Abstract
Aims
Ketones have been proposed to be a ‘thrifty’ fuel for the heart and increasing cardiac ketone oxidation can be cardioprotective. However, it is unclear how much ketone oxidation can contribute to energy production in the heart, nor whether increasing ketone oxidation increases cardiac efficiency. Therefore, our goal was to determine to what extent high levels of the ketone body, β-hydroxybutyrate (βOHB), contributes to cardiac energy production, and whether this influences cardiac efficiency.
Methods and results
Isolated working mice hearts were aerobically perfused with palmitate (0.8 mM or 1.2 mM), glucose (5 mM) and increasing concentrations of βOHB (0, 0.6, 2.0 mM). Subsequently, oxidation of these substrates, cardiac function, and cardiac efficiency were assessed. Increasing βOHB concentrations increased myocardial ketone oxidation rates without affecting glucose or fatty acid oxidation rates where normal physiological levels of glucose (5 mM) and fatty acid (0.8 mM) are present. Notably, ketones became the major fuel source for the heart at 2.0 mM βOHB (at both low or high fatty acid concentrations), with the elevated ketone oxidation rates markedly increasing tricarboxylic acid (TCA) cycle activity, producing a large amount of reducing equivalents and finally, increasing myocardial oxygen consumption. However, the marked increase in ketone oxidation at high concentrations of βOHB was not accompanied by an increase in cardiac work, suggesting that a mismatch between excess reduced equivalents production from ketone oxidation and cardiac adenosine triphosphate production. Consequently, cardiac efficiency decreased when the heart was exposed to higher ketone levels.
Conclusions
We demonstrate that while ketones can become the major fuel source for the heart, they do not increase cardiac efficiency, which also underscores the importance of recognizing ketones as a major fuel source for the heart in times of starvation, consumption of a ketogenic diet or poorly controlled diabetes.
Keywords: Ketone, Energy metabolism, Heart, Uncoupling, Perfusion
1. Introduction
The heart is the most metabolically demanding organ in the body.1 To sustain contractile function, the heart meets its high demand for energy [adenosine triphosphate (ATP)] by metabolizing an array of fuels—fatty acids, glucose, lactate, ketones, and amino acids.2,3 A healthy, insulin-sensitive heart is metabolically versatile and can dynamically adapt to various physiological states (fed vs. fasted) by shifting its reliance primarily between fatty acids and glucose.2 For example, during a post-prandial state, increases in circulating insulin levels prime the heart to use glucose for energy.2 Conversely, in a fasted state where circulating levels of blood glucose are low, the heart increases its reliance on fatty acids for energy.2 Maintenance of this cardiac ‘metabolic flexibility’ (shifting fuel use from fatty acids to glucose and vice versa) is also attributed to the Randle cycle phenomenon, whereby fatty acid and glucose metabolism are reciprocally regulated.4 Loss of this metabolic flexibility, such as that seen in diabetes and heart failure, can lead to perturbed cardiac metabolic profiles and contractile dysfunction.5,6
Ketones are a source of energy that our bodies primarily use during fasting/starvation,7 though ketones are also a prominent fuel source during consumption of a ketogenic diet,8 prolonged exercise,9 or in poorly controlled diabetics.10 There are three different types of ketone bodies: β-hydroxybutyrate (βOHB), acetoacetate, and acetone,11 with the predominant ketone body found in our circulation being βOHB.12 While most studies have focused on the involvement of fatty acids and carbohydrates in cardiac intermediary energy metabolism, there has recently been a growing appreciation for ketones as an important cardiac fuel source.3 This has become increasingly apparent in light of the proposed concept that ketones are a potential ‘super-fuel’ for the heart in a diabetic setting.13,14 The notion that ketones are a ‘super-fuel’ was suggested following the demonstration that empagliflozin, an SGLT2 inhibitor used to treat type 2 diabetes, exhibited profound cardioprotective effects in diabetic patients with high cardiovascular risk.15 It has been proposed that empagliflozin-mediated increases in ketone levels could explain the cardioprotection seen in these patients by improvements in the cardiac metabolic state,13,14 specifically an increase in cardiac efficiency. However, we have shown that neither ketones nor empagliflozin improve cardiac efficiency in diabetic mice.16 Ketones have also been brought to the forefront in the setting of heart failure where we, alongside several other labs, have shown that the failing heart has increased reliance on ketones as a fuel source.17–21
The heart can be exposed to increased ketone concentrations under numerous physiological (fasting, exercise), supra-physiological (administering ketone esters, SGLT2 inhibitors, or a ketogenic diet) and pathological states (heart failure, uncontrolled diabetes). However, it is not clear to what extent ketones can be used as a source of energy by the heart, what effect increased ketone concentrations have on the metabolism of other cardiac energy substrates, or what effect increased ketones directly have on cardiac efficiency. In this study, we investigated what effect increasing ketone concentrations, specifically βOHB, has on cardiac energetics, to definitively underline its effects on cardiac metabolism, function, and efficiency in the healthy heart.
2. Methods
2.1 Animal care
All protocols involving our mice were approved by the University of Alberta Institutional Animal Care and Use Committee. All mice received treatment and care abiding by the guidelines set out by the Canadian Council on Animal Care and all procedures conformed to the guidelines from Directive 2010/63/EU of the European Parliament. All mice were kept in a temperature-controlled housing unit with a 12-h light/dark cycle. Furthermore, mice had access to water and regular chow diet ad libitum. C57BL/6J male mice were purchased from Jackson Laboratories at 12-weeks of age and after an intraperitoneal injection of sodium pentobarbital (60 mg/kg), their hearts were subjected to an isolated working heart perfusion.
2.2 Isolated working heart perfusion
Isolated working hearts from C57BL/6J male mice were perfused aerobically for 60-min, with either a low or high concentration of radiolabelled palmitate pre-bound to 3% albumin (low, 0.8 mM or high, 1.2 mM), glucose (5 mM), and three increasing concentrations of βOHB (0, 0.6, and 2.0 mM). The βOHB levels used are representative of βOHB concentrations that can acutely change under physiological conditions (fasting, intense exercise) and supra-physiological conditions (ex. with a ketone ester22) of healthy hearts with an intact insulin axis. Moreover, all hearts were perfused with and without insulin to assess βOHB’s effect on cardiac insulin sensitivity. Alongside ex vivo cardiac function, oxidation of these substrates was measured as previously described.17 In light of the pressing question of whether ketones can improve cardiac efficiency, we also measured cardiac efficiency by cannulating the pulmonary artery, and oxygen concentrations difference in the buffer were measured between the left atria and the cannulated pulmonary artery.
2.3 CoA/nucleotide levels
Frozen heart tissue was homogenized using a 6% perchloric acid (PCA)/1 mM dithiothreitol (DTT)/0.5 mM ethylenediaminetetraacetic acid (EDTA) solution and after centrifugation at 12 000×g, half of the supernatant was weighed and subjected to Ultra Performance Liquid Chromatography (UPLC) for CoA quantification. The other half of the supernatant had its pH brought to 7 using K2CO3, after which it was centrifuged, and the supernatant was weighed and subjected to UPLC for nucleotide quantification.
