Abstract
Purpose of review:
This review seeks to provide an overview of the role of inflammation and metabolism in tendon cell function, tendinopathy, and tendon healing. We have summarized the state of knowledge in both tendon and enthesis.
Recent Findings:
Recent advances in the field include a substantial improvement in our understanding of tendon cell biology, including the heterogeneity of the tenocyte environment during homeostasis, the diversity of the cellular milieu during in vivo tendon healing, and the effects of inflammation and altered metabolism on tendon cell function in vitro. In addition, the mechanisms by which altered systemic metabolism, such as Diabetes, disrupt tendon homeostasis continue to be better understood.
Summary:
A central conclusion of this review is the critical need to better define fundamental cellular and signaling mechanisms of inflammation and metabolism during tendon homeostasis, tendinopathy, and tendon healing in order to identify therapies to enhance or maintain tendon function.
Keywords: Tendon, enthesis, inflammation, metabolism, tendinopathy, tendon healing
INTRODUCTION
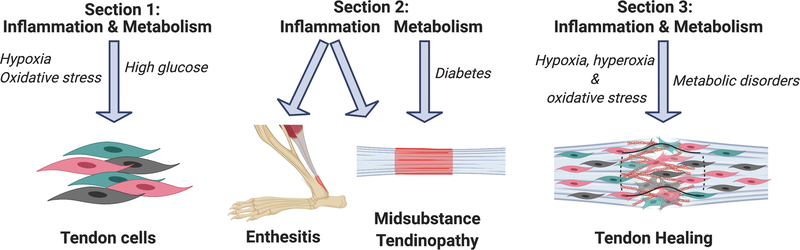
The basal metabolic rate of tendon is relatively low, likely due to the high degree of quiescence observed in tendon cells during tissue homeostasis. However, multiple factors can lead to the loss of cell quiescence, alterations in tendon cell functions, and altered tendon function, with inflammation being a key regulator of these processes. This review will summarize the central inflammatory and metabolic mechanisms that regulate i) tendon cell function; ii) disruptions in tendon homeostasis (e.g. tendinopathy); and iii) healing of acute tendon injuries, and how inflammation can interact with and modulate metabolism in these contexts (Figure 1). In addition, within the respective sections on tendinopathy and healing of acute tendon injuries, a distinction is made between the tendon mid-substance and the tendon-bone junction (enthesis) as appropriate, given that there are important region-specific differences in the tendon response to inflammation and altered metabolic function.
Figure 1.
Overview of the review. Section 1 summarizes the effects of inflammation and metabolic alterations on in vitro tendon cell function, with a particular emphasis on the role of inflammation-induced hypoxia and oxidative stress, as well as the impact of high glucose on tendon cell function. Section 2 summarizes the impact of inflammation on both the tendon enthesis and mid-substance tendinopathy, as well as how alterations in systemic metabolic function, including Diabetes can promote tendinopathy. Section 3 focuses on the effects of inflammation, including hypoxia, hyperoxia and oxidative stress on the healing process, and the effects of systemic metabolic disorders on the tendon healing process. This figure was created using Biorender.com.
1. METABOLISM, INFLAMMATION & IN VITRO TENDON CELL BIOLOGY
It has long been established that tendon cells are capable of both glycolysis and mitochondrial respiration for energy production (1, 2). Recently, it has been shown that loss of Scleraxis expression in tendon cells leads to upregulated oxidative phosphorylation and mitochondrion organization, suggesting that putative tendon markers affect tendon cell metabolism (3). In contrast to tendon cells isolated during homeostasis, tendon cells isolated from injured human flexor tendons exhibited increased glycolytic pathway flux and the capacity for differentiation down both tenogenic and chondrogenic pathways (4). When treated with a glycolysis inhibitor, chondrogenic differentiation was inhibited and tenogenic differentiation was stimulated, suggesting that glycolysis pushed tendon cells along a non-tenogenic fate (4). Furthermore, treatment of tendon cells with the inflammatory cytokine IL-1β drives lactate production, which results in decreased collagen and decreased expression of key tendon markers (5). This demonstrates that inflammatory signaling can directly modulate tendon cell metabolism.
Hypoxia
Numerous studies have explored the effects of oxygen deprivation (hypoxia, typically 1–5% O2) on tendon cells in vitro. Tendon cells cultured in hypoxic conditions have limited mitochondrial energy production accompanied by elevated glycolytic flux and lactate production, demonstrating that hypoxia drastically changes metabolic processes in tendon cells (6). For example, hypoxia drives tendon cell proliferation and prevents multilineage differentiation potential while upregulating stem cell markers such as NANOG and OCT-4, compared to normoxic culture conditions (~20% O2)(7–11). Hypoxia also drives increased expression of tendon markers, such as tenomodulin(7). Importantly, tendon cells cultured in hypoxic conditions and subsequently cultured under normoxic conditions regain their multilineage differentiation potential, demonstrating that the effects of hypoxia on tendon cells are reversible (7). Hypoxia also limits the formation of primary cilia on tendon cells, potentially limiting their mechanosensory properties (12). Moreover, highly hypoxic conditions (~0.1% O2) and anoxia lead to tendon cell death via apoptosis (13, 14).
Previous studies have demonstrated that aged tendon cells have impaired proliferative capacity, decreased expression of tendon markers, increased adipogenic differentiation potential, increased mineralized fibrocartilage phenotype, and increased senescence relative to young tendon cells (15–18). Interestingly, culturing aged tendon cells with the conditioned media from young, hypoxic tendon cells increases proliferative capacity and migration rate of the aged cells, increases expression of tendon markers, and decreases the number senescent cells, suggesting that paracrine signaling via secreted factors from hypoxic tendon cells reverses the aged tendon cell phenotype (17). Furthermore, hypoxic tendon cell co-cultures drive other cell types, such as adipose-derived mesenchymal stem cells, along a tendon cell fate. Recent studies show that hypoxia triggers tendon cells to produce exosomes containing upregulated extracellular matrix related genes, further supporting the idea of paracrine, cell-cell mediated communication during hypoxia (19, 20).
