Abstract
Across Latin American and Caribbean countries (LACs), the fight against dementia faces pressing challenges, such as heterogeneity, diversity, political instability, and socioeconomic disparities. These can be addressed more effectively in a collaborative setting that fosters open exchange of knowledge. In this work, the Latin American and Caribbean Consortium on Dementia (LAC‐CD) proposes an agenda for integration to deliver a Knowledge to Action Framework (KtAF). First, we summarize evidence‐based strategies (epidemiology, genetics, biomarkers, clinical trials, nonpharmacological interventions, networking, and translational research) and align them to current global strategies to translate regional knowledge into transformative actions. Then we characterize key sources of complexity (genetic isolates, admixture in populations, environmental factors, and barriers to effective interventions), map them to the above challenges, and provide the basic mosaics of knowledge toward a KtAF. Finally, we describe strategies supporting the knowledge creation stage that underpins the translational impact of KtAF.
Keywords: biomarkers, clinical trials, dementia, Latin America, epidemiology, evidence‐based recommendations, genetics, knowledge to action framework, networking and translational research, nonpharmacological interventions
1. INTRODUCTION
Despite its global scope, 1 , 2 , 3 , 4 , 5 , 6 dementia poses distinctive perils for Latin American and Caribbean countries (LACs). 1 , 7 , 8 , 9 , 10 This region has a high and increasing prevalence of dementia (between 7.1% and 11.5% among individuals 65+ years of age, compared with lower, stable, or decreased prevalence from Europe and the United States). 11 In addition, it presents various relevant risk factors, including a remarkable heterogeneity of genetics and social determinants of health (SDH). Regional clinical trials and non‐pharmacological interventions are limited and biomarker research is lagging behind. More coordinated networking and translational research will help address existing and new challenges, such as those brought by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) pandemic, which will likely burden vulnerable populations across LACs. 12 , 13 The goal of this Policy Forum is to propose a Knowledge to Action Framework (KtAF) allowing regional challenges to be addressed through both local initiatives and global dementia strategies (see Panels for specific goals of this article). In addition, the proposed KtAF should enable regional knowledge to more reliably inform such global strategies.
The Latin American and Caribbean Consortium on Dementia (LAC‐CD1, an initiative supported by the Alzheimer's Association and the Global Brain Health Institute, encompassing over 250 experts, see below) has identified priority areas (see Figure 1), which present distinctive challenges and unique opportunities for the region. 1 In order of priority, these areas include: (1) risk factors for dementia and non‐pharmacological interventions, (2) epidemiological and genetic studies, (3) biomarkers for dementia, (4) clinical trials, and (5) networking and translational research. These areas and their associated challenges are in accordance with knowledge available and regional needs previously highlighted by LAC‐CD (see Supplementary Material 1).
FIGURE 1.
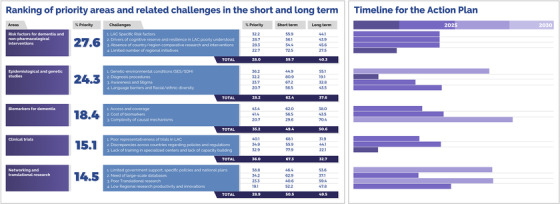
Priority levels assigned to core areas and challenges via a knowledge inquiry and related actions timelines. Co‐authors of this work were presented with a survey and were asked to rank the five areas and associated challenges in order of priority. We calculated the percentage of respondents who rated these within the top two priorities and used these to rank both areas and challenges. The right inset shows the timeline for the proposed actions described in Section 4. Experts were also asked to deliver their views about a feasible timeline to address these challenges and actions (0‐5 or 5‐10 years) (% = Mean % of responses)
We have organized our proposal based on the knowledge‐to‐action approach. 14 An initial step consists of integrating the creation and application of relevant knowledge. To this end, we briefly introduce the origins of LAC‐CD and explain the need for a regional KtAF. To make a case for a LAC KtAF, we rely on two levels of evidence. One supports the most relevant challenges linked to each priority area (Section 3). The second supports recommendations drawn from relevant regional and global initiatives (Panel 2 summarizes these main recommendations in order of priority). Based on these, we introduce the plan formulation (Section 4), assessing the implementation and action cycle of the KtAF. By integrating levels of evidence and contextual challenges that will need to be addressed as part of the KtAF's action cycle (Supplementary Material 3), we propose five workgroups. We performed a regional review (see the search strategy and selection criteria in Panel 3) presented in Supplementary file 5 (Annex 1: epidemiologic studies, Annex 2: clinical trials, Annex 3: local and regional research initiatives, and Annex 4: initiatives for public policies and awareness); these are discussed across the manuscript and summarized in Figure 2. Annexes provide an extended source of information supporting the current policy narrative. In addition, they offer a systematic and integrated reference point for clinicians, researchers, and policymakers working on epidemiological studies, clinical trials, research initiatives, and public policies in the region. A glossary of definitions and terms is provided in Supplementary Material 4.
FIGURE 2.
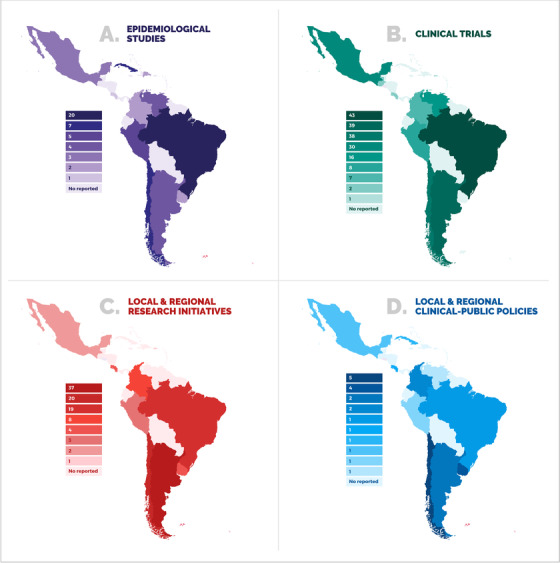
Geographical distribution of (A) epidemiological studies, (B) clinical trials, (C) local and regional research initiatives, and (D) government policies to support dementia in LACs. Data used to build this figure can be found in the Annexes 1 to 4
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature using traditional sources (eg, PubMed, see Panel 3). Although an integrated strategy for facing dementia in Latin American Countries (LACs) has not yet been developed, recent publications describe different aspects of dementia in LACs that will be important to address in an integrated strategy.
Interpretation: We present t recommendations for the knowledge creation stage of the knowledge‐to‐action framework (KtAF), including: (a) identifying risk factors and nonpharmacological interventions, (b) accurate epidemiological data, (c) gathering biomarker data, (d) enhancing capacity for clinical trials, and (e) networking and translational research. We first synthesize available knowledge and challenges, and then discuss strategies to tailor and implement actions that can promote the use of knowledge (evidence‐based actions and recommendations).
Future directions: We propose a framework for the generation of a KtAF, as well as steps needed to monitor knowledge use and develop an outcome evaluation process.
2. THIS POLICY FORUM
2.1. Setting the context for a KtAF
A key step in delivering a KtAF is knowledge inquiry, a process resulting in natural, largely unrefined, first‐generation knowledge. 14 LAC‐CD's inquiry efforts began in 2015, when a group of dementia experts from the region came together to discuss the barriers faced by practitioners (see Annexes 1 to 4 and Figure 2 for a brief overview of current initiatives). Thereupon, LAC‐CD has explored the realities of the region and used such knowledge to propose the action plan presented here (see Supplementary Material 1 for more information about the history of LAC‐CD).
Such first‐generation knowledge was synthesized, leading to second‐generation knowledge. 14 The latter constitutes the foundations of the present action plan. To effectively implement our KtAF we also need third‐generation knowledge. This entails specific tools that can guide processes involved in knowledge generation and use. 14 Via workgroups focusing on the priority areas identified here, the KtAF will ensure consistency in the use of knowledge across LACs, its communication to relevant stakeholders, its mapping to global strategies, and the timely incorporation of new ensuing knowledge to the KtAF. The lack of such a knowledge‐based structure has long precluded harmonization of research and clinical practices in LACs, preventing regional initiatives from attaining necessary and well‐deserved visibility. With such foundations in place, LAC‐CD can enter an action cycle. We will discuss how such a cycle (the driving force of the KtAF) will allow stakeholders to adapt knowledge, monitor its use, and generate new knowledge. We envisage that this proposal will become the roadmap for the convergence of regional and global dementia initiatives, allowing the former to eventually inform the latter. It will also provide a tool for monitoring regional progress in the fight against dementia.
2.2. Operationalizing LAC‐CD's KtAF
The key areas of LAC‐CD's KtAF have been appraised by all co‐authors. Their priority has been ranked and their associated challenges rated. The resulting knowledge and syntheses have been endorsed by a large network, paving the way for third‐generation knowledge, that is, knowledge tools. 14 To this end, we propose a plan that outlines and describes core actions of the KtAF, grounded in planned‐action theories (ie, identifying and controlling factors that impact the likelihood of effecting change in each target problem 15 ). This plan will help LAC‐CD to enter an action cycle following theses milestones: (a) adapting knowledge to local contexts; (b) assessing challenges to knowledge access and use; (c) selecting, tailoring, and implementing actions; (d) monitoring knowledge use; (e) evaluating outcomes; and (f) sustaining the KtAF. 14
3. KNOWLEDGE CREATION
This section addresses the challenges and knowledge gaps in each priority area. Figure 1 shows the priority areas, related challenges, and timeliness of proposed actions. Priority levels were assigned to core areas and challenges via knowledge inquiry. Co‐authors of this work ranked the five areas and associated challenges in order of priority. The values in the figure indicate the percentage of respondents from the total number of experts (coauthors) who rated these within the top two priorities, as these would more likely capture areas needing urgent actions. Respondents also rated whether each challenge needs to be addressed in the short (0‐5 years) or long (5‐10 years) term. For the latter, we calculated the average proportion of responses within the two time periods.
