19.
Most importantly, the current pandemic is particularly severe, with higher case fatality in individuals with non-communicable diseases (NCDs). 20–51% of COVID-19 patients suffered at least one comorbidity, like diabetes (10–20%), hypertension (10–15%), and cardio-and cerebrovascular diseases (7–40%) (85). Many studies and anecdotal reports suggest obesity as a notable risk factor for COVID-19, primarily in younger patients (86). Moreover, people with diabetes need a balanced diet to control glycemia and maintain immune function. However, hypo-nutrition aggravates impaired immunity in COVID-19 (87). On the other hand, probiotics may reduce cardiovascular and diabetes risk factors such as elevated blood pressure, serum cholesterol, obesity, and insulin resistance. Growing evidence reveals that probiotics can lower elevated blood pressure, glycemia, low-density lipoproteins (LDL)-cholesterol, LDL/high-density lipoproteins (HDL) ratio, inflammatory mediators, and body mass index (88). Modulating the gut microbiota composition by probiotics modify central energy metabolism and alter satiety hormone levels, exerting anti-obesity effects. A study in mice demonstrated that a carboxypeptidase derived from Paenibacillus sp.B38 suppressed angiotensin-II-induced hypertension, cardiac hypertrophy, and fibrosis (89). COVID-19 frequently presents with coagulopathy and thrombotic complications. COVID-19 associated coagulopathy (CAC), is emerging as a key survival indicator in SARS-CoV-2 infection (90). The probiotic lactic acid bacteria (LAB) can produce a beneficial effect during IFV, respiratory syncytial virus, and Streptococcus pneumonia infection via modulating the immunocoagulative response in the host (91). Zelaya H, et al. demonstrated that Lactobacillus rhamnosus CRL1505 administered nasally in adult immunocompetent mice, significantly modulates inflammation, maintains homeostasis, and decreases lung tissue damage after pneumococcal infection (91). The induction of IFN-γ, IL-10, and von Willebrand factor (vWF) via the TLR 2 signalling pathway appears to have a vital role in the ‘immunobiotic’ effect of probiotic strains. The probiotics enhance the release of TNF-α, IFN-γ, and IL-10 during pneumococcal infection and reduce tissue factor (TF). Immunobiotic therapy significantly decreases the permeability of the bronchoalveolar-capillary barrier, reducing lung damage, and general cytotoxicity. Therefore, corrective measures of immunomodulation, including probiotic supplementation, can reinstate innate and adaptive immunity, offering a promising therapeutic adjunct for COVID-19 (92).
Neurological manifestations of COVID-19 are mounting in parallel with the expanding pandemic (93). These could be direct effects of SARS-CoV-2 on the nervous system, para- or post-infectious immune-mediated illness, and neurological complications of the systemic impacts of COVID-19. A UK-wide surveillance study reported psychiatric or neurological disorders in 125 COVID-19 patients, with altered mental status (39;31%), including encephalopathy (16;13%) and neuropsychiatric illness (23;18%) such as psychosis (10; 8%), neurocognitive (dementia-like) syndrome (6;5%), and an affective disorder (4;3%) (94). Remarkably, the patient data showed cerebrovascular event (77;62%) including ischaemic strokes (57;46%), intracerebral haemorrhages (9;7%), CNS vasculitis (1;<1%), and other cerebrovascular events (10;8%). The significant role of probiotics in the prevention and treatment of neurologic disorders involves multiple pathways such as neural, immunological, and metabolic via the brain-gut axis. Preclinical evidence shows probiotics have neuroprotective effects against ischemic/reperfusion injury, possibly via antioxidant and antiapoptotic effects, anxiolytic effects, improved cognition, attenuation of inflammatory response and elevated serotoninergic precursors and antidepressant effects (95). Indeed, in an RCT, the oral administration of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175, for one month, decreased psychological distress and depression in healthy volunteers (96). Likewise, in another RCT, fermented milk with Bifidobacterium animalis subsp. lactis, Streptococcus thermophiles, Lactobacillus bulgaricus and Lactococcus lactis subsp. lactis affected regions of the brain that regulate the central processing of emotion and sensation in healthy women (97). Similarly, oral administration of Lactobacillus casei strain Shirota for two months in chronic fatigue syndrome reduced anxiety significantly more than controls. Probiotic metabolites can interact with and play a role in behaviour. Dinan TG, et al. (98) showed that physical or psychological stress is directly associated with the microbiota-brain-gut axis imbalance. Moreover, probiotics also diminish the frequency and severity of migraine headache attacks (99).
