Abstract
Acute lymphoblastic leukemia (ALL) is a hematological malignancy characterized by the malignant clonal expansion of lymphoid hematopoietic precursors. It is regulated by various signaling molecules such as cytokines and adhesion molecules in its microenvironment. Chemokines are chemotactic cytokines that regulate migration, positioning and interactions of cells. Many chemokine axes such as CXCL12/CXCR4 and CCL25/CCR9 have been proved to play important roles in leukemia microenvironment and further affect ALL outcomes. In this review, we summarize the chemokines that are involved in ALL progression and elaborate on their roles and mechanisms in leukemia cell proliferation, infiltration, drug resistance and disease relapse. We also discuss the potential of targeting chemokine axes for ALL treatments, since many related inhibitors have shown promising efficacy in preclinical trials, and some of them have entered clinical trials.
Keywords: Acute lymphoblastic leukemia, Chemokine, Microenvironment, Therapeutic targets
Background
Acute lymphoblastic leukemia (ALL) is the most common cancer among children with 25% chance of disease relapse [1], while in adults the chance is much higher [2]. According to the cell of origin, ALL can be further subclassified into B-ALL and T-ALL [3]. In clinical, B-ALL accounts for about 80% of pediatric leukemia where it is by far the most common malignancy, with a peak incidence around 2–5 years of age [4], but only accounts for 20% of adult leukemia. By contrast, T-ALL accounts for 10–15% and 25% in pediatric and adult ALL, respectively [5]. The five-year overall survival (OS) is about 85% and 40% in pediatric and adult B-ALL patients, respectively [6–9]. The 5-year event-free survival (EFS) of pediatric T-ALL is 70–75%, while it is 30–40% for adult T-ALL below 60 years of age, and only 10% above this age with intensified multi-agent chemotherapy [10]. Despite the progress in optimization of chemotherapy and the availability of hematopoietic stem cell transplantation, there is still a considerable scope for improving therapeutic outcome by developing novel biomarkers that can predict and refine prognosis in ALL patients.
Chemokines and their receptors have shown great potential in tumor-targeted therapy due to their active roles in remodeling tumor microenvironment [11]. Leukemia cells “hijack” the proliferative vascular niche and inhibit the osteoblastic niche to reshape the healthy bone marrow (BM) microenvironment into “leukemic” microenvironment, thereby destroying normal hematopoietic function and promoting its own proliferation and survival. Chemoresistance and relapse of acute leukemias are believed to be driven by leukemia initiating cells (LICs), which at least are partly dependent on signals from leukemic microenvironment to evade chemotherapy-induced death and acquire drug resistance [4]. With the progress of understanding the roles of signals like chemokines and their receptors in ALL, the use of chemokines or their receptors as novel targets to regulate leukemia cellular signal pathways will become a powerful new method for the therapy of ALL.
In this review, we aim to elaborate on the roles of chemokines and their receptors in ALL leukemic microenvironment, and how they contribute to the process of leukemogenesis and chemotherapy resistance. We also conclude potential chemokine-targeted ALL treatments and provide new insights into novel targeted therapy.
ALL and its microenvironment
ALL is a malignant clonal disease associated with the abnormal proliferation of hematopoietic stem cells (HSCs) and progenitor cells. In ALL, abnormal progenitor cells and immature cells multiply rapidly in the BM, accumulate in the BM and inhibit normal hematopoietic function, and eventually they may infiltrate extramedullary organs such as the central nervous system (CNS), liver, spleen and lymph nodes.
It has been clearly confirmed that BM microenvironment plays a significant role in the occurrence and development of ALL, whose cellular composition includes HSCs, endothelial cells, osteoblasts, osteoclasts and mesenchymal stem cells (MSCs) [12]. BM microenvironment which includes osteoblastic (endosteal) and vascular niches secretes specific factors and then regulates the number and status of healthy HSCs [13–15]. The osteoblastic niches located in the endosteum are mainly composed of osteoblasts, osteoclasts and glial unmyelinated Schwann cells and regulatory T (Treg) cells. The vascular niches in the sinusoidal walls are mainly composed of CXCL12-abundant reticular cells, endothelial cells, nestin-positive MSCs and leptin receptor positive perivascular stromal cells. However, these niches also provide shelter where subsets of leukemic cells escape chemotherapy-induced death and acquire a drug-resistant phenotype, and leukemia cells are able to remodel the BM niches into malignant niches which better support neoplastic cell survival and proliferation [4]. Chemokines and their receptors play an important role in this process. For example, the stimulation of CXCR4 by CXCL12 produced by stromal cells in the BM is important for the maintenance of T-ALL cells [16].
In addition to the extensively studied BM microenvironment, the extramedullary microenvironments are also critical in the development and relapse of ALL [17]. CNS can protect blast cells from systemic chemotherapy [18], resulting in 10–30% of CNS relapse [19]. In T-ALL, chemokine receptors CCR7 and CXCR4 were associated with CNS infiltration of T-ALL cells [20, 21]. Furthermore, the hepatic microenvironment also provides an extramedullary niche for leukemic cells, which are maintained by the CXCL12/CXCR4 axis [22]. In splenic microenvironment, T-ALL cells could be stimulated to express a higher level of MIP-3β (CCL19), a ligand of CCR7, which further stimulates the proliferation and migration of T-ALL cells [23].
Therefore, abnormal microenvironment is the key contributor in the development of leukemia. In this process, various factors, headed by chemokines, play important roles in the progression of ALL via responding to abnormal expression of genes and affecting microenvironments [24]. We further summarize the mechanisms of chemokines and their receptors in ALL.
The roles of chemokines and their receptors in ALL
Chemokines, named because of their ability to induce directional chemotaxis in nearby reactive cells, are small cytokines or signaling proteins secreted by cells [25]. They are categorized into four main subfamilies: CXC, CC, CX3C and XC. All of these proteins exert their biological effects by interacting with G protein-bound transmembrane receptors (chemokines receptors), which are selectively expressed on the surface of target cells [26]. Consequently, chemokines play a central role in the development and homeostasis of the immune system [27, 28]. In ALL, different chemokines interacting with their receptors have great effects on the leukemogenesis, progression and relapse. Such kind of relationships between chemokines and the biological process of ALL are exhibited in Fig. 1.
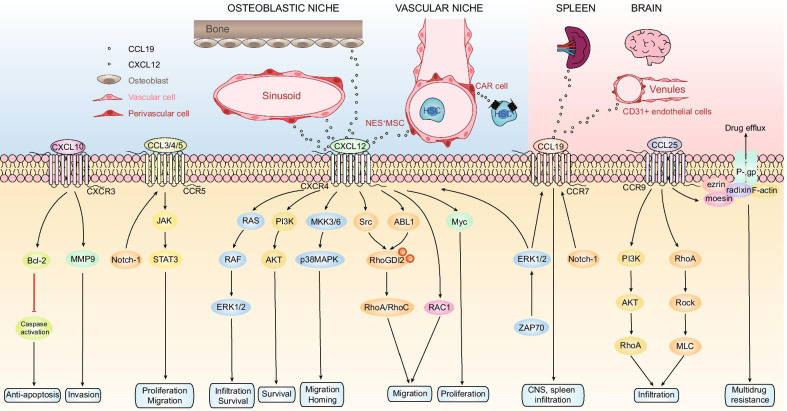
Fig. 1.
Chemokines and their receptors’ roles in acute lymphoblastic leukemia. In osteoblastic niche, T-ALL cells are in direct, stable contact with vascular cells in the BM that produce CXCL12, while in vascular niche CXCL12 is secreted by several stromal cell types, especially CAR cells. Apart from the BM microenvironment, extramedullary organs such as the brain and spleen showed high expression of CCL19, which is involved in leukemic cells’ infiltration to such sites. CXCL12/CXCR4 triggers the activation of downstream kinases Src and ABL1 which are responsible for the phosphorylation of RhoGDI2, which released RhoA and RhoC, leading to subsequent cytoskeleton redistribution and assembly in the process of migration. CXCR4 could activate both ERK1/2 and PI3K/Akt pathways to promote the survival of leukemic blasts. In the homing of B cell progenitor ALL cells to the BM, CXCL12-mediated activation of p38MAPK was required and ZAP70 kinase can control the expression of CXCR4 and CCR7 via ERK1/2, which is correlated with CNS infiltration during T-ALL. CXCR4 could also activate RAC1 to mediate migration and engraftment of B-ALL cells in the BM or testicles. In addition, CXCR4 can induce the proliferation of T-ALL cells via Myc. CXCL10/CXCR3 axis may increase survival rate of leukemic cells during treatment through stabilizing Bcl-2 and inhibiting caspase activation. CXCL10 has also been proved to promote migration of leukemic cells via MMP9. The expression of CCR5 and CCR7 is regulated by Notch-1. CCR5 regulate leukemic cell’s proliferation and anti-apoptosis through JAK/STAT3 pathway. CCL25/CCR9 axis increase drug efflux-induced resistance by activating the binding of P-gp and ERM. It also facilitates the infiltration through RhoA-Rock-MLC and PI3K/AKT-RhoA pathway
CXCL12/CXCR4
Stromal cell-derived factor-1 (SDF-1)/CXCL12 functions as the chemoattractant for both committed and primitive hematopoietic progenitors and regulates embryonic development including organ homeostasis [29]. CXCR4 is the cognate receptor of CXCL12, widely expressed in numerous tissues including immature osteoblasts and endothelial cells in the BM, epithelial cells of many organs, CNS and hematopoietic cells [30]. CXCL12/CXCR4 axis provides the stimulus for proliferation, survival, self-renewal, differentiation and functional activation in normal hematopoietic cells [31] and plays the role of retaining normal developing B and myeloid cells in the BM [32].
However, the activation of CXCL12/CXCR4 axis has been found to be involved in various aspects of ALL progression, which includes proliferation, migration, infiltration and chemoresistance. CXCR4 is highly expressed on the surface of mouse and human ALL cells [16, 33–35], and its high expression predicts poor prognosis of ALL patients [36–38]. CXCR4 deletion or treatment with the CXCR4 antagonist could significantly reduce T-ALL burden, indicating that it may be a promising target for ALL therapy [16, 33, 39].
Lots of research has shown that CXCL12/CXCR4 was able to accelerate the proliferation of ALL cells, but the specific mechanism still needs to be further studied [40–44]. Juarez J et al. found that CXCL12 synergized with interleukin (IL) -7 or IL-3 was associated with enhanced phosphorylation of the mitogen-activated protein kinases (MAPK), extracellular signal-related kinase 1/2 (ERK1/2) and p38, and AKT, implicating these pathways in the proliferation of B-ALL [45]. A study based on ALL patients suggested that CXCL12 might enhance proliferation of precursor B-ALL via the signal transducer and activator of transcription 5 (STAT5) activation [46]. Knockout CXCR4 could reduce the expression of Myc, which was responsible for the proliferation of T-ALL cells [16].
