Abstract
Venous stenting has become a common treatment option for central deep venous outflow obstructions and postthrombotic syndrome. Following successful recanalization and stenting, stent patency is endangered by in‐stent thrombosis and recurrent venous thromboembolism. Antithrombotic therapy might reduce patency loss. This systematic review summarizes the literature on antithrombotic therapy following (post)thrombotic venous stenting. A systematic PubMed, MEDLINE, EMBASE, and Cochrane search was performed for studies addressing antithrombotic therapy prescribed following venous stenting of the iliofemoral tract indicated by acute or chronic thrombotic pathology. A total of 277 articles was identified of which 64 (56 original studies) were selected. Overall, a mean primary patency rate of 82.3% was seen 1 year after the intervention, which decreased to 73.3% after 2 years. In the majority (43 of 56 studies, 77%), treatment was based on use of vitamin K antagonists, either with (18%) or without (59%) use of antiplatelet drugs. Only two studies (4%) directly assessed the effect of antithrombotic therapy on treatment outcomes. The impact of postinterventional antithrombotic therapy on stent patency remains unknown because of limited and insufficient data available in current literature. Further clinical research should more clearly address the role of antithrombotic therapy for preservation of long‐term patency following venous stenting.
Keywords: antithrombotic agents, deep vein thrombosis, postthrombotic syndrome, venous stenting, vascular patency
Essentials.
Venous stenting has become a more common treatment for (thrombotic) venous outflow obstructions.
Despite its presumed importance, evidence on post‐procedural antithrombotic management is lacking.
Consistent and comparable reporting of treatment outcomes is scant yet suggests a beneficial effect.
In order to formulate evidence‐based recommendations there is an urgent need for clinical trials.
1. INTRODUCTION
Over time, venous stent placement has become a more commonly used treatment modality for symptomatic central venous obstructions. Stenting is applied in the acute thrombotic phase in addition to thrombus removal (eg, following catheter‐directed thrombolysis [CDT] in acute iliofemoral deep vein thrombosis [IFDVT]) to alleviate symptomatology, restore and preserve patency, and in an attempt to prevent the postthrombotic syndrome (PTS). Furthermore, stenting is used in the treatment of existing chronic venous pathology associated with venous hypertension in patients with PTS or nonthrombotic venous obstructions. Unfortunately, in‐stent thrombosis (IST) is a frequent and clinically important complication. 1 , 2 , 3 IST may induce recurrence of symptoms and complaints that, as a result, necessitate reinterventions. Therefore, preservation of venous patency after a successful venous stent placement is important.
Many factors (eg, venous flow, stent characteristics, stent localization) may affect venous patency following stent placement and thus the risk of adverse outcomes. The role of periprocedural antithrombotic management in the preservation of stent patency and prevention of PTS is not clearly defined. Current international guidelines provide no specific recommendations with regard to postinterventional antithrombotic management. 4 The increased use and expanding possibilities of venous stent placement combined with the introduction of novel anticoagulant treatment options resulted in a large variety of postinterventional antithrombotic treatment regimens. 5 A previous systematic review concluded that none of these treatment regimens are evidence‐based because of a lack of relevant studies on this subject. 6 Given the increasing use of venous stenting as a treatment modality for (post)thrombotic venous obstructions and the more frequent application of direct oral anticoagulants (DOAC) we set out to perform an up‐to‐date analysis to assess the available evidence on postinterventional antithrombotic management after venous stenting of the (post)thrombotic iliofemoral tract.
2. METHODS
2.1. Study selection
Before the literature search, the research question was formulated using the PICO format. Inclusion criteria for eligible studies were specified and documented in a protocol that was registered at the International Prospective Register of Systematic Reviews (PROSPERO, protocol number: 147 539).
The primary objective for this review is to assess and summarize the antithrombotic treatment regimens prescribed (including agent, dosing, intensity, and duration) in patients receiving venous stent placement of the iliofemoral tract following deep vein thrombosis (DVT).
Treatment indications could be either acute IFDVT or chronic postthrombotic lesions. Treatment combinations with complementary procedures (eg, CDT, percutaneous mechanical thrombectomy, percutaneous transluminal balloon angioplasty, endophlebectomy, creation of an arteriovenous fistula) were permitted. Postinterventional antithrombotic treatment could be based on the use of anticoagulants (vitamin K antagonists [VKA], DOAC, unfractionated heparin, low molecular weight heparin [LMWH], and fondaparinux), antiplatelet drugs (cyclooxygenase inhibitors and ADP‐receptor antagonists), or a combination of both. Only original articles reporting postinterventional antithrombotic therapy were eligible. There were no restrictions regarding the duration of follow‐up.
The outcome of interest was the postinterventional antithrombotic treatment regimen prescribed in patients receiving venous stenting. Furthermore, patency rates and the occurrence of recurrent deep vein thrombosis (reDVT), pulmonary embolism (PE), IST, major bleeding, and PTS during follow‐up were assessed. Different definitions for these outcomes could be applied in the respective studies.
2.2. Data sources and searches
The final search was performed in week 14 of 2020 (31 March) using PubMed, MEDLINE, EMBASE, and Cochrane databases. We used the search terms as presented in a previously published systematic review by our group 6 because modification of the search by adding alternative search terms did not influence the search results. The search was limited to English articles that were available in full text. No restrictions regarding publication date were imposed. The exact search strategy can be found in Supplementary Information. The first selection of search results was performed by one researcher (P.N.) assessing title and abstract for relevance in relation to the research question. Subsequently, a full appraisal of the selected publications and hand search of the reference lists was performed by two researchers independently (P.N. and A.t.C.H.). Decisions regarding eligibility needed to be unanimous and reasons for exclusion were registered.
2.3. Data extraction and quality assessment
A prespecified form was used to record data regarding study eligibility, study design, study characteristics, and relevant study outcomes. Data extraction was performed by a single researcher (P.N.) and checked for accuracy by a second researcher (A.t.C.H.). Furthermore, quality assessment of the selected publications was performed by two researchers independently (A.t.C.H. and P.N.). Randomized controlled trials were assessed using the Cochrane risk‐of‐bias tool. 7 For the assessment of nonrandomized studies, a previously adapted version of the Newcastle‐Ottawa Scale 8 , 9 was used. 6 To meet the specific needs of this review, it included a selection of five relevant qualitative study features of which each item can be graded 1 point, leading to a maximum total score of 5 ( Supplementary Information).
2.4. Data analysis
Treatment indications as well as study outcomes were classified and reported using a wide range of definitions. Moreover, outcomes were rarely specified for patients receiving venous stenting of the (post)thrombotic iliofemoral tract. Because of the lack of comparable data in the selected publications, a comparative meta‐analysis could not be performed. Available data regarding the outcomes were tabulated and a systematic analysis was provided. Outcomes were clustered for publications specifically aimed at intervention during the acute phase and publications that (also) included interventions performed for chronic pathology.
3. RESULTS
3.1. Search results
A total of 277 articles was identified in our search of the PubMed (n = 236), MEDLINE (n = 1), EMBASE (n = 5), and Cochrane databases (n = 9) in combination with articles identified by hand searching of the reference lists (n = 26). Appraisal of title and abstract resulted in a first selection of 65 articles. Subsequently, an extensive full content review of these articles was performed that resulted in the selection of 64 articles that were derived from 56 original studies relevant for this review (Figure 1).
FIGURE 1.
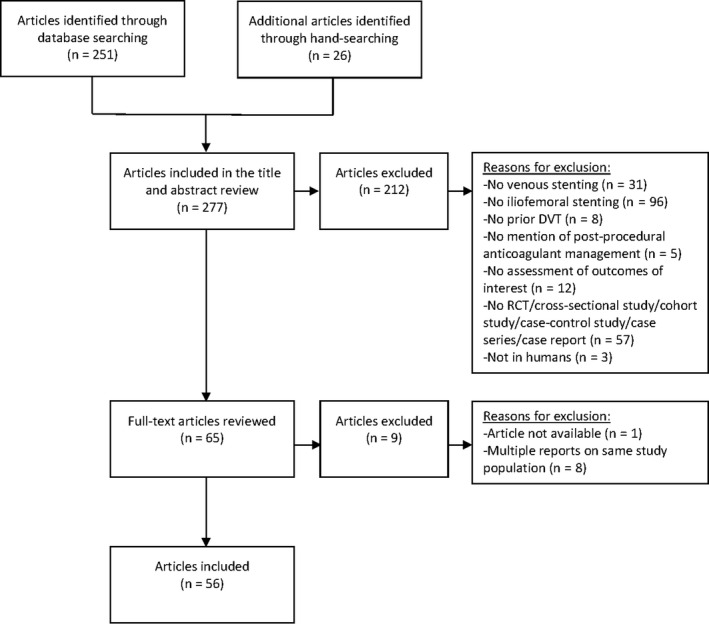
PRISMA flow chart: Summary of evidence search and selection. DVT, deep vein thrombosis; RCT, randomized controlled trial
3.2. General aspects of the studies
The selected studies included six randomized controlled trials (11 publications 2 , 19 ), 32 cohort studies (35 publications; eight prospective 3 , 20 , 21 , 22 , 23 , 24 , 25 , 26 and 27 retrospective 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 ), eight case series, 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 and 10 case reports. 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 These studies included a total of 5153 patients, of which 3235 (62.8%) were stented. In 26 of 56 studies (46%), the complete study population was stented. The study population in 43 of the 56 studies (77%) consisted exclusively of patients with a (history of) DVT: 30 studies (36 publications) of which reported on interventions performed during the acute phase, 12 (12 publications) on treatment of chronic (postthrombotic) pathology and obstructions, and one (one publication) entailed a combined study population with acute and/or chronic postthrombotic sequelae. The remaining 13 studies (23%) reported on stenting for thrombotic as well as nonthrombotic indications such as iliac vein compression syndrome. A summary of the selected publications is shown in Table 1.
TABLE 1.
Study characteristics
| Publication | Treatment Indication | Study Population | Study Population Demographics: Age, Sex, Postthrombotic status, Risk Factors (ie, Thrombophilia, Cancer, MTS) | Sample Size: Total Patient Number; Number of Stented Patients (%) | Intervention: Eventual Comparison of Treatment Groups | Intervention: Postinterventional Antithrombotic Therapy a | ||
|---|---|---|---|---|---|---|---|---|
| Randomized controlled trials | ||||||||
| Enden, 2012 10 (CaVenT) | Acute DVT | Patients aged 18‐75 y with a first time objectively verified iliofemoral DVT (± popliteal and calf vein thrombosis) and symptom duration <21 d |
CDT (n = 90) vs. STND (n = 99)
|
N = 189 Not all stented. Stented: N = 15 (7.9%; 15/90 = 16.7% of CDT patients) |
Standard treatment with additional CDT (n = 90) vs. standard treatment alone (n = 99). |
Start LMWH (200 IU/kg dalteparin or 1.5 mg/kg enoxaparin) at day of diagnosis in both groups. ‐STND group: LMWH was to be continued for a minimum of 5 d or until adequate INR on warfarin. ‐CDT group: One hour after removal of the catheters, treatment with therapeutic weight‐adjusted dose of LMWH 2 each day and concurrent warfarin was initiated. In both groups, warfarin was prescribed for at least 6 mo with a target INR of 2.0‐3.0. |
||
|
Enden, 2009 11 (CaVenT) |
Acute DVT | Prespecified interim results: 6‐mo follow‐up |
CDT (n = 50) vs. STND (n = 53)
|
N = 103 Not all stented. Stented: N = 8 (7.8%; 8/50 = 16% of CDT patients ) |
Standard treatment with additional CDT (n = 50) vs. standard treatment alone (n = 53) | See Enden 2012 | ||
|
Haig, 2013 12 (CaVenT) |
Acute DVT | Subgroup analysis: patients from the CDT group at 24 mo follow‐up |
CDT (n = 92)
|
N = 92 Not all stented. Stented: N = 16 (17.4%) |
Standard treatment with additional CDT. | See Enden 2012 | ||
|
Haig, 2016 13 (CaVenT) |
Acute DVT | Prespecified sub analysis: 5‐year follow‐up |
CDT (n = 87) vs. STND (n = 89)
|
N = 176 Not all stented. Stented: N = not specified |
Standard treatment with additional CDT (n = 87) vs. standard treatment alone (n = 89). | See Enden 2012 | ||
| Sharifi, 2012 14 (TORPEDO) | Acute DVT | Patients with acute femoropopliteal (or more proximal) DVT with severe complaints (ie, edema, erythema, induration, pain, tenderness) |
PEVI (n = 91) vs. STND (n = 92)
|
N = 183 Not all stented, N = 27 (14.8%, 27/91 = 29.7% of PEVI‐patients) |
Percutaneous endovenous intervention (one or more of a combination of thrombectomy, balloon venoplasty, stenting, and/or local low‐dose thrombolytic therapy) with standard anticoagulation (n = 91) vs. standard treatment alone (n = 92). |
Initiation of warfarin (target INR 2.0‐3.0) with concurrent use of LMWH (enoxaparin 2 each day 1 mg/kg or UFH IV [loading dose: 80 u/kg, continuous infusion: 18 U/kg/h]). ‐STND: LMWH or UFH had to be continued for at least 5 d with 1‐day overlap of therapeutic INR. ‐CDT: Parenteral anticoagulation was stopped as soon as INR became therapeutic. Additionally, Aspirin 81 mg 1 each day for at least 6 mo was prescribed. In case of femoropopliteal stenting with a low risk of bleeding, clopidogrel 75 mg 1 each day was also prescribed for 2‐4 wk. |
||
|
Sharifi, 2010 15 (TORPEDO) |
Acute DVT | Prespecified interim results: 6‐mo follow‐up |
PEVI (n = 91) vs. STND (n = 92)
|
N = 183 Not all stented, N = 27 (14.8%, 27/91 = 29.7% of PEVI patients) |
Percutaneous endovenous intervention (one or more of a combination of thrombectomy, balloon venoplasty, stenting, and/or local low‐dose thrombolytic therapy) with standard anticoagulation (n = 91) vs. standard anticoagulation alone (n = 92). | See Sharifi 2012 | ||
| Cakir, 2014 16 | Acute IFDVT | Patients with acute iliofemoral‐popliteal DVT |
PAT (n = 21) vs. STND (n = 21)
|
N = 42 Not all stented. Stented: N = 14 (33.3%; 14/21 = 66.7% of PAT patients) |
Additional percutaneous aspiration Thrombectomy (n = 21) vs. standard anticoagulation alone (n = 21). | Initiation of warfarin (target INR 2.5‐3.0) at the day of diagnosis with concurrent use of LMWH for at least 5 d. Procedures were performed at the first or second day of anticoagulation. | ||
| Zhang, 2014 17 | Subacute IFDVT (≤4 wk) |
IFDVT (CFV or more cranial) patients lacking effective treatment in the acute phase |
CDT (n = 190) vs. CDT + PTA (n = 186)
|
N = 386 Not all stented. Stented: CDT vs. CDT + PTA: N = 44 (23.2%) vs. N = 37 (19.9%) |
Additional catheter‐directed thrombolysis vs. additional catheter‐directed thrombolysis with balloon dilatation. (n = 186) |
Initiation of warfarin (target INR 2.0‐3.0, treatment duration 6‐12 mo) within 6 h of diagnosis with concurrent LMWH for 5‐7 d. LMWH were only discontinued when INR reached ≥2 for 2 consecutive days. Use of NSAIDs and antiplatelets was discouraged. | ||
| Vedantham, 2017 18 (ATTRACT) | Acute DVT | Patients with symptomatic proximal deep vein thrombosis involving the femoral, common femoral, or iliac vein (with or without other involved ipsilateral veins) |
CDT (n = 336) vs. STND (n = 355)
|
N = 691 Not all stented. Stented: N = 82 (11.9%; 82/336 = 24.4% of CDT patients) |
Standard treatment with additional pharmacomechanical thrombolysis (catheter‐mediated or device‐mediated intrathrombus delivery of rtPA and thrombus aspiration or maceration, with or without stenting (n = 336) vs. standard treatment alone (n = 355). |
Both groups were initiated on warfarin (or DOAC when they became available) with concurrent LMWH immediately at diagnosis according to international guidelines. During thrombolysis, oral anticoagulation was discontinued and replaced with either therapeutic doses of LMWH or UFH IV. Within 2 h after hemostasis following removal of the catheters, oral anticoagulation was reinstalled according to the same guidelines. |