We found 14 RCTs and one prospective observational study with probiotics registered in clinical trial registries around the world (Supplementary Table1).
Conflicting Evidence on Probiotics
Differences persist in the physiology and metabolism among probiotic strains of various species, and consequently, their effects are different on the human body. Even different strains of the same species may have different health effects (100). The dose also matters, and a probiotic consumed at a higher dose may not be as good as a lower dose. Similarly, different doses of the same probiotic strain can produce diverse effects. Besides, the same probiotic strain can function differently in different hosts. Hence, the functions of probiotics should be proved at the strain level to confirm efficacy. Therefore, probiotics should be cautiously selected for optimal benefit.
There have been adverse reports of probiotics. According to Single-cell RNA sequencing (scRNA-Seq) analysis, Feng Z, et al. (101) demonstrated the ACE2, SARS-Cov-2 receptor, could be elevated in the presence of both invasive bacteria Salmonella enterica and its counterpart, Segmented Filamentous Bacteria as probiotics in the mouse small intestine (102) and human enterocytes (101). In another study, both Lactobacillus acidophilus and Bacillus clausii also failed to decrease the expression of coronavirus receptors compared to control after Salmonella infection in the murine intestine (103). The efficacy, as well as the safety of probiotics, have been contentious. Although adverse effects of probiotics appear to be mild (mainly digestive belching or bloating), severe consequences have been noticed in individuals with underlying health issues. There are reports of fungaemia (104) and bacteraemia (105) following probiotic administration. A Salmonella-colitis mice model has demonstrated that probiotic therapy in the absence of VDR expression produce severe infection (106). The dependence of probiotic function on VDR status, may explain the disparity in the clinical response of some IBD patients. The rationale for introducing probiotics for COVID-19 comes from indirect evidence (107). Therefore, blindly using conventional probiotics is not warranted until we clearly understand SARS-CoV-2 pathogenesis, its effect on gut microbiota, and vice-versa.
Conclusion
Evidence supports probiotics’ role in regulating the immune system, suggesting a definitive role for probiotics in viral infections. Probiotics supplementation could reduce the severity of COVID-19 morbidity and mortality. Probiotics can inhibit cytokine storm by simultaneously boosting the innate immunity and evading the exaggeration of adaptive immunity, which is challenged to respond quickly to the viral onslaught. Probiotics-induced suppression of the inflammatory cytokine response may prevent both the severity and the occurrence of ARDS, making probiotics an attractive adjunct. Inventing effective therapy will transform the impact of the pandemic on lives as well as economies across the globe. Therefore, supplementation of probiotics in high risk and severely ill patients, and frontline health workers, might limit the infection and flatten the COVID-19 curve. However, currently, there are no RCTs to demonstrate conclusive evidence. On the other hand, circumstantial evidence has supported the presumption that probiotic supplementation decreases the severity of COVID-19 responses, including mortality. Many clinical trials are underway globally to delineate the role of probiotics in both prevention and treatment of COVID-19.
Acknowledgement
The authors express their gratitude to Manipal College of Pharmaceutical Sciences, Manipal Academy of Higher Education (MAHE), Manipal for the support and facilities. The first author is thankful to Dr. TMA Pai Ph.D. scholarship program, MAHE, Manipal.
Source of Financial Support
None
Competing Interests
The authors report no conflicts of interest
Authors' Contribution
Dr.Shilia Jacob Kurian and Dr.Sonal Sekhar Miraj conducted the literature search and wrote the manuscript. Dr. B Shrikar Reddy drew the figure I. Dr. Mazhuvancheny Kesavan Unnikrishnan, Dr. Debasis Bagchi, Dr. Mithu Banerjce. Dr. Gabriel Sunil Rodrigues, Dr. Mohan K Manu, Dr. Kavitha Saravu, Dr. Chiranjay Mukhopadhyay and Dr. Mahadev Rao critically evaluated the manuscript. All the authors participated in literature collection and review. All the authors approved the final draft of the manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.arcmed.2021.03.002.
