Significance
Rapid communication between neurons relies on the precise coordination of several presynaptic active-zone proteins. Munc13/UNC-13 is the centerpiece of this deeply conserved process, and several domains of this large cytoplasmic protein serve specific roles such as coordinating SNARE protein assembly, integrating calcium and lipid signals, and bringing synaptic vesicles close to the plasma membrane, although a mechanistic understanding of these critical steps is lacking. Here, we describe for the first time a novel C2 domain (HC2M) at the C terminus of UNC-13 that helps to bind synaptic vesicles to the plasma membrane in preparation for fusion. We demonstrate the impact of HC2M loss on nervous system function and characterize its structural, biochemical, and evolutionarily conserved features.
Keywords: Munc13, Caenorhabditis elegans, synaptic vesicle, C2, unc-13
Abstract
Neurotransmitter release during synaptic transmission comprises a tightly orchestrated sequence of molecular events, and Munc13-1 is a cornerstone of the fusion machinery. A forward genetic screen for defects in neurotransmitter release in Caenorhabditis elegans identified a mutation in the Munc13-1 ortholog UNC-13 that eliminated its unique and deeply conserved C-terminal module (referred to as HC2M) containing a Ca2+-insensitive C2 domain flanked by membrane-binding helices. The HC2M module could be functionally replaced in vivo by protein domains that localize to synaptic vesicles but not to the plasma membrane. HC2M is broadly conserved in other Unc13 family members and is required for efficient synaptic vesicle priming. We propose that the HC2M domain evolved as a vesicle/endosome adaptor and acquired synaptic vesicle specificity in the Unc13ABC protein family.
Chemical synaptic transmission is the primary mode of cellular communication within the nervous system. The presynaptic piece of this process encompasses a remarkable set of sequential and highly regulated interactions between a host of proteins, synaptic vesicles (SV), the plasma membrane, and calcium ions (Ca2+). Fusion of neurotransmitter-containing vesicles with the presynaptic plasma membrane is driven by the assembly of the neuronal SNAREs SNAP-25 and Syntaxin 1 on the plasma membrane and Synaptobrevin-2/VAMP2 on the SV. The assembly process and its coupling to intracellular Ca2+ are choreographed by a deeply conserved group of proteins including Munc13, Munc18, Synaptotagmin 1, and Complexin (1–4). Together with the SNAREs, these proteins form the core of the fusion apparatus across all metazoan nervous systems (5–7).
First identified in a landmark genetic screen for nervous system mutants in the nematode Caenorhabditis elegans, UNC-13 is the founding member of the highly conserved metazoan Unc13 secretory protein family that includes Unc13ABC in humans (Munc13-1/2/3 in mice) (8–10). Munc13-1/UNC-13 localizes to the presynaptic active zone and is implicated in numerous presynaptic functions including initiation of release site assembly, SV docking and priming, Ca2+- and lipid-dependent forms of short-term synaptic plasticity, opening and positioning Syntaxin 1 for SNARE assembly, and protecting SNARE complexes from disassembly by NSF/alpha-SNAP (3, 11–13). Loss of Munc13-1 orthologs in the nervous system almost entirely eliminates all forms of chemical synaptic transmission, establishing the Unc13 family as essential to this process (14–16). All UNC-13 orthologs contain a large Syntaxin-binding MUN domain flanked by a Ca2+- and lipid-binding C1-C2 module and an additional C2 domain on its C terminus referred to as C2C (5, 10, 17).
The C-terminal end of UNC-13 is the least understood domain within the Unc13 protein family in terms of both structure and mechanism (18, 19). Recent work on the MUN and C2C domains of Munc13-1 both in vitro and in cultured hippocampal synapses supports the notion that the MUN-C2C region attaches Munc13-1 to SVs as a means of preparing SVs for fusion (20, 21), but several questions remain unresolved. Is the SV interaction mediated by direct membrane binding? Does the C2C domain itself bind to SVs or does the MUN domain serve this role? Does either domain provide cargo specificity as part of the priming process? Interestingly, the C-terminal end of the MUN domain of CAPS, another Unc13 family member, can bind dense-core vesicles (DCVs) although it lacks a C-terminal C2 domain (22). Moreover, the MUN domain without the C2C domain has also been demonstrated to bind liposomes through an interaction with Synaptobrevin 2 (23). These observations bring up several possibilities for interactions with the C terminus of Munc13 including direct MUN–membrane interactions, C2C–membrane interactions, or protein–protein interactions involving either or both domains. Other Unc13 family members possessing a MUN domain with a C-terminal C2 domain such as Unc13D/Munc13-4 and BAIAP3 have been proposed to tether specific cargo such as endosomes, secretory granules, and large DCVs (24, 25). How Unc13 proteins select among different cargos remains largely unanswered (24, 26, 27).
Through behavioral, electrophysiological, biochemical, and genetic approaches, we uncover a deeply conserved C-terminal membrane-binding domain within Munc13-1/UNC-13 termed the Munc13 C-terminal (MCT) domain. This region, together with C2C and a neighboring N-terminal helix fold together into a stable membrane-binding protein domain in vitro, and loss of any part of this module in vivo impairs SV priming and nervous system function. Moreover, the C-terminal domain can be replaced by foreign domains that bind SVs but not the plasma membrane, demonstrating a role in SV interactions at the synapse. Phylogenetic protein sequence comparisons suggest that the ancestral Unc13/BAIAP3 homolog possessed a similar C-terminal domain prior to the emergence of metazoa, and subsequently, the UNC-13ABC subfamily domain evolved as an SV adaptor that plays a critical role in neurotransmission in all animals.
Results
Deletion of Either the C2C or the MCT Domain of UNC-13 Impairs Nervous System Function and Neurotransmitter Release.
To examine the molecular mechanisms underlying neurotransmitter release, we conducted a chemical mutagenesis screen in C. elegans and isolated a mutant harboring a stop mutation near the end of the unc-13 gene (W1663Stop) resulting in the expression of a truncated UNC-13/Munc13-1 variant lacking its final ∼200 residues including the C-terminal C2 domain (C2C) (Fig. 1A). Hereafter, we refer to the W1663Stop mutation as C2C-Stop. Worm UNC-13 and mouse Munc13-1 exhibit high sequence conservation throughout the C2C domain (Fig. 1A). Sequence conservation extends past the C2C into the final ∼50 to 60 residues of the Unc13 gene family across metazoa, which we refer to as the MCT domain (SI Appendix, Fig. S1). Because the C2C-Stop protein lacks both the C2C and MCT domains, we created two additional unc-13 mutants using CRISPR-Cas9 gene editing (28). In the ΔC2C strain, the C2C domain was deleted while leaving the MCT domain in place, while in the ΔMCT strain, the MCT domain was deleted, leaving the C2C domain intact. For all of these unc-13 alleles, mutants were assessed for functional defects using three approaches: spontaneous locomotion speed as a measure of nervous system dysfunction, sensitivity to inhibition of cholinesterases via aldicarb as a crude measure of acetylcholine (ACh) secretion, and direct electrophysiological recordings of stimulus-evoked ACh release at the neuromuscular junction, as described previously (14, 29). Compared with wild type (WT), loss of either or both C-terminal domains significantly decreased movement, with average locomotory speed decreased by 70 to 80% (SI Appendix, Fig. S2 A and B). While the decrease in spontaneous movement was considerable, all three mutants moved substantially better than unc-13(s69) null mutants, suggesting that UNC-13 function was diminished but not eliminated. To control for the possibility that deleting regions of the UNC-13 C terminus might decrease protein stability and/or trafficking, synaptic UNC-13 abundance was quantified in animals harboring an endogenous mScarlet tag using CRISPR. Deletion of the C2C domain had little impact on UNC-13 abundance or distribution throughout axons (SI Appendix, Fig. S2 C and D). Moreover, UNC-13 levels were down by ∼30% in hemizygous animals expressing only one copy of full-length UNC-13 while behavior was unaffected, indicating that 70% of normal UNC-13 protein levels is sufficient for full functionality. Thus, perturbations of the UNC-13 C terminus affected its function rather than its abundance or trafficking. To assess the functional conservation of C2C between worm and mammals, the unc-13 gene was edited using CRISPR to replace the endogenous C2C domain with the corresponding domain from mouse Munc13-1, hereafter designated unc-13(mC2C). These animals were indistinguishable from WT worms by locomotion. Replacement of worm MCT with the mouse MCT and complete replacement of worm C2C-MCT with the mouse equivalent was not attempted in the present study.