Infiltration and chemoresistance are major causes of ALL relapse and correlate with poor prognosis [4, 5]. In the critical process of infiltration, transendothelial migration of leukemic cells is required to exit the blood stream into targeted organs, which is the prerequisite for infiltration [5]. High expression of CXCR4 by leukemic blasts and activation of the CXCL12/CXCR4 axis are involved in leukemia migration [47, 48]. CXCL12/CXCR4 triggered the activation of downstream kinases Src and ABL1 which were responsible for the phosphorylation of Rho GDP-dissociation inhibitor 2 (RhoGDI2) on Y153, Y24 and Y130. Phosphorylation of RhoGDI2 on Y24 and Y153 released RhoA and RhoC from RhoGDI2, which led to subsequent cytoskeleton redistribution and assembly in the process of migration [49, 50]. Besides, p38MAPK signaling was shown to be involved in CXCR4-mediated migration but not proliferation in ALL [40, 51]. CD9 could modulate CXCR4-mediated migration via RAC1 signaling, which may modulate the chemotactic response to CXCL12 by regulating the internalization of CXCR4 [52, 53].
Accumulating data have demonstrated that high expression of CXCR4 is associated with infiltration into spleen, liver, lymph nodes, CNS and testicles in ALL [21, 38]. In the homing of B cell progenitor ALL cells to the BM, CXCL12-mediated activation of p38MAPK was required [51], and those with high CXCR4 expression had more significant liver or spleen infiltration than those with low CXCR4 expression in pediatric B-ALL patients [46]. Infiltration of the CNS is a deteriorate trait of T-ALL [54] and T-ALL neuropathology was found to result from meningeal infiltration through CXCR4-mediated BM colonization [21]. Zeta-chain-associated protein 70 (ZAP70) kinase controlled the expression of CCR7 and CXCR4 via ERK1/2 in B-ALL and T-ALL, which was correlated with CNS infiltration in T-ALL patients [55]. CXCR4 could also activate RAC1 to mediate migration and engraftment of B-ALL cells in the BM or testicles, which was enhanced by CD9 [52].
CXCL12/CXCR4 could induce the chemoresistance of leukemia cells. LICs are thought to be responsible for resistance to treatment and relapse following chemotherapy, and CXCR4 was found to be essential to the LICs activity of T-ALL [43]. In BM leukemic niches, interactions of stromal cells and extracellular matrix with leukemic blasts can generate antiapoptotic signals that provide a sanctuary for subpopulations of leukemic cells to evade chemotherapy-induced death and allow acquisition of a drug-resistant phenotype [47, 56]. Overexpression of CXCR4 granted leukemic blasts a higher capacity to seed into BM niches, and the inhibition of CXCR4 could mobilize the leukemic cells to overcome the therapeutic resistance [57–59]. BM MSCs can protect leukemic cells from chemotherapy via the interplay between CXCL12 and CXCR4. β1-integrin/hERG1/CXCR4 complex was found to participate in the interaction between MSCs and ALL cells, activating both the ERK1/2 and the phosphoinositide 3-kinase (PI3K)/Akt pathways to promote the survival of leukemic blasts [60]. The CXCR4 antagonist could effectively reverse MSC-mediated drug resistance and sensitize leukemic cells from refractory/relapsed ALL patients to chemotherapy drugs [61]. Therefore, blocking the CXCL12/CXCR4 axis may have implications for improving the therapeutic effect of ALL patients.
As for the upstream regulation of CXCR4 expression in leukemic cells, runt-related transcription factor 2 which was upregulated in high-risk T-ALL could directly regulate the transcription of CXCR4 gene, thereby promoted homing to medullary and extramedullary sites [62]. Besides, Kruppel-like factor 4 which was identified as an important negative regulator in T-ALL could directly bind to the promoter of CXCR4 and suppress its expression [63]. Except for transcription factors, ghrelin as a hormone could induce CXCR4 expression via the SIRT1/AMP-activated protein kinase axis in ALL cell lines [64]. CXCR4 could also be suppressed by miRNA-139 which was lowly expressed, whereas CXCR4 was highly expressed in T-ALL cell lines and patient samples [44]. CXCR4 cell surface expression was regulated by cortactin, an actin-binding protein implicated in the regulation of cytoskeleton dynamics, and the expression of cortactin was dependent on calcineurin [43].
CCL25/CCR9
CCR9 is mainly distributed in immature T lymphocytes and on the surface of intestinal cells, and it plays a role in T lymphocyte development and tissue-specific homing when bound to its specific ligand [65]. CCL25, which is the only ligand for CCR9, is mainly expressed by epithelial cells in the thymus as well as small intestine and acts as an important chemoattractant for T cells in the gut [65–67].
To our knowledge, we are the first to report that CCR9 is highly expressed on T-ALL CD4+ T cells, and rarely expressed on normal CD4+ T cells [68]. Later studies have found that CCL25/CCR9 axis plays an important role in several aspects of T-ALL progression.
CCR9 is closely related to the infiltration of leukemia cells. Our studies have shown that CCL25 induces MOLT4 cells (human T-ALL cell line with naturally high expression of CCR9) polarization and microvilli absorption to participate in leukemia infiltration and trafficking via the RhoA-Rock-MLC and ezrin pathway [69, 70]. CCL25/CCR9 has also been shown to upregulate the expression of Wnt5a by promoting the expression and activation of protein kinase C, thereby enhancing MOLT4 cells migration, invasion, actin polarization, and lamellipodium and filopodia formation via PI3K/Akt-RhoA pathway activation [71]. We also found that the combined use of IL-2 and IL-4 promoted the internalization of CCR9 and therefore attenuated leukemia cell infiltration and metastasis [72]. Furthermore, Miething C et al. reported that leukemia infiltration into the intestine was dependent on CCR9, which was amplified by PTEN loss, since CCL25 stimulation had little impact on PI3K signaling in the presence of PTEN [73].
CCL25/CCR9 could also induce the chemoresistance of T-ALL. We found that CCL25/CCR9 involvement in the resistance of TNF-α-induced apoptosis in T-ALL depended on Livin, suggesting that CCL25/CCR9 plays an antiapoptotic role [74]. Furthermore, we obtained a multi-resistant T-ALL cell line which was derived from MOLT4 through doxorubicin dosing screening. Then, we investigated this multi-resistant cell line and found that CCR9 induced resistance to chemotherapy drugs, which could be blocked by CCR9 antibodies. Mechanistically, CCL25/CCR9 activated the binding of P-glycoprotein (P-gp) and the cytoskeleton protein ERM to increase P-gp efflux, thus mediating multidrug resistance of T-ALL cells [75].
As for the regulatory mechanism of CCR9 overexpression in T-ALL, it is reported that Notch1 pathway activation could boost the expression of CCR9 [76]. Moreover, we found that certain non-coding RNAs, such as miRNA and lncRNA, may also mediate the expression of CCR9 and further affect its biological function in T-ALL (the relevant work is ongoing).
Therefore, inhibiting CCL25/CCR9 may be a potential therapeutic strategy for treating leukemia patients, and it is of great significance to further explore the role of CCL25/CCR9 in leukemia.
CXCL10/CXCR3
CXCR3 is preferentially expressed on the surface of monocytes, T cells, NK cells, dendritic cells and cancer cells. CXCL9, CXCL10 and CXCL11 are selective ligands for CXCR3 [77], but so far only the role of the CXCL10/CXCR3 axis has been noted in ALL.
ALL relapse is associated with the survival of blasts in organs such as the CNS or the testicles, where levels of antileukemic drugs are diminished [78]. CXCR3 is highly expressed in cerebrospinal fluid (CSF) leukocytes, and its ligand CXCL10 is upregulated in the CSF of multiple sclerosis patients [79], suggesting that CXCR3 may play an important role in the chemotaxis of cells to CNS.
In T-ALL, the levels of CXCL10 in CSF were found to be significantly higher among patients with CNS relapses. Treating the leukemic mice model with CXCR3 antagonist AMG487 could significantly reduce leukemic infiltration of the CNS [80]. Besides, Williams MT et al. also found that IL-15 might upregulate CXCR3 in precursor B-ALL, and leukemic cells could migrate toward the CXCL10 which is detectable in CSF samples from patients with ALL [81]. These results indicate that CXCL10/CXCR3 axis may promote the CNS infiltration of ALL cells.
In addition, in precursor B-ALL, CXCL10 released by monocytes promoted migration and invasive capacity of CXCR3+ precursor B-ALL cells and possibly led to metastatic spread. CXCL10 could induce MMP9 expression and activity in precursor B-ALL cells, which may explain its role in promoting their invasion [82].
CXCL10/CXCR3 axis may also lead to recurrence of ALL by increasing the survival rate of ALL cells during treatment. Mechanistically, CXCL10 enhanced the viability and drug resistance of leukemia cells by stabilizing Bcl-2 and then inhibiting the activation of the caspase cascade induced by drugs such as cytarabine [80]. Therefore, blocking the CXCL10/CXCR3 axis may have a positive effect on reducing relapses in ALL patients.
CCL19/CCR7
CCR7 and its two ligands, CCL19 and CCL21, play important but apparently opposing roles in cancer. On the one hand, expressed on dendritic cells, T helper cells, Treg cells, B cells and central memory T cells, CCR7 is involved in the modulation of the immune response to a growing tumor. On the other hand, CCR7 also plays a key role in promoting metastasis via the lymphatic system, and it is involved in leukemic cells’ migration to the CNS [83].
Studies have demonstrated that CCR7 could be upregulated by Notch1 and ZAP70/ERK pathway and plays a key role in ALL CNS infiltration [20, 55, 84]. It is worth noting that CCL19 but not CCL21 could be detected in the brain sections of leukemic mice. More specifically, CCL19 is mainly produced by CD31+ endothelial cells of brain venules, thereby mediating CNS infiltration in T-ALL [20].
Besides, CCL19 was also highly expressed in the splenic microenvironment, thereby recruiting T-ALL cells with high expression of CCR7. This kind of microenvironment could promote the proliferation of T-ALL cells, stimulate the expression of CCR7 and enhance the migration ability of T-ALL cells to increase the degree of T-ALL malignancy [23].
Moreover, a clinical research indicated that CCR7 was highly expressed in adult acute leukemia cells, and the expression of CCR7 was related to extramedullary infiltration in ALL [85].
