Abstract
Medulloblastoma is the most common malignant pediatric brain tumor. Amplification of c-MYC is a hallmark of a subset of poor-prognosis medulloblastoma. MYC upregulates glutamine metabolism across many types of cancer. We modified the naturally occurring glutamine antagonist 6-diazo-5-oxo-l-norleucine (DON) by adding 2 promoeities to increase its lipophilicity and brain penetration creating the prodrug isopropyl 6-diazo-5-oxo-2-(((phenyl (pivaloyloxy) methoxy) - carbonyl) amino) hexanoate, termed JHU395. This prodrug was shown to have a 10-fold improved CSF-to-plasma ratio and brain-to-plasma ratio relative to DON. We hypothesized that JHU395 would have superior cell penetration compared with DON and would effectively and more potently kill MYC-expressing medulloblastoma. JHU395 treatment caused decreased growth and increased apoptosis in multiple human high-MYC medulloblastoma cell lines at lower concentrations than DON. Parenteral administration of JHU395 in Nu/Nu mice led to the accumulation of micromolar concentrations of DON in brain. Treatment of mice bearing orthotopic xenografts of human MYC-amplified medulloblastoma with JHU395 increased median survival from 26 to 45 days compared with vehicle control mice (p < 0.001 by log-rank test). These data provide preclinical justification for the ongoing development and testing of brain-targeted DON prodrugs for use in medulloblastoma.
Keywords: 6-diazo-5-oxo-l-norleucine, Cancer metabolism, DON, Pediatric brain tumor, Prodrug
INTRODUCTION
Abnormal metabolism is a hallmark of cancer. High-level expression of MYC increases glutamine transport and use (1). Human and mouse studies using FDG-glutamine demonstrated increased glutamine uptake in cancer cells compared with normal brain (2). MYC-amplification defines a subtype of medulloblastoma, which is the most common pediatric brain tumor. MYC-amplified medulloblastoma has a worse outcome compared with non-MYC amplified medulloblastoma (3). MYC-amplification is found in large cell/anaplastic medulloblastoma, and this histologic feature is associated with increased risk of death (4). A magnetic resonance spectroscopy study found that medulloblastoma patients with high levels of glutamate in their tumors, potentially indicative of increased glutamine use, had significantly worse survival than patients with low glutamate levels (5).
Drugs that antagonize glutamine are increasingly being tested against MYC-driven malignancies (6–8). The naturally occurring compound DON (6-diazo-5-oxo-l-norleucine) was tested in adult and pediatric clinical trials, but was never systematically tested against MYC-driven brain tumors (9). To increase DON’s applicability to clinical use, we modified DON to improve oral bioavailability and showed that the DON prodrug JHU083 had activity against MYC-amplified medulloblastoma in vitro and in vivo (10). We have subsequently further modified DON to improve brain penetration, and we hypothesized that this new brain-targeted DON prodrug called JHU395 would kill MYC-driven medulloblastoma cells.
MATERIALS AND METHODS
JHU395
The synthesis of JHU395 prodrug (Dracen Pharmaceuticals, New York, NY), isopropyl 6-diazo-5-oxo-2-(((phenyl (pivaloyloxy) methoxy) - carbonyl) amino) hexanoate (JHU395), was conducted as detailed previously (11). For in vitro and in vivo testing, JHU395 was dissolved in 100% sterile dimethyl sulfoxide (DMSO) (Millipore Sigma, Burlington, MA).
Cell Culture
The patient-derived medulloblastoma cell lines D283MED and D425MED, first established at Duke University, were grown in MEM media (Gibco, Waltham, MA) supplemented with 5% fetal bovine serum (FBS) (Gibco) and 1% non-essential amino acids (NEAA) (Gibco) (12). The MED211 patient-derived xenograft was obtained from the Brain Tumor Resource Lab and has been previously described (10, 13). A cell line was derived from the MED211 PDX model by removing tumor tissue from tumor-bearing mice at the time of death and passing tumor through a P1000 pipette. Cells were grown in epidermal growth factor (EGF) and fibroblast growth factor (FGF) (Peprotech, Rocky Hill, NJ) media. We used lentivirus containing MYC, R248W TP53 or BMI1 to immortalize human neural stem cells, as previously described (14, 15). We subsequently transduced these cells with constitutively active AKT and hTERT creating immortalized cells harboring MYC/DNp53/AKT/hTERT or BMI1/DNp53/AKT/hTERT. Expression of oncogenes was confirmed by Western blot (15). All cells were verified to be mycoplasma-free by PCR testing. Cell line identity testing was performed by the Johns Hopkins Genetic Resources Core Facility and is included in Supplementary Data Figure S1.
Cell Growth Assay
Experiments were performed in 96-well plates. Equal numbers of cells were plated in triplicate for each treatment. 20 μL of CellTiterBlue (Promega, Madison, WI) was added to each well for every 100 μL of media and incubated at 37°C for 3 hours. Absorbance was measured on a plate reader with TECAN read Infinite M1000 PRO on days 0, 3, and 5. Growth between vehicle and drug-treated cells was statistically analyzed on day 5 with 1-way analysis of variance (ANOVA) with Dunnet’s multiple comparison test using GraphPad Prism.