Fig. 1.
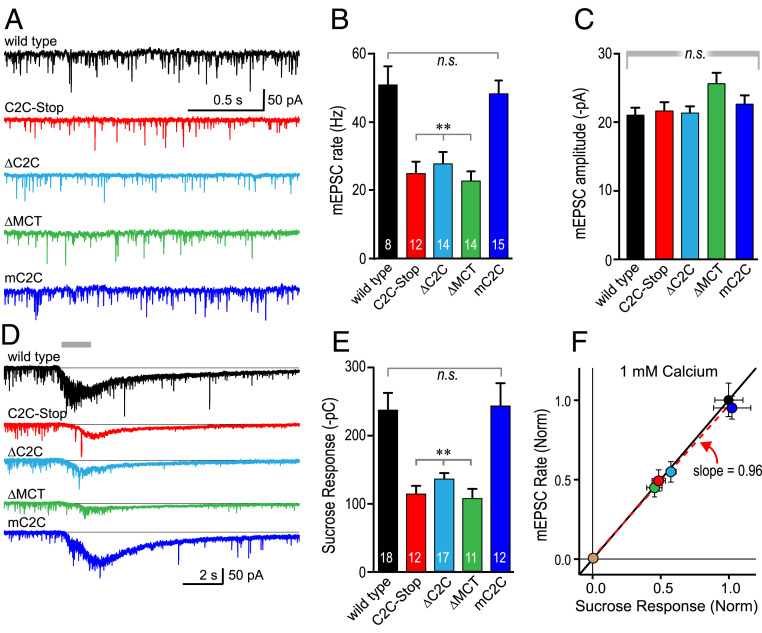
Deletion of UNC-13 C2C impairs nervous system function and neurotransmitter release. (A) Protein sequence alignment for the C terminus of C. elegans (Ce) UNC-13 and mouse (Mm) Munc13-1. The C2C domains are highly conserved (blue = identical and red = similar). The final two MUN helices are labeled a and b (orange). There were 45 residues following C2C designated as MCT domain (green). Trp → stop identified in the tau1 allele of unc-13 in red (C2C-Stop). (B) Cartoon of the C. elegans NMJ depicting release of ACh (gray) into the synaptic cleft, ACh esterase (AChE, green), and the cholinesterase inhibitor aldicarb (red). (C) Paralysis time courses for WT (black), C2C-Stop (red), ΔC2C (cyan), and ΔMCT (green). (D) Paralysis after 120 min for the same four strains. (E) Representative stimulus-evoked EPSCs for WT (black), C2C-Stop (red), ΔC2C (cyan), ΔMCT (green), and mC2C (dark blue). Average EPSC peak amplitude (F) and cumulative charge transfer (G) for the same five genotypes. Error bars are mean ± SEM, and sample numbers are indicated on the bars. For statistical comparisons, ** indicate significant difference from WT without a significant difference within the bracket group with P < 0.01. Not significant, n.s. Data were compared using ANOVA, and the Tukey–Kramer test for multiple comparisons was used to generate P values. Strains: N2, BC168, JSD0557, JSD1193, JSD1200, and JSD1234.
Previous studies have established that impaired ACh release renders mutant animals resistant to acute pharmacological inhibition of cholinesterases (30, 31). All three C-terminal unc-13 mutants displayed similar resistance to paralysis during a 2-h exposure to aldicarb (Fig. 1 B–D), suggesting comparable defects in ACh secretion. Finally, Ca2+-triggered SV fusion was directly assessed at the neuromuscular junction (NMJ) in dissected worms using whole-cell voltage-clamp recording of synaptic currents in body wall muscles in response to a single electrical stimulus (Fig. 1E), as described previously (19, 32, 33). Based on peak current and total synaptic charge transfer, stimulus-evoked ACh release was reduced more than fivefold in C2C-Stop, ΔC2C, and ΔMCT animals (Fig. 1 E–G). Notably, ACh release was unaffected in unc-13(mC2C) mutants, consistent with their normal locomotory behavior. Taken together, the behavioral and electrophysiological data point to a major conserved synaptic role for the C2C-MCT module of UNC-13.
Deletion of the UNC-13 HC2M Decreases Spontaneous SV Fusion and SV Priming.
Which aspect of neurotransmitter release is defective in the C-terminal deletion mutants? To address the site of action for the C2C-MCT module of UNC-13, we recorded spontaneous SV fusion events in the absence of external Ca2+ at both cholinergic and GABAergic synapses. All three deletion mutations strongly reduced the spontaneous rate of fusion in both cholinergic and GABAergic NMJs with no impact on quantal amplitude while the mouse C2C substitution displayed only a small reduction (SI Appendix, Fig. S3). These observations support a presynaptic role for the C2C-MCT module prior to Ca2+-triggered fusion that does not impact SV size or vesicular ACh concentration. Endogenous neural activity drives tonic Ca2+-triggered ACh release in dissected worm neuromuscular preparations (32–35). Tonic SV fusion events were recorded in the four mutant strains to examine their impact at cholinergic synapses (Fig. 2A). Similar to the other modes of synaptic transmission, tonic release was significantly decreased in all three deletion mutants and unaffected in the mouse C2C substitution mutant (Fig. 2 B and C). Only a subset of all SVs at a synapse are docked at the plasma membrane in a fusion-competent state at the moment Ca2+ enters the presynaptic terminal, and synaptic transmission is initially limited to this “readily releasable” pool (RRP) of primed vesicles (36, 37). The Unc13ABC protein family plays a critical role in SV priming and is thus a major determinant of RRP size (3, 5, 12, 14, 19, 37–39). We utilized hypertonic sucrose to estimate the RRP size in WT animals compared with the various HC2M mutants (14, 19, 36, 40, 41) (Fig. 2 D and E). Notably, the response to hypertonic sucrose and the tonic ACh release rate were reduced in almost exactly the same proportions across the unc-13 mutants (Fig. 2F), suggesting that nearly all of the impairment could be ascribed to the SV pool size rather than to some additional aspect of Ca2+ coupling during fusion. A recent study in mouse Munc13-1 also found that deletion of the C2C domain decreased both the RRP size and the number of morphologically docked vesicles in cultured hippocampal synapses, supporting a role for this region of Munc13-1 in efficient priming of SVs (21). An additional and independent assessment of pool size was made using optical stimulus trains in animals expressing the channelrhodopsin variant ChIEF. Similarly, a large decrease in the extrapolated pool size was observed in the C2C deletion but not in the mouse C2C substitution (SI Appendix, Fig. S4). Taken together, these measures of SV fusion and pool size indicate a crucial role for the UNC-13/Munc13-1 C terminus in the formation of primed SVs but do not provide a mechanistic understanding of the C2C-MCT protein module and its properties.
Fig. 2.
UNC-13 C2C domain is required for efficient priming of SVs. (A) Tonic EPSCs were recorded from body wall muscle of adult worms in 1 mM external Ca2+. mEPSC traces from WT (black), C2C-Stop (red), ΔC2C (cyan), ΔMCT (green), and mouse C2C (dark blue). Average of the frequency (B) and amplitude (C) of the mEPSCs from the same genotypes. (D) Responses to a brief pulse of 0.5 M sucrose for the same genotypes. (E) Average charge transfer from the sucrose-evoked fusion events. (F) Average tonic EPSC rate is plotted versus average sucrose-evoked charge for all five genotypes (both normalized to WT) as indicated by color. Linear regression produced a slope of 0.96 (dashed red line) compared with a slope of 1 (black solid line). Error bars are mean ± SEM, and recording numbers are indicated in the bars. For statistical comparisons, **P < 0.01 versus WT. *P < 0.05 versus WT. Data were compared using ANOVA, and the Tukey–Kramer test for multiple comparisons was used to generate P values. Strains: N2, JSD0557, JSD1200, JSD1193, and JSD1234.
The Last 200 Residues of Munc13-1/UNC-13 Form a Stable Membrane-Binding Protein Domain.
