Abstract
Human respiratory virus infections lead to a spectrum of respiratory symptoms and disease severity, contributing to substantial morbidity, mortality and economic losses worldwide, as seen in the COVID-19 pandemic. Belonging to diverse families, respiratory viruses differ in how easy they spread (transmissibility) and the mechanism (modes) of transmission. Transmissibility as estimated by the basic reproduction number (R0) or secondary attack rate is heterogeneous for the same virus. Respiratory viruses can be transmitted via four major modes of transmission: direct (physical) contact, indirect contact (fomite), (large) droplets and (fine) aerosols. We know little about the relative contribution of each mode to the transmission of a particular virus in different settings, and how its variation affects transmissibility and transmission dynamics. Discussion on the particle size threshold between droplets and aerosols and the importance of aerosol transmission for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and influenza virus is ongoing. Mechanistic evidence supports the efficacies of non-pharmaceutical interventions with regard to virus reduction; however, more data are needed on their effectiveness in reducing transmission. Understanding the relative contribution of different modes to transmission is crucial to inform the effectiveness of non-pharmaceutical interventions in the population. Intervening against multiple modes of transmission should be more effective than acting on a single mode.
Subject terms: Influenza virus, Viral transmission, SARS-CoV-2, Policy and public health in microbiology, Epidemiology
In this Review, Leung provides an overview of the transmissibility and modes of transmission of respiratory viruses, the viral, host and environmental determinants of transmission, and common non-pharmaceutical interventions for mitigating respiratory virus transmission. She also discusses the recent controversies over aerosol transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19.
Introduction
Human respiratory viruses include a broad range of viruses that infect cells of the respiratory tract, elicit respiratory and other symptoms, and are transmitted mainly by respiratory secretions of infected persons. Respiratory virus infections often cannot be differentiated clinically. Respiratory viruses belong to diverse virus families that differ in viral and genomic structures, populations susceptible to infection, disease severity, seasonality of circulation, transmissibility and modes of transmission. Together, they contribute to substantial morbidity1, mortality2 and concomitant economic losses3 annually worldwide. In addition, occasional pandemics cause extreme disruption to societies and economies as exemplified by the current COVID-19 pandemic. Until effective treatments or vaccines for COVID-19 are available, we have to rely heavily on population-based and individual-based public health measures to mitigate transmission. The effectiveness and the suitability of a non-pharmaceutical intervention (NPI) to mitigate transmission depends substantially on the ease of transmission (transmissibility) and the mechanism of transmission (modes of transmission) specific to that virus, as these interventions can target some but not all potential modes of transmission. Therefore, understanding how to evaluate the transmissibility and evidence supporting different modes of transmission will aid in the control of respiratory virus transmission.
Previous reviews and commentaries discussed the transmissibility of influenza virus4,5; methods for studying transmission, including animal models4–7, human models6,8 and epidemiological studies9; the mechanism and evidence for different modes of transmission4,6,7,9–12; factors affecting transmission4,5,11,13; controversies regarding the relative importance of different modes of transmission14,15; pharmaceutical interventions4 and NPIs11,16–18 for mitigating transmission16–19; and guidelines from public health agencies on infection prevention and control recommendations for respiratory viruses9,15. These various aspects of transmissibility and transmission have been more comprehensively studied for influenza virus4–7,10,14,17,19 than for other respiratory viruses9,11,12,15,16,18. In this Review, I will bring these discussions together to provide a broad overview of the transmissibility and modes of transmission of respiratory viruses, the approaches used to make these assessments, the viral, host and environmental determinants of transmission, and common NPIs for mitigating respiratory virus transmission, in the hope of illustrating the common approaches for studying respiratory virus transmission as well as the interconnection and differences between these discussions. I also discuss recent controversies regarding the role of aerosols in transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and the difficulties in evaluating the relative contribution of each mode to the transmission of respiratory viruses.
Transmissibility
In the control of a novel pandemic, one of the most important early questions is how easily the disease will spread from an infected person to a susceptible person; that is, how transmissible the disease is. Transmissibility is determined by the infectivity of the pathogen, the contagiousness of the infected individual, the susceptibility of the exposed individual, the contact patterns between the infected individual and the exposed individual, and the environmental stress exerted on the pathogen during transmission. These will determine the scale and intensity of control measures needed to suppress transmission. In animal models, volunteer transmission studies, modelling studies and observational as well as interventional epidemiological studies, although the number of successful transmission events (that is, infection in the exposed individual) is often used as an outcome measure, these study designs answer different research questions when evaluating the transmissibility of a respiratory virus.
Evaluating transmissibility in animals and volunteers
Animal models are often used to compare the transmissibility of respiratory viruses with different naturally occurring or engineered genomic constructs to identify viral molecular determinants of increased transmissibility, or to compare the transmissibility between different modes of transmission (Box 1). For example, for influenza virus, animal transmission studies have been used to evaluate the molecular determinants of transmissibility20, the airborne transmission potential of emerging viruses21 or drug-resistant viruses22, the relative importance of droplets and aerosols to transmission23 and the anatomical site that drives the different routes of transmission24. Alternatively, volunteer transmission studies, where transmission is observed in susceptible volunteers who are exposed to other volunteers who are either experimentally or naturally infected8, may be used to provide important information on the effectiveness of interventions and the importance of presymptomatic or asymptomatic transmission in a controlled setting25. However, these studies can be challenging and expensive to conduct, and may be criticized as too artificial8.
Box 1 Evaluating transmissibility in animal transmission studies.
In the simplest form of animal transmission studies, naive (‘infector’ or ‘donor’) animals are first inoculated with a candidate virus, with infection confirmed by the recovery of virus in respiratory specimens; other naive (‘infectee’ or ‘contact’) animals are then co-housed with the infected animals for some period to observe whether the contact animal subsequently acquires infection. The number of donor and contact animals used may vary, or multiple donor–contact pairs of animals are used, and transmissibility is assessed by counting the proportion of contact animals infected204. This basic design can be adjusted to study transmissibility for different modes of transmission: direct contact transmission studies are done by co-housing donor and contact animals in the same cage to allow all major modes of transmission to occur; airborne transmission studies are done by physically separating the donor and contact animals usually by an air-permeable barrier, with some distance between the cages or no distance, so only aerosol route, with or without the droplet route, of transmission can occur205; and fomite transmission studies are done by putting contact animals in the cage that was previously used to house the donor animals108. Depending on the specific respiratory virus studied, costs, availability of reagents and desired pathogenesis, different animal models are available for transmission studies204,206,207, including mice, guinea pigs, ferrets, Syrian hamsters and non-human primates. Transgenic or transduced mouse models may also be used to better mimic human pathogenesis when access to non-human primates is limited; for example, transgenic mice expressing the human ACE2 receptor as a model for coronavirus infection208. In animal transmission studies, it has been suggested that a minimum of four pairs of donor–contact animals per group is required for sufficient power to detect differences in transmissibility between experimental groups209, and this is usually considered as an accepted number to report in studies of influenza virus210.
Evaluating transmissibility in the population
Mathematical or statistical models are often used to estimate transmissibility of a respiratory virus in the population, especially during pandemics to assess the extent of transmission. With use of data from surveillance, observational and interventional epidemiological studies, or simulation from modelling studies, transmissibility is usually assessed by the estimation of the basic reproduction number (R0) or secondary attack rate (SAR) (Box 2). In addition, by comparing the two simultaneously, one can assess the role of specific populations (for example, households or schools)26 or superspreading events27 in driving community transmission.
Box 2 Basic reproduction number and secondary attack rate.
Basic reproduction number
The reproduction number (denoted as R) is defined as the average number of successful transmissions per infectious individual in a population and can be estimated from mathematical models that describe the natural history of disease. The effective reproduction number (Rt) represents R at any time (t) during an epidemic. The basic reproduction number (R0) is R at the start of an epidemic and represents the average number of secondary infections caused by a primary infection after its introduction to a completely susceptible population211. Therefore, R0 is an important quantity that reflects the capacity of a virus to be transmitted (that is, transmissibility) and will inform the potential ease or difficulty in controlling transmission of the disease. Reported estimates of R0 were heterogenous between viruses and even for the same virus: R0 for rhinovirus212,213, parainfluenza virus212 and adenovirus212,214 was usually slightly above 1 to 5, R0 for coronaviruses213,215 could be up to 8 (also based on a recent preprint213), whereas R0 for respiratory syncytial virus212,213, influenza viruses216, varicella zoster virus217 and measles virus218 may be as low as around 1 but could go up to above 5 or even above 10 (Table 1). Advanced model structures (for example, ‘susceptible–exposed–infectious–recovered’) or further compartmentation (for example, age, contact patterns or vaccination status) allow a more complex description of disease or transmission dynamics (for example, presymptomatic transmission95, asymptomatic infection, waning immunity219 or seasonality or contact patterns220), the prediction of impacts of interventions, or identifying the key factors required for such predictions221. Incorporation of phylogenetic data into epidemiological models has identified important factors that drive influenza virus transmission at the population level222,223 and evaluated the transmissibility of emerging severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants224. Furthermore, the effect of superspreading events on transmission may be described by a dispersion parameter (k), where for a disease with low dispersion most secondary infections are from only a small number of the most infectious individuals225,226, and estimating ‘individual-based’ R instead of ‘population-based (mean)’ R could account for individual variations in contagiousness225. Therefore, R0 takes on different values for different populations and scenarios216–218 and whether superspreading events are considered227, and comparing transmissibility between respiratory viruses directly on the basis of R0 estimated from different studies will be challenging. An important application of R0 is determining the herd immunity threshold needed for an epidemic to end228. During an epidemic when Rt < 1, which occurs when the proportion of immune individuals in the population reaches the herd immunity threshold, transmission decreases over time and the epidemic eventually ends. The modes of transmission and their virus, host and environmental determinants211 influence transmissibility by modulating how effective the contact allows transmission, once contact between susceptible and infectious individuals is established.
Secondary attack rate
The secondary attack rate (SAR) is defined as the proportion infected among those susceptible in contact with the primary case229. Some suggested calling it ‘secondary infection risk’, as the quantity refers to a proportion and not a rate230, because infection may not necessarily lead to symptomatic illness, and ‘symptomatic secondary infection risk’ could be used instead when one is referring to the risk of symptomatic infection231. SAR is most frequently used to estimate the transmission risk in households232,233, and sometimes in outbreaks if the index (primary) cases introducing the infection are known and supplemented with contact tracing27,234; that is, case-ascertained studies where exposed individuals are followed up to observe them for infection once a primary case is ascertained235, either by identifying symptomatic illness or by systematic collection of a respiratory or serum specimen regardless of symptoms for laboratory-confirmed infection. The proportion of exposed household contacts with infection (that is, the household SAR) is then used to describe the transmission risk from the index member to household members. Similarly to R0, reported estimates of household SAR were heterogenous, ranging between 1% and 38% for influenza virus230, and estimates were lower if infections were ascertained only in contacts with symptomatic illness excluding asymptomatic infections231,236,237, or if only laboratory-confirmed illnesses were included238. For other respiratory viruses, household SARs for respiratory syncytial virus237,239 and coronaviruses240 generally fall in a similar range as for influenza virus, and are higher for rhinovirus238, parainfluenza virus237,241, varicella zoster virus232,242 and measles virus232,243 (Table 1), but direct comparison between viruses is again challenging. Case-ascertained studies can be used to evaluate factors affecting transmission — for example, virus type/subtype, age, types of contact244, asymptomatic or presymptomatic transmission244,245 and pre-infection immunity237,238,242,245 — or the effectiveness of interventions, such as the postexposure prophylaxis use of antivirals246 and non-pharmaceutical interventions125, and vaccine efficacy247. Individual-based hazard models and Bayesian Markov chain Monte Carlo techniques can account for multiple index cases, unobserved transmission or multiple covariates, for example, incorporating symptom onset data to estimate transmission risk within households versus outside households248, or viral shedding data to estimate the effectiveness of face masks and hand hygiene in reducing transmission249.
Modes of transmission
Respiratory viruses are transmitted between individuals when the virus is released from the respiratory tract of an infected person and is transferred through the environment, leading to infection of the respiratory tract of an exposed and susceptible person. There are a number of different routes (or modes) through which transmission could occur, the chance of which is modified by viral, host and environmental factors. Although there is evidence in support of individual modes of transmission, the relative contribution of different modes to a successful transmission event, and the relative effect of each factor on each mode or multiple modes simultaneously, is often unknown.
Direct contact, indirect contact, droplet and aerosol
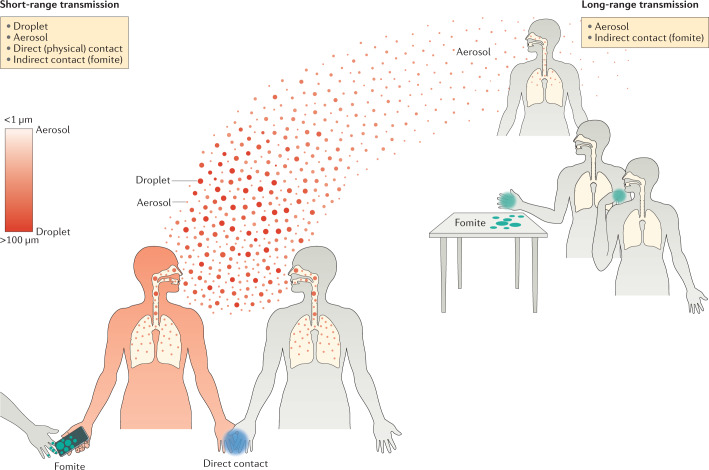
Respiratory viruses can be transmitted via respiratory secretions over multiple routes independently and simultaneously. Traditionally, it is believed that respiratory viruses are transmitted directly via physical contact between an infected individual (infector) and a susceptible individual (infectee), indirectly via contact with contaminated surfaces or objects (fomites) or directly through the air from one respiratory tract to another via large respiratory droplets or via fine respiratory aerosols6,7 (Fig. 1). These four major modes of transmission (direct contact, indirect contact/fomite, droplet and aerosol) are often the foci of transmission control; for example, infection prevention and control measures in health-care settings are designed specifically for each mode28. Some respiratory viruses, including influenza viruses, coronaviruses and rhinoviruses, can be recovered from faeces29,30 or infect cells in the gastrointestinal tract29, suggesting infection may spread via faeces; for example, via aerosolization during toilet flushing31. Studies have shown SARS-CoV-2 in ocular secretions32 and influenza virus infection by ocular exposure33, suggesting respiratory viruses might also be transmitted via exposure to the eyes.
Fig. 1. Major modes of transmission of respiratory viruses during short-range and long-range transmission.
During an acute respiratory virus infection, an infected individual (infector; red) may shed virus in exhaled breath droplets and aerosols, and may also contaminate their immediate bodily surfaces (for example, skin and clothes) or surrounding objects and surfaces (for example, tables) with their respiratory secretions. In general, if a susceptible individual (infectee; grey) is close to the infector, short-range transmission may occur when the infectee breathes in the virus-laden droplets or aerosols released by the infector, during direct (physical) contact with the infector or during physical contact with objects or surfaces contaminated (fomite) by the infector. If the infectee is at a distance from the infector, long-range transmission may occur when the infectee breathes in the virus-laden aerosols released by the infector or during physical contact with a fomite. However, the terminology and the defining features of each mode of respiratory virus transmission, especially regarding redefining the particle size threshold between droplets and aerosols, is under active discussion (see the section Terminology and defining features of modes of transmission).
