Abstract
Purpose
To perform a systematic review and meta-analysis to calculate the pooled upgrade rate of pure flat epithelial atypia (FEA) diagnosed at core needle biopsy (CNB).
Materials and Methods
A PubMed and Embase database search was performed in December 2019. Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines were followed. Study quality and publication bias were assessed. The upgrade rate of pure FEA to cancer, invasive carcinoma, and ductal carcinoma in situ (DCIS), as well as the co-occurrence rate of atypical ductal hyperplasia (ADH), with 95% CIs were calculated. A random effect model was used to integrate the proportions and their corresponding 95% CI. Study heterogeneity was calculated using τ2 and I2.
Results
A total of 2482 cases of pure FEA across 42 studies (mean age range, 46–59 years) met inclusion criteria to be analyzed. Significant study heterogeneity was identified (τ2 = 0.001, I2 = 67%). The pooled upgrade rates reported for pure FEA were 5% (95% CI: 3%, 6%) for breast cancer, 1% (95% CI: 0%, 2%) for invasive carcinoma, and 2% (95% CI: 1%, 3%) for DCIS. When more than 90% of calcifications were removed at CNB, the pooled upgrade rate was 0% (95% CI: 0%, 2%). The pooled co-occurrence rate of ADH at surgical excision was 17% (95% CI: 12%, 21%). Study quality was medium to high with a risk of publication bias (P < .01).
Conclusion
Pure FEA diagnosed at CNB should be surgically excised due to the pooled upgrade rate of 5% for breast cancer. If more than 90% of the targeted calcifications are removed by CNB for pure FEA, close imaging follow-up is recommended.
Keywords: Biopsy/Needle Aspiration, Breast, Mammography
Supplemental material is available for this article.
© RSNA, 2021
Summary
Surgical excision is recommended for pure flat epithelial atypia diagnosed at core needle biopsy, as the upgrade rate is greater than 2% as recommended by the Breast Imaging Reporting and Data System.
Key Points
■ Meta-analysis of 2482 cases of pure flat epithelial atypia (FEA) across 42 studies demonstrated a pooled upgrade rate of 5% to all breast cancers, 1% to invasive carcinoma, and 2% to ductal carcinoma in situ.
■ When more than 90% of targeted calcifications were removed by core needle biopsy (CNB), the pooled upgrade rate to breast cancer was 0%.
■ The co-occurrence rate of atypical ductal hyperplasia and FEA at surgical excision after a diagnosis of pure FEA at CNB was reported at 17%.
Introduction
Flat epithelial atypia (FEA) of the breast is the neoplastic equivalent of a benign columnar cell lesion (1). FEA is a neoplastic alteration of acini, characterized by replacement of cuboidal epithelium by one to several layers of columnar cells, loss of nuclear polarity, and low-grade cytologic atypia as defined by the World Health Organization (typical tissue architecture is shown in Fig E1 [supplement]) (1,2). FEA is associated with intraluminal microcalcifications and secretions. It often coexists with atypical ductal hyperplasia (ADH), lobular neoplasia, ductal carcinoma in situ (DCIS), and invasive ductal carcinoma (3,4). Pure FEA is diagnosed when FEA is the most advanced lesion at core needle biopsy (CNB) with no associated invasive ductal carcinoma, DCIS, ADH, lobular neoplasia, or other atypia.
At mammography, the majority of FEA cases manifest as grouped amorphous microcalcifications, but FEA can also manifest as fine pleomorphic or punctate calcifications (Fig E2a [supplement]) (2,5–7). Less often, FEA may appear as a mass, asymmetry, or architectural distortion. At US, it often appears as an irregular mass with microlobulated margins and hypoechoic or complex echotexture (Fig E2b, E2c [supplement]) (7). Nonmass enhancement is the most common manifestation of FEA at MRI (7,8). Although FEA is rare, it is increasingly diagnosed at CNB due to its association with microcalcifications and accounts for 1%–8% of all CNB diagnoses (6,9).
The treatment of patients diagnosed with FEA as the most abnormal lesion at CNB is controversial because the reported upgrade rates to malignancy upon excision are widely varied. In addition, the co-occurrence of FEA and ADH may warrant additional treatment therapy such as risk-reducing medication (3). The primary aim of the study was to identify the upgrade rates for invasive breast cancers and DCIS at surgical excision or at 2-year imaging follow-up after a CNB demonstrates pure FEA. We performed a systematic review and meta-analysis to assess these pooled upgrade rates. The results of this study can assist in guiding the management recommendation (surgical excision vs close imaging follow-up) for patients diagnosed with pure FEA at CNB.
Materials and Methods
Study Protocol and Quality Assessment
Institutional review board approval was not needed for this study. The study was registered on Open Science Framework-Center for Open Science (https://osf.io/cb5jg). We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement to conduct this study. The Newcastle-Ottawa Scale was used to appraise the quality of the included studies by two breast radiologists with 6 and 30 years of experience (R.A.W. and S.J.L., respectively).
Literature Search
A systematic search was performed by our academic and research services librarian in conjunction with two authors (M.E.M. and R.A.W.) in December of 2019 by using PubMed and Embase for studies reporting the upgrade rate of pure FEA diagnosed at CNB. The keywords and search strings used for PubMed and Embase were: (“flat epithelial atypia management” OR “Flat Epithelial atypia upgrade” OR “Flat epithelial atypia” OR “Pure Flat Epithelial Atypia”)) AND ((((((“Breast Neoplasms/etiology”[Mesh]) OR “Breast Neoplasms/diagnosis”[Mesh]) OR “Breast Neoplasms/surgery”[Mesh]) OR “Breast Neoplasms/pathology”[Mesh]) OR “Breast/pathology”[Mesh]) OR “Breast/chemistry”[Mesh])) AND ((“Biopsy, Needle”[Mesh]) OR “Biopsy, Large-Core Needle”[Mesh]). The number of studies identified with this search was documented. Table E1 (supplement) shows additional details on the Embase search string.