Oxidative Stress
In addition to hypoxia, several other factors such as diet and environmental stimuli, can drive oxidative stress. Many studies have examined the effects of oxidative stress in the form of reactive oxygen species (ROS) on tendon cells in vitro. Treatment of tendon cells with ROS results in decreases in cellular proliferation, migration, viability, and stemness(21, 22). Activation of mitochondrial aldehyde dehydrogenase 2 (ALDH2), a known reliever of oxidative stress, in tendon cells prevents H2O2-induced cell death and prevents depolarization of mitochondrial membrane potential(23). In addition, multiple vitamins have antioxidant properties. Treatment of tendon cells with vitamin C also decreases NO synthesis by tendon cells(24). Treatment of tendon cells with low-dose vitamin C increases cell proliferation, viability, and migration(22, 24). Tendon cell proliferation is further enhanced by co-treatment of vitamin C and thyroid hormone T3 (24). Similarly, treatment with vitamin D increases cell proliferation and reduces production of reactive oxygen species(25, 26). However, vitamin D also reduces gene expression of type I collagen(25). Retinoic acid, a metabolite of vitamin A, induces nuclear localization of Scleraxis and aids in the maintenance of tendon stem cell properties(27).
High Glucose
Supplementation of culture medium with high levels of glucose modulates many aspects of tenocyte function. More specifically, tenocytes in high glucose demonstrate altered inflammatory signaling via IL-6 and COX2, increased ROS production, decreased proliferation, decreased migration, decreased mitochondrial membrane potential, and increased apoptosis(28–34). Moreover, high glucose decreases the expression of tendon genes, promotes simultaneous decreases in Type I Collagen and increases in Type III Collagen expression, increases the expression and activity of multiple MMPs and TIMPS, and stimulates adipogenic transdifferentiation(28–34). In addition, the effects of high glucose are exacerbated in the presence of Advanced Glycation Endproducts (AGEs), as the combination leads to reduced proliferation, reduced ATP production, decreased electron transport efficiency, and alterations in collagen and MMP gene expression(34). Consistent with the effects of high glucose supplementation, tendon cells isolated from diabetic rat patellar tendons exhibit decreased proliferative ability, decreased expression of tendon markers, and increased osteogenic and chondrogenic differentiation ability (35), mimicking many effects of high glucose culture conditions, and suggesting that diabetic cells retain some ‘memory’ and, therefore, functional alterations of the in vivo environment. Interestingly, mechanotransduction can suppress several aspects of the effects of high glucose on tendon cells. Mechanical stretch prevents adipogenic transdifferentiation, increases tendon cell migration, and enhances fibroblastic-like morphology of cells under high glucose conditions, suggesting that mechanotransduction can ameliorate some of the negative outcomes associated with hyperglycemia(30).
Other metabolic mediators of in vitro tendon cell function
Cholesterol can also affect tendon cell function. Recent studies have shown that high cholesterol suppresses tendon cell proliferation, cell migration, and Scleraxis gene expression, while increasing ROS production(36, 37). The effects of female sex hormones on tendon cells has also been investigated. Estrogen increases cell proliferative ability and decreases adipogenic differentiation potential of tendon cells(38). Additionally, treatment with estradiol-17β increases tendon cell proliferation but decreases Scleraxis gene expression(25). Finally, there is some evidence that treatment with platelet rich plasma (PRP) induces inflammation-related changes in tendon cell function. PRP treatment of tendon cells upregulates TNF-α-induced NF-κB signaling pathway, downregulates the expression of extracellular matrix genes, and induces the expression of autophagy-related and ROS-related genes(39).
2. INFLAMMATION AND METABOLISM IN TENDINOPATHY
Inflammation and Mid-substance Tendinopathy
Loss of tendon homeostasis, resulting in tendinopathy, is characterized by inflammation and degeneration of the native tendon tissue. Tendinopathy represents a major clinical burden, decreases patient quality of life, and increases the risk of tendon rupture(40). Our current understanding of the pathophysiology of tendinopathy is a combination of supraphysiological overloading and inflammation(41–43). Rather than a single overloading event, cyclic overloading is required for the rapid expression of inflammatory mediators that are observed in tendinopathies (44). These inflammatory mediators include Alarmins (such as HIF-1α, IL-33, S100a8/9, and HMGB1), which have been implicated in the earliest phases of tendinopathy development (43, 45, 46). Moreover, there is strong evidence that many of the pathological changes observed in tendinopathy are driven largely by extrinsic factors(47). More specifically, immune cell involvement is thought to play a central role in tendinopathy development, although there is limited direct evidence for this contribution. Indeed, a recent review of inflammatory mediators of tendinopathy(48) defines a high degree of heterogeneity in terms of the extent of the inflammatory cell environment that has been characterized in tendinopathy studies. However, samples from both Achilles and supraspinatus tendinopathy patients demonstrate an influx of pro-inflammatory monocyte-derived macrophages, with increased macrophage content occurring with increased disease severity (41, 49), suggesting that severity of tendinopathy may be characterized by examination of the inflammation signature.
In terms of modulating inflammation to treat tendinopathy, anti-inflammatory approaches have had mixed success but have also highlighted the effects of treatment timing on treatment efficacy. While some in vitro studies have suggested that aspirin can promote tenogenesis via GDF7/Smad1/5 signaling(50), in vivo studies have not demonstrated the same promise. For example, Heinemeier et al., found that one-week treatment with ibuprofen elicited no change in adult human chronic tendinopathic tissues(51), while Bittermann et al. showed that ibuprofen treatment during the inflammatory phase actually blunted healing in a model of murine Achilles tendinopathy (52). A potential alternative anti-inflammatory approach involves the use of lipid mediators of aspirin-induced eicosanoid metabolism, such as 15-epi LXA4. These have proven effective in other chronic inflammatory diseases, such as pulmonary inflammation and eczema and, therefore, may also hold promise in resolving tendon inflammation without the damaging side effects (41, 53).