2.4 Triacylglycerol levels
Frozen heart tissue was homogenized in 2:1 chloroform:methanol solution, after which samples were centrifuged at 3500 rpm and the supernatant was quantified and transferred to new tubes. CaCl2 was then added to the supernatant to separate the mixture into two phases. Following centrifugation at 2400 rpm, the upper phase was removed and the interface was rinsed with pure solvent three times. Next, methanol was added to get one phase and samples were dried under N2 gas at 60°C. Finally, samples were re-dissolved in 3:2 tert-butyl alcohol:triton X-100/methyl alcohol (1:1) mixture and then triacylglycerol (TAG) levels were determined using the Wako Triglyceride E Kit.
2.5 Immunoblotting
Frozen myocardial tissue was homogenized using a ‘TissueLyser’, and lysate was extracted after which a Bradford protein assay was carried out. After samples were prepared, they were resolved via gel electrophoresis [sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE)] and following transfer to a nitrocellulose membrane, immunoblotting was carried out.
2.6 Statistical analysis
Data are presented as mean ± standard error of the mean (SEM). Statistically significant differences were determined by an unpaired, two-tailed Student’s t-test, one-way or two-way analysis of variance (ANOVA) followed by a Bonferroni post hoc test. Commercially available software was used to conduct the statistical analysis (GraphPad Prism V7). Differences were deemed statistically significant when P < 0.05.
3. Results
3.1 Ketones are an unregulated fuel source in the heart and are oxidized in proportion to their availability independent of the Randle cycle
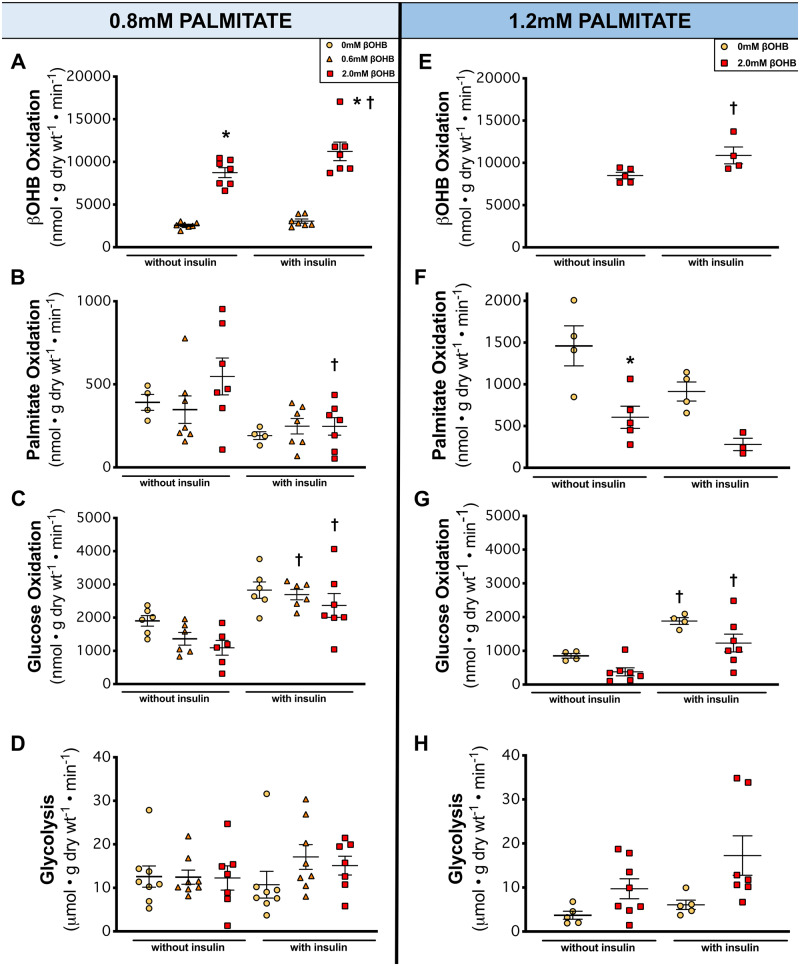
Circulating ketone levels have the capacity to significantly increase under various physiological and pathophysiological conditions.23 Using methodology we developed to directly measure ketone oxidation in the heart,17 we subjected isolated working mice hearts to increasing concentrations of βOHB that were physiologically relevant to a mildly ketotic (0.6 mM) and a higher ketotic state (2.0 mM).23 Furthermore, to mimic a ketogenic condition (high fat), we perfused hearts at two concentrations of palmitate, 0.8 and 1.2 mM.24 This was also an important and intentional aspect of the study because physiologically speaking, one would not see high blood ketone levels unless high fat was available.25 Increasing βOHB concentrations increased myocardial ketone oxidation rates in a first-order kinetics-manner (Figure 1A,E), agreeably with previous findings from Sultan et al.26 To our surprise though, the absolute rates of βOHB oxidation reached 11 233 nmol/g dry weight/min in hearts perfused with 2.0 mM βOHB with insulin, a considerably high oxidative rate compared to previous reports on myocardial ketone oxidation.26,27 The addition of insulin to assess myocardial insulin sensitivity did increase myocardial ketone oxidation rates at 2.0 mM βOHB, interestingly suggesting that myocardial ketone oxidation may be positively regulated by insulin at higher ketone concentrations (Figure 1A,E).
Figure 1.
The metabolic profile of the healthy heart perfused at increasing concentrations of βOHB in the presence of 0.8 mM or 1.2 mM palmitate. (A, E) βOHB (ketone body) oxidation (n = 4–7). (B, F) Palmitate (fatty acid) oxidation (n = 3–7). (C, G) Glucose oxidation (n = 6–7). (D, H) Glycolysis (n = 5–9). Hearts perfused with 0 mM βOHB are represented by yellow circles, 0.6 mM βOHB by orange triangles, and 2.0 mM βOHB by red squares. A one-way ANOVA with Bonferroni correction for multiple comparisons was carried out for each panel in this figure. Data are expressed as mean ± SEM. *P < 0.05 compared to the 0 mM βOHB group except for A where *P < 0.05 compared to the 0.6 mM βOHB. †P < 0.05 compared to the without insulin group.
As expected, increasing the fatty acid concentration to 1.2 mM palmitate resulted in elevated cardiac palmitate oxidation rates (Figure 1F) compared to 0.8 mM palmitate (Figure 1B), although this increase in palmitate concentration did not affect βOHB oxidation rates (Figure 1E vs. A). However, glucose oxidation rates were lower at 1.2 mM palmitate (Figure 1G) than at 0.8 mM palmitate (Figure 1C), consistent with classical Randle cycle regulation.4 The addition of insulin in these hearts also confirmed that these hearts were insulin sensitive as seen by significant increases in glucose oxidation rates (Figure 1C,G) and decreases in palmitate oxidation rates (Figure 1B,F). It should also be noted that our cardiac energy metabolism profiles in the presence of low/high βOHB or palmitate concentrations were independent of changes in the content of short chain CoA esters (e.g. succinyl CoA, malonyl CoA, acetyl CoA, etc.) (Supplementary material online, Figure S1).