||
| Comerota, 2019 19 (ATTRACT) | Acute DVT | Subgroup analysis: patients with IFDVT |
CDT (n = 196) vs. STND (n = 195)
|
N = 391 Not all stented. Stented: N = 70 (17.9%; 70/196 = 35.7% of CDT‐patients) |
Standard treatment with additional pharmacomechanical thrombolysis (catheter‐mediated or device‐mediated intrathrombus delivery of rtPA and thrombus aspiration or maceration, with or without stenting (n = 196) vs. standard treatment alone (n = 195). | See Vedantham 2017 | ||
| Notten, 2020 (CAVA) 2 | Acute IFDVT | Patients aged 18‐85 y with a first‐time acute iliofemoral deep vein thrombosis and symptoms for no more than 14 d. |
CDT (n = 77) vs. STND (n = 75)
|
N = 152 Not all stented: N = 35 (23.0%; 35/77 = 45.5% of CDT patients) |
Standard treatment with additional UACDT (n = 77) vs. standard treatment alone (n = 75) |
For both groups, anticoagulation therapy was performed according to international guidelines using either VKA (acenocoumarol or phenprocoumon; installed with concurrent use of LMWH for at least 5 d until therapeutic range of 2.0‐3.0 was reached), DOACs (rivaroxaban, apixaban, or dabigatran), or LMWH. ‐CDT group: Oral anticoagulants were replaced with therapeutic dose LMWH for the duration of CDT only to be reinstalled 1 h after removal of the catheter. |
||
| Cohort studies, prospective | ||||||||
| AbuRahma, 2001 20 | Acute IFDVT | Patients with acute IFDVT (<2 wk) |
STND (n = 33) vs. MULTI (n = 18)
|
N = 51 Not all stented. Stented: N = 10 (19.6%, 10/18 = 55.6% of MULTI‐patients) |
Standard treatment vs. additional multimodal treatment. ‐Standard therapy was performed in all patients and consisted of systemic heparinization (UFH IV, loading dose 5000‐10 000 IU followed by continuous infusion of 1000‐2000 IU/h for 5‐7 d) concurrent with initiation of warfarin (started within 48‐72 h after start of heparinization and to be continued at a target INR of 2.0‐3.0 for 6 mo unless PE, [9‐12 mo] hypercoagulability [indefinitely], or recurrent DVT [indefinitely]), limb elevation, and gradient compression stockings. ‐Multimodal treatment could entail additional lytic therapy (urokinase, loading dose 4500 U/kg followed by infusion of 4500 U/kg/h for 24‐48 h. During the study, urokinase was replaced with rtPA [loading dose 4‐8 mg, infusion 2‐4 mg/h]), PTA, and percutaneous stenting (indicated if underlying stenosis of ≥50%). If stents were placed, warfarin was indicated indefinitely. |
All patients were treated with systemic heparinization (UFH IV, loading dose 5000‐10 000 IU followed by continuous infusion of 1000‐2000 IU/h for 5‐7 d) concurrent with initiation of warfarin (started within 48‐72 h after start of heparinization and to be continued at a target INR of 2.0‐3.0 for 6 mo unless PE, [9‐12 mo] hypercoagulability [indefinitely], recurrent DVT [indefinitely]), or performed stenting [indefinitely]. | ||
| Grommes, 2011 21 | Acute DVT | Patients with acute DVT treated with additional UACDT |
|
N = 12 (13 limbs) Not all stented. Stented: N = 3 (25.0%) |
Standard treatment with additional UACDT (EKOS‐system; EKOS Corporation) using rtPA (10/13 = 76.9%) or urokinase (3/13 = 23.1%). | VKA was prescribed for 3 mo in case of provoked DVT and 6 mo in case of idiopathic DVT. In was initiated with concurrent LMWH and targeted at an INR of 2.0‐3.0. | ||
| Manninen, 2012 22 | Acute IFDVT | Patients with acute DVT including the iliofemoral vein (with or without caval involvement) or high femoral vein (with or without popliteal‐crural involvement |
Age: 48 y b Male: 26 (46%) All post‐DVT. N = 56, 100%. Acute: 100% Thrombophilia: 19 (33.9%) Cancer: 3 (5%) |
N = 56 Not all stented. Stented: N = 9 (16.1%, all iliac) |
Selective thrombolysis with PTA and percutaneous stenting. | Initiation of warfarin with concurrent UFH IV. Warfarin was prescribed for at least 6 mo. | ||
| Raju, 2014 23 | Chronic obstruction (iliac) | Patients stented with cavo‐iliac vein obstruction treated with Wallstents using the Z‐technique in cavoiliac veins |
|
N = 217 limbs All stented. N = 217 (100%) |
PTA and percutaneous stent placement in the cavo‐iliac veins using Wallstents and the Z‐technique. | Patients with pre‐interventional indications for long‐term anticoagulation (thrombophilia, recurrent thrombosis, unprovoked thrombosis) continued their anticoagulant treatment. All other patients received LMWH for up to 6 wk followed by long‐term use of aspirin. | ||
| Srinivas, 2014 24 | Subacute DVT (1‐8 wk) | Patients with DVT existing 1‐8 wk |
CDT (n = 27) vs. STND (n = 28)
|
N = 55 Not all stented. Stented: N = 6 (10.9%; 6/27 = 22.2% in CDT‐group) |
Standard therapy with additional CDT (mechanical thrombus aspiration and streptokinase infusion [1 lakh units/h; two‐thirds through the catheter and one‐third through the intravenous sheath) along with UFH [loading dose: 5000 IU; continuous infusion 1000 IU/h], n = 27) vs. standard anticoagulation alone (n = 28) |
All patients started warfarin or acenocoumarol on the day of the DVT diagnosis and was continued for 6 mo. ‐STND: UFH IV 1000 IU/h for 48 h followed by 5 d of bolus UFH (5000 IU 6 hourly) or LMWH (1 mg/kg). |
||
| Sarici, 2014 25 | PTS | Patients with chronic PTS (symptoms and signs of CVI in a leg previously affected by DVT [>6 mo ago]) receiving PTA and stenting |
|
N = 52 (59 limbs) All stented. N = 52 (100%) |
PTA and percutaneous stenting | Following the intervention, patients received UFH IV 1000 IU/h for 1 d. Subsequently, 2 mo of clopidogrel and life‐long use of aspirin was indicated. Patients with thrombophilia were treated with life‐long warfarin (target INR 2.0‐3.0). | ||
| Sebastian, 2018 26 | Acute IFDVT | All patients with acute IFDVT treated with CDT and/or PMT followed by nitinol stent placement |
VKA (n = 73) vs. rivaroxaban (n = 38)
|
N = 111 (119 limbs) All stented. N = 111 (100%) |
Postinterventional treatment with 3 mo of VKA (n = 73) vs. rivaroxaban (n = 38) | Within 24 h after the intervention UFH IV was converted to either VKA (with concurrent LMWH for at least 5 d and until a stable target INR of 2.0‐3.0 was reached) or rivaroxaban. Both treatments were prescribed for at least 3 mo. | ||
| Notten, 2020 3 | Obstruction (cavo‐iliofemoral; post‐thrombotic (acute or chronic) or IVCS) | Patients with acute cavo‐iliofemoral DVT, chronic deep venous obstruction resulting from the presence of postthrombotic sequelae (ie, PTS with postthrombotic synechiae), or (nonthrombotic) IVCS treated with PTA and venous stent placement |
Low target INR (2.0‐3.5, n = 40, 50.4%) vs. high target INR (2.5‐4.0, n = 39, 49.6%)
|
N = 79 All stented. N = 79 (100%) |
Postinterventional target INR “low” (2.0‐3.5. n = 40 (50.6%)) vs. “high” (2.5‐4.0. n = 39 (49.4%)) | VKA therapy was continued for at least 6 mo in patients with preinterventional antithrombotic treatment. In all other patients, LMWH was given directly following the procedure and VKA therapy was initiated according to international guidelines at the first postinterventional day. Treatment was continued for at least 6 mo. Target INR (2.0‐3.5 or 2.5‐4.0) and treatment duration was at the discretion of the treating physician. | ||
| Cohort studies, retrospective | ||||||||
| O'Sullivan 2000 27 | Chronic obstruction (IVCS) | Patients with endovascular treatment of IVCS (acute or chronic) |
|
N = 39 Not all stented. Stented: N = 35 (89.7%) |
PTA and percutaneous stenting with additional CDT (urokinase 120 000‐180 000 IU/h), in thrombotic patients. During the last 3 y of the study, thrombotic patients with a symptom duration >4 weeks were treated with PTA and stenting alone. | Warfarin (target INR 2.0‐3.0) for at least 6 mo. | ||
| Kölbel, 2007 28 | Acute IFDVT | Patients with acute iliocaval DVT treated with CDT (and stent placement) |
|
N = 37 (44 limbs) Not all stented. Stented: N = 31 (83.8%; 36 limbs: 81.8%) |
Additional CDT (alteplase, continuous infusion 1‐2 mg/h) with or without percutaneous stenting. | Warfarin (target INR 2.0‐3.0) for 6 mo. | ||
| Knipp, 2007 29 | Chronic obstruction (IVCS) | Patients with IVCS treated with PTA and stenting |
|
N = 58 All stented. N = 58 (100%) |
PTA and percutaneous stenting (with/without adjunctive chemical thrombolysis, mechanical thrombus fragmentation, AVF creation, IVC filter placement) | There was no protocol on postinterventional anticoagulant treatment: warfarin with variable treatment durations was prescribed in 42 (72.4%) patients, antiplatelets (aspirin, clopidogrel, or both) for a minimum of 6 wk in 11 (19.0%) patients, and in 5 (8.6%) patients’ postinterventional anticoagulant treatment was unknown. | ||
|
Neglén, 2007 30 (Neglén cohort) |
Chronic obstruction (femoro‐ilio‐caval) | Patients with chronic nonmalignant obstruction of the femoroiliocaval veins treated with endovascular stent placement. |
|
N = 870 (982 limbs) All stented. N = 870 (100%; 982 limbs: 100%) |
PTA and percutaneous stenting |
Dalteparin 2500 IU was given directly after the procedure as well as the next morning. An additional 30 mg ketorolac was given before discharge. Aspirin 81 mg 1 each day was indicated indefinitely for all patients. Patients with thrombophilia or preinterventional use of VKA were treated with life‐long warfarin. During the extended study period, the following amendments were made regarding postinterventional antithrombotic therapy: discontinuation of warfarin 2 d before the procedure until the day of the procedure; dosage of postinterventional dalteparin was changed into 5000 IU 2 each day for the first 36‐48 h after the procedure; 30 mg Toradol was administered at the moment of recanalization and at 8‐hour intervals until discharge; aspirin was dosed at 81 mg twice weekly in patients with concomitant warfarin; warfarin was (re)started in patients with thrombophilia, recurrent VTE, or other preexisting indications; patients with homocystinemia were treated with aspirin, vitamin B6, and folate therapy. |
||
|
Neglén, 2000 31 (Neglén cohort) |
Chronic obstruction (femoro‐ilio‐caval) | Subgroup analysis: first 137 patients of cohort (chronic primary or postthrombotic venous iliac vein obstructions treated with endovascular stent placement) |
|
N = 137 (139 limbs) All stented. N = 137 (100%) |
See Neglén 2007 | See Neglén 2007 | ||
| Hartung, 2009 32 | Chronic obstruction (iliocaval) | Patients with endovenous stenting for chronic iliocaval obstructive lesions |
|
N = 89 (96 limbs) Not all stented. Stented: N = 87 (97.8%) |
PTA and percutaneous stenting. | Up to 2003 all patients received 6 mo of warfarin (initiated with LMWH). Thereafter, patients stented for MTS received LMWH for 15 d and antiplatelets (not specified) for at least 1 year. Patients with complex lesions (ie, postthrombotic and recanalization mainly. N = 52, 58.4%) were treated with oral anticoagulation for a minimum of 12 mo. | ||
| Kölbel, 2009 33 | Chronic obstruction (iliac) | Patients with endovenous stenting for chronic iliac occlusions |
|
N = 59 (66 limbs) All stented. N = 59 (100%) |
PTA and percutaneous stenting | Warfarin (target INR 2.0‐3.0) for at least 6 mo. | ||
| Raju, 2009 34 | Chronic obstruction (postthrombotic) | Patients with postthrombotic chronic total occlusions of femoro‐iliocaval vein segments treated with percutaneous recanalization |
|
N = 159 (167 limbs) Not all stented. Stented: N = 131 (82.3%; 139 limbs: 83.2%) |
PTA and percutaneous stenting | In the beginning of the study, aspirin (or warfarin in case of thrombophilia) was prescribed as postinterventional anticoagulation. Later, this changed into injection of dalteparin 2500 IU (before, directly afterwards, and 3‐5 d following the procedure) combined with prophylactic dosage of fondaparinux sodium for 4‐6 wk. Therapeutic dosage of fondaparinux as well as long‐term warfarin was prescribed if recanalization comprised ≥3 vein segments, suprarenal stent placement, thrombophilia, or other indications for long‐term anticoagulants. | ||
| Baekgaard, 2010 35 (Gentofte‐cohort) | Acute IFDVT | Patients with IFDVT treated with CDT |
|
N = 101 (103 limbs) Not all stented. Stented: N = 57 (56.4%) |
Additional CDT, PTA, and percutaneous stenting. In the first 9 patients CDT was performed using the Mewissen Infusion Catheter (Boston Scientific; loading dose 1 mg alteplase with 1000‐5000 IU UFH followed by continuous infusion of 1 mg/h alteplase and 1000 IU/h UFH). Thereafter, patients were treated with a pulse‐spray technique (injection of 10 mg alteplase with 1000‐5000 IU UFH for 15‐30 min using the Pro Infusion Catheter; AngioDynamics) before thrombolysis as described for the first 9 patients. |
Warfarin (initiated with concurrent Tinzaparin [100 U/kg 2 each day for 14 d]) for at least 12 mo or lifelong if at high risk for recurrent thrombosis (eg, serious coagulant defects: antithrombin deficiency, homozygous FVL, protein C and S deficiency). | ||
|
Sillesen, 2005 36 (Gentofte‐cohort) |
Acute IFDVT | Subgroup analysis: first 45 patients of cohort |
|
N = 45 Not all stented. Stented: N = 30 (66.7%) |
See Baekgaard 2010 | See Baekgaard 2010 | ||
| Jeon, 2010 37 | Acute DVT (with MTS) | Patients with acute (<2 wk) IFDVT from MTS treated with CDT and stenting of the left CIV |
|
N = 30 All stented. N = 30 (100%) |
Endovascular intervention (ie, CDT, PAT, PTA, and percutaneous stenting) | Warfarin (target INR 2.0‐3.0, at least 6 mo) initiated with concurrent LMWH or UFH IV. | ||
| Rosales, 2010 38 | Chronic obstruction (iliofemoral, post‐thrombotic) | Patients with chronic postthrombotic cavo‐iliofemoral occlusions receiving endovascular interventions |
|
N = 34 Not all stented. Stented: N = 32 (94.1%) |
PTA and percutaneous stenting | Initiation of warfarin with concurrent dalteparin 100 U/kg 2 each day. Warfarin was prescribed at least 6 mo, indefinitely in case of thrombophilia, and tailor‐made in other patients. | ||
| Titus, 2010 39 | Obstruction (iliofemoral) | Patients receiving iliofemoral venous PTA and stenting for symptomatic iliofemoral occlusive venous disease |
|
N = 36 (40 limbs) All stented. N = 36 (100%) |
PTA and percutaneous stenting | Warfarin (target INR 2.0‐3.0) or enoxaparin for at least 6 mo. | ||
| Wahlgren, 2010 40 | Chronic obstruction (femoro‐ilio‐caval, post‐thrombotic) | Patients with chronic postthrombotic femoro‐iliocaval venous disease |
|
N = 50 (51 limbs) Not all stented. Stented: N = 16 (32%) |
Additional endovascular treatment including percutaneous stenting. | In the beginning of the study, warfarin was initiated with concurrent UFH IV. In time, this changed into concurrent use of LMWH until therapeutic levels were reached. Warfarin was continued for at least 6 mo in all patients. Additional aspirin 75 mg 1 each day was prescribed for 1 mo in patients with stent placement. | ||
| Nayak, 2012 41 | PTS | Patients with chronic PTS |
|
N = 44 Not all stented. Stented: N = 39 (88.6%, 45 limbs) |
Endovascular interventions (with/without percutaneous stenting). Adjunctive EVLA was performed in case of saphenous reflux. | All patients received aspirin (81 mg 1 each day following the intervention. If patients were already on anticoagulants before the intervention, there were continued thereafter. | ||
| Blanch, 2013 42 | Chronic obstruction (iliofemoral, post‐thrombotic) | Patients with postthrombotic chronic iliofemoral flow obstruction secondary to stenotic or occlusive lesions with a clinical CEAP ≥3 or venous pain receiving percutaneous stent placement |
|
N = 36 (41 limbs) Not all stented. Stented: N = 34 (94.4%; 39 limbs: 95.1%) |
