Abstract
Background and aim:
A rectal ointment containing 3% of sucralfate and herbal extracts (calendula, witch hazel leaf (hamamelis), chamomile), became available in Italy in 2019 for the treatment of symptoms associated with haemorrhoidal disease. This survey evaluated the effect of the mentioned sucralfate ointment, on quality of life (QoL) and symptom frequency in participants seeking treatment for haemorrhoidal disease from community pharmacies in Italy.
Methods:
EMOCARE was a multicentre prospective survey conducted at community pharmacies in Italy. Eligible participants (≥18 years) were those with haemorrhoidal symptoms in the last 7 days and were willing to initiate a treatment with the sucralfate ointment and herbal extracts (calendula, witch hazel leaf (hamamelis), chamomile). A survey was administered by the investigating pharmacists at the beginning and end (~14 days) of treatment. The primary endpoint was the change in HEMO-FISS-QoL scores.
Results:
Of the 290 (mean age 53.1 years old; 58.3% female) enrolled, 287 attended the follow-up visit. After a mean duration of 13 days, the sucralfate ointment significantly improved total HEMO-FISS-QoL scores (mean change from baseline: –10.41; 95%CI –11.95, –8.86; P<0.001) and mean scores for all domains of the HEMO-FISS-QoL scale (–11.13 [95%CI –12.95, –9.30] for physical disorders, –6.14 [95%CI –7.42, –4.85] for psychology, –18.79 [95% CI –21.67, –15.90] for defaecation, and –6.46 [95%CI –8.40, –4.51] for sexuality; all P<0.001 versus baseline). At the end of treatment, 39.4% of participants reported that they no longer had haemorrhoidal symptoms and the frequency of all assessed symptoms were reduced significantly from baseline (all P<0.05).
Conclusions:
After a mean 13 days of treatment the sucralfate ointment with herbal extracts improved HEMO-FISS-QoL scores and reduced symptoms in people with haemorrhoidal disease. (www.actabiomedica.it)
Keywords: clinical pharmacist, community pharmacy, haemorrhoidal disease, minor disease, quality of life, sucralfate ointment
Introduction
Haemorrhoidal disease is a very common proctological condition; however, the exact prevalence is difficult to determine as many patients do not seek medical advice (1). A large international web-based survey found that, based on self-reported signs and symptoms, the estimated prevalence of haemorrhoidal disease was 11% and only 41% of patients consulted a physician for their first step of treatment (2). Other sources of treatment information included pharmacies and healthcare stores, medical websites or forums, and asking friends or relatives.
Common symptoms of haemorrhoidal disease include pain, anal bleeding, discomfort, itching, swelling, rectal prolapse, soiling and faecal incontinence (1, 2). For early-stage haemorrhoidal disease, treatment is generally aimed at controlling or relieving symptoms, rather than curing the disease (3). Because constipation and high body mass index are often associated with the recurrence and severity of haemorrhoidal disease (1), lifestyle and dietary changes are commonly recommended; however, these changes require high patient compliance and improvements may take some time to become apparent (4).
Phlebotonic drugs have proven efficacy in treating symptoms of haemorrhoidal disease, particularly bleeding and itching (5-7). There are also a large number of topical ointments available (used by ~70% of patients as their first treatment step (2)), which are often self-prescribed by patients with the aim of achieving rapid symptom relief (4). However, there is a paucity of quality data supporting the effectiveness of many of these topical treatments (3, 4, 8), so it is important that such studies are conducted. Furthermore, prolonged use of topical agents containing anaesthetics, antiseptic and steroids may lead to sensitisation or the development of local reactions (3, 4).
Emoflon®, a medical device manufactured by Egis is a rectal ointment containing 3% of sucralfate and herbal extracts (calendula, witch hazel leaf (hamamelis), chamomile) that became available in Italy in 2019, and is intended for the treatment of symptoms associated with haemorrhoidal disease and its complications (9). The ointment creates a physical barrier on the anal epithelium covers and protects the anal epidermis, and provides care to the inflamed, itchy skin, helping to promote skin regeneration. It diminishes the drying out of the skin, which helps in wound healing, and reduces the risk of fissure and injury caused by defaecation. The aim of the EMOCARE survey was to evaluate the effect of this sucralfate ointment on quality of life (QoL) and symptom frequency in participants seeking treatment for haemorrhoidal disease from community pharmacies in Italy.
