Abstract
Previous research indicates that tailoring lifestyle interventions to participant characteristics optimizes intervention effectiveness. Our objective was to assess whether the effects of a preconception lifestyle intervention in obese infertile women depended on women's exposure to adversity in childhood. A follow‐up of a preconception lifestyle intervention randomized controlled trial (the LIFEstyle study) was conducted in the Netherlands among 577 infertile women (age 18–39 years) with a body mass index (BMI) ≥29 kg/m2 at time of randomization; N = 110 (19%) consented to the follow‐up assessment, 6 years later. A 6‐month preconception lifestyle intervention aimed weight loss through improving diet and increasing physical activity. The control group received care as usual. Outcome measures included weight, BMI, waist and hip circumference, body fat percentage, blood pressure and metabolic syndrome. The potential moderator, childhood adversity, was assessed with the Life Events Checklist‐5 questionnaire. Among the 110 women in our follow‐up study, n = 65 (59%) reported no childhood adverse events, n = 28 (25.5%) reported 1 type of childhood adverse events and n = 17 (15.5%) reported ≥2 types of childhood adverse events. Regression models showed significant interactions between childhood adversity and effects of lifestyle intervention at the 6‐year follow‐up. Among women who experienced childhood adversity, the intervention significantly reduced weight (−10.0 [95% CI −18.5 to −1.5] kg, p = 0.02), BMI (−3.2 [−6.1 to −0.2] kg/m2, p = 0.04) and body fat percentage (−4.5 [95% CI −7.2 to −1.9] p < 0.01). Among women without childhood adversity, the intervention did not affect these outcomes (2.7 [−3.9 to 9.4] kg, p = 0.42), (0.9 [−1.4 to 3.3] kg/m2, p = 0.42) and (1.7 [95% CI −0.3 to 3.7] p = 0.10), respectively. Having a history of childhood adversity modified the effect of a preconception lifestyle intervention on women's body composition. If replicated, it may be important to consider childhood adversity as a determinant of lifestyle intervention effectiveness.
Keywords: childhood adversity, effectiveness, lifestyle intervention, obesity
1. INTRODUCTION
Obesity is an increasing problem worldwide, with serious negative health effects (Ng et al., 2014). The first step in the treatment of obesity is lifestyle modification through behavioural change. Lifestyle interventions aim at improving diet and/or increasing physical activity and are less invasive compared to bariatric surgery. Lifestyle interventions aiming at the combination of improving diet and increasing physical activity have been most effective in terms of weight reduction (Ketola, Sipilä, & Mäkelä, 2000), although studies have demonstrated that lifestyle interventions show moderate effects on weight loss in short‐ and long term, and that motivation of participants may play a significant role in lifestyle intervention effectiveness (Lloyd‐Jones et al., 2010; Wu, Gao, Chen, & van Dam, 2009). Lifestyle interventions of >12 months duration showed more consistent weight loss results compared to interventions of <6 months duration (Naslund et al., 2017). We have shown that a lifestyle intervention, offered during the preconception period when women are particularly receptive to lifestyle advice (McBride, Emmons, & Lipkus, 2003), is effective in improving lifestyle (van Elten et al., 2018), reducing body weight and improving cardiometabolic health (van Dammen et al., 2018).
Obesity is a complex condition, with numerous risk factors implicated in its development (Cecchini et al., 2010; Weinsier, Hunter, Heini, Goran, & Sell, 1998), including experiencing childhood adversity (Williamson, Thompson, Anda, Dietz, & Felitti, 2002). Stressful and traumatic experiences during childhood, including physical and sexual abuse, loss of a parent or parental neglect, are associated with higher rates of obesity as well as cardiovascular disease, cancer and even mortality in adulthood (Bellis et al., 2015; Felitti et al., 1998; Shonkoff & Garner, 2012; Suglia et al., 2017). Childhood adversity is linked to obesity in adulthood through a variety of mediating mechanisms. Childhood adversity is associated with the development of post‐traumatic stress disorder (PTSD) symptoms and depressive symptoms, which in turn, are linked to higher rates of overweight and obesity (Farr et al., 2015; Michopoulos et al., 2015). PTSD and depressive symptoms may be linked to overweight and obesity through direct physiological pathways and indirect psychological pathways. The direct pathways include higher levels of glucocorticoids, which are known to promote weight gain, and immunological dysregulation; proinflammatory cytokines have a negative impact on both mood and eating behaviour (Aaseth, Roer, Lien, & Bjørklund, 2019; Markowitz, Friedman, & Arent, 2008). Indirect psychological pathways are lower adherence or motivation to maintain a healthy lifestyle in those who experience PTSD or depressive symptoms (Aaseth et al., 2019; Markowitz et al., 2008). Another mechanism involves hypothalamic–pituitary–adrenal axis functioning, which may be altered in those who have experienced childhood adversity and is also associated with the development of overweight and obesity (Miller, Arbel, Shapiro, Han, & Margolin, 2018). Altered eating behaviour such as stress‐induced eating and night eating syndrome could also explain how childhood adversity and obesity are linked (Palmisano, Innamorati, & Vanderlinden, 2016). Given the increased risk of obesity in adulthood in individuals who experienced childhood adversity, and the heterogeneity in who sees positive results after lifestyle interventions (Gaalema, Elliott, Morford, Higgins, & Ades, 2017; Karsten et al., 2018), it is possible that the psychological and physiological mechanisms identified in the link between early life adversity and obesity may reduce the effectiveness of lifestyle interventions among individuals who experienced childhood adversity. In this study, we assessed whether the effectiveness of a preconception lifestyle intervention differed for participants who experienced childhood adversity and participants who did not experience childhood adversity. We hypothesized that the intervention may be less effective in improving body composition among women who experienced childhood adversity than in women who did not experience childhood adversity.