The initial selection of studies was based on titles and abstracts, which were screened by two authors (M.E.M. and R.A.W.) with 2 and 6 years of experience in breast imaging, respectively. Articles that reported an upgrade rate for pure FEA in the abstract or tables and/or figures within the full text were considered eligible for full review. Only studies written in English were included. Studies that used all types of CNBs (US, MRI, stereotactic, or tomosynthesis) were included. No limit was set for a minimum or maximum number of patients. Discrepancies in study selection were adjudicated by a third reviewer who has 30 years of experience in breast imaging (S.J.L.). The full texts of eligible articles were reviewed by the authors.
Eligible studies for inclusion in the meta-analysis met the following criteria: (a) pure FEA diagnosed with CNB, (b) surgical excision performed for the biopsied pure FEA or 2 years of breast imaging follow-up was documented, and (c) documented upgrade to in situ and/or invasive carcinoma. We defined an “upgrade” as the diagnosis of in situ or invasive carcinoma upon surgical excision or 2-year imaging follow-up. We did not consider lobular neoplasia, ADH, or other high-risk breast lesions to represent an upgrade. Studies were excluded if: (a) pure FEA was not evaluated, (b) studies did not differentiate pure FEA from other high-risk breast lesions, or (c) the lesion did not undergo surgical excision or lacked documented 2-year imaging follow-up.
Data Extraction
For each study included, the following data were recorded: publication date, number of cases of pure FEA at CNB, number of cases of pure FEA that had surgical excision or 2-year imaging follow-up, number of pure FEA with upgrade to invasive carcinoma and/or DCIS, number of pure FEA and ADH co-occurrences at surgical excision, gauge of the core biopsy needle, number of cases with more than 90% of the target lesion removed at CNB, and authors’ recommendation for management of FEA.
Data were extracted by two authors with 2 and 6 years of experience (M.E.M. and R.A.W., respectively) in breast imaging, and disagreements were resolved by a third author with 30 years of experience (S.J.L.) in breast imaging. Reviewers were not blinded to authors’ affiliations or journal names.
Statistical Analysis
The primary outcome for the study was to determine the overall upgrade rate of pure FEA diagnosed at CNB to breast cancer at surgical excision or imaging follow-up. The overall upgrade rate included invasive carcinoma and DCIS combined. Secondary outcomes included the pooled upgrade rates to invasive carcinoma and DCIS, respectively. A separate analysis was performed to determine the overall upgrade to carcinoma when at least 90% of the targeted lesion had been removed. Last, the rate of co-occurrence of ADH with FEA at surgical excision was determined.
The overall upgrade rate, the upgrade rates to invasive carcinoma and DCIS separately, and the co-occurrence rate of ADH with 95% CIs were calculated for each study. A random effect model was used to integrate the proportions and their corresponding 95% CI. Forest plots illustrate the results from the random effect model. To assess statistical heterogeneity, we calculated τ2. τ2 is the extent of variation among the effects observed in different studies, meaning the variance of the effect size parameters across the population of studies, and it reflects the variance of the true effect sizes. We also calculated the I2 statistic, which estimates the proportion of variability in the meta-analysis caused by differences between studies rather than sampling error. An I2 of more than 50% indicated the presence of statistical heterogeneity. I2 and its 95% CI was reported for each outcome.
Sensitivity analysis was performed by omitting the included studies one at a time and for different subgroups. Funnel plots were used to assess publication bias by visually inspecting for asymmetry in funnel plots of treatment effect against its standard error and with formal statistical procedures. The Egger test was used to examine possible small study effects.
All statistical analyses were performed using statistical software R version 3.6.3 (R Foundation for Statistical Computing). Packages meta, metafor, and funnelR were used for R. A P value less than .05 was considered statistically significant.
Results
Literature Search
The literature search selection process is outlined in Figure 1. From the 307 articles identified by the search engines (66 from PubMed and 241 from Embase), 42 studies, published between 2004 and 2020, met the inclusion criteria and were analyzed (2,4–44). Detailed characteristics of the included studies are provided in the Table. All studies were retrospective. A total of 2881 pure FEA cases were reported in the studies, of which 2482 had the data meeting the study criteria and were analyzed. Most patients were treated with surgical excision, and 104 patients had 2 years of breast imaging follow-up. All patients were women, and the mean age range was 46–59 years. A single study did not report the mean age (44).
Figure 1:
Flow diagram of study selection. Of the 307 initially retrieved articles, 176 were excluded as they did not meet the inclusion criteria, and 42 were included the final analysis.
Overview of Study Quality Scores and Characteristics

Study Characteristics
Most of the studies (n = 29) reported using 8–11-gauge CNB devices. Use of a 14-gauge or larger CNB device was reported by six studies (6,11,19,34,36,43), and one study reported the use of a 12-gauge needle (39). The CNB device was not reported in six studies (4,9,12,14,32,40). All but one study distinguished invasive carcinoma from DCIS (8). This study was included in the overall upgrade rate to breast cancer but was excluded from the secondary analysis separating invasive carcinoma and DCIS (8). Eleven studies reported the extent of target lesion removal with a corresponding upgrade to DCIS or invasive carcinoma at surgical excision (2,4,7,12,22,23,25–27,33,41). Regarding management recommendations, 16 studies described criteria for management with surgical excision versus close imaging follow-up, 17 studies recommended surgical excision, six studies recommended close imaging follow-up, and three studies made no management recommendations.
Overall Upgrade Rate to Breast Cancer
The overall upgrade rate was defined as the upgrade of pure FEA diagnosed at CNB to breast cancer, including invasive carcinoma and DCIS, at surgical excision or 2-year imaging follow-up. The observed proportions of upgrade to breast cancer varied widely among the studies, ranging from 0% to 38% (Fig 2a). The pooled overall upgrade rate to breast cancer by the random effect model was 5% (95% CI: 3%, 6%). Significant study heterogeneity was found when we considered the overall upgrade rate (τ2 = 0.001; I2 = 67% [95% CI: 54%, 76%], P < .01).
