Abstract
The current COVID-19 pandemic is caused by the severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2). By late October 2020, more than 43 million cases of infections, including over 1.15 million deaths, have been confirmed worldwide. This review focuses on our current understanding of SARS-CoV-2 from the perspective of the three-dimensional (3D) structures of SARS-CoV-2 viral proteins and their implications on therapeutics development against COVID-19.
Keywords: Structure, SARS-CoV-2, Spike protein, Main protease, RdRp, Nsp
1. Introduction
SARS-CoV-2 is a member of the Betacoronavirus genus that also includes severe acute respiratory syndrome coronavirus (SARS-CoV), murine hepatitis coronavirus (MHV) and Middle East respiratory syndrome coronavirus (MERS-CoV), etc. The single-stranded positive-sense RNA genome of SARS-CoV-2 is about 30 kb and consists of six major open reading frames (ORFs) and several other accessory genes (Fig. 1 A). Polyprotein (pp)1a/1ab, translated from ORF1a and ORF1b, are cleaved into 16 non-structural proteins (nsps) through the collaboration of papain-like protease (PLpro) and main protease (Mpro) (Fig. 1B). The remaining of the genome are translated to four major structural proteins: Spike (S), Envelope (E), Membrane (M), and Nucleocapsid (N) proteins and several accessory proteins (Fig. 1A) (Lu et al., 2020, Zhou et al., 2020b, Zhu et al., 2020).
Fig. 1.
Schematic diagram of the SARS-CoV-2 genome. (A) SARS-CoV-2 genome. (B) Pp1a, polyprotein 1a; pp1ab, polyprotein 1ab. Proteins with no reported 3D structures are in grey.
Since the SARS-CoV-2 genome was sequenced, scientists all over the world have raced to solve the structures of SARS-CoV-2 proteins in order to reveal the structural basis of the SARS-CoV-2 viral infection, replication and assembly. We summarized here our current knowledge of their structures, especially the key drug targets, including S protein, Mpro, RNA-dependent RNA polymerase (RdRp), and their implications in therapeutics development.
2. Structures of SARS-CoV-2 proteins and their implications in therapeutics development
2.1. S protein
The infection mechanisms of the SARS-CoV-2 have been extensively studied. For all enveloped viruses, membrane fusion is considered to play a key role in the viral infection through a dramatic conformational change of the S protein. The S protein belongs to the class I fusion glycoproteins which mediate the attachment of virus to a variety of cell-surface receptors and subsequent fusion between viral and host cell surface (Belouzard et al., 2012). The SARS-CoV-2 S protein is a homotrimer, and each protomer consists of S1 and S2 subunits. S1 folds into 4 subdomains which are N-terminal domain (NTD), receptor binding domain (RBD), C-terminal domain 1 (CTD1) and C-terminal domain 2 (CTD2) to protect the trimerized pre-fusion S2 core. S2 includes fusion peptide (FP), heptad repeat 1 (HR1), central helix (CH), connector domain (CD), heptad repeat 2 (HR2), transmembrane domain (TM) and C-terminal domain (CT) (Fig. 2 A) (Wrapp et al., 2020b).
Fig. 2.
SARS-CoV-2 S protein in different conformations. (A) Domain structure of the full-length SARS-CoV-2 S protein. (B) Structural model of SARS-CoV-2 S trimer. Three protomers are colored in green, blue and purple, with A in “RBD-erect” and B, C in “RBD-down” conformations, respectively (PDB code: 6VSB). (C) Structural components of SARS-CoV-2 S trimer in the same color scheme as Fig. 2A. The S1/S2 cleavage site and the S2’ cleavage site are also indicated (PDB code: 6VSB). (D) Structural rearrangement in the process of membrane fusion. From the left to right are the S trimer in closed conformation (PDB code: 6VXX), the 1 RBD “erect” conformation (6VSB), and ACE2 bounded S protein. After the S1 dissociation, S2 components were exposed and the post-fusion intermediate was sequentially formed. Finally, folding back of HR2 formed the post-fusion conformation (PBD code: 6XRA) and brings the viral envelope close to the host cell membrane. The ACE2 bounded S obtained by superimposed with ACE2/RBD complex. The conformations masked in gray were modelled to illustrate the proposed membrane fusion process. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.1.1. Mechanism of membrane fusion through the S protein
SARS-CoV-2 uses human angiotensin converting enzyme 2 (ACE2) as the key cellular receptor to enter cells, the same as SARS-CoV (Letko et al., 2020, Zhou et al., 2020b). Structures of the RBD of SARS-CoV-2 S protein in complex with ACE2 were determined in the early stage of the epidemic (Lan et al., 2020, Shang et al., 2020b, Wang et al., 2020c, Yan et al., 2020), which showed how ACE2 might mediate SARS-CoV-2 entry into cells, and explained SARS-CoV-2’s higher affinity for ACE2 compared to SARS-CoV. Another study revealed heparan sulfate as a key element for SARS-CoV-2 infection, which interacted with RBD and promoted the S-ACE2 interaction (Clausen et al., 2020).
These structural studies provide a first look at how the virus is recognized by the host cell, but still cannot explain the process of membrane fusion. The S protein is originally produced as a trimerized precursor and cleaved into the receptor binding subunit and membrane fusion subunit at S1/S2 site (Belouzard et al., 2012). Compared to SARS-CoV S protein, SARS-CoV-2 S harbors a four amino acids insertion at the S1/S2 cleavage site which can be cleaved during biosynthesis by the proprotein convertase furin (Walls et al., 2020). This process decreases the stability of SARS-CoV-2 S and facilitates the S trimer to the open conformation which is required for ACE2 binding (Wrobel et al., 2020). In addition, the highly cleavable S1/S2 site is required for efficient proteolysis of the S proteins and cell-virus fusion, as well as reducing the viral dependence on target cell proteases for cell entry (Hoffmann et al., 2020a, Shang et al., 2020a). Furthermore, SARS-CoV-2 is proteolytically cleaved at the S2’ site by a serine like protease TMPRSS2 to facilitate the membrane fusion (Hoffmann et al., 2020b).
In order to clarify the process of membrane fusion and further guide the development of vaccines and therapeutics, researchers have made great efforts to determine the structure of the S trimer protein. Earlier structures of the SARS-CoV-2 S ectodomain protein in pre-fusion conformation were determined by single-particle Cryo-Electron Microscopy (Cryo-EM), with S trimer proteins either in closed conformation or 1 RBD “erect” conformation (Fig. 2B, 2C) (Walls et al., 2020, Wrapp et al., 2020b). Comparison of pre- and post-fusion conformation of SARS-CoV-2 S protein reveals that S protein undergoes a dramatically conformational change in the process of membrane fusion include dissociation of S1 fragment and refolding of S2 fragment (Fig. 2D). Cryo-EM study of furin-cleaved SARS-CoV-2 S mixed with ACE2 showed that ACE2 binding events destabilize the S trimer. The rearrangement of S1 domain following ACE2 binding disrupts interactions between S1 and S2, which un-shields trimeric S2 core and facilitates exposure of second proteolytic site (S2’) (Benton et al., 2020). Cleavage at S2’ site unleashes the constraints on the fusion peptide (FP) which is required for the fusion peptide release. Then the HR1 region of S2 fragment undergoes a “jackknife” transition, that allows the FP insertion into the host cell membrane which forms the fusion intermediate state, and the folding back of HR2 brings the viral envelope close to the host cell membrane and leads to the membrane fusion (Cai et al., 2020).
2.1.2. Conformational distributions of SARS-CoV-2 S protein
Because the RBD “erect” conformation is required for S protein mediated fusion of the virion membrane with the host cell membrane (Tortorici and Veesler, 2019), the conformational distribution of S trimer proteins is considered as an important feature to evaluate the viral infectivity. Based on single-particle cryo-EM data, it was proposed that about 50–70% of the particles from the cryo-EM data belong to the RBD “erect” conformations (Walls et al., 2020, Wrapp et al., 2020b). However, some researchers doubted that virus concentration, protein purification and other factors may lead to the artifacts of the S protein conformational distribution. Consequently, the structure and conformational distribution of S trimers in situ on the virion surface were studied using the Cryo-Electron Tomography (Cryo-ET) method. It was shown that about 73% of S trimers were in the pre-cleaved form on the virion surface, with 97% of S trimers in the pre-fusion state, and 3% in the post-fusion state. For the pre-fusion S trimers, 31% of them were in closed conformation, 55% in 1 RBD “erect” conformation and 14% with 2 RBDs “erect” conformation, which indicated that the opening of the RBD observed in recombinant S trimers was largely consistent with S trimers on the virion (Ke et al., 2020). The Cryo-ET structure of the authentic SARS-CoV-2 virus also showed that the native structures of S trimer proteins were mainly in closed, 1 RBD “erect” and post-fusion conformations (Yao et al., 2020). However, there are still factors that may affect the conformational distributions of SARS-CoV-2 S trimers like some mutations in the key sites and the influence of ligands.
Many subtypes of SARS-CoV-2 viruses with various mutations were involved in COVID-19 (Morais et al., 2020). Among them, the D614G mutation in the S protein renders the virus higher infectivity and predominates in the infected population over time (Daniloski et al., 2020, Yurkovetskiy et al., 2020). The D614G mutation likely enhances the infectivity of the virus by reducing S1 shedding and increasing total S protein incorporation into the virion (Zhang et al., 2020b). Consistently, structural studies showed that D614 formed a salt bridge with K854 (Cai et al., 2020) and formed hydrogen bonds with the T859 side chain in the adjacent protomer (Yurkovetskiy et al., 2020). Therefore, the D614G mutation weakens the stability of the CTD and the S trimer and increases the probability of open conformation SARS-CoV-2 S trimers, while only 5% of the particles of the D614G S trimers were in closed conformation (Yurkovetskiy et al., 2020).
In addition to D614G mutation, binding of heparan sulfate to RBD also shifts the S structure to opening conformation and facilitate the ACE2 binding (Clausen et al., 2020). Furthermore, free fatty acid linoleic acid (LA) can also change the conformational distribution of SARS-CoV-2 S protein. In the presence of LA, the probability of closed conformation of SARS-CoV-2 S protein increases to 70%, which indicates that LA binding stabilizes the closed conformation and may reduce the S-ACE2 interaction and infection (Toelzer et al., 2020).