2. METHODS
2.1. LIFEstyle study
The LIFEstyle study, a randomized controlled trial, was carried out in 23 hospitals throughout the Netherlands from 2009 to 2012. The trial was registered in the Netherlands Trial Registry (NTR 1530) and a detailed description of the lifestyle intervention and the primary and secondary outcomes can be found in the original study protocol (Mutsaerts et al., 2010). Participants were infertile women between 18 and 39 years of age with a body mass index (BMI) ≥29 kg/m2. Women with premature ovarian insufficiency, endocrinopathy, severe endometriosis, untreated pre‐existing hypertension, or women with a history of hypertension‐related pregnancy complications were not eligible for participation. In total, 577 women provided written informed consent, of whom 290 were assigned to a 6‐month lifestyle intervention aimed at improving diet and physical activity before they could start with infertility treatment. The control group did not receive a lifestyle intervention and could start with infertility treatment as indicated. Participants were approximately 30 years old at time of randomization and had a mean BMI of 36 kg/m2 (range 29–51). Participants in the lifestyle intervention group were coached by trained nurses through six individual face‐to‐face sessions and four telephone calls. The intervention aimed at 5%–10% weight loss or a BMI ≤29 kg/m2. An individualized behavioural modification plan was made. Intervention coaches counselled patients about lifestyle leading to overweight and infertility, and formulated a ‘patient contract’, together with the patient, describing her individual goals. The coaches also provided information regarding relapse after weight loss and how to prevent relapse. In addition to the individualized behavioural modifications, the intervention focused on a healthy diet using an online diary. The goal was a daily caloric intake reduction of 600 kcal with a minimum intake of 1200 kcal per day. The coaches also counselled patients about increasing physical activity to a minimum of two or three times per week at moderate intensity level. A pedometer was provided, and patients were encouraged to take 10,000 steps per day. If the participant reached 5%–10% weight loss or BMI ≤29 kg/m2 or completed the 6‐month intervention period, she could start with infertility treatment as indicated.
Six months post randomization, body weight (kg), height (cm), waist and hip circumference (cm) and blood pressure (mmHg, in seated position) were measured by research nurses in non‐pregnant participants who were clothed and not sober. On a different day, fasting blood samples were collected and levels of glucose, triglycerides and high‐density lipoprotein cholesterol (HDL‐C) were obtained from biochemical analyses performed in the laboratory from the University Medical Centre Groningen. Polycystic ovary syndrome was diagnosed at baseline according to Rotterdam 2003 criteria (Rotterdam ESHRE/ASRM‐Sponsored PCOS Consensus Workshop Group, 2004).
The primary outcome of the LIFEstyle study was the vaginal birth of a healthy singleton after ≥37 weeks of gestation, of which the results were published in 2016, showing similar rates in the intervention and control group (Mutsaerts et al., 2016). The effects of the lifestyle intervention on weight loss and cardiometabolic health were published in 2018 (van Dammen et al., 2018). In the short term (6 months after randomization), the lifestyle intervention led to a significant lower body weight, decreased waist and hip circumference, blood pressure, a lower prevalence of metabolic syndrome and improved physical quality of life (van Dammen et al., 2018).
2.2. Follow‐up visit
A follow‐up visit of the LIFEstyle study was conducted after a median of 6 years (IQR 5.2–6.7) after randomization (van de Beek et al., 2018). In total 564 women, who were originally recruited at 23 different hospitals, were eligible for participation in this follow‐up study, and of 550 women the latest contact information could be determined. These women were asked if they were willing to fill out questionnaires and participate in physical measurements.
2.3. Questionnaire
The 17‐item Life Events Checklist for DSM‐5 was used to evaluate adverse events during childhood and adolescence. This questionnaire is characterized by adequate psychometric properties, based on previous research (Gray, Litz, Hsu, & Lombardo, 2004). In order to establish childhood adversity (between birth and 18 years of age), the questionnaire was modified slightly. For events that a person experienced or witnessed, the year in which the event took place was asked. This year was later used to calculate age at exposure. A total score was calculated with all items summed (if a woman reported any type of event occurring once or more before the age of 19 years, she received a score of one for experiencing that type of event during childhood). The scores were categorized in two different ways. For the first score, the dichotomous childhood adversity score, women who did not experience any childhood adversity were coded as zero, and women who experienced one or more types of childhood adverse events received a score of one. To be able to also investigate a potential graded effect of the number of adverse event types, we additionally computed a second score, the childhood adversity groups score. For this score, we divided women into three categories as follows: a group that did not experience any type of event; a group that experienced one type of event; and a group that experienced two or more types of events.
2.4. Physical measurements
All women were examined in a mobile research vehicle, parked near the participant's house, as part of the follow‐up. Weight, height, waist and hip circumference were measured twice, and a third time if there was a difference of either >0.5 kg or >0.5 cm for weight and height, respectively, or >1 cm for waist and hip circumference. Seated blood pressure was measured three times, after a 5‐min resting period. Mean values were calculated based on all available measurements. Body fat percentage was measured with arm‐to‐leg bioelectrical impedance analysis using the Bodystat 1500 (Bodystat Ltd.). Fasting blood samples were collected by trained nurses and blood plasma was stored at −80°C. Levels of glucose, triglycerides and HDL‐C were obtained through biochemical analyses at the AMC Clinical Chemistry Laboratory.
The presence of metabolic syndrome 6 months and 6 years post randomization was assessed with cut‐off values for obesity, and cut‐off values or medication use for hyperglycaemia, dyslipidaemia and hypertension, based on the US National Cholesterol Education Programme Adult Treatment Panel III (NCEP ATP III) criteria (Grundy et al., 2005).