Figure 2a:
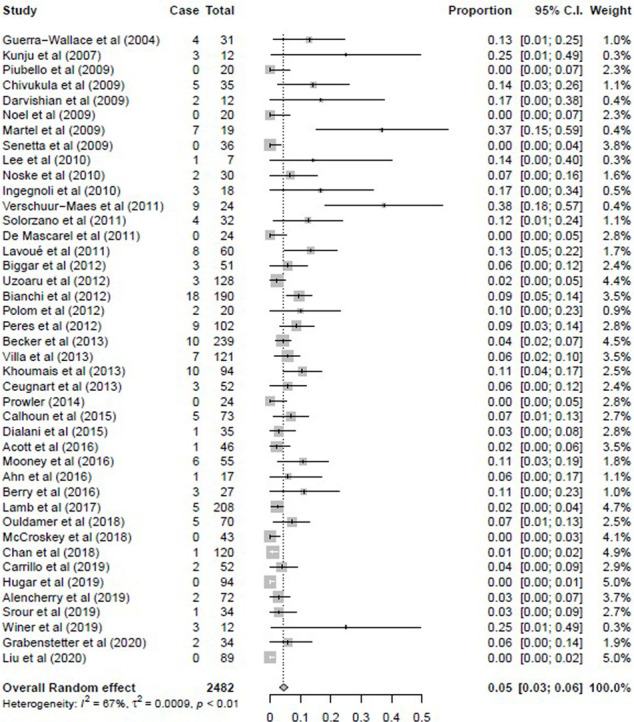
Forest plots of the (a) overall upgrade rate to breast cancer, (b) upgrade rate to invasive breast cancer, and (c) upgrade rate to ductal carcinoma in situ at surgical excision after pure flat epithelial atypia was diagnosed at core needle biopsy. (a) Pooled upgrade rate to breast cancer by random-effects model (shown in last row) was 5% (95% CI: 3%, 6%). (b) Pooled upgrade rate to invasive breast cancer by random-effects model (shown in last row) was 1% (95% CI: 0%, 2%). (c) Pooled upgrade rate to ductal carcinoma in situ by random-effects model (shown in last row) was 2% (95% CI: 2%, 3%).
With the significant heterogeneity identified, two subgroup analyses were subsequently performed. The 14 studies that reported fewer than 30 pure FEA cases in the study were removed, and the remainder of the studies were analyzed (Fig E3 [supplement]). The overall upgrade rate for this subanalysis was 4% (95% CI: 3%, 5%) with a study heterogeneity of 68%. In the second subanalysis, the 12 studies reporting use of a CNB gauge of larger than 12 for most of their biopsies and those in which no CNB gauge was reported were removed, and the remainder of the studies were analyzed. The overall upgrade rate for this subanalysis was 4% (95% CI: 3%, 5%) with a study heterogeneity of 61% (Fig E4 [supplement]).
Upgrade Rate to Invasive Carcinoma
Heterogeneity among the studies was low when we considered the pooled upgrade rate for invasive breast cancer (τ2 < 0.0001; I2 = 31% [95% CI: 0%, 53%], P = .03). The observed proportions with upgrade to cancer varied widely among the studies, ranging from 0% to 37% (Fig 2b). The pooled upgrade rate to invasive breast cancer by random effects model was 1% (95% CI: 0%, 2%) at surgical excision or 2-year imaging follow-up.
Figure 2b:
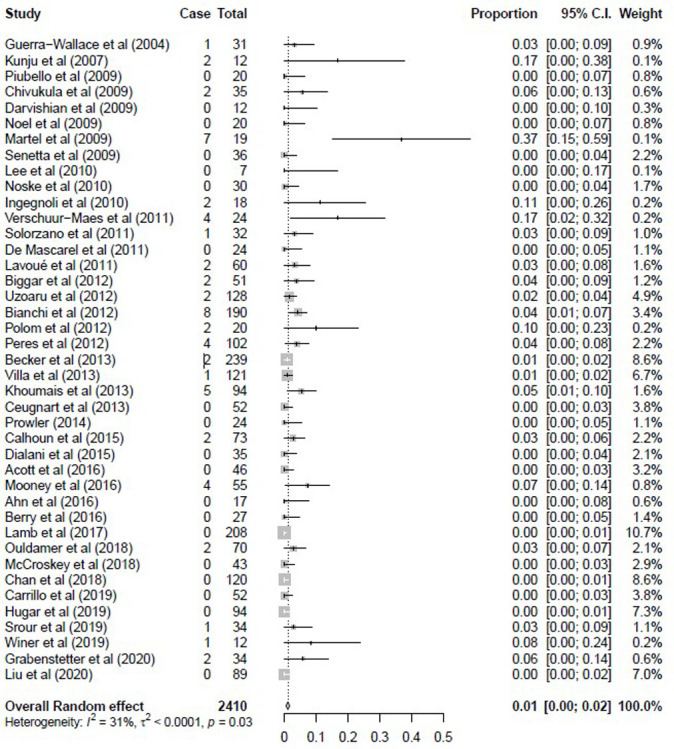
Forest plots of the (a) overall upgrade rate to breast cancer, (b) upgrade rate to invasive breast cancer, and (c) upgrade rate to ductal carcinoma in situ at surgical excision after pure flat epithelial atypia was diagnosed at core needle biopsy. (a) Pooled upgrade rate to breast cancer by random-effects model (shown in last row) was 5% (95% CI: 3%, 6%). (b) Pooled upgrade rate to invasive breast cancer by random-effects model (shown in last row) was 1% (95% CI: 0%, 2%). (c) Pooled upgrade rate to ductal carcinoma in situ by random-effects model (shown in last row) was 2% (95% CI: 2%, 3%).
Upgrade Rate to DCIS
Heterogeneity among the studies was low when we considered the pooled upgrade rate for DCIS (τ2 = 0.0002; I2 = 38% [95% CI: 1%, 58%], P < .01). The observed proportions with upgrade to DCIS did not vary as widely as those for invasive cancer among the studies, ranging from 0% to 21% (Fig 2c). The pooled upgrade rate to DCIS by random effects model was 2% (95% CI: 1%, 3%) at surgical excision or 2-year imaging follow-up.
Figure 2c:

Forest plots of the (a) overall upgrade rate to breast cancer, (b) upgrade rate to invasive breast cancer, and (c) upgrade rate to ductal carcinoma in situ at surgical excision after pure flat epithelial atypia was diagnosed at core needle biopsy. (a) Pooled upgrade rate to breast cancer by random-effects model (shown in last row) was 5% (95% CI: 3%, 6%). (b) Pooled upgrade rate to invasive breast cancer by random-effects model (shown in last row) was 1% (95% CI: 0%, 2%). (c) Pooled upgrade rate to ductal carcinoma in situ by random-effects model (shown in last row) was 2% (95% CI: 2%, 3%).