Others
In addition to CXCR4 mentioned above, CXCR7 can also bind to CXCL12, and there is increasing evidence that CXCR7 can activate a series of intracellular signaling pathways by forming a heterodimer with CXCR4 [86, 87], or by clearing and controlling the gradient of CXCL12 to adjust the signal transduction of CXCL12/CXCR4 [88]. CXCR7 was found to be highly expressed in ALL cells, and CXCR7 silencing inhibited migration of T-ALL cells, but did not affect proliferation and apoptosis [89]. However, another study thought that miR-101 targeted CXCR7/STAT3 axis to reduce T-ALL growth and metastasis [90].
The expression levels of CCL2 and IL-8 (CXCL8) were found to be increased in ALL BM microenvironments. These chemokines have adverse effects on normal HSCs by promoting the survival, proliferation and adhesion of BM MSCs, establishing a malignant BM microenvironment, and then resulting in a poor prognosis [91]. Further studies indicated that BM MSCs-derived periostin promoted B-ALL cell proliferation via NF-κB/CCL2 pathway and that B-ALL cells-derived CCL2 increased periostin level in BM MSCs via STAT3 activation [92]. These studies implied critical crosstalk between leukemia cells and stromal cells via CCL2 in ALL progression. Except for the BM microenvironment, clinical samples analysis also indicated that CNS involvement in ALL was associated with significantly higher levels of CCL2 during therapy [93].
CCR5 was also found to be upregulated by Notch1 pathway in ALL cells [76], and the CCR5 selective inhibitor maraviroc can inhibit the proliferation and migration of ALL cells and induce apoptosis by inhibiting the Janus kinase (JAK)/STAT3 pathway [94].
The expression of CCL18, CCL23 could also be seen increased in ALL plasma, which possibly reflects tumor/host cell interactions in the circulation [95]. Gutiérrez-Aguirre CH et al. found that the higher serum XCL1 levels at diagnosis and their progressive decline throughout chemotherapy might be correlated with higher survival, but its mechanism was still unknown [96].
In summary, chemokines and their receptors play an extremely important role in the progression and relapse of ALL. Therefore, exploring the specific mechanisms of chemokines, chemokine receptors and microenvironment, is beneficial to find more drug targets, which can provide a new and effective way for the treatment of ALL patients (Table 1).
Table 1.
Roles and pathways of chemokines and their receptors in ALL
| Pathways | Roles | References | |
|---|---|---|---|
| CXCL12/CXCR4 | Myc | Proliferation | [16] |
| Src and ABL1/RhoGDI2/RhoA and RhoC | Migration | [49, 50] | |
| P38MAPK | Migration, homing | [40, 51] | |
| RAC1 | Migration | [52, 53] | |
| ERK1/2 | Infiltration, survival | [55] | |
| PI3K/Akt | Survival | [60] | |
| CCL25/CCR9 | RhoA-Rock-MLC and ezrin | Infiltration | [69, 70] |
| PI3K/Akt-RhoA | Infiltration | [71] | |
| P-gp/ERM/F-actin | Drug resistance | [75] | |
| CXCL10/CXCR3 | Bcl-2/caspases | Survival | [80] |
| MMP9 | Invasion | [82] | |
| CCR5 | JAK/STAT3 | Proliferation, migration | [94] |
Novel ALL treatment targeting chemokines and their receptors
The first-line treatment for ALL typically includes four phases over 2–3 years: induction, consolidation, intensification and long-term maintenance. Routine CNS prophylaxis is recommended in conjunction with systemic chemotherapy and allogeneic hemopoietic cell transplantation remains the standard consolidation treatment in patients at high risk who are fit and have an available donor [2].
Although these therapies have considerably improved outcomes in patients, the main crux in current treatments is the drug resistance of leukemia cells and the high recurrence rate, especially for refractory/relapsed T-ALL, whose therapeutic options are much more restricted [97, 98]. The inefficiency in treatments is closely related to the microenvironment which provides leukemia cells with an ideal environment for survival and proliferation, and thus enables the leukemia cells to escape chemotherapy-induced death [99]. Besides, in the migration and homing of leukemia cells, chemokines and their receptors also play important roles [100]. Based on this view, many inhibitors/biologics targeting chemokine axes, as we have shown in Fig. 2, have shown promising efficacy in preclinical models, and some of them have entered clinical trials.
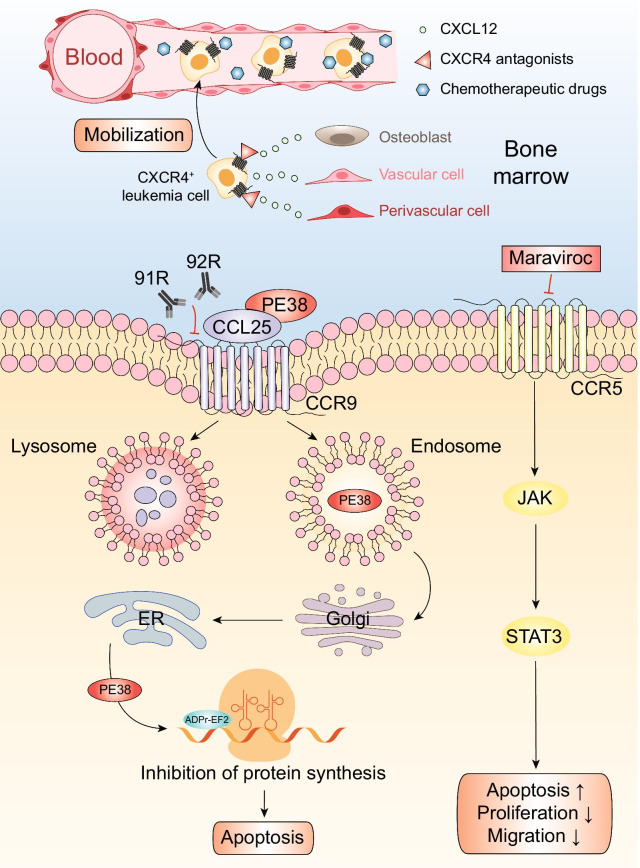
Fig. 2.
Acute lymphoblastic leukemia’s potential treatments targeting chemokines and their receptors. Chemokine antagonists and immunotoxins combined with chemotherapy may significantly optimize prognosis of ALL patients. CXCR4 antagonists have been proved to inhibit CXCL12-mediated chemotaxis and reverse drug resistance. AMD3100, TC14012 and BL-8040 can block the chemotactic function of the CXCL12/CXCR4 axis, interfere with the bone marrow microenvironment on which leukemia cells depend to survive, and mobilize these leukemia cells into the peripheral circulation, thereby increasing the sensitivity of leukemia cells to chemotherapeutic drugs. CCL25-PE38 fusion protein could effectively induce the apoptosis of CCR9 positive T-ALL cells. Immunotoxin PE38 is internalized via the endolysosomal system, first transported to the Golgi and further to the ER where it is activated. Activated PE38 ribosylates ADP and inactivate EF2, thus halting protein synthesis and eventually leads to apoptosis. Monoclonal antibodies 91R and 92R could inhibit the growth of T-ALL cells transplanted into immunodeficient mice. Competitive antagonist of CCR5 maraviroc inhibits CCR5-activated signaling proteins JAK and STAT3, which may lead to apoptosis, and inhibition of survival, proliferation and migration
Inhibitors of the CXCL12/CXCR4 axis
As the most studied chemokine axis, drugs targeting the CXCL12/CXCR4 axis are the most common and diverse. Some CXCR4 inhibitors are undergoing phased clinical trials. The mechanisms of inhibitors targeting CXCR4 can be summarized as (1) disrupting interactions between adhesion matrices that provide survival signals and drug tolerance signals for leukemia cells; (2) mobilizing tumor cells and making them better accessible to conventional therapy; (3) preventing tumor migration and dissemination; and (4) blocking a series of activation signaling caused by CXCL12/CXCR4 axis activation [32]. Here, we will introduce the drugs separately:
AMD3100 (Plerixafor)
AMD3100 is a highly specific antagonist of CXCR4 and blocks binding of CXCL12 by interacting with the carboxylic acid group of Asp171 and Asp262 at each end of the main ligand-binding crevice [101]. It was shown that AMD3100 specifically inhibited signal transduction initiated by the interaction of CXCL12 with CXCR4 (as monitored by calcium flux) and inhibited CXCL12-induced endocytosis of CXCR4 [101, 102].
In leukemia, AMD3100 can block the chemotactic function of the CXCL12/CXCR4 axis, interfere with the microenvironment on which leukemia cells depend to survive, and mobilize these leukemia cells into the peripheral circulation, thereby increasing the sensitivity of leukemia cells to chemotherapeutic drugs and promoting the apoptosis of leukemia cells [103]. AMD3100 could also effectively reverse MSCs-mediated drug resistance and sensitize leukemic cells from refractory/relapsed ALL patients to chemotherapy drugs [61]. Moreover, AMD3100 increased the proportion of activated parts of the cell cycle, which may increase the sensitivity of cell cycle-dependent drugs in the treatment of ALL, such as vincristine [104].
AMD3100 inhibited the proliferation of extramedullary ALL cells after chemotherapy and greatly improved the OS rate of AMD3100-treated mice, and reduced the recurrence rate of ALL [22]. It was also found to inhibit CXCL12 induced migration and phosphorylation of ERK 1/2 in leukemia mouse models [105].
In a phase 1 study (NCT01319864), pediatric patients with refractory/relapsed acute myeloid leukemia (AML), ALL or myelodysplastic syndrome (MDS) were administered AMD3100 and combination chemotherapy consisting of etoposide and cytarabine daily for five days. However, there were only moderate clinical responses in three patients with AML and no responses were observed in patients with ALL or MDS [106]. Main defects of AMD3100 include a short half-life and limited method of administration, which is only injection-administered [107].
AMD11070
AMD11070 is a non-cyclamin orally administrable CXCR4 antagonist that can inhibit CXCL12-induced signaling pathways in ALL cells and block the migration of ALL cells towards CXCL12 and stroma. Combination treatment of AMD11070 with chemotherapeutic drugs reduces recovery from treatment [108]. In vitro experiments confirmed that the same concentration of AMD11070 showed stronger efficiency than AMD3100 in the recognition of CXCR4 and the chemotaxis effects of ALL cells to CXCL12 [108]. A comparative study of AMD3100 and AMD11070 proved that these two compounds bond to overlapping but not identical residues in the receptor-binding regions [109]. Therefore, AMD11070 and AMD3100 have different receptor affinities, binding constants and abilities to block multiple downstream effects in which the receptor CXCR4 participates.
A phase I study confirmed that the use of AMD11070 did not cause safety problems [110], but pathological damage to the liver had been observed in long-term animal experiments [111]. Therefore, the optimal clinical dose of AMD11070 still needs further determinations.