PTA and percutaneous stenting | Prophylactic dosage LMWH at 6 and 24 h after procedure with Aspirin 100 mg 1 each day for long‐term use in 5 patients (14.7%). The other 29 patients (85.3%) were treated with therapeutic dosage LMWH and long‐term oral anticoagulation because of thrombophilia, stents comprising ≥3 vein segments, or previous indication for anticoagulation. | ||
| Stanley, 2013 43 | Acute or chronic DVT | Patients with acute or chronic DVT of the CIV, EIV, CFV, FV or PoplV |
|
N = 80 Not all stented. Stented: N = 52 (65.0%) |
Either immediate PMT with/without UACDT, primary UACDT with subsequent PMT, or UACDT alone. Ten minutes before start of the procedure the tenecteplase (TNKase) was injected. PMT was performed using AngioJet (10 mg TNKase), Trellis (mean 6.5 mg TNKase), or Omniwave (mean 6.0 mg TNKase). UACDT was performed through the EKOS system (continuous infusion 0.25 mg/h TNKase for 12 h) in combination with UFH IV. |
All chronic patients were on systemic anticoagulation at presentation. In case of acute DVT, patients were admitted immediately and started with UFH, LMWH, or argatroban. After the procedure all patients were prescribed warfarin (target INR 2.0‐3.0) or LMWH for at least 6 mo. Treatment duration depended on hypercoagulable state, residual clot, and recurrent events. Stented patients continued life‐long antiplatelet therapy after discontinuation of warfarin. |
||
| Liu, 2014 44 | Chronic obstruction (IVCS) | Patients with IVCS (visualization of >50% reduction in luminal diameter, formation of collateral circulation, pressure gradient >2 mmHg across stenosis while in supine position) receiving PTA and stenting |
N = 48 Not all stented. Stented: N = 46 (95.8%) |
PTA and percutaneous stenting | For the first 3 postinterventional days 4000 IU LMWH was given twice daily. Concurrently, warfarin was installed and continued for at least 6 mo (≥12 mo for postthrombotic patients). | |||
| Park, 2014 45 | Acute DVT (with MTS) | Patients with acute (<2 wk) IFDVT from MTS treated with CDT and iliac stenting. |
|
N = 51 All stented. N = 51 (100%) |
CDT, PTA, and percutaneous stenting | Warfarin (target INR 2.0‐3.0) was prescribed for at least 3 mo and until symptom relief. Followed by another 3‐6 mo of antiplatelet therapy (aspirin or clopidogrel). | ||
| Sang, 2014 46 | PTS | Patients with endovascular stenting for PTS |
|
N = 67 Not all stented. Stented: N = 63 (94.0%) |
PTA and percutaneous stenting. Ultimately, only 36 of 63 procedures could be performed using only endovascular techniques. | Initiation of warfarin with concurrent enoxaparin 4000 IU twice daily until INR was stabilized at 2.0‐2.5. Warfarin was to be continued for at least 6 mo. | ||
| Ye, 2014 47 | Chronic obstruction (iliofemoral, post‐thrombotic) | Patients with endovascular PTA and stent placement for postthrombotic chronic total occlusion of the iliofemoral vein |
|
N = 110 (118 limbs) Not all stented. Stented: N = 104 (94.5%; 112 limbs: 94.9%) |
PTA and percutaneous stenting | Initiation of warfarin (target INR 2.0‐3.0) with concurrent LMWH 4000 IU twice daily. Warfarin was prescribed for at least 6 mo or long‐term in case of thrombophilia. | ||
|
Catarinella, 2015 48 (MUMC‐cohort) |
Chronic obstruction | Patients with severe venous symptoms (CEAP 4‐6) or venous claudication combined with deep venous obstruction (partial or complete) on DUS or MRV |
|
N = 153 All stented. N = 153 (100%) |
PTA and percutaneous stenting (with or without endophlebectomy and/or AVF creation) | VKA (target INR 2.5‐3.5) initiated with concurrent LMWH for 5 d. VKA were to be continued for at least 6 mo. | ||
|
deWolf, 2013 49 (MUMC‐cohort) |
Chronic obstruction | Subgroup analysis: first 63 patients of the cohort |
|
N = 63 All stented. N = 63 (100%) |
See Catarinella 2015 | See Catarinella 2015 | ||
| Shi, 2016 50 | Chronic obstruction (IVCS) | All patients with IVCS who received endovascular treatment |
|
N = 233 Not all stented: N = 225 (96.6%) |
PTA and percutaneous stenting for subacute/chronic DVT and non‐thrombotic pathology. In acute DVT adjunctive procedures such as CDT (500 000‐700 000 IU Urokinase IV per day, maximum of 3‐5 d), PMT, and thrombectomy were performed. | Initiation of warfarin (target INR 2.0‐3.0) with concurrent LMWH. Warfarin was prescribed for 6 mo. | ||
| Comerota, 2019 51 | Chronic obstruction (iliofemoral, post‐thrombotic) | Patients with incapacitating postthrombotic iliofemoral obstruction involving the CFV who underwent hybrid operative procedures to restore unobstructed venous drainage from the involved leg to the patent vena cava |
|
N = 31 (36 limbs) All stented. N = 31 (100%) |
Hybrid intervention including endophlebectomy of the CFV and endovascular reconstruction of cranial vein segments. | Warfarin (3 d) and DOACs (1 d) were discontinued before the intervention. Aspirin 81 mg 1 each day and clopidogrel 75 mg 1 each day (as well as cilostazol 100 mg 2 each day if placement of a prosthetic graft was anticipated) were started 3 d before the intervention. In the last 14 patients, a sheath was placed in the ipsilateral popliteal vein for peri‐procedural anticoagulation. Following the intervention patients received UFH IV concurrent with initiation of warfarin (target INR 2.0‐3.0). Warfarin as well as aspirin were indicated indefinitely. If a prosthetic graft was used, cilostazol was also continued indefinitely. Clopidogrel was continued for 8 wk. In the last 14 patients, UFH was continued for 5 d at a continuous rate of 600‐700 IU/h. Furthermore, the target INR was increased to 3.0‐4.0 for the first 6‐12 mo. Subsequently to be reduced to 2.0‐3.0 or to convert to treatment with direct oral Xa inhibitors. Cilostazol had to be discontinued after 8 wk if anticipated PTFE graft was not used. | ||
| Dumantepe, 2018 52 | Subacute DVT (<1 mo) | All patients with acute (<1 mo) massive lower extremity DVT |
|
N = 68 Not all stented: N = 11, 16.2% (including all 4 MTS‐patients) |
Rheolytic thrombectomy with percutaneous stenting | Rivaroxaban 15 mg 2 each day for 3 wk, followed by 20 mg 1 each day for 3‐6 mo. | ||
| Endo, 2018 53 | Chronic compression and/or acute DVT | Patients with successful endovascular iliocaval stent placement |
|
N = 62 (71 limbs) All stented. N = 62 (100%) |
Percutaneous stenting | Following the intervention 24 patients (38.7%) used anticoagulation alone, 2 patients (3.2%) used antiplatelets alone, and 36 patients (58.1%) used both anticoagulants and antiplatelets. In 22 patients (35.5%), multiple anticoagulants were used or a change between anticoagulants was made. Use as specified per agent: warfarin (48.4%, n = 30), enoxaparin (62.9%, n = 39), oral DOAC (rivaroxaban, apixaban. 25.8%, n = 16), aspirin (n = 26, 41.9%), clopidogrel (n = 8, 12.9%), aspirin with clopidogrel (n = 4, 6.4%). | ||
| Case series | ||||||||
| Acharya, 2005 54 | Subacute DVT (≤3 wk ≤6 wk postpartum) | Patients with symptomatic acute (<3 wk) DVT within 42 d of childbirth treated with CDT |
|
N = 5 Not all stented. Stented: N = 2 (40%) |
Additional CDT (Alteplase [loading dose 5 mg in 10 mL 0.9%NaCl; continuous infusion 0.01 mg/kg/h] and UFH [loading dose: 5000 IU; continuous infusion 300 IU/kg/d]) | Warfarin for 1 year or indefinitely when stented. | ||
| Dayal, 2005 55 | Critical chronic compression and/or acute DVT | Patients with critical venous occlusive disease (acute or chronic) |
|
N = 25 Not all stented. Stented: N = 15 (60%) |
CDT (urokinase or alteplase) and concurrent UFH IV combined with additional endovascular interventions (mechanical thrombectomy [AngioJet], transluminal venoplasty, or [nitinol] stent placement). | Long‐term systemic anticoagulant treatment (not specified). | ||
| Husmann, 2007 56 | Acute DVT (with MTS) | Patients with acute IFDVT (<1 wk) with underlying venous spur (from MTS) treated with a combination of surgical thrombectomy of the iliac veins and locoregional thrombolysis of veins below the groin |
|
N = 11 All stented. N = 11 (100%) |
Additional locoregional thrombolysis, surgical thrombectomy, and percutaneous stenting | Patients received UFH IV for 12 h following the procedure. Subsequently, coumarins were initiated with concurrent LMWH. Treatment was targeted at an INR of 2.0‐3.0 and was continued for 6 mo. | ||
| Murphy, 2009 57 | Acute DVT (with MTS and initiation of oral contraceptives) | Patients with DVT following initiation of oral contraceptives and unknown underlying MTS treated with CDT, stent placement and 6 mo of warfarin |
|
N = 7 All stented. N = 7 (100%) |
Additional CDT, mechanical thrombectomy, PTA, and stent placement. | Postinterventional use of acenocoumarol (initiated with concurrent LMWH, target INR 2.0‐3.0 for 6 mo) and aspirin (indefinitely) was prescribed. | ||
| Oguzkurt, 2011 58 | Phlegmasia cerulea dolens (from IFDVT) | Patients with phlegmasia cerulea dolens from acute IFDVT treated with manual aspiration thrombectomy |
|
N = 7 Not all stented. Stented: N = 3 (42.9%, all MTS‐patients) |
Percutaneous manual aspiration thrombectomy | Warfarin for 6 mo. | ||
| Bloom, 2015 59 | Acute IFDVT (during pregnancy or ≤6 wk postpartum) | Patients treated with PMT for symptomatic IFDVT during pregnancy or ≤6 wk postpartum |
|
N = 11 Not all stented. Stented: N = 8 (72.7%) |
PMT and percutaneous stenting | Warfarin (target INR 2.0‐3.0) was initiated with LMWH. Additionally, low‐dose aspirin was prescribed for 3 mo in patients after stent placement. | ||
| Langwieser, 2016 60 | Chronic obstruction (iliofemoral, postthrombotic) | Patients with postthrombotic iliofemoral venous obstructions |
|
N = 9 (10 limbs) All stented. N = 9 (100%) |
Percutaneous stenting | All patients were prescribed rivaroxaban 20 mg 1 each day and clopidogrel 75 mg every other day (depending on individual drug response). At 6 mo, clopidogrel was stopped in all patients. Rivaroxaban was continued in 3 (33.3%), stopped in 3 (33.3%), and switched to acetylsalicylic acid in 3 (33.3%) patients, respectively. | ||
| Ming, 2017 61 | Acute IFDVT (with IVCS) | All patients with IFDVT combined with IVCS |
No PTS (n = 173) vs. PTS (n = 74) |
N = 247 Not all stented. Stented: N = 116 (47.0%) |
CDT (urokinase: loading dose 100 000‐300 000 IU/h for 1 h; continuous infusion: 16 000‐25 000 IU/h) with percutaneous stenting | Initiation of warfarin (target INR 2.0‐3.0) with minimally 5 d of concurrent LMWH treatment. Warfarin was continued for 6 mo. | ||
| Case reports | ||||||||
| Kapranov, 2003 62 | PTS (after IFDVT) | Patient with PTS (continuing complaints of pain, heaviness, and edema as well as absent recanalization) 11 mo after IFDVT |
|
N = 1 All stented. N = 1 (100%) |
Percutaneous stenting | Acenocoumarol (2 mg/d) was initiated with concurrent enoxaparin 60 mg/d for 6 d. | ||
| Oguzkurt, 2008 63 | Phlegmasia cerulea dolens (from IFDVT with MTS) | Patient with phlegmasia cerulea dolens as a result of IFDVT with MTS |
|
N = 1 All stented. N = 1 (100%) |
Manual aspiration thrombectomy and percutaneous stenting | Warfarin for 6 mo. | ||
| Salam, 2010 64 | Acute IFDVT (with EIV stenosis from repetitive microtrauma) | Patient with IFDVT based on repetitive microtrauma of the EIV stenosis from cycling |
|
N = 1 All stented. N = 1 (100%) |
CDT (Alteplase [loading dose 2.0 mg; continuous infusion 0.5 mg/h] with UFH 500 IU/h) and percutaneous stenting | Warfarin (target INR 2.0‐3.0) was initiated with concurrent enoxaparin. Warfarin was continued for 3 mo. Additionally, aspirin was indicated indefinitely. | ||
| Sharifi, 2010 65 | Acute IFDVT | Patient with IFDVT and worsening presentation under anticoagulation treatment |
|
N = 1 All stented. N = 1 (100%) |
CDT (tPA [1.0 mg/h] and UFH [12 IU/kg/h]) and percutaneous stenting (stent expansion) | Following the intervention, warfarin was initiated (with concurrent use of enoxaparin), Aspirin 81 mg 1 each day as well as 2 wk of clopidogrel 75 mg 1 each day. | ||
| Wormald, 2012 66 | Acute IFDVT (with MTS) | Patient with acute IFDVT (and MTS) |
|
N = 1 All stented. N = 1 (100%) |
Mechanical thrombectomy (Trellis device; Covidien) and percutaneous stenting | Warfarin for 6 mo. | ||
| Singh, 2017 67 | Acute IFDVT (with MTS and pelvic mass) and PE | Patient with IFDVT based on MTS complicated with PE and spontaneous retroperitoneal hematoma |
|
N = 1 All stented. N = 1 (100%) |
Percutaneous stenting | Following the intervention, UFH IV was continued and later switched to apixaban 5 mg 2 each day. | ||
| Kohler, 2018 68 | Severe PTS (after cavo‐iliacal DVT) | Patient with severe PTS 11 y after cavo‐bilateral DVT |
|
N = 1 All stented. N = 1 (100%) |
Percutaneous stenting | Initially: rivaroxaban 15 mg 2 q.d. and clopidogrel (loading dose of 600 mg, maintenance of 75 mg 1 each day. Because of recurrent IST, multiple regimens were tried (UFH IV with clopidogrel, dabigatran with clopidogrel, dabigatran and prasugrel) before successful anticoagulant treatment was found with prasugrel with phenprocoumon. | ||
| Lakha, 2018 69 | Acute IFDVT (with MTS) | Patient known with Behcet's disease presenting with IFDVT and underlying MTS |
|
N = 1 All stented. N = 1 (100%) |
PMT, thrombectomy, and percutaneous stenting | Rivaroxaban and aspirin. | ||
| Rohr, 2019 70 | Acute IFDVT | Insufficient relief following IFDVT despite 1 wk of enoxaparin treated with attempted single‐session CDT using the JETi device |
|
N = 1 All stented. N = 1 (100%) |
Single‐session CDT (tPA, 6 mg) using the JETi device and percutaneous stenting | Therapeutic dosage of enoxaparin 1 mg/kg twice daily was continued following the intervention. This was converted to apixaban 5 mg 2 each day combined with aspirin 325 mg 1 each day after 2 wk. At 9 mo, full‐dose apixaban was discontinued as aspirin was continued indefinitely. | ||
| Barge, 2020 71 | Acute cavo‐bi‐iliacal DVT (also involving left renal vein) | Extensive acute DVT involving the ICV down to the popliteal veins bilaterally as well as the left renal vein treated with a combination of endovascular treatment modalities |
|
N = 1 (2 limbs) All stented. N = 1 (100%) |
UACDT (Alteplase; 2.0 mg/h) and percutaneous stenting | Dalteparin 7500 IU 2 each day was continued for 2 wk following the intervention before converting to warfarin (target INR 2.0‐3.0) for 6 mo. Subsequently, this was switched to apixaban 5 mg twice daily for a remaining 6 mo. | ||
The shaded (dark gray) rows represent the outcomes of (pre specified) sub analyses regarding the study population from the primary study. The primary study is reported between brackets and its results are presented in the first unshaded row above.
All outcomes reported in bold represent data specified for the number of patients with post‐DVT (acute or chronic) treatment indications receiving venous stent placement.
Abbreviations: AVF, arteriovenous fistula; CDT, catheter‐directed thrombolysis; CEAP, clinical‐etiology‐anatomy‐pathophysiology; CFV, common femoral vein; CIV, common iliac vein; CVI, chronic venous insufficiency; DUS, duplex ultrasound; DVT, deep vein thrombosis; DOAC, direct oral anticoagulants; EIV, external iliac vein; EVLA, endovascular laser ablation; FV, femoral vein; IFDVT, iliofemoral deep‐vein thrombosis; ICV, inferior caval vein; INR, international normalized ratio; IU, international units; IV, intravenous; IVCS, iliac vein compression syndrome; LMWH, low molecular weight heparin; MRV, magnetic resonance venography; MTS, May‐Thurner syndrome; MULTI, multimodal treatment; PAT, percutaneous aspiration thrombectomy; PE, pulmonary embolism; PEVI, percutaneous endovenous intervention; PoplV, popliteal vein; PMT, pharmacomechanical (catheter‐directed) thrombolysis; PTA, percutaneous transluminal angioplasty; PTS, postthrombotic syndrome; rtPA, recombinant tissue plasminogen activator; STND, standard treatment; TNKase, tenecteplase; tPA, tissue plasminogen activator; UACDT, ultrasound‐accelerated catheter‐directed thrombolysis; UFH, unfractionated heparin; VKA, vitamin K antagonist.