Inflammation and enthesitis
In addition to impacting the integrity and homeostasis of the tendon mid-substance, inflammation also impacts the integrity of the tendon-bone interface (enthesis). Enthesitis, or inflammation of the enthesis, is predominantly associated with arthritis (54) but can also be initiated by repeated bouts of high mechanical stress, which induces an inflammatory response (55), consistent with mid-substance tendinopathy development. For example, while mice overexpressing TNF are highly susceptible to enthesitis and arthritis, hindlimb suspension inhibited arthritis development and blunted the TNF-mediated inflammatory response, compared to controls (56). Importantly, enthesitis is a hallmark of Spondyloarthritis (SpA), particularly juvenile cases (57), which often go undiagnosed due to lack of diagnostic criteria. As such, increasing our fundamental understanding of the cellular and molecular mechanisms that drive and initiate increased enthesitis in the context of SpA could have a substantial impact of both disease diagnosis and treatment.
Metabolism and Tendinopathy
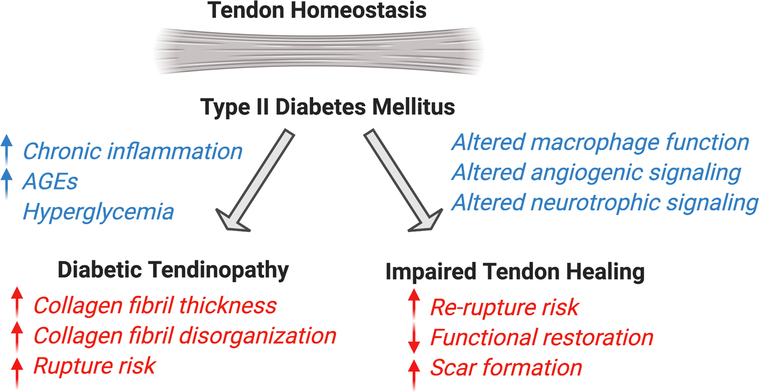
In addition to inflammation and mechanical overloading, a variety of metabolic diseases can also initiate tendinopathy development. While metabolic syndrome encompasses many co-morbidities, including high blood pressure, high blood glucose, and obesity, very few studies have examined the collective effect of metabolic syndrome on tendon pathology, but have more commonly looked at the impact of individual co-morbidities. However, there is a strong association between metabolic syndrome and the development of trigger finger (58). In terms of individual conditions, Type II diabetes greatly increases the risk of tendon pathology (59–62) (Summarized in Figure 2), likely due to a combination of chronic, low-grade inflammation and a high glucose environment. Consistent with this, there is some evidence that alterations in glucose metabolism may represent a metabolic marker of tendinopathy(6). Moreover, diabetic tendons are thicker and demonstrate fibril disorganization at homeostasis, changes that are thought to be due to a combination of hyperglycemia and accumulation of AGEs (30, 34). AGEs promote increased cross-linking with age or diabetes, and inhibit normal sliding of tendon fascicles, leaving them prone to pathology(63). Other perturbations in metabolism have also been shown to influence development of pathological conditions, such as elevated cholesterol levels (37, 64, 65) though these effects are less well-characterized.
Figure 2.
Summary of the impact of Type II Diabetes on tendon homeostasis and healing of acute tendinopathy. This figure was created using Biorender.com.
3. METABOLIC AND INFLAMMATORY MEDIATORS OF ACUTE TENDON HEALING
Metabolic Disorders
Metabolic disorders, such as Diabetes Mellitus, dramatically affect the tendon healing process (Figure 2). For example, rotator cuff repairs in diabetic patients are up to two times more likely to re-rupture than non-diabetic patients and show evidence of diminished healing (66, 67), including slower or decreased improvements in pain and function(68). Moreover, murine and rat models of Type II Diabetes demonstrate decreased biomechanical properties following tendon injury, compared to non-diabetic animals(69–71). Healing Type II Diabetic tendons also exhibit increased scar tissue formation(71), aberrations in macrophage polarization(71), and detrimental effects to angiotrophic and neurotrophic signaling pathways(72). Interestingly, Type I Diabetes also disrupts tendon healing. Streptozotocin-induced Type I diabetes suppresses immune cell infiltration (including macrophages), decreases cell proliferation, and decreases biomechanical properties during healing, relative to non-diabetic controls (73–75). To better understand the effects of high blood glucose on Achilles tendon healing, non-diabetic rats were supplemented with a high glucose diet, which did not elevated blood glucose levels even after 4 weeks of treatment. Glucose supplementation resulted in increased tendon thickness and stiffness, as well as an altered gait pattern(76). In addition, cell proliferation was increased, as was expression of chondrogenic markers (Sox9, Col2a1, Acan, Comp), and cartilage-like areas were detected within the repair tissue(76), suggesting that high glucose alters in vivo tenogenic function and may promoter aberrant chondrogenic differentiation in the healing tendon.
Interaction of Metabolism and Inflammation
Acute tendon injuries contain elevated levels of metabolites, including glutamate, lactate, and pyruvate, compared to uninjured tendon(77–79). Recently, 13C-glucose labeling was utilized to examine glycolytic and TCA cycle activity during murine Achilles tendon healing following transection. While glycolysis was elevated at both 1 and 4 weeks post-injury, the flux through the TCA cycle was significantly increased at 1 week post-repair (78). This indicates that multiple metabolic processes are highly active during the early, inflammatory phases of healing, while glycolysis persists into the later remodeling phase. A clinical study in humans demonstrated that glutamate, lactate, and pyruvate are elevated at 2 weeks post-Achilles tendon injury(79). Weight-bearing mobilization of the injured tendon further increased glutamate, which was significantly correlated with elevated procollagen type I levels(79). Interestingly, metabolic activity, as measured by glucose uptake remains elevated in healing human tendon through at least 12 months post-injury, with metabolic activity being highest shortly after injury and slowly decreasing over time(80). Patient-reported tendon functional scores were negatively correlated with high glucose uptake, suggesting that sustained metabolic activity may negatively impact healing(80). In contrast, an observational study examining human Achilles tendon rupture duration of operating time (DOT) found that patients who experienced longer DOT had higher levels of glutamate, which was significantly associated with improved functional outcomes(81).