Appendix. Supplementary materials
References
- 1.World Health Organization. Rolling updates on coronavirus disease (COVID-19). [updated 31st July 2020]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen (Accesed Aug 12, 2020).
- 2.COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. [updated 12th August 2020]. Available from: https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6. (Accessed Aug 12, 2020).
- 3.World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. [updated 12th August 2020]. Available from: https://covid19.who.int/. (Accessed Aug 12, 2020).
- 4.Daneshkhah A, Agrawal V, Eshein A, et al.. The possible role of vitamin D in suppressing cytokine storm and associated mortality in COVID-19 patients. MedRxiv 2020.04.08.20058578; Available from: https://www.medrxiv.org/content/10.1101/2020.04.08.20058578v4. (Accessed Aug 10, 2020).
- 5.Undela K, Gudi SK. Assumptions for disparities in case-fatality rates of coronavirus disease (COVID-19) across the globe. Eur Rev Med Pharmacol Sci. 2020;24:5180–5182. doi: 10.26355/eurrev_202005_21215. [DOI] [PubMed] [Google Scholar]
- 6.Zhao X, Zhang B, Li P, et al. Incidence, clinical characteristics, and prognostic factor of patients with COVID-19: a systematic review and meta-analysis. MedRxiv 2020.03.17.20037572; Available from: https://www.medrxiv.org/content/10.1101/2020.03.17.20037572v1. (Accessed Aug 10, 2020).
- 7.Shivakumar S, Smibert OC, Trubiano JA, et al. Immunosuppression for COVID‑19: repurposing medicines in a pandemic. Aust. Prescr. 2020;43:106–107. doi: 10.18773/austprescr.2020.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horby P, Landray M. Oxford University News Release; 2020. Low-cost dexamethasone reduces death by up to one third in hospitalised patients with severe respiratory complications of COVID-19 (RECOVERY Trial)https://www.recoverytrial.net/files/recovery_dexamethasone_statement_160620_v2final.pdf Available from: (Accessed June 16) [Google Scholar]
- 9.Tim Locke. UK COVID-19 Update: Dexamethasone praise & scepticism - Medscape. Available from: https://www.medscape.com/viewarticle/932477 (Accessed Aug 12, 2020).
- 10.Azad MAK, Sarker M, Wan D. Immunomodulatory effects of probiotics on cytokine profiles. Biomed Res Int. 2018;2018 doi: 10.1155/2018/8063647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morshedi M, Hashemi R, Moazzen S, et al. Immunomodulatory and anti-inflammatory effects of probiotics in multiple sclerosis: a systematic review. J Neuroinflammation. 2019;16:231. doi: 10.1186/s12974-019-1611-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Wu Y, Wang Y, et al. Antioxidant properties of probiotic bacteria. Nutrients. 2017;9:521. doi: 10.3390/nu9050521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eguchi K, Fujitani N, Nakagawa H, et al. Prevention of respiratory syncytial virus infection with probiotic lactic acid bacterium Lactobacillus gasseri SBT2055. Sci Rep. 2019;9:4812. doi: 10.1038/s41598-019-39602-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dumas A, Bernard L, Poquet Y, et al. The role of the lung microbiota and the gut-lung axis in respiratory infectious diseases. Cell Microbiol. 2018;20:e12966. doi: 10.1111/cmi.12966. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy EA, King KY, Baldridge MT. Mouse microbiota models: comparing germ-free mice and antibiotics treatment as tools for modifying gut bacteria. Front Physiol. 2018;9:1534. doi: 10.3389/fphys.2018.01534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dabke K, Hendrick G, Devkota S. The gut microbiome and metabolic syndrome. J Clin Invest. 2019;129:4050–4057. doi: 10.1172/JCI129194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishida A, Inoue R, Inatomi O, et al. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol. 2018;11:1–10. doi: 10.1007/s12328-017-0813-5. [DOI] [PubMed] [Google Scholar]