AMD3465
AMD3465 antagonizes the binding of CXCL12 and inhibits the CXCL12-mediated signal transmission process including GTP binding, calcium flux and chemotaxis. At the same time, it has been shown to cause leukocytosis in animal experiments of mice and dogs, suggesting that it may possess the potential for HSC mobilization [112].
Pitt LA et al. confirmed that AMD3465 alone could effectively reduce the number of ALL cells in the spleen, thymus, BM and lymph nodes in ALL mice, thus implicating the antileukemia effect of this CXCR4 antagonist even when administered alone, providing a potential treatment strategy for ALL [16].
T140, T134 and TC14012
The polyphemusin II analogs T140, T134 and TC14012 are potent inhibitors of CXCL12-mediated chemotaxis and the BM-dependent B-ALL progenitor cells proliferation [113]. T140 attenuated the migration of pre-B-ALL cells into BM stromal layers in vitro, while TC14012 significantly inhibited stroma-dependent proliferation and enhanced the cytotoxic and antiproliferative effects of the cytotoxic agents vincristine and dexamethasone [113]. In the mouse model, CXCR4 antagonists AMD3100 and TC14012 can interfere with B-ALL progenitor cells and their supporting niches in vivo by blocking the interaction of CXCL12/CXCR4 and mobilize the leukemia cells rapidly into the peripheral blood therefore reducing their numbers at the local lesion [114].
BL-8040
The T140 analog BL-8040 is a synthetic peptide antagonist with a high affinity and a slow dissociation rate from CXCR4 [115]. It has been reported that BL-8040 potently suppresses T-ALL cell growth in xenotransplantation models of T-ALL. In an ongoing phase II trial (NCT02763384) involving patients with refractory/relapsed T-ALL, BL-8040 is being administered in combination with nelarabine. Five of nine patients enrolled in the study have achieved a complete remission with an overall response rate of 56%. The study also showed the sustained blockade of CXCR4 on leukemic blasts at 24 h after administration along with mobilization of ALL blasts into the peripheral blood [116].
POL5551
The protein epitope mimetic POL5551 is a novel CXCR4 antagonist [117]. This medium-sized, fully synthetic cyclic peptide-like molecule functions via mimicking the two most critical motifs in protein interactions, β-hairpins and α-helices [118]. Sison EA et al. first reported POL5551 in the study of pediatric ALL, showing that POL5551 was an inhibitor more potent than plerixafor. POL5551 exerted its effects through binding to CXCR4 surface at the 12G5- (and thus CXCL12-) binding site, resulting in the attenuation of CXCL12-mediated phosphorylation of ERK1/2, inhibition of CXCL12 induced chemotaxis, and restoration of chemosensitivity in a stroma co-culture model [119]. Thus, POL5551 may improve the outcomes in high-risk refractory pediatric ALL.
Inhibitors of the CCL25/CCR9 axis
CCL25-Pseudomonas exotoxin (PE38) fusion protein
We previously developed a CCL25-PE38 fusion protein using genetic engineering, which was proved to be able to attract the migration of MOLT4 cells and then induce the apoptosis of these cells thus suppressing the growth of CCR9+ tumors [120].
The induction of apoptosis is achieved through the internalization of PE38 when bound via the endolysosomal system, by which it is first transported to the Golgi and further to the endoplasmic reticulum and get activated. The activated PE38 ribosylates ADP and inactivates elongation factor 2 (EF2), thus halting protein synthesis and eventually leads to apoptosis [65, 121].
CCL25-PE38 could induce the specific killing of CCR9+ cells, but shown no cytotoxicity on CCR9− cells [120]. Optimization of the structure of CCL25-PE38 as well as increasing its activity and stability may make CCL25-PE38 a superior reagent in the treatment of T-ALL.
Monoclonal antibodies 91R, 92R
Monoclonal antibodies have been a standard component of cancer therapy, which work through several mechanisms, including antibody-dependent cell-mediated cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC) and direct induction of apoptosis [122, 123].
Monoclonal antibodies 91R (mouse antihuman CCR9 IgG2b) [124] and 92R (mouse antihuman CCR9 IgG2a) [125] were shown to inhibits the growth of T-ALL cells transplanted into immunodeficient mice, and both of them were able to elicit CDC and ADCC in vitro against CCR9+ cells. These antibodies have therapeutic potential for the targeted elimination of CCR9+ tumor cells, used either alone or in combination with other therapies.
Inhibitor of CCR5
CCR5 inhibitor maraviroc has been widely used in the treatment of HIV [126, 127], and recent studies have shown that it has potent inhibitory effects on many different tumors [128]. Treatment with maraviroc could inhibit the survival, proliferation and migration of ALL cells in vitro and in vivo, which may be achieved by inhibition of JAK/STAT3 pathway, a major signaling pathway that plays a role in the development of leukemia [94]. Therefore, the CCR5 inhibitor maraviroc may be a potential human ALL therapeutic agent.
Others
CXCR7 has showed great relevance to CXCR4, which indicates the inhibitor of CXCR7 may accomplish surprising outcomes. Ando N et al. found that application of CXCR7 inhibitor can accelerate the apoptosis of ALL cells with MLL gene rearrangements [88].
Besides, Tan Q et al. developed diphtheria-toxin-based antihuman CCR4 immunotoxins, which significantly prolonged the survival of tumor-bearing mice injected with human CCR4+ ALL cells [129].
Therefore, combining chemokine antagonists to the conventional therapy may significantly optimize prognosis of ALL patients [56]. Table 2 summarizes the potential chemokine antagonists for the treatment of ALL.
Table 2.
Potential therapies targeting chemokines and their receptors in ALL
| Targets | Drugs | Clinical stages/NCT numbers | Functions | References |
|---|---|---|---|---|
| CXCL12/CXCR4 axis | AMD3100 (Plerixafor) | Phase 1/ NCT01319864 | Increasing the sensitivity of chemotherapeutic drugs and promoting the apoptosis of ALL cells | [103] |
| AMD11070 | NA | Inhibiting CXCL12-induced signaling pathways and block the migration of ALL cells | [108] | |
| AMD3465 | NA | Suppressing the growth and infiltration of T-ALL | [16] | |
| T140, T134 &TC14012 | NA | Inhibiting proliferation and migration, enhancing the effects of the cytotoxic agents | [113, 114] | |
| BL-8040 | Phase 2/ NCT02763384 | Suppressing T-ALL cell growth in xenotransplantation models and mobilizing of leukemic blasts into the peripheral blood in T-ALL patients | [116] | |
| POL5551 | NA | Attenuating CXCL12-mediated phosphorylation of ERK1/2, inhibiting CXCL12-induced chemotaxis, and increasing chemosensitivity of ALL cells | [119] | |
| CCL25/CCR9 axis | CCL25-PE38 | NA | Inducing apoptosis and inhibiting the growth of CCR9+ cells | [120] |
| 91R, 92R | NA | Inhibiting CCR9+ T-ALL tumor growth through CDC, ADCC | [124, 125] | |
| CCR5 | Maraviroc | NA | Inhibiting the proliferation of ALL cells by inhibiting the JAK/STAT3 pathway | [94] |
NA: Not applicable
Conclusions
ALL is the most common childhood malignancy, accounting for 20% of all cancers before 20 years of age [130]. With the risk stratification based on biological features of ALL cells and response to treatment, the treatment modification adapted from pharmacodynamics and pharmacogenomics, and the improved supportive care, 5-year OS in pediatric ALL has improved to roughly 90% in clinical trials [131]. However, relapse and refractory of ALL remain obstacles, making it necessary to look for the clarification of the mechanism of ALL occurrence and relapse.
Infiltration and chemoresistance of ALL cells are main causes of the relapse, which at least are partly dependent on signals between the interactions of leukemic microenvironment and chemokines. In general, with the existing of chemokines and their receptors, leukemia cells tend to have the traits of migration and infiltration, and at the same time, the leukemic microenvironment can provide shelter where leukemic cells escape chemotherapy-induced death and acquire a drug-resistant phenotype. In addition, the refractory nature of ALL is also related to the protective effect of the microenvironment, where chemokines rebuild the healthy one into a “leukemic” one, thereby destroying normal hematopoietic function. Therefore, using chemokines and their receptors as targets for new drugs will become an effective method for the improvement of therapeutic efficacy and patient prognosis.
However, the physiological functions of chemokines and their receptors cannot be ignored, which may be correlated with the side effects of targeting chemokines. For example, CXCL12/CXCR4 is also important to HSCs maintenance within BM, and antagonism of CXCL12/CXCR4 could lead to HSCs mobilization out of BM into the peripheral blood, so the safety of the drugs should also be carefully evaluated [132].
Although many studies have shown the important roles of chemokines and their receptors in ALL, the exact mechanisms remain to be further elucidated and more clinical trials are needed to confirm their effects. In general, with the deeper understanding of chemokines roles in ALL, we believe that more and more ALL patients will benefit from the development of novel targeted therapies in the near future.
Acknowledgements
Not applicable.
Abbreviations
- ALL
Acute lymphoblastic leukemia
- OS
Overall survival
- EFS
Event-free survival
- BM
Bone marrow
- HSCs
Hematopoietic stem cells
- CNS
Central nervous system
- MSCs
Mesenchymal stem cells
- Treg cells
Regulatory T cells
- SDF-1
Stromal cell-derived factor-1
- IL
Interleukin
- MAPK
Mitogen-activated protein kinases
- ERK1/2
Extracellular signal-related kinase 1/2
- STAT
Signal transducer and activator of transcription
- RhoGDI2
Rho GDP-dissociation inhibitor 2
- LICs
Leukemia-initiating cells
- PI3K
Phosphoinositide 3-kinase
- P-gp
P-glycoprotein
- CSF
Cerebrospinal fluid
- JAK
Janus kinase
- AML
Acute myeloid leukemia
- MDS
Myelodysplastic syndrome
- PE38
Pseudomonas exotoxin
- EF2
Inactivates elongation factor 2
- ADCC
Antibody-dependent cell-mediated cytotoxicity
- CDC
Complement-dependent cytotoxicity
Authors' contributions
ZH, ZW and TX drafted the manuscript and created figures and tables. LF drafted part of the manuscript. JS, FZ and MJ revised the manuscript and provided critical advice. LS and QZ provided direction and guidance throughout the preparation of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No.81770180) and Basic-clinical Transformation Medicine Fund Project of Zhongnan Hospital, Wuhan University (ZNLH201902).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zixi Hong, Zimeng Wei, Tian Xie and Lin Fu have contributed equally to this work
Contributor Information
Qiuping Zhang, Email: qpzhang@whu.edu.cn.
Liang Shao, Email: liangsmd@163.com.