There has been relatively little exploration into how modulation of metabolic pathways affects tendon healing. However, inhibition of lactate synthesis (a metabolite of the glycolysis product pyruvate) was positively correlated with decreased width and cross-sectional area of the healing tendon, increased biomechanical properties, improved collagen fiber alignment, and reduced mineralization of the injury site, suggestive of improved healing(78). Interestingly, the effects of acute injury and healing on mitochondrial function are unclear. For example, enhanced mitochondrial activity is observed during rat rotator cuff healing (82), while diminished mitochondrial activity was observed following rotator cuff tenotomy in a sheep model (83). Moreover, photobiomodulation, a non-ionizing laser-therapy that stimulates mitochondrial energy production, resulted in mild improvements in mechanical properties of Achilles tendons after injury (84). Collectively, these studies demonstrate the need for additional work to understand the specific contributions of metabolic processes throughout the various stages of tendon healing and to determine how modification of mitochondrial function may impact tendon healing.
Hypoxia, Hyperoxia and Oxidative Stress
While alterations in tendon cell function as a result of oxygen concentration are well characterized in vitro, the in vivo effects are less clear. However, the importance of oxygen concentration and consumption is demonstrated by clinical studies in the rotator cuff, which demonstrate decreased cellular activity and oxygen consumption in large tears, and are associated with worse outcomes, relative to smaller tears (85). A such, a few studies have examined the effects of hyperbaric oxygen treatment on tendon healing, although no consensus has yet emerged. In a rat patellar tendon injury model, hyperbaric oxygen supplementation did not alter the healing process in one study(86), while another reported increased collagen gene expression at 1–2 weeks post-injury(87). Recent work demonstrated increased fibrotic tissue during Achilles tendon healing following supplementation with hyperbaric oxygen in a rat model (88).
Given the effects of oxidative stress on tendon cell function in vitro, substantial investigation has been conducted into the efficacy of Vitamin C due to its antioxidant functions and role in collagen formation (89). Vitamin C supplementation increases angiogenesis and type I collagen deposition in a rat Achilles tendon injury model (90). Moreover, administration of a supplement containing mucopolysaccharides, vitamin C, and collagen during Achilles tendon healing did not alter biomechanical properties or collagen production but did increase cell proliferation and TGF-β1 production in endotenon fibroblasts (91). Local administration of vitamin C following Achilles tendon repair improved mechanical properties (92) and functional outcomes (93) of healing tendons, while a combination of vitamin C and thyroid hormone T3 also enhanced healing(94).
While oxygen concentration and consumption, as well as oxidative stress, impact tendon cell function and in vivo healing, more work is needed to determine if and how these pathways can be utilized to enhance tendon healing.
CONCLUSIONS AND KEY KNOWLEDGE GAPS
Given the frequency of tendon injuries, including those induced by tendinopathy, and the substantial subsequent complications that can occur, understanding the underlying mechanisms that regulate healing is critical to the identification of therapeutic targets. Inflammation is central to successful healing; however, aberrant, excessive or insufficient inflammation all have profound effects on the tendon healing process. As such, future work is needed to better define i) the central inflammatory signaling cascades; ii) how inflammatory and immune cells interact with resident tendon cells and extrinsic cells to mediate the healing process, and iii) how inflammation modulates cell metabolism and therefore cell function during healing. In terms of tendon homeostasis, while the effects of altered systemic metabolism (e.g. Type II Diabetes) on the tendon are clear (Figure 2), there is a gap in knowledge in terms of our fundamental understanding of how metabolism regulates tendon cell function to maintain homeostasis, and how different aspects of metabolism may be targeted to restore normal tenogenic function and therefore tendon homeostasis in the context of altered systemic metabolism (e.g. metabolic syndrome or Diabetes). Collectively, while much remains unknown about the molecular and cellular-level impact of altered inflammation and metabolism on tendon homeostasis and healing, the recent and rapid maturation of the tendon field suggests it is only a matter of time until these processes are more clearly defined and can therefore be leveraged to prevent or reverse tendon pathology and improve healing.
Footnotes
Conflict of Interest
Jessica Ackerman, Katherine Best, Samantha Muscat and Alayna Loiselle declare that they have no conflicts of interest.
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any primary data with human or animal subjects performed by any of the authors.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Floridi A, Ippolito E, Postacchini F. Age-related changes in the metabolism of tendon cells. Connect Tissue Res 1981;9(2):95–7. [DOI] [PubMed] [Google Scholar]
- 2.Birch HL, Rutter GA, Goodship AE. Oxidative energy metabolism in equine tendon cells. Res Vet Sci 1997;62(2):93–7. [DOI] [PubMed] [Google Scholar]
- 3.Nichols AEC, Settlage RE, Werre SR, Dahlgren LA. Novel roles for scleraxis in regulating adult tenocyte function. BMC Cell Biol 2018;19(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Izumi S, Otsuru S, Adachi N, Akabudike N, Enomoto-Iwamoto M. Control of glucose metabolism is important in tenogenic differentiation of progenitors derived from human injured tendons. PloS one. 2019;14(3):e0213912.* This study examines how metabolic flux through glycolysis and the TCA cycle differently influence differentiation potential of cells derived from human injured tendons.
- 5.Zhang K, Asai S, Yu B, Enomoto-Iwamoto M. IL-1β irreversibly inhibits tenogenic differentiation and alters metabolism in injured tendon-derived progenitor cells in vitro. Biochem Biophys Res Commun 2015;463(4):667–72.* This study demonstrated that pro-inflammatory cytokine IL-1β enhanced lactate production, directly affecting expression of tendon marker Scx and matrix gene Col1.
- 6.Sikes KJ, Li J, Gao SG, Shen Q, Sandy JD, Plaas A, et al. TGF-b1 or hypoxia enhance glucose metabolism and lactate production via HIF1A signaling in tendon cells. Connect Tissue Res 2018;59(5):458–71.** This study used a TGF-β1-induced murine Achilles tendinopathy model to demonstrate that glycolytic reprogramming may play a role in the development of pathology.
