Abstract
Many patients with chronic fatigue syndrome (CFS) fail to derive benefit from evidence-based treatments such as cognitive–behavioural therapy (CBT) and graded exercise therapy leading to permanent disability. To discover whether a repeat prescription of modafinil might potentiate the benefits of CBT leading to social recovery as defined by 2 or more point improvement in energy and muscular pain/concentration and return to work or full-time training. Three patients with treatment-resistant CFS (mean duration 17.66 years) treated with modafinil and CBT in a Liaison Psychiatry clinic were retrospectively reviewed. Progress was reviewed at baseline, 4–6 months and 10–24 months. Patients rated their fatigue, pain and concentration using 10-point Likert scales. 2/3 achieved clinically meaningful improvements in energy and pain/concentration and 3/3 achieved social recovery. Modafinil, when prescribed over the medium term, would appear to be a potentially useful potentiating agent when added to CBT.
Keywords: psychiatry (drugs and medicines), cognitive behavioural psychotherapy
Background
The defining symptoms of chronic fatigue syndrome (CFS) are marked physical and mental fatigue with associated muscular pain.1 The prevalence of CFS varies from 0.4% to <2% and it particularly affects women.2 An estimated 250 000 individuals in the UK3 and up to 4 million individuals in the USA are afflicted with CFS at any one point in time.4 Diagnosis can be difficult leading to treatment delay.5 Approximately, 25% of afflicted individuals are disabled for 6 or more months.6 7 The economic cost is between £75.5 and £128.9 million in the UK,8 and US$18 to US$24 billion annually.9 In general, there is a lack of evidence-based treatments in CFS. The cognitive model as applied to CFS has been clearly described and cognitive–behavioural therapy (CBT) is accepted as being an evidence based treatment. The question is what can be done if CBT does not lead to symptomatic improvement and chronic symptoms develop. CBT effectively reduces fatigue, anxiety and depression and improves physical functioning.10 CBT is thought to work by changing avoidance behaviour and related beliefs leading to dichotomous thinking11 and by improving cognitive confidence.12 There is also encouraging evidence of benefit with graded exercise therapy (GET); however, GET is often less acceptable to patients than other management approaches such as rest or pacing.13 In terms of pharmacological treatments, the antidepressant fluoxetine has been shown to be of little or no benefit in treating the fatigue of CFS in randomised controlled trials.14 Clearly, more effective treatments and potentiating agents need to be identified.15 Modafinil is a central nervous system stimulant used in the treatment of narcolepsy and obstructive sleep apnoea syndrome, and also to enhance wakefulness in chronic shift workers.16–18
Chronic stress leads to selective desensitisation of alpha 1 adrenoceptors in CFS and modafinil has agonist properties which may lead to a clinically significant antidepressant effect.19 Modafinil differs from other stimulants like amphetamines as its action is more localised in the paraventricular and suprachiasmatic nuclei, anterior hypothalamus, amygdala, and tuberomammilary nucleus.20–22 Modafinil also increases glutamate release in the hippocampal formation and thalamus which may contribute to increased alertness.23 24 It may also act through modulation of the hypocretin system.25 Modafinil in case studies has shown some benefit in improving the fatigue secondary to many neurological disorders, including multiple sclerosis, Parkinson’s disease, motor neuron disease, stroke and post-polio syndrome.26 27 Use of modafinil in CFS was first reported in a case study.28
Case presentation
The aims of this case series were to investigate retrospectively whether adding a longer course of modafinil for patients with refractory CFS potentiated the benefit of CBT leading to clinically meaningful improvement on a priori criteria on energy and muscular pain/concentration and to investigate whether social recovery followed.
Three patients with treatment-resistant CFS, with a mean age of 41.33 years (range 38–47 years) and average illness duration of 17.66 years (range 13–20 years) are included in this review. The diagnosis was confirmed using the ICD-10 (International Classification of Diseases 10th edition) checklist.29 Patients, at mean follow-up periods of 4–6 and 10–12 months, were asked to score their core symptoms of fatigue, pain and concentration on 10 point numerical rating scales (1–10). Informed consent to treatment was obtained from all subjects prior to modafinil initiation which included disclosure that modafinil is an off-licence treatment for CFS. Clinically relevant improvement was defined a priori as a two or more point improvement in energy, pain and concentration.