References
- 1.Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med. 2015;373(16):1541–1552. doi: 10.1056/NEJMra1400972. [DOI] [PubMed] [Google Scholar]
- 2.Malard F, Mohty M. Acute lymphoblastic leukaemia. Lancet. 2020;395(10230):1146–1162. doi: 10.1016/S0140-6736(19)33018-1. [DOI] [PubMed] [Google Scholar]
- 3.Terwilliger T, Abdul-Hay M. Acute lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer J. 2017;7(6):e577. doi: 10.1038/bcj.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiarini F, Lonetti A, Evangelisti C, Buontempo F, Orsini E, Evangelisti C, Cappellini A, Neri LM, McCubrey JA, Martelli AM. Advances in understanding the acute lymphoblastic leukemia bone marrow microenvironment: From biology to therapeutic targeting. Biochem Biophys Acta. 2016;1863(3):449–463. doi: 10.1016/j.bbamcr.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 5.Vadillo E, Dorantes-Acosta E, Pelayo R, Schnoor M. T cell acute lymphoblastic leukemia (T-ALL): new insights into the cellular origins and infiltration mechanisms common and unique among hematologic malignancies. Blood Rev. 2018;32(1):36–51. doi: 10.1016/j.blre.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Hodby KA, Marks DI. Recent advances in the management of acute lymphoblastic leukaemia. Curr Treat Options Oncol. 2020;21(3):23. doi: 10.1007/s11864-020-0712-8. [DOI] [PubMed] [Google Scholar]
- 7.Pui CH, Yang JJ, Hunger SP, Pieters R, Schrappe M, Biondi A, Vora A, Baruchel A, Silverman LB, Schmiegelow K, et al. Childhood acute lymphoblastic leukemia: progress through collaboration. J Clin Oncol: Off J Am Soc Clin Oncol. 2015;33(27):2938–2948. doi: 10.1200/JCO.2014.59.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ratti S, Lonetti A, Follo MY, Paganelli F, Martelli AM, Chiarini F, Evangelisti C. B-ALL complexity: is targeted therapy still a valuable approach for pediatric patients? Cancers. 2020;12(12):3498. doi: 10.3390/cancers12123498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao J, Song Y, Liu D. Recent advances on blinatumomab for acute lymphoblastic leukemia. Exp Hematol Oncol. 2019;8:28. doi: 10.1186/s40164-019-0152-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park KM, Yang EJ, Lee JM, Hah JO, Park SK, Park ES, Lim JY, Kim JY, Park J, Shim YJ, et al. Treatment outcome in pediatric acute lymphoblastic leukemia with hyperleukocytosis in the Yeungnam Region of Korea: a multicenter retrospective study. J Pediatr Hematol Oncol. 2020;42(4):275–280. doi: 10.1097/MPH.0000000000001771. [DOI] [PubMed] [Google Scholar]
- 11.Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. 2017;17(9):559–572. doi: 10.1038/nri.2017.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrison SJ, Spradling AC. Stem cells and niches: Mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132(4):598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taichman RS, Reilly MJ, Emerson SG. Human osteoblasts support human hematopoietic progenitor cells in in vitro bone marrow cultures. Blood. 1996;87(2):518–524. doi: 10.1182/blood.V87.2.518.bloodjournal872518. [DOI] [PubMed] [Google Scholar]
- 14.Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118(2):149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Miyamoto K, Yoshida S, Kawasumi M, Hashimoto K, Kimura T, Sato Y, Kobayashi T, Miyauchi Y, Hoshi H, Iwasaki R, et al. Osteoclasts are dispensable for hematopoietic stem cell maintenance and mobilization. J Exp Med. 2011;208(11):2175–2181. doi: 10.1084/jem.20101890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pitt LA, Tikhonova AN, Hu H, Trimarchi T, King B, Gong Y, Sanchez-Martin M, Tsirigos A, Littman DR, Ferrando AA, et al. CXCL12-producing vascular endothelial niches control acute T cell leukemia maintenance. Cancer Cell. 2015;27(6):755–768. doi: 10.1016/j.ccell.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaudichon J, Jakobczyk H, Debaize L, Cousin E, Galibert MD, Troadec MB, Gandemer V. Mechanisms of extramedullary relapse in acute lymphoblastic leukemia: Reconciling biological concepts and clinical issues. Blood Rev. 2019;36:40–56. doi: 10.1016/j.blre.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Gossai NP, Gordon PM. The role of the central nervous system microenvironment in pediatric acute lymphoblastic leukemia. Front Pediatr. 2017;5:90. doi: 10.3389/fped.2017.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scharff B, Modvig S, Marquart HV, Christensen C. Integrin-mediated adhesion and chemoresistance of acute lymphoblastic leukemia cells residing in the bone marrow or the central nervous system. Front Oncol. 2020;10:775. doi: 10.3389/fonc.2020.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buonamici S, Trimarchi T, Ruocco MG, Reavie L, Cathelin S, Mar BG, Klinakis A, Lukyanov Y, Tseng JC, Sen F, et al. CCR7 signalling as an essential regulator of CNS infiltration in T-cell leukaemia. Nature. 2009;459(7249):1000–U1129. doi: 10.1038/nature08020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jost TR, Borga C, Radaelli E, Romagnani A, Perruzza L, Omodho L, Cazzaniga G, Biondi A, Indraccolo S, Thelen M, et al. Role of CXCR4-mediated bone marrow colonization in CNS infiltration by T cell acute lymphoblastic leukemia. J Leukoc Biol. 2016;99(6):1077–1087. doi: 10.1189/jlb.5MA0915-394R. [DOI] [PubMed] [Google Scholar]
- 22.Kato I, Niwa A, Heike T, Fujino H, Saito MK, Umeda K, Hiramatsu H, Ito M, Morita M, Nishinaka Y, et al. Identification of hepatic niche harboring human acute lymphoblastic leukemic cells via the SDF-1/CXCR4 axis. PLoS ONE. 2011;6(11):e27042. doi: 10.1371/journal.pone.0027042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma S, Shi Y, Pang Y, Dong F, Cheng H, Hao S, Xu J, Zhu X, Yuan W, Cheng T, et al. Notch1-induced T cell leukemia can be potentiated by microenvironmental cues in the spleen. J Hematol Oncol. 2014;7:71. doi: 10.1186/s13045-014-0071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karantanou C, Godavarthy PS, Krause DS. Targeting the bone marrow microenvironment in acute leukemia. Leuk Lymphoma. 2018;59(11):2535–2545. doi: 10.1080/10428194.2018.1434886. [DOI] [PubMed] [Google Scholar]
- 25.Hughes CE, Nibbs RJB. A guide to chemokines and their receptors. FEBS J. 2018;285(16):2944–2971. doi: 10.1111/febs.14466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36(5):705–716. doi: 10.1016/j.immuni.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hutchings CJ, Koglin M, Olson WC, Marshall FH. Opportunities for therapeutic antibodies directed at G-protein-coupled receptors. Nat Rev Drug Discov. 2017;16(9):661. doi: 10.1038/nrd.2017.173. [DOI] [PubMed] [Google Scholar]
- 28.Koenen RR, Weber C. Therapeutic targeting of chemokine interactions in atherosclerosis. Nat Rev Drug Discov. 2010;9(2):141–153. doi: 10.1038/nrd3048. [DOI] [PubMed] [Google Scholar]
- 29.Ratajczak MZ, Zuba-Surma E, Kucia M, Reca R, Wojakowski W, Ratajczak J. The pleiotropic effects of the SDF-1-CXCR4 axis in organogenesis, regeneration and tumorigenesis. Leukemia. 2006;20(11):1915–1924. doi: 10.1038/sj.leu.2404357. [DOI] [PubMed] [Google Scholar]
- 30.Chotinantakul K, Leeanansaksiri W. Hematopoietic stem cell development, niches, and signaling pathways. Bone Marrow Res. 2012;2012:270425. doi: 10.1155/2012/270425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Lourdes PA, Amarante MK, Guembarovski RL, de Oliveira CE, Watanabe MA. CXCL12/CXCR4 axis in the pathogenesis of acute lymphoblastic leukemia (ALL): a possible therapeutic target. Cell Mol Life Sci. 2015;72(9):1715–1723. doi: 10.1007/s00018-014-1830-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burger JA, Peled A. CXCR4 antagonists: targeting the microenvironment in leukemia and other cancers. Leukemia. 2009;23(1):43–52. doi: 10.1038/leu.2008.299. [DOI] [PubMed] [Google Scholar]
- 33.Randhawa S, Cho BS, Ghosh D, Sivina M, Koehrer S, Müschen M, Peled A, Davis RE, Konopleva M, Burger JA. Effects of pharmacological and genetic disruption of CXCR4 chemokine receptor function in B-cell acute lymphoblastic leukaemia. Br J Haematol. 2016;174(3):425–436. doi: 10.1111/bjh.14075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corcione A, Arduino N, Ferretti E, Pistorio A, Spinelli M, Ottonello L, Dallegri F, Basso G, Pistoia V. Chemokine receptor expression and function in childhood acute lymphoblastic leukemia of B-lineage. Leuk Res. 2006;30(4):365–372. doi: 10.1016/j.leukres.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 35.Dürig J, Schmücker U, Dührsen U. Differential expression of chemokine receptors in B cell malignancies. Leukemia. 2001;15(5):752–756. doi: 10.1038/sj.leu.2402107. [DOI] [PubMed] [Google Scholar]
- 36.Konoplev S, Jorgensen JL, Thomas DA, Lin E, Burger J, Kantarjian HM, Andreeff M, Medeiros LJ, Konopleva M. Phosphorylated CXCR4 is associated with poor survival in adults with B-acute lymphoblastic leukemia. Cancer. 2011;117(20):4689–4695. doi: 10.1002/cncr.26113. [DOI] [PubMed] [Google Scholar]
- 37.Ko SY, Park CJ, Park SH, Cho YU, Jang S, Seo EJ, Kim N, Kim DY, Koh KN, Im HJ, et al. High CXCR4 and low VLA-4 expression predicts poor survival in adults with acute lymphoblastic leukemia. Leuk Res. 2014;38(1):65–70. doi: 10.1016/j.leukres.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 38.Crazzolara R, Kreczy A, Mann G, Heitger A, Eibl G, Fink FM, Mohle R, Meister B. High expression of the chemokine receptor CXCR4 predicts extramedullary organ infiltration in childhood acute lymphoblastic leukaemia. Br J Haematol. 2001;115(3):545–553. doi: 10.1046/j.1365-2141.2001.03164.x. [DOI] [PubMed] [Google Scholar]
