Abstract
BACKGROUND AND PURPOSE: Flow-sensitive alternating inversion recovery (FAIR) MR imaging is a technique for depicting cerebral perfusion without contrast enhancement. Our purpose was to determine whether quantification at FAIR imaging can be used to assess regional cerebral blood flow (rCBF) in a manner similar to [iodine 123]-iodoamphetamin (123I-IMP) single photon emission CT (SPECT).
METHODS: Nine patients with internal carotid or major cerebral arterial stenosis underwent 123I-IMP SPECT and FAIR imaging (single section, different TIs, 1.5 T) at rest and after acetazolamide (Diamox) stress. FAIR and 123I-IMP rCBF values were compared and correlated. Receiver operating characteristic analysis was conducted to detect hypoperfused segments on FAIR images.
RESULTS: rCBF values of normally perfused segments were 41.53 and 51.91 mL/100 g/min for pre- and post-acetazolamide 123I-IMP studies, respectively. Corresponding values for pre- and post-acetazolamide FAIR images, respectively, were 46.64 and 59.60 mL/100 g/min with a TI of 1200 milliseconds and 53.23 and 68.17 mL/100 g/min with a TI of 1400 milliseconds. 123I-IMP and FAIR resultswere significantly correlated, with both pre- and post-acetazolamide images. Sensitivity (86%) in detecting hypoperfused segments was significantly higher with post-acetazolamide images (TI, 1400 milliseconds), and specificity (82–85%) and accuracy (80–82%) were higher with all pre- and post-acetazolamide images (all TIs).
CONCLUSIONS: The significant correlation, high specificity and accuracy in detecting hypoperfused segments, similar increases in flow on both post-acetazolamide images, and absence of the need for contrast enhancement suggest that FAIR imaging, like nuclear medicine study, is complementary to routine MR imaging in the assessment of cerebral perfusion.
The assessment of cerebral perfusion by using nuclear medicine techniques is well established with both single photon emission CT (SPECT) and positron emission tomography (PET). Moreover, regional cerebral blood flow (rCBF) measurements with various brain imaging agents and nuclear medicine techniques have a good correlation with the criterion standard rCBF measurements obtained with either radioactive microspheres or xenon 133 (1–5). Measurements of rCBF with [iodine 123]-iodoamphetamin (123I-IMP) by using autoradiographic and modified autoradiographic (table look-up) methods have a good correlation with those obtained at PET, microspheric assessment of cerebral blood flow, and 133Xe studies (3, 6–8).
With advancements in MR imaging hardware and software, easy image acquisition with new and faster sequences is possible. In recent years, several new MR pulse sequences with arterial spin labeling have been developed to assess cerebral perfusion and rCBF without contrast enhancement (9–12). Flow-sensitive alternating inversion recovery (FAIR) imaging is one of the techniques that can be used to predict cerebral perfusion without the use of contrast agents (13). A few preliminary reports (12, 14) have shown the usefulness of such sequences in functional brain imaging. The measurement of quantitative rCBF by using FAIR imaging is possible by measuring the T1 and M0 values in the brain tissue in each case (14). However, to our knowledge, no study of the direct correlation with these findings with those of conventional methods for assessing cerebral perfusion or rCBF has been conducted. Although the effect of acetazolamide (Diamox) has recently been assessed with FAIR imaging (15), we found no correlative study of SPECT or PET.
The purpose of this study was to determine whether quantification with FAIR images can be used to predict rCBF values in a manner similar to that of 123I-IMP SPECT at rest and after the induction of stress with the administration of acetazolamide. The accuracy in detecting hypoperfused segments on FAIR images was assessed by means of receiver operating characteristic (ROC) analysis.
Methods
Nine patients (six men, three women; age range, 48–69 years; mean age, 59.2 years) were enrolled in this comparative study (Table 1). Seven patients had various degrees of internal carotid artery and/or other major cerebral artery stenosis (assessed with MR angiography in three patients, with digital subtraction angiography in two, and with both modalities in two). Two patients had transient ischemic attacks and dizziness, but neither MR imaging nor SPECT images showed abnormalities regarding the brain or cerebral perfusion, respectively. All patients were referred to the nuclear medicine division of our radiology department to undergo pre- and post-acetazolamide 123I-IMP SPECT to assess cerebral perfusion and the reserve capacity of major cerebral arteries in view of possible bypass surgery. After thorough discussion and informed consent was obtained from both the referring physician and patients, all patients were asked to undergo pre- and post-acetazolamide FAIR imaging just before the post-acetazolamide 123I-IMP SPECT. The study protocol was planned in such a way that a single injection of acetazolamide was sufficient for both post-acetazolamide FAIR imaging and 123I-IMP SPECT. The FAIR imaging protocol was approved, and it was in accordance with the guidelines of the university hospital ethical committee. No adverse reactions or patient complaints occurred during the study.
