Abstract
While there is an increasing agreement that hypertension is associated with cerebrovascular compromise, relationships between blood pressure and cerebral blood flow are not fully understood. It is not known what blood pressure level, and consequently what therapeutic goal, is optimal for brain perfusion. Moreover, there is limited data on how blood pressure affects hippocampal perfusion, a structure critically involved in memory.
We conducted a cross-sectional (n=445) and longitudinal (n=185) study of adults and elderly without dementia or clinically apparent stroke, who underwent clinical examination and brain perfusion assessment (age 69.2±7.5 years, 62% women, 45% hypertensive). Linear models were used to test baseline blood pressure-blood flow relationship and to examine how changes in blood pressure influence changes in perfusion.
In the entire group, systolic blood pressure was negatively related to cortical (β=−0.13, p=0.005) and hippocampal blood flow (β=−0.12, p=0.01). Notably, this negative relationship was apparent already in subjects without hypertension.
Hypertensive subjects showed a quadratic relationship between systolic blood pressure and hippocampal blood flow (β=−1.55, p=0.03): Perfusion was the highest in subjects with mid-range systolic blood pressure around 125 mmHg. Longitudinally, in hypertensive subjects perfusion increased with increased systolic blood pressure at low baseline systolic blood pressure, but increased with decreased systolic blood pressure at high baseline systolic blood pressure.
Cortical and hippocampal perfusion decrease with increasing systolic blood pressure across the entire blood pressure spectrum. However, in hypertension, there appears to be a window of mid-range systolic blood pressure which maximizes perfusion.
Keywords: cerebral blood flow, blood pressure, hypertension, brain, hippocampus, Glodzik, Systolic blood pressure, cerebral perfusion
Introduction
Hypertension (HTN) affects nearly 30% of the US population1 and its prevalence increases with advancing age: over half of the population over 60 years old is hypertensive2. The brain is among many organs damaged by high blood pressure (BP). HTN accelerates plaque formation in large vessels, leading to stroke3. It also causes vascular remodeling, lumen narrowing and rarefaction of small vessels. These, in turn, result in decreased cerebral blood flow (CBF)4, 5 and impaired cerebral autoregulation6, 7 in hypertensive subjects compared to their normotensive peers. Despite our growing understanding of the relationship between BP and CBF there are important unanswered questions:
First, the seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC7) introduced the concept of prehypertension, defined as BP values 120–139/80–89 mmHg2. Since then, ample data has confirmed that prehypertension infers a greater risk for cerebrovascular and cardiovascular morbidity than lower BP levels8. However, it is unknown if prehypertension is related to impaired CBF.
Second, there is limited data on the relationship between BP and CBF within the hypertensive group. While high BP may cause progressive decrease in CBF due to structural changes in the vasculature it appears that excessive lowering of BP may also lead to hypoperfusion, due to the shift of the autoregulatory curve to the right6, 7. The exact functional relationship between BP and CBF is unknown and it remains to be determined if an optimal BP value exists to maximize CBF.
Third, most studies have examined the relationship between BP and global cerebral perfusion. Much less is known about regional changes, in particular the impact of BP on hippocampal perfusion. The hippocampus plays a prominent role in cognition and it is one of the earliest brain structures affected during the progression of Alzheimer’s disease (AD)9. Given that HTN increases the risk of future cognitive decline10, including AD11, it would be of great value to examine the relationship between BP and hippocampal perfusion.
Accordingly we undertook a large prospective study of cortical and hippocampal CBF in adults and elderly without dementia. Our key aim was to analyze and model both cross-sectional and longitudinal relationships between BP and brain perfusion.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Subjects were recruited between January 2008 and November 2017 at the NYU School of Medicine, Center for Brain Health. They included volunteers responding to advertisement, those interested in research participation, and family members of cognitively impaired patients. All individuals signed Institutional Review Board (ethical committee)-approved consent forms. Excluded were subjects with previous neocortical stroke, brain tumor, and life-long psychotic or depressive disorders.
From a pool of 489 subjects with perfusion data, we report here on 445 participants without dementia, over 50 years old, with technically adequate ASL (arterial spin labeling) MRI and available clinical information (see Figure S1 for exclusion criteria and participant flow). Dementia was excluded based on a physician-administered interview using the Brief Cognitive Rating Scale, rating on the Global Deterioration Scale12, and Clinical Dementia Rating13.