2.5. Statistical analyses
Participant characteristics were analysed with ANOVA tests, t‐tests, chi‐square tests and Kruskal–Wallis tests, as appropriate. Baseline differences of participants and non‐participants in the follow‐up analyses were assessed with t‐tests, Fisher‐Freeman‐Halton tests and chi‐square tests. The change in weight, BMI, waist and hip circumference and blood pressure was calculated, and an interaction term was calculated as the product of randomization group and the dichotomous childhood adversity score. The differences between the intervention and control group in changes in body composition and blood pressure were assessed with univariate regression models, with adjustment for baseline levels. Univariate regression models were used to assess the effect of the interaction between randomization group (intervention and control group) and dichotomous childhood adversity score (no adversity vs. any adversity), covarying for the baseline measurement of that outcome. The regression analyses were also carried out separately for the intervention and control group and dichotomous childhood adversity groups, for each outcome, to assess the main effects. These univariate regression models were repeated with childhood adversity categorized in three groups (no adversity, 1 type of event and ≥2 types of events).
3. RESULTS
The baseline differences between participants and those who did not participate in the follow‐up analyses are described in Table 1. Results showed that both control and intervention participants were more often Caucasian and more often recruited at a general hospital compared to non‐participants. No other differences were observed. A total of 121 women provided written informed consent; of these, six women declined to participate and five women had no physical examination, thus complete data were available for 110 women (Figure S1). In total, 65 women (n = 34 intervention group and n = 31 control group) reported no childhood adverse events, 28 (n = 10 intervention group, n = 18 control group) reported 1 type of adverse event and 17 (n = 6 intervention group, n = 11 control group) reported two or more types of adverse events during childhood. The most common adverse event was a traffic accident (n = 18) including car, boat, train and plane accidents. Physical assault (n = 11) was the second most common reported event, followed by sexual assault (n = 8), unwanted sexual experiences (n = 8), life threatening illness/injury (n = 7), severe illness/injury (n = 6) and sudden unexpected death of someone close (n = 6). Table 2 shows the participant characteristics, first divided into childhood adversity exposure groups and then divided by randomization group. Women with a history of childhood adversity were significantly younger compared to women without childhood adversity. No differences were observed in ethnicity and education level, smoking status, baseline polycystic ovary syndrome diagnosis, childlessness and BMI between childhood adversity groups or randomization groups.
TABLE 1.
Baseline characteristics participants and non‐participants in the analyses
| Participants (n = 110) | Non‐participants in the analyses (n = 464) | p‐value | |
|---|---|---|---|
| Age (mean [SD]) | 30.3 (4.1) | 29.6 (4.7) | 0.10 |
| Race (Caucasian, n [%]) | 104 (94.5) | 398 (85.8) | 0.01 |
| Current smoker (n [%]) | 25 (22.9) | 111 (24.1) | 0.79 |
| Education level (n [%]) | 0.30 | ||
| Primary school | 1 (1.0) | 26 (5.9) | |
| Secondary education | 25 (23.8) | 106 (23.9) | |
| Intermediate vocational education | 52 (49.5) | 214 (48.2) | |
| Advanced vocational education or university | 27 (25.7) | 98 (22.1) | |
| Type of hospital | 0.03 | ||
| University medical centre (n [%]) | 22 (20) | 141 (30.4) | |
| General hospital (n [%]) | 88 (80) | 323 (69.6) | |
| Polycystic ovary syndrome (n [%]) | 43 (39.1) | 158 (34.2) | 0.33 |
| Weight in kg (mean [SD]) | 103.3 (12.1) | 103.2 (13.3) | 0.94 |
| BMI kg/m2 (mean [SD]) | 35.7 (3.0) | 36.1 (3.5) | 0.23 |
| Waist circumference in cm (mean [SD]) | 106.7 (10.1) | 108.3 (9.2) | 0.10 |
| Hip circumference in cm (mean [SD]) | 124.0 (8.3) | 125.3 (8.9) | 0.16 |
| Systolic blood pressure in mmHg (mean [SD]) | 125.4 (12.4) | 126.6 (14.3) | 0.42 |
| Diastolic blood pressure in mmHg (mean [SD]) | 80.6 (7.6) | 79.6 (9.4) | 0.29 |
Abbreviations: BMI, body mass index; SD, standard deviation.
TABLE 2.
Characteristics participants (N = 110) at follow‐up
| No childhood adversity (n = 65) | Childhood adversity (n = 45) | p‐value | Intervention group (n = 50) | Control group (n = 60) | p‐value | |
|---|---|---|---|---|---|---|
| Age (mean [SD]) | 36.7 (4.3) | 34.7 (4.1) | 0.02 | 35.8 (4.5) | 35.9 (4.2) | 0.89 |
| Race (Caucasian, n [%]) a | 59 (91) | 45 (100) | 0.08 | 48 (96) | 56 (93) | 0.54 |
| Current smoker (n [%]) | 7 (11.9) | 9 (20.9) | 0.42 | 7 (12.7) | 9 (19.1) | 0.66 |
| Education level (n [%]) | 0.35 | 0.47 | ||||
| Primary school | 0 (0) | 2 (4.7) | 0 (0) | 2 (3.6) | ||
| Secondary education | 11 (18.6) | 10 (23.3) | 12 (25.5) | 9 (16.4) | ||
| Intermediate vocational education | 30 (50.8) | 20 (46.5) | 23 (48.9) | 27 (49.1) | ||
| Advanced vocational education or university | 18 (30.5) | 11 (25.6) | 12 (25.5) | 17 (30.9) | ||
| Polycystic ovary syndrome (n [%]) a | 26 (40.0) | 17 (37.8) | 0.81 | 20 (40.0) | 23 (38.3) | 0.86 |
| No child (n [%]) | 12 (21.1) | 8 (18.6) | 0.76 | 12 (26.7) | 8 (14.5) | 0.13 |
| Body mass index in kg/m2 (mean [SD]) a | 35.6 (3.1) | 35.9 (2.9) | 0.61 | 35.5 (2.9) | 35.9 (3.2) | 0.57 |
Abbreviation: SD, standard deviation.