Western Blots
Protein was extracted from cell pellets using RIPA buffer (Millipore Sigma) and quantified using a Bradford Assay. Antibody against cleaved-PARP is from Cell Signaling Technologies (no. 9541) (Danvers, MA). Antibody against Beta ACTIN (sc-47778) is from Santa Cruz Biotechnologies (Dallas, TX). The following dilutions were used cleaved-PARP (1:1000), Beta ACTIN (1:1000). Peroxidase-labeled secondary antibodies are from Cell Signaling Technologies and used at a 1:3500 dilution. Bands were quantified using ImageJ.
BrdU and Cleaved Caspase-3 Immunofluorescence
Following 72-hour drug treatment, cells were incubated with bromo-deoxyuridine (BrdU) (Cell Signaling Technologies) for 6 hours and fixed in cytospin fluid and spun onto glass slides using a cytocentrifuge as described previously (16). Cells were permeabilized in 0.1% TritonX, blocked in 5% normal goat serum, and incubated with anti-Brdu and anticleaved caspase-3 antibody diluted 1:400 (Cell Signaling Technology, clone 5A1E), followed by a secondary antibody conjugated to Cy3 diluted 1:500 (Jackson ImmunoResearch, West Grove, PA). 4′,6-diamidino-2-phenylindole (DAPI) (Roche Diagnostics, Mannheim, Germany) was used as a counterstain. Five DAPI and the corresponding Cy3 images were taken for each slide. The number of DAPI- and Cy3-positive cells were counted using Imagescope. For each pair of images, the percent of Cy3-positive cells was calculated. The average Cy3 positivity was determined by calculating the average of at least 5 pairs of images for each treatment. Images were blinded before counting. Vehicle and drug-treated cells were compared by Student t-test.
Tumor Cell-to-Plasma Partitioning
D425MED cells grown under standard conditions were centrifuged and washed with Dulbecco’s phosphate buffered saline (PBS) (Gibco, Cat. no. 14-190-144) and resuspended in human plasma (Innovative Research, Inc., Novi, MI) to obtain a cell density of 10 million cells/mL of plasma. For analysis of cell partitioning, 1 mL of the cell-plasma suspension was preincubated at 37°C for 5 minutes; then, JHU395 was added to a final concentration of 20 μM. Cells were reincubated at 37°C for 1 hour. Following incubation, the cell suspension was centrifuged at 10 000 g for 10 minutes at 4°C and supernatant plasma was collected and stored at −80°C until bioanalysis. The cell pellet was washed once with ice-cold Dulbecco’s PBS (Gibco), followed by centrifugation and stored at −80°C for intact JHU395 and DON bioanalysis. Briefly, the calibration curves for quantification of analytes were prepared using naïve D425 medulloblastoma cells or human plasma. Calibration curves were constructed over the range 0.03–100 nmol/mL for DON and 10–20 μmol/mL for JHU395. For extraction of analytes, pellets were weighed and suspended in a known quantity of water. To 50 μL of cell suspension or plasma, ice-cold methanol containing internal standards (losartan: 0.5 μM and glutamate-d5: 10 μM) was added in a 5:1 ratio. Samples were vortex-mixed, and centrifuged at 10 000g for 10 minutes at 4°C. The supernatant was collected and analyzed for both DON and intact prodrug as previously described (17, 18).
Animal Studies
After induction of general anesthesia with ketamine/xylazine in Nu/Nu mice, a burr hole was made in the skull of female Nu/Nu mice Charles River (Wilmington, MA) 1 mm to the right of and 2 mm posterior to the lambdoid suture with an 18-gauge needle. The needle of a Hamilton syringe was inserted to a depth of 2.5 mm into the cerebellum using a needle guard, and 100,000 D425MED cells in 3 μL of media were injected. JHU395 was prepared DMSO and administered by IP injection on day 10 after surgery with 15 mg/kg in a 100 μL bolus twice weekly on Tuesdays and Fridays. Animals were monitored daily. Symptomatic individuals were killed, brains removed and fixed in formalin. Survival curves were analyzed using a Log-rank test. For the JHU395 brain penetration pharmacokinetic study, athymic nude mice without brain tumors were given a single DON-equivalent dose of 20 mg/kg JHU395 dissolved in DMSO by IP. Exactly 1-hour postdose, mice were killed and the brain regions manually dissected and flash frozen. Dosing of the animals was staggered to ensure each animal was only exposed to the drug for 1 hour. Extraction and quantification of DON was performed as previously described (19). For animal care and anesthesia, “Principles of laboratory animal care” (NIH publication No. 8623, revised 1985) was followed, using a protocol approved by the Johns Hopkins Animal Care and Use Committee, in compliance with the United States Animal Welfare Act regulations and Public Health Service Policy.
RESULTS
Structure of JHU395
To enhance brain penetration of DON, we created a prodrug with increased lipophilicity. Figure 1 shows the structure of this prodrug, JHU395. The intracellular environment coverts JHU395 to active DON (11, 17).
FIGURE 1.