One hundred eighty five individuals completed follow-up ASL exam and were subject to longitudinal CBF analyses. All subjects, but one, have been cognitively re-assessed at follow-up. None of 184 individuals converted to dementia. Reasons for lack of follow-up are detailed in the Supplement.
Clinical assessment
All subjects underwent medical, psychiatric, and neurological assessments, blood tests, ECG, and MRI examinations. Blood samples taken in a fasting state were examined for complete blood count, liver function tests, metabolic and lipid panel. Blood pressure was taken in a sitting position, after 5 minutes of rest. It was measured on the left upper arm using a manual sphyngomanometer.
Medication:
The use of and the type of antihypertensive medications was recorded. The categories were: angiotensin receptor blockers, angiotensin converting enzyme inhibitors (ARBs), beta-blockers, diuretics, and calcium channel blockers. For longitudinal analyses we used an additional variable “Change in antihypertensive medication” – defined as increase, decrease or no change in number of medications over the interval. We also recorded the use of statins and glucose-lowering drugs.
Body mass index (BMI) was calculated as weight/height2 [kilograms]/[meters]2.
Global Coronary Heart Disease risk (GCHD) was calculated based on the Framingham Heart Study equation. The heart disease was ascertained by medical interview. Details for both assessments are given in the Supplement.
Study groups
Hypertension (HTN) was defined as current antihypertensive treatment or BP ≥140/90 mmHg2. Two hundred subjects were classified as hypertensive. 158 subjects were taking medication and 42 were un-medicated with high BP recorded during their in-office visit.
Prehypertension (Pre-HTN) was defined as BP of 120–139/80–89 mmHg and no antihypertensive treatment.
Normotension (NTN) was defined as BP: <120/80 mmHg and no antihypertensive treatment.
NTN and Pre-HTN subjects were analyzed together as a group without HTN.
MR Imaging
All MR imaging was performed on the 3T system (Siemens, Erlangen, Germany). Imaging protocol consisted of sagittal T1-weighted Magnetization Prepared Rapid Acquisition Gradient Echo, axial fluid attenuation inversion recovery and perfusion Arterial Spin Labeling (ASL) sequences. Details of MRI acquisition are given in the Supplement.
Structural MRI processing.
Gray matter (GM) and intracranial volumes (ICV) were estimated using Statistical Parametric Mapping segmentation procedure (SPM, version 8, with “New-Segment” extension)14. Left and right hippocampal volumes were obtained with FreeSurfer version 6.015. All volumes are presented as ratios to the ICV.
The presence of white matter lesions (WML) was determined on FLAIR images. WMH were graded from 0–3 on the Fazekas scale16. Periventricular (PWML) and deep white matter lesions (DWML) were graded separately.
CBF sampling.
Hippocampal, cortical, and white mater (WM) region of interest (ROIs) were defined directly on high-resolution ASL images (to minimize partial volume errors) (Figure 1, bottom right), using an in-house-developed software (https://wp.nyu.edu/firevoxel/). Cortical ROI encompassed temporal, parietal and in some cases also occipital cortex (Figure 1). For brevity we will refer to this ROI as ‘cortical’ as opposed to ‘hippocampal’. The process entailed: a) choosing a seed region in GM and WM, b) constructing a WM ROI within 10% of the WM seed, restricted to the largest connected components and refined by automatic boundary erosion, c) constructing GM ROI by intensity thresholding followed by automatic boundary erosion and removal of nonbrain tissue17, d) delineating right and left hippocampal regions. Each ROI was visually confirmed by investigators knowledgeable about the studied anatomy (LG, HJK) who were blind to subject’s age and hypertension status. A batch process was then run to generate GM and hippocampal CBF. In the final step, all voxels with CBF >150 ml/(100g min) were deemed to contain large blood vessels and were excluded from ROIs18.
Figure 1.