Upgrade Rate to Carcinoma with Near-Complete Target Removal
Near-complete target removal was defined as at least 90% of the target lesion removed with CNB. Eleven studies reported that more than 90% of the target was removed during CNB, which were all cases of calcifications. Complete removal of other lesions, such as nonmass enhancement or asymmetry, was not reported. These 11 studies were analyzed for an overall upgrade rate to carcinoma, including both DCIS and invasive cancer at surgical excision or 2-year imaging follow-up (Fig 3). The observed proportions with overall upgrade to breast cancer ranged from 0% to 12%, with a pooled upgrade rate of 0% (95% CI: 0%, 2%) by the random effect model.
Figure 3:

Forest plot of the overall upgrade rate to breast cancer at surgical excision when more than 90% of the targeted calcifications were removed at core needle biopsy with a diagnosis of pure flat epithelial atypia. Pooled upgrade rate by random-effects model (shown in last row) was 0% (95% CI: 0%, 2%).
Co-Occurrence Rate of ADH at Surgery
The rate of ADH found at surgical excision after a diagnosis of pure FEA at CNB was analyzed. A total of 1502 cases were included from 25 studies. Heterogeneity among the studies was high when we considered the pooled co-occurrence rate for ADH (τ2 = 0.0096; I2 = 91% [95% CI: 89%, 94%], P < .01). The observed proportions for the co-occurrence of ADH ranged from 0% to 42% (Fig E5 [supplement]). The pooled co-occurrence rate by random effects model was 17% (95% CI: 12%, 21%).
Two subgroup analyses were performed. The five studies that reported fewer than 30 pure FEA cases were removed, and the 20 remaining studies were analyzed (Fig E6 [supplement]) (19,24,34,37,43). The pooled co-occurrence rate for ADH at surgical excision by the random effect model for this subanalysis was 16% (95% CI: 11%, 21%), with a study heterogeneity of 93%. In the second subanalysis, the seven studies reporting a CNB gauge of larger than 12 for majority of biopsies or no CNB gauge reported were removed, and the 18 remaining studies were analyzed (Fig E7 [supplement]) (4,6,11,12,19,34,43). The pooled co-occurrence rate for ADH at surgical excision by the random effect model for this subanalysis was 16% (95% CI: 11%, 21%), with a study heterogeneity of 92%.
Cumulative Meta-Analysis
Cumulative meta-analysis was performed according to the studies’ publication times (Fig 4a–4d and Fig E8 [supplement]). The cumulative meta-analysis showed a stability in FEA upgrade rates to overall cancer, invasive cancer, DCIS, overall upgrade rate to breast cancer at surgical excision when more than 90% of the targeted calcifications were removed at CNB, as well as co-occurrence of ADH at surgery over time, and corresponding 95% CIs did become narrower with the studies added one by one. This indicates that the current studies’ cumulative data lead to a safe conclusion on overall upgrade rate to cancer, invasive cancer, DCIS, overall upgrade rate to breast cancer at surgical excision when more than 90% of the targeted calcifications were removed at CNB, as well as co-occurrence of ADH at surgery over time.
Figure 4a:
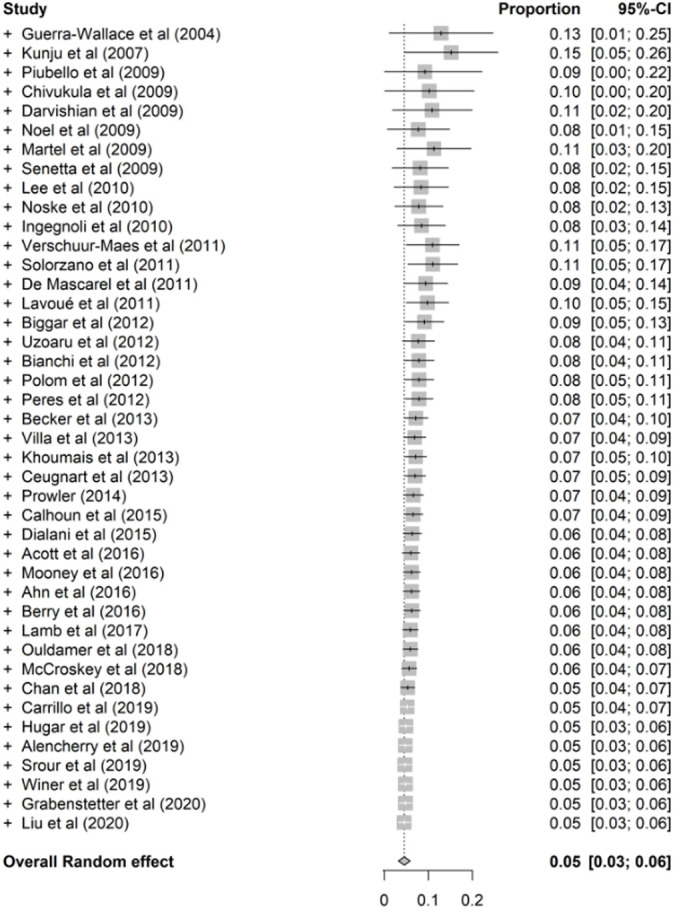
Cumulative meta-analysis plots of the (a) overall upgrade rate to breast cancer, (b) upgrade rate to invasive breast cancer, (c) upgrade rate to ductal carcinoma in situ at surgical excision after pure flat epithelial atypia was diagnosed at core needle biopsy, and (d) overall upgrade rate to breast cancer at surgical excision when more than 90% of the targeted calcifications were removed at core needle biopsy.
Figure 4d:

Cumulative meta-analysis plots of the (a) overall upgrade rate to breast cancer, (b) upgrade rate to invasive breast cancer, (c) upgrade rate to ductal carcinoma in situ at surgical excision after pure flat epithelial atypia was diagnosed at core needle biopsy, and (d) overall upgrade rate to breast cancer at surgical excision when more than 90% of the targeted calcifications were removed at core needle biopsy.