Measured at baseline.
3.1. Intervention effects 6 months after randomization and childhood adversity
Among women participating in the follow‐up, weight change between baseline and 6 months after randomization was not different for the intervention and control group (−1.78 [95% CI −4.83 to 1.26] p = 0.24). Similarly, no differences were observed between the groups for BMI, waist and hip circumference, systolic and diastolic blood pressure change (−0.53 [95% CI −1.61 to 0.56] p = 0.33), (−1.13 [95% CI −5.85 to 3.58] p = 0.63), (0.86 [95% CI −5.12 to 6.84] p = 0.77), (−3.71 [95% CI −9.59 to 2.17] p = 0.21) and (−0.50 [95% CI −5.75 to 4.75] p = 0.85), respectively. The prevalence of metabolic syndrome was similar for the intervention and control group 6 months post randomization (OR = 1.16 [95% CI 0.19–8.36] p = 0.81). The univariate regression models showed that the intervention effect on body composition outcomes 6 months after randomization did not differ for women with and without childhood adversity, although the groups were small (Table 3). Women exposed to childhood adversity were not significantly different in BMI, waist and hip circumference, systolic blood pressure or odds for metabolic syndrome than women not exposed to childhood adversity.
TABLE 3.
Differences between childhood adversity exposure groups in body‐composition and blood pressure change from baseline to 6 months post randomization
| Intervention group | Control group | |||||
|---|---|---|---|---|---|---|
| No childhood adversity (n = 16) | Childhood adversity (n = 5) | p‐value | No childhood adversity (n = 18) | Childhood adversity (n = 13) | p‐value | |
| Δ weight baseline‐6 months | −4.1 (7.7) | −4.0 (5.9) | 0.96 | −1.3 (3.5) | −3.3 (3.4) | 0.16 |
| Δ BMI baseline‐6 months | −1.0 (2.7) | −1.8 (2.3) | 0.66 | −0.4 (1.2) | −1.1 (1.1) | 0.09 |
| Δ waist circumference baseline‐6 months | −2.1 (8.4) | −4.0 (10.8) | 0.71 | −0.9 (9.4) | −1.0 (4.3) | 0.96 |
| Δ hip circumference baseline‐6 months | −17.6 (12.1) | −20.5 (11.4) | 0.75 | −17.9 (7.6) | −20.8 (11.5) | 0.61 |
| Δ Systolic Blood Pressure baseline‐6 months | −2.5 (11.3) | −7.8 (14.3) | 0.49 | −4.0 (13.2) | −2.1 (11.0) | 0.25 |
| Δ Diastolic Blood Pressure baseline‐6 months | −2.7 (6.9) | 0.2 (8.7) | 0.48 | −1.9 (9.5) | −1.0 (8.7) | 0.83 |
| Odds ratio metabolic syndrome a | 0.51 (95% CI 0.02 to 15.03) | 0.70 | 3.40 (95% CI 0.31 to 36.91) | 0.32 | ||
Abbreviation: BMI, body mass index.
Odds ratio 6 months post randomization adjusted for baseline prevalence.
3.2. Intervention effects 6 years after randomization
Weight change between baseline and 6 years after randomization across the entire sample that participated in the follow‐up was not different between the intervention and control group (−1.79 [95% CI −3.36 to 6.95] p = 0.49). There was also no difference between groups in change from baseline to 6 years later regarding BMI (−0.60 [95% CI −1.22 to 2.42] p = 0.51), waist and hip circumference (−0.78 [95% CI −3.43 to 4.98] p = 0.72) and (−0.51 [95% CI −3.00 to 4.03] p = 0.77), respectively, systolic and diastolic blood pressure (−0.20 [95% CI −5.46 to 5.05] p = 0.94) and (−0.54 [95% CI −4.03 to 2.97] p = 0.76), respectively, body fat percentage (−0.73 [95% CI −2.38 to 0.92] p = 0.38) or prevalence of metabolic syndrome (OR = 0.41 [95% CI 0.13–1.27] p = 0.12).
3.3. Intervention effects 6 years after randomization by exposure to childhood adversity
The intervention effect 6 years after randomization on body composition outcomes differed significantly for women with and without childhood adversity and have been described in Table 4. In Figure 1, the results of the models testing the interactions utilizing the three category childhood adversity groups (no adversity, 1 type of event and ≥2 type of events) show similar results as were found analysing childhood adversity as a dichotomous exposure variable.
TABLE 4.
Intervention effects 6 years after randomization by exposure to childhood adversity
| No childhood adversity (n = 65) | Childhood adversity (n = 45) | Interaction effect | |
|---|---|---|---|
| Weight (kg) | 2.7 (−3.9 to 9.4) p = 0.42 | −10.0 (−18.5 to −1.5) p = 0.02 | −12.7 (−23.3 to −2.1) p = 0.02 |
| BMI (kg/m2) | 0.9 (−1.4 to 3.3) p = 0.42 | −3.2 (−6.1 to −0.2) p = 0.04 | −4.3 (−8.1 to −0.6) p = 0.02 |
| Waist circumference (cm) | 1.7 (−3.7 to 7.1) p = 0.53 | −7.9 (−15.1 to −0.7) p = 0.03 | −7.7 (−16.5 to 1.1) p = 0.09 |
| Hip circumference (cm) | 2.7 (−1.8 to 7.2) p = 0.23 | −6.8 (−12.6 to −1.0) p = 0.02 | −9.3 (−16.5 to −2.1) p = 0.01 |
| Body fat percentage | 1.7 (−0.3 to 3.7) p = 0.10 | −4.5 (−7.2 to −1.9) p < 0.01 | −6.2 (−9.4 to −2.9) p < 0.01 |
FIGURE 1.