There were no adapted anticoagulation regimens used in specific patient groups (ie, stented patients) unless explicitly specified.
Mean value.
Median value.
Patients’ age ranged from 13 to 96 years, 23 , 53 with an overall median age of 48 years (43‐53). With the exception of the randomized trials, study populations were predominantly female: 35 of 56 studies (63%). The prevalence of important prothrombotic risk factors was assessed in various studies: 37 studies (66%) reported on hypercoagulability, 19 studies (34%) on active malignancies, and 30 studies (54%) on underlying deep venous pathology such as iliac vein compression syndromes.
3.3. Quality assessment
Quality assessment was performed on all selected studies with the exception of case series and case reports (Supplementary Information). Using the Cochrane risk‐of‐bias tool for randomized trials (2.0 7 ), three randomized trials were considered to have a high risk of bias. 14 , 16 , 17 The maximum score of 5 points on the modified Newcastle‐Ottawa scale was awarded to seven cohort studies, 3 , 20 , 23 , 32 , 43 , 48 , 50 and two studies 44 , 52 were awarded with the lowest score of 2 points. In five studies, 25 , 26 , 37 , 45 , 51 the studied cohort was considered a true representative for patients receiving (post)thrombotic iliofemoral stent placement. Additional selection criteria were used in another five studies. 29 , 33 , 35 , 44 , 52 A follow‐up time of ≥ 24 months was seen in 15 studies 3 , 38 , 43 , 46 , 47 , 48 , 50 with reported losses to follow‐up ranging from 0.0% to 34.4%. 38
4. OUTCOMES
4.1. Antithrombotic management
All studies performed treatment of acute or chronic thrombotic iliofemoral venous obstructions using venous stent placement (including adjunctive procedures) and all studies provided information on the postinterventional antithrombotic therapy prescribed (Table 1). Details on preinterventional and peri‐interventional antithrombotic treatment were provided in 28 (50%) 2 , 3 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 26 , 31 , 35 , 36 , 37 , 43 , 45 , 51 , 54 , 55 , 57 , 59 , 60 , 62 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 and 46 (82%) 2 , 3 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 30 , 31 , 32 , 34 , 35 , 36 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 63 , 64 , 65 , 67 , 69 , 70 , 71 studies, respectively. Information about eventual (dis)continuation of existing antithrombotic therapy during the intervention was provided in 19 studies (34%). 2 , 3 , 18 , 19 , 24 , 26 , 27 , 30 , 43 , 51 , 63 , 64 , 65 , 67 , 68 , 69 , 70
A broad variety of postinterventional antithrombotic treatment regimens was reported in the selected studies. This variation applied to antithrombotic drug of choice as well as to prescribed dosage and treatment duration. However, full details on the postinterventional antithrombotic regimen including type of antithrombotic agent, dosage, frequency, treatment intensity, treatment duration, and eventual indications for adjustments were rarely reported (Table 2). Full‐dose anticoagulant treatment based on VKA or DOAC was prescribed in 33 studies (59%). 2 , 3 , 10 , 11 , 12 , 13 , 24 , 26 , 27 , 28 , 33 , 35 , 36 , 37 , 38 , 39 , 44 , 46 , 47 , 48 , 49 , 50 , 52 , 54 , 55 , 56 , 58 , 61 , 62 , 63 , 66 , 71 Generally, VKA in the acute phase was initiated with concurrent use of LMWH for a limited number of days or until the international normalized ratio was stabilized at an intensity of 2.0 to 3.0. Predominantly, treatment was continued for a minimum of 6 months with alternative durations of treatment in case of hypercoagulability, recurrent venous thromboembolic events (reVTE), postthrombotic lesions, stenting, or preexisting indications for antithrombotic treatment. 20 , 21 , 35 , 38 , 44 , 47 , 54 Concomitant or subsequent use of antiplatelet drugs was prescribed in another 10 studies (18%), 14 , 15 , 40 , 43 , 45 , 57 , 59 , 60 , 64 , 65 , 69 stent placement being the principal reason for additional antiplatelet therapy. There was only one study 41 (2%) that prescribed antiplatelet drugs as single postinterventional antithrombotic treatment in (post)thrombotic patients. However, if antithrombotic treatment with VKA was indicated before the intervention, which was the case in 95.5% of the population, it was continued accordingly. In six additional studies (11%), 29 , 32 , 34 , 51 , 53 , 68 various treatments or treatment combinations were prescribed depending on the complexity of the lesion or the extensiveness of the procedure (eg, mere stent placement or with adjunctive procedures), the location and the extent of the affected trajectory, documented hypercoagulability, or preexisting indications for anticoagulation. Antithrombotic treatment consisted of LMWH followed by antiplatelet drugs or DOAC in six studies (11%) 23 , 25 , 30 , 31 , 42 , 67 , 70 with VKA only prescribed in case of hypercoagulability, recurrent or unprovoked thrombosis, extensive stenting, or preinterventional use. 23 , 25 , 30 , 31 , 42
TABLE 2.
Postinterventional antithrombotic regimens
| Publication | Population | Intervention: Postinterventional Antithrombotic Therapy |
|---|---|---|
| Full‐dose anticoagulant treatment (VKA or DOAC) | ||
| Randomized controlled trials | ||
| Enden, 2012 10 | Acute DVT | VKA (target INR 2.0‐3.0), treatment duration ≥6 mo |
| Cakir, 2014 16 | Acute DVT | VKA (target INR 2.5‐3.0), treatment duration not specified |
| Zhang, 2014 17 | Subacute DVT (≤4 wk) |
VKA (target INR 2.0‐3.0), treatment duration not specified Use of NSAIDs and antiplatelets was discouraged |
| Vedantham, 2017 18 | Acute DVT | VKA, treatment duration ≥3 mo |
| Notten, 2020 2 | Acute DVT | VKA (target INR 2.0‐3.0), treatment duration ≥3 mo (82.2% of population) or DOAC (11.8% of population: rivaroxaban, apixaban, or dabigatran) or LMWH (0.7% of population) |
| Cohort study, prospective | ||
| AbuRahma, 2001 20 | Acute DVT | VKA (target INR 2.0‐3.0), treatment duration ≥6 mo a |
| Grommes, 2011 21 | Acute DVT | VKA (target INR 2.0‐3.0), treatment duration ≥3 mo a |
| Manninen, 2012 22 | Acute DVT | VKA, treatment duration 6 mo |
| Srinivas, 2014 24 | Subacute DVT (1‐8 wk) |
VKA, treatment duration 6 mo
|
| Sebastian, 2018 26 | Acute DVT | VKA (target INR 2.0‐3.0) in 34.2% of population or Rivaroxaban in 65.8% of population, treatment duration 3 mo |
| Notten, 2020 3 | Obstruction, acute or chronic |
VKA (target INR 2.0‐3.5 or 2.5‐4.0), treatment duration ≥6 mo
|
| Cohort study, retrospective | ||
| O'Sullivan 2000 27 | Chronic obstruction | VKA (target INR 2.0‐3.0), treatment duration ≥6 mo |
| Kölbel, 2007 28 | Acute DVT | VKA (target INR 2.0‐3.0), treatment duration ≥6 mo |
| Kölbel, 2009 33 | Chronic obstruction | VKA (target INR 2.0‐3.0), treatment duration ≥6 mo |
| Baekgaard, 2010 35 | Acute DVT | VKA (target INR 2.0‐3.0), treatment duration ≥6 mo a |
| Jeon, 2010 37 | Acute DVT | VKA (target INR 2.0‐3.0), treatment duration ≥6 mo |
| Rosales, 2010 38 | Chronic obstruction | VKA (target INR 2.0‐3.0), treatment duration ≥6 mo a |
| Titus, 2010 39 | Chronic obstruction | VKA (target INR 2.0‐3.0) or LMWH, treatment duration ≥6 mo |
| Liu, 2014 44 | Chronic obstruction | VKA (target INR 2.0‐3.0), treatment duration ≥6 mo a |
| Sang, 2014 46 | PTS | VKA (target INR 2.0‐2.5), treatment duration ≥6 mo |
| Ye, 2014 47 | Chronic obstruction | VKA (target INR 2.0‐3.0), treatment duration ≥6 mo a |
| Catarinella, 2015 48 | Chronic obstruction | VKA (target INR 2.5‐3.5), treatment duration ≥6 mo |
| Shi, 2016 50 | Chronic obstruction | VKA (target INR 2.0‐3.0), treatment duration 6 mo |
| Dumantepe, 2018 52 | Subacute DVT (<1 mo) | Rivaroxaban (15 mg 2 q.d. for 3 wk, followed by 20 mg 1 each day for 3‐6 mo) |
| Case series | ||
| Acharya, 2005 54 | Subacute DVT (≤3 wk ≤6 wk postpartum) | VKA, treatment duration 12 mo a |
| Dayal, 2005 55 | Obstruction, acute or chronic | Long‐term systemic anticoagulant treatment. No further specification |
| Husmann, 2007 56 | Acute DVT | VKA (target INR 2.0‐3.0), treatment duration 6 mo |
| Oguzkurt, 2011 58 | Acute DVT | VKA, treatment duration 6 mo |
| Ming, 2017 61 | Acute DVT | VKA (target INR 2.0‐3.0), treatment duration 6 mo |
| Case reports | ||
| Kapranov, 2003 62 | PTS | VKA, treatment duration not specified |
| Oguzkurt, 2008 63 | Acute DVT | VKA, treatment duration 6 mo |
| Wormald, 2012 66 | Acute DVT | VKA, treatment duration 6 mo |
| Barge, 2020 71 | Acute DVT | VKA (target INR 2.0‐3.0) for 6 mo followed by apixaban (5 mg 2 each day) for another 6 mo. |
| Full‐dose anticoagulant treatment (VKA or DOAC) in combination with or followed by APT | ||
| Randomized controlled trials | ||
| Sharifi, 2012 14 | Acute DVT |
VKA (target INR 2.0‐3.0), treatment duration not specified
|
| Cohort study, retrospective | ||
| Wahlgren, 2010 40 | Chronic obstruction |
VKA (target INR 2.0‐3.0), treatment duration ≥6 mo
|
| Stanley, 2013 43 | Obstruction, acute or chronic |
VKA (target INR 2.0‐3.0) OR LMWH, treatment duration ≥6 mo
|
| Park, 2014 45 | Acute DVT | VKA (target INR 2.0‐3.0) for ≥3 mo followed by 3‐6 mo of antiplatelets (aspirin or clopidogrel) |
| Case series | ||
| Murphy, 2009 57 | Acute DVT | VKA (target INR 2.0‐3.0) for ≥6 mo and aspirin indefinitely |
| Bloom, 2015 59 | Acute DVT |
VKA (target INR 2.0‐3.0), treatment duration not specified
|
| Langwieser, 2016 60 | Chronic obstruction | Rivaroxaban (20 mg 1 each day) and clopidogrel (75 mg once a day or once every other day) for 6 mo. After 6 mo, clopidogrel was stopped. Rivaroxaban was continued in 33% of the population, switched to acetylsalicylic acid in 33% of the population, and stopped in 33% of the population. |
| Case report | ||
| Salam, 2010 64 | Acute DVT | VKA (target INR 2.0‐3.0) for 3 mo followed by aspirin for life |
| Sharifi, 2010 65 | Acute DVT | VKA (target INR 2.0‐3.0), aspirin (81 mg 1 each day), and 2 wk of clopidogrel (75 mg 1 each day) |
| Lakha, 2018 69 | Acute DVT | Rivaroxaban and aspirin |
| APT | ||
| Cohort study, retrospective | ||
| Nayak, 2012 41 | PTS |
Aspirin (81 mg 1 each day indefinitely) after discharge.
|
| Mixed or various treatments (between or within groups) | ||
| Cohort study, retrospective | ||
| Knipp, 2007 29 | Chronic obstruction | VKA (72.4% of population) with variable treatment durations or ≥6 wk of antiplatelets (19.0% of population: aspirin, clopidogrel, or both) |
| Hartung, 2009 32 | Chronic obstruction |
VKA, treatment duration 6 mo
|
| Raju, 2009 34 | Chronic obstruction |
Aspirin or VKA (in case of thrombophilia)
|
| Comerota, 2019 51 | Chronic obstruction |
VKA (target INR 2.0‐3.0) and aspirin (81 mg 1 each day). for life with clopidogrel (75 mg 1 each day) for 8 wk. In case of a prosthetic graft placement, cilostazol (100 mg 2 each day) was also indicated for life.
|
| Endo, 2018 53 | Obstruction, acute or chronic | VKA, LMWH, DOAC, or antiplatelets. No further specification |
| Case report | ||
| Kohler, 2018 68 | PTS |
Rivaroxaban (15 mg 2 q.d.) with clopidogrel (75 mg 1 each day and a loading dose of 600 mg).
|
| Heparin followed by APT and/or DOAC | ||
| Cohort study, prospective | ||
| Raju, 2014 23 | Chronic obstruction |
LMWH for up to 6 wk followed by long‐term aspirin.
|
| Sarici, 2014 25 | PTS |
Continuous infusion of UFH IV was given the first day following the intervention. Then clopidogrel (2 mo) and aspirin (indefinite) were prescribed.
|
| Cohort study, retrospective | ||
| Neglén, 2007 30 | Chronic obstruction |
Dalteparin (2 gifts) and ketorolac (1 gift) during admission. Aspirin (indefinitely) after discharge.
|
| Blanch, 2013 42 | Chronic obstruction |
Two gifts of LMWH following the intervention and indefinite use of aspirin (100 mg 1 each day).
|
| Case report | ||
| Singh, 2017 67 | Acute DVT | UFH IV followed by apixaban (5 mg 2 each day) |
| Rohr, 2019 70 | Acute DVT | LMWH was converted to treatment with apixaban (5 mg 2 each day) for 9 mo and aspirin (325 mg 1 each day) for life. |
Abbreviations: APT, antiplatelet therapy; CDT, catheter‐directed thrombolysis; DVT, deep vein thrombosis; DOAC, direct oral anticoagulants; INR, international normalized ratio; IU, international units; IV, intravenous; LMWH, low molecular weight heparin; MTS, May‐Thurner syndrome; NSAID, nonsteroidal anti‐inflammatory drugs; PE, pulmonary embolism; PTS, postthrombotic syndrome; UFH, unfractionated heparin; VKA, vitamin K antagonist.
Alternative treatment durations were specified for patients with hypercoagulability, 20 , 35 , 38 , 47 recurrent venous thromboembolic events, 20 , 35 idiopathic venous thromboembolic events, 21 stent placement, 20 , 54 treatment of postthrombotic lesions, 44 or in case of individually tailored treatments. 38
Only two studies directly assessed the impact of different postinterventional antithrombotic management regimens following iliofemoral venous stent placement on postinterventional clinical outcomes. 3 , 26 The first study, 26 a prospective cohort study in patients with acute IFDVT treated with CDT and stenting, found that treatment with rivaroxaban (n = 73) or VKA (n = 38) for a minimum of 3 months following the intervention was equally effective in preserving stent patency at 24 months. A total of 15 reVTE occurred: one PE (1.4%) and seven IST (9.6%) in the rivaroxaban‐group vs one contralateral DVT (2.6%) and six IST (15.8%) in the VKA group. Five of these IST developed within 30 days of the procedure (three in the rivaroxaban group vs two in the VKA group, P = .78); the other eight occurred after more than 30 days (four events in both groups, P = .33). There was no difference in the occurrence of major bleeding (one in each group, P = .64) or the number of patients free from PTS at the last follow‐up visit (57 [85%] vs 29 [88%], P = .76). The second study 3 was a cohort study of patients stented for thrombotic (acute or chronic) or nonthrombotic obstructions of the cavo‐iliofemoral venous tract and identified the time within therapeutic range (TTR), an indicative measure of the quality of antithrombotic treatment with VKA, as an important determinant for the development of IST. An increased risk was seen if the TTR was less than the cutoff value of 49.9%, which could further be specified for patients treated with stenting during the acute phase of thrombosis (cutoff value 69.4%) or for chronic postthrombotic sequelae (cutoff value 45.9%). IST developed in 16 of the 74 (21.6%) patients stented for thrombotic pathology of which four received stenting during the acute phase. No reDVT or PE was seen.
4.2. Patency rates
Patency rates were reported in 40 studies (71%) (Table 3). Multiple definitions for patency were used (eg, primary patency, assisted primary patency, secondary patency, a maximum percentage of residual stenosis, a combination with the absence of reflux) and variable durations of follow‐up were reported. Results were often not specified for patients with thrombotic (acute or chronic) or nonthrombotic treatment indications.
TABLE 3.
