Significance
Ecological processes that enhance or dampen the likelihood of shifts between top-down (i.e., predator-driven) and bottom-up (i.e., resource-driven) forcing underpin community regulation, functioning, and stability. Here, we demonstrate how the behavioral response of an apex predator to changes in prey behavior and condition (i.e., energetic profitability) can dramatically alter the strength of top-down forcing and enhance the role (or relative contribution) of bottom-up community regulation, depending on the spatial organization of diverse ecosystem states. These results highlight the role of consumer and predator trait-mediated responses to resource mosaics that are common throughout the natural world and enhance understanding of reciprocal feedbacks between top-down and bottom-up forcing on the regional stability of ecosystems.
Keywords: community regulation, ecosystem functioning, trophic cascade, stability, species interactions
Abstract
Consumer and predator foraging behavior can impart profound trait-mediated constraints on community regulation that scale up to influence the structure and stability of ecosystems. Here, we demonstrate how the behavioral response of an apex predator to changes in prey behavior and condition can dramatically alter the role and relative contribution of top-down forcing, depending on the spatial organization of ecosystem states. In 2014, a rapid and dramatic decline in the abundance of a mesopredator (Pycnopodia helianthoides) and primary producer (Macrocystis pyrifera) coincided with a fundamental change in purple sea urchin (Strongylocentrotus purpuratus) foraging behavior and condition, resulting in a spatial mosaic of kelp forests interspersed with patches of sea urchin barrens. We show that this mosaic of adjacent alternative ecosystem states led to an increase in the number of sea otters (Enhydra lutris nereis) specializing on urchin prey, a population-level increase in urchin consumption, and an increase in sea otter survivorship. We further show that the spatial distribution of sea otter foraging efforts for urchin prey was not directly linked to high prey density but rather was predicted by the distribution of energetically profitable prey. Therefore, we infer that spatially explicit sea otter foraging enhances the resistance of remnant forests to overgrazing but does not directly contribute to the resilience (recovery) of forests. These results highlight the role of consumer and predator trait-mediated responses to resource mosaics that are common throughout natural ecosystems and enhance understanding of reciprocal feedbacks between top-down and bottom-up forcing on the regional stability of ecosystems.
The role of trophic interactions in creating and maintaining the structure and functioning of natural communities remains a central issue in ecology. In particular, there are now many examples of the importance of top-down (i.e., predator-driven) and bottom-up (i.e., resource-driven) processes that determine the structure and stability of communities (1–3). Although odd or even numbers of trophic levels can define the relative importance of top-down versus bottom-up community regulation, the addition or loss of entire trophic levels is uncommon relative to changes in the strength of interactions between trophic levels. Such changes can result from environmental disturbances (e.g., severe storms or drought) or from shifts in the abundance or traits (e.g., foraging behavior or size structure) of populations (3–5). Therefore, empirical evaluations of ecological processes that enhance or dampen the likelihood of shifts between top-down and bottom-up forcing are essential to understanding the potential for cascading effects that can underpin community structure, functioning, and stability (1, 6, 7). Moreover, the processes that facilitate these alternations at ecologically relevant scales may only be revealed through opportunistic and significant disturbance events, especially in the form of herbivore outbreaks (8–10).
While trophic cascades have traditionally focused on the direct role of apex predators on lower trophic-level species, it is now clear that both predator foraging behavior and prey attributes (e.g., morphological, physiological, and behavioral) can impart profound trait-mediated constraints on community regulation (11). For example, prey condition (e.g., age or health) may influence the strength of top-down control by altering predator foraging strategies and the capacity for predators to optimize prey acquisition (5). Prey may also respond to the mere presence of predators by reducing their grazing activity, thereby enhancing the productivity of primary producers (2). However, bottom-up trait-mediated interactions can also dictate community dynamics through changes in energy transfer between primary producers and higher trophic level consumers (3, 12). Therefore, understanding how shifts in prey condition and behavior resulting from changes in primary production reciprocally influence the foraging preferences of predators is essential to predicting when, where, and under what conditions communities vary in the relative influence of top-down or bottom-up processes and how such interactions influence the state of communities and ecosystems.
In kelp forest ecosystems around the world, active sea urchin grazing has repeatedly transformed forested reefs to sea urchin “barrens” that are void of macroalgae (13). Fundamental changes in sea urchin foraging behavior (from passive foraging on drift algae, to active foraging on live kelp and other macroalgae) have been attributed to the loss of sea urchin predators (14–18) or a reduction in food availability (19). In kelp forests along the northeastern Pacific, sea urchins reside in the refuge of cracks and crevices, primarily forage on drift algae, and invest energy into reproductive gonad production (20). However, when sea urchins shift to an active foraging modality and emerge from the refuge of cracks and crevices, they have the capacity to drastically reduce kelp abundance, at which point gonad condition can decline because of reduced food availability (19). While many studies have focused on the role of apex predators in sea urchin population control, less attention is given to how predators respond to variation in both the behavior (i.e., cryptic or exposed) and gonad condition (i.e., energetic profitability) of sea urchin prey resulting from changes in the abundance of macroalgae.
In this study, we examined how a shift in grazer behavior and energetic profitability led to a fundamental change in predator foraging behavior, thereby altering both the role and contribution of top-down forcing on the system. Along the central coast of California, forests of giant kelp (Macrocystis pyrifera, hereafter “kelp”) had persisted for decades because purple sea urchins (Strongylocentrotus purpuratus, hereafter “urchins”) were controlled by the top predator, the southern sea otter (Enhydra lutris nereis, hereafter “sea otter”) and various mesopredators. However, in 2014, an unprecedented decline in kelp resulted from a series of climatic stressors similar to those observed farther north (21). This decline in kelp production coincided with a widespread sea star wasting disease that decimated local populations of the urchin predator, Pycnopodia helianthoides [hereafter, “Pycnopodia” (22)]. Both of these factors likely contributed to a shift in sea urchin foraging behavior from cryptic passive grazing to active grazing of live macroalgae, transforming a once expansive kelp forest to a patchy-mosaic landscape of remnant kelp forests interspersed with sea urchin barrens.
