Abstract
Glaucoma is a complex group of optic neuropathies that affects both humans and animals. Intraocular pressure (IOP) elevation is a major risk factor that results in the loss of retinal ganglion cells (RGCs) and their axons. Currently, lowering IOP by medical and surgical methods is the only approved treatment for primary glaucoma, but there is no cure, and vision loss often progresses despite therapy. Recent technologic advances provide us with a better understanding of disease mechanisms and risk factors; this will permit earlier diagnosis of glaucoma and initiation of therapy sooner and more effectively. Gene and cell therapies are well suited to target these mechanisms specifically with the potential to achieve a lasting therapeutic effect. Much progress has been made in laboratory settings to develop these novel therapies for the eye. Gene and cell therapies have already been translated into clinical application for some inherited retinal dystrophies and age-related macular degeneration (AMD). Except for the intravitreal application of ciliary neurotrophic factor (CNTF) by encapsulated cell technology for RGC neuroprotection, there has been no other clinical translation of gene and cell therapies for glaucoma so far. Possible application of gene and cell therapies consist of long-term IOP control via increased aqueous humor drainage, including inhibition of fibrosis following filtration surgery, RGC neuroprotection and neuroregeneration, modification of ocular biomechanics for improved IOP tolerance, and inhibition of inflammation and neovascularization to prevent the development of some forms of secondary glaucoma.
Keywords: cell therapy, gene therapy, glaucoma, intraocular pressure (IOP), neuroprotection, ocular biomechanics
1. GLAUCOMA – DEFINITION AND CURRENT THERAPIES
Glaucoma is a complex group of optic neuropathies defined by loss of retinal ganglion cells (RGCs) and their axons within the optic nerve.1,2 Intraocular pressure (IOP) is a major risk factor that is commonly elevated. Glaucoma is a leading cause of vision loss in both humans and animals, especially dogs.1–6 Depending on absence or presence of a detectable underlying disease process resulting in impaired aqueous humor drainage through the iridocorneal angle (ICA) and subsequent IOP increase, glaucoma is defined as either primary or secondary. Individuals affected with primary glaucoma have a genetic predisposition. Primary glaucoma can be further categorized as either open-angle or angle-closure glaucoma based on ICA morphology.1,2 Possible causes of secondary glaucoma include the impairment of aqueous humor outflow through the ICA by invading neoplasms or neovascularization and formation of a preiridal fibrovascular membrane (PIFVM) as induced by chronic anterior uveitis.4,5,7 In dogs, lens-induced uveitis associated with cataract formation and surgery are common causes of secondary, neovascular glaucoma.8–14 Regardless of the underlying etiology, the final common pathway for all forms of glaucoma consists of loss of RGCs and their axons with characteristic atrophy and cupping of the optic nerve head (ONH).
Except for addressing known underlying causes in secondary glaucoma, currently accepted treatments to slow disease progression are limited to lowering IOP by medical and surgical methods. These occur by lowering production and increasing drainage of aqueous humor. The most used drug classes by veterinary ophthalmologists, under consideration of species-specific efficacy differences, include prostaglandin-analogues, carbonic anhydrase inhibitors, and beta-adrenergic antagonists.15–25 Unfortunately, the extent and duration of drug efficacy tends to be limited, resulting in disease progression despite therapy.1,2 The development of new IOP-lowering glaucoma drugs is slow, and they are generally optimized for human rather than veterinary application.26–28
A major limitation of medical glaucoma therapy, which has been investigated more thoroughly in human than veterinary patients, is poor adherence. Glaucomatous optic neuropathy may progress because eye drops are not administered as frequently as recommended.29–31 Poor adherence, especially in elderly patients, can be based on several factors, including forgetfulness or technical difficulties administering eye drops. As an alternative, a number of pharmaceutical companies are working on biodegradable intracameral drug implants that continuously release prostaglandin-analogues inside the eye.32 In March 2020, the U.S. Food and Drug Administration (FDA) approved the bimatoprost implant Durysta™ by Allergan plc to lower IOP in human open-angle glaucoma (OAG) and ocular hypertension patients. Other implants for sustained intraocular drug release are in preclinical and clinical trials.32,33
Surgical methods to reduce aqueous humor production consists of transscleral or endoscopic laser cyclophotocoagulation, and aqueous humor outflow can be increased via filtration surgery with or without the use of gonioimplants.1,2,34,35 While technical advances have been made, degree and duration of efficacy still need to be improved considering significant failure rates in some forms of glaucoma as well as animal species and breeds.2,35
Unless the underlying disease mechanisms can be identified and eliminated, such as in some forms of secondary glaucoma, a cure is not available even with apparently well-controlled IOP. There is a great need for more effective therapies that target specific disease mechanisms and prevent the progressive loss of RGCs and their axons. Gene and cell therapies have the potential to provide more effective and lasting treatments or even cures by addressing specific disease mechanisms. While not yet approved for clinical application, these treatments showed great potential in laboratory settings and several glaucoma animal models and will be reviewed here.
2. UNDERSTANDING DISEASE MECHANISMS AND EARLY DIAGNOSIS
Gene and cell therapies are well suited to be tailored for specific genetic defects or disease pathways and have the potential to be adjustable for individual patients; a strategy defined as personalized or precision medicine. Initial progress in the investigation of canine glaucoma genetics included the identification of mutations in the ADAMTS10, ADAMTS17, and OLFML3 genes causing primary glaucoma in a number of breeds.36–43 There is also ongoing progress to predict more accurately glaucoma progression based on ICA morphology and aqueous humor outflow pathways; these efforts are facilitated by improvements in high-resolution imaging techniques.44–49
One of the major challenges in the successful translation of novel therapies from the laboratory bench into the clinic is the limited ability to diagnose glaucoma early so that treatment can be initiated more effectively before too much damage has occurred. Many of the novel gene and cell therapies discussed here were tested under experimental conditions with treatment beginning simultaneously or even before the onset of optic nerve damage.50–58 This experimental setup does not represent clinical reality. In human patients with primary open-angle glaucoma (POAG), visual field loss is not diagnosed until 50% of RGC axons have already been lost.59,60 A recent study of canine eyes at risk of developing primary angle-closure glaucoma (PACG) showed significant loss of total retinal and nerve fiber layer thickness measured by high-resolution imaging with optical coherence tomography (OCT) before the occurrence of IOP increase and other clinical signs of glaucoma.61 Moving forward, our ability to diagnose glaucoma early will be facilitated by a better understanding of disease mechanisms and improved diagnostic technologies, most importantly high-resolution imaging of the anterior and posterior ocular segments, continuous tonometry, and genetic testing.2
3. BASIC PRINCIPLES OF GENE AND CELL THERAPIES
3.1. Gene therapy
Ocular gene therapy is defined as the introduction of genetic material, DNA or RNA, into cells of the eye to modify gene expression and achieve a therapeutic effect. A common, effective method to administer the genetic material into the eye is using a viral vector, such as adeno-associated virus (AAV) or lentivirus.62,63 Generally, the gene therapy vector is administered in close proximity to the cells to be treated – the specific injection techniques, most importantly intracameral and intravitreal, will be discussed throughout this article. In addition to the injection technique, the ability to transduce and target transgene expression to a certain cell type is determined by the cell tropism of the viral vector, which is influenced by the viral capsid and serotype, and the promoter regulatory sequence that is placed in front of the transgene.62,63 A genetic switch, for example glucocorticoid response elements (GRE), can be included so that the transgene can be turned on and off by initiating or discontinuing the use of particular drugs.64
The advantages of gene therapy include the ability to specifically target known disease mechanisms and cells. Furthermore, if target cells are post-mitotic, i.e. not replaced, a one-time treatment will result potentially in a long-term therapeutic effect and possibly even a cure. Gene therapy cannot replace cells that have already been lost by disease. Overall, the eye is well suited for gene and cell therapies because it forms a separate compartment that is immune privileged.65
Most ocular gene therapies have been developed for the treatment of inherited diseases. Gene therapy can be used to address genetic risk factors by replacing, silencing, or editing defective genes that contribute to the development and progression of disease.62,66 Most ocular gene therapies have been developed for autosomal recessive traits by replacing the mutated, non-functional gene with a normal or wild type copy.67,68 The treatment of autosomal dominant traits is more challenging because the mutated gene producing a potentially toxic protein may have to be silenced or knocked down first and then replaced with a normal or wild type copy of the gene.69 The treatment of non-Mendelian, complex traits or acquired, non-genetic disorders may be more challenging, but possible if specific molecular disease pathways are identified that can be targeted. Gene therapy can also be applied to enable cells within the eye to produce proteins with a therapeutic effect.62
Several ocular gene therapies have already moved into clinical trials for human patients, mostly for the treatment of blinding retinal diseases.68 Generally, the eye injections to deliver gene therapies are well established and tolerated. In December 2017, the U.S. FDA approved the first viral ocular gene therapy, Luxturna™ by Spark Therapeutics, Inc., for the treatment of one form of retinal childhood blindness.
3.2. Cell therapy
Cell therapies are defined as the administration of cells with the purpose of achieving a therapeutic effect. Two main strategies are being investigated: (1) The use of stem cells to replace cells that have been lost in a degenerative disease process – this is referred to as regenerative medicine; and (2) the use of genetically modified cells or stem cells as a mean to continuously synthesize and release proteins with a therapeutic effect on adjacent cells and tissues.70
Stem cells are undifferentiated or partially differentiated cells with the ability to proliferate and differentiate into many different types of specialized cells. The ability to induce pluripotent stem cells (iPSCs) from differentiated adult cells, such as skin or blood cells, has boosted stem cell research for clinical application since it replaced the need of using human embryologic stem cells (hESCs), which have been associated with some ethical concerns.70
Despite much progress in laboratory settings and some ongoing human clinical trials, there is currently no approved ocular stem cell therapy for clinical application in humans or animals. Nevertheless, autologous adult stem cells, for instance hematopoietic stem cells harvested from bone marrow and mesenchymal stem cells harvested from fat tissue, are being used commercially for clinical application in humans and animals. Intraocular administration of these cells is not approved by the U.S. FDA and have not been scientifically proven to provide a safe, therapeutic effect (https://www.fda.gov/consumers/consumer-updates/fda-warns-about-stem-cell-therapies). Recent human case reports have demonstrated the potential adverse effects of such non-approved intraocular stem cell therapies, including intraocular hemorrhage, retinal detachment, and vision loss.71–73
3.3. Potential applications of gene and cell therapies for glaucoma
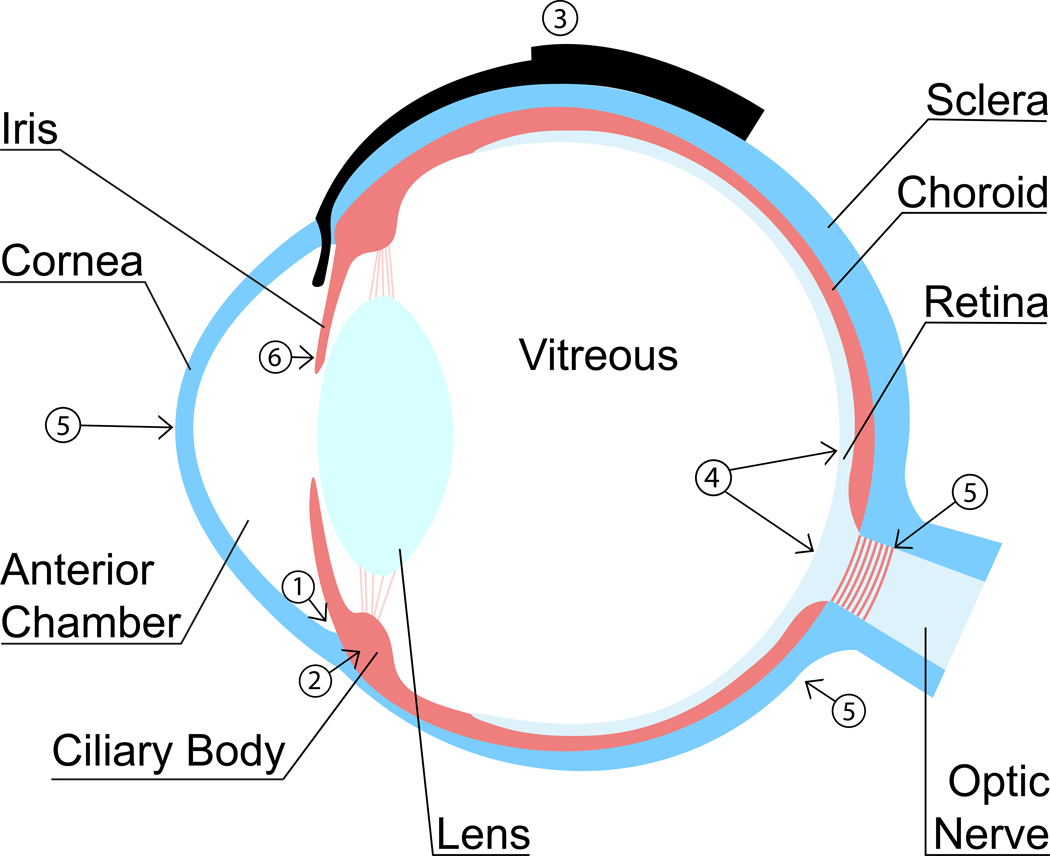
Both gene and cell therapies are rapidly evolving in laboratory settings and offer great potential advantages over many conventional drugs, including longer duration with continuous drug release and a therapeutic effect that can be tailored to specific molecular disease pathways within an individual human or animal patient. The following potential applications could be considered for improved glaucoma therapy and will be discussed here (Figure 1): improved IOP control by increased aqueous humor drainage (1), decreased aqueous humor production (2), and/or prevention of gonioimplant bleb fibrosis (3), neuroprotection and neuroregeneration of RGCs and their axons (4), modification of the ocular biomechanical properties for improved IOP tolerance (5), and inhibition of inflammation and neovascularization to prevent the formation of PIFVM and secondary, neovascular glaucoma (6).