- 18.Meijnikman AS, Gerdes VE, Nieuwdorp M, Herrema H. Evaluating causality of gut microbiota in obesity and diabetes in humans. Endocr Rev. 2018;39:133–153. doi: 10.1210/er.2017-00192. [DOI] [PubMed] [Google Scholar]
- 19.Gurung M, Li Z, You H, et al. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine. 2020;51 doi: 10.1016/j.ebiom.2019.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang WH, Kitai T, Hazen SL. Gut microbiota in cardiovascular health and disease. Circ Res. 2017;120:1183–1196. doi: 10.1161/CIRCRESAHA.117.309715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang C, Li G, Huang P, et al. The gut microbiota and Alzheimer's disease. J Alzheimers Dis. 2017;58:1–15. doi: 10.3233/JAD-161141. [DOI] [PubMed] [Google Scholar]
- 22.Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Enaud R, Prevel R, Ciarlo E, et al. The gut-lung axis in health and respiratory diseases: a place for inter-organ and inter-kingdom crosstalks. Front Cell Infect Microbiol. 2020;10:9. doi: 10.3389/fcimb.2020.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ver Heul A, Planer J, Kau AL. The human microbiota and asthma. Clin Rev Allergy Immunol. 2019;57:350–363. doi: 10.1007/s12016-018-8719-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mammen MJ, Sethi S. COPD and the microbiome. Respirology. 2016;21:590–599. doi: 10.1111/resp.12732. [DOI] [PubMed] [Google Scholar]
- 26.Namasivayam S, Sher A, Glickman MS, Wipperman MF. The microbiome and tuberculosis: early evidence for cross talk. mBio. 2018;9:e01418–e01420. doi: 10.1128/mBio.01420-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Budden KF, Gellatly SL, Wood DL, et al. Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol. 2017;15:55–63. doi: 10.1038/nrmicro.2016.142. [DOI] [PubMed] [Google Scholar]
- 28.Carabotti M, Scirocco A, Maselli MA, et al. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28:203–209. [PMC free article] [PubMed] [Google Scholar]
- 29.Dickson RP, Arbor A. The microbiome and critical illness. Lancet Respir Med. 2017;4:59–72. doi: 10.1016/S2213-2600(15)00427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagpal R, Mainali R, Ahmadi S, et al. Gut microbiome, and aging: physiological and mechanistic insights. Nutr Healthy Aging. 2018;4:267–285. doi: 10.3233/NHA-170030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thaiss CA, Zmora N, Levy M, et al. The microbiome and innate immunity. Nature. 2016;535:65–74. doi: 10.1038/nature18847. [DOI] [PubMed] [Google Scholar]
- 32.Wang L, Zhu L, Qin S. Gut microbiota modulation on intestinal mucosal adaptive immunity. J Immunol Res. 2019;2019 doi: 10.1155/2019/4735040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ostaff MJ, Stange EF, Wehkamp J. Antimicrobial peptides and gut microbiota in homeostasis and pathology. EMBO Mol Med. 2013;5:1465–1483. doi: 10.1002/emmm.201201773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhar D, Mohanty A. Gut microbiota and covid-19- possible link and implications. Virus Res. 2020;285 doi: 10.1016/j.virusres.2020.198018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Negi S, Das DK, Pahari S, et al. Potential role of gut microbiota in induction and regulation of innate immune memory. Front Immunol. 2019;10:2441. doi: 10.3389/fimmu.2019.02441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kishino E, Takemura N, Masaki H, et al. Dietary lactosucrose suppresses influenza A (H1N1) virus infection in mice. Biosci Microbiota Food Health. 2015;34:67–76. doi: 10.12938/bmfh.2015-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rooks MG., Garrett W.S. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16:341–352. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gill HS. Enhancement of immunity in the elderly by dietary supplementation with the probiotic Bifidobacterium lactis HN019. Am J Clin Nutr. 2001;74:833–839. doi: 10.1093/ajcn/74.6.833. [DOI] [PubMed] [Google Scholar]