2.1.3. Antibodies and therapeutics targeting the S protein
Discovering the structural details of antibodies in complex with S protein of SARS-CoV-2 is critical for vaccine design and immunotherapeutic development. CR3022 is the first structurally characterized antibody, previously isolated from the plasma of convalescent SARS individuals, and interacts with the RBD domain of SARS-CoV-2 S protein (Yuan et al., 2020b). Unfortunately, although it could interact with SARS-CoV S, CR3022 could not effectively neutralize SARS-CoV-2. The crystal structure shows that CR3022 targets a highly conserved epitope from RBD, and enables cross-reactive binding between SARS-CoV and SARS-CoV-2. However, the epitope can only be accessed by CR3022 when at least 2 RBDs on the trimeric S protein are in the erect conformation, which is rare for SARS-CoV-2 (Yuan et al., 2020b).
Numerous antibodies have been structurally characterized that contains many distinct epitopes, including various regions in the RBD at different S conformations, as well as many different non-RBD epitopes such like NTD (Barnes et al., 2020a, Barnes et al., 2020b, Brouwer et al., 2020, Chi et al., 2020). These antibodies can be classified into four categories by their neutralizing mechanisms: (I) those target the RBD and neutralize SARS-CoV-2 by competing with ACE2; (II) those target the RBD and lock the S protein in the closed conformation; (III) those bind to epitopes outside the RBD; (IV) those presumably neutralize virus through the unclear mechanism. Class I is the major category including most of the neutralizing antibodies, all of which recognize the epitopes around the top of the RBD and occludes ACE2 binding, even though their epitopes are slightly different (Fig. 3 A, 3B) (Brouwer et al., 2020, Cao et al., 2020b, Du et al., 2020, Hansen et al., 2020, Ju et al., 2020, Lv et al., 2020, Shi et al., 2020, Wec et al., 2020, Yuan et al., 2020a, Zhou et al., 2020a). Class II antibodies behave like a lock that fixes the S trimers in the closed conformation and blocks ACE2 interaction (Fig. 3C) (Liu et al., 2020, Tortorici et al., 2020). Class III antibodies bind to regions outside RBD, such as NTD of the S protein. They may restrain conformational changes of the S protein needed for membrane fusion (Fig. 3D) (Chi et al., 2020, Liu et al., 2020). Class IV antibodies recognize epitopes also on the RBD of SARS-CoV-2 S but far away from the ACE2 binding site and generate no collision with ACE2. The underlying neutralizing mechanism of Class IV antibodies awaits further investigation (Fig. 3E) (Pinto et al., 2020).
Fig. 3.
Antibodies targeting the S protein of SARS-CoV-2. The PDB codes of related structures are listed. (A)The complex structure of human ACE2 shown as orange cartoon and SARS-CoV-2 Spike RBD shown as gray surface. (B) Antibodies targeting RBD and neutralizing SARS-CoV-2 by competing with ACE2 (Class I). (C) Antibodies neutralizing SARS-CoV-2 by locking the S protein in closed conformation (Class II). (D) Antibody binding to the epitopes outside the RBD (Class III). (E) Antibody targeting RBD and neutralizing SARS-CoV-2 without steric hindrance to ACE2 (Class IV). (F) Antibody cocktails which can potently neutralizing SARS-CoV-2 and successfully rescue mutation-induced neutralization escapes. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Antibodies targeting the S protein of SARS-CoV-2 especially the RBD are broadly expected to play a key role in combating the COVID-19 pandemic. However, mutations in RBD rapidly appeared following the viral passaging, and resulted in the loss of antibody neutralization activity. Consequently, researchers reckon that a therapeutic antibody cocktail would have the potential to decrease the influence by virus mutations. The ideal antibody cocktail partners should bind to non-overlapping epitopes on the S protein which can prevent the virus escape mutants (Baum et al., 2020). For example, REGN10933 and REGN10987 have great potential to form an antibody cocktail (Hansen et al., 2020) as well as BD-368-2/BD-629 which can also successfully rescue mutation-induced neutralization escapes (Fig. 3F) (Du et al., 2020).
Besides the antibodies, researchers discovered many nanobodies that potently disrupt the cell entry of SARS-CoV-2. There are five structurally characterized nanobodies: three of them bind to the top of RBD and block the interaction with ACE2, function like the Class I antibodies (Hanke et al., 2020, Huo et al., 2020); another one locks the S protein in the closed conformation like the Class II antibodies (Schoof et al., 2020); the last one is similar with Class IV antibodies, the epitope of which is on the side of RBD and far away from ACE2 binding site (Wrapp et al., 2020a).
In addition to antibodies and nanobodies, de novo protein design, a rapidly developing interdisciplinary field (Huang et al., 2016), has also brought new hopes to the therapeutics. Based on the structures of ACE2 and SARS-CoV-2 S protein, researchers used two major strategies to de novo design of RBD binders which can block the interaction with ACE2: (1) Incorporating the major elements of ACE2 which form the interactions with RBD into small proteins and redesigning the small proteins to attain higher neutralizing ability to the RBD (Cao et al., 2020a, Huang et al., 2020, Linsky et al., 2020); (2) Docking the RBD against a previously generated mini-protein libraries (Chevalier et al., 2017) to find new binding patterns, subsequently the sequences of the target mini-proteins are designed to optimize the binding affinity, neutralizing ability and stability (Cao et al., 2020a). These de novo designed mini-proteins have provided many great starting points for the SARS-CoV-2 therapeutics.
2.2. Mpro
Mpro (also known as 3CLpro) with 3C-like proteinase activity from coronavirus is a cysteine protease. The genomic Mpro of SARS-CoV-2 is located at nsp5 region in pp1a/pp1ab. Its prominent function is to cleave pp1a and pp1ab at 11 or more sites for viral replication, transcription and immune regulation (Kiemer et al., 2004). It cleaves the polyproteins mostly at the L-Q↓-(S/A/G) site. The lack of a similar protease in human ensures relatively low toxicity of the Mpro inhibitors (Zhang et al., 2020c), making the Mpro of SARS-CoV-2 an attractive drug target against SARS-CoV-2. The Mpro of SARS-CoV-2 shares over 96% sequence homology with SARS-CoV (Jin et al., 2020a), despite the fact that their associated viral behavior and pathological performance vary significantly (Lu et al., 2020).
Structural analyses have been performed with SARS-CoV-2 Mpro and its complexes with various ligands for drug discovery. SARS-CoV-2 Mpro is composed of three domains. Domain I and II contain six-strand antiparallel β barrels, and domain III contains five helices (Fig. 4 A) (Dai et al., 2020, Jin et al., 2020a, Zhang et al., 2020c). In the absence of ligands, active SARS-CoV-2 Mpro forms homodimers mainly through interactions between the domain III of the two protomers, which is critical for its catalytic activity by forming substrate-binding pocket (Fig. 4B) (Dai et al., 2020, Kneller et al., 2020, Zhang et al., 2020c). The Cys145 and His41 catalytic dyad and the substrate binding site are located in the cleft between domains I and II (Fig. 4A) (Dai et al., 2020, Jin et al., 2020a, Zhang et al., 2020c). Structural comparison revealed that residues Thr285 and Ile286 in SARS-CoV Mpro were replaced by Ala and Leu in SARS-CoV-2 respectively, which resulted in a more closed conformation of the two domain III in the Mpro dimer and may explain the increased catalytic activity in SARS-CoV-2 Mpro (Zhang et al., 2020c).
Fig. 4.
Structures of Mpro and structures of RdRp complex. (A) Cartoon representation of the Mpro protomer in color scheme. The catalytic dyad that located in the cleft between domain I and domain II are shown as hotpink sticks. (B) Surface representation of the Mpro homodimer. Protomer A is in pink, and protomer B is in cyan. The PDB codes of the related structures are listed. (C) The overall structure of the RdRp complex in color scheme. The PDB codes of the related structures are listed. (D) Illustration of the inhibitory mechanism of Remdesivir based on structural modeling. The RDV-MP and Ser861 are shown as sticks. A steric clash between the incorporated RDV-MP and Ser861 would prevent the incorporation of the nucleic acid at position “i + 4”. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
To discover lead compounds, the crystal structure of SARS-CoV-2 Mpro with the broad-spectrum antiviral inhibitor N3 were determined and elucidated their binding details (Jin et al., 2020a), and the binding mode was similar to these of SARS-CoV-2 Mpro with other ligands (Dai et al., 2020). In the symmetric complex composed of two Mpro protomers and one N3 inhibitor, N3 binds covalently with Cys145 in the substrate binding pocket, and its kinetic analysis confirmed an irreversible inhibitory mechanism (Jin et al., 2020a). Furthermore, researchers turned attention to designing and modifying specific compounds based on structures and worked on repurposing approved drugs or operating high-throughput screening from natural compounds or databases. For example, the covalent interactions between Mpro and antineoplastic carmofur were revealed with crystal structure (Jin et al., 2020b).
Since the studies mentioned above, structural studies of SARS-CoV-2 Mpro interacting with different types of inhibitors such as covalent, non-covalent or non-peptide inhibitors, continue to emerge, according to the PDB database (https://www.rcsb.org/). These studies have provided deep insights into structure-based drug design targeting Mpro.
2.3. RdRp
The genome of SARS-CoV-2 consists of a single-stranded positive-sense RNA, which is directly translated by host cell factors (Faheem et al., 2020). In contrast, the viral nsp12 protein, also knowns as RdRp, acts as the core component in RNA synthesis instead of host polymerases (Eskier et al., 2020). Cofactors nsp7/nsp8 are required for full function to promote the binding between nsp12 and RNA template (Yin et al., 2020).
The complex structures of nsp12/nsp7/nsp8 of SARS-CoV-2 have been determined (Fig. 4C) (Gao et al., 2020b, Yin et al., 2020). The first successfully determined cryo-EM structure of SARS-CoV-2 nsp12/nsp7/nsp8 complex (PDB: 6 M71; 7BTF), showed the complex structure of nsp12 with one nsp7-nsp8 pair and one nsp8 monomer. Compared with the nsp8 in the nsp7-nsp8 pair, the conformation of the nsp8 monomer was shifted. The nsp7-nsp8 pair was similar to the SARS-CoV nsp7-nsp8 pair, which showed the conservation between them. In addition, a unique “hairpin” domain at N terminal of RdRp was discovered, which stabilized the overall structure by forming a set of close contacts (Gao et al., 2020b). To investigate the specific function of cofactors, conformations of the complex in the stable extension stage were captured (PDB code: 6YYT; 7C2K). N-terminal regions of the two nsp8 subunits formed long α-helical extensions flanking exiting RNA duplex, which offered a place on upstream exist pathway for product RNA. The processivity of RdRp may be explained by the extension conformation of nsp8 and its interactions with RNA (Hillen et al., 2020, Wang et al., 2020b).
