Abstract
The constitutive androstane receptor (CAR, NR3I1) belongs to nuclear receptor superfamily. It was reported that CAR agonist TCPOBOP induces hepatomegaly but the underlying mechanism remains largely unknown. Yes-associated protein (YAP) is a potent regulator of organ size. The aim of this study is to explore the role of YAP in CAR activation-induced hepatomegaly and liver regeneration. TCPOBOP-induced CAR activation on hepatomegaly and liver regeneration was evaluated in wild-type (WT) mice, liver-specific YAP-deficient mice, and partial hepatectomy (PHx) mice. The results demonstrate that TCPOBOP can increase the liver-to-body weight ratio in wild-type mice and PHx mice. Hepatocytes enlargement around central vein (CV) area was observed, meanwhile hepatocytes proliferation was promoted as evidenced by the increased number of KI67+ cells around portal vein (PV) area. The protein levels of YAP and its downstream targets were upregulated in TCPOBOP-treated mice and YAP translocation can be induced by CAR activation. Co-immunoprecipitation results suggested a potential protein–protein interaction of CAR and YAP. However, CAR activation-induced hepatomegaly can still be observed in liver-specific YAP-deficient (Yap–/–) mice. In summary, CAR activation promotes hepatomegaly and liver regeneration partially by inducing YAP translocation and interaction with YAP signaling pathway, which provides new insights to further understand the physiological functions of CAR.
KEY WORDS: Constitutive androstane receptor, Nuclear receptors, Hepatomegaly, Liver enlargement, Liver regeneration, Yes-associated protein, Protein–protein interaction, Partial hepatectomy
Abbreviations: AhR, aryl hydrocarbon receptor; ALB, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; ANKRD1, ankyrin repeat domain 1; AST, aspartate transaminase; CAR, constitutive androstane receptor; CCNA1, cyclin A1; CCND1, cyclin D1; CCNE1, cyclin E1; CITCO, 6-(4-chlorophenyl)imidazo[2,1-b][1,3]thiazole-5-carbaldehyde O-(3,4-dichlorobenzyl)oxime; Co-IP, co-immunoprecipitation; CTGF, connective tissue growth factor; CTNNB1, β-catenin; CV, central vein; CYR61, cysteine-rich angiogenic inducer 61; EGFR, epidermal growth factor receptor; FOXM1, forkhead box M1; FXR, farnesoid X receptor; H&E, haematoxylin and eosin; PHx, partial hepatectomy; PPARα, peroxisome proliferators-activated receptor alpha; PV, portal vein; TBA, total bile acid; TBIL, total bilirubin; TCPOBOP, 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene; TEAD, TEA domain family member; YAP, yes-associated protein
Graphical abstract
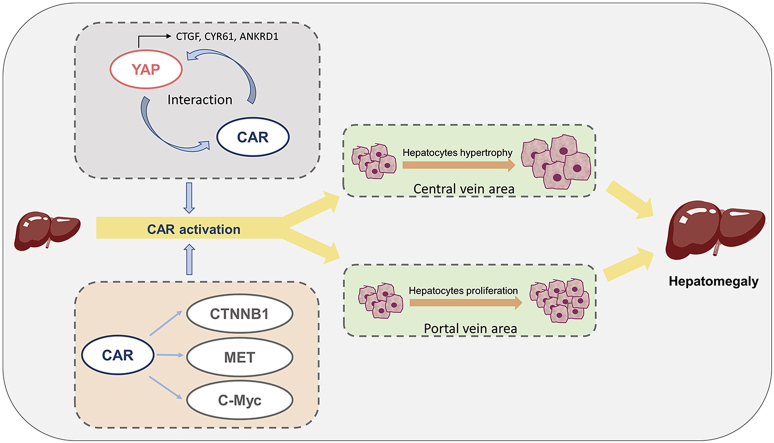
Constitutive androstane receptor (CAR) activation promotes hepatomegaly partially by interaction with yes-associated protein (YAP) signaling pathway, and C-Myc, β-catenin and MET are also involved in CAR-induced hepatomegaly.
1. Introduction
The constitutive androstane receptor (CAR, NR3I1), is a xenobiotic-sensing nuclear receptor, and a member of the nuclear receptor superfamily1. CAR is composed of a DNA-binding domain, a large carboxy-terminal ligand-binding domain, and a poorly conserved amino-terminal domain2. It is localized in the cytoplasm by forming a complex with HSP90 and the cytoplasmic CAR retention protein. Once activated, CAR dissociates from the cytoplasmic complex and is translocated into the nucleus where it forms a heterodimer with the retinoid X receptor alpha (RXRα) and activates the transcription of its downstream targets3.
CAR activates transcription of its target genes encoding various enzymes and proteins involved in drug metabolism and transport when exposed to xenobiotics1,4,5. In addition to xenobiotics, CAR can also regulate the elimination of toxic endobiotics such as bilirubin and bile acids, so one major function of CAR is detoxification6,7. However, recent studies have also unveiled the crucial role of CAR in various physiological and pathophysiological processes in the liver including gluconeogenesis, metabolism of fatty acids, metabolism of lipids, hormonal regulation, proliferation of hepatocytes, and hepatocarcinogenesis8. CAR is also involved in metabolic diseases including jaundice, cholestasis, thyroid homeostasis and obesity9,10.
1,4-Bis[2-(3,5-dichloropyridyloxy)] benzene (TCPOBOP), a classical and specific CAR agonist, was reported to induce robust hepatocyte proliferation and hepatomegaly in mouse livers11. Moreover, the rapid proliferation response induced by TCPOBOP can protect liver from failure even after massive tissue loss by 91% hepatectomy12. The mechanism of TCPOBOP-induced hepatomegaly was studied in previous reports. C-Myc and its downstream target forkhead box protein M1 (FOXM1) are crucial in TCPOBOP-induced direct liver hyperplasia13. β-Catenin (CTNNB1) deficiency decreases the impact of CAR activation on hepatocytes proliferation14. Disruption of epidermal growth factor receptor (EGFR) and MET signaling results in significant impairment of the TCPOBOP-induced proliferation without altering CAR activation15.
Yes-associated protein (YAP), a key downstream factor of Hippo signaling pathway, is a potent regulator of organ size and tissue homeostasis16. When Hippo signaling is ON, YAP is phosphorylated by LATS1/2 and binds with 14-3-3 in cytoplasm. When Hippo signaling is OFF, YAP can be dephosphorylated and translocated into nucleus where it binds with TEA domain family member (TEAD) to activate transcription of genes involved in cell survival, growth, and proliferation17,18. According to previous studies, YAP increases organ size and causes aberrant tissue expansion in mice. YAP activation reversibly increases liver size more than 4-fold19. Removal of Mst1 and Mst2 (the upstream of YAP) in liver, which causes the YAP activation, results in significant liver enlargement and dysplasia20. In addition, YAP also participates in liver regeneration after partial hepatectomy (PHx). The expression of nuclear YAP increased in parallel with hepatocyte proliferative activity after PHx. Deleting Yap from hepatocytes reduced the nuclear accumulation of pSmad2, the EMT-like response and the proliferative response21. Inhibition of MST1 and MST2 augments liver repair and regeneration by activating the downstream effector YAP and promoting cell growth22.