Terminology and defining features of each mode of transmission
The lack of standardization of terminology and the defining features of each major mode of respiratory virus transmission, in particular the difficulty to differentiate between ‘droplets’ and ‘aerosols’, has caused much confusion34. Although the direct contact route traditionally refers to transmission via direct physical contact between infectors and infectees6,7,9, some consider exposure to infectious (large) droplets as an additional form of contact transmission28,35,36, sometimes using the term ‘droplet contact’ to describe transmission via droplets37 and ‘direct contact’35 or ‘close contact’36 to describe transmission via both physical contact and exposure to droplets. The WHO uses ‘direct, indirect, or close contact’ to describe ‘contact and droplet transmission’, although it is unclear whether ‘close contact’ refers to transmission via droplets alone, direct (physical) contact alone or both38,39. Some attempted to define ‘close contact transmission’ by proposing three subroutes as ‘short-range airborne’, ‘large droplets’ and ‘immediate body-surface contact’ to describe transmission in close proximity, where the last refers to an infectee in contact with the infector’s immediate contaminated bodily surfaces (for example, skin and clothes), and is to be distinguished from the (distant) fomite route, which involves delayed and less frequent touching from a greater distance40. Some use ‘airborne’ to describe transmission via droplets and aerosols as both can travel through the air9, whereas others use it to describe transmission via aerosols only6,12,28,39,41.
In the 1930s, William F. Wells, who studied air bacteriology and the transmission of respiratory tuberculosis, proposed that the particle size of exhaled respiratory droplets influences how they are transported in the air, and could be classified as ‘aerosols’ or ‘droplets’, with different implications to disease transmission42 (Box 3). Subsequently, animal studies, experimental volunteer studies and observational epidemiological studies were conducted to study the transmission of respiratory syncytial virus (RSV), rhinovirus and influenza virus in homes and health-care settings43,44. In recent years, on the basis of different aspects of particle behaviours45, various particle size cut-offs, often in the range between 5 and 20 µm, have been used to differentiate particles as ‘aerosols’ or ‘droplets’. For example, the cut-off at 5 µm used by many regulatory bodies35,38 is based on early studies of pulmonary tuberculosis that believed particles smaller than 5 µm would deposit in the pulmonary/alveolar region of the lung by settlement and initiate infection, whereas particles larger than 5 µm deposit in the nasal cavity by centrifugal force28,46,47. Other studies put similar emphasis on the region of particle deposition in the human respiratory tract, and suggested that particles smaller than 10 µm reach and deposit in the pulmonary region12,45,48,49, and particles of size between 10 and 100 µm are inspired but deposit in the head airways or tracheobronchial regions49,50. Some consider particles smaller than 20 µm important for aerosol transmission as particles of 20 µm were estimated to take 4 minutes to settle on the ground from a height of 3 m, whereas particles smaller than 3 µm practically do not settle due to regular resuspension by air currents51.
A recent workshop on the airborne transmission of SARS-CoV-2 suggested referring to ‘aerosols’ as a stable suspension of solid and/or liquid particles in air, with a particle size cut-off set to an order of magnitude larger than previously thought (that is, ~30–100 µm), and ‘droplets’ as liquid particles that are larger than aerosols52. Some argued such dichotomization based on particle size alone has not accounted for the influence of expiratory activities on particle behaviour and proposed a turbulent gas cloud model to describe exhaled particle behaviour53. The model describes exhaled air as primarily a multiphase turbulent gas cloud (‘puff’) that consists of clusters of exhaled particles mixed with ambient air, where the particles have sizes on a continuous scale and are carried forwards by the momentum of expiratory activities53. The behaviour of exhaled particles is then influenced by conditions of both the ambient and the exhaled air, such as composition, temperature, humidity and airflow, highlighting the chance of larger droplets to be propelled further away than would be expected on the basis of particle size alone53,54.
Box 3 Initial recognition of the importance of aerosols in disease transmission.
Engineers coined the term ‘aerosol’ in the 1920s and defined it as a two-phase system consisting of a collection of solid or liquid particles and the gas in which they are suspended, exemplified by a wide range of products from combustion processes or meteorological phenomena such as dust, fume, mist and clouds, with particle size ranges from about 0.002 µm to more than 100 µm (in aerodynamic diameter)48. A related term, ‘bioaerosol’, describes aerosols of biological origin such as viruses, bacteria, fungi, fungal spores and pollen48. The term ‘aerosol’ is commonly used when ‘bioaerosol’ is actually meant; for example, the transmission of virus-laden aerosol particles is referred to as ‘aerosol transmission’. In the 1930s William F. Wells, who studied air bacteriology and the transmission of respiratory tuberculosis, proposed the differences between droplets and aerosols and their implications for transmission42. Wells suggested respiratory droplets expelled from the nose or mouth undergo evaporation, with smaller droplets evaporating almost immediately, while larger droplets (which may also be referred to as ‘drops’34) settle to the ground rapidly without much evaporation42. He therefore hypothesized that “Transmission of infection through the air may therefore take one of two forms, depending on the size of the infected droplet. The more obvious form, recognized by Flügge, is droplet infection proper. It applies to droplets larger than 0.1 mm in diameter, which are rapidly removed from the air by gravity before they can dry and within a short distance from the source. The second form may be called air-borne infection and deals with the dried residues of infected droplets, or droplet nuclei, derived directly from droplets less than 0.1 mm in diameter, depending primarily on air for the buoyancy that keeps them suspended for longer times and carries them longer distances”250. In the 1940s he subsequently demonstrated the use of ultraviolet germicidal irradiation to prevent airborne transmission of measles in schools196, and in the 1950s together with Richard L. Riley demonstrated the airborne transmission of tuberculosis168.
Increased concern for aerosol transmission
To many, bioaerosols (Box 3) pose particular concerns in transmission control. As bioaerosols can remain airborne for a prolonged period and travel through the air over long distances55–60, this potentiates disease transmission over long distances and therefore requires additional measures (for example, larger spatial separation between hospital beds28). Individuals with higher bioaerosol production might contribute to superspreading events61,62. Some hypothesize that the propensity of aerosol deposition in the lower respiratory tract45 might require a lower infectious dose and lead to severer disease. Volunteer challenge studies showed that influenza virus and adenovirus infection initiated by the inhalation of infectious bioaerosols required a lower infectious dose51,63,64, whereas rhinovirus might require a similar or a higher infectious dose51. Infection initiated by inhaling infectious influenza virus bioaerosols led to higher risk of fever compared with intranasal inoculation65, although some studies alternatively suggested that infection initiated by droplets leads to severer disease due to a higher infectious dose66. Alternatively, a narrower bottleneck of the minimum infectious virions required for transmission via aerosols may reduce virus diversity in the population and therefore the chance of a pandemic strain emerging67, whereas a wider bottleneck via the contact route may allow the propagation of minor variants, such as drug-resistant viruses68.
Determinants of transmission
Virus, host and environmental factors influence whether a successful transmission occurs by governing the infectivity of the respiratory virus, the contagiousness of the infector, the susceptibility of the exposed individual and the environmental stress on the virus (Fig. 2). These determinants may have different relative effects on each mode of transmission69.
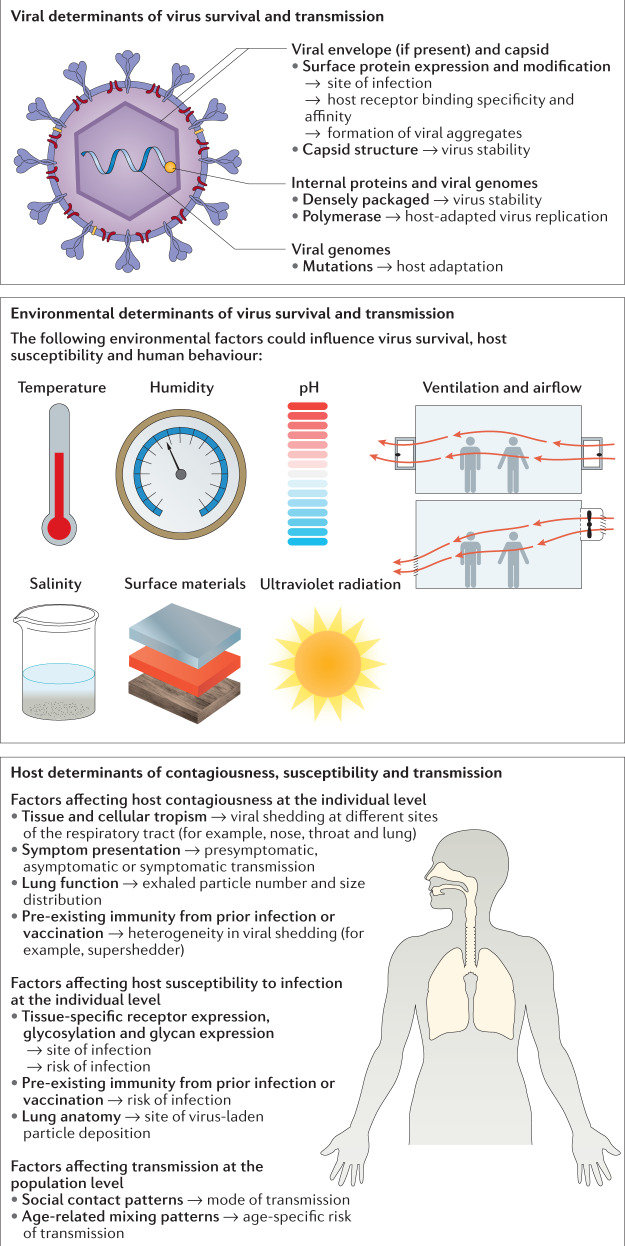
Fig. 2. Viral, environmental and host determinants of respiratory virus transmission.
Virus, environmental and host factors influence whether a successful transmission occurs by governing the infectivity of the respiratory virus, the contagiousness of the infected person, the environmental stress on the virus, which affects its persistence and survival during transmission, and the susceptibility of the exposed person.
Viral determinants
The propensity for respiratory viruses to be transmitted is affected by virus stability under environmental stress70,71, which in turn is influenced by the composition and structure of the virus envelope72,73, capsid74, internal proteins and genomes75 as well as the formation of viral aggregates76. For instance, DNA viruses such as herpesviruses (for example, varicella zoster virus (VZV)) with a more densely packaged genome have a stronger capsid structure that may prevent premature release of the viral genome before infection77. RNA virus genomes such as those of influenza virus have higher mutation rates, giving rise to diverse viral genomic variants (quasispecies) in infected individuals78, which may allow faster host adaptation of a virus strain that is efficiently transmitted via respiratory droplets79. In addition to virus stability, other viral factors, such as viral protein expression and modification, also influence transmission. Viral surface and internal proteins can affect transmissibility by determining the site of infection and interacting with specific host receptors with differing binding specificity and affinity. In studies of the pandemic potential of avian influenza viruses, human adapted haemagglutinin (HA) and polymerase subunit PB2, which exhibit a preferential binding to ɑ2,6-linked sialic acids and support viral genome replication in the lower-temperature environment of the mammalian airway, respectively, conferred efficient transmission over the respiratory droplet route in ferrets80. Similarly, an optimal ratio of HA to neuraminidase (NA) was essential for efficient transmission over the respiratory droplet route20, which is hypothesized to be associated with the release of single viral particles instead of aggregates with increased NA activity81. The loss of glycosylation sites at specific HA amino acid positions also conferred transmissibility to an avian influenza virus in a mammalian model82, thereby increasing its pandemic potential.
Environmental determinants
Environmental determinants could, on one hand, affect transmissibility by influencing the survival and persistence of respiratory viruses in respiratory droplets or fomites after their release to the environment, or on the other hand, modulate transmission by modulating host factors such as viral shedding and human behaviour. These effects could differ across different transmission modes and settings, and may favour one mode over another. As demonstrated mostly for influenza virus, environmental factors that may affect virus survival include temperature, humidity, salinity, pH, the medium or materials of the contaminated objects or surfaces, ventilation, airflow and ultraviolet radiation83,84. Their effects on survival may differ between viruses85, and their effects on transmission may be assessed in animal, epidemiological or modelling studies86. Interestingly, although a higher temperature is usually associated with lower influenza virus survival, different studies have suggested that the association between influenza virus survival and relative humidity may follow a monotonic inverse or a U-shaped relationship13. For transmission, transmission risk assessment suggested non-fabric surface materials, compared with fabric surfaces, favour fomite transmission for RSV and rhinovirus but not for influenza virus in hospital rooms and aircraft cabins87. In guinea pig models, a cold and dry environment was shown to favour influenza virus transmission, with the contact route dominating at higher temperatures88. Alternatively, to explain the difference in seasonality of influenza virus circulation across regions with temperate, subtropical and tropical climates, ecological studies suggested that influenza virus transmission was favoured in a cold-and-dry climate if a lower threshold of humidity and temperature was reached; otherwise transmission was favoured in a humid-and-rainy climate when precipitation was greatest89. However, it was unclear how much was due to changes in virus survival, host susceptibility, indoor crowding or the dominant route of transmission89,90.
Host determinants
Host determinants in both infectors and infectees could affect the propensity of transmission or the preferential routes of transmission. For infectors, tissue and cellular tropism for productive virus replication in the respiratory tract determines the site of release of virus progeny. Compared with SARS-CoV, which replicates mainly in alveolar epithelium, SARS-CoV-2 replicates extensively in both bronchial and alveolar epithelia, which, together with other factors, might explain its more efficient transmission91. Host viral shedding could determine the contagiousness of the infector. However, nasal or throat viral shedding alone was inadequate to explain influenza A virus transmission in households92, suggesting the importance of other host factors (for example, variability in symptom presentation93 or lung function94) that may lead to heterogeneity in contagiousness and partially explain the presence of superspreaders and superspreading events. For SARS-CoV-2, some studies showed presymptomatic viral shedding and transmission95, and similar levels of viral shedding96 in asymptomatic and symptomatic infected individuals, demonstrating substantial ‘silent’ presymptomatic transmission or transmission from a substantial fraction of infected individuals who are asymptomatic97 is possible. Pre-existing immunity98 and vaccination history93,99 may also modulate virus shedding in infectors. For infectees, tissue-specific expression of viral receptors100 or glycosylation and glycan expression101 along the respiratory tract determines the site of infection and may affect the preferential route of infection. Interestingly, despite the aerodynamic tendency of aerosols to deposit in the lower respiratory tract102, a preferential expression of ACE2 and the observation that virus-laden aerosols deposited mostly in the nose may indicate that virus-laden aerosols may initiate SARS-CoV-2 infection in the nasal cavity100. Although host genetics is suggested to modulate infection severity upon virus exposure, less evidence is available for its role in the transmissibility and modes of transmission of respiratory viruses103. At the population level, heterogeneous social contacts and age-related mixing patterns between infected and susceptible individuals drives transmission in specific groups or favours a particular route of transmission in different settings104.