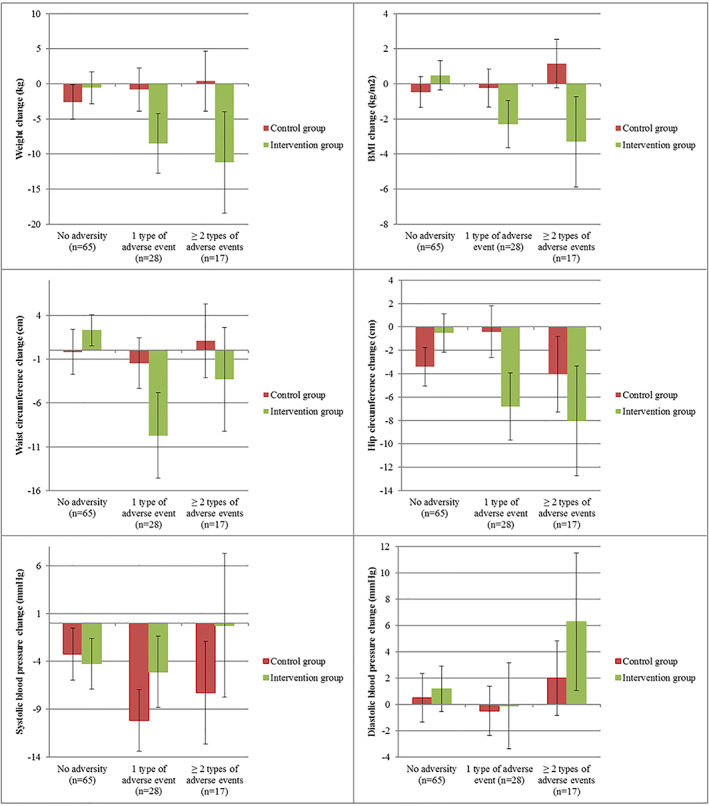
Regression results of the association between weight, body mass index (BMI), waist and hip circumference and systolic and diastolic blood pressure change from baseline to 6 years post randomization (estimated marginal means) and childhood adversity for the control and intervention group, adjusted for baseline weight or BMI, waist or hip circumference or systolic and diastolic blood pressure
There were no significant interactions between childhood adversity and randomization group on systolic and diastolic blood pressure, or metabolic syndrome, 6 years post randomization (all p values ≥0.05). All analyses were also run adjusted for age, as women with childhood adversity exposure were younger compared to women without childhood adversity exposure, which did not alter the results (data not shown).
4. DISCUSSION
In this study, we found that a preconception lifestyle intervention in obese infertile women was only effective in improving body composition 6 years after randomization in women who experienced childhood adversity, compared to women without childhood adversity. This suggests childhood adversity may be an important determinant of long‐term lifestyle intervention effectiveness.
Since we hypothesized that the intervention would be less effective in improving body composition among women who experienced childhood adversity due to psychological and physiological mechanisms, our findings were surprising. To the best of our knowledge, our study is the first to examine childhood adversity as a determinant of lifestyle intervention effectiveness, and we cannot compare our results to other findings. However, our findings are aligned with ‘differential susceptibility’ frameworks (Belsky & van Ijzendoorn, 2015; Boyce, 2016; Obradovic, Bush, Stamperdahl, Adler, & Boyce, 2010) that suggest that childhood adversity may render individuals more susceptible or sensitive to experiences in their environment, including sensitivity to intervention (Belsky & van Ijzendoorn, 2015; Boyce, 2016; Obradovic et al., 2010). To date, evidence supporting these intervention‐sensitivity theories is based on allelic differences in ‘sensitivity genes’ historically understood to increase individuals' risk for negative outcomes but that have been more recently demonstrated to be associated with those same individuals being most susceptible to positive effects of interventions (Belsky & van Ijzendoorn, 2015). For example, interventions to improve early literacy skills or reduce depressive symptoms, including parent training, psychotherapy and computer‐assisted learning, have been found to be more effective for children, adolescents and adults with variants of ‘sensitivity genes’ formerly thought to promote risk for problem behaviour (Cicchetti, Toth, & Handley, 2015; Plak, Kegel, & Bus, 2015; van Ijzendoorn & Bakermans‐Kranenburg, 2015). Based on differential susceptibility theories, it is plausible that early life adversity‐induced sensitivity to the environment would play a similarly moderating role. In our study, we speculate that women who have been exposed to childhood adversity may be particularly sensitive to the positive social components of the lifestyle intervention, including components of motivational counselling; these women may have experienced the intervention as providing the care and personal attention that was lacking to some extent during their childhood, making it particularly meaningful. Individuals who have experienced childhood adversity tend to appraise situations as out of their control and report low self‐efficacy (Southwick & Charney, 2012). The lifestyle intervention may have increased feelings of control and self‐efficacy, resulting in better health outcomes. Future research could provide insight in the role of underlying mechanisms including increased feelings of control and self‐efficacy.
Children who have experienced childhood adversity more often develop mental and physical health problems (Felitti et al., 1998), as well as obesity (Greenfield & Marks, 2009). In order to reduce the risk of cardiovascular disease associated obesity (Burke et al., 2008; Felitti et al., 1998), lifestyle interventions to reduce weight are important, and may be especially effective in individuals who experienced childhood adversity. Although our preconception lifestyle intervention improved body composition among women who were exposed to childhood adversity, no effects were observed for blood pressure or metabolic syndrome 6 years after randomization. The small sample size in the analyses with blood pressure and metabolic syndrome as outcomes led to low statistical power, and the positive effects of improved body composition on cardiovascular risk may take more time to develop. Further follow‐up could shed light on the potential long‐term effects of improved body composition after lifestyle intervention on cardiovascular risk in women exposed to childhood adversity.