- 39.Hanada S, Pirzadeh M, Carver KY, et al. Respiratory viral infection-induced microbiome alterations and secondary bacterial pneumonia. Front Immunol. 2018;9:2640. doi: 10.3389/fimmu.2018.02640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zuo T, Zhang F, Lui GCY, et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020;159:944–955. doi: 10.1053/j.gastro.2020.05.048. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu F, Ye S, Zhu X, et al. Gastrointestinal disturbance and effect of fecal microbiota transplantation in discharged COVID-19 patients. J Med Case Rep. 2021;15:1–9. doi: 10.1186/s13256-020-02583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeoh YK, Zuo T, Lui GC, et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;0:1–9. doi: 10.1136/gutjnl-2020-323020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheung KS, Hung IFN, Chan PPY, et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology. 2020;159:81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tian Y, Rong L, Nian W, et al. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020;51:843–851. doi: 10.1111/apt.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan R, Zhang Y, Li Y, et al. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang H, Kang Z, Gong H, et al. The digestive system is a potential route of 2019-nCov infection: a bioinformatics analysis based on single-cell transcriptomes. bioRxiv 2020.01.30.927806; Available from: https://www.biorxiv.org/content/10.1101/2020.01.30.927806v1. (Accessed Aug 11, 2020).
- 47.Cole-Jeffrey CT, Liu M, Katovich MJ, et al. ACE2 and Microbiota: emerging targets for cardiopulmonary disease therapy. J Cardiovasc Pharmacol. 2015;66:540–550. doi: 10.1097/FJC.0000000000000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lake MA. What we know so far: COVID-19 current clinical knowledge and research. Clin Med Lond (Lond) 2020;20:124–127. doi: 10.7861/clinmed.2019-coron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gou W, Fu Y, Yue L, et al. Gut microbiota may underlie the predisposition of healthy individuals to COVID-19. medRxiv 2020.04.22.20076091; Available from: https://www.medrxiv.org/content/10.1101/2020.04.22.20076091v1. (Accessed Aug 11, 2020).
- 50.Gu S, Chen Y, Wu Z, et al. Alterations of the gut microbiota in patients with COVID-19 or H1N1 influenza. Clin Infect Dis. 2020;71:2669–2678. doi: 10.1093/cid/ciaa709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hill C, Guarner F, Reid G, et al. Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 52.Zeng W, Shen J, Bo T, et al. Cutting edge: probiotics and fecal microbiota transplantation in immunomodulation. J Immunol Res. 2019;2019 doi: 10.1155/2019/1603758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bermudez-Brito M, Plaza-Díaz J, Muñoz-Quezada S, et al. Probiotic mechanisms of action. Ann Nutr Metab. 2012;61:160–174. doi: 10.1159/000342079. [DOI] [PubMed] [Google Scholar]
- 54.Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A. Mechanisms of action of probiotics. Adv Nutr. 2019;10(suppl_1):S49–S66. doi: 10.1093/advances/nmy063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shang M, Sun J. Vitamin D/VDR, probiotics, and gastrointestinal diseases. Curr Med Chem. 2017;24:876–887. doi: 10.2174/0929867323666161202150008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Konieczna P, Groeger D, Ziegler M, et al. Bifidobacterium infantis 35624 administration induces Foxp3 T regulatory cells in human peripheral blood: potential role for myeloid and plasmacytoid dendritic cells. Gut. 2012;61:354–366. doi: 10.1136/gutjnl-2011-300936. [DOI] [PubMed] [Google Scholar]