Here, we examine how sea otters (predator) respond to changes in urchin (prey) behavior and condition (i.e., gonad index) to better understand how the contribution and role of predator-driven impacts (top-down forcing) and resource abundance (bottom-up forcing) drive the spatial dynamics of community structure. First, we test two hypotheses related to prey behavior and condition as a function of resource availability: 1) a shift in sea urchin behavior from passive to active foraging is associated with a decline in kelp availability, and 2) active sea urchin foraging behavior is associated with a decline in prey condition (gonad volume). We build on these hypotheses by exploring whether a top predator responds numerically and functionally to changes in sea urchin behavior and condition. Specifically, we test for a numerical response by exploring 3) whether a population-level increase in sea otter abundance is explained by an increase in the density of exposed prey (purple sea urchin). We then explore the functional response of sea otters by testing for 4) an increase in the dietary contribution of urchins. Finally, we test the following hypothesis: 5) the spatial distribution of sea otter foraging effort for urchin prey is not directly linked to high prey density but rather is predicted by the distribution of energetically profitable (gonad rich) prey.
Results
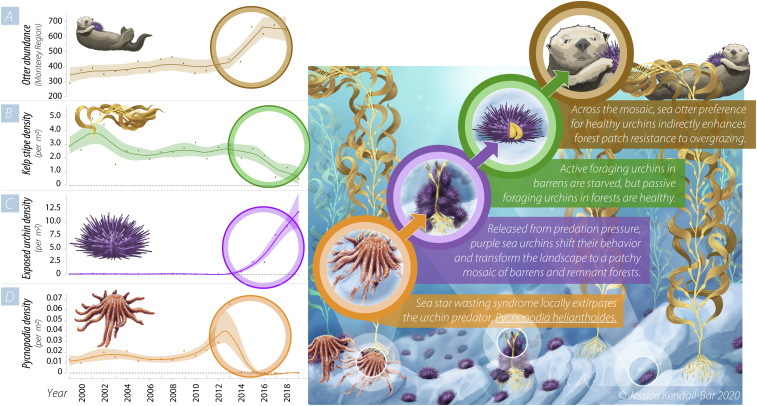
Variation in the relative density of Pycnopodia revealed that purple sea urchins, kelp, and sea otter abundance were tightly coupled, with evidence of a synchronous increase in urchins and otters, and a sharp decline in kelp and Pycnopodia, beginning in 2014 (Fig. 1). These dynamics initiated the transformation of a once expansive kelp forest to a patchy mosaic of remnant forests interspersed with sea urchin barrens (SI Appendix, Figs. S1 and S2).
Fig. 1.
Temporal dynamics of sea otters, kelp, sea urchins, and Pycnopodia. (Left) Annual changes in sea otter abundance in the Monterey study region (A) and relative density of kelp stipes (B), exposed sea urchins (C), and Pycnopodia (D). The trend line in A is corrected for observer error and fit with a Bayesian state-space model (SI Appendix, Supplementary Methods) to the time series of raw survey counts of independent sea otters. B–D represent annual mean observed densities fit with a cubic spline (λ = 0.05). Each shaded region across A–D represents the 95% credible interval. (Right) A conceptual illustration of the dynamics that initiated the formation of the mosaic of remnant kelp forests interspersed with sea urchin barrens. See SI Appendix, Supplementary Methods for expanded time series analyses. We used published data for A from the US Geological Survey (available at https://doi.org/10.3133/ds1097) and subtidal data for B–D from the Partnership for Interdisciplinary Studies of Coastal Oceans subtidal surveys (available at https://doi.org/10.6085/AA/PISCO_SUBTIDAL.151.2).
Shift in Urchin Behavior and Condition.
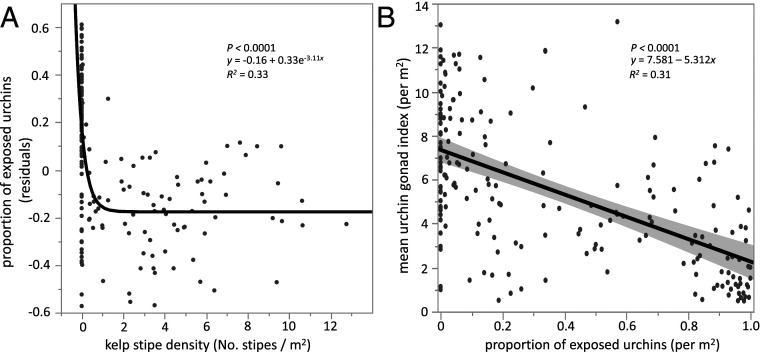
The log-transformed proportion of exposed urchins was positively associated with urchin density (P < 0.0001, degrees of freedom [DF] = 1, R2 = 0.40). After controlling for the positive effect of density on urchin exposure, variation in the residuals was further explained by a negative exponential relationship with kelp stipe density (P < 0.0001, R2 = 0.33; Fig. 2A). The proportion of exposed urchins was greatest in areas with no kelp, and crevice occupancy increased with increasing kelp density to the point where most individuals were concealed. The asymptotic projection of the model indicated that most urchins were concealed where the mean kelp stipe density was greater than one stipe per square meter.
Fig. 2.
Sea urchin foraging behavior (exposed, concealed) and condition (gonad index) as a function of kelp density. (A) Residuals from a linear regression on the log-transformed proportion of exposed urchins (to account for urchin density) fit with a negative exponential decay function with kelp stipe density. (B) The relationship between mean gonad index (per square meter) and the proportion of exposed sea urchins. The gray shaded area represents the 95% confidence of fit.
A linear regression on mean urchin gonad index as a function of the proportion of exposed urchins suggests that gonad index declines with increasing urchin exposure (i.e., active foraging behavior). Gonad index was greatest in areas with high kelp density, where urchins were mainly occupying crevices and assumed to be passively foraging on drift kelp, but gonad index declined linearly with increasing urchin exposure (P < 0.0001, R2 = 0.31; Fig. 2B).
Sea Otter Numerical Response to Density of Exposed Prey.
We found strong support at the population level for a positive numerical response in sea otter abundance that coincided with the onset of an increase in the density of exposed purple urchins. Sea otter numbers around the Monterey peninsula were relatively stable over the period 2000 to 2013 (Fig. 1A) (23); a pattern that has been interpreted as being reflective of a population that had reached local carrying capacity (24–26). However, results from our mixed model suggest that otter abundance increased significantly in the Monterey region (Seaside to Pt. Sur; P < 0.001) following the 2014 urchin outbreak, from 269 (SD ± 77) individuals to 432 (SD ± 123) individuals (mixed model P < 0.001). Increased abundance in Monterey during this latter period was associated with an initial spike in the ratio of pups to independent otters, followed by a rapid increase in the number of independent otters (SI Appendix, Fig. S3). A demographic model fit to the survey data suggests a sharp uptick in survival of both pups and subadults after 2013 (SI Appendix, Fig. S4), a pattern consistent with greater prey availability (27). The decline in pup ratio after 2015 reflects the dramatic increase in the number of surviving subadults (i.e., prereproductive animals) and thus a decrease in the proportional representation of reproductive-age females.