Outcomes
| Publication | Treatment Indication | Outcomes | |||||
|---|---|---|---|---|---|---|---|
| Patency (% and Term) | ReVTE | IST | Major Bleeding | PTS | FU | ||
| Randomized controlled trials | |||||||
| Enden, 2012 (CaVenT) 10 | Acute DVT | Not reported |
ReVTE: 28/189 (14.8%) PE: None (0/15) |
Not reported | 3/189 (1.6%) |
CDT vs. STND § 37/90 (41.1%) vs. 55/99 (55.6%). ARR 14.4% (0.2‐27.9) |
24 mo |
| Sharifi, 2012 14 (TORPEDO) | Acute DVT | Not reported |
PEVI vs. STND ReVTE: 4/88 (4.5%) vs. 13/81 (16.0%), P = .02. PE: 0/88 (0%) vs. 4/81 (4.9%). All patients from PEVI group received ICV filters of which 10/91 (11%) showed thrombi. |
1/27 (3.7%) | Not reported |
PEVI vs. STND** 6/88 (6.8%) vs. 24/81 (29.6%), P < .001. |
30 mo a |
| Cakir, 2014 16 | Acute IFDVT |
At 1 mo: patent 13/21 (61.9%) vs. 0/21 (0%), partial thrombosis 8/21 (38.1%) vs. 5/21 (23.8%), full thrombosis 1/21 (4.8%) vs. 16/21 (76.2%), (P < .001). Stented patients at 1 mo: patent 10/14 (71.4%), partial thrombosis 3/14 (21.4%), full thrombosis 1/14 (7.2%). At 3 mo: patent 12/21 (57.1%) vs. 0/21 (0%), partial thrombosis 8/21 (38.1%) vs. 6/21 (28.6%), full thrombosis 1/21 (4.8%) vs. 15/21 (71.4%), (P < .001). Stented patients at 3 mo: patent 10/14 (71.4%), partial thrombosis 2/14 (14.3%), full thrombosis 2/14 (14.3%). At 12 mo: patent 12/21 (57.1%) vs. 1/21 (4.8%), partial thrombosis 8/21 (38.1%) vs. 15/21 (71.4%), full thrombosis 1/21 (4.8%) vs. 5/21 (23.8%), (P < .001). Stented patients at 12 mo: patent 10/14 (71.4%), partial thrombosis 2/14 (14.3%), full thrombosis 2/14 (14.3%). |
PAT vs. STND ReDVT: 1/21 (4.8%) vs. 0/21 (0%). In stented patients: None (0/14) PE: 1/21 (4.8%) vs. 4/21 (19.0%). ICV filters placed in 2/21 PAT patients (9.5%). |
2/14 (14.3%) | Not reported | Not reported | 12 mo |
| Zhang, 2014 17 | Subacute IFDVT (≤4 wk) | Not reported |
CDT vs. CDT + PTA ReDVT: 3/186 (1.6%) vs. 4/190 (2.1%). PE: None (0/81). All patients received ICV filters |
Not reported | None (0/81) | Not reported | 24 mo |
| Vedantham, 2017 18 (ATTRACT) | Acute DVT | Not reported |
CDT vs. STND ReVTE (reDVT, IST or PE), overall: 42/337 (12.5%) vs. 30/355 (8.5%). ‐Within first 10 d: 6/337 (1.8%) vs. 4/355 (1.1%). |
Not reported |
CDT vs. STND Overall: 19/337 (5.6%) vs. 13/355 (3.7%). ‐Within first 10 d: 6/337 (1.8%) vs. 1/355 (0.3%). |
CDT vs. STND § 157/336 (46.6%) vs. 171/355 (48.2%). |
24 mo |
| Notten, 2020 2 (CAVA) | Acute IFDVT | Not reported |
CDT vs. STND ReVTE (reDVT, PE, or IST): 24 (17 vs. 7) events in 20 (14 vs. 6) patients. ‐ReDVT: 5/77 (6.5%) vs. 5/75 (6.7%). ‐PE: 0/77 (0%) vs. 2/75 (2.7%). |
12/77 (15.6%) |
CDT vs. STND 4/77 (5.2%) vs. 0/75 (0%). |
CDT vs. STND ‐Original score: 22/77 (28.6%) vs. 26/75 (34.7%). ‡ ‐ISTH method: 32/77 (41.6%) vs. 33/75 (44.0%). § |
12 mo b |
| Cohort studies, prospective | |||||||
| AbuRahma, 2001 20 | Acute IFDVT |
STND vs. MULTI: ‐At 1 mo: 3% vs. 83% (P < .001). ‐At 6 mo: 24% vs. 83% (P < .001). ‐At 1 yr: 24% vs. 83% (P < .01). ‐At 3 y: 18% vs. 69% (P < .01). ‐At 5 yr: 18% vs. 69% (P < .01). |
STND vs. MULTI ReVTE: 2/33 (6.1%) vs. 2/18 (11.1%). PE: 2/33 (6.1%) vs. 0/18 (0%). No ICV filters used |
Not reported | STND vs. MULTI: 2/33 (6.1%) vs. 2/18 (11.1%) | Not reported | 63 mo a vs. 51 mo a |
| Grommes, 2011 21 | Acute DVT | Not reported |
ReVTE: 4/13 (30%). In stented patients: None (0/3) PE: None (0/3) |
None (0/3) | None (0/3) | Not reported | 7 mo a |
| Manninen, 2012 22 | Acute IFDVT | At 3 y: 41/47 (87%) |
ReDVT: 2/56 (3.6%). PE: PE 1/56 (1.8%). ICV‐filter placed in 5 (8.9%) |
Not reported | 1/56 (1.8%) | 4/47 (8.5%)** | 42 mo a |
| Raju, 2014 23 | Chronic obstruction (iliac) | PP and SP: 69% and 93% |
ReDVT: ‐Early (<30 d): 4% ‐Late (>30 d): 1% |
8/217 (3.7%) | Not reported | Not reported | 24 mo |
| Srinivas, 2014 24 | Subacute DVT (1‐8 wk) |
CDT vs. STND 20/25 (80.0%) vs. 7/26 (26.9%). |
CDT vs. STND PE: 14.8% (4/27) vs. 21.4% (6/28). ICV filters placed in 5/27 patients from the CDT group (18.5%) |
Not reported | None (0/6) |
CDT vs. STND § 5/25 (20.0%) vs. 19/26 (73.1%) |
6 mo |
| Sarici, 2014 25 | PTS |
If primary disease: 86% If secondary disease: 90%. |
Not reported | 5/52 (9.6%) | Not reported | Not reported | 6 mo |
| Sebastian, 2018 26 | Acute IFDVT |
DOAC vs. VKA PP, aPP, and SP: ‐At 12 mo: 90% vs. 85%; 91% vs. 88%; 97% vs. 94% ‐At 24 mo: 87% vs. 72%; 89% vs. 88%; 95% vs. 94% |
ReVTE (DVT, PE, or IST): 15/111 (14%) ‐ReDVT: 1/111 (1%; VKA group) ‐PE: 1/111 (1%; DOAC group) |
13/111 (11.7%) ‐Early: 5/111 (4.5%). DOAC vs. VKA: 3/73 (4%) vs. 2/38 (5%) ‐Late: 8/111 (7.2%). DOAC vs. VKA: 4/73 (5%) vs. 4/38 (11%) |
2/111 (2%). DOAC vs. VKA: 1/73 (1%) vs. 1/38 (3%) |
DOAC vs. VKA ¶ Free of PTS: 85% vs. 88% |
24 mo a |
| Notten, 2020 3 | Obstruction (cavo‐iliofemoral; postthrombotic (acute or chronic) or IVCS) | Not reported |
ReDVT: None (0/74) PE: None (0/74) |
16/79 (20.3%). In post‐DVT patients: 16/74 (21.6%; acute n = 4 (25.0%) vs. chronic n = 12 (75.0%)) | 2/79 (2.5%). In post‐DVT patients: 2/74 (2.7%) | Not reported | 39 mo b |
| Cohort studies, retrospective | |||||||
| O'Sullivan 2000 27 | Chronic obstruction (IVCS) |
PP at 1 d, 1 mo, and 1 yr: ‐Overall: 97%, 93.6%, 93.6%. ‐Acute DVT: 100%, 93.1%, and 93.1%. ‐IVCS: 93.9%, 93.9%, and 93.9% |
PE: None | 2/35 (5.7%) | None | Not reported | 12 mo b |
| Kölbel, 2007 28 | Acute IFDVT | PP, aPP, and SP at 16 mo: 34/44 (77.3%), 38/44 (86.4%), and 39/44 (88.6%) |
ReDVT: 1/36 (2.8%) PE: None (0/36). ICV filters implanted in all patients before start of intervention. All were removed afterwards. |
5/44 limbs (11.4%) | 3/36 (8.3%) | Not reported |
Patency: 16 mo b Clinical: 27 mo b |
| Knipp, 2007 29 | Chronic obstruction (IVCS) |
PP, aPP, and SP: ‐At 1 y: 74.1%, 79.7%, 85.8% ‐At 5 y: 38.1%, 62.8%, 73.8% |
Not reported | Not reported | 1 (1.7%) | Not reported | 30 mo a |
| Neglén, 2007 30 (Neglén‐cohort) | Chronic obstruction (femoro‐ilio‐caval) |
PP, aPP, and SP at 72 mo: Overall: 67%, 89%, and 93% ‐NIVL: 79%, 100%, and 100% ‐Post‐DVT: 57%, 80%, and 86%. |
ReVTE (reDVT or IST): 47/982 (4.8%): ‐ReDVT: 16/982 (1.6%) ‐Early (<30 d): 7/982 (0.7%) ‐Late (>30 d): 9/982 (0.9%) |
31/982 (3.2%) ‐Early (<30 d): 8/982 (0.8%) ‐Late (>30 d): 23/982 (2.3%) |
2/982 (0.2%) | Not reported | 22 mo a |
| Hartung, 2009 32 | Chronic obstruction (iliocaval) | PP, aPP, and SP at 3 and 10 y: 83%, 89%, 93% |
ReVTE: 5/89 (5.6%) ‐In‐hospital: 2/89 (2.2%) ‐During FU: 3/89 (3.4%). |
Not reported | Not reported | Not reported | 38 mo b |
| Kölbel, 2009 33 | Chronic obstruction (iliac) | PP, aPP, and SP: 67%, 75%, and 79% | ReVTE: 3/59 (5.1%) | Not reported | 2/62 (3.2%) | Not reported | 25 mo b |
| Raju, 2009 34 | Chronic obstruction (postthrombotic) | SP at 4 y: 66% | Not reported |
39/139 (28.1%) ‐Within 30 d: 10/139 (7.2%) ‐After 30 d: 29/139 (20.9%) |
None (0/129) | Not reported | Not reported |
|
Baekgaard, 2010 35 (Gentofte‐cohort) |
Acute IFDVT | Patency without reflux at 6 y: 82% |
ReVTE (DVT, IST): 6/101 (5.9%) ‐ReDVT: 5/101 (5.0%) ‐Early (<1 wk): 2/101 (2.0%) ‐Late: 3/101 (3.0%) PE: None (0/57). ICV‐filter placed in 7 patients. |
1/101 (1.0%) ‐Early (<1 wk): 1/101 (1.0%) ‐Late: 0/101 (0%) |
1/101 (1.0%) | Not reported | 50 mo b |
| Jeon, 2010 37 | Acute DVT (with MTS) | PP and SP at 1 yr: 83.3% and 90% | Not reported | 4/30 (13.3%) | Not reported | Not reported | Not reported |
| Rosales, 2010 38 | Chronic obstruction (iliofemoral, postthrombotic) | PP, aPP, and SP at 2 y: 14/21 (67%), 16/21 (76%), 19/21 (90%) |
ReVTE: 13/32 (40.6%) ‐Early (<1 mo): 2/32 (6.3%) ‐Late: 11/32 (34.4%). |
Not reported | Not reported | Not reported | 33 mo b |
| Titus, 2010 39 | Obstruction (iliofemoral) |
PP, aPP, and SP: ‐At 6 mo: 88.1%, 92.5%, 100.0% ‐At 12 mo: 78.3%, 82.7%, 95.0%. ‐At 24 mo: 78.3%, 82.7%, 95.0%. |
PE: None. ICV filters already placed in 9 (25%) patients and in 2 (5.6%) patients as part of this study. |
6/36 (16.7%) ‐Early: 1/36 (2.8%) ‐Late: 5/36 (13.9%) |
None | Not reported | 10 mo a |
| Wahlgren, 2010 40 | Chronic obstruction (femoro‐ilio‐caval, post‐thrombotic) | PP and aPP/SP at 12 mo: 61% and 81% | Not reported |
7/16 (43.8%) ‐Early: 3/16 (18.8%) ‐Late: 4/16 (25.0%) |
None (0/16) | Not reported | 23 mo |
| Nayak, 2012 41 | PTS | Not reported | PE: None (0/39) | 4/39 (10.3%) | None (0/39) | Not reported | 41.7 ± 13.2 d (range 20‐108 d) a |
| Blanch, 2013 42 | Chronic obstruction (iliofemoral, postthrombotic) | PP, aPP, and SP at 33 mo: 74%, 87%, 89% | Not reported |
9/39 limbs (23.1%) ‐Early (<4 wk): 5/39 (12.8%) ‐‐Stenosis: 2/39 (5.1%) ‐‐Occlusion: 3/39 (7.7%) ‐Late (>4 wk): 4/39 (10.3%) ‐‐Occlusion: 4/39 (10%). |
Not reported | Not reported | 21 mo a |
| Stanley, 2013 43 | Acute or chronic DVT |
Acute vs. chronic ‐At 1 mo: 96% vs. 93% ‐At 6 mo: 92% vs. 89% ‐At 46 mo: 94% vs. 82% |
PE: 6/80 (7.5%). ICV filter in 49/80 (61.3%) | Not reported | 3/80 (3.8%) | Not reported | 46 mo a |
| Liu, 2014 44 | Chronic obstruction (IVCS) |
PP at 12 mo: 93.0% ‐Post‐DVT vs. nonthrombotic: 81.8% vs. 96.9% |
ReDVT: 1/46 (2.2%) PE: None (0/12) |
2/46 (4.3%) | 1/46 (2.2%) | Not reported | 12 mo |
| Park, 2014 45 | Acute DVT (with MTS) | PP at 6, 12, and 24 mo: 95.8%, 87.5%, and 84.3% |
ReVTE (DVT or IST): 4/51 (7.8%) ‐ReDVT: 1/51 (2.0%) ‐PE: Not reported. All patients received ICV filters |
3/51 (5.9%) | 1/51 (2.0%) | Not reported | 16 mo a |
| Sang, 2014 46 | PTS |
PP and SP: ‐At 12 mo: 87.9% and 93.1% ‐At 36 mo: 70.7% and 82.8% |
PE: None (0/63) |
11/63 (14.5%) ‐Early (<30 d): 7/63 (11.1%) ‐Late (>30 d): 4/63 (6.3%) |
None (0/63) | Not reported | 36 mo a |
| Ye, 2014 47 | Chronic obstruction (iliofemoral, postthrombotic) | PP, aPP, and SP at 3 y: 70%, 90%, and 94% | PE: None (0/112) | 20/110 (18.2%; 21/112 limbs = 18.8%) | None (0/112) | Not reported | 25 mo b |
|
Catarinella, 2015 48 (MUMC) |
Chronic obstruction |
PP, aPP, and SP: 65%, 78%, and 89% |
Not reported | Not reported | Not reported | Not reported | 24 mo |
| Shi, 2016 50 | Chronic obstruction (IVCS) |
PP and SP: ‐At 1 yr: 93.2% and 100% ‐At 3 y: 84.3% and 93.3% ‐At 5 y: 74.5% and 92.0% |
ReDVT: 11/225 (4.9%) PE: Not reported. IVC filter placed in 95 (40.9%) patients, all being post‐DVT (95/110 = 86.3%) |
37/225 (16.4%) ‐Caudal: 22/37 (59.5%) ‐Complete tract 15/37 (40.5%) |
2/225 (0.9%) | Not reported | 34 mo b |
| Comerota, 2019 51 | Chronic obstruction (iliofemoral, postthrombotic) | Not reported |
Old vs. new method ReDVT: 5/17 (29.4%) vs. 0/14 (0%) |
Not reported |
Old vs. new method 4/17 (23.5%) vs. 1/14 (7.1%) |
Not reported | Not reported |
| Dumantepe, 2018 52 | Subacute DVT (<1 mo) | At 12 mo: 59/65 (90.7%) | PE: Not reported. IVC filter placed in 10/68 (14.7%) | Not reported | 1/68 (1.5%) | At 12 mo: 5/68 (7.3%) § | 16 mo a |
| Endo, 2018 53 | Chronic compression and/or acute DVT | PP and SP: 70.0% and 92.4%. | Not reported |
17/62 (27.4%) ‐Stenosis: 5/62 (8.1%) ‐Occlusion: 12/62 (19.3%). |
3/62 (4.8%) | Not reported | 12 mo b |
| Case series | |||||||
| Acharya, 2005 54 | Subacute DVT (≤3 wk ≤6 wk postpartum) | Not reported |
ReDVT: None (0/2) PE: None (0/2) |
None (0/2) | None (0/2) | Not reported | Not reported |
| Dayal, 2005 55 | Critical chronic compression and/or acute DVT | Not reported |
ReVTE (reDVT or IST): 13/25 (48%) ‐Within 14 d: 1/25 (4%) ‐After 14 d: 12/25 (48%) ‐ReDVT: 7/25 (28%) PE: None. ICV filters placed in 11 patients |
6/15 (40%) | 3/25 (12%) | Not reported | 11 mo a |
| Husmann, 2007 56 | Acute DVT (with MTS) | PP and aPP: 81% (9/11) and 91% (10/11) | Not reported |
2/11 (18.2%) ‐In‐hospital: 1/11 (9.1%) ‐During FU: 1/11 (9.1%) |
None (0/11) | Not reported | 22 mo a |
| Murphy, 2009 57 | Acute DVT (with MTS and initiation of oral contraceptives) | PP: 100% | ReDVT: None (0/7) | None (0/7) | 1/7 (14.3%) | None (0/7) †† | 16 mo a |
| Oguzkurt, 2011 58 | Phlegmasia cerulea dolens (from IFDVT) | Not reported |
ReDVT: 2/7 (28.0%) ‐Early: 2/7 (28.0%). In stented patients: 0/3 (0%) ‐During FU: None (0/3) PE: None (0/3). ICV filters were placed in 3 (42.9%) patients. All were removed and none had emboli inside. |
None (0/3) | None (0/3) | Not reported | 4 mo |
| Bloom, 2015 59 | Acute IFDVT (during pregnancy or ≤6 wk postpartum) | PP: 87.5% |
ReDVT, early: 2/8 (25.0%) PE: None (0/8). All patients received an ICV filter. Thrombus was found inside the filter in 2 patients of which 1 with stent (1/8, 12.5%) |
Not reported | None (0/8) | None (0/8) § | 20 mo b |
| Langwieser, 2016 60 | Chronic obstruction (iliofemoral, postthrombotic) | PP: 100% | ReDVT: None (0/9) | None (0/9) | None (0/9) | Not reported | 14 mo b |
| Ming, 2017 61 | Acute IFDVT (with IVCS) | Not reported | Not reported | Not reported | Not reported |
74/247 (30%). § Stented vs. not stented: 25/116 (21.6%) vs. 49/131 (37.4%) |
Not reported |
| Case reports | |||||||
| Kapranov, 2003 62 | PTS (after IFDVT) | 100% | Not reported | None (0/1) | Not reported | Not reported | 3 mo |
| Oguzkurt, 2008 63 | Phlegmasia cerulea dolens (from IFDVT with MTS) | 100% | ReDVT: None (0/1) | None (0/1) | None (0/1) | None (0/1) ‡‡ | 3 mo |
| Salam, 2010 64 | Acute IFDVT (with EIV stenosis from repetitive microtrauma) | Not reported | Not reported | None (0/1) | Not reported | Not reported | 1 mo |
| Sharifi, 2010 65 | Acute IFDVT | 100% | Not reported | None (0/1) | Not reported | None (0/1) ‡‡ | 6 mo |
| Wormald, 2012 66 | Acute IFDVT (with MTS) | 100% | ReDVT: None (0/1) | None (0/1) | Not reported | None (0/1) ‡‡ | 6 mo |
| Singh, 2017 67 | Acute IFDVT (with MTS and pelvic mass) and PE | 100% | PE: None. Temporary ICV filter placed, removed after 4 d. | Not reported | Not reported | Not reported | 6 mo |
| Kohler, 2018 68 | Severe PTS (after cavo‐iliacal DVT) | Not reported | Not reported | Multiple IST | Not reported | Not reported | 10 mo |
| Lakha, 2018 69 | Acute IFDVT (with MTS) | Not reported | Not reported | Symptomatic IST and new stenosis of the left VIC distal to the stent at 5‐month FU | Not reported | Not reported | 17 mo |
| Rohr, 2019 70 | Acute IFDVT | 100% | ReDVT: None (0/1) | None (0/1) | None (0/1) | Not reported | 9 mo |
| Barge, 2020 71 | Acute cavo‐bi‐iliacal DVT (also involving left renal vein) | 100% | Not reported | None (0/1) | Not reported | None (0/1) †† | 6 mo |
All outcomes reported in bold represent data specified for the number of patients with post‐DVT (acute or chronic) treatment indications receiving venous stent placement. In the reporting of PTS, various definitions were used. These included the original definition 72 (‡), the ISTH consensus method 72 , 73 (§), a combination of the Venous Clinical Severity Score 75 and a revised Villalta‐score 74 (¶), or alternative definitions (**). In some studies, no definition was specified (††). Furthermore, some studies reported an absence of symptoms (‡‡).