Figure 4b:

Cumulative meta-analysis plots of the (a) overall upgrade rate to breast cancer, (b) upgrade rate to invasive breast cancer, (c) upgrade rate to ductal carcinoma in situ at surgical excision after pure flat epithelial atypia was diagnosed at core needle biopsy, and (d) overall upgrade rate to breast cancer at surgical excision when more than 90% of the targeted calcifications were removed at core needle biopsy.
Figure 4c:
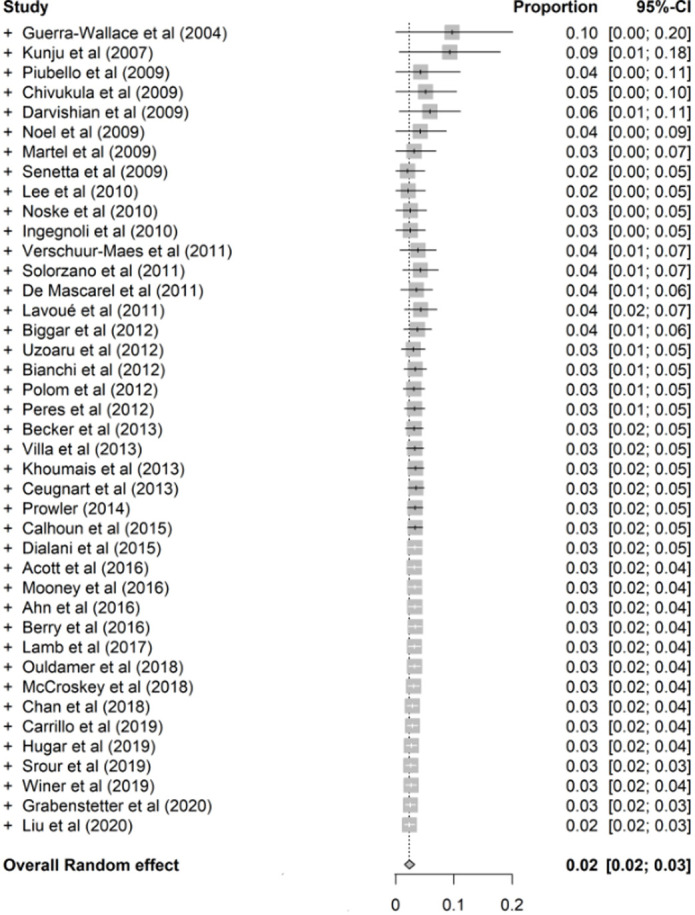
Cumulative meta-analysis plots of the (a) overall upgrade rate to breast cancer, (b) upgrade rate to invasive breast cancer, (c) upgrade rate to ductal carcinoma in situ at surgical excision after pure flat epithelial atypia was diagnosed at core needle biopsy, and (d) overall upgrade rate to breast cancer at surgical excision when more than 90% of the targeted calcifications were removed at core needle biopsy.
Sensitivity Analysis
Sensitivity analysis was performed due to significant heterogeneity. One study at a time was removed from the random effect model to evaluate the effect of that study on the pooled estimate. The overall random effect ratios with 95% CIs were reported in Table E2 (supplement). All pooled estimates were 0.04 or 0.05, which was similar to the results using all studies, indicating the stability of the current results.
Risk of Publication Bias and Study Quality
Inspection of the funnel plots showed high asymmetry, indicating evidence of a small study effect for overall upgrade rate to FEA (Egger test: P < .01) (Fig 5). Of the 42 studies, 69% (29 of 42) received a score of 8, 26% (11 of 42) received a score 7, and 5% (2 of 42) received a score of 6 by the Newcastle-Ottawa scale, representing medium study quality (Table).
Figure 5:
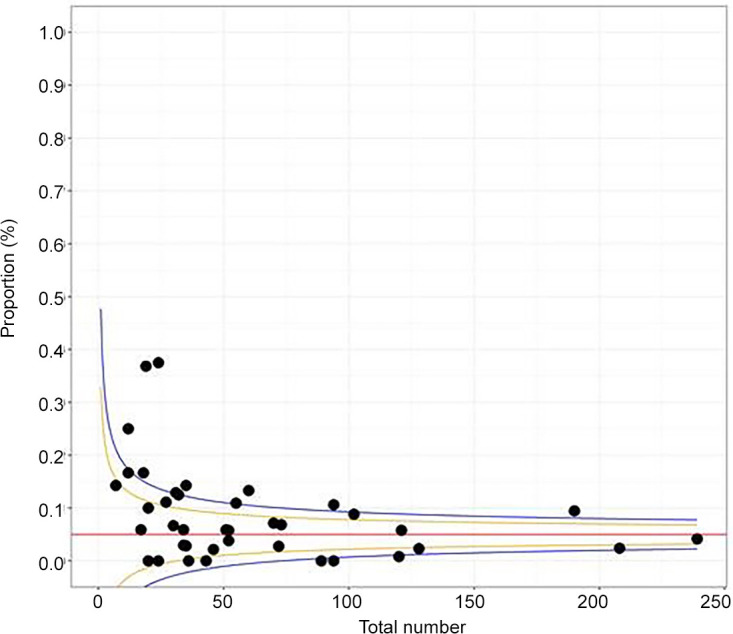
Funnel plot demonstrates asymmetry, indicating a publication bias (P < .01, Egger test). Each study is represented with a dot; x axis represents total number of pure flat epithelial atypia cases.
Discussion
With the development of improved breast imaging techniques, particularly in the detection of calcifications at mammography, as well as greater understanding of FEA as defined by the World Health Organization, there has been an increase in the diagnosis of FEA at CNB over the last decade (1,45,46). The challenge that clinicians face is how to treat these patients, given the highly variable upgrade rates of pure FEA reported in the literature (46). With pure FEA accounting for approximately 1%–8% of all CNB diagnoses, establishing management guidelines with studies containing a limited number of cases is difficult (2,6). We performed a systematic review and meta-analysis to maximize the number of cases of pure FEA to provide more robust evaluation.