Evidence and relative importance of modes of transmission
Various approaches, including environmental sampling, experimental animal and volunteer transmission studies, and epidemiological observations (mostly from outbreak investigations), have been used to provide evidence in support of each individual mode of transmission for different respiratory viruses, although for each, some may criticize their relevance6,7. Furthermore, although attempts have been made to classify each mode as ‘obligate’, ‘preferential’ or ‘opportunistic’15,50, limited research was done to quantify the relative importance of each mode to transmission9.
Evidence supporting individual modes of transmission
There are different types of evidence in support of individual modes of transmission of common respiratory viruses in humans (Table 1). For the direct (physical) contact and the fomite routes, experimental studies demonstrated the survival of respiratory viruses on surfaces, although higher viral doses than would usually be identified in natural settings are usually used105,106. Virus genetic material, and much less often infectious viruses, were recovered from patients’ hands or naturally contaminated objects in homes, workplaces, day-care centres, nursing homes and hospitals105–107. Experimental animal studies68,108 and limited experimental human109–111 or epidemiological112 studies were able to demonstrate disease transmission via fomites in the absence of direct contact, droplets and aerosols. For the droplet and aerosol routes, collection of exhaled breath from healthy individuals suggested human expiratory activities release respiratory droplet particles in a continuum of particle size, covering droplets or aerosols, via the mouth and nose. The particle sizes and their respective concentrations depend on the expiratory activities involved and the original sites of particle generation in the respiratory tract113. Although many recognize the generation of respiratory droplets and aerosols via talking, coughing and sneezing, additionally studies have demonstrated the exhalation of aerosols during normal breathing; such generation varies considerably between individuals94,113,114. Furthermore, studies showed that exhaled particles could contain respiratory viruses115. Viral RNA (for example, influenza virus, rhinovirus and coronavirus RNA) was recovered from both exhaled breath droplets and aerosols of symptomatic infected individuals93,99,115, but infectious virus has so far been only found in aerosols and not in droplets for influenza virus93,99,116. Experimental animal studies of influenza virus108, experimental human studies of rhinovirus117 and epidemiological studies of SARS-CoV118 have demonstrated transmission via respiratory droplets (including both droplets and aerosols); however, experimental animal studies of influenza virus23,119 and SARS-CoV-2 (ref.120), experimental human studies of Coxsackie virus63 (which belongs to the same family as rhinovirus) and epidemiological studies of influenza virus121 and rhinovirus122 have demonstrated that transmission likely occurs via aerosols. Epidemiological studies observed reduced SARS-CoV transmission associated with the use of masks and respirators in health-care settings123 and reduced influenza virus transmission after disinfection of room air by ultraviolet light124, suggestive of the role of aerosols and/or droplets in the transmission. Notably, few studies have demonstrated transmission via droplets only in the absence of aerosols, direct physical contact and fomites7,62.
Table 1.
Transmissibility of, modes of transmission of and transmission-based precautions for common respiratory viruses in humans
| Transmissibility and transmission | HCoV | IV | MeV | PIV | RSV | HMPV | VZV | RhV | HAdVa |
|---|---|---|---|---|---|---|---|---|---|
| Transmissibilityb | |||||||||
| Basic reproduction number (R0) | 0.5–8.0 | 1.0–21.0 | 1.4–770 | 2.3–2.7 | 0.9–21.9 | – | 1.2–16.9 | 1.2–2.7 | 2.3–5.1 |
| Household SAR (%) | 0–38.2 | 1.4–38.0 | 52.0–84.6 | 36.0–67.0 | 11.6–39.3 | – | 61.0–78.1 | 28.0–58.0 | – |
| Evidence for direct contact transmissionc | |||||||||
| Infectious virus survival on experimentally contaminated handsd | ✓ | ✓ | – | ✓ | ✓ | – | ✓ | ✓ | – |
| Virus genetic material recovered on naturally contaminated hands | – | – | – | – | – | – | ✓ | ✓ | – |
| Infectious virus recovered on naturally contaminated hands | – | – | – | – | – | – | – | ✓ | – |
| Transfer of virus genetic material between hands experimentally | – | ✓ | – | – | – | – | – | – | – |
| Transfer of infectious virus between hands experimentally | – | – | – | – | ✓ | – | – | ✓ | – |
| Infection initiated via exposure to infectious virus on hands demonstrated in volunteer studies | – | – | – | – | – | – | – | ✓ | – |
| Transmission of laboratory-confirmed infection via hands demonstrated in observational studies | ✓ | ✓ | ✓ | – | ✓ | – | – | – | ✓ |
| Transmission of laboratory-confirmed infection via hands demonstrated in volunteer studies | – | ✓ | – | – | – | – | – | ✓ | – |
| Evidence for indirect contact (fomite) transmissionc | |||||||||
| Infectious virus survival on experimentally contaminated surfacesd | ✓ | ✓ | – | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Virus genetic material recovered on naturally contaminated surfaces | ✓ | ✓ | ✓ | ✓ | – | – | ✓ | ✓ | ✓ |
| Infectious virus recovered on naturally contaminated surfaces | ✓ | ✓ | – | – | – | – | – | ✓ | ✓ |
| Transfer of virus genetic material between hands and surfaces experimentally | – | ✓ | – | – | – | – | – | ✓ | – |
| Transfer of infectious virus between hands and surfaces experimentally | – | ✓ | – | ✓ | ✓ | – | – | ✓ | ✓ |
| Infection initiated via exposure to infectious virus on surfaces demonstrated in volunteer studies | – | – | – | – | – | – | – | – | – |
| Transmission of laboratory-confirmed infection via surfaces demonstrated in observational studies | ✓ | – | – | – | – | – | ✓ | – | – |
| Transmission of laboratory-confirmed infection via surfaces demonstrated in volunteer studies | – | – | – | – | ✓ | – | – | ✓ | – |
| Evidence for droplet transmissionc,e | |||||||||
| Infectious virus survival in experimentally generated droplets | – | ✓ | – | (✓) | – | – | – | – | – |
| Virus genetic material recovered in droplets in human exhaled breathf | (✓) | (✓) | – | (✓) | (✓) | (✓) | – | (✓) | – |
| Infectious virus recovered in droplets in human exhaled breath | – | (✓) | – | – | – | – | – | – | – |
| Virus genetic material recovered in droplets in the air | (✓) | (✓) | (✓) | – | (✓) | – | – | (✓) | (✓) |
| Infectious virus recovered in droplets in the air | – | – | – | – | (✓) | – | – | – | – |
| Infection initiated via exposure to infectious virus in droplets demonstrated in volunteer studies | ✓ | ✓ | – | – | ✓ | ✓ | – | ✓ | (✓) |
| Transmission of laboratory-confirmed infection via droplets demonstrated in observational studies | – | – | – | – | – | – | – | – | – |
| Transmission of laboratory-confirmed infection via droplets demonstrated in volunteer studies | – | – | – | – | – | – | – | – | – |
| Evidence for aerosol transmissionc,e | |||||||||
| Infectious virus survival in experimentally generated aerosols | ✓ | ✓ | ✓ | ✓ | ✓ | – | – | – | ✓ |
| Virus genetic material recovered in aerosols in human exhaled breathf | ✓ | ✓ | – | ✓ | ✓ | ✓ | – | ✓ | – |
| Infectious virus recovered in aerosols in human exhaled breath | – | ✓ | – | – | – | – | – | – | – |
| Virus genetic material recovered in aerosols in the air | ✓ | ✓ | ✓ | ✓ | ✓ | – | ✓ | ✓ | ✓ |
| Infectious virus recovered in aerosols in the air | ✓ | ✓ | – | – | ✓ | – | – | – | – |
| Infection initiated via exposure to infectious virus in aerosols demonstrated in volunteer studies | – | ✓ | – | – | – | – | – | ✓ | ✓ |
| Transmission of laboratory-confirmed infection via aerosols demonstrated in observational studies | ✓ | ✓ | ✓ | – | – | – | ✓ | – | – |
| Transmission of laboratory-confirmed infection via aerosols demonstrated in volunteer studies | – | – | ✓ | – | – | – | ✓ | ✓ | – |
| Transmission-based precautions in health-care settingsg | |||||||||
| Contact precautionsh | Y | Y | N | Y | Y | Y | N | Y | Y |
| Droplet precautions | Y | Y | N | Y | Y | Y | N | Y | Y |
| Airborne precautions | N | N | Y | N | N | N | Y | N | N |
See Supplementary Table 1 for supporting references as well as evidence stratified by coronavirus types or influenza virus (IV) types/subtypes. Human bocavirus is not shown due to a lack of evidence regarding all modes of transmission. HAdV, human adenovirus; HCoV, human coronavirus; HMPV, human metapneumovirus; MeV, measles virus; N, not recommended; PIV, parainfluenza virus; RhV, rhinovirus; RSV, respiratory syncytial virus; SAR, secondary attack rate; VZV, varicella zoster virus; Y, recommended; ✓, evidence identified; (✓), evidence identified only in particles with aerodynamic diameter between 5 and 100 µm (applicable to droplet transmission only); –, evidence not found. aHAdV types that are considered mainly respiratory (but not enteric) are included. bThe range of reported estimates of the mean or median is provided. Estimates of household SAR in the absence of interventions were extracted where possible. cObservational studies include epidemiological or outbreak investigations, whereas volunteer studies include challenge studies or randomized (controlled) trials. dData include contamination by direct virus inoculation or contamination by volunteers who were experimentally infected. eParticles with aerodynamic diameter larger than 5 µm are traditionally defined as droplets, whereas those with a smaller aerodynamic diameter are defined as aerosols. However, there is ongoing discussion on redefining the particle size threshold between droplets and aerosols (see the section Terminology and defining features of modes of transmission). Therefore, for evidence on droplet transmission, evidence is provided in parentheses if evidence of virus recovery is identified only in particles with aerodynamic diameter between 5 and 100 µm. Air samples collected without size fractionation but that were collected more than 2 m from a known source (for example, an infected individual) are considered as evidence suggestive of aerosols. fEvidence for virus genetic material recovered in droplets or aerosols in human exhaled breath for PIV, RSV and HMPV is based on the author’s own additional data of the published study93. gEach precaution represents a set of infection prevention and control practices and personal protective equipment recommended by the WHO for health-care workers during routine patient care (excluding aerosol-generating procedures) within health-care facilities, with consideration of the current understanding on the modes of transmission of the respective pathogen. hFor IV, contact precautions are recommended for zoonotic IV only but not for endemic or pandemic IV.
Relative importance of modes of transmission
Very few experimental human transmission or epidemiological studies have evaluated the relative importance of different modes of transmission in the same study25,117,125. In a human challenge transmission study of rhinovirus, the authors observed that droplet and aerosol routes alone were sufficient to allow rhinovirus transmission to occur, whereas transmission via fomites was not observed (in the article ‘aerosols’ probably refers to both droplets and aerosols)117. In a recent similar study of influenza virus25, by attributing its failure to achieve the targeted SAR to a higher ventilation rate when compared with an earlier proof-of-concept study126, the authors suggested aerosol transmission was more important than transmission via the large droplet and contact routes.
Alternatively, the relative importance of different modes of transmission in different circumstances may be evaluated using mathematical mechanistic models, simulations and risk analyses; for example, outbreaks in aircraft36, on cruise ships127 and in health-care settings61 or during patient care128 (for example, in households49,129). By describing the efficiency of virus transfer at each step of a transmission route and coupled with a dose–response model with reference to the minimal infectious virus dose needed to initiate infection, one may estimate the relative infection risk between routes, thereby identifying the major transmission route in a particular circumstance. Furthermore, these approaches can also inform the likely values of the impact of individual determinants130 on each route, and the potential effectiveness of route-specific interventions such as face coverings128, and allow comparison of the major transmission modes between viruses36. However, a particular challenge for this approach is to identify the minimal infectious dose required for the virus to establish infection via a specific transmission mode.
Aerosol transmission of SARS-CoV-2 and influenza virus
Historically, national and international agencies commonly consider respiratory viruses to be transmitted over the contact and/or droplet route, and exercise caution when one is considering the possibility of aerosol transmission28,131. However in recent years, more researchers have advocated the recognition of the importance of aerosol transmission12,132. The recent controversy regarding aerosol transmission of SARS-CoV-2 reflects the perspectives and long-standing challenges in evaluating the relative importance of each mode of transmission for respiratory viruses.
The report by the WHO–China Joint Mission on Coronavirus Disease 2019 (ref.133) published on 28 February 2020 stated that “airborne spread has not been reported for COVID-19 and it is not believed to be a major driver of transmission based on available evidence; however, it can be envisaged if certain aerosol-generating procedures are conducted in health-care facilities”. The initial perception was that droplets and fomites were the major routes of transmission for SARS-CoV-2. However, as more data on SARS-CoV-2 transmission became available, on 27 March, the WHO published another scientific brief specifically on the modes of SARS-CoV-2 transmission38. It described droplet transmission as transmission that occurs through infective respiratory droplets of diameter larger than 5 µm to 10 µm or fomites in the immediate environment around the infected person, and airborne transmission through infective droplet nuclei smaller than 5 µm (ref.38). Addressing the findings of SARS-CoV-2 remaining infectious in artificially generated aerosols for 3 hours or more134 and the absence of SARS-CoV-2 in a small number of air samples collected near symptomatic individuals with COVID-19 (refs135,136), the WHO continued to recommend droplet and contact precautions in the absence of aerosol-generating procedures (that is, airborne transmission was not considered to be a major route).
On 6 July 2020, a group of 239 multidisciplinary scientists published an open letter advocating the recognition of potential airborne transmission of SARS-CoV-2 (ref.132), citing the latest studies of SARS-CoV-2 as well as previous studies of influenza virus, coronaviruses and other respiratory viruses. These studies included mechanistic evidence showing generation of (non-virus-containing) aerosols from human expiratory activities113,137, infectious influenza virus in cough aerosols99,138, that exhaled droplets of 60–100 µm can be carried less than 1 m away by breathing and more than 2 m by coughing139, and survival of SARS-CoV-2 in artificially generated aerosols (the same study referenced by the WHO)134. Furthermore, these studies provided evidence of SARS-CoV-2 viral RNA140, or infectious RSV141, Middle East respiratory syndrome-related coronavirus142 and SARS-CoV-2 (ref.143) in aerosols from the air near infected individuals. They also cited epidemiological observations of outbreaks or infection clusters of SARS and COVID-19, sometimes supported by additional modelling studies, which suggested transmission occurs mainly via aerosols. These studies included the large community outbreak of SARS in Amoy Gardens in Hong Kong59; clusters of SARS-CoV-2 infections in a restaurant in Guangzhou54,144 and a shopping mall in Wenzhou145, China, and a choir in Skagit County, Washington, United States144,146,147; and a modelling study with tracer gas measurement to simulate the spread of exhaled respiratory droplets from the suspected index patient for the SARS-CoV-2 restaurant outbreak in Guangzhou148. Some argued149,150 that the current epidemiological data and clinical observations for COVID-19 do not support the letter’s claim of the importance of aerosols in SARS-CoV-2 transmission; for example, the lack of observed long-range transmission or observed increased risk of infection among health-care workers in the absence of airborne precautions150, which the authors of the open letter responded to151. On 9 July, the WHO issued a updated scientific brief39, citing some of the evidence described in the open letter132 and extensive subsequent evidence on SARS-CoV-2 transmission. Addressing studies that identified low quantities of SARS-CoV-2 RNA in the exhaled breath of infected individuals152 or in air samples collected from health-care facilities in the absence of aerosol-generating procedures140,153–156, the WHO commented these do not necessarily indicate a sufficient dose of infectious virus for transmission to occur157. Addressing outbreak reports54,147,158,159, the WHO acknowledged possible SARS-CoV-2 transmission through aerosols in indoor crowded spaces in the absence of aerosol-generating procedures, although transmission through droplets or fomites cannot be ruled out. Therefore, the WHO concluded that SARS-CoV-2 is transmitted primarily through direct, indirect or close contact with infected persons or their respiratory droplets that are expelled during coughing, sneezing, talking or singing, that transmission via fomites is likely and that transmission via aerosols is possible in indoor crowded spaces39.
