Abstract
SUMMARY: A programmable CSF shunt valve was assessed for magnetic field interactions, heating (transmit-receive body radio-frequency coil; whole-body averaged specific absorption rate, 2.1 W/kg), functional alterations, and artifacts at 3T. The programmable valve showed minor magnetic field interactions and heating was not excessive (+0.8°C). The function of the programmable valve was not altered by multiple exposures to the 3T scanner or from exposure to various MR imaging conditions. Therefore, this implant is safe for a patient undergoing MR imaging at 3T or less when the radiologist follows specific safety guidelines. Artifacts for the programmable valve were relatively large in relation to the size and shape of the valve; this finding may impact the diagnostic use of MR imaging if the area of interest is in proximity to this implant.
CSF shunts are frequently used to treat hydrocephalus. The shunt is positioned to enable CSF to be drained from the cerebral ventricles or subarachnoid spaces into another absorption site (eg, the right atrium of the heart or the peritoneal cavity) through a system of small flexible catheters.1 A valve is often inserted into the catheter pathway to keep CSF flowing away from the brain and to moderate the pressure or flow rate.1 Some valves are fixed pressure valves (ie, monopressure valves), whereas others are adjustable or programmable valves.1–6 The use of a programmable valve allows the surgeon to noninvasively change the opening pressure, negating the need for revision surgery to alter the valve pressure.2–6 Furthermore, the programmability of the valve may allow the patient to undergo a specialized or individualized treatment regime.
Most programmable valves incorporate a magnetically activated component, which could be problematic for patients subjected to MR imaging, especially if performed at 3T.7,8 In fact, there are well-known electromagnetic field–related hazards that involve programmable valves.9,10 Exposure to the powerful magnetic field of the scanner could move the valve, alter the programmed settings, or permanently damage the device.3–10 Excessive MR imaging–related heating and substantial artifacts are also of concern for metallic implants, such as magnetically activated devices.7,8 Therefore, this study evaluated magnetic field interactions, heating, function, and artifacts for a programmable CSF shunt valve relative to the use of a 3T MR imaging system.
Materials and Methods
Programmable Valve
The programmable valve (proGAV, Aesculap, Inc., Center Valley, Pa) evaluated in this study consists of a circular chamber with inlet and outlet connection ports at opposite ends. The inlet is the location of the valve, which has a ball and cone design with a leaf spring mechanism. The spring force on the ball dictates the opening pressure of the valve. The spring tension (and, thus, the pressure) is controlled by a center-mounted rotor with small magnets on either side. Rotation of the magnets in the wheel changes the pressure setting. The setting is locked by a braking mechanism that prohibits the wheel from rotating. The brake can be released by external pressure applied to a detent pin in the center that allows the wheel to rotate as the inner magnets align themselves with the external alignment device. The external alignment device is a manual device similar in appearance to a pen. There is a center-mounted rod that is used to unlock the breaking mechanism inside this programmable shunt valve. There are 2 magnets in the external device, which can be manually set to realign the magnets. The user selects the desired pressure setting on the external device, places it next to the patient’s skin centered directly over the programmable shunt, and pushes the center button (connected to the rod). Another external device is used to verify that the desired pressure has been set. Materials for this programmable shunt valve include titanium alloy (TiAl6V4) for the housing, rotor, and spring; a 2-mm sapphire ball (sapphire Al203), and small magnets (NdFeB) 1 mm in diameter and 1.30 mm in length, integrated in the valve rotor (Fig 1).
Fig 1.
The programmable valve that underwent evaluation for MR imaging safety at 3T.
Magnetic Field Interactions
Magnetic field interactions were determined for the programmable valve by using a shielded 3T MR imaging system (Magnetom Trio, Siemens Medical Solutions, Malvern, Pa; active-shielded horizontal field scanner).
Translational Attraction.