Figure 1.
Schematic cross-section of an eye showing potential targets for gene and cell glaucoma therapies: (1) Increased aqueous humor outflow, (2) decreased aqueous humor production, (3) preventing gonioimplant bleb fibrosis, (4) RGC neuroprotection and neuroregeneration, (5) modification of biomechanical properties of sclera, lamina cribrosa, and cornea, and (6) inhibition of inflammation and PIFVM formation.
4. INTRAOCULAR PRESSURE (IOP) CONTROL
4.1. Increasing aqueous humor outflow by treatment of outflow pathways
Lowering IOP is the only approved treatment strategy and will remain the main focus of glaucoma therapy in the foreseeable future. Current treatments consist of medical and surgical techniques to lower production and increase drainage of aqueous humor; however, there are limitations in the extent and duration of IOP-lowering treatments.2 As we gain a better understanding of the physiology and pathophysiology of aqueous humor outflow resistance in different species, breeds, and forms of glaucoma, including genetic risk factors, more effective, lasting therapies can be developed. Gene and cell therapies have great potential to be tailored to these specific molecular disease pathways.
Major advances have been made in the robust and safe targeting of transgene expression to the trabecular meshwork and the aqueous humor outflow pathways within the ICA of different animal species and human cell and organ cultures.62 While transgene expression can be targeted to the trabecular meshwork with naked nucleic acid,74 the use of viral vectors generally provides more robust transduction. Both intracameral and intravitreal administration of adenovirus, lentivirus, adeno-associated virus (AAV), and herpes simplex virus (HSV) gene therapy vectors have been performed successfully to target aqueous humor outflow pathways, with the intracameral route being preferred in larger animal species (Figure 2).62,75–80 Traditionally, intracamerally injected adenovirus was most effective as shown in several species, including mouse, rat, rabbit, dog, cynomolgus monkey, and human cell and organ cultures, and it appears to be the preferred viral vector to target the murine trabecular meshwork via intracameral or intravitreal injection.81–86 Because adenovirus is more immunogenic and tends to cause more uveitis compared to AAV and lentivirus, it is rarely used in larger animal species.62,82 Lentivirus vectors provide robust targeting of the aqueous humor outflow pathways in cats, cynomolgus monkeys, and cultured human donor eyes, as shown most commonly with modified feline and human immunodeficiency virus.87–89
Figure 2.
Schematic cross-section of an eye showing possible methods for intraocular administration of gene and cell therapies for glaucoma. (1) Gene therapy vectors or cells for the treatment of the aqueous humor outflow pathways within the ICA are administered by intracameral injection. (2) Injections through the pars plana of the ciliary body into the anterior vitreous are used to target the ciliary body epithelium; reagents will also reach the anterior chamber and aqueous humor outflow pathways. (3) Gene therapy vectors or cells for neuroprotective therapy are injected through the pars plana into the posterior vitreous, close to the surface of the retina and ONH. (4) Capsules with genetically modified cells, that release neuroprotective reagents, are attached to the sclera at the pars plana and reach into the anterior vitreous. (5) Transplantation of RGCs to restore vision are injected into the posterior vitreous or (not shown) underneath the retina’s inner limiting membrane. If successful, the cells embed in the retina and form axons that extend through the optic nerve and connect with targets in the brain.
While AAV tends to be the preferred vector for ocular gene therapy with great safety and efficacy in preclinical and clinical trials, including FDA approved Luxturna™, the first commercially available viral ocular gene therapy, it has not shown great results in initial attempts to target the trabecular meshwork.62,90,91 The use of the self-complementary AAV vectors and the recent development of novel capsid mutated AAV particles resulted in an expanded toolkit for targeting of the trabecular meshwork and other tissues within the anterior segment of the eye (Figure 3).62,77,78,80,92–94,95 The self-complementary AAV genome facilitates the generation of double-stranded DNA for more efficient and long-term transduction of trabecular meshwork cells in several species, with long-lasting effects for at least 2 years in monkeys.62,77,91 Capsid mutant AAV2 (AAV of serotype 2) showed the highest transduction of the trabecular meshwork, followed by AAV5, AAV6 and AAV8 serotypes as shown in the mouse, rat, and cultured human trabecular meshwork cells and perfused anterior chambers.78,80,93 AAV capsid mutations even resulted in improved trabecular meshwork targeting without the use of self-complementary genome, thereby permitting the insertion of bigger transgenes.80,93
Figure 3.
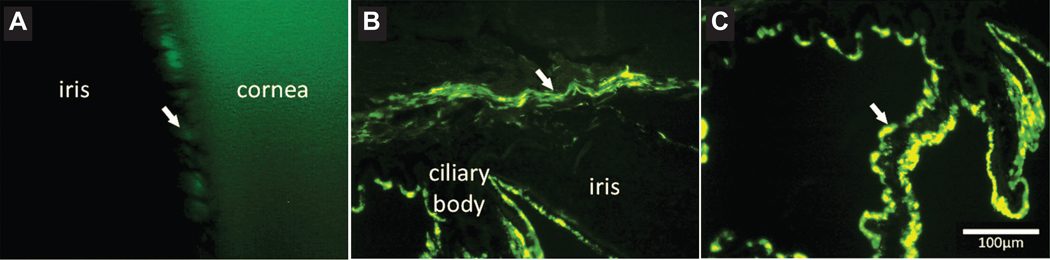
Targeting of GFP transgene expression to the canine anterior ocular segment by intravitreal injection of capsid mutated AAV2 (triple Y-F+T-V). (A) Fluorescent gonioscopic imaging showing fluorescence within the ciliary cleft 6 weeks post intravitreal AAV injection. Note the pectinate ligament fibrils demonstrated by the dark bands crossing the region of fluorescence. (B) Immunohistochemical labeling of cryosections of the canine anterior segment shows GFP within the trabecular meshwork (arrow). (C) GFP expression is also seen within the ciliary body epithelium (arrow). GFP, green fluorescent protein; triple Y-F + T-V, AAV2 based capsid was mutated by substitution of three surface-exposed capsid tyrosine (Y) residues with phenylalanine (F) and one threonine (T) residue with valine (V). Scale bar, 100 μm. Boyd et al. 2016, with permission.94
Numerous studies in different animal species have provided proof of concept that gene therapy-mediated modification of gene expression within the trabecular meshwork can result in an IOP change. Two types of studies were performed in animal models and perfused human anterior segment cultures: (1) development of potential treatments by lowering IOP, 62,64,96–105 and (2) development of glaucoma animal models by increasing IOP.62,97,106–113 Sustained IOP reduction, for example, was achieved for at least 5 months in normal cats and monkeys by lentivirus-mediated gene therapy of the trabecular meshwork with either prostaglandin biosynthesis enzyme cyclooxygenase-2 (COX-2) and prostaglandin F2alpha receptor (FPR), or prostaglandin F synthase (PGFS).104,105
An alternative approach to trabecular meshwork-targeted gene therapy is cell therapy. Trabecular meshwork-like cells can be created from iPSC and injected into the anterior chamber in order to regenerate trabecular meshwork cells that are lost with chronic glaucoma (Figure 2). Proof of concept for this treatment method was provided in transgenic, myocilin (MYOC)-mutant mice with POAG, where the cell therapy resulted in improved outflow facility, IOP control, and halted RGC loss, even in animals with advanced stages of glaucoma.114,115 Interestingly, the main therapeutic effect of the transplantation occurred via induction of proliferation of the remaining endogenous trabecular meshwork cells. These results were verified in human eye perfusion culture.116 Potential future clinical application may involve the collection of skin cells from glaucoma patients, followed by creation of iPSCs and induction of trabecular meshwork cells. These cells can then be administered intracamerally back into the same patients to achieve a therapeutic effect.
4.2. Decreasing aqueous humor production by treating the ciliary body epithelium
Even though the elevation of IOP in glaucoma patients occurs because of increased aqueous humor outflow resistance, glaucoma treatment strategies include the reduction of aqueous humor production by medical (e.g., carbonic anhydrase inhibitors) and surgical (i.e., cyclophotocoagulation) therapies.1,2,34 While most glaucoma gene therapy efforts aimed at lowering IOP by treatment of the aqueous humor outflow pathways, there were a few attempts to lower aqueous humor production by the ciliary body epithelium. The advantage of any such gene or cell therapy would be the replacement of daily eye drops for a more effective, sustained control of IOP following a one-time vector injection. Since the underlying disease process is not being addressed with this treatment approach, it is possible that disease progression may only be slowed but not prevented. The ciliary body epithelium can be reached by gene therapy vectors via intravitreal injection (Figure 2 and Figure 3).94,117 Recently, a team of investigators achieved a reduction of aqueous humor production in experimental mouse models of corticosteroid and microbead-induced ocular hypertension following an intravitreal AAV administration that resulted in disruption of the aquaporin 1 (AQP1) gene within the ciliary epithelium by use of CRISPR-Cas9 gene editing.117
4.3. Increasing aqueous humor outflow by inhibiting fibrosis of conjunctival filtration bleb
Glaucoma filtration surgery is commonly used in humans and dogs when IOP cannot be controlled by maximized medical therapy.1,2,35 Aqueous humor drainage is increased typically by diversion under the conjunctiva and Tenon’s capsule and the formation of a filtration bleb.118 The most common techniques are trabeculectomy in humans and gonioimplants, for example Ahmed valve or Baerveldt glaucoma drainage device, in humans and dogs.2,35,119,120 Filtration surgery failures are not uncommon and usually due to excessive wound healing in the subconjunctival space resulting in fibrosis of the filtration bleb, inhibition of aqueous humor drainage, and IOP increase.2,35,118,121,122 Susceptibility to bleb failure appears to vary between species, with dogs showing more fibrosis than humans, and even between individual canine breeds indicating potential genetic risk factors that could be addressed by gene therapies.2 Antimitotic drugs, most commonly mitomycin C (MMC), are routinely used to soak the conjunctival pocket during surgery and minimize bleb fibrosis.121,123–128 Duration of antimitotic drug application and subsequent copious irrigation are critical to minimize adverse effects.118 There is a delicate balance between bleb failure due to fibrosis and toxic effects of antimetabolite overuse, most often conjunctival dehiscence with bleb leakage as well as scleral and conjunctival necrosis.118,123,126,129–135
The search for new, improved and safer antifibrotic treatment strategies (‘scar wars’) has been ongoing for many years and continues to be a high priority.2,118 Numerous studies in several animal species, including rabbits, rats, dogs, and non-human primates, were conducted to determine the molecular and cellular disease mechanisms of conjunctival bleb fibrosis so that they can be targeted more specifically and effectively.118,136–142
Gene therapy has a great potential to specifically target and modify these pathways for safe and long-term prevention of bleb fibrosis. For example, small interfering RNA (siRNA) was developed to silence transcription factors involved in conjunctival tissue fibrosis: the myocardin-related transcription factor/serum response factor (MRTF/SRF) pathway or secreted protein acidic and rich in cysteine (SPARC).143–145 Intravitreal antisense oligonucleotides against transforming growth factor-beta2 (TGF-beta2) showed promising results in human subjects with OAG undergoing trabeculectomy.146 Gene therapy was also successfully performed in rabbits and ocular hypertensive monkeys by recombinant adenovirus with the human p21 transgene (encoding the CDKN1A protein), either by presurgical subconjunctival injection or by topical administration onto the surgical field, in order to modulate wound healing after glaucoma trabeculectomy surgery.147 The intention was to cause cell cycle arrest of surrounding cells rather than their destruction as with MMC. Another adenovirus-mediated gene therapy method tested in mice consisted of blocking p38 mitogen-activated protein kinase (MAPK) on post-injury conjunctival scarring; it resulted in inhibition of the fibrogenic reaction induced by the subconjunctival fibroblasts.148
5. NEUROPROTECTION AND NEUROREGENERATION
5.1. Neuroprotection
In many human and animal glaucoma patients the optic nerve continues to degenerate despite apparently effective IOP control within its physiologic range.1,2 The reasons and mechanisms for the continuing RGC death by apoptosis are not fully understood and may not all be associated with IOP-related biomechanical axon damage at the lamina cribrosa within the ONH.149–152 Some mechanisms that have been identified and studied include excitotoxicity caused by excessive excitatory amino acid release, most importantly glutamate and aspartate,153,154 neurotrophin deprivation from blockage of retrograde axonal transport,155–158 excessive intracellular calcium,159 compromised blood flow to the ONH and retina,160–166 oxidative stress,167–171 inflammation and autoimmunity against retinal and optic nerve antigens,172–174 and reactive gliosis.175–178 There is a general understanding among glaucoma clinicians and vision scientists that a more effective glaucoma therapy must include neuroprotective strategies to protect the RGCs and their axons.1,2,179
Several compounds were identified with neuroprotective properties in laboratory settings, mostly in rodent models of optic nerve injury, acute ocular hypertension, and experimental hypertensive glaucoma.180,181 So far, translation of these treatments into the clinical application with proven efficacy failed. Most prominently, the N-methyl-D-aspartate (NMDA) receptor antagonist memantine, which counters the toxic effect of excessive glutamate in the extracellular space, reduced RGC death and functional loss in experimental glaucoma in rats and primates.182–184 Unfortunately, two phase 3 clinical trials failed to demonstrate the protection of visual function by memantine in human glaucoma patients.185
Gene therapy is well suited for the development of neuroprotective treatments of RGCs because (1) many transgenes can be easily incorporated into viral vectors for specific modification of molecular disease pathways, and (2) the RGCs located in the inner retina can be directly targeted via intravitreal vector administration (Figure 2). The latter has been demonstrated mostly with AAV in several animal species, including mice, rats, dogs, and monkeys with differences in transduction efficiency partially due to species-specific variations in retinal inner limiting membrane thickness (Figure 4).94,186–192 Techniques for sub-inner limiting membrane injection of AAV have been described to improve transgene delivery to the retina.193,194 While other intraocular injection methods could also be considered, for example subretinal and suprachoroidal techniques, the intravitreal administration of viral vector appears to be the most effective to target RGCs.191,195
Figure 4.