To rule out the potentially confounding effects of an aggregative response (i.e., sea otter movement between regions) on sea otter abundance, we compared abundance dynamics in adjacent neighboring regions to the north and south of the Monterey study area. A contrast test on the region and time period interaction term revealed a less-dramatic but significant increase in sea otter abundance in the region to the south (Big Sur, P < 0.0001, F = 17, degrees of freedom in the denominator [DenDF] = 105), and no significant change in abundance in Santa Cruz (although there appeared to be a declining trend after 2012). Considering both neighboring areas together, there was almost no net change in abundance after versus before 2014, suggesting that sea otters were not simply redistributing into the study area (SI Appendix, Fig. S5).
Prevalence of Urchins in Sea Otter Diets.
A k-means cluster analysis on the diet composition of sea otters revealed an urchin specialist cluster defined by a high composition of urchin prey (>40%). While there were no detectable changes in the mean proportion of urchins in the diets of individual sea otters that specialize on urchins, the overall proportion of consumed urchins significantly increased both at the population level and in sea otters specializing on all other prey types (SI Appendix, Figs. S6 and S7; P < 0.0001, DF = 7). At the population level, there was a significant increase in the frequency of sea otters specializing on urchin prey following the 2014 increase in the density of exposed sea urchins (P < 0.01, DF = 4).
Urchin Condition and Sea Otter Selectivity.
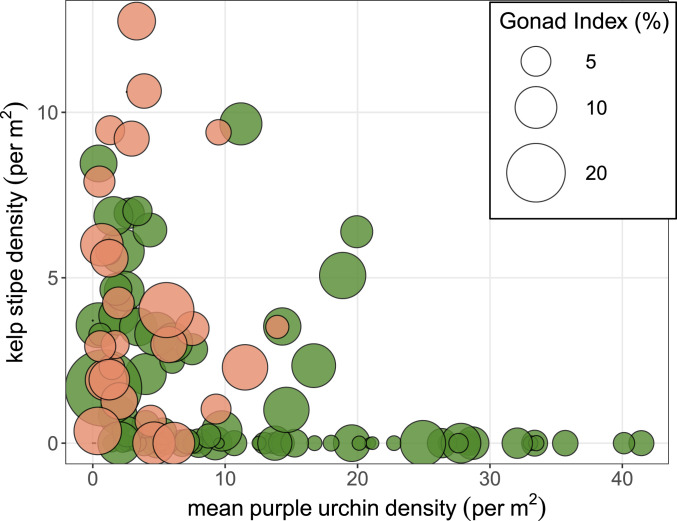
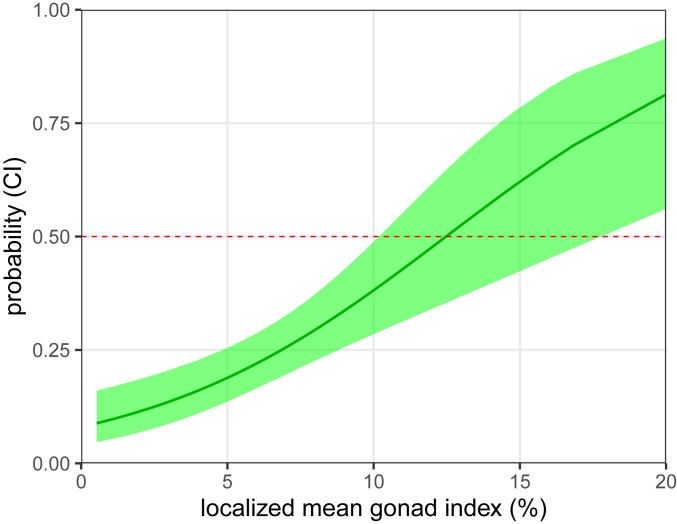
We found support for the hypothesis that the spatial distribution of sea otter foraging effort on urchin prey is best predicted by energetically profitable prey patches, indicating a strong level of spatially explicit foraging selectivity (Fig. 3). Model selection for the full population-level logistic regression revealed gonad index, depth, and patch type (barren, forest) as the three most influential factors driving patch selection by sea otters (R2 = 0.47, P < 0.0001, Corrected Akaike Information Criterion [AICc] = 73). The resulting logit-transformed probability coefficients indicated that gonad index was the only positive predictor of selection probability (βGI = 0.14), while depth (βDepth = −0.32) and the urchin barren patch type (βPatch = −0.66) were negatively associated with the likelihood of focal patch choice. The threshold estimate of gonad index required to affect a positive selection of a sea otter focal patch was 12%/m2 (Fig. 4).
Fig. 3.
Gonad index of sea urchins in focal patches where sea otters were actively foraging on sea urchins (orange circles) and reference sites (green circles) where otters were not foraging on sea urchins. Also depicted is the density of urchins and kelp at each patch.
Fig. 4.
Probability of sea otter focal patch selection by urchin gonad index. Model predicted foraging preference (with 95% CIs shaded in green) using the localized mean urchin gonad index (mean gonad index/square meter). Probability values (green line) are translated from the logit-transformed logged odds. The red dashed line indicates the 50% transition threshold.
Discussion
Our results demonstrate how the behavioral response of an apex predator to changes in prey behavior and condition can erode the strength of top-down forcing and enhance the role of bottom-up community regulation, depending on the spatial organization of diverse ecosystem states. In 2014, a rapid and dramatic decline in the abundance of a mesopredator (P. helianthoides) and primary producer (M. pyrifera) coincided with a fundamental change in urchin foraging behavior and condition. This trait-mediated response of urchins to a decline in a primary resource (kelp) and an important benthic mesopredator (Pycnopodia) initiated further declines in kelp abundance, resulting in a spatial mosaic of remnant kelp forests interspersed with patches of sea urchin barrens. The mosaic of forests and barrens provided us a unique opportunity to explore the numerical and functional responses of sea otters when given the choice to forage in adjacent alternative states of the ecosystem and to evaluate the relative contribution and reciprocal dynamics of top-down or bottom-up control across the landscape. Our findings add to a growing body of literature surrounding trait-mediated trophic cascades by revealing that predator and prey behavioral responses to spatially distributed mosaics of resources can underpin community functioning and regional stability.