Abbreviations: aPP, assisted primary patency; ARR, absolute risk reduction; CDT, catheter‐directed thrombolysis; CIV, common iliac vein; DOAC, direct oral anticoagulants; DVT, deep vein thrombosis; EIV, external iliac vein; FU, follow‐up; ICV, inferior caval vein; IFDVT, iliofemoral deep vein thrombosis; IST, in‐stent thrombosis; ISTH, International Society of Thrombosis and Haemostasis; IVCS, iliac vein compression syndrome; MTS, May‐Thurner syndrome; MULTI, multimodal treatment; NIVL, nonthrombotic iliac vein lesions; PAT, percutaneous aspiration thrombectomy; PE, pulmonary embolism; PEVI, percutaneous endovenous intervention; PP, primary patency; PTA, percutaneous transluminal angioplasty; PTS, postthrombotic syndrome; reVTE, recurrent venous thromboembolic event; SP, secondary patency; STND, standard treatment; VKA, vitamin K antagonist.
Mean value.
Median value.
Nineteen studies (34%) 16 , 52 , 56 , 57 , 59 , 63 , 65 , 66 , 67 , 70 , 71 included only patients who received an intervention for acute DVT. Five studies 26 , 28 , 37 , 45 , 56 reported patency using the definitions primary patency, assisted primary patency, and/or secondary patency. Results were specified for stented patients in four studies, 26 , 37 , 45 , 56 of which three reported on stenting for acute DVT associated with underlying May‐Thurner syndrome. 37 , 45 , 56 In the stented patients of these four studies, with a follow‐up duration between 12 and 22 months, the mean primary patency was 82% (standard deviation [SD] 6.6), ranging from 72% 26 to 90% 26 ; the mean assisted primary patency was 89% (SD 1.7), ranging from 87% 26 , 45 to 91% 26 , 56 ; and the mean secondary patency was 93% (SD 3.1), ranging from 89% 45 to 97%. 26 Postinterventional antithrombotic treatment with either 3 months of DOAC or 3 months of VKA did not impact patency rates differently (P > .10). 26 The study that did not specify results for stented patients reported an overall primary patency of 77.3%, assisted primary patency of 86.4%, and a secondary patency of 88.6% 16 months after an intervention for acute IFDVT. 28
In the study by AbuRahma et al 20 patency was defined as a ≤30% residual stenosis. A significant difference between standard treatment and additional multimodality treatment (including CDT, percutaneous transluminal balloon angioplasty, and/or stenting) was reported after 1 month, 3 months, 1 year, 3 years, and 5 years. It was not specified whether patients were stented for thrombotic or other indications. Another study 52 used a cutoff value of <50% restenosis to define patency. At 1 year, they reported a patency rate of 90.7% in patients with a subacute (complaints for less than 1 month) DVT. Baekgaard et al used a definition for patency which included the absence of reflux. The analysis of the first 45 patients included in the cohort showed a patency rate of 100% at 2 years of follow‐up. 36 After extension of the cohort and the follow‐up duration to 6 years, patency was seen in 82% of patients. 35
Cakir et al 16 differentiated between completely patent, partially patent, and thrombosed. They found a significantly higher complete patency rate in patients randomized to additional percutaneous aspiration thrombectomy compared with standard treatment alone following acute iliofemoral‐popliteal DVT at 1 month, at 3 months, and at 1 year (all P < .001). Specified for stented patients, complete patency was seen in 71.4%.
Vein segments were classified as either patent or occluded in four studies. 22 , 24 , 57 , 59 Manninen et al 22 found 87% of vein segments to be patent 3 years after an intervention. Srinivas et al 24 compared additional CDT to standard treatment alone in subacute DVT and found a significant difference at 6 months: 80.0% versus 26.9%, P < .01. Specifically, patency was 100.0% in stented patients with accompanying May‐Thurner syndrome 57 and 87.5% in patients stented after an acute IFDVT during pregnancy or the first 6 weeks postpartum. 59 All six case reports reported patent stents 63 , 65 , 70 , 71 or stents without (residual) signs of DVT. 66 , 67
Another 21 studies 23 , 25 , 38 , 39 , 40 , 42 , 43 , 44 , 46 , 47 , 48 , 49 , 50 , 53 , 60 , 62 included a mixed case load with patients treated for thrombotic (acute or chronic) or nonthrombotic venous pathology. Outcomes were mostly reported as primary patency, assisted primary patency, and secondary patency. Overall, the primary patency rates in these studies had a mean of 82.5% (SD 12.7) and ranged from 61% 40 to 100% 60 1 year after the intervention. 27 , 29 , 39 , 40 , 44 , 46 , 49 , 50 , 53 , 60 After 2 years, 23 , 33 , 38 , 39 , 48 a mean primary patency of 69.3% (SD 5.2) was found ranging from 65% 48 to 78.3%. 39 This remained stable after 3 years, 32 , 42 , 46 , 47 , 50 with a mean primary patency of 76.4% (SD 6.8) and a range from 70.0% 47 to 84.3%. 50 Mean primary patency dropped to 56.3% (SD 25.7) after 5 years with a range from 38.1% 29 to 74.5%. 50 Long‐term follow‐up data on patency rates are scarce: one study 30 reported 67% primary patency after 6 years, and another study 32 reported 83% primary patency after 10 years. Reported secondary patency rates varied from 81% 40 to 100% 50 after 1 year with a combined mean of 91.9% (SD 6.5), 29 , 39 , 40 , 46 , 49 , 50 , 53 which appeared to remain stable at longer durations of follow‐up: 89.2% (SD 6.2; range 79% 33 to 95% 39 ), 23 , 33 , 38 , 39 , 48 90.4% (SD 4.7; range 82.8% 46 to 94% 47 ), 32 , 42 , 46 , 47 , 50 and 82.9% (SD 12.9; range 73.8% 29 to 92.0% 50 ) after 2, 3, and 5 years, respectively.
Some studies presented patency rates per‐treatment indication being either primary or secondary 25 , 27 , 30 , 31 , 44 resulting in a combined mean primary patency of 83.2% (SD 14.7) versus 74.8% (SD 19.0). No statistically significant differences were seen for acute versus chronic thrombotic pathology at 46 months of follow‐up (P = .12). 43
4.3. Recurrent venous thromboembolism
Information regarding reVTE was provided in 41 studies (73%) 3 , 43 , 44 , 46 , 47 , 49 , 50 , 51 , 55 , 60 ; 23 of these studies included patients treated for acute DVT. 2 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 24 , 26 , 28 , 35 , 36 , 45 , 52 , 54 , 57 , 63 , 66 , 67 , 70 (Table 3). Studies used different definitions for reVTE, which could include reDVT, IST, PE, and/or the use of inferior caval vein filters. Although specification of results into these different entities was often not performed, when provided, these data will also be presented separately here.
The incidence of reVTE was reported in 16 studies (29%). 2 , 10 , 26 , 30 , 32 , 33 , 35 , 36 , 38 , 45 , 50 , 51 , 55 Nine studies 2 , 26 , 35 , 36 , 45 included patients treated for acute venous pathology only. In two of these studies, the incidences for reVTE were specified for patients receiving stents: 0.0% in the study by Grommes et al 21 and 7.8% in the study by Park et al 45 Overall, incidences for reVTE ranged from 5.9% 35 to 30%, 21 with significant differences reported depending on received treatment but not on duration of follow‐up. In the publication by Sharifi et al, treatment with additional venous interventions was compared with standard anticoagulant therapy that resulted in recurrence rates of 2.3% versus 14.8%, P = .003, after 6 months of follow‐up, 15 whereas these rates were 4.8% versus 16.0%, P = .02, after a mean follow‐up of 30 months. 14 Notten et al 2 assessed the number of events including IST and found events in 22.1% versus 9.3% (P = .05) of patients, respectively. For the seven studies that also included interventions for chronic pathologies, 30 , 32 , 33 , 38 , 50 , 51 , 55 the overall incidences varied from 4.8% 30 to 48% 55 without correlation to duration of follow‐up.
ReDVT was registered as a separate entity in 24 studies (43%): 17 including only interventions for acute DVT 2 , 35 , 45 , 54 , 57 , 58 , 59 , 63 , 66 , 70 and seven studies (also) including chronic treatment indications. 3 , 23 , 27 , 30 , 44 , 55 , 60 In the studies including acute pathology only, incidence had a mean of 6.0% (SD 8.1) versus 4.6% (SD 9.6) in studies also including patients treated for chronic venous obstructions without influence of follow‐up duration.
In 13 studies incidences could be specified for patients with (a history of) DVT who underwent venous stent placement 16 , 26 , 28 , 45 , 54 , 57 , 58 , 63 , 66 , 70 ; reDVT was only seen in studies that included patients treated during the acute phase of DVT: Sebastian et al (1%), 26 Park et al (2.0%), 45 and Kölbel et al (2.8%). 28 ReDVT was absent in the other seven studies (including three case reports) 16 , 54 , 57 , 58 , 63 , 66 , 70 with an acute indication for intervention as well as in the other three studies with mixed treatment indications including both acute and chronic pathology. 3 , 27 , 60
PE was reported in 25 studies (45%): 16 addressing interventions performed during the acute phase only, 2 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 20 , 21 , 22 , 24 , 26 , 28 , 35 , 54 , 58 , 59 , 67 and nine also including interventions during the chronic thrombotic phase. 3 , 39 , 41 , 43 , 44 , 46 , 47 , 49 , 55 Overall, including the total study population of these studies, incidences of PE ranged from 0.0% to 21.4%, 24 with two studies 16 , 24 reporting an incidence of more than 10%.
In 18 studies (10 10 , 17 , 21 , 26 , 28 , 35 , 54 , 58 , 59 , 67 solely directed at treatment of acute DVT vs eight 3 , 39 , 41 , 44 , 46 , 47 , 49 , 55 including chronic pathology) results were specified for stented patients. PE was absent in all but one study: Srinivas et al reported an incidence of 1%. 24 The use of (temporary) inferior caval vein filters was addressed in 16 studies (29%). 14 , 15 , 16 , 17 , 43 , 45 , 50 , 52 , 55 , 58 , 59 , 67
4.4. In‐stent thrombosis
IST was reported as a separate entity in 41 studies (73%): 20 studies in which interventions were performed in the acute thrombotic phase 2 , 14 , 15 , 16 , 45 , 54 , 56 , 57 , 58 , 63 , 64 , 65 , 66 , 69 , 70 , 71 and 21 studies (also) including interventions for chronic thrombotic pathology. 3 , 23 , 44 , 46 , 47 , 49 , 50 , 53 , 55 , 60 , 62 , 68 (Table 3).
Specification of outcomes for patients stented following thrombosis was possible in all 20 studies reporting on interventions performed during the acute phase. Occurrence of IST was seen in 11 of these studies 2 , 14 , 16 , 26 , 28 , 35 , 37 , 45 , 56 , 57 and ranged from 1.0% 35 to 18.2%. 56 IST was absent in the remainder cohort study, 21 two case series, 54 , 58 and six case reports. 63 , 64 , 65 , 66 , 70 , 71 Differentiation of the occurrence rate into early and late IST was possible in the studies by Sebastian et al (4.5% vs 7.2%, P = .57) 26 and Husmann et al (9.1% vs 9.1%, P > .99). 56 No difference was seen when treated with 3 months of VKA or rivaroxaban: P > .99 for the early IST and P = .54 for the late IST. 26
If study populations also included patients receiving interventions for chronic pathology, 3 , 47 , 55 , 60 the occurrence of IST in patients with (post)thrombotic stenting ranged from 0.0% 60 , 62 to 43.8% 40 next to the multiple recurrences resulting from nonresponsiveness to antithrombotic treatment in one 68 of the two case reports. 62 , 68 Early occurrences ranged from 5.4% 3 to 18.8% 40 and late occurrences from 3.8% 31 to 34.4%. 38 In studies that did not allow specification of outcomes for patients with thrombotic or nonthrombotic chronic pathology, 23 , 27 , 30 , 39 , 44 , 49 , 50 , 53 the overall risk of IST ranged from 3.2% 30 to 27.4%. 53 Several studies also mentioned the occurrence of (in‐stent) stenosis and occlusion without clarifying its cause being thrombotic or nonthrombotic. 32 , 38 , 42 , 46 , 53
4.5. Major bleeding
In 40 studies (71%), the occurrence of major bleedings was reported, 21 studies 2 , 10 , 11 , 12 , 13 , 24 , 26 , 28 , 35 , 36 , 45 , 52 , 54 , 56 , 57 , 58 , 59 , 63 , 70 aimed at interventions in the acute phase and 19 studies including chronic treatment indications 3 , 27 , 43 , 44 , 46 , 47 , 49 , 50 , 51 , 53 , 55 , 60 (Table 3). In studies comparing additional interventional treatment for thrombus removal to standard treatment alone, there appeared to be a higher risk of major bleeding in the intervention groups: 2.2% versus 1.1% during a mean hospitalization of 2.7 ± 1.1 versus 5.8 ± 1.3 days (P = .57), 15 1.8% versus 0.3% (P = .049) 18 and 1.5% versus 0.5% during the first 10 days after the intervention (P = .32), 19 5.2% versus 0.0% at 1 year of follow‐up (P = .06), 2 and 11.1% versus 6.1% at long term (P = .89). 20
The incidence of major bleeding could be specified for stented (post)thrombotic patients in 24 studies. 3 , 13 , 39 , 40 , 41 , 45 , 46 , 47 , 51 , 54 , 56 , 57 , 58 , 59 , 60 , 63 , 70 Major bleedings were absent in 10 of the 14 studies on acute pathology. 13 , 17 , 21 , 24 , 54 , 56 , 58 , 59 , 63 , 70 This included the two case reports addressing this complication. 63 , 70 Only a single study showed an incidence of more than 2.0%: the case series by Murphy et al found an incidence of 14.3%. 57 Of the studies including chronic venous pathology, eight found an incidence of 0.0% 27 , 34 , 39 , 40 , 41 , 46 , 47 , 60 and one of 2.7%. 3 The study by Comerota et al 51 compared incidences between patients treated according to their original peri‐interventional protocol and after modifications that resulted in a significant risk reduction: 23.5% versus 7.1% (P = .47).