- 39.Passaro D, Irigoyen M, Catherinet C, Gachet S, De Jesus CD, Lasgi C, Quang CT, Ghysdael J. CXCR4 is required for leukemia-initiating cell activity in T cell acute lymphoblastic leukemia. Cancer Cell. 2015;27(6):769–779. doi: 10.1016/j.ccell.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 40.Bendall LJ, Baraz R, Juarez J, Shen W, Bradstock KF. Defective p38 mitogen-activated protein kinase signaling impairs chemotaxic but not proliferative responses to stromal-derived factor-1alpha in acute lymphoblastic leukemia. Cancer Res. 2005;65(8):3290–3298. doi: 10.1158/0008-5472.CAN-04-3402. [DOI] [PubMed] [Google Scholar]
- 41.Catusse J, Wollner S, Leick M, Schröttner P, Schraufstätter I, Burger M. Attenuation of CXCR4 responses by CCL18 in acute lymphocytic leukemia B cells. J Cell Physiol. 2010;225(3):792–800. doi: 10.1002/jcp.22284. [DOI] [PubMed] [Google Scholar]
- 42.Habringer S, Lapa C, Herhaus P, Schottelius M, Istvanffy R, Steiger K, Slotta-Huspenina J, Schirbel A, Hänscheid H, Kircher S, et al. Dual targeting of acute leukemia and supporting niche by CXCR4-directed theranostics. Theranostics. 2018;8(2):369–383. doi: 10.7150/thno.21397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Passaro D, Irigoyen M, Catherinet C, Gachet S, De Jesus CDC, Lasgi C, Tran Quang C, Ghysdael J. CXCR4 is required for leukemia-initiating cell activity in T cell acute lymphoblastic leukemia. Cancer Cell. 2015;27(6):769–779. doi: 10.1016/j.ccell.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 44.Qin L, Deng HY, Chen SJ, Wei W, Zhang YT. miR-139 acts as a tumor suppressor in T-cell acute lymphoblastic leukemia by targeting CX chemokine receptor 4. Am J Transl Res. 2017;9(9):4059–4070. [PMC free article] [PubMed] [Google Scholar]
- 45.Juarez J, Baraz R, Gaundar S, Bradstock K, Bendall L. Interaction of interleukin-7 and interleukin-3 with the CXCL12-induced proliferation of B-cell progenitor acute lymphoblastic leukemia. Haematologica. 2007;92(4):450–459. doi: 10.3324/haematol.10621. [DOI] [PubMed] [Google Scholar]
- 46.Mowafi F, Cagigi A, Matskova L, Björk O, Chiodi F, Nilsson A. Chemokine CXCL12 enhances proliferation in pre-B-ALL via STAT5 activation. Pediatr Blood Cancer. 2008;50(4):812–817. doi: 10.1002/pbc.21370. [DOI] [PubMed] [Google Scholar]
- 47.Ayala F, Dewar R, Kieran M, Kalluri R. Contribution of bone microenvironment to leukemogenesis and leukemia progression. Leukemia. 2009;23(12):2233–2241. doi: 10.1038/leu.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spiegel A, Kollet O, Peled A, Abel L, Nagler A, Bielorai B, Rechavi G, Vormoor J, Lapidot T. Unique SDF-1-induced activation of human precursor-B ALL cells as a result of altered CXCR4 expression and signaling. Blood. 2004;103(8):2900–2907. doi: 10.1182/blood-2003-06-1891. [DOI] [PubMed] [Google Scholar]
- 49.Luo J, Wang J, Zheng H, Wang L. Rho GDP-dissociation inhibitor 2 inhibits C-X-C chemokine receptor type 4-mediated acute lymphoblastic leukemia cell migration. Front Oncol. 2020;10:1512. doi: 10.3389/fonc.2020.01512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luo J, Li D, Wei D, Wang X, Wang L, Zeng X. RhoA and RhoC are involved in stromal cell-derived factor-1-induced cell migration by regulating F-actin redistribution and assembly. Mol Cell Biochem. 2017;436(1–2):13–21. doi: 10.1007/s11010-017-3072-3. [DOI] [PubMed] [Google Scholar]
- 51.Juarez JG, Thien M, Dela Pena A, Baraz R, Bradstock KF, Bendall LJ. CXCR4 mediates the homing of B cell progenitor acute lymphoblastic leukaemia cells to the bone marrow via activation of p38MAPK. Br J Haematol. 2009;145(4):491–499. doi: 10.1111/j.1365-2141.2009.07648.x. [DOI] [PubMed] [Google Scholar]
- 52.Arnaud MP, Vallée A, Robert G, Bonneau J, Leroy C, Varin-Blank N, Rio AG, Troadec MB, Galibert MD, Gandemer V. CD9, a key actor in the dissemination of lymphoblastic leukemia, modulating CXCR4-mediated migration via RAC1 signaling. Blood. 2015;126(15):1802–1812. doi: 10.1182/blood-2015-02-628560. [DOI] [PubMed] [Google Scholar]
- 53.Freret M, Gouel F, Buquet C, Legrand E, Vannier JP, Vasse M, Dubus I. Rac-1 GTPase controls the capacity of human leukaemic lymphoblasts to migrate on fibronectin in response to SDF-1α (CXCL12) Leuk Res. 2011;35(7):971–973. doi: 10.1016/j.leukres.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 54.Pui CH, Howard SC. Current management and challenges of malignant disease in the CNS in paediatric leukaemia. Lancet Oncol. 2008;9(3):257–268. doi: 10.1016/S1470-2045(08)70070-6. [DOI] [PubMed] [Google Scholar]
- 55.Alsadeq A, Fedders H, Vokuhl C, Belau NM, Zimmermann M, Wirbelauer T, Spielberg S, Vossen-Gajcy M, Cario G, Schrappe M, et al. The role of ZAP70 kinase in acute lymphoblastic leukemia infiltration into the central nervous system. Haematologica. 2017;102(2):346–355. doi: 10.3324/haematol.2016.147744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Konopleva M, Tabe Y, Zeng Z, Andreeff M. Therapeutic targeting of microenvironmental interactions in leukemia: mechanisms and approaches. Drug Resist Updat. 2009;12(4–5):103–113. doi: 10.1016/j.drup.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peled A, Klein S, Beider K, Burger JA, Abraham M. Role of CXCL12 and CXCR4 in the pathogenesis of hematological malignancies. Cytokine. 2018;109:11–16. doi: 10.1016/j.cyto.2018.02.020. [DOI] [PubMed] [Google Scholar]
- 58.Parameswaran R, Yu M, Lyu MA, Lim M, Rosenblum MG, Groffen J, Heisterkamp N. Treatment of acute lymphoblastic leukemia with an rGel/BLyS fusion toxin. Leukemia. 2012;26(8):1786–1796. doi: 10.1038/leu.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sison EA, Rau RE, McIntyre E, Li L, Small D, Brown P. MLL-rearranged acute lymphoblastic leukaemia stem cell interactions with bone marrow stroma promote survival and therapeutic resistance that can be overcome with CXCR4 antagonism. Br J Haematol. 2013;160(6):785–797. doi: 10.1111/bjh.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pillozzi S, Masselli M, De Lorenzo E, Accordi B, Cilia E, Crociani O, Amedei A, Veltroni M, D'Amico M, Basso G, et al. Chemotherapy resistance in acute lymphoblastic leukemia requires hERG1 channels and is overcome by hERG1 blockers. Blood. 2011;117(3):902–914. doi: 10.1182/blood-2010-01-262691. [DOI] [PubMed] [Google Scholar]
- 61.Wang S, Wang X, Liu S, Zhang S, Wei X, Song Y, Yin Q. The CXCR4 antagonist, AMD3100, reverses mesenchymal stem cell-mediated drug resistance in relapsed/refractory acute lymphoblastic leukemia. Onco Targets Ther. 2020;13:6583–6591. doi: 10.2147/OTT.S249425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matthijssens F, Sharma ND, Nysus M, Nickl CK, Kang H, Perez DR, Lintermans B, Van Loocke W, Roels J, Peirs S, et al. RUNX2 regulates leukemic cell metabolism and chemotaxis in high-risk T cell acute lymphoblastic leukemia. J Clin Investig. 2021 doi: 10.1172/JCI141566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li W, Jiang Z, Li T, Wei X, Zheng Y, Wu D, Yang L, Chen S, Xu B, Zhong M, et al. Genome-wide analyses identify KLF4 as an important negative regulator in T-cell acute lymphoblastic leukemia through directly inhibiting T-cell associated genes. Mol Cancer. 2015;14:26. doi: 10.1186/s12943-014-0285-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heshmati M, Soltani A, Sanaei MJ, Nahid-Samiei M, Shirzad H, Jami MS, GhatrehSamani M. Ghrelin induces autophagy and CXCR4 expression via the SIRT1/AMPK axis in lymphoblastic leukemia cell lines. Cell Signal. 2020;66:109492. doi: 10.1016/j.cellsig.2019.109492. [DOI] [PubMed] [Google Scholar]
- 65.Tu Z, Xiao R, Xiong J, Tembo KM, Deng X, Xiong M, Liu P, Wang M, Zhang Q. CCR9 in cancer: oncogenic role and therapeutic targeting. J Hematol Oncol. 2016;9:10. doi: 10.1186/s13045-016-0236-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wurbel MA, Philippe JM, Nguyen C, Victorero G, Freeman T, Wooding P, Miazek A, Mattei MG, Malissen M, Jordan BR, et al. The chemokine TECK is expressed by thymic and intestinal epithelial cells and attracts double- and single-positive thymocytes expressing the TECK receptor CCR9. Eur J Immunol. 2000;30(1):262–271. doi: 10.1002/1521-4141(200001)30:1<262::AID-IMMU262>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 67.Campbell DJ, Butcher EC. Intestinal attraction: CCL25 functions in effector lymphocyte recruitment to the small intestine. J Clin Invest. 2002;110(8):1079–1081. doi: 10.1172/JCI0216946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qiuping Z, Qun L, Chunsong H, Xiaolian Z, Baojun H, Mingzhen Y, Chengming L, Jinshen H, Qingping G, Kejian Z, et al. Selectively increased expression and functions of chemokine receptor CCR9 on CD4+ T cells from patients with T-cell lineage acute lymphocytic leukemia. Cancer Res. 2003;63(19):6469–6477. [PubMed] [Google Scholar]