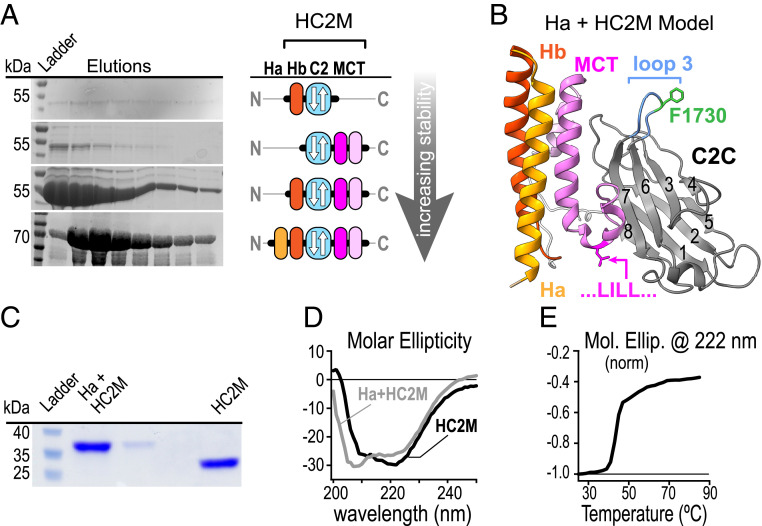
Are C2C and MCT two distinct domains or parts of a single domain? Previous studies have suggested that the ∼800-residue MUN-C2C region tethers Munc13-1 to vesicles via direct membrane binding (20, 21). To our knowledge, the biochemical properties of isolated C2C domains (∼130 residues) from the Unc13ABC proteins have not been characterized. We generated a series of constructs containing the UNC-13 C2C domain and assessed their expression, stability, and folding in vitro. Interestingly, the isolated C2C domain could not be produced as a stable well-folded protein whereas inclusion of the MCT domain and one or two neighboring N-terminal helices (Ha & Hb) fully stabilized the protein (Fig. 3 A and B). The resulting protein displayed spectroscopic signatures of both beta sheets and alpha helices by circular dichroism (CD), and inclusion of Ha in addition to Hb did not significantly change stability, folding, or yield (Fig. 3 C–E). Similar results were observed with the mouse Munc13-1 C2C domain (SI Appendix, Fig. S7). Thus, the C2C domain flanked by one or two N-terminal alpha helices and the MCT may form a single functional protein domain at the C terminus of UNC-13. We propose that this Helix-C2C-MCT domain (HC2M) is the minimal structural unit of the UNC-13 C terminus.
Fig. 3.
C2C together with N-terminal helices and the MCT form a membrane-binding protein domain. (A) Protein gels of C2C domain elutions (Left) and domain cartoons (Right) for several constructs differing in the amount of neighboring protein included in the expression construct assuming a type-I topology for the C2 domain. (B) A proposed model for HC2M structure (reference SI Appendix for details). Protein gel (C) and CD spectra (D) of recombinant worm Hb-C2C-MCT (black) and Ha-Hb-C2C-MCT (gray) following cleavage from maltose-binding protein. The minimal stable domain (Hb-C2C-MCT) is referred to as HC2M. (E) Thermal melting plot of WT HC2M (black).
To generate a working model for HC2M, sequence alignment and predicted secondary structure were compared with known C2 domain topologies. These comparisons indicated that C2C adopts a type-I topology similar to Synaptotagmin 1 and distinct from the UNC-13/Munc13-1 C2A and C2B domains (SI Appendix, Fig. S5). Modeling of the MCT and two helices on the N-terminal side of C2C predicted the formation of an alpha helical bundle, which was then docked onto the C2C domain (see SI Appendix for details). The resulting HC2M domain provides a rationale for the dependence on both C2C and MCT for a well-folded C-terminal domain (Fig. 3B). While nothing definitive can be concluded about the molecular organization of HC2M in the absence of direct structural data, the prediction served as a working model for designing several perturbations of the UNC-13 C terminus throughout this study.
Past studies have proposed a role for the MUN-C2C in anchoring Munc13 to SVs, but neither the membrane binding by MUN versus C2C nor the degree of membrane/cargo specificity are known (20, 21). Perhaps the C2C domain specifically binds SVs via specific lipid headgroups, Ca2+, or through a curvature preference (42). The lipid- and Ca2+-binding properties of the isolated HC2M domain were examined via liposome cosedimentation with small unilamellar vesicles (SUVs) laden with sucrose. Following incubation of the protein and SUVs, followed by centrifugation to pellet the SUVs, ∼60% of total protein was recovered in the pellet, consistent with liposome binding (SI Appendix, Fig. S6A). Importantly, this association did not depend on lipid headgroup charge, vesicle curvature, Ca2+ concentration, or the presence of PI4P (SI Appendix, Fig. S6 C–F). Because some of the cosedimented HC2M may have settled due to aggregation rather than lipid binding, the association of HC2M with liposomes was also examined by flotation in a sucrose gradient (SI Appendix, Fig. S6B). Consistent with the cosedimentation results, ∼50% of the protein was recovered in the liposome fraction at the top of the gradient following centrifugation. Similar observations were made with the mouse Munc13-1 HC2M (SI Appendix, Fig. S7). Thus, the isolated HC2M indeed binds directly to membranes, but this interaction is not specific for a particular membrane composition or curvature and does not depend on Ca2+.
HC2M Binds Membranes via a Predicted Alpha Helix in MCT.
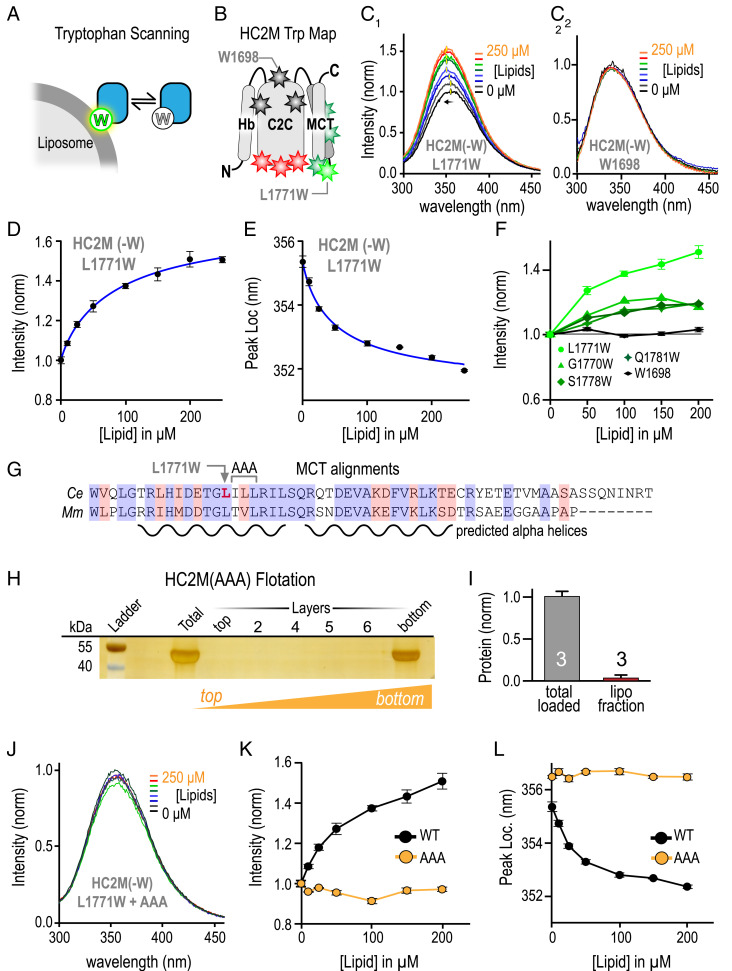
As an independent measure of HC2M membrane binding, we analyzed tryptophan (Trp) fluorescence using the fact that Trp fluorescence undergoes a blue shift and an increase in intensity as Trp enters a lower dielectric environment such as a lipid bilayer (Fig. 4A) (43). Trp was substituted into various locations in HC2M, and its fluorescence emission was monitored as a function of liposome concentration (44, 45) (Fig. 4B). Seven locations throughout HC2M were assayed, while three additional Trp substitutions depicted in red disrupted protein stability and were not analyzed further (Fig. 4B). All viable Trp substitutions in C2C failed to show lipid-dependent alterations in fluorescence (Fig. 4 B and C, 2 and SI Appendix, Fig. S8). By contrast, a strong lipid-dependent fluorescence response was observed at position L1771 in MCT (using UNC-13L residue numbering) altering both peak emission wavelength and peak intensity (Fig. 4 C, 1 and D–F). Weaker changes in intensity were observed with other MCT Trp substitutions (Fig. 4F and SI Appendix, Fig. S8). As a notable comparison, an endogenous Trp in C2C loop 2 (W1698) did not display lipid-induced fluorescence changes (Fig. 4 C, 2). Thus, systematic Trp scanning revealed lipid interactions mediated by the MCT domain but not the C2C domain.