Methods
EMOCARE was a multicentre prospective survey conducted at community pharmacies in Italy. Participants were recruited by the investigating pharmacist at the pharmacy counter when a consultation on haemorrhoids and related symptoms was sought and a request for a product to manage the haemorrhoids was made. Eligible participants were male and female aged at least 18 years, had haemorrhoidal symptoms, and were willing to initiate treatment with the sucralfate ointment and herbal extracts. Once the criteria for inclusion had been verified, and the participant provided written informed consent, they were shown the survey and offered to participate.
At the beginning of the survey, pharmacists gave participants instructions on the application of the sucralfate ointment. Participants were advised that the ointment was to be applied around the anus, or inserted into the rectum in small quantities using the applicator provided. Participants were also advised to apply the ointment 1 to 2 times a day (depending on the severity of their symptoms) for approximately 14 days, or until symptom resolution. The use of the sucralfate ointment was discouraged in participants with bleeding haemorrhoids although patients could have spotting.
Participants were assessed by pharmacists at the beginning of the survey and again at the end of treatment (approximately 14 days later). The primary endpoint was the change in QoL scores from baseline to end of treatment. QoL was assessed using an Italian version of the validated HEMO-FISS-QoL questionnaire (10), which is specifically designed to evaluate QoL in people with haemorrhoidal disease and anal fissures (Supplement 1). This questionnaire consists of 23 questions in four domains (physical disorders, psychology, defaecation, sexuality). Responses can range from 1 (never) to 5 (always), where a higher score represents a worse QoL. For each question there is also a 6th option: not applicable. Participant-reported frequency (never, rarely, sometimes, very often, always) of seven predefined most common haemorrhoidal symptoms (pain, bleeding, swelling, prolapse, itching, soiling, faecal incontinence) at baseline and end of treatment, the proportion of participants who reported improved, no change or worsened symptom frequency at end of treatment, and safety were also assessed. Improved symptom frequency was defined as a reduction in the frequency of symptoms or symptom resolution while worsened symptom frequency was defined as an increase in the frequency of symptoms.
The survey was carried out in accordance with the ethical standards set out in the Declaration of Helsinki in 1964 and its subsequent amendments. The protocol was approved on December 3, 2019 by the Azienda Tutela della Salute Sardegna ethics committee. All participants provided informed consent for personal data processing, and pharmacists did not keep participant data after the survey.
Statistical analysis
Using a paired data distribution, the estimated sample size was 230 participants, which would have 90% power to detect an effect size of 4.3, the difference between the maximum and minimum value of the scores obtained in the primary endpoint (HEMO-FISS-QoL Total scores), with a standard deviation of 20 and an alpha significance level of 0.05 (two tailed). However, based on previous experience, it was anticipated that 20% of participants may withdraw from the survey, so it was decided to enrol 280 participants. There was no defined numerical target of participants to enrol from each centre, only an overall number. As such, when the total number of participants was reached, recruitment was closed regardless of the number of participants recruited at each centre.
Categorical variables are presented as frequencies, and continuous variables as mean, standard deviation (SD), 95% confidence intervals (CI), median, and minimum and maximum values. Variations in the final overall QoL score were verified with Wilcoxon’s rank sign tests. For any missing data on the HEMO-FISS-QoL total or individual domain scores (i.e. when participants have used the “not applicable” option), the value that could not be calculated was replaced by the average of the population participating in the survey (10). The changes from baseline to end of treatment in the patient-reported frequency of the most common haemorrhoidal symptoms was also assessed using a sign test. For all hypothesis testing the level of statistical significance was P<0.05. The analyses were performed using the statistical processing software SPSS version 20 (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.).