3.1. Risk factors for dementia and non‐pharmacological interventions
LAC‐specific risk factors (Priority Rating: 32.2%, Time window: next 0 to 5 years). Thirty‐five percent of dementia cases around the world seem to be explained by risk factors 17 , 18 such as childhood education, midlife hearing loss, hypertension, obesity, unhealthy diets, later‐life smoking, depression, physical inactivity, social isolation, and diabetes. However, in LACs, the percentage increases to 56%, 18 seemingly due to a combination of cultural, political, and economic factors, among others. In particular, the vast socioeconomic inequalities within countries (and across the region) reduce access to basic human needs and health services, which, combined with a higher exposure to multiple risk factors, seemingly increase the prevalence of dementia. Data from Brazil suggest that by modifying risk factors such as low educational attainment, physical inactivity, midlife hypertension, midlife obesity, depression, smoking, and diabetes mellitus, the country could potentially reduce the prevalence of dementia by 16.2% by 2050. 19 Prospective studies 21 , 22 have provided evidence on the differential effects of alcohol use, education, lifetime sanitary conditions, and cerebrovascular risk factors. Although characterizing specific risks factors affecting LACs is beyond the scope of this work, we provide a brief summary of risk factors for dementia in LACs in Supplementary Material 2.
Drivers of cognitive reserve and resilience in LACs poorly understood (Priority Rating: 28.5%, Time window: next 0‐5 years). Cognitive reserve is affected by the interaction of genetic and environmental factors. 22 The admixture of Amerindians, Europeans, and Africans found in LACs is thought to influence cognitive reserve in ways that differ from those identified in high‐income countries (HICs). Recent reports highlight the unequal impact of diverse environmental factors on LACs. 12 , 24 There seems to be a consensus that available models of cognitive and brain reserve, which consider high educational levels, high intellectual functioning, and healthy lifestyles as their building blocks, 24 may not provide valid accounts to explain trajectories of cognitive aging in low and middle income countries (LMICs). 25 Factors modulating cognitive reserve are poorly understood in the context of low educational levels 24 and limited access to socio‐educational programs. Nevertheless, the prevalence and incidence of dementia is higher among illiterate people. 26 With the 56% of dementia cases in LACs being accounted for by modifiable risk factors, 12 the potential of the region to inform cognitive reserve models and support dementia prevention initiatives is unique.
Absence of country/region comparative research and interventions (Priority Rating: 28.3%, Time window: next 0‐5 years). Most ongoing interventions in the region (described in Annex 4) do not consider critical risk factors or systematic comparisons between countries. Thus the scarcity of national/regional comparative research and interventions preclude the identification of more detailed country‐specific and cross‐country risk factors and the development of interventions that can be either tailored or harmonized. For instance, education, literacy, verbal fluency, and motor sequencing are outcomes linked to protection against the onset of dementia in LMICs. 27 Because these factors also protect against dementia onset in high‐income countries (HICs), they may provide tools to develop protocols that can be better harmonized across regional and global initiatives.
Limited number of regional initiatives (Priority Rating: 22.7%, Time window: next 0‐5 years). Of 93 regional initiatives identified (see Annex 3 for a description of current national and regional research initiatives), only 4 included the word “risk” in the title. Moreover, only 3 initiatives addressed issues around “cognitive training” in dementia and related disorders. Despite its relatively low priority, 72.5% of respondents considered that this challenge needs to be addressed in the short‐term (0‐5 years). The preventive World Wide FINGERS study has been recently extended to LACs. 28 Another prototype example is the Strengthening Responses to Dementia in Developing Countries (STRiDE) running in different LACs (see Panel 2). Hence, there is a positive context in the region to promote initiatives aimed at better characterizing and addressing risk factors for dementia.
3.2. Epidemiological and genetic studies
Genetic‐environmental conditions (Priority Rating: 36.2%, Time window: next 5‐10 years). Lifelong exposure to interacting genetic and environmental factors associated with increased risk of dementia 30 , 31 , 32 , 33 could drive the phenotypic heterogeneity of dementia in LACs. 1 , 7 , 8 , 9 , 10 , 34 At the genetic level, LACs host population isolates of rare gene mutations 2 , 4 , 5 , 6 , 34 that cause neurodegenerative diseases (eg, familial AD, Huntington disease, ataxia, Parkinson disease, and frontotemporal dementia, and so on 7 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 ). Such highly informative groups may be more common than currently known due to ancestry and admixed genetic backgrounds. 1 , 8 , 36 Genetic studies of immigrants from LACs have confirmed the influence of such isolates. 9 , 57 Recent Polygenic Risk Scores (PRSs) to identify individuals at risk for dementia are promising 58 , 59 , 60 , 61 but < 3% have been developed from Latino, admixed, or indigenous populations. 61 Large consortia have assessed genetic susceptibility mostly in HICs, but other regions, including LACs, 1 , 3 remain understudied. Thus it is expected that different levels of genetic risk may selectively affect the prevalence of dementia in this region. For instance, the impact of rare variants (TREM2, PLCG2, and ABI3) on the Argentinian population highlights different features of LAC admixed populations. 62 At the environmental level, socioeconomic status (SES) and social determinants of health (SDH), which signficantly influence the prevalence of dementia, 63 reveal larger inequalities in LACs than in HICs. SDH are strong predictors of brain health, 64 and, together with SES, 65 increase the risk for dementia in Latinos. 66 Relatedly, social stress, social deprivation, among other factors, also contribute to the high dementia prevalence in LACs. 68 , 69
Diagnosis procedures (Priority Rating: 32.2%, Time window: next 0‐5 years). Dementia diagnosis requires well‐developed connections between primary and secondary care, a need currently unmet in LACs. 1 Compared to European and North American countries, this region witnesses an unprecedented growth of aging and dementia rates. 69 In the 65‐69 year age group, dementia prevalence is twice as high in LACs compared with developed countries. 26 Some authors 12 acknowledged that the use of paper and pencil tests developed in HICs to detect cognitive impairment in low educated individuals may impact diagnosis, even with education‐adjusted cut‐off scores. Lack of cultural validity across assessment tools, together with limited understanding of and training in dementia diagnosis, present LACs with such significant challenges which, for the majority of experts (80.9%), require immediate actions (0‐5 years).
Awareness and Stigma (Priority Rating: 23.7%, Time window: next 0‐5 years). Low awareness and stigma have proved a barrier to early characterization of dementia. 1 , 26 , 27 , 71 , 72 , 73 Both factors are pervasive in LACs. 73 Senior Latinos find it difficult to talk about cognitive evaluation because discussing cognitive and mental health is considered a taboo. 74 Messages used to promote awareness are often found to be vague and produce feelings of guilt. Poor awareness is also found among health care providers. In Chile, 51% of health professionals from different professional areas considered that their knowledge about dementia was insufficient and 81% did not feel prepared to care for a person with dementia. 71
Language barriers and racial/ethnic diversity (Priority Rating: 20.7%, Time window: next 0‐5 years). The cultural and ethnic heterogeneity of LACs has long hindered several dementia strategies. 1 , 26 , 76 Although Spanish and Portuguese are the two most widely spoken languages, Latin America is a hotspot of linguistic diversity. There are 455 living languages in the region. The linguistic diversity in LACs stands in stark contrast to that of Europe, where 287 have been recorded. One area particularly impacted by this linguistic diversity is cognitive assessment. Tools used to support diagnosis have been translated rather than locally developed, thus lacking in cultural validity. In 2010, there were 42 million indigenous people living in the continent, corresponding to 8% of the total population. About 34 million (80.1%) live in Bolivia, Guatemala, Mexico, and Peru. Most of these individuals live in urban areas and are bilingual, but their educational level is lower than other groups. 12 The prevalence of dementia among indigenous populations appears to be higher than in non‐indigenous populations, 76 and they are more vulnerable to cognitive disorders. 12 , 78
3.3. Biomarkers for dementia in LACs
The biomarkers agenda in the region faces important challenges. The survey revealed that only 18.4% of the experts rated this area in the two top priorities. Considering recent international consensuses, which stress the importance of biomarker evidence to support the biological definition of dementia, 79 , 80 , 81 this low percentage is noteworthy. The challenges prioritized by LAC‐CD experts provide insight on the underlying reasons.
Access and Coverage (Priority Rating: 43.4%, Time window: next 0‐5 years). To gather the evidence required by the NIA‐AA biomarker framework (ie, amyloid beta [Aβ] and pathologic tau), sophisticated diagnostic procedures are needed. The LAC‐CD's knowledge inquiry reveals that several LAC research centers will not have access to these biomarkers anytime soon. 1 , 26 Although highly relevant, the biomarker framework 78 for AD will pose greater challenges to LMIC. For instance, one of the most expensive and least available techniques, positron emission tomography (PET), is available in a few centers across only 13 LACs (and only nine have cyclotrons for the production of radiotracers). Other advanced biomarker procedures, such as MRI/fMRI neuroimaging, are available only in specialized centers typically located in major cities.