Translational attraction was assessed for the programmable valve by using the deflection angle method, as previously described.11–14 The programmable valve was attached to a test fixture to measure the deflection angle in the 3T MR imaging system. The test fixture has a protractor with 1° graduated markings mounted to the top of the structure and included a bubble level to ensure proper orientation in the scanner for the test procedure. The programmable valve was suspended from the protractor by a 20-cm length of string (weight, <1% of the weight of the implant) that was attached at the 0° indicator.11–14 Measurements were obtained at the position in the 3T MR imaging system that produced the greatest magnetically induced deflection angle.11–15 This point was determined by using gauss line plots, magnetic field measurements, and visual inspection to identify the location of the highest spatial gradient. For the 3T MR imaging system, the direction of the static magnetic field is horizontal, and the highest spatial gradient, 525 gauss/m, occurs at a position that is 78 cm from the isocenter. The programmable valve was held on the test fixture so that the string was vertical and then released. The deflection angle from the vertical direction to the nearest 1° was measured 3 times, and an average value was calculated.11–15
Torque.
Magnetic field–induced torque was assessed qualitatively for the programmable valve by using previously described methodology.12,13 This procedure used a flat plastic material with a millimeter grid on the bottom. The programmable valve was placed on the test platform in an orientation that was 45° relative to the static magnetic field of the 3T scanner. The test apparatus with the programmable valve was then positioned in the center of the scanner, where the effect of torque is the greatest (based on the known characteristics of the 3T MR imaging system) and observed for alignment or rotation.12,13 Next, the programmable valve was moved 45° relative to its previous position and again observed for alignment or rotation. This process was repeated to encompass a 360° rotation of positions for the programmable valve in the 3T scanner. A qualitative scale was applied to the results to characterize torque12,13: 0, no torque; +1, mild or low torque, the implant slightly changed orientation but did not align to the magnetic field; +2, moderate torque, the implant aligned gradually to the magnetic field; +3, strong torque, the implant showed rapid and forceful alignment to the magnetic field; +4, very strong torque, the implant showed very rapid and very forceful alignment to the magnetic field.
MR imaging–Related Heating
An in vitro experiment was performed at 3T (Magnetom Trio, Software syngo MR 2004A, Siemens Medical Solutions, Malvern, Pa) to determine MR imaging–related heating for the programmable valve according to a previously described protocol.11–13 The programmable valve was placed to approximate its intended in vivo position in a plastic head/torso phantom (head portion: width, 16.5 cm; length, 29.2 cm; height, 16.5 cm; torso portion: width, 43.2 cm; length, 61.0 cm; height, 16.5 cm.11–13 The phantom was filled to a depth of 10 cm with a gelling agent in an aqueous solution (ie, 0.8 g/L of NaCl plus 5.85 g/L of polyacrylic acid in distilled water).11–13,16 A plastic frame placed on the bottom of the phantom had small adjustable posts that were used to position the programmable valve (Fig 2).
Fig 2.
The experimental setup used to assess MR imaging–related heating for the programmable valve, showing the implant in position on the plastic frame placed on the bottom of the head/torso phantom. Note the cables going to the fluoroptic thermometry probes.
Temperatures were recorded by using a fluoroptic thermometry system (Model 3100, Luxtron, Santa Clara, Calif). Fluoroptic thermometry probes (Model SFF, 0.5 mm in diameter) were positioned on the programmable valve to record positions on this implant that would be associated with the greatest heating during MR imaging (ie, based on results from pilot experiments) as follows: probe 1, placed in direct contact with one end of the programmable valve; probe 2, placed in direct contact with the other end of the programmable valve; and probe 3, placed in direct contact with the middle portion of the programmable valve. The positions of the fluoroptic thermometry probes were verified immediately before and after the heating experiment.