Results
Participant demographics and baseline characteristics
Between 16 December 2019 and 5 July 2020, 290 participants were enrolled at 36 pharmacies, and 287 attended the follow-up visit. Participants were aged between 19 and 96 (mean [SD] 53.1 [15.9]) years, and 58.3% were female (Table 1). Concomitant constipation was reported by 132 participants (45.5%). Just over half (52.4%) of the participants had consulted a doctor for haemorrhoidal disease, and 21.4% reported using laxatives. Of the 115 participants (39.7%) using systemic treatment for haemorrhoidal disease, 92 (80%) were taking micronized purified flavonoid fraction at baseline. Furthermore, during the survey period, five participants (1.7%) used other topical haemorrhoid treatments in addition to the sucralfate ointment.
Table 1.
Baseline patients’ demographics and characteristics
| Characteristics | N = 290 |
| Female, n (%) | 169 (58.3) |
| Age, years | |
| Mean (SD) | 53.1 (15.9) |
| Median (range) | 53.0 (19–96) |
| Concomitant constipation, n (%) | |
| Never | 142 (49.0) |
| <18 months | 39 (13.4) |
| 19 months to 5 years | 31 (10.7) |
| >5 years | 62 (21.4) |
| Don’t know | 16 (5.5) |
| Consulted a doctor for haemorrhoids, n (%) | 152 (52.4) |
| Laxative use, n (%) | 62 (21.4) |
| Systemic haemorrhoid treatment, n (%) | 115 (39.7) |
| SD, standard deviation | |
The mean duration of treatment with the sucralfate ointment was 13 days, and the average number of applications was 1.6/day.
Quality of Life
QoL data were available for all 290 participants at baseline and for 287 patients at follow-up. With sucralfate ointment use, the HEMO-FISS-QoL mean overall score and the mean score for all domains of the scale significantly improved (P<0.001) from baseline to end of treatment (Fig. 1). Mean change from baseline in scores was –11.13 (95%CI –12.95, –9.30) for the physical disorders domain, –6.14 (95%CI –7.42, –4.85) for the psychology domain, –18.79 (95% CI –21.67, –15.90) for the defaecation domain, –6.46 (95%CI –8.40, –4.51) for the sexuality domain, and –10.41 (95%CI –11.95, –8.86) for the overall QoL score. The improvements in mean QoL scores remained significant (P<0.001) when participants who used additional topical haemorrhoid treatments were excluded from the analysis (data not shown).
Figure 1.
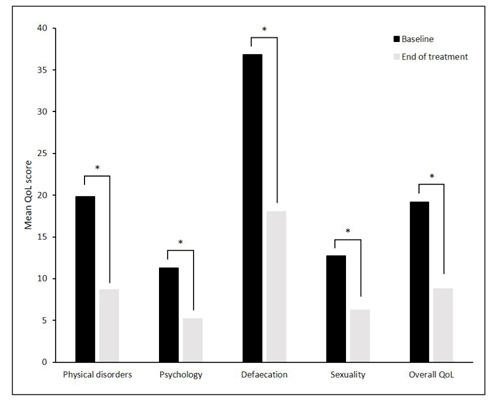
Change from baseline to end of treatment in mean quality of life (QoL) scores. A reduction in score indicates an improvement. *P<0.001.
Effectiveness
Per protocol, at baseline all participants had haemorrhoidal symptoms in the past 7 days. The frequencies of these symptoms are outlined in Table 2. Overall, the most common symptoms were pain and swelling, both reported (at any frequency) by >80% of participants.
Table 2.