TABLE 1:
Patient profiles, examinations, and diagnoses
| Patient No./Age (y)/Sex | Delay between Pre- and Post-Acetazolamide 128I-IMP SPECT (d)* | Diagnosis† |
|---|---|---|
| 1/68/M | 7 | Right ICA severe stenosis at MRA and DSA |
| 2/63/M | 3 | Right ICA severe stenosis (90%) and left ICA stenosis (40%) at MRA and DSA |
| 3/48/M | 4 | Right MCA (M1) occulusion with collateral circulation at DSA |
| 4/51M | 3 | TIA, normal findings at MR imaging and SPECT |
| 5/53/F | 8 | Right ICA occulsion, left ICA stenosis, left ACA occulsion at DSA |
| 6/69/F | 7 | Bilateral mild ICA stenosis with lacunar infarctions at MRA and MR imaging |
| 7/57/F | 7 | Dizziness, normal flow and perfusion at MRA and SPECT |
| 8/55/M | 4 | Right ICA, MCA, and ACA stenosis at MRA; decreased perfusion at SPECT |
| 9/69/M | 4 | Right ICA stenosis at MRA, mildly decreased perfusion at SPECT |
Both pre- and post-acetazolamide FAIR studies were performed on the day of post-acetazolamide 123I-IMP SPECT.
ACA indicates anterior cerebral artery; DSA, digital subtraction angiography; ICA, internal carotid artery; MCA, middle cerebral artery; MRA, magnetic resonance angiography; and TIA, transient ischemic attack.
Study Protocol
The at-rest 123I-IMP SPECT study was usually performed 3–8 days before the acetazolamide stress study. On the day of the acetazolamide stress study, both FAIR and 123I-IMP SPECT images were acquired as follows: spin-echo echo-planar imaging (SE-EPI) with different TIs (200, 400, and 800 milliseconds), then at-rest FAIR imaging (TI, 1200 and then 1400 milliseconds), the intravenous administration of 1000 mg of acetazolamide, a waiting period of 8 minutes, FAIR imaging (TI, 1400 milliseconds), the intravenous administration of 123I-IMP, FAIR imaging (TI, 1200 milliseconds), a waiting period of 6 minutes 25 seconds, arterial blood collection, and transfer of the patient to the nuclear medicine department for SPECT (20 minutes after the administration of 123I-IMP).
FAIR Imaging
On the day of the acetazolamide-stress 123I-IMP study, patients underwent FAIR imaging with a 1.5-T MR imaging system (Horizon; GE Medical Systems, Milwaukee, WI). Single-section FAIR studies with different TIs (1200 and 1400 milliseconds) were performed before and after the administration of acetazolamide, according to the method presented by Kwong et al (13), by using SE-EPI sequences (2000/20.6/100 [TR/TE/NEX] [pair of 50 images]; bandwidth, 83 kHz; field of view, 24 × 24 cm; matrix, 96 × 96; section thickness, 10 mm) with a head coil. To calculate quantitative rCBF values from FAIR images obtained at rest and after acetazolamide stress, both T1 and M0 values in gray matter and white matter were measured in all patients by acquiring SE-EPI images with different non–section-selective TIs (200, 400, and 800 milliseconds). After two pre-acetazolamide FAIR images were acquired (TIs, 1200 and 1400 milliseconds), 1000 mg of acetazolamide was intravenously administered over 60–90 seconds. Approximately 8 minutes after the administration of acetazolamide, FAIR images (TIs, 1400 and then 1200 milliseconds) were acquired. Both pre- and post-acetazolamide FAIR images were acquired in one session without moving the patient’s head from the headrest. Images were acquired at the mid section of the cerebrum (along the basal ganglia and thalamus) in each patient. The total time required to acquire one image was 3 minutes 36 seconds. After transferring the images (both at-rest and acetazolamide-stress images) to a workstation, 15 regions of interest (ROIs) were created over the entire cerebrum; cerebral hemispheres; thalami; basal ganglia; and frontal, posterior parietal, temporal, and occipital regions on both sides to record the average signal intensity for the measurement of rCBF in these regions. The size of the ROI was at least 10 voxels. Care was taken not to include the high-intensity areas in the sinuses and peripheral vessels. After the FAIR images were acquired and arterial blood was drawn, patients were transported to the nuclear medicine division for 123I-IMP SPECT.