- 69.Zhou B, Leng J, Hu M, Zhang L, Wang Z, Liu D, Tong X, Yu B, Hu Y, Deng C, et al. Ezrin is a key molecule in the metastasis of MOLT4 cells induced by CCL25/CCR9. Leuk Res. 2010;34(6):769–776. doi: 10.1016/j.leukres.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 70.Zhang L, Yu B, Hu M, Wang Z, Liu D, Tong X, Leng J, Zhou B, Hu Y, Wu R, et al. Role of Rho-ROCK signaling in MOLT4 cells metastasis induced by CCL25. Leuk Res. 2011;35(1):103–109. doi: 10.1016/j.leukres.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 71.Deng X, Tu Z, Xiong M, Tembo K, Zhou L, Liu P, Pan S, Xiong J, Yang X, Leng J, et al. Wnt5a and CCL25 promote adult T-cell acute lymphoblastic leukemia cell migration, invasion and metastasis. Oncotarget. 2017;8(24):39033–39047. doi: 10.18632/oncotarget.16559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tong X, Zhang L, Zhang L, Hu M, Leng J, Yu B, Zhou B, Hu Y, Zhang Q. The mechanism of chemokine receptor 9 internalization triggered by interleukin 2 and interleukin 4. Cell Mol Immunol. 2009;6(3):181–189. doi: 10.1038/cmi.2009.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miething C, Scuoppo C, Bosbach B, Appelmann I, Nakitandwe J, Ma J, Wu G, Lintault L, Auer M, Premsrirut PK, et al. PTEN action in leukaemia dictated by the tissue microenvironment. Nature. 2014;510(7505):402–406. doi: 10.1038/nature13239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qiuping Z, Jei X, Youxin J, Wei J, Chun L, Jin W, Qun W, Yan L, Chunsong H, Mingzhen Y, et al. CC chemokine ligand 25 enhances resistance to apoptosis in CD4+ T cells from patients with T-cell lineage acute and chronic lymphocytic leukemia by means of livin activation. Cancer Res. 2004;64(20):7579–7587. doi: 10.1158/0008-5472.CAN-04-0641. [DOI] [PubMed] [Google Scholar]
- 75.Zhang L, Xiao R, Xiong J, Leng J, Ehtisham A, Hu Y, Ding Q, Xu H, Liu S, Wang J, et al. Activated ERM protein plays a critical role in drug resistance of MOLT4 cells induced by CCL25. PLoS ONE. 2013;8(1):e52384. doi: 10.1371/journal.pone.0052384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mirandola L, Chiriva-Internati M, Montagna D, Locatelli F, Zecca M, Ranzani M, Basile A, Locati M, Cobos E, Kast WM, et al. Notch1 regulates chemotaxis and proliferation by controlling the CC-chemokine receptors 5 and 9 in T cell acute lymphoblastic leukaemia. J Pathol. 2012;226(5):713–722. doi: 10.1002/path.3015. [DOI] [PubMed] [Google Scholar]
- 77.Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol. 2011;89(2):207–215. doi: 10.1038/icb.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martínez-Laperche C, Gómez-García AM, Lassaletta Á, Moscardó C, Vivanco JL, Molina J, Fuster JL, Couselo JM, de Toledo JS, Bureo E, et al. Detection of occult cerebrospinal fluid involvement during maintenance therapy identifies a group of children with acute lymphoblastic leukemia at high risk for relapse. Am J Hematol. 2013;88(5):359–364. doi: 10.1002/ajh.23407. [DOI] [PubMed] [Google Scholar]
- 79.Balashov KE, Rottman JB, Weiner HL, Hancock WW. CCR5(+) and CXCR3(+) T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions. Proc Natl Acad Sci U S A. 1999;96(12):6873–6878. doi: 10.1073/pnas.96.12.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gomez AM, Martinez C, Gonzalez M, Luque A, Melen GJ, Martinez J, Hortelano S, Lassaletta A, Madero L, Ramirez M. Chemokines and relapses in childhood acute lymphoblastic leukemia: a role in migration and in resistance to antileukemic drugs. Blood Cells Mol Dis. 2015;55(3):220–227. doi: 10.1016/j.bcmd.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 81.Williams MT, Yousafzai Y, Cox C, Blair A, Carmody R, Sai S, Chapman KE, McAndrew R, Thomas A, Spence A, et al. Interleukin-15 enhances cellular proliferation and upregulates CNS homing molecules in pre-B acute lymphoblastic leukemia. Blood. 2014;123(20):3116–3127. doi: 10.1182/blood-2013-05-499970. [DOI] [PubMed] [Google Scholar]
- 82.Lee Y, Chittezhath M, Andre V, Zhao H, Poidinger M, Biondi A, D'Amico G, Biswas SK. Protumoral role of monocytes in human B-cell precursor acute lymphoblastic leukemia: involvement of the chemokine CXCL10. Blood. 2012;119(1):227–237. doi: 10.1182/blood-2011-06-357442. [DOI] [PubMed] [Google Scholar]
- 83.Salem A, Alotaibi M, Mroueh R, Basheer HA, Afarinkia K. CCR7 as a therapeutic target in Cancer. Biochim Biophys Acta. 2021;1875(1):188499. doi: 10.1016/j.bbcan.2020.188499. [DOI] [PubMed] [Google Scholar]
- 84.Redondo-Munoz J, Garcia-Pardo A, Teixido J. Molecular Players in Hematologic Tumor Cell Trafficking. Front Immunol. 2019;10:156. doi: 10.3389/fimmu.2019.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li SQ, Guo R, Gan SL, Jiang ZX, Yue BH, Ma J, Liu YF, Xie XS, Sun H. Expression and clinical significance of CC-chemokine receptor 7 in adult acute leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2016;24(2):311–315. doi: 10.7534/j.issn.1009-2137.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 86.Levoye A, Balabanian K, Baleux F, Bachelerie F, Lagane B. CXCR7 heterodimerizes with CXCR4 and regulates CXCL12-mediated G protein signaling. Blood. 2009;113(24):6085–6093. doi: 10.1182/blood-2008-12-196618. [DOI] [PubMed] [Google Scholar]
- 87.Scala S. Molecular pathways: targeting the CXCR4-CXCL12 axis-untapped potential in the tumor microenvironment. Clin Cancer Res. 2015;21(19):4278–4285. doi: 10.1158/1078-0432.CCR-14-0914. [DOI] [PubMed] [Google Scholar]
- 88.Ando N, Furuichi Y, Kasai S, Tamai M, Harama D, Kagami K, Abe M, Goi K, Inukai T, Sugita K. Chemosensitivity is differentially regulated by the SDF-1/CXCR4 and SDF-1/CXCR7 axes in acute lymphoblastic leukemia with MLL gene rearrangements. Leuk Res. 2018;75:36–44. doi: 10.1016/j.leukres.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 89.Melo RCC, Longhini AL, Bigarella CL, Baratti MO, Traina F, Favaro P, de Melo CP, Saad ST. CXCR7 is highly expressed in acute lymphoblastic leukemia and potentiates CXCR4 response to CXCL12. PLoS ONE. 2014;9(1):e85926. doi: 10.1371/journal.pone.0085926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang XY, Sheng Y. miR-101 represses T-cell acute lymphoblastic leukemia by targeting CXCR7/STAT3 axis. Oncol Res. 2019;27(9):997–1006. doi: 10.3727/096504018X15439207752093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.de Vasconcellos JF, Laranjeira ABA, Zanchin NIT, Otubo R, Vaz TH, Cardoso AA, Brandalise SR, Yunes JA. Increased CCL2 and IL-8 in the bone marrow microenvironment in acute lymphoblastic leukemia. Pediatr Blood Cancer. 2011;56(4):568–577. doi: 10.1002/pbc.22941. [DOI] [PubMed] [Google Scholar]
- 92.Ma Z, Zhao X, Deng M, Huang Z, Wang J, Wu Y, Cui D, Liu Y, Liu R, Ouyang G. Bone marrow mesenchymal stromal cell-derived periostin promotes B-ALL progression by modulating CCL2 in leukemia cells. Cell Rep. 2019;26(6):1533–1543. doi: 10.1016/j.celrep.2019.01.034. [DOI] [PubMed] [Google Scholar]
- 93.Eisenkraft A, Keidan I, Bielorai B, Keller N, Toren A, Paret G. MCP-1 in the cerebrospinal fluid of children with acute lymphoblastic leukemia. Leuk Res. 2006;30(10):1259–1261. doi: 10.1016/j.leukres.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 94.Zi J, Yuan S, Qiao J, Zhao K, Xu L, Qi K, Xu K, Zeng L. Treatment with the C-C chemokine receptor type 5 (CCR5)-inhibitor maraviroc suppresses growth and induces apoptosis of acute lymphoblastic leukemia cells. Am J Cancer Res. 2017;7(4):869–880. [PMC free article] [PubMed] [Google Scholar]
- 95.Struyf S, Schutyser E, Gouwy M, Gijsbers K, Proost P, Benoit Y, Opdenakker G, Van Damme J, Laureys G. PARC/CCL18 is a plasma CC chemokine with increased levels in childhood acute lymphoblastic leukemia. Am J Pathol. 2003;163(5):2065–2075. doi: 10.1016/S0002-9440(10)63564-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gutiérrez-Aguirre CH, Flores-Jiménez JA, Alatorre-Ricardo J, Cantú-Rodríguez OG, Rosas-Taraco A, Salazar-Riojas R, Jaime-Pérez JC, Sánchez-Cárdenas M, López-Silva L, Martínez-Castilla AM, et al. The prognostic significance of serum XCL1 concentration in patients with acute lymphoblastic leukemia: a pilot study. Ann Hematol. 2017;96(12):2015–2024. doi: 10.1007/s00277-017-3142-3. [DOI] [PubMed] [Google Scholar]
- 97.Frismantas V, Dobay MP, Rinaldi A, Tchinda J, Dunn SH, Kunz J, Richter-Pechanska P, Marovca B, Pail O, Jenni S, et al. Ex vivo drug response profiling detects recurrent sensitivity patterns in drug-resistant acute lymphoblastic leukemia. Blood. 2017;129(11):e26–e37. doi: 10.1182/blood-2016-09-738070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu D, Zhao J, Song Y, Luo X, Yang T. Clinical trial update on bispecific antibodies, antibody-drug conjugates, and antibody-containing regimens for acute lymphoblastic leukemia. J Hematol Oncol. 2019;12(1):15. doi: 10.1186/s13045-019-0703-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Konopleva MY, Jordan CT. Leukemia stem cells and microenvironment: biology and therapeutic targeting. J Clin Oncol. 2011;29(5):591–599. doi: 10.1200/JCO.2010.31.0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ahmadzadeh A, Kast RE, Ketabchi N, Shahrabi S, Shahjahani M, Jaseb K, Saki N. Regulatory effect of chemokines in bone marrow niche. Cell Tissue Res. 2015;361(2):401–410. doi: 10.1007/s00441-015-2129-4. [DOI] [PubMed] [Google Scholar]
- 101.Hatse S, Princen K, Bridger G, De Clercq E, Schols D. Chemokine receptor inhibition by AMD3100 is strictly confined to CXCR4. FEBS Lett. 2002;527(1–3):255–262. doi: 10.1016/S0014-5793(02)03143-5. [DOI] [PubMed] [Google Scholar]