Since RdRp plays a vital role in the life cycle of SARS-CoV-2, it is proposed to be the target of a class of antiviral drugs that are nucleotide analogues, including Remdesivir. Remdesivir is an investigational nucleotide prodrug of a 1′-cyano-substituted adenosine analog, which was initially developed for the treatment of Ebola virus. Although Remdesivir failed in a clinical trial during an Ebola outbreak, it has been recognized as a promising drug against RNA viruses including SARS-CoV, MERS-CoV and SARS-CoV-2 (Pardo et al., 2020, Wang et al., 2020a). Structural studies were performed with RdRp in complex with RNA and Remdesivir, because Remdesivir in triphosphate (RDV-TP) format can compete with ATP and be incorporated into synthesis complex in Remdesivir monophosphate (RDV-MP) format. Structure and sequence alignments suggest that the mechanism of template RNA recognition and Remdesivir inhibition is highly conserved in multiple RdRp complexes from various RNA viruses. To gain insight into the mechanism of SARS-CoV-2 RNA replication and its inhibition by Remdesivir, 3D structures of SARS-CoV-2 RdRp-RNA complex with incorporated RDV-MP (PDB:7C2K; 7BV2) were determined (Wang et al., 2020b, Yin et al., 2020). Combined with biochemical evidence, the mechanism of Remdesivir-mediated RNA synthesis inhibition was proposed. Despite different structure resolutions and different RDV-MP insertion states, both studies agreed with previously proposed delayed chain termination hypothesis. The incorporation of RDV-MP into the product RNA chain at position “i” would cause the termination of the RNA synthesis at position “i + 3”, because the 1’–CN substituent of the incorporated RDV-MP would encounter a steric clash with the sidechain of Ser861 in RdRp and hamper further translocation, probably leading to a significant distortion of the positioning of the RNA and intercepting RNA replication (Fig. 4D) (Gordon et al., 2020a, Wang et al., 2020b, Yin et al., 2020).
The structural studies suggest the therapeutic potential of nucleotide analogues. The efficiency of incorporation of RDV-TP with its natural counterpart ATP and other RdRp inhibitors were compared, showing Favipiravir triphosphate and Ribavirin triphosphate were less well-incorporated by SARS-CoV-2 RdRp (Gordon et al., 2020a). Another nucleotide analog with broad-spectrum antiviral activity against SARS-CoV-2, MERS-CoV and SARS-CoV, β-D-N4-hydroxycytidine (NHC; EIDD-1931), showed increased potency against coronaviruses bearing resistance mutations to Remdesivir (Sheahan et al., 2020).
In addition to screening therapeutic drugs through experimental methods, computational approaches targeting RdRp complex catalytic cavity (including Asp760, and Asp761) were also wildly used in potential antiviral drug screening and design (Elfiky, 2020, Iftikhar et al., 2020).
2.4. Other potential drug targets with known structures
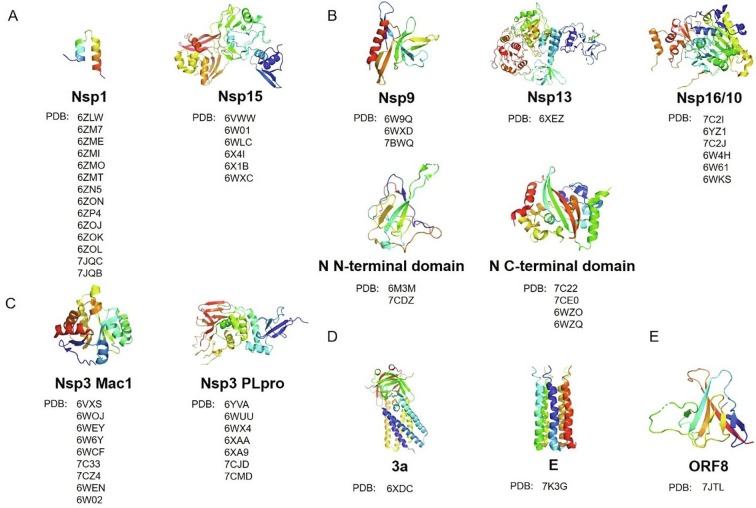
Although S, Mpro, and RdRp proteins are the most essential drug targets against SARS-CoV-2, extending the range of drug targets may expedite the process of therapeutic discovery and expand the diversity of available drugs, which may alleviate the probable antiviral drug-resistance problem. Here we summarize the current structural knowledge of other potential drug targets of SARS-CoV-2 as follows. They were grouped based on their functions (Fig. 5 ).
Fig. 5.
Representative structures of other potential drug targets shown in cartoon with gradient colors, and the lists of PDB codes of their related structures. (A) Representative structures of proteins that involve in host response suppression. (B) Representative structures of proteins that involve in replication, transcription, and RNA capping. (C) Representative structures of Mac1 and PLpro domains of nsp3, which involves in double-membrane vesicles formation. (D) Representative structures of proteins that involve in putative ion channels. (E) Representative structure of ORF8.
2.4.1. Host response suppression
Nsp1 proteins are the major virulence factors of alpha- and beta-coronavirus that show similar biological functions in suppressing host gene expression, facilitating efficient viral replication and immune evasion. SARS-CoV-2 nsp1 shows 84% amino acid sequence identity with SARS-CoV. Structural analysis of nsp1-40S ribosomal subunit complex revealed that the C terminus of nsp1, comprising two α-helices and two short loops, locates close to the “latch” between the head and body domains of the 40S ribosomal subunit and obstructs the mRNA entry channel, effectively blocking RIG-I-dependent innate immune responses (Fig. 5A) (Schubert et al., 2020, Thoms et al., 2020, Yuan et al., 2020c). Therefore, the structural characterization of the inhibitory mechanism of nsp1, provided a valuable insight into viral protein synthesis, and may aid structure-based drug design against SARS-CoV-2.
Nsp15 is a uridylate-specific endoribonuclease that preferentially cleaves both double- and single-stranded RNA substrates 3′ of uridines with different efficiencies, regulating the viral RNA to make it escape from the host’s immune recognition to evade antiviral defense of the host cell (Deng et al., 2019, Hackbart et al., 2020). The UTP bound structure of SARS-CoV-2 nsp15 showed it as a hexamer consisting of a dimer of trimers with key residues in the active site, which can recognize uridine and promote the catalysis of phosphodiester bonds, making the uridine derivatives like Tipiracil plausible therapeutic candidates (Fig. 5A) (Kim et al., 2020a, Kim et al., 2020b).
2.4.2. Replication, transcription, and RNA capping
The RdRp complex works with a number of accessory factors during the viral life cycle including nsp9 and key factors including nsp13. Nsp9 is a single-stranded RNA-binding subunit that assists the RNA-dependent RNA replicase machinery of coronaviruses to replicate and transcribe viral RNA. SARS-CoV-2 nsp9 shares a 97% amino acid sequence identity with SARS-CoV (Zhang et al., 2020a). The crystal structure of SARS-CoV-2 nsp9 is a horseshoe-shaped tetramer with two stable dimeric interfaces, providing a structural basis for understanding the self-assembly mechanism of RNA binding proteins (Fig. 5B) (Zhang et al., 2020a). However, it remains to be investigated that how nsp9 dimerization may affect interactions with other replicase machinery subunits. In addition, there is a peptide binding site close to the dimer interface, but the identity and binding affinity of the peptide need to be determined (Littler et al., 2020, Zhang et al., 2020a). Several molecules, such as conivaptan and telmisartan, were identified as potent inhibitors using a computational approach of structure-based drug repurposing, and the efficacy of these compounds needs further verification (Chandel et al., 2020).
Nsp13 is a member of superfamily 1B helicase that has RNA and DNA duplex-unwinding activities with 5′-to-3′ polarity using the energy of nucleotide hydrolysis. In addition to helicase activity, nsp13 also has RNA 5′-triphosphatase activity, which may play an important role in formation of the 5′ cap structure of viral RNA. The SARS-CoV2 nsp13 shares a 99.8% sequence identity with SARS-CoV with only one different residue (White et al., 2020). Biochemical and biophysical studies suggested that nsp13 and nsp12 can work together to improve the efficiency of viral replication. Structural studies showed that nsp13 binds to RdRp complex with NTPase domain sitting in front of the replication-transcription complex (RTC), making the RTC dynamically retrospective for proofreading or changing template during the process of RNA transcription by backtracking. In this model, RdRp complex and nsp13 translocate on the same template RNA strand but in the opposite direction. When the translocation of nsp13 is dominant, which might push the RdRp backward on the template RNA, but the model needs to be verified experimentally (Fig. 5B) (Chen et al., 2020). Simeprevir, paritaprevir and grazoprevir were identified as potential inhibitors by a comparative homology modelling approach, and more biological assays needs to further confirm the finding (Gurung, 2020).
After the replication and transcription process, the RNA transcripts are further capped at the 5′ end. The capping of mRNA involves nsp13, nsp14, and nsp16/nsp10, which promotes initiation of translation, protects mRNAs and helps virus hijack the host's translation machinery to produce viral proteins. The cap is added to the 5′ hydroxyl group of first nucleotide of the growing mRNA strand in four sequential steps (Bouvet et al., 2010). Firstly, the 5′ triphosphate group is hydrolyzed by the triphosphatase nsp13 to make the diphosphate-RNA. Secondly, guanylic acid is added by unknown guanylyltransferase to produce the guanosine cap. Thirdly, a methyl group is transferred by the methyltransferase nsp14 to the guanosine cap to yield 7-methylguanosine cap. Lastly, nsp16/nsp10 complex, a cap ribose 2′-O-RNA methyltransferase, uses S-adenosyl methionine (SAM) as substrate to methylate the first nucleotide of the nascent mRNA at the ribose 2′-O position, causing the host cell fail to distinguish the non-self mRNA. By transforming viral mRNA from the Cap-0 state to the Cap-1 state, nsp16/nsp10 complex enhances the efficiency of translation and helps coronavirus destroy host innate immune responses by escaping the type I interferon suppression. The structures of SARS-CoV-2 nsp16/nsp10 in complex with the pan-methyltransferase inhibitor sinefungin or SAM demonstrate the catalytic mechanism of mRNA capping, making nsp16 a potential target to limit pathogenesis (Fig. 5B) (Krafcikova et al., 2020, Lin et al., 2020b, Rosas-Lemus et al., 2020, Viswanathan et al., 2020).
Additionally, the N protein, which is highly conserved among all coronaviruses, is a multifunctional RNA-binding protein. It binds the viral RNA and forms helical ribonucleoprotein, which is vital for viral RNA replication and packaging (Zhou et al., 2020c). In addition, it can interact with numerous host cell proteins to modulate the metabolism of infected cells (Gordon et al., 2020b). The N- and C-terminal structures of SARS-CoV-2N protein reveals the surface electrostatic potential characteristics and self-assembly properties, explaining the mechanism of its binding ability to single-stranded RNA (Fig. 5B) (Kang et al., 2020, Peng et al., 2020, Ye et al., 2020, Zhou et al., 2020c). Furthermore, compound PJ34 targeted to HCoV-OC43 and 5-benzyloxygramine targeted to MERS-CoV may be potential drugs to block SARS-CoV-2 replication, transcription and virus assembly based on conserved key residues in structures (Lin et al., 2020c, Peng et al., 2020).
