Abstract
BACKGROUND AND PURPOSE: Tracer studies have demonstrated that 100% oxygen inhalation causes a small cerebral blood flow (CBF) decrease. This study was performed to determine whether arterial spin-labeling (ASL), a noninvasive MR imaging technique, could image these changes with clinically reasonable imaging durations.
Materials and METHODS: Continuous ASL imaging was performed in 7 healthy subjects before, during, and after 100% oxygen inhalation. ASL difference signal intensity (ΔM, control − label), CBF, and CBF percentage change were measured. A test-retest paradigm was used to calculate the variability of the initial and final room air CBF measurements.
RESULTS: During oxygen inhalation, ΔM decreased significantly in all regions (eg, global ΔM decreased by 23 ± 11%, P < .01, all values mean ± SD). Accounting for the reduced T1 of hyperoxygenated blood, we found a smaller CBF decrease, which did not reach significance in any of the regions. Global CBF dropped from 50 ± 10 mL per 100 g/minute to 47 ± 10 mL per 100 g/minute following 100% oxygen inhalation, a decrease of 5 ± 14% (P > .17). The root-mean-square variability of the initial and final room air CBF measurements was 7–8 mL per 100 g/minute.
CONCLUSIONS: The ΔM signal intensity decreased significantly with oxygen inhalation; however, after accounting for changes in blood T1 with oxygen, CBF decreases were small. Such measurements support the use of hyperoxia as an MR imaging contrast agent and may be helpful to interpret hyperoxia-based stroke trials.
One hundred percent oxygen inhalation causes arterial vasoconstriction with an associated small (10%–15%) reduction in cerebral blood flow (CBF), as initially demonstrated by using nitrous oxide inhalation methods.1 Although the original methods evaluated only global CBF, tomographic methods offer the potential to measure regional CBF changes, such as those due to tissue type (ie, gray versus white matter) or focally in the setting of cerebrovascular disease. Some of these methods, such as positron-emission tomography (PET) or stable xenon CT, involve radiation and are not as widely available as MR imaging. Arterial spin-labeling (ASL) is a noninvasive MR imaging technique that measures CBF by magnetically labeling water protons in the arterial blood, which are subsequently transported and extracted into brain tissue.2,3 By modifying the Bloch equations to account for flow, CBF can be measured in physiologic units of milliliters per 100 g/minute, and these values are comparable to prior gold standard methods.4 Because ASL does not require a contrast agent, it can be easily repeated under different physiologic conditions during the same imaging examination. Also, it avoids the use of gadolinium-based contrast agents; this advantage has become more desirable given the possible association of nephrogenic systemic fibrosis with gadolinium-based contrast agents in some patients.5
Quantifying CBF changes that occur while breathing oxygen is important for several reasons. A recent pilot study of normobaric 100% oxygen inhalation for patients with acute ischemic stroke 6 demonstrated transient improvement in MR imaging biomarkers, such as diffusion, relative CBF, and cerebral blood volume (CBV); however, this small study—as another large study before it7—showed no long-term benefit, which may relate to the CBF decrease that occurs with increased arterial oxygenation. Global CBF changes make interpretation of relative measures extracted from bolus contrast techniques difficult, and thus knowledge of quantitative CBF changes might help clarify the true hemodynamic status of the brain.8 Also, there is interest in using inhaled oxygen as an MR imaging contrast agent, both for measuring CBV as well as for direct oxygenation by using T1-based techniques.9–11 Because CBF changes alter both CBV (the so-called “Grubb relationship”)12 and apparent tissue T1,13 it is important to understand the magnitude and distribution of CBF changes associated with 100% oxygen inhalation.
There is controversy in the MR imaging literature regarding the magnitude of the CBF decrease with 100% hyperoxia. Two studies using cardiac-gated velocity-encoded phase contrast (VEPC) measured global CBF decreases ranging between 16% and 27%,14,15 greater than that measured with tracer methods. Two previous ASL-based measurements have reported markedly different CBF changes, one measuring a 7% decrease,11 the other a 33% decrease.16 Thus, the main aim of this study was to determine the magnitude of CBF changes in hyperoxia by using ASL and to compare them with prior literature measurements. A second aim of the study was to demonstrate the usefulness of ASL for imaging CBF during physiologic challenge paradigms.