Fig. 4.
HC2M binds membranes via MCT. (A) Cartoon of Trp residue penetrating into the SUV membrane. (B) Cartoon of the HC2M domain depicting the placement of Trp (W) residues at various locations with color indicating response to liposomes (red = unfolded, black = no response, dark green = small response in peak intensity, and light green = large response in both peak amplitude and blue shift). HC2M(-W) is a variant of HC2M with all four endogenous Trp residues removed. Emission spectra for L1771W (C, 1) and W1698 (C, 2) for a series of liposome concentrations. The location (D) and normalized intensity (E) of the emission peak for L1771W versus lipid concentration with simple binding model fits (blue). (F) Trp emission intensity versus lipid concentration for Trp locations in MCT (green) along with W1698 in C2C (black). (G) Sequence alignment of the MCT domain for C. elegans (Ce) UNC-13 and mouse (Mm) Munc13-1 indicating the residues mutated to alanines (ILL to AAA) and the predicted helix content. (H) Liposome flotation for GST-HC2M (AAA) mutant. (I) Quantification of the liposome binding assay normalized for the total protein loaded with n = 3 independent replicates. (J) Trp emission spectra for HC2M(-W) + L1771W + AAA. The emission peak (K) and peak location (L) plotted versus lipid for WT (black) and AAA (orange) variants of L1771W. Error bars are mean ± SEM.
The L1771W substitution provided the largest lipid-binding response among the seven viable Trp substitutions, and this well-conserved region of the MCT is predicted to adopt a helical conformation, perhaps with a turn near L1771 (Figs. 3B and 4G). The three hydrophobic residues following L1771 may participate directly in membrane binding. To test this hypothesis, these residues were replaced with alanines (A) in the WT protein, and this HC2M(AAA) variant was subjected to liposome flotation and cosedimentation assays. In stark contrast to WT, this well-folded protein failed to associate with liposomes with little to no protein recovered in the liposome layer (Fig. 4 H and I), supporting the idea that MCT helices strongly contribute to membrane binding. As an independent measure of lipid binding, Trp fluorescence at position L1771W in HC2M(AAA) was tested for lipid-dependent changes. Unlike WT L1771W, the AAA variant displayed no shift in emission peak intensity or location over a wide range of lipid concentration (Fig. 4 J–L). These experiments indicate that the MCT mediates membrane binding and that the triple point mutation eliminates detectable membrane binding in vitro without disrupting overall HC2M folding or stability.
Loss of Membrane Binding via HC2M Does Not Impact Nervous System Function.
If membrane binding by HC2M is required for proper function of UNC-13 in supporting synaptic transmission, introducing the triple point mutation into the WT unc-13 gene would likely phenocopy the Δ-MCT and Δ-C2C mutants. We used CRISPR to create HC2M(AAA) mutant animals, and unexpectedly, the triple point mutant animals appeared indistinguishable from WT in both locomotion and sensitivity to aldicarb (Fig. 5 A and B). Consistent with the behavioral assays, recordings from the AAA mutant NMJs revealed normal tonic- and stimulus-evoked synaptic transmission (Fig. 5 C–H). While there may have been some residual lipid-binding affinity in MCT that was undetectable in vitro, these in vivo assays demonstrated that direct membrane binding by HC2M is surprisingly not critical for its synaptic function.
Fig. 5.
Loss of membrane binding in the UNC-13 C-terminal domain does not impact nervous system function. (A) Average paralysis time course on 1 mM aldicarb for WT (black), ILL/AAA triple substitution mutant (AAA, orange), and artificial alpha helix substitution (hsHC2M, red) with the predicted AAA substitution location based on HC2M model (Inset). (B) Paralysis after 120 min for the same three strains as well as strains lacking either C2C (ΔC2C, cyan) or MCT (ΔMCT, green). (C) Tonic EPSCs were recorded from body wall muscle of adult worms in 1 mM external Ca2+. mEPSC traces from WT (black), AAA mutant (orange), and hsHC2M (red). Average of the frequency (D) and amplitude (E) of the mEPSCs from the same genotypes. (F) Representative stimulus-evoked EPSCs for WT (black), AAA (orange), and hsHC2M (red). Average EPSC peak amplitude (G) and cumulative charge transfer (H) for the same three genotypes. (I) First predicted alpha helix in MCT and a plot of the predicted alpha helicity for the WT helix (black) and artificial alpha helix substitution (red) using Agadir (68). (Inset) CD spectra for WT HC2M (black), AAA (orange), and hsHC2M (red). Strains: N2, JSD1193, JSD1200, JSD1288, and JSD1311.
To further explore the MCT domain, we designed a stable alpha helix spanning 14 residues (hsHC2M) and substituted the helical region surrounding L1771 in MCT to examine its impact on HC2M folding and stability (Fig. 5I) (46, 47). The hsHC2M protein was well folded and stable in vitro, indicating that the high sequence conservation of MCT is not critical for HC2M folding. The helix substitution (hsHC2M) was introduced into worms via CRISPR editing, and the resulting unc-13(hsHC2M) mutants were tested for behavioral and electrophysiological defects. In contrast to the AAA mutant, unc-13(hsHC2M) mutants phenocopied ΔMCT and ΔC2C mutants by displaying strongly impaired locomotion and profound aldicarb resistance (Fig. 5 A and B). Moreover, both tonic- and stimulus-evoked synaptic transmission were impacted to a similar degree (Fig. 5 C–H). Together, these observations indicate that the functional impact of the conserved MCT helical motif in UNC-13 cannot be explained by its ability to stabilize the C2C domain or by its membrane binding affinity.
Loop 3 of C2C Is Essential for HC2M Function In Vivo but Does Not Mediate Membrane Binding.
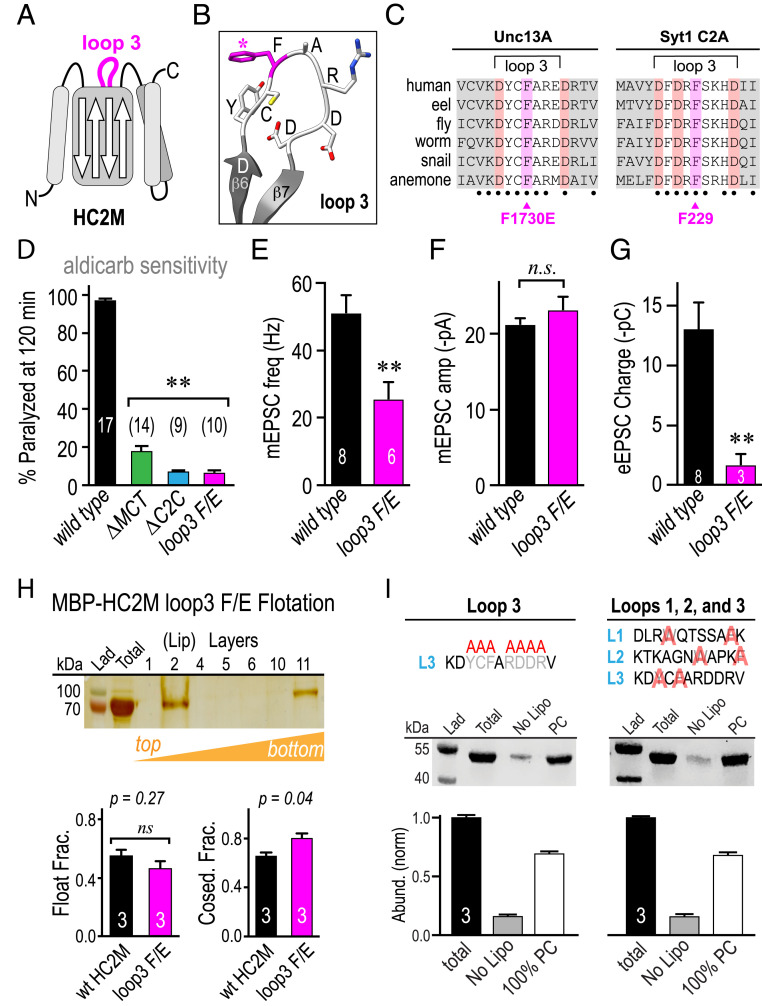
Another highly conserved region in the C-terminal domain is loop 3 in the C2C domain between beta strands 6 and 7 where Ca2+-dependent membrane binding occurs in Synaptotagmin 1 (Syt1) C2 domains (Fig. 6 A–C). The 9-residue motif DYCFAR[D/E]DR is almost perfectly preserved across the Unc13A subfamily (ranging from primates to cnidaria but not sponges), and a recent study proposed that this region of C2C directly mediates membrane interactions analogous to Syt1 C2 domains, noting that mutating a conserved phenylalanine (F) could disrupt membrane binding (Fig. 6C) (21). Although the UNC-13 C2C loop 3 contains two aspartates, Ca2+-dependent membrane binding was not detected (SI Appendix, Fig. S6C). To examine the impact of C2C loop 3 on UNC-13 function in the worm, unc-13(s69) null animals were rescued with an UNC-13L transgene encoding a glutamate (E) substitution in place of the conserved loop 3 F (F/E). Locomotion and aldicarb sensitivity were strongly impacted by the F/E mutant (Fig. 6D). Moreover, tonic- and stimulus-evoked synaptic transmission were impaired to a comparable degree as the ΔC2C and ΔMCT mutants (Fig. 6 E–G), consistent with loop 3 playing a critical role in HC2M function in both mice and worms. Given that membrane binding in HC2M mapped to the MCT helices rather than C2C loops (Fig. 4B), we examined the impact of the F/E substitution on HC2M lipid binding in vitro using liposome cosedimentation and flotation assays. The substitution did not cause a detectable change in lipid binding by either assay (Fig. 6H). Indeed, neither selective replacement of hydrophobic residues across C2C loops 1, 2, and 3 nor complete replacement of the entire C2C loop 3 with alanines impacted membrane association based on liposome cosedimentation (Fig. 6I), supporting the notion that MCT rather than C2C mediates membrane binding. Taken together, these in vitro and in vivo experiments indicate that the conserved loop 3 plays a critical role in HC2M function, but this role does not rely on direct membrane binding. Since the loop 3 sequence is almost perfectly conserved across metazoa but does not mediate membrane binding, perhaps this reflects a critical loop 3 protein–protein interaction in contrast to Syt1 C2 domains.