This is not the first time that the same evidence base has led different scientists or regulatory bodies to different conclusions on the importance of the aerosol route of transmission, with a similar discussion for influenza virus6,10,14,160, where there is evidence both for and against the importance of aerosol transmission. In support, infectious influenza virus was detected in aerosols in exhaled breath and coughs93,99 and in the air161, infectious aerosols could initiate infection in volunteers51,64, long-range transmission followed airflow162 and epidemiological studies showed increased transmission with less ventilation121 or decreased transmission with upper air disinfection by ultraviolet light124. Additional evidence includes influenza virus survival in artificially generated aerosols163, suggestive of infectiousness in aerosols in nature; however, this work has been criticized as being too artificial6, and influenza virus RNA recovery in the air in both community settings164 and health-care settings55,56,165 (even beyond 1.5 m from the source55,56) has been criticized because infectious virus was not identified. Some argued that there is insufficient evidence of influenza virus aerosol transmission6, such as evidence for infectious virus recovery far from the source, infection initiated by inhaling air from patient rooms, transmission over long distances, association between airflow and disease spread after removal of the source patient, and effectiveness of ultraviolet irradiation in reducing transmission; moreover, aerosol transmission is argued against as outbreaks in aircraft still occur despite their being well ventilated. Some researchers have explicitly considered that transmission via the aerosol route is essentially a long-range transmission6 and have argued that influenza virus transmission is mostly observed at close range and particularly with prolonged close contact. Importantly, some researchers have argued that since the goal of recognizing the importance of aerosol transmission is to minimize the risk of transmission in health-care settings by airborne precautions15, such recognition may be counterproductive due to resource limitations, logistics challenges and low compliance6.
In summary, some researchers argued that the ability to be transmitted over long distances is a prerequisite for aerosol transmission6, as was shown for measles virus57,58, VZV166,167 and Mycobacterium tuberculosis168, the three respiratory pathogens that are widely accepted to be transmitted mainly via aerosols12. They also require evidence of transmission via aerosols in the absence of all other routes6. The latest WHO scientific brief on modes of transmission of SARS-CoV-2 also reflects the emphasis of identifying infectious virus, and not viral RNA alone, in air samples39. However, although transmission over longer distances through the air is possible for some respiratory viruses57,166, this would require large numbers of viruses to be produced at the source and could be prevented by air dilution via ventilation or virus inactivation by environmental determinants. A failure to observe long-range transmission is therefore not evidence against aerosol transmission7, as it could also be explained by low rates of virus emission at the source or by effective dilution or inactivation. The observation that influenza virus viral load in aerosols decreased substantially with increasing distance from the source, possibly because of dilution of virus concentration further from the source56,169, suggests that if aerosol transmission does occur, it will occur mostly at close range and rarely at long range170. Furthermore, transmission via the droplet route in the absence of all other routes has yet to be observed (Table 1), raising the concern of the available evidence that supports placing relatively more emphasis on droplets over aerosols.
Non-pharmaceutical interventions
At the early stage of pandemics, virus-specific pharmaceutical interventions such as vaccines and therapeutics are not available, and in resource-limited settings, such interventions are rarely readily available. Furthermore, owing to constant viral evolution, new viral strains emerge or resistance is gained such that pharmaceutical interventions can soon become outdated. Therefore, at the early stages of a pandemic, NPIs become the most important public health measures that individuals or communities can adopt to reduce respiratory virus transmission. Common NPIs include the use of personal protective equipment (PPE) or hygiene practices at the individual level, environmental disinfection or dilution at a systemic level, and social distancing measures at the community level, which reduce transmission by interfering with a single mode or multiple modes of transmission. In particular, in health-care settings, different NPIs constitute part of standard precautions or transmission-based precautions (that is, contact, droplet or airborne precautions) (Table 1), which represent different sets of practices and PPE that health-care workers adopt to lower the risk of nosocomial transmission. Although many NPIs have demonstrated mechanistically the ability to inactivate or reduce the amount of respiratory viruses in experimental or natural settings, the effectiveness of these NPIs in preventing infection at the individual level or mitigating transmission at the population level depends on a number of factors: the overall risk of transmission in a specific setting (for example, dining in restaurants versus playing sports outdoor) or population group (for example, health-care workers versus institutionalized individuals); the risk of transmission through the specific modes which the NPIs act on, and whether the virus could be transmitted via alternative modes after intervention; and individual adherence or population-wide adoption of the NPIs (Table 2).
Table 2.
Mechanistic evidence and effectiveness of common non-pharmaceutical interventions
| Non-pharmaceutical intervention | Targeted mode of transmissiona | Mechanism of action | Mechanistic evidenceb | Effectivenessc | |
|---|---|---|---|---|---|
| PPE and hygiene practice | Hand hygiene | Contact |
Soaps remove organic substances by detergent properties Alcohol denatures proteins in the presence of water |
Alcohol had higher viricidal activity on enveloped viruses than on non-enveloped viruses Alcohol-based hand sanitizers are more efficacious than soaps with regard to pathogen inactivation in vivo |
Multiple systematic reviews suggested hand hygiene alone is significantly associated with reduced respiratory illness but not influenza virus infection in community settings Studies on the effectiveness of hand hygiene in reducing respiratory virus transmission in health-care settings were not identified Insufficient studies to compare the efficacies of soaps versus alcohol-based hand sanitizers against respiratory infections |
| Face coverings | Droplet and aerosol (contact) |
As source control: when worn by an infected individual, reduce virus release to the environment by filtration and immediate virus exposure of nearby healthy individuals by deflection As protection: when worn by a healthy individual, reduce exposure to virus-laden droplets and aerosols in the air Might also reduce contact transmission by reducing the frequency of hands touching respiratory mucosa |
As source control: surgical masks efficaciously reduced influenza virus and coronavirus release from infected individuals by filtration (efficacies on exhaled droplets and aerosols may differ between viruses) Studies using mannequins suggested deflection is also important in reducing virus release As protection against close-range transmission: cloth masks, surgical masks and respirators were efficacious against artificial bacteriophage or influenza virus aerosol challenge by filtration As protection against long-range transmission: in the absence of environmental airflow only 1% of radiolabeled saline aerosols generated from the source mannequin reached the exposed mannequin 3 feet apart, where only fitted respirators but not surgical masks reduced exposure to aerosols |
Multiple systematic reviews of observational studies or randomized trials mostly suggested the use of face coverings alone, or in combination with other non-pharmaceutical interventions, is effective in reducing the risk of respiratory illness or respiratory virus transmission in health-care and high-risk community settings Low adherence to use of a face shield during high-risk procedures associated with higher risk of respiratory illnesses in health-care workers Preliminary evidence suggested face mask use by household members before the person with the primary case developed symptoms is significantly associated with reduced SARS-CoV-2 household transmission |
|
| Environmental disinfection and dilution | Surface cleaning | Contact (droplet and aerosol) |
Common disinfectants in health-care settings: 0.1 M sodium hydroxide, 70% ethanol, 70% 1-propanol, ethylene oxide and sodium hypochlorite Common household cleaning agents: liquid soap, 1% bleach and antimicrobial or antiviral wipes Both disinfect contaminated surfaces by virus inactivation Might also reduce droplet or aerosol transmission by reducing fomites available for resuspension |
Common disinfectants in health-care settings effectively inactivated influenza virus and coronaviruses on surfaces in experimental settings Common household cleaning agents effectively inactivated (enveloped) influenza virus, but were less effective for (non-enveloped) adenovirus in experimental settings Biweekly disinfection of toys significantly reduced the presence of virus genetic material in the environment for adenovirus, rhinovirus and RSV, but not coronaviruses, parainfluenza virus and bocavirus, in nurseries in a randomized trial |
A systematic review found limited epidemiological studies on the effectiveness of surface and object cleaning in reducing community respiratory virus transmission during pandemics Biweekly disinfection of toys did not reduce respiratory illness in nurseries in a randomized trial The combined use of an alcohol-based hand sanitizer and chloride wipes, compared with hand washing, did not reduce respiratory illness in elementary school students in a randomized trial. Daily household cleaning was significantly associated with reduced household transmission of SARS-CoV-2 |
| Air dilution by ventilation and directional airflow | Droplet and aerosol |
Ventilation is the intentional introduction of outdoor air into a building by mechanical ventilation (for example, fans, ductwork or air conditioning) or natural ventilation (for example, windows) Directional airflow provides clean air from the cleanest area to less clean areas |
Lower ventilation associated with rhinovirus RNA detection in the air in an office environment in an observational study |
Multiple systematic reviews suggested strong and sufficient evidence supporting the association between indoor ventilation and airflow patterns with transmission of SARS-CoV, influenza virus, measles virus and varicella zoster virus Directional airflow may reduce the risk of airborne infection in vulnerable individuals or transmission in health-care and community settings |
|
| Air and surface disinfection by UVGI | Aerosol and contact |
Use of UV light in the germicidal range (200–320 nm), especially UV-C (200–280 nm), to crosslink nucleic acids Air disinfection: in upper-room UVGI, irradiation is confined to the area above the occupants’ heads to minimize direct exposure, but requires good vertical air movement in the room; in in-duct UVGI, air passes through ventilation systems and is irradiated inside before recirculation or exhaustion Surface disinfection: UVGI is used on internal surfaces of ventilation systems, or surfaces and equipment |
UV-C efficiently inactivated experimentally generated aerosols containing influenza virus or coronaviruses, but was less effective for adenovirus At higher relative humidity, increased susceptibility to UV-C was observed for experimentally generated aerosols containing adenovirus, but decreased susceptibility to UV-C was observed for influenza virus and vaccinia virus UV-C efficiently inactivated experimentally generated MERS-CoV on glass slides Upper-room UVGI efficiently reduced infectious vaccina virus aerosols in a simulated hospital room UVGI significantly inactivated experimentally inoculated influenza virus on respirators |
Upper-room UVGI was shown to prevent airborne transmission of measles virus in schools Upper-room UVGI was associated with reduced influenza virus infections among individuals with tuberculosis Randomized trials evaluating the effectiveness of UVGI for air or surface disinfection in reducing respiratory virus transmission were not identified. |
|
See Supplementary Table 2 for supporting references. MERS-CoV, Middle East respiratory syndrome-related coronavirus; PPE, personal protective equipment; RSV, respiratory syntactical virus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; UV, ultraviolet; UVGI, ultraviolet germicidal irradiation. aThe mode or modes of transmission listed in parentheses indicate possible but presumably less important transmission via that mode. bMechanistic evidence with regard to virus reduction or inactivation. cEffectiveness with regard to prevention of respiratory illness or respiratory virus transmission.
PPE and personal hygiene practices
Hand hygiene: soaps and alcohol-based hand sanitizers
Since Semmelweis first demonstrated in the 1840s that health-care workers, by adopting hand hygiene practices, reduced mortality in parturient women, hand hygiene has probably been the most widely adopted NPI for mitigating disease transmission (targeting the physical contact route) and is recommended as part of standard precautions for all patient care in health-care facilities171. Common hand hygiene practices used in health-care and community settings include handwashing with plain (non-antimicrobial) soaps or hand rub using alcohol-based hand sanitizers172 (Table 2). Alcohol-based hand sanitizers are useful in situations where sinks are not readily available, but are not recommended when hands are visibly dirty171. There is mechanistic evidence demonstrating bacterium or virus inactivation by hand hygiene173, and a number of systematic reviews of observational studies or randomized trials together suggest that hand hygiene alone is significantly associated with reducing respiratory illnesses; however, it is unclear whether hand hygiene is effective against laboratory-confirmed influenza virus infections17–19, possibly due to insufficiently large study sample size or weak adherence to the intervention125,174.
Face coverings: cloth masks, surgical masks, respirators, face shields and eye protection
Surgical face masks, face shields and eye protection are commonly used by health-care workers during routine patient care or when they are performing high-risk procedures as protection against splashes of bodily fluids or respiratory secretions, and respirators are commonly used as protection against aerosols15,131,175. In the community, the COVID-19 pandemic has not only led to community-wide adoption of surgical face masks; the extremely high demand has also resulted in (reusable) cloth masks being advocated as an alternative to surgical masks176. Apart from mitigating droplet and aerosol transmission, these face coverings might also reduce contact transmission by reducing the frequency of hands touching respiratory mucosa177. Mechanistically, face coverings can act either as protection against infection by reducing exposure to a virus when worn by a healthy individual or as source control by filtration93 and deflection when worn by an infected person178,179 (Table 2). On the basis of systematic reviews of observational studies123,180 and randomized trials16,18,123, many believe there is sufficient evidence supporting the effectiveness of the use of face coverings alone, or in combination with other NPIs, in reducing the risk of respiratory illness or virus transmission in health-care settings123,180 and high-risk community settings16, whereas some do not18. Mechanistic data from one study preliminarily suggest the effectiveness of surgical face masks may differ between viruses93. The relatively lower infection risk in the community compared with health-care settings, the requirement for fit testing and lower adherence argue against the use of respirators in the community181. Although surgical face masks as source control are likely applicable to most settings, as protection against infection they may have more utility in close encounters178 and crowded indoor settings; however, more research is needed. More research on the use of reusable masks, including cloth masks, in community settings either as source control or as protection is also urgently needed, including key parameters for assessment and standardization to address the diversity of materials available.
Environmental disinfection and dilution
Surface cleaning
Surface cleaning by disinfectants used in health-care settings182 or household cleaning agents183 (Table 2) mitigates transmission via the fomite route and might also block the droplet or aerosol route by reducing fomites available for resuspension due to various activities (for example, walking or door opening)184. Although supported by mechanistic evidence on virus inactivation, limited epidemiological studies have evaluated its effectiveness in reducing respiratory virus transmission17. One randomized trial in day-care nurseries suggested biweekly disinfection of toys significantly reduced the detection of adenovirus, rhinovirus and RSV, but not common cold coronaviruses, parainfluenza virus and bocavirus, in the environment; however, surface cleaning did not reduce the incidence of respiratory illness185, suggesting transmission may have occurred via routes other than the fomite route.