Surprisingly, despite an intact Randle cycle and cardiac metabolic flexibility in response to insulin, increasing βOHB to 2.0 mM (which markedly increased ketone oxidation rates) had no effect on glucose oxidation rates (Figure 1C,G), although there was a trend to a decrease glucose oxidation rates at 2.0 mM but did not reach the threshold of statistical significance (P > 0.05). Glycolysis rates were also unaffected by increasing βOHB concentrations (Figure 1D,H). Palmitate oxidation rates were also unaffected by increasing concentrations of βOHB at 0.8 mM palmitate (Figure 1B), but at 1.2 mM palmitate, 2.0 mM βOHB did have an inhibitory effect on palmitate oxidation rates (Figure 1F). Since endogenous TAG turnover is a potential source of fatty acids for β-oxidation, we also measured the TAG content in the hearts at the end of the perfusion (Supplementary material online, Figure S2A,D), as well as radiolabelled fatty acid content of palmitate in myocardial TAG (Supplementary material online, Figure S2B,E). No changes in TAG content or the radiolabelled content in TAG was seen with high ketones, suggesting that high ketone concentrations were not affecting TAG contribution of fatty acids for β-oxidation (Supplementary material online, Figure S2).
Since cardiac work is an important determinant of the heart’s oxidative capacity and oxidative rates,28,29 we normalized all absolute metabolic rates (Figure 1) to cardiac work (Supplementary material online, Figure S3) and found no significant differences between absolute and normalized metabolic rates.
While glucose oxidation and fatty acid oxidation, both of which had substantially lower absolute rates than βOHB oxidation (Figure 1B,C,F–G), are reciprocally regulated via the Randle cycle, myocardial ketone oxidation appears to be unregulated by this cycle, and consequently the heart has a powerful capacity to increase the oxidation of ketones in proportion to its availability.
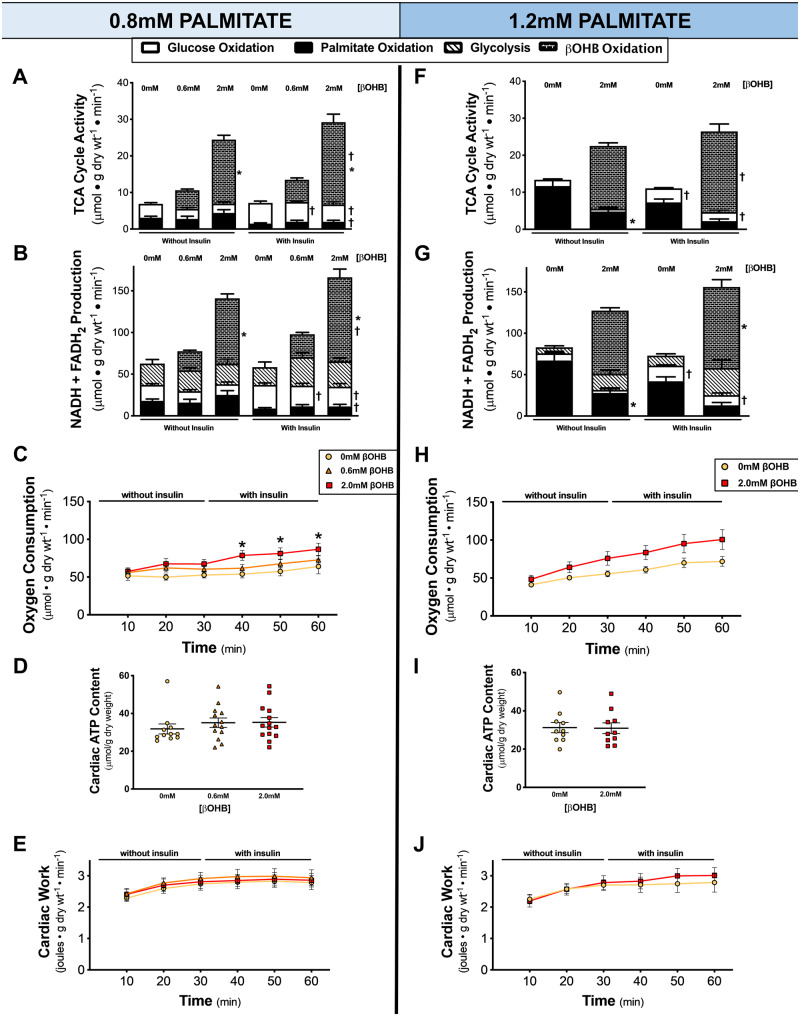
3.2 Increasing ketone concentrations markedly increases cardiac TCA cycle activity and ketones can become the major fuel of the heart
Since our isolated working heart perfusion method of assessing cardiac energy metabolism allows for direct quantification of acetyl CoA entering the TCA cycle, the consequence of increasing βOHB concentration’s on TCA cycle activity was directly assessed. Increasing βOHB concentration to 2.0 mM resulted in a three-fold increase in total TCA cycle activity, with βOHB oxidation having the greatest contribution of acetyl CoA for the TCA cycle at both 0.8 mM and 1.2 mM palmitate (Figure 2A,F). This robust increase in myocardial ketone oxidation rates at higher ketone concentrations (Figures1A and2E) were translated into marked increases in TCA cycle activity and NADH + FADH2 production (Figure 2B,G). As a result, at high ketone concentrations, ketones became the major fuel of the heart (Supplementary material online, Figure S4).
Figure 2.
The effect of increasing ketone levels on TCA cycle activity, oxygen consumption, cardiac ATP content and cardiac work. (A, F) TCA cycle activity from glucose oxidation (n = 4–7), palmitate oxidation (n = 3–7), or βOHB oxidation (n = 4–7). (B, G) Total NADH and FADH2 produced as calculated from glycolysis (n = 5–8), glucose oxidation (n = 4–7), palmitate oxidation (n = 3–7), or βOHB oxidation (n = 4–7) rates. (C, H) Myocardial oxygen consumption rates at 10-min intervals for the duration of the perfusion (n = 9–13). (D, I) Total cardiac adenosine triphosphate (ATP) content as determined from heart tissue snap-frozen at the end of the perfusion by UPLC (n = 10–14). (E, J) Ex vivo cardiac work measured at each time interval during the perfusion (n = 10–15). Hearts perfused with 0 mM βOHB are represented by yellow circles, 0.6 mM βOHB by orange triangles, and 2.0 mM βOHB by red squares. A two-way ANOVA with Bonferroni correction for multiple comparisons was carried out for each panel in this figure. Data are expressed as mean ± SEM. *P < 0.05 compared to the 0 mM βOHB group (except in A, B, F, G where *P < 0.05 compared to the 0.6 mM βOHB group when comparing βOHB oxidation). †P < 0.05 compared to the without insulin group.