When CBT was potentiated with a more prolonged course of modafinil, 2/3 (66.67%) of the patients with treatment-resistant CFS showed clinically relevant improvement in fatigue and pain or concentration symptoms. Also, 3/3 patients achieved social recovery as defined by return to work or full-time training. Changes in numerical ratings are outlined in table 1 for baseline, and follow-up intervals of 4–6 months and 10–12 months. Higher scores for energy and concentration indicate improvement, whereas lower pain scores represent pain reductions.
Table 1.
Individual scores on energy, pain and concentration as cognitive–behavioural therapy techniques are potentiated with modafinil in refractory chronic fatigue syndrome
| Energy* | Pain* | Concentration* | Modafinil dosage (daily) |
Social recovery | |||||||||
| Baseline | 4–6 months | 10–12 months | Baseline | 4–6 months | 10–12 months | Baseline | 4–6 months | 10–12 months | Baseline | 4–6 months | 10–12 months | ||
| Case 1 | 4.5 | 7.0 | 8.5 | 5.5 | 2.5 | 1.5 | 2.0 | 5.0 | 7.0 | 100 mg | 300 mg | 300 mg | Returned to work |
| Case 2 | 4.5 | 5.0 | 5.0 | 5.0 | 7.0 | 7.0 | 6.0 | 6.0 | 6.5 | 100 mg | 200 mg | 200 mg | Returned to work in a different post. |
| Case 3 | 3.5 | 6.0 | 6.5 | 6.5 | 0 | 2.0 | 6.5 | 6.5 | 6.5 | 25 mg increased to 50 mg in 2 weeks | 50 mg | 50 mg | Returned to full-time training. |
*Numbers represent ratings on a 0–10 scale, with 0=none and 10=highest energy, pain or concentration, respectively.
Case 1
Our first case is a 39-year-old man referred as an outpatient with a 20-year history of CFS and associated occupational disability. There were no comorbidities, and he was taking no other medication when seen. Prior treatment with activity management, pacing, fluoxetine, GET and CBT yielded little improvement. Modafinil was started in a dose of 100 mg/day which was gradually increased over a period of 3 months to 300 mg one time a day. He continued on this dosage for 10 months and was advised to keep a weekly record of his energy, pain and concentration. He was advised to again attempt to use CBT and graded exercise. A clinically relevant four-point improvement was reported in his levels of energy and pain, and concentration was improved by five points. Improvement was maintained after discontinuing modafinil. He successfully returned to a full-time career and used CBT management skills successfully which had failed to help prior to the prescription of modafinil.
Case 2
The second case, a 38-year-old woman with a diagnosis of CFS and a 13–14 year illness duration had received previous treatments of activity management, analgesics, amitriptyline, pacing, GET and CBT all of which provided only minimal benefit. This woman had been on high-dose fluoxetine (80 mg per day) for several years for the treatment of bulimia nervosa and this continued during the period of modafinil treatment. She was referred to the Liaison Clinic and was started on modafinil in a dosage of 100 mg mane which was gradually increased to 400 mg in the morning. She was encouraged to attempt to use her CBT techniques again. At follow-up, there was a subthreshold improvement in energy and concentration in the first 10 months, but muscular pain was more severe. The patient was able to resume work in her previous profession.
Case 3
Our last case is a 47-year-old man referred to the Liaison Clinic with a diagnosis of CFS for 20 years; he had no comorbid disorders and was on no other medication. He had been treated unsuccessfully with activity management, pacing, painkillers, fluoxetine, GET and CBT. A small daily dose of modafinil (25 mg) initially was increased to 50 mg after 2 weeks. At 10–12 month follow-up, there were clinically relevant improvements in energy and pain. The patient was able to return to full-time training with sustained social recovery.
Comment on prior treatments. In all three cases, graded exercise, fluoxetine and CBT were reported as treatments undertaken prior to commencement of modafinil. The graded exercise was given by non-expert therapists. However, the CBT was given in adequate dose (16–20 sessions) by expert therapists. During modafinil treatment, all patients were encouraged to continue with the CBT and graded exercise techniques they had learnt.
Treatment
All three patients had previously received a full course of expert CBT and GET. The minimum starting dose of modafinil was 25 mg/ day which was gradually titrated up to the maximum of 300 mg/day depending on response.