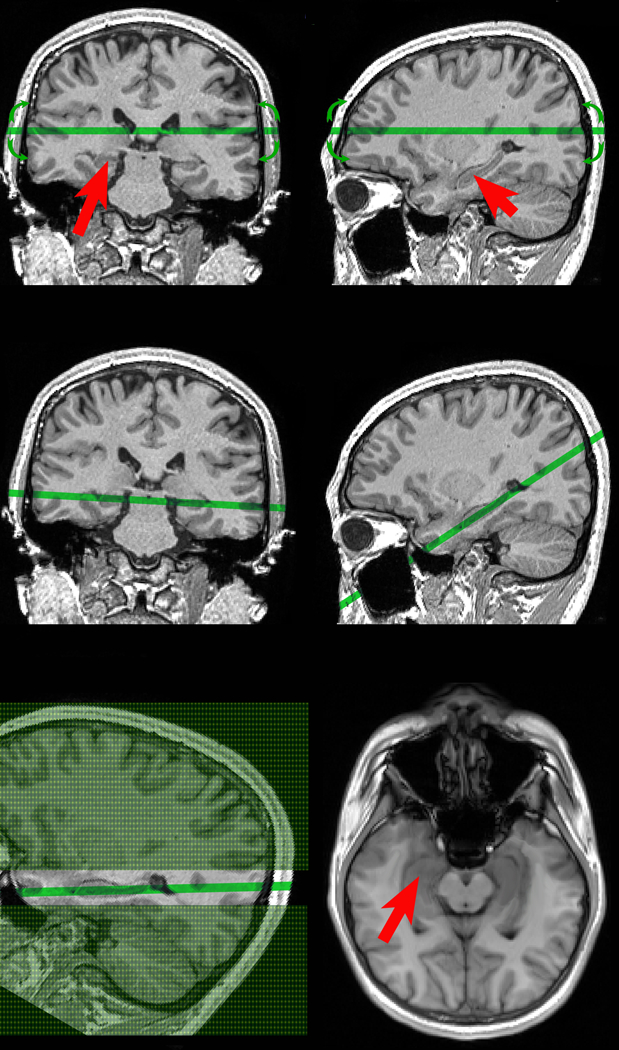
Selection of oblique hippocampal/temporal cortex plane at the MRI console.
Top: A trained operator with experience in neuroanatomy is presented with coronal & sagittal views of 3D MPRAGE volume. Red arrows indicate the right and the left hippocampus. The operator manipulates the position and the orientation of oblique acquisition plane while monitoring the 3D anatomical alignment (green line and green arrows).
Middle: The ASL exam begins after selecting the optimal plane through both the left and the right hippocampus.
Bottom left: the green area represents tagging, and the difference between slice non-selective (shaded in green) and slice selective slab (not shaded). Note that slice selective slab is 3.5 times thicker than imaged slice.
Bottom right: The resulting balanced steady-state free precession image used to measure CBF. Red arrows indicate the hippocampus.
Statistics
General
Categorical variables were compared with χ2 tests. T-test, analysis of variance (ANOVA) or analysis of covariance (ANCOVA) was used to compare group means for continuous variables. When appropriate, non-parametric U Mann Whitney or Kruskal-Wallis ANOVA was used. All post-hoc pair-wise comparisons were carried out with Bonferroni correction. Correlations were checked using Pearson or Spearmen coefficient.
CBF data were acquired over the span of 9 years a 3T magnet that underwent significant hardware and software upgrades. Since the variability that corresponds to the five temporal epochs could contaminate our analyses, the perfusion values were z scored separately by epoch, recentered and rescaled to eliminate this fixed bias. Please see the Supplement for more information about rescaling and its validation.
In all analyses, right and left hippocampal CBF and volume data were averaged.
Testing BP – CBF relationship at baseline
Relationships between continuous CBF (dependent variable) and BP were tested with separate stepwise (backward) linear regression. They were tested in the entire groups, as well as in subjects with and without HTN separately. We first tested relationship between CBF and individual variables: age, gender, PWML, DWML, GCHD score, heart disease, statin and antihypertensive medication use. Variables significantly associated with CBF were included into regression models.
We hypothesized that in the HTN group an optimal BP may exist, which maximizes perfusion. To test for the existence of the peak, the linear BP term was followed by entry of the quadratic term (BP2) in regression models examining the relationship to CBF. When a quadratic relationship CBF = aBP2 + bBP + c was confirmed (Figure 2d), the critical (maximum/minimum) BP value xc is given by equation:
| [1] |
For linear regression we report standardized beta values (β), as well unstandardized coefficient B with its 95% confidence interval (CI).