Myocardial oxygen consumption was also assessed throughout the heart perfusions. Increasing βOHB concentration to 2.0 mM resulted in a significant increase in oxygen consumption at 0.8 mM palmitate (Figure 2C) and a trend to also increase oxygen consumption at 1.2 mM palmitate (Figure 2H). However, while myocardial oxygen consumption proportionally increased alongside the increased TCA cycle activity (Figure 2A,F) and NADH + FADH2 production (Figure 2B,G), this was not translated into changes in cardiac ATP levels (Figure 2D,I) or cardiac work (Figure 2E,J). As a result, βOHB-mediated TCA cycle activity increases in reducing equivalents were accompanied by increases in myocardial oxygen consumption but did not translate into increases in cardiac ATP content or cardiac function.
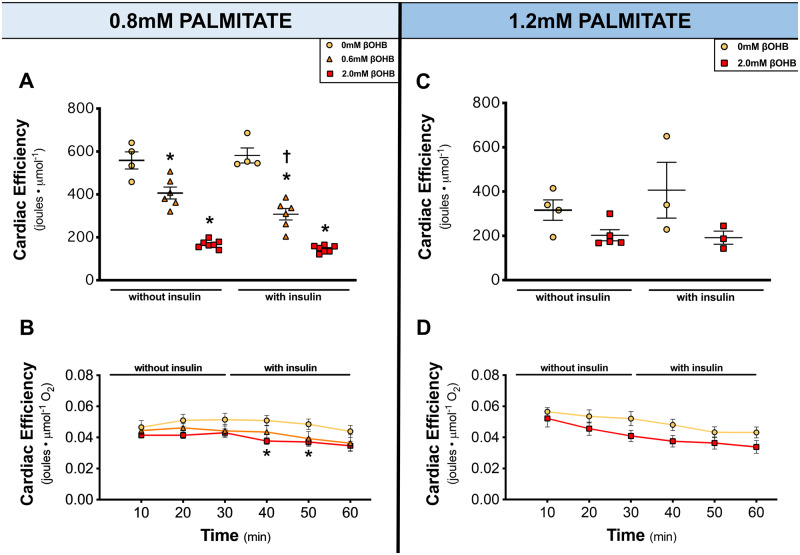
3.3 Increasing ketone concentrations decreases cardiac efficiency
Since significant increases in myocardial ketone oxidation rates, TCA cycle activity and NADH + FADH2 production were uncoupled from changes in cardiac work, we analysed cardiac efficiency. Cardiac efficiency was determined by two ways: cardiac work normalized to total TCA cycle activity and cardiac work normalized to oxygen consumption. As βOHB concentration increased, cardiac efficiency significantly decreased (Figure 3A,B) at 0.8 mM palmitate and trended to decrease at 1.2 mM palmitate (Figure 3C,D). This shows that while the heart can readily oxidize ketones, it is not a more efficient source of fuel.
Figure 3.
Ketones decrease cardiac efficiency. (A, C) Cardiac efficiency as calculated by cardiac work normalized to total TCA cycle activity (n = 3–7). (B, D) Cardiac efficiency as calculated by cardiac work normalized to oxygen consumption (n = 8–13). Hearts perfused with 0 mM βOHB are represented by yellow circles, 0.6 mM βOHB by orange triangles, and 2.0 mM βOHB by red squares. A one-way ANOVA with Bonferroni correction for multiple comparisons was carried out for (A, C) while a two-way ANOVA with Bonferroni correction for multiple comparisons was carried out for (B, D). Data are expressed as mean ± SEM. *P < 0.05 compared to the 0 mM βOHB group. †P < 0.05 compared to the without insulin group.
4. Discussion
This study provides several important novel observations. First, through direct measurement of myocardial ketone oxidation rates and competing energy substrate rates, we found that ketones can become the major fuel of the healthy heart. Second, our experimental model is novel in that it allows for the first time measurement of the coupling of myocardial NADH and FADH2 production and oxygen consumption to contractile function in the heart. Third, while our study confirms that glucose and fatty acid metabolism are reciprocally regulated, ketones had no inhibitory effect on glucose oxidation and its oxidation increases in an unregulated manner proportional to its availability. Fourth, through direct measurement of reducing equivalent production rates simultaneously with cardiac oxygen consumption and cardiac work, we found that increasing ketone availability to the heart was not accompanied by changes in cardiac function despite the heart’s high capacity to oxidize ketones. As a result, we show that increasing ketone availability to the heart decreases cardiac efficiency, challenging the notion that ketones are a ‘thrifty fuel’ for the heart. Fifth, we demonstrate that when the heart is provided with an excess of energy substrates, such as high ketones, the enhanced supply of reducing equivalents becomes uncoupled from ATP production rates, leading to a decrease in cardiac efficiency.
Despite a growing and overwhelming interest in the role of ketones in the failing and diabetic heart, no previous study has directly assessed the actual contribution of ketones to cardiac energy metabolism. The importance of this is further implicated when considering that many scenarios can physiologically, supra-physiologically, and pathologically increase circulating ketone levels and thus, its availability to the heart. Ketones have also been proposed to be a ‘thrifty fuel’ or ‘super fuel’ for the heart,13,14 although we have previously challenged this concept.30 In this study, we demonstrate that the heart does have a powerful capacity to oxidize ketones in proportion to its availability, and at high concentrations, ketones can actually become the major fuel of the heart. Of importance, we also show that despite marked increases in the contribution of ketone oxidation to TCA cycle activity at high concentrations of ketones, this was not accompanied by decreases in the contribution of glucose oxidation or fatty acid oxidation to TCA cycle activity. This could have important implications in the setting of heart failure, where providing ketones could be a potential extra source of energy for a starved heart,31 without the concern that other sources of energy production would be inhibited. A previous study by Stanley et al.32 investigated the impact of co-infusion of βOHB with fat emulsion on myocardial fatty acid uptake in vivo using an anaesthetized pig model. An important point of this study is that the measurement of myocardial fatty acid oxidation was based on the assumption that fatty acid uptake equals fatty acid oxidation. However, a growing body of evidence has shown that this is not always the case and that enhanced myocardial uptake of fatty acid does not always mean it is going to be oxidized by the heart since it can be accumulated in the myocardium or stored at TAG. Considering that neither cardiac ATP nor TAG were measured in that study, it is not clear how these changes in myocardial uptake fatty acid and βOHB actually influenced cardiac fatty acid and βOHB oxidation, cardiac ATP levels or endogenous stores of fatty acids in the heart. However, the Stanley et al.32 study does supports the novel findings of our submitted study that ketone’s contribution to cardiac ATP production can be increased by enhancing circulating levels of ketone with no inhibitory effect on cardiac glucose oxidation or no changes to cardiac function.
We also demonstrate that ketones are not a more efficient energy substrate compared to other carbon substrates, and that increases in ketone oxidation in the healthy heart are actually accompanied by decreases in cardiac efficiency. Increasing ketone concentrations in the healthy heart had no impact on cardiac function despite the fact that both TCA cycle activity and oxygen consumption increased, the result of which was an actual decrease in cardiac efficiency. In the failing heart, we showed that the increase in TCA cycle and oxygen consumption seen with increasing ketone concentrations is matched by an increase in cardiac work, resulting in no change in cardiac efficiency.17 Combined, these results suggest that ketones are a potential extra source of fuel for the heart, albeit without increasing cardiac efficiency.
