Abstract
Purpose –
We examined patients in a large clinical registry to assess factors associated with laser trabeculoplasty (LTP) responses.
Design –
Retrospective cohort study.
Methods –
Study population: LTP patients in the Intelligent Research in Sight (IRIS®) Registry, 2013–2018.
Observation: IRIS® Registry data were extracted if the eye had a procedural code for LTP and a glaucoma diagnosis. Eyes were excluded if LTP laterality or baseline IOP could not be determined. Following LTP, “nonresponders” were those with < 20% IOP reduction after 8 weeks, while “responders” were those with ≥ 20% IOP reduction.
Main Outcome Measures: proportion of responders, odds ratios (OR) of pre-LTP factors associated with being a nonresponder.
Results –
A total of 263,480 eyes were included, with mean age 71.4 +/−11.7 years. Mean baseline IOP was 19.1 +/− 5.0 mmHg, mean number of pre-LTP medications was 2.1 +/− 1.5. Response rate was 36.9% overall and 68.8% for those with baseline IOP > 24 mmHg. Higher baseline IOP was associated with reduced odds of nonresponse (OR = 0.60, P < 0.0001 for a 3 mm Hg increase). Angle recession, uveitis, and aphakia increased the odds of a nonresponse (ORs 2.46, 1.50, (both P < 0.0001), and 1.55 (P = 0.0259), respectively). In nonresponders with at least one medication at baseline, 76.3% of eyes had fewer medications postoperatively.
Conclusions –
Lower baseline IOP, angle recession, uveitis, and aphakia were associated with increased odds of nonresponse. Future studies that analyze LTP responder survival and implementation lag would facilitate resource optimization in glaucoma therapy.
Introduction
Laser trabeculoplasty (LTP) is one of the most frequently performed ophthalmic interventions. In 2014, it was performed in approximately 150,000 patients and comprised 40% of all glaucoma surgical interventions.1 From prior studies of modestly-sized cohorts, LTP reduced mean IOP 20–30% from baseline,2–5 and the efficacy was maintained in about 80% of patients after 2 years.5 Reduction of IOP occurred in approximately 80% of patients (responders), with the remaining 20% (nonresponders) having little or no treatment effect.6 To maximize LTP utility, the ability to offer the procedure selectively to those who are likely to respond would be crucial. Several factors have been examined in modest-sized cohorts to predict LTP outcomes, including baseline IOP, age, and prostaglandin analogue therapy, although the findings were inconsistent.7–15 As the utilization of LTP is likely to increase, the characterization of its treatment effect is a priority to determine which patients are most likely to benefit from the procedure,1, 16 and analysis of a larger cohort is needed to assess these potential predictive factors.
The Intelligent Research in Sight (IRIS®) Registry is an electronic health record (EHR)-based clinical data registry. As of January 1, 2019, 14,945 physicians (ophthalmologists plus eligible clinicians) from 3,120 practices had signed up for EHR integration, and the IRIS® Registry database contains 31.63 million visits representing 52.97 million unique patients that captured fields including patient demographics, payer types, social history, ocular examination laterality and values, diagnoses, procedures and medications.17 Recently, analyses of the IRIS® Registry have provided “real-world” clinical insight to several important ophthalmologic diagnoses and treatments including: the prevalence and treatment patterns of myopic choroidal neovascularization, the incidence of post-cataract surgery endophthalmitis, and outcomes of age-related macular degeneration treatment, macular hole surgery, and strabismus surgery.18–24 In this study, we analyzed a large cohort of ophthalmic patients using the IRIS® Registry to assess potential predictive factors of LTP treatment outcomes.
Methods
This is a retrospective cohort studying using the IRIS® Registry database. The study protocol was reviewed and exempted by the Institutional Review Board of the University of Miami Miller School of Medicine as it did not meet the criteria of research involving human subjects.
Data Source
The IRIS® Registry data acquisition have been described elsewhere (https://www.aao.org/iris-registry/about).17 Study eyes met the following inclusion and exclusion criteria – Inclusion: 1) Current Procedural Terminology (CPT) code for LTP (65855); 2) All entries up to August 31, 2018; 3) Eyes with a glaucoma or glaucoma suspect diagnosis (see Supplemental Table 1) not excluded below. Exclusion : 1) Entries without LTP laterality (coded as “unspecified”) in a patient with two sighted eyes; 2) LTP eye that had angle-closure International Classification of Diseases codes (9th and 10th editions): 365.2X, H40.2X; 3) Eyes with no light perception; 4) Eyes without visual acuity and/or pretreatment baseline IOP measurements (defined below) prior to LTP; 5) Eyes that have reached an “exclusion event,” as defined below. All data referred to below were for the study eyes, except as noted. The number of medications refer to the number of topical or systemic IOP-lowering agents, with fixed-dosed combination medications counted based on their constituent agents. Medications recorded in the IRIS® Registry database are not eye-specific, and every glaucoma medication for a patient was attributed to the study eye.
Study Definitions
Defining treatment groups.
Each study eye was classified into one of two groups based on the sequence of LTP procedures. “Treatment” refers to the entirety of the management protocol; “procedure” refers to each individual LTP episode. 1) “Single LTP” was one LTP procedure without an additional LTP within 8 weeks. Treatment Date (TD) was the date of the procedure. 2) “Double LTP” was an initial LTP procedure followed by one or more additional LTP procedures within 8 weeks. Dates of the first and last procedures were recorded, as “early procedure date” (EPD) and “later procedure date” (LPD). LPD was designated as the TD.
Defining IOP baseline and treatment responses.
Pretreatment baseline IOP was defined as follows: If two or more IOP measurements were available within the 3 months prior to LTP TD (TD – 3 months), pretreatment baseline IOP was the average of the immediate two (or more if these were all on the same day) measurements prior to LTP (before LTP TD in “Single LTP,” or before EPD in “Double LTP”). If only one IOP measurement was available within TD – 3 months, then that single IOP measurement was the pretreatment baseline IOP.
Following LTP treatment, some eyes had “response unknown” if an exclusion event occurred prior to 8 weeks following the LTP TD or if no IOP data were available between 8 weeks and 6 months (inclusive) following LTP TD. All other eyes were classified with a treatment outcome of nonresponder or responder (Figure 1):
Figure 1.