Previous research suggests there might be a U‐shaped relationship between adversity exposure and health, such that moderate exposure is associated with better outcomes than either no exposure or severe exposure (Dienstbier, 1992; Seery, 2011; Seery, Holman, & Silver, 2010). In our sample, it was not possible to analyse the effects of severe exposure on intervention effectiveness, because of the limited number of women with severe exposure in our sample. The most common adverse event in our sample was a traffic accident, whereas studies suggesting the U‐shaped relationship describe bereavement and losing someone close as the most frequently reported events (Seery et al., 2010). The exposure to childhood adversity in our sample may be described as moderate compared to other studies.
Our study has several limitations. Our hypothesis was explorative in nature and our findings were contrary to our hypothesis. Another limitation to the findings reported here was attrition. Roughly 20% of the women who were randomized participated in the follow‐up, which reduced statistical power and resulted in a selective, non‐representative sample. Participants were more often Caucasian and more often recruited at a general hospital compared to non‐participants, reducing the generalizability of our results. Furthermore, childhood adversity was assessed at a mean age of 36 years, and recall bias may have led to an overestimation or underestimation of childhood adversity. However, reports of childhood adversity have shown to be relatively stable over time and reliable in adulthood in previous research, limiting the role of recall bias in the results (Yancura & Aldwin, 2009). No statistically significant differences were observed on body composition outcomes 6 months after the start of the intervention between women who were exposed to childhood adversity and those who were not exposed to childhood adversity. However, these analyses were based on very small numbers as there was only a small subgroup of women with cardiometabolic data available at both 6 months and 6 years post randomization. In general, the direction of the non‐significant results 6 months post randomization was in line with the statistically significant results observed 6 years post randomization.
Our results show that women assigned to a lifestyle intervention who reported being exposed to childhood adversity had improved body composition 6 years later, whereas intervention women without childhood adversity did not improve their body composition. These explorative findings suggest that childhood adversity may be an important determinant of lifestyle intervention effectiveness, especially if these findings will be replicated in other samples in the future.
CONFLICT OF INTEREST
The authors report no conflicts of interest.
ETHICS STATEMENT
All participants provided written informed consent which included a statement about publishing anonymous data. No individual data has been published. The study protocol was approved by the University Medical Centre Groningen ethics committee.
DATA AVAILABILITY STATEMENT
An anonymized data set has been made available as supporting information.
Supporting information
Figure S1: Flowchart participants in the LIFEstyle study and follow‐up
ACKNOWLEDGEMENTS
We thank all women who participated in the LIFEstyle study and the follow‐up visit. We thank all students, PhD students, research nurses and other research personnel involved in the LIFEstyle study and follow‐up visit. This research was supported by The Netherlands Organization for Health Research and Development (50‐50110‐96‐518), the Dutch Heart Foundation (grant number: 2013T085) and the European Commission (Horizon2020 project ‘DynaHealth’, 633595). The Department of Obstetrics and Gynaecology from the UMCG receives an unrestricted educational grant from Ferring Pharmaceutical BV the Netherlands, unrelated to the present study.
References
REFERENCES
- Aaseth, J. , Roer, G. E. , Lien, L. , & Bjørklund, G. (2019). Is there a relationship between PTSD and complicated obesity? A review of the literature. Biomedicine & Pharmacotherapy, 117, 108834. 10.1016/j.biopha.2019.108834 [DOI] [PubMed] [Google Scholar]
- Bellis, M. A. , Hughes, K. , Leckenby, N. , Hardcastle, K. A. , Perkins, C. , & Lowey, H. (2015). Measuring mortality and the burden of adult disease associated with adverse childhood experiences in England: A national survey. Journal of Public Health, 37(3), 445–454. 10.1093/pubmed/fdu065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky, J. , & van Ijzendoorn, M. H. (2015). What works for whom? Genetic moderation of intervention efficacy. Development and Psychopathology, 27(1), 1–6. 10.1017/s0954579414001254 [DOI] [PubMed] [Google Scholar]
- Bush, N. R. , & Boyce, T. W. (2016). Differential sensitivity to context: Implications for developmental psychopathology. Development and Psychopathology, 10.1002/9781119125556.devpsy203 [DOI] [Google Scholar]
- Burke, G. L. , Bertoni, A. G. , Shea, S. , Tracy, R. , Watson, K. E. , Blumenthal, R. S. , … Carnethon, M. R. (2008). The impact of obesity on cardiovascular disease risk factors and subclinical vascular disease: The multi‐ethnic study of atherosclerosis. Archives of Internal Medicine, 168(9), 928–935. 10.1001/archinte.168.9.928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchini, M. , Sassi, F. , Lauer, J. A. , Lee, Y. Y. , Guajardo‐Barron, V. , & Chisholm, D. (2010). Tackling of unhealthy diets, physical inactivity, and obesity: Health effects and cost‐effectiveness. The Lancet, 376(9754), 1775–1784. 10.1016/S0140-6736(10)61514-0 [DOI] [PubMed] [Google Scholar]