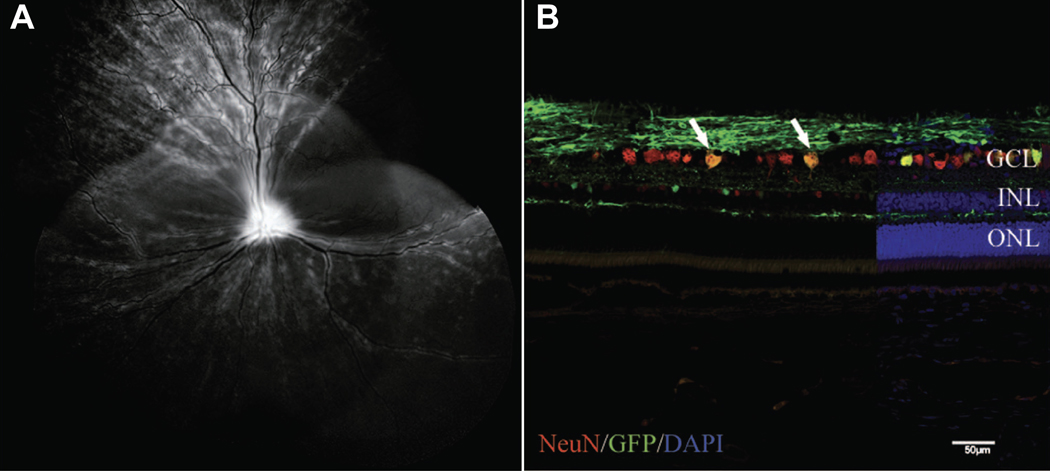
Transduction of canine RGC following intravitreal injection of capsid mutated AAV2 (triple Y-F + T-V) with GFP transgene. (A) Representative confocal scanning laser ophthalmoscopy image obtained at 5 weeks post injection demonstrated widespread GFP fluorescence. (B) Immunohistochemical labeling of retinal cryosection with neuronal nuclei (NeuN) antibody (red) to label RGCs demonstrates a high number of cells colabeling with GFP (green). Cell nuclei are shown in blue with DAPI.
DAPI, 4’,6-diamidino-2-phenylindole; GCL, ganglion cell layer; GFP, green fluorescent protein; INL, inner nuclear layer; ONL, outer nuclear layer; triple Y-F+T-V, AAV2 based capsid was mutated by substitution of three surface-exposed capsid tyrosine (Y) residues with phenylalanine (F) and one threonine (T) residue with valine (V). Scale bar, 50 μm. Boyd et al. 2016, with permission.94
Tropism for RGCs varies between different viral vectors with AAV2 being superior compared to adenovirus.62 The specific targeting of RGCs by gene therapy vectors may not be critical for all neuroprotective strategies; some transgenes encode for secreted proteins that have a regional protective effect following transduction of Müller glia.196,197 An alternative, less practical approach to target RGCs is by administering the gene therapy vector to specific areas of the brain with the transgene being transported to the RGCs via retrograde axonal transport.198
Several neuroprotective gene therapy strategies to support RGC survival were successfully tested in rodent models with spontaneous glaucoma (DBA/2J mice), experimental hypertensive glaucoma, acute IOP elevation, or optic nerve injury (optic nerve crush and transection); this was achieved mostly by intravitreal administration of AAV.62
Neurotrophins have been investigated extensively as a possible neuroprotective treatment to promote RGC survival in glaucoma and traumatic optic neuropathies.62,199 Neurotrophins are essential proteins for differentiation and maintenance of RGCs, but some neurotrophins and their receptors are downregulated in glaucoma and traumatic optic neuropathies.200,201 Since neurotrophins are proteins, the effect of their direct intravitreal injection is short-lived with a need for sustained production and release that could be achieved by gene or cell therapies.202 Protection of RGCs was demonstrated by viral vector-mediated overexpression of brain-derived neurotrophic factor (BDNF) and its TrkB receptor, ciliary neurotrophic factor (CNTF), glial cell-line derived neurotrophic factor (GDNF), and their downstream pathways.50–52,57,196,197,201,203–205
In addition to neurotrophins, other successfully tested neuroprotective gene therapy strategies for RGCs included increased expression of antiapoptotic,53,206–208 antioxidant,54–56, and anti-inflammatory genes,58,209,210 as well as inhibition of proapoptotic genes.211 Intramuscular injection of AAV5 with the mutant erythropoietin (EPO) transgene EpoR76E provided RGC neuroprotection in glaucomatous DBA/2J mice and mice with experimental hypertensive glaucoma via the systemic release of EPO from the skeletal muscle.212–214
A less developed strategy for delivery of non-selective neuroprotective and/or immune-modulatory factors to the RGCs is the intravitreal administration of multipotent stem cells, ocular progenitor cells, or other, genetically modified cells; this technique is time-limited by the viability and secretory capacity of the transplanted cells (Figure 2).70 The cells are injected as a single cell suspension but subsequently aggregate in vivo to form small clusters that are free-floating in the vitreous and provide neurotrophic support to the retina without the need for integration into the host tissue.70
A well-tested alternative to the intravitreal injection of cells for neuroprotection is the use of encapsulated cell technology (Figure 2). This approach was previously tested in animals and human patients with inherited retinal dystrophies, including Irish Setter dogs with rod-cone dystrophy 1 (rcd1).215,216 The NT-501 capsule was developed by Neurotech Pharmaceuticals, Inc., and contains genetically modified human cells that synthesize and continuously release the neurotrophic factor CNTF through a semipermeable membrane into the vitreous. The implant is anchored to the sclera at the pars plana of the ciliary body. The continuous release of CNTF by intravitreal encapsulated cell therapy was tested recently in a phase 1 clinical trial on human patients with POAG (ClinicalTrials.gov NCT01408472) and ischemic optic neuropathy (NCT01411657), but results have not yet been published. Two phase 2 clinical trials for the treatment of glaucoma are currently ongoing (NCT02862938 and NCT04577300).
5.2. Neuroregeneration
Like other neurons in the fully developed mammalian central nervous system (CNS), RGCs and their axons within the optic nerve are unable to regenerate after injury, such as glaucoma.217 This is different from peripheral neurons and CNS neurons of other animal species, for instance fish and amphibians, which have the ability to regenerate throughout their lifespan.218 The failure of optic nerve axons to regenerate is based on a combination of environmental factors within the optic nerve, including inhibition by CNS-specific myelin proteins, as well as reactive scarring and inflammation, and other cellular and molecular responses mediated by glial cells.218,219 If a RGC is still alive, gene therapy strategies could promote regrowth of its axon within the optic nerve, but a major challenge is the ability of these axons to reach and properly connect with targets in the brain in order to restore functional visual capacity.51,218,219 While the clinical application is not available yet, major advances have been made in understanding the mechanisms regulating RGC survival and optic nerve axon regeneration following injury.217,219 Supportive gene therapies and other strategies are being investigated. Promising work was done in adult mice with optic nerve crush injury in which a combination of gene therapy-induced modification of gene expression resulted in RGC axon regeneration and partial restoration of visual function.220
The replacement of dead and lost RGCs is associated with a number of additional major challenges: lost cells have to be replaced, followed by extension and growth of dendrites and axons, and functional connections with the proper targets in the brain.221 Three main strategies are being pursued for RGC replacement therapy: (1) syngeneic transplantation of adult iPSCs programmed to assume the RGC phenotype, (2) allogeneic transplantation of RGCs from healthy donor eyes, and (3) reprogramming endogenous Müller glial cells into RGCs by gene therapy approach.218 The transplantation of cells could potentially occur via sub-inner limiting membrane or intravitreal injection, as long as the cells are able to penetrate the retinal inner limiting membrane and become integrated into the retina (Figure 2).192–194 Regeneration of the optic nerve is a high priority of several funding agencies, including the U.S. National Eye Institute (NEI)/National Institutes of Health (NIH) and U.S. Department of Defense.222 Improvements in optic nerve regeneration may even permit whole eye transplantations in the future.219
While RGC replacement therapies have not yet reached human patients, clinical trials were conducted or are currently underway using hESCs and iPSCs for dry age-related macular degeneration (AMD) and retinitis pigmentosa (NCT04339764).223
6. INFLUENCING THE BIOMECHANICAL PROPERTIES OF THE EYE
There are strong indications that an individual’s susceptibility to IOP increase and variation is determined by the biomechanical properties of the eye, most importantly its fibrous layer consisting of cornea, sclera, and lamina cribrosa.224,225 In some eyes, the RGC axons are not sufficiently supported by the lamina cribrosa and the peripapillary sclera, so that even physiologic IOP can be harmful.1,2 This may be the case in human patients with normotensive OAG and progressive optic nerve damage despite consistent IOP measurements below 21 mmHg.226,227 Disease progression can be slowed or prevented in these patients by a 30% reduction in IOP.228 Veterinary ophthalmologists also observe individual variations in IOP susceptibility, which tends to be species- and breed-related.2 While proof still needs to be provided in dogs that tissue biomechanical properties are linked to IOP susceptibility, initial studies in ADAMTS10-mutant Beagles with OAG showed that their posterior sclera is weaker with reduced fibrous collagen density, possibly explaining the slower optic nerve degeneration with chronic, severe IOP increases compared to dogs of other breeds.45,229–231 Similar findings can be expected in other ADAMTS10- and ADAMTS17-mutant dogs with OAG, for example Norwegian Elkhound (ADAMTS10),36 Petit Basset Griffon Vendéen (ADAMTS17),38,232 Basset Fauve de Bretagne (ADAMTS17),40 and Chinese Shar-Pei (ADAMTS17).41,232 Some transgenic mice are also resistant to glaucomatous damage, while hardening of the sclera with cross-linking agents worsens IOP-related damage to the RGC axons.224
Once we gain a better understanding of which biomechanical properties of the fibrous layer are advantageous, therapeutic tools can be developed to modify these properties to achieve a protective effect from IOP increases. Technologies for specific targeting of transgene expression to the sclera and lamina cribrosa still need to be developed. The peripapillary sclera could potentially be reached by gene therapy vector via suprachoroidal or retrobulbar injections.195,233–236 Targeting the lamina cribrosa by gene therapy without damaging the RGC axons will be a more challenging undertaking.