Cost of biomarkers (Priority Rating: 41.4%, Time window: next 0‐5 years). Biomarker procedures are expensive and such costs are not routinely covered by public health care providers in LACs. Available biomarkers are currently recommended for research purposes. 78 Yet the costs of these procedures are prohibitive even for national funding agencies in LACs. Regional studies on the validity of dementia biomarkers rarely attract funds from national agencies and they are restricted to major research centers. Often such studies are co‐funded or fully supported by international grants, which are also awarded to research groups hosted by major research centers. Hence, challenges linked to access, coverage, and costs of biomarker in LACs seem to overlap.
Complexity of causal mechanisms (Priority Rating: 20.7%, Time window: next 5‐10 years). A third challenge, not restricted to LACs, is the reliability of available biomarkers to unveil pathognomonic features of dementia. Biomarkers seem to be more reliable only in centers with high pre‐test dementia prevalence. 81 This normally applies to specialized centers in LACs that host memory clinics. Because only a limited number of patients will have access to such services, the feasibility of costly and still non‐specific dementia biomarkers in LACs needs to be further assessed. For instance, conversion rates to Alzheimer's disease (AD) after 5‐year follow‐up is about 50% for biomarkers positive (Amyloid + Tau + Neurodegeneration: A‐T‐N+ patients). 82 Inspections revealed that only two patients had the A‐T‐N+ profile, one of whom received the diagnosis of hippocampal sclerosis probably due to TDP43 proteinopathy and the other was inconclusive. However, other authors 83 recently reported that some individuals with amnestic MCI progressed to clinical AD dementia within all four major A/T/N groups including A‐T + N+ and A‐T‐N‐. They suggested that when selecting individuals for research, a combination of the A/T/N framework and clinical status may be necessary because the prognostic value of the former depends on the latter. An interesting single case was recently published that sheds new light on the causal mechanisms of AD dementia and the A/T/N framework. 84
3.4. Clinical trials in LACs
Poor regional representativeness of trials in LACs (Priority Rating: 40.1%, Time window: next 5‐10 years). Our review of clinical trials in LACs (see Annex 2 for a review of availably initiatives identified in clinicaltrials.gov and Figure 2.A) identified 10 involved countries. The issue, then, is not only a limited number of clinical trials running in LACs but also the poor representativeness of such trials. Of the identified clinical trials, 89% were linked to five countries. According to clinicaltrials.gov, the majority of clinical trials (in general) are conducted in Brazil, Mexico, Colombia, and Argentina. LACs are considered one of the unexplored regions for Clinical Trials, harboring about 6% of the total active studies in the world and about 11% of total studies.
Discrepancies across countries regarding policies and regulations (Priority Rating: 34.9%, Time window: next 5‐10 years). Significant discrepancies are linked to institutional review boards. 85 For instance, regulatory agencies in Brazil and Argentina, two LACs hosting the largest number of clinical trials (Figure 2.B), impose additional restrictions beyond those coming from international agreements. In Chile, the country with the third highest number of clinical trials (Figure 2.B), article 23 of Law 20584 (law on patients’ rights and duties), prohibits people who are unable to provide consent from participating in scientific research studies. 1 Of interest, countries reporting such barriers are at the forefront of clinical trials for dementia in the region, thus suggesting additional drivers of poor representativeness. Unfortunately, there are scant tools available for comparison and coordination in LAC, such as regional registries or common multicentric protocols.
Lack of training in specialized centers and lack of capacity building (Priority Rating: 32.9%, Time window: next 5‐10 years). Clinical trials in LACs seem restricted to a few centers with competitive infrastructure and capacity. Such is the case of the Neuroscience Group of the University of Antioquia in Colombia, which launched the Alzheimer's Prevention Initiative (API). 86 This collaborative multi‐partner initiative supports an autosomal dominant AD trial and seeks other AD treatments. Beyond such examples, most clinical and research centers do not meet basic requirements for clinical trials, such as the use of harmonized practices, adherence to standardized diagnostic procedures, and availability of genetic counseling. Although this area was not rated as a priority, there seems to be consensus that its associated challenges need prioritization. As an unexplored region for clinical trials, LACs harbor opportunities that are still unknown. Hence, training and capacity building are key priorities.
3.5. Networking and translational research
Finally, only 14.5% of the experts rated Networking and Translational Research within the top two priority areas. Within this area, challenges included, in order of relevance, (a) limited government support, specific policies and national plans; (b) need of large‐scale databases; (c) poor translational research; and (d) low regional research productivity and innovations.
Health research, mainly concerning neurology and dementia, has been systematically overlooked in LACs. For many decades, regional research has been limited by lower levels of production, restricted financial and human resources, lack of government support, and almost absent policies, interventions, or national plans. 88 , 89 With some exceptions, most active groups develop short‐term research agendas and work in isolation without support from international initiatives. 89 Among some selected regional initiatives identified in this work (see Annex 3 for representative research projects in the region, and Figure 2.C), we found isolated efforts, replication of studies from HICs without adequate harmonization and adaptation to local contexts, and a lack of regional research targeting dementia policies. Translational research has emerged as a dominant driving force in diagnostic and therapeutic advances in dementia, 91 , 92 but only a few examples can be found in LACs. Similarly, large‐scale databases have been successfully implemented in other regions 93 , 94 but no similar initiatives have been developed in LACs. To our knowledge, only an ongoing project from the Inter American Developmental bank is developing a shared basic registry for Argentina, Chile, and Uruguay. Thus an urgent call for more systematic networking and translational research is needed.
An interesting outcome from the survey is that the top two areas (detailed in Sections 3.1 and 3.2) comprise challenges that can be largely addressed through coordinated and joint research efforts. However, areas that attracted lower ratings (Sections 3.3‐3.5) comprise challenges whose solutions are more reliant on political and governmental support. This suggests that low expectations could drive such ratings, as LACs have long witnessed considerable political and economic turmoil.
4. PLAN FORMULATION
4.1. Implementation of the KtAF
The knowledge base presented above paves the way toward the development and implementation of a KtAF. 14 Long‐term actions based on our KtAF include specific planning for (a) promoting integration at a regional level, (b) liaising with government representatives, (c) improving health systems, (d) supporting the implementation of networking and translational initiatives, and (e) allocating available regional resources to the LAC‐KtAF (see Supplementary Material 3 for an analysis of these actions). Such an integration requires an increase in the participation of affected people and their caregivers, government agencies, and various stakeholders in activities focusing on co‐design and co‐production. 94 More interaction is needed between researchers and policymakers to increase and improve health system provisions, and enhance networking and translational initiatives that enjoy support from government, legal, and policy agencies. 95 These recommendations are aligned with the Alzheimer's Disease International's From Plan to Impact call. 97 , 98 For instance, discrepancies between the number of research initiatives (eg, epidemiological studies, clinical trials, and research, Figure 2A‐C) and governmental policies aimed at supporting these initiatives (Figure 2.D) are striking. This indicates that more work needs to be done with government and stakeholders to ensure that available regional resources are allocated, which will be key to effectively developing and sustaining the KtAF proposed here. The following sections address direct actions that constitute the operational architecture of the KtAF, supporting its transformative influences and own growth through a dynamic knowledge cycle (ie, new knowledge production). The suggested actions stem from the evidence supporting the challenges identified in Section 3, the recommendations summarized in Panel 2, and the future strategies to set the context for the KtAF (Supplementary Material 3).
4.2. Action cycle of the KtAF
The action cycle is presented in Panel 2 (detailing proposed actions in order of priority) and summarized in Figure 3. These actions are presented considering the timelines provided in Figure 1.
FIGURE 3.
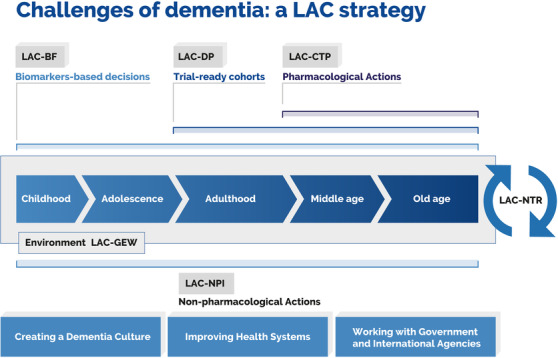
Knowledge‐to‐action framework. The diagram captures challenges posed by dementia and the related mapping of key actions. Such actions may be linked to specific working groups that have been included in the framework. This approach comprises a biomarker framework (LAC‐BF), genetics and epidemiology workgroup (LAC‐GEW), dementia platform (LAC‐DP), clinical trial program (LAC‐CTP), nonpharmacological interventions (LAC‐NPI), and an LAC network for translational research (LAC‐NTR)
4.2.1. The non‐pharmacological interventions workgroup
The Non‐pharmacological Interventions Workgroup (LAC‐NPI) will focus on better characterizing and reducing risk factors associated with noncommunicable diseases (NCDs). To these aims, an initial set of evidence‐based recommendations (Panel 2.A) will focus on challenges presented in Figure 1 and discussed in Section 3 above. The proposed actions are:
Expanding experiences from LACs involved in international initiatives shown in Panel 2.A to other countries in the region (Challenge: LAC‐specific risk factors).
Supporting LACs to build and strengthen risk factor surveillance capacity by incorporating the World Health Organization (WHO) STEPwise approach to NCD (Challenge: LAC Specific risk factors).
Supporting the development of National Dementia Plans capitalizing on experiences from LACs that have launched their plans and accrued evidence of their feasibility and impact (see Panel 2.A; Challenges: LAC specific risk factors, drivers of cognitive reserve and resilience in LAC poorly understood, absence of country/region comparative research and interventions, limited number of regional initiatives).