2.4.3. Double-membrane vesicles formation
Coronavirus genome replication is related with virus-induced cytosolic double-membrane vesicles (DMVs), which can offer an isolated micro-environment in the infected cell for viral RNA synthesis (Snijder et al., 2020). Similar to SARS-CoV infected cells, DMVs were observed in cells when co-expressing nsp3, nsp4 and nsp6. However, the DMVs were not observed when nsp3 lacking its C-terminal (residues 1319–1922) was co-expressed with nsp4 and nsp6 (Angelini et al., 2013). Additionally, the co-expression of nsp4 and C-terminal of nsp3 (residues 1256–1922) can drive the formation of the ER membrane rearrangements where the coronavirus RTC assemble in MHV or SARS-CoV infected cells (Hagemeijer et al., 2014). The structure of SARS-CoV-2 DMV is still elusive. The low-resolution structure of DMV in MHV resolved by Cryo-ET showed that a molecular pore complex that spans both membranes of the DMV, and nsp3 is the main component of the pore structure (Wolff et al., 2020).
Except forming the DMV, the multi-domain and multifunctional nsp3, which is highly conserved between SARS-CoV and SARS-CoV-2, plays various roles in coronavirus life. Nsp3 can process viral polyproteins, which is essential for viral replication. It can also remove post-translational modifications on host proteins to evade host antiviral innate immune response, and interact with host proteins to support virus survival. Nsp3 consists several domains including Ubl1, HVR, Mac1, Mac2, Mac3, DPUP, Ubl2, PLpro, NAB, βSM, TM, Ecto, AH1, Y1 and CoV-Y, in which not every domain has clear structure features and functions (Lei et al., 2018). A few domain structures of SARS-CoV-2 nsp3 have been solved including Mac1 domain and PLpro domain (Fig. 5C). Mac1 domain can remove ADP-ribose from ADP-ribosylated proteins and the structures in the apo form and in complexes with ligands including 2-(N-morpholino)-ethanesulfonic acid, AMP and ADP-ribose have been solved, respectively (Alhammad et al., 2020, Frick et al., 2020, Lin et al., 2020a, Michalska et al., 2020). PLpro domain recognizes the consensus cleavage sequence LXGG to process three cleavage sites at the boundaries of nsp1/2, nsp2/3 and nsp3/4, and to remove the ubiquitin and ISG15 from host proteins, showing deubiquitinating and deISGylating activities to antagonize innate immune response (Lei et al., 2018). The inhibitors of PLpro may provide a dual therapeutic effect – suppressing virus replication and enhancing antiviral immune response in the host. Small molecule inhibitors have been developed targeting SARS-CoV PLpro, including thiopurine compounds, tanshinones, diarylheptanoids, geranylated flavonoids, zinc ion/zinc conjugate compounds, and naphthalene-based compounds (Baez-Santos et al., 2015). Since the SARS-CoV-2 PLpro and SARS-CoV PLpro share 83% sequence identity, these inhibitors may accelerate the drug development against SARS-CoV-2. However, PLpro is a cysteine protease which belongs to ubiquitin specific protease family (USP) class with 56 members in human, and the catalytic domains among the USP class are highly conserved (Baez-Santos et al., 2015). As a result, it would be difficult to design the inhibitors selectively against PLpro but not human USPs, and the structure-based drug design approaches may help solve this problem. The structures of the SARS-CoV-2 PLpro domain in apo form, and its C111S mutant, in complex with ubiquitin, ISG15 or inhibitors have been solved, respectively (Gao et al., 2020a, Klemm et al., 2020, Rut et al., 2020, Shin et al., 2020), providing the structural basis of their substrate specificities. These inhibitors including VIR250, VIR251 and GRL0617, and rac5c, were identified by a combination of substrate library and activity analysis or in a repurposing approach, and the efficacy of these inhibitors needs to be further verified (Gao et al., 2020a, Klemm et al., 2020, Rut et al., 2020). As a key component for coronavirus replication and survival, other structure features and functions of nsp3 worth further investigation.
It should be noted that the structures and functions of nsp4 and nsp6 remain poorly understood. Currently, there is not any known 3D structure for any coronavirus nsp6 protein, and only one 3D structure for the C-terminal cytoplasmic domain of nsp4 from MHV (Xu et al., 2009).
2.4.4. Putative ion channels
The SARS-CoV-2 genome encodes two putative ion channels, 3a and E, which are highly conserved within the beta-coronavirus subgenus Sarbecovirus including bat coronaviruses (Kern et al., 2020, Srinivasan et al., 2020), suggesting that they are potential therapeutic targets to cure COVID-19.
SARS-CoV 3a forms an ion channel involved in viral release, and necrotic cell death, which is required for SARS-CoV replication and virulence (Castano-Rodriguez et al., 2018). SARS-CoV-2 3a forms large conductance cation channels in dimeric or tetrameric state and the high resolution structure may provide the structural basis for antiviral drugs design (Fig. 5D) (Kern et al., 2020).
SARS-CoV E protein forms cation-selective ion channels in endoplasmic reticulum - Golgi intermediate compartment and/or Golgi membranes that are permeable to calcium ions, which mediates the process of budding and releasing of progeny viruses and may activate the host immune response (Schoeman and Fielding, 2019). SARS-CoV-2 E forms a five-helix bundle surrounding a narrow central pore which can interact with ions, drugs and other host and viral proteins. The amantadine, known to inhibit the proton channels of influenza A virus and HIV-1, and hexamethylene amiloride both bind to polar residues in the N-terminal lumen, establishing the structural basis for designing E inhibitors against SARS-CoV-2 (Fig. 5D) (Mandala et al., 2020).
2.4.5. Orf8
ORF8 is one of the most rapidly evolving accessory proteins in all beta-coronaviruses and the SARS-CoV-2 ORF8 has less than 20% sequence identity to SARS-CoV ORF8 (Mohammad et al., 2020), which may be vital for understanding SARS-CoV-2 as a promptly spread human pathogen. SARS-CoV-2 ORF8 is presumably a secreted protein (Hachim et al., 2020) that interacts with a variety of host proteins, which inhibits type I interferon signaling pathway (Li et al., 2020) and mediates immune evasion through potently downregulating MHC-I (Zhang et al., 2020d). SARS-CoV-2 ORF8 has two novel dimer interfaces that can form unique large-scale assemblies which is impossible for SARS-CoV ORF8, potentially providing essential immune evasion activities (Fig. 5E) (Flower et al., 2020). The understanding of structure of SARS-CoV-2 ORF8 is very helpful to the development of novel therapies.
3. Perspectives
Although many structures of SARS-CoV-2 proteins have been solved by X-ray crystallography, Cryo-EM and NMR methods, and have provided much-needed structural basis for developing effective therapeutics against COVID19, including inhibitors, antibodies and vaccines, the pathogenesis of SARS-CoV-2 infection and the molecular mechanism of virus-host interaction are still unclear. It is urgent to further study the host immune response to SARS-CoV-2 and the processes of SARS-CoV-2 infection, replication and assembly, which will lay a foundation for formulating prevention and treatment strategies against COVID-19 and future pandemics.
Funding
The work in the authors’ laboratory is currently supported by the ShanghaiTech University Startup grant to WX and the Shanghai Pujiang program (Grant # 20PJ1410500 to ZW) from Science and Technology Commission of Shanghai Municipality and Shanghai Municipal Human Resources and Social Security Bureau.
Author contributions
ZW conceived the review. GS, LX, QH, YZ, WX & ZW wrote the manuscript.
Declaration of Competing Interest
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The manuscript was prepared in late October. During the period of manuscript submission and revision, new studies on SARS-CoV-2 continued to emerge. The authors regret that they cannot cite those works. The authors thank Ms. Zhizhuo Dai for her critical reading and comments on the manuscript.
Data availability
No data was used for the research described in the article.
References
- Alhammad, Y.M.O., Kashipathy, M.M., Roy, A., Johnson, D.K., McDonald, P., Battaile, K.P., Gao, P., Lovell, S., Fehr, A.R., 2020. The SARS-CoV-2 conserved macrodomain is a highly efficient ADP-ribosylhydrolase enzyme. bioRxiv. [DOI] [PMC free article] [PubMed]
- Angelini M.M., Akhlaghpour M., Neuman B.W., Buchmeier M.J., Moscona A. Severe acute respiratory syndrome coronavirus nonstructural proteins 3, 4, and 6 induce double-membrane vesicles. mBio. 2013;4(4) doi: 10.1128/mBio.00524-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baez-Santos Y.M., St John S.E., Mesecar A.D. The SARS-coronavirus papain-like protease: structure, function and inhibition by designed antiviral compounds. Antiviral Res. 2015;115:21–38. doi: 10.1016/j.antiviral.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes, C.O., Jette, C.A., Abernathy, M.E., Dam, K.A., Esswein, S.R., Gristick, H.B., Malyutin, A.G., Sharaf, N.G., Huey-Tubman, K.E., Lee, Y.E., Robbiani, D.F., Nussenzweig, M.C., West, A.P., Bjorkman, P.J., 2020a. Structural classification of neutralizing antibodies against the SARS-CoV-2 spike receptor-binding domain suggests vaccine and therapeutic strategies. bioRxiv.