Corneal biomechanical properties not only affect tonometry measurements but also appear to influence susceptibility to glaucomatous damage.225,237 Corneal hysteresis, a clinical parameter of the cornea’s viscoelastic properties, has emerged as an important risk factor for glaucoma progression in humans.238 In contrast to sclera and lamina cribrosa, where gene therapy has not yet been performed, corneal AAV-mediated targeting of transgene expression and gene therapy was recently performed successfully in several animal species and disease models, such as dogs with Mucopolysaccharidoses I (MPS I).239 Transgene expression can be targeted to the cornea by intrastromal, intracameral, or subconjunctival injection, as well as topical eye drop application following removal of the corneal epithelium.240 Based on the available technologies, corneal gene therapy could be considered an additional possible tool to decrease an individual’s susceptibility to glaucomatous optic nerve damage.241
7. INHIBITION OF ANTERIOR UVEAL NEOVASCULARIZATION AND INFLAMMATION
While some forms of secondary glaucoma can be successfully treated by identifying and addressing the underlying cause, other forms continue to progress despite symptomatic therapy with IOP-lowering and anti-inflammatory medications. A major concern in veterinary ophthalmology is the development of secondary glaucoma in dogs following phacoemulsification surgery.2 While the underlying molecular disease mechanisms are still largely unknown, and may include genetic predisposition of certain breeds, the IOP increase in many affected dogs is based on the formation of PIFVM that covers the aqueous humor drainage pathways, resulting in IOP increase.10 An upregulation of vascular endothelial growth factor (VEGF) expression associated with canine lens-induced uveitis likely contributes to PIFVM formation post cataract surgery.242 Anti-neovascular gene therapies of the anterior chamber may be an effective treatment. Several such gene therapy strategies have already been developed for the posterior ocular segment in human patients with neovascular disease, most importantly neovascular AMD and diabetic retinopathy, and could be considered for use in dogs.243,244 These include the gene transfer of endostatin/angiostatin by lentivirus (RetinoStat® by Oxford BioMedica plc; clinicaltrials.gov: NCT01301443) or anti-VEGF antibodies by AAV8 (RGX-314 by RegenxBio®; clinicaltrials.gov: NCT03999801, NCT04567550, NCT04514653, and NCT03066258).243
More specific and effective gene therapies for anterior uveitis of unknown origin, including non-infectious, autoimmune uveitis, would also help to decrease the incidence of secondary glaucoma. A recently published study showed that intravitreal administration of AAV encoding human leukocyte antigen G1/5 (HLA-G1/5) reduced the severity of experimental autoimmune uveitis in rats.245
7. CONCLUSIONS
Much progress has been made in recent years in the development and clinical testing of ocular gene and cell therapies. Promising novel methods have been developed in the laboratory for the comprehensive treatment of glaucoma by reducing IOP and providing neuroprotection of the retina and optic nerve. As we gain a better understanding of glaucoma disease mechanisms, we will be able to target them specifically using gene and cell therapies in order to achieve safe, effective, and long-term prevention of vision loss. Early diagnosis of glaucoma will be critical for better treatment outcomes; this will be facilitated by improvements in diagnostic tools, for example high-resolution imaging and continuous tonometry. A major obstacle is the translation of new therapies from the laboratory into the clinical setting, especially for veterinary ophthalmic application, due to limited resources for developing animal-specific treatments.
ACKNOWLEDGMENT
Funding of the authors’ glaucoma research was provided by the National Eye Institute/National Institutes of Health (AMK: R01-EY025752; SAP: K08-EY030950), the BrightFocus Foundation (AMK: G2017185), and the Michigan State University College of Veterinary Medicine Endowed Research Funds (AMK).
CONFLICT OF INTEREST
The authors have the following potential conflicts of interest: CRISPR Therapeutics (AMK: research funding) and PolyActiva Pty Ltd (AMK: research funding).
REFERENCES
- 1.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311(18):1901–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Komaromy AM, Bras D, Esson DW, et al. The future of canine glaucoma therapy. Veterinary ophthalmology. 2019;22(5):726–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gelatt KN, MacKay EO. Prevalence of the breed-related glaucomas in pure-bred dogs in North America. Veterinary ophthalmology. 2004;7(2):97–111. [DOI] [PubMed] [Google Scholar]
- 4.Gelatt KN, MacKay EO. Secondary glaucomas in the dog in North America. Veterinary ophthalmology. 2004;7(4):245–259. [DOI] [PubMed] [Google Scholar]
- 5.Strom AR, Hassig M, Iburg TM, Spiess BM. Epidemiology of canine glaucoma presented to University of Zurich from 1995 to 2009. Part 2: secondary glaucoma (217 cases). Veterinary ophthalmology. 2011;14(2):127–132. [DOI] [PubMed] [Google Scholar]
- 6.Strom AR, Hassig M, Iburg TM, Spiess BM. Epidemiology of canine glaucoma presented to University of Zurich from 1995 to 2009. Part 1: Congenital and primary glaucoma (4 and 123 cases). Veterinary ophthalmology. 2011;14(2):121–126. [DOI] [PubMed] [Google Scholar]
- 7.Johnsen DA, Maggs DJ, Kass PH. Evaluation of risk factors for development of secondary glaucoma in dogs: 156 cases (1999–2004). Journal of the American Veterinary Medical Association. 2006;229(8):1270–1274. [DOI] [PubMed] [Google Scholar]
- 8.Newbold GM, Kelch WJ, Chen T, Ward DA, Hendrix DVH. Phacoemulsification outcomes in Boston terriers as compared to non-Boston terriers: a retrospective study (2002–2015). Veterinary ophthalmology. 2018. [DOI] [PubMed] [Google Scholar]
- 9.Foote BC, Pederson SL, Welihozkiy A, et al. Retinal detachment and glaucoma in the Boston Terrier and Shih Tzu following phacoemulsification (135 patients): 2000–2014. Veterinary ophthalmology. 2018;21(3):240–248. [DOI] [PubMed] [Google Scholar]
- 10.Scott EM, Esson DW, Fritz KJ, Dubielzig RR. Major breed distribution of canine patients enucleated or eviscerated due to glaucoma following routine cataract surgery as well as common histopathologic findings within enucleated globes. Veterinary ophthalmology. 2013;16 Suppl 1:64–72. [DOI] [PubMed] [Google Scholar]
- 11.Moeller E, Blocker T, Esson D, Madsen R. Postoperative glaucoma in the Labrador Retriever: incidence, risk factors, and visual outcome following routine phacoemulsification. Veterinary ophthalmology. 2011;14(6):385–394. [DOI] [PubMed] [Google Scholar]
- 12.Sigle KJ, Nasisse MP. Long-term complications after phacoemulsification for cataract removal in dogs: 172 cases (1995–2002). Journal of the American Veterinary Medical Association. 2006;228(1):74–79. [DOI] [PubMed] [Google Scholar]
- 13.Biros DJ, Gelatt KN, Brooks DE, et al. Development of glaucoma after cataract surgery in dogs: 220 cases (1987–1998). Journal of the American Veterinary Medical Association. 2000;216(11):1780–1786. [DOI] [PubMed] [Google Scholar]
- 14.Lannek EB, Miller PE. Development of glaucoma after phacoemulsification for removal of cataracts in dogs: 22 cases (1987–1997). Journal of the American Veterinary Medical Association. 2001;218(1):70–76. [DOI] [PubMed] [Google Scholar]
- 15.Gum GG, Larocca RD, Gelatt KN, Mead JP, Gelatt JK. The effect of topical timolol maleate on intraocular pressure in normal beagles and beagles with inherited glaucoma. Progress in Veterinary and Comparative Ophthalmology. 1991;1:141–150. [Google Scholar]
- 16.Wilkie DA, Latimer CA. Effects of topical administration of timolol maleate on intraocular pressure and pupil size in dogs. American journal of veterinary research. 1991;52(3):432–435. [PubMed] [Google Scholar]
- 17.Maehara S, Ono K, Ito N, et al. Effects of topical nipradilol and timolol maleate on intraocular pressure, facility of outflow, arterial blood pressure and pulse rate in dogs. Veterinary ophthalmology. 2004;7(3):147–150. [DOI] [PubMed] [Google Scholar]
- 18.Plummer CE, MacKay EO, Gelatt KN. Comparison of the effects of topical administration of a fixed combination of dorzolamide-timolol to monotherapy with timolol or dorzolamide on IOP, pupil size, and heart rate in glaucomatous dogs. Veterinary ophthalmology. 2006;9(4):245–249. [DOI] [PubMed] [Google Scholar]
- 19.Smith LN, Miller PE, Felchle LM. Effects of topical administration of latanoprost, timolol, or a combination of latanoprost and timolol on intraocular pressure, pupil size, and heart rate in clinically normal dogs. American journal of veterinary research. 2010;71(9):1055–1061. [DOI] [PubMed] [Google Scholar]
- 20.Cawrse MA, Ward DA, Hendrix DV. Effects of topical application of a 2% solution of dorzolamide on intraocular pressure and aqueous humor flow rate in clinically normal dogs. American journal of veterinary research. 2001;62(6):859–863. [DOI] [PubMed] [Google Scholar]
- 21.Gelatt KN, MacKay EO. Changes in intraocular pressure associated with topical dorzolamide and oral methazolamide in glaucomatous dogs. Veterinary ophthalmology. 2001;4(1):61–67. [DOI] [PubMed] [Google Scholar]
- 22.Scardillo A, Pugliese M, De Majo M, Niutta PP, Pugliese A. Effects of topical 0.5% levobunolol alone or in association with 2% dorzolamide compared with a fixed combination of 0.5% timolol and 2% dorzolamide on intraocular pressure and heart rate in dogs without glaucoma. Vet Ther. 2010;11(3):E1–6. [PubMed] [Google Scholar]
- 23.Studer ME, Martin CL, Stiles J. Effects of 0.005% latanoprost solution on intraocular pressure in healthy dogs and cats. American journal of veterinary research. 2000;61(10):1220–1224. [DOI] [PubMed] [Google Scholar]
- 24.Gelatt KN, MacKay EO. Effect of different dose schedules of latanoprost on intraocular pressure and pupil size in the glaucomatous Beagle. Veterinary ophthalmology. 2001;4(4):283–288. [DOI] [PubMed] [Google Scholar]
- 25.Tofflemire KL, Whitley EM, Allbaugh RA, et al. Comparison of two- and three-times-daily topical ophthalmic application of 0.005% latanoprost solution in clinically normal dogs. American journal of veterinary research. 2015;76(7):625–631. [DOI] [PubMed] [Google Scholar]
- 26.Gelatt KN, MacKay EO. Effect of single and multiple doses of 0.2% brimonidine tartrate in the glaucomatous Beagle. Veterinary ophthalmology. 2002;5(4):253–262. [DOI] [PubMed] [Google Scholar]
- 27.Yang VY, Miller PE, Keys DA, La Croix NC. Effects of 0.02% netarsudil ophthalmic solution on intraocular pressure of normotensive dogs. Veterinary ophthalmology. 2020. [DOI] [PubMed] [Google Scholar]
- 28.Leary KA, Lin KT, Steibel JP, Harman CD, Komaromy AM. Safety and efficacy of topically administered netarsudil (Rhopressa) in normal and glaucomatous dogs with ADAMTS10-open-angle glaucoma (ADAMTS10-OAG). Veterinary ophthalmology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okeke CO, Quigley HA, Jampel HD, et al. Adherence with topical glaucoma medication monitored electronically the Travatan Dosing Aid study. Ophthalmology. 2009;116(2):191–199. [DOI] [PubMed] [Google Scholar]
- 30.Friedman DS, Quigley HA, Gelb L, et al. Using pharmacy claims data to study adherence to glaucoma medications: methodology and findings of the Glaucoma Adherence and Persistency Study (GAPS). Investigative ophthalmology & visual science. 2007;48(11):5052–5057. [DOI] [PubMed] [Google Scholar]
- 31.Miller PE, Schmidt GM, Vainisi SJ, Swanson JF, Herrmann MK. The efficacy of topical prophylactic antiglaucoma therapy in primary closed angle glaucoma in dogs: a multicenter clinical trial. Journal of the American Animal Hospital Association. 2000;36(5):431–438. [DOI] [PubMed] [Google Scholar]
- 32.Miller PE, Eaton JS. Medical anti-glaucoma therapy: Beyond the drop. Veterinary ophthalmology. 2020. [DOI] [PubMed] [Google Scholar]
- 33.Kompella UB, Hartman RR, Patil MA. Extraocular, periocular, and intraocular routes for sustained drug delivery for glaucoma. Progress in retinal and eye research. 2020:100901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bras D, Maggio F. Surgical Treatment of Canine Glaucoma: Cyclodestructive Techniques. The Veterinary clinics of North America Small animal practice. 2015;45(6):1283–1305, vii. [DOI] [PubMed] [Google Scholar]