Fig. 6.
The conserved loop 3 region plays a critical functional role. (A) Cartoon of C2 loop 3 within the HC2M domain indicated in magenta. (B) Model of the loop 3 residues with F1730 indicated (magenta). (C) Loop 3 sequences alignment for representative vertebrate and invertebrate species for Unc13A HC2M and Syt1 C2A. Beta strands indicated in gray, aspartates highlighted in red, and loop 3 F1730 in UNC-13 and loop 3 F229 of Syt1 C2A highlighted in magenta. (D) Summary of percentage paralyzed in 1 mM aldicarb after 120 min for WT (black), C2C (cyan), MCT (green), and F1730E (magenta). Average tonic mEPSC frequency (E) and amplitude (F) in 1 mM Ca2+ for WT (black) and loop 3 F1730E (magenta). (G) Stimulus-evoked charge transfer for the same genotypes. (H) Liposome flotation in a sucrose gradient for MBP-HC2M(F1730E) and quantification of coflotation and cosedimentation assays normalized to total protein loaded for WT (black) and F1730E (magenta). (I) Liposome cosedimentation with representative protein gel and protein band quantification for the total protein loaded (total), protein only (no lipo), and 100% phosphatidylcholine liposomes (PC) for a complete replacement of the C2C loop residues with alanines. Left: Y1728A, C1729A, F1730A, R1732A, D1733A, D1734A, and R1735A, and targeted substitutions of the hydrophobic residues in loops 1, 2, and 3. Right: W1663A, F1669A, W1698A, F1702A, Y1728A, and F1730A. Error bars are mean ± SEM. n is indicated in or above the bars. (**P < 0.01; n.s., by ANOVA and Tukey–Kramer test for multiple comparisons). Strains: N2, JSD1193, JSD1200, and JSD1298.
Artificial Synaptic Vesicle Tethers Functionally Replace the UNC-13 HC2M Domain.
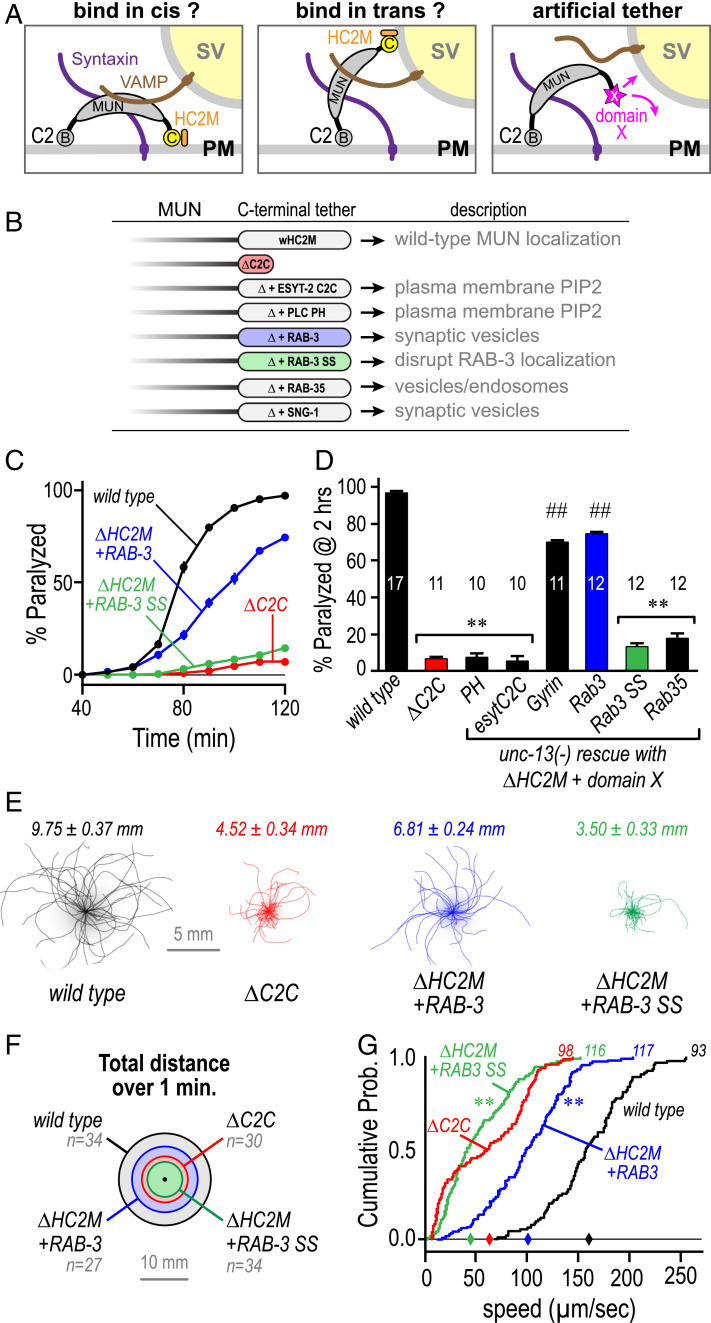
The large C-terminal domain of UNC-13 comprising MUN and C2C has been proposed to bind to SVs thereby tethering them to the plasma membrane (20, 21). However, the in vitro and in vivo results presented here thus far indicate that direct membrane binding by HC2M is not the likely mechanism for attaching the MUN domain to SVs. To explore HC2M mechanisms in vivo, HC2M was replaced with a series of small proteins known to associate with either the plasma membrane or SVs while assessing whether anchoring the MUN domain to a particular membrane could restore UNC-13 function at the synapse (Fig. 7 A and B). For example, the C-terminal C2 domain of the endoplasmic reticulum (ER) resident protein extended synaptotagmin ESYT-2 binds PI(4,5)P2 on the cytoplasmic leaflet of the plasma membrane thereby helping to tether the plasma membrane to ER tubules (48, 49). In contrast, RAB-3 is an SV-associated protein anchored via its geranylgeranylated C-terminal cysteines (50, 51). We asked whether these and other membrane-binding modules could restore UNC-13 function when fused to the C terminus of an UNC-13 ΔHC2M protein expressed in unc-13(s69) null mutant animals (Fig. 7B). Remarkably, there was a strong correlation between function and SV targeting based on aldicarb sensitivity; proteins that target the plasma membrane or other intracellular compartments failed to restore function, whereas RAB-3 and SNG-1 synaptogyrin both restored ∼70% of WT aldicarb sensitivity (Fig. 7 C and D). RAB-3 rescue was specific to its anchoring mechanism since replacement of the prenylated cysteines with serines (Rab3 SS) eliminated rescue (Fig. 7 C and D). As an independent test of functional rescue, spontaneous locomotion of the Rab3 and Rab3 SS tether transgenic animals was quantified and compared with WT and ΔC2C (Fig. 7E). Compared with loss of the C2C domain, the RAB-3 tether shifted the distribution of speeds toward WT whereas the SS variant failed to improve locomotion (Fig. 7 F and G). These artificial membrane anchor experiments strongly support the importance of an interaction between HC2M and SVs. Given the high degree of HC2M conservation across Unc13A, BAIAP3, and Unc13D subfamilies, we speculate that their HC2M domains share common features and that this domain has evolved specialized cargo-specific tethering roles in distinct trafficking pathways within metazoan cells (SI Appendix, Figs. S9 and S10).