Outcome and follow-up
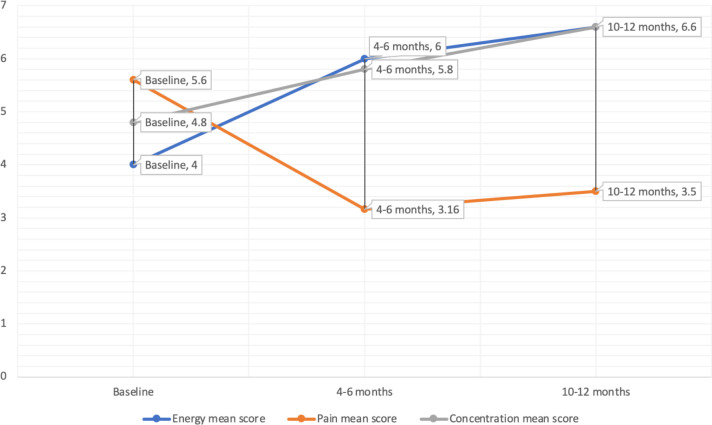
Summary results are contained in figure 1 which show mean scores for energy, muscular pain and concentration of all three patients over a period of 10–12 months.
Figure 1.
Mean scores for energy, pain and concentration at baseline, 4–6 months and 10–12 months.
Discussion
These results describing the benefits of the potentiation of CBT with modafinil are preliminary but encouraging. Two out of 3 patients with refractory, disabling CFS, achieved clinically relevant improvement in energy and pain/concentration. Three out of three achieved social recovery as defined by return to work or full-time training. The patients were closely monitored over the medium term. Medium-term prescribing of modafinil should be considered when chronic disability has supervened in CFS with failure of other evidence-based treatment modalities. However, there are limitations in this preliminary case series. The sample size of three clients with refractory CFS does not lead to generalisable results and the findings may be explained by regression to the mean, placebo effect or the natural course of the illness. Shorter illness duration has been shown to be a significant predictor of sustained remission, and thus early detection and effective treatment of CFS is of the utmost importance.30 However, this group treated with modafinil was severely disabled and treatment resistant with 3/3 reporting illness duration greater than a decade. For the sake of simplicity, we used numerical ratings scales; however, using validated scales in future studies would be indicated. In a preliminary study of 14 patients with CFS treated with doses of 200 mg and 400 mg of modafinil for 20 days, mixed effects were reported on two cognitive tasks. The authors suggested that unclear cognitive effects may have occurred by chance, or that a subgroup of patients with daytime sleepiness would have shown greater benefits.31 The use of modafinil over the medium term in chronic severe disabling CFS was first described in a case report.31 In that case, clinically significant improvement in all symptoms and return to a good quality of life was reported. In a subsequent randomised controlled trial involving 115 patients with multiple sclerosis, there was no improvement in fatigue with modafinil versus placeb.32 In the only other study we found in this area, 14 patients with CFS were treated for 20 days with a dosage of 200–400 mg of modafinil or placebo. No effects were observed on the performance of psychometric tests or on self-ratings of fatigue, quality of life or mood, but this may have been due to insufficient statistical power33 or the brevity of the course of modafinil. The unique pharmacodynamics of modafinil have led to concern about its potential to become a drug of abuse. As such, the European Medicines Agency in 2010 advised that modafinil was no longer indicated as a treatment for obstructive sleep apnoea or shift work sleep disorder.34 The benefits now only outweigh its risks in the treatment of narcolepsy. It is only due to the severity and unresponsive nature of the disability of CFS that the authors recommend further investigation of the use of modafinil. Double blind placebo controlled studies with adequate power and long duration of follow-up need to be done to provide more definitive evidence in this area.
Patient’s perspective.
‘I’ve got my life and business back due to the Modafinil’
‘Modafinil did enough to kick start activities again but pain got worse’
‘The Modafinil saved my career.’
Learning points.
Modafinil may potentiate the benefits of cognitive–behavioural therapy (CBT) in chronic fatigue syndrome (CFS).
Modafinil may need to be prescribed over a prolonged period of months.
Modafinil may improve not only energy but also concentration.
The analgesic effect of modafinil and CBT may be mediated by increased activity.
Modafinil may lead to social and occupational recovery even after many years of treatment failure and disability.