Methods
The study was approved by the Committee on Human Research of our institution. Seven healthy volunteers (mean age, 34 years; range, 24–40 years) were imaged at 1.5T (Intera; Philips Medical Systems, Cleveland, Ohio). Amplitude-modulated continuous ASL17 using spin-echo echo-planar imaging (EPI) readout was performed with the following parameters: TR/TE/label time/postlabel delay, 4300/32/2500/1480 ms; FOV, 24 cm; matrix, 64 × 64; section thickness/skip, 8/2 mm; 8 sections. Thirty-four pairs of label and control images were acquired for a total imaging time of 4′52" per CBF map. A single axial section just superior to the lateral ventricles, which encompassed the centrum semiovale white matter and cortical gray matter, was chosen for analysis. The rationale for limiting analysis to this single section was as follows: 1) This easily identified section increases the uniformity between subjects and avoids the susceptibility artifacts that can occur with EPI images near the skull base, 2) it represents a section with a relatively long postlabel delay time (1480 ms), which will mitigate any errors from prolonged blood arrival times, 3) it corresponds to the location where intracranial oxygen and flow sensors are typically placed, and 4) there is excellent demarcation of gray and white matter, which permits more robust segmentation.
Systolic and diastolic arterial blood pressure, heart rate, and arterial blood oxygen saturation were obtained before, during, and after breathing of 100% oxygen, which was administered at 15 L/minute by using a nonrebreather facemask (Model 1060; Hudson RCI, Temecula, Calif) for 30 minutes. The ASL images were acquired before, 15 minutes after 100% oxygen inhalation, and 15 minutes after resumption of room air breathing. Quantitative CBF maps were produced from the ASL time series by using the general kinetic model, with CSF signal intensity as a reference for proton attenuation. CBF was derived from the following equation as described by Buxton et al18 and Chalela et al19:
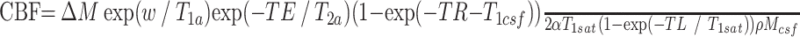 |
where ΔM is the ASL difference signal intensity (the subtraction of all the label images from the control images); T1a is the arterial blood T1, taken to be 1230 ms and 1050 ms for normoxia and 100% hyperoxia, respectively20,21; T2a is the arterial blood T2, taken to be 240 ms21; T1sat is the T1 of the tissue measured in the presence of off-resonance radio-frequency irradiation (0.75 and 0.5 seconds for gray and white matter, respectively); TL is the labeling time; w is the postlabel delay; TR/TE are the repetition and echo times; T1csf is the T1 relaxation time of the CSF (4200 ms); α is the labeling efficiency, which for amplitude modulated continuous ASL has been measured to be 0.7117; ρ is the brain attenuation (1.05 g/mL); and Mcsf is the measured CSF signal intensity acquired from averaged control and label acquisitions.
Gray and white matter was segmented by using SPM2 (Wellcome Department of Imaging Neuroscience, University College London, United Kingdom, http://www.fil.ion.ucl.ac.uk/spm), by using a proton-density modality. Voxels with a confidence threshold of 70% or greater were used to generate the gray and white matter regions of interest. Statistical analysis for significance was performed by using analysis of variance; individual paired 2-tailed t tests by using the Bonferroni correction were used to determine significant differences at the P < .05 level.
Reproducibility was determined by using a test-retest paradigm, by using the initial and final CBF maps, which were both acquired with the subject breathing room air, and separated in time by 45 minutes. Absolute and percentage CBF root-mean-square (RMS) changes were calculated in each subject, and the average value and SD are reported for global, gray matter, and white matter regions of interest.
Results
Physiologic monitoring of the subjects is shown in Table 1, and no significant differences were found between 100% oxygen inhalation and either room air breathing period. ASL difference (control − label) images [ΔM]) for all 7 subjects are shown before, during, and following 100% oxygen inhalation in Fig 1. The section level shown is immediately superior to the lateral verticals and is the section used for quantitative analysis. This figure provides a qualitative overview of the quality, consistency, and signal intensity-to-noise ratio (SNR) of the data. ΔM decreased significantly in all subjects in all regions; for example, ΔM decreased by 23 ± 11% (P < .01) between the initial room air and 100% oxygen measurements in the global region of interest. However, when corrected for the arterial blood T1 decrease, there was no significant CBF change during oxygen inhalation in any region, but rather a trend in all regions toward a decrease. Global CBF while breathing room air was 50 ± 10 mL per 100 g/minute, during 100% oxygen inhalation was 47 ± 10 mL per 100 g/minute (a decrease of 5 ± 14%), and 15 minutes following resumption of room air breathing was 50 ± 9 mL per 100 g/min. This data are summarized in detail in Table 2.