Fig. 7.
Artificial SV tethers can functionally replace the UNC-13 HC2M module. (A) Cartoon depicting models of HC2M as either a plasma membrane tether (PM, cis) or a SV tether (trans). Replacing the HC2M module with a foreign tethering domain (magenta) will drive the MUN domain to one of these possible locations. (B) Schematic of the foreign tether strategy listing all of the membrane-binding proteins screened: extended synaptotagmin ESYT-2 C2C domain (esytC2C), PLC-δ2 PH domain (PH), RAB-3 (Rab3), SNG-1 synaptogyrin (Gyrin), RAB-35 (Rab35), and RAB-3 CC → SS variant (Rab3 SS). Functionality was assessed by expression of these chimeric constructs in the unc-13(s69) null. (C) Average paralysis time course on 1 mM aldicarb for WT (black), ΔC2C (red), RAB-3 (blue solid circles), and RAB-3 SS (green). (D) Summary of percentage paralyzed at 120 min for these strains as well as PH, Rab35, esytC2C, and Gyrin (black). (E) Locomotion trajectories collected over 1 min plotted for WT (gray), ΔC2C (red), ΔHC2M+Rab-3 (blue), and ΔHC2M+Rab-3 SS (green) with average total distance covered indicated above. (F) Total distance covered over 1 min from the origin represented as circle diameter for the same four genotypes. (G) Cumulative probability distributions of locomotion speed for the same four genotypes with the total number of animals indicated at the top of the curve and median values along the horizontal axis (diamonds). Error bars are mean ± SEM, and n is indicated in the bars. **P < 0.01 versus WT; ##P < 0.01 versus WT and bracket group. Significance for the aldicarb assay was generated from ANOVA and Tukey–Kramer test for multiple comparisons. The Kolmogorov–Smirnov test was used to compare velocity distributions. Strains: N2, JSD1200, JSD0981, JSD0949, JSD1329, JSD1331, JSD1333, and JSD1334.
Discussion
The findings described in this study were initiated by a whole-genome suppressor screen to better understand the molecular mechanisms underlying SV fusion, and the first identified mutant corresponded to a selective deletion of the C-terminal domain of UNC-13. A combination of behavioral, genetic, electrophysiological, and biochemical approaches led to four major findings. First, loss of the C2C domain predominantly impaired SV priming rather than some step downstream of Ca2+ entry during stimulus-evoked fusion. Second, we report the HC2M domain as a critical feature of the Unc13 protein family structure and function. Third, we establish the pure lipid-binding properties of the isolated HC2M domain, map the lipid binding location, and determine that this direct mode of membrane association is in fact not critical for HC2M function at the synapse. And fourth, HC2M can be functionally replaced in vivo with foreign protein domains that steer the MUN domain to SVs but not to the plasma membrane.
Comparisons with Previous Studies.
The striking protein-sequence conservation across the C-terminal half of Unc13 family members including a C-terminal C2 domain was originally noted by Brose et al. (9). The first studies to establish functional importance for the C2C domain in secretion found that loss of a C-terminal region containing C2C impaired synaptic transmission in worm as well as vesicle fusion in chromaffin cells (18, 19). Another study in C. elegans systematically explored NMJ synaptic transmission in an allelic series of unc-13 mutants including a point substitution at the end of the MUN domain nearby C2C (14). The unc-13(e2813) mutant harbors an S1574R point mutation within a conserved helical region at the C terminus of the MUN domain, and a similar impairment of synaptic transmission was observed in this variant compared with deletion of C2C-MCT described here (14, 52, 53). Thus, several studies have previously established that perturbing the C-terminal end of Munc13-1/UNC-13 has deleterious functional consequences at the synapse.
The concept that the MUN-C2C region of Munc13 connects Munc13 to SVs was put forward based on an extensive series of rigorous in vitro experiments as well as synaptic recordings in cultured hippocampal autapses (20, 21). Many of the results presented here broadly support and extend these findings; particularly, the HC2M domain appears to serve as an attachment site between the MUN domain and SVs, and this association is required for efficient SV priming. Moreover, the fact that loss of the C2C domain was identified here in an unbiased forward genetics screen further highlights its importance for controlling synaptic transmission in a behaving nervous system. One interesting and notable difference in the current study compared with previous Munc13 work is the isolation of HC2M away from the bulk of the large MUN domain. We find that HC2M autonomously binds liposomes via a predicted helix in MCT but that disruption of this membrane interaction is unexpectedly inconsequential in the animal. In addition, an F/E substitution in the highly conserved C2C loop 3 does not disrupt membrane binding by HC2M in vitro while profoundly disrupting nervous system function, similar to that described previously (21). Moreover, elimination of all hydrophobic residues in loops 1, 2, and 3 of C2C failed to perturb liposome interactions, suggesting that C2C does not function as a membrane-binding domain on its own. Based on these observations, as well as the precise loop 3 sequence conservation observed from cnidaria to primates, we propose that this loop is not explicitly involved in membrane binding per se but in a highly conserved intra- or intermolecular protein interaction with a partner yet to be determined. Because the HC2M module can be largely bypassed with a foreign tether like RAB-3, a logical candidate would be some SV-associated protein. Indeed, a recent investigation of MUN domain binding partners proposed that VAMP2 binds to the C-terminal helices of the MUN nearby C2C, although C2C and MCT were not included in that study (23). Future exploration of HC2M interactions will provide a more accurate picture of loop 3 function.
Conservation of HC2M and C-Terminal Tethering across the Extended Unc13 Family.
Sequence comparisons across Unc13 homologs broadly divide them into four subfamilies: Unc13ABC (synaptic), BAIAP3 (general trafficking), Unc13D (immune cells), and CAPS (neurons and neuroendocrine cells) (SI Appendix, Fig. S10 and Table S3). The extended Unc13 family likely diverged from an ancestral Unc13 gene prior to the emergence of metazoa because several Unc13 homologs are found in single-celled choanoflagellates. Which features of HC2M are most deeply conserved? The predicted helices of MCT with their specific sequence motifs are identifiable even in the sponge Amphimedon queenslandica. However, the critical C2 loop 3 is conserved in ctenophora and cnidaria but not sponges, corresponding to the origins of true neurons (54–56). The five Ca2+-binding aspartates found in loops 1 and 3 of type-I C2 domains are generally present in BAIAP3 and Unc13D but mostly absent in Unc13A (SI Appendix, Fig. S9). Thus, the regulation of HC2M may have diverged as the subfamilies evolved distinct roles in membrane trafficking in metazoa, and Ca2+ regulation of HC2M was specifically lost in synaptic Unc13A homologs. Perhaps all Unc13 family members act as membrane tethers, with the HC2M domain diverging between subfamilies (SI Appendix, Fig. S10). For example, the C-terminal region of Unc13D/Munc13-4 is crucial for its proper function in the immune system. Specifically, the C2 domain of Unc13D HC2M has been proposed to aid in regulated fusion of cytotoxic granules via membrane and Ca2+ binding. Interestingly, an early stop mutation in human Unc13D lacking its HC2M domain causes familial hemophagocytic lymphohistiocytosis type 3 associated with an impairment in cytotoxic lymphocyte degranulation (25). While HC2M is conspicuously absent in the CAPS subfamily, CAPS still participates in DCV tethering and fusion via its MUN domain, indicating that the MUN domain itself may play a specific role in target membrane recognition and binding (22, 57). HC2M is found at the C terminus of most Unc13 family members, but its sequence conservation is considerably higher in Unc13A compared with the other family members (SI Appendix, Fig. S10A), indicating a strong evolutionary pressure operating on Unc13A HC2M. We suggest that, in neurons, the HC2M module evolved into an SV adaptor contributing to the precise and highly regulated properties of synaptic transmission during the evolution of protonervous systems.