Footnotes
Contributors: All authors: conception or design of the work; acquisition, analysis or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Fukuda K, Straus SE, Hickie I, et al. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International chronic fatigue syndrome Study Group. Ann Intern Med 1994;121:953–9. 10.7326/0003-4819-121-12-199412150-00009 [DOI] [PubMed] [Google Scholar]
- 2.Prins JB, van der Meer JWM, Bleijenberg G. Chronic fatigue syndrome. Lancet 2006;367:346–55. 10.1016/S0140-6736(06)68073-2 [DOI] [PubMed] [Google Scholar]
- 3.Hutchinson CV, Maltby J, Badham SP, et al. Vision-related symptoms as a clinical feature of chronic fatigue syndrome/myalgic encephalomyelitis? Evidence from the DePaul symptom questionnaire. Br J Ophthalmol 2014;98:144–5. 10.1136/bjophthalmol-2013-304439 [DOI] [PubMed] [Google Scholar]
- 4.Reeves WC, Jones JF, Maloney E, et al. Prevalence of chronic fatigue syndrome in metropolitan, urban, and rural Georgia. Popul Health Metr 2007;5:5. 10.1186/1478-7954-5-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke JN, James S. The radicalized self: the impact on the self of the contested nature of the diagnosis of chronic fatigue syndrome. Soc Sci Med 2003;57:1387–95. 10.1016/S0277-9536(02)00515-4 [DOI] [PubMed] [Google Scholar]
- 6.Marshall R, Paul L, Wood L. The search for pain relief in people with chronic fatigue syndrome: a descriptive study. Physiother Theory Pract 2011;27:373–83. 10.3109/09593985.2010.502554 [DOI] [PubMed] [Google Scholar]
- 7.Anderson VR, Jason LA, Hlavaty LE, et al. A review and meta-synthesis of qualitative studies on myalgic Encephalomyelitis/Chronic fatigue syndrome. Patient Educ Couns 2012;86:147–55. 10.1016/j.pec.2011.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collin SM, Crawley E, May MT, et al. The impact of CFS/ME on employment and productivity in the UK: a cross-sectional study based on the CFS/ME national outcomes database. BMC Health Serv Res 2011;11:217. 10.1186/1472-6963-11-217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jason L, Richman J. How science can stigmatize: the case of chronic fatigue syndrome. J Chronic Fatigue Syndr 2008;14:85–103. 10.1080/10573320802092146 [DOI] [Google Scholar]
- 10.Fernie BA, Murphy G, Wells A, et al. Treatment outcome and metacognitive change in CBT and GET for chronic fatigue syndrome. Behav Cogn Psychother 2016;44:397–409. 10.1017/S135246581500017X [DOI] [PubMed] [Google Scholar]
- 11.Deale A, Chalder T, Wessely S. Illness beliefs and treatment outcome in chronic fatigue syndrome. J Psychosom Res 1998;45:77–83. 10.1016/S0022-3999(98)00021-X [DOI] [PubMed] [Google Scholar]
- 12.Fernie BA, Murphy G, Wells A, et al. Treatment outcome and metacognitive change in CBT and GET for chronic fatigue syndrome. Behav Cogn Psychother 2016;44:397–409. 10.1017/S135246581500017X [DOI] [PubMed] [Google Scholar]
- 13.Edmonds M, McGuire H, Price J. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst Rev 2004:CD003200. 10.1002/14651858.CD003200.pub2 [DOI] [PubMed] [Google Scholar]
- 14.Alegre de Miquel C, Alejandra-Pereda C, Betina-Nishishinya M, et al. [Systematic review of pharmacologic treatment in fibromyalgia]. Med Clin 2005;125:784–7. 10.1016/s0025-7753(05)72190-4 [DOI] [PubMed] [Google Scholar]
- 15.Van Houdenhove B, Pae C-U, Luyten P. Chronic fatigue syndrome: is there a role for non-antidepressant pharmacotherapy? Expert Opin Pharmacother 2010;11:215–23. 10.1517/14656560903487744 [DOI] [PubMed] [Google Scholar]
- 16.Ballard RD. Management of patients with obstructive sleep apnea. J Fam Pract 2008;57:S24–30. [PubMed] [Google Scholar]
- 17.Ballon JS, Feifel D. A systematic review of modafinil: potential clinical uses and mechanisms of action. J Clin Psychiatry 2006;67:554–66. 10.4088/jcp.v67n0406 [DOI] [PubMed] [Google Scholar]