Table 1:
Physiologic parameters before, during, and after 100% oxygen inhalation*
| Condition | Heart Rate (bpm) | Systolic BP (mm Hg) | Diastolic BP (mm Hg) | HgbO2 Sat (%) |
|---|---|---|---|---|
| Before | 65 ± 11 | 113 ± 10 | 74 ± 16 | 98 ± 1 |
| During | 59 ± 10 | 113 ± 12 | 71 ± 10 | 99 ± 0 |
| After | 67 ± 13 | 114 ± 14 | 71 ± 9 | 98 ± 1 |
Note:—bpm indicates beats per minute; BP, blood pressure; HgbO2 Sat, saturation.
Noninvasive physiological monitoring of subjects (n = 7). There are no significant changes between the different breathing conditions (P > .05). Ninety-nine percent was the highest number our oxygen saturation monitoring equipment could record.
Fig 1.
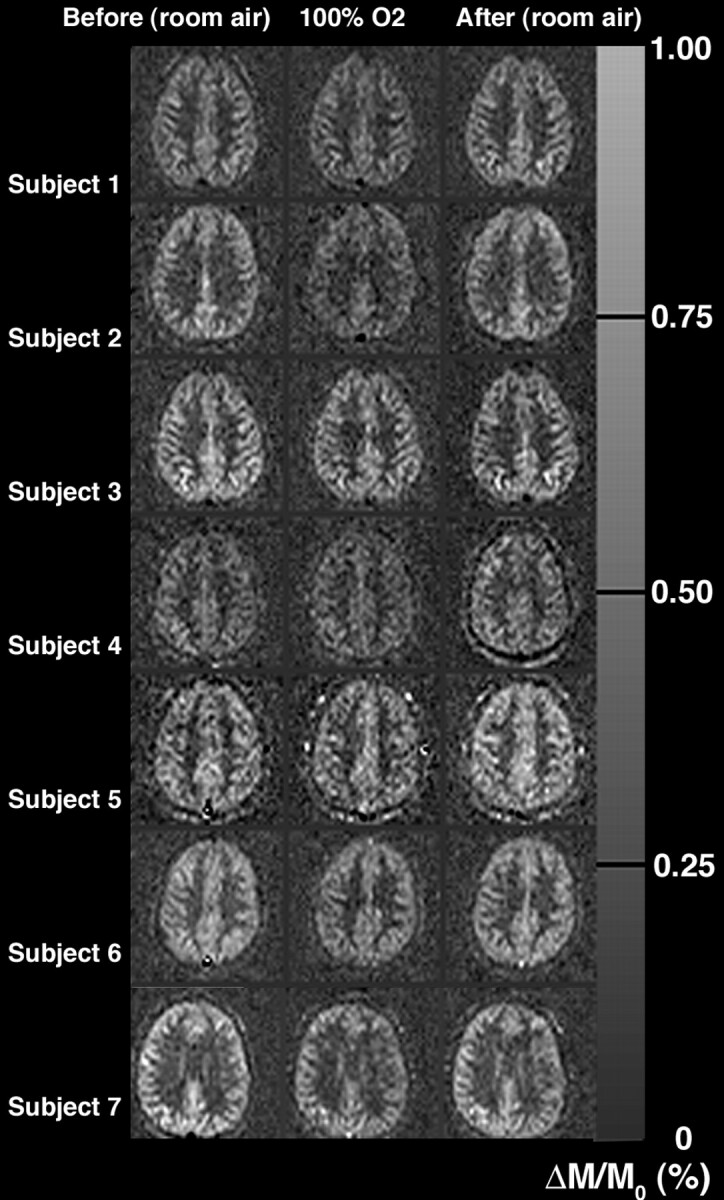
ASL signal-intensity difference (ΔM, control − label) images in all subjects. Decreased ΔM signals were seen in all subjects in all regions of interest during the 100% oxygen inhalation. However, accounting for the reduced hyperoxygenated blood T1, we found that only small CBF decreases were measured with oxygen.