Figure 2.
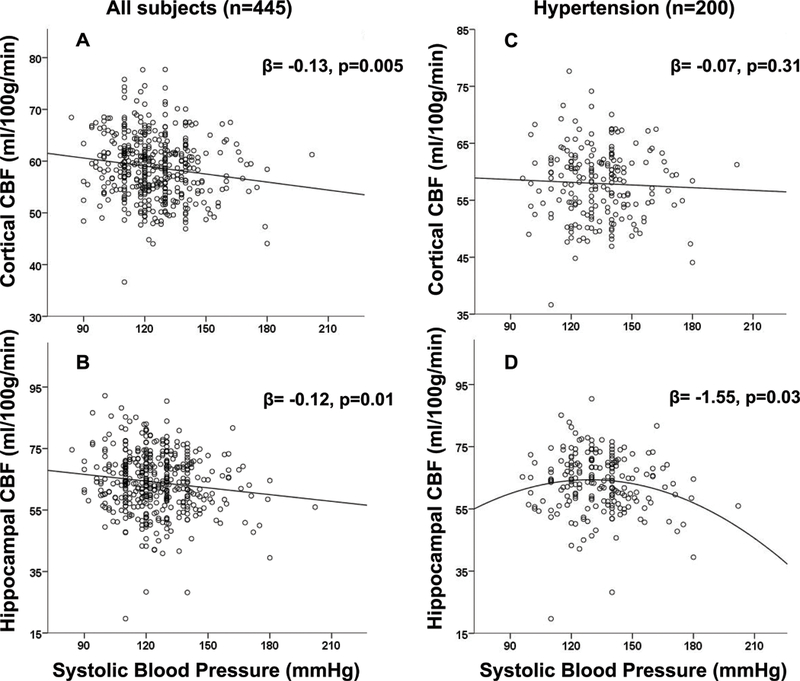
Cortical (A,C) and hippocampal (B,D) CBF as a function of SBP in the entire group (A,B) and among HTN patients (C,D). For panels A, B, C β values from linear regression model with gender included. For panel D from the model with statin use.
Testing BP- CBF relationship longitudinally
These relationships were tested using mixed model repeated measures (MMRM). CBF was the dependent variable; continuous baseline BP, BP and interaction between them were fixed terms; intercept was set as random. Subjects’ visits were set as repeated term. We also tested a model where BP was set as random. The model with the better fit (compared using likelihood ratio test) was chosen. Age, gender, medication use, change in number of antihypertensive medications, and the presence of heart disease at baseline were tested and retained when significant.
To confirm the results we repeated our analyses using linear regression. For each subject we created slopes for BP and CBF change over time. CBF slope was a dependent variable predicted by continuous baseline BP, BP slope and interaction between them. The same covariates were tested in the models. Interaction terms were built with lower terms centered on their means. Collinearity was examined by assessing variance inflation factors.
Analyses were conducted in the entire group as well as in subjects with and without HTN, separately.
Finally, to explore the relationship between BP and CBF changes over different baseline BP, we fit linear (first-order) and quadratic (second-order) polynomial surfaces to the 3 dimensional data of baseline BP, BP slope, and CBF slope, using the first two variables as inputs (x,y), and the last as output (z). Here we have tested a parsimonious 4-parameter model:
| [2] |
which for our data appeared to have the same explanatory power as the general 2nd degree model with 6 parameters:
| [3] |
The best model as indicated by Akaike information criterion (AIC) was chosen. The surface fitting was carried out by the Curve Fitting Toolbox in Matlab (ver. 2015a, Mathworks Inc. USA). To estimate the baseline BP value marking the point where relationship between CBF slope and BP slope was changing, we calculated critical point (xc) from:
| [4] |
Linear models were checked for violations of model assumptions (distribution of the residuals, correct specification of the variance structure and linear relationship between the response and the linear predictor). If necessary, Box Cox procedure was used to determine most appropriate transformation of the variables. Violations were reported and models retested using transformed data. For all the analyses, the most parsimonious model was chosen, defined as including only significant or necessary (main effects when interaction was present) terms.
Statistical significance was defined as a p value <0.05. SPSS (version 23, SPSS, Inc., Chicago, IL) software was used for all analyses.