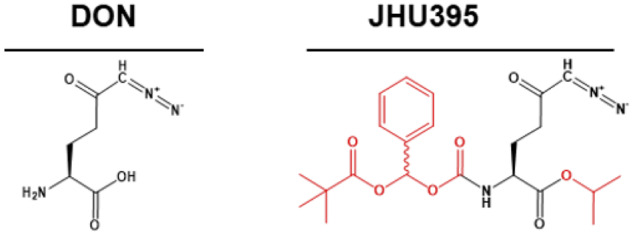
Chemical structure of 6-diazo-5-oxo-l-norleucine and (isopropyl 6-diazo-5-oxo-2-(((phenyl (pivaloyloxy) methoxy) - carbonyl) amino) hexanoate).
DON Prodrug JHU395 Suppresses MYC-Amplified Medulloblastoma Growth
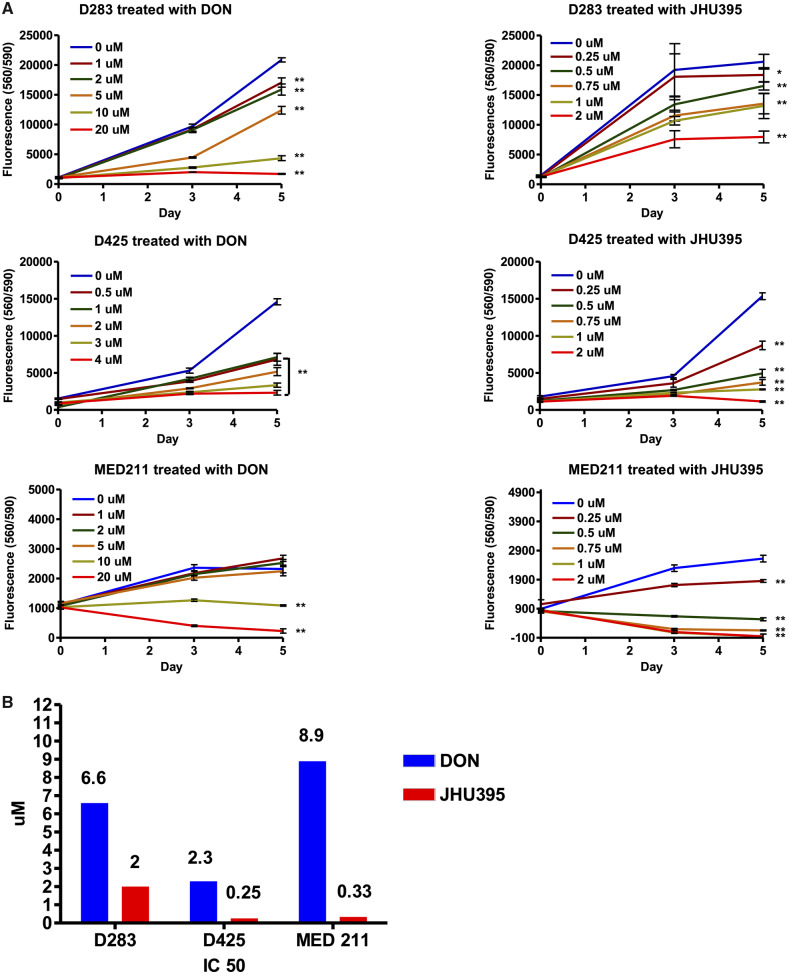
Treatment with JHU395 significantly inhibited the growth of multiple MYC-amplified medulloblastoma cell lines at lower concentrations compared with DON, as analyzed on day 5 with 1-way ANOVA with Dunnet’s multiple comparison test. In D283MED, DON inhibited growth at 1 µM and above (p < 0.01 compared with vehicle control). In contrast, 0.25 and 0.5 µM JHU395 suppressed growth in this cell line (p < 0.05 and 0.01 respectively; Fig. 2A). Similar results were seen when we treated D425MED cells with DON, with suppression of growth at 0.5 µM (p < 0.01) while JHU395 suppressed growth starting at 0.25 µM (p < 0.01). In MED211, DON inhibited growth at 10 µM and above (p < 0.01), while JHU395 suppressed growth starting at 0.25 µM (p < 0.01).
FIGURE 2.
Isopropyl 6-diazo-5-oxo-2-(((phenyl (pivaloyloxy) methoxy) - carbonyl) amino) hexanoate (JHU395) decreases growth of multiple high MYC-expressing medulloblastoma cell lines. (A) CelltiterBlue growth assays showing JHU395 decreased growth of multiple cell lines expressing MYC at lower concentrations compared to 6-diazo-5-oxo-l-norleucine (DON). Asterisks indicate p < 0.05, double asterisks indicate p < 0.01 by 1-way ANOVA with Dunnet’s multiple comparison test compared with control on day 5. Representative graphs are shown. Each experiment was repeated a minimum of 3 times with similar results. (B) IC50 of DON and JHU395 were calculated by GraphPad Prism on different high MYC-expressing medulloblastoma cell lines.
Half-maximal inhibitory concentrations (IC50) values were calculated for all 3 MYC-amplified cell lines at day 5 of treatment. For D283MED, the IC50 of DON was 6.6 µM versus 2 µM for JHU395. In D425MED, the IC50 of DON was 2.3 µM while the IC50 of JHU395 was 0.25 µM. In MED211, the IC50 of DON was 8.9 µM and the IC50 of JHU395 was 0.33 µM (Fig. 2B).