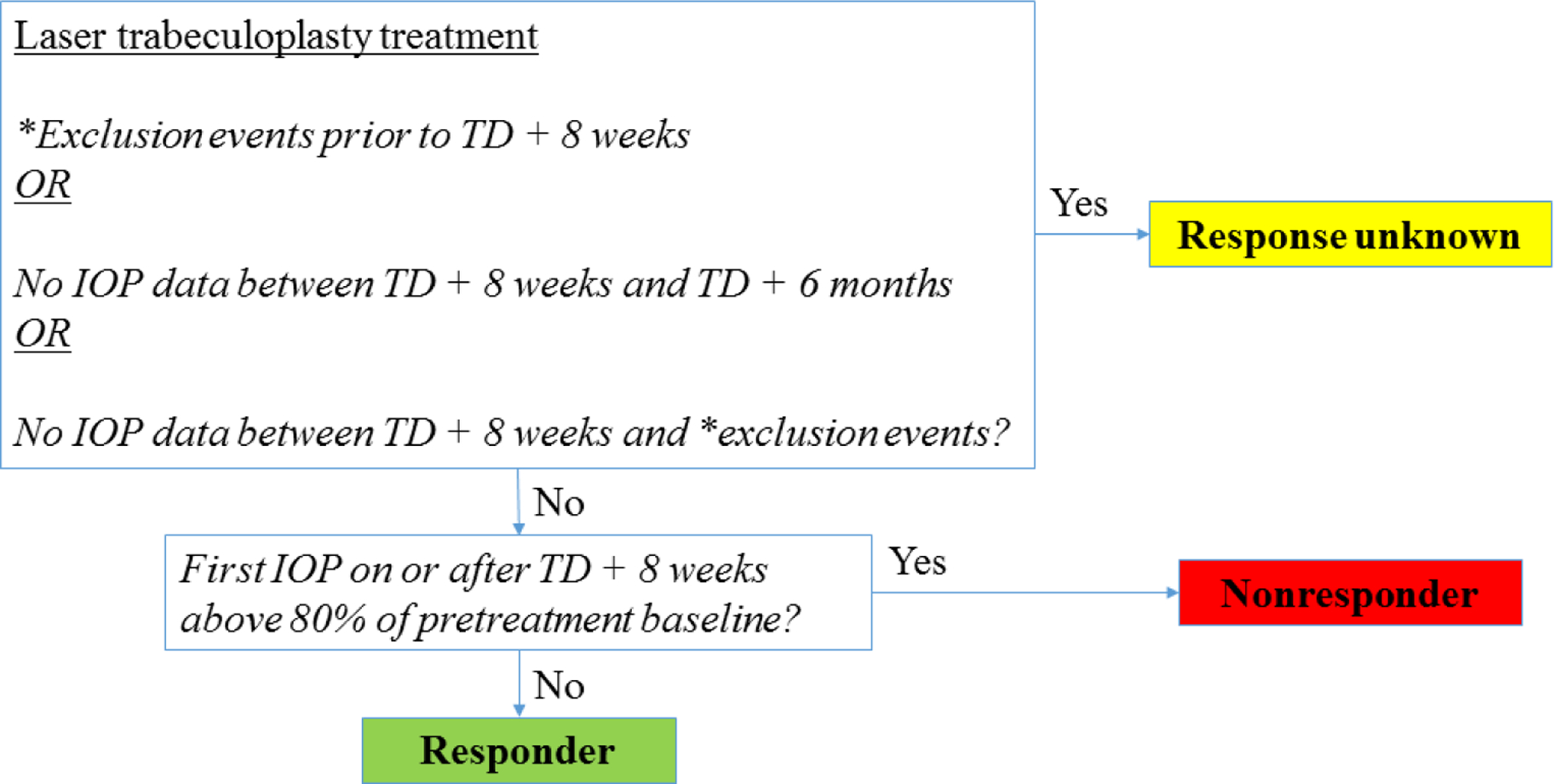
Algorithm for defining treatment response groups. Following laser trabeculoplasty treatment, the treatment outcome will be either nonresponder, responder, or response unknown. Key – TD (treatment date), IOP (intraocular pressure).
*Exclusion events see Defining an “exclusion event” in the text.
Nonresponders – eyes whose first day’s mean IOP measurement on or after 8 weeks post treatment was above 80% of the pretreatment baseline IOP;
Responders – eyes whose first day’s mean IOP measurement on or after 8 weeks post treatment was at or below 80% of the pretreatment baseline IOP.
Some or many eyes with at least one pretreatment medication may have post-treatment IOP above 80% of the pretreatment IOP but requiring fewer medications. Nevertheless, given that medication is not laterality nor dosage specific, classifying these eyes as nonresponders ensures a conservative assessment of LTP efficacy. The impact of LTP on the number of medications in nonresponders was analyzed.
Defining an “exclusion event”
An exclusion event occurs (and excludes an eye from analysis) on the first date following LTP TD when 1) IOP-lowering medication was added and/or 2) An IOP-lowering procedure (CPT 658XX, 661XX, 665XX, 666XX, 667XX) was performed on the study eye (or if procedure laterality was unspecified) and/or 3) Cataract surgery (CPT 668XX, 6698X) was performed on the study eye (or if the procedure laterality was unspecified) and/or 4) Reaching the end of IRIS® Registry followup.
Given that medication is not laterality specific, to exclude whenever medication was added ensured a conservative assessment of LTP efficacy.
Statistical methods
Continuous data were summarized as mean +/− standard deviation (SD), while categorical data are summarized with counts and/or percentages. Odds ratios (ORs) were calculated using multivariable logistic regression with the Generalized Estimating Equations method to account for the correlation between two eyes of a patient. All analyses were performed using SAS (Cary, NC) version 9.4. A p-value of ≤ 0.050 was considered statistically significant, and an OR ≥ 1.5 or ≤ 0.67 was considered clinically significant to avoid unnecessarily emphasizing weak (but statistically significant) associations that are likely to result from a large database.25, 26
Results
Search algorithm results and baseline characteristics
The initial CPT code search yielded 668,128 eyes. After applying the exclusion criteria, 380,957 eyes were included for analysis (Figure 2). There were 117,477 eyes categorized as “response-unknown.” Of the remaining 263,480 eyes, 74.4% aged > 65 years (mean 71.4 +/−11.7 years); 56.0% female; 64.8% white, 11.8% black, 97.1% single LTP. 73.1% of diagnoses were primary open angle glaucoma and 18.6% were glaucoma suspect. Mean baseline pre-LTP IOP was 19.1 +/− 5.0 mmHg, mean number of pre-LTP medications was 2.1 +/− 1.5. Baseline descriptive statistics of the sample may be found in Table 1.
Figure 2.