- 57.Fujii T, Ohtsuka Y, Lee T, et al. Bifidobacterium breve enhances transforming growth factor beta1 signaling by regulating Smad7 expression in preterm infants. J Pediatr Gastroenterol Nutr. 2006;43:83–88. doi: 10.1097/01.mpg.0000228100.04702.f8. [DOI] [PubMed] [Google Scholar]
- 58.Xie J, Nie S, Yu Q, et al. Lactobacillus plantarum NCU116 attenuates cyclophosphamide-induced immunosuppression and regulates Th17/Treg cell immune responses in mice. J Agric Food Chem. 2016;64:1291–1297. doi: 10.1021/acs.jafc.5b06177. [DOI] [PubMed] [Google Scholar]
- 59.Wu Q, Liu MC, Yang J, et al. Lactobacillus rhamnosus GR-1 Ameliorates Escherichia coli-induced inflammation and cell damage via attenuation of ASC-independent NLRP3 inflammasome activation. Appl Environ Microbiol. 2015;82:1173–1182. doi: 10.1128/AEM.03044-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.West CE, Jenmalm MC, Prescott SL. The gut microbiota and its role in the development of allergic disease: a wider perspective. Clin Exp Allergy. 2015;45:43–53. doi: 10.1111/cea.12332. [DOI] [PubMed] [Google Scholar]
- 61.Jayawardena R, Sooriyaarachchi P, Chourdakis M, et al. Enhancing immunity in viral infections, with special emphasis on COVID-19: a review. Diabetes Metab Syndr. 2020;14:367–382. doi: 10.1016/j.dsx.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jones ML, Martoni CJ, Prakash S. Oral supplementation with probiotic L. reuteri NCIMB 30242 increases mean circulating 25-hydroxyvitamin D: a post hoc analysis of a randomized controlled trial. J Clin Endocrinol Metab. 2013;98:2944–2951. doi: 10.1210/jc.2012-4262. [DOI] [PubMed] [Google Scholar]
- 63.Sonia Yoon SW, Yong-guo Zhang, Rong Lu, et al. Probiotic regulation of vitamin D receptor in intestinal inflammation. Gastroenterology. 2011;140:S–19. [Google Scholar]
- 64.Wu S, Yoon S, Zhang YG, et al. Vitamin D receptor pathway is required for probiotic protection in colitis. Am J Physiol Gastrointest Liver Physiol. 2015;309:G341–G349. doi: 10.1152/ajpgi.00105.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chai W, Burwinkel M, Wang Z, et al. Antiviral effects of a probiotic Enterococcus faecium strain against transmissible gastroenteritis coronavirus. Arch Virol. 2013;158:799–807. doi: 10.1007/s00705-012-1543-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li J, Zhao J, Wang X, et al. Novel angiotensin-converting enzyme-inhibitory peptides from fermented bovine milk started by Lactobacillus helveticus KLDS.31 and Lactobacillus casei KLDS.105: purification, identification, and interaction mechanisms. Front Microbiol. 2019;10:2643. doi: 10.3389/fmicb.2019.02643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rai AK, Sanjukta S, Jeyaram K. Production of angiotensin I converting enzyme inhibitory (ACE-I) peptides during milk fermentation and their role in reducing hypertension. Crit Rev Food Sci Nutr. 2017;57:2789–2800. doi: 10.1080/10408398.2015.1068736. [DOI] [PubMed] [Google Scholar]
- 68.Chen Y, Zuo W, Xue J, et al. Angiotensin-converting enzyme inhibitory activity of Lactobacillus helveticus strains from traditional fermented dairy foods and antihypertensive effect of fermented milk of strain H9. J Dairy Sci. 2014;97:6680–6692. doi: 10.3168/jds.2014-7962. [DOI] [PubMed] [Google Scholar]