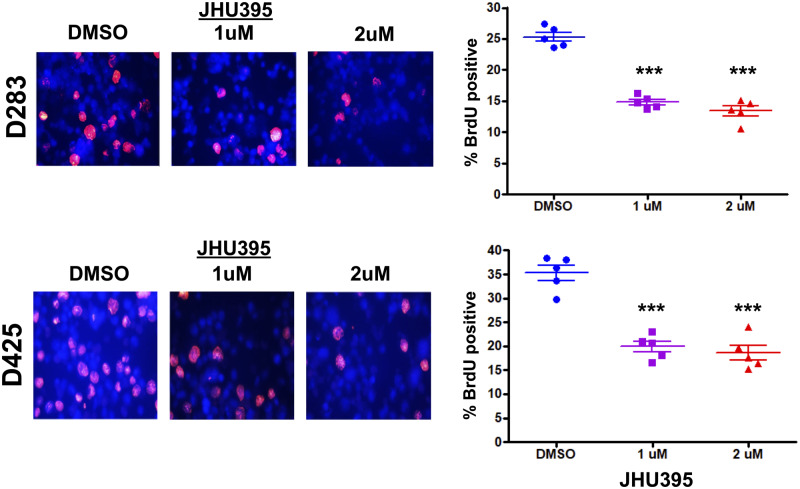
Cells treated with JHU395 at 1 and 2 µM for 72 hours showed significantly decreased BrdU expression as measured by immunofluorescence staining, statistically analyzed by Student t-test compared with vehicle control. In D283MED, BrdU positivity decreased from 25% in vehicle-treated cells to 14.8% and 13.3% when treated with 1 and 2 µM JHU395, respectively (p < 0.01). Similar results were seen in D425MED, in which vehicle-treated cells were 35.2% BrdU-positive while treatment with 1 and 2 μM JHU395 led to 19.8% and 18.5% BrdU positivity, respectively (p < 0.01; Fig. 3).
FIGURE 3.
Isopropyl 6-diazo-5-oxo-2-(((phenyl (pivaloyloxy) methoxy) - carbonyl) amino) hexanoate (JHU395) decreases cell proliferation. Cells treated with 1 or 2 μM JHU395 for 72 hours show decreased incorporation of BrdU by immunofluorescence. Top: 200× photomicrographs of BrdU immunofluorescence (red) of D283 medulloblastoma cells treated with JHU395 for 72 hours. DAPI counterstains nuclei blue. Bottom: BrdU immunofluorescence of D425 medulloblastoma treated with JHU395 for 72 hours. Graphs at right show quantification. Bars represent range with each replicate result represented by a solid dot. ***p < 0.001 by Student t-test (n = 5 biological replicates).
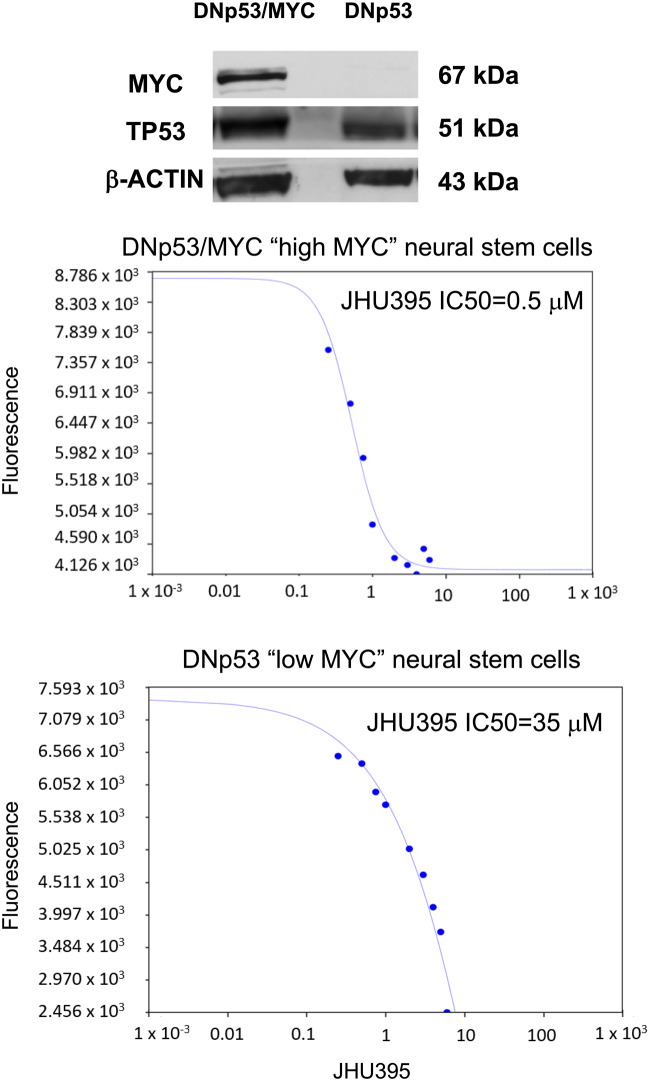
Because MYC controls many aspects of glutamine metabolism, we hypothesized that JHU395 would have greater effect on high-MYC cells compared with low-MYC cells (1). JHU395 inhibited the growth of neural stem cells transduced with MYC and R248W p53 (high MYC cells) to a greater extent than neural stem cells transduced with BMI1 and R248W p53 (low MYC cells). The IC50 of JHU395 was 0.5 µM for high MYC cells as compared with 35 µM for low MYC cells (Fig. 4).