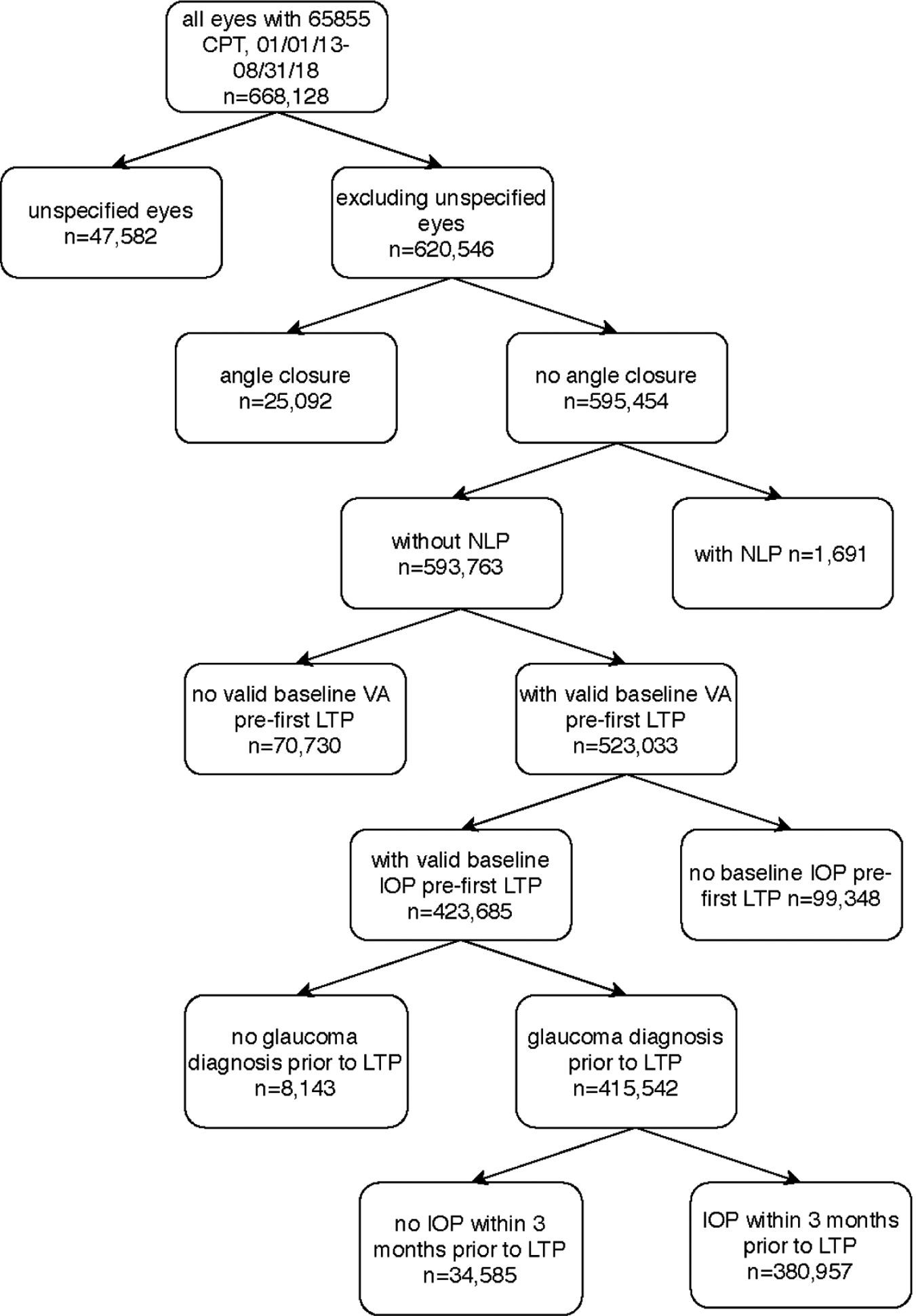
Applying exclusion criteria to eyes with Intelligent Research In Sight Registry database. Key – CPT (Current Procedural Terminology code), date (month/date/year), IOP (intraocular pressure), LTP (laser trabeculoplasty), n (sample size), NLP (no light perception), VA (visual acuity).
Table 1:
Baseline descriptive statistics of the IRIS Registry Eyes in the Laser Trabeculoplasty Study
| Categorical Variables and Baseline Values | n | % | |
|---|---|---|---|
| Age Group (in years)2 | 18–39 | 2,968 | 1.1% |
| 40–64 | 63,322 | 24.0% | |
| 65–79 | 127,298 | 48.3% | |
| 80+ | 68,802 | 26.1% | |
| Not Recorded | 1,090 | 0.4% | |
| Sex1 | Male | 81,992 | 43.8% |
| Female | 104,951 | 56.0% | |
| Not Recorded | 400 | 0.2% | |
| Race1 | Asian | 4,066 | 2.2% |
| Black | 22,101 | 11.8% | |
| Not Recorded | 12,264 | 6.5% | |
| White | 121,420 | 64.8% | |
| Hispanic | 9,460 | 5.0% | |
| Other/Multi-Racial | 18,032 | 9.6% | |
| Insurance1 | Dual Medicaid & Medicare | 23,813 | 12.7% |
| Medicaid | 3,970 | 2.1% | |
| Medicare Advantage | 20,984 | 11.2% | |
| Medicare Fee-For-Service | 84,065 | 44.9% | |
| Military | 1,072 | 0.6% | |
| Other Government | 309 | 0.2% | |
| Private | 38,600 | 20.6% | |
| Not Recorded | 14,530 | 7.8% | |
| Region2 | Midwest | 61,358 | 23.3% |
| Northeast | 47,025 | 17.8% | |
| South | 103,101 | 39.1% | |
| West | 46,828 | 17.8% | |
| Not Recorded | 5,168 | 2.0% | |
| Diabetes1 | Yes | 36,127 | 19.3% |
| Hypertension1 | Yes | 5,432 | 2.9% |
| LTP Type2 | Single | 256,129 | 97.2% |
| Double | 7,351 | 2.8% | |
| Angle Recession2 | Yes | 560 | 0.2% |
| Uveitis2 | Yes | 2,706 | 1.0% |
| Glaucoma Procedure2 | 2.0% | ||
| Yes | 5,214 | ||
| Lens Surgery2 | Yes | 19,011 | 7.2% |
| Intravitreal Injection/Surgery2 | Yes | 6,479 | 2.5% |
| Glaucoma | 50,506 | 19.2% | |
| Provider Specialty2 | Cataract | 116,531 | 44.2% |
| Other | 92,966 | 35.3% | |
| Unknown | 3,477 | 1.3% | |
| Provider Laser Trabeculoplasty Count2 | <=50 | 95,941 | 36.4% |
| 51–99 | 53,302 | 20.2% | |
| 100–499 | 101,641 | 38.6% | |
| 500+ | 12,596 | 4.8% | |
| Glaucoma Type2 | Glaucoma Suspect | 49,054 | 18.6% |
| Primary open angle glaucoma | 192,707 | 73.1% | |
| Trauma/Other Eye Disorders | 1,200 | 0.5% | |
| Inflammation/Drugs | 695 | 0.3% | |
| Other Glaucoma | 503 | 0.2% | |
| Unspecified Glaucoma | 19,321 | 7.3% | |
| Glaucoma Severity2 | Mild | 47,165 | 17.9% |
| Moderate | 59,123 | 22.4% | |
| Severe | 36,605 | 13.9% | |
| Indeterminate | 6,355 | 2.4% | |
| Not Recorded | 45,354 | 17.2% | |
| Not Applicable | 68,878 | 26.1% | |
| Lens Status2 | Phakic/Cataract | 91,505 | 34.7% |
| Pesudophakia | 21,319 | 8.1% | |
| Aphakia | 733 | 0.3% | |
| Not Recorded | 149,923 | 56.9% | |
| Continuous Baseline Varaibles | mean(SD) | min-max | |
| Mean IOP (mm Hg)2 | 19.1 (5.0) | 1–68 | |
| Mean LogMAR Visual Acuity2 | 0.22 (0.28) | −0.30–2.00 | |
| Number Glaucoma Medication Categories2 | 2.1 (1.5) | 0–7 | |
| Age2 | 71.4 (11.7) | 18–99 | |
Note:
Variable is per patient (n = 187,343)
Variable is per eye (n = 263,480).
