Key Points
Question
What will be the short-term budget impact of a gene therapy for sickle cell disease among Medicaid programs with the highest prevalence of the disease?
Findings
This budget impact analysis estimated a mean 1-year budget impact of $29.96 million per state Medicaid program, or $1.91 per member per month increase in spending, in the 10 states of interest.
Meaning
This study suggests that gene therapy for sickle cell disease will likely present affordability challenges to several Medicaid plans.
Abstract
Importance
Hundreds of gene therapies are undergoing clinical testing and are likely to be priced more than $1 million per course of treatment. The association that high prices will have with insurance coverage of gene therapy remains unclear. Gene therapy for sickle cell disease has shown early success and would be one of the first gene therapies available for a relatively large population.
Objectives
To estimate the budget impact and affordability of a gene therapy for severe sickle cell disease from the perspective of US Medicaid programs with the highest prevalence of sickle cell disease while exploring the impact of an annuity payment model.
Design, Setting, and Participants
A budget impact analysis was performed from January 1 to May 31, 2020, for a sickle cell disease gene therapy from the perspective of 10 state Medicaid plans with a 5-year time horizon, using state-level disease prevalence data from 2012. Disease prevalence, Medicaid enrollment, and expenditures were derived from the available literature. The eligible population was based on modified clinical trial inclusion criteria including individuals aged 13 to 45 years with severe disease.
Exposures
The gene therapy was assumed to be administered to 7% of the eligible population annually and was curative (no subsequent disease-related expenditures). The gene therapy price was $1.85 million in the base case, and baseline disease-related expenditures were $42 200 per year.
Main Outcomes and Measures
The main outcomes were total budget impact and budget impact per member per month in years 1 through 5. One-way sensitivity analysis was used to evaluate uncertainty of market diffusion, size of eligible population, price of therapy, and cost of routine care.
Results
An estimated 5464 Medicaid enrollees would be eligible for the gene therapy nationally, with 2315 individuals in the 10 Medicaid programs of interest (16 per 100 000 enrollees). The model projected a mean 1-year budget impact of $29.96 million per state Medicaid program in the sample ($1.91 per member per month). A 5-year annuity payment reduced the short-term budget impact.
Conclusions and Relevance
This study suggests that a gene therapy for severe sickle cell disease is likely to produce a considerable budget impact for many Medicaid plans while potentially offering substantial benefit to patients. Payers may need to take steps to ensure affordability and access. Gene therapy for sickle cell disease is likely to provide an early demonstration of the unique financial challenges associated with this emerging drug class.
This economic evaluation estimates the budget impact and affordability of a gene therapy for severe sickle cell disease from the perspective of US Medicaid programs with the highest prevalence of sickle cell disease while exploring the impact of an annuity payment model.
Introduction
Many gene therapies under development may soon provide novel curative options for a host of malignant and nonmalignant conditions. Hundreds of such therapies are undergoing clinical testing and an estimated 40 to 50 will enter the market by 2030.1 Currently, the commercially available therapies are indicated for a small number of patients while having exceptionally high prices—exceeding $1 million per treatment. The unparalleled price and potentially curative single administration presents various challenges to the existing reimbursement system in the US.2 As demonstrated by direct-acting antivirals (DAAs) for hepatitis C, a cost-effective therapy may still create affordability challenges for payers balancing short-term budgets.3 The assessment of value (cost effectiveness) and affordability (budget impact) do not need to occur in tandem and, in some circumstances, early consideration of affordability may be essential.
Although clinical benefit and accrued savings may make a given therapy financially tenable in the long run, what if a patient changes health plans after a chronic disease is cured with a high-cost durable therapy? Is the original insurer entitled to the long-term accrued savings? Although several alternative payment models have been proposed, much uncertainty remains and there is unlikely to be a one-size-fits-all approach.4,5
Sickle cell disease (SCD) affects approximately 100 000 individuals in the US.6 Despite improvements with comprehensive care and hydroxyurea treatment, quality of life remains inferior when compared with many other chronic diseases,7,8 and median survival is less than 50 years for individuals with hemoglobin SS or hemoglobin Sß0 genotypes.9 Only allogeneic hematopoietic stem cell transplantation (HSCT) offers a cure, although its use is limited owing to toxic effects and difficulty finding well-matched donors. The recent approvals of l-glutamine, voxelotor, and crizanlizumab for SCD also deserve acknowledgment, although their clinical utility appears modest.10,11,12 The profound morbidity from SCD is associated with significant spending, with lifetime SCD-related health care expenditures exceeding $550 000 per person in 2020 US dollars.13
Gene therapy for severe SCD appears promising, with one lentiviral-based therapy demonstrating early efficacy in the ongoing phase 1/2 trial HGB-206.14,15 The early data suggest that this therapy may markedly improve the lives of individuals with SCD. A gene therapy using the same lentiviral vector received conditional approval from the European Medicines Agency in 2019 for the treatment of transfusion-dependent β-thalassemia. It appears that a gene therapy for SCD may soon enter the US market, presenting an option for a relatively large population in comparison with existing gene therapies for other diseases. However, this therapy is likely to cost more than $1 million, presenting a variety of fiscal challenges to US health care payers covering large numbers of patients with SCD. At least 55 000 patients with SCD are enrolled in Medicaid nationally.16
We sought to perform a budget impact analysis to estimate the short-term affordability of a gene therapy for severe SCD from the perspective of US Medicaid programs—a payer group likely to experience the greatest budget impact. Our objectives were to (1) estimate the potential short-term budget impact of a gene therapy for severe SCD from the perspective of the 10 state Medicaid plans with the highest prevalence of SCD; (2) describe how uncertainty in the size of the eligible population, market diffusion, and pricing are associated with budget impact; and (3) examine how an alternative payment model is associated with short-term cost.
Methods
Overview
A budget impact analysis was performed from January 1 to May 31, 2020, for an SCD gene therapy from the perspective of 10 state Medicaid plans with a 5-year time horizon. The annual budget impact was calculated as the estimated one-time cost of the gene therapy less the annual savings from patients having previously received the intervention. Key model assumptions included the percentage of patients with SCD with a severe phenotype, cost of the gene therapy, and annual market diffusion rate for the therapy (Table 1).13,17,18 State-level disease burden based on published Centers for Medicare & Medicaid Services data are detailed in the eTable in the Supplement.16 The state-level SCD prevalence data are from 2012 (most recent available). The total budget for each Medicaid program was available via The Kaiser Family Foundation.19 No institutional review board approval was required because the analysis used publicly available data with no protected health information.
Table 1. Key Model Inputs.
| Parameter | Value | Range considered in sensitivity analysis | Source |
|---|---|---|---|
| Patients with SCD with severe phenotype, % | 25 | 10-40 | Assumed |
| Baseline annual expenditure for patient with SCD, $ | 42 200a | 17 100-67 250 | Kauf et al,13 Arnold et al17 |
| Cost of single gene therapy treatment per person, $ | 1 850 000 | 1 440 000-2 170 000 | Beasley and Mathias18 |
| Annual market diffusion rate for gene therapy, % | 7 | 2-15 | Assumed |
Abbreviation: SCD, sickle cell disease.
Mean of estimates in associated reference.
Perspective and Population
The 10 state Medicaid programs with the highest prevalence of SCD included in the study were (descending order of prevalence): Mississippi; Alabama; South Carolina; Georgia; Washington, DC; Louisiana; Virginia; North Carolina; Florida; and Maryland. Six of the 10 states have not adopted Medicaid expansion under the Patient Protection and Affordable Care Act. An analysis was also performed for all US Medicaid programs in aggregate. International Society for Pharmacoeconomics and Outcomes Research (ISPOR) practice guidelines informed our design.20
The population estimated to be eligible for the gene therapy was based on criteria for HGB-206 trial eligibility.21 This estimation was intended to approximate potential product labeling and criteria for coverage. Adapted eligibility criteria were individuals with SCD aged 13 to 45 years with a phenotype of severe SCD (at least 4 severe pain episodes in 24 months). Our age range for eligibility differs from the clinical trial (12-50 years), but provides a more accurate and conservative estimate of the population size by conforming to Centers for Medicare & Medicaid Services age strata. As per the HGB-206 trial, patients with hemoglobin SCD were excluded using the assumption that 25% of individuals with SCD have the SC genotype.
Intervention Mix and Time Horizon
The intervention was a theoretical, one-time gene therapy based on betibeglogene autotemcel (HGB-206 trial). All individuals entered the model in the standard of care state—representing routine preventive and acute SCD care, in aggregate. Allogenic HSCT was not considered a competing therapy under the assumption that an efficacious autologous gene therapy would be preferred (and the 7% diffusion rate leaves adequate market share for transplantation among those with severe disease). Other noncurative therapies were not included as competing treatments. A 5-year time horizon was used to allow for modeling of a 5-year annuity payment.
Analytic Framework Description
A static cohort entered the model receiving standard of care (eFigure 1 in the Supplement). Each year, a subset of this cohort (defined via the market diffusion rate) received the gene therapy and incurred no SCD-related costs in subsequent years (therapy is fully curative and durable). All individuals remaining in the standard of care state incurred SCD-related expenditures. The model considers only direct medical expenditures associated with SCD and the cost of gene therapy.
The model provided the annual net budget impact and per-member per-month (PMPM) cost. The PMPM cost was calculated as the annual budget impact divided by the total number of plan enrollees, divided by 12 months.
Input Data
The proportion of patients with SCD and a severe phenotype was 25% in the base case and varied from 10% to 40% in the sensitivity analysis. These parameters were determined via opinion as no data were available to inform the estimate.
The annual market diffusion rate of the gene therapy was 7% in the base case across all 5 years. There were no data to inform this estimate given the novelty of this drug class; therefore, we aimed for a conservative estimate. A range of the annual market diffusion rate from 2% to 15% was considered in the sensitivity analysis.
The baseline SCD-related health expenditure was $42 200 per year in the base case. There is significant variation among published data for annual SCD-related expenditures. The base case was calculated as the mean of 2 prior studies representing low and high ends of the expenditure range: the low estimate ($17 100) came from a study of Florida Medicaid patients with SCD13 and the high estimate ($67 250) came from a study of costs in the year preceding HSCT.17 These estimates provided the upper and lower bounds for the sensitivity analysis. All costs are updated to 2019 US dollars using the Consumer Price Index.
The cost of the gene therapy was $1.85 million in 2019 US dollars for the single administration in the base case—derived from initial pricing ($1.78 million) of betibeglogene autotemcel in the European Union for β-thalassemia plus $70 000 for 1 admission for myeloablative conditioning followed by gene therapy infusion.18,22 The cost of incident admission for autologous stem cell transplantation ($120 000) was derived from commercial payer data and adjusted to $70 000 in the assumption that Medicaid pays 57% of the commercial rate for inpatient care.23,24 The range considered for sensitivity analysis ($1.44 million-$2.17 million) represents a 23% discount from the base case (Medicaid best-price guarantee for drug) with the upper bound as the “intrinsic value” of the treatment as proposed by the manufacturer.18 The list price often overestimates true cost but is widely used for similar purposes. No costs associated with prehospitalization or posthospitalization care or adverse effects were included. The model was intended to provide a conservative estimate of the cost of the therapy.
In addition to full upfront payment, a 5-year 20% annuity payment was evaluated (20% of price paid annually). The manufacturer of betibeglogene autotemcel established an outcome-based annuity in Germany and suggests this as an option in other markets.25 Our model used a 5-year annuity with no outcome-based component given assumed 100% effectiveness and durability.
Sensitivity Analyses and Uncertainty
The uncertainty of model inputs was evaluated using 1-way sensitivity analysis for the following parameters: proportion with a severe phenotype, market diffusion rate, price of the gene therapy, and annual costs of standard of care. We report mean PMPM cost as the outcome of interest across the 10-state sample.
Results
An estimated 5464 Medicaid enrollees with SCD would be eligible for the therapy nationally, with 2315 individuals in the 10 state Medicaid programs of interest (16 per 100 000 enrollees). The model projected a mean 1-year budget impact of $29.96 million per state Medicaid program (Table 2) with a mean of $1.91 PMPM (0.31% of program budget). Florida demonstrated the highest absolute 1-year cost among the states evaluated, at $67.43 million ($1.48 PMPM), whereas Washington, DC, had the lowest, at $5.71 million ($1.81 PMPM). Relative to each program’s total budget, Mississippi’s spending was the highest and represented 0.45% of the budget with $3.07 PMPM in year 1.
Table 2. Budget Impact Analysis, Medicaid Perspective for US and Selected State Programs.
| Estimates | US | Alabama | Washington, DC | Florida | Georgia | Louisiana | Maryland | Mississippi | North Carolina | South Carolina | Virginia |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total No. eligible for gene therapy | 5464 | 182 | 44 | 521 | 353 | 245 | 170 | 184 | 260 | 203 | 153 |
| No. to receive therapy, year 1 | 383 | 13 | 3 | 36 | 25 | 17 | 12 | 13 | 18 | 14 | 11 |
| No. treated per 100 000 members, year 1 | 0.52 | 1.41 | 1.18 | 0.96 | 1.36 | 1.11 | 0.90 | 1.99 | 1.02 | 1.37 | 1.04 |
| Budget impact, year 1 (million USD), $ | 707.63 | 23.58 | 5.71 | 67.43 | 45.67 | 31.66 | 22.02 | 23.77 | 33.65 | 26.32 | 19.76 |
| Budget impact, year 1 (% total Medicaid spending) | 0.12 | 0.42 | 0.20 | 0.29 | 0.41 | 0.29 | 0.19 | 0.45 | 0.25 | 0.42 | 0.21 |
| Cost per member per month, year 1, $ | 0.80 | 2.18 | 1.81 | 1.48 | 2.09 | 1.81 | 1.38 | 3.07 | 1.57 | 2.11 | 1.60 |
| Cost per member per month, year 5, $ | 0.53 | 1.45 | 1.21 | 0.98 | 1.39 | 1.20 | 0.92 | 2.04 | 1.05 | 1.41 | 1.06 |
| Budget impact, year 5 (million USD), $ | 471.25 | 15.70 | 3.80 | 44.91 | 30.42 | 21.09 | 14.67 | 15.83 | 22.41 | 17.53 | 13.16 |
Abbreviation: USD, US dollars.
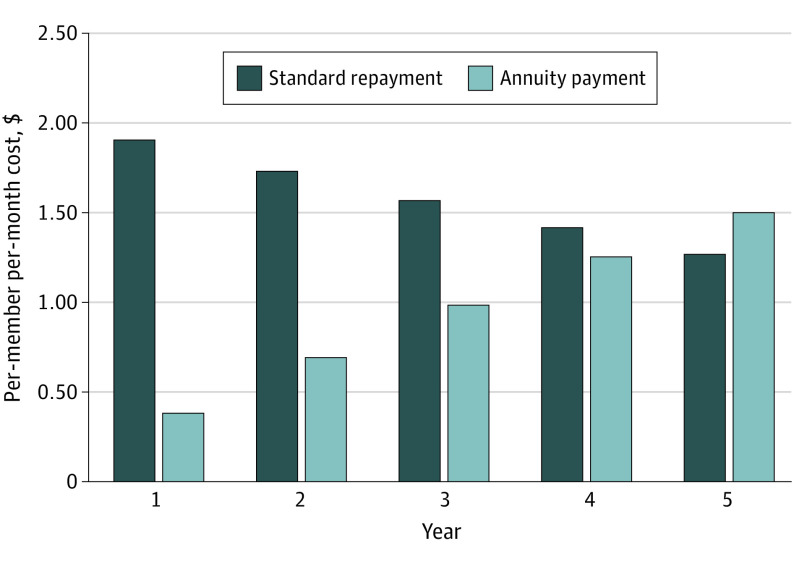
Over time, savings accrued and fewer individuals remained eligible for the therapy. An annual decrease in budget impact occurred across the 5-year time horizon. For Mississippi, the annual cost for years 1 through 5 was $23.8, $21.6, $19.5, $17.6, and $15.8 million (PMPM decreasing from $3.07 in year 1 to $2.04 in year 5). The mean budget impact across the 10 state perspectives decreased from $1.91 PMPM in year 1 to $1.27 in year 5.
Annuity Payment Model
Compared with the standard payment scenario, the 5-year annuity payment decreased the short-term budget impact during the first 4 years by deferring some spending beyond the 5-year horizon (Figure 1). By year 5, the annual budget impact in the annuity model exceeded that of the standard repayment. The same number of patients received the therapy in both scenarios. eFigures 2 to 12 in the Supplement demonstrate the PMPM cost for the standard vs annuity payment for each of the 10 states and for all US Medicaid programs in aggregate.
Figure 1. Standard vs Annuity Payment Model: Mean Per-Member Per-Month Budget Impact of Gene Therapy for 10-State Sample.
Sensitivity Analysis
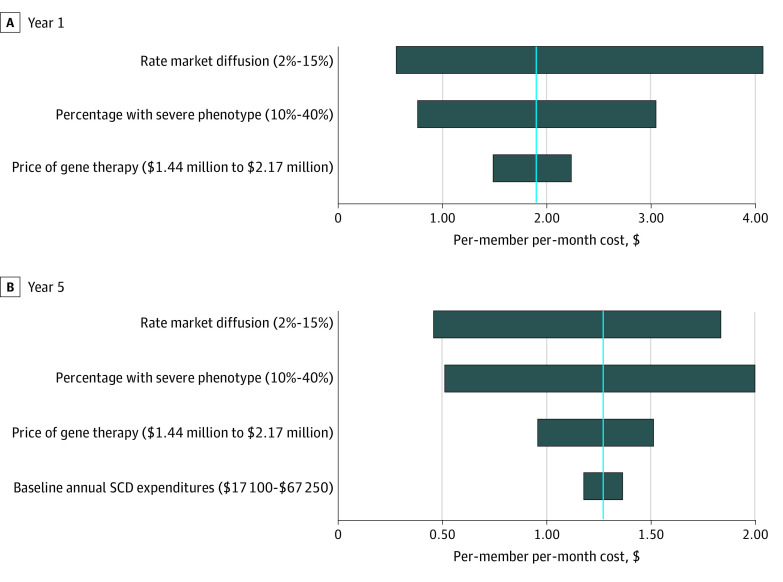
Figure 2 demonstrates the result of the sensitivity analysis at years 1 and 5 for the mean PMPM cost across the sample. Baseline SCD-related expenditures had no association with year 1 PMPM spending (no savings from averted expenditures in the first year), so this parameter is only in Figure 2B. The budget impact was most sensitive to changes in the rate of market diffusion. Conversely, list price of the therapy and baseline SCD-related expenditures had a more modest impact. For the gene therapy price range considered ($1.44 million to $2.17 million), the year 1 mean PMPM cost was $1.49 to $2.24.
Figure 2. Univariate Sensitivity Analysis: Mean Per-Member Per-Month Budget Impact for 10-State Sample.
Blue line indicates mean cost per member per month. SCD indicates sickle cell disease.
Discussion
Our results indicate that a commercially available gene therapy for severe SCD will likely generate a considerable short-term budget impact and potentially present affordability challenges for many Medicaid programs. For the 10 states with the highest prevalence of SCD, our results suggest a 1-year budget impact of nearly $2 PMPM. These findings demonstrate how, even under ideal circumstances (fully curative therapy with minimal associated costs of administration), this emerging class of durable one-time therapies may create unique financial challenges. We sought to focus on states with the highest prevalence of SCD, but from a national perspective, the budget impact is not trivial, at $0.80 PMPM for all US Medicaid programs in aggregate.
Although there is no threshold for budgetary significance for Medicaid plans, any new technology adding $1 to $3 PMPM is noteworthy, especially when applyed to few enrollees. No existing therapies are suitable for direct comparison, although gene therapy for spinal muscular atrophy provides context. One recent budget impact analysis estimated a PMPM cost of less than $0.10 for a US payer including the gene therapy onasemnogene abeparvovec-xioi on the formulary.26 Alternatively, we considered Medicaid spending on the DAA sofosbuvir in 2014. We estimated an average PMPM cost of $1.54 in the 10 Medicaid programs included in our analysis (in 2019 dollars).27 Our results indicate that gene therapy for SCD could present an affordability challenge comparable with that presented by the DAAs for some Medicaid plans.
It is unclear if this sizable budget impact would force Medicaid plans to implement strategies to limit access. As a society, we would need to consider the lack of alternative therapies and inequity in research funding for SCD compared with other diseases.28 Furthermore, the population with SCD may be especially marginalized and lacking the degree of organized advocacy seen in other disease areas such as cystic fibrosis. Several Medicaid programs have faced litigation in response to access restrictions for the cost-effective DAAs for hepatitis C.3 Extrapolating from the DAA experience would be tenuous, as the financing of prescription benefits differs from medical benefits. Because SCD will likely present the first of many affordability challenges in this emerging drug class, more research is needed evaluating potential reimbursement strategies.
These findings represent a theoretical 5-year period in which access to gene therapy for SCD is unencumbered by market entry delays owing to manufacturing or coverage negotiations, both of which have occurred in Europe with this therapy for β-thalassemia.29,30 The uptake in the first year after approval may be more limited than our 7% rate owing to new regulatory and manufacturing challenges. In addition, SCD affects racial/ethnic groups experiencing barriers to accessing care, systemic racism, and a history of exploitation within the US health care system. Even if the initial years after approval demonstrate limited diffusion, it is possible that annual demand may exceed the 166 individuals treated in year 1 of our model.
Upfront and 5-year annuity payment models were explored given uncertainty regarding the payment model to be used in the US. As seen in Figure 1, the annuity payment decreases the short-term budget impact, partly by moving some expense beyond the 5-year horizon. Such an approach may be desirable for payers looking to minimize short-term uncertainty and risk, but it provides no inherent savings or benefit when assuming a societal perspective. In Germany, a 5-year installment plan has been offered, with payment being contingent on patients with β-thalassemia remaining transfusion independent.31
For US payers, outcome-based contracting may be appealing for mitigating risk, although experience implementing such contracting is limited. Moreover, health system incentives and infrastructure may impede large-scale implementation of outcome-based reimbursements. Although there is uncertainty regarding alternative financing methods in the US, many agree that a nonstandard approach is needed.32,33 As evidenced by the controversy surrounding volume-based managed entry of the DAAs to the market for hepatitis C, such an approach for a curative therapy for SCD is unlikely to be accepted.
One-way sensitivity analysis demonstrated that the rate of market diffusion and the percentage of patients meeting eligibility criteria were both highly associated with the budget impact (Figure 2). Although the potential price of the therapy has received much attention, the price has a relatively modest impact when much uncertainty exists regarding the size of target population and market diffusion.
Our analyses synthesize available data and are not meant to suggest coverage decisions for Medicaid programs. Rather, they are meant to inform preexisting methods guiding coverage decisions based on states’ unique context. Payers will need to make decisions in the context of the disease burden among enrollees, as well as availability of alternative treatments (ie, comprehensive care and allogeneic HSCT).
Limitations
This study has some limitations. As with any theoretical analysis, the validity of the model inputs determines the validity of the findings. For example, our estimation of the proportion of patients with the severe phenotype meeting eligibility criteria was imprecise, with no data available to inform the parameter. The market diffusion rate was also a challenging parameter given the paucity of data for this drug class. Patients with SCD may be reluctant to be early adopters or early commercial success may increase demand. In addition, the eligibility criteria used may differ from the eventual product label as one trial plans to enroll children aged 2 to 14 years.25
The therapy’s price was extrapolated from European pricing for a different indication. We presumably underestimated the true cost of the therapy by omitting ancillary costs associated with preparation, administration, and follow-up.15 We assumed that the therapy cured all recipients and prevented any SCD-related costs in subsequent years. Even if the therapy proves to be this effective, organ damage from SCD may require ongoing care. Future analyses need to consider such health care use as clinical trial data mature. We intended to demonstrate how even an ideal therapy may still present short-term fiscal challenges when limited to those with severe disease. Last, our study does not consider potential improvements in quality of life or other meaningful outcomes—here we encourage future inquiry, including cost-effectiveness analysis.
Our findings cannot be generalized to other payer perspectives given the variable prevalence of SCD. In addition, variation in payer contracting and financing will influence affordability.
Conclusions
A gene therapy for severe SCD is likely to produce a considerable budget impact for many Medicaid plans while early clinical trial data suggest that this therapeutic class may considerably improve the lives of patients. With payers needing to balance short-term budgets, we expect some action will need to be taken to ensure affordability. This may prompt Medicaid plans to increase revenue, restrict access, or establish alternative reimbursement methods. Gene therapy for SCD is likely to provide an early demonstration of the unique financial challenges associated with this emerging drug class.
eFigure 1. Flow Diagram of the Analytic Framework
eTable. Disease Burden Among Medicaid Enrollees
eFigure 2. Standard vs Annuity Payment Model: PMPM for all US Medicaid Programs in Aggregate
eFigure 3. Standard vs Annuity Payment Model: PMPM for Alabama
eFigure 4. Standard vs Annuity Payment Model: PMPM for Washington, DC
eFigure 5. Standard vs Annuity Payment Model: PMPM for Florida
eFigure 6. Standard vs Annuity Payment Model: PMPM for Georgia
eFigure 7. Standard vs Annuity Payment Model: PMPM for Louisiana
eFigure 8. Standard vs Annuity Payment Model: PMPM for Maryland
eFigure 9. Standard vs Annuity Payment Model: PMPM for Mississippi
eFigure 10. Standard vs Annuity Payment Model: PMPM for North Carolina
eFigure 11. Standard vs Annuity Payment Model: PMPM for South Carolina
eFigure 12. Standard vs Annuity Payment Model: PMPM for Virginia
References
- 1.Quinn C, Young C, Thomas J, Trusheim M; MIT NEWDIGS FoCUS Writing Group . Estimating the clinical pipeline of cell and gene therapies and their potential economic impact on the US healthcare system. Value Health. 2019;22(6):621-626. doi: 10.1016/j.jval.2019.03.014 [DOI] [PubMed] [Google Scholar]
- 2.The FoCUS Project . Survey results: Payer perspectives on financing and reimbursement of one-time high-cost durable treatments. Massachusetts Institute of Technology Center for Biomedical Innovation. Published October 11, 2019. Accessed January 20, 2020. https://newdigs.mit.edu/sites/default/files/MIT FoCUS Payer Perspectives 2019F210v044.pdf
- 3.Wyden R, Grassley C. The price of Sovaldi and its impact on the U.S. health care system. Committee on Finance, United States Senate. Published December 2015. Accessed January 20, 2020. https://www.finance.senate.gov/imo/media/doc/1%20The%20Price%20of%20Sovaldi%20and%20Its%20Impact%20on%20the%20U.S.%20Health%20Care%20System%20(Full%20Report).pdf
- 4.Trusheim MR, Cassidy WM, Bach PB. Alternative state-level financing for hepatitis C treatment—the “Netflix model”. JAMA. 2018;320(19):1977-1978. doi: 10.1001/jama.2018.15782 [DOI] [PubMed] [Google Scholar]
- 5.Seeley E, Chimonas S, Kesselheim AS. Can outcomes-based pharmaceutical contracts reduce drug prices in the US? a mixed methods assessment. J Law Med Ethics. 2018;46(4):952-963. doi: 10.1177/1073110518821995 [DOI] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Data & statistics on sickle cell disease. Updated October 21, 2019. Accessed April 1, 2020. https://www.cdc.gov/ncbddd/sicklecell/data.html
- 7.Panepinto JA, Bonner M. Health-related quality of life in sickle cell disease: past, present, and future. Pediatr Blood Cancer. 2012;59(2):377-385. doi: 10.1002/pbc.24176 [DOI] [PubMed] [Google Scholar]
- 8.Ballas SK, Barton FB, Waclawiw MA, et al. Hydroxyurea and sickle cell anemia: effect on quality of life. Health Qual Life Outcomes. 2006;4:59. doi: 10.1186/1477-7525-4-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeBaun MR, Ghafuri DL, Rodeghier M, et al. Decreased median survival of adults with sickle cell disease after adjusting for left truncation bias: a pooled analysis. Blood. 2019;133(6):615-617. doi: 10.1182/blood-2018-10-880575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niihara Y, Miller ST, Kanter J, et al. ; Investigators of the Phase 3 Trial of l-Glutamine in Sickle Cell Disease . A phase 3 trial of l-glutamine in sickle cell disease. N Engl J Med. 2018;379(3):226-235. doi: 10.1056/NEJMoa1715971 [DOI] [PubMed] [Google Scholar]
- 11.Vichinsky E, Hoppe CC, Ataga KI, et al. ; HOPE Trial Investigators . A phase 3 randomized trial of voxelotor in sickle cell disease. N Engl J Med. 2019;381(6):509-519. doi: 10.1056/NEJMoa1903212 [DOI] [PubMed] [Google Scholar]
- 12.Ataga KI, Kutlar A, Kanter J, et al. Crizanlizumab for the prevention of pain crises in sickle cell disease. N Engl J Med. 2017;376(5):429-439. doi: 10.1056/NEJMoa1611770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kauf TL, Coates TD, Huazhi L, Mody-Patel N, Hartzema AG. The cost of health care for children and adults with sickle cell disease. Am J Hematol. 2009;84(6):323-327. doi: 10.1002/ajh.21408 [DOI] [PubMed] [Google Scholar]
- 14.Kanter J, Tisdale JF, Mapara MY, et al. Resolution of sickle cell disease manifestations in patients treated with lentiglobin gene therapy: updated results from the phase 1/2 Hgb-206 group C study. Blood. 2019;134(suppl_1):990. doi: 10.1182/blood-2019-128894 [DOI] [Google Scholar]
- 15.Walters M, Locatelli F, Thrasher A, et al. Safety of autologous hematopoietic stem cell transplantation with gene addition therapy for transfusion dependent beta-thalassemia, sickle cell disease and cerebral adrenoleukodystrophy. Presented at: Transplantation and Cellular Therapy Meeting, American Society for Transplantation and Cellular Therapy; February 5, 2020; Orlando, Florida. [Google Scholar]
- 16.Wilson-Frederick SH, Hulihan M, Anderson KK. Prevalence of sickle cell disease among Medicaid beneficiaries in 2012. Centers for Medicare & Medicaid Services Office of Minority Health. Published June 2019. Accessed November 10, 2019. https://www.cms.gov/About-CMS/Agency-Information/OMH/Downloads/Data-Highlight-16-Sickle-Cell-Disease.pdf
- 17.Arnold SD, Brazauskas R, He N, et al. Clinical risks and healthcare utilization of hematopoietic cell transplantation for sickle cell disease in the USA using merged databases. Haematologica. 2017;102(11):1823-1832. doi: 10.3324/haematol.2017.169581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beasley DM, Mathias T. Bluebird prices gene therapy at 1.58 million euros over 5 years. Reuters Health News. Published June 14, 2019. Accessed November 10, 2019. https://www.reuters.com/article/us-bluebird-bio-gene-therapy-price/bluebird-prices-gene-therapy-at-1-575-million-euros-over-five-years-idUSKCN1TF1HP
- 19.The Kaiser Family Foundation . State health facts: total Medicaid spending FY 2018. Updated August 2019. Accessed November 1, 2019. https://www.kff.org/medicaid/state-indicator/total-medicaid-spending/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D
- 20.Sullivan SD, Mauskopf JA, Augustovski F, et al. Budget impact analysis—principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health. 2014;17(1):5-14. doi: 10.1016/j.jval.2013.08.2291 [DOI] [PubMed] [Google Scholar]
- 21.Clinicaltrials.gov. A study evaluating the safety and efficacy of bb1111 in severe sickle cell disease. Published May 16, 2014. Accessed November 10, 2019. https://clinicaltrials.gov/ct2/show/NCT02140554
- 22.Leschly N. In pricing our gene therapy, Bluebird weighed value, shared risk, and a lifetime cap. STAT. Published November 26, 2019. Accessed February 1, 2020. https://www.statnews.com/2019/11/26/gene-therapy-pricing-bluebird-value-shared-risk-lifetime-cap/
- 23.Broder MS, Quock TP, Chang E, et al. The cost of hematopoietic stem-cell transplantation in the United States. Am Health Drug Benefits. 2017;10(7):366-374. [PMC free article] [PubMed] [Google Scholar]
- 24.Selden TM, Karaca Z, Keenan P, White C, Kronick R. The growing difference between public and private payment rates for inpatient hospital care. Health Aff (Millwood). 2015;34(12):2147-2150. doi: 10.1377/hlthaff.2015.0706 [DOI] [PubMed] [Google Scholar]
- 25.bluebird bio . bluebird bio Reports fourth quarter and full year 2019 financial results and highlights operational progress. Published February 18, 2020. Accessed April 1, 2020. https://investor.bluebirdbio.com/news-releases/news-release-details/bluebird-bio-reports-fourth-quarter-and-full-year-2019-financial
- 26.Malone D, Miller B, Dean R, et al. Use of single dose gene-replacement therapy for the treatment of spinal muscular atrophy type 1: a United States payer budget impact analysis. Value Health. 2019;22:S336-S337. doi: 10.1016/j.jval.2019.04.1644 [DOI] [Google Scholar]
- 27.Liao JM, Fischer MA. Early patterns of sofosbuvir utilization by state Medicaid programs. N Engl J Med. 2015;373(13):1279-1281. doi: 10.1056/NEJMc1506108 [DOI] [PubMed] [Google Scholar]
- 28.Farooq F, Mogayzel PJ, Lanzkron S, Haywood C, Strouse JJ. Comparison of US federal and foundation funding of research for sickle cell disease and cystic fibrosis and factors associated with research productivity. JAMA Netw Open. 2020;3(3):e201737-e201737. doi: 10.1001/jamanetworkopen.2020.1737 [DOI] [PubMed] [Google Scholar]
- 29.bluebird bio . bluebird bio Reports third quarter 2019 financial results and highlights operational progress. Published October 31, 2019. Accessed April 6, 2020. https://investor.bluebirdbio.com/news-releases/news-release-details/bluebird-bio-reports-third-quarter-2019-financial-results-and
- 30.National Institute for Health and Care Excellence . Betibeglogene autotemcel for treating transfusion-dependent beta-thalassemia [ID968]. Updated April 6, 2020. Accessed April 6, 2020. https://www.nice.org.uk/guidance/indevelopment/gid-ta10334
- 31.bluebird bio . bluebird bio Announces launch in Germany of Zynteglo (autologous CD34+ cells encoding βA-T87Q-globin gene) gene therapy for patients 12 years and older with transfusion-dependent β-thalassemia who do not have β0/β0 genotype. Published January 13, 2020. Accessed April 6, 2020. https://investor.bluebirdbio.com/news-releases/news-release-details/bluebird-bio-announces-launch-germany-zynteglotm-autologous-cd34
- 32.The FoCUS Project . Precision financing solutions for durable/potentially curative therapies. Massachusetts Institute of Technology Center for Biomedical Innovation website. Published January 24, 2019. Accessed November 10, 2020. https://newdigs.mit.edu/sites/default/files/MIT%20FoCUS%20Precision%20Financing%202019F201v023.pdf
- 33.Basu A. Financing cures in the United States. Expert Rev Pharmacoecon Outcomes Res. 2015;15(1):1-4. doi: 10.1586/14737167.2015.990887 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Flow Diagram of the Analytic Framework
eTable. Disease Burden Among Medicaid Enrollees
eFigure 2. Standard vs Annuity Payment Model: PMPM for all US Medicaid Programs in Aggregate
eFigure 3. Standard vs Annuity Payment Model: PMPM for Alabama
eFigure 4. Standard vs Annuity Payment Model: PMPM for Washington, DC
eFigure 5. Standard vs Annuity Payment Model: PMPM for Florida
eFigure 6. Standard vs Annuity Payment Model: PMPM for Georgia
eFigure 7. Standard vs Annuity Payment Model: PMPM for Louisiana
eFigure 8. Standard vs Annuity Payment Model: PMPM for Maryland
eFigure 9. Standard vs Annuity Payment Model: PMPM for Mississippi
eFigure 10. Standard vs Annuity Payment Model: PMPM for North Carolina
eFigure 11. Standard vs Annuity Payment Model: PMPM for South Carolina
eFigure 12. Standard vs Annuity Payment Model: PMPM for Virginia