Endorsing regional collaboration to increase understanding of cognitive/brain maintenance, reserve, compensation, and resilience in older populations. Secondary data analysis capitalizing on local databases and expanding such protocols to other countries constitute a first action (Challenge: drivers of cognitive reserve and resilience in LAC poorly understood).
Improving training and educational programs. The Atlantic Fellows program for Health Equity of the Global Brain Health Institute (GBHI), in coordination with other ongoing initiatives from the Alzheimer's Association aimed at increasing leadership capacity, can promote a dementia ambassador program that would assess awareness of and changes in lifestyle factors across LACs, and refine the recommendations for an action plan (Challenges: LAC‐specific risk factors, drivers of cognitive reserve and resilience in LACs that are poorly understood, absence of country/region comparative research and interventions, limited number of regional initiatives).
Exploring opportunities for and barriers to implementing cognitive training and stimulation programs that use technology (eg, digital care, virtual/augmented reality) as well as other emerging NPI (eg, reminiscence approaches such as music therapy, sporting memories, or animal assisted interventions) (challenge: absence of country/region comparative research and interventions, limited number of regional initiatives).
4.2.2. The genetic and epidemiology workgroup
A Genetics and Epidemiology Workgroup (LAC‐GEW) would focus on recommendations presented in Panel 2.B, which will allow more LACs to be better represented in the current epidemiological landscape of dementia. The actions to be promoted by LAC‐GEW are:
Implementation of pan‐LAC epidemiological studies and generation of workshops on epidemiology and related issues supported by experts outside the region. 16 These approaches will help boost critical regional needs, such as the identification of cognitively vulnerable or resilient 98 common and unknown risk factors (such as obesity, smoking, sanitary conditions, depression, gender, genetics, and cardiovascular and cerebrovascular diseases, 21 , 100 among others) for dementia (Challenges: genetic‐environmental conditions, diagnosis procedures).
To identify the influence of lifelong factors on neurocognitive development, LACs would benefit from strategies offered by behavioral insight approaches 100 and systematic assessments of multimodal sources of heterogeneity. A recent survey in >3000 health professionals working in aging suggest a strong interest in behavioral insights and multimodal regional research 73 (Challenges: genetic‐environmental conditions, awareness and stigma, language barriers and racial/ethnic diversity).
To incorporate assessment protocols such as those developed by the Neuroscience Group of Antioquia, Colombia through which more genetic variants of dementing illnesses (eg, Ile416Thr, a variant in PSEN1 101 ) will be identified across the region. The implementation of a regional manual for best practices for the dementia diagnosis such those developed by LAC‐CD 102 (particularly from the Multi‐partner Consortium to Expand Dementia Research in Latin America, ReDLat, see Panel 2.C for specific recommendations) can help to harmonize procedures in local contexts (Challenge: diagnosis procedures).
Implementation of training/education programs to increase knowledge and practice in topics relevant to mental health and dementia. The ReDLat training and certification program (currently for six LACs), included training on DNA procedures, clinical diagnosis and assessment, cognitive evaluation, and neuroimaging recordings and preprocessing, needs to be expanded to other countries (Challenge: diagnosis procedures).
Development of a digital platform (regional database, Figure 3) to collect and share data across LACs following harmonized procedures. An Inter‐American Development Bank project is creating an initial registry in Argentina, Chile, and Uruguay, with potential expansions to other countries (Challenges: diagnosis procedures, language barriers and racial/ethnic diversity)
To incorporate recommendations from ADI's Report on Stigmas 70 and work with LACs that participated in the generation of this report to develop stigma reduction strategies. These can focus on social media campaigns (eg, #StillHere campaign by Alzheimer's Society UK) or inter‐generational approaches 103 (Challenge: awareness and stigma).
Increase understanding of the impact of genetic heterogeneity 105 , 106 , 107 on people living with dementia in LACs and address issues related to genetic disclosure and counselling (Challenges: genetic‐environmental conditions, diagnosis procedures, awareness and stigma).
Promote support from local governments and international agencies to establish an LAC dementia observatory that can contribute to the global dementia observatory set up by the WHO (Challenges: genetic‐environmental conditions, diagnosis procedures, awareness and stigma, language barriers and racial/ethnic diversity).
4.2.3. The biomarker framework
The KtAF includes a Biomarker Framework (LAC‐BF, Figure 3, Panel 2.B) that will support the validation of novel methodologies complemented and validated with current biomarker pipelines. 78 The proposed actions are:
Validation of this framework in hubs of the LAC‐CD that can provide the level of evidence required by the Amyloid + Tau + Neurodegeneration (A/T/N) framework. These protocols, which have been preliminarily tested in LACs, can be easily implemented in institutions hosting the required infrastructure, where validation of these novel approaches against canonical biomarkers can be undertaken. LAC‐CD has currently implemented an ongoing survey, which aims to characterize the biomarker landscape in LAC. This will pave the way to future strategies of the LAC‐BF. Through this process, these protocols will continue to be refined to reflect appropriate approaches for biomarker use (Challenges: access and coverage, cost of biomarkers).
Strengthening LAC‐CD partnership with the Alzheimer's Association and the National Institute of Health/National Institute on Aging (NIH/NIA) toward the validation of the LAC‐BF. The combination of these stakeholders, as well as local leadership, will help address shortage of local funds and also expand available networks. One example is the Multi‐partner Consortium to Expand Dementia Research in Latin America (ReDLat), supported by the Alzheimer's Association, the NIH/NIA, the Tau Consortium, and the GBHI. This platform aims to identify the genetic and socioeconomic/SDH risks of dementia (the cohort involves >4000 participants from LACs and a US team). It will also provide access to blood biomarkers and is already supporting local parallel grant applications in Argentina, Chile, Colombia, Brazil, Peru, and Mexico. Available initiatives from NIA/NIH in the region can be synergistically connected with other available multinational stakeholders (ie, Horizon 2020, Newton UK‐Latin American programs). For instance, the Newton funds program has developed bilateral opportunities to address social priorities for LACs (Brazil, Chile, Colombia, Mexico, and Peru). Collaboration with the NIA/NIH, the Alzheimer's Association, and the GBHI in a global context will be key to validate and disseminate the LAC‐BF (Challenges: access and coverage, cost of biomarkers).
Introduction of complementary affordable biomarkers based on cognitive assessment, eye‐tracking, noninvasive peripheral markers (ie,plasma markers such as AB‐42, ptau1, 80 , and so on), and multimodal neuroimaging 108 , 109 , 110 (eg, EEG, MRI, fMRI, DTI), combined with machine‐ and deep‐learning algorithms. In a second stage, newly validated tools and technical knowledge will be made freely available via online platforms (eg, LAC‐CD website, local institutions) (Challenges: access and coverage, cost of biomarkers).
LAC‐BF will help identify new causal mechanisms and improve our understanding of the relationship between genotypes, clinical phenotypes, and the severity of neurodegeneration via ongoing and forthcoming initiatives (eg, DIAN Latin America, ADNI, RedLat). (Challenge: complexity of causal mechanisms).
4.2.4. The clinical trial program
The Clinical Trial Program (LAC‐CTP) will focus on the challenges presented in Section 3.4 and supported by evidence‐based recommendations shown in Panel 2.D. The proposed actions are:
Identifying countries where prevention trials could be carried out. The experience gained by countries such as Colombia, together with access to new populations affected, will allow for the expansion of initiatives such as API in the region. The country has developed a state‐of‐the‐art infrastructure and professional training program that can be expanded to other hubs in the region (Challenge: poor representativeness of trials in LAC).
Linking regional clinical trial programs (Figure 2.B) with national regulatory agencies to harmonize regional policies on future multi‐country clinical trials (Challenge: discrepancies across countries regarding policies and regulations).
Promoting the development of trial‐ready cohorts capitalizing on data gathered by the Genetics and Epidemiology Workgroup (LAC‐GEW) and hosted by the dementia platform (LAC‐DP). LAC‐CTP should rely on experiences from international (eg, EPAD, DIAN) and regional initiatives such as FLENI experience in ADNI (Argentina), DIAN in several LACs, and API in Colombia (Challenge: poor representativeness of trials in LAC).
Accelerating research relying on LACs’ brain banks. Local available brain banks could be more extensively connected across the network for translational research (LAC‐NTR, Figure 2: Challenge poor representativeness of trials in LAC).
Launching a Clinical Trial Training Program led by reference centers in the region (eg, Neuroscience Group from Antioquia, Colombia, FLENI from Argentina) and supported by international initiatives such as API to improve local infrastructure and expertise (Challenge: lack of training in specialized centers and lack of capacity building).
4.2.5. The network for translational research
The implementation of a network for translational research (LAC‐NTR) will require the harmonization of resources and logistics, formulation of regional standardized data‐sharing guidelines, 110 training of human resources, and promotion of a culture of collaboration. Some research groups are already working on relevant clinical issues in dementia 8 , 34 (Figure 2.C, Annex 3). The actions promoted by LAC‐NTR are:
Establishing a Latin American network for translational research (LAC‐NTR), which would articulate the efforts of scientists, clinicians, pharmaceutical leaders, and government representatives. One representative case is the public‐private Geroscience program in Chile, which integrates basic and clinic research, as well as animal and human models of aging and neurodegeneration (Challenges: limited government support, specific policies and national plans, need for large‐scale databases, poor translational research, low regional research productivity, and innovations).
Implementing IT platforms to collaborate and share resources related to registries, regional brain banks, telecare, training, and post‐diagnosis support (Challenge: specific policies and national plans, need of large‐scale databases).