% = percentage; IOP = intra-ocular pressure; IRIS = Intelligent Research in Sight; LogMAR = logarithm of the minimum angle of resolution; LTP = Laser Trabeculoplasty; max = maximum; min = minimum; mm Hg = millimeters of mercury; n = number; NPI = National Provider Identifier; SD = standard deviation;
LTP response rate and factors associated with responders vs. nonresponders
Overall, there were 97,148 (36.9%) responders and 166,332 (63.1%) nonresponders. The main outcome IOP measurement occurred at a mean +/− SD of 104.14 +/− 36.44 days (median 98; minimum 56, maximum 180, interquartile range 70–134 days). Among those with baseline IOP > 24 mmHg (34,271, 13.0%), 68.8% were responders; baseline IOP between 18 and 24 mmHg (123,261, 46.8%), 42.4% were responders; baseline IOP < 18 mmHg (105,948, 40.2%), 20.1% were responders. Angle recession, uveitis, and aphakia significantly and relevantly increased the odds of a nonresponse (ORs 2.46, 1.50, (both P < 0.0001), 1.55 (P = 0.0259), respectively), while higher baseline IOP reduced the odds of a nonresponse (OR = 0.60 for a 3 mmHg increase), in multivariable analysis. Provider specialty, prior surgeon LTP counts, and single- vs double-LTP were not clinically significant factors. A complete list of the variables that were included in the multivariable model, along with their ORs and p-values may be found in Table 2. Variables included in univariable modeling may be found in Supplemental Table 2.
Table 2:
Multivariable Model Results: Odds Ratios for Nonresponse from the IRIS Registry database
| Categorical Variables | Reference Group | Comparison Group | Odds Ratio | 95% CI for the Estimated Odds Ratio | p-value | |
|---|---|---|---|---|---|---|
| Sex | Male | Female | 1.22 | 1.20 | 1.25 | <0.0001* |
| Sex | Male | Not Recorded | 0.60 | 0.49 | 0.72 | <0.0001* |
| Age Group (years) | 65–79 | 18–39 | 1.29 | 1.17 | 1.42 | <0.0001* |
| Age Group (years) | 65–79 | 40–64 | 1.06 | 1.03 | 1.09 | 0.0001* |
| Age Group (years) | 65–79 | 80+ | 0.93 | 0.91 | 0.96 | <0.0001* |
| Age Group (years) | 65–79 | Not Recorded | 0.97 | 0.83 | 1.14 | 0.7190 |
| Region | South | Midwest | 1.07 | 1.05 | 1.10 | <0.0001* |
| Region | South | Northeast | 1.08 | 1.05 | 1.12 | <0.0001* |
| Region | South | West | 0.92 | 0.82 | 1.04 | 0.7261 |
| Region | South | Unknown | 1.00 | 0.97 | 1.02 | 0.2032 |
| Race | White | Black | 0.91 | 0.85 | 0.98 | <0.0001* |
| Race | White | Asian | 0.94 | 0.91 | 0.97 | 0.0093* |
| Race | White | Multiple Races/Other | 0.96 | 0.92 | 1.00 | 0.9970 |
| Race | White | Hispanic | 1.00 | 0.97 | 1.03 | 0.0616 |
| Race | White | Not Recorded | 0.79 | 0.76 | 0.82 | <0.0001* |
| Insurance | Private | Dual Insurance | 0.93 | 0.89 | 0.96 | 0.0001* |
| Insurance | Private | Medicaid | 0.95 | 0.89 | 1.02 | 0.1866 |
| Insurance | Private | Medicare | 0.90 | 0.86 | 0.93 | 0.0027* |
| Insurance | Private | Medicare | 0.95 | 0.93 | 0.98 | <0.0001* |
| Insurance | Private | Military | 0.88 | 0.78 | 1.00 | 0.0515 |
| Insurance | Private | Other | 0.98 | 0.78 | 1.23 | 0.8660 |
| Insurance | Private | Not Recorded | 1.11 | 1.06 | 1.16 | <0.0001* |
| Provider Specialty | Glaucoma | Cataract | 1.08 | 1.04 | 1.11 | <0.0001* |
| Provider Specialty | Glaucoma | Other | 0.98 | 0.95 | 1.00 | 0.0938 |
| Provider Specialty | Glaucoma | Unknown | 0.91 | 0.78 | 1.06 | 0.2151 |
| Glaucoma Procedure | NO | YES | 1.14 | 1.06 | 1.22 | 0.0004* |
| Lens Surgery | NO | YES | 1.03 | 1.00 | 1.08 | 0.0814 |
| Intravitreal Inj./Surg. | NO | YES | 1.03 | 0.97 | 1.09 | 0.3723 |
| Diabetes | NO | YES | 1.21 | 1.18 | 1.24 | <0.0001* |
| Hypertension | NO | YES | 0.89 | 0.84 | 0.94 | <0.0001* |
| Angle Recession | NO | YES | 2.46 | 1.80 | 3.35 | <0.0001* |
| Uveitis | NO | YES | 1.50 | 1.34 | 1.68 | <0.0001* |
| Provider LTP Count | < 50 | 51–99 | 0.92 | 0.90 | 0.95 | 0.5816 |
| Provider LTP Count | < 50 | 100–499 | 1.19 | 1.13 | 1.25 | <0.0001* |
| Provider LTP Count | < 50 | 500+ | 0.99 | 0.97 | 1.02 | <0.0001* |
| Glaucoma Severity | Mild | Moderate | 0.89 | 0.84 | 0.96 | <0.0001* |
| Glaucoma Severity | Mild | Severe | 1.09 | 1.06 | 1.13 | <0.0001* |
| Glaucoma Severity | Mild | Indeterminate | 0.83 | 0.80 | 0.85 | 0.0010* |
| Glaucoma Severity | Mild | Not Recorded | No odds ratio estimated | |||
| Glaucoma Severity | Mild | Not Applicable | 0.75 | 0.73 | 0.78 | <0.0001* |
| Glaucoma Type | Suspect | POAG | 0.93 | 0.77 | 1.12 | <0.0001* |
| Glaucoma Type | Suspect | Trauma/OED | 0.76 | 0.62 | 0.94 | 0.0005* |
| Glaucoma Type | Suspect | Inflammation/Drugs | 0.91 | 0.88 | 0.94 | 0.4415 |
| Glaucoma Type | Suspect | Other Glaucoma | 1.29 | 1.12 | 1.49 | 0.0105* |
| Glaucoma Type | Suspect | Unspecified Glaucoma | 0.84 | 0.80 | 0.87 | <0.0001* |
| Lens Status | Phakic/Cataract | Pseudophakia | 1.23 | 1.02 | 1.47 | <0.0001* |
| Lens Status | Phakic/Cataract | Aphakia | 1.55 | 1.49 | 1.61 | 0.0259* |
| Lens Status | Phakic/Cataract | Not Recorded | 0.81 | 0.79 | 0.83 | <0.0001* |
| LTP Type | Single | Double | 1.05 | 0.99 | 1.11 | 0.0900 |
| Continuous Variables (increase for odds ratio) | ||||||
| Age at Baseline | 10 year increase in age | 0.74 | 0.71 | 0.78 | <0.0001* | |
| Mean Baseline IOP | 3 mm Hg increase in BL IOP | 0.60 | 0.59 | 0.60 | <0.0001* | |
| Mean BL LogMAR VA | 1 unit increase in LogMAR VA | 1.03 | 1.00 | 1.07 | 0.0617 | |
| Glaucoma Meds | 1 additional glaucoma med cat | 1.04 | 1.03 | 1.05 | <0.0001* | |
Statistically significant p-values are flagged with an asterik
Possible clinically significant odds ratios are bolded. % = percentage; BL = baseline; cat = categories; CI = confidence interval; Inj. = Injection; IOP = intraocular pressure; IRIS = Intelligent Research in Sight; LogMAR = logarithm of the minimum angle of resolution; LTP = Laser Trabeculoplasty; Meds = medications categories; NPI = National Provider Identifier; OED = Other Eye Disorders; POAG = Primary open angle glaucoma; Surg. = Surgery; VA = visual acuity.