Most recently, YAP/TEAD activation was found to participate in CAR-dependent proliferation of murine hepatocytes23. However, this study used an in vitro cell-based system and an animal experiment of verteporfin (an inhibitor of YAP/TEAD interaction) treatment. The mechanistic influence of CAR activation on YAP signaling has not been clarified and the relationship between CAR and YAP remains unknown. Therefore, the current study aims to elucidate the role of YAP in CAR activation-induced hepatomegaly using liver-specific YAP-deficient mice, to investigate whether YAP is associated with CAR activation-promoted liver regeneration, and further explore the relationship between CAR and YAP.
2. Materials and methods
2.1. Materials
Antibodies used in this study include anti-β-catenin (BD Biosciences, Cat# 610153, RRID: AB_397554), anti-KI67 (Abcam, Cat# ab15580, RRID: AB_443209), anti-YAP (Cell Signaling Technology, Cat# 14074, RRID: AB_2650491), anti-CTGF (Sangon Biotech, Cat# D260212, Shanghai, China), anti-CYR61 (Cell Signaling Technology, Cat# 39382, RRID: AB_2799154), anti-ANKRD1 (Sangon Biotech, Cat# D121628, RRID: AB_2819216), anti-CCNA1 (Sangon biotech, Cat# D220507, RRID: AB_2819214), anti-CCND1 (Sangon biotech, Cat# D220509), anti-CCNE1 (Sangon biotech, Cat# D151593), anti-p-YAP Ser127 (Cell Signaling Technology, Cat# 13008, RRID: AB_2650553), anti-β-actin (Cell Signaling Technology, Cat# 8457, RRID: AB_10950489), anti-lamin B (Cell Signaling Technology, Cat# 13435, RRID: AB_2737428), anti-c-Myc (Cell Signaling Technology, Cat# 9402, RRID: AB_2151827), anti-CTNNB1 (Sangon biotech, Cat# D199519), anti-MET (Sangon biotech, Cat# D160981) and anti-FOXM1 (Proteintech, Cat# 13147-1-AP, RRID: AB_2106213, Rosemont, IL, USA).
2.2. Animal experiment
Male C57BL/6 mice (8- to 9-week-old) were obtained from Guangdong Medical Laboratory Animal Center (Foshan, China). Liver-specific YAP-deficient mice and paired wild-type (WT) mice were generated by Shanghai Model Organisms Center, Inc. (Shanghai, China). Male C57BL/6 mice were injected intraperitoneally with the vehicle corn oil (Aladdin, Cat# C116025, Shanghai, China) or 3 mg/kg TCPOBOP per day (Sigma–Aldrich, Cat# T1443, St. Louis, MO, USA). Liver tissue and serum samples were harvested after 5 and 10 days. WT mice were injected intraperitoneally with vehicle or 3 mg/kg TCPOBOP per day following PHx. Tissue and serum samples were collected at 2 and 5 days after the surgery. Male WT and Yap–/– mice (8- to 9-week-old) were injected intraperitoneally with corn oil or 3 mg/kg TCPOBOP per day and the tissue samples were collected at Day 10. Serum and liver samples were snap frozen in liquid nitrogen, then stored at −80 °C for further use. A portion of liver was immediately fixed in 10% formalin for histological section. The animal treatments were approved by the Institutional Animal Care and Use Committee at Sun Yat-sen University, Guangzhou, China. The entire study stuck to the 3Rs principle of animal experiments, under the guideline of ARRIVE guidelines with respect.
2.3. Histological and biochemical assessment
According to our previous publication24, liver samples were fixed, embedded, sectioned, and then stained by haematoxylin and eosin (H&E staining). Paraffin-embedded sections were stained with β-catenin antibody (BD Biosciences, Cat# 610153, San Jose, CA, USA) and KI67 antibody (Abcam, Cat# ab15580, Cambridge, UK). For CTNNB1 staining quantification, the number of hepatocytes was counted, then the area of the whole visual field was measured by Image J software (National Institutes of Health, Bethesda, USA). After that, the central vein (CV) area was subtracted from whole visual field and the average area of each hepatocyte was calculated. For KI67 staining quantification, the total number of hepatocytes and the number of KI67+ cells were counted, respectively. And then, the percentage of KI67+ cells was calculated.
The levels of serum alanine aminotransferase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), total bile acid (TBA) and total bilirubin (TBIL) were measured by commercially available kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) following the manufacturer's instruction.
2.4. Quantitative real-time PCR analysis
As described in our previous report22, Trizol reagent was used for the extraction of total RNA from the liver. Primer Script RT reagent Kit (Takara, Kusatsu, Shiga, Japan) was used for the synthesis of cDNA. All sequence of primers used for quantitative RNA analysis were listed in Supporting Information. Real-time PCR reactions were performed in Biosystems 7500 using SYBR Premix Ex-Taq II Kit (Takara) according to the manufactory's instructions. The fold changes of mRNA levels were analyzed by ΔΔCt method.
2.5. Western blot analysis
The total proteins were extracted and the levels were determined by Western blot analysis according to our previous procedures22. Briefly, protein samples were separated by 10% SDS-PAGE gels and then transferred onto membranes. After blocking for 1 h, the blots were incubated with different antibodies overnight at 4 °C, followed by secondary anti-rabbit (Cell Signaling Technology, Cat# 7077, RRID: AB_10694715, Danvers, MA, USA) or anti-mouse antibodies (Cell Signaling Technology, Cat# 7076, RRID: AB_330924) at room temperature for 1 h on the next day. The ECL Detection Kit (Millipore, Darmstadt, Germany) was employed to develop the blots. The intensity of the bands was quantified by Quantity One software (Bio-Rad, Hercules, CA, USA).
2.6. Co-immunoprecipitation (Co-IP)
HepG2 cells (ATCC, Cat# HB-8065, RRID: CVCL_0027, VA, USA) were cultured in DMEM containing 10% FBS. Co-immunoprecipitation (Co-IP) was conducted using Thermo Scientific Pierce co-IP kit (Thermo Scientific) as described in our previous report24. Samples were analyzed by Western blot using anti-IgG (Abcam, Cat# ab133470), anti-CAR (Abcam, Cat# ab62590, RRID: AB_956175) and anti-YAP (Cell Signaling Technology, Cat# 14074, RRID: AB_2650491).
2.7. Co-localization assay
According to previous publications24, HepG2 cells (ATCC, Cat# HB-8065, RRID: CVCL_0027) were cultured in DMEM containing 10% FBS and then treated with DMSO or 10 μmol/L CITCO (Sigma–Aldrich, Cat# C6240) for 48 h. After incubation, cells were fixed in 4% paraformaldehyde and 0.5% Triton X-100, respectively. Then, cells were incubated with rabbit polyclonal anti-CAR (Abcam, Cat# ab62590, RRID: AB_956175) and mouse monoclonal anti-YAP (R&D Systems, Cat# MAB8094, RRID: AB_2819224, MN, USA) overnight at 4 °C. Cells were stained with secondary antibodies including anti-mouse IgG Alexa Fluor 488 (Cell Signaling Technology, Cat# 4408, RRID: AB_10694704) and anti-rabbit IgG Alexa Fluor 647 (Cell Signaling Technology, Cat# 4414, RRID: AB_10693544). Images were acquired using a confocal microscope (Olympus FV3000, Tokyo, Japan).