- 35.Maggio F, Bras D. Surgical Treatment of Canine Glaucoma: Filtering and End-Stage Glaucoma Procedures. The Veterinary clinics of North America Small animal practice. 2015;45(6):1261–1282, vi-vii. [DOI] [PubMed] [Google Scholar]
- 36.Ahonen SJ, Kaukonen M, Nussdorfer FD, Harman CD, Komaromy AM, Lohi H. A novel missense mutation in ADAMTS10 in Norwegian Elkhound primary glaucoma. PloS one. 2014;9(11):e111941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuchtey J, Olson LM, Rinkoski T, et al. Mapping of the disease locus and identification of ADAMTS10 as a candidate gene in a canine model of primary open angle glaucoma. PLoS genetics. 2011;7(2):e1001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forman OP, Pettitt L, Komaromy AM, Bedford P, Mellersh C. A Novel Genome-Wide Association Study Approach Using Genotyping by Exome Sequencing Leads to the Identification of a Primary Open Angle Glaucoma Associated Inversion Disrupting ADAMTS17. PloS one. 2015;10(12):e0143546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Komaromy AM, Petersen-Jones SM. Genetics of Canine Primary Glaucomas. The Veterinary clinics of North America Small animal practice. 2015;45(6):1159–1182, v. [DOI] [PubMed] [Google Scholar]
- 40.Oliver JA, Forman OP, Pettitt L, Mellersh CS. Two Independent Mutations in ADAMTS17 Are Associated with Primary Open Angle Glaucoma in the Basset Hound and Basset Fauve de Bretagne Breeds of Dog. PloS one. 2015;10(10):e0140436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oliver JAC, Rustidge S, Pettitt L, et al. Evaluation of ADAMTS17 in Chinese Shar-Pei with primary open-angle glaucoma, primary lens luxation, or both. American journal of veterinary research. 2018;79(1):98–106. [DOI] [PubMed] [Google Scholar]
- 42.Oliver JAC, Wright H, Massidda PA, Burmeister LM, Mellersh CS. A variant in OLFML3 is associated with pectinate ligament abnormality and primary closed-angle glaucoma in Border Collies from the United Kingdom. Veterinary ophthalmology. 2020;23(1):25–36. [DOI] [PubMed] [Google Scholar]
- 43.Pugh CA, Farrell LL, Carlisle AJ, et al. Arginine to Glutamine Variant in Olfactomedin Like 3 (OLFML3) Is a Candidate for Severe Goniodysgenesis and Glaucoma in the Border Collie Dog Breed. G3 (Bethesda). 2019;9(3):943–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dubin AJ, Bentley E, Buhr KA, Miller PE. Evaluation of potential risk factors for development of primary angle-closure glaucoma in Bouviers des Flandres. Journal of the American Veterinary Medical Association. 2017;250(1):60–67. [DOI] [PubMed] [Google Scholar]
- 45.Grozdanic SD, Kecova H, Harper MM, Nilaweera W, Kuehn MH, Kardon RH. Functional and structural changes in a canine model of hereditary primary angle-closure glaucoma. Investigative ophthalmology & visual science. 2010;51(1):255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hasegawa T, Kawata M, Ota M. Ultrasound biomicroscopic findings of the iridocorneal angle in live healthy and glaucomatous dogs. The Journal of veterinary medical science / the Japanese Society of Veterinary Science. 2016;77(12):1625–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kagemann L, Wollstein G, Ishikawa H, et al. Visualization of the conventional outflow pathway in the living human eye. Ophthalmology. 2012;119(8):1563–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kagemann L, Wollstein G, Ishikawa H, et al. 3D visualization of aqueous humor outflow structures in-situ in humans. Experimental eye research. 2011;93(3):308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Almazan A, Tsai S, Miller PE, et al. Iridocorneal angle measurements in mammalian species: normative data by optical coherence tomography. Veterinary ophthalmology. 2013;16(2):163–166. [DOI] [PubMed] [Google Scholar]
- 50.Martin KR, Quigley HA, Zack DJ, et al. Gene therapy with brain-derived neurotrophic factor as a protection: retinal ganglion cells in a rat glaucoma model. Investigative ophthalmology & visual science. 2003;44(10):4357–4365. [DOI] [PubMed] [Google Scholar]
- 51.Leaver SG, Cui Q, Plant GW, et al. AAV-mediated expression of CNTF promotes long-term survival and regeneration of adult rat retinal ganglion cells. Gene therapy. 2006;13(18):1328–1341. [DOI] [PubMed] [Google Scholar]
- 52.Pease ME, Zack DJ, Berlinicke C, et al. Effect of CNTF on retinal ganglion cell survival in experimental glaucoma. Investigative ophthalmology & visual science. 2009;50(5):2194–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilson AM, Chiodo VA, Boye SL, Brecha NC, Hauswirth WW, Di Polo A. Inhibitor of apoptosis-stimulating protein of p53 (iASPP) is required for neuronal survival after axonal injury. PloS one. 2014;9(4):e94175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen B, Tang L. Protective effects of catalase on retinal ischemia/reperfusion injury in rats. Experimental eye research. 2011;93(5):599–606. [DOI] [PubMed] [Google Scholar]
- 55.Xiong W, MacColl Garfinkel AE, Li Y, Benowitz LI, Cepko CL. NRF2 promotes neuronal survival in neurodegeneration and acute nerve damage. The Journal of clinical investigation. 2015;125(4):1433–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang W, Tang L, Zeng J, Chen B. Adeno-associated virus mediated SOD gene therapy protects the retinal ganglion cells from chronic intraocular pressure elevation induced injury via attenuating oxidative stress and improving mitochondrial dysfunction in a rat model. Am J Transl Res. 2016;8(2):799–810. [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou Y, Pernet V, Hauswirth WW, Di Polo A. Activation of the extracellular signal-regulated kinase 1/2 pathway by AAV gene transfer protects retinal ganglion cells in glaucoma. Molecular therapy : the journal of the American Society of Gene Therapy. 2005;12(3):402–412. [DOI] [PubMed] [Google Scholar]
- 58.Kwong JM, Gu L, Nassiri N, et al. AAV-mediated and pharmacological induction of Hsp70 expression stimulates survival of retinal ganglion cells following axonal injury. Gene therapy. 2015;22(2):138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Quigley HA, Addicks EM, Green WR, Maumenee AE. Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Archives of ophthalmology. 1981;99(4):635–649. [DOI] [PubMed] [Google Scholar]
- 60.Harwerth RS, Wheat JL, Fredette MJ, Anderson DR. Linking structure and function in glaucoma. Progress in retinal and eye research. 2010;29(4):249–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Graham KL, McCowan CI, Caruso K, Billson FM, Whittaker CJG, White A. Optical coherence tomography of the retina, nerve fiber layer, and optic nerve head in dogs with glaucoma. Veterinary ophthalmology. 2020;23(1):97–112. [DOI] [PubMed] [Google Scholar]
- 62.Borras T. The Pathway From Genes to Gene Therapy in Glaucoma: A Review of Possibilities for Using Genes as Glaucoma Drugs. Asia Pac J Ophthalmol (Phila). 2017;6(1):80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lundstrom K. Viral Vectors in Gene Therapy. Diseases. 2018;6(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Borras T, Buie LK, Spiga MG. Inducible scAAV2.GRE.MMP1 lowers IOP long-term in a large animal model for steroid-induced glaucoma gene therapy. Gene therapy. 2016;23(5):438–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Verdera HC, Kuranda K, Mingozzi F. AAV Vector Immunogenicity in Humans: A Long Journey to Successful Gene Transfer. Molecular therapy : the journal of the American Society of Gene Therapy. 2020;28(3):723–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kumar SR, Markusic DM, Biswas M, High KA, Herzog RW. Clinical development of gene therapy: results and lessons from recent successes. Mol Ther Methods Clin Dev. 2016;3:16034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aguirre GD. Concepts and Strategies in Retinal Gene Therapy. Investigative ophthalmology & visual science. 2017;58(12):5399–5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garafalo AV, Cideciyan AV, Heon E, et al. Progress in treating inherited retinal diseases: Early subretinal gene therapy clinical trials and candidates for future initiatives. Progress in retinal and eye research. 2019:100827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cideciyan AV, Sudharsan R, Dufour VL, et al. Mutation-independent rhodopsin gene therapy by knockdown and replacement with a single AAV vector. Proceedings of the National Academy of Sciences of the United States of America. 2018;115(36):E8547–E8556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singh MS, Park SS, Albini TA, et al. Retinal stem cell transplantation: Balancing safety and potential. Progress in retinal and eye research. 2020;75:100779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saraf SS, Cunningham MA, Kuriyan AE, et al. Bilateral Retinal Detachments After Intravitreal Injection of Adipose-Derived ‘Stem Cells’ in a Patient With Exudative Macular Degeneration. Ophthalmic Surg Lasers Imaging Retina. 2017;48(9):772–775. [DOI] [PubMed] [Google Scholar]
- 72.Turner L. Direct-to-consumer marketing of stem cell interventions by Canadian businesses. Regen Med. 2018;13(6):643–658. [DOI] [PubMed] [Google Scholar]
- 73.Kuriyan AE, Albini TA, Townsend JH, et al. Vision Loss after Intravitreal Injection of Autologous “Stem Cells” for AMD. The New England journal of medicine. 2017;376(11):1047–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Comes N, Borras T. Functional delivery of synthetic naked siRNA to the human trabecular meshwork in perfused organ cultures. Molecular vision. 2007;13:1363–1374. [PubMed] [Google Scholar]
- 75.Spencer B, Agarwala S, Miskulin M, Smith M, Brandt CR. Herpes simplex virus-mediated gene delivery to the rodent visual system. Investigative ophthalmology & visual science. 2000;41(6):1392–1401. [PubMed] [Google Scholar]
- 76.Liu X, Brandt CR, Gabelt BT, Bryar PJ, Smith ME, Kaufman PL. Herpes simplex virus mediated gene transfer to primate ocular tissues. Experimental eye research. 1999;69(4):385–395. [DOI] [PubMed] [Google Scholar]
- 77.Buie LK, Rasmussen CA, Porterfield EC, et al. Self-complementary AAV virus (scAAV) safe and long-term gene transfer in the trabecular meshwork of living rats and monkeys. Investigative ophthalmology & visual science. 2010;51(1):236–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bogner B, Boye SL, Min SH, et al. Capsid Mutated Adeno-Associated Virus Delivered to the Anterior Chamber Results in Efficient Transduction of Trabecular Meshwork in Mouse and Rat. PloS one. 2015;10(6):e0128759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dang Y, Loewen R, Parikh HA, Roy P, Loewen NA. Gene transfer to the outflow tract. Experimental eye research. 2017;158:73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang L, Xiao R, Andres-Mateos E, Vandenberghe LH. Single stranded adeno-associated virus achieves efficient gene transfer to anterior segment in the mouse eye. PloS one. 2017;12(8):e0182473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Budenz DL, Barton K, Gedde SJ, et al. Five-year treatment outcomes in the Ahmed Baerveldt comparison study. Ophthalmology. 2015;122(2):308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Borras T, Tamm ER, Zigler JS Jr. Ocular adenovirus gene transfer varies in efficiency and inflammatory response. Investigative ophthalmology & visual science. 1996;37(7):1282–1293. [PubMed] [Google Scholar]
- 83.Andrawiss M, Maron A, Beltran W, et al. Adenovirus-mediated gene transfer in canine eyes: a preclinical study for gene therapy of human uveal melanoma. The journal of gene medicine. 2001;3(3):228–239. [DOI] [PubMed] [Google Scholar]
- 84.Borras T, Gabelt BT, Klintworth GK, Peterson JC, Kaufman PL. Non-invasive observation of repeated adenoviral GFP gene delivery to the anterior segment of the monkey eye in vivo. The journal of gene medicine. 2001;3(5):437–449. [DOI] [PubMed] [Google Scholar]
- 85.Kee C, Sohn S, Hwang JM. Stromelysin gene transfer into cultured human trabecular cells and rat trabecular meshwork in vivo. Investigative ophthalmology & visual science. 2001;42(12):2856–2860. [PubMed] [Google Scholar]
- 86.Borras T, Matsumoto Y, Epstein DL, Johnson DH. Gene transfer to the human trabecular meshwork by anterior segment perfusion. Investigative ophthalmology & visual science. 1998;39(8):1503–1507. [PubMed] [Google Scholar]