Supporting collaborative work in the region with specific brain banks with ongoing initiatives such as UndoAD (a Colombo‐German consortium hosting brain banks of rare gene mutations) or the Biobank for Aging Studies of the University of São Paulo. Such resources are unique assets for supporting drug discovery programs. (Challenge: need of large‐scale databases).
Working with relevant partners such as the Alzheimer's Association and the GBHI to launch the Latin‐American Congress on Dementia. These partnerships will also provide an educational program for a new generation of leaders in dementia (Challenge: low regional research productivity and innovations).
5. MONITORING KNOWLEDGE USE AND GENERATION VIA THE KTAF
Evaluation of the outcomes based upon the knowledge and establishment of strategies to sustain ongoing applications are two key steps of planned change theories that should guide the KtAF actions. 15 , 16 The different workgroups supporting such architecture will become the channels through which advances will be deployed and new knowledge generated. Via such tools, LAC‐CD will monitor knowledge and its use, evaluate the outcomes of using such knowledge, and feed these outcomes back into the operational architecture of the KtAF to strengthen it and expand its scope. To make LAC‐CD's KtAF operational, additional strategies will be needed (these are summarized in Supplementary Material 3).
It is worth noting that the challenges, supporting evidence, and ensuing actions here proposed are far from exhaustive. LACs host vast challenges, which are beyond the scope of this work. The actions here presented are aimed at providing an initial set of tools that can allow regional teams to use available knowledge and address priorities. By implementing these actions and revising their outcomes, we anticipate our agenda will allow us to explore the feasibility of the KtAF to address the challenges here discussed and to unveil new challenges.
6. CONCLUDING REMARKS
The key actions proposed here closely adhere to a rapidly changing landscape of dementia at a global level and provide a specific framework for the LAC regional workforce. The framework shall become the engine of a sustained dialogue to translate differences into shared concerns and to map these onto regional actions. The framework will raise awareness of where knowledge and resources should be more quickly deployed, promote equitable growth of those regions in the least favorable positions, and thus guide more balanced regional development through organized and coordinated agendas that effectively link local, regional, and global needs to local, regional, and global support.
7. PANELS
7.1. Panel 1 Aims of this Policy Forum
-
‐
To describe a set of specific LAC priority areas, actions, and recommendations that stem from well‐characterized challenges.
-
‐
To present a knowledge‐to‐action framework built on the complex interaction of barriers and needs specific to LACs.
-
‐
To assess the alignment of LACs’ needs and their potential links to the local and global dementia challenge.
-
‐
To suggest specific actions and recommendations for the future management of dementia in LACs, highlighting how challenges can be turned into unprecedented opportunities, and to provide recommendations to policymakers, funding agencies, and other stakeholders for future investments that can shift the regional dementia context.
8. Panel 2 EVIDENCE‐BASED RECOMMENDATIONS TOWARD THE PLAN FORMULATION OF THE LAC‐CD'S KTAF
A. Risk factors and non‐pharmacological interventions
New initiatives have been recently launched in partnership with international projects such as Strengthening Responses to Dementia in Developing Countries (STRiDE) across several LACs. The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), now extended worldwide through World Wide FINGERS, is also involved in LACs via the support of the Alzheimer's Association and the WW‐FINGERS leadership. There is great potential in LACs to support WW‐FINGERS’ remit.
Based on ADI's 2019 report on Latin America, only Chile, Costa Rica, Cuba, Mexico, and Puerto Rico have a national dementia plan (in Argentina the plan has been discontinued). Such experiences can be used to support the advancement of new dementia plans in other LACs.
LACs offer a suitable context for further expanding theories on cognitive reserve and resilience and their impact on the neurobiology of dementia by weighting the differential impact of unequal SDH. Some research centers and groups in LAC have developed local databases (eg, Neuroscience Group of Antioquia) and have been systematically collecting data from populations that hold different risks for dementia.
Although computerized cognitive training programs have yielded mixed findings, available results are promising. However, efforts are needed in LACs to overcome geographical, socioeconomic, and cultural barriers that can pose further challenges to technology‐driven interventions.
B. Toward accurate epidemiological data
A lifelong approach to dementia should be adopted, akin to the life course model proposed by the Lancet International Commission on Dementia Prevention and Care, but it should focus on key features of LACs that can contribute to the understanding of the epidemiology and genetics of dementia at a more global level. Accurate genetic and epidemiological information will help raise awareness and promote a positive dementia culture.
The influence of diversity and disparities (based on genetic, linguistic, ethnic, cultural, literacy, and education factors) is a growing area of interest, which is still poorly understood. The racial, ethnic, and linguistic diversity of LACs, along with the marked heterogeneity of educational backgrounds, make this region attractive for accelerating research on the interplay of sociodemographic factors.
Regarding genetics, LACs have pioneered strategies leading to the establishment of worldwide initiatives, such as the API. Lessons learned from such strategies should be expanded to other LACs. For example, as part of the API, the Neuroscience Group of Antioquia, Colombia, has developed pre‐screening tools and genealogical interviews to increase recruitment in clinical trials. In addition, in 2014, Argentina implemented a translational medicine program for the research, diagnosis, and treatment of AD in public institutions (PBIT/09). In this context, the Alzheimer Genetics in Argentina consortium was created. Registries of genetic presentation may also help better characterize prevalence and boost future drug discovery and prevention.
C. Situating the biomarkers agenda in the region
LAC‐CD has access to unique populations of individuals who carry autosomal dominant mutations that lead to various forms of neurodegenerative diseases that cause dementia. By capitalizing on such populations, LAC‐CD can support the validation of low‐cost biomarkers, which can help in the preclinical detection of dementia.
Moreover, by capitalizing on such populations, LAC‐CD can contribute unique knowledge about the causal mechanisms of dementia and the relationships between genotypes and phenotypes across neurodegenerative diseases. For example, The Multi‐partner Consortium to Expand Dementia Research in Latin America (RedLat, supported by the supported by the National Institute on Aging, the Alzheimer's Association, the Tau Consortium, and the Global Brain Health Institute [GBHI]), aims to provide accurate and representative evidence of genetic and sociocultural drivers of dementia phenotypes in LAC.
LACs host expertise to embark on initiatives that can help harmonize biomarker practices. LAC centers hosting such an expertise can become hubs of the biomarker agenda, which can support training and validation.
Emerging initiatives that rely on affordable methodologies such as EEG can support the validation of low‐cost biomarkers in LAC (eg, the UK–Latin America Brain Connectivity Research Network).
New innovative industry‐led methodologies are emerging, which hold cultural validity and can be easily introduced in primary care settings in LACs.
D. Enhancing LACs’ capacity to conduct clinical trials
Recommendations highlight the need to interact with national regulatory agencies to harmonize policies on future clinical trials in the region
LACs hold unique strengths to develop trial‐ready cohorts. By capitalizing on international experiences (eg, EPAD) and ongoing regional initiatives (eg, ADNI and DIAN, API in Colombia), such cohorts can grant access to unique populations that would become appealing to pharmacological trials focusing on secondary prevention such as those supported by the API.
LAC hosts unique brain banks that can support translational research in the region and beyond.
E. Networking and translational research
Via LAC‐CD and other ongoing regional initiatives, LACs have reached an unprecedented momentum that can not only leverage dementia research but also allow knowledge emerging from this region to inform global dementia strategies. Similar networking initiatives are being launched elsewhere. The KtAF here proposed can offer unique opportunities to bridge such global initiatives and contribute to the dementia challenge with true global strategies.
The establishment of networking and translational research is essential to link basic neuroscience with the development of diagnostic and therapeutic advances that can improve patient and caregiver quality of life. 24 In addition to the unique opportunities offered by LACs’ biobanks, there is great potential in the region to support research on animal models. Such research could benefit from including a novel model (eg, the Octodon degus, the only rodent model with natural AD from the region, with exceptional longevity) tested at multiple sites.
Despite the impact that translational research could have on LACs, funding and commitment from local governments to support these initiatives are still too limited. LAC funding bodies will need to establish partnerships with international funding agencies to implement translational research agendas. LAC‐CDs have succeeded in attracting funding and support from international organizations such as the Alzheimer's Association and the Global Brain Health Institute (or GBHI). These partnerships are providing a solid context to further expand its networking strategies.
The Alzheimer's Association has launched the AAIC Satellite Symposia, which is allowing researches from LACs to engage with the wider international community, especially early career researchers. Two have been held in LACs (Buenos Aires, Argentina in 2018 and Sao Paulo, Brazil, in 2019). Such platforms have proved invaluable to promote a regional integration.
9. Panel 3 SEARCH STRATEGY AND SELECTION CRITERIA
References for this work were identified by searching the PubMed database between 1997 and June 2020. The search terms combined relevant keywords (eg, “Latin America,” “Latin American countries,” “South America,” “Caribbean,” “Argentina,” “Bolivia,” “Brazil,” “Chile,” “Colombia,” “Costa Rica,” “Cuba,” “Dominican Republic,” “Ecuador,” “El Salvador,” “Guatemala,” “Honduras,” “Mexico,” “Nicaragua,” “Panama,” “Paraguay,” “Peru,” OR “Uruguay,” “Venezuela” each in combination (AND) with “aging,” “dementia” OR “neurodegeneration,” “neurodegenerative diseases,” “older adults,” “brain health,” “Alzheimer's disease,” “frontotemporal dementia,” “Parkinson's disease,” and “vascular dementia”). Only papers published in English, Portuguese, and Spanish were reviewed. Additional pertinent references related to the topics discussed were also selected by the authors and included in the manuscript.