SPECT Studies
rCBF was measured by using the modified autoradiographic (table look-up) method with 123I-IMP SPECT images, according to the method presented by Iida et al (7, 8). Both early and delayed at-rest and acetazolamide-stress 123I-IMP images were obtained in all patients (within 3–8 days of each other). For the acetazolamide-stress study, 123I-IMP was injected after the first post-acetazolamide FAIR image was acquired (TI, 1400 milliseconds), which was usually 12 minutes after the administration of acetazolamide (8 minutes + 3 minutes 36 seconds). Early SPECT scans were acquired 20 minutes after 123I-IMP (167–222 MBq) was injected for 20 min (midpoint of the scanning time was 30 minutes after injection) A Toshiba GCA3000A/DI triple-head gamma camera mounted with fan beam collimators was used. The images were acquired in a 128 × 128 matrix with continuous rotation (10 rotations, 2 minutes per rotation) and 4° steps by using the triple energy-window method. SPECT scans were reconstructed from the back projected data by using Butterworth and Ramp filters after proper scatter correction. The thickness of the axial sections was 6.8 mm. Delayed scans were acquired with similar acquisition parameters at 3 hours after the injection of 123I-IMP. Delayed SPECT scans were necessary to calculate the distribution volume and global cerebral blood flow with the modified autoradiographic method to measure rCBF. A single-point brachial arterial blood sample was collected in each patient at 10 minutes after the injection of 123I-IMP, and the radioactivity was measured with an auto gamma well-counter. The blood sample was collected from the side contralateral to that of the 123I-IMP injection. The mid section of the cerebrum (along the basal ganglia and thalamus) was selected in each patient to measure the rCBF. rCBF values of the entire cerebrum; cerebral hemispheres; thalami; basal ganglia; and frontal, posterior parietal, temporal, and occipital regions on both sides were measured by creating ROIs. In each patient, rCBF values in 15 regions at rest and after acetazolamide stress were measured. The size of the ROI was at least 10 pixels.
Image Selection and Analysis
All at-rest and acetazolamide-stress FAIR images were obtained during the same session without moving the patient’s head from the headrest. Although image coregistration was not applied to select the cerebral blood flow images of the 123I-IMP study, a matching section along the mid section of cerebrum was selected from the 123I-IMP study in all patients. One investigator (A.S.A.) selected all the image sections and drew the ROIs to minimize false registration.
ROC Analysis
A total of 108 segments (bilateral occipital, parietal, temporal, frontal, basal ganglia, and thalami) from nine patients were used in the ROC analysis. Eighty of the 108 segments showed normal perfusion on 123I-IMP SPECT scans. Any segment that had an rCBF of 30 mL/100 g/min or more at rest with an increase in flow after acetazolamide administration was considered normal, and none of the segments had atrophy or high signal intensity on previously obtained T2-weighted MR images. Three (S.A., K.T., T.U.) independent readers analyzed all FAIR images before and after acetazolamide administration. None of the readers was aware of the patient’s history, and none participated in consensus readings of the images. Pre- and post-acetazolamide images were presented randomly to the readers for analysis. The readers were asked to score the segments on FAIR images in each patient with a confidence level of 1–5 based on their observations, as follows: 1 indicated definitely normal; 2, probably normal; 3, equivocal; 4, probably hypoperfusion; and 5 = definite hypoperfusion or absent perfusion.
For each FAIR imaging technique used before and after the induction of acetazolamide stress, a binormal ROC curve was fitted to each reader’s confidence rating data by using a maximum-likelihood estimation (16). The diagnostic accuracy of each imaging technique was determined by calculating the area under each reader-specific binormal ROC curve (Az index) plotted on the previously designated square (17). Differences between the ROC curves of the individual readers were tested for significance by using a bivariate method of the ROCKIT algorithm with the χ2 test (18). Composite ROC curves for each imaging technique were calculated by averaging the binormal parameter values of the three readers for that technique. Differences between the imaging techniques in terms of Az indices were analyzed statistically by means of a post hoc analysis of variance (Bonferroni) test. A P values of less than .0083 was considered to indicate a significant difference with this table of data.
Any segment on a FAIR image that had a confidence level of 4 or more was considered an abnormally perfused area. The sensitivity, specificity, and accuracy of detecting abnormally perfused segments with each FAIR imaging technique were calculated for each reader. A post hoc analysis of variance (Bonferroni) test was applied to assess the significance of differences among the readers. A P value of less than .0083 was considered to indicate a significant difference.
Calculation of rCBF from FAIR Images
Two inversion recovery images are acquired by using the FAIR technique: one with a non–section-selective inversion pulse and the other with a section-selective inversion pulse (9, 13). The longitudinal magnetization of the tissue-water proton M after an inversion pulse can be described by a Bloch equation (Eq 1), in the presence of flow components and with an assumption of instantaneous blood and tissue spin exchange, as follows:
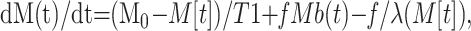 |
1) |
where t is the TI, M0 is the fully relaxed longitudinal magnetization of the tissue-water proton, T1 is the longitudinal relaxation time of the tissue-water proton after correcting flow effects, ƒ is the cerebral blood flow (in units of milliliter per gram per second), Mb is the incoming arterial blood-water proton and λ is the tissue-to-blood partition coefficient (0.9 mL/g) (14).