Long-term monitoring observations around our study area on the central coast of California indicate a simultaneous decline in the giant kelp and an increase in the density of exposed purple sea urchins well beyond historic records (28). These changes coincided with the 2014 onset of a marine heatwave and decline in the sea urchin predator, Pycnopodia. Similarly, in 2014 an unprecedented decline in bull kelp (Nereocystis luetkeana) along the northern coast of California coincided with the marine heatwave event and the decline of Pycnopodia (21, 22). Despite a region-wide increase in the density of exposed sea urchins, we found that urchins in patches of forests were more cryptic and had higher gonadal indices than those in barrens. This pattern is consistent with other studies that identified the abundance of predators (16, 29–31) and food availability (19, 32) as drivers of urchin behavior and nourishment.
While predator control of herbivores is widely cited as a fundamental mechanism driving community stability (4, 8, 10), far less is known about predator behavioral responses resulting from resource-driven variability in herbivore condition and behavior. The sea otter–sea urchin–kelp forest trophic cascade in the northern Pacific is perhaps the most well-known example of predator-driven recovery, where the reclamation of historical range by sea otters reduced the abundance of herbivorous sea urchins, thereby enhancing the recovery of kelp forests (14). Our study documents an unusual example of a sea urchin outbreak in an area where sea otters were near their projected local carrying capacity (24, 26, 33). A broadly similar urchin increase was reported in the 1980s prior to the start of sea otter census surveys but when sea otter densities were lower (34). The unanticipated herbivore outbreak that began in 2014 helped reveal the consequences of predator and prey trait-mediated responses on community regulation. Our results suggest that because sea otters mostly ignore urchins in barrens, they are unlikely to directly contribute to the recovery of forests in barren areas. However, spatially explicit sea otter foraging for energetically profitable urchins in forested areas enhances patch resistance to overgrazing. This latter response has important implications for the recovery of barrens to the forested state because these remnant forests protected by sea otters are the spore sources to ultimately replenish and facilitate recovery of forests in barren areas.
Concurrent with the 2014 increase in the density of exposed urchins, sea otter abundance increased well beyond levels seen since the repatriation of otters to the Monterey region in the early 1960s (27). Given the large area of each of our three survey regions (Big Sur, Monterey, and Santa Cruz), population-level dynamics within each region are expected to be primarily driven by demographic processes rather than by immigration or emigration (35, 36). Because sea otter birth rates do not vary over time (33, 37), higher pup recruitment and higher survival of juvenile and subadult sea otters are the most likely demographic mechanisms for the observed numerical response; a scenario consistent with the sequential spikes in pup ratio and numbers of independents and supported by the results of a state-space model fit to these data (SI Appendix, Supplementary Methods) (33). Sea otters have been reported to be food limited in the Monterey region prior to 2014 (27, 38, 39), with a net annual growth rate of just 1% per year from 2000 to 2014 (Fig. 1A). Our demographic analysis suggests that a 60% reduction in the instantaneous mortality rates of pups and subadults, and a smaller reduction in adult mortality, was sufficient to explain an increase in annual growth rate to ∼15% per year between 2014 and 2016. This growth rate is consistent with trends reported for other areas of California where prey resources are more abundant (28, 40). However, urchins are not the only prey type to have increased after 2014; we also observed a sharp uptick in the intake of mussels at this time, which may also have contributed to increased otter survivorship (SI Appendix, Fig. S8). Finally, concurrent studies of tagged otters during this time period showed no evidence of significant movements of animals between regions (24), and combined with the lack of a net decline in numbers in neighboring regions this would argue against redistribution (i.e., an aggregative response) as a plausible explanation for the increase in numbers around Monterey.
Asynchronous trends in sea otter dynamics between the southerly regions (Monterey and Big Sur) and the northern Santa Cruz region may be explained by differences in sea otter mortality factors. Santa Cruz is located within 8 km of Point Año Nuevo, which is a white shark hunting location (41). Sea otter shark-bite mortality has increased substantially over the past 15 y (42), but this increase has been less pronounced in Monterey than in regions to the north and the south (27, 43). There has also been a reduction in protozoal encephalitis since the earlier 2000s, possibly driven in part by drier years and reduced runoff input of pathogens from watersheds (44). Thus, spatial differences in several mortality factors likely contribute to variation in observed sea otter trends across the surveyed regions (45).
At the community level, this study demonstrates strong functional responses in a top predator as a result of changes in prey behavior and condition. Many studies have quantified functional responses of predators (46, 47); however, fewer have examined the multiple pathways by which trait-mediated interactions can erode or amplify trophic cascades (48, 49). Our analysis of long-term sea otter diet composition across the study area revealed a clear and rapid increase in population-level specialization and selectivity in response to increases in prey density and variation in prey energetic profitability. In particular, as the density of exposed sea urchins increased, the prevalence of sea urchin prey in sea otter diets also increased, indicating a rapid population-level functional response. This response reflected in part an increase in the relative numbers of urchin specialists within the population and in part an increase in the numbers of urchins consumed by nonurchin specialists. However, the spatial distribution of foraging effort for urchin prey was not directly linked to the density of exposed prey but rather was predicted by the distribution of energetically profitable (gonad rich) prey. As such, both the role and contribution of top-down control of community structure was dramatically altered by these trait-mediated interactions.
The results presented in this study have far-reaching implications to the field of community ecology that enhance understanding of how the strengths of trait-mediated interactions can reorganize community regulation. While shifts in density-mediated interactions are a mechanism for trophic cascades (2, 50, 51), our study suggests that community dynamics also depend on the relative magnitude of behaviorally mediated interactions, as well as the temporal and spatial scales over which population responses occur. Our study demonstrates that the barrens ecosystem state is maintained by bottom-up processes driven by intense grazing pressure as a result of a reduction in the availability of a primary producer and a shift in sea urchin behavior to active foraging. In contrast, abundant stands of macroalgae in kelp forest patches promote cryptic and passive-foraging behavior in sea urchins that translates to higher energetic profitability. In a patchy mosaic landscape of kelp forests interspersed with sea urchin barrens, spatially explicit top-down control by sea otter foraging on energetically profitable sea urchins may indirectly maintain the kelp forest state of the ecosystem by promoting stability of kelp forest patches within the mosaic.
This study highlights the underexplored role of consumer and predator foraging behavior on community functioning and stability. We suspect that the patterns here are not unique to kelp forest ecosystems but are reflective of how predators and prey respond to mosaics of resources that are common throughout ecosystems around the world. Greater consideration of consumer and predator behavioral responses to resource mosaics may, therefore, present new ways of understanding how trait-mediated interactions and reciprocal feedbacks between top-down and bottom-up forcing affect community dynamics and ultimately underpin the regional stability of ecosystems.