MR imaging was performed at 3T on the gelled saline-filled phantom with the programmable valve by using a transmit radio-frequency (RF) body coil to produce a relatively high level of RF energy as follows: fast (turbo) spin-echo pulse sequence; axial plane; TR/TE, 986/12 ms; echo-train length, 5; flip angle, 180°; bandwidth, 199 Hz/pixel; field of view, 40 cm; imaging matrix, 256 × 256; section thickness, 10 mm; number of section locations, 20; phase direction, anterior to posterior; transmitter gain, 180; imaging time, 15 minutes. These imaging parameters produced an MR imaging system–reported value of 2.1 W/kg for the whole-body averaged specific absorption rate (SAR). The land-marking position (ie, the center position or anatomic region for the MR imaging procedure) and section locations were selected to encompass the entire area of the programmable valve. The room temperature and bore temperature of the scanner were at a constant level throughout the heating experiment. After recording baseline temperatures (5 minutes), we performed MR imaging for 15 minutes with temperatures recorded at 10-second intervals.
Effects of MR Imaging on the Function of the Programmable Valve
Experiments were conducted to determine the effects of exposing the programmable valve to the 3T MR imaging environment (ie, the static magnetic field, alone) and to various MR imaging conditions selected to be representative of typical techniques used for clinical scanning performed at 3T. These experiments involved a range of pressure settings for multiple samples of the programmable valves, along with different orientations of the valves in relation to the MR imaging system to cover a variety of possible scenarios (Tables 1 and 2).
Table 1:
Conditions used to expose the programmable valves to the 3T MR system
| Test | Pressure Setting | Orientation | Arrow Direction |
|---|---|---|---|
| 1 | 0 | Sagittal | Up |
| 2 | 0 | Sagittal | Down |
| 3 | 0 | Axial | Up |
| 4 | 0 | Axial | Down |
| 5 | 0 | Coronal | In |
| 6 | 0 | Coronal | Out |
| 7 | 8 | Sagittal | Up |
| 8 | 8 | Sagittal | Down |
| 9 | 8 | Axial | Up |
| 10 | 8 | Axial | Down |
| 11 | 8 | Coronal | In |
| 12 | 8 | Coronal | Out |
| 13 | 20 | Sagittal | Up |
| 14 | 20 | Sagittal | Down |
| 15 | 20 | Axial | Up |
| 16 | 20 | Axial | Down |
| 17 | 20 | Coronal | In |
| 18 | 20 | Coronal | Out |
| 19 | 5 | Simulated patient | In |
| 20 | 5 | Simulated patient | Out |
Note:—All conditions were in-out × 5. There were no changes under any conditions.
Table 2:
MR imaging parameters used in the assessment of the effects of MRI at 3T on the programmable valve
| Pulse Sequence |
||||||||
|---|---|---|---|---|---|---|---|---|
| T1-SE | T2-SE | T1-FSE | T2-FSE | GRE, 3D | GFRE, 3D | GRE, MTC | EPI | |
| TR (ms) | 741 | 3000 | 700 | 5180 | 20 | 163 | 628 | 3400 |
| TE (ms) | 7 | 100 | 9 | 113 | 5 | 4 | 10 | 103 |
| Flip angle | N/A | N/A | N/A | N/A | 25 | N/A | 25 | N/A |
| Field of view (cm) | 30 | 30 | 30 | 30 | 12 | 30 | 30 | 30 |
| Matrix size | 256 × 256 | 256 × 256 | 256 × 256 | 256 × 256 | 256 × 256 | 256 × 256 | 256 × 256 | 256 × 256 |
| Section Thickness (mm) | 10 | 10 | 10 | 10 | 3 | 3 | 10 | 10 |
| Section gap (mm) | 1 | 1 | 1 | 1 | 0.6 | 0.6 | 1 | 1 |
| Imaging plane | Axial | Axial | Axial | Axial | Volume | Volume | Axial | Axial |
| Imaging time | 1:00 | 1:00 | 1:00 | 1:00 | 1:00 | 1:00 | 1:00 | 1:00 |
| SAR, whole body (W/kg) | 2.0 | 0.6 | 2.3 | 1.1 | 0.8 | 0.3 | 1.5 | 0.5 |
Note:—T1-SE indicates T1-weighted spin-echo; T2-SE, T2-weighted spin-echo; T1-FSE, T1-weighted fast spin-echo; T2-FSE, T2-weighted fast spin-echo; GRE, gradient echo; FGRE, fast gradient echo; MTC, magnetization transfer contrast; EPI, echo-planar imaging; N/A, not applicable; SAR, specific absorption rate.