Although these odds ratios were not within our chosen clinically significant range, the following groups did have increased odds of a nonresponse that might be important to consider when selecting treatment: females compared to males OR = 1.22, patients aged 18–39 compared to those aged 65–79 OR = 1.29, patients with diabetes compared to those without OR = 1.21, patients with other glaucoma diagnoses compared to those who were glaucoma suspect OR = 1.29, and patients who were pseudophakic compared to those who were phakic OR = 1.23. Also, the following groups had decreased odds of a nonresponse that might be important to consider when selecting treatment: patients with indeterminate compared to those with mild glaucoma severity OR = 0.83, patients with “Trauma/Other Eye Disorder” glaucoma diagnoses compared to those who were glaucoma suspect OR = 0.76, and patients with a 10-year increase in age compared to younger patients OR = 0.74. Of particular note, these results indicate that there is a statistically significant increase in the odds of being a nonresponder for patients who were 18–39 years old compared to patients who were 65–79 years old in addition to (i.e. even when adjusting for) a statistically significant linear effect of greater age decreasing the odds of being a nonresponder. Uncovering such complex associations are precisely the purpose for which the IRIS® Registry was created.
Changes in the number of medications in nonresponders
Out of the 380,957 eyes in the full cohort, 74,550 (19.6%) had zero pretreatment medications. In all nonresponders with at least one medication at baseline (139,337 of 166,332 nonresponders, 83.8%), 76.3% of eyes had fewer medications postoperatively (74.3% in baseline IOP > 24 mmHg; 75.9% in baseline IOP between 18 and 24 mmHg, and 76.9% in baseline IOP < 18 mmHg).
Discussion
The IRIS® Registry represents a real-world sampling of clinical data from across the United States, which provides a unique opportunity to assess medical resource utilization by gauging treatment outcomes and practice patterns in an ophthalmologic registry. In glaucoma treatment, more than 50% of the total cost are attributed to medications,27–30 with combination therapy costing more than monotherapy.27–29, 31 Medication cost is also a major barrier in therapy adherence32, 33 which in turn is a major factor in progressive glaucomatous damage.34–36 Thus, in order to minimize the cost and maximize the treatment outcomes in glaucoma management, finding strategies to decrease medication dependence is key. Specifically, office-based IOP-lowering procedures such as LTP are highly relevant given the rich evidence of safety and efficacy.3, 5, 6, 37–39 Several high-quality clinical studies have shown LTP to be efficacious in eyes with high pressure,40 comparably efficacious to topical medications,37, 41–43 and may be more cost-effective as the initial treatment of primary open angle glaucoma compare to topical medications.37, 43, 44
Ideally, an evidence-based, economically optimized treatment algorithm for primary open angle glaucoma would involve offering an initial treatment such as LTP (prior to medications) in eyes with high IOP, to reduce IOP and to reduce or eliminate medication burden. However, the IRIS® Registry data reflect some notable differences in the providers’ real-world practice results. The overall LTP response rate of 36.9% in the IRIS® Registry is lower than that reported by prior studies,5, 6 which is likely due to the lower mean baseline IOP of 19.1 +/− 5.0 mmHg. Based on the IRIS® Registry data capture strategy, we cannot discount the possibility of over-representing responders if IOP-lowering medications were added post-LTP but were not recorded, and thus failed to exclude eyes that were otherwise “response unknown.” Only 13% of IRIS® Registry study eyes had baseline IOP > 24 mmHg, while approximately 40% had baseline IOP < 18 mmHg. This suggests that IRIS® Registry providers are more likely to offer LTP when the IOP is not overtly elevated, despite evidence showing high pretreatment IOP correlating with high treatment success.38, 45, 46 The subset of eyes in the IRIS® Registry with baseline IOP > 24 mmHg had a response rate of 68.8%, which is comparable to the response rate (66–82%) of several prior studies with mean baseline IOP ranging between 23.9 and 26.8 mmHg.47–49 Angle recession, uveitis and aphakia were significant predictors of LTP nonresponse, which is consistent with previous studies.50–52 Provider status (glaucoma specialist, nonglaucoma anterior segment surgeon, and others) and experience (total number of LTP performed in the 12 months preceding the study date) did not significantly influence outcome, which implies that the technical demands of LTP are modest, and the outcomes are somewhat surgeon-independent, in contrast to traditional glaucoma procedures such as trabeculectomy.53–55 In the IRIS® Registry, 19.6% of study eyes were medication-free at the time treatment, while 80.4% of eyes had at least one IOP-lowering medication at baseline. This suggests that LTP may be relatively underutilized in medication-free patients. LTP has been shown to be safe and efficacious as initial medical therapy,39, 56 while several studies that compared LTP to medication as initial treatment showed comparable efficacies.37, 42, 44 Outcome of the first major randomized clinical trial comparing LTP and topical medication as initial treatment in primary open angle glaucoma, the Glaucoma Laser Trial (GLT), was published in 1995. GLT authors reported eyes initially treated with laser had lower IOP and better visual field and optic disc status than fellow eyes treated initially with topical medication.42 In the two decades following the GLT, LTP utilization increased between 2001 and 2006 (possibly due to the introduction of selective laser trabeculoplasty over the traditional argon LTP), and decreased between 2006 and 2012 (possibly attributed to a decline in allowed Medicare charge for the procedure over the same period),1, 57 while the procedure’s safety and efficacy as initial glaucoma therapy was confirmed in several additional trials.37, 44, 56