If blood T1 is assumed to be equal to tissue T1, T1 becomes the true tissue T1 with a nonselective inversion pulse, whereas T1 becomes T1app with a section-selective inversion pulse; this is a function of the true tissue T1 and flow. According to Kwong et al (13), this can be expressed as follows (Eq 2):
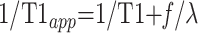 |
2) |
.
Considering that T1app varies according to the application of either non–section-selective or section-selective inversion sequences, a perfusion contrast image can be obtained by simply subtracting a flow-insensitive image (with non–section-selective inversion) from a flow-sensitive image (with section-selective inversion) (13). According to the theory derived by Kim and Tsekos (14), the intensity on the subtracted image is expressed with the following function (Eq 3):
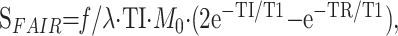 |
3) |
where SFAIR is the signal intensity on the subtraction FAIR images, TI is 1200 and 1400 milliseconds, and TR is 2000 milliseconds. This equation shows that, by measuring T1 and M0 from T1-weighted images obtained with non–section-selective inversion, absolute cerebral blood flow can be determined on FAIR images. Values of M0 and T1 were measured by plotting T1-weighted images obtained with non–section-selective inversion by using the following Equation 4 on a workstation:
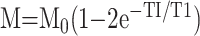 |
4) |
.
The measured T1 values of gray matter and white matter were 0.829 seconds ± 0.04 and 0.553 seconds ± 0.03, respectively (gray matter–white matter ratio, 1.5). The measured M0 was 1198.31 ± 129.3.
Data Analysis
rCBF values measured on FAIR and 123I-IMP images in all patients were analyzed for correlation. The simple linear regression method was applied. A P value of less than .05 was considered to indicate a significant difference.
Results
ROC Analysis
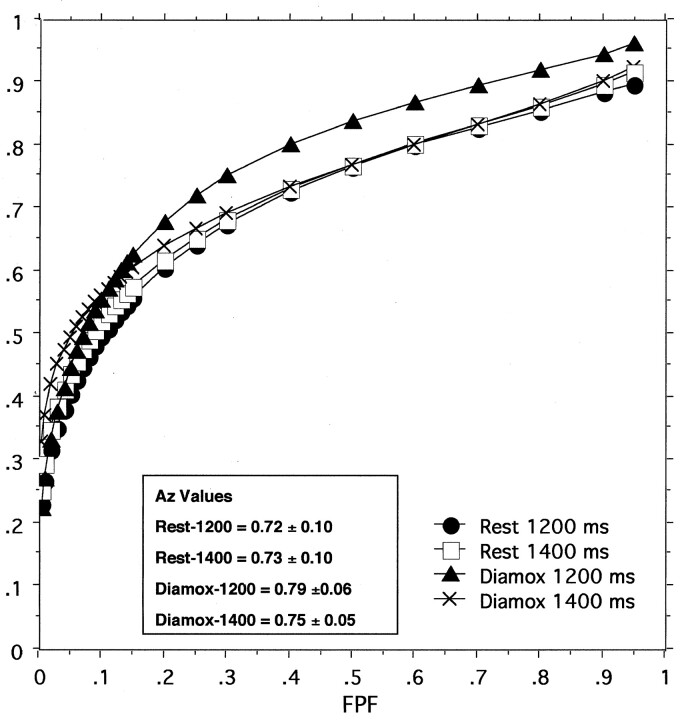
Figure 1 shows the composite ROC curves for each method of image acquisition. All readers improved their performance in detecting lesions on acetazolamide-stress FAIR images, especially with an TI of 1200 milliseconds (data not shown). However, no significant differences in Az values were found among the readers for any of the TIs at rest or after the induction of acetazolamide stress. The areas under the composite ROC curves (Az index) of the three readers were 0.725 ± 0.098 for at-rest images with an TI of 1200 milliseconds, 0.735 ± 0.097 for at-rest images with an TI of 1400 milliseconds, 0.817 ± 0.062 for acetazolamide-stress images with an TI of 1200 milliseconds, and 0.747 ± 0.055 for acetazolamide-stress images with an TI of 1400 milliseconds. No significant differences were found among the Az values of composite curves, for all TIs.
Fig 1.
Composite ROC curves and Az values of the areas under the curves for the detection of abnormal segments on FAIR images obtained with different TIs show no significant difference among the Az values. TPF indicates true-positive fraction; FPF, false-positive fraction.
Table 2 shows the cumulative sensitivity, specificity, and accuracy in detecting hypoperfused segments on images obtained with different TIs before and after the administration of acetazolamide. Significantly higher sensitivity (86% ± 5%) was observed with a post-acetazolamide TI of 1400 milliseconds, whereas 67–69% sensitivity was found with a pre- and post-acetazolamide TI of 1200 millisecond and a pre-acetazolamide TI of 1400 milliseconds. However, similarly high specificity (82–85%) and accuracy (80–82%) were observed with all TIs before and after the administration of acetazolamide.