Results
The total sample of 445 subjects consisted of 61.6% women (age 68.4±7.7 years (mean ± standard deviation); education 16.6±2.4 years); and 38.4% men (age 70.6±7.1 years; education 17.0±2.3 years) (Table 1). The racial composition was: 87.9% Caucasian, 8.8% African American, 1.3% Asian, 2% other races. In the longitudinal group of 185 subjects proportion of women (age 68.8±7.9 years; education 16.7±2.0 years) and men (age 71.2±5.9 years; education 17.2±2.2 years) was the same as in the entire sample. The mean time between examinations was 2.2±0.51 years. Supplemental tables list the characteristics of the longitudinal group alone (S1) and compared with subjects studied only at baseline (S2).
Table 1.
Baseline characteristics of the entire study group (n=445).
| Variable | NTN (n=120) | Pre-HTN (n=125) | HTN (n=200) | p | |
|---|---|---|---|---|---|
| Age (years) | 65.7±7.4 | 69.7±7.1* | 71.0±7.1* | <0.001 | |
| Age range | 50.1–82.3 | 51.9–87.9 | 51.1–91.2 | ||
| Education (years) | 16.8±2.3 | 16.7±2.1 | 16.7±2.5 | 0.90 | |
| Gender (n, %female) | 88, 73% | 76, 61% | 110, 55%* | 0.005 | |
| SBP (mmHg) | 108.2±7.7 | 125.1±7.2 | 133.6±17.6 | N/a | |
| DBP (mmHg) | 66.1±7.3 | 74.8±7.8 | 77.2±11.0 | N/a | |
| Mean Arterial Pressure (mmHg) | 80.0±6.6 | 91.5±5.8* | 95.9±11.6*† | <0.001 | |
| Pulse Pressure (mmHg) | 42.1±7.5 | 50.2±10.4* | 56.4±14.6*† | <0.001 | |
| BMI* | 24.6±4.0 | 26.1±4.4* | 27.7±6.1* | <0.001 | |
| Total cholesterol† (mg/dL) | 203.7±33.2 | 196.2±35.2 | 187.7±35.3* | <0.001 | |
| High-density lipoprotein cholesterol† (mg/dL) | 70.0±19.9 | 64.5±17.6 | 60.6±15.6* | <0.001 | |
| Low-density lipoprotein cholesterol‡ (mg/dL) | 115.7±29.1 | 112.8±32.7 | 107.2±29.0* | 0.04 | |
| Triglycerides§ (mg/dL) | 89.5±39.9 | 95.9±49.1 | 100.7±45.6 | 0.06 | |
| Glucose|| (mg/dL) | 81.0±11.7 | 80.9±13.1 | 87.7±18.1*† | <0.001 | |
| Antihypertensive medication (n, %) | NA | NA | 158, 79% | N/a | |
| Statins (n, %) | 21, 17% | 33, 26% | 94, 47%*† | <0.001 | |
| Glucose lowering medications (n, %) | 1, 0.8% | 2, 1.6% | 17, 8.5%*† | 0.001 | |
| GCHD risk¶ (%) | 5.2±3.7 | 8.2±4.1* | 10.8±6.5*† | <0.001 | |
| Heart disease# (n, %) | 5, 4% | 4, 3% | 24, 12%† | 0.004 | |
| Cortical CBF** (ml/100g/min) | 60.0±0.54 | 58.8±0.52 | 58.1±0.41* | 0.02 | |
|
Hippocampal CBF** (ml/100g/min) |
64.9±0.87 | 64.1±0.85 | 63.6±0.67 | 0.49 | |
| Gray matter volume†† (% ICV) | 40.7±0.33 | 41.1±0.32 | 39.8±0.25† | 0.005 | |
| Hippocampal volume‡‡ (%ICV) | 0.263±0.003 | 0.262±0.003 | 0.256±0.002 | 0.05 | |
| PWML§§ (n, %) | 26, 22% | 37, 30% | 69, 35%* | 0.04 | |
| DWML§§ (n, %) | 17, 14% | 24, 19% | 50, 26% | 0.05 | |
Data is presented as mean ± standard deviation unless otherwise indicated. P values come from Kruskal-Wallis ANOVA or ANOVA (age). For categorical variables χ2 was used. Post hoc comparisons are performed with Bonferroni correction.