Methods
To address our five hypotheses, we combine spatially explicit observations of numerical and functional responses of sea otters with changes in sea urchin behavior and condition to reveal the relative contribution of top-down and bottom-up control in the structure of kelp forest communities.
Study Area.
This study was conducted along the Monterey Peninsula in California (SI Appendix, Fig. S1). The study region is ∼300 ha, and all marine mammals, algae, and invertebrates are protected from harvest (since 2007) within marine protected areas. The subtidal habitat covers a range of low to high topographic relief comprised of continuous igneous rock that extends from the shore to ∼23 m depth, where it becomes expansive sandy bottom strewn with small rocky outcrops. In the nineteenth century, the southern sea otter was locally hunted to near extinction, but a recovering sea otter population repatriated the area in the early 1960s. The local sea otter population increased rapidly over the course of the following 30 y, reaching an apparent equilibrium by the late 1990s (26, 27). In 2014, the region shifted from a once expansive kelp forest to a mosaic of remnant forests interspersed with patches of sea urchin barrens that range in size from ∼30 to 60 ha (SI Appendix, Fig. S2). The formation of the mosaic was initiated in part by the loss of a sea urchin mesopredator (P. helianthoides) that coincided with an unprecedented marine heatwave (resulting in reduced kelp productivity), an outbreak of purple sea urchins, and an increase in the abundance of sea otters well beyond levels recorded since the early 1960s (Fig. 1).
Shifts in Urchin Behavior and Condition.
A total of 236 underwater surveys (hereafter, “reference sites”) were conducted from May to September in 2017 (n = 71), 2018 (n = 92), and 2019 (n = 73) in order to evaluate the spatial extent and temporal dynamics of kelp forests and urchin barren patches across the study area, and to determine urchin foraging behavior and condition. Survey sites were randomly selected on hard substratum between 5 and 20 m of water (based on diving limitations). All surveys were conducted between the hours of 9:00 AM and 1:00 PM. Each site was sampled using eight 5-m-long transects with two randomly placed 1-m2 quadrats (16 quadrats per site) fixed with a high-resolution camera. A single transect was assigned to each cardinal (north, east, south, and west) and intercardinal (northeast, northwest, southeast, and southwest) direction around the survey site (eight total transects). The positions of the quadrats along each transect were weighted using a randomly stratified design so that the quadrats were not biased toward either the center or outer edge of the sampling circle (SI Appendix, Fig. S9). Therefore, each survey site represents an independent replicate sample.
The density of purple urchins was recorded in situ within each 1-m2 quadrat by carefully searching for urchins, including those seeking refuge in cracks and crevices. Still photographs were taken of each quadrat for estimates of urchin behavior. In the laboratory, photographs were analyzed for the presence of exposed, actively foraging urchins. Urchins with a test diameter visible by 50% or more were quantified as exposed. We selected the 50% visibility threshold to account for actively foraging urchins at intermediate ranges of exposure, and because a subsample of urchins from a representative sample of 505 quadrats quantified at the 100% exposure level was not statistically different from the 50% level. The ratio between the number of urchins quantified as exposed and the total number of urchins quantified in situ represents our proportional estimate of urchins employing active foraging behavior. At each site, kelp density was quantified as the number of stipes in the entire survey area (per 78 m2).
To test the hypothesis that a shift in urchin behavior from passive to active foraging is associated with a decline in kelp availability, we used a sequential model fitting approach to assess whether the mean proportion of exposed urchins was related to kelp density. First, we conducted a linear regression with the proportion of exposed urchins as a function of the log-transformed urchin density to control for the positive effect of urchin density on foraging behavior (i.e., urchins are more likely to be exposed at higher densities). We then regressed the residuals from that model against kelp stipe density to determine the relationship between the proportion of exposed urchins and kelp density. We took the sequential approach instead of using multiple regressions because it was clear from initial analyses that both relationships (proportion versus urchin density and proportion versus kelp density) were nonlinear such that a simultaneous (and linear) approach would mask the actual relationships. Finally, to rule out refuge availability as a confounding effect of behavior, we regressed field estimates of rugosity collected using Risk’s chain-and-tape method (52) against site location, kelp density, and the proportion of exposed urchins, for which we found no effect (i.e., rugosity was relatively uniform across all sites).
Gonadal indices were constructed to comparatively evaluate the hypothesis that active urchin foraging behavior is associated with a decline in condition (i.e., gonad quality). At each of the survey sites above, a maximum of 32 urchins were randomly collected (two per quadrat from adjacent fixed positions) and brought to the laboratory for dissection (n = 4,408). Urchins were placed on ice immediately after collection to slow digestive and reproductive processes. Soon after returning to the laboratory, urchins were injected with 2 to 12 mL 10% neutral buffered formalin (depending on the size of urchin). Urchins were injected through the peristomial membrane and placed in a venting room for a minimum of 24 h to allow fixation of tissues and gonads. After fixation, gonads were blotted dry and weighed to the nearest 0.01 g. To compare gonad mass across individuals, gonad indices were calculated as follows:
| [1] |
Because the fixation process results in variable amounts of perivisceral fluid loss, an equation relating wet mass to test diameter was generated following the methods of Harrold and Reed (19). A biomass equation was fitted to a previous sample of over 400 purple sea urchins. The r2 for Eq. 2 was 0.97, and the partitioned sum of squares gave P < 0.0001.
| [2] |
Sea Otter Numerical Response to Density of Exposed Prey.
We examined support for a numerical response by testing for a temporal change in the mean annual sea otter abundance within the focal study area (Monterey) before (2000 to 2013) and after (2014 to 2018) the 2014 urchin outbreak. We used published annual abundance surveys for southern sea otters (53) to test for variation in abundance over time. Specifically, we evaluated support for a temporal change in sea otter abundance within the focal study area (Monterey) by comparing the mean annual abundances for two time periods (2000 to 2013 and 2014 to 2018). In order to distinguish a numerical response due to increased survivorship (as opposed to a redistribution or migration), we compared sea otter abundance dynamics in the regions immediately to the north (Pigeon Pt. to Seaside, hereafter “Santa Cruz”) and south (Pt. Sur to San Simeon, hereafter “Big Sur”) of the study area (SI Appendix, Fig. S10). Abundance dynamics were evaluated between regions and across time periods using a generalized mixed model, treating abundance estimates as a gamma-distributed response variable and with time period, region, and their interaction as fixed effects and segment (repeated annual survey area) as a random effect.