Air dilution by ventilation and directional airflow
Ventilation and directional airflow, although usually used to provide thermal comfort and clean air, could also help in mitigating droplet and aerosol transmission by dilution, especially indoors. Ventilation is an intentional mechanical or natural introduction of outdoor air into a building (Table 2). Natural ventilation, if properly designed, is valuable especially in resource-limited settings, but can be used only in locations where climatic conditions are favourable186. The ventilation rate is usually described as either per building or per room as the number of air changes per hour, or per occupant in the space as outdoor air rate per person. The minimal ventilation required differs depending on the level of infection risk expected or protection needed; for example, six air changes per hour in patient rooms and 12 air changes per hour in airborne-infection isolation rooms187. Separately, directional airflow provides clean air from the cleanest patient care areas to less clean patient care areas. Although limited data demonstrate reduced virus recovery in the air with increased ventilation or the presence of directional airflow122, it has been suggested there is ‘strong and sufficient’ evidence supporting the association between ventilation and airflow patterns in buildings and transmission of respiratory viruses, including SARS-CoV, influenza virus, measles virus and VZV41,188, although this may require further validation by intervention studies or randomized trials86. Furthermore, directional airflow may reduce the risk of airborne infection in vulnerable individuals or nosocomial transmission in health-care settings187,189, and also in community settings (for example, aircraft cabins190). Some suggested that for high levels of virus exposure in crowded indoor areas, increasing indoor mechanical ventilation may be less effective or less cost-effective to achieve sufficient risk reduction191, and that it might increase aerosol dispersion and infection risk for individuals further away from the source190; an uninterrupted air stream from the source to the exhaust may then have a more important role in reducing transmission192.
Air and surface disinfection by ultraviolet germicidal irradiation
Concern over aerosol and fomite transmission of SARS-CoV-2 has renewed interest in the use of ultraviolet germicidal irradiation (UVGI)193 — that is, the use of ultraviolet light in the germicidal range of wavelengths (200–320 nm) — for the disinfection of air and surfaces194. For air disinfection, upper-room UVGI and in-duct UVGI are usually used195 (Table 2). The use of UVGI to prevent airborne transmission was pioneered by Wells for the control of tuberculosis; Wells also demonstrated its use to prevent measles virus transmission in schools196. Upper-room UVGI was associated with reduced influenza virus infections among individuals with tuberculosis124. Surface disinfection with UVGI was initially used for bacterial decontamination197. Studies evaluating the inactivation of respiratory viruses on surfaces using UVGI in experimental settings198 are scarce and would be strengthened by studies using infectious virus recovery from naturally contaminated surfaces as the outcome measure. Randomized trials evaluating the effectiveness of UVGI for air or surface disinfection in reducing respiratory virus transmission are also lacking. Some proposed disinfecting surgical masks and respirators199 with UVGI to allow their reuse in resource-limited settings200. Although UVGI is not considered carcinogenic, its domestic use (for example, consumer products advertised for control of COVID-19) is cautioned against as it requires expert knowledge of the dosage required, and the efficacies of these consumer products are in doubt201.
Conclusions
The complexity of the control of respiratory virus transmission is reflected by the cross-disciplinary efforts to estimate the transmissibility of a respiratory virus, to evaluate the relative importance of modes of transmission and factors affecting transmission, to evaluate the efficacy and effectiveness of NPIs in different settings, and in turn how these translate to reduced transmissibility in the general and specific populations. Although population-based estimates of transmissibility (R0) could inform the combined effectiveness of multiple interventions in reducing transmission, the household-based estimates (SAR) in randomized trials could inform the effectiveness of individual interventions. As shown, relative transmissibility between respiratory viruses may be different depending on whether R0 or SAR is used to describe transmissibility, amid heterogeneities in estimates of the same virus (Table 1). Studies comparing the transmissibility of different respiratory viruses in parallel in the same study, perhaps in case-ascertained household studies, where study settings are more controlled, would be useful to identify which respiratory viruses are more transmissible than others.
The controversies regarding the role of aerosols in the transmission of SARS-CoV-2 and influenza virus highlight our very limited understanding on the relative importance of different modes of transmission. This includes the lack of consensus on the defining features of each mode of transmission, especially the difficulty in differentiating between droplets and aerosols; the different levels of scrutiny when evidence supporting each mode is being evaluated; the technical challenges in recovering infectious virus from the environment; the challenges in identifying the minimal infectious dose required to establish infection in susceptible or immunized individuals; and the lack of quantitative risk assessment for different modes of transmission. Given the different types of qualitative evidence available in support of individual modes of transmission (Table 1), discussion may be warranted in assigning priority or strength of evidence to these different types of evidence in support of each mode; for example, whether the demonstration of transmission via aerosols alone in the absence of other routes is essential to support the importance of the aerosol route, as suggested for measles virus57 and VZV166, but which was not done in support of the importance of the droplet route for RSV. These study designs may be possible for aerosols and fomites, but it will be more challenging to demonstrate transmission via direct contact and droplets in the absence of (close-range) aerosols. For rhinovirus, airborne precautions are not required despite the evidence of aerosol transmission in the absence of other routes63,117; in addition, as rhinovirus transmission has been demonstrated independently via direct contact202, fomite109 and aerosols63,117, efforts should be made to quantitatively evaluate the relative contribution of each mode to transmission and their determinants in different settings130. However, the relative contribution of different modes at the population level likely varies between different settings, populations and interventions in place, and at the individual level varies between individuals due to heterogeneity in contagiousness and susceptibility, which also changes over time. There is also minimal research on the modes of transmission of bocavirus and metapneumovirus (Supplementary Table 1). In general, there is a lack of studies demonstrating droplet transmission alone in the absence of other routes for all respiratory viruses (Table 1), and such studies are urgently needed to support the importance of droplets over aerosols and will have important implications for the choice of NPIs for mitigating transmission. Alternatively, aerosol transmission does not necessarily indicate a higher intrinsic transmissibility of the virus (Table 1) nor long-range transmission, as transmissibility depends on multiple factors, including the degree of presymptomatic transmission, the contagiousness of the infector, the susceptibility of the infectee, the contact patterns between them and the environmental determinants of transmission in the shared space; and long-range transmission depends on rates of virus emission at the source or effective dilution or inactivation by environmental determinants. Moving beyond deciding on the adoption of an NPI mostly on the basis of the perceived importance of a particular mode of transmission would provide an incentive for public health practitioners to recognize the importance of aerosol transmission.
For common NPIs, although we have mechanistic evidence supporting their efficacy with regard to virus reduction or inactivation, we have limited knowledge of their effectiveness in reducing transmission in the population both in health-care settings and in community settings. This may be because we do not yet know the relative contribution of different modes of transmission in a particular setting and whether the different modes can partially compensate for each other if a mode is absent174. If the latter is true, studies evaluating the effectiveness of one intervention alone, which targets a specific mode, may underestimate its potential effectiveness because a reduction in transmission via a specific mode by the NPI might be compensated by transmission via another mode174. Given effectiveness is demonstrated, the eventual adoption of NPIs would also depend on the perceived severity of the disease15, the infection risk in a particular setting175,180, the accessibility of the resources, the purpose of interventions6 and the economic or societal costs of implementing the intervention. In particular, the choice of which transmission-based precautions to adopt for a particular respiratory virus in health-care settings depends on the perceived major modes of transmission for the pathogen15, the level of caution and the resources likely available if the recommendation is made15, and therefore could differ between countries. Intervening against multiple modes of transmission would be more effective than acting against a single mode. For example, although effectiveness of the use of face masks or hand hygiene alone in mitigating community transmission of laboratory-confirmed respiratory virus infection was not demonstrated, possibly due to various experimental challenges, their combined use has been shown to be effective in reducing influenza virus transmission and should be considered203. A clear public health message accounting for these uncertainties will help to gain public confidence and support public health efforts.
Supplementary information
Acknowledgements
This work was supported by the Theme-based Research Scheme (project no. T11-712/19-N) from the Research Grants Council and the Collaborative Research Fund (project no. C7025-16G) from the University Grants Committee of Hong Kong. The author acknowledges B. Cowling for helpful discussions.
Competing interests
The author declares no competing interests.
Footnotes
Peer review information
Nature Reviews Microbiology thanks J. Tang, T. Tumpey and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41579-021-00535-6.
References
- 1.James SL, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth GA, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fendrick AM, Monto AS, Nightengale B, Sarnes M. The economic burden of non-influenza-related viral respiratory tract infection in the United States. Arch. Intern. Med. 2003;163:487–494. doi: 10.1001/archinte.163.4.487. [DOI] [PubMed] [Google Scholar]
- 4.Belser JA, Maines TR, Tumpey TM, Katz JM. Influenza A virus transmission: contributing factors and clinical implications. Expert Rev. Mol. Med. 2010;12:e39. doi: 10.1017/S1462399410001705. [DOI] [PubMed] [Google Scholar]
- 5.Richard M, Fouchier RA. Influenza A virus transmission via respiratory aerosols or droplets as it relates to pandemic potential. FEMS Microbiol. Rev. 2015;40:68–85. doi: 10.1093/femsre/fuv039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brankston G, Gitterman L, Hirji Z, Lemieux C, Gardam M. Transmission of influenza A in human beings. Lancet Infect. Dis. 2007;7:257–265. doi: 10.1016/S1473-3099(07)70029-4. [DOI] [PubMed] [Google Scholar]
- 7.Killingley B, Nguyen-Van-Tam J. Routes of influenza transmission. Influenza Other Respir. Viruses. 2013;7(Suppl. 2):42–51. doi: 10.1111/irv.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Killingley B, et al. Potential role of human challenge studies for investigation of influenza transmission. Lancet Infect. Dis. 2011;11:879–886. doi: 10.1016/S1473-3099(11)70142-6. [DOI] [PubMed] [Google Scholar]
- 9.Kutter JS, Spronken MI, Fraaij PL, Fouchier RAM, Herfst S. Transmission routes of respiratory viruses among humans. Curr. Opin. Virol. 2018;28:142–151. doi: 10.1016/j.coviro.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tellier R. Aerosol transmission of influenza A virus: a review of new studies. J. R. Soc. Interface. 2009;6(Suppl. 6):S783–S790. doi: 10.1098/rsif.2009.0302.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang JW, Li Y, Eames I, Chan PK, Ridgway GL. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J. Hosp. Infect. 2006;64:100–114. doi: 10.1016/j.jhin.2006.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tellier R, Li Y, Cowling BJ, Tang JW. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect. Dis. 2019;19:101. doi: 10.1186/s12879-019-3707-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marr LC, Tang JW, Van Mullekom J, Lakdawala SS. Mechanistic insights into the effect of humidity on airborne influenza virus survival, transmission and incidence. J. R. Soc. Interface. 2019;16:20180298. doi: 10.1098/rsif.2018.0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemieux C, Brankston G, Gitterman L, Hirji Z, Gardam M. Questioning aerosol transmission of influenza. Emerg. Infect. Dis. 2007;13:173–174. doi: 10.3201/eid1301.061202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shiu EYC, Leung NHL, Cowling BJ. Controversy around airborne versus droplet transmission of respiratory viruses: implication for infection prevention. Curr. Opin. Infect. Dis. 2019;32:372–379. doi: 10.1097/QCO.0000000000000563. [DOI] [PubMed] [Google Scholar]
- 16.MacIntyre CR, Chughtai AA. Facemasks for the prevention of infection in healthcare and community settings. BMJ. 2015;350:h694. doi: 10.1136/bmj.h694. [DOI] [PubMed] [Google Scholar]
- 17.Xiao J, et al. Nonpharmaceutical measures for pandemic influenza in nonhealthcare settings-personal protective and environmental measures. Emerg. Infect. Dis. 2020;26:967–975. doi: 10.3201/eid2605.190994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jefferson, T. et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst. Rev.10.1002/14651858.CD006207.pub5 (2020). [DOI] [PMC free article] [PubMed]
- 19.Saunders-Hastings P, Crispo JAG, Sikora L, Krewski D. Effectiveness of personal protective measures in reducing pandemic influenza transmission: A systematic review and meta-analysis. Epidemics. 2017;20:1–20. doi: 10.1016/j.epidem.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Yen HL, et al. Hemagglutinin-neuraminidase balance confers respiratory-droplet transmissibility of the pandemic H1N1 influenza virus in ferrets. Proc. Natl Acad. Sci. USA. 2011;108:14264–14269. doi: 10.1073/pnas.1111000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herfst S, et al. Airborne transmission of influenza A/H5N1 virus between ferrets. Science. 2012;336:1534–1541. doi: 10.1126/science.1213362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouvier NM, Lowen AC, Palese P. Oseltamivir-resistant influenza A viruses are transmitted efficiently among guinea pigs by direct contact but not by aerosol. J. Virol. 2008;82:10052–10058. doi: 10.1128/JVI.01226-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou J, et al. Defining the sizes of airborne particles that mediate influenza transmission in ferrets. Proc. Natl Acad. Sci. USA. 2018;115:E2386–E2392. doi: 10.1073/pnas.1716771115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richard M, et al. Influenza A viruses are transmitted via the air from the nasal respiratory epithelium of ferrets. Nat. Commun. 2020;11:766. doi: 10.1038/s41467-020-14626-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen-Van-Tam JS, et al. Minimal transmission in an influenza A (H3N2) human challenge-transmission model within a controlled exposure environment. PLoS Pathog. 2020;16:e1008704. doi: 10.1371/journal.ppat.1008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Y, et al. The transmissibility and control of pandemic influenza A (H1N1) virus. Science. 2009;326:729–733. doi: 10.1126/science.1177373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Eggo RM, Kucharski AJ. Secondary attack rate and superspreading events for SARS-CoV-2. Lancet. 2020;395:e47. doi: 10.1016/S0140-6736(20)30462-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siegel JD, Rhinehart E, Jackson M, Chiarello L. 2007 guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am. J. Infect. Control. 2007;35:S65–S164. doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao F, et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833 e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minodier L, et al. Clinical and virological factors associated with gastrointestinal symptoms in patients with acute respiratory infection: a two-year prospective study in general practice medicine. BMC Infect. Dis. 2017;17:729. doi: 10.1186/s12879-017-2823-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson D, Lynch R, Marshall C, Mead K, Hirst D. Aerosol generation by modern flush toilets. Aerosol Sci. Technol. 2013;47:1047–1057. doi: 10.1080/02786826.2013.814911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colavita F, et al. SARS-CoV-2 isolation from ocular secretions of a patient with COVID-19 in Italy with prolonged viral RNA detection. Ann. Intern. Med. 2020 doi: 10.7326/M20-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bischoff WE, Reid T, Russell GB, Peters TR. Transocular entry of seasonal influenza-attenuated virus aerosols and the efficacy of N95 respirators, surgical masks, and eye protection in humans. J. Infect. Dis. 2011;204:193–199. doi: 10.1093/infdis/jir238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milton DK. A rosetta stone for understanding infectious drops and aerosols. J. Pediatric Infect. Dis. Soc. 2020;9:413–415. doi: 10.1093/jpids/piaa079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. Guidelines for environmental infection control in health-care facilities. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). https://www.cdc.gov/infectioncontrol/guidelines/environmental/index.html (2003). [PubMed]
- 36.Lei H, et al. Routes of transmission of influenza A H1N1, SARS CoV, and norovirus in air cabin: comparative analyses. Indoor Air. 2018;28:394–403. doi: 10.1111/ina.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.British Columbia Centre for Disease Control. About COVID-19: How it Spreads. http://www.bccdc.ca/health-info/diseases-conditions/covid-19/about-covid-19/how-it-spreads (2020).