CONFLICTS OF INTEREST
The Expert Meeting was supported by Alzheimer's Society UK grants awarded to MP in collaboration with AI (AS‐R42303, AS‐SF‐14‐008). AS is supported by CONICYT / FONDAP /15150012; Conicyt/ Fondecyt Regular/ 1140423 and Basal Funds for Centers of Excellence, Project FB 0003 from the Associative Research Program of CONICYT. FL is supported by a grant API COLOMBIA funded by GENENTECH and Banner Institute. FK is supported by a National Health and Medical Research Council of Australia (NHMRC)‐Australian Research Council Dementia Research Development Fellowship (APP1097026). OP is supported by an NHMRC Senior Research Fellowship (APP1103258). FFDO is supported by FAPESP ‐ The State of São Paulo Research Foundation (grant #2015/10109‐5). The support from Alzheimer´s Scotland Dementia Research and the Centre for Cognitive Ageing and Cognitive Epidemiology part of the cross council Lifelong Health and Wellbeing Initiative (MR/K026992/1) both from the University of Edinburgh is also acknowledged. FS is supported by Geroscience Center for Brain Health and Metabolism (FONDAP‐15150012) and Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT, No. 1150766). RAV is supported by CONICYT AFB 170008 and by Faculty of Sciences, University of Chile, PAIFAC grant. PC is supported by CNPq, Brazil (bolsa de produtividade em pesquisa). PL is supported by FONDAP Program Grant 15150012 & by Conicyt/Fondecyt Regular/ 1160940. STF is supported by National Institute for Translational Neuroscience (Brazil, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil) and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ/Brazil). BCB is supported by Alzheimer's Association and Global Brain Health Institute (GBHI_ALZ‐18‐542347). LTG is supported by NIH K24AG053435. MLFC is supported by CNPq (bolsa de produtividade em pesquisa). RDR is supported by FAPESP (2016/24326‐0) and the Alzheimer´s Association (AARF‐18‐566005). LD is partially supported by Neuromedicenter. RF is supported by Alzheimer's Society (grant # 284). LCS is supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil). MSY is supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil) and São Paulo Research Foundation (FAPESP/BRAZIL). MACB is supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil) and Minas Gerais Research Foundation (FAPEMIG/BRAZIL). IA receives National public grant level 2 from the National Council for Scientific and Technological Development (Ministry of Science, Technology, Innovation and Communications, Brazil). HMS is a full‐time employee of the Alzheimer's Association. AI is partially supported by grants from CONICET, FONCyT‐PICT 2017‐1818, FONCyT‐PICT 2017‐1820, FONDAP 15150012, the Interamerican Development Bank (IDB), Alzheimer's Association GBHI ALZ UK‐20‐639295, and the MULTI‐PARTNER CONSORTIUM TO EXPAND DEMENTIA RESEARCH IN LATIN AMERICA [ReDLat, supported by National Institutes of Health, National Institute on Aging (R01 AG057234), Alzheimer's Association (SG‐20‐725707), Tau Consortium, and Global Brain Health Institute)]. The contents of this publication are solely the responsibility of the authors and do not represent the official views of these Institutions. The other authors declare no conflict of interests.
AUTHORS CONTRIBUTION
Mario Alfredo Parra and Agustin Ibanez designed the proposal. Mario Alfredo Parra, Agustin I, Sandra Baez, Lucas Sedeno, Cecilia Gonzalez Campo, and Hernando Santamaria‐Garcia wrote the drafts, discussed contributions from all co‐authors, and approved the final version. Mario Alfredo Parra, Sandra Baez, Lucas Sedeno, Cecilia Gonzalez Campo, Hernando Santamaria‐Garcia, and Agustin Ibanez prepared Panels and Figures 1, 2, 3. All authors searched the literature, participated in discussing the contents of the paper, contributed to editing, and approved the final version of the article.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
The authors thank Laura Bleiler from the Alzheimer's Association (AA) for proofreading the article. The authors also thank the Inter‐American Development Bank (IDB) and the the MULTI‐PARTNER CONSORTIUM TO EXPAND DEMENTIA RESEARCH IN LATIN AMERICA [ReDLat, supported by National Institutes of Health, National Institutes of Aging (R01 AG057234), Alzheimer's Association (SG‐20‐725707), Tau Consortium, and Global Brain Health Institute] and the grant Alzheimer's Association GBHI ALZ UK‐20‐639295. The contents of this publication are solely the responsibility of the authors and do not represent the official views of these institutions.
Parra MA, Baez S, Sedeño L, et al. Dementia in Latin America: Paving the way towards a regional action plan. Alzheimer's Dement. 2021;17:295–313. 10.1002/alz.12202
Sandra Baez, Lucas Sedeño, Cecilia Gonzalez Campo, and, Hernando Santamaría‐García equal contribution to this study.
Footnotes
REFERENCES
- 1. Parra MA, Baez S, Allegri R, et al. Dementia in Latin America: assessing the present and envisioning the future. Neurology. 2018;90(5):222‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Addressing global dementia. Lancet. 2014;383(9936):2185. [DOI] [PubMed] [Google Scholar]
- 3. Alladi S, Hachinski V. World dementia: one approach does not fit all. Neurology. 2018;91(6):264‐270. [DOI] [PubMed] [Google Scholar]
- 4. Dartigues JF. Alzheimer's disease: a global challenge for the 21st century. Lancet Neurol. 2009;8(12):1082‐1083. [DOI] [PubMed] [Google Scholar]
- 5. Shah H, Albanese E, Duggan C, et al. Research priorities to reduce the global burden of dementia by 2025. Lancet Neurol. 2016;15(12):1285‐1294. [DOI] [PubMed] [Google Scholar]
- 6. Wu YT, Beiser AS, Breteler MMB, et al. The changing prevalence and incidence of dementia over time—current evidence. Nat Rev Neurol. 2017;13(6):327‐339. [DOI] [PubMed] [Google Scholar]
- 7. Aguirre‐Acevedo DC, Lopera F, Henao E, et al. Cognitive decline in a colombian kindred with autosomal dominant alzheimer disease: a retrospective cohort study. JAMA Neurol. 2016;73(4):431‐438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baez S, Ibanez A. Dementia in Latin America: an emergent silent tsunami. Front Aging Neurosci. 2016;8:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reitz C, Mayeux R. Genetics of Alzheimer's disease in Caribbean Hispanic and African American populations. Biol Psychiatry. 2014;75(7):534‐541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tosto G, Bird TD, Tsuang D, et al. Polygenic risk scores in familial Alzheimer disease. Neurology. 2017;88(12):1180‐1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ibanez A, Sedeno L, Garcia AM, Deacon RMJ, Cogram P. Editorial: human and animal models for translational research on neurodegeneration: challenges and opportunities from South America. Front Aging Neurosci. 2018;10:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nitrini R, Barbosa MT, Dozzi Brucki SM, Yassuda MS, Caramelli P. Current trends and challenges on dementia management and research in Latin America. J Glob Health. 2020;10(1):010362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ibanez A, Santamaria‐Garcia H, Barragan AG, et al. The impact of SARS‐CoV‐2 in dementia across Latin America: A call for an urgent regional plan and coordinated response. Alzheimer's Dement. 2020;e12092. 10.1002/trc2.12092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ibanez A, Kosik KS. COVID‐19 in older people with cognitive impairment in Latin America. Lancet Neurol. 2020;19(9):719‐721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Graham ID, Logan J, Harrison MB, et al. Lost in knowledge translation: time for a map? J Continuing Educ Health Professions. 2006;26(1):13‐24. [DOI] [PubMed] [Google Scholar]
- 16. Tiffany CR. Analysis of planned change theories. Nurs Manage. 1994;25(2):60‐62. [PubMed] [Google Scholar]
- 17. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673‐2734. [DOI] [PubMed] [Google Scholar]
- 18. Orgeta V, Mukadam N, Sommerlad A, Livingston G. The lancet commission on dementia prevention, intervention, and care: a call for action. Ir J Psychol Med. 2019;36(2):85‐88. [DOI] [PubMed] [Google Scholar]
- 19. Mukadam N, Sommerlad A, Huntley J, Livingston G. Population attributable fractions for risk factors for dementia in low‐income and middle‐income countries: an analysis using cross‐sectional survey data. Lancet Glob Health. 2019;7(5):e596‐e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oliveira D, Jun Otuyama L, Mabunda D, et al. Reducing the number of people with dementia through primary prevention in mozambique, brazil, and portugal: an analysis of population‐based data. J Alzheimer's Dis. 2019;70(s1):S283‐s291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de Oliveira FF, de Almeida SS, Chen ES, Smith MC, Naffah‐Mazzacoratti MDG, Bertolucci PHF. Lifetime risk factors for functional and cognitive outcomes in patients with Alzheimer's disease. J Alzheimer's Dis. 2018;65(4):1283‐1299. [DOI] [PubMed] [Google Scholar]
- 22. Oliveira FF, Chen ES, Smith MC, Bertolucci PH. Predictors of cognitive and functional decline in patients with Alzheimer disease dementia from Brazil. Alzheimer Dis Assoc Disord. 2016;30(3):243‐250. [DOI] [PubMed] [Google Scholar]