TABLE 2:
Combined sensitivity, specificity and accuracy at ROC analysis involving three independent readers*
| Image and TI (ms) | Sensitivity | Specificity | Accuracy |
|---|---|---|---|
| Rest, 1200 | 67.0 ± 4.58 | 83.7 ± 2.31 | 80.0 ± 0.58 |
| Rest, 1400 | 69.7 ± 2.89 | 84.0 ± 1.73 | 81.7 ± 1.15 |
| Acetazolamide, 1200 | 67.3 ± 2.52 | 85.7 ± 2.51 | 81.3 ± 1.15 |
| Acetazolamide, 1400 | 86.0 ± 5.00† | 82.0 ± 1.73 | 82.7 ± 0.58 |
In the detection of abnormally perfused segments on images obtained with different TIs pre- and post-acetazolamide administration.
Significant difference from results on images obtained with other TIs.
Quantification with FAIR Images
Eighty of 108 segments were normally perfused on 123I-IMP images. The rCBF values of the normally perfused segments were 41.53 mL/100 g/min ± 8.56 and 51.91 mL/100 g/min ± 9.66 (25% increase) on pre- and post-acetazolamide 123I-IMP images, respectively. On FAIR images, the rCBF values of the normally perfused segments were 46.64 mL/100 g/min ± 10.0 and 59.60 mL/100 g/min ± 15.30 (28% increase) on pre- and post-acetazolamide images, respectively, with a TI of 1200 milliseconds. However, the rCBF values of the normally perfused segments were 53.23 mL/100 g/min ± 12.0 and 68.17 mL/100 g/min ± 17.18 (28% increase) on pre- and post-acetazolamide FAIR images, respectively, with a TI of 1400 milliseconds. Both 123I-IMP and FAIR images showed similarly increased flow in normal segments after the administration of acetazolamide.
Figures 2 and 3 show representative FAIR and 123I-IMP images obtained before and after acetazolamide administration in a patient with almost normal perfusion and in another patient with right middle cerebral artery occlusion, respectively. Abnormal linear signal intensities can be seen in the hypoperfused temporoparietal region (along the sulci) on the FAIR images; these might represent either collateral flows or vessels with notably delayed flow.
Fig 2.
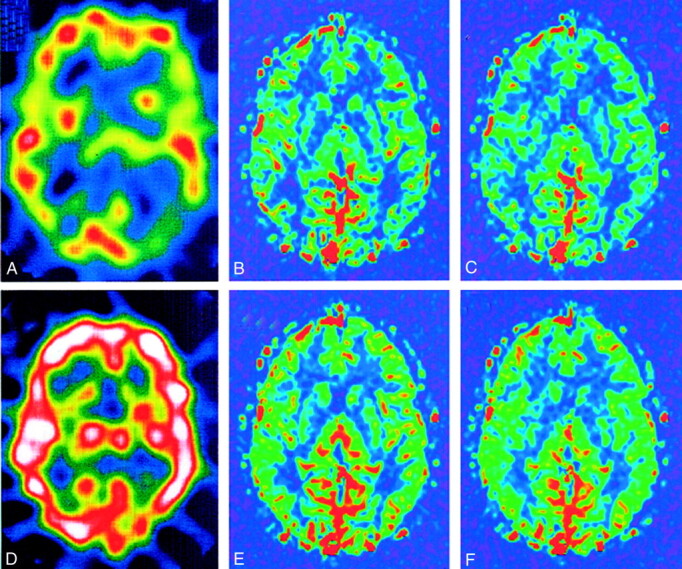
123I-IMP images in a representative case show normal perfusion. Although relatively decreased perfusion is seen at the left parietal-occipital region in A, quantitative analysis showed flow of more than 30 mL/100 g/min, with markedly increased flow after acetazolamide administration. Note similar cerebral blood flow depictions on both pre- and post-acetazolamide FAIR images.
A, Pre-acetazolamide 123I-IMP image.
B, Pre-acetazolamide FAIR image obtained with a TI of 1200 milliseconds.
C, Pre-acetazolamide FAIR image obtained with a TI of 1400 milliseconds.
D, Post-acetazolamide 123I-IMP image.
E, Post-acetazolamide FAIR image obtained with a TI of 1200 milliseconds.
F, Post-acetazolamide FAIR image obtained with a TI of 1400 milliseconds.
Fig 3.