- 38.World Health Organization. Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations: scientific brief, 27 March 2020. https://apps.who.int/iris/handle/10665/331616 (2020).
- 39.World Health Organization. Transmission of SARS-CoV-2: implications for infection prevention precautions: scientific brief, 9 July 2020. https://apps.who.int/iris/handle/10665/333114 (2020).
- 40.Zhang N, et al. Close contact behavior in indoor environment and transmission of respiratory infection. Indoor Air. 2020;30:645–661. doi: 10.1111/ina.12673. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, et al. Role of ventilation in airborne transmission of infectious agents in the built environment - a multidisciplinary systematic review. Indoor Air. 2007;17:2–18. doi: 10.1111/j.1600-0668.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- 42.Wells WF. On air-borne infection: study II. Droplets and droplet nuclei. Am. J. Hyg. 1934;20:611–618. [Google Scholar]
- 43.Goldmann DA. Epidemiology and prevention of pediatric viral respiratory infections in health-care institutions. Emerg. Infect. Dis. 2001;7:249–253. doi: 10.3201/eid0702.010220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldmann DA. Transmission of viral respiratory infections in the home. Pediatr. Infect. Dis. J. 2000;19:S97–S102. doi: 10.1097/00006454-200010001-00002. [DOI] [PubMed] [Google Scholar]
- 45.Gralton J, Tovey E, McLaws ML, Rawlinson WD. The role of particle size in aerosolised pathogen transmission: a review. J. Infect. 2011;62:1–13. doi: 10.1016/j.jinf.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duguid JP. The size and the duration of air-carriage of respiratory droplets and droplet-nuclei. J. Hyg. 1946;44:471–479. doi: 10.1017/s0022172400019288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hatch, T. F. Behavior of microscopic particles in the air and in the respiratory system in Aerobiology (ed. Forest R. Moulton) 102–105 (American Association for the Advancement of Science, 1942).
- 48.Hinds, W. C. Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles (John Wiley & Sons, 2012).
- 49.Nicas M, Jones RM. Relative contributions of four exposure pathways to influenza infection risk. Risk Anal. 2009;29:1292–1303. doi: 10.1111/j.1539-6924.2009.01253.x. [DOI] [PubMed] [Google Scholar]
- 50.Roy CJ, Milton DK. Airborne transmission of communicable infection - The elusive pathway. N. Engl. J. Med. 2004;350:1710–1712. doi: 10.1056/NEJMp048051. [DOI] [PubMed] [Google Scholar]
- 51.Knight V. Viruses as agents of airborne contagion. Ann. N. Y. Acad. Sci. 1980;353:147–156. doi: 10.1111/j.1749-6632.1980.tb18917.x. [DOI] [PubMed] [Google Scholar]
- 52.National Academies of Sciences, Engineering, Medicine. Airborne Transmission of SARS-CoV-2: Proceedings of a Workshop — in Brief (eds Shelton-Davenport, M., Pavlin, J., Saunders, J. & Staudt, A.) (The National Academies Press, 2020). [PubMed]
- 53.Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID-19. JAMA. 2020;323:1838–1838. doi: 10.1001/jama.2020.4756. [DOI] [PubMed] [Google Scholar]
- 54.Lu J, et al. COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerg. Infect. Dis. 2020;26:1628–1631. doi: 10.3201/eid2607.200764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leung NH, et al. Quantification of influenza virus RNA in aerosols in patient rooms. PLoS ONE. 2016;11:e0148669. doi: 10.1371/journal.pone.0148669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bischoff WE, Swett K, Leng I, Peters TR. Exposure to influenza virus aerosols during routine patient care. J. Infect. Dis. 2013;207:1037–1046. doi: 10.1093/infdis/jis773. [DOI] [PubMed] [Google Scholar]
- 57.Bloch AB, et al. Measles outbreak in a pediatric practice: airborne transmission in an office setting. Pediatrics. 1985;75:676–683. doi: 10.1542/peds.75.4.676. [DOI] [PubMed] [Google Scholar]
- 58.Remington PL, Hall WN, Davis IH, Herald A, Gunn RA. Airborne transmission of measles in a physician’s office. JAMA. 1985;253:1574–1577. doi: 10.1001/jama.1985.03350350068022. [DOI] [PubMed] [Google Scholar]
- 59.Yu IT, et al. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N. Engl. J. Med. 2004;350:1731–1739. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]
- 60.Sawyer MH, Chamberlin CJ, Wu YN, Aintablian N, Wallace MR. Detection of varicella-zoster virus DNA in air samples from hospital rooms. J. Infect. Dis. 1994;169:91–94. doi: 10.1093/infdis/169.1.91. [DOI] [PubMed] [Google Scholar]
- 61.Xiao S, Li Y, Sung M, Wei J, Yang Z. A study of the probable transmission routes of MERS-CoV during the first hospital outbreak in the Republic of Korea. Indoor Air. 2018;28:51–63. doi: 10.1111/ina.12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fennelly KP. Particle sizes of infectious aerosols: implications for infection control. Lancet Respir. Med. 2020;8:P914–924. doi: 10.1016/S2213-2600(20)30323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Couch RB, Cate TR, Douglas RG, Gerone PJ, Knight V. Effect of route of inoculation on experimental respiratory viral disease in volunteers and evidence for airborne transmission. Bacteriol. Rev. 1966;30:517–529. doi: 10.1128/br.30.3.517-529.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alford RH, Kasel JA, Gerone PJ, Knight V. Human influenza resulting from aerosol inhalation. Proc. Soc. Exp. Biol. Med. 1966;122:800–804. doi: 10.3181/00379727-122-31255. [DOI] [PubMed] [Google Scholar]
- 65.Henle W, Henle G, Stokes J, Jr., Maris EP. Experimental exposure of human subjects to viruses of influenza. J. Immunol. 1946;52:145–165. doi: 10.4049/jimmunol.52.2.145. [DOI] [PubMed] [Google Scholar]
- 66.Teunis PF, Brienen N, Kretzschmar ME. High infectivity and pathogenicity of influenza A virus via aerosol and droplet transmission. Epidemics. 2010;2:215–222. doi: 10.1016/j.epidem.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 67.Varble A, et al. Influenza A virus transmission bottlenecks are defined by infection route and recipient host. Cell Host Microbe. 2014;16:691–700. doi: 10.1016/j.chom.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Frise R, et al. Contact transmission of influenza virus between ferrets imposes a looser bottleneck than respiratory droplet transmission allowing propagation of antiviral resistance. Sci. Rep. 2016;6:29796. doi: 10.1038/srep29793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spicknall IH, et al. Informing optimal environmental influenza interventions: how the host, agent, and environment alter dominant routes of transmission. PLoS Comput. Biol. 2010;6:e1000969. doi: 10.1371/journal.pcbi.1000969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Minhaz Ud-Dean SM. Structural explanation for the effect of humidity on persistence of airborne virus: seasonality of influenza. J. Theor. Biol. 2010;264:822–829. doi: 10.1016/j.jtbi.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 71.Ausar SF, et al. Analysis of the thermal and pH stability of human respiratory syncytial virus. Mol. Pharm. 2005;2:491–499. doi: 10.1021/mp0500465. [DOI] [PubMed] [Google Scholar]
- 72.Ijaz MK, Brunner AH, Sattar SA, Nair RC, Johnson-Lussenburg CM. Survival characteristics of airborne human coronavirus 229E. J. Gen. Virol. 1985;66:2743–2748. doi: 10.1099/0022-1317-66-12-2743. [DOI] [PubMed] [Google Scholar]
- 73.Bajimaya S, Frankl T, Hayashi T, Takimoto T. Cholesterol is required for stability and infectivity of influenza A and respiratory syncytial viruses. Virology. 2017;510:234–241. doi: 10.1016/j.virol.2017.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mateu MG. Assembly, stability and dynamics of virus capsids. Arch. Biochem. Biophys. 2013;531:65–79. doi: 10.1016/j.abb.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 75.Saha B, Wong CM, Parks RJ. The adenovirus genome contributes to the structural stability of the virion. Viruses. 2014;6:3563–3583. doi: 10.3390/v6093563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gerba CP, Betancourt WQ. Viral aggregation: impact on virus behavior in the environment. Environ. Sci. Technol. 2017;51:7318–7325. doi: 10.1021/acs.est.6b05835. [DOI] [PubMed] [Google Scholar]
- 77.Bauer DW, et al. Exploring the balance between DNA pressure and capsid stability in herpesviruses and phages. J. Virol. 2015;89:9288–9298. doi: 10.1128/JVI.01172-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Poon LL, et al. Quantifying influenza virus diversity and transmission in humans. Nat. Genet. 2016;48:195–200. doi: 10.1038/ng.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Russell CA, et al. The potential for respiratory droplet-transmissible A/H5N1 influenza virus to evolve in a mammalian host. Science. 2012;336:1541–1547. doi: 10.1126/science.1222526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Van Hoeven N, et al. Human HA and polymerase subunit PB2 proteins confer transmission of an avian influenza virus through the air. Proc. Natl Acad. Sci. USA. 2009;106:3366–3371. doi: 10.1073/pnas.0813172106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schrauwen EJ, Fouchier RA. Host adaptation and transmission of influenza A viruses in mammals. Emerg. Microbes Infect. 2014;3:e9. doi: 10.1038/emi.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gao Y, et al. Identification of amino acids in HA and PB2 critical for the transmission of H5N1 avian influenza viruses in a mammalian host. PLoS Pathog. 2009;5:e1000709. doi: 10.1371/journal.ppat.1000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pica N, Bouvier NM. Environmental factors affecting the transmission of respiratory viruses. Curr. Opin. Virol. 2012;2:90–95. doi: 10.1016/j.coviro.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weber TP, Stilianakis NI. Inactivation of influenza A viruses in the environment and modes of transmission: a critical review. J. Infect. 2008;57:361–373. doi: 10.1016/j.jinf.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sattar SA, et al. Activity of an alcohol-based hand gel against human adeno-, rhino-, and rotaviruses using the fingerpad method. Infect. Control. Hosp. Epidemiol. 2000;21:516–519. doi: 10.1086/501796. [DOI] [PubMed] [Google Scholar]
- 86.Luongo JC, et al. Role of mechanical ventilation in the airborne transmission of infectious agents in buildings. Indoor Air. 2016;26:666–678. doi: 10.1111/ina.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sze-To GN, Yang Y, Kwan JK, Yu SC, Chao CY. Effects of surface material, ventilation, and human behavior on indirect contact transmission risk of respiratory infection. Risk Anal. 2014;34:818–830. doi: 10.1111/risa.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lowen AC, Steel J, Mubareka S, Palese P. High temperature (30 degrees C) blocks aerosol but not contact transmission of influenza virus. J. Virol. 2008;82:5650–5652. doi: 10.1128/JVI.00325-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tamerius JD, et al. Environmental predictors of seasonal influenza epidemics across temperate and tropical climates. PLoS Pathog. 2013;9:e1003194. doi: 10.1371/journal.ppat.1003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moriyama M, Hugentobler WJ, Iwasaki A. Seasonality of respiratory viral infections. Annu. Rev. Virol. 2020;7:83–101. doi: 10.1146/annurev-virology-012420-022445. [DOI] [PubMed] [Google Scholar]
- 91.Hui KPY, et al. Tropism, replication competence, and innate immune responses of the coronavirus SARS-CoV-2 in human respiratory tract and conjunctiva: an analysis in ex-vivo and in-vitro cultures. Lancet Respir. Med. 2020;8:687–695. doi: 10.1016/S2213-2600(20)30193-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tsang TK, et al. Influenza a virus shedding and infectivity in households. J. Infect. Dis. 2015;212:1420–1428. doi: 10.1093/infdis/jiv225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Leung NHL, et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat. Med. 2020;26:676–680. doi: 10.1038/s41591-020-0843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schwarz K, Biller H, Windt H, Koch W, Hohlfeld JM. Characterization of exhaled particles from the healthy human lung–a systematic analysis in relation to pulmonary function variables. J. Aerosol Med. Pulm. Drug Deliv. 2010;23:371–379. doi: 10.1089/jamp.2009.0809. [DOI] [PubMed] [Google Scholar]
- 95.He X, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 96.Cevik M, et al. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe. 2020;2:E12–E22. doi: 10.1016/S2666-5247(20)30172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Byambasuren O, et al. Estimating the extent of asymptomatic COVID-19 and its potential for community transmission: systematic review and meta-analysis. J. Assoc. Med. Microbiol. Infect. Dis. Can. 2020 doi: 10.1101/2020.05.10.20097543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maier HE, et al. Pre-existing anti-neuraminidase antibodies are associated with shortened duration of influenza A (H1N1)pdm virus shedding and illness in naturally infected adults. Clin. Infect. Dis. 2019;70:2290–2297. doi: 10.1093/cid/ciz639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yan J, et al. Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community. Proc. Natl Acad. Sci. USA. 2018;115:1081–1086. doi: 10.1073/pnas.1716561115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hou YJ, et al. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell. 2020;182:429–446.e14. doi: 10.1016/j.cell.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Long JS, Mistry B, Haslam SM, Barclay WS. Host and viral determinants of influenza A virus species specificity. Nat. Rev. Microbiol. 2018;17:67–81. doi: 10.1038/s41579-018-0115-z. [DOI] [PubMed] [Google Scholar]
- 102.Smith, H. Human Respiratory Tract Model for Radiological Protection. Report No. 66, (The International Commission on Radiological Protection, 1994). [PubMed]