4.6. Postthrombotic syndrome
PTS was assessed in 15 studies (27%), 2 , 24 , 26 , 52 , 57 , 59 , 61 , 63 , 65 , 66 , 71 all of which addressed venous interventions during the acute phase (Table 3). The definition for PTS varied among the different studies. The original definition, 72 , 73 requiring an elevated Villalta score on two separate measurements at least 3 months apart or the occurrence of venous ulceration, was used in one study 2 only. Comparing additional ultrasound‐accelerated CDT (which included stenting in 45.5% of patients) to standard treatment alone, PTS developed in 28.6% and 34.7%, respectively. Most frequently used (seven studies 2 , 10 , 12 , 13 , 18 , 19 , 24 , 52 , 59 , 61 ) was the definition according to the ISTH consensus method 73 : a single elevated Villalta score (≥5) 72 at 6 months or later after the acute event or the presence of venous ulceration. In four of these studies, outcomes were compared between groups treated with standard treatment alone or additional interventions, which sometimes included venous stenting. The reported incidence of PTS in these groups were 55.6% versus 41.1% (P = .047), 10 73.1% versus 20.0% (P < .01), 24 48.2% versus 46.6% (P = .56), 18 and 44.0% versus 41.6% (P = .76), 2 respectively. However, the incidence of PTS was not specified separately for patients who received venous stenting. Sebastian et al 26 used a revised Villalta score 74 and the Venous Clinical Severity Score 75 when comparing the use of DOAC to the use of VKA following (post)thrombotic stenting. Patients were reported to be free of PTS in 85% versus 88% of cases (P = .76). Alternative definitions were used by Sharifi et al 14 , 15 (presence of ≥two symptoms [leg burning, pain, aches, discomfort, restlessness, and tingling] combined with edema and venous reflux [classified as mild PTS], skin hyperpigmentation or lipodermatosclerosis [classified as moderate PTS], or an active or healed ulcer [classified as severe PTS]) and Manninen et al 22 (abnormal functioning of the venous system because of valvular incompetence with or without associated venous obstruction in a limb with a prior objectified DVT). In one study 57 and four case reports, 63 , 65 , 66 , 71 the definition of PTS was not specified but only the absence of the PTS was reported. Three of these case reports merely described that patients were free of symptoms during follow‐up. 63 , 65 , 66
5. DISCUSSION
With the increasing numbers of venous interventions performed and the introduction of (dedicated) venous stents over the recent years, we expected that the available literature reporting on peri‐interventional antithrombotic management would have expanded accordingly. We therefore set out to perform an update of a systematic review addressing the issue of peri‐interventional antithrombotic management related to venous stenting published by our group 6 years ago. 6 At the time, we concluded that there was little information and much uncertainty on the role of antithrombotic treatment in the context of venous stenting. Unfortunately, not much has changed.
Our up‐to‐date search showed that in the year 2020, there still is no or little attention for antithrombotic treatment surrounding venous stent placement given that only two studies directly assessed this issue when reporting on study outcomes. Only one of these studies addressed the direct impact of antithrombotic treatment on IST following stenting procedures. 3 This study suggested that the risk of IST decreases with increased quality of antithrombotic treatment with VKA, expressed as TTR.
The majority of postinterventional antithrombotic regimens prescribed in the studies included in this review entail temporary use of VKA with concomitant LMWH during the initiation phase. The use of DOAC was still limited in the selected studies despite their convenience, presumed advantages in limiting PTS, and a lower risk of major bleeding. Partially, this could be explained by the fact that most studies in this systematic review are older and DOAC were not yet incorporated into daily practice. Specification of antithrombotic treatment for stented patients in particular was rarely provided. If antithrombotic treatment was described for stented patients specifically, it most often entailed the concomitant use of antiplatelet drugs. Furthermore, sole treatment with antiplatelet drugs, sometimes with LMWH directly following the intervention, was frequent in patients stented for chronic pathology. Overall, it appears that choices in postinterventional antithrombotic management are driven by the patient's history of DVT and follow current guidelines for thrombotic management rather than to specifically adjust treatment if stenting is performed.
Following the increased acceptance of the “open vein hypothesis” and the rapid evolution of new treatment techniques, venous stent placement has become a more and more prominent treatment modality for both acute and chronic thrombotic as well as nonthrombotic deep venous pathology in an attempt to prevent or reduce the severity of symptoms and complaints. Subsequently, preservation of acquired patency is presumed essential to prevent the development of PTS or the recurrence of symptoms requiring reinterventions leading to additional health care costs. The value of peri‐interventional antithrombotic treatment to achieve optimal long‐term results may be presumed from Virchow's triad. This may explain why the apparent higher recurrence rate in nonthrombotic (eg, May‐Thurner syndrome) patients is lower than in patients with prior thrombosis. In principle, anticoagulation corrects the hypercoagulability from preexisting prothrombotic risk factors and spans the time needed for the healing of endothelial perturbation following recanalization. It is striking to note that, although the influence of antithrombotic treatment on treatment outcomes is widely recognized, it still receives so little attention. This lack of attention extends to other potential confounders on outcomes surrounding the intervention, including selection of patients, thrombotic status, stent characteristics, and the interventional techniques used. 51 Moreover, study outcomes are reported using various definitions and classifications, which makes it difficult to extract specific results and leads to limited availability of comparable data.
Over time, the outcomes of interest reported seem to have shifted from the reporting of mainly technical (ie, patency rates) outcomes to inclusion of more clinical outcomes (ie, PTS). However, the correlation between technical success and clinical outcome remains uncertain.
Besides the many weaknesses that have to be taken into consideration when interpreting the reported study outcomes, one should also consider what is the minimal effect that should be deemed acceptable. Regarding the primary patency rate an overall mean of 82.3% (SD 10.8) was seen at 1‐year of follow‐up, which reduced to 73.3% (SD 7.9) at 2 years, and 76.4% (SD 6.8) at 3 years of follow‐up. This means that in about 25% of patients, patency could not be preserved, potentially resulting in recurrence of symptoms or the need for a reintervention. Data on patency rates at longer durations of follow‐up are limited. Second, one should assess whether and where adaptations can and should be made. Improvements could be targeted at either the stenting material, the interventional technique, or the peri‐interventional circumstances including treatment indications and antithrombotic treatment. Over the years, outcomes for primary patency did not improve considerably despite progression and innovation regarding venous stenting procedures, suggesting the influence of stenting materials and interventional techniques to be less important. Parallel to the optimization of antithrombotic treatment regimen supporting stenting procedures in the arterial setting, one would expect that optimization of antithrombotic therapy could possibly also contribute to the reduction of IST and improved clinical outcomes in the venous setting. Although a meta‐analysis could not be performed, an indication for a protective effect of postinterventional antithrombotic treatment was suspected. This is in particular based on the study by Notten et al 3 showing that a more adequately executed antithrombotic treatment could protect against the development of IST.
The overall quality of the studies reported is reasonable. However, there seems to be a discrepancy between acquired quality assessment score and our intuitive appraisal of the data. Therefore, we suggest the introduction of common data elements for the reporting of venous interventions and stenting to overcome these impediments. We propose that a clinical trial comparing outcomes in groups with different yet clearly defined and well‐documented antithrombotic treatment regimens is necessary to gather valid data regarding its influence on long‐term outcomes after (post)thrombotic iliofemoral venous stent placement. In addition, results should be presented after consideration of the various factors surrounding the intervention possibly intervening with its outcomes.
In conclusion, the results of this review show that there is a persistent hiatus in knowledge regarding antithrombotic management surrounding venous stent placement even though its clinical importance can be presumed. Future studies addressing this issue should be performed using specified treatment protocols and report on clearly defined study outcomes while also taking into consideration the broad variety of possible confounding factors surrounding an intervention.
CONFLICT OF INTEREST
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organization for the submitted work. Dr. ten Cate has received research grants and/or honorariums from Bayer, Pfizer, LEO Pharma, Gideon Pharmaceuticals, Alveron Pharma, and Coagulation profile outside the submitted work; no other relationships or activities that could appear to have influenced the submitted work.
AUTHOR CONTRIBUTION
Arina J. ten Cate‐Hoek is the guarantor of this manuscript; she accepts full responsibility for the finished manuscript, had access to any data, and controlled the decision to publish. In addition, she originated the idea for this systematic review, supervised literature search and data collection, and contributed to data interpretation, composition of figures and tables, and writing of the manuscript. Pascale Notten contributed to the literature search, data collection, data analysis, data interpretation, composition of figures and tables, and writing of the manuscript. Hugo ten Cate contributed to critical review of the manuscript.
ETHICAL APPROVAL
No ethical approval was required for the conduct of this systematic review since it did not include patient participation.
Supporting information
Supplementary Material
Notten P, ten Cate H, ten Cate‐Hoek AJ. Postinterventional antithrombotic management after venous stenting of the iliofemoral tract in acute and chronic thrombosis: A systematic review. J Thromb Haemost.2021;19:753–796. 10.1111/jth.15197
Manuscript handled by: Sabine Eichinger
Final decision: Sabine Eichinger, 23 November 2020
DATA AVAILABILITY STATEMENT
Request for access to the data underlying the reported results should be directed to the corresponding author Arina J. ten Cate‐Hoek (arina.tencate@maastrichtuniversity.nl).
REFERENCES
- 1. van Vuuren T, de Wolf MAF, Arnoldussen C et al. Editor's Choice ‐ reconstruction of the femoro‐ilio‐caval outflow by percutaneous and hybrid interventions in symptomatic deep venous obstruction. Eur J Vasc Endovas. 2017;54:495‐503. [DOI] [PubMed] [Google Scholar]
- 2. Notten P, Ten Cate‐Hoek AJ, Arnoldussen C, et al. Ultrasound‐accelerated catheter‐directed thrombolysis versus anticoagulation for the prevention of post‐thrombotic syndrome (CAVA): a single‐blind, multicentre, randomised trial. Lancet Haematol. 2020;7:e40‐e49. 10.1016/s2352-3026(19)30209-1 [DOI] [PubMed] [Google Scholar]
- 3. Notten P, van Laanen JHH, Eijgenraam P, et al. Quality of anticoagulant therapy and the incidence of in‐stent thrombosis after venous stenting. Res Pract Thromb Haemost. 2020;4:594‐603. 10.1002/rth2.12330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149:315‐352. 10.1016/j.chest.2015.11.026 [DOI] [PubMed] [Google Scholar]
- 5. Milinis K, Thapar A, Shalhoub J, Davies AH. Antithrombotic therapy following venous stenting: International Delphi Consensus. Eur J Vas Endovasc Surg. 2018;55:537‐544. 10.1016/j.ejvs.2018.01.007 [DOI] [PubMed] [Google Scholar]
- 6. Eijgenraam PCH, ten Cate‐Hoek AJ. Venous stenting after deep venous thrombosis and antithrombotic therapy: a systematic review. Rev Vasc Med. 2014;2:88‐97. [Google Scholar]
- 7. Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 8. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25:603‐605. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 9. Wells GASB, O’Connell D, Peterson J, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta‐analyses. 2014.