2.8. Statistical analysis
All values were presented as the mean±standard deviation (SD). Two-tailed Student's t tests were used for statistical test by SPSS 23.0 software (IBM Analytics, Armonk, USA) and GraphPad Prism 7.0 software (GraphPad Software, San Diego, CA USA). A P value <0.05 was considered as significantly different.
3. Results
3.1. CAR activation induces hepatomegaly in mice liver
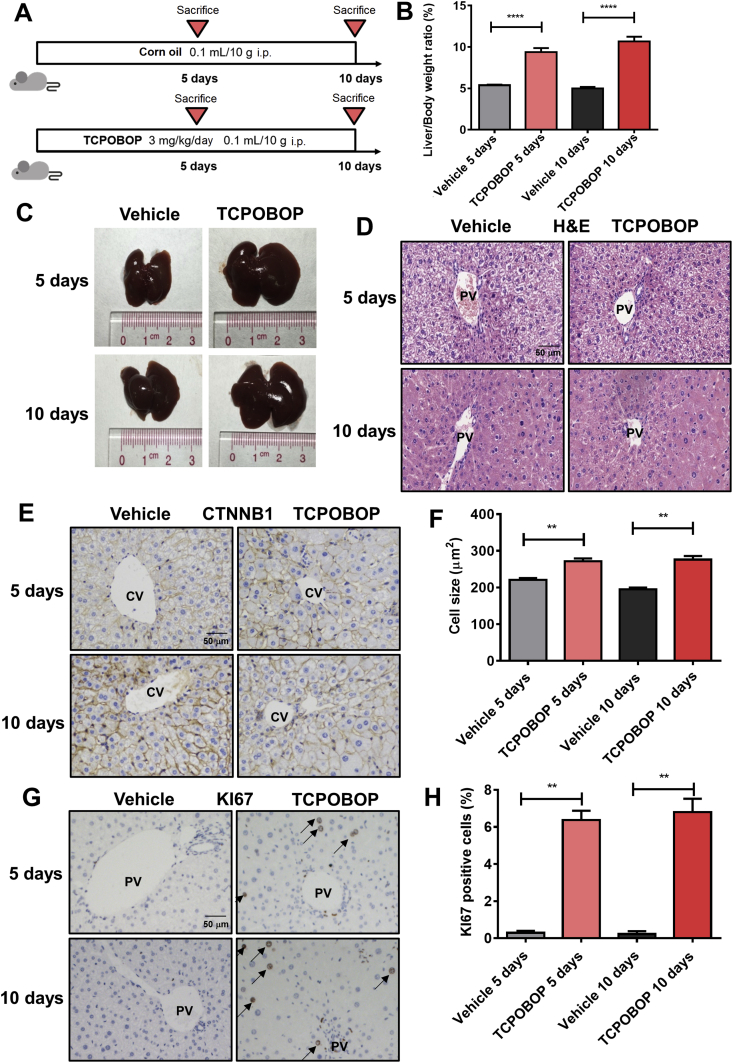
To evaluate the effect of hepatomegaly induced by CAR, mice were treated for 5 or 10 days with the CAR specific agonist TCPOBOP (Fig. 1A). The results show that liver/body weight ratios in TCPOBOP-treated groups were significantly higher (1.74-fold, P<0.0001 after treated for 5 days; 2.14-fold, P<0.0001 after treated for 10 days) than that of the vehicle groups (Fig. 1B). Liver morphology also shows an obvious enlargement both 5 and 10 days after TCPOBOP treatment (Fig. 1C). H&E staining does not reveal significant liver injury (Fig. 1D). Serum ALT, AST and ALP were also examined and no significant difference was observed between the TCPOBOP- and vehicle-treated groups (Supporting Information Fig. S1A), suggesting that the hepatomegaly was not induced by liver injury.
Figure 1.
CAR activation induces hepatomegaly and increase the liver-to-body weight ratio. (A) Male C57BL/6 mice were treated by TCPOBOP for 5 or 10 days. (B) The liver-to-body weight ratio significantly increased in TCPOBOP-treated group. (C) Liver morphology also shows a liver hypertrophy. (D) H&E staining suggests that there was no liver injury. Scale bar = 50 μm. (E) CTNNB1 staining was performed to measure the cell size, which indicated an increase of cell size around CV area. Scale bar = 50 μm. (F) Quantification of CTNNB1 staining in TCPOBOP-treated mice. (G) The expression of KI67 was measured by KI67 staining, the number of KI67+ cells was increased in PV area after treated by TCPOBOP. KI67+ cells were indicated by arrows. Scale bar = 50 μm. (H) Quantification of KI67 staining in TCPOBOP-treated mice. Data are presented as mean±SD, n = 5; ∗∗P<0.01, ∗∗∗∗P<0.0001, significantly different to the control; Student's t test.
It was reported that TCPOBOP can induce hepatocyte proliferation and the increase of hepatocyte cell size13. To characterize the size of hepatocytes, CTNNB1 staining was performed and an increase of cell size around the central vein (CV) area was observed after TCPOBOP-treatment for 5 days (1.23-fold versus vehicle group, P<0.01) or 10 days (1.42-fold versus vehicle group, P < 0.01; Fig. 1E and F), while there was no significant change in cell size around the portal vein (PV) area (Fig. S1B). Expression of KI67 was determined by immunohistochemistry to assess hepatocyte proliferation. The percentage of KI67+ cells around the PV area was dramatically increased after 5 days (21.3-fold versus vehicle group, P<0.01) or 10 days (34.0-fold versus vehicle group, P<0.01) of TCPOBOP treatment, indicating a high level of cell proliferation induced by CAR (Fig. 1G and H). However, the KI67+ cells did not appear around the CV area (Fig. S1C).