Table 2:
ASL difference signal (ΔM, control − label images), CBF, and CBF changes before, after 15 minutes 100% oxygen inhalation, and 15 minutes following resumption of room air breathing in 7 healthy young subjects
| Room Air Baseline (before) | 100% Oxygen (during) | Room Air (after) | ||
|---|---|---|---|---|
| T1a | Blood | 1230 ms | 1050 ms | 1230 ms |
| ΔM change (% from baseline) | Global | – | −23 ± 11* | 1 ± 17 |
| Gray | – | −21 ± 11* | 2 ± 15 | |
| White | – | −28 ± 19† | −3 ± 35 | |
| CBF (ml/100 g/min) | Global | 50 ± 10 | 47 ± 10 | 50 ± 9 |
| Gray | 62 ± 11 | 60 ± 13 | 63 ± 9 | |
| White | 30 ± 9 | 25 ± 3 | 29 ± 7 | |
| CBF change (% from baseline) | Global | – | −5 ± 14 | 0 ± 17 |
| Gray | – | −2 ± 14 | 1 ± 15 | |
| White | – | −12 ± 23 | −3 ± 39 |
Note:— –, indicates data not available.
P < .01.
P < .05. All values are mean ± SD. The SDs are across subjects.
We also explored the extent to which our ΔM maps and measured CBF values returned to baseline values after 100% oxygen inhalation. Table 3 shows the root-mean-square differences between the initial and final CBF values, showing that the reproducibility of the CBF measurement over the interval of 45 minutes was between 7 and 8 mL per 100 g/min for regions of interest ranging in size between 27 ± 5 mL (white matter) and 76 ± 13 mL (global).
Table 3:
Reproducibility of the CASL perfusion method*
| Gray matter | White matter | Global | |
|---|---|---|---|
| Region of interest size (mL) | 49 ± 11 | 27 ± 5 | 76 ± 13 |
| Quantitative (mL/100 g/min) | 8 ± 8 | 8 ± 9 | 7 ± 8 |
| % difference (% baseline) | 12 ± 7 | 29 ± 24 | 14 ± 8 |
Root-mean-square variability measured for both quantitative CBF (mL/100 g/min) and % CBF change for 7 healthy subjects breathing room air. Comparison is between measurements acquired before oxygen inhalation and 15 minutes after the inhalation of oxygen ceased; they were separated in time by 45 minutes
Discussion
ASL images CBF noninvasively, by using endogenous water as a diffusible tracer. This particular implementation (continuous ASL [CASL]) has the highest SNR of current ASL techniques and is thus suited to imaging at clinical field strengths in reasonable imaging times.22 In CASL, brain images are acquired with and without adiabatically induced magnetic inversion of arterial blood water at the base of the skull during the labeling period.23 This water then flows distally and is subsequently delivered to and extracted into the brain parenchyma. The difference in magnetization between images collected with and without labeling is small (approximately 0.5%–2% of the equilibrium blood magnetization) and is proportional to CBF. Knowledge of the degree and duration of labeling as well as the blood T1 permits conversion to quantitative CBF.24 A well-known pitfall is the underestimation of CBF in regions with prolonged blood-arrival times, which can be partially mitigated by using long postlabel delays, as was done in the current study.25 Despite this, the ASL CBF measurement method compares well in measures of accuracy, repeatability, and reproducibility when compared with gold standard measurements such as PET.4 Its noninvasive nature and repeatability are features particularly attractive for measuring the physiologic response to inhalational and pharmacologic challenges.
This study found a significant decrease in the ΔM signal intensity for subjects breathing room air or 100% oxygen, which was on the order of 20% (Fig 1, Table 2). However, the ΔM signal intensity is affected not only by CBF but also by the decay of the label during its transit between the labeling plane and the site of extraction into the tissue. Several groups have reported significant decreases in arterial blood T1 during hyperoxia,20,21 which is likely due to the increase in dissolved molecular oxygen, which acts as a weak paramagnetic contrast agent. This increased decay of the label in the arterial blood will cause a decreased ΔM signal intensity even in the absence of a CBF change. Because direct measurement of T1 changes in the pulsatile rapidly flowing cerebral circulation is quite challenging, prior studies11 as well as the current study have estimated the T1 values on the basis of prior work. However, uncertainty in the exact level of dissolved oxygen is a potential source of error in the interpretation of CBF changes in challenges that involve inhalation of gasses with supranormal levels of oxygen.