Conditions for Static Magnetic Field Exposures.
Eighteen programmable valves underwent a comprehensive assessment to evaluate all functional aspects before the static magnetic field–exposure experiments. The 18 valves were then attached to a cylinder-shaped foam rubber phantom that allowed consistent orthogonal (sagittal, axial, and coronal) positioning within the 3T MR imaging system. For a given exposure scenario, all samples were placed in the same direction (ie, on the basis of the direction of the arrow indicator on the valve; 10 valves set to a higher breaking force; 8 valves set to a lower breaking force) by taping them to 2 plastic frames with double-sided tape. These frames were covered by additional plastic frames to prevent possible movement during the exposure tests (Fig 3). The 2 frames with the samples were then placed together and attached to the cylinder-shaped foam rubber phantom. The pressure settings were 0, 8, and 20 cm H2O. The orientations were axial, coronal, and sagittal. The directions of the arrows for the valves were up/down (for the axial and sagittal orientations) and in/out (for the coronal orientation). In addition, a positioning scenario was used to simulate the position of these samples on the patient (ie, typically implanted behind the patient’s ear or retroauricular region) with the pressure setting at 5 cm H2O and the arrows in the in/out directions (Fig 4). Table 1 shows a summary of the orientations used for the exposure tests used for the samples.
Fig 3.
The 18 samples of the programmable valve shown attached to the cylinder-shaped phantom in preparation for the static magnetic field exposures (sagittal orientation).
Fig 4.
The 18 samples of the programmable valve shown attached to the cylinder-shaped phantom in preparation for the static magnetic field exposures (simulated patient orientation).
For the exposure tests, the samples attached to the cylinder-shaped foam rubber phantom were placed on the patient table of the 3T MR imaging system and inserted into (ie, past the isocenter and out the back of the scanner to the farthest point) and out of (ie, approximately 0.5 m past the opening of the bore of the MR imaging system) 5 times in each of the indicated orientations shown in Table 1. After each exposure condition, functional testing was performed on each programmable valve.
Conditions for MR imaging Exposures.
Next, experiments were performed to determine the effects of various MR imaging conditions on the programmable valves (Table 2). Before exposure to the MR imaging conditions, all valves underwent functional assessment. All samples were adjusted to a pressure setting of 5 cm H2O. The plastic frames with the programmable valves were attached to a phantom by using the positioning scenario to simulate in situ placement on a patient (ie, typically implanted behind the patient’s ear) with all arrow indicators facing “in” relative to the MR imaging system. The phantom was placed in a plastic rectangular box-shaped container (40 cm, length; 30-cm, width; 22 cm, height) that was filled with gelled saline. MR imaging was conducted on the phantom with the programmable valves by using a transmit-receive body RF coil and 8 different pulse sequences, running sequentially, for approximately 1 minute per sequence (Table 2). The multiple section locations were selected to encompass all the programmable valves to ensure thorough exposure to these MR imaging conditions. Function was evaluated for the programmable valves immediately after MR imaging.
Artifacts
Artifacts were assessed for the programmable valve by performing MR imaging at 3T (Magnetom Trio, Software syngo MR 2004A) with the implant placed inside a gadolinium-doped saline fluid–filled phantom, as previously described.11–13 The programmable valve was attached to a plastic frame to facilitate positioning and MR imaging within this phantom.