3.2. CAR activation promotes liver regeneration after partial hepatectomy
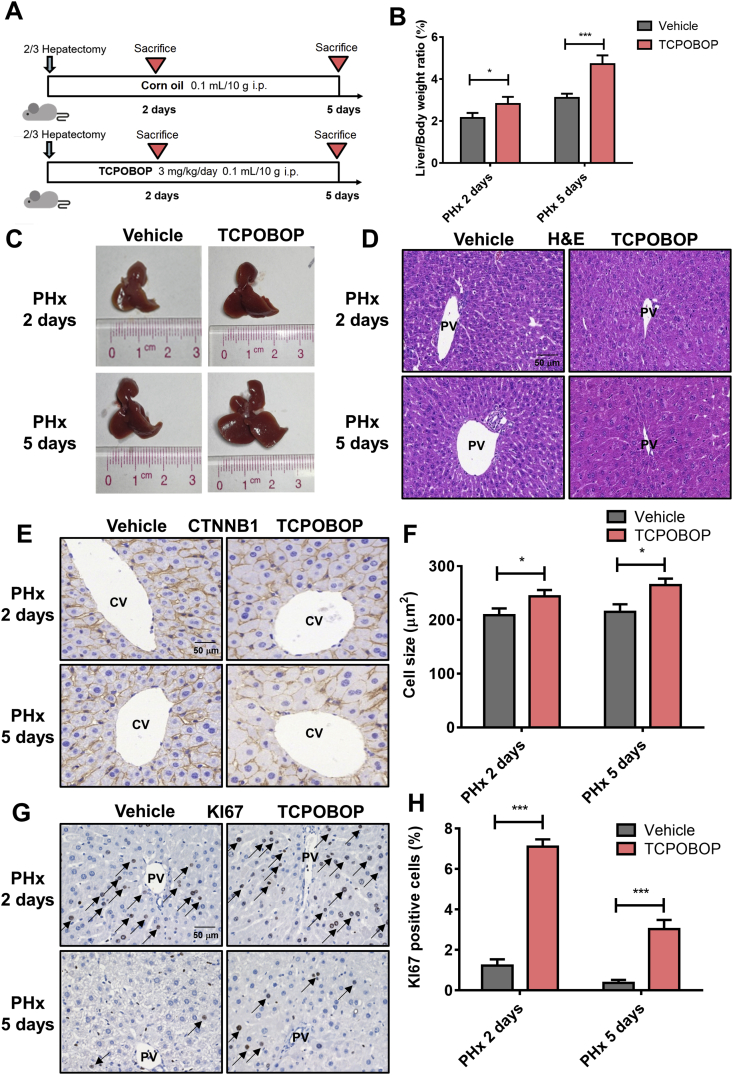
To explore whether CAR activation can promote liver regeneration, a 2/3 hepatectomy model was used and treated with TCPOBOP (Fig. 2A). Significant increase of liver size and liver/body weight ratios (1.33-fold, P<0.05 after treated for 2 days; 1.52-fold, P<0.001 after treated for 5 days) were observed in TCPOBOP-treated group (Fig. 2B and C).
Figure 2.
CAR activation promotes liver regeneration and facilitates the restore of liver weight after partial hepatectomy. (A) Mice after partial hepatectomy were treated with TCPOBOP for 2 or 5 days. (B) The liver-to-body weight ratio was much higher in TCPOBOP-treated group. (C) Liver morphology suggested an increase of liver size. (D) H&E staining suggested that no liver injury occurred. Scale bar = 50 μm. (E) Hepatocyte size was quantified by CTNNB1 staining which showed hepatocytes hypertrophy around CV area. Scale bar = 50 μm. (F) Quantification of CTNNB1 staining in TCPOBOP-treated mice after PHx. (G) The number of KI67+ cells significantly was increased around PV area which indicated an increase of proliferative response. KI67+ cells were indicated by arrows. Scale bar = 50 μm. (H) Quantification of KI67 staining in TCPOBOP-treated mice after PHx. Data are presented as mean±SD, n = 5; ∗P<0.05, ∗∗∗P<0.001, significantly different to the control; Student's t test.
H&E staining revealed no liver injury occurred (Fig. 2D). Serum ALT, AST, TBIL and TBA didn't change significantly after TCPOBOP treatment, indicating normal liver function (Supporting Information Fig. S2A). CTNNB1 and KI67 staining were also performed. Hepatocytes around the CV area showed an enlargement (1.17-fold, P<0.05 after treated for 2 days; 1.23-fold, P<0.05 after treated for 5 days; Fig. 2E, quantification shown in Fig. 2F) which was absent around the PV area (Fig. S2B). The percentage of KI67+ cells was apparently increased around the PV area after treatment with TCPOBOP (5.92-fold, P<0.05 after treated for 2 days; 7.50-fold, P<0.05 after treated for 5 days; Fig. 2G and H) while there was no expression around the CV area (Fig. S2C). These data suggest that CAR activation promotes hepatocyte enlargement around the CV area and proliferation around the PV area, which further facilitates liver regeneration.
3.3. YAP pathway is involved in CAR activation-induced hepatomegaly and liver regeneration
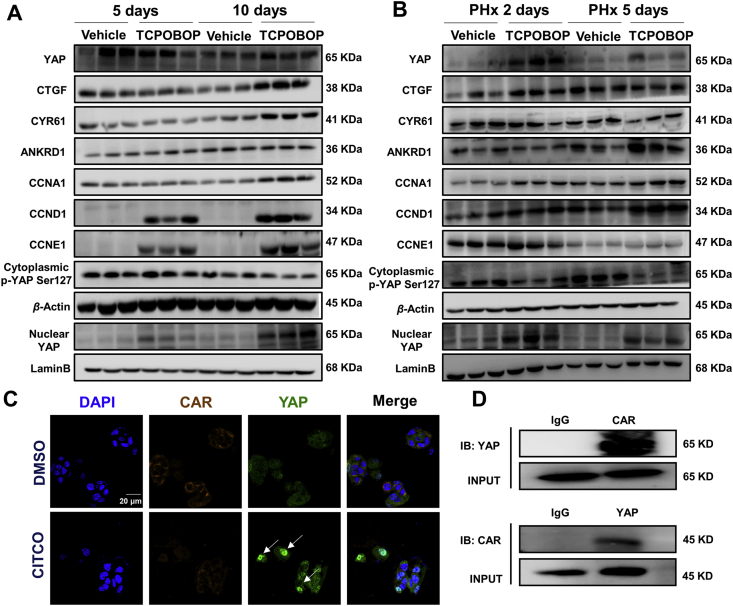
YAP acts as a regulator of organ size and tissue homeostasis16. The role of YAP/TEAD in CAR activation-induced hepatomegaly was also investigated. The protein expression levels of total YAP and its downstream targets CTGF, ANKRD1 and CYR61 were measured in hepatomegaly and liver regeneration models. All of these proteins were upregulated in TCPOBOP-treated mice, especially at Day 10 (Fig. 3A). The proliferation-related proteins such as CCNA1, CCND1 and CCNE1 were all significantly upregulated after TCPOBOP treatment. The upregulation of nuclear YAP and the downregulation of cytoplasmic p-YAP suggested that the YAP pathway was activated in this process (Supporting Information Fig. S3A).
Figure 3.
YAP participates in CAR activation-induced hepatomegaly and liver regeneration. (A) Total YAP, nuclear YAP and YAP downstream targets were all upregulated, while the cytoplasmic p-YAP was downregulated, which indicated the activation of YAP pathway. Proliferation-related proteins were also upregulated after treated by TCPOBOP. (B) In PHx model, total YAP, nuclear YAP and YAP downstream targets CTGF, ANKRD1 were upregulated, cytoplasmic p-YAP was downregulated. Meanwhile proliferation-related proteins such as CCND1 and CCNA1 were upregulated. (C) The intensity of nuclear YAP was much higher after treated by 10 μmol/L CITCO for 48 h in HepG2 cells, indicating that CAR activation promotes the nuclear translocation of YAP. (D) Co-immunoprecipitation results suggested the potential protein-protein interaction between CAR and YAP. Scale bar = 20 μm.
In the PHx mice model, the protein expression levels of YAP and its downstream targets ANKRD1 and CYR61 were upregulated, while CTGF showed a slight increase in TCPOBOP-treated mice. The proliferation-related proteins such as CCNA1, CCND1 and CCNE1 were all significantly upregulated after TCPOBOP treatment, especially at Day 2 after PHx (Fig. 3B). Similarly, the nuclear YAP and the cytoplasmic p-YAP showed the same trend as the hepatomegaly model, which indicated that the YAP pathway was activated in these processes (Fig. S3B).