- 23. Scarmeas N, Stern Y. Cognitive reserve: implications for diagnosis and prevention of Alzheimer's disease. Curr Neurol Neurosci Rep. 2004;4(5):374‐380. Not in File. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kivipelto M, Mangialasche F, Snyder HM, et al. World‐wide FINGERS network: a global approach to risk reduction and prevention of dementia. Alzheimer's Dement. 2020;16(7):1078‐1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stern Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol. 2012;11(11):1006‐1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Parra MA, Butler S, McGeown WJ, Brown Nicholls LA, Robertson DJ. Globalising strategies to meet global challenges: the case of ageing and dementia. J Glob Health. 2019;9(2):020310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Custodio N, Wheelock A, Thumala D, Slachevsky A. Dementia in Latin America: epidemiological evidence and implications for public policy. review. Front Aging Neurosci. 2017;9(221). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Prince M, Acosta D, Ferri CP, et al. Dementia incidence and mortality in middle‐income countries, and associations with indicators of cognitive reserve: a 10/66 Dementia Research Group population‐based cohort study. Lancet. 2012;380(9836):50‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. The Lancet N . Latin America takes centre stage in dementia prevention. Lancet Neurol. 2020;19(9):711. [DOI] [PubMed] [Google Scholar]
- 30. Bertola L, Ávila RT, Bicalho MAC, Malloy‐Diniz LF. Semantic memory, but not education or intelligence, moderates cognitive aging: a cross‐sectional study. Braz J Psychiatry. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de Oliveira FF, Bertolucci PH, Chen ES, Smith MC. Risk factors for age at onset of dementia due to Alzheimer's disease in a sample of patients with low mean schooling from Sao Paulo, Brazil. Int J Geriatr Psychiatry. 2014;29(10):1033‐1039. [DOI] [PubMed] [Google Scholar]
- 32. Farfel JM, Nitrini R, Suemoto CK, et al. Very low levels of education and cognitive reserve: a clinicopathologic study. Neurology. 2013;81(7):650‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oliveira FF, Chen ES, Smith MC, Bertolucci PH. Associations of cerebrovascular metabolism genotypes with neuropsychiatric symptoms and age at onset of Alzheimer's disease dementia. Braz J Psychiatry. 2017;39(2):95‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ibáñez A, Sedeño L, García AM, Deacon RMJ, Cogram P. Human and Animal Models for Translational Research on Neurodegeneration: Challenges and Opportunities From South America. 2018. [DOI] [PMC free article] [PubMed]
- 35. Arboleda‐Velasquez JF, Lopera F, Lopez E, et al. C455R notch3 mutation in a Colombian CADASIL kindred with early onset of stroke. Neurology. 2002;59(2):277‐279. [DOI] [PubMed] [Google Scholar]
- 36. Cardona‐Gomez GP, Dementia Lopera F., Preclinical studies in neurodegeneration and its potential for translational medicine in South America. Front Aging Neurosci. 2016;8:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cornejo‐Olivas M, Torres L, Velit‐Salazar MR, et al. Variable frequency of LRRK2 variants in the Latin American research consortium on the genetics of Parkinson's disease (LARGE‐PD), a case of ancestry. NPJ Parkinson's Dis. 2017;3:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cornejo‐Olivas MR, Yu CE, Mazzetti P, et al. Clinical and molecular studies reveal a PSEN1 mutation (L153V) in a Peruvian family with early‐onset Alzheimer's disease. Neurosci Lett. 2014;563:140‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Giraldo M, Lopera F, Siniard AL, et al. Variants in triggering receptor expressed on myeloid cells 2 are associated with both behavioral variant frontotemporal lobar degeneration and Alzheimer's disease. Neurobiol Aging. 2013;34(8):2077.e11‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gomez‐Isla T, Growdon WB, McNamara MJ, et al. The impact of different presenilin 1 andpresenilin 2 mutations on amyloid deposition, neurofibrillary changes and neuronal loss in the familial Alzheimer's disease brain: evidence for other phenotype‐modifying factors. Brain. 1999;122(Pt 9):1709‐1719. [DOI] [PubMed] [Google Scholar]
- 41. Lee SE, Khazenzon AM, Trujillo AJ, et al. Altered network connectivity in frontotemporal dementia with C9orf72 hexanucleotide repeat expansion. Brain. 2014;137(Pt 11):3047‐3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lemere CA, Lopera F, Kosik KS, et al. The E280A presenilin 1 Alzheimer mutation produces increased A beta 42 deposition and severe cerebellar pathology. Nat Med. 1996;2(10):1146‐1150. [DOI] [PubMed] [Google Scholar]
- 43. Moreno DJ, Pino S, Rios A, et al. Genetic ancestry and susceptibility to late‐onset Alzheimer disease (LOAD) in the admixed colombian population. Alzheimer Dis Assoc Disord. 2017;31(3):225‐231. [DOI] [PubMed] [Google Scholar]
- 44. Norton DJ, Amariglio R, Protas H, et al. Subjective memory complaints in preclinical autosomal dominant Alzheimer disease. Neurology. 2017;89(14):1464‐1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Parra MA, Saarimaki H, Bastin ME, et al. Memory binding and white matter integrity in familial Alzheimer's disease. Brain. 2015;138(Pt 5):1355‐1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pastor P, Roe CM, Villegas A, et al. Apolipoprotein Eepsilon4 modifies Alzheimer's disease onset in an E280A PS1 kindred. Ann Neurol. 2003;54(2):163‐169. [DOI] [PubMed] [Google Scholar]
- 47. Pietto M, Parra MA, Trujillo N, et al. Behavioral and electrophysiological correlates of memory binding deficits in patients at different risk levels for Alzheimer's disease. J Alzheimer's Dis. 2016;53(4):1325‐1340. [DOI] [PubMed] [Google Scholar]
- 48. Quiroz YT, Budson AE, Celone K, et al. Hippocampal hyperactivation in presymptomatic familial Alzheimer's disease. Ann Neurol. 2010;68(6):865‐875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Quiroz YT, Sperling RA, Norton DJ, et al. Association between amyloid and tau accumulation in young adults with autosomal dominant Alzheimer disease. JAMA Neurol. 2018;75(5):548‐556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Roxburgh RH, Smith CO, Lim JG, Bachman DF, Byrd E, Bird TD. The unique co‐occurrence of spinocerebellar ataxia type 10 (SCA10) and Huntington disease. J Neurol Sci. 2013;324(1‐2):176‐178. [DOI] [PubMed] [Google Scholar]
- 51. Sepulveda‐Falla D, Barrera‐Ocampo A, Hagel C, et al. Familial Alzheimer's disease‐associated presenilin‐1 alters cerebellar activity and calcium homeostasis. J Clin Invest. 2014;124(4):1552‐1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Takada LT, Bahia VS, Guimaraes HC, et al. GRN and MAPT Mutations in 2 Frontotemporal Dementia Research Centers in Brazil. Alzheimer Dis Assoc Disord. 2016;30(4):310‐317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Takada LT, Pimentel ML, Dejesus‐Hernandez M, et al. Frontotemporal dementia in a Brazilian kindred with the c9orf72 mutation. Arch Neurol. 2012;69(9):1149‐1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Velez JI, Lopera F, Sepulveda‐Falla D, et al. APOE*E2 allele delays age of onset in PSEN1 E280A Alzheimer's disease. Mol Psychiatry. 2016;21(7):916‐924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Velez JI, Rivera D, Mastronardi CA, et al. A Mutation in DAOA Modifies the Age of Onset in PSEN1 E280A Alzheimer's Disease. Neural Plasticity. 2016;2016:9760314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zabetian CP, Mata IF. LARGE‐PD: Examining the genetics of Parkinson's disease in Latin America. Movement Disord. 2017;32(9):1330‐1331. [DOI] [PubMed] [Google Scholar]
- 57. Blue EE, Horimoto A, Mukherjee S, Wijsman EM, Thornton TA. Local ancestry at APOE modifies Alzheimer's disease risk in Caribbean Hispanics. Alzheimer's Dement. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Desikan RS, Fan CC, Wang Y, et al. Genetic assessment of age‐associated Alzheimer disease risk: development and validation of a polygenic hazard score. PLoS Med. 2017;14(3):e1002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lacour A, Espinosa A, Louwersheimer E, et al. Genome‐wide significant risk factors for Alzheimer's disease: role in progression to dementia due to Alzheimer's disease among subjects with mild cognitive impairment. Mol Psychiatry. 2017;22(1):153‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tan CH, Fan CC, Mormino EC, et al. Polygenic hazard score: an enrichment marker for Alzheimer's associated amyloid and tau deposition. Acta Neuropathol. 2018;135(1):85‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tan CH, Hyman BT, Tan JJX, et al. Polygenic hazard scores in preclinical Alzheimer disease. Ann Neurol. 2017;82(3):484‐488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Duncan L, Shen H, Gelaye B, et al. Analysis of polygenic risk score usage and performance in diverse human populations. Nat Commun. 2019;10(1):3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dalmasso MC, Brusco LI, Olivar N, et al. Transethnic meta‐analysis of rare coding variants in PLCG2, ABI3, and TREM2 supports their general contribution to Alzheimer's disease. Transl Psychiatry. 2019;9(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Resende EPF, Llibre Guerra JJ, Miller BL. Health and socioeconomic inequities as contributors to brain health. JAMA Neurol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Santamaría‐García H, Baez S, Gómez C, et al. The role of social cognition skills and social determinants of health in predicting symptoms of mental illness. Transl Psychiatry. 2020;10(1):165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Resende EPF, Llibre Guerra JJ, Miller BL. Health and socioeconomic inequities as contributors to brain health. JAMA Neurol. 2019;76(6):633‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Vega IE, Cabrera LY, Wygant CM, Velez‐Ortiz D, Counts SE. Alzheimer's disease in the latino community: intersection of genetics and social determinants of health. J Alzheimer's Dis. 2017;58(4):979‐992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Romieu I, Gouveia N, Cifuentes LA, et al. Multicity study of air pollution and mortality in Latin America (the ESCALA study). Res Rep Health Effects Institute. 2012;(171):5‐86. [PubMed] [Google Scholar]