Frequency of symptoms reported at baseline (N=290)
| Symptom | Frequency, n (%) | ||||
| Never | Rarely | Sometimes | Very often | Always | |
| Pain | 50 (17.2) | 54 (18.6) | 89 (30.7) | 66 (22.8) | 31 (10.7) |
| Bleeding | 120 (41.4) | 63 (21.7) | 67 (23.1) | 31 (10.7) | 9 (3.1) |
| Swelling | 51 (17.6) | 42 (14.5) | 91 (31.4) | 70 (24.1) | 36 (12.4) |
| Prolapse | 163 (56.2) | 29 (10.0) | 51 (17.6) | 22 (7.6) | 25 (8.6) |
| Itching | 91 (31.4) | 43 (14.8) | 70 (24.1) | 56 (19.3) | 30 (10.3) |
| Soiling | 207 (71.4) | 33 (11.4) | 34 (11.7) | 12 (4.1) | 4 (1.4) |
| Faecal incontinence | 261 (90.0) | 16 (5.5) | 4 (1.4) | 7 (2.4) | 2 (0.7) |
At the end of treatment, of the 287 participants available for follow-up, 113 (39.4%) reported that they no longer had haemorrhoidal symptoms. Furthermore, with the sucralfate ointment use, the frequency of all assessed symptoms was reduced significantly from baseline (Table 3). For pain, 72.1% of participants reported they were improved, 23.3% reported no change and 4.5% had worsened symptom frequency (P<0.001). Respective values for swelling were an improvement in 68.3% of patients, no change in 25.8%, and worsening in 5.9% (P<0.001, Table 3). Itching improved in 53.3% of patients, did not change in 36.2%, and worsened in 10.5% (P<0.001, Table 3).
Table 3.
Change in the frequency of symptoms from baseline to end of treatment (N=287)
| Symptom | Change from baseline, n (%) | P-value | ||
| Improved | No change | Worsened | ||
| Pain | 207 (72.1) | 67 (23.3) | 13 (4.5) | <0.001 |
| Bleeding | 134 (46.7) | 139 (48.4) | 14 (4.9) | <0.001 |
| Swelling | 196 (68.3) | 74 (25.8) | 17 (5.9) | <0.001 |
| Prolapse | 95 (33.1) | 182 (63.4) | 10 (3.5) | <0.001 |
| Itching | 153 (53.3) | 104 (36.2) | 30 (10.5) | <0.001 |
| Soiling | 71 (24.7) | 206 (71.8) | 10 (3.5) | <0.001 |
| Faecal incontinence | 21 (7.3) | 259 (90.2) | 7 (2.4) | 0.014 |
For all assessed symptoms, the proportion of participants reporting any frequency of symptoms was reduced from baseline to the end of treatment (Fig. 2A), as was the proportion reporting the frequency as ‘always’ or ‘very often’ (Fig. 2B). The number of participants using systemic treatment decreased from 39.7% baseline to 23.7% after the sucralfate ointment use.
Figure 2.
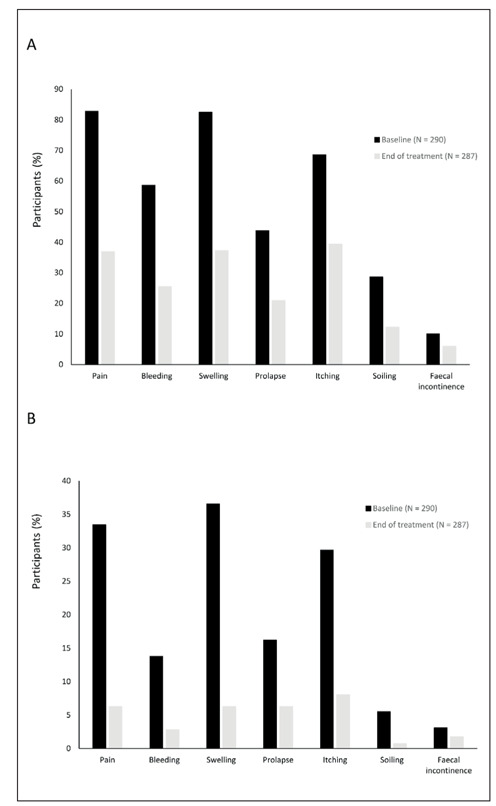
Change from baseline to end of treatment in the percentage of participants reporting symptoms A) of any frequency (rarely, sometimes, very often or always) and B) very often or always.
Ease of use
The majority of participants (>92%) found the application of the sucralfate ointment very easy, easy or neither easy nor hard to use. Just over half of participants (53.3%) used the provided applicator and almost all participants (94.4%) found the consistency of the sucralfate ointment ‘absolutely’ or ‘quite’ appropriate for use.