- 87.Loewen N, Fautsch MP, Peretz M, et al. Genetic modification of human trabecular meshwork with lentiviral vectors. Human gene therapy. 2001;12(17):2109–2119. [DOI] [PubMed] [Google Scholar]
- 88.Loewen N, Fautsch MP, Teo WL, Bahler CK, Johnson DH, Poeschla EM. Long-term, targeted genetic modification of the aqueous humor outflow tract coupled with noninvasive imaging of gene expression in vivo. Investigative ophthalmology & visual science. 2004;45(9):3091–3098. [DOI] [PubMed] [Google Scholar]
- 89.Barraza RA, Rasmussen CA, Loewen N, et al. Prolonged transgene expression with lentiviral vectors in the aqueous humor outflow pathway of nonhuman primates. Human gene therapy. 2009;20(3):191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Borras T. Advances in glaucoma treatment and management: gene therapy. Investigative ophthalmology & visual science. 2012;53(5):2506–2510. [DOI] [PubMed] [Google Scholar]
- 91.Borras T, Xue W, Choi VW, et al. Mechanisms of AAV transduction in glaucoma-associated human trabecular meshwork cells. The journal of gene medicine. 2006;8(5):589–602. [DOI] [PubMed] [Google Scholar]
- 92.Borras T. Gene therapy strategies in glaucoma and application for steroid-induced hypertension. Saudi journal of ophthalmology : official journal of the Saudi Ophthalmological Society. 2011;25(4):353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rodriguez-Estevez L, Asokan P, Borras T. Transduction optimization of AAV vectors for human gene therapy of glaucoma and their reversed cell entry characteristics. Gene therapy. 2020;27(3–4):127–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Boyd RF, Boye SL, Conlon TJ, et al. Reduced retinal transduction and enhanced transgene-directed immunogenicity with intravitreal delivery of rAAV following posterior vitrectomy in dogs. Gene therapy. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Asokan A, Schaffer DV, Samulski RJ. The AAV vector toolkit: poised at the clinical crossroads. Molecular therapy : the journal of the American Society of Gene Therapy. 2012;20(4):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tan J, Wang X, Cai S, et al. C3 Transferase-Expressing scAAV2 Transduces Ocular Anterior Segment Tissues and Lowers Intraocular Pressure in Mouse and Monkey. Mol Ther Methods Clin Dev. 2020;17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aga M, Bradley JM, Wanchu R, Yang YF, Acott TS, Keller KE. Differential effects of caveolin-1 and −2 knockdown on aqueous outflow and altered extracellular matrix turnover in caveolin-silenced trabecular meshwork cells. Investigative ophthalmology & visual science. 2014;55(9):5497–5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gerometta R, Spiga MG, Borras T, Candia OA. Treatment of sheep steroid-induced ocular hypertension with a glucocorticoid-inducible MMP1 gene therapy virus. Investigative ophthalmology & visual science. 2010;51(6):3042–3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kumar S, Shah S, Tang HM, Smith M, Borras T, Danias J. Tissue plasminogen activator in trabecular meshwork attenuates steroid induced outflow resistance in mice. PloS one. 2013;8(8):e72447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vittitow JL, Garg R, Rowlette LL, Epstein DL, O’Brien ET, Borras T. Gene transfer of dominant-negative RhoA increases outflow facility in perfused human anterior segment cultures. Molecular vision. 2002;8:32–44. [PubMed] [Google Scholar]
- 101.Rao PV, Deng P, Maddala R, Epstein DL, Li CY, Shimokawa H. Expression of dominant negative Rho-binding domain of Rho-kinase in organ cultured human eye anterior segments increases aqueous humor outflow. Molecular vision. 2005;11:288–297. [PubMed] [Google Scholar]
- 102.Liu X, Hu Y, Filla MS, et al. The effect of C3 transgene expression on actin and cellular adhesions in cultured human trabecular meshwork cells and on outflow facility in organ cultured monkey eyes. Molecular vision. 2005;11:1112–1121. [PubMed] [Google Scholar]
- 103.Borras T, Buie LK, Spiga MG, Carabana J. Prevention of nocturnal elevation of intraocular pressure by gene transfer of dominant-negative RhoA in rats. JAMA ophthalmology. 2015;133(2):182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Barraza RA, McLaren JW, Poeschla EM. Prostaglandin pathway gene therapy for sustained reduction of intraocular pressure. Molecular therapy : the journal of the American Society of Gene Therapy. 2010;18(3):491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee ES, Rasmussen CA, Filla MS, et al. Prospects for lentiviral vector mediated prostaglandin F synthase gene delivery in monkey eyes in vivo. Current eye research. 2014;39(9):859–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang WH, McNatt LG, Pang IH, et al. Increased expression of the WNT antagonist sFRP-1 in glaucoma elevates intraocular pressure. The Journal of clinical investigation. 2008;118(3):1056–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Buie LK, Karim MZ, Smith MH, Borras T. Development of a model of elevated intraocular pressure in rats by gene transfer of bone morphogenetic protein 2. Investigative ophthalmology & visual science. 2013;54(8):5441–5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Robertson JV, Golesic E, Gauldie J, West-Mays JA. Ocular gene transfer of active TGF-beta induces changes in anterior segment morphology and elevated IOP in rats. Investigative ophthalmology & visual science. 2010;51(1):308–318. [DOI] [PubMed] [Google Scholar]
- 109.Shepard AR, Millar JC, Pang IH, Jacobson N, Wang WH, Clark AF. Adenoviral gene transfer of active human transforming growth factor-{beta}2 elevates intraocular pressure and reduces outflow facility in rodent eyes. Investigative ophthalmology & visual science. 2010;51(4):2067–2076. [DOI] [PubMed] [Google Scholar]
- 110.Junglas B, Kuespert S, Seleem AA, et al. Connective tissue growth factor causes glaucoma by modifying the actin cytoskeleton of the trabecular meshwork. The American journal of pathology. 2012;180(6):2386–2403. [DOI] [PubMed] [Google Scholar]
- 111.Giovingo M, Nolan M, McCarty R, et al. sCD44 overexpression increases intraocular pressure and aqueous outflow resistance. Molecular vision. 2013;19:2151–2164. [PMC free article] [PubMed] [Google Scholar]
- 112.Pattabiraman PP, Rinkoski T, Poeschla E, Proia A, Challa P, Rao PV. RhoA GTPase-induced ocular hypertension in a rodent model is associated with increased fibrogenic activity in the trabecular meshwork. The American journal of pathology. 2015;185(2):496–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang Z, Dhaliwal AS, Tseng H, et al. Outflow tract ablation using a conditionally cytotoxic feline immunodeficiency viral vector. Investigative ophthalmology & visual science. 2014;55(2):935–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhu W, Gramlich OW, Laboissonniere L, et al. Transplantation of iPSC-derived TM cells rescues glaucoma phenotypes in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(25):E3492–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhu W, Jain A, Gramlich OW, Tucker BA, Sheffield VC, Kuehn MH. Restoration of Aqueous Humor Outflow Following Transplantation of iPSC-Derived Trabecular Meshwork Cells in a Transgenic Mouse Model of Glaucoma. Investigative ophthalmology & visual science. 2017;58(4):2054–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhu W, Godwin CR, Cheng L, Scheetz TE, Kuehn MH. Transplantation of iPSC-TM stimulates division of trabecular meshwork cells in human eyes. Scientific reports. 2020;10(1):2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wu J, Bell OH, Copland DA, et al. Gene Therapy for Glaucoma by Ciliary Body Aquaporin 1 Disruption Using CRISPR-Cas9. Molecular therapy : the journal of the American Society of Gene Therapy. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chong RS, Crowston JG, Wong TT. Experimental models of glaucoma filtration surgery. Acta Ophthalmol. 2020. [DOI] [PubMed] [Google Scholar]
- 119.Burr J, Azuara-Blanco A, Avenell A, Tuulonen A. Medical versus surgical interventions for open angle glaucoma. Cochrane Database Syst Rev. 2012(9):CD004399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hitchings R. Initial treatment for open-angle glaucoma- medical, laser, or surgical? Surgery is the treatment of choice for open-angle glaucoma. Archives of ophthalmology. 1998;116(2):241–242. [DOI] [PubMed] [Google Scholar]
- 121.Lama PJ, Fechtner RD. Antifibrotics and wound healing in glaucoma surgery. Survey of ophthalmology. 2003;48(3):314–346. [DOI] [PubMed] [Google Scholar]
- 122.Georgoulas S, Dahlmann-Noor A, Brocchini S, Khaw PT. Modulation of wound healing during and after glaucoma surgery. Progress in brain research. 2008;173:237–254. [DOI] [PubMed] [Google Scholar]
- 123.Al Habash A, Aljasim LA, Owaidhah O, Edward DP. A review of the efficacy of mitomycin C in glaucoma filtration surgery. Clin Ophthalmol. 2015;9:1945–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fan Gaskin JC, Nguyen DQ, Soon Ang G, O’Connor J, Crowston JG. Wound Healing Modulation in Glaucoma Filtration Surgery-Conventional Practices and New Perspectives: The Role of Antifibrotic Agents (Part I). J Curr Glaucoma Pract. 2014;8(2):37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bergstrom TJ, Wilkinson WS, Skuta GL, Watnick RL, Elner VM. The effects of subconjunctival mitomycin-C on glaucoma filtration surgery in rabbits. Archives of ophthalmology. 1991;109(12):1725–1730. [DOI] [PubMed] [Google Scholar]
- 126.Wilkins M, Indar A, Wormald R. Intra-operative mitomycin C for glaucoma surgery. Cochrane Database Syst Rev. 2005(4):CD002897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Green E, Wilkins M, Bunce C, Wormald R. 5-Fluorouracil for glaucoma surgery. Cochrane Database Syst Rev. 2014(2):CD001132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Westermeyer HD, Hendrix DV, Ward DA. Long-term evaluation of the use of Ahmed gonioimplants in dogs with primary glaucoma: nine cases (2000–2008). Journal of the American Veterinary Medical Association. 2011;238(5):610–617. [DOI] [PubMed] [Google Scholar]
- 129.Akova YA, Koc F, Yalvac I, Duman S. Scleromalacia following trabeculectomy with intraoperative mitomycin C. Eur J Ophthalmol. 1999;9(1):63–65. [DOI] [PubMed] [Google Scholar]
- 130.Fourman S. Scleritis after glaucoma filtering surgery with mitomycin C. Ophthalmology. 1995;102(10):1569–1571. [DOI] [PubMed] [Google Scholar]
- 131.Mietz H, Krieglstein GK. Three-year follow-up of trabeculectomies performed with different concentrations of mitomycin-C. Ophthalmic Surg Lasers. 1998;29(8):628–634. [PubMed] [Google Scholar]
- 132.Group TFFS. Fluorouracil Filtering Surgery Study one-year follow-up. The Fluorouracil Filtering Surgery Study Group. American journal of ophthalmology. 1989;108(6):625–635. [DOI] [PubMed] [Google Scholar]
- 133.Greenfield DS, Suner IJ, Miller MP, Kangas TA, Palmberg PF, Flynn HW Jr., Endophthalmitis after filtering surgery with mitomycin. Archives of ophthalmology. 1996;114(8):943–949. [DOI] [PubMed] [Google Scholar]
- 134.Higginbotham EJ, Stevens RK, Musch DC, et al. Bleb-related endophthalmitis after trabeculectomy with mitomycin C. Ophthalmology. 1996;103(4):650–656. [DOI] [PubMed] [Google Scholar]
- 135.Wells AP, Cordeiro MF, Bunce C, Khaw PT. Cystic bleb formation and related complications in limbus- versus fornix-based conjunctival flaps in pediatric and young adult trabeculectomy with mitomycin C. Ophthalmology. 2003;110(11):2192–2197. [DOI] [PubMed] [Google Scholar]
- 136.Maruichi M, Takai S, Sugiyama T, et al. Role of chymase on growth of cultured canine Tenon’s capsule fibroblasts and scarring in a canine conjunctival flap model. Experimental eye research. 2004;79(1):111–118. [DOI] [PubMed] [Google Scholar]
- 137.Kojima S, Sugiyama T, Takai S, et al. Effects of Gelatin Hydrogel Loading Mitomycin C on Conjunctival Scarring in a Canine Filtration Surgery Model. Investigative ophthalmology & visual science. 2015;56(4):2601–2605. [DOI] [PubMed] [Google Scholar]
- 138.Esson DW, Neelakantan A, Iyer SA, et al. Expression of connective tissue growth factor after glaucoma filtration surgery in a rabbit model. Investigative ophthalmology & visual science. 2004;45(2):485–491. [DOI] [PubMed] [Google Scholar]
- 139.Esson DW, Popp MP, Liu L, Schultz GS, Sherwood MB. Microarray analysis of the failure of filtering blebs in a rat model of glaucoma filtering surgery. Investigative ophthalmology & visual science. 2004;45(12):4450–4462. [DOI] [PubMed] [Google Scholar]