- 69.Garcia-Crespo KE, Chan CC, Gabryszewski SJ, et al. Lactobacillus priming of the respiratory tract: heterologous immunity and protection against lethal pneumovirus infection. Antiviral Res. 2013;97:270–279. doi: 10.1016/j.antiviral.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kumova OK, Fike AJ, Thayer JL, et al. Lung transcriptional unresponsiveness and loss of early influenza virus control in infected neonates is prevented by intranasal Lactobacillus rhamnosus GG. PLoS Pathog. 2019;15 doi: 10.1371/journal.ppat.1008072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harata G, He F, Hiruta N, et al. Intranasal administration of Lactobacillus rhamnosus GG protects mice from H1N1 influenza virus infection by regulating respiratory immune responses. Lett Appl Microbiol. 2010;50:597–602. doi: 10.1111/j.1472-765X.2010.02844.x. [DOI] [PubMed] [Google Scholar]
- 72.Jung YJ, Lee YT, Ngo VL, et al. Heat-killed Lactobacillus casei confers broad protection against influenza A virus primary infection and develops heterosubtypic immunity against future secondary infection. Sci Rep. 2017;7:17360. doi: 10.1038/s41598-017-17487-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park MK, Ngo V, Kwon YM, et al. Lactobacillus plantarum DK119 as a probiotic confers protection against influenza virus by modulating innate immunity. PLoS One. 2013;8:e75368. doi: 10.1371/journal.pone.0075368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Evrard B, Coudeyras S, Dosgilbert A, et al. Dose-dependent immunomodulation of human dendritic cells by the probiotic Lactobacillus rhamnosus Lcr35. PLoS One. 2011;6:e18735. doi: 10.1371/journal.pone.0018735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weiss G, Rasmussen S, Zeuthen LH, et al. Lactobacillus acidophilus induces virus immune defence genes in murine dendritic cells by a Toll-like receptor-2-dependent mechanism. Immunology. 2010;131:268–281. doi: 10.1111/j.1365-2567.2010.03301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Waki N, Matsumoto M, Fukui Y, et al. Effects of probiotic Lactobacillus brevis KB290 on incidence of influenza infection among schoolchildren: an open-label pilot study. Lett Appl Microbiol. 2014;59:565–571. doi: 10.1111/lam.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee DK, Kang JY, Shin HS, et al. Antiviral activity of Bifidobacterium adolescentis SPM0212 against hepatitis B virus. Arch Pharm Res. 2013;36:1525–1532. doi: 10.1007/s12272-013-0141-3. [DOI] [PubMed] [Google Scholar]
- 78.Kang JY, Lee DK, Ha NJ, et al. Antiviral effects of Lactobacillus ruminis SPM0211 and Bifidobacterium longum SPM1205 and SPM1206 on rotavirus-infected Caco-2 cells and a neonatal mouse model. J Microbiol. 2015;53:796–803. doi: 10.1007/s12275-015-5302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pourhossein M, Moravejolahkami AR. Probiotics in viral infections, with a focus on COVID-19: a systematic review. Prepints 2020. doi: 10.22541/au.158938616.61042433. [DOI]
- 80.Adnan ML, Dewi MD. Potential effects immunomodulators on probiotics in COVID-19 preventing infection in the future. A narrative review. Int J Med Stud 2020. doi: 10.5195/ijms.2020.486. [DOI]
- 81.Xu K, Cai H, Shen Y, et al. Management of corona virus disease-19 (COVID-19): the Zhejiang experience. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49:147–157. doi: 10.3785/j.issn.1008-9292.2020.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kang EJ, Kim SY, Hwang IH, et al. The effect of probiotics on prevention of common cold: a meta-analysis of randomized controlled trial studies. Korean J Fam Med. 2013;34:2–10. doi: 10.4082/kjfm.2013.34.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Su M, Jia Y, Li Y, et al. Probiotics for the prevention of ventilator-associated pneumonia: a meta-analysis of randomized controlled trials. Respir Care. 2020;65:673–685. doi: 10.4187/respcare.07097. [DOI] [PubMed] [Google Scholar]
- 84.Fornell D. Kawasaki-like inflammatory disease affects children with COVID-19. Diagnostic and Interventional Cardiology. Available from: https://www.dicardiology.com/article/kawasaki-inflammatory-disease-affects-children-covid-19. (Accessed May 30, 2020).