- 7.Lee WY, Lui PP, Rui YF. Hypoxia-mediated efficient expansion of human tendon-derived stem cells in vitro. Tissue Eng Part A. 2012;18(5–6):484–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li P, Xu Y, Gan Y, Song L, Zhang C, Wang L, et al. Role of the ERK1/2 Signaling Pathway in Osteogenesis of Rat Tendon-Derived Stem Cells in Normoxic and Hypoxic Cultures. International journal of medical sciences. 2016;13(8):629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu Y, Lin L, Zhou Y, Lu X, Shao X, Lin C, et al. Effect of Hypoxia on Self-Renewal Capacity and Differentiation in Human Tendon-Derived Stem Cells. Medical science monitor : international medical journal of experimental and clinical research. 2017;23:1334–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Wang JH. Human tendon stem cells better maintain their stemness in hypoxic culture conditions. PloS one. 2013;8(4):e61424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Wang B, Zhang WJ, Zhou G, Cao Y, Liu W. Enhanced proliferation capacity of porcine tenocytes in low O2 tension culture. Biotechnol Lett 2010;32(2):181–7. [DOI] [PubMed] [Google Scholar]
- 12.Lavagnino M, Oslapas AN, Gardner KL, Arnoczky SP. Hypoxia inhibits primary cilia formation and reduces cell-mediated contraction in stress-deprived rat tail tendon fascicles. Muscles Ligaments Tendons J 2016;6(2):193–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott A, Khan KM, Duronio V. IGF-I activates PKB and prevents anoxic apoptosis in Achilles tendon cells. J Orthop Res 2005;23(5):1219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang M, Cornell HR, Zargar Baboldashti N, Thompson MS, Carr AJ, Hulley PA. Regulation of hypoxia-induced cell death in human tenocytes. Advances in orthopedics. 2012;2012:984950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Z, Akinbiyi T, Xu L, Ramcharan M, Leong DJ, Ros SJ, et al. Tendon-derived stem/progenitor cell aging: defective self-renewal and altered fate. Aging Cell. 2010;9(5):911–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai WC, Chang HN, Yu TY, Chien CH, Fu LF, Liang FC, et al. Decreased proliferation of aging tenocytes is associated with down-regulation of cellular senescence-inhibited gene and up-regulation of p27. J Orthop Res 2011;29(10):1598–603. [DOI] [PubMed] [Google Scholar]
- 17.Jiang D, Jiang Z, Zhang Y, Wang S, Yang S, Xu B, et al. Effect of young extrinsic environment stimulated by hypoxia on the function of aged tendon stem cell. Cell biochemistry and biophysics. 2014;70(2):967–73. [DOI] [PubMed] [Google Scholar]
- 18.McBeath R, Edwards RW, O’Hara BJ, Maltenfort MG, Parks SM, Steplewski A, et al. Tendinosis develops from age- and oxygen tension-dependent modulation of Rac1 activity. Aging Cell. 2019;18(3):e12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thankam FG, Agrawal DK. Hypoxia-driven secretion of extracellular matrix proteins in the exosomes reflects the asymptomatic pathology of rotator cuff tendinopathies. Can J Physiol Pharmacol 2020. [DOI] [PubMed] [Google Scholar]
- 20.Thankam FG, Chandra I, Diaz C, Dilisio MF, Fleegel J, Gross RM, et al. Matrix regeneration proteins in the hypoxia-triggered exosomes of shoulder tenocytes and adipose-derived mesenchymal stem cells. Mol Cell Biochem. 2020;465(1–2):75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen H, Ge HA, Wu GB, Cheng B, Lu Y, Jiang C. Autophagy Prevents Oxidative Stress-Induced Loss of Self-Renewal Capacity and Stemness in Human Tendon Stem Cells by Reducing ROS Accumulation. Cell Physiol Biochem. 2016;39(6):2227–38.* This study demonstrated that human tendon-derived cells treated with oxidative stressor H2O2 resulted in reactive oxygen species accumulation and impaired self-renewal capacity and stemness, and also provided evidence that autophagy reverses these phenotypes.
- 22.Lee YW, Fu SC, Yeung MY, Lau CML, Chan KM, Hung LK. Effects of Redox Modulation on Cell Proliferation, Viability, and Migration in Cultured Rat and Human Tendon Progenitor Cells. Oxidative medicine and cellular longevity. 2017;2017:8785042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu YC, Wang HL, Huang YZ, Weng YH, Chen RS, Tsai WC, et al. Alda-1, an activator of ALDH2, ameliorates Achilles tendinopathy in cellular and mouse models. Biochemical pharmacology. 2020;175:113919. [DOI] [PubMed] [Google Scholar]
- 24.di Giacomo V, Berardocco M, Gallorini M, Oliva F, Colosimo A, Cataldi A, et al. Combined supplementation of ascorbic acid and thyroid hormone T(3) affects tenocyte proliferation. The effect of ascorbic acid in the production of nitric oxide. Muscles Ligaments Tendons J 2017;7(1):11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maman E, Somjen D, Maman E, Katzburg S, Sharfman ZT, Stern N, et al. The response of cells derived from the supraspinatus tendon to estrogen and calciotropic hormone stimulations: in vitro study. Connect Tissue Res 2016;57(2):124–30. [DOI] [PubMed] [Google Scholar]
- 26.Min K, Lee JM, Kim MJ, Jung SY, Kim KS, Lee S, et al. Restoration of Cellular Proliferation and Characteristics of Human Tenocytes by Vitamin D. J Orthop Res 2019;37(10):2241–8. [DOI] [PubMed] [Google Scholar]
- 27.Webb S, Gabrelow C, Pierce J, Gibb E, Elliott J. Retinoic acid receptor signaling preserves tendon stem cell characteristics and prevents spontaneous differentiation in vitrox. Stem Cell Res Ther 2016;7:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurosawa T, Mifune Y, Inui A, Nishimoto H, Ueda Y, Kataoka T, et al. Evaluation of apocynin in vitro on high glucose-induced oxidative stress on tenocytes. Bone Joint Res 2020;9(1):23–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai WC, Liang FC, Cheng JW, Lin LP, Chang SC, Chen HH, et al. High glucose concentration up-regulates the expression of matrix metalloproteinase-9 and −13 in tendon cells. BMC musculoskeletal disorders. 2013;14:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu YF, Wang HK, Chang HW, Sun J, Sun JS, Chao YH. High glucose alters tendon homeostasis through downregulation of the AMPK/Egr1 pathway. Sci Rep 2017;7:44199.* This study revealed that rat Achilles tendon cells exposed to high glucose levels had reduced expression of key tendon-related genes, which may be mediated through an AMPK/Egr1 mechanism.