- Barnes C.O., West A.P., Jr., Huey-Tubman K.E., Hoffmann M.A.G., Sharaf N.G., Hoffman P.R., Koranda N., Gristick H.B., Gaebler C., Muecksch F., Lorenzi J.C.C., Finkin S., Hägglöf T., Hurley A., Millard K.G., Weisblum Y., Schmidt F., Hatziioannou T., Bieniasz P.D., Caskey M., Robbiani D.F., Nussenzweig M.C., Bjorkman P.J. Structures of human antibodies bound to SARS-CoV-2 spike reveal common epitopes and recurrent features of antibodies. Cell. 2020;182(4):828–842.e816. doi: 10.1016/j.cell.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum A., Fulton B.O., Wloga E., Copin R., Pascal K.E., Russo V., Giordano S., Lanza K., Negron N., Ni M., Wei Y., Atwal G.S., Murphy A.J., Stahl N., Yancopoulos G.D., Kyratsous C.A. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science. 2020;369(6506):1014–1018. doi: 10.1126/science.abd0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belouzard S., Millet J.K., Licitra B.N., Whittaker G.R. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4(6):1011–1033. doi: 10.3390/v4061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton D.J., Wrobel A.G., Xu P., Roustan C., Martin S.R., Rosenthal P.B., Skehel J.J., Gamblin S.J. Receptor binding and priming of the spike protein of SARS-CoV-2 for membrane fusion. Nature. 2020 doi: 10.1038/s41586-020-2772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvet M., Debarnot C., Imbert I., Selisko B., Snijder E.J., Canard B., Decroly E. In vitro reconstitution of SARS-coronavirus mRNA cap methylation. PLoS Pathog. 2010;6(4) doi: 10.1371/journal.ppat.1000863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer P.J.M., Caniels T.G., van der Straten K., Snitselaar J.L., Aldon Y., Bangaru S., Torres J.L., Okba N.M.A., Claireaux M., Kerster G., Bentlage A.E.H., van Haaren M.M., Guerra D., Burger J.A., Schermer E.E., Verheul K.D., van der Velde N., van der Kooi A., van Schooten J., van Breemen M.J., Bijl T.P.L., Sliepen K., Aartse A., Derking R., Bontjer I., Kootstra N.A., Wiersinga W.J., Vidarsson G., Haagmans B.L., Ward A.B., de Bree G.J., Sanders R.W., van Gils M.J. Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability. Science. 2020;369(6504):643–650. doi: 10.1126/science.abc5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y., Zhang J., Xiao T., Peng H., Sterling S.M., Walsh R.M., Jr., Rawson S., Rits-Volloch S., Chen B. Distinct conformational states of SARS-CoV-2 spike protein. Science. 2020;369(6511):1586–1592. doi: 10.1126/science.abd4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L., Goreshnik I., Coventry B., Case J.B., Miller L., Kozodoy L., Chen R.E., Carter L., Walls A.C., Park Y.J., Strauch E.M., Stewart L., Diamond M.S., Veesler D., Baker D. De novo design of picomolar SARS-CoV-2 miniprotein inhibitors. Science. 2020;370(6515):426–431. doi: 10.1126/science.abd9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Su B., Guo X., Sun W., Deng Y., Bao L., Zhu Q., Zhang X., Zheng Y., Geng C., Chai X., He R., Li X., Lv Q., Zhu H., Deng W., Xu Y., Wang Y., Qiao L., Tan Y., Song L., Wang G., Du X., Gao N., Liu J., Xiao J., Su X.D., Du Z., Feng Y., Qin C., Qin C., Jin R., Xie X.S. Potent Neutralizing Antibodies against SARS-CoV-2 Identified by High-Throughput Single-Cell Sequencing of Convalescent Patients' B Cells. Cell. 2020;182(1):73–84.e16. doi: 10.1016/j.cell.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castano-Rodriguez C., Honrubia J.M., Gutierrez-Alvarez J., DeDiego M.L., Nieto-Torres J.L., Jimenez-Guardeno J.M., Regla-Nava J.A., Fernandez-Delgado R., Verdia-Baguena C., Queralt-Martin M., Kochan G., Perlman S., Aguilella V.M., Sola I., Enjuanes L. Role of severe acute respiratory syndrome coronavirus viroporins E3a, 2018 and 8a in replication and pathogenesis. mBio. 2018;9(3) doi: 10.1128/mBio.02325-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandel V., Sharma P.P., Raj S., Choudhari R., Rathi B., Kumar D. Structure-based drug repurposing for targeting Nsp9 replicase and spike proteins of severe acute respiratory syndrome coronavirus 2. J. Biomol. Struct. Dyn. 2020:1–14. doi: 10.1080/07391102.2020.1811773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Malone B., Llewellyn E., Grasso M., Shelton P.M.M., Olinares P.D.B., Maruthi K., Eng E.T., Vatandaslar H., Chait B.T., Kapoor T.M., Darst S.A., Campbell E.A. Structural basis for helicase-polymerase coupling in the SARS-CoV-2 replication-transcription complex. Cell. 2020;182(6):1560–1573 e1513. doi: 10.1016/j.cell.2020.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier A., Silva D.A., Rocklin G.J., Hicks D.R., Vergara R., Murapa P., Bernard S.M., Zhang L., Lam K.H., Yao G., Bahl C.D., Miyashita S.I., Goreshnik I., Fuller J.T., Koday M.T., Jenkins C.M., Colvin T., Carter L., Bohn A., Bryan C.M., Fernandez-Velasco D.A., Stewart L., Dong M., Huang X., Jin R., Wilson I.A., Fuller D.H., Baker D. Massively parallel de novo protein design for targeted therapeutics. Nature. 2017;550(7674):74–79. doi: 10.1038/nature23912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi X., Yan R., Zhang J., Zhang G., Zhang Y., Hao M., Zhang Z., Fan P., Dong Y., Yang Y., Chen Z., Guo Y., Zhang J., Li Y., Song X., Chen Y., Xia L., Fu L., Hou L., Xu J., Yu C., Li J., Zhou Q., Chen W. A neutralizing human antibody binds to the N-terminal domain of the Spike protein of SARS-CoV-2. Science. 2020;369(6504):650–655. doi: 10.1126/science.abc6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T.M., Sandoval D.R., Spliid C.B., Pihl J., Perrett H.R., Painter C.D., Narayanan A., Majowicz S.A., Kwong E.M., McVicar R.N., Thacker B.E., Glass C.A., Yang Z., Torres J.L., Golden G.J., Bartels P.L., Porell R.N., Garretson A.F., Laubach L., Feldman J., Yin X., Pu Y., Hauser B.M., Caradonna T.M., Kellman B.P., Martino C., Gordts P., Chanda S.K., Schmidt A.G., Godula K., Leibel S.L., Jose J., Corbett K.D., Ward A.B., Carlin A.F., Esko J.D. SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2. Cell. 2020 doi: 10.1016/j.cell.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W., Zhang B., Jiang X.M., Su H., Li J., Zhao Y., Xie X., Jin Z., Peng J., Liu F., Li C., Li Y., Bai F., Wang H., Cheng X., Cen X., Hu S., Yang X., Wang J., Liu X., Xiao G., Jiang H., Rao Z., Zhang L.K., Xu Y., Yang H., Liu H. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science. 2020;368(6497):1331–1335. doi: 10.1126/science.abb4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniloski, Z., Guo, X., Sanjana, N.E., 2020. The D614G mutation in SARS-CoV-2 Spike increases transduction of multiple human cell types. bioRxiv. [DOI] [PMC free article] [PubMed]
- Deng X., van Geelen A., Buckley A.C., O'Brien A., Pillatzki A., Lager K.M., Faaberg K.S., Baker S.C. Coronavirus endoribonuclease activity in porcine epidemic diarrhea virus suppresses type I and type III interferon responses. J. Virol. 2019;93(8) doi: 10.1128/JVI.02000-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du S., Cao Y., Zhu Q., Yu P., Qi F., Wang G., Du X., Bao L., Deng W., Zhu H., Liu J., Nie J., Zheng Y., Liang H., Liu R., Gong S., Xu H., Yisimayi A., Lv Q., Wang B., He R., Han Y., Zhao W., Bai Y., Qu Y., Gao X., Ji C., Wang Q., Gao N., Huang W., Wang Y., Xie X.S., Su X.D., Xiao J., Qin C. Structurally resolved SARS-CoV-2 antibody shows high efficacy in severely infected hamsters and provides a potent cocktail pairing strategy. Cell. 2020 doi: 10.1016/j.cell.2020.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfiky A.A. SARS-CoV-2 RNA dependent RNA polymerase (RdRp) targeting: an in silico perspective. J. Biomol. Struct. Dyn. 2020:1–9. doi: 10.1080/07391102.2020.1761882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskier D., Karakülah G., Suner A., Oktay Y. RdRp mutations are associated with SARS-CoV-2 genome evolution. PeerJ. 2020;8 doi: 10.7717/peerj.9587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faheem B.K., Sekhar K., Kunjiappan S., Jamalis J., Balaña-Fouce R., Tekwani B.L., Sankaranarayanan M. Druggable targets of SARS-CoV-2 and treatment opportunities for COVID-19. Bioorg. Chem. 2020;104 doi: 10.1016/j.bioorg.2020.104269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flower, T.G., Buffalo, C.Z., Hooy, R.M., Allaire, M., Ren, X., Hurley, J.H., 2020. Structure of SARS-CoV-2 ORF8, a rapidly evolving coronavirus protein implicated in immune evasion. bioRxiv. [DOI] [PMC free article] [PubMed]
- Frick D.N., Virdi R.S., Vuksanovic N., Dahal N., Silvaggi N.R. Molecular basis for ADP-ribose binding to the mac1 domain of SARS-CoV-2 nsp3. Biochemistry. 2020;59(28):2608–2615. doi: 10.1021/acs.biochem.0c00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Qin B., Chen P., Zhu K., Hou P., Wojdyla J.A., Wang M., Cui S. Crystal structure of SARS-CoV-2 papain-like protease. Acta Pharm Sin B. 2020 doi: 10.1016/j.apsb.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Yan L., Huang Y., Liu F., Zhao Y., Cao L., Wang T., Sun Q., Ming Z., Zhang L., Ge J., Zheng L., Zhang Y., Wang H., Zhu Y., Zhu C., Hu T., Hua T., Zhang B., Yang X., Li J., Yang H., Liu Z., Xu W., Guddat L.W., Wang Q., Lou Z., Rao Z. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science. 2020;368(6492):779–782. doi: 10.1126/science.abb7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon C.J., Tchesnokov E.P., Woolner E., Perry J.K., Feng J.Y., Porter D.P., Götte M. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. The Journal of biological chemistry. 2020;295(20):6785–6797. doi: 10.1074/jbc.RA120.013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., White K.M., O'Meara M.J., Rezelj V.V., Guo J.Z., Swaney D.L., Tummino T.A., Huttenhain R., Kaake R.M., Richards A.L., Tutuncuoglu B., Foussard H., Batra J., Haas K., Modak M., Kim M., Haas P., Polacco B.J., Braberg H., Fabius J.M., Eckhardt M., Soucheray M., Bennett M.J., Cakir M., McGregor M.J., Li Q., Meyer B., Roesch F., Vallet T., Mac Kain A., Miorin L., Moreno E., Naing Z.Z.C., Zhou Y., Peng S., Shi Y., Zhang Z., Shen W., Kirby I.T., Melnyk J.E., Chorba J.S., Lou K., Dai S.A., Barrio-Hernandez I., Memon D., Hernandez-Armenta C., Lyu J., Mathy C.J.P., Perica T., Pilla K.B., Ganesan S.J., Saltzberg D.J., Rakesh R., Liu X., Rosenthal S.B., Calviello L., Venkataramanan S., Liboy-Lugo J., Lin Y., Huang X.P., Liu Y., Wankowicz S.A., Bohn M., Safari M., Ugur F.S., Koh C., Savar N.S., Tran Q.D., Shengjuler D., Fletcher S.J., O'Neal M.C., Cai Y., Chang J.C.J., Broadhurst D.J., Klippsten S., Sharp P.P., Wenzell N.A., Kuzuoglu-Ozturk D., Wang H.Y., Trenker R., Young J.M., Cavero D.A., Hiatt J., Roth T.L., Rathore U., Subramanian A., Noack J., Hubert M., Stroud R.M., Frankel A.D., Rosenberg O.S., Verba K.A., Agard D.A., Ott M., Emerman M., Jura N., von Zastrow M., Verdin E., Ashworth A., Schwartz O., d'Enfert C., Mukherjee S., Jacobson M., Malik H.S., Fujimori D.G., Ideker T., Craik C.S., Floor S.N., Fraser J.S., Gross J.D., Sali A., Roth B.L., Ruggero D., Taunton J., Kortemme T., Beltrao P., Vignuzzi M., Garcia-Sastre A., Shokat K.M., Shoichet B.K., Krogan N.J. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583(7816):459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung A.B. In silico structure modelling of SARS-CoV-2 Nsp13 helicase and Nsp14 and repurposing of FDA approved antiviral drugs as dual inhibitors. Gene Rep. 2020;21 doi: 10.1016/j.genrep.2020.100860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachim A., Kavian N., Cohen C.A., Chin A.W.H., Chu D.K.W., Mok C.K.P., Tsang O.T.Y., Yeung Y.C., Perera R., Poon L.L.M., Peiris J.S.M., Valkenburg S.A. ORF8 and ORF3b antibodies are accurate serological markers of early and late SARS-CoV-2 infection. Nat. Immunol. 2020;21(10):1293–1301. doi: 10.1038/s41590-020-0773-7. [DOI] [PubMed] [Google Scholar]