The baseline global CBF level of 50 mL per 100 g/minute in healthy subjects breathing room air measured in this study is consistent with prior ASL studies and other gold standard techniques. After accounting for the expected reduction in the arterial blood T1 due to breathing 100% oxygen, we observed only a trend toward a global CBF decrease (−5 ± 14%, P > .17). This finding is consistent with the original nitrous oxide tracer kinetic methods and a previous MR imaging measurement of a 7% decrease (also not statistically significant) by Bulte et al11 by using a pulsed ASL technique at 3T. It is smaller than the 33% decrease measured in another report by using continuous ASL,16 though these authors do not indicate whether their results were corrected for the T1 decrease of hyperoxygenated arterial blood. Finally, the small CBF change measured in the current study is also at odds with the 16%–27% decrease measured with cardiac-gated VEPC MR imaging.14,15 Because VEPC images taken of the major vessels near the circle of Willis should represent a reasonable estimate of global supratentorial CBF,26 more investigation of this apparent discrepancy is warranted.
Because ASL is an imaging technique, it is possible to measure CBF separately in gray and white matter. The percentage decrease in the ΔM signal intensity (Table 2) was not statistically different between gray and white matter, though there was a trend toward a greater percentage CBF decrease in white matter. This agrees with the results of Floyd et al,16 who also demonstrated no significant difference. Bulte et al9 chose not to measure white matter CBF, because the baseline levels are so low that they approach the noise level of the measurement. We chose to include such data, because we did observe a significant ΔM decrease (−28 ± 19%, P < .05) in the white matter in our study.
The inclusion of a final CBF image after the subjects switched back to room air breathing allowed us to test the reproducibility of the CBF measurement in the same person by using a test-retest paradigm. ASL CBF levels measured approximately 45 minutes apart during the same imaging session have an RMS variability of between 7 and 8 mL per 100 g/minute, which is similar to prior reports for both PET27,28 and ASL-based MR imaging.29–31 Although this is probably adequate to assess changes in global or gray matter CBF, because white matter CBF at baseline is low, the percentage variability is high (29 ± 24%) and limits interpretation of changes. More robust imaging of white matter CBF changes probably requires higher field strengths (3T and above) and longer imaging times than those in the current study.32
Knowledge of the magnitude of CBF decrease during hyperoxia may be relevant to interpret clinical trials of normobaric oxygen in acute stroke.6,7 There is renewed interest in re-evaluating normobaric oxygen as a potential extender of the therapeutic window for thrombolysis.6 In theory, quantitative CBF imaging (such as with ASL) is preferable to techniques that measure relative changes, such as bolus susceptibility contrast perfusion-weighted imaging. However, for challenge studies before and during oxygen inhalation in healthy subjects, the current study shows that absolute CBF changes are small; however, this may not be true for patients with either baseline global or focal perfusion deficits. Also, new intravascular devices (such as the NeuroFlo balloon; CoAxia, Maple Grove, Minn) that partially occlude the infrarenal aorta are believed to augment cerebral perfusion pressure and thus improve CBF33; therefore, such devices would be expected to cause global CBF changes that may confound relative CBF measurement between affected and unaffected brain. Thus, the ability to image quantitative CBF changes noninvasively during both respiratory gas alteration and intravascular device use makes ASL a potentially important clinical technique for stroke trials. Finally, oxygen inhalation has been proposed as a contrast agent to estimate CBV9 or to alter T2*.34 Because CBF and CBV are coupled in the normal brain (the Grubb relationship12), CBF changes with oxygen should be considered when comparing these techniques with gold standard techniques obtained during room air breathing. The small CBF decrease observed in the current study supports the use of these oxygen-based contrast methods as a means of probing baseline physiology in healthy subjects.