To assess whether the observed changes in abundance were explicable by biologically feasible changes in vital rates, and to generate estimates of regional trends that reduce the effects of observer error in the raw counts (23), we fit a Bayesian state space model to the time series of survey counts for pups and independent otters (SI Appendix, Supplementary Methods). The model structure followed previously described age-structured models of sea otter demographics (54, 55), and allowed us to infer the underlying changes in survival rates that explained population trends over the study period.
Prevalence of Urchins in Sea Otter Diets.
To test for a sea otter functional response to increases in prey abundance, we evaluated the dietary prevalence of urchins in sea otter diets before and after the 2014 increase in the abundance of exposed urchins. Long-term sea otter observational foraging data were collected from 2000 to 2018 to determine diet composition and the spatial extent and frequency of urchin captures. Observational foraging data were collected primarily from radio-tagged sea otters that were captured and monitored as part of long-term population studies (24, 55). Observation sessions were conducted from shore to collect foraging data and were somewhat opportunistic and haphazard (i.e., based on availability of candidate animals foraging within visible distance of shore), although consistent efforts were made to obtain balanced sample sizes of foraging observations from each tagged study animal in each season (target = 200 observed dives per otter, per season) and to distribute observations for each animal throughout its home range, to avoid spatial biases. Once a feeding sea otter was selected for observation, the observing team used a high powered telescope, stopwatch, Global Positioning System, compass, and laser range finders to record the following variables for each dive in the feeding bout (contiguous series of dives made by a single otter): geolocation (computed based on observer’s location and direction/distance to otter), dive duration, interdive interval, dive outcome (success or no prey), prey type (to the lowest taxonomic level possible), prey size, number of prey items, prey handling time, and various other fields (56, 57).
For purposes of analyzing individual sea otter diets, we restricted analysis to tagged sea otters for which we recorded a minimum of 10 bouts comprising 300 or more feeding dives over a 1- to 3-y period. We assembled information on diameter–biomass relationships and calorific densities for each of the most common prey types (58). For the population as a whole, and for each tagged study animal, we then estimated diet composition on the basis of consumed wet edible biomass using a Monte Carlo, resampling algorithm designed to account for uncertainty and potential biases inherent in the raw data (56, 57). Briefly, the analysis utilizes empirically derived relationships between recorded variables (prey handling time, prey size, and number of items per dive) to correct for those dives with missed data points. The Monte Carlo analysis results in bias-corrected estimates of consumption rates (g/minute) for each prey type and thus proportional contribution of each prey type to individual diets and to the population-level diet. We then used k-means cluster analysis to test for natural groupings in the diet composition data for individual sea otters, as previous analyses of sea otter diets on the central coast of California have found strong evidence for individual diet specialization (56–58). We used the silhouette method combined with examination of elbow plots (59) to determine the optimal number of clusters. To interpret cluster assignments, we identified the most common prey type for sea otters assigned to each cluster [previous analyses have found that a single prey type generally comprises 35% or more of the diets of individuals assigned to a given cluster (60)]. Urchin specialists were identified as sea otters belonging to the cluster where urchins were the most common prey type.
The dietary prevalence of urchins was compared using the proportional contribution of urchins to individual sea otter diets across two time periods: 2000 to 2013 and 2014 to 2018 (one period before and one after the increase in the abundance of exposed urchins). We used beta regression to account for the proportional (0 to 1) response variable (61, 62), with time period and diet specialization (urchin specialists versus all other diet types) as categorical predictor variables and allowing for an interaction between these main effects. We then used Fisher’s exact test (63) to evaluate whether there were differences across time periods in the relative frequency of urchin specialists in the population.
Urchin Condition and Sea Otter Selectivity.
To test the hypothesis that the spatial distribution of sea otter foraging effort for urchin prey is predicted by the distribution of energetically profitable sea urchins, we compared patterns in sea otter foraging behavior with spatially explicit urchin gonadal indices. For this analysis, a sea otter foraging subbout is considered any number of dives made within a 10-m diameter zone of the starting dive’s geolocation. Subbouts where three or more consecutive feeding dives resulting in successful capture and consumption of urchins were identified as “non-random urchin foraging patches” (hereafter, “focal patch”).
Additional underwater surveys were conducted at each focal patch in 2017 (n = 22) and 2018 (n = 29) following the sampling protocol above to construct spatially explicit gonadal indices. These surveys were conducted within a 2-wk interval from the initial shore observation of sea otter foraging to link urchin gonad condition with sea otter foraging behavior in near-to-real time. Because a subbout includes dives made by an otter within a 10-m diameter zone, an underwater radial sampling design allowed for seamless integration between the underwater surveys and the shore observations of sea otter foraging behavior (SI Appendix, Fig. S9).
A stepwise conditional logistic regression was used to test whether sea otter focal patches are predicted by the spatial distribution of energetically profitable urchins. We examined population-level preferences using a binary categorical response variable (defined as 0 [nonfocal patch] or 1 [focal patch]) for patch selection across 51 focal patches (where otters were foraging on urchins) and 163 randomly sampled reference sites (where otters were not foraging on urchins). The model terms included gonad index, urchin density, rugosity, temperature, depth, kelp density, and a categorical assignment of patch type (barren or kelp forest). Models were forward selected and evaluated using Akaike’s Information Criterion.
Ethics.
Study animal collection was approved by the California Department of Fish and Wildlife permit no. SC-389. Sea otter surveys were approved by the US Fish and Wildlife Service no. MA672624-20. All experiments and surveys were undertaken with approval from the University of California Santa Cruz Institutional Animal Care and Use Committee.
Supplementary Material
Acknowledgments
We are indebted to C. de Jong, S. Garcia, L. Gaspar, T. Gorra, C. Jarman, C. Juliussen, M. Lovos, and dozens of other researchers and volunteers who contributed to fieldwork and laboratory assays. We deeply thank M. Carr, P. Raimondi, A. Salomon, J. Estes, and members of the Raimondi–Carr laboratory for their helpful comments and feedback. We also thank J. Kendall-Bar for illustrations of the study animals. Finally, we thank two anonymous reviewers whose comments helped improve the manuscript. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the US government. This research was supported by the NSF Grant OCE-1538582, the NSF Graduate Research Internship Program, and the Future Leaders in Coastal Science Award at the University of California Santa Cruz. J.G.S. was supported by a NSF Graduate Research Fellowship. Operational and logistical support for sea otter studies were provided by the US Geological Survey, Monterey Bay Aquarium, and California Department of Fish and Wildlife.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. R.S.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2012493118/-/DCSupplemental.