- 85.Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55 doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou Y, Chi J, Lv W, et al. Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (COVID-19) Diabetes Metab Res Rev. 2021;37:e3377. doi: 10.1002/dmrr.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Briguglio M, Pregliasco FE, Lombardi G, et al. The malnutritional status of the host as a virulence factor for new coronavirus SARS-CoV-2. Front Med (Lausanne) 2020;7:146. doi: 10.3389/fmed.2020.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thushara RM, Gangadaran S, Solati Z, et al. Cardiovascular benefits of probiotics: a review of experimental and clinical studies. Food Funct. 2016;7:632–642. doi: 10.1039/c5fo01190f. [DOI] [PubMed] [Google Scholar]
- 89.Minato T, Nirasawa S, Sato T, et al. B38-CAP is a bacteria-derived ACE2-like enzyme that suppresses hypertension and cardiac dysfunction. Nat Commun. 2020;11:1058. doi: 10.1038/s41467-020-14867-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Becker RC. COVID-19 update: Covid-19-associated coagulopathy. J Thromb Thrombolysis. 2020;50:54–67. doi: 10.1007/s11239-020-02134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zelaya H, Villena J, Lopez AG, et al. Modulation of the inflammation-coagulation interaction during pneumococcal pneumonia by immunobiotic Lactobacillus rhamnosus CRL1505: role of Toll-like receptor 2. Microbiol Immunol. 2014;58:416–426. doi: 10.1111/1348-0421.12163. [DOI] [PubMed] [Google Scholar]
- 92.Di Renzo L, Merra G, Esposito E, et al. Are probiotics effective adjuvant therapeutic choice in patients with COVID-19? Eur Rev Med Pharmacol Sci. 2020;24:4062–4063. doi: 10.26355/eurrev_202004_20977. [DOI] [PubMed] [Google Scholar]
- 93.Ellul MA, Benjamin L, Singh B, et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19:767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7:875–882. doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Umbrello G, Esposito S. Microbiota and neurologic diseases: potential effects of probiotics. J Transl Med. 2016;14:298. doi: 10.1186/s12967-016-1058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Messaoudi M, Lalonde R, Violle N, et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr. 2011;105:755–764. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- 97.Tillisch K, Labus J, Kilpatrick L, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144:1394–1401. doi: 10.1053/j.gastro.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dinan TG, Quigley EM, Ahmed SM, et al. Hypothalamic-pituitary gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker? Gastroenterology. 2006;130:304–311. doi: 10.1053/j.gastro.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 99.Dai YJ, Wang HY, Wang XJ, et al. Potential beneficial effects of probiotics on human migraine headache: A literature review. Pain Physician. 2017;20:E251–E255. [PubMed] [Google Scholar]
- 100.International Scientific Association for probiotics and prebiotics (ISAPP). Probiotics Available from: https://isappscience.org/for-scientists/resources/probiotics/ (Accessed Aug 12, 2020). [DOI] [PMC free article] [PubMed]
- 101.Feng Z, Wang Y, Qi W. The small intestine, an underestimated site of SARS-CoV-2 infection: from red queen effect to probiotics. Preprints.org 2020. doi: 10.20944/preprints202003.0161.v1.
- 102.Liang W, Feng Z, Rao S, et al. Diarrhoea may be underestimated: a missing link in 2019 novel coronavirus. Gut. 2020;69:1141–1143. doi: 10.1136/gutjnl-2020-320832. [DOI] [PubMed] [Google Scholar]
- 103.Pradhan B, Guha D, Naik AK, et al. Probiotics L. acidophilus and B. clausii modulate gut microbiota in Th1- and Th2- biased mice to ameliorate Salmonella typhimurium-induced diarrhea. Probiotics Antimicrob Proteins. 2019;11:887–904. doi: 10.1007/s12602-018-9436-5. [DOI] [PubMed] [Google Scholar]
- 104.Appel-da-Silva MC, Narvaez GA, Perez LR, et al. Saccharomyces cerevisiae var. boulardii fungemia following probiotic treatment. Med Mycol Case Rep. 2017;18:15–17. doi: 10.1016/j.mmcr.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gargar JD, Divinagracia RM. When good things go bad: a case series of bacteremia from probiotics. Chest. 2019;155:92A. [Google Scholar]
- 106.Yoon S, Wu S, Zhang YG, et al. Probiotic regulation of vitamin D receptor in intestinal inflammation. Gastroenterology. 2011;140 S-19. [Google Scholar]
- 107.Mak JWY, Chan FKL, Ng SC. Probiotics and COVID-19: one size does not fit all. Lancet Gastroenterol Hepatol. 2020;5:644–645. doi: 10.1016/S2468-1253(20)30122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.