Nevertheless, the adaptation of LTP as initial treatment in real-world practice remained uncertain despite its clear efficacy.37, 41, 44 There are several potential barriers to the implementation of LTP as initial therapy. At the physician level, compared to LTP, initiating a topical medication has the immediate advantages of treating both eyes at the same time, lowering IOP in a matter of hours to days (rather than weeks), shorter encounter time, and no postoperative visits. In addition, while all providers are able to prescribe medications, not all providers have the training and access to the proper equipment to perform LTP. At the patient level, any short-term aversion to risks associated with the procedure (no matter how remote) may result in them choosing a regiment (e.g. topical medications) that is considered “safer,” albeit less effective long-term, when both options are offered by the physician.58
Among nonresponders on at least one medication at baseline, despite a lack of robust IOP reduction, approximately 75% of eyes had fewer medications postoperatively. In the IRIS® Registry cohort with baseline IOP < 18 mmHg, the response rate of 20.1% is comparable to that of a cohort of normal tension glaucoma patients (baseline IOP 14.3 +/− 3.4 mmHg, mean 1.5 +/− 0.8 medications, post-washout IOP 16.2 +/− 2.2 mmHg), which had a response rate (defined as ≥ 20% reduction of IOP without the addition of medications) of 22.0%.59 In both instances, there were significant reductions in the number of medications despite a modest IOP-lowering effect. While medication is not captured in a laterality-specific fashion, for the IRIS-captured data to reflect a decrease in medication, one of three scenarios must occur: 1. The medication was used only in the LTP eye and is now discontinued; 2. The medication was used in both eyes and is now discontinued in both the LTP and non-LTP eyes; and 3. The medication was used in the non-LTP eye only, and is now discontinued from the non-LTP eye. There are no compelling reason to associate LTP in one eye with discontinuation of medication only in the fellow eye, thus we believe this finding to be valid, and the true effect (e.g. medication discontinue in the LTP eye but continued in the fellow eye) may perhaps be even larger than reflected in our study. This suggests that IRIS® Registry providers may be offering LTP as a means of reducing medication burden, which has potential cost-saving implications. However, offering LTP as a means of reducing medication burden is a distinct approach from offering LTP as an initial therapy, and it was not possible to categorize eyes based on the clinical objective of LTP treatment. Therefore, we cannot determine to what extent these results apply to using LTP as an initial or subsequent glaucoma therapy. Based on these results, future IRIS® Registry research might explore a more complex and nuanced definition of a responder (including both IOP and medication reduction), but such an analysis was beyond the scope of our original proposal. In a Markov model of patients with two medications, offering LTP decreased the 5-year treatment cost by approximately 26% when compared to adding a third medication.30 Hence, by offering LTP in patients with normal/borderline IOP can potentially achieve further IOP-lowering and decrease medication burden.
This study has several notable limitations. First, we excluded eyes with secondary open angle that were miscoded as angle closure (previously closed angle that had opened following peripheral iridotomy), and the findings do not apply to this subset of diagnoses. Second, the CPT code for LTP does not differentiate between argon versus selective laser trabeculoplasties, though prior studies showed comparable efficacy between the two LTP subtypes.40 Third, the IRIS® Registry data is an observational data source derived from EHR, are not subjected to the same rigorous validation as clinical trial data, and the medication laterality information is not available for detailed analysis. However, the direct extraction of longitudinal clinical information from the EHR at a scale that would not be practical through other means makes IRIS® Registry the best large-scale real-world database for assessing ophthalmology treatment outcomes and practice patterns. Nevertheless, clinicians should recognized the limitations of such registries, as information, selection and confounding biases are possible.26 Lastly, with a large database such as the IRIS® Registry, many statistically significant associations are weak, and subjective interpretation is required to regard each statistically significant finding as being clinically significant. We have arbitrarily defined OR >1.5 or <0.67 as clinically significant in order not to emphasize weak associations but have made available the entire output of the uni- and multivariable analyses such that the readers may draw their own conclusions. Factors such as cost-benefit and/or risk-benefit ratios and physician/patient preference could be considered in defining the “clinical significance” of statistically significant findings. With the growing utilization of large database analyses, this interpretation issue is likely to persist. To overcome it, a formalized, practical metric of clinical significance based on agreed-upon criteria would be vital.
In conclusion, this analysis of 380,957 eyes in the IRIS® Registry revealed a modest overall LTP response rate. High baseline IOP is associated with being a responder, while angle recession, uveitis and aphakia increase the odds of nonresponse. In order to optimize LTP utilization, policy should encourage a strategy of offering LTP as initial therapy, to patients with high baseline IOP, and as a means of decreasing medication burden even when further IOP-lowering may not be required. It is possible that there are unidentified barriers in the implementation of evidence-based practices such as financial incentives, time, psychologic/behavioral economic considerations, or a combination of these factors. Future studies that analyze LTP responder survival and implementation lag and barriers of clinical evidence would facilitate resource optimization in glaucoma therapy.
Supplementary Material
Highlight.