- 31.Lin YC, Li YJ, Rui YF, Dai GC, Shi L, Xu HL, et al. The effects of high glucose on tendon-derived stem cells: implications of the pathogenesis of diabetic tendon disorders. Oncotarget 2017;8(11):17518–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ueda Y, Inui A, Mifune Y, Sakata R, Muto T, Harada Y, et al. The effects of high glucose condition on rat tenocytes in vitro and rat Achilles tendon in vivo. Bone Joint Res 2018;7(5):362–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwan CK, Fu SC, Yung PS. A high glucose level stimulate inflammation and weaken pro-resolving response in tendon cells - A possible factor contributing to tendinopathy in diabetic patients. Asia-Pacific journal of sports medicine, arthroscopy, rehabilitation and technology. 2020;19:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel SH, Yue F, Saw SK, Foguth R, Cannon JR, Shannahan JH, et al. Advanced Glycation End-Products Suppress Mitochondrial Function and Proliferative Capacity of Achilles Tendon-Derived Fibroblasts. Sci Rep 2019;9(1):12614.** This study demonstrated that advanced glycation end-products significantly impaired rat Achilles tendon cell energy production and proliferative capacity.
- 35.Shi L, Li YJ, Dai GC, Lin YC, Li G, Wang C, et al. Impaired function of tendon-derived stem cells in experimental diabetes mellitus rat tendons: implications for cellular mechanism of diabetic tendon disorder. Stem Cell Res Ther 2019;10(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li K, Deng Y, Deng G, Chen P, Wang Y, Wu H, et al. High cholesterol induces apoptosis and autophagy through the ROS-activated AKT/FOXO1 pathway in tendon-derived stem cells. Stem Cell Res Ther 2020;11(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li K, Deng G, Deng Y, Chen S, Wu H, Cheng C, et al. High cholesterol inhibits tendon-related gene expressions in tendon-derived stem cells through reactive oxygen species-activated nuclear factor-κB signaling. J Cell Physiol 2019;234(10):18017–28. [DOI] [PubMed] [Google Scholar]
- 38.Bian X, Liu T, Zhou M, He G, Ma Y, Shi Y, et al. Absence of estrogen receptor beta leads to abnormal adipogenesis during early tendon healing by an up-regulation of PPARγ signalling. J Cell Mol Med 2019;23(11):7406–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hudgens JL, Sugg KB, Grekin JA, Gumucio JP, Bedi A, Mendias CL. Platelet-Rich Plasma Activates Proinflammatory Signaling Pathways and Induces Oxidative Stress in Tendon Fibroblasts. Am J Sports Med 2016;44(8):1931–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abate M, Salini V. Mid-portion Achilles tendinopathy in runners with metabolic disorders. European journal of orthopaedic surgery & traumatology : orthopedie traumatologie. 2019;29(3):697–703. [DOI] [PubMed] [Google Scholar]
- 41.Dakin SG, Martinez FO, Yapp C, Wells G, Oppermann U, Dean BJ, et al. Inflammation activation and resolution in human tendon disease. Science translational medicine. 2015;7(311):311ra173.** A thorough characterization of the inflammatory signature for different stages of tendinopathy using human supraspinatus tendon.
- 42.Tran PHT, Malmgaard-Clausen NM, Puggaard RS, Svensson RB, Nybing JD, Hansen P, et al. Early development of tendinopathy in humans: Sequence of pathological changes in structure and tissue turnover signaling. Faseb j 2020;34(1):776–88. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Deng XH, Lebaschi AH, Wada S, Carballo CB, Croen B, et al. Expression of alarmins in a murine rotator cuff tendinopathy model. J Orthop Res 2020. [DOI] [PubMed] [Google Scholar]
- 44.Spiesz EM, Thorpe CT, Chaudhry S, Riley GP, Birch HL, Clegg PD, et al. Tendon extracellular matrix damage, degradation and inflammation in response to in vitro overload exercise. J Orthop Res 2015;33(6):889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akbar M, Gilchrist DS, Kitson SM, Nelis B, Crowe LAN, Garcia-Melchor E, et al. Targeting danger molecules in tendinopathy: the HMGB1/TLR4 axis. RMD Open. 2017;3(2):e000456.* Human tendon-derived cells were used to show that blocking HMGB1 signaling via silencing TLR4 reversed inflammatory and matrix changes observed in tendinopathies.
- 46.Crowe LAN, McLean M, Kitson SM, Melchor EG, Patommel K, Cao HM, et al. S100A8 & S100A9: Alarmin mediated inflammation in tendinopathy. Sci Rep 2019;9(1):1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wunderli SL, Blache U, Beretta Piccoli A, Niederöst B, Holenstein CN, Passini FS, et al. Tendon response to matrix unloading is determined by the patho-physiological niche. Matrix biology : journal of the International Society for Matrix Biology. 2020;89:11–26.* This study used a tendon explant model to look at hypervascularity-driven changes involved in tendinopathy progression.