- Cicchetti, D. , Toth, S. L. , & Handley, E. D. (2015). Genetic moderation of interpersonal psychotherapy efficacy for low‐income mothers with major depressive disorder: Implications for differential susceptibility. Development and Psychopathology, 27(1), 19–35. 10.1017/S0954579414001278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienstbier, R. A. (1992). Mutual impacts of toughening on crises and losses. In Montada L., Philipp S. H., & Lerner M. J. (Eds.), Life crises and experiences of loss in adulthood (pp. 367–384). Hillsdale, NJ, US: Lawrence Erlbaum Associates, Inc. [Google Scholar]
- Farr, O. M. , Ko, B. J. , Joung, K. E. , Zaichenko, L. , Usher, N. , Tsoukas, M. , … Mantzoros, C. S. (2015). Posttraumatic stress disorder, alone or additively with early life adversity, is associated with obesity and cardiometabolic risk. Nutrition, Metabolism, and Cardiovascular Diseases, 25(5), 479–488. 10.1016/j.numecd.2015.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti, V. J. , Anda, R. F. , Nordenberg, D. , Williamson, D. F. , Spitz, A. M. , Edwards, V. , … Marks, J. S. (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The adverse childhood experiences (ACE) study. American Journal of Preventive Medicine, 14, 245–258. 10.1016/S0749-3797(98)00017-8 [DOI] [PubMed] [Google Scholar]
- Gaalema, D. E. , Elliott, R. J. , Morford, Z. H. , Higgins, S. T. , & Ades, P. A. (2017). Effect of socioeconomic status on propensity to change risk behaviors following myocardial infarction: Implications for healthy lifestyle medicine. Progress in Cardiovascular Diseases, 60(1), 159–168. 10.1016/j.pcad.2017.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray, M. J. , Litz, B. T. , Hsu, J. L. , & Lombardo, T. W. (2004). Psychometric properties of the life events checklist. Assessment, 11(4), 330–341. 10.1177/1073191104269954 [DOI] [PubMed] [Google Scholar]
- Greenfield, E. A. , & Marks, N. F. (2009). Violence from parents in childhood and obesity in adulthood: Using food in response to stress as a mediator of risk. Social Science & Medicine, 68(5), 791–798. 10.1016/j.socscimed.2008.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy, S. M. , Cleeman, J. I. , Daniels, S. R. , Donato, K. A. , Eckel, R. H. , Franklin, B. A. , … Costa, F. (2005). Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation, 112(17), 2735–2752. 10.1161/circulationaha.105.169404 [DOI] [PubMed] [Google Scholar]
- Karsten, M. D. A. , van Oers, A. M. , Groen, H. , Mutsaerts, M. A. Q. , van Poppel, M. N. M. , Geelen, A. , … de Bruin, J. P. (2018). Determinants of successful lifestyle change during a 6‐month preconception lifestyle intervention in women with obesity and infertility. European Journal of Nutrition, 58, 2463–2475. 10.1007/s00394-018-1798-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketola, E. , Sipilä, R. , & Mäkelä, M. (2000). Effectiveness of individual lifestyle interventions in reducing cardiovascular disease and risk factors. Annals of Medicine, 32(4), 239–251. 10.3109/07853890009011767 [DOI] [PubMed] [Google Scholar]
- Lloyd‐Jones, D. M. , Hong, Y. , Labarthe, D. , Mozaffarian, D. , Appel, L. J. , Van Horn, L. , … Rosamond, W. D. (2010). Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association's strategic impact goal through 2020 and beyond. Circulation, 121(4), 586–613. 10.1161/circulationaha.109.192703 [DOI] [PubMed] [Google Scholar]
- Markowitz, S. , Friedman, M. A. , & Arent, S. M. (2008). Understanding the relation between obesity and depression: Causal mechanisms and implications for treatment. Clinical Psychology: Science and Practice, 15(1), 1–20. 10.1111/j.1468-2850.2008.00106.x [DOI] [Google Scholar]
- McBride, C. M. , Emmons, K. M. , & Lipkus, I. M. (2003). Understanding the potential of teachable moments: The case of smoking cessation. Health Education Research, 18(2), 156–170. [DOI] [PubMed] [Google Scholar]
- Michopoulos, V. , Powers, A. , Moore, C. , Villarreal, S. , Ressler, K. J. , & Bradley, B. (2015). The mediating role of emotion dysregulation and depression on the relationship between childhood trauma exposure and emotional eating. Appetite, 91, 129–136. 10.1016/j.appet.2015.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, K. F. , Arbel, R. , Shapiro, L. S. , Han, S. C. , & Margolin, G. (2018). Does the cortisol awakening response link childhood adversity to adult BMI? Health Psychology, 37(6), 526–529. 10.1037/hea0000601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutsaerts, M. A. Q. , Groen, H. , ter Bogt, N. C. W. , Bolster, J. H. T. , Land, J. A. , Bemelmans, W. J. E. , … Hoek, A. (2010). The LIFESTYLE study: Costs and effects of a structured lifestyle program in overweight and obese subfertile women to reduce the need for fertility treatment and improve reproductive outcome. A randomised controlled trial. BMC Women's Health, 10(1), 22. 10.1186/1472-6874-10-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutsaerts, M. A. Q. , van Oers, A. M. , Groen, H. , Burggraaff, J. M. , Kuchenbecker, W. K. H. , Perquin, D. A. , … Hoek, A. (2016). Randomized trial of a lifestyle program in obese infertile women. New England Journal of Medicine, 374(20), 1942–1953. 10.1056/NEJMoa1505297 [DOI] [PubMed] [Google Scholar]