- Hackbart M., Deng X., Baker S.C. Coronavirus endoribonuclease targets viral polyuridine sequences to evade activating host sensors. Proc. Natl. Acad. Sci. USA. 2020;117(14):8094–8103. doi: 10.1073/pnas.1921485117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemeijer M.C., Monastyrska I., Griffith J., van der Sluijs P., Voortman J., van Bergen en Henegouwen P.M., Vonk A.M., Rottier P.J., Reggiori F., de Haan C.A. Membrane rearrangements mediated by coronavirus nonstructural proteins 3 and 4. Virology. 2014;458–459:125–135. doi: 10.1016/j.virol.2014.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke L., Vidakovics Perez L., Sheward D.J., Das H., Schulte T., Moliner-Morro A., Corcoran M., Achour A., Karlsson Hedestam G.B., Hällberg B.M., Murrell B., McInerney G.M. An alpaca nanobody neutralizes SARS-CoV-2 by blocking receptor interaction. Nat. Commun. 2020;11(1):4420. doi: 10.1038/s41467-020-18174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J., Baum A., Pascal K.E., Russo V., Giordano S., Wloga E., Fulton B.O., Yan Y., Koon K., Patel K., Chung K.M., Hermann A., Ullman E., Cruz J., Rafique A., Huang T., Fairhurst J., Libertiny C., Malbec M., Lee W.Y., Welsh R., Farr G., Pennington S., Deshpande D., Cheng J., Watty A., Bouffard P., Babb R., Levenkova N., Chen C., Zhang B., Romero Hernandez A., Saotome K., Zhou Y., Franklin M., Sivapalasingam S., Lye D.C., Weston S., Logue J., Haupt R., Frieman M., Chen G., Olson W., Murphy A.J., Stahl N., Yancopoulos G.D., Kyratsous C.A. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science. 2020;369(6506):1010–1014. doi: 10.1126/science.abd0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillen H.S., Kokic G., Farnung L., Dienemann C., Tegunov D., Cramer P. Structure of replicating SARS-CoV-2 polymerase. Nature. 2020;584(7819):154–156. doi: 10.1038/s41586-020-2368-8. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Pohlmann S. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol. Cell. 2020;78(4):779–784 e775. doi: 10.1016/j.molcel.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280 e278. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P.S., Boyken S.E., Baker D. The coming of age of de novo protein design. Nature. 2016;537(7620):320–327. doi: 10.1038/nature19946. [DOI] [PubMed] [Google Scholar]
- Huang X., Pearce R., Zhang Y. De novo design of protein peptides to block association of the SARS-CoV-2 spike protein with human ACE2. Aging (Albany NY) 2020;12(12):11263–11276. doi: 10.18632/aging.103416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo J., Le Bas A., Ruza R.R., Duyvesteyn H.M.E., Mikolajek H., Malinauskas T., Tan T.K., Rijal P., Dumoux M., Ward P.N., Ren J., Zhou D., Harrison P.J., Weckener M., Clare D.K., Vogirala V.K., Radecke J., Moynié L., Zhao Y., Gilbert-Jaramillo J., Knight M.L., Tree J.A., Buttigieg K.R., Coombes N., Elmore M.J., Carroll M.W., Carrique L., Shah P.N.M., James W., Townsend A.R., Stuart D.I., Owens R.J., Naismith J.H. Neutralizing nanobodies bind SARS-CoV-2 spike RBD and block interaction with ACE2. Nat. Struct. Mol. Biol. 2020;27(9):846–854. doi: 10.1038/s41594-020-0469-6. [DOI] [PubMed] [Google Scholar]
- Iftikhar H., Ali H.N., Farooq S., Naveed H., Shahzad-Ul-Hussan S. Identification of potential inhibitors of three key enzymes of SARS-CoV2 using computational approach. Comput. Biol. Med. 2020;122 doi: 10.1016/j.compbiomed.2020.103848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y., Zhang B., Li X., Zhang L., Peng C., Duan Y., Yu J., Wang L., Yang K., Liu F., Jiang R., Yang X., You T., Liu X., Yang X., Bai F., Liu H., Liu X., Guddat L.W., Xu W., Xiao G., Qin C., Shi Z., Jiang H., Rao Z., Yang H. Structure of M(pro) from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582(7811):289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- Jin Z., Zhao Y., Sun Y., Zhang B., Wang H., Wu Y., Zhu Y., Zhu C., Hu T., Du X., Duan Y., Yu J., Yang X., Yang X., Yang K., Liu X., Guddat L.W., Xiao G., Zhang L., Yang H., Rao Z. Structural basis for the inhibition of SARS-CoV-2 main protease by antineoplastic drug carmofur. Nat. Struct. Mol. Biol. 2020;27(6):529–532. doi: 10.1038/s41594-020-0440-6. [DOI] [PubMed] [Google Scholar]
- Ju B., Zhang Q., Ge J., Wang R., Sun J., Ge X., Yu J., Shan S., Zhou B., Song S., Tang X., Yu J., Lan J., Yuan J., Wang H., Zhao J., Zhang S., Wang Y., Shi X., Liu L., Zhao J., Wang X., Zhang Z., Zhang L. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020;584(7819):115–119. doi: 10.1038/s41586-020-2380-z. [DOI] [PubMed] [Google Scholar]
- Kang S., Yang M., Hong Z., Zhang L., Huang Z., Chen X., He S., Zhou Z., Zhou Z., Chen Q., Yan Y., Zhang C., Shan H., Chen S. Crystal structure of SARS-CoV-2 nucleocapsid protein RNA binding domain reveals potential unique drug targeting sites. Acta Pharm. Sin. B. 2020;10(7):1228–1238. doi: 10.1016/j.apsb.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Z., Oton J., Qu K., Cortese M., Zila V., McKeane L., Nakane T., Zivanov J., Neufeldt C.J., Cerikan B., Lu J.M., Peukes J., Xiong X., Krausslich H.G., Scheres S.H.W., Bartenschlager R., Briggs J.A.G. Structures and distributions of SARS-CoV-2 spike proteins on intact virions. Nature. 2020 doi: 10.1038/s41586-020-2665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern, D.M., Sorum, B., Hoel, C.M., Sridharan, S., Remis, J.P., Toso, D.B., Brohawn, S.G., 2020. Cryo-EM structure of the SARS-CoV-2 3a ion channel in lipid nanodiscs. bioRxiv.
- Kiemer L., Lund O., Brunak S., Blom N. Coronavirus 3CLpro proteinase cleavage sites: possible relevance to SARS virus pathology. BMC Bioinf. 2004;5:72. doi: 10.1186/1471-2105-5-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Jedrzejczak R., Maltseva N.I., Wilamowski M., Endres M., Godzik A., Michalska K., Joachimiak A. Crystal structure of Nsp15 endoribonuclease NendoU from SARS-CoV-2. Protein Sci. 2020;29(7):1596–1605. doi: 10.1002/pro.3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y., Wower, J., Maltseva, N.I., Chang, C.K., Jedrzejczak, R., Wilamowski, M., Kang, S., Nicolaescu, V., Randall, G., Michalska, K., Joachimiak, A., 2020b. Tipiracil binds to uridine site and inhibits Nsp15 endoribonuclease NendoU from SARS-CoV-2. bioRxiv. [DOI] [PMC free article] [PubMed]
- Klemm T., Ebert G., Calleja D.J., Allison C.C., Richardson L.W., Bernardini J.P., Lu B.G., Kuchel N.W., Grohmann C., Shibata Y., Gan Z.Y., Cooney J.P., Doerflinger M., Au A.E., Blackmore T.R., van der Heden van Noort G.J., Geurink P.P., Ovaa H., Newman J., Riboldi-Tunnicliffe A., Czabotar P.E., Mitchell J.P., Feltham R., Lechtenberg B.C., Lowes K.N., Dewson G., Pellegrini M., Lessene G., Komander D. Mechanism and inhibition of the papain-like protease, PLpro, of SARS-CoV-2. EMBO J. 2020;39(18) doi: 10.15252/embj.2020106275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneller D.W., Phillips G., O’Neill H.M., Jedrzejczak R., Stols L., Langan P., Joachimiak A., Coates L., Kovalevsky A. Structural plasticity of SARS-CoV-2 3CL Mpro active site cavity revealed by room temperature X-ray crystallography. Nat. Commun. 2020;11(1):3202. doi: 10.1038/s41467-020-16954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krafcikova P., Silhan J., Nencka R., Boura E. Structural analysis of the SARS-CoV-2 methyltransferase complex involved in RNA cap creation bound to sinefungin. Nat. Commun. 2020;11(1):3717. doi: 10.1038/s41467-020-17495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., Zhang Q., Shi X., Wang Q., Zhang L., Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581(7807):215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- Lei J., Kusov Y., Hilgenfeld R. Nsp3 of coronaviruses: Structures and functions of a large multi-domain protein. Antiviral Res. 2018;149:58–74. doi: 10.1016/j.antiviral.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020;5(4):562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.Y., Liao C.H., Wang Q., Tan Y.J., Luo R., Qiu Y., Ge X.Y. The ORF6, ORF8 and nucleocapsid proteins of SARS-CoV-2 inhibit type I interferon signaling pathway. Virus Res. 2020;286 doi: 10.1016/j.virusres.2020.198074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M.H., Chang S.C., Chiu Y.C., Jiang B.C., Wu T.H., Hsu C.H. Structural, biophysical and biochemical elucidation of the SARS-CoV-2 nonstructural protein 3 macro domain. ACS Infect. Dis. 2020 doi: 10.1021/acsinfecdis.0c00441. [DOI] [PubMed] [Google Scholar]
- Lin S., Chen H., Ye F., Chen Z., Yang F., Zheng Y., Cao Y., Qiao J., Yang S., Lu G. Crystal structure of SARS-CoV-2 nsp10/nsp16 2'-O-methylase and its implication on antiviral drug design. Signal Transduct Target Ther. 2020;5(1):131. doi: 10.1038/s41392-020-00241-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S.M., Lin S.C., Hsu J.N., Chang C.K., Chien C.M., Wang Y.S., Wu H.Y., Jeng U.S., Kehn-Hall K., Hou M.H. Structure-based stabilization of non-native protein-protein interactions of coronavirus nucleocapsid proteins in antiviral drug design. J. Med. Chem. 2020;63(6):3131–3141. doi: 10.1021/acs.jmedchem.9b01913. [DOI] [PubMed] [Google Scholar]
- Linsky, T.W., Vergara, R., Codina, N., Nelson, J.W., Walker, M.J., Su, W., Hsiang, T.Y., Esser-Nobis, K., Yu, K., Hou, Y.J., Priya, T., Mitsumoto, M., Pong, A., Lau, U.Y., Mason, M.L., Chen, J., Chen, A., Berrocal, T., Peng, H., Clairmont, N.S., Castellanos, J., Lin, Y.R., Josephson-Day, A., Baric, R., Walkey, C.D., Swanson, R., Gale, M., Blancas-Mejia, L.M., Yen, H.L., Silva, D.A., 2020. De novo design of ACE2 protein decoys to neutralize SARS-CoV-2. bioRxiv.