Overall laser trabeculoplasty (LTP) response rate is 36.9%
In the subgroup with pretreatment IOP > 24 mmHg response rate is 68.8%
Angle recession, uveitis and aphakia increased the risk of nonresponse
Only 19.6% of eyes had zero preoperative medications
LTP reduced number of medications in 76.3% of nonresponders
Acknowledgment
Funding/Support: The project was supported by NIH Center Core Grant P30EY014801, Research to Prevent Blindness Unrestricted Grant, The 2019 University of Miami Institute for Advanced Study of the Americas Pilot Grant, 2018 IRIS-Registry-AGS Research Initiative Grant, and Grant Number UL1TR002736, Miami Clinical and Translational Science Institute, from the National Center for Advancing Translational Sciences and the National Institute on Minority Health and Health Disparities. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Financial Disclosures: No financial disclosures for any of the authors
Relevant Financial Interests: None
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Prior Presentations: Poster presentation at the American Glaucoma Society 2020 Annual Meeting
Conflict of interest: None
References
- 1.Arora KS, et al. , Use of Various Glaucoma Surgeries and Procedures in Medicare Beneficiaries from 1994 to 2012. Ophthalmology, 2015. 122(8): p. 1615–24. [DOI] [PubMed] [Google Scholar]
- 2.Latina MA, et al. , Q-switched 532-nm Nd:YAG laser trabeculoplasty (selective laser trabeculoplasty): a multicenter, pilot, clinical study. Ophthalmology, 1998. 105(11): p. 2082–8; discussion 2089–90. [DOI] [PubMed] [Google Scholar]
- 3.Damji KF, et al. , Selective laser trabeculoplasty v argon laser trabeculoplasty: a prospective randomised clinical trial. Br J Ophthalmol, 1999. 83(6): p. 718–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melamed S, Ben Simon GJ, and Levkovitch-Verbin H, Selective laser trabeculoplasty as primary treatment for open-angle glaucoma: a prospective, nonrandomized pilot study. Arch Ophthalmol, 2003. 121(7): p. 957–60. [DOI] [PubMed] [Google Scholar]
- 5.Odberg T and Sandvik L, The medium and long-term efficacy of primary argon laser trabeculoplasty in avoiding topical medication in open angle glaucoma. Acta Ophthalmol Scand, 1999. 77(2): p. 176–81. [DOI] [PubMed] [Google Scholar]
- 6.McIlraith I, et al. , Selective laser trabeculoplasty as initial and adjunctive treatment for open-angle glaucoma. J Glaucoma, 2006. 15(2): p. 124–30. [DOI] [PubMed] [Google Scholar]
- 7.Ayala M and Chen E, Predictive factors of success in selective laser trabeculoplasty (SLT) treatment. Clin Ophthalmol, 2011. 5: p. 573–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruen R, Lesk MR, and Harasymowycz P, Baseline Factors Predictive of SLT Response: A Prospective Study. J Ophthalmol, 2012. 2012: p. 642869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chun M, et al. , Selective laser trabeculoplasty for early glaucoma: analysis of success predictors and adjusted laser outcomes based on the untreated fellow eye. BMC Ophthalmol, 2016. 16(1): p. 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirn C, et al. , Long-term efficacy of selective laser trabeculoplasty in patients on prostaglandin therapy. Klin Monbl Augenheilkd, 2014. 231(4): p. 351–6. [DOI] [PubMed] [Google Scholar]
- 11.Hodge WG, et al. , Baseline IOP predicts selective laser trabeculoplasty success at 1 year post-treatment: results from a randomised clinical trial. Br J Ophthalmol, 2005. 89(9): p. 1157–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mao AJ, et al. , Development of a prediction rule to estimate the probability of acceptable intraocular pressure reduction after selective laser trabeculoplasty in open-angle glaucoma and ocular hypertension. J Glaucoma, 2008. 17(6): p. 449–54. [DOI] [PubMed] [Google Scholar]
- 13.Martow E, Hutnik CM, and Mao A, SLT and adjunctive medical therapy: a prediction rule analysis. J Glaucoma, 2011. 20(4): p. 266–70. [DOI] [PubMed] [Google Scholar]
- 14.Realini T, et al. , West Indies Glaucoma Laser Study (WIGLS) 3. Anterior Chamber Inflammation Following Selective Laser Trabeculoplasty in Afro-Caribbeans with Open-angle Glaucoma. J Glaucoma, 2019. 28(7): p. 622–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song J, et al. , High failure rate associated with 180 degrees selective laser trabeculoplasty. J Glaucoma, 2005. 14(5): p. 400–8. [DOI] [PubMed] [Google Scholar]
- 16.Stein JD, et al. , Comparison of Outcomes of Laser Trabeculoplasty Performed by Optometrists vs Ophthalmologists in Oklahoma. JAMA Ophthalmol, 2016. 134(10): p. 1095–1101. [DOI] [PubMed] [Google Scholar]
- 17.Chiang MF, et al. , The 2016 American Academy of Ophthalmology IRIS((R)) Registry (Intelligent Research in Sight) Database: Characteristics and Methods. Ophthalmology, 2018. 125(8): p. 1143–1148. [DOI] [PubMed] [Google Scholar]
- 18.Willis J, et al. , Treatment Patterns for Myopic Choroidal Neovascularization in the United States: Analysis of the IRIS Registry. Ophthalmology, 2017. 124(7): p. 935–943. [DOI] [PubMed] [Google Scholar]
- 19.Rao P, et al. , Real-World Vision in Age-Related Macular Degeneration Patients Treated with Single Anti-VEGF Drug Type for 1 Year in the IRIS Registry. Ophthalmology, 2018. 125(4): p. 522–528. [DOI] [PubMed] [Google Scholar]
- 20.Mehta H, et al. , Real-world outcomes in patients with neovascular age-related macular degeneration treated with intravitreal vascular endothelial growth factor inhibitors. Prog Retin Eye Res, 2018. 65: p. 127–146. [DOI] [PubMed] [Google Scholar]
- 21.Atchison EA, et al. , The Real-World Effect of Intravitreous Anti-Vascular Endothelial Growth Factor Drugs on Intraocular Pressure: An Analysis Using the IRIS Registry. Ophthalmology, 2018. 125(5): p. 676–682. [DOI] [PubMed] [Google Scholar]
- 22.Parke DW 3rd and Lum F, Return to the Operating Room after Macular Surgery: IRIS Registry Analysis. Ophthalmology, 2018. 125(8): p. 1273–1278. [DOI] [PubMed] [Google Scholar]
- 23.Repka MX, Lum F, and Burugapalli B, Strabismus, Strabismus Surgery, and Reoperation Rate in the United States: Analysis from the IRIS Registry. Ophthalmology, 2018. 125(10): p. 1646–1653. [DOI] [PubMed] [Google Scholar]
- 24.Pershing S, et al. , Endophthalmitis after Cataract Surgery in the United States: A Report from the Intelligent Research in Sight Registry, 2013–2017. Ophthalmology, 2019. [DOI] [PubMed] [Google Scholar]
- 25.Tai V, Grey A, and Bolland MJ, Results of observational studies: analysis of findings from the Nurses’ Health Study. PLoS One, 2014. 9(10): p. e110403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nathan H and Pawlik TM, Limitations of claims and registry data in surgical oncology research. Ann Surg Oncol, 2008. 15(2): p. 415–23. [DOI] [PubMed] [Google Scholar]