- 140.Popp MP, Liu L, Timmers A, et al. Development of a microarray chip for gene expression in rabbit ocular research. Molecular vision. 2007;13:164–173. [PMC free article] [PubMed] [Google Scholar]
- 141.Sherwood MB, Esson DW, Neelakantan A, Samuelson DA. A new model of glaucoma filtering surgery in the rat. Journal of glaucoma. 2004;13(5):407–412. [DOI] [PubMed] [Google Scholar]
- 142.Chang L, Wong T, Ohbayashi M, et al. Increased mast cell numbers in the conjunctiva of glaucoma patients: a possible indicator of preoperative glaucoma surgery inflammation. Eye. 2009;23(9):1859–1865. [DOI] [PubMed] [Google Scholar]
- 143.Yu-Wai-Man C, Tagalakis AD, Manunta MD, Hart SL, Khaw PT. Receptor-targeted liposome-peptide-siRNA nanoparticles represent an efficient delivery system for MRTF silencing in conjunctival fibrosis. Scientific reports. 2016;6:21881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fernando O, Tagalakis AD, Awwad S, et al. Development of Targeted siRNA Nanocomplexes to Prevent Fibrosis in Experimental Glaucoma Filtration Surgery. Molecular therapy : the journal of the American Society of Gene Therapy. 2018;26(12):2812–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Seet LF, Tan YF, Toh LZ, et al. Targeted therapy for the post-operative conjunctiva: SPARC silencing reduces collagen deposition. The British journal of ophthalmology. 2018;102(10):1460–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pfeiffer N, Voykov B, Renieri G, et al. First-in-human phase I study of ISTH0036, an antisense oligonucleotide selectively targeting transforming growth factor beta 2 (TGF-beta2), in subjects with open-angle glaucoma undergoing glaucoma filtration surgery. PloS one. 2017;12(11):e0188899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Perkins TW, Faha B, Ni M, et al. Adenovirus-mediated gene therapy using human p21WAF-1/Cip-1 to prevent wound healing in a rabbit model of glaucoma filtration surgery. Archives of ophthalmology. 2002;120(7):941–949. [DOI] [PubMed] [Google Scholar]
- 148.Yamanaka O, Saika S, Ohnishi Y, Kim-Mitsuyama S, Kamaraju AK, Ikeda K. Inhibition of p38MAP kinase suppresses fibrogenic reaction in conjunctiva in mice. Molecular vision. 2007;13:1730–1739. [PubMed] [Google Scholar]
- 149.John SW, Smith RS, Savinova OV, et al. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Investigative ophthalmology & visual science. 1998;39(6):951–962. [PubMed] [Google Scholar]
- 150.Quigley HA, Nickells RW, Kerrigan LA, Pease ME, Thibault DJ, Zack DJ. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Investigative ophthalmology & visual science. 1995;36(5):774–786. [PubMed] [Google Scholar]
- 151.Cordeiro MF, Guo L, Luong V, et al. Real-time imaging of single nerve cell apoptosis in retinal neurodegeneration. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(36):13352–13356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kerrigan LA, Zack DJ, Quigley HA, Smith SD, Pease ME. TUNEL-positive ganglion cells in human primary open-angle glaucoma. Archives of ophthalmology. 1997;115(8):1031–1035. [DOI] [PubMed] [Google Scholar]
- 153.Seki M, Lipton SA. Targeting excitotoxic/free radical signaling pathways for therapeutic intervention in glaucoma. Progress in brain research. 2008;173:495–510. [DOI] [PubMed] [Google Scholar]
- 154.Brooks DE, Garcia GA, Dreyer EB, Zurakowski D, Franco-Bourland RE. Vitreous body glutamate concentration in dogs with glaucoma. American journal of veterinary research. 1997;58(8):864–867. [PubMed] [Google Scholar]
- 155.Pease ME, McKinnon SJ, Quigley HA, Kerrigan-Baumrind LA, Zack DJ. Obstructed axonal transport of BDNF and its receptor TrkB in experimental glaucoma. Investigative ophthalmology & visual science. 2000;41(3):764–774. [PubMed] [Google Scholar]
- 156.Knox DL, Eagle RC Jr., Green WR. Optic nerve hydropic axonal degeneration and blocked retrograde axoplasmic transport: histopathologic features in human high-pressure secondary glaucoma. Archives of ophthalmology. 2007;125(3):347–353. [DOI] [PubMed] [Google Scholar]
- 157.Salinas-Navarro M, Alarcon-Martinez L, Valiente-Soriano FJ, et al. Ocular hypertension impairs optic nerve axonal transport leading to progressive retinal ganglion cell degeneration. Experimental eye research. 2010;90(1):168–183. [DOI] [PubMed] [Google Scholar]
- 158.Fahy ET, Chrysostomou V, Crowston JG. Mini-Review: Impaired Axonal Transport and Glaucoma. Current eye research. 2016;41(3):273–283. [DOI] [PubMed] [Google Scholar]
- 159.Ward NJ, Ho KW, Lambert WS, Weitlauf C, Calkins DJ. Absence of transient receptor potential vanilloid-1 accelerates stress-induced axonopathy in the optic projection. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34(9):3161–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Agarwal R, Gupta SK, Agarwal P, Saxena R, Agrawal SS. Current concepts in the pathophysiology of glaucoma. Indian J Ophthalmol. 2009;57(4):257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Flammer J, Haefliger IO, Orgul S, Resink T. Vascular dysregulation: a principal risk factor for glaucomatous damage? Journal of glaucoma. 1999;8(3):212–219. [PubMed] [Google Scholar]
- 162.Michelson G, Langhans MJ, Harazny J, Dichtl A. Visual field defect and perfusion of the juxtapapillary retina and the neuroretinal rim area in primary open-angle glaucoma. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 1998;236(2):80–85. [DOI] [PubMed] [Google Scholar]
- 163.Chung HS, Harris A, Kagemann L, Martin B. Peripapillary retinal blood flow in normal tension glaucoma. The British journal of ophthalmology. 1999;83(4):466–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gelatt KN, Miyabayashi T, Gelatt-Nicholson KJ, MacKay EO. Progressive changes in ophthalmic blood velocities in Beagles with primary open angle glaucoma. Veterinary ophthalmology. 2003;6(1):77–84. [DOI] [PubMed] [Google Scholar]
- 165.Gelatt-Nicholson KJ, Gelatt KN, MacKay EO, Brooks DE, Newell SM. Comparative Doppler imaging of the ophthalmic vasculature in normal Beagles and Beagles with inherited primary open-angle glaucoma. Veterinary ophthalmology. 1999;2(2):97–105. [DOI] [PubMed] [Google Scholar]
- 166.Brooks DE, Samuelson DA, Gelatt KN. Ultrastructural changes in laminar optic nerve capillaries of beagles with primary open-angle glaucoma. American journal of veterinary research. 1989;50(6):929–935. [PubMed] [Google Scholar]
- 167.Hondur G, Goktas E, Yang X, et al. Oxidative Stress-Related Molecular Biomarker Candidates for Glaucoma. Investigative ophthalmology & visual science. 2017;58(10):4078–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tezel G. Oxidative stress in glaucomatous neurodegeneration: mechanisms and consequences. Progress in retinal and eye research. 2006;25(5):490–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Moreno MC, Campanelli J, Sande P, Sanez DA, Keller Sarmiento MI, Rosenstein RE. Retinal oxidative stress induced by high intraocular pressure. Free Radic Biol Med. 2004;37(6):803–812. [DOI] [PubMed] [Google Scholar]
- 170.Mozaffarieh M, Grieshaber MC, Orgul S, Flammer J. The potential value of natural antioxidative treatment in glaucoma. Survey of ophthalmology. 2008;53(5):479–505. [DOI] [PubMed] [Google Scholar]
- 171.Liu Q, Ju WK, Crowston JG, et al. Oxidative stress is an early event in hydrostatic pressure induced retinal ganglion cell damage. Investigative ophthalmology & visual science. 2007;48(10):4580–4589. [DOI] [PubMed] [Google Scholar]
- 172.Wax MB, Tezel G. Immunoregulation of retinal ganglion cell fate in glaucoma. Experimental eye research. 2009;88(4):825–830. [DOI] [PubMed] [Google Scholar]
- 173.Bell K, Gramlich OW, Von Thun Und Hohenstein-Blaul N, et al. Does autoimmunity play a part in the pathogenesis of glaucoma? Progress in retinal and eye research. 2013;36:199–216. [DOI] [PubMed] [Google Scholar]
- 174.Pumphrey SA, Pizzirani S, Pirie CG, Anwer MS, Logvinenko T. Western blot patterns of serum autoantibodies against optic nerve antigens in dogs with goniodysgenesis-related glaucoma. American journal of veterinary research. 2013;74(4):621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bringmann A, Pannicke T, Grosche J, et al. Muller cells in the healthy and diseased retina. Progress in retinal and eye research. 2006;25(4):397–424. [DOI] [PubMed] [Google Scholar]
- 176.Son JL, Soto I, Oglesby E, et al. Glaucomatous optic nerve injury involves early astrocyte reactivity and late oligodendrocyte loss. Glia. 2010;58(7):780–789. [DOI] [PubMed] [Google Scholar]
- 177.Inman DM, Horner PJ. Reactive nonproliferative gliosis predominates in a chronic mouse model of glaucoma. Glia. 2007;55(9):942–953. [DOI] [PubMed] [Google Scholar]
- 178.Neufeld AH, Liu B. Glaucomatous optic neuropathy: when glia misbehave. Neuroscientist. 2003;9(6):485–495. [DOI] [PubMed] [Google Scholar]
- 179.Fechtner RD, Weinreb RN. Mechanisms of optic nerve damage in primary open angle glaucoma. Survey of ophthalmology. 1994;39(1):23–42. [DOI] [PubMed] [Google Scholar]
- 180.He S, Stankowska DL, Ellis DZ, Krishnamoorthy RR, Yorio T. Targets of Neuroprotection in Glaucoma. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2018;34(1–2):85–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Naik S, Pandey A, Lewis SA, Rao BSS, Mutalik S. Neuroprotection: A versatile approach to combat glaucoma. Eur J Pharmacol. 2020;881:173208. [DOI] [PubMed] [Google Scholar]
- 182.Hare WA, WoldeMussie E, Lai RK, et al. Efficacy and safety of memantine treatment for reduction of changes associated with experimental glaucoma in monkey, I: Functional measures. Investigative ophthalmology & visual science. 2004;45(8):2625–2639. [DOI] [PubMed] [Google Scholar]
- 183.Hare WA, WoldeMussie E, Weinreb RN, et al. Efficacy and safety of memantine treatment for reduction of changes associated with experimental glaucoma in monkey, II: Structural measures. Investigative ophthalmology & visual science. 2004;45(8):2640–2651. [DOI] [PubMed] [Google Scholar]
- 184.WoldeMussie E, Yoles E, Schwartz M, Ruiz G, Wheeler LA. Neuroprotective effect of memantine in different retinal injury models in rats. Journal of glaucoma. 2002;11(6):474–480. [DOI] [PubMed] [Google Scholar]
- 185.Weinreb RN, Liebmann JM, Cioffi GA, et al. Oral Memantine for the Treatment of Glaucoma: Design and Results of 2 Randomized, Placebo-Controlled, Phase 3 Studies. Ophthalmology. 2018;125(12):1874–1885. [DOI] [PubMed] [Google Scholar]
- 186.Hellstrom M, Ruitenberg MJ, Pollett MA, et al. Cellular tropism and transduction properties of seven adeno-associated viral vector serotypes in adult retina after intravitreal injection. Gene therapy. 2009;16(4):521–532. [DOI] [PubMed] [Google Scholar]
- 187.Martin KR, Klein RL, Quigley HA. Gene delivery to the eye using adeno-associated viral vectors. Methods. 2002;28(2):267–275. [DOI] [PubMed] [Google Scholar]
- 188.Yin L, Greenberg K, Hunter JJ, et al. Intravitreal injection of AAV2 transduces macaque inner retina. Investigative ophthalmology & visual science. 2011;52(5):2775–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Cehajic-Kapetanovic J, Le Goff MM, Allen A, Lucas RJ, Bishop PN. Glycosidic enzymes enhance retinal transduction following intravitreal delivery of AAV2. Molecular vision. 2011;17:1771–1783. [PMC free article] [PubMed] [Google Scholar]
- 190.Hanlon KS, Chadderton N, Palfi A, et al. A Novel Retinal Ganglion Cell Promoter for Utility in AAV Vectors. Front Neurosci. 2017;11:521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Dudus L, Anand V, Acland GM, et al. Persistent transgene product in retina, optic nerve and brain after intraocular injection of rAAV. Vision research. 1999;39(15):2545–2553. [DOI] [PubMed] [Google Scholar]
- 192.Peynshaert K, Devoldere J, Minnaert AK, De Smedt SC, Remaut K. Morphology and Composition of the Inner Limiting Membrane: Species-Specific Variations and Relevance toward Drug Delivery Research. Current eye research. 2019;44(5):465–475. [DOI] [PubMed] [Google Scholar]