Data Availability
Source code and datasets used in this article are available on Dryad (64).
References
- 1.Estes J. A., Tinker M. T., Williams T. M., Doak D. F., Killer whale predation on sea otters linking oceanic and nearshore ecosystems. Science 282, 473–476 (1998). [DOI] [PubMed] [Google Scholar]
- 2.Schmitz O. J., Direct and indirect effects of predation and predation risk in old-field interaction webs. Am. Nat. 151, 327–342 (1998). [DOI] [PubMed] [Google Scholar]
- 3.Elderd B. D., Bottom-up trait-mediated indirect effects decrease pathogen transmission in a tritrophic system. Ecology 100, e02551 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Pace M. L., Cole J. J., Carpenter S. R., Kitchell J. F., Trophic cascades revealed in diverse ecosystems. Trends Ecol. Evol. 14, 483–488 (1999). [DOI] [PubMed] [Google Scholar]
- 5.Grant J., Hopcraft C., Sinclair A. R. E., Packer C., Planning for success: Serengeti lions seek prey accessibility. J. Anim. Ecol. 74, 559–566 (2005). [Google Scholar]
- 6.Christianou M., Ebenman B., Keystone species and vulnerable species in ecological communities: Strong or weak interactors? J. Theor. Biol. 235, 95–103 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Nichols K. D., Segui L., Hovel K. A., Effects of predators on sea urchin density and habitat use in a southern California kelp forest. Mar. Biol. 162, 1227–1237 (2015). [Google Scholar]
- 8.Power M. E., Mathews W. J., Stewart A. J., Grazing minnows, pscivorous bass, and stram algae: Dynamics of a strong interaction. Ecology 66, 1448–1456 (1985). [Google Scholar]
- 9.Hunter M. D., Price P. W., Playing chutes and ladders: Heterogeneity and the relative roles of bottom-up and top-down forces in natural communities. Ecology 73, 724–732 (1992). [Google Scholar]
- 10.Terborgh J., Estes J., Trophic Cascades: Predators, Prey and the Changing Dynamics of Nature (Island Press, 2010). [Google Scholar]
- 11.Werner E. E., Peacor S. D., A review of trait-mediated indirect interactions in ecological communities. Ecology 84, 1083–1100 (2003). [Google Scholar]
- 12.Lynam C. P., et al., Interaction between top-down and bottom-up control in marine food webs. Proc. Natl. Acad. Sci. U.S.A. 114, 1952–1957 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filbee-Dexter K., Scheibling R. E., Sea urchin barrens as alternative stable states of collapsed kelp ecosystems. Mar. Ecol. Prog. Ser. 495, 1–25 (2014). [Google Scholar]
- 14.Estes J. A., Palmisano J. F., Sea otters: Their role in structuring nearshore communities. Science 185, 1058–1060 (1974). [DOI] [PubMed] [Google Scholar]
- 15.Mann K. H., Kelp, sea urchins and predators: A review of strong interactions in rocky subtidal systems of eastern Canada, 1970-1980. Neth. J. Sea Res. 16, 414–423 (1982). [Google Scholar]
- 16.Cowen R. K., Ecology on red sea urchin (Strongylocentrotus franciscanus) populations: An experimental analysis. Oecologia 58, 249–255 (1983). [DOI] [PubMed] [Google Scholar]
- 17.Scheibling R. E., The role of predation in regulating sea urchin populations in eastern Canada. Oceanol. Acta 19, 421–430 (1996). [Google Scholar]
- 18.Burt J. M., et al., Sudden collapse of a mesopredator reveals its complementary role in mediating rocky reef regime shifts. Proc. Biol. Sci. 285, 20180553 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrold C., Reed D. C., Food availability, sea urchin grazing, and kelp forest community structure. Ecology 66, 1160–1169 (1985). [Google Scholar]
- 20.Conor J. J., Gonad growth in the sea urchin, Strongylocentrotus purpuratus (Stimpson) (echinodermata: Echinoidea) and the assumptions of gonad index methods. J. Exp. Mar. Biol. Ecol. 10, 89–103 (1972). [Google Scholar]
- 21.Rogers-Bennett L., Catton C. A., Marine heat wave and multiple stressors tip bull kelp forest to sea urchin barrens. Sci. Rep. 9, 15050 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harvell C. D., et al., Disease epidemic and a marine heat wave are associated with the continental-scale collapse of a pivotal predator (Pycnopodia helianthoides). Sci. Adv. 5, eaau7042 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hatfield B. B., Tinker M. T., “California sea otter (Enhydra lutris nereis) census results, spring 2017” (US Geological Survey Report No. 1067, 2017).
- 24.Tinker M. T., et al., “Southern sea otter (Enhydra lutris nereis) population biology at Big Sur and Monterey, California–Investigating the consequences of resource abundance and anthropogenic stressors for sea otter recovery” (US Geological Survey Report No. 1022, 2019).
- 25.Thometz N. M., et al., Trade-offs between energy maximization and parental care in a central place forager, the sea otter. Behav. Ecol. 27, 1552–1566 (2016). [Google Scholar]
- 26.Tinker M. T.et al., Habitat features predict carrying capacity of a recovering marine carnivore. J. Wildl. Manage. 85, 303–323 (2021). [Google Scholar]
- 27.Hatfield B. B., Yee J. L., Kenner M. C., Tomoleoni J. A., Tinker M. T., “California sea otter (Enhydra lutris nereis) census results, spring 2018” (US Geological Survey Report No. 1097, 2018).