Signs in superscript:
n= 435 (NTN=118, Pre-HTN=122, HTN=195)
n= 427 (NTN=119, Pre-HTN=120, HTN=188)
n= 420 (NTN=114, Pre-HTN=119, HTN=187)
n= 426 (NTN=118, Pre-HTN=120, HTN=188)
n= 429 (NTN=119, Pre-HTN=121, HTN=189)
n= 419 (NTN=114, Pre-HTN=119, HTN=186)
n= 444 (NTN=120, Pre-HTN=125, HTN=199)
values presented as mean ± standard errors (SE), p values from ANCOVA after accounting for gender
n= 444, (NTN=120, Pre-HTN=124, HTN=200), values presented as mean±SE, p values from ANCOVA after accounting for age, gender and acquisition type
n= 442 (NTN=120, Pre-HTN=124, HTN=198), values presented as mean±SE, p values from ANCOVA after accounting for age, gender and acquisition type
n= 437 (NTN=118, Pre-HTN=124, HTN=195), data presented as % subject rated as moderate or severe lesions (score of 2 or 3 on the Fazekas scale)
For comparison between groups:
different from NTN group at p<0.05 corrected
different from Pre-HTN group at p<0.05 corrected
Relationships between BP and CBF at baseline
In the entire study group higher systolic blood pressure (SBP) was associated with reduced cortical (β= −0.13, p=0.005 (B=−0.047, 95% CI −0.080,−0.015), entire model F2,444=13.0, p<0.001, gender included, Figure 2a) and hippocampal CBF (β= −0.12, p=0.01 (B=−0.069, 95% CI −0.121,−0.016), entire model F2,444=7.1, p=0.001, gender included, Figure 2b).
Among subjects without HTN (n=245), again, SBP was associated with reduced cortical (β= −0.15, p=0.02 (B=−0.078, 95% CI −0.141,−0.014), entire model F1,244=5.8, p=0.02) and hippocampal CBF (β= −0.13, p=0.04 (B=−0.111, 95% CI −0.217,−0.005), entire model F2,244=4.5, p=0.01, heart disease included).
Cortical CBF differed between the NTN, Pre-HTN and HTN subjects (F3,444=4.2, p=0.02, after adjusting for gender). NTN group had significantly higher cortical CBF than HTN (p=.01, 3% difference). Although CBF for Pre-HTN was in between NTN and HTN values it did not differ from either group. Hippocampal perfusion did not differ between groups (Table 1).
Within the HTN subgroup (n=200), there was no relationship between SBP and cortical CBF (Figure 2c). For the hippocampal CBF quadratic SBP2 term was significant (β=−1.55, p=0.03 (B=−0.003, 95% CI −0.006, 0.000), entire model F3,199=4.0, p=0.008, statin use included), (Figure 2b). The analysis of transformed (squared) data confirmed the results (β=−1.53, p=0.03 (B=−0.18, 95% CI −0.343,−0.017), entire model F3,199=4.1, p=0.008). The optimal SBP value calculated from eq.1 was 125 mmHg (see Supplemental data).
Diastolic blood pressure (DBP) was not related to CBF.
Longitudinal relationship between BP and CBF
In the longitudinal group (n=185) the interaction baseline SBP* SBP (F=12.8, p<0.001) was a significant predictor of CBF in the MMRM. Thus, the relationship between change in SBP and change in CBF differed depending on the baseline SBP value. The model included gender, ARBs, diuretics, and the presence of heart disease at baseline. Linear regression predicting the cortical CBF slope confirmed the results with a significant baseline SBP*SBP slope interaction (see Supplemental data).
Further analyses showed that the relationship in the entire group was driven by the HTN group, as there was no relationship between change in CBF and change in SBP in subjects without HTN (n=106).