- 69. Russ TC, Murianni L, Icaza G, Slachevsky A, Starr JM. Geographical variation in dementia mortality in Italy, New Zealand, and Chile: the Impact of latitude, vitamin D, and air pollution. Dement Geriatr Cogn Disord. 2016;42(1‐2):31‐41. [DOI] [PubMed] [Google Scholar]
- 70. Nitrini R, Bottino CM, Albala C, et al. Prevalence of dementia in Latin America: a collaborative study of population‐based cohorts. Int Psychogeriatrics. 2009;21(4):622‐630. Not in File. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. International AsD. World Alzheimer Report 2012: Overcoming the stigma of dementia. 2012. 2012. https://www.alz.co.uk/research/world-report-2012
- 72. Olavarría L, Mardones C, Delgado C, Slachevsky Ch A. Chilean healthcare professionals' perception of knowledge about dementia. Revista Medica De Chile. Oct 2016;144(10):1365‐1368. Percepción de conocimiento sobre las demencias en profesionales de la salud de Chile. [DOI] [PubMed] [Google Scholar]
- 73. Farina N, Suemoto CK, Burton JK, Oliveira D, Frost R. Perceptions of dementia amongst the general public across Latin America: a systematic review. Aging Mental Health. 2020;2020:1‐10. [DOI] [PubMed] [Google Scholar]
- 74. Ibanez A, Flichtentrei D, Hesse E, et al. The power of knowledge about dementia in Latin America across health professionals working on aging. Alzheimer's Dement; 2020;12:e12117. 10.1002/dad2.12117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Moraes Balbim G, Aguirre K, Zavala M, Davila K, Marquez D. Evaluation of public health messages to promote early detection of dementia among adult Latino children. Innov Aging. 2018;2(Suppl 1):931‐932. [Google Scholar]
- 76. Parra MA. Overcoming barriers in cognitive assessment of Alzheimer's disease. Dement Neuropsychol. 2014;8(2):95‐98. Not in File. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Warren LA, Shi Q, Young K, Borenstein A, Martiniuk A. Prevalence and incidence of dementia among indigenous populations: a systematic review. Int Psychogeriatr. 2015;27(12):1959‐1970. [DOI] [PubMed] [Google Scholar]
- 78. de Souza‐Talarico JN, de Carvalho AP, Brucki SMD, Nitrini R, Ferretti‐Rebustini REdL. Dementia and cognitive impairment prevalence and associated factors in indigenous populations: a systematic review. Alzheimer Dis Assoc Disord. 2016;30(3):281‐287. [DOI] [PubMed] [Google Scholar]
- 79. Jack CR, Jr. , Bennett DA, Blennow K, et al. NIA‐AA research framework: toward a biological definition of Alzheimer's disease. Alzheimer's Dement. 2018;14(4):535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Dubois B, Hampel H, Feldman HH, et al. Preclinical Alzheimer's disease: Definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12(3):292‐323. Not in File. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Jack CR, Bennett DA, Blennow K, et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87(5):539‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lee SAW, Sposato LA, Hachinski V, Cipriano LE. Cost‐effectiveness of cerebrospinal biomarkers for the diagnosis of Alzheimer's disease. Alzheimer's Res Ther. 2017;9(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Allegri RF, Chrem Méndez P, Calandri I, et al. Prognostic value of ATN Alzheimer biomarkers: 60‐month follow‐up results from the Argentine Alzheimer's Disease Neuroimaging Initiative. Alzheimer's Dement. 2020;12(1):e12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Grøntvedt GR, Lauridsen C, Berge G, et al. The Amyloid, Tau, and Neurodegeneration (A/T/N) Classification Applied to a Clinical Research Cohort with Long‐Term Follow‐Up. J Alzheimer's Dis. 2020;74(3):829‐837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Arboleda‐Velasquez JF, Lopera F, O'Hare M, et al. Resistance to autosomal dominant Alzheimer's disease in an APOE3 Christchurch homozygote: a case report. Nat Med. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Allegri RF, Bagnati P, Brucki S, Nitrini R. Chapter 13—South AmericaGÇÖs AD clinical trials experience: lessons learned from Argentina and Brazil A2—Bairu, Menghis. In: Weiner MW, ed. Global Clinical Trials for Alzheimer's Disease. Academic Press; 2014:219‐230. [Google Scholar]
- 87. Reiman EM, Langbaum JBS, Fleisher AS, et al. Alzheimer's Prevention Initiative: a plan to accelerate the evaluation of presymptomatic treatments. J Alzheimer's Dis. 2011;26 Suppl 3:321‐329. Not in File. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Fiestas F, Gallo C, Poletti G, et al. Improving mental and neurological health research in Latin America: a qualitative study. BMC Public Health. 2009;9(1):334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Moloney A. Latin America faces hurdles in health research. Lancet. 2009;374(9695):1053‐1054. [DOI] [PubMed] [Google Scholar]
- 90. Ibáñez A, Sedeño L, García AM, Deacon RMJ, Cogram P. Editorial: human and animal models for translational research on neurodegeneration: challenges and opportunities from South America. Editorial. Front Aging Neurosci. 2018;10(95). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Honig LS. Translational research in neurology: dementia. Arch Neurol. 2012;69(8):969‐977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lunn JS, Sakowski SA, Hur J, Feldman EL. Stem cell technology for neurodegenerative diseases. Ann Neurol. 2011;70(3):353‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Bockholt HJ, Scully M, Courtney W, et al. Mining the mind research network: a novel framework for exploring large scale, heterogeneous translational neuroscience research data sources. Front Neuroinformatics. 2010;3:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Brueggen K, Grothe MJ, Dyrba M, et al. The European DTI Study on Dementia ‐ A multicenter DTI and MRI study on Alzheimer's disease and Mild Cognitive Impairment. NeuroImage. 2017;144(Pt B):305‐308. [DOI] [PubMed] [Google Scholar]
- 95. Rodgers PA. Co‐designing with people living with dementia. CoDesign. 2018;14(3):188‐202. [Google Scholar]
- 96. Uzochukwu B, Onwujekwe O, Mbachu C, et al. The challenge of bridging the gap between researchers and policy makers: experiences of a Health Policy Research Group in engaging policy makers to support evidence informed policy making in Nigeria. Glob Health. 2016;12(1):67‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. International AsD. From plan to impact: Progress towards targets of the global action plan on dementia. 2018.
- 98. International AsD. From Plan to Impact II: The urgent need for action. 2019;
- 99. Borelli WV, Carmona KC, Studart‐Neto A, Nitrini R, Caramelli P, da Costa JC. Operationalized definition of older adults with high cognitive performance. Dement Neuropsychol. 2018;12(3):221‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Suemoto CK, Nitrini R, Grinberg LT, et al. Atherosclerosis and dementia: a cross‐sectional study with pathological analysis of the carotid arteries. Stroke. 2011;42(12):3614‐3615. [DOI] [PubMed] [Google Scholar]
- 101. Co‐operation OfE, Development. Behavioural Insights and Public Policy: Lessons from Around the World: OECD. 2017.
- 102. Ramirez Aguilar L, Acosta‐Uribe J, Giraldo MM, et al. Genetic origin of a large family with a novel PSEN1 mutation (Ile416Thr). Alzheimer's Dement. 2019;15(5):709‐719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. New manual aims to create common standards for dementia diagnosis across Latin America. Alzheimer's Dement. 2020;7(16):1099. [DOI] [PubMed] [Google Scholar]
- 104. Evans S, Atkinson T. An intergenerational approach to addressing stigma. Innov Aging. 2017;1(suppl_1):694‐694. [Google Scholar]
- 105. Belbin GM, Nieves‐Colon MA, Kenny EE, Moreno‐Estrada A, Gignoux CR. Genetic diversity in populations across Latin America: implications for population and medical genetic studies. Curr Opin Genet Development. 2018;53:98‐104. [DOI] [PubMed] [Google Scholar]
- 106. Conley AB, Rishishwar L, Norris ET, et al. A Comparative analysis of genetic ancestry and admixture in the colombian populations of choco and medellin. G3 (Bethesda, Md). 2017;7(10):3435‐3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Norris ET, Wang L, Conley AB, et al. Genetic ancestry, admixture and health determinants in Latin America. BMC Genomics. 2018;19(Suppl 8):861‐861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Donnelly‐Kehoe PA, Pascariello GO, García AM, et al. Robust automated computational approach for classifying frontotemporal neurodegeneration: Multimodal/multicenter neuroimaging. Alzheimers Dement (Amst). 2019;11:588‐598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. García‐Cordero I, et al. Feeling, learning from, and being aware of inner states: Interoceptive dimensions in neurodegeneration and stroke. Philos Trans R Soc Lond B Biol Sci. 2016;371(1708):20160006; Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Melloni M, Billeke P, Baez S, et al. Your perspective and my benefit: multiple lesion models of self‐other integration strategies during social bargaining. Brain. 2016;139(11):3022‐3040. [DOI] [PubMed] [Google Scholar]
- 111. Khachaturian AS, Meranus DH, Kukull WA, Khachaturian ZS. Big data, aging, and dementia: pathways for international harmonization on data sharing. Alzheimer's Dement. 2013;9(5 Suppl):S61‐S62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