MR imaging was performed by using a send-receive RF head coil and the following pulse sequence parameters11–13: T1-weighted spin-echo pulse sequence; TR/TE, 500/20 ms; matrix size, 256 × 256; section thickness, 5 mm; field of view, 24 cm; number of excitations, 2; and gradient-echo pulse sequence; TR/TE, 100/15 ms; flip angle, 30°; matrix size, 256 × 256; section thickness, 5 mm; field of view, 24 cm; number of excitations, 2. The imaging planes were oriented to encompass the long axis and short axis of the programmable valve. The frequency-encoding direction was parallel to the plane of imaging. Section locations were selected through the programmable valve from multiple scout MR images to represent the largest or worst case artifacts for this implant, as previously described.11–13 Image display parameters (ie, window and level settings, magnification, etc.) were used in a consistent manner to facilitate valid measurements of artifact size. Planimetry software was used to measure (accuracy and resolution ± 10%) the cross-sectional area of the largest artifact size for each pulse sequence and for each orientation of the section location.11–13
Results
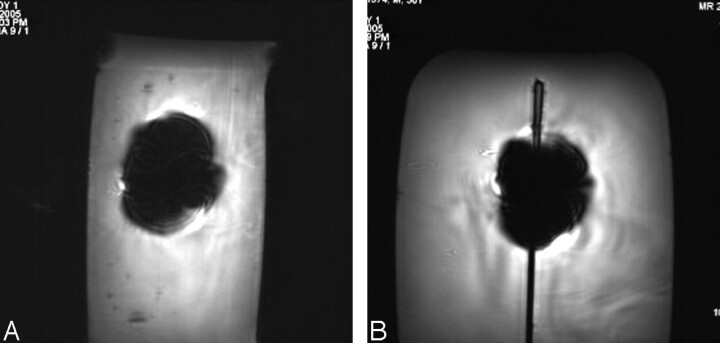
The average deflection angle was 9°, and the qualitative torque value was 0 (no torque) for the programmable valve. Findings for the MR imaging–related heating experiment showed that the highest temperature changes measured by probes 1, 2, and 3 were 0.5°C, 0.6°C, and 0.8°C, respectively. On the basis of a comparison between the pre-MR imaging and post-MR imaging functional assessment of the programmable valves, the samples were unaffected (ie, no damage and fully operational) by multiple exposures to the 3T static magnetic field and to the various MR imaging conditions. Artifact test results are summarized in Table 3. The artifacts were seen as signal intensity voids that were larger than the size and shape of the programmable valve, with the gradient-echo pulse sequence showing larger artifacts than the T1-weighted spin-echo pulse sequence (Fig 5).
Table 3:
Artifact size for the programmable valve associated with MR imaging at 3T
| Pulse Sequence | Plane Orientation* | Signal Void (mm2) |
|---|---|---|
| T1-SE | Long axis | 1,359 |
| Short axis | 1,165 | |
| GRE | Long axis | 3,483 |
| Short axis | 3,156 |
Note:—T1-SE indicates T1-weighted spin-echo; GRE, gradient echo.
Imaging plane relative to the programmable valve.
Fig 5.
Examples of MR images showing artifacts for the programmable valve (gradient-echo pulse sequence; TR/TE, 100/15; flip angle, 30°; section thickness, 5 mm; field of view, 24 cm) at 3T. A, Section location oriented to the long axis of the programmable valve. B, Section location oriented to the short axis of the programmable valve.
Discussion
Magnetic Field Interactions.
The programmable valve (proGAV) exhibited minor magnetic field interactions (9° deflection angle and no torque) in association with exposure to the 3T scanner. As such, there is no concern with regard to displacement of this device. The 9° deflection angle for the proGAV is well below the acceptable level stated by the American Society for Testing Materials International15 as follows: “… if the implant deflects less than 45°, then the magnetically induced deflection force is less than the force on the implant due to gravity (its weight).” Because no torque was shown by the qualitative test for this programmable valve, it was unnecessary to conduct a quantitative evaluation of torque.13 Notably, a patient with the proGAV would be allowed to undergo MR imaging at 3T or less immediately following implantation because of the lack of substantial magnetic field interactions.
MR Imaging–Related Heating.
The highest temperature change measured for the programmable valve during MR imaging performed at a whole-body averaged SAR of 2.1 W/kg was 0.8°C. This temperature elevation occurred in a “static” phantom model and, thus, simulates an extreme condition with regard to MR imaging–related heating for the programmable valve. This minor degree of heating will not have any physiologic consequence for a patient with the proGAV. Furthermore, limiting MR imaging procedures to a whole-body averaged SAR of 2.1 W/kg for 15 minutes or less will prevent possible excessive heating of the programmable valve with an acceptable margin of safety (most MR imaging examinations that involve the area of the body in which the proGAV is implanted can be accomplished easily without exceeding this SAR level).