Our analysis included 42 published studies, with a total of 2482 cases of pure FEA. We identified a pooled upgrade rate to breast cancer, which included invasive carcinoma and DCIS, of 5% (95% CI: 3%, 6%), a pooled upgrade rate for invasive carcinoma of 1% (95% CI: 0%, 2%), and a pooled upgrade rate for DCIS of 2% (95% CI: 1%, 3%). The meta-analysis was used to combine results of different studies, particularly those with a small sample size. For the analysis, a common statistical measure was identified that was shared among the studies, and a weighted average was calculated for each common measure. Typically, in a meta-analysis, each study and each outcome have different weights. This measure then reflects the effect of an upgrade, adherence, or other intervention on average across all studies. If studies do not report each individual outcome, the weights of these outcomes will be different. For example, in our study, Alencherry et al did not report individual upgrade rates for invasive ductal carcinoma or DCIS but did report an overall upgrade to cancer (8). Therefore, the overall upgrade to cancer (5%) will not equal the sum of individual upgrade rates (invasive carcinoma 1% and DCIS 2%), as each study will be weighted differently on the number and type of reported upgrades. The Breast Imaging Reporting and Data System (BI-RADS) (47) guidelines recommend surgical excision rather than imaging follow-up for lesions with an upgrade rate of greater than 2%. Therefore, our results indicate that FEA diagnosed at CNB should be managed with surgical excision. Similar to our study, Rudin et al performed a systematic review and meta-analysis of pure FEA of 1517 cases, reporting an upgrade rate of 7.5% to cancer and recommending surgical excision (3).
Removal of more than 90% of the target lesion at CNB was reported for 312 cases of pure FEA in 11 studies (2,4,7,12,22,23,25–27,33,41). The pooled upgrade rate was 0% for these lesions. The targeted lesions in all these studies were calcifications. These studies used a 12-gauge or smaller needle, with the exception of two studies that did not report a needle size (4,12). Use of a larger sized needle allows for nearly complete removal of calcifications because more tissue can be collected. Thus, if more than 90% of a group of calcifications is removed at CNB, these women can be closely followed with imaging. However, as no studies reported upgrades after 90% lesion removal for other target lesions such as asymmetries, masses, or nonmass enhancement, the same management recommendations do not apply.
The co-occurrence of ADH and FEA is a common finding at surgical excision (6,7,9). We found a co-occurrence rate of 17%; however, there was significant study heterogeneity. The needle gauge employed at CNB will impact the co-occurrence of FEA and ADH at CNB (3,48). A larger needle will allow for a larger tissue sample at CNB, which will increase the likelihood of capturing FEA and ADH in the CNB. In addition, the interobserver agreement between pathologists for the diagnosis of FEA can be highly variable, as demonstrated by Samples et al (49). It is important that pathologists adhere to the strict definition of FEA to make an accurate diagnosis at CNB. Both underreporting and overdiagnosing FEA with ADH at CNB occurs (40,50). Darvishian et al and O’Malley et al, however, demonstrated acceptable interobserver agreement after improved training and tutorials for the pathologists (40,50). An accurate pathologic diagnosis of pure FEA and FEA with ADH at CNB is critical to making correct management recommendations (40,50). The presence of ADH is known to increase a woman’s overall risk of breast cancer, and patients may benefit from increased screening or risk reduction strategies (3). Thus, identifying ADH is clinically important. The high co-occurrence rate further supports surgical excision of pure FEA to identify these at-risk women.
Our study had some limitations. There was significant study heterogeneity, likely related to the relatively small number of FEA cases reported in the literature. This is understandable, as FEA only accounts for 1%–8% of CNB diagnoses (2,6). Furthermore, there was publication bias evident. Another limitation was the lack of definitive 2-year follow-up, as recommended by BI-RADS, for several studies (47). Studies typically provided a range for follow-up, with the lower limit less than 2 years for some reports. Not all studies described complete target removal of calcifications, and no studies described complete target removal for other lesions, such as asymmetry, mass, or nonmass enhancement. A prospective multi-institutional study would be needed, with a uniform biopsy protocol and follow-up, to further investigate upgrade rates to DCIS and invasive ductal carcinoma of pure FEA diagnosed at CNB.
While there is no consensus on the management of pure FEA diagnosed at CNB, most published studies recommend excision of pure FEA over imaging follow-up. The results of our meta-analysis support such recommendation, with a pooled upgrade rate of 5% to breast cancer at surgical excision or 2-year imaging follow-up of pure FEA diagnosed at CNB. In addition, there was a high co-occurrence rate of ADH at surgery. If more than 90% of targeted calcifications are removed by CNB for pure FEA, close imaging follow-up can be performed instead of surgical excision.
SUPPLEMENTAL TABLES
SUPPLEMENTAL FIGURES
Authors declared no funding for this work.
Disclosures of Conflicts of Interest: R.A.W. disclosed no relevant relationships. S.J.L. disclosed no relevant relationships. M.EM. disclosed no relevant relationships. B.Z. disclosed no relevant relationships. M.C.M. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: author is board member of the Radiological Society of North America and the American College of Radiology (ACR); author’s institution received Innovation Grant from ACR; author received royalties from Elsevier for breast textbook; author is editorial board member of Contemporary Diagnostic Radiology. Other relationships: disclosed no relevant relationships.