- 102.Donzella GA, Schols D, Lin SW, Esté JA, Nagashima KA, Maddon PJ, Allaway GP, Sakmar TP, Henson G, De Clercq E, et al. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat Med. 1998;4(1):72–77. doi: 10.1038/nm0198-072. [DOI] [PubMed] [Google Scholar]
- 103.Zhang Y, Patel S, Abdelouahab H, Wittner M, Willekens C, Shen S, Betems A, Joulin V, Opolon P, Bawa O, et al. CXCR4 inhibitors selectively eliminate CXCR4-expressing human acute myeloid leukemia cells in NOG mouse model. Cell Death Disease. 2012;3:e396. doi: 10.1038/cddis.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Welschinger R, Liedtke F, Basnett J, Dela Pena A, Juarez JG, Bradstock KF, Bendall LJ. Plerixafor (AMD3100) induces prolonged mobilization of acute lymphoblastic leukemia cells and increases the proportion of cycling cells in the blood in mice. Exp Hematol. 2013;41(3):293–302. doi: 10.1016/j.exphem.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 105.Kawaguchi A, Orba Y, Kimura T, Iha H, Ogata M, Tsuji T, Ainai A, Sata T, Okamoto T, Hall WW, et al. Inhibition of the SDF-1alpha-CXCR4 axis by the CXCR4 antagonist AMD3100 suppresses the migration of cultured cells from ATL patients and murine lymphoblastoid cells from HTLV-I Tax transgenic mice. Blood. 2009;114(14):2961–2968. doi: 10.1182/blood-2008-11-189308. [DOI] [PubMed] [Google Scholar]
- 106.Cooper TM, Sison EAR, Baker SD, Li L, Ahmed A, Trippett T, Gore L, Macy ME, Narendran A, August K, et al. A phase 1 study of the CXCR4 antagonist plerixafor in combination with high-dose cytarabine and etoposide in children with relapsed or refractory acute leukemias or myelodysplastic syndrome: A Pediatric Oncology Experimental Therapeutics Investigators' Consortium study (POE 10-03) Pediatr Blood Cancer. 2017;64(8):e26414. doi: 10.1002/pbc.26414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hendrix CW, Flexner C, MacFarland RT, Giandomenico C, Fuchs EJ, Redpath E, Bridger G, Henson GW. Pharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR-4 chemokine receptor, in human volunteers. Antimicrob Agents Chemother. 2000;44(6):1667–1673. doi: 10.1128/AAC.44.6.1667-1673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Parameswaran R, Yu M, Lim M, Groffen J, Heisterkamp N. Combination of drug therapy in acute lymphoblastic leukemia with a CXCR4 antagonist. Leukemia. 2011;25(8):1314–1323. doi: 10.1038/leu.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wong RS, Bodart V, Metz M, Labrecque J, Bridger G, Fricker SP. Comparison of the potential multiple binding modes of bicyclam, monocylam, and noncyclam small-molecule CXC chemokine receptor 4 inhibitors. Mol Pharmacol. 2008;74(6):1485–1495. doi: 10.1124/mol.108.049775. [DOI] [PubMed] [Google Scholar]
- 110.Stone ND, Dunaway SB, Flexner C, Tierney C, Calandra GB, Becker S, Cao YJ, Wiggins IP, Conley J, MacFarland RT, et al. Multiple-dose escalation study of the safety, pharmacokinetics, and biologic activity of oral AMD070, a selective CXCR4 receptor inhibitor, in human subjects. Antimicrob Agents Chemother. 2007;51(7):2351–2358. doi: 10.1128/AAC.00013-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moyle G, DeJesus E, Boffito M, Wong RS, Gibney C, Badel K, MacFarland R, Calandra G, Bridger G, Becker S. Proof of activity with AMD11070, an orally bioavailable inhibitor of CXCR4-tropic HIV type 1. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2009;48(6):798–805. doi: 10.1086/597097. [DOI] [PubMed] [Google Scholar]
- 112.Bodart V, Anastassov V, Darkes MC, Idzan SR, Labrecque J, Lau G, Mosi RM, Neff KS, Nelson KL, Ruzek MC, et al. Pharmacology of AMD3465: a small molecule antagonist of the chemokine receptor CXCR4. Biochem Pharmacol. 2009;78(8):993–1000. doi: 10.1016/j.bcp.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 113.Juarez J, Bradstock KF, Gottlieb DJ, Bendall LJ. Effects of inhibitors of the chemokine receptor CXCR4 on acute lymphoblastic leukemia cells in vitro. Leukemia. 2003;17(7):1294–1300. doi: 10.1038/sj.leu.2402998. [DOI] [PubMed] [Google Scholar]
- 114.Juarez J, Dela Pena A, Baraz R, Hewson J, Khoo M, Cisterne A, Fricker S, Fujii N, Bradstock KF, Bendall LJ. CXCR4 antagonists mobilize childhood acute lymphoblastic leukemia cells into the peripheral blood and inhibit engraftment. Leukemia. 2007;21(6):1249–1257. doi: 10.1038/sj.leu.2404684. [DOI] [PubMed] [Google Scholar]
- 115.Tamamura H, Fujisawa M, Hiramatsu K, Mizumoto M, Nakashima H, Yamamoto N, Otaka A, Fujii N. Identification of a CXCR4 antagonist, a T140 analog, as an anti-rheumatoid arthritis agent. FEBS Lett. 2004;569(1–3):99–104. doi: 10.1016/j.febslet.2004.05.056. [DOI] [PubMed] [Google Scholar]
- 116.Uy GL, Kadia TM, Stock W, Brammer JE, Bohana-Kashtan O, Vainstein A, Sorani E, Chen H, DiPersio JF, Link DC. CXCR4 inhibition with BL-8040 in combination with nelarabine in patients with relapsed or refractory T-cell acute lymphoblastic leukemia/lymphoblastic lymphoma. Blood. 2019;134(Supplement_1):2630–2630. doi: 10.1182/blood-2019-127121. [DOI] [Google Scholar]
- 117.Xiang J, Hurchla MA, Fontana F, Su X, Amend SR, Esser AK, Douglas GJ, Mudalagiriyappa C, Luker KE, Pluard T, et al. CXCR4 protein epitope mimetic antagonist POL5551 disrupts metastasis and enhances chemotherapy effect in triple-negative breast cancer. Mol Cancer Ther. 2015;14(11):2473–2485. doi: 10.1158/1535-7163.MCT-15-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Luther A, Moehle K, Chevalier E, Dale G, Obrecht D. Protein epitope mimetic macrocycles as biopharmaceuticals. Curr Opin Chem Biol. 2017;38:45–51. doi: 10.1016/j.cbpa.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 119.Sison EA, Magoon D, Li L, Annesley CE, Romagnoli B, Douglas GJ, Tuffin G, Zimmermann J, Brown P. POL5551, a novel and potent CXCR4 antagonist, enhances sensitivity to chemotherapy in pediatric ALL. Oncotarget. 2015;6(31):30902–30918. doi: 10.18632/oncotarget.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hu Y, Zhang L, Wu R, Han R, Jia Y, Jiang Z, Cheng M, Gan J, Tao X, Zhang Q. Specific killing of CCR9 high-expressing acute T lymphocytic leukemia cells by CCL25 fused with PE38 toxin. Leuk Res. 2011;35(9):1254–1260. doi: 10.1016/j.leukres.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 121.Weldon JE, Xiang L, Zhang J, Beers R, Walker DA, Onda M, Hassan R, Pastan I. A recombinant immunotoxin against the tumor-associated antigen mesothelin reengineered for high activity, low off-target toxicity, and reduced antigenicity. Mol Cancer Ther. 2013;12(1):48–57. doi: 10.1158/1535-7163.MCT-12-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Weiner GJ. Building better monoclonal antibody-based therapeutics. Nat Rev Cancer. 2015;15(6):361–370. doi: 10.1038/nrc3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jabbour E, O'Brien S, Ravandi F, Kantarjian H. Monoclonal antibodies in acute lymphoblastic leukemia. Blood. 2015;125(26):4010–4016. doi: 10.1182/blood-2014-08-596403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chamorro S, Vela M, Franco-Villanueva A, Carramolino L, Gutiérrez J, Gómez L, Lozano M, Salvador B, García-Gallo M, Martínez AC, et al. Antitumor effects of a monoclonal antibody to human CCR9 in leukemia cell xenografts. MAbs. 2014;6(4):1000–1012. doi: 10.4161/mabs.29063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Somovilla-Crespo B, Martín Monzón MT, Vela M, Corraliza-Gorjón I, Santamaria S, Garcia-Sanz JA, Kremer L. 92R monoclonal antibody inhibits human CCR9(+) Leukemia cells growth in NSG mice xenografts. Front Immunol. 2018;9:77. doi: 10.3389/fimmu.2018.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Woollard SM, Kanmogne GD. Maraviroc: a review of its use in HIV infection and beyond. Drug Des Dev Ther. 2015;9:5447–5468. doi: 10.2147/DDDT.S90580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dolin R. A new class of anti-HIV therapy and new challenges. N Engl J Med. 2008;359(14):1509–1511. doi: 10.1056/NEJMe0806598. [DOI] [PubMed] [Google Scholar]
- 128.Tan Q, Zhu Y, Li J, Chen Z, Han GW, Kufareva I, Li T, Ma L, Fenalti G, Li J, et al. Structure of the CCR5 chemokine receptor-HIV entry inhibitor maraviroc complex. Science. 2013;341(6152):1387–1390. doi: 10.1126/science.1241475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang Z, Wei M, Zhang H, Chen H, Germana S, Huang CA, Madsen JC, Sachs DH, Wang Z. Diphtheria-toxin based anti-human CCR4 immunotoxin for targeting human CCR4(+) cells in vivo. Mol Oncol. 2015;9(7):1458–1470. doi: 10.1016/j.molonc.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dixon SB, Chen Y, Yasui Y, Pui CH, Hunger SP, Silverman LB, Ness KK, Green DM, Howell RM, Leisenring WM, et al. Reduced morbidity and mortality in survivors of childhood acute lymphoblastic leukemia: a report from the childhood cancer survivor study. J Clin Oncol. 2020;38(29):3418–3429. doi: 10.1200/JCO.20.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Inaba H, Greaves M, Mullighan CG. Acute lymphoblastic leukaemia. Lancet. 2013;381(9881):1943–1955. doi: 10.1016/S0140-6736(12)62187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Karpova D, Bonig H. Concise review: CXCR4/CXCL12 signaling in immature hematopoiesis-lessons from pharmacological and genetic models. Stem Cells. 2015;33(8):2391–2399. doi: 10.1002/stem.2054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.