Effects of MR Imaging on Function.
The results from tests involving multiple proGAV devices placed in various orientations during exposure to a 3T scanner and subjected to 8 different pulse sequences revealed that there was no apparent damage or alteration in the functional aspects of this programmable valve. Notably, the specific setting was unchanged by these conditions. By comparison, other programmable valves that use magnets have been reported to undergo accidental alterations in pressure settings after exposure to electromagnetic environments, including MR imaging systems.3–6,9,10
In fact, various reports indicate that the settings of programmable valves that incorporate magnetic mechanisms for pressure adjustments can be changed by exposure to magnetic fields,3–6,9,10 even at intensities as low as those associated with toy magnets.9 Obviously, an unwanted valve setting could cause either rapid and excessive increases in intracranial pressure or overdrainage, resulting in serious clinical outcomes.1,9,10 However, if the valve has a locking mechanism such as the one used in the proGAV (ie, the setting is locked by a braking mechanism), the setting may be secured despite exposure to even a 3T static magnetic field, as has been reported by Ludemann et al.2 Accordingly, Schneider, et al10 has stated that programmable valves should be developed with a feature that requires a high magnetic flux attenuation to alter the setting and/or with a locking mechanism to prevent inadvertent changes. In lieu of this change, it is generally recommended to constantly monitor the pressure level for a magnetically activated programmable valve, especially if exposure to an electromagnetic field has occurred.3,7,9,10
Although there was no evidence that exposure to the 3T MR imaging environment with regard to the static magnetic field and various MR imaging conditions altered the pressure settings for the proGAV, it would be prudent to follow a policy whereby the setting for this device is checked immediately before and after MR imaging to ensure patient safety until appropriate clinical data are obtained that may indicate this procedure is unnecessary.
Artifacts.
The assessment of artifacts indicated that the extent of the signal-intensity void created by the proGAV was relatively large in relation to the size and shape of this implant. This is not surprising given the fact that this programmable valve uses NdFeB magnets. Materials with high magnetic susceptibility will greatly impact MR images and produce large signal-intensity voids that are dependent on imaging parameters and other factors.9–13 Therefore, if the imaging area of interest is close to the proGAV, the size of the artifact associated with this implant could create problems for the diagnostic use of MR imaging.
Recommendations
In consideration of the results of the tests conducted to evaluate the programmable valve with regard to MR imaging and to take proper precautions to ensure patient safety, the following guidelines are recommended for scanning a patient with this device:
(1) A patient with the proGAV may undergo MR imaging at 3T or less immediately after implantation.
(2) Before MR imaging, the proGAV setting should be determined by appropriate personnel by using proper equipment.
(3) The exposure to RF energy should be limited to a whole-body averaged SAR of 2.1 W/kg for 15 minutes.
(4) After MR imaging, the programmable valve setting should be determined and reset as needed.
Summary and Conclusions
MR imaging safety issues and other concerns were assessed for the programmable valve, proGAV, in relation to the use of a 3T scanner. Magnetic field interactions were minor, and MR imaging–related heating will not cause an increased risk with this device to a patient who is undergoing an MR imaging procedure according to the conditions used for this evaluation(ie, whole-body-averaged SAR of 2.1 W/kg for 15 minutes). The functional aspects of the proGAV were shown to be unaffected by multiple exposures to the 3T MR imaging system and by 8 different pulse sequences that may be used for clinical MR imaging procedures. Artifacts for the programmable valve were relatively large in relation to the size and shape of the implant, which may impact the diagnostic use of MR imaging if the area of interest is in proximity to this implant.
Footnotes
Supported by an unrestricted research grant from Aesculap, Inc., Center Valley, Pa.