- Littler D.R., Gully B.S., Colson R.N., Rossjohn J. Crystal Structure of the SARS-CoV-2 Non-structural Protein 9, Nsp9. iScience. 2020;23(7) doi: 10.1016/j.isci.2020.101258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, L., Wang, P., Nair, M.S., Yu, J., Rapp, M., Wang, Q., Luo, Y., Chan, J.F., Sahi, V., Figueroa, A., Guo, X.V., Cerutti, G., Bimela, J., Gorman, J., Zhou, T., Chen, Z., Yuen, K.Y., Kwong, P.D., Sodroski, J.G., Yin, M.T., Sheng, Z., Huang, Y., Shapiro, L., Ho, D.D., 2020. Potent Neutralizing Monoclonal Antibodies Directed to Multiple Epitopes on the SARS-CoV-2 Spike. bioRxiv.
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Z., Deng Y.Q., Ye Q., Cao L., Sun C.Y., Fan C., Huang W., Sun S., Sun Y., Zhu L., Chen Q., Wang N., Nie J., Cui Z., Zhu D., Shaw N., Li X.F., Li Q., Xie L., Wang Y., Rao Z., Qin C.F., Wang X. Structural basis for neutralization of SARS-CoV-2 and SARS-CoV by a potent therapeutic antibody. Science. 2020;369(6510):1505–1509. doi: 10.1126/science.abc5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala V., McKay M., Shcherbakov A., Dregni A., Kolocouris A., Hong M. Structure and drug binding of the SARS-CoV-2 envelope protein in phospholipid bilayers. Res Sq. 2020 doi: 10.1038/s41594-020-00536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalska K., Kim Y., Jedrzejczak R., Maltseva N.I., Stols L., Endres M., Joachimiak A. Crystal structures of SARS-CoV-2 ADP-ribose phosphatase: from the apo form to ligand complexes. IUCrJ. 2020;7(Pt 5):814–824. doi: 10.1107/S2052252520009653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad S., Bouchama A., Mohammad Alharbi B., Rashid M., Saleem Khatlani T., Gaber N.S., Malik S.S. SARS-CoV-2 ORF8 and SARS-CoV ORF8ab: genomic divergence and functional convergence. Pathogens. 2020;9(9) doi: 10.3390/pathogens9090677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais I.J., Polveiro R.C., Souza G.M., Bortolin D.I., Sassaki F.T., Lima A.T.M. The global population of SARS-CoV-2 is composed of six major subtypes. Sci. Rep. 2020;10(1):18289. doi: 10.1038/s41598-020-74050-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo J., Shukla A.M., Chamarthi G., Gupte A. The journey of remdesivir: from Ebola to COVID-19. Drugs. Context. 2020;9 doi: 10.7573/dic.2020-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y., Du N., Lei Y., Dorje S., Qi J., Luo T., Gao G.F., Song H. Structures of the SARS-CoV-2 nucleocapsid and their perspectives for drug design. EMBO J. 2020;39(20) doi: 10.15252/embj.2020105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D., Park Y.J., Beltramello M., Walls A.C., Tortorici M.A., Bianchi S., Jaconi S., Culap K., Zatta F., De Marco A., Peter A., Guarino B., Spreafico R., Cameroni E., Case J.B., Chen R.E., Havenar-Daughton C., Snell G., Telenti A., Virgin H.W., Lanzavecchia A., Diamond M.S., Fink K., Veesler D., Corti D. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020;583(7815):290–295. doi: 10.1038/s41586-020-2349-y. [DOI] [PubMed] [Google Scholar]
- Rosas-Lemus, M., Minasov, G., Shuvalova, L., Inniss, N.L., Kiryukhina, O., Wiersum, G., Kim, Y., Jedrzejczak, R., Maltseva, N.I., Endres, M., Jaroszewski, L., Godzik, A., Joachimiak, A., Satchell, K.J.F., 2020. The crystal structure of nsp10-nsp16 heterodimer from SARS-CoV-2 in complex with S-adenosylmethionine. bioRxiv.
- Rut W., Lv Z., Zmudzinski M., Patchett S., Nayak D., Snipas S.J., El Oualid F., Huang T.T., Bekes M., Drag M., Olsen S.K. Activity profiling and crystal structures of inhibitor-bound SARS-CoV-2 papain-like protease: A framework for anti-COVID-19 drug design. Sci. Adv. 2020;6(42) doi: 10.1126/sciadv.abd4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol J. 2019;16(1):69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoof, M., Faust, B., Saunders, R.A., Sangwan, S., Rezelj, V., Hoppe, N., Boone, M., Billesbølle, C.B., Zimanyi, M., Deshpande, I., Liang, J., Anand, A.A., Dobzinski, N., Zha, B.S., Barsi-Rhyne, B., Belyy, V., Barile-Hill, A.W., Gupta, S., Simoneau, C.R., Leon, K., White, K.M., Nock, S., Liu, Y., Krogan, N.J., Ralston, C.Y., Swaney, D.L., García-Sastre, A., Ott, M., Vignuzzi, M., Walter, P., Manglik, A., 2020. An ultra-high affinity synthetic nanobody blocks SARS-CoV-2 infection by locking Spike into an inactive conformation. bioRxiv.
- Schubert K., Karousis E.D., Jomaa A., Scaiola A., Echeverria B., Gurzeler L.A., Leibundgut M., Thiel V., Muhlemann O., Ban N. SARS-CoV-2 Nsp1 binds the ribosomal mRNA channel to inhibit translation. Nat. Struct. Mol. Biol. 2020;27(10):959–966. doi: 10.1038/s41594-020-0511-8. [DOI] [PubMed] [Google Scholar]
- Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., Li F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA. 2020;117(21):11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., Geng Q., Auerbach A., Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581(7807):221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T.P., Sims A.C., Zhou S., Graham R.L., Pruijssers A.J., Agostini M.L., Leist S.R., Schäfer A., Dinnon K.H., 3rd, Stevens L.J., Chappell J.D., Lu X., Hughes T.M., George A.S., Hill C.S., Montgomery S.A., Brown A.J., Bluemling G.R., Natchus M.G., Saindane M., Kolykhalov A.A., Painter G., Harcourt J., Tamin A., Thornburg N.J., Swanstrom R., Denison M.R., Baric R.S. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci. Transl. Med. 2020;12(541) doi: 10.1126/scitranslmed.abb5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi R., Shan C., Duan X., Chen Z., Liu P., Song J., Song T., Bi X., Han C., Wu L., Gao G., Hu X., Zhang Y., Tong Z., Huang W., Liu W.J., Wu G., Zhang B., Wang L., Qi J., Feng H., Wang F.S., Wang Q., Gao G.F., Yuan Z., Yan J. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature. 2020;584(7819):120–124. doi: 10.1038/s41586-020-2381-y. [DOI] [PubMed] [Google Scholar]
- Shin D., Mukherjee R., Grewe D., Bojkova D., Baek K., Bhattacharya A., Schulz L., Widera M., Mehdipour A.R., Tascher G., Geurink P.P., Wilhelm A., van der Heden van Noort G.J., Ovaa H., Müller S., Knobeloch K.P., Rajalingam K., Schulman B.A., Cinatl J., Hummer G., Ciesek S., Dikic I. Papain-like protease regulates SARS-CoV-2 viral spread and innate immunity. Nature. 2020 doi: 10.1038/s41586-020-2601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder E.J., Limpens R., de Wilde A.H., de Jong A.W.M., Zevenhoven-Dobbe J.C., Maier H.J., Faas F., Koster A.J., Barcena M. A unifying structural and functional model of the coronavirus replication organelle: Tracking down RNA synthesis. PLoS Biol. 2020;18(6) doi: 10.1371/journal.pbio.3000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S., Cui H., Gao Z., Liu M., Lu S., Mkandawire W., Narykov O., Sun M., Korkin D. Structural Genomics of SARS-CoV-2 Indicates Evolutionary Conserved Functional Regions of Viral Proteins. Viruses. 2020;12(4) doi: 10.3390/v12040360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoms M., Buschauer R., Ameismeier M., Koepke L., Denk T., Hirschenberger M., Kratzat H., Hayn M., Mackens-Kiani T., Cheng J., Straub J.H., Stürzel C.M., Fröhlich T., Berninghausen O., Becker T., Kirchhoff F., Sparrer K.M.J., Beckmann R. Structural basis for translational shutdown and immune evasion by the Nsp1 protein of SARS-CoV-2. Science. 2020;369(6508):1249–1255. doi: 10.1126/science.abc8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toelzer C., Gupta K., Yadav S.K.N., Borucu U., Davidson A.D., Kavanagh Williamson M., Shoemark D.K., Garzoni F., Staufer O., Milligan R., Capin J., Mulholland A.J., Spatz J., Fitzgerald D., Berger I., Schaffitzel C. Free fatty acid binding pocket in the locked structure of SARS-CoV-2 spike protein. Science. 2020 doi: 10.1126/science.abd3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorici M.A., Beltramello M., Lempp F.A., Pinto D., Dang H.V., Rosen L.E., McCallum M., Bowen J., Minola A., Jaconi S., Zatta F., De Marco A., Guarino B., Bianchi S., Lauron E.J., Tucker H., Zhou J., Peter A., Havenar-Daughton C., Wojcechowskyj J.A., Case J.B., Chen R.E., Kaiser H., Montiel-Ruiz M., Meury M., Czudnochowski N., Spreafico R., Dillen J., Ng C., Sprugasci N., Culap K., Benigni F., Abdelnabi R., Foo S.C., Schmid M.A., Cameroni E., Riva A., Gabrieli A., Galli M., Pizzuto M.S., Neyts J., Diamond M.S., Virgin H.W., Snell G., Corti D., Fink K., Veesler D. Ultrapotent human antibodies protect against SARS-CoV-2 challenge via multiple mechanisms. Science. 