Safety
Of the participants available for follow-up, four reported burning (which was described as severe by two participants and associated with pain in another) possibly associated with the sucralfate ointment and one patient each reported a sensation of warmth and rash. Overall, six (2.1%) participants reported one of these conditions. None of these were considered serious; four conditions resolved upon discontinuation of treatment and two resolved spontaneously.
Discussion
The prospective, multicentre EMOCARE survey showed that the sucralfate ointment and herbal extracts (calendula, witch hazel leaf (hamamelis), chamomile) significantly improved QoL and reduced proctological symptoms in participants with haemorrhoidal disease. The baseline QoL scores show that the impact of haemorrhoidal disease on the QoL of the participants included in this survey was relatively low, although the ‘defaecation’ domain was more severely impacted than the other three domains. This result possibly indicates that the type of participants who self-prescribe or seek help from a pharmacist do not have severe or debilitating symptoms; however, this assumption cannot be confirmed as our survey was not designed to include grading of the severity of haemorrhoidal disease or its symptoms. Nevertheless, treatment with the sucralfate ointment for approximately 14 days significantly improved the mean total HEMO-FISS-QoL score, as well as the mean scores for each of the four individual domains.
Per protocol, at baseline all participants in our survey had had haemorrhoidal disease symptoms in the past 7 days. Symptoms (of any frequency) reported by >50% of participants at baseline were pain (82.8%), swelling (82.4%), itching (68.6%) and bleeding (58.6%). Although at different incidences, these four symptoms were also among the most common in an international web-based survey of 1725 participants with self-reported haemorrhoidal disease (pain [60%], bleeding [47%], discomfort [43%], itching [35%] and swelling [33%] (2)) and the most common in the large international CHORUS study (Chronic venous and HemORrhoidal diseases evalUation and Scientific research, N=5617). In the latter study, bleeding was reported by 71.8% of patients, pain by 67.4%, swelling by 55.0% and itching by 44.1% (1). The difference in incidence of these four main symptoms in CHORUS and our survey may be attributable to different patient characteristics due to differing enrolment criteria and patient demographics. CHORUS enrolled patients who consulted a physician, rather than a pharmacist, for haemorrhoidal disease complaints. Our survey was conducted in Italy, whereas CHORUS included patients from Russia, India, Pakistan, Mexico, Slovenia, Thailand and Belgium (1). A higher proportion of patients in CHORUS reported bleeding when consulting their physician than those in our survey who sought help from a pharmacist, which seems to confirm our assumption that participants in our study were less likely to have severe symptoms. In our survey, at the end of treatment with the sucralfate ointment , 39.4% of participants no longer had any haemorrhoidal disease symptoms, and the frequency of all assessed symptoms had decreased significantly. The proportion of participants reporting very frequent symptoms (very often or always) was also reduced for all assessed symptoms; with very frequent pain reduced from 33.5% to 6.3% of participants, swelling from 36.6% to 6.3%, and itching from 29.67% to 8.0%. This is the first set of data to describe the potential effectiveness of a 3% sucralfate-containing ointment in people with self-reported haemorrhoid symptoms; most evidence to date of its use is to alleviate pain in the post-surgical setting (after haemorrhoidectomy (11-13)) with a higher concentration of sucralfate.
As one would expect, treatment adherence is a key factor in achieving good therapeutic outcomes (14). In EMOCARE, participants applied the sucralfate ointment an average of 1.6 times a day, which is close to the optimal treatment of 1 to 2 applications/day. This good treatment adherence may in part be explained by the fact that >90% of participants had no difficulties applying the sucralfate ointment and found the consistency of the product appropriate, as these factors have been shown to impact compliance with topical treatments (15).
Patients with haemorrhoidal disease often self-medicate with topical treatments to achieve symptom relief; however, prolonged use of these treatments could cause local reactions or skin sensitization, especially when they contain corticosteroids or anaesthetics (3). In EMOCARE, 2.1% of participants reported pain, a sensation of burning/warmth and rash; none of which were considered serious and all resolved upon discontinuation of treatment or spontaneously. The safety of prolonged use of the sucralfate ointment, i.e. beyond the recommended 14-day period, will need to be further investigated.