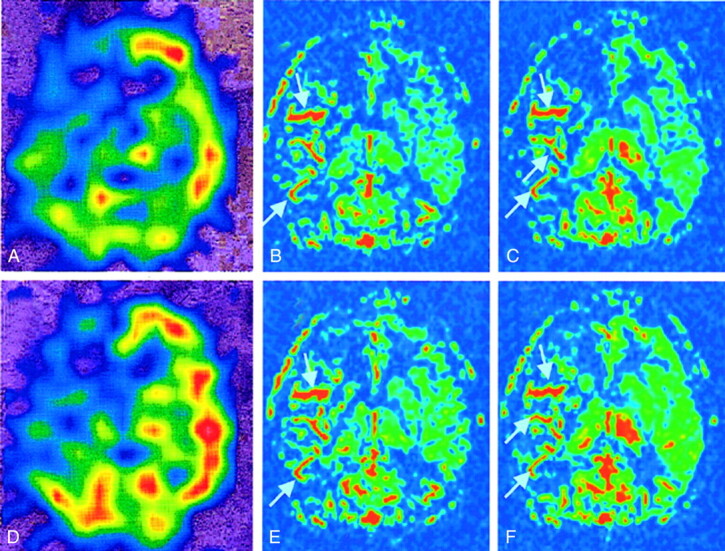
Images in a patient with right middle cerebral artery occlusion. Note the linear high-signal intensity areas (arrows) along the major sulci at right temporoparietal regions; these may represent collateral flow or vessels with notably delayed flow.
A, Pre-acetazolamide 123I-IMP image.
B, Pre-acetazolamide FAIR image obtained with a TI of 1200 milliseconds.
C, Pre-acetazolamide FAIR image obtained with a TI of 1400 milliseconds.
D, Post-acetazolamide 123I-IMP image.
E, Post-acetazolamide FAIR image obtained with a TI of 1200 milliseconds.
F, Post-acetazolamide FAIR image obtained with a TI of 1400 milliseconds.
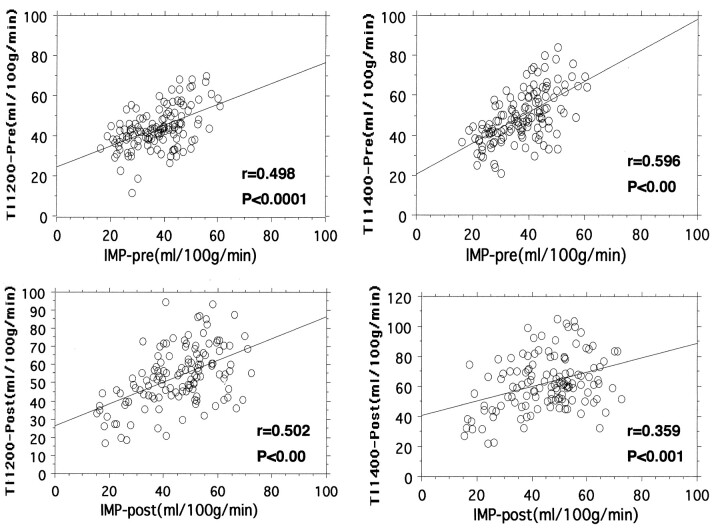
Figure 4 shows the correlation among the rCBF values measured on 123I-IMP and FAIR (with different TIs) images obtained before and after the administration of acetazolamide. Significant correlations were found between 123I-IMP and FAIR results, on both pre- and post-acetazolamide images. However, the distribution of data became random on post-acetazolamide regression plots, especially with images obtained with a TI of 1400 milliseconds.
Fig 4.
Regression plots of rCBF values measured on pre-acetazolamide (-pre) and post-acetazolamide (-post) 123I-IMP and FAIR images. All plots show a significant correlation between 123I-IMP and FAIR results. A better correlation coefficient is observed between the findings on pre-acetazolamide 123I-IMP images and FAIR images obtained with a TI of 1400 milliseconds. On post-acetazolamide images, a higher correlation is observed between 123I-IMP images and FAIR images obtained with a TI of 1200 milliseconds.
Discussion
For more than a decade, measurements of rCBF and perfusion images have been obtained primarily with SPECT or PET because these imaging methods can be used to predict cerebral perfusion at almost physiologic levels with a negligible contribution from intravascular blood. Because rCBF values measured with 123I-IMP images had a good correlation with that those obtained with radioactive microsphere or 133Xe studies (1–5), rCBF measured at 123I-IMP imaging was considered the standard for this investigation. However, the spatial resolution of SPECT is lower than that of MR imaging, and because of this advantage of unique spatial resolution, new MR sequences are being developed to enable the measurement of cerebral perfusion along with its anatomic details. Contrast-enhanced perfusion MR imaging is being used to quantify perfusion in the brain. However, to our knowledge, no acceptable level of success has been achieved, because perfusion MR imaging renders images of mostly cerebral blood volume rather than cerebral blood flow (19, 20). Recently, from work related to functional imaging, FAIR and other similar imaging sequences have been developed to measure perfusion changes (9–13). In a recent report (21), the ratio of signal intensities on FAIR images were significantly correlated with the corresponding ratio of rCBF values at 123I-IMP imaging (21). However, to our knowledge, no correlation of the quantitative rCBF values measured on FAIR and 123I-IMP studies has been published.