Through reciprocal regulation of glucose and palmitate oxidation, one would expect that an increase in ketone oxidation, as with increased fatty acid oxidation, would result in competition for acetyl CoA supply for the TCA cycle. However, to our surprise, significantly increasing ketone oxidation did not result in any form of glucose oxidation inhibition, and palmitate oxidation was only inhibited at 1.2 mM palmitate by 2.0 mM βOHB. This suggests that ketones are not subjected to the classic Randle Cycle phenomena that glucose and fatty acids are. Indeed, while raising fatty acid concentrations significantly decreased glucose oxidation as expected (Figure 1C,G), both in the presence or absence of insulin, raising ketone concentrations did not alter glucose oxidation rates. Interestingly, we found that increasing βOHB or palmitate concentration had no effect on the phosphorylation of pyruvate dehydrogenase, a key ‘feedback inhibited’ enzyme that contributes to the interplay between glucose and palmitate oxidation (Supplementary material online, Figure S5). Regardless, the fact that ketones had no effect on glucose oxidation or the phosphorylation of its rate-limiting enzyme (pyruvate dehydrogenase), has potential significance in the setting of heart failure, where stimulating glucose oxidation can benefit the failing heart.5 It suggests that increasing ketone oxidation may not compromise glucose oxidation, and therefore heart function, in the failing heart. Our findings, while in a healthy setting, are not consistent with a recent study claiming that the failing myocardium treated with empagliflozin (increases circulating ketone levels) switches its fuel use away from glucose to ketones. Their findings suggest that ketones decrease glucose use, and this results in an increase in cardiac efficiency. However, the authors do not measure glucose oxidation and only measure glucose uptake.33 Therefore, further studies are still needed to directly assess the interaction between ketone oxidation and glucose oxidation in the failing heart.
We also demonstrate that the main determinant of how much ketones are used as an energy source by the heart is the concentration of ketones to which the heart is exposed. Insulin is an important regulator of energy metabolism in the heart, with increasing insulin concentrations resulting in an increase in glucose oxidation (Figure 1C,G) and a decrease in fatty acid oxidation (Figure 1B,F).2,34 In contrast, at lower concentrations of ketones, insulin had no effect on ketone oxidation rates (Figure 1A).26,35 Interestingly, insulin did have a mild stimulatory effect on ketone oxidation at high ketone concentrations (Figure 1A,B), although the mechanism by which this occurred is not clear.
Notably, increasing βOHB concentrations resulted in an uninhibited three-fold increase in TCA cycle activity and at 2.0 mM βOHB, ketones became the major fuel source for the heart (Supplementary material online, Figure S4). Our results support early work from Jeffrey et al.36 who also demonstrated that acetoacetate (the second most abundant ketone body found in our circulation) contributed up to 78% of acetyl-CoA in an isolated working rat heart in a fasted state where ketone levels were also elevated. However, in addition to not having used βOHB as the substrate, Jeffrey et al.36 did not report any glucose oxidation rates as they were too low and entirely suppressed by their competing substrates. In contrast, we did not recapitulate these findings and instead, present novel data that despite βOHB becoming the major fuel for the heart, βOHB did not exhibit any effects on myocardial glucose oxidation rates.
The consequence of an unregulated and increased reliance on ketones for energy ultimately resulted in depressed cardiac efficiency (Figure 3). Through the metabolism of glucose, palmitate and βOHB, the hydrogen carriers NAD+ and FAD are reduced to NADH and FADH2, respectively. The reduced equivalents then carry the hydrogens to the electron transport chain where they are used to reduce oxygen to water. Their oxidation also results in proton pumping and the subsequent proton motive force which is used for ATP synthesis. We found high βOHB causes excess supply of NADH and FADH2 that was directly measured for the first time in this study. Interestingly, this excess supply was not accompanied with an increase in ATP levels (Figure 2I), cardiac function (Figure 2J) or additionally quantified nucleotides (Supplementary material online, Figure S6). We also found that there was an increase in myocardial oxygen consumption in the high βOHB perfused hearts, which supports an increase in electron transport chain (ETC) activity that accompanied the increase in NADH and FADH2 supply. Interestingly, this increase in oxygen consumption was not accompanied by an increase or a decrease in cardiac work, suggesting there is no change in ATP use by the contractile proteins. Together, there are two possibilities that may explain this disassociation between the excess supply of reducing equivalents and no change in cardiac ATP levels. One possibility is that ATP production increased in the presence of high levels of βOHB, but that high ßOHB’s are decreasing the efficiency of the contractile proteins causing an excessive ATP hydrolysis. The second possibility is that the increase in ETC activity, evidenced by an increase supply of NADH and FADH2 and oxygen consumption, becomes uncoupled from ATP production. However, we believe the first possibility is unlikely, and, to the best of our knowledge, there is no evidence in the literature that supports this hypothesis. Cardiac work, which is the major consumer of ATP in the heart, did not change in the presence of excess ßOHB levels, and there no evidence in the literature that ketones decrease the efficiency of the contractile proteins. As a result, this suggests that excess NADH and FADH2 supply from the high ketone oxidation rates became uncoupled from ATP production in the ETC. In line with our findings, perfusing rat hearts with high levels of lactate (8 mM) and palmitate (1.2 mM) also causes an excessive supply of reducing equivalents, with no effect on the heart’s contractile function.37 Therefore, it seems plausable to suggest that the heart is equipped with a machinery to uncouple reduced equivalent supply from ATP production to handle the excessive supply of energy in times of ‘super plenty’ (excessive supply of reducing equivalents).
While mitochondrial uncoupling has been well described,38 it is still unclear exactly how this occurs. A likely scenario is that the proton motive force that normally drives ATP production is reduced by allowing H+ to flow to the mitochondrial matrix, instead of building up in the mitochondrial intermembrane space. Uncoupling proteins (UCPs) are mitochondrial inner membrane proteins that are regulated proton channels. UCPs are thus capable of dissipating the proton gradient generated by reducing equivalents-powered pumping of protons between mitochondrial intermembrane space and the mitochondrial matrix. UCPs have also been implicated in regulating the mitochondrial membrane potential by channelling H+ to the mitochondrial matrix. UCP 2 and 3 are present in the heart mitochondria and are classically known for their roles in thermogenesis and regulation of mitochondrial production of reactive oxygen species, energy expenditure, and obesity.39–41 The role of UCPs in the healthy heart is not likely to be to promote gross thermogenesis, since the heart is not a ‘thermogenic organ’ like brown adipose tissue. Instead, UCP2 and UCP3’s roles in fatty acid export and attenuation of mitochondrial reactive oxygen species are thought to be important in protecting the heart in pathological conditions and can affect cardiac efficiency.42,43 Taken together, UCPs are potential candidates that can allow H+ to flow to the mitochondrial matrix to decrease the proton motive force and consequently ATP production in times of super plenty such as high ketone supply to the heart (Figure 4). In a number of conditions expression of UCPs increases under conditions suggestive of mitochondrial uncoupling.42,44,45 However, in our study, increasing ßOHB supply resulted in an immediate increase in reduced equivalent supply, suggesting that an increased expression of UCPs could not explain the proposed uncoupling that we observed. The possibility exists that excess ketone supply could directly affect the activity of the UCPs, although this has yet to be determined.