- 18.Happe S, Pirker W, Sauter C, et al. Successful treatment of excessive daytime sleepiness in Parkinson’s disease with modafinil. J Neurol 2001;248:632–4. 10.1007/s004150170148 [DOI] [PubMed] [Google Scholar]
- 19.Stone EA, Cotecchia S, Lin Y, et al. Role of brain alpha 1B-adrenoceptors in modafinil-induced behavioral activity. Synapse 2002;46:269–70. 10.1002/syn.10127 [DOI] [PubMed] [Google Scholar]
- 20.Engber TM, Koury EJ, Dennis SA, et al. Differential patterns of regional c-Fos induction in the rat brain by amphetamine and the novel wakefulness-promoting agent modafinil. Neurosci Lett 1998;241:95–8. 10.1016/S0304-3940(97)00962-2 [DOI] [PubMed] [Google Scholar]
- 21.Lin JS, Hou Y, Jouvet M. Potential brain neuronal targets for amphetamine-, methylphenidate-, and modafinil-induced wakefulness, evidenced by c-fos immunocytochemistry in the cat. Proc Natl Acad Sci U S A 1996;93:14128–33. 10.1073/pnas.93.24.14128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scammell TE, Estabrooke IV, McCarthy MT, et al. Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci 2000;20:8620–8. 10.1523/JNEUROSCI.20-22-08620.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferraro L, Antonelli T, O'Connor WT, et al. The antinarcoleptic drug modafinil increases glutamate release in thalamic areas and hippocampus. Neuroreport 1997;8:2883–7. 10.1097/00001756-199709080-00016 [DOI] [PubMed] [Google Scholar]
- 24.Ferraro L, Antonelli T, O'Connor WT, et al. Modafinil: an antinarcoleptic drug with a different neurochemical profile to d-amphetamine and dopamine uptake blockers. Biol Psychiatry 1997;42:1181–3. 10.1016/S0006-3223(97)00353-3 [DOI] [PubMed] [Google Scholar]
- 25.Pérez de la Mora M, Aguilar-García A, Ramon-Frías T, et al. Effects of the vigilance promoting drug modafinil on the synthesis of GABA and glutamate in slices of rat hypothalamus. Neurosci Lett 1999;259:181–5. 10.1016/S0304-3940(98)00905-7 [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Gao XB, Sakurai T, et al. Hypocretin/Orexin excites hypocretin neurons via a local glutamate neuron-A potential mechanism for orchestrating the hypothalamic arousal system. Neuron 2002;36:1169–81. 10.1016/S0896-6273(02)01132-7 [DOI] [PubMed] [Google Scholar]
- 27.Happe S, Pirker W, Sauter C, et al. Successful treatment of excessive daytime sleepiness in Parkinson's disease with modafinil. J Neurol 2001;248:632–4. 10.1007/s004150170148 [DOI] [PubMed] [Google Scholar]
- 28.Rammohan KW, Rosenberg JH, Lynn DJ, et al. Efficacy and safety of modafinil (Provigil) for the treatment of fatigue in multiple sclerosis: a two centre phase 2 study. J Neurol Neurosurg Psychiatry 2002;72:179–83. 10.1136/jnnp.72.2.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO . The ICD-10 classification of mental and behavioural disorders. Geneva: World Health Organisation (WHO); 1992. [Google Scholar]
- 30.Turkington D, Hedwat D, Rider I, et al. Recovery from chronic fatigue syndrome with modafinil. Hum Psychopharmacol 2004;19:63–4. 10.1002/hup.554 [DOI] [PubMed] [Google Scholar]
- 31.Nisenbaum R, Jones JF, Unger ER, et al. A population-based study of the clinical course of chronic fatigue syndrome. Health Qual Life Outcomes 2003;1:49. 10.1186/1477-7525-1-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stankoff B, Waubant E, Confavreux C, et al. Modafinil for fatigue in MS: a randomized placebo-controlled double-blind study. Neurology 2005;64:1139–43. 10.1212/01.WNL.0000158272.27070.6A [DOI] [PubMed] [Google Scholar]
- 33.Randall DC, Cafferty FH, Shneerson JM, et al. Chronic treatment with modafinil may not be beneficial in patients with chronic fatigue syndrome. J Psychopharmacol 2005;19:647–60. 10.1177/0269881105056531 [DOI] [PubMed] [Google Scholar]
- 34.European Medicines Agency . Modafinil: European medicines Agency recommends restricted use. Drug Saftey Update 2010;3. [Google Scholar]