Abbreviations:
- ADH
- atypical ductal hyperplasia
- BI-RADS
- Breast Imaging Reporting and Data System
- CNB
- core needle biopsy
- DCIS
- ductal carcinoma in situ
- FEA
- flat epithelial atypia
References
- 1.Sinn HP, Kreipe H. A Brief Overview of the WHO Classification of Breast Tumors, 4th Edition, Focusing on Issues and Updates from the 3rd Edition. Breast Care (Basel) 2013;8(2):149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grabenstetter A, Brennan S, Salagean ED, Morrow M, Brogi E. Flat Epithelial Atypia in Breast Core Needle Biopsies With Radiologic-Pathologic Concordance: Is Excision Necessary? Am J Surg Pathol 2020;44(2):182–190.31609784 [Google Scholar]
- 3.Rudin AV, Hoskin TL, Fahy A, et al. Flat Epithelial Atypia on Core Biopsy and Upgrade to Cancer: a Systematic Review and Meta-Analysis. Ann Surg Oncol 2017;24(12):3549–3558. [DOI] [PubMed] [Google Scholar]
- 4.Acott AA, Mancino AT. Flat epithelial atypia on core needle biopsy, must we surgically excise? Am J Surg 2016;212(6):1211–1213. [DOI] [PubMed] [Google Scholar]
- 5.Becker AK, Gordon PB, Harrison DA, et al. Flat ductal intraepithelial neoplasia 1A diagnosed at stereotactic core needle biopsy: is excisional biopsy indicated? AJR Am J Roentgenol 2013;200(3):682–688. [DOI] [PubMed] [Google Scholar]
- 6.Khoumais NA, Scaranelo AM, Moshonov H, et al. Incidence of breast cancer in patients with pure flat epithelial atypia diagnosed at core-needle biopsy of the breast. Ann Surg Oncol 2013;20(1):133–138. [DOI] [PubMed] [Google Scholar]
- 7.Solorzano S, Mesurolle B, Omeroglu A, et al. Flat epithelial atypia of the breast: pathological-radiological correlation. AJR Am J Roentgenol 2011;197(3):740–746. [DOI] [PubMed] [Google Scholar]
- 8.Alencherry E, Goel R, Gore S, et al. Clinical, imaging, and intervention factors associated with the upgrade of isolated flat epithelial atypia. Clin Imaging 2019;54:21–24. [DOI] [PubMed] [Google Scholar]
- 9.Martel M, Barron-Rodriguez P, Tolgay Ocal I, Dotto J, Tavassoli FA. Flat DIN 1 (flat epithelial atypia) on core needle biopsy: 63 cases identified retrospectively among 1,751 core biopsies performed over an 8-year period (1992-1999). Virchows Arch 2007;451(5):883–891. [DOI] [PubMed] [Google Scholar]
- 10.Liu C, Dingee CK, Warburton R, et al. Pure flat epithelial atypia identified on core needle biopsy does not require excision. Eur J Surg Oncol 2020;46(2):235–239. [DOI] [PubMed] [Google Scholar]
- 11.Carrillo M, Maturana G, Maiz C, et al. Breast lesions with atypia in percutaneous biopsies, managed with surgery in the last 10 years. Ecancermedicalscience 2019;13:923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hugar SB, Bhargava R, Dabbs DJ, Davis KM, Zuley M, Clark BZ. Isolated Flat Epithelial Atypia on Core Biopsy Specimens Is Associated With a Low Risk of Upgrade at Excision. Am J Clin Pathol 2019;151(5):511–515. [DOI] [PubMed] [Google Scholar]
- 13.Srour MK, Donovan C, Chung A, et al. Flat epithelial atypia on core needle biopsy does not always mandate excisional biopsy. Breast J 2020;26(4):679–684. [DOI] [PubMed] [Google Scholar]
- 14.Winer LK, Hinrichs BH, Lu S, et al. Flat epithelial atypia and the risk of sampling error: Determining the value of excision after image-guided core-needle biopsy. Am J Surg 2019;218(4):730–736. [DOI] [PubMed] [Google Scholar]
- 15.Chan PMY, Chotai N, Lai ES, et al. Majority of flat epithelial atypia diagnosed on biopsy do not require surgical excision. Breast 2018;37:13–17. [DOI] [PubMed] [Google Scholar]
- 16.McCroskey Z, Sneige N, Herman CR, et al. Flat epithelial atypia in directional vacuum-assisted biopsy of breast microcalcifications: surgical excision may not be necessary. Mod Pathol 2018;31(7):1097–1106. [DOI] [PubMed] [Google Scholar]
- 17.Ouldamer L, Poisson E, Arbion F, et al. All pure flat atypical atypia lesions of the breast diagnosed using percutaneous vacuum-assisted breast biopsy do not need surgical excision. Breast 2018;40:4–9. [DOI] [PubMed] [Google Scholar]
- 18.Lamb LR, Bahl M, Gadd MA, Lehman CD. Flat Epithelial Atypia: Upgrade Rates and Risk-Stratification Approach to Support Informed Decision Making. J Am Coll Surg 2017;225(6):696–701. [DOI] [PubMed] [Google Scholar]
- 19.Ahn HS, Jang M, Kim SM, et al. Diagnosis of Columnar Cell Lesions and Atypical Ductal Hyperplasia by Ultrasound-Guided Core Biopsy: Findings Associated with Underestimation of Breast Carcinoma. Ultrasound Med Biol 2016;42(7):1457–1463. [DOI] [PubMed] [Google Scholar]
- 20.Berry JS, Trappey AF, Vreeland TJ, et al. Analysis of Clinical and Pathologic Factors of Pure, Flat Epithelial Atypia on Core Needle Biopsy to Aid in the Decision of Excision or Observation. J Cancer 2016;7(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mooney KL, Bassett LW, Apple SK. Upgrade rates of high-risk breast lesions diagnosed on core needle biopsy: a single-institution experience and literature review. Mod Pathol 2016;29(12):1471–1484. [DOI] [PubMed] [Google Scholar]
- 22.Calhoun BC, Sobel A, White RL, et al. Management of flat epithelial atypia on breast core biopsy may be individualized based on correlation with imaging studies. Mod Pathol 2015;28(5):670–676. [DOI] [PubMed] [Google Scholar]
- 23.Dialani V, Venkataraman S, Frieling G, Schnitt SJ, Mehta TS. Does isolated flat epithelial atypia on vacuum-assisted breast core biopsy require surgical excision? Breast J 2014;20(6):606–614. [DOI] [PubMed] [Google Scholar]
- 24.Prowler VL, Joh JE, Acs G, et al. Surgical excision of pure flat epithelial atypia identified on core needle breast biopsy. Breast 2014;23(4):352–356. [DOI] [PubMed] [Google Scholar]
- 25.Ceugnart L, Doualliez V, Chauvet MP, et al. Pure flat epithelial atypia: is there a place for routine surgery? Diagn Interv Imaging 2013;94(9):861–869. [DOI] [PubMed] [Google Scholar]