The improvement in the ROC analysis regarding the detection of lesions on acetazolamide-stress FAIR images by all readers indicates the usefulness of the post-acetazolamide FAIR study. Nonsignificant differences among Az values for images with different TIs and among readers also indicate nonsignificant interobserver variability. Although the Az values of composite ROC curves did not significantly differ among the FAIR images obtained with different TIs, the significantly higher sensitivity in the detection of hypoperfused segments on post-acetazolamide FAIR images with a TI of 1400 milliseconds indicates its advantage compared with images obtained with a TI of 1200 milliseconds. The higher specificity and accuracy of FAIR images obtained with all TIs indicate that FAIR images acquired with proper TIs may be complementary investigation of cerebral perfusion.
When rCBF was quantitatively calculated from FAIR images, a similar cerebral perfusion value was observed in normally perfused segments between FAIR (TI, 1200 milliseconds) and 123I-IMP images before and after acetazolamide stress. Although the rCBF was higher on FAIR images obtained with a TI of 1400 milliseconds than that on 123I-IMP images, the significant correlation indicated that FAIR images acquired with a TI of 1400 milliseconds was equally useful in the measurement of rCBF. In nuclear medicine, various methods and modalities (PET or SPECT) are being used to assess rCBF, but the established normal range of values for each modality and method are different (6, 8, 22) despite a significant correlation among them. In spite of differences in rCBF with TIs of 1200 and 1400 milliseconds, FAIR images obtained with both could be used to assess rCBF. However, a normal range of rCBF values should be established with different TIs for each MR system.
Although the ratios of intensity on FAIR and 123I-IMP images were correlated in a previous study (21), absolute rCBF values was not measured on FAIR images. In this study, rCBF values assessed with both FAIR and 123I-IMP images obtained before and after acetazolamide administration were measured quantitatively. The significant correlation (although correlation coefficients were relatively low) of rCBF values among the 123I-IMP and FAIR images also indicates the usefulness and complementary role of FAIR imaging in the depiction normally perfused and hypoperfused segments in the brain. As we expected and as others hypothesized (14), a better correlation coefficient was observed with a longer TI (1400 milliseconds) on the pre-acetazolamide FAIR images. This result may reflect perfusion rather than the intravascular blood volume, but the decrease in the correlation coefficient with the post-acetazolamide FAIR images acquired with an TI of 1400 milliseconds might have been due to the earlier acquisition of images (at 8 minutes) after acetazolamide administration, when the vessels might not have been optimally dilated. The increase in the correlation coefficient on FAIR images obtained with a TI of 1200 milliseconds (image acquisition after 12 minutes in the administration of acetazolamide) indicates that the late acquisition of images with an TI of 1400 milliseconds would have resulted in a better correlation coefficient. Although findings from the FAIR and 123I-IMP images were significantly correlated, the random distribution of the data and relatively low correlation coefficient may have been due to the effect of the signal intensity from large vessels on the FAIR images. Any technique that eliminates the signal intensity from large vessels on FAIR images may provide more accurate rCBF assessment.
The linear high signal intensity on FAIR images in the hypoperfused segments (Fig 3) might have been due to delayed flow or flow through collateral vessels that corresponded to the time of acquisition (TIs). In all cases, these linear high signal intensities in the hypoperfused segments were easily identified and could be separated from flow through small arterioles and capillary beds in the tissue. Although signals from larger arteries at peripheral parts were not included within the ROI, these linear signals influenced the rCBF values calculated from average signal intensity in an ROI. The addition of a sequence with FAIR imaging to suppress flow effects might eliminate this problem. The development of software to eliminate high signal intensities by means of threshold (upper threshold) fitting may be useful.
Although we calculated absolute rCBF values from the FAIR images by using two TIs, the number of patients enrolled in this study was small. Healthy volunteers and those without cerebrovascular disease were not included to measure their rCBF values with both FAIR and 123I-IMP imaging. A further investigation with larger series of patients, volunteers, and/or animal models is needed to more completely validate these findings. Moreover, to validate the rCBF values measured from FAIR images against the criterion standard, further studies with xenon, microspheres, or PET are important.
Conclusion
Significant correlation with 123I-IMP results, higher specificity and accuracy in detecting hypoperfused segments, similar increases in flow on both post-acetazolamide images, and absence of the need for contrast enhancement suggest that FAIR imaging, in addition to nuclear medicine technology, may be complementary to routine MR imaging in the assessment of cerebral perfusion.