Figure 4.
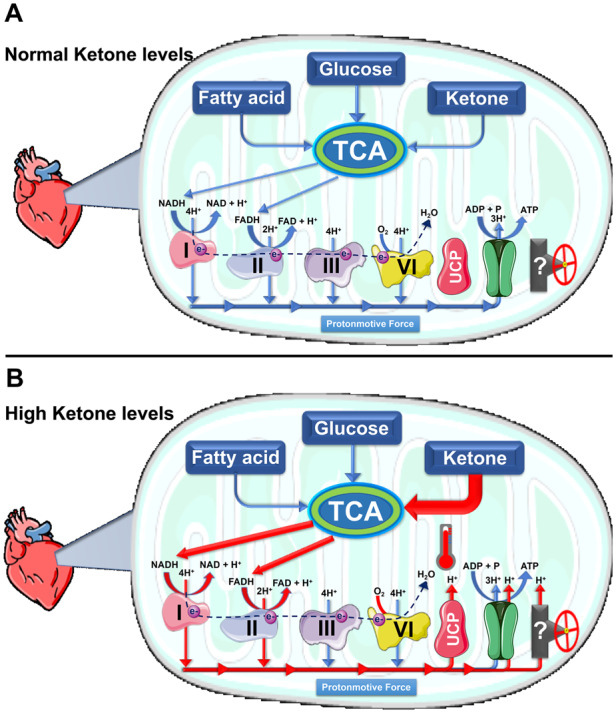
Proposed mechanism for preserving the capacity of ATP production in the event of high ketone-derived excess of reducing equivalents and the absence of cardiac work-load. (A) The flow of reducing equivalents to the electron transport chain to be oxidized to provide hydrogen (H+) and electron to build up the proton motive force that drives the production ATP. (B) Our proposed role of uncoupling proteins (UCPs) and/or ATP synthase in preserving the mitochondrial capacity of ATP production in the event of a high supply of reducing equivalents in the absence of cardiac work demand for ATP production. We proposed that such excess in reducing equivalents supply, derived from unregulated oxidation of substrates like ketones, triggers the activity of UCPs and/or ATP synthase to reduce proton motive force and allow H+ to flow to the mitochondrial matrix. This will prevent excessive production of ATP when it is not needed and preserve the limit, unreplaceable amount of nucleotide (i.e. ADP) that is available for ATP production. This proposed mechanism do not exclude the possibility that there could be another undefined candidate(s) that can be responsible for mitigating the sudden supraphysiological supply of reducing equivalents due to an unregulated fuel source (such as ketones) when there is no cardiac work demand. ADP, adenosine diphosphate.
Another potential candidate contributing to uncoupling is the mitochondrial permeability transition pore (PTP). PTP is a non-selective channel between the inner membrane and mitochondrial matrix that allows the passage of molecules <1.5 kDa including H+,46–48 and it regulates mitochondrial membrane potential.49,50 Although still controversial, recent studies have suggested that PTP could be an uncoupling channel within the c-subunit ring of the mitochondrial ATP synthase (Complex V) that allows H+ to flow to the mitochondrial matrix in time of stress.51,52 While ATP synthase normally functions as a H+ pore that couples proton motive force to ATP synthesis, this H+ flux may theoretically become uncoupled from ATP synthesis. It is possible that ATP synthase allows H+ to flow from the intermembrane space to the mitochondrial matrix uncoupled from ATP synthesis in time of super plenty, such as excess ketone oxidation rates. This is an exciting area to be explored in future investigations.
These proposed mechanisms do not exclude the possibility that there could be another undefined candidate(s) which can be responsible for mitigating the sudden supraphysiological supply of reducing equivalents due to an unregulated fuel source (such as ketones) when there is no increase in cardiac work demand. The fact that cardiac work neither increased nor decreased with the addition of ketones suggests that the increases in the oxidation of this excess supply of reducing equivalents resulted in a subsequent ‘mild’ uncoupling in which there was a lowered proton motive force, but ATP synthesis was still normal. As the law of thermodynamics states that energy cannot be created or destroyed,53 the ‘mild’ decrease of this proton motive force is likely dissipated as heat (Figure 4).
While our study looked at the acute effects of βOHB on cardiac metabolism, function, and efficiency, it is important to note that scenarios that result in chronic elevations in ketone levels (as with a ketogenic diet or long-term ketone ester consumption) introduce another layer of complexity due to eventual physiological compensatory adaptations in transcriptional machinery as previously reported by Wentz et al.27 Instead, the acute design of our study allows for us to definitively assess the effect of ketones on cardiac energetics independent of transcriptional compensatory changes. Therefore, regardless of what ketones are doing acutely or chronically, the direct effect of βOHB on cardiac efficiency still stands—it does not improve cardiac efficiency.
To conclude, we present novel data in an animal model showing that βOHB is oxidized in an unregulated manner proportional to its delivery. Importantly, increasing βOHB concentration resulted in marked increases to TCA cycle activity without any significant decrease in glucose oxidation or palmitate oxidation. As a result, increasing βOHB led to an accumulation of reduced equivalents, and increases in myocardial oxygen consumption, all of which were uncoupled from ATP production and cardiac function, ultimately decreasing cardiac efficiency. Our findings not only indicate that acute ketotic milieus can significantly impact cardiac energetics but also underscore the importance of appreciating ketones as a major fuel source for the heart that do not exhibit substrate competition or increase cardiac efficiency.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author (Dr Gary D. Lopaschuk).
Translational perspective
Recent clinical interest has focused on ketones as a potential fuel source for the failing heart, primarily because ketones have been popularized as a ‘thrifty’ fuel that may increase cardiac efficiency. However, we have directly assessed cardiac ketone oxidation rates alongside their competing energy substrates and found that: not only can ketones become the major fuel of the heart with no inhibitory effect on cardiac glucose oxidation, but they can provide the healthy heart with an excess energy supply, with no changes to cardiac function, resulting in a mismatch between reducing equivalents supply and cardiac ATP production, ultimately contributing to a decrease in cardiac efficiency.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Authors’ contributions
K.H., Q.K., C.W., L.Z., K.V., T.A., G.U., carried out the experiments, analysed data, and contributed to the writing of the manuscript. J.U. and G.L. designed the study, planned the experiments, analysed data, and contributed to the writing of the manuscript. Dr Gary D. Lopaschuk is the primary person (guarantor) for the contents of the article.
Supplementary Material
Acknowledgements
We would like to acknowledge Ms Teresa Leone and Dr. Daniel Kelly (Penn Cardiovascular Institute and Perelman School of Medicine, University of Pennsylvania, Pennsylvania, USA) as well as Dr. Deborah Muoio (Duke Molecular Physiology Institute and Sarah W. Stedman Nutrition and Metabolism Center, Durham, NC, USA) for their help and contribution to this study.