- 48.Jomaa G, Kwan CK, Fu SC, Ling SK, Chan KM, Yung PS, et al. A systematic review of inflammatory cells and markers in human tendinopathy. BMC musculoskeletal disorders. 2020;21(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dakin SG, Newton J, Martinez FO, Hedley R, Gwilym S, Jones N, et al. Chronic inflammation is a feature of Achilles tendinopathy and rupture. Br J Sports Med 2018;52(6):359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y, He G, Tang H, Shi Y, Zhu M, Kang X, et al. Aspirin promotes tenogenic differentiation of tendon stem cells and facilitates tendinopathy healing through regulating the GDF7/Smad1/5 signaling pathway. J Cell Physiol 2020;235(5):4778–89. [DOI] [PubMed] [Google Scholar]
- 51.Heinemeier KM, Øhlenschlæger TF, Mikkelsen UR, Sønder F, Schjerling P, Svensson RB, et al. Effects of anti-inflammatory (NSAID) treatment on human tendinopathic tissue. J Appl Physiol (1985). 2017;123(5):1397–405. [DOI] [PubMed] [Google Scholar]
- 52.Bittermann A, Gao S, Rezvani S, Li J, Sikes KJ, Sandy J, et al. Oral Ibuprofen Interferes with Cellular Healing Responses in a Murine Model of Achilles Tendinopathy. J Musculoskelet Disord Treat 2018;4(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dakin SG, Colas RA, Newton J, Gwilym S, Jones N, Reid HAB, et al. 15-Epi-LXA4 and MaR1 counter inflammation in stromal cells from patients with Achilles tendinopathy and rupture. FASEB J 2019;33(7):8043–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaeley GS, Eder L, Aydin SZ, Gutierrez M, Bakewell C. Enthesitis: A hallmark of psoriatic arthritis. Semin Arthritis Rheum 2018;48(1):35–43. [DOI] [PubMed] [Google Scholar]
- 55.McGonagle D, Lories RJ, Tan AL, Benjamin M. The concept of a “synovio-entheseal complex” and its implications for understanding joint inflammation and damage in psoriatic arthritis and beyond. Arthritis Rheum 2007;56(8):2482–91. [DOI] [PubMed] [Google Scholar]
- 56.Jacques P, Lambrecht S, Verheugen E, Pauwels E, Kollias G, Armaka M, et al. Proof of concept: enthesitis and new bone formation in spondyloarthritis are driven by mechanical strain and stromal cells. Ann Rheum Dis 2014;73(2):437–45. [DOI] [PubMed] [Google Scholar]
- 57.Kehl AS, Corr M, Weisman MH. Review: Enthesitis: New Insights Into Pathogenesis, Diagnostic Modalities, and Treatment. Arthritis & rheumatology. 2016;68(2):312–22.** This review focuses on how enthesitis is central to spondyloarthritis and discusses causes of enthesitis: genetic susceptibility, microbial factors, proinflammatory cytokines, etc.
- 58.Junot HSN, Hertz A, Vasconcelos G, da Silveira D, Nelson P, Almeida SF. Epidemiology of Trigger Finger: Metabolic Syndrome as a New Perspective of Associated Disease. Hand (N Y). 2019:1558944719867135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Collins KH, Herzog W, MacDonald GZ, Reimer RA, Rios JL, Smith IC, et al. Obesity, Metabolic Syndrome, and Musculoskeletal Disease: Common Inflammatory Pathways Suggest a Central Role for Loss of Muscle Integrity. Frontiers in physiology. 2018;9:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Partridge L, Rajbhandari S. Achilles Tendon in Diabetes. Curr Diabetes Rev 2017;13(4):424–7. [DOI] [PubMed] [Google Scholar]
- 61.Studentsova V, Mora KM, Glasner MF, Buckley MR, Loiselle AE. Obesity/Type II Diabetes Promotes Function-limiting Changes in Murine Tendons that are not reversed by Restoring Normal Metabolic Function. Sci Rep 2018;8(1):9218.* Loss of normal tendon homeostasis occurs caused by obesity-induced type II diabetes is shown to be irreversible.
- 62.Ursini F, Arturi F, D’Angelo S, Amara L, Nicolosi K, Russo E, et al. High Prevalence of Achilles Tendon Enthesopathic Changes in Patients with Type 2 Diabetes Without Peripheral Neuropathy. J Am Podiatr Med Assoc 2017;107(2):99–105. [DOI] [PubMed] [Google Scholar]
- 63.Lee JM, Veres SP. Advanced glycation end-product cross-linking inhibits biomechanical plasticity and characteristic failure morphology of native tendon. J Appl Physiol (1985). 2019;126(4):832–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soslowsky LJ, Fryhofer GW. Tendon Homeostasis in Hypercholesterolemia. Advances in experimental medicine and biology. 2016;920:151–65. [DOI] [PubMed] [Google Scholar]
- 65.Taylor B, Cheema A, Soslowsky L. Tendon Pathology in Hypercholesterolemia and Familial Hypercholesterolemia. Current rheumatology reports. 2017;19(12):76. [DOI] [PubMed] [Google Scholar]
- 66.Mall NA, Tanaka MJ, Choi LS, Paletta GA Jr. Factors affecting rotator cuff healing. J Bone Joint Surg Am 2014;96(9):778–88. [DOI] [PubMed] [Google Scholar]
- 67.Cho NS, Moon SC, Jeon JW, Rhee YG. The influence of diabetes mellitus on clinical and structural outcomes after arthroscopic rotator cuff repair. Am J Sports Med 2015;43(4):991–7. [DOI] [PubMed] [Google Scholar]
- 68.Clement ND, Hallett A, MacDonald D, Howie C, McBirnie J. Does diabetes affect outcome after arthroscopic repair of the rotator cuff? J Bone Joint Surg Br 2010;92(8):1112–7. [DOI] [PubMed] [Google Scholar]
- 69.Ahmed AS, Schizas N, Li J, Ahmed M, Ostenson CG, Salo P, et al. Type 2 diabetes impairs tendon repair after injury in a rat model. J Appl Physiol (1985). 2012;113(11):1784–91. [DOI] [PubMed] [Google Scholar]
- 70.David MA, Jones KH, Inzana JA, Zuscik MJ, Awad HA, Mooney RA. Tendon repair is compromised in a high fat diet-induced mouse model of obesity and type 2 diabetes. PloS one. 2014;9(3):e91234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ackerman JE, Geary MB, Orner CA, Bawany F, Loiselle AE. Obesity/Type II diabetes alters macrophage polarization resulting in a fibrotic tendon healing response. PloS one. 2017;12(7):e0181127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ahmed AS, Li J, Abdul AM, Ahmed M, Östenson CG, Salo PT, et al. Compromised Neurotrophic and Angiogenic Regenerative Capability during Tendon Healing in a Rat Model of Type-II Diabetes. PloS one. 2017;12(1):e0170748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chbinou N, Frenette J. Insulin-dependent diabetes impairs the inflammatory response and delays angiogenesis following Achilles tendon injury. American journal of physiology Regulatory, integrative and comparative physiology. 2004;286(5):R952–7. [DOI] [PubMed] [Google Scholar]
- 74.Egemen O, Ozkaya O, Ozturk MB, Sen E, Akan M, Sakiz D, et al. The biomechanical and histological effects of diabetes on tendon healing: experimental study in rats. J Hand Microsurg. 2012;4(2):60–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mohsenifar Z, Feridoni MJ, Bayat M, Masteri Farahani R, Bayat S, Khoshvaghti A. Histological and biomechanical analysis of the effects of streptozotocin-induced type one diabetes mellitus on healing of tenotomised Achilles tendons in rats. Foot Ankle Surg 2014;20(3):186–91. [DOI] [PubMed] [Google Scholar]
- 76.Korntner S, Kunkel N, Lehner C, Gehwolf R, Wagner A, Augat P, et al. A high-glucose diet affects Achilles tendon healing in rats. Sci Rep 2017;7(1):780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Greve K, Domeij-Arverud E, Labruto F, Edman G, Bring D, Nilsson G, et al. Metabolic activity in early tendon repair can be enhanced by intermittent pneumatic compression. Scand J Med Sci Sports. 2012;22(4):e55–63. [DOI] [PubMed] [Google Scholar]
- 78.Zhang K, Hast MW, Izumi S, Usami Y, Shetye S, Akabudike N, et al. Modulating Glucose Metabolism and Lactate Synthesis in Injured Mouse Tendons: Treatment With Dichloroacetate, a Lactate Synthesis Inhibitor, Improves Tendon Healing. Am J Sports Med 2018;46(9):2222–31.** This study examined metabolic flux during mouse Achilles tendon healing and specifically examined the function of lactate synthesis during tendon healing.