In the HTN group (n=79) the MMRM predicting cortical CBF confirmed the significant interaction baseline SBP*SBP (F=9.7, p=0.002). The model included ARBs. Similarly, linear regression predicting the cortical CBF slope showed a significant baseline SBP*SBP slope interaction (see Supplemental data). The best model corresponded to a simplified second-order polynomial that contained the x*y product (baseline SBP*SBP slope) and linear terms but in which x2 and y2 were null. The resulting model captures the key characteristics of our data: a positive correlation of SBP and CBF slopes (increased CBF with increased SBP) at low baseline SBP, a negative correlation of SBP and CBF slopes (increased CBF with decreased SBP) at high baseline SBP, and a smooth transition in the middle baseline SBP (Figure 3). The xc calculated from eq.4 revealed a saddle point in the fitted quadratic function and corresponded to 128 mmHg (see Supplemental data). Although relationship between changes in SBP and changes in hippocampal CBF did not reach significance, we observed a similar pattern (Figure S3).
Figure 3.
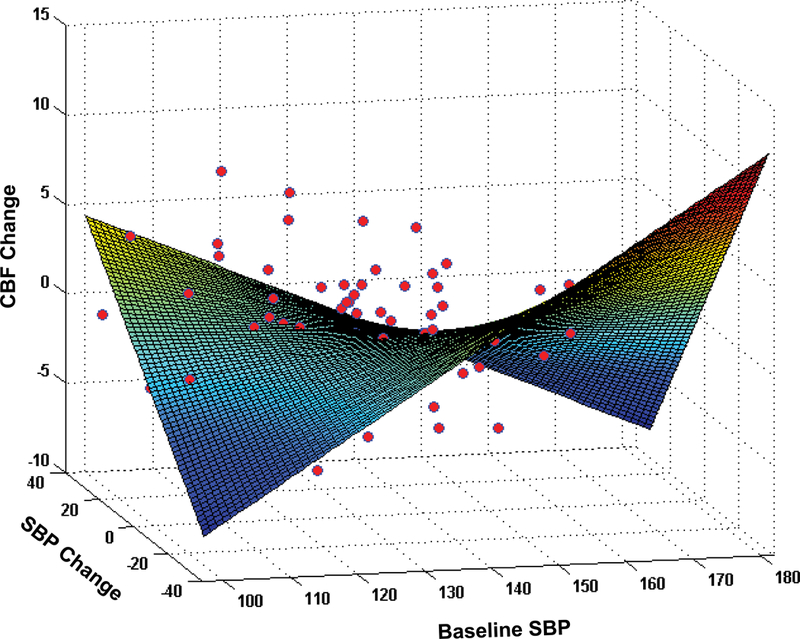
The results of 3D surface fitting depicting the relationship between baseline SBP, change in SBP (slope: mmHg/ year) and change in cortical CBF (slope: ml/100g/min/ year). The graph shows a positive correlation of SBP and CBF slopes (increased CBF with increased SBP) at low baseline SBP and a negative correlation of SBP and CBF slopes (increased CBF with decreased SBP) at high baseline SBP.
DBP changes were not related to CBF changes.
Additional data regarding the relationships between mean BP, pulse pressure and CBF changes, cerebrovascular resistance, and blood gases during image acquisition are presented in the Supplement.
Discussion
Retrospective and epidemiologic studies demonstrate the importance of adequate cerebral perfusion in healthy aging. Hypoperfusion has been suggested to lead to pathologic and atrophic brain changes19, 20. Unfortunately older studies suffered from limited resolution that confounds hypoperfusion and atrophy. In this prospective examination of a large cohort of normal aging we have used high-resolution ASL-MRI to show that hypoperfusion is linked to high SBP. We found that cortical and hippocampal CBF declines with increasing pressure across the entire spectrum of BP. Although Pre-HTN group did not differ in CBF from normotensive subjects, a significant negative association between SBP and CBF was seen already when HTN subjects were excluded from analyses. Moreover, CBF values in the Pre-HTN group were closer to HTN, while their brain volumes were closer to volumes of subjects with normal BP. Our results strongly support the premise that CBF impairments precede structural changes since bSSFP ASL method is minimally affected by partial volume effects. Our observations are in line with earlier studies showing that prehypertension confers higher risk of stroke as compared to lower BP21 and suggest early treatment of elevated BP.
Based on increasing evidence from the literature it appears that impaired endothelial function and atherosclerotic changes in vessel walls cause reduced perfusion. The reduction in hippocampal perfusion may mediate a connection between HTN and memory decline.
DBP was not related to CBF. This study supports the view that SBP is a more important vascular risk factor than DBP in subjects over 50 years old2.