References
- 1.McHenry LC Jr, Merory J, Bass E, et al. Xenon-133 inhalation method for regional cerebral blood flow measurements: normal values and test-retest results. Stroke 1978;9:396–399 [DOI] [PubMed] [Google Scholar]
- 2.Nakano S, Kinoshita K, Jinnouchi S, Hoshi H, Watanabe K. Comparative study of regional cerebral blood flow images by SPECT using xenon-133, iodine-123 IMP, and technetium-99m HM-PAO. J Nucl Med 1989;30:157–164 [PubMed] [Google Scholar]
- 3.Nakano S, Kinoshita K, Jinnouchi S, Hoshi H, Watanabe K. Critical cerebral blood flow thresholds studied by SPECT using xenon-133 and iodine-123 iodoamphetamin. J Nucl Med 1989;30:337–342 [PubMed] [Google Scholar]
- 4.Greenberg JH, Kushner M, Rango M, Alavi A, Reivich M. Validation studies of iodine-123-iodoamphetamine as a cerebral blood flow tracer using emission tomography. J Nucl Med 1990;31:1364–1369 [PubMed] [Google Scholar]
- 5.Matsuda H, Higashi S, Tsuji S, et al. A new noninvasive quantitative assessment of cerebral blood flow using N-isopropyl-(iodine 123)p-iodoamphetamine. Am J Physiol Imaging 1987;2:49–55 [PubMed] [Google Scholar]
- 6.Ohkubo M, Odano I, Takahashi M. A comparative study of simple methods to measure regional cerebral blood flow using iodine-123-IMP SPECT. J Nucl Med 1997;38:597–601 [PubMed] [Google Scholar]
- 7.Iida H, Akutsu T, Endo K, et al. A multicenter validation of regional cerebral blood flow quantitation using [123I]iodoamphetamine (IMP) and single photon emission computed tomography. J Cereb Blood Flow Metab 1996;16:781–793 [DOI] [PubMed] [Google Scholar]
- 8.Iida H, Itou H, Nakazawa M, et al. Quantitative mapping of regional cerebral blood flow using iodine-123-IMP and SPECT. J Nucl Med 1994;35:2019–2030 [PubMed] [Google Scholar]
- 9.Kim SG. Quantification of relative cerebral blood flow change by flow-sensitive alternating inversion recovery (FAIR) technique: application to functional mapping. Magn Reson Med 1995;34:293–301 [DOI] [PubMed] [Google Scholar]
- 10.Roberts DA, Detre JA, Bolinger L, Insko EK, Leigh JS Jr. Quantitative magnetic resonance imaging of human brain perfusion at 1.5 T using steady-state inversion of arterial water. Proc Natl Acad Sci U S A 1994;91:33–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edelman RR, Siewert B, Darby DG, et al. Quantitative mapping of cerebral blood flow and functional localization with echo-planar MR imaging and signal targeting with alternating radio frequency. Radiology 1994;192:513–520 [DOI] [PubMed] [Google Scholar]
- 12.Chen Q, Siewert B, Bly BM, Warach S, Edelman RR. STAR-HASTE: perfusion imaging without magnetic susceptibility artifact. Magn Reson Med 1997;38:404–408 [DOI] [PubMed] [Google Scholar]
- 13.Kwong KK, Chesler DA, Weisskoff RM, et al. MR perfusion studies with T1-weighted echo planar imaging. Magn Reson Med 1995;34:878–887 [DOI] [PubMed] [Google Scholar]
- 14.Kim SG, Tsekos NV. Perfusion imaging by a flow-sensitive alternating inversion recovery (FAIR) technique: application to functional brain imaging. Magn Reson Med 1997;37:425–435 [DOI] [PubMed] [Google Scholar]
- 15.Shirouzu I, Machida T, Noda M, et al. Cerebral perfusion imaging with acetazolamide challenge using FAIR in arterial occlusive cerebrovascular diseases [abstr]. Radiology 1999;213:54510551239 [Google Scholar]
- 16.Metz CE. ROC methodology in radiologic imaging. Invest Radiol 1986;21:720–733 [DOI] [PubMed] [Google Scholar]
- 17.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982;143:29–36 [DOI] [PubMed] [Google Scholar]
- 18.Metz CE, Herman BA, Roe CA. Statistical comparison of two ROC-curve estimations obtained from partially-paired datasets. Med Decis Making 1998;18:110–121 [DOI] [PubMed] [Google Scholar]
- 19.Ernst T, Chang L, Itti L, Speck O. Correlation of regional cerebral blood flow from perfusion MRI and SPECT in normal subjects. Magn Reson Imaging 1999;17:349–354 [DOI] [PubMed] [Google Scholar]
- 20.Muller TB, Jones RA, Haraldseth O, Westby J, Unsgard G. Comparison of MR perfusion imaging and microsphere measurements of regional cerebral blood flow in a rat model of middle cerebral artery occlusion. Magn Reson Imaging 1996;14:1177–1183 [DOI] [PubMed] [Google Scholar]
- 21.Arbab AS, Aoki S, Toyama K, et al. Brain perfusion measured by flow-sensitive alternating inversion recovery (FAIR) and dynamic susceptibility contrast-enhanced magnetic resonance imaging: comparison with nuclear medicine technique. Eur Radiol 2001;11:635–641 [DOI] [PubMed] [Google Scholar]
- 22.Koyama M, Kawashima R, Ito H, et al. SPECT imaging of normal subjects with technetium-99m-HMPAO and technetium-99m-ECD. J Nucl Med 1997;38:587–592 [PubMed] [Google Scholar]