Conclusion
HC2M function is merely one facet of the large multifunctional UNC-13/Munc13-1 protein. Distinct from the priming of docked SVs, HC2M may play a part in Munc13-dependent nucleation of release sites in hippocampal synapses (11). Our current data do not distinguish between site nucleation, SV docking, and SV priming, but recent synaptic data from a ΔC2C truncated Munc13-1 in mouse neurons indicates that SV docking is strongly impaired (21), consistent with either docking or site nucleation roles for this domain. The N-terminal half of UNC-13/Munc13-1 interacts with active-zone proteins as well as calmodulin and varies widely across evolution and across isoforms within a species (5, 58–61). The UNC-13/Munc13-1 C1-C2B module integrates lipid and Ca2+ signaling to modulate vesicle fusogenicity, potentially in response to past synaptic activity (3, 39, 40, 62–64). And the defining feature of this protein family, the 20 nm long MUN domain, regulates Syntaxin 1 incorporation into the neuronal SNARE complex in the proper orientation (4, 17, 23, 65–67). Remarkably, these diverse presynaptic roles are performed by a single gene product that dates back to the origins of synaptic transmission more than 600 Mya. Future studies will no doubt continue to reveal important features of all these Unc13 family functions in regulated exocytosis.
Materials and Methods
Strains and Constructs.
Strains were maintained and genetically manipulated as previously described (8). Animals were raised at 20 °C on nematode growth media seeded with OP50. The following primary strains were used: N2 and unc-13(s69). Reference SI Appendix, Table S1 for reagents, reference SI Appendix, Table S2 for full list of transgenic animals and constructs used, and reference SI Appendix, Supplementary Methods for descriptions of locomotion, aldicarb sensitivity, and imaging assays.
Electrophysiology.
Whole-cell voltage-clamp recordings were performed on body-wall muscles of dissected adult worms superfused in an extracellular solution containing (in mM) 127 NaCl, 5 KCl, 26 NaHCO3, 1.25 NaH2PO4, 20 glucose, 1 CaCl2, and 4 MgCl2, bubbled with 5% CO2 and 95% O2 at 20 °C. Ca2+ ions were replaced with 1 mM MgCl2 to record miniature excitatory postsynaptic currents (mEPSCs) and miniature inhibitory postsynaptic currents (mIPSCs) in 0 mM Ca2+. Whole-cell recordings were carried out at −60 mV using an internal solution containing (in mM) 105 CsCH3SO3, 10 CsCl, 15 CsF, 4 MgCl2, 5 EGTA, 0.25 CaCl2, 10 Hepes, and 4 Na2ATP, adjusted to pH 7.2 using CsOH. Under these conditions, we only observed endogenous ACh EPSCs. Further details are provided in SI Appendix, Supplementary Methods.
Protein and Liposome Purification, Trp Fluorescence, and CD Spectroscopy.
UNC-13 HC2M and its variants listed in the table of recombinant proteins (SI Appendix, Table S2) were cloned into PGEX-KG vector or P202:His-MBP vectors and transformed into T7 Express lysY/Iq-competent Escherichia coli cells for protein expression. Lipids were obtained from Avanti polar lipids and stored at −20 °C. Trp fluorescence measurements were made at 20 °C using a spectrofluorometer. Protein samples were mixed with liposomes at different concentrations in buffer Tris HCl pH 8.0 with 250 mM NaCl and incubated at room temperature for 30 min. The samples were excited at 280 nm, and emission spectra were collected from 300 to 460 nm with the slit widths 4 nm and 1 nm, respectively. Protein-liposome fluorescence spectra were corrected for fluorescence in liposomes alone. The data were analyzed using the software IGOR Pro (WaveMetrics). Far ultraviolet CD spectroscopy experiments were performed on an AVIV Biomedical Model 410 CD spectrometer. Buffers, purification details, and liposome cosedimentation and coflotation protocols are described in SI Appendix, Supplementary Methods.
Supplementary Material
Acknowledgments
We thank Tim Ryan, Joshua Kaplan, David Eliezer, Hengyi Xie, and members of the J.S.D. laboratory for help, advice, and for critically reading the manuscript. We thank the Kaplan laboratory for providing the KP9537 strain. We also acknowledge the High Performance Computing Center at Texas Tech University at Lubbock for providing computing resources that have contributed to the research reported here: http://cmsdev.ttu.edu/hpcc. This work was supported by NIH Grant R01-NS116747 (J.S.D.) and Australian Research Council Grant DP160100849 (Z.H.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. E.R.C. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2016276118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or supporting information.
References
- 1.Südhof T. C., Neurotransmitter release: The last millisecond in the life of a synaptic vesicle. Neuron 80, 675–690 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dittman J. S., Ryan T. A., The control of release probability at nerve terminals. Nat. Rev. Neurosci. 20, 177–186 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Rizo J., Mechanism of neurotransmitter release coming into focus. Protein Sci. 27, 1364–1391 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunger A. T., et al., The pre-synaptic fusion machinery. Curr. Opin. Struct. Biol. 54, 179–188 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dittman J. S., Unc13: A multifunctional synaptic marvel. Curr. Opin. Neurobiol. 57, 17–25 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burkhardt P., et al., Primordial neurosecretory apparatus identified in the choanoflagellate Monosiga brevicollis. Proc. Natl. Acad. Sci. U.S.A. 108, 15264–15269 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varoqueaux F., Fasshauer D., Getting nervous: An evolutionary overhaul for communication. Annu. Rev. Genet. 51, 455–476 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Brenner S., The genetics of Caenorhabditis elegans. Genetics 77, 71–94 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brose N., Hofmann K., Hata Y., Südhof T. C., Mammalian homologues of Caenorhabditis elegans unc-13 gene define novel family of C2-domain proteins. J. Biol. Chem. 270, 25273–25280 (1995). [DOI] [PubMed] [Google Scholar]
- 10.Koch H., Hofmann K., Brose N., Definition of Munc13-homology-domains and characterization of a novel ubiquitously expressed Munc13 isoform. Biochem. J. 349, 247–253 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakamoto H., et al., Synaptic weight set by Munc13-1 supramolecular assemblies. Nat. Neurosci. 21, 41–49 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Richmond J. E., Weimer R. M., Jorgensen E. M., An open form of syntaxin bypasses the requirement for UNC-13 in vesicle priming. Nature 412, 338–341 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunger A. T., Choi U. B., Lai Y., Leitz J., Zhou Q., Molecular mechanisms of fast neurotransmitter release. Annu. Rev. Biophys. 47, 469–497 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richmond J. E., Davis W. S., Jorgensen E. M., UNC-13 is required for synaptic vesicle fusion in C. elegans. Nat. Neurosci. 2, 959–964 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aravamudan B., Fergestad T., Davis W. S., Rodesch C. K., Broadie K., Drosophila UNC-13 is essential for synaptic transmission. Nat. Neurosci. 2, 965–971 (1999). [DOI] [PubMed] [Google Scholar]
- 16.Augustin I., Rosenmund C., Südhof T. C., Brose N., Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature 400, 457–461 (1999). [DOI] [PubMed] [Google Scholar]
- 17.Rizo J., Xu J., The synaptic vesicle release machinery. Annu. Rev. Biophys. 44, 339–367 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Stevens D. R., et al., Identification of the minimal protein domain required for priming activity of Munc13-1. Curr. Biol. 15, 2243–2248 (2005). [DOI] [PubMed] [Google Scholar]
- 19.Madison J. M., Nurrish S., Kaplan J. M., UNC-13 interaction with syntaxin is required for synaptic transmission. Curr. Biol. 15, 2236–2242 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Liu X., et al., Functional synergy between the Munc13 C-terminal C1 and C2 domains. eLife 5, e13696 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quade B., et al., Membrane bridging by Munc13-1 is crucial for neurotransmitter release. eLife 8, e42806 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grishanin R. N., et al., Membrane association domains in Ca2+-dependent activator protein for secretion mediate plasma membrane and dense-core vesicle binding required for Ca2+-dependent exocytosis. J. Biol. Chem. 277, 22025–22034 (2002). [DOI] [PubMed] [Google Scholar]