Conflict of interest: G.D.L. is a shareholder of Metabolic Modulators Research Ltd and has received grant support from Servier, Boehringer Ingelheim, Sanofi and REMED Biopharmaceuticals. The other authors have no additional conflicts of interest relevant to this article to disclose.
Funding
This work was supported by the Canadian Institutes of Health Research [Foundation Grant to Dr. G. Lopaschuk]. K.H. is a student supported by the Alberta Innovates Graduate Studentship, Motyl Graduate Studentship in Cardiac Sciences from the University of Alberta Faculty of Medicine and Dentistry and has also received funding from the Alberta Diabetes Institute. Q.K. is supported by Alberta Innovates Postgraduate Fellowship in Health Innovation.
Time for primary review: 23 days
References
- 1. Wang Z, Ying Z, Bosy-Westphal A, Zhang J, Schautz B, Later W, Heymsfield SB, Müller MJ.. Specific metabolic rates of major organs and tissues across adulthood: evaluation by mechanistic model of resting energy expenditure. Am J Clin Nutr 2010;92:1369–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lopaschuk GD, Ussher JR, Folmes CDL, Jaswal JS, Stanley WC.. Myocardial fatty acid metabolism in health and disease. Physiol Rev 2010;90:207–258. [DOI] [PubMed] [Google Scholar]
- 3. Lopaschuk GD, Ussher JR.. Evolving concepts of myocardial energy metabolism. Circ Res 2016;119:1173–1176. [DOI] [PubMed] [Google Scholar]
- 4. Randle PJ, Garland PB, Hales CN, Newsholme EA.. The glucose fatty-acid cycle its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963;281:785–789. [DOI] [PubMed] [Google Scholar]
- 5. Lopaschuk GD. Metabolic modulators in heart disease: past, present, and future. Can J Cardiol 2017;33:838–849. [DOI] [PubMed] [Google Scholar]
- 6. Karwi QG, Uddin GM, Ho KL, Lopaschuk GD.. Loss of metabolic flexibility in the failing heart. Front Cardiovasc Med 2018;5:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cahill GF Jr, Veech RL.. Ketoacids? Good medicine? Trans Am Clin Climatol Assoc 2003;114:143–149. [PMC free article] [PubMed] [Google Scholar]
- 8. Gilbert DL, Pyzik PL, Freeman JM.. The ketogenic diet: seizure control correlates better with serum beta-hydroxybutyrate than with urine ketones. J Child Neurol 2000;15:787–790. [DOI] [PubMed] [Google Scholar]
- 9. Koeslag JH, Noakes TD, Sloan AW.. Post-exercise ketosis. J Physiol 1980;301:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev 1999;15:412–426. [DOI] [PubMed] [Google Scholar]
- 11. Puchalska P, Crawford PA.. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab 2017;25:262–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Newman JC, Verdin E.. Ketone bodies as signaling metabolites. Trends Endocrinol Metab 2014;25:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mudaliar S, Alloju S, Henry RR.. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME study? A unifying hypothesis. Diabetes Care 2016;39:1115–1122. [DOI] [PubMed] [Google Scholar]
- 14. Ferrannini E, Mark M, Mayoux E.. CV Protection in the EMPA-REG OUTCOME Trial: a “thrifty substrate” hypothesis. Diabetes Care 2016;39:1108–1114. [DOI] [PubMed] [Google Scholar]
- 15. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE.. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 16. Verma S, Rawat S, Ho KL, Wagg CS, Zhang L, Teoh H, Dyck JE, Uddin GM, Oudit GY, Mayoux E, Lehrke M, Marx N, Lopaschuk GD.. Empagliflozin increases cardiac energy production in diabetes: novel translational insights into the heart failure benefits of SGLT2 inhibitors. JACC Basic Transl Sci 2018;3:575–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ho KL, Zhang L, Wagg C, Al Batran R, Gopal K, Levasseur J, Leone T, Dyck JRB, Ussher JR, Muoio DM, Kelly DP, Lopaschuk GD.. Increased ketone body oxidation provides additional energy for the failing heart without improving cardiac efficiency. Cardiovasc Res 2019;115:1606–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aubert G, Martin OJ, Horton JL, Lai L, Vega RB, Leone TC, Koves T, Gardell SJ, Krüger M, Hoppel CL, Lewandowski ED, Crawford PA, Muoio DM, Kelly DP.. The failing heart relies on ketone bodies as a fuel. Circulation 2016;133:698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bedi KC, Snyder NW, Brandimarto J, Aziz M, Mesaros C, Worth AJ, Wang LL, Javaheri A, Blair IA, Margulies KB, Rame JE.. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation 2016;133:706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Uchihashi M, Hoshino A, Okawa Y, Ariyoshi M, Kaimoto S, Tateishi S, Ono K, Yamanaka R, Hato D, Fushimura Y, Honda S, Fukai K, Higuchi Y, Ogata T, Iwai-Kanai E, Matoba S.. Cardiac-specific Bdh1 overexpression ameliorates oxidative stress and cardiac remodeling in pressure overload-induced heart failure. Circ Heart Fail 2017;10. [DOI] [PubMed] [Google Scholar]
- 21. Horton JL, Davidson MT, Kurishima C, Vega RB, Powers JC, Matsuura TR, Petucci C, Lewandowski ED, Crawford PA, Muoio DM, Recchia FA, Kelly DP.. The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight 2019;4: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cox Pete J, Kirk T, Ashmore T, Willerton K, Evans R, Smith A, Murray Andrew J, Stubbs B, West J, McLure Stewart W, King MT, Dodd Michael S, Holloway C, Neubauer S, Drawer S, Veech Richard L, Griffin Julian L, Clarke K.. Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Metab 2016;24:256–268. [DOI] [PubMed] [Google Scholar]
- 23. Miller VJ, Villamena FA, Volek JS.. Nutritional ketosis and mitohormesis: potential implications for mitochondrial function and human health. J Nutr Metab 2018;2018:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meidenbauer JJ, Ta N, Seyfried TN.. Influence of a ketogenic diet, fish-oil, and calorie restriction on plasma metabolites and lipids in C57BL/6J mice. Nutr Metab (Lond) 2014;11:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Veech RL, Chance B, Kashiwaya Y, Lardy HA, Cahill GF. Jr. Ketone bodies, potential therapeutic uses. IUBMB Life 2001;51:241–247. [DOI] [PubMed] [Google Scholar]
- 26. Sultan AM. Effects of diabetes and insulin on ketone bodies metabolism in heart. Mol Cell Biochem 1992;110:17–23. [DOI] [PubMed] [Google Scholar]
- 27. Wentz AE, d'Avignon DA, Weber ML, Cotter DG, Doherty JM, Kerns R, Nagarajan R, Reddy N, Sambandam N, Crawford PA.. Adaptation of myocardial substrate metabolism to a ketogenic nutrient environment. J Biol Chem 2010;285:24447–24456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bing RJ, Siegel A, Ungar I, Gilbert M.. Metabolism of the human heart. II. Studies on fat, ketone and amino acid metabolism. Am J Med 1954;16:504–515. [DOI] [PubMed] [Google Scholar]