- 27.Rouland JF, et al. , Naturalistic, prospective study of glaucoma and ocular hypertension treatment in France: Strategies, clinical outcomes, and costs at 2 years. Eur J Ophthalmol, 2005. 15(5): p. 562–580. [DOI] [PubMed] [Google Scholar]
- 28.Rouland JF, Le Pen C, and S. Ophthalmologists of the Glaucoma, Naturalistic, prospective study of glaucoma and ocular hypertension treatment in France: strategies, clinical outcomes, and costs at 1 year. Eur J Ophthalmol, 2003. 13 Suppl 4: p. S5–20. [DOI] [PubMed] [Google Scholar]
- 29.Schmier JK, Halpern MT, and Jones ML, The economic implications of glaucoma: a literature review. Pharmacoeconomics, 2007. 25(4): p. 287–308. [DOI] [PubMed] [Google Scholar]
- 30.Cantor LB, et al. , Economic evaluation of medication, laser trabeculoplasty and filtering surgeries in treating patients with glaucoma in the US. Curr Med Res Opin, 2008. 24(10): p. 2905–18. [DOI] [PubMed] [Google Scholar]
- 31.Abelson MB, Netland PA, and Chapin MJ, Switching patients with glaucoma or ocular hypertension from dual therapy to monotherapy: evaluation of brimonidine as a model. Adv Ther, 2001. 18(6): p. 282–97. [DOI] [PubMed] [Google Scholar]
- 32.Sleath B, et al. , Patient-reported behavior and problems in using glaucoma medications. Ophthalmology, 2006. 113(3): p. 431–6. [DOI] [PubMed] [Google Scholar]
- 33.Tsai JC, A comprehensive perspective on patient adherence to topical glaucoma therapy. Ophthalmology, 2009. 116(11 Suppl): p. S30–6. [DOI] [PubMed] [Google Scholar]
- 34.Rossi GC, et al. , Do adherence rates and glaucomatous visual field progression correlate? Eur J Ophthalmol, 2011. 21(4): p. 410–4. [DOI] [PubMed] [Google Scholar]
- 35.Sleath B, et al. , The relationship between glaucoma medication adherence, eye drop technique, and visual field defect severity. Ophthalmology, 2011. 118(12): p. 2398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newman-Casey PA, et al. , The Association between Medication Adherence and Visual Field Progression in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology, 2020. 127(4): p. 477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gazzard G, et al. , Selective laser trabeculoplasty versus eye drops for first-line treatment of ocular hypertension and glaucoma (LiGHT): a multicentre randomised controlled trial. Lancet, 2019. 393(10180): p. 1505–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khawaja AP, et al. , Real-World Outcomes of Selective Laser Trabeculoplasty in the United Kingdom. Ophthalmology, 2019. [DOI] [PubMed] [Google Scholar]
- 39.Realini T, Selective laser trabeculoplasty for the management of open-angle glaucoma in St. Lucia. JAMA Ophthalmol, 2013. 131(3): p. 321–7. [DOI] [PubMed] [Google Scholar]
- 40.Samples JR, et al. , Laser trabeculoplasty for open-angle glaucoma: a report by the american academy of ophthalmology. Ophthalmology, 2011. 118(11): p. 2296–302. [DOI] [PubMed] [Google Scholar]
- 41.Gazzard G, et al. , Selective laser trabeculoplasty versus drops for newly diagnosed ocular hypertension and glaucoma: the LiGHT RCT. Health Technol Assess, 2019. 23(31): p. 1–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.The Glaucoma Laser Trial (GLT) and glaucoma laser trial follow-up study: 7. Results. Glaucoma Laser Trial Research Group. Am J Ophthalmol, 1995. 120(6): p. 718–31. [DOI] [PubMed] [Google Scholar]
- 43.Stein JD, et al. , Cost-effectiveness of medications compared with laser trabeculoplasty in patients with newly diagnosed open-angle glaucoma. Arch Ophthalmol, 2012. 130(4): p. 497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garg A, et al. , Primary Selective Laser Trabeculoplasty for Open-Angle Glaucoma and Ocular Hypertension: Clinical Outcomes, Predictors of Success, and Safety from the Laser in Glaucoma and Ocular Hypertension Trial. Ophthalmology, 2019. 126(9): p. 1238–1248. [DOI] [PubMed] [Google Scholar]
- 45.Alaghband P, et al. , Predictors of selective laser trabeculoplasty success in open angle glaucoma or ocular hypertension: does baseline tonography have a predictive role? Br J Ophthalmol, 2020. [DOI] [PubMed] [Google Scholar]
- 46.Pillunat KR, et al. , Preoperative intraocular pressure as a predictor of selective laser trabeculoplasty efficacy. Acta Ophthalmol, 2016. 94(7): p. 692–696. [DOI] [PubMed] [Google Scholar]
- 47.Cvenkel B, One-year follow-up of selective laser trabeculoplasty in open-angle glaucoma. Ophthalmologica, 2004. 218(1): p. 20–5. [DOI] [PubMed] [Google Scholar]
- 48.Lai JS, et al. , Five-year follow up of selective laser trabeculoplasty in Chinese eyes. Clin Exp Ophthalmol, 2004. 32(4): p. 368–72. [DOI] [PubMed] [Google Scholar]
- 49.Rozsival P, Kana V, and Hovorkova M, [Selective laser trabeculoplasty]. Cesk Slov Oftalmol, 2004. 60(4): p. 267–74. [PubMed] [Google Scholar]
- 50.Goldberg I, Argon laser trabeculoplasty and the open-angle glaucomas. Aust N Z J Ophthalmol, 1985. 13(3): p. 243–8. [DOI] [PubMed] [Google Scholar]
- 51.Robin AL and Pollack IP, Argon laser trabeculoplasty in secondary forms of open-angle glaucoma. Arch Ophthalmol, 1983. 101(3): p. 382–4. [DOI] [PubMed] [Google Scholar]
- 52.Schwartz AL, Wilson MC, and Schwartz LW, Efficacy of argon laser trabeculoplasty in aphakic and pseudophakic eyes. Ophthalmic Surg Lasers, 1997. 28(3): p. 215–8. [PubMed] [Google Scholar]
- 53.Walkden A, et al. , Trabeculectomy training in England: are we safe at training? Two year surgical outcomes. Eye (Lond), 2018. 32(7): p. 1253–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abe RY, et al. , Primary Trabeculectomy Outcomes by Glaucoma Fellows in a Tertiary Hospital in Brazil. J Glaucoma, 2017. 26(11): p. 1019–1024. [DOI] [PubMed] [Google Scholar]
- 55.Connor MA, et al. , Trainee glaucoma surgery: experience with trabeculectomy and glaucoma drainage devices. Ophthalmic Surg Lasers Imaging, 2010. 41(5): p. 523–31. [DOI] [PubMed] [Google Scholar]
- 56.Realini T, et al. , West Indies Glaucoma Laser Study (WIGLS): 1. 12-Month Efficacy of Selective Laser Trabeculoplasty in Afro-Caribbeans With Glaucoma. Am J Ophthalmol, 2017. 184: p. 28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jampel HD, et al. , Trends over time and regional variations in the rate of laser trabeculoplasty in the Medicare population. JAMA Ophthalmol, 2014. 132(6): p. 685–90. [DOI] [PubMed] [Google Scholar]
- 58.Chang TC, Vanner EA, and Parrish RK, Glaucoma surgery preferences when the surgeon adopts the role of the patient. Eye, 2019. 33(10): p. 1577–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee JW, et al. , Efficacy of selective laser trabeculoplasty for normal tension glaucoma: 1 year results. BMC Ophthalmol, 2015. 15: p. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