- 28.Carr M., Caselle J., Data from “PISCO: Subtidal community swath surveys.” 10.6085/AA/PISCO_SUBTIDAL.151.2. Accessed 29 May 2020. [DOI]
- 29.Lafferty K. D., Fishing for lobsters indirectly increases epidemics in sea urchins. Ecol. Appl. 14, 1566–1573 (2004). [Google Scholar]
- 30.Hamilton S. L., Caselle J. E., Exploitation and recovery of a sea urchin predator has implications for the resilience of southern California kelp forests. Proc. Biol. Sci. 282, 20141817 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selden R. L., Gaines S. D., Hamilton S. L., Warner R. R., Protection of large predators in a marine reserve alters size-dependent prey mortality. Proc. Biol. Sci. 284, 20161936 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Livore J. P., Connell S. D., Effects of food origin and availability on sea urchin condition and feeding behaviour. J. Sea Res. 68, 1–5 (2012). [Google Scholar]
- 33.Riedman M. L., Estes J. A., Staedler M. M., Giles A. A., Carlson D. R., Breeding patterns and reproductive success of California sea otters. J. Wildl. Manage. 58, 391 (1994). [Google Scholar]
- 34.Watanabe J. M., Harrold C., Destructive grazing by sea urchins Strongylocentrotus spp. in a central California kelp forest: Potential roles of recruitment, depth, and predation. Mar. Ecol. Prog. Ser. 71, 125–141 (1991). [Google Scholar]
- 35.Tinker M. T., Doak D. F., Estes J. A., Using demography and movement behavior to predict range expansion of the southern sea otter. Ecol. Appl. 18, 1781–1794 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Raymond W. W., et al., Location-specific factors influence patterns and effects of subsistence sea otter harvest in Southeast Alaska. Ecosphere 10, e02874 (2019). [Google Scholar]
- 37.Thometz N. M., Tinker M. T., Staedler M. M., Mayer K. A., Williams T. M., Energetic demands of immature sea otters from birth to weaning: Implications for maternal costs, reproductive behavior and population-level trends. J. Exp. Biol. 217, 2053–2061 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Thometz N. M., et al., Trade-offs between energy maximization and parental care in a central place forager, the sea otter. Behav. Ecol. 27, 1552–1566 (2016). [Google Scholar]
- 39.Chinn S. M., et al., The high cost of motherhood: End-lactation syndrome in southern sea otters (enhydra lutris nereis) on the central California coast, USA. J. Wildl. Dis. 52, 307–318 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Tinker M. T., et al., “Southern sea otter range expansion and habitat use in the Santa Barbara channel, California” (US Geological Survey Report No. 2017-1001, 2017).
- 41.Klimley A. P., Le Boeuf J., Cantara K. M., Davis S. F., Kelly J. T., Hunting strategy of great whites on seals. Mar. Biol. 138, 617–636 (2001). [Google Scholar]
- 42.Tinker M. T., Hatfield B. B., Harris M. D., Ames J. A., Dramatic increase in sea otter mortality from white sharks in California. Mar. Mamm. Sci. 32, 309–326 (2016). [Google Scholar]
- 43.Nicholson T. E., et al., Gaps in kelp cover may threaten the recovery of California sea otters. Ecography 41, 1751–1762 (2018). [Google Scholar]
- 44.Burgess T. L., et al., Defining the risk landscape in the context of pathogen pollution: Toxoplasma gondii in sea otters along the pacific Rim. R. Soc. Open Sci. 5, 171178 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller M. A., et al., Predators, disease, and environmental change in the nearshore ecosystem: Mortality in southern sea otters (Enhydra lutris nereis) from 1998-2012. Front. Mar. Sci. 7, 582 (2020). [Google Scholar]
- 46.Holling C. S., Some characteristics of simple types of predation and parasitism. Can. Entomol. 91 (1959). [Google Scholar]
- 47.Real L. A., Ecological determinants of functional response. Ecology 60, 481–485 (1979). [Google Scholar]
- 48.Trussell G. C., Matassa C. M., Ewanchuk P. J., Moving beyond linear food chains: Trait-mediated indirect interactions in a rocky intertidal food web. Proc. Biol. Sci. 284, 1–9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Witman J. D., Smith F., Novak M., Experimental demonstration of a trophic cascade in the Galápagos rocky subtidal: Effects of consumer identity and behavior. PLoS One 12, e0175705 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosenzweig M. L., Exploitation in three trophic levels. Am. Nat. 107, 275–294 (1973). [Google Scholar]
- 51.Oksanen L., Fretwell S. D., Arruda J., Niemela P., Exploitation ecosystems in gradients of primary productivity. Am. Nat. 118, 240–261 (1981). [Google Scholar]
- 52.Michael J. Risk , Fish diversity on a coral reef in the Virgin Islands. Atoll Res. Bull. 153, 1–4 (1972). [Google Scholar]
- 53.Hatfield B. B., Yee J. L., Kenner M. C., Tomoleoni J. A., Tinker M. T., Data from “California sea otter (Enhydra lutris nereis) census results, spring 2018” (US Geological Survey Data Series No. 1097). 10.3133/ds1097. Deposited 24 September 2018. [DOI]
- 54.Gerber L. R., Tinker M. T., Doak D. F., Estes J. A., Jessup D. A., Mortality sensitivity in life-stage simulation analysis: A case study of southern sea otters. Ecol. Appl. 14, 1554–1565 (2004). [Google Scholar]
- 55.Tinker M. T., et al., Incorporating diverse data and realistic complexity into demographic estimation procedures for sea otters. Ecol. Appl. 16, 2293–2312 (2006). [DOI] [PubMed] [Google Scholar]
- 56.Tinker M. T., Bentall G., Estes J. A., Food limitation leads to behavioral diversification and dietary specialization in sea otters. Proc. Natl. Acad. Sci. U.S.A. 105, 560–565 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tim Tinker M., et al., Structure and mechanism of diet specialisation: Testing models of individual variation in resource use with sea otters. Ecol. Lett. 15, 475–483 (2012). [DOI] [PubMed] [Google Scholar]
- 58.Newsome S. D.et al., Using stable isotopes to investigate individual diet specialization in California sea otters (Enhydra lutris nereis). Ecology 90, 961–974 (2009). [DOI] [PubMed] [Google Scholar]
- 59.Kaufman L., Rousseeuw P., Finding Groups in Data: An Introduction to Cluster Analysis (John Wiley & Sons, 2009), vol. 344. [Google Scholar]
- 60.Fujii J. A., Ralls K., Tinker M. T., Food abundance, prey morphology, and diet specialization influence individual sea otter tool use. Behav. Ecol. 28, 1206–1216 (2017). [Google Scholar]
- 61.Cribari-Neto F., Zeileis A., Beta regression in R. J. Stat. Softw. 34, 1–24 (2010). [Google Scholar]
- 62.Ferrari S. L. P., Cribari-Neto F., Beta regression for modelling rates and proportions. J. Appl. Stat. 31, 799–815 (2004). [Google Scholar]
- 63.Mehta C. R., Patel N. R., A network algorithm for performing Fisher’s exact test in r × c contingency tables. J. Am. Stat. Assoc. 78, 427–434 (1983). [Google Scholar]
- 64.Smith J. G., et al., “Behavioral responses across a mosaic of ecosystem states restructure a sea otter-urchin trophic cascade” (Dryad). Dataset, 10.7291/D1566H. Deposited November 6, 2020. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Source code and datasets used in this article are available on Dryad (64).