- 103.Horby P, Nguyen NY, Dunstan SJ, Baillie JK. An updated systematic review of the role of host genetics in susceptibility to influenza. Influenza Other Respir. Viruses. 2013;7(Suppl. 2):37–41. doi: 10.1111/irv.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mossong J, et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008;5:e74. doi: 10.1371/journal.pmed.0050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Boone SA, Gerba CP. Significance of fomites in the spread of respiratory and enteric viral disease. Appl. Environ. Microbiol. 2007;73:1687–1696. doi: 10.1128/AEM.02051-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Otter JA, et al. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J. Hosp. Infect. 2016;92:235–250. doi: 10.1016/j.jhin.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ikonen N, et al. Deposition of respiratory virus pathogens on frequently touched surfaces at airports. BMC Infect. Dis. 2018;18:437. doi: 10.1186/s12879-018-3150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mubareka S, et al. Transmission of influenza virus via aerosols and fomites in the guinea pig model. J. Infect. Dis. 2009;199:858–865. doi: 10.1086/597073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gwaltney JM, Hendley JO. Transmission of experimental rhinovirus infection by contaminated surfaces. Am. J. Epidemiol. 1982;116:828–833. doi: 10.1093/oxfordjournals.aje.a113473. [DOI] [PubMed] [Google Scholar]
- 110.Barker J, Stevens D, Bloomfield SF. Spread and prevention of some common viral infections in community facilities and domestic homes. J. Appl. Microbiol. 2001;91:7–21. doi: 10.1046/j.1365-2672.2001.01364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hall CB. Respiratory syncytial virus: its transmission in the hospital environment. Yale J. Biol. Med. 1982;55:219–223. [PMC free article] [PubMed] [Google Scholar]
- 112.Heung LC, Li T, Mak SK, Chan WM. Prevalence of subclinical infection and transmission of severe acute respiratory syndrome (SARS) in a residential care home for the elderly. Hong. Kong Med. J. 2006;12:201–207. [PubMed] [Google Scholar]
- 113.Morawska L, et al. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J. Aerosol Sci. 2009;40:256–269. doi: 10.1016/j.jaerosci.2008.11.002. [DOI] [Google Scholar]
- 114.Fabian P, Brain J, Houseman EA, Gern J, Milton DK. Origin of exhaled breath particles from healthy and human rhinovirus-infected subjects. J. Aerosol Med. Pulm. Drug Deliv. 2011;24:137–147. doi: 10.1089/jamp.2010.0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gralton J, Tovey ER, McLaws ML, Rawlinson WD. Respiratory virus RNA is detectable in airborne and droplet particles. J. Med. Virol. 2013;85:2151–2159. doi: 10.1002/jmv.23698. [DOI] [PubMed] [Google Scholar]
- 116.Milton DK, Fabian MP, Cowling BJ, Grantham ML, McDevitt JJ. Influenza virus aerosols in human exhaled breath: particle size, culturability, and effect of surgical masks. PLoS Pathog. 2013;9:e1003205. doi: 10.1371/journal.ppat.1003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dick EC, Jennings LC, Mink KA, Wartgow CD, Inhorn SL. Aerosol transmission of rhinovirus colds. J. Infect. Dis. 1987;156:442–448. doi: 10.1093/infdis/156.3.442. [DOI] [PubMed] [Google Scholar]
- 118.Olsen SJ, et al. Transmission of the severe acute respiratory syndrome on aircraft. N. Engl. J. Med. 2003;349:2416–2422. doi: 10.1056/NEJMoa031349. [DOI] [PubMed] [Google Scholar]
- 119.Andrewes C, Glover R. Spread of infection from the respiratory tract of the ferret. I. Transmission of influenza A virus. Br. J. Exp. Pathol. 1941;22:91. [Google Scholar]
- 120.Sia SF, et al. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature. 2020;583:834–838. doi: 10.1038/s41586-020-2342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Moser MR, et al. An outbreak of influenza aboard a commercial airliner. Am. J. Epidemiol. 1979;110:1–6. doi: 10.1093/oxfordjournals.aje.a112781. [DOI] [PubMed] [Google Scholar]
- 122.Myatt TA, et al. Detection of airborne rhinovirus and its relation to outdoor air supply in office environments. Am. J. Respir. Crit. Care Med. 2004;169:1187–1190. doi: 10.1164/rccm.200306-760OC. [DOI] [PubMed] [Google Scholar]
- 123.Offeddu V, Yung CF, Low MSF, Tam CC. Effectiveness of masks and respirators against respiratory infections in healthcare workers: a systematic review and meta-analysis. Clin. Infect. Dis. 2017;65:1934–1942. doi: 10.1093/cid/cix681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jordan WS., Jr. The mechanism of spread of Asian influenza. Am. Rev. Respir. Dis. 1961;83:29–40. doi: 10.1164/arrd.1961.83.2P2.29. [DOI] [PubMed] [Google Scholar]
- 125.Cowling BJ, et al. Facemasks and hand hygiene to prevent influenza transmission in households: a cluster randomized trial. Ann. Intern. Med. 2009;151:437–446. doi: 10.7326/0003-4819-151-7-200910060-00142. [DOI] [PubMed] [Google Scholar]
- 126.Killingley B, et al. Use of a human influenza challenge model to assess person-to-person transmission: proof-of-concept study. J. Infect. Dis. 2012;205:35–43. doi: 10.1093/infdis/jir701. [DOI] [PubMed] [Google Scholar]
- 127.Azimi P, Keshavarz Z, Cedeno Laurent JG, Stephens B, Allen JG. Mechanistic transmission modeling of COVID-19 on the Diamond Princess cruise ship demonstrates the importance of aerosol transmission. Proc. Natl Acad. Sci. USA. 2021;118:e2015482118. doi: 10.1073/pnas.2015482118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jones RM. Relative contributions of transmission routes for COVID-19 among healthcare personnel providing patient care. J. Occup. Environ. Hyg. 2020;17:408–415. doi: 10.1080/15459624.2020.1784427. [DOI] [PubMed] [Google Scholar]
- 129.Atkinson MP, Wein LM. Quantifying the routes of transmission for pandemic influenza. Bull. Math. Biol. 2008;70:820–867. doi: 10.1007/s11538-007-9281-2. [DOI] [PubMed] [Google Scholar]
- 130.Jones RM, Adida E. Influenza infection risk and predominate exposure route: uncertainty analysis. Risk Anal. 2011;31:1622–1631. doi: 10.1111/j.1539-6924.2011.01600.x. [DOI] [PubMed] [Google Scholar]
- 131.World Health Organization. Infection prevention and control of epidemic-and pandemic prone acute respiratory infections in health care - WHO guidelines. http://www.who.int/csr/bioriskreduction/infection_control/publication/en/ (2014). [PubMed]
- 132.Morawska L, Milton DK. It is time to address airborne transmission of COVID-19. Clin. Infect. Dis. 2020;71:2311–2312. doi: 10.1093/cid/ciaa939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.World Health Organization. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). https://www.who.int/publications-detail/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19) (2020).
- 134.van Doremalen N, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cheng VCC, et al. Escalating infection control response to the rapidly evolving epidemiology of the coronavirus disease 2019 (COVID-19) due to SARS-CoV-2 in Hong Kong. Infect. Control. Hosp. Epidemiol. 2020;41:493–498. doi: 10.1017/ice.2020.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ong SWX, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020;323:1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Somsen GA, van Rijn C, Kooij S, Bem RA, Bonn D. Small droplet aerosols in poorly ventilated spaces and SARS-CoV-2 transmission. Lancet Respir. Med. 2020;8:658–659. doi: 10.1016/S2213-2600(20)30245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lindsley WG, et al. Viable influenza a virus in airborne particles from human coughs. J. Occup. Environ. Hyg. 2015;12:107–113. doi: 10.1080/15459624.2014.973113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xie X, Li Y, Chwang AT, Ho PL, Seto WH. How far droplets can move in indoor environments–revisiting the Wells evaporation-falling curve. Indoor Air. 2007;17:211–225. doi: 10.1111/j.1600-0668.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- 140.Liu Y, et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582:557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- 141.Kulkarni H, et al. Evidence of respiratory syncytial virus spread by aerosol. time to revisit infection control strategies? Am. J. Respir. Crit. Care Med. 2016;194:308–316. doi: 10.1164/rccm.201509-1833OC. [DOI] [PubMed] [Google Scholar]
- 142.Kim SH, et al. Extensive viable Middle East respiratory syndrome (MERS) coronavirus contamination in air and surrounding environment in MERS isolation wards. Clin. Infect. Dis. 2016;63:363–369. doi: 10.1093/cid/ciw239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lednicky JA, et al. Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patients. Int. J. Infect. Dis. 2020;100:476–482. doi: 10.1016/j.ijid.2020.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Buonanno G, Morawska L, Stabile L. Quantitative assessment of the risk of airborne transmission of SARS-CoV-2 infection: Prospective and retrospective applications. Environ. Int. 2020;145:106112. doi: 10.1016/j.envint.2020.106112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cai J, et al. Indirect virus transmission in cluster of COVID-19 cases, Wenzhou, China, 2020. Emerg. Infect. Dis. 2020;26:1343–1345. doi: 10.3201/eid2606.200412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Miller SL, et al. Transmission of SARS-CoV-2 by inhalation of respiratory aerosol in the Skagit Valley Chorale superspreading event. Indoor Air. 2020 doi: 10.1111/ina.12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hamner L, et al. High SARS-CoV-2 attack rate following exposure at a choir practice - Skagit County, Washington, March 2020. MMWR. 2020;69:606–610. doi: 10.15585/mmwr.mm6919e6. [DOI] [PubMed] [Google Scholar]
- 148.Li, Y. et al. Evidence for probable aerosol transmission of SARS-CoV-2 in a poorly ventilated restaurant. Build. Environ.10.1016/j.buildenv.2021.107788 (2021). [DOI] [PMC free article] [PubMed]
- 149.Thomas BR. Does expert opinion trump evidence? Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chagla Z, Hota S, Khan S, Mertz D. Re: it is time to address airborne transmission of COVID-19. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Morawska L, Milton DK. Reply to Chagla et al., and Thomas. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1121. [DOI] [PubMed] [Google Scholar]
- 152.Ma, J. et al. Coronavirus disease 2019 patients in earlier stages exhaled millions of severe acute respiratory syndrome coronavirus 2 Per Hour. Clin. Infect. Dis.10.1093/cid/ciaa1283 (2020). [DOI] [PMC free article] [PubMed]
- 153.Chia PY, et al. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat. Commun. 2020;11:2800. doi: 10.1038/s41467-020-16670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Guo ZD, et al. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg. Infect. Dis. 2020;26:1583–1591. doi: 10.3201/eid2607.200885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Barclay WS, et al. Investigating SARS-CoV-2 surface and air contamination in an acute healthcare setting during the peak of the COVID-19 pandemic in London. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Santarpia JL, et al. Aerosol and surface contamination of SARS-CoV-2 observed in quarantine and isolation care. Sci. Rep. 2020;10:12732. doi: 10.1038/s41598-020-69286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bullard J, et al. Predicting infectious SARS-CoV-2 from diagnostic samples. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jang S, Han SH, Rhee JY. Cluster of coronavirus disease associated with fitness dance classes, South Korea. Emerg. Infect. Dis. 2020;26:1917–1920. doi: 10.3201/eid2608.200633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Leclerc Q, et al. What settings have been linked to SARS-CoV-2 transmission clusters? [version 1; peer review: 1 approved with reservations] Wellcome Open Res. 2020 doi: 10.12688/wellcomeopenres.15889.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tellier R. Review of aerosol transmission of influenza A virus. Emerg. Infect. Dis. 2006;12:1657–1662. doi: 10.3201/eid1211.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Xie C, et al. Detection of influenza and other respiratory viruses in air sampled from a university campus: a longitudinal study. Clin. Infect. Dis. 2019;70:580–858. doi: 10.1093/cid/ciz296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wong BC, et al. Possible role of aerosol transmission in a hospital outbreak of influenza. Clin. Infect. Dis. 2010;51:1176–1183. doi: 10.1086/656743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wells WF, Brown HW. Recovery of influenza virus suspended in air. Science. 1936;84:68–69. doi: 10.1126/science.84.2168.68-b. [DOI] [PubMed] [Google Scholar]
- 164.Coleman KK, et al. Bioaerosol sampling for respiratory viruses in Singapore’s mass rapid transit network. Sci. Rep. 2018;8:17476. doi: 10.1038/s41598-018-35896-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lindsley WG, et al. Distribution of airborne influenza virus and respiratory syncytial virus in an urgent care medical clinic. Clin. Infect. Dis. 2010;50:693–698. doi: 10.1086/650457. [DOI] [PubMed] [Google Scholar]
- 166.Leclair JM, Zaia JA, Levin MJ, Congdon RG, Goldmann DA. Airborne transmission of chickenpox in a hospital. N. Engl. J. Med. 1980;302:450–453. doi: 10.1056/NEJM198002213020807. [DOI] [PubMed] [Google Scholar]
- 167.Thomson F. The aerial conveyance of infection. Lancet. 1914;183:1669–1673. doi: 10.1016/S0140-6736(01)58406-8. [DOI] [Google Scholar]
- 168.Riley RL, et al. Aerial dissemination of pulmonary tuberculosis. A two-year study of contagion in a tuberculosis ward. Am. J. Hyg. 1959;70:185–196. [Google Scholar]
- 169.Wong TW, et al. Cluster of SARS among medical students exposed to single patient, Hong Kong. Emerg. Infect. Dis. 2004;10:269–276. doi: 10.3201/eid1002.030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liu L, Li Y, Nielsen PV, Wei J, Jensen RL. Short-range airborne transmission of expiratory droplets between two people. Indoor Air. 2017;27:452–462. doi: 10.1111/ina.12314. [DOI] [PubMed] [Google Scholar]
- 171.World Health Organization. Epidemic-prone and pandemic-prone acute respiratory diseases. Summary guidance: Infection prevention & control in health-care facilities. https://www.who.int/csr/resources/publications/aidememoireepidemicpandemid/en/ (2007).
- 172.World Health Organization. WHO Guidelines on Hand Hygiene in Health Care (World Health Organization, 2009).
- 173.Suchomel M, Steinmann J, Kampf G. Efficacies of the original and modified WHO-recommended hand rub formulations. J. Hosp. Infect. 2020;106:264–270. doi: 10.1016/j.jhin.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cowling BJ, et al. Aerosol transmission is an important mode of influenza A virus spread. Nat. Commun. 2013;4:1935. doi: 10.1038/ncomms2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.World Health Organization. Infection prevention and control during health care when novel coronavirus (nCoV) infection is suspected: interim guidance (March 2020). https://www.who.int/publications/i/item/10665-331495 (2020).
- 176.World Health Organization. Advice on the use of masks in the context of COVID-19: Interim guidance (5 June 2020). Report no. WHO/2019-nCov/IPC_Masks/2020.4 (World Health Organization, 2020).