In addition to suggesting that the sucralfate ointment is an effective treatment for the symptoms of haemorrhoidal disease, the EMOCARE survey corroborated the clinical profile of patients reported in other studies. As shown previously in patients with haemorrhoidal disease (1, 16), constipation was common in EMOCARE, afflicting almost half of the participants. Additionally, just over half of EMOCARE participants had consulted a physician for haemorrhoidal disease, which is in line with other studies showing that people with haemorrhoidal disease often do not seek, or delay seeking, treatment from doctors (2, 16).
There are some limitations to this survey. As EMOCARE was designed to mirror normal clinical practice in pharmacies, a control group was not included, making it difficult to draw firm conclusions regarding the effectiveness of the sucralfate ointment. In addition, the pharmacists involved in the survey were not equipped to make a diagnosis, meaning some participants may have incorrectly self-diagnosed haemorrhoidal disease. A further limitation is imputation of data for missing values. Nevertheless, our survey results reflect a real-world population of participants seeking treatment with over-the-counter topical agents, and was conducted in the community pharmacy setting, opening a new way for the clinical pharmacist to be involved in the conduct of clinical investigations of medicines. Finally, as with all investigations of this nature, there may be some selection bias due to how participants were recruited for this survey.
Conclusions
The results of this Italian multicentre prospective survey, EMOCARE, conducted at community pharmacies suggest that the treatment with a sucralfate ointment and herbal extracts (calendula, witch hazel leaf (hamamelis), chamomile) used for around 2 weeks improves QoL, reduces symptoms, and is safe for use in people with symptoms associated with haemorrhoidal disease. Furthermore, treatment adherence was good, and participants’ qualitative feedback regarding use of the product was positive. Further controlled studies would be useful to confirm these results.
Acknowledgements
We would like to thank Toni Dando who wrote the outline on behalf of Springer Healthcare Communications and Simone Tait of Springer Healthcare Communications who wrote the first draft. This medical writing assistance was funded by Servier.
Founding:
The EMOCARE survey was sponsored by Servier Italia
Author contributions:
C.G. and A.P.contributed to the study design. N.F., F.R., and the SGCP collaborators enrolled participants. L.M performed the statistical analyses. C.G., A.P., N.F., F.R., and L.M. read and approved all drafts of the manuscript.
Conflict of Interest:
C.G., L.M., A.P., N.F., and F.R. have no conflicts of interest to declare.
Collaborators:
SIFAC Group of Clinical Community Pharmacists (SGCP): Matteo Baio, Lisa Boschetti, Cesare Cecchini, Benedetta Fofi, Valentina Gianotti, Enrico Keber, Alberto Lepore, Stefano Miggos, Michele Modugno, Margherita Tafuri and Giusi Arcifa, Michela Barbisan, Francesco Benetti, Alfredo Carruba, Maria Laura Deidda, Giuseppe Di Bonaventura, Ivonne Falcione, Greta Giannantonio, Eleonora Giorgino, Piera Loiacono, Andrea Lunardini, Silvia Magagnotto, Francesca Marson, Andrea Marzini, Rita Meloni, Agnese Offidani, Dario Oggioni, Margherita Parisi, Benedetta Poggetti, Lucia Sacco, Filippos Spyridon Sagias, Cristiano Tavani, Lorenzo Turati, Concetta Vazzana, Diego Zorzetto
References