FIGURE 4.
MYC transformed cells are more sensitive to isopropyl 6-diazo-5-oxo-2-(((phenyl (pivaloyloxy) methoxy) - carbonyl) amino) hexanoate (JHU395). JHU395 inhibited the growth of neural stem cells transduced with MYC and R248W p53 (high MYC cells) to a greater extent than neural stem cells transduced with BMI1 and R248W p53 (low MYC cells). The IC50 of JHU395 was 0.5 µM for high MYC cells as compared with 35 µM for low MYC cells.
JHU395 Treatment Increases Apoptosis in MYC-Expressing Medulloblastoma Cell Lines
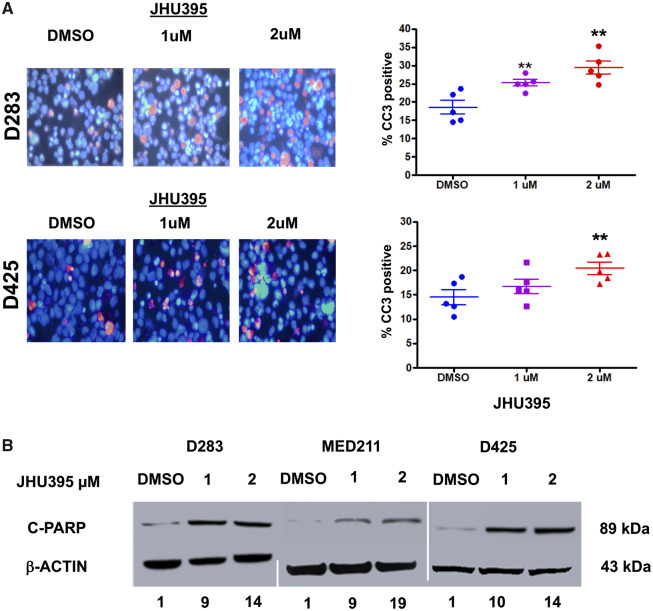
JHU395 treatment of medulloblastoma cell lines for 72 hours caused an increase in the expression of cleaved caspase-3 (CC3) as measured by immunofluorescence, as analyzed by Student t-test compared with vehicle control (Fig. 5A). In D283MED, CC3 expression increased to 25.2% and 29.4% when treated with 1 and 2 µM JHU395, respectively, compared with 18.5% in vehicle (p < 0.01). Similar patterns were seen in D425MED. When treated with 1 and 2 µM JHU395, CC3 expression increased to 16.8% and 20.4%, respectively, compared with 14.4% in vehicle control (p < 0.01). JHU395 treatment also led to increased apoptotic cell death as measured by cleaved-PARP Western blot. We detected a 9- to-19-fold increase in cPARP as measured by Western blot with JHU395 treatment compared with vehicle control (Fig. 5B).
FIGURE 5.
Isopropyl 6-diazo-5-oxo-2-(((phenyl (pivaloyloxy) methoxy) - carbonyl) amino) hexanoate (JHU395) treatment induces cell death by apoptosis. (A) 200× magnification photomicrographs of cleaved caspase-3 immunofluorescence of medulloblastoma cells treated for 72 hours with 1 and 2 μM JHU395 or DMSO control. DAPI counterstains nuclei blue. Quantification of results is represented in graphs at right. **p < 0.01, ***p < 0.001 by Student t-test compared with vehicle control (n = 5 biological replicates). Bars represent range with each replicate result represented by a solid dot. (B) Treatment with 1 and 2 μM JHU395 causes increased cleaved-PARP expression as determined by Western blot. Numbers below the blot indicate fold-increase of cleaved-PARP expression compared with vehicle control. Cleaved-PARP expression was normalized to Actin.
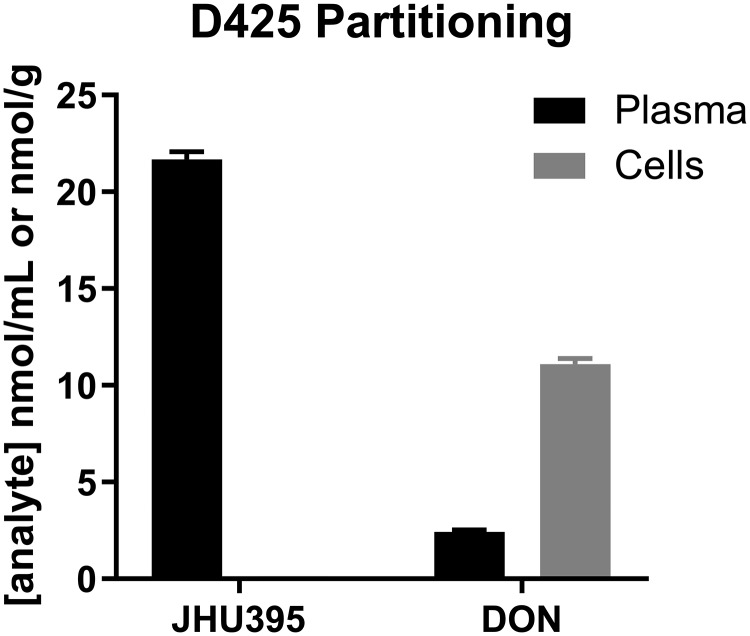
To understand JHU395 prodrug delivery of DON into high-MYC medulloblastoma cells, we investigated the partitioning of JHU395 into D425MED cells incubated in human plasma as previously described (17). After 1-hour incubation with 20 μM JHU395 in D425MED cells in human plasma, the intact prodrug was detected primarily in plasma (21 nmol/mL), and there was a minimal amount in cells (0.1 nmol/g). In contrast, DON release from JHU395 was higher in cells (11 nmol/g) versus plasma (2.5 nmol/L) with cell-to-plasma ratio of ∼4.5 (Fig. 6).