- 29. Olson RE, Schwartz WB.. Myocardial metabolism in congestive heart failure. Medicine 1951;30:21–42. [DOI] [PubMed] [Google Scholar]
- 30. Lopaschuk GD, Verma S.. Empagliflozin’s fuel hypothesis: not so soon. Cell Metabolism 2016;24:200–202. [DOI] [PubMed] [Google Scholar]
- 31. Neubauer S. The failing heart—an engine out of fuel. N Engl J Med 2007;356:1140–1151. [DOI] [PubMed] [Google Scholar]
- 32. Stanley WC, Meadows SR, Kivilo KM, Roth BA, Lopaschuk GD.. beta-Hydroxybutyrate inhibits myocardial fatty acid oxidation in vivo independent of changes in malonyl-CoA content. Am J Physiol Heart Circ Physiol 2003;285:H1626–1631. [DOI] [PubMed] [Google Scholar]
- 33. Santos-Gallego CG, Requena-Ibanez JA, San Antonio R, Ishikawa K, Watanabe S, Picatoste B, Flores E, Garcia-Ropero A, Sanz J, Hajjar RJ, Fuster V, Badimon JJ.. Empagliflozin ameliorates adverse left ventricular remodeling in nondiabetic heart failure by enhancing myocardial energetics. J Am Coll Cardiol 2019;73:1931–1944. [DOI] [PubMed] [Google Scholar]
- 34. Ussher JR, Koves TR, Jaswal JS, Zhang L, Ilkayeva O, Dyck JR, Muoio DM, Lopaschuk GD.. Insulin-stimulated cardiac glucose oxidation is increased in high-fat diet-induced obese mice lacking malonyl CoA decarboxylase. Diabetes 2009;58:1766–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Karwi QG, Zhang L, Wagg CS, Wang W, Ghandi M, Thai D, Yan H, Ussher JR, Oudit GY, Lopaschuk GD.. Targeting the glucagon receptor improves cardiac function and enhances insulin sensitivity following a myocardial infarction. Cardiovasc Diabetol 2019;18:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jeffrey FM, Diczku V, Sherry AD, Malloy CR.. Substrate selection in the isolated working rat heart: effects of reperfusion, afterload, and concentration. Basic Res Cardiol 1995;90:388–396. [DOI] [PubMed] [Google Scholar]
- 37. Onay-Besikci A. Impact of lactate in the perfusate on function and metabolic parameters of isolated working rat heart. Mol Cell Biochem 2007;296:121–127. [DOI] [PubMed] [Google Scholar]
- 38. Divakaruni AS, Brand MD.. The regulation and physiology of mitochondrial proton leak. Physiology (Bethesda) 2011;26:192–205. [DOI] [PubMed] [Google Scholar]
- 39. Brand MD, Esteves TC.. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab 2005;2:85–93. [DOI] [PubMed] [Google Scholar]
- 40. Murray AJ, Anderson RE, Watson GC, Radda GK, Clarke K.. Uncoupling proteins in human heart. Lancet 2004;364:1786–1788. [DOI] [PubMed] [Google Scholar]
- 41. Tian XY, Ma S, Tse G, Wong WT, Huang Y.. Uncoupling protein 2 in cardiovascular health and disease. Front Physiol 2018;9:1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Murray AJ, Cole MA, Lygate CA, Carr CA, Stuckey DJ, Little SE, Neubauer S, Clarke K.. Increased mitochondrial uncoupling proteins, respiratory uncoupling and decreased efficiency in the chronically infarcted rat heart. J Mol Cell Cardiol 2008;44:694–700. [DOI] [PubMed] [Google Scholar]
- 43. Boudina S, Han YH, Pei S, Tidwell TJ, Henrie B, Tuinei J, Olsen C, Sena S, Abel ED.. UCP3 regulates cardiac efficiency and mitochondrial coupling in high fat-fed mice but not in leptin-deficient mice. Diabetes 2012;61:3260–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McLeod CJ, Aziz A, Hoyt RF Jr, McCoy JP Jr, Sack MN.. Uncoupling proteins 2 and 3 function in concert to augment tolerance to cardiac ischemia. J Biol Chem 2005;280:33470–33476. [DOI] [PubMed] [Google Scholar]
- 45. Safari F, Anvari Z, Moshtaghioun S, Javan M, Bayat G, Forosh SS, Hekmatimoghaddam S.. Differential expression of cardiac uncoupling proteins 2 and 3 in response to myocardial ischemia-reperfusion in rats. Life Sci 2014;98:68–74. [DOI] [PubMed] [Google Scholar]
- 46. Bernardi P, Vassanelli S, Veronese P, Colonna R, Szabo I, Zoratti M.. Modulation of the mitochondrial permeability transition pore. Effect of protons and divalent cations. J Biol Chem 1992;267:2934–2939. [PubMed] [Google Scholar]
- 47. Halestrap AP. Calcium-dependent opening of a non-specific pore in the mitochondrial inner membrane is inhibited at pH values below 7. Implications for the protective effect of low pH against chemical and hypoxic cell damage. Biochem J 1991;278:715–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Szabo I, Bernardi P, Zoratti M.. Modulation of the mitochondrial megachannel by divalent cations and protons. J Biol Chem 1992;267:2940–2946. [PubMed] [Google Scholar]
- 49. Petronilli V, Cola C, Bernardi P.. Modulation of the mitochondrial cyclosporin A-sensitive permeability transition pore. II. The minimal requirements for pore induction underscore a key role for transmembrane electrical potential, matrix pH, and matrix Ca2 +. J Biol Chem 1993;268:1011–1016. [PubMed] [Google Scholar]
- 50. Bernardi P. Modulation of the mitochondrial cyclosporin A-sensitive permeability transition pore by the proton electrochemical gradient. Evidence that the pore can be opened by membrane depolarization. J Biol Chem 1992;267:8834–8839. [PubMed] [Google Scholar]
- 51. Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, Glick GD, Petronilli V, Zoratti M, Szabo I, Lippe G, Bernardi P.. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci USA 2013;110:5887–5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park HA, Licznerski P, Li H, Nabili P, Hockensmith K, Graham M, Porter GA Jr, Jonas EA.. An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc Natl Acad Sci USA 2014;111:10580–10585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gibbs CL. Cardiac energetics. Physiol Rev 1978;58:174–254. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author (Dr Gary D. Lopaschuk).
Translational perspective
Recent clinical interest has focused on ketones as a potential fuel source for the failing heart, primarily because ketones have been popularized as a ‘thrifty’ fuel that may increase cardiac efficiency. However, we have directly assessed cardiac ketone oxidation rates alongside their competing energy substrates and found that: not only can ketones become the major fuel of the heart with no inhibitory effect on cardiac glucose oxidation, but they can provide the healthy heart with an excess energy supply, with no changes to cardiac function, resulting in a mismatch between reducing equivalents supply and cardiac ATP production, ultimately contributing to a decrease in cardiac efficiency.