- 1.Godeberge P, Sheikh P, Zagriadskiĭ E, et al. Hemorrhoidal disease and chronic venous insufficiency: Concomitance or coincidence; results of the CHORUS study (Chronic venous and HemORrhoidal diseases evalUation and Scientific research) J Gastroenterol Hepatol. 2020;35(4):577–85. doi: 10.1111/jgh.14857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheikh P, Régnier C, Goron F, Salmat G. The prevalence, characteristics and treatment of hemorrhoidal disease: results of an international web-based survey. J Comp Eff Res. 2020;9(17):1219–32. doi: 10.2217/cer-2020-0159. [DOI] [PubMed] [Google Scholar]
- 3.Gallo G, Martellucci J, Sturiale A, et al. Consensus statement of the Italian society of colorectal surgery (SICCR): management and treatment of hemorrhoidal disease. Tech Coloproctol. 2020;24(2):145–64. doi: 10.1007/s10151-020-02149-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altomare DF, Giannini I. Pharmacological treatment of hemorrhoids: a narrative review. Expert Opin Pharmacother. 2013;14(17):2343–9. doi: 10.1517/14656566.2013.836181. [DOI] [PubMed] [Google Scholar]
- 5.Alonso-Coello P, Zhou Q, Martinez-Zapata MJ, et al. Meta-analysis of flavonoids for the treatment of haemorrhoids. Br J Surg. 2006;93(8):909–20. doi: 10.1002/bjs.5378. [DOI] [PubMed] [Google Scholar]
- 6.Perera N, Liolitsa D, Iype S, et al. Phlebotonics for haemorrhoids. Cochrane Database Syst Rev. 2012;8:CD004322. doi: 10.1002/14651858.CD004322.pub3. [DOI] [PubMed] [Google Scholar]
- 7.Sheikh P, Lohsiriwat V, Shelygin Y. Micronized Purified Flavonoid Fraction in Hemorrhoid Disease: A Systematic Review and Meta-Analysis. Adv Ther. 2020;37(6):2792–812. doi: 10.1007/s12325-020-01353-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amaturo A, Meucci M, Mari FS. Treatment of haemorrhoidal disease with micronized purified flavonoid fraction and sucralfate ointment. Acta Biomed. 2020;91(1):139–41. doi: 10.23750/abm.v91i1.9361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Servier Italia S.p.A. Emoflon™ pomata rettale - Istruzioni per l’Uso [in Italian] https://emoflon.it/Content/documents/emoflon-foglietto-illustrativo.pdf . Accessed 26 November 2020. [Google Scholar]
- 10.Abramowitz L, Bouchard D, Siproudhis L, et al. Psychometric properties of a questionnaire (HEMO-FISS-QoL) to evaluate the burden associated with haemorrhoidal disease and anal fissures. Colorectal Dis. 2019;21(1):48–58. doi: 10.1111/codi.14393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vejdan AK, Khosravi M, Amirian Z, et al. Evaluation of the efficacy of topical sucralfate on healing haemorrhoidectomy incision wounds and reducing pain severity: a randomised clinical trial. Int Wound J. 2020;17(4):1047–51. doi: 10.1111/iwj.13369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta PJ, Heda PS, Kalaskar S, Tamaskar VP. Topical sucralfate decreases pain after hemorrhoidectomy and improves healing: a randomized, blinded, controlled study. Dis Colon Rectum. 2008;51(2):231–4. doi: 10.1007/s10350-007-9092-4. [DOI] [PubMed] [Google Scholar]
- 13.Ala S, Saeedi M, Eshghi F, et al. Efficacy of 10% sucralfate ointment in the reduction of acute postoperative pain after open hemorrhoidectomy: a prospective, double-blind, randomized, placebo-controlled trial. World J Surg. 2013;37(1):233–8. doi: 10.1007/s00268-012-1805-8. [DOI] [PubMed] [Google Scholar]
- 14.DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: a meta-analysis. Med Care. 2002;40(9):794–811. doi: 10.1097/00005650-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Zschocke I, Mrowietz U, Lotzin A, Karakasili E, Reich K. Assessing adherence factors in patients under topical treatment: development of the Topical Therapy Adherence Questionnaire (TTAQ) Arch Dermatol Res. 2014;306(3):287–97. doi: 10.1007/s00403-014-1446-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tournu G, Abramowitz L, Couffignal C, et al. Prevalence of anal symptoms in general practice: a prospective study. BMC Fam Pract. 2017;18(1):78. doi: 10.1186/s12875-017-0649-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