FIGURE 6.
Tumor cell-to-plasma partitioning. Intact Isopropyl 6-diazo-5-oxo-2-(((phenyl (pivaloyloxy) methoxy) - carbonyl) amino) hexanoate (JHU395) and 6-diazo-5-oxo-l-norleucine (DON) quantification (nmol/mL plasma or nmol/g cell) in D425 medulloblastoma cells following 1-hour incubation with 20 μmol/L JHU395. After 1-hour incubation with JHU395 in D425 medulloblastoma cells, the intact prodrug was detected in plasma (21 nmol/L) with minimal amount in cells (0.1 nmol/g). DON release from JHU395 was higher in cells (11 nmol/g) versus plasma (2.5 nmol/L) with cell- to-plasma ratio of ∼4.5.
Systemic Administration of JHU395 Achieved Micromolar Concentrations of DON in the Brain
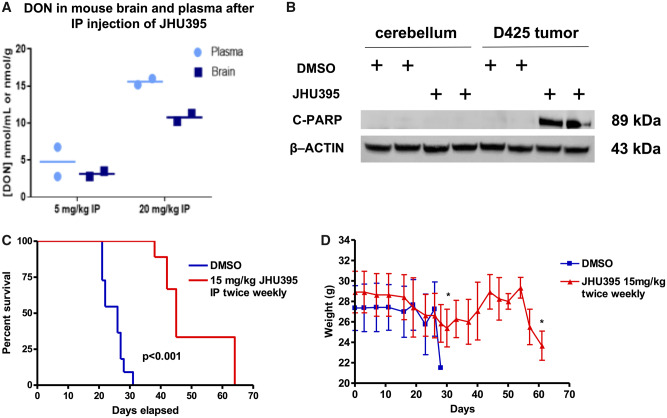
To determine plasma/brain ratio of DON delivered by JHU395, we administered JHU395 intraperitoneally to Nu/Nu mice. After 1 hour, the animals were killed and the brains removed. Intact prodrug and DON were measured by LC/MS-MS as we have previously described (11, 18). IP dosing resulted in an average 0.66 brain-to-plasma ratio of DON (Fig. 7A). We measured 4 μM concentration of DON in the brains of mice that received a dose of 5 mg/kg JHU-395. A higher 20 mg/kg IP dose led to an average concentration of 11.3 µM of DON in the brain.
FIGURE 7.
Isopropyl 6-diazo-5-oxo-2-(((phenyl (pivaloyloxy) methoxy) - carbonyl) amino) hexanoate (JHU395) administration leads to micromolar levels of 6-diazo-5-oxo-l-norleucine (DON) in brain as measured by mass spectrometry with resulting increase in survival of mice bearing medulloblastoma orthotopic xenografts. (A) Parenteral administration of JHU395 dosing at 5 or 20 mg/kg in Nu/Nu mice led to the accumulation of micromolar concentrations of DON in brain as measured by mass spectrometry. IP dosing resulted in an average 0.66 brain-to-plasma ratio. The brain had an average concentration of 11.3 nmol/ml of DON after 20 mg/kg JHU395 intraperitoneal administration. (B) Single treatment of JHU395 after 72 hours showed significant effect in inducing apoptotic cells death specifically on D425MED but not on normal cerebellum. (C) Twice weekly 15 mg/kg dosing of JHU395 significantly extended the survival of athymic nude mice with D425MED tumors. Red line, JHU-395; blue line, vehicle. p < 0.001 comparing treated vs vehicle control as determined by Log-rank test n = 10 animals in the control group and n = 9 animals in the treatment group). (D) Representative graph shows the mouse weights were stable during the treatment course. At week 3 of the study, mean mouse weight reduced around 10% but then subsequently recovered back to baseline. Both control and treatment mice lost weight in the few days immediately before death due to progression of tumor.
Because 2 µM JHU395 successfully reduced growth of MYC-expressing medulloblastoma cell lines (Figs. 2 and 3) and induced apoptosis (Fig. 4), we hypothesized that a 15 mg/kg IP dose of JHU395 would be effective in treating orthotropic human medulloblastoma xenografts in mice with an intermittent dosing schedule.
JHU395 Induces Apoptosis in MYC-Driven Medulloblastoma Orthotopic Xenografts
To determine if JHU395 could also induce apoptosis in vivo in high-MYC medulloblastoma, we treated mice bearing orthotopic D425 MED tumors with a single dose of JHU395 (15 mg/kg) or DMSO and killed mice 72 hours later. We extracted protein from tumor-involved cerebellum as well as the uninvolved contralateral cerebellum. Western blot for cPARP showed a single dose of JHU395 induced apoptosis in high MYC-amplified medulloblastoma but not in normal control cerebellum (Fig. 7B).