- 177.Kwok YLA, Gralton J, McLaws M-L. Face touching: a frequent habit that has implications for hand hygiene. Am. J. Infect. Control. 2015;43:112–114. doi: 10.1016/j.ajic.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Diaz KT, Smaldone GC. Quantifying exposure risk: surgical masks and respirators. Am. J. Infect. Control. 2010;38:501–508. doi: 10.1016/j.ajic.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 179.Ueki H, et al. Effectiveness of face masks in preventing airborne transmission of SARS-CoV-2. mSphere. 2020 doi: 10.1128/mSphere.00637-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chu DK, et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bakhit M, et al. Downsides of face masks and possible mitigation strategies: a systematic review and meta-analysis. BMJ Open. 2021 doi: 10.1101/2020.06.16.20133207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020;104:246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Greatorex JS, et al. Effectiveness of common household cleaning agents in reducing the viability of human influenza A/H1N1. PLoS ONE. 2010;5:e8987. doi: 10.1371/journal.pone.0008987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wei J, Li Y. Airborne spread of infectious agents in the indoor environment. Am. J. Infect. Control. 2016;44:S102–S108. doi: 10.1016/j.ajic.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ibfelt T, Engelund EH, Schultz AC, Andersen LP. Effect of cleaning and disinfection of toys on infectious diseases and micro-organisms in daycare nurseries. J. Hosp. Infect. 2015;89:109–115. doi: 10.1016/j.jhin.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Atkinson, J. et al. Natural ventilation for infection control in health-care settings: WHO guidelines 2009. https://www.who.int/water_sanitation_health/publications/natural_ventilation/en/ (2009). [PubMed]
- 187.Sehulster, L. M. et al. Guidelines for Environmental Infection Control in Health-Care Facilities. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). https://www.cdc.gov/infectioncontrol/pdf/guidelines/environmental-guidelines-P.pdf (2004). [PubMed]
- 188.Sundell J, et al. Ventilation rates and health: multidisciplinary review of the scientific literature. Indoor Air. 2011;21:191–204. doi: 10.1111/j.1600-0668.2010.00703.x. [DOI] [PubMed] [Google Scholar]
- 189.Chen C, Zhao B, Yang X, Li Y. Role of two-way airflow owing to temperature difference in severe acute respiratory syndrome transmission: revisiting the largest nosocomial severe acute respiratory syndrome outbreak in Hong Kong. J. R. Soc. Interface. 2011;8:699–710. doi: 10.1098/rsif.2010.0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sze To GN, Wan MP, Chao CYH, Fang L, Melikov A. Experimental study of dispersion and deposition of expiratory aerosols in aircraft cabins and impact on infectious disease transmission. Aerosol Sci. Technol. 2009;43:466–485. doi: 10.1080/02786820902736658. [DOI] [Google Scholar]
- 191.Nardell EA, Keegan J, Cheney SA, Etkind SC. Airborne infection. Theoretical limits of protection achievable by building ventilation. Am. Rev. Respir. Dis. 1991;144:302–306. doi: 10.1164/ajrccm/144.2.302. [DOI] [PubMed] [Google Scholar]
- 192.Memarzadeh F, Xu W. Role of air changes per hour (ACH) in possible transmission of airborne infections. Build. Simul. 2012;5:15–28. doi: 10.1007/s12273-011-0053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Nardell EA, Nathavitharana RR. Airborne spread of SARS-CoV-2 and a potential role for air disinfection. JAMA. 2020;324:141–142. doi: 10.1001/jama.2020.7603. [DOI] [PubMed] [Google Scholar]
- 194.Centers for Disease Control and Prevention. Environmental Control for Tuberculosis: Basic Upper-Room Ultraviolet Germicidal Irradiation Guidelines for Healthcare Settings. https://www.cdc.gov/niosh/docs/2009-105/default.html (2009).
- 195.Reed NG. The History of ultraviolet germicidal irradiation for air disinfection. Public. Health Rep. 2010;125:15–27. doi: 10.1177/003335491012500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wells WF, Wells MW, Wilder TS. The environmental control of epidemic contagion: I. An epidemiologic study of radiant disinfection of air in day schools. Am. J. Epidemiol. 1942;35:97–121. doi: 10.1093/oxfordjournals.aje.a118789. [DOI] [Google Scholar]
- 197.Dancer SJ. Controlling hospital-acquired infection: focus on the role of the environment and new technologies for decontamination. Clin. Microbiol. Rev. 2014;27:665–690. doi: 10.1128/CMR.00020-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bedell K, Buchaklian AH, Perlman S. Efficacy of an automated multiple emitter whole-room ultraviolet-C disinfection system against coronaviruses MHV and MERS-CoV. Infect. Control. Hosp. Epidemiol. 2016;37:598–599. doi: 10.1017/ice.2015.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Mills D, Harnish DA, Lawrence C, Sandoval-Powers M, Heimbuch BK. Ultraviolet germicidal irradiation of influenza-contaminated N95 filtering facepiece respirators. Am. J. Infect. Control. 2018;46:e49–e55. doi: 10.1016/j.ajic.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Seresirikachorn K, et al. Decontamination and reuse of surgical masks and N95 filtering facepiece respirators during the COVID-19 pandemic: a systematic review. Infect. Control. Hosp. Epidemiol. 2020;42:25–30. doi: 10.1017/ice.2020.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.International Commission on Illumination. CIE position statement on ultraviolet (UV) radiation to manage the risk of COVID-19 transmission. http://cie.co.at/publications/cie-position-statement-use-ultraviolet-uv-radiation-manage-risk-covid-19-transmission (2020).
- 202.Gwaltney JM, Jr., Moskalski PB, Hendley JO. Interruption of experimental rhinovirus transmission. J. Infect. Dis. 1980;142:811–815. doi: 10.1093/infdis/142.6.811. [DOI] [PubMed] [Google Scholar]
- 203.Wong VW, Cowling BJ, Aiello AE. Hand hygiene and risk of influenza virus infections in the community: a systematic review and meta-analysis. Epidemiol. Infect. 2014;142:922–932. doi: 10.1017/S095026881400003X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Thangavel RR, Bouvier NM. Animal models for influenza virus pathogenesis, transmission, and immunology. J. Immunol. Methods. 2014;410:60–79. doi: 10.1016/j.jim.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lowen AC, Mubareka S, Tumpey TM, Garcia-Sastre A, Palese P. The guinea pig as a transmission model for human influenza viruses. Proc. Natl Acad. Sci. USA. 2006;103:9988–9992. doi: 10.1073/pnas.0604157103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sutton TC, Subbarao K. Development of animal models against emerging coronaviruses: from SARS to MERS coronavirus. Virology. 2015;479-480:247–258. doi: 10.1016/j.virol.2015.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lakdawala SS, Menachery VD. The search for a COVID-19 animal model. Science. 2020;368:942–943. doi: 10.1126/science.abc6141. [DOI] [PubMed] [Google Scholar]
- 208.McCray PB, Jr., et al. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J. Virol. 2007;81:813–821. doi: 10.1128/JVI.02012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Nishiura H, Yen HL, Cowling BJ. Sample size considerations for one-to-one animal transmission studies of the influenza A viruses. PLoS ONE. 2013;8:e55358. doi: 10.1371/journal.pone.0055358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Belser JA, Maines TR, Katz JM, Tumpey TM. Considerations regarding appropriate sample size for conducting ferret transmission experiments. Future Microbiol. 2013;8:961–965. doi: 10.2217/fmb.13.64. [DOI] [PubMed] [Google Scholar]
- 211.Delamater PL, Street EJ, Leslie TF, Yang YT, Jacobsen KH. Complexity of the basic reproduction number (R0) Emerg. Infect. Dis. 2019;25:1–4. doi: 10.3201/eid2501.171901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Reis J, Shaman J. Simulation of four respiratory viruses and inference of epidemiological parameters. Infect. Dis. Model. 2018;3:23–34. doi: 10.1016/j.idm.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Spencer, J. et al. Epidemiological parameter review and comparative dynamics of influenza, respiratory syncytial virus, rhinovirus, human coronavirus, and adenovirus. Preprint at 10.1101/2020.02.04.20020404v1 (2020).
- 214.Guo Z, et al. Epidemiological analysis of an outbreak of an adenovirus type 7 infection in a boot camp in China. PLoS ONE. 2020;15:e0232948. doi: 10.1371/journal.pone.0232948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Park M, Cook AR, Lim JT, Sun Y, Dickens BL. A systematic review of COVID-19 epidemiology based on current evidence. J. Clin. Med. 2020;9:967. doi: 10.3390/jcm9040967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Biggerstaff M, Cauchemez S, Reed C, Gambhir M, Finelli L. Estimates of the reproduction number for seasonal, pandemic, and zoonotic influenza: a systematic review of the literature. BMC Infect. Dis. 2014;14:480. doi: 10.1186/1471-2334-14-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Nardone A, et al. The comparative sero-epidemiology of varicella zoster virus in 11 countries in the European region. Vaccine. 2007;25:7866–7872. doi: 10.1016/j.vaccine.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 218.Guerra FM, et al. The basic reproduction number (R0) of measles: a systematic review. Lancet Infect. Dis. 2017;17:e420–e428. doi: 10.1016/S1473-3099(17)30307-9. [DOI] [PubMed] [Google Scholar]
- 219.Goeyvaerts N, et al. Estimating dynamic transmission model parameters for seasonal influenza by fitting to age and season-specific influenza-like illness incidence. Epidemics. 2015;13:1–9. doi: 10.1016/j.epidem.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 220.Zhang J, et al. Changes in contact patterns shape the dynamics of the COVID-19 outbreak in China. Science. 2020;368:1481–1486. doi: 10.1126/science.abb8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368:860–868. doi: 10.1126/science.abb5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Muller NF, et al. Characterising the epidemic spread of influenza A/H3N2 within a city through phylogenetics. PLoS Pathog. 2020;16:e1008984. doi: 10.1371/journal.ppat.1008984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lemey P, et al. Unifying viral genetics and human transportation data to predict the global transmission dynamics of human influenza H3N2. PLoS Pathog. 2014;10:e1003932. doi: 10.1371/journal.ppat.1003932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.van Dorp L, et al. No evidence for increased transmissibility from recurrent mutations in SARS-CoV-2. Nat. Commun. 2020;11:5986. doi: 10.1038/s41467-020-19818-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lloyd-Smith JO, Schreiber SJ, Kopp PE, Getz WM. Superspreading and the effect of individual variation on disease emergence. Nature. 2005;438:355–359. doi: 10.1038/nature04153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Adam DC, et al. Clustering and superspreading potential of SARS-CoV-2 infections in Hong Kong. Nat. Med. 2020;26:1714–1719. doi: 10.1038/s41591-020-1092-0. [DOI] [PubMed] [Google Scholar]
- 227.Riley S, et al. Transmission dynamics of the etiological agent of SARS in Hong Kong: Impact of public health interventions. Science. 2003;300:1961–1966. doi: 10.1126/science.1086478. [DOI] [PubMed] [Google Scholar]
- 228.Randolph HE, Barreiro LB. Herd immunity: understanding COVID-19. Immunity. 2020;52:737–741. doi: 10.1016/j.immuni.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Halloran, M. E. Secondary attack rate. In Encyclopedia of Biostatistics (eds Armitage, P. & Colton, T.) (Wiley, 2005).
- 230.Tsang TK, Lau LL, Cauchemez S, Cowling BJ. Household transmission of influenza virus. Trends Microbiol. 2016;24:123–133. doi: 10.1016/j.tim.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Lau LL, et al. Household transmission of 2009 pandemic influenza A (H1N1): a systematic review and meta-analysis. Epidemiology. 2012;23:531–542. doi: 10.1097/EDE.0b013e31825588b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Simpson RE. Infectiousness of communicable diseases in the household (measles, chickenpox, and mumps) Lancet. 1952;2:549–554. doi: 10.1016/S0140-6736(52)91357-3. [DOI] [PubMed] [Google Scholar]
- 233.Cowling BJ, et al. Comparative epidemiology of pandemic and seasonal influenza A in households. N. Engl. J. Med. 2010;362:2175–2184. doi: 10.1056/NEJMoa0911530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Leitmeyer K, Adlhoch C. Review article: influenza transmission on aircraft: a systematic literature review. Epidemiology. 2016;27:743–751. doi: 10.1097/EDE.0000000000000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Klick B, Leung GM, Cowling BJ. Optimal design of studies of influenza transmission in households. I: case-ascertained studies. Epidemiol. Infect. 2012;140:106–114. doi: 10.1017/S0950268811000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Leung NH, Xu C, Ip DK, Cowling BJ. Review article: the fraction of influenza virus infections that are asymptomatic: a systematic review and meta-analysis. Epidemiology. 2015;26:862–872. doi: 10.1097/EDE.0000000000000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Cooney MK, Fox JP, Hall CE. The Seattle Virus Watch. VI. Observations of infections with and illness due to parainfluenza, mumps and respiratory syncytial viruses and Mycoplasma pneumoniae. Am. J. Epidemiol. 1975;101:532–551. doi: 10.1093/oxfordjournals.aje.a112125. [DOI] [PubMed] [Google Scholar]
- 238.Fox JP, Cooney MK, Hall CE. The Seattle Virus Watch. V. Epidemiologic observations of rhinovirus infections, 1965–1969, in families with young children. Am. J. Epidemiol. 1975;101:122–143. doi: 10.1093/oxfordjournals.aje.a112078. [DOI] [PubMed] [Google Scholar]
- 239.Hall CB, et al. Respiratory syncytial virus infections within families. N. Engl. J. Med. 1976;294:414–419. doi: 10.1056/NEJM197602192940803. [DOI] [PubMed] [Google Scholar]
- 240.Madewell ZJ, Yang Y, Longini IM, Jr., Halloran ME, Dean NE. Household transmission of SARS-CoV-2: a systematic review and meta-analysis. JAMA Netw. Open. 2020;3:e2031756. doi: 10.1001/jamanetworkopen.2020.31756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Banatvala JE, Anderson TB, Reiss BB. Parainfluenza infections in the community. BMJ. 1964;1:537–540. doi: 10.1136/bmj.1.5382.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Seward JF, Zhang JX, Maupin TJ, Mascola L, Jumaan AO. Contagiousness of varicella in vaccinated cases: a household contact study. JAMA. 2004;292:704–708. doi: 10.1001/jama.292.6.704. [DOI] [PubMed] [Google Scholar]
- 243.Top FH. Measles in Detroit, 1935 — I, factors influencing the secondary attack rate among susceptibles at risk. Am. J. Public Health Nations Health. 1938;28:935–943. doi: 10.2105/AJPH.28.8.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Zhang W, et al. Secondary transmission of coronavirus disease from presymptomatic persons, China. Emerg. Infect. Dis. 2020;26:1924–1926. doi: 10.3201/eid2608.201142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Cauchemez S, et al. Determinants of influenza transmission in South East Asia: insights from a household cohort study in Vietnam. PLoS Pathog. 2014;10:e1004310. doi: 10.1371/journal.ppat.1004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Welliver R, et al. Effectiveness of oseltamivir in preventing influenza in household contacts: a randomized controlled trial. JAMA. 2001;285:748–754. doi: 10.1001/jama.285.6.748. [DOI] [PubMed] [Google Scholar]
- 247.McCormick JB, Halsey N, Rosenberg R. Measles vaccine efficacy determined from secondary attack rates during a severe epidemic. J. Pediatr. 1977;90:13–16. doi: 10.1016/S0022-3476(77)80756-7. [DOI] [PubMed] [Google Scholar]
- 248.Cauchemez S, et al. Household transmission of 2009 pandemic influenza A (H1N1) virus in the United States. N. Engl. J. Med. 2009;361:2619–2627. doi: 10.1056/NEJMoa0905498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lau MS, Cowling BJ, Cook AR, Riley S. Inferring influenza dynamics and control in households. Proc. Natl Acad. Sci. USA. 2015;112:9094–9099. doi: 10.1073/pnas.1423339112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Wells W, Wells M. Air-borne infection. J. Am. Med. Assoc. 1936;107:1698–1703. doi: 10.1001/jama.1936.02770470016004. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.