Nuclear translocation is essential both for CAR and YAP to activate their downstream targets, thus the possible co-localization of CAR and YAP was examined. HepG2 cells were treated with hCAR selective agonist CITCO. The result suggests that the CAR activation enhanced translocation of YAP (Fig. 3C). Co-immunoprecipitation (Co-IP) experiment was then performed to confirm the potential protein–protein interaction of CAR and YAP and a clear interaction between CAR and YAP was observed (Fig. 3D).
3.4. CAR activation-induced hepatomegaly is partially due to YAP signaling
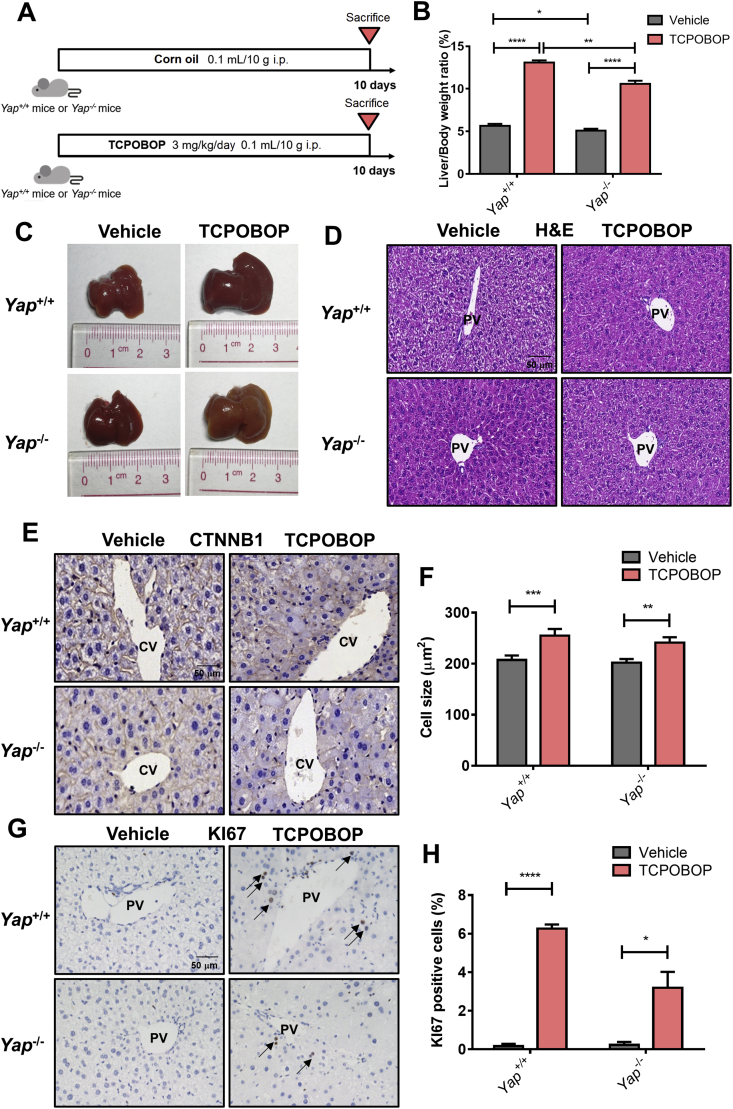
To further explore the role of YAP in TCPOBOP-induced hepatomegaly, Yap–/– mice were used and treated with TCPOBOP for 10 days (Fig. 4A). The liver/body weight ratios still showed an increase in TCPOBOP treated Yap–/– mice (2.08-fold, P<0.0001) compared with the vehicle group, but the average value of liver/body weight ratios in TCPOBOP-treated Yap–/– mice was lower than that of TCPOBOP-treated WT mice (10.6% versus 13.1%, P<0.01), suggesting that YAP may be partially involved in CAR activation-induced hepatomegaly (Fig. 4B). A slight shrinkage of liver was noted in Yap–/– mice in the treated group compared with the counterpart in WT mice (Fig. 4C).
Figure 4.
CAR activation-induced hepatomegaly is partially via YAP pathway. (A) Wild-type or Yap–/– mice were treated by TCPOBOP for 10 days. (B) The liver-to-body weight ratio in TCPOBOP-treated Yap–/– mice was lower than TCPOBOP-treated wild-type mice, but was still much higher than vehicle group of Yap–/– mice. (C) Liver morphology suggested that liver size was increased even in Yap–/– mice. (D) H&E staining suggested that no liver injury occurred. Scale bar = 50 μm. (E) CTNNB1 staining showed that there was still a slight increase of hepatocytes size around CV area in Yap–/– mice. Scale bar = 50 μm. (F) Quantification of CTNNB1 staining in wild-type or Yap–/– mice. (G) The number of KI67+ cells still was increased in Yap–/– mice around PV area. KI67+ cells were indicated by arrows. Scale bar = 50 μm. (H) Quantification of KI67 staining in wild-type or Yap–/– mice. Data are presented as mean±SD, n = 5; ∗P<0.05, ∗∗P<0.01, ∗∗∗∗P<0.0001, significantly different to the control; Student's t test.
Serum ALT, AST and ALP indicated no significant difference between the treatment and vehicle group (Supporting Information Fig. S4A). H&E staining suggests that there was no liver injury (Fig. 4D). The YAP protein and its downstream targets together with proliferation-related proteins were also measured (Fig. S4B and S4C). YAP was totally depleted in Yap–/– mice, YAP downstream targets CTGF, ANKRD1 and proliferation-related protein CCND1 were upregulated in TCPOBOP-treated group both in WT and Yap–/– mice.
Immunohistochemical staining of CTNNB1 and KI67 was conducted revealing that hepatocyte size in the CV area was still enlarged (1.19-fold, P<0.01; Fig. 4E and F), while the number of KI67+ cells was increased in the PV area (16.0-fold, P<0.05) in TCPOBOP-treated Yap–/– mice (Fig. 4G and H), but the effect was weaker compared with TCPOBOP-treated WT mice. CTNNB1 staining in the PV area and KI67 staining in the CV area are shown in Fig. S4D and S4E.