Statistical data modeling showed a quadratic relationship between hippocampal CBF and SBP in the HTN group. Perfusion was maximized at around 125 mmHg. This sweet-spot was confirmed by longitudinal data, which revealed that moving away from the optimum SBP (128 mmHg, not far from 125 mmHg estimated from cross-sectional data) in both low and high BP direction was related to CBF reduction.
When SBP is above the optimum, hypoperfusion is likely caused by continued deleterious consequences of high BP: vascular wall hardening and narrowing. Recently Foster-Dingley et al. examined CBF in hypertensive (mean SBP=145 mm Hg) subjects in their eighties22. Their finding of lack of CBF increase anticipated after a discontinuation of BP lowering treatment and consequent BP increase is consistent with our observations. The interpretation of hypoperfusion below the optimum in HTN is more speculative. It is presumably caused by preexisting vessel damage resulting from previously high BP. It manifests as a failure of dilation in response to reduced perfusion pressure. Our results add to many previous reports that low BP or its sharp decline are linked in various populations to increased risk of dementia23, brain atrophy24 or poorer self-rating of mental and physical wellbeing25. They also corroborate the notion that BP lowering may not be beneficial in all instances, and support the existence of much debated optimal BP which would guarantee the best organ perfusion.
Reduction in vascular risk burden may be responsible for declining incidence of dementia26. It is known that that lower resting CBF predicts cognitive decline27. Our results suggest that perfusion improvement is a possible mechanism behind this risk reduction.
A quadratic relationship between SBP and CBF in the HTN group was evident in the hippocampus, but not in the neocortex. An earlier study showed that aging may be associated with increased vulnerability of posterior circulation to BP fluctuations28. Hippocampal blood supply comes mostly from the posterior cerebral artery (PCA)29. Although the hippocampus has an arcade-like anastomosis system involving the anterior choroidal artery, this system is quite variable29, 30. A study of patients with PCA occlusion showed that in subjects with better collateralizations, hippocampal infarct occurred less frequently31. This predominantly single artery supply or regional differences in cerebral autoregulation32 may explain our results. Finally, singularity of posterior circulation is also suggested by recent discovery showing that vertebral artery hypoplasia plays a role in triggering systemic hypertension to maintain adequate brain perfusion33.
We found that hippocampal perfusion was consistently higher than cortical CBF. This is consistent with recent findings of higher vascular density in the hippocampus as compared to the cortex34. It is also possible that spillover of ASL signal from big vessels passing near the hippocampus (larger than penetrating arteries in temporal cortex) was the reason for this discrepancy. Despite our exclusion of voxels with CBF >150 ml/(100g min) (see Methods), the bias might have persisted.
There are several limitations of our study. First, our group was predominantly Caucasian with relatively low levels of vascular comorbidity, so generalizability is uncertain. Second, we did not have reliable information about the duration of HTN. Third, subjects were assigned to the HTN group based on baseline/screening in-office BP measurement. Should our HTN group incorrectly include some subjects with “white coat hypertension”, the real difference between groups would have been even greater. HTN is a strong risk factor for stroke, which can affect CBF. We excluded subjects with clinically apparent or cortical stroke. As for white matter lesions, although their load was the highest in the HTN group, the overall burden was low across all subjects. Throughout the manuscript, we assumed that higher perfusion signifies better outcome. Some reports indicate that cognitive decline in associated with biphasic CBF changes, i.e compensatory increase followed by CBF reduction35. Given that our group was cognitively stable, we believe this possibility did not bias our results. Resting CBF is affected by many regulatory mechanisms. We could not control for all of them. We believe, however, that large number of participants helps overcome this potential problem.
Hippocampus, being a smaller structure, makes it more challenging to measure CBF. Higher variability of hippocampal CBF as compared to cortical values speaks in favor of this explanation. This variability could have bearing on our results by leading to type II error -- failure to observe the relationship between BP and CBF, but it does not invalidate statistically significant findings. Validity of our results is also supported by increased recognition that CBF measured with ASL correlates well with gold standard H215O PET method36.
Finally, BP values where perfusion was the highest may differ from person to person and between different populations. Our results should rather be seen as a proof of concept that such point may exist than a fixed value ready to