JHU395 Extends the Survival of Animals with MYC-Driven Medulloblastoma Orthotopic Xenografts
Nude mice treated with 15 mg/kg twice weekly of JHU395 (15 mg/kg twice weekly) had a median survival 45 days compared with 24 days for vehicle-control-treated mice (p < 0.001 by Log-rank test; Fig. 7C). Mouse weights were stable during the treatment course. At week 3 of the study, median mouse weight was reduced around 10% in the JHU-395 group, but the mice then gained back the lost weight after 1 week (Fig. 7D). Both groups lost weight when symptomatic from eventual tumor progression, leading to death.
DISCUSSION
The abnormal expression of MYC occurs in many types of cancers (20). MYC drives growth by promoting signal transduction pathways and upregulating the metabolism of both glucose and the amino acid glutamine to accumulate biomass. Glutamine is a nonessential amino acid and is the primary nitrogen source for synthesis of nucleic acids, other amino acids, and hexosamines. Glutamine can also be used to replenish TCA cycle intermediates in an anaplerosis reaction (21, 22). One of the most well-established roles of MYC in cancers is upregulating glutamine metabolism, which occurs across many types of cancers (23–26). This makes cancer cells reliant on glutamine to grow and proliferate and renders them vulnerable to glutamine depletion in vitro (27). We found that the DON prodrug JHU395 killed MYC-driven medulloblastoma at concentrations 450-fold lower (0.25–2 vs 900 μM) than what was recently reported for DON against the non-MYC medulloblastoma cell lines UW228 and DAOY (28), suggesting that the presence of MYC may render cells more sensitive to glutamine inhibitors. We further extended this finding to show that human neural stem cells transduced with MYC and mutant p53 were more sensitive to JHU395 than human neural stem cells transduced with BMI1 and mutant p53 (Fig. 4).
MYC-amplified medulloblastoma is highly resistant to our current therapy, with <50% of patients having long-term survival (29). New therapies are desperately needed. Targeting abnormal metabolism downstream of MYC may disrupt key metabolic pathways in MYC-driven medulloblastoma that may provide a significant therapeutic index. We showed that a single dose of JHU395 induces apoptosis as measured by cPARP in D425MED orthotopic xenografts but does not induce apoptosis in normal brain from the same animal, highlighting the on-target effects of JHU395 on tumor cells in vivo (Fig. 7B).
We here demonstrate that a novel prodrug of DON has increased potency against MYC-amplified medulloblastoma and suppresses the growth of MYC-amplified medulloblastoma orthotopic xenografts. We find that JHU395 has higher potency compared with DON in suppressing and killing MYC-driven medulloblastoma. We have previously noted the efficacy of another DON prodrug, JHU083, in high-MYC medulloblastoma (10). JHU395 differs from JHU083 in that it is significantly more hydrophobic, which leads to improved penetration into cells and into the brain (11).
The increased potency of JHU-395 against MYC-amplified medulloblastoma compared with DON suggests that lower doses may be efficacious in the clinic. Although DON was generally well tolerated in pediatric early clinical trials (9), gastrointestinal toxicity was a dose-limiting finding in adult clinical trials (30). The brain penetration of DON in humans is unclear, so engineering prodrugs that can deliver DON efficiently to the brain and limit GI exposure may enhance applicability to the clinic. We anticipate that on-going engineering of glutamine antagonist prodrugs will enhance brain penetration and reduce systemic exposure, thereby further broadening the therapeutic index.
Supplementary Material
This study was supported by NINDS 1R01NS103927 (B.S.S. and E.H.R.); NCI R01 R01CA229451 (B.S.S.), the Spencer Grace Foundation (E.H.R.), the Ace for a Cure Foundation (E.H.R.) Giant Food Pediatric Cancer Research Fund; National Cancer Institute Core Grant to the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center (P30CA006973).
Under a license agreement between Dracen Pharmaceuticals, Inc. and the Johns Hopkins University, Dr Slusher, Dr Rais, and Mr Alt are entitled to royalty distributions related to technology used in the research described in this publication. In addition, Dr Slusher and Dr Rais are founders of and hold equity in Dracen Pharmaceuticals, Inc. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies.
Supplementary Data can be found at http://academic.oup.com/jnen.