3.5. Other factors are involved in CAR activation-induced hepatomegaly
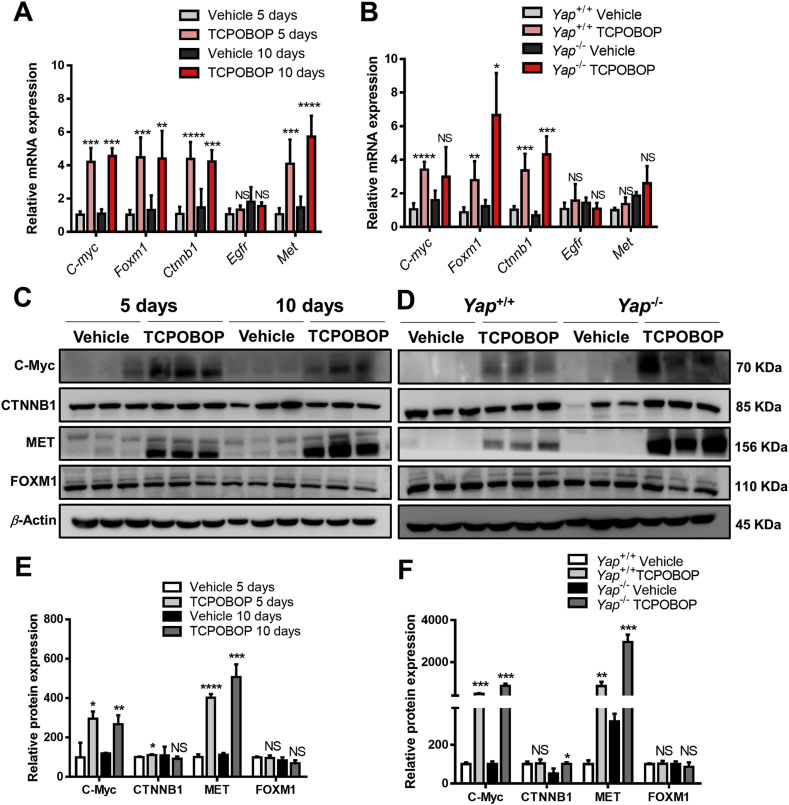
To investigate other possible factors involved in CAR activation-induced liver enlargement, the mRNA expression of C-myc, Foxm1, Ctnnb1, Egfr and Met were measured in TCPOBOP-treated WT mice and Yap–/– mice (Fig. 5A and B). The results suggested that C-myc, Foxm1, Ctnnb1 and Met mRNA were upregulated in TCPOBOP-treated WT mice and TCPOBOP-treated Yap–/– mice. The protein expression levels of C-Myc, CTNNB1 and MET were significantly upregulated in TCPOBOP-treated WT mice and Yap–/– mice, indicating that these signaling pathways also contribute to CAR activation-induced liver enlargement when Yap was absent (Fig. 5C and D). These data suggested that many different pathways were involved in CAR -induced hepatomegaly, the knockout of Yap only relieve the liver enlargement to some extent but cannot totally eliminate the proliferation effect caused by other pathways such as C-Myc, CTNNB1 and MET signaling.
Figure 5.
Other factors are involved in CAR activation-induced hepatomegaly and liver regeneration. (A) The mRNA level of other related genes in wild-type mice treated by TCPOBOP or corn oil for 5 or 10 days. (B) The mRNA level of other related genes in wild-type or Yap–/– mice. (C) The protein levels of C-Myc, FOXM1 and MET were upregulated after treated by TCPOBOP for 5 or 10 days. (D) The protein levels of C-Myc, FOXM1, CTNNB1 and MET were upregulated after treated by TCPOBOP in Yap−/− mice. (E) Quantification of Western blot results in wild-type mice. (F) Quantification of Western blot results in Yap−/− mice. Data are presented as mean±SD, n = 5; ∗P<0.05, ∗∗P<0.01, ∗∗∗P<0.001, ∗∗∗∗P<0.0001, significantly different to control; Student's t test.
4. Discussion
CAR plays a critical role in the control of endogenous and xenobiotic compound metabolism. CAR activation by TCPOBOP induces hepatomegaly and an increase in both hepatocyte growth and proliferation in mice1. Since YAP is a potent factor in the control of liver size, its role in the hepatoproliferative effects of CAR was investigated. Most recently, YAP/TEAD activation was found to play a key role in CAR activation-promoted proliferation in murine hepatocytes23. However, the role of YAP in CAR activation-induced hepatomegaly and liver regeneration, and the relationship between CAR and YAP remains unknown. In the current study, CAR activation significantly promoted YAP translocation and directly interacted with YAP, which consequently promotes hepatomegaly and liver regeneration thus indicating a potential role for YAP in CAR activation-induced hepatomegaly and liver regeneration. These findings provide new insights to further understand the physiological functions of CAR.
It was reported that CAR activation via TCPOBOP can induce robust hepatocyte proliferation and hepatomegaly in mouse livers and promote liver regeneration after liver tissue loss11,12. In the current study, CAR activation-induced hepatomegaly and liver regeneration were confirmed in WT and the PHx mouse model. All mice were male in order to eliminate the gender difference, since CAR can be activated by estrogens, although to a lesser degree than by exogenous ligands such as TCPOBOP8,25. CAR activation-induced hepatomegaly and liver regeneration in this study is mainly caused by hepatocyte enlargement and proliferation. Hepatocytes proliferation can be observed only in the PV area, while the increase of cell size was only shown in the CV area. A possible reason for this phenomenon is that CAR activation induces the expression of enzymes and perhaps the enlargement of subcellular structures, since enzymes related to glycolysis, glycogen synthesis, ketogenesis, lipogenesis, and detoxication are preferentially situated in the CV area24,26.
YAP, a key downstream factor in the Hippo signaling pathway, possesses a critical role in the control of organ size16. A previous study demonstrated that another nuclear receptor, pregnane X receptor (PXR), can also induce liver enlargement and promote liver regeneration via activation of YAP24. Both PXR and CAR belong to the superfamily of nuclear receptors27, suggesting that CAR may activate or interact YAP as well. A previous cell-based study suggested that there is a functional crosstalk between CAR and YAP in the nucleus of hepatocytes, but there is no evidence suggesting a functional relationship between CAR and YAP23. In this study, CAR activation can significantly promote the nuclear translocation of YAP and then upregulate the downstream targets of YAP. Furthermore, Co-IP assays showed a potential interaction of CAR and YAP, which suggested the important role of YAP in CAR activation-induced liver enlargement and regeneration. However, additional evidence such as surface plasmon resonance and isothermal titration calorimetry is needed to confirm their interactions, and to determine the amino acids that lead to the CAR and YAP interaction.
Yap liver-specific knockout mice revealed that CAR activation-induced hepatomegaly was only partially dependent on the YAP pathway, suggesting other regulatory factors may be involved in CAR activation-induced liver enlargement. Many regulators were reported to be involved in CAR activation-induced hepatomegaly such as C-Myc, FOXM1, CTNNB1, EGFR and MET. According to previous reports, the activation of CAR can still promote hepatomegaly in Ctnnb1 knockout mice14. The depletion of C-Myc also only partially restored the enlargement of liver13. Similarly, liver enlargement can still be observed in EGFRi+MET double knockout mice after treated with TCPOBOP for 10 days15. These studies indicated complicated mechanisms in CAR activation-induced liver enlargement and regeneration process. In the current study, the mRNA and protein levels of related factors involved in hepatocytes enlargement and proliferation were measured. In WT mice, the expression of C-Myc and MET were significantly upregulated after treated by TCPOBOP. Interestingly, the level of these proteins was even higher in TCPOBOP-treated Yap–/– mice than WT mice. We hypothesize that these proteins were upregulated to a higher extent to compensate the proliferative response reduced by the loss of Yap. CTNNB1 was not significantly changed consistent with a previous report14, but can be downregulated in Yap–/– mice, and then be induced by the activation of CAR, suggesting that there may be a crosstalk between the CTNNB1 and YAP pathways. On the other hand, a previous study revealed that PXR can also promote hepatomegaly and liver regeneration via YAP pathway. However, the activation of PXR failed to induce hepatomegaly when YAP was depleted, indicating that PXR-induced liver enlargement is totally in a YAP dependent manner24.