- 79.Valkering KP, Aufwerber S, Ranuccio F, Lunini E, Edman G, Ackermann PW. Functional weight-bearing mobilization after Achilles tendon rupture enhances early healing response: a single-blinded randomized controlled trial. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2017;25(6):1807–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eliasson P, Couppé C, Lonsdale M, Svensson RB, Neergaard C, Kjær M, et al. Ruptured human Achilles tendon has elevated metabolic activity up to 1 year after repair. European journal of nuclear medicine and molecular imaging. 2016;43(10):1868–77.** This study demonstrated that glucose uptake remains elevated in healing human tendon for at least 12 months and revealed that glucose uptake was related to poor clinical outcomes.
- 81.Svedman S, Westin O, Aufwerber S, Edman G, Nilsson-Helander K, Carmont MR, et al. Longer duration of operative time enhances healing metabolites and improves patient outcome after Achilles tendon rupture surgery. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2018;26(7):2011–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thankam FG, Chandra IS, Kovilam AN, Diaz CG, Volberding BT, Dilisio MF, et al. Amplification of Mitochondrial Activity in the Healing Response Following Rotator Cuff Tendon Injury. Sci Rep 2018;8(1):17027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fluck M, Fitze D, Ruoss S, Valdivieso P, von Rechenberg B, Bratus-Neuenschwander A, et al. Down-Regulation of Mitochondrial Metabolism after Tendon Release Primes Lipid Accumulation in Rotator Cuff Muscle. Am J Pathol 2020;190(7):1513–29. [DOI] [PubMed] [Google Scholar]
- 84.Locke RC, Lemmon EA, Dudzinski E, Kopa SC, Wayne JM, Soulas JM, et al. Photobiomodulation does not influence maturation and mildly improves functional healing of mouse achilles tendons. J Orthop Res 2020;38(8):1866–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Matthews TJ, Smith SR, Peach CA, Rees JL, Urban JP, Carr AJ. In vivo measurement of tissue metabolism in tendons of the rotator cuff: implications for surgical management. J Bone Joint Surg Br 2007;89(5):633–8. [DOI] [PubMed] [Google Scholar]
- 86.Sieg R, Garcia EJ, Schoenfeld AJ, Collins T, Owens BD. Effects of supplemental oxygen and hyperbaric oxygen on tendon healing in a rat model. Journal of surgical orthopaedic advances. 2011;20(4):225–9. [PubMed] [Google Scholar]
- 87.Ishii Y, Miyanaga Y, Shimojo H, Ushida T, Tateishi T. Effects of hyperbaric oxygen on procollagen messenger RNA levels and collagen synthesis in the healing of rat tendon laceration. Tissue engineering. 1999;5(3):279–86. [DOI] [PubMed] [Google Scholar]
- 88.Kuran FD, Pekedis M, Yıldız H, Aydın F, Eliyatkın N. Effect of hyperbaric oxygen treatment on tendon healing after Achilles tendon repair: an experimental study on rats. Acta Orthop Traumatol Turc 2012;46(4):293–300. [DOI] [PubMed] [Google Scholar]
- 89.Murad S, Grove D, Lindberg KA, Reynolds G, Sivarajah A, Pinnell SR. Regulation of collagen synthesis by ascorbic acid. Proc Natl Acad Sci U S A. 1981;78(5):2879–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Omeroğlu S, Peker T, Türközkan N, Omeroğlu H. High-dose vitamin C supplementation accelerates the Achilles tendon healing in healthy rats. Archives of orthopaedic and trauma surgery. 2009;129(2):281–6. [DOI] [PubMed] [Google Scholar]
- 91.Gemalmaz HC, Sarıyılmaz K, Ozkunt O, Gurgen SG, Silay S. Role of a combination dietary supplement containing mucopolysaccharides, vitamin C, and collagen on tendon healing in rats. Acta Orthop Traumatol Turc 2018;52(6):452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dincel YM, Adanir O, Arikan Y, Caglar AK, Dogru SC, Arslan YZ. Effects of high-dose vitamin C and hyaluronic acid on tendon healing. Acta ortopedica brasileira. 2018;26(2):82–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Souza M, Moraes SAS, de Paula DR, Maciel AA, Batista EJO, Silva DGF, et al. Local treatment with ascorbic acid accelerates recovery of post-sutured Achilles tendon in male Wistar rats. Brazilian journal of medical and biological research 2019;52(9):e8290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oliva F, Maffulli N, Gissi C, Veronesi F, Calciano L, Fini M, et al. Combined ascorbic acid and T(3) produce better healing compared to bone marrow mesenchymal stem cells in an Achilles tendon injury rat model: a proof of concept study. Journal of orthopaedic surgery and research. 2019;14(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]