- 193.Gamlin PD, Alexander JJ, Boye SL, Witherspoon CD, Boye SE. SubILM Injection of AAV for Gene Delivery to the Retina. Methods in molecular biology. 2019;1950:249–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Boye SE, Alexander JJ, Witherspoon CD, et al. Highly Efficient Delivery of Adeno-Associated Viral Vectors to the Primate Retina. Human gene therapy. 2016;27(8):580–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Peden MC, Min J, Meyers C, et al. Ab-externo AAV-mediated gene delivery to the suprachoroidal space using a 250 micron flexible microcatheter. PloS one. 2011;6(2):e17140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Di Polo A, Aigner LJ, Dunn RJ, Bray GM, Aguayo AJ. Prolonged delivery of brain-derived neurotrophic factor by adenovirus-infected Muller cells temporarily rescues injured retinal ganglion cells. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(7):3978–3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Isenmann S, Klocker N, Gravel C, Bahr M. Short communication: protection of axotomized retinal ganglion cells by adenovirally delivered BDNF in vivo. The European journal of neuroscience. 1998;10(8):2751–2756. [DOI] [PubMed] [Google Scholar]
- 198.Garcia-Valenzuela E, Rayanade R, Perales JC, Davidson CA, Hanson RW, Sharma SC. Axon-mediated gene transfer of retinal ganglion cells in vivo. J Neurobiol. 1997;32(1):111–122. [DOI] [PubMed] [Google Scholar]
- 199.Unsicker K. Neurotrophic molecules in the treatment of neurodegenerative disease with focus on the retina: status and perspectives. Cell and tissue research. 2013;353(2):205–218. [DOI] [PubMed] [Google Scholar]
- 200.Johnson EC, Guo Y, Cepurna WO, Morrison JC. Neurotrophin roles in retinal ganglion cell survival: lessons from rat glaucoma models. Experimental eye research. 2009;88(4):808–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Cheng L, Sapieha P, Kittlerova P, Hauswirth WW, Di Polo A. TrkB gene transfer protects retinal ganglion cells from axotomy-induced death in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22(10):3977–3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Klocker N, Kermer P, Weishaupt JH, Labes M, Ankerhold R, Bahr M. Brain-derived neurotrophic factor-mediated neuroprotection of adult rat retinal ganglion cells in vivo does not exclusively depend on phosphatidyl-inositol-3’-kinase/protein kinase B signaling. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20(18):6962–6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Weise J, Isenmann S, Klocker N, et al. Adenovirus-mediated expression of ciliary neurotrophic factor (CNTF) rescues axotomized rat retinal ganglion cells but does not support axonal regeneration in vivo. Neurobiology of disease. 2000;7(3):212–223. [DOI] [PubMed] [Google Scholar]
- 204.Schmeer C, Straten G, Kugler S, Gravel C, Bahr M, Isenmann S. Dose-dependent rescue of axotomized rat retinal ganglion cells by adenovirus-mediated expression of glial cell-line derived neurotrophic factor in vivo. The European journal of neuroscience. 2002;15(4):637–643. [DOI] [PubMed] [Google Scholar]
- 205.Ren R, Li Y, Liu Z, Liu K, He S. Long-term rescue of rat retinal ganglion cells and visual function by AAV-mediated BDNF expression after acute elevation of intraocular pressure. Investigative ophthalmology & visual science. 2012;53(2):1003–1011. [DOI] [PubMed] [Google Scholar]
- 206.McKinnon SJ, Lehman DM, Tahzib NG, et al. Baculoviral IAP repeat-containing-4 protects optic nerve axons in a rat glaucoma model. Molecular therapy : the journal of the American Society of Gene Therapy. 2002;5(6):780–787. [DOI] [PubMed] [Google Scholar]
- 207.Malik JM, Shevtsova Z, Bahr M, Kugler S. Long-term in vivo inhibition of CNS neurodegeneration by Bcl-XL gene transfer. Molecular therapy : the journal of the American Society of Gene Therapy. 2005;11(3):373–381. [DOI] [PubMed] [Google Scholar]
- 208.Krishnan A, Fei F, Jones A, et al. Overexpression of Soluble Fas Ligand following Adeno-Associated Virus Gene Therapy Prevents Retinal Ganglion Cell Death in Chronic and Acute Murine Models of Glaucoma. Journal of immunology. 2016;197(12):4626–4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Stankowska DL, Minton AZ, Rutledge MA, et al. Neuroprotective effects of transcription factor Brn3b in an ocular hypertension rat model of glaucoma. Investigative ophthalmology & visual science. 2015;56(2):893–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zhou X, Li F, Kong L, Chodosh J, Cao W. Anti-inflammatory effect of pigment epithelium-derived factor in DBA/2J mice. Molecular vision. 2009;15:438–450. [PMC free article] [PubMed] [Google Scholar]
- 211.Lingor P, Koeberle P, Kugler S, Bahr M. Down-regulation of apoptosis mediators by RNAi inhibits axotomy-induced retinal ganglion cell death in vivo. Brain. 2005;128(Pt 3):550–558. [DOI] [PubMed] [Google Scholar]
- 212.Bond WS, Hines-Beard J, GoldenMerry YL, et al. Virus-mediated EpoR76E Therapy Slows Optic Nerve Axonopathy in Experimental Glaucoma. Molecular therapy : the journal of the American Society of Gene Therapy. 2016;24(2):230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Sullivan TA, Geisert EE, Hines-Beard J, Rex TS. Systemic adeno-associated virus-mediated gene therapy preserves retinal ganglion cells and visual function in DBA/2J glaucomatous mice. Human gene therapy. 2011;22(10):1191–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hines-Beard J, Bond WS, Backstrom JR, Rex TS. Virus-mediated EpoR76E gene therapy preserves vision in a glaucoma model by modulating neuroinflammation and decreasing oxidative stress. J Neuroinflammation. 2016;13:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Tao W, Wen R, Goddard MB, et al. Encapsulated cell-based delivery of CNTF reduces photoreceptor degeneration in animal models of retinitis pigmentosa. Investigative ophthalmology & visual science. 2002;43(10):3292–3298. [PubMed] [Google Scholar]
- 216.Wen R, Tao W, Li Y, Sieving PA. CNTF and retina. Progress in retinal and eye research. 2012;31(2):136–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Williams PR, Benowitz LI, Goldberg JL, He Z. Axon Regeneration in the Mammalian Optic Nerve. Annu Rev Vis Sci. 2020;6:195–213. [DOI] [PubMed] [Google Scholar]
- 218.Laha B, Stafford BK, Huberman AD. Regenerating optic pathways from the eye to the brain. Science. 2017;356(6342):1031–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Benowitz LI, He Z, Goldberg JL. Reaching the brain: Advances in optic nerve regeneration. Experimental neurology. 2017;287(Pt 3):365–373. [DOI] [PubMed] [Google Scholar]
- 220.de Lima S, Koriyama Y, Kurimoto T, et al. Full-length axon regeneration in the adult mouse optic nerve and partial recovery of simple visual behaviors. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(23):9149–9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Tanaka T, Yokoi T, Tamalu F, Watanabe S, Nishina S, Azuma N. Generation of retinal ganglion cells with functional axons from human induced pluripotent stem cells. Scientific reports. 2015;5:8344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Goldberg JL, Guido W, Agi Workshop P. Report on the National Eye Institute Audacious Goals Initiative: Regenerating the Optic Nerve. Investigative ophthalmology & visual science. 2016;57(3):1271–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Siqueira RC, Messias A, Messias K, et al. Quality of life in patients with retinitis pigmentosa submitted to intravitreal use of bone marrow-derived stem cells (Reticell -clinical trial). Stem Cell Res Ther. 2015;6:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Quigley HA. The contribution of the sclera and lamina cribrosa to the pathogenesis of glaucoma: Diagnostic and treatment implications. Progress in brain research. 2015;220:59–86. [DOI] [PubMed] [Google Scholar]
- 225.Sayah DN, Mazzaferri J, Descovich D, Costantino S, Lesk MR. The Association Between Ocular Rigidity and Neuroretinal Damage in Glaucoma. Investigative ophthalmology & visual science. 2020;61(13):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Sommer A, Tielsch JM, Katz J, et al. Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans. The Baltimore Eye Survey. Archives of ophthalmology. 1991;109(8):1090–1095. [DOI] [PubMed] [Google Scholar]
- 227.Klein BE, Klein R, Sponsel WE, et al. Prevalence of glaucoma. The Beaver Dam Eye Study. Ophthalmology. 1992;99(10):1499–1504. [DOI] [PubMed] [Google Scholar]
- 228.Group CN-TGS. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Collaborative Normal-Tension Glaucoma Study Group. American journal of ophthalmology. 1998;126(4):487–497. [DOI] [PubMed] [Google Scholar]
- 229.Boote C, Palko JR, Sorensen T, et al. Changes in posterior scleral collagen microstructure in canine eyes with an ADAMTS10 mutation. Molecular vision. 2016;22:503–517. [PMC free article] [PubMed] [Google Scholar]
- 230.Palko JR, Iwabe S, Pan X, Agarwal G, Komaromy AM, Liu J. Biomechanical properties and correlation with collagen solubility profile in the posterior sclera of canine eyes with an ADAMTS10 mutation. Investigative ophthalmology & visual science. 2013;54(4):2685–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Palko JR, Morris HJ, Pan X, et al. Influence of Age on Ocular Biomechanical Properties in a Canine Glaucoma Model with ADAMTS10 Mutation. PloS one. 2016;11(6):e0156466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Jeanes EC, Oliver JAC, Ricketts SL, Gould DJ, Mellersh CS. Glaucoma-causing ADAMTS17 mutations are also reproducibly associated with height in two domestic dog breeds: selection for short stature may have contributed to increased prevalence of glaucoma. Canine Genet Epidemiol. 2019;6(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Yiu G, Chung SH, Mollhoff IN, et al. Suprachoroidal and Subretinal Injections of AAV Using Transscleral Microneedles for Retinal Gene Delivery in Nonhuman Primates. Mol Ther Methods Clin Dev. 2020;16:179–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ding K, Shen J, Hafiz Z, et al. AAV8-vectored suprachoroidal gene transfer produces widespread ocular transgene expression. The Journal of clinical investigation. 2019;129(11):4901–4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Hannon BG, Luna C, Feola AJ, et al. Assessment of Visual and Retinal Function Following In Vivo Genipin-Induced Scleral Crosslinking. Translational vision science & technology. 2020;9(10):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Shen J, Kim J, Tzeng SY, et al. Suprachoroidal gene transfer with nonviral nanoparticles. Sci Adv. 2020;6(27). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Simcoe MJ, Khawaja AP, Hysi PG, Hammond CJ, Eye UKB, Vision C. Genome-wide association study of corneal biomechanical properties identifies over 200 loci providing insight into the genetic etiology of ocular diseases. Human molecular genetics. 2020;29(18):3154–3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Zimprich L, Diedrich J, Bleeker A, Schweitzer JA. Corneal Hysteresis as a Biomarker of Glaucoma: Current Insights. Clin Ophthalmol. 2020;14:2255–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Song L, Bower JJ, Llanga T, Salmon JH, Hirsch ML, Gilger BC. Ocular Tolerability and Immune Response to Corneal Intrastromal AAV-IDUA Gene Therapy in New Zealand White Rabbits. Mol Ther Methods Clin Dev. 2020;18:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Song L, Bower JJ, Hirsch ML. Preparation and Administration of Adeno-associated Virus Vectors for Corneal Gene Delivery. Methods in molecular biology. 2020;2145:77–102. [DOI] [PubMed] [Google Scholar]
- 241.Rahman N, O’Neill E, Irnaten M, Wallace D, O’Brien C. Corneal Stiffness and Collagen Cross-Linking Proteins in Glaucoma: Potential for Novel Therapeutic Strategy. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2020;36(8):582–594. [DOI] [PubMed] [Google Scholar]
- 242.Sandberg CA, Herring IP, Huckle WR, LeRoith T, Pickett JP, Rossmeisl JH. Aqueous humor vascular endothelial growth factor in dogs: association with intraocular disease and the development of pre-iridal fibrovascular membrane. Veterinary ophthalmology. 2012;15 Suppl 1:21–30. [DOI] [PubMed] [Google Scholar]
- 243.Campochiaro PA, Lauer AK, Sohn EH, et al. Lentiviral Vector Gene Transfer of Endostatin/Angiostatin for Macular Degeneration (GEM) Study. Human gene therapy. 2017;28(1):99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Heier JS, Kherani S, Desai S, et al. Intravitreous injection of AAV2-sFLT01 in patients with advanced neovascular age-related macular degeneration: a phase 1, open-label trial. Lancet. 2017;390(10089):50–61. [DOI] [PubMed] [Google Scholar]
- 245.Crabtree E, Song L, Llanga T, et al. AAV-mediated expression of HLA-G1/5 reduces severity of experimental autoimmune uveitis. Scientific reports. 2019;9(1):19864. [DOI] [PMC free article] [PubMed] [Google Scholar]