YAP downstream targets, such as CTGF and ANKRD1, were upregulated even in Yap–/– mice. We hypothesize that these proteins might be regulated by other factors simultaneously. CTGF belongs to CCN family, which is involved in diverse biological processes such as cell adhesion, proliferation, and angiogenesis. Previous studies reported that CTGF can be regulated by the WNT/β-catenin signaling pathway28,29. Meanwhile, it is also a potential target of WNT and BMP signaling30. ANKRD1 is a potent regulator of early cardiac development, it can be significantly upregulated in cardiac hypertrophy and heart failure31. ANKRD1 can be induced by both the TGF-β and WNT pathways in epithelial cells. The upregulation of ANKRD1 can be observed in models of WNT/β-catenin-induced tumors32. Our results suggest that the β-catenin signaling pathway was activated in Yap–/– mice, and thus CTGF and ANKRD1 were still significantly upregulated in Yap–/– mice.
Cyclin D and cyclin E are two major classes of cyclins during the G1 phase of the cell cycle33. Protein levels of CCND1 and CCNE1 were upregulated significantly at both time points in TCPOBOP-treated WT mice. This suggested that CAR activation induces hepatocyte proliferation by promoting G1-S transition. Since DNA synthesis in hepatocytes was terminated 4–5 days after PHx, the liver mass was restored so the proliferative response was much weaker at Day 524. Thus, in the PHx vehicle group, the expression of CCNE1 was downregulated at Day 5 while CCND1 didn't change significantly.
Liver possesses the ability of regeneration in response to injury. Liver regeneration can be defined as compensatory hyperplasia with the remaining liver tissue expands to meet the metabolic needs of the organism. However, the expanding liver does not regain its original gross anatomical structure. The pathways participated in liver regeneration include growth factors, cytokines and metabolic networks. The most common model is that of liver regeneration following partial hepatectomy, which 3 of 5 liver lobes (2/3 of the liver mass) is removed34. It was reported that CAR deficiency impaired liver regeneration, while the CAR activation by pharmacological means can prevent or rescue the experimental SFSS (small-for-size-syndrome), which is a modified version of extended hepatectomy in mice (eHx, 86% removed). Meanwhile, huCAR mice (transgenic mice bearing a human CAR) developed spontaneous hepatomegaly in response to CITCO. CITCO also improved liver weight gain, steatosis and hyperbilirubinemia in huCAR mice after eHx, suggesting that CAR activation may be effective against human SFSS. CITCO can also mitigate liver failure in huCAR mice and exerts proregenerative and protective effects on human liver slices12. The present study shows that YAP, a critical factor of liver regeneration, was activated in TCPOBOP-promoted liver regeneration in PHx model, which aids the liver to regain its normal functions.
5. Conclusions
The current study demonstrates that CAR activation can promote liver enlargement and liver regeneration partially by inducing YAP translocation and interaction with YAP. These findings provide new insights to further understand the physiological functions of CAR and indicate the potential for manipulation of liver size. CAR can be a potential target to promote liver regeneration after liver surgery and rescue SFSS during the transplantation of liver. The investigation of protein-protein interaction of CAR and YAP may provide therapeutic strategy that can avoid the side effect of CAR activation and promote its clinical use in the future.
Acknowledgments
The work was supported by the Natural Science Foundation of China (Grant numbers: 82025034 and 81973392), the National Key Research and Development Program (Grant number: 2017YFE0109900, China), the Shenzhen Science and Technology Program (Grant number: KQTD20190929174023858, China), the Natural Science Foundation of Guangdong (Grant number: 2017A030311018, China), the 111 project (Grant number: B16047, China), the Key Laboratory Foundation of Guangdong Province (Grant number: 2017B030314030, China), the Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (Grant number: 2017BT01Y093, China), and the National Engineering and Technology Research Center for New drug Druggability Evaluation (Seed Program of Guangdong Province, Grant number: 2017B090903004, China).
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2020.11.021.
Contributor Information
Min Huang, Email: huangmin@mail.sysu.edu.cn.
Huichang Bi, Email: bihchang@mail.sysu.edu.cn.
Author contribution
Huichang Bi and Min Huang conceived and designed the project. Yue Gao, Shicheng Fan, Xinpeng Yao, Ruimin Wang, and Jianing Tian performed the experiments. Hua Li and Shuguang Zhu contributed to the animal models. Yiming Jiang, Xiao Yang, and Frank J. Gonzalez participated in the scientific discussion and research design. Huichang Bi, Yue Gao, and Frank J. Gonzalez wrote and revised the manuscript.
Conflicts of interest
The authors declared there is no competing interest exists.
Appendix ASupplementary data
The following are the Supplementary data to this article:
References
- 1.Konno Y., Negishi M., Kodama S. The roles of nuclear receptors CAR and PXR in hepatic energy metabolism. Drug Metabol Pharmacokinet. 2008;23:8–13. doi: 10.2133/dmpk.23.8. [DOI] [PubMed] [Google Scholar]
- 2.Willson T.M., Kliewer S.A. PXR, CAR and drug metabolism. Nat Rev Drug Discov. 2002;1:259–266. doi: 10.1038/nrd753. [DOI] [PubMed] [Google Scholar]
- 3.Kodama S., Negishi M. Phenobarbital confers its diverse effects by activating the orphan nuclear receptor car. Drug Metab Rev. 2006;38:75–87. doi: 10.1080/03602530600569851. [DOI] [PubMed] [Google Scholar]
- 4.Wei P., Zhang J., Egan-Hafley M., Liang S.G., Moore D.D. The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism. Nature. 2000;407:920–923. doi: 10.1038/35038112. [DOI] [PubMed] [Google Scholar]
- 5.Lv C., Huang L. Xenobiotic receptors in mediating the effect of sepsis on drug metabolism. Acta Pharm Sin B. 2020;10:33–41. doi: 10.1016/j.apsb.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugatani J., Yamakawa K., Yoshinari K., Machida T., Takagi H., Mori M. Identification of a defect in the UGT1A1 gene promoter and its association with hyperbilirubinemia. Biochem Biophys Res Commun. 2002;292:492–497. doi: 10.1006/bbrc.2002.6683. [DOI] [PubMed] [Google Scholar]
- 7.Eloranta J.J., Kullak-Ublick G.A. Coordinate transcriptional regulation of bile acid homeostasis and drug metabolism. Arch Biochem Biophys. 2005;433:397–412. doi: 10.1016/j.abb.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 8.Kachaylo E.M., Pustylnyak V.O., Lyakhovich V.V., Gulyaeva L.F. Constitutive androstane receptor (CAR) is a xenosensor and target for therapy. Biochemistry (Mosc) 2011;76:1087–1097. doi: 10.1134/S0006297911100026. [DOI] [PubMed] [Google Scholar]