- 10. Enden T, Haig Y, Klow NE, et al. Long‐term outcome after additional catheter‐directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet. 2012;379:31‐38. 10.1016/s0140-6736(11)61753-4 [DOI] [PubMed] [Google Scholar]
- 11. Enden T, Klow NE, Sandvik L, et al. Catheter‐directed thrombolysis vs. anticoagulant therapy alone in deep vein thrombosis: results of an open randomized, controlled trial reporting on short‐term patency. J Thrombosis Haemostasis. 2009;7:1268‐1275. 10.1111/j.1538-7836.2009.03464.x [DOI] [PubMed] [Google Scholar]
- 12. Haig Y, Enden T, Slagsvold CE, Sandvik L, Sandset PM, Klow NE. Determinants of early and long‐term efficacy of catheter‐directed thrombolysis in proximal deep vein thrombosis. J Vasc Int Radiol. 2013;24:17‐24; quiz 6. 10.1016/j.jvir.2012.09.023 [DOI] [PubMed] [Google Scholar]
- 13. Haig Y, Enden T, Grotta O, et al. Post‐thrombotic syndrome after catheter‐directed thrombolysis for deep vein thrombosis (CaVenT): 5‐year follow‐up results of an open‐label, randomised controlled trial. Lancet Haematol. 2016;3:e64‐71. 10.1016/s2352-3026(15)00248-3 [DOI] [PubMed] [Google Scholar]
- 14. Sharifi M, Bay C, Mehdipour M, Sharifi J. Thrombus Obliteration by Rapid Percutaneous Endovenous Intervention in Deep Venous Occlusion (TORPEDO) trial: midterm results. J Endovasc Therapy. 2012;19:273‐280. 10.1583/11-3674mr.1 [DOI] [PubMed] [Google Scholar]
- 15. Sharifi M, Mehdipour M, Bay C, Smith G, Sharifi J. Endovenous therapy for deep venous thrombosis: the TORPEDO trial. Catheterization Cardiovasc intervent. 2010;76:316‐325. 10.1002/ccd.22638 [DOI] [PubMed] [Google Scholar]
- 16. Cakir V, Gulcu A, Akay E, et al. Use of percutaneous aspiration thrombectomy vs. anticoagulation therapy to treat acute iliofemoral venous thrombosis: 1‐year follow‐up results of a randomised, clinical trial. Cardiovasc Intervent Radiol. 2014;37:969‐976. 10.1007/s00270-014-0925-y [DOI] [PubMed] [Google Scholar]
- 17. Zhang X, Ren Q, Jiang X, et al. A prospective randomized trial of catheter‐directed thrombolysis with additional balloon dilatation for iliofemoral deep venous thrombosis: a single‐center experience. Cardiovasc Intervent Radiol. 2014;37:958‐968. 10.1007/s00270-013-0747-3 [DOI] [PubMed] [Google Scholar]
- 18. Vedantham S, Goldhaber SZ, Julian JA, et al. Pharmacomechanical catheter‐directed thrombolysis for deep‐vein thrombosis. N Engl J Med. 2017;377:2240‐2252. 10.1056/NEJMoa1615066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Comerota AJ, Kearon C, Gu CS, et al. Endovascular thrombus removal for acute iliofemoral deep vein thrombosis. Circulation. 2019;139:1162‐1173. 10.1161/circulationaha.118.037425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. AbuRahma AF, Perkins SE, Wulu JT, Ng HK. Iliofemoral deep vein thrombosis: conventional therapy versus lysis and percutaneous transluminal angioplasty and stenting. Ann Surg. 2001;233:752‐760. 10.1097/00000658-200106000-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grommes J, Strijkers R, Greiner A, Mahnken AH, Wittens CH. Safety and feasibility of ultrasound‐accelerated catheter‐directed thrombolysis in deep vein thrombosis. Eur J Vasc Endovasc Surg. 2011;41:526‐532. 10.1016/j.ejvs.2010.11.035 [DOI] [PubMed] [Google Scholar]
- 22. Manninen H, Juutilainen A, Kaukanen E, Lehto S. Catheter‐directed thrombolysis of proximal lower extremity deep vein thrombosis: a prospective trial with venographic and clinical follow‐up. Eur J Radiol. 2012;81:1197‐1202. 10.1016/j.ejrad.2011.03.068 [DOI] [PubMed] [Google Scholar]
- 23. Raju S, Ward M Jr, Kirk O. A modification of iliac vein stent technique. Ann Vasc Surg. 2014;28:1485‐1492. 10.1016/j.avsg.2014.02.026 [DOI] [PubMed] [Google Scholar]
- 24. Srinivas BC, Patra S, Nagesh CM, Reddy B, Manjunath CN. Catheter‐directed thrombolysis along with mechanical thromboaspiration versus anticoagulation alone in the management of lower limb deep venous thrombosis‐a comparative study. Int J Angiol. 2014;23:247‐254. 10.1055/s-0034-1382157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sarici IS, Yanar F, Agcaoglu O, et al. Our early experience with iliofemoral vein stenting in patients with post‐thrombotic syndrome. Phlebology. 2014;29:298‐303. 10.1177/0268355513477641 [DOI] [PubMed] [Google Scholar]
- 26. Sebastian T, Hakki LO, Spirk D, et al. Rivaroxaban or vitamin‐K antagonists following early endovascular thrombus removal and stent placement for acute iliofemoral deep vein thrombosis. Thromb Res. 2018;172:86‐93. 10.1016/j.thromres.2018.10.027 [DOI] [PubMed] [Google Scholar]
- 27. O'Sullivan GJ, Semba CP, Bittner CA, et al. Endovascular management of iliac vein compression (May‐Thurner) syndrome. J Vasc Intervent Radiol. 2000;11:823‐836. 10.1016/s1051-0443(07)61796-5 [DOI] [PubMed] [Google Scholar]
- 28. Kolbel T, Lindh M, Holst J, et al. Extensive acute deep vein thrombosis of the iliocaval segment: midterm results of thrombolysis and stent placement. J Vasc Intervent Radiol. 2007;18:243‐250. 10.1016/j.jvir.2006.12.002 [DOI] [PubMed] [Google Scholar]
- 29. Knipp BS, Ferguson E, Williams DM, et al. Factors associated with outcome after interventional treatment of symptomatic iliac vein compression syndrome. J Vasc Surg. 2007;46:743‐749. 10.1016/j.jvs.2007.05.048 [DOI] [PubMed] [Google Scholar]
- 30. Neglen P, Hollis KC, Olivier J, Raju S. Stenting of the venous outflow in chronic venous disease: long‐term stent‐related outcome, clinical, and hemodynamic result. J Vasc Surg. 2007;46:979‐990. 10.1016/j.jvs.2007.06.046 [DOI] [PubMed] [Google Scholar]
- 31. Neglen P, Berry MA, Raju S. Endovascular surgery in the treatment of chronic primary and post‐thrombotic iliac vein obstruction. Eur J Vasc Endovasc 2000;20:560‐571. 10.1053/ejvs.2000.1251 [DOI] [PubMed] [Google Scholar]
- 32. Hartung O, Loundou AD, Barthelemy P, Arnoux D, Boufi M, Alimi YS. Endovascular management of chronic disabling ilio‐caval obstructive lesions: long‐term results. Eur J Vas Endovasc Surg. 2009;38:118‐124. 10.1016/j.ejvs.2009.03.004 [DOI] [PubMed] [Google Scholar]
- 33. Kolbel T, Lindh M, Akesson M, Wasselius J, Gottsater A, Ivancev K. Chronic iliac vein occlusion: midterm results of endovascular recanalization. J Endovasc Therapy. 2009;16:483‐491. 10.1583/09-2719.1 [DOI] [PubMed] [Google Scholar]
- 34. Raju S, Neglen P. Percutaneous recanalization of total occlusions of the iliac vein. J Vasc Surg. 2009;50:360‐368. 10.1016/j.jvs.2009.01.061 [DOI] [PubMed] [Google Scholar]
- 35. Baekgaard N, Broholm R, Just S, Jorgensen M, Jensen LP. Long‐term results using catheter‐directed thrombolysis in 103 lower limbs with acute iliofemoral venous thrombosis. Eur J Vasc Endovasc Surg. 2010;39:112‐117. 10.1016/j.ejvs.2009.09.015 [DOI] [PubMed] [Google Scholar]
- 36. Sillesen H, Just S, Jorgensen M, Baekgaard N. Catheter directed thrombolysis for treatment of ilio‐femoral deep venous thrombosis is durable, preserves venous valve function and may prevent chronic venous insufficiency. Eur J Vasc Endovas Surg. 2005;30:556‐562. 10.1016/j.ejvs.2005.06.012 [DOI] [PubMed] [Google Scholar]
- 37. Jeon UB, Chung JW, Jae HJ, et al. May‐Thurner syndrome complicated by acute iliofemoral vein thrombosis: helical CT venography for evaluation of long‐term stent patency and changes in the iliac vein. AJR Am J Roentgenol. 2010;195:751‐757. 10.2214/ajr.09.2793 [DOI] [PubMed] [Google Scholar]
- 38. Rosales A, Sandbaek G, Jorgensen JJ. Stenting for chronic post‐thrombotic vena cava and iliofemoral venous occlusions: mid‐term patency and clinical outcome. Eur J Vasc Endovasc Surg. 2010;40:234‐240. 10.1016/j.ejvs.2010.04.016 [DOI] [PubMed] [Google Scholar]
- 39. Titus JM, Moise MA, Bena J, Lyden SP, Clair DG. Iliofemoral stenting for venous occlusive disease. J Vasc Surg. 2011;53:706‐712. 10.1016/j.jvs.2010.09.011 [DOI] [PubMed] [Google Scholar]
- 40. Wahlgren CM, Wahlberg E, Olofsson P. Endovascular treatment in postthrombotic syndrome. Vasc Endovasc Surg. 2010;44:356‐360. 10.1177/1538574410369710 [DOI] [PubMed] [Google Scholar]
- 41. Nayak L, Hildebolt CF, Vedantham S. Postthrombotic syndrome: feasibility of a strategy of imaging‐guided endovascular intervention. J Vasc Intervent Radiol. 2012;23:1165‐1173. 10.1016/j.jvir.2012.06.012 [DOI] [PubMed] [Google Scholar]
- 42. Blanch Alerany M, Izquierdo Lamoca LM, Ramirez Ortega M, Lago Rivas I, Zotta Desboeufs R, Stefanov KS. Endovascular treatment of iliofemoral chronic post‐thrombotic venous flow obstruction. J Vasc Surg Venous Lymphat Disord. 2014;2:2‐7. 10.1016/j.jvsv.2013.07.003 [DOI] [PubMed] [Google Scholar]
- 43. Stanley GA, Murphy EH, Plummer MM, Chung J, Modrall JG, Arko FR 3rd. Midterm results of percutaneous endovascular treatment for acute and chronic deep venous thrombosis. J Vasc Surg Venous Lymphat Dis. 2013;1:52‐58. 10.1016/j.jvsv.2012.04.002 [DOI] [PubMed] [Google Scholar]
- 44. Liu Z, Gao N, Shen L, et al. Endovascular treatment for symptomatic iliac vein compression syndrome: a prospective consecutive series of 48 patients. Ann Vasc Surg. 2014;28:695‐704. 10.1016/j.avsg.2013.05.019 [DOI] [PubMed] [Google Scholar]
- 45. Park JY, Ahn JH, Jeon YS, Cho SG, Kim JY, Hong KC. Iliac vein stenting as a durable option for residual stenosis after catheter‐directed thrombolysis and angioplasty of iliofemoral deep vein thrombosis secondary to May‐Thurner syndrome. Phlebology. 2014;29:461‐470. 10.1177/0268355513491724 [DOI] [PubMed] [Google Scholar]
- 46. Sang H, Li X, Qian A, Meng Q. Outcome of endovascular treatment in postthrombotic syndrome. Ann Vasc Surg. 2014;28:1493‐1500. 10.1016/j.avsg.2014.03.031 [DOI] [PubMed] [Google Scholar]
- 47. Ye K, Lu X, Jiang M, et al. Technical details and clinical outcomes of transpopliteal venous stent placement for postthrombotic chronic total occlusion of the iliofemoral vein. J Vasc Intervent Radiol. 2014;25:925‐932. 10.1016/j.jvir.2014.02.031 [DOI] [PubMed] [Google Scholar]
- 48. Catarinella FS, Nieman FH, de Wolf MA, Toonder IM, de Graaf R, Wittens CH. Quality‐of‐life in interventionally treated patients with post‐thrombotic syndrome. Phlebology. 2015;30:89‐94. 10.1177/0268355515569431 [DOI] [PubMed] [Google Scholar]
- 49. Friedrich de Wolf MA, Arnoldussen CW, Grommes J, et al. Minimally invasive treatment of chronic iliofemoral venous occlusive disease. J Vasc Surg Venous Lymphat Dis. 2013;1:146‐153. 10.1016/j.jvsv.2012.07.002 [DOI] [PubMed] [Google Scholar]
- 50. Shi WY, Gu JP, Liu CJ, He X, Lou WS. Endovascular treatment for iliac vein compression syndrome with or without lower extremity deep vein thrombosis: a retrospective study on mid‐term in‐stent patency from a single center. Eur J Radiol. 2016;85:7‐14. 10.1016/j.ejrad.2015.10.021 [DOI] [PubMed] [Google Scholar]
- 51. Comerota AJ, Lurie F, Assi Z. The contemporary hybrid operative procedure for incapacitating post‐thrombotic iliofemoral and vena caval obstruction improves procedural outcomes. J Vasc Surg Venous Lymphat Disorder. 2019;7:65‐73. 10.1016/j.jvsv.2018.07.012 [DOI] [PubMed] [Google Scholar]
- 52. Dumantepe M, Uyar I. The effect of Angiojet rheolytic thrombectomy in the endovascular treatment of lower extremity deep venous thrombosis. Phlebology. 2018;33:388‐396. 10.1177/0268355517711792 [DOI] [PubMed] [Google Scholar]
- 53. Endo M, Jahangiri Y, Horikawa M, et al. Antiplatelet therapy is associated with stent patency after iliocaval venous stenting. Cardiovasc Intervent Radiol. 2018;41:1691‐1698. 10.1007/s00270-018-2062-5 [DOI] [PubMed] [Google Scholar]
- 54. Acharya G, Singh K, Hansen JB, Kumar S, Maltau JM. Catheter‐directed thrombolysis for the management of postpartum deep venous thrombosis. Acta Obstet Gynecol Scand. 2005;84:155‐158. 10.1111/j.0001-6349.2005.00565.x [DOI] [PubMed] [Google Scholar]
- 55. Dayal R, Bernheim J, Clair DG, et al. Multimodal percutaneous intervention for critical venous occlusive disease. Ann Vasc Surg. 2005;19:235‐240. 10.1007/s10016-004-0167-6 [DOI] [PubMed] [Google Scholar]
- 56. Husmann MJ, Heller G, Kalka C, et al. Stenting of common iliac vein obstructions combined with regional thrombolysis and thrombectomy in acute deep vein thrombosis. Eur J Vasc Endovasc Surg. 2007;34:87‐91. 10.1016/j.ejvs.2007.01.006 [DOI] [PubMed] [Google Scholar]
- 57. Murphy EH, Davis CM, Journeycake JM, DeMuth RP, Arko FR. Symptomatic ileofemoral DVT after onset of oral contraceptive use in women with previously undiagnosed May‐Thurner Syndrome. J Vasc Surg. 2009;49:697‐703. 10.1016/j.jvs.2008.10.002 [DOI] [PubMed] [Google Scholar]
- 58. Oguzkurt L, Ozkan U, Demirturk OS, Gur S. Endovascular treatment of phlegmasia cerulea dolens with impending venous gangrene: manual aspiration thrombectomy as the first‐line thrombus removal method. Cardiovasc Intervent Radiol. 2011;34:1214‐1221. 10.1007/s00270-010-0042-5 [DOI] [PubMed] [Google Scholar]
- 59. Bloom AI, Farkas A, Kalish Y, Elchalal U, Spectre G. Pharmacomechanical catheter‐directed thrombolysis for pregnancy‐related iliofemoral deep vein thrombosis. J Vasc Intervent Radiol. 2015;26:992‐1000. 10.1016/j.jvir.2015.03.001 [DOI] [PubMed] [Google Scholar]
- 60. Langwieser N, Bernlochner I, Wustrow I, et al. Combination of factor Xa inhibition and antiplatelet therapy after stenting in patients with iliofemoral post‐thrombotic venous obstruction. Phlebology. 2016;31:430‐437. 10.1177/0268355515596289 [DOI] [PubMed] [Google Scholar]
- 61. Ming ZB, Li WD, Yuan RF, Li XQ, Ding WB. Effectiveness of catheter directed thrombolysis and stent implantation on iliofemoral vein thrombosis caused by iliac vein compression. J Thromb Thrombolysis. 2017;44:254‐260. 10.1007/s11239-017-1515-z [DOI] [PubMed] [Google Scholar]
- 62. Kapranov SA, Gavrilov SG, Cherkashin MA. The first experience with endovascular stenting of the iliac veins in patients suffering from post‐thrombophlebitic disease. Angiol Vasc Surg. 2003;9:29‐34. [PubMed] [Google Scholar]
- 63. Oguzkurt L, Tercan F, Ozkan U. Manual aspiration thrombectomy with stent placement: rapid and effective treatment for phlegmasia cerulea dolens with impending venous gangrene. Cardiovasc Intervent Radiol. 2008;31:205‐208. 10.1007/s00270-007-9156-9 [DOI] [PubMed] [Google Scholar]
- 64. Salam A, Chung J, Milner R. External iliac vein stenosis owing to prolonged cycling. Vascular. 2010;18:111‐115. 10.2310/6670.2010.00005 [DOI] [PubMed] [Google Scholar]
- 65. Sharifi M, Mehdipour M. Percutaneous therapy of acute on chronic lower extremity venous occlusive disease. Catheterization Cardiovasc Intervent. 2010;75:685‐689. 10.1002/ccd.22377 [DOI] [PubMed] [Google Scholar]
- 66. Wormald JR, Lane TR, Herbert PE, Ellis M, Burfitt NJ, Franklin IJ. Total preservation of patency and valve function after percutaneous pharmacomechanical thrombolysis using the Trellis(R)‐8 system for an acute, extensive deep venous thrombosis. Ann R Coll Surg Engl. 2012;94:e103‐e105. 10.1308/003588412x13171221589496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Singh B, Bharadwaj P, Bajaj N, Chadha D. Endovascular management of a case of spontaneous retroperitoneal haematoma complicated with deep vein thrombosis and pulmonary embolism. BMJ Case Rep. 2017. 10.1136/bcr-2017-222217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kohler C, Fuss T, Schweizer R, Baumgartner I, Kucher N, Schindewolf M. Haemostaseological complication management in caval and iliac venous stenting. VASA Zeitschrift fur Gefasskrankheiten. 2018;47:243‐246. 10.1024/0301-1526/a000686 [DOI] [PubMed] [Google Scholar]
- 69. Lakha S, Png CYM, Chun K, Ting W. Recurrent iliofemoral venous thrombosis in the setting of May‐Thurner syndrome as the presenting symptom of Behcet's disease. Ann Vasc Surg. 2018;49:315. 10.1016/j.avsg.2017.11.058 [DOI] [PubMed] [Google Scholar]
- 70. Rohr AM, Kuo WT. Single‐session pharmacomechanical catheter‐directed thrombolysis using the JETi thrombectomy device for acute iliofemoral deep vein thrombosis refractory to therapeutic anticoagulation. J Vasc Intervent Radiol. 2019;30:1682‐1685. 10.1016/j.jvir.2019.04.042 [DOI] [PubMed] [Google Scholar]
- 71. Barge TF, Wilton E, Wigham A. Endovascular treatment of an extensive iliocaval and renal vein thrombosis secondary to inferior vena cava stenosis and May‐Thurner type iliac vein compression: a case report. Vasc Endovasc Surg. 2020;54:297‐300. 10.1177/1538574419900613 [DOI] [PubMed] [Google Scholar]
- 72. Villalta SBP, Piccioli A, Lensing AW, Prins MH, Prandoni P. Assessment of validity and reproducibility of a clinical scale for the post‐thrombotic syndrome. Haemostasis. 1994;1994:158. [Google Scholar]
- 73. Kahn SR, Partsch H, Vedantham S, Prandoni P, Kearon C. Definition of post‐thrombotic syndrome of the leg for use in clinical investigations: a recommendation for standardization. J Thrombosis Haemostasis. 2009;7:879‐883. 10.1111/j.1538-7836.2009.03294.x [DOI] [PubMed] [Google Scholar]
- 74. Vasquez MA, Rabe E, McLafferty RB, et al. Revision of the venous clinical severity score: venous outcomes consensus statement: special communication of the American Venous Forum Ad Hoc Outcomes Working Group. J Vasc Surg. 2010;52:1387‐1396. 10.1016/j.jvs.2010.06.161 [DOI] [PubMed] [Google Scholar]
- 75. Rutherford RB, Padberg FT Jr, Comerota AJ, Kistner RL, Meissner MH, Moneta GL. Venous severity scoring: an adjunct to venous outcome assessment. J Vasc Surg. 2000;31:1307‐1312. 10.1067/mva.2000.107094 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
Request for access to the data underlying the reported results should be directed to the corresponding author Arina J. ten Cate‐Hoek (arina.tencate@maastrichtuniversity.nl).