Conclusions
The ASL signal-intensity difference between control and label acquisitions decreased significantly on the order of 20% when healthy subjects breathed 100% oxygen. However, after accounting for the decreased T1 of hyperoxygenated blood, global CBF showed only a slight 5% decrease, which was not significantly different from zero. Quantitative CBF levels are in accordance with prior literature values. There is good reproducibility of ASL-based CBF measurements obtained 45 minutes apart in the same subject during the same imaging session. We believe that the combination of noninvasiveness, quantitation, and repeatability of ASL-based MR imaging measurements of CBF will be useful to document clinically relevant physiologic changes during respiratory, interventional, or therapeutic challenges.
References
- 1.Kety S, Schmidt C. Effects of alterations in the tensions of carbon dioxide and oxygen on the cerebral blood flow and cerebral oxygen consumption of normal young men. Fed Proc 1946;5:55. [PubMed] [Google Scholar]
- 2.Dixon WT, Du LN, Faul DD, et al. Projection angiograms of blood labelled by adiabatic fast passage. Magn Reson Med 1986;3:454–62 [DOI] [PubMed] [Google Scholar]
- 3.Detre JA, Leigh JS, Williams DS, et al. Perfusion imaging. Magn Reson Med 1992;23:37–45 [DOI] [PubMed] [Google Scholar]
- 4.Ye FQ, Berman KF, Ellmore T, et al. H(2)(15)O PET validation of steady-state arterial spin tagging cerebral blood flow measurements in humans. Magn Reson Med 2000;44:450–56 [DOI] [PubMed] [Google Scholar]
- 5.Grobner T. Gadolinium: a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant 2006;21:1104–08 [DOI] [PubMed] [Google Scholar]
- 6.Singhal AB, Benner T, Roccatagliata L, et al. A pilot study of normobaric oxygen therapy in acute ischemic stroke. Stroke 2005;36:797–802 [DOI] [PubMed] [Google Scholar]
- 7.Ronning OM, Guldvog B. Should stroke victims routinely receive supplemental oxygen? A quasi-randomized controlled trial. Stroke 1999;30:2033–37 [DOI] [PubMed] [Google Scholar]
- 8.Yonas H, Pindzola RR, Meltzer CC, et al. Qualitative versus quantitative assessment of cerebrovascular reserves. Neurosurgery 1998;42:1005–10, discussion 1011–12 [DOI] [PubMed] [Google Scholar]
- 9.Bulte DP, Chiarelli PA, Wise RG, et al. Measurement of Cerebral Blood Volume Using Hyperoxic Contrast: Proceeding of the International Society for Magnetic Resonance in Medicine, Berlin, Germany, May 19–25, 2007. Berlin, Germany: ISMRM;2007. :781
- 10.Hsu J-J, Glover GH. Quantitative Analysis of Brain Functional Hemodynamics with Time-Series T1 Mapping: Proceeding of the International Society for Magnetic Resonance in Medicine, Berlin, Germany, May 19–25, 2007. Berlin, Germany: ISMRM;2007. :1952
- 11.Bulte DP, Chiarelli PA, Wise RG, et al. Cerebral perfusion response to hyperoxia. J Cereb Blood Flow Metab 2007;27:69–75 [DOI] [PubMed] [Google Scholar]
- 12.Grubb RL, Phelps ME, Raichle ME, et al. The effects of arterial blood pressure on the regional cerebral blood volume by x-ray fluorescence. Stroke 1973;4:390–99 [DOI] [PubMed] [Google Scholar]
- 13.Kwong KK, Chesler DA, Weisskoff RM, et al. MR perfusion studies with T1-weighted echo planar imaging. Magn Reson Med 1995;34:878–87 [DOI] [PubMed] [Google Scholar]
- 14.Rostrup E, Larsson HB, Toft PB, et al. Signal changes in gradient echo images of human brain induced by hypo- and hyperoxia. NMR Biomed 1995;8:41–47 [DOI] [PubMed] [Google Scholar]
- 15.Watson NA, Beards SC, Altaf N, et al. The effect of hyperoxia on cerebral blood flow: a study in healthy volunteers using magnetic resonance phase-contrast angiography. Eur J Anaesthesiol 2000;17:152–59 [DOI] [PubMed] [Google Scholar]