- 9.Dash A.K., Yende A.S., Kumar S., Singh S.K., Kotiya D., Rana M. The constitutive androstane receptor (CAR): A nuclear receptor in health and disease. J Endocrinol Reprod. 2014;18:59–74. [Google Scholar]
- 10.Qatanani M., Moore D.D. CAR, the continuously advancing receptor, in drug metabolism and disease. Curr Drug Metabol. 2005;6:329–339. doi: 10.2174/1389200054633899. [DOI] [PubMed] [Google Scholar]
- 11.Costa R.H., Kalinchenko V.V., Tan Y.J., Wang I.C. The CAR nuclear receptor and hepatocyte proliferation. Hepatology. 2005;42:1004–1008. doi: 10.1002/hep.20953. [DOI] [PubMed] [Google Scholar]
- 12.Tschuor C., Kachaylo E., Limani P., Raptis D.A., Linecker M., Tian Y. Constitutive androstane receptor (CAR)-driven regeneration protects liver from failure following tissue loss. J Hepatol. 2016;65:66–74. doi: 10.1016/j.jhep.2016.02.040. [DOI] [PubMed] [Google Scholar]
- 13.Blanco-Bose W.E., Murphy M.J., Ehninger A., Offner S., Dubey C., Huang W. C-Myc and its target FoxM1 are critical downstream effectors of constitutive androstane receptor (CAR) mediated direct liver hyperplasia. Hepatology. 2008;48:1302–1311. doi: 10.1002/hep.22475. [DOI] [PubMed] [Google Scholar]
- 14.Ganzenberg K., Singh Y., Braeuning A. The time point of beta-catenin knockout in hepatocytes determines their response to xenobiotic activation of the constitutive androstane receptor. Toxicology. 2013;308:113–121. doi: 10.1016/j.tox.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Bhushan B., Stoops J.W., Mars W.M., Orr A., Bowen W.C., Paranjpe S. TCPOBOP-induced hepatomegaly and hepatocyte proliferation are attenuated by combined disruption of MET and EGFR signaling. Hepatology. 2019;69:1702–1718. doi: 10.1002/hep.30109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kowalik M.A., Saliba C., Pibiri M., Perra A., Ledda-Columbano G.M., Sarotto I. Yes-associated protein regulation of adaptive liver enlargement and hepatocellular carcinoma development in mice. Hepatology. 2011;53:2086–2096. doi: 10.1002/hep.24289. [DOI] [PubMed] [Google Scholar]
- 17.Patel S.H., Camargo F.D., Yimlamai D. Hippo signaling in the liver regulates organ size, cell fate, and carcinogenesis. Gastroenterology. 2017;152:533–545. doi: 10.1053/j.gastro.2016.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao C., Zeng C., Ye S., Dai X., He Q., Yang B. Yes-associated protein (YAP) and transcriptional coactivator with a PDZ-binding motif (TAZ): A nexus between hypoxia and cancer. Acta Pharm Sin B. 2020;10:947–960. doi: 10.1016/j.apsb.2019.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camargo F.D., Gokhale S., Johnnidis J.B., Fu D., Bell G.W., Jaenisch R. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 20.Song H., Mak K.K., Topol L., Yun K., Hu J., Garrett L. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc Natl Acad Sci U S A. 2010;107:1431–1436. doi: 10.1073/pnas.0911409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh S.H., Swiderska-Syn M., Jewell M.L., Premont R.T., Diehl A.M. Liver regeneration requires Yap1-TGFb-dependent epithelial-mesenchymal transition in hepatocytes. J Hepatol. 2018;69:359–367. doi: 10.1016/j.jhep.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan F., He Z., Kong L.L., Chen Q., Yuan Q., Zhang S. Pharmacological targeting of kinases MST1 and MST2 augments tissue repair and regeneration. Sci Transl Med. 2016;8:352ra108. doi: 10.1126/scitranslmed.aaf2304. [DOI] [PubMed] [Google Scholar]
- 23.Abe T., Amaike Y., Shizu R., Takahashi M., Kano M., Hosaka T. Role of YAP activation in nuclear receptor CAR-mediated proliferation of mouse hepatocytes. Toxicol Sci. 2018;165:408–419. doi: 10.1093/toxsci/kfy149. [DOI] [PubMed] [Google Scholar]
- 24.Jiang Y., Feng D., Ma X., Fan S., Gao Y., Fu K. Pregnane X receptor regulates liver size and liver cell fate by Yes-associated protein activation in mice. Hepatology. 2019;69:343–358. doi: 10.1002/hep.30131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Min G., Kim H., Bae Y., Petz L., Kemper J.K. Inhibitory cross-talk between estrogen receptor (ER) and constitutively activated androstane receptor (CAR). CAR inhibits ER-mediated signaling pathway by squelching p160 coactivators. J Biol Chem. 2002;277:34626–34633. doi: 10.1074/jbc.M205239200. [DOI] [PubMed] [Google Scholar]
- 26.Jungermann K., Katz N. Functional specialization of different hepatocyte populations. Physiol Rev. 1989;69:708–764. doi: 10.1152/physrev.1989.69.3.708. [DOI] [PubMed] [Google Scholar]
- 27.Chai X., Zeng S., Xie W. Nuclear receptors PXR and CAR: Implications for drug metabolism regulation, pharmacogenomics and beyond. Expet Opin Drug Metabol Toxicol. 2013;9:253–266. doi: 10.1517/17425255.2013.754010. [DOI] [PubMed] [Google Scholar]
- 28.Li Z.Q., Ding W., Sun S.J., Li J., Pan J., Zhao C. Cyr61/CCN1 is regulated by Wnt/beta-Catenin signaling and plays an important role in the progression of hepatocellular carcinoma. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deng Y.Z., Chen P.P., Wang Y., Yin D., Koeffler H.P., Li B.J. Connective tissue growth factor is overexpressed in esophageal squamous cell carcinoma and promotes tumorigenicity through beta-catenin-T-cell factor/Lef signaling. J Biol Chem. 2007;282:36571–36581. doi: 10.1074/jbc.M704141200. [DOI] [PubMed] [Google Scholar]
- 30.Luo Q., Kang Q., Si W.K., Jiang W., Park J.K., Peng Y. Connective tissue growth factor (CTGF) is regulated by Wnt and bone morphogenetic proteins signaling in osteoblast differentiation of mesenchymal stem cells. J Biol Chem. 2004;279:55958–55968. doi: 10.1074/jbc.M407810200. [DOI] [PubMed] [Google Scholar]
- 31.Kojic S., Nestorovic A., Rakicevic L., Belgrano A., Stankovic M., Divac A. A novel role for cardiac ankyrin repeat protein Ankrd1/CARP as a co-activator of the p53 tumor suppressor protein. Arch Biochem Biophys. 2010;502:60–67. doi: 10.1016/j.abb.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 32.Labbe E., Lock L., Letamendia A., Gorska A.E., Gryfe R., Gallinger S. Transcriptional cooperation between the transforming growth factor-beta and wnt pathways in mammary and intestinal tumorigenesis. Cancer Res. 2007;67:75–84. doi: 10.1158/0008-5472.CAN-06-2559. [DOI] [PubMed] [Google Scholar]
- 33.Geng Y., Whoriskey W., Park M.Y., Bronson R.T., Medema R.H., Li T.S. Rescue of cyclin D1 deficiency by knockin cyclin E. Cell. 1999;97:767–777. doi: 10.1016/s0092-8674(00)80788-6. [DOI] [PubMed] [Google Scholar]
- 34.Mao S.A., Glorioso J.M., Nyberg S.L. Liver regeneration. Transl Res. 2014;163:352–362. doi: 10.1016/j.trsl.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.