References
- 1.Czosnyka Z, Czosnyka M, Richards H, et al. Hydrodynamic properties of hydrocephalus shunts. Acta Neurochir Suppl 1998;71:334–39 [DOI] [PubMed] [Google Scholar]
- 2.Ludemann W, Rosahl SK, Kaminsky J, et al. Reliability of a new adjustable shunt device without the need for readjustment following 3-Tesla MRI. Childs Nerv Syst 2005;21:227–29 [DOI] [PubMed] [Google Scholar]
- 3.Miwa K, Kondo H, Sakai N. Pressure changes observed in Codman-Medos programmable valves following magnetic exposure and flipping. Childs Nerv Syst 2001;17:150–53 [DOI] [PubMed] [Google Scholar]
- 4.Ortler M, Kostron H, Felber S. Transcutaneous pressure-adjustable valves and magnetic resonance imaging: an ex vivo examination of the Codman-Medos programmable valve and the Sophy adjustable pressure valve. Neurosurgery 1997;40:1050–57 [DOI] [PubMed] [Google Scholar]
- 5.Fransen P, Dooms G, Thauvoy C. Safety of the adjustable pressure ventricular valve in magnetic resonance imaging: problems and solutions. Neuroradiology 1992;34:508–09 [DOI] [PubMed] [Google Scholar]
- 6.Fransen P. Transcutaneous pressure-adjustable valves and magnetic resonance imaging: an ex vivo examination of the Codman-Medos programmable valve and the Sophy adjustable pressure valve. Neurosurgery 1998;42:430. [DOI] [PubMed] [Google Scholar]
- 7.Shellock FG, Crues JV. MR procedures: biologic effects, safety, and patient care. Radiology 2004;232:635–52 [DOI] [PubMed] [Google Scholar]
- 8.Shellock FG. Reference Manual For Magnetic Resonance Safety, Implants, and Devices: 2006 Edition. Los Angeles, Calif; Biomedical Research Publishing Group;2006
- 9.Anderson RC, Walker ML, Viner JM, et al. Adjustment and malfunction of a programmable valve after exposure to toy magnets. J Neurosurg 2004;101:222–25 [DOI] [PubMed] [Google Scholar]
- 10.Schneider T, Knauff U, Nitsch J, et al. Electromagnetic field hazards involving adjustable shunt valves in hydrocephalus. J Neurosurg 2002;96:331–34 [DOI] [PubMed] [Google Scholar]
- 11.Shellock FG, Cosendai G, Park SM, et al. Implantable microstimulator: magnetic resonance safety at 1.5-Tesla. Invest Radiol 2004;39:591–99 [DOI] [PubMed] [Google Scholar]
- 12.Shellock FG, Gounis M, Wakhloo A. Detachable Coil for cerebral aneurysms: in vitro evaluation of magnet field interactions, heating, and artifacts at 3-Tesla. AJNR Am J Neuroradiol 2005;26:363–66 [PMC free article] [PubMed] [Google Scholar]
- 13.Shellock FG, Forder J. Drug eluting coronary stent: in vitro evaluation of magnet resonance safety at 3-Tesla. J Cardiovasc Magn Reson 2005;7:415–1915881523 [Google Scholar]
- 14.Shellock FG. Biomedical implants and devices: assessment of magnetic field interactions with a 3.0-Tesla MR system. J Magn Reson Imaging 2002;16:721–32 [DOI] [PubMed] [Google Scholar]
- 15.American Society for Testing and Materials (ASTM) International: F2052. Standard test method for measurement of magnetically induced displacement force on passive implants in the magnetic resonance environment. In: Annual Book of ASTM Standards: Medical Devices and Services. Vol.13.01 . West Conshohocken, Pa: American Society for Testing and Materials;2001. :1576–80 [Google Scholar]
- 16.American Society for Testing and Materials (ASTM) International: F2182–02 Test method for measurement of radio-frequency induced heating near passive implants during magnetic resonance imaging. Annual Book of ASTM Standards: Medical Devices and Services. Vol.13.01 . West Conshohocken, Pa: American Society for Testing and Materials;2003 [Google Scholar]