- 16.Floyd TF, Clark JM, Gelfand R, et al. Independent cerebral vasoconstrictive effects of hyperoxia and accompanying arterial hypocapnia at 1 ATA. J Appl Physiol 2003;95:2453–61 [DOI] [PubMed] [Google Scholar]
- 17.Alsop DC, Detre JA. Multisection cerebral blood flow MR imaging with continuous arterial spin labeling. Radiology 1998;208:410–16 [DOI] [PubMed] [Google Scholar]
- 18.Buxton RB, Frank LR, Wong EC, et al. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med 1998;40:383–96 [DOI] [PubMed] [Google Scholar]
- 19.Chalela JA, Alsop DC, Gonzalez-Atavales JB, et al. Magnetic resonance perfusion imaging in acute ischemic stroke using continuous arterial spin labeling. Stroke 2000;31:680–87 [DOI] [PubMed] [Google Scholar]
- 20.Tadamura E, Hatabu H, Li W, et al. Effect of oxygen inhalation on relaxation times in various tissues. J Magn Reson Imaging 1997;7:220–25 [DOI] [PubMed] [Google Scholar]
- 21.Noseworthy MD, Kim JK, Stainsby JA, et al. Tracking oxygen effects on MR signal in blood and skeletal muscle during hyperoxia exposure. J Magn Reson Imaging 1999;9:814–20 [DOI] [PubMed] [Google Scholar]
- 22.Wong EC, Buxton RB, Frank LR. A theoretical and experimental comparison of continuous and pulsed arterial spin labeling techniques for quantitative perfusion imaging. Magn Reson Med 1998;40:348–55 [DOI] [PubMed] [Google Scholar]
- 23.Roberts DA, Detre JA, Bolinger L, et al. Quantitative magnetic resonance imaging of human brain perfusion at 1.5 T using steady-state inversion of arterial water. Proc Natl Acad Sci U S A 1994;91:33–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buxton RB. Quantifying CBF with arterial spin labeling. J Magn Reson Imaging 2005;22:723–26 [DOI] [PubMed] [Google Scholar]
- 25.Alsop DC, Detre JA. Reduced transit time sensitivity in noninvasive magnetic resonance imaging of human cerebral blood flow. J Cereb Blood Flow Metab 1996;16:1236–49 [DOI] [PubMed] [Google Scholar]
- 26.Zhao M, Amin-Hanjani S, Ruland S, et al. Regional cerebral blood flow using quantitative MR angiography. AJNR Am J Neuroradiol 2007;28:1470–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matthew E, Andreason P, Carson RE, et al. Reproducibility of resting cerebral blood flow measurements with H2(15)O positron emission tomography in humans. J Cereb Blood Flow Metab 1993;13:748–54 [DOI] [PubMed] [Google Scholar]
- 28.Coles JP, Fryer TD, Bradley PG, et al. Intersubject variability and reproducibility of 15O PET studies. J Cereb Blood Flow Metab 2006;26:48–57 [DOI] [PubMed] [Google Scholar]
- 29.Parkes LM, Rashid W, Chard DT, et al. Normal cerebral perfusion measurements using arterial spin labeling: reproducibility, stability, and age and gender effects. Magn Reson Med 2004;51:736–43 [DOI] [PubMed] [Google Scholar]
- 30.Yen Y-F, Field AS, Martin EM, et al. Test-retest reproducibility of quantitative CBF measurements using FAIR perfusion MRI and acetazolamide challenge. Magn Reson Med 2002;47:921–28 [DOI] [PubMed] [Google Scholar]
- 31.Floyd TF, Ratcliffe SJ, Wang J, et al. Precision of the CASL-perfusion MRI technique for the measurement of cerebral blood flow in whole brain and vascular territories. J Magn Reson Imaging 2003;18:649–55 [DOI] [PubMed] [Google Scholar]
- 32.van Gelderen P, de Zwart JA, Duyn JH. On the Feasibility of White Matter Arterial Spin-Labeling Measurements: Proceeding of the International Society for Magnetic Resonance in Medicine, Berlin, Germany, May 19–25, 2007. Berlin, Germany: ISMRM;2007. :1416
- 33.Lylyk P, Vila JF, Miranda C, et al. Partial aortic obstruction improves cerebral perfusion and clinical symptoms in patients with symptomatic vasospasm. Neurological Research 2005;27 (suppl 1):S129–35 [DOI] [PubMed] [Google Scholar]
- 34.Losert C, Peller M, Schneider P, et al. Oxygen-enhanced MRI of the brain. Magn Reson Med 2002;48:271–77 [DOI] [PubMed] [Google Scholar]