- 23.Wang S., et al., Munc18 and Munc13 serve as a functional template to orchestrate neuronal SNARE complex assembly. Nat. Commun. 10, 69 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X., et al., BAIAP3, a C2 domain-containing Munc13 protein, controls the fate of dense-core vesicles in neuroendocrine cells. J. Cell Biol. 216, 2151–2166 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elstak E. D., et al., A novel Dutch mutation in UNC13D reveals an essential role of the C2B domain in munc13-4 function. Pediatr. Blood Cancer 58, 598–605 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Boswell K. L., et al., Munc13-4 reconstitutes calcium-dependent SNARE-mediated membrane fusion. J. Cell Biol. 197, 301–312 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.James D. J., Martin T. F., CAPS and Munc13: CATCHRs that SNARE vesicles. Front. Endocrinol. (Lausanne) 4, 187 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dickinson D. J., Ward J. D., Reiner D. J., Goldstein B., Engineering the Caenorhabditis elegans genome using Cas9-triggered homologous recombination. Nat. Methods 10, 1028–1034 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dittman J. S., Kaplan J. M., Behavioral impact of neurotransmitter-activated G-protein-coupled receptors: Muscarinic and GABAB receptors regulate Caenorhabditis elegans locomotion. J. Neurosci. 28, 7104–7112 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rand J. B., Russell R. L., Molecular basis of drug-resistance mutations in C. elegans. Psychopharmacol. Bull. 21, 623–630 (1985). [PubMed] [Google Scholar]
- 31.Miller K. G., et al., A genetic selection for Caenorhabditis elegans synaptic transmission mutants. Proc. Natl. Acad. Sci. U.S.A. 93, 12593–12598 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richmond J. E., Jorgensen E. M., One GABA and two acetylcholine receptors function at the C. elegans neuromuscular junction. Nat. Neurosci. 2, 791–797 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin J. A., Hu Z., Fenz K. M., Fernandez J., Dittman J. S., Complexin has opposite effects on two modes of synaptic vesicle fusion. Curr. Biol. 21, 97–105 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Q., Hollopeter G., Jorgensen E. M., Graded synaptic transmission at the Caenorhabditis elegans neuromuscular junction. Proc. Natl. Acad. Sci. U.S.A. 106, 10823–10828 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z. W., Saifee O., Nonet M. L., Salkoff L., SLO-1 potassium channels control quantal content of neurotransmitter release at the C. elegans neuromuscular junction. Neuron 32, 867–881 (2001). [DOI] [PubMed] [Google Scholar]
- 36.Rosenmund C., Stevens C. F., Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron 16, 1197–1207 (1996). [DOI] [PubMed] [Google Scholar]
- 37.Imig C., et al., The morphological and molecular nature of synaptic vesicle priming at presynaptic active zones. Neuron 84, 416–431 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Lipstein N., et al., Dynamic control of synaptic vesicle replenishment and short-term plasticity by Ca(2+)-calmodulin-Munc13-1 signaling. Neuron 79, 82–96 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Rosenmund C., et al., Differential control of vesicle priming and short-term plasticity by Munc13 isoforms. Neuron 33, 411–424 (2002). [DOI] [PubMed] [Google Scholar]
- 40.Michelassi F., Liu H., Hu Z., Dittman J. S., A C1-C2 module in Munc13 inhibits calcium-dependent neurotransmitter release. Neuron 95, 577–590.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li L., et al., A hyperactive form of unc-13 enhances Ca2+ sensitivity and synaptic vesicle release probability in C. elegans. Cell Rep. 28, 2979–2995.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corbalan-Garcia S., Gómez-Fernández J. C., Signaling through C2 domains: More than one lipid target. Biochim. Biophys. Acta 1838, 1536–1547 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Ladokhin A. S., White S. H., Alphas and taus of tryptophan fluorescence in membranes. Biophys. J. 81, 1825–1827 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.White S. H., Wimley W. C., Ladokhin A. S., Hristova K., Protein folding in membranes: Determining energetics of peptide-bilayer interactions. Methods Enzymol. 295, 62–87 (1998). [DOI] [PubMed] [Google Scholar]
- 45.Chapman E. R., Davis A. F., Direct interaction of a Ca2+-binding loop of synaptotagmin with lipid bilayers. J. Biol. Chem. 273, 13995–14001 (1998). [DOI] [PubMed] [Google Scholar]
- 46.Marqusee S., Baldwin R. L., Helix stabilization by Glu-...Lys+ salt bridges in short peptides of de novo design. Proc. Natl. Acad. Sci. U.S.A. 84, 8898–8902 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Radoff D. T., et al., The accessory helix of complexin functions by stabilizing central helix secondary structure. eLife 3, e04553 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stefan C. J., Manford A. G., Emr S. D., ER-PM connections: Sites of information transfer and inter-organelle communication. Curr. Opin. Cell Biol. 25, 434–442 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saheki Y., De Camilli P., The extended-synaptotagmins. Biochim. Biophys. Acta Mol. Cell Res. 1864, 1490–1493 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fukuda M., Regulation of secretory vesicle traffic by Rab small GTPases. Cell. Mol. Life Sci. 65, 2801–2813 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nonet M. L., et al., Caenorhabditis elegans rab-3 mutant synapses exhibit impaired function and are partially depleted of vesicles. J. Neurosci. 17, 8061–8073 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kohn R. E., et al., Expression of multiple UNC-13 proteins in the Caenorhabditis elegans nervous system. Mol. Biol. Cell 11, 3441–3452 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yook K. J., Proulx S. R., Jorgensen E. M., Rules of nonallelic noncomplementation at the synapse in Caenorhabditis elegans. Genetics 158, 209–220 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burkhardt P., Sprecher S. G., Evolutionary origin of synapses and neurons–Bridging the gap. Bioessays 39, 1–10 (2017). [DOI] [PubMed] [Google Scholar]
- 55.King N., Rokas A., Embracing uncertainty in reconstructing early animal evolution. Curr. Biol. 27, R1081–R1088 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liebeskind B., Hofmann H., Hillis D., Zakon H., Futuyma D., Evolution of animal neural systems. Annu. Rev. Ecol. Evol. Syst. 48, 377–398 (2017). [Google Scholar]
- 57.Kabachinski G., Kielar-Grevstad D. M., Zhang X., James D. J., Martin T. F., Resident CAPS on dense-core vesicles docks and primes vesicles for fusion. Mol. Biol. Cell 27, 654–668 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu Z., Tong X. J., Kaplan J. M., UNC-13L, UNC-13S, and Tomosyn form a protein code for fast and slow neurotransmitter release in Caenorhabditis elegans. eLife 2, e00967 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu H., et al., Heterodimerization of UNC-13/RIM regulates synaptic vesicle release probability but not priming in C. elegans. eLife 8, e40585 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lipstein N., et al., Nonconserved Ca(2+)/calmodulin binding sites in Munc13s differentially control synaptic short-term plasticity. Mol. Cell. Biol. 32, 4628–4641 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reddy-Alla S., et al., Stable positioning of Unc13 restricts synaptic vesicle fusion to defined release sites to promote synchronous neurotransmission. Neuron 95, 1350–1364.e12 (2017). [DOI] [PubMed] [Google Scholar]
- 62.Lou X., Korogod N., Brose N., Schneggenburger R., Phorbol esters modulate spontaneous and Ca2+-evoked transmitter release via acting on both Munc13 and protein kinase C. J. Neurosci. 28, 8257–8267 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rhee J. S., et al., Beta phorbol ester- and diacylglycerol-induced augmentation of transmitter release is mediated by Munc13s and not by PKCs. Cell 108, 121–133 (2002). [DOI] [PubMed] [Google Scholar]
- 64.Shin O. H., et al., Munc13 C2B domain is an activity-dependent Ca2+ regulator of synaptic exocytosis. Nat. Struct. Mol. Biol. 17, 280–288 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu J., et al., Mechanistic insights into neurotransmitter release and presynaptic plasticity from the crystal structure of Munc13-1 C1C2BMUN. eLife 6, e22567 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang X., et al., Syntaxin opening by the MUN domain underlies the function of Munc13 in synaptic-vesicle priming. Nat. Struct. Mol. Biol. 22, 547–554 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li W., et al., The crystal structure of a Munc13 C-terminal module exhibits a remarkable similarity to vesicle tethering factors. Structure 19, 1443–1455 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Muñoz V., Serrano L., Development of the multiple sequence approximation within the AGADIR model of alpha-helix formation: Comparison with zimm-bragg and lifson-roig formalisms. Biopolymers 41, 495–509 (1997). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information.