- 26.Villa A, Chiesa F, Massa T, et al. Flat epithelial atypia: comparison between 9-gauge and 11-gauge devices. Clin Breast Cancer 2013;13(6):450–454. [DOI] [PubMed] [Google Scholar]
- 27.Bianchi S, Bendinelli B, Castellano I, et al. Morphological parameters of flat epithelial atypia (FEA) in stereotactic vacuum-assisted needle core biopsies do not predict the presence of malignancy on subsequent surgical excision. Virchows Arch 2012;461(4):405–417. [DOI] [PubMed] [Google Scholar]
- 28.Biggar MA, Kerr KM, Erzetich LM, Bennett IC. Columnar cell change with atypia (flat epithelial atypia) on breast core biopsy-outcomes following open excision. Breast J 2012;18(6):578–581. [DOI] [PubMed] [Google Scholar]
- 29.Peres A, Barranger E, Becette V, Boudinet A, Guinebretiere JM, Cherel P. Rates of upgrade to malignancy for 271 cases of flat epithelial atypia (FEA) diagnosed by breast core biopsy. Breast Cancer Res Treat 2012;133(2):659–666. [DOI] [PubMed] [Google Scholar]
- 30.Polom K, Murawa D, Murawa P. Flat epithelial atypia diagnosed on core needle biopsy-Clinical challenge. Rep Pract Oncol Radiother 2012;17(2):93–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uzoaru I, Morgan BR, Liu ZG, et al. Flat epithelial atypia with and without atypical ductal hyperplasia: to re-excise or not. Results of a 5-year prospective study. Virchows Arch 2012;461(4):419–423. [DOI] [PubMed] [Google Scholar]
- 32.de Mascarel I, Brouste V, Asad-Syed M, Hurtevent G, Macgrogan G. All atypia diagnosed at stereotactic vacuum-assisted breast biopsy do not need surgical excision. Mod Pathol 2011;24(9):1198–1206. [DOI] [PubMed] [Google Scholar]
- 33.Lavoué V, Roger CM, Poilblanc M, et al. Pure flat epithelial atypia (DIN 1a) on core needle biopsy: study of 60 biopsies with follow-up surgical excision. Breast Cancer Res Treat 2011;125(1):121–126. [DOI] [PubMed] [Google Scholar]
- 34.Verschuur-Maes AH, Witkamp AJ, de Bruin PC, van der Wall E, van Diest PJ. Progression risk of columnar cell lesions of the breast diagnosed in core needle biopsies. Int J Cancer 2011;129(11):2674–2680. [DOI] [PubMed] [Google Scholar]
- 35.Ingegnoli A, d’Aloia C, Frattaruolo A, et al. Flat epithelial atypia and atypical ductal hyperplasia: carcinoma underestimation rate. Breast J 2010;16(1):55–59. [DOI] [PubMed] [Google Scholar]
- 36.Lee TY, Macintosh RF, Rayson D, Barnes PJ. Flat epithelial atypia on breast needle core biopsy: a retrospective study with clinical-pathological correlation. Breast J 2010;16(4):377–383. [DOI] [PubMed] [Google Scholar]
- 37.Noël JC, Buxant F, Engohan-Aloghe C. Immediate surgical resection of residual microcalcifications after a diagnosis of pure flat epithelial atypia on core biopsy: a word of caution. Surg Oncol 2010;19(4):243–246. [DOI] [PubMed] [Google Scholar]
- 38.Noske A, Pahl S, Fallenberg E, et al. Flat epithelial atypia is a common subtype of B3 breast lesions and is associated with noninvasive cancer but not with invasive cancer in final excision histology. Hum Pathol 2010;41(4):522–527. [DOI] [PubMed] [Google Scholar]
- 39.Chivukula M, Bhargava R, Tseng G, Dabbs DJ. Clinicopathologic implications of “flat epithelial atypia” in core needle biopsy specimens of the breast. Am J Clin Pathol 2009;131(6):802–808. [DOI] [PubMed] [Google Scholar]
- 40.Darvishian F, Singh B, Simsir A, Ye W, Cangiarella JF. Atypia on breast core needle biopsies: reproducibility and significance. Ann Clin Lab Sci 2009;39(3):270–276. [PubMed] [Google Scholar]
- 41.Piubello Q, Parisi A, Eccher A, Barbazeni G, Franchini Z, Iannucci A. Flat epithelial atypia on core needle biopsy: which is the right management? Am J Surg Pathol 2009;33(7):1078–1084. [DOI] [PubMed] [Google Scholar]
- 42.Senetta R, Campanino PP, Mariscotti G, et al. Columnar cell lesions associated with breast calcifications on vacuum-assisted core biopsies: clinical, radiographic, and histological correlations. Mod Pathol 2009;22(6):762–769. [DOI] [PubMed] [Google Scholar]
- 43.Kunju LP, Kleer CG. Significance of flat epithelial atypia on mammotome core needle biopsy: Should it be excised? Hum Pathol 2007;38(1):35–41. [DOI] [PubMed] [Google Scholar]
- 44.Guerra-Wallace MM, Christensen WN, White RL Jr. A retrospective study of columnar alteration with prominent apical snouts and secretions and the association with cancer. Am J Surg 2004;188(4):395–398. [DOI] [PubMed] [Google Scholar]
- 45.Luiten JD, Korte B, Voogd AC, et al. Trends in frequency and outcome of high-risk breast lesions at core needle biopsy in women recalled at biennial screening mammography, a multiinstitutional study. Int J Cancer 2019;145(10):2720–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ. 4th ed. WHO Classification of Tumours of the Breast. Geneva, Switzerland: World Health Organization, 2012. [Google Scholar]
- 47.D’Orsi CJ, Sickles EA, Mendelson EB, Morris EA. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. Reston, Va: American College of Radiology, 2013. [Google Scholar]
- 48.Darling ML, Smith DN, Lester SC, et al. Atypical ductal hyperplasia and ductal carcinoma in situ as revealed by large-core needle breast biopsy: results of surgical excision. AJR Am J Roentgenol 2000;175(5):1341–1346. [DOI] [PubMed] [Google Scholar]
- 49.Samples LS, Rendi MH, Frederick PD, et al. Surgical implications and variability in the use of the flat epithelial atypia diagnosis on breast biopsy specimens. Breast 2017;34:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Malley FP, Mohsin SK, Badve S, et al. Interobserver reproducibility in the diagnosis of flat epithelial atypia of the breast. Mod Pathol 2006;19(2):172–179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