- Naslund, J. A. , Whiteman, K. L. , McHugo, G. J. , Aschbrenner, K. A. , Marsch, L. A. , & Bartels, S. J. (2017). Lifestyle interventions for weight loss among overweight and obese adults with serious mental illness: A systematic review and meta‐analysis. General Hospital Psychiatry, 47, 83–102. 10.1016/j.genhosppsych.2017.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng, M. , Fleming, T. , Robinson, M. , Thomson, B. , Graetz, N. , Margono, C. , … Gakidou, E. (2014). Global, regional, and national prevalence of overweight and obesity in children and adults during 1980‐2013: A systematic analysis for the global burden of disease study 2013. Lancet, 384(9945), 766–781. 10.1016/s0140-6736(14)60460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obradovic, J. , Bush, N. R. , Stamperdahl, J. , Adler, N. E. , & Boyce, W. T. (2010). Biological sensitivity to context: The interactive effects of stress reactivity and family adversity on socioemotional behavior and school readiness. Child Development, 81(1), 270–289. 10.1111/j.1467-8624.2009.01394.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmisano, G. L. , Innamorati, M. , & Vanderlinden, J. (2016). Life adverse experiences in relation with obesity and binge eating disorder: A systematic review. Journal of Behavioral Addictions, 5(1), 11–31. 10.1556/2006.5.2016.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plak, R. D. , Kegel, C. A. T. , & Bus, A. G. (2015). Genetic differential susceptibility in literacy‐delayed children: A randomized controlled trial on emergent literacy in kindergarten. Development and Psychopathology, 27(1), 69–79. 10.1017/S0954579414001308 [DOI] [PubMed] [Google Scholar]
- Rotterdam ESHRE/ASRM‐Sponsored PCOS Consensus Workshop Group . (2004). Revised 2003 consensus on diagnostic criteria and long‐term health risks related to polycystic ovary syndrome. Fertility and Sterility, 81(1), 19–25. 10.1016/j.fertnstert.2003.10.004 [DOI] [PubMed] [Google Scholar]
- Seery, M. D. (2011). Resilience: A silver lining to experiencing adverse life events? Current Directions in Psychological Science, 20(6), 390–394. 10.1177/0963721411424740 [DOI] [Google Scholar]
- Seery, M. D. , Holman, E. A. , & Silver, R. C. (2010). Whatever does not kill us: Cumulative lifetime adversity, vulnerability, and resilience. Journal of Personality and Social Psychology, 99(6), 1025. [DOI] [PubMed] [Google Scholar]
- Shonkoff, J. P. , & Garner, A. S. (2012). The lifelong effects of early childhood adversity and toxic stress. Pediatrics, 129, e232–e246. 10.1542/peds.2011-2663 [DOI] [PubMed] [Google Scholar]
- Southwick, S. M. , & Charney, D. S. (2012). The science of resilience: Implications for the prevention and treatment of depression. Science, 338(6103), 79. 10.1126/science.1222942 [DOI] [PubMed] [Google Scholar]
- Suglia, S. F. , Koenen, K. C. , Boynton‐Jarrett, R. , Chan, P. S. , Clark, C. J. , Danese, A. , … Zachariah, J. P. (2017). Childhood and adolescent adversity and cardiometabolic outcomes: A scientific statement from the American Heart Association. Circulation, 137(5), e15–e28. 10.1161/cir.0000000000000536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dammen, L. , Wekker, V. , van Oers, A. M. , Mutsaerts, M. A. Q. , Painter, R. C. , Zwinderman, A. H. , … on behalf of the LIFEstyle study group. (2018). Effect of a lifestyle intervention in obese infertile women on cardiometabolic health and quality of life: A randomized controlled trial. PLoS One, 13(1), e0190662. 10.1371/journal.pone.0190662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Beek, C. , Hoek, A. , Painter, R. C. , Gemke, R. J. B. J. , van Poppel, M. N. M. , Geelen, A. , … Roseboom, T. J. (2018). Women, their offspring and improving lifestyle for better cardiovascular health of both (WOMB project): A protocol of the follow‐up of a multicentre randomised controlled trial. BMJ Open, 8(1). Retrieved from http://bmjopen.bmj.com/content/8/1/e016579.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Elten, T. M. , Karsten, M. D. A. , Geelen, A. , van Oers, A. M. , van Poppel, M. N. M. , Groen, H. , … on behalf of the LIFEstyle study group. (2018). Effects of a preconception lifestyle intervention in obese infertile women on diet and physical activity; A secondary analysis of a randomized controlled trial. PLoS One, 13(11), e0206888. 10.1371/journal.pone.0206888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ijzendoorn, M. H. , & Bakermans‐Kranenburg, M. J. (2015). Genetic differential susceptibility on trial: Meta‐analytic support from randomized controlled experiments. Development and Psychopathology, 27(1), 151–162. 10.1017/S0954579414001369 [DOI] [PubMed] [Google Scholar]
- Weinsier, R. L. , Hunter, G. R. , Heini, A. F. , Goran, M. I. , & Sell, S. M. (1998). The etiology of obesity: Relative contribution of metabolic factors, diet, and physical activity. The American Journal of Medicine, 105(2), 145–150. 10.1016/S0002-9343(98)00190-9 [DOI] [PubMed] [Google Scholar]
- Williamson, D. F. , Thompson, T. J. , Anda, R. F. , Dietz, W. H. , & Felitti, V. (2002). Body weight and obesity in adults and self‐reported abuse in childhood. International Journal of Obesity, 26, 1075. 10.1038/sj.ijo.080203 [DOI] [PubMed] [Google Scholar]
- Wu, T. , Gao, X. , Chen, M. , & van Dam, R. M. (2009). Long‐term effectiveness of diet‐plus‐exercise interventions vs. diet‐only interventions for weight loss: A meta‐analysis. Obesity Reviews, 10(3), 313–323. 10.1111/j.1467-789X.2008.00547.x [DOI] [PubMed] [Google Scholar]
- Yancura, L. A. , & Aldwin, C. M. (2009). Stability and change in retrospective reports of childhood experiences over a 5‐year period: Findings from the Davis Longitudinal Study. Psychology and Aging, 24(3), 715–721. 10.1037/a0016203 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Flowchart participants in the LIFEstyle study and follow‐up
Data Availability Statement
An anonymized data set has been made available as supporting information.