2020 doi: 10.1126/science.abe3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorici M.A., Veesler D. Structural insights into coronavirus entry. Adv. Virus Res. 2019;105:93–116. doi: 10.1016/bs.aivir.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan T., Arya S., Chan S.H., Qi S., Dai N., Misra A., Park J.G., Oladunni F., Kovalskyy D., Hromas R.A., Martinez-Sobrido L., Gupta Y.K. Structural basis of RNA cap modification by SARS-CoV-2. Nat. Commun. 2020;11(1):3718. doi: 10.1038/s41467-020-17496-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181(2):281–292 e286. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Wu J., Wang H., Gao Y., Liu Q., Mu A., Ji W., Yan L., Zhu Y., Zhu C., Fang X., Yang X., Huang Y., Gao H., Liu F., Ge J., Sun Q., Yang X., Xu W., Liu Z., Yang H., Lou Z., Jiang B., Guddat L.W., Gong P., Rao Z. Structural Basis for RNA Replication by the SARS-CoV-2 Polymerase. Cell. 2020;182(2):417–428.e413. doi: 10.1016/j.cell.2020.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Zhang Y., Wu L., Niu S., Song C., Zhang Z., Lu G., Qiao C., Hu Y., Yuen K.Y., Wang Q., Zhou H., Yan J., Qi J. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell. 2020;181(4):894–904.e899. doi: 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wec A.Z., Wrapp D., Herbert A.S., Maurer D.P., Haslwanter D., Sakharkar M., Jangra R.K., Dieterle M.E., Lilov A., Huang D., Tse L.V., Johnson N.V., Hsieh C.L., Wang N., Nett J.H., Champney E., Burnina I., Brown M., Lin S., Sinclair M., Johnson C., Pudi S., Bortz R., 3rd, Wirchnianski A.S., Laudermilch E., Florez C., Fels J.M., O'Brien C.M., Graham B.S., Nemazee D., Burton D.R., Baric R.S., Voss J.E., Chandran K., Dye J.M., McLellan J.S., Walker L.M. Broad neutralization of SARS-related viruses by human monoclonal antibodies. Science. 2020;369(6504):731–736. doi: 10.1126/science.abc7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, M.A., Lin, W., Cheng, X., 2020. Discovery of COVID-19 Inhibitors Targeting the SARS-CoV2 Nsp13 Helicase. bioRxiv. [DOI] [PMC free article] [PubMed]
- Wolff G., Limpens R., Zevenhoven-Dobbe J.C., Laugks U., Zheng S., de Jong A.W.M., Koning R.I., Agard D.A., Grunewald K., Koster A.J., Snijder E.J., Barcena M. A molecular pore spans the double membrane of the coronavirus replication organelle. Science. 2020;369(6509):1395–1398. doi: 10.1126/science.abd3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrapp D., De Vlieger D., Corbett K.S., Torres G.M., Wang N., Van Breedam W., Roose K., van Schie L., Hoffmann M., Pöhlmann S., Graham B.S., Callewaert N., Schepens B., Saelens X., McLellan J.S. Structural basis for potent neutralization of betacoronaviruses by single-domain camelid antibodies. Cell. 2020;181(5):1004–1015.e1015. doi: 10.1016/j.cell.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrobel A.G., Benton D.J., Xu P., Roustan C., Martin S.R., Rosenthal P.B., Skehel J.J., Gamblin S.J. SARS-CoV-2 and bat RaTG13 spike glycoprotein structures inform on virus evolution and furin-cleavage effects. Nat. Struct. Mol. Biol. 2020;27(8):763–767. doi: 10.1038/s41594-020-0468-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Lou Z., Ma Y., Chen X., Yang Z., Tong X., Zhao Q., Xu Y., Deng H., Bartlam M., Rao Z. Crystal structure of the C-terminal cytoplasmic domain of non-structural protein 4 from mouse hepatitis virus A59. PLoS ONE. 2009;4(7) doi: 10.1371/journal.pone.0006217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H., Song Y., Chen Y., Wu N., Xu J., Sun C., Zhang J., Weng T., Zhang Z., Wu Z., Cheng L., Shi D., Lu X., Lei J., Crispin M., Shi Y., Li L., Li S. Molecular Architecture of the SARS-CoV-2 Virus. Cell. 2020 doi: 10.1016/j.cell.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, Q., West, A.M.V., Silletti, S., Corbett, K.D., 2020. Architecture and self-assembly of the SARS-CoV-2 nucleocapsid protein. bioRxiv. [DOI] [PMC free article] [PubMed]
- Yin W., Mao C., Luan X., Shen D.D., Shen Q., Su H., Wang X., Zhou F., Zhao W., Gao M., Chang S., Xie Y.C., Tian G., Jiang H.W., Tao S.C., Shen J., Jiang Y., Jiang H., Xu Y., Zhang S., Zhang Y., Xu H.E. Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir. Science. 2020;368(6498):1499–1504. doi: 10.1126/science.abc1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M., Liu H., Wu N.C., Lee C.D., Zhu X., Zhao F., Huang D., Yu W., Hua Y., Tien H., Rogers T.F., Landais E., Sok D., Jardine J.G., Burton D.R., Wilson I.A. Structural basis of a shared antibody response to SARS-CoV-2. Science. 2020;369(6507):1119–1123. doi: 10.1126/science.abd2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M., Wu N.C., Zhu X., Lee C.D., So R.T.Y., Lv H., Mok C.K.P., Wilson I.A. A highly conserved cryptic epitope in the receptor binding domains of SARS-CoV-2 and SARS-CoV. Science. 2020;368(6491):630–633. doi: 10.1126/science.abb7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, S., Peng, L., Park, J.J., Hu, Y., Devarkar, S.C., Dong, M.B., Wu, S., Chen, S., Lomakin, I., Xiong, Y., 2020c. Nonstructural protein 1 of SARS-CoV-2 is a potent pathogenicity factor redirecting host protein synthesis machinery toward viral RNA. bioRxiv. [DOI] [PMC free article] [PubMed]
- Yurkovetskiy L., Wang X., Pascal K.E., Tomkins-Tinch C., Nyalile T.P., Wang Y., Baum A., Diehl W.E., Dauphin A., Carbone C., Veinotte K., Egri S.B., Schaffner S.F., Lemieux J.E., Munro J.B., Rafique A., Barve A., Sabeti P.C., Kyratsous C.A., Dudkina N.V., Shen K., Luban J. Structural and Functional Analysis of the D614G SARS-CoV-2 Spike Protein Variant. Cell. 2020 doi: 10.1016/j.cell.2020.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Chen Y., Li L., Yang Y., He J., Chen C., Su D. Structural basis for the multimerization of nonstructural protein nsp9 from SARS-CoV-2. Molecular Biomedicine. 2020;1(1) doi: 10.1186/s43556-020-00005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L., Jackson, C.B., Mou, H., Ojha, A., Rangarajan, E.S., Izard, T., Farzan, M., Choe, H., 2020b. The D614G mutation in the SARS-CoV-2 spike protein reduces S1 shedding and increases infectivity. bioRxiv.
- Zhang L., Lin D., Sun X., Curth U., Drosten C., Sauerhering L., Becker S., Rox K., Hilgenfeld R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. 2020;368(6489):409–412. doi: 10.1126/science.abb3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., Zhang, J., Chen, Y., Luo, B., Yuan, Y., Huang, F., Yang, T., Yu, F., Liu, J., Liu, B., Song, Z., Chen, J., Pan, T., Zhang, X., Li, Y., Li, R., Huang, W., Xiao, F., Zhang, H., 2020d. The ORF8 Protein of SARS-CoV-2 Mediates Immune Evasion through Potently Downregulating MHC-I. bioRxiv.
- Zhou D., Duyvesteyn H.M.E., Chen C.P., Huang C.G., Chen T.H., Shih S.R., Lin Y.C., Cheng C.Y., Cheng S.H., Huang Y.C., Lin T.Y., Ma C., Huo J., Carrique L., Malinauskas T., Ruza R.R., Shah P.N.M., Tan T.K., Rijal P., Donat R.F., Godwin K., Buttigieg K.R., Tree J.A., Radecke J., Paterson N.G., Supasa P., Mongkolsapaya J., Screaton G.R., Carroll M.W., Gilbert-Jaramillo J., Knight M.L., James W., Owens R.J., Naismith J.H., Townsend A.R., Fry E.E., Zhao Y., Ren J., Stuart D.I., Huang K.A. Structural basis for the neutralization of SARS-CoV-2 by an antibody from a convalescent patient. Nat. Struct. Mol. Biol. 2020;27(10):950–958. doi: 10.1038/s41594-020-0480-y. [DOI] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R., Zeng R., von Brunn A., Lei J. Structural characterization of the C-terminal domain of SARS-CoV-2 nucleocapsid protein. Mol. Biomed. 2020;1(1) doi: 10.1186/s43556-020-00001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D.Y., Wang W.L., Li X.W., Yang B., Song J.D., Zhao X., Huang B.Y., Shi W.F., Lu R.J., Niu P.H., Zhan F.X., Ma X.J., Wang D.Y., Xu W.B., Wu G.Z., Gao G.G.F., Tan W.J., Coronavirus C.N. A novel coronavirus from patients with pneumonia in China, 2019. New Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.