REFERENCES
- 1. Dang CV. Rethinking the Warburg effect with Myc micromanaging glutamine metabolism. Cancer Res 2010;70:859–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Venneti S, Dunphy MP, Zhang H, et al. Glutamine-based PET imaging facilitates enhanced metabolic evaluation of gliomas in vivo. Sci Transl Med 2015;7:274ra17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eberhart CG, Kratz J, Wang Y, et al. Histopathological and molecular prognostic markers in medulloblastoma: c-myc, N-myc, TrkC, and anaplasia. J Neuropathol Exp Neurol 2004;63:441–9 [DOI] [PubMed] [Google Scholar]
- 4. Eberhart CG, Kepner JL, Goldthwaite PT, et al. Histopathologic grading of medulloblastomas: A Pediatric Oncology Group study. Cancer 2002;94:552–60 [DOI] [PubMed] [Google Scholar]
- 5. Wilson M, Gill SK, MacPherson L, et al. Non-invasive detection of glutamate predicts survival in pediatric medulloblastoma. Clin Cancer Res 2014;20:4532–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang J, Guo Y, Seo W, et al. Targeting cellular metabolism to reduce head and neck cancer growth. Sci Rep 2019;9:4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Han T, Guo M, Zhang T, et al. A novel glutaminase inhibitor-968 inhibits the migration and proliferation of non-small cell lung cancer cells by targeting EGFR/ERK signaling pathway. Oncotarget 2017;8:28063–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gross MI, Demo SD, Dennison JB, et al. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol Cancer Ther 2014;13:890–901 [DOI] [PubMed] [Google Scholar]
- 9. Sullivan MP, Nelson JA, Feldman S, et al. Pharmacokinetic and phase I study of intravenous DON (6-diazo-5-oxo-L-norleucine) in children. Cancer Chemother Pharmacol 1988;21:78–84 [DOI] [PubMed] [Google Scholar]
- 10. Hanaford AR, Alt J, Rais R, et al. Orally bioavailable glutamine antagonist prodrug JHU-083 penetrates mouse brain and suppresses the growth of MYC-driven medulloblastoma. Transl Oncol 2019;12:1314–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nedelcovych MT, Tenora L, Kim BH, et al. N-(pivaloyloxy)alkoxy-carbonyl prodrugs of the glutamine antagonist 6-diazo-5-oxo-l-norleucine (DON) as a potential treatment for HIV associated neurocognitive disorders. J Med Chem 2017;60:7186–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. He XM, Wikstrand CJ, Friedman HS, et al. Differentiation characteristics of newly established medulloblastoma cell lines (D384 Med, D425 Med, and D458 Med) and their transplantable xenografts. Lab Invest 1991;64:833–43 [PubMed] [Google Scholar]
- 13. Brabetz S, Leary SES, Grobner SN, et al. A biobank of patient-derived pediatric brain tumor models. Nat Med 2018;24:1752–61 [DOI] [PubMed] [Google Scholar]
- 14. Hanaford AR, Archer TC, Price A, et al. DiSCoVERing innovative therapies for rare tumors: Combining genetically accurate disease models with in silico analysis to identify novel therapeutic targets. Clin Cancer Res 2016;22:3903–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Taylor I, Ahsan S, Price A, et al. Abstract 494: MYC, dominant negative p53 and AKT transform human neural stem cells into primitive neuro-ectodermal tumors sensitive to glutaminase inhibitors. Cancer Res 2015;75:494–494. [Google Scholar]
- 16. Weingart MF, Roth JJ, Hutt-Cabezas M, et al. Disrupting LIN28 in atypical teratoid rhabdoid tumors reveals the importance of the mitogen activated protein kinase pathway as a therapeutic target. Oncotarget 2015;6:3165–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tenora L, Alt J, Dash RP, et al. Tumor-targeted delivery of 6-diazo-5-oxo-l-norleucine (DON) using substituted acetylated lysine prodrugs. J Med Chem 2019;62:3524–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lemberg KM, Zhao L, Wu Y, et al. The novel glutamine antagonist prodrug JHU395 has antitumor activity in malignant peripheral nerve sheath tumor. Mol Cancer Ther 2020;19:397–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu X, Nedelcovych MT, Thomas AG, et al. JHU-083 selectively blocks glutaminase activity in brain CD11b(+) cells and prevents depression-associated behaviors induced by chronic social defeat stress. Neuropsychopharmacology 2019;44:683–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dang CV. MYC on the path to cancer. Cell 2012;149:22–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McKeehan WL. Glycolysis, glutaminolysis and cell proliferation. Cell Biol Int Rep 1982;6:635–50 [DOI] [PubMed] [Google Scholar]
- 22. Lunt SY, Vander Heiden MG. Aerobic glycolysis: Meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol 2011;27:441–64 [DOI] [PubMed] [Google Scholar]
- 23. DeBerardinis RJ, Mancuso A, Daikhin E, et al. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A 2007;104:19345–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wise DR, Thompson CB. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci 2010;35:427–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wise DR, DeBerardinis RJ, Mancuso A, et al. MYC regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A 2008;105:18782–187827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu W, Le A, Hancock C, et al. Reprogramming of proline and glutamine metabolism contributes to the proliferative and metabolic responses regulated by oncogenic transcription factor c-MYC. Proc Natl Acad Sci U S A 2012;109:8983–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dang CV. Therapeutic targeting of Myc-reprogrammed cancer cell metabolism. Cold Spring Harb Symp Quant Biol 2011;76:369–74 [DOI] [PubMed] [Google Scholar]
- 28. Niklison-Chirou MV, Erngren I, Engskog M, et al. TAp73 is a marker of glutamine addiction in medulloblastoma. Genes Dev 2017;31:1738–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martin AM, Raabe E, Eberhart C, et al. Management of pediatric and adult patients with medulloblastoma. Curr Treat Options Oncol 2014;15:581–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rahman A, Smith FP, Luc PT, et al. Phase I study and clinical pharmacology of 6-diazo-5-oxo-L-norleucine (DON). Invest New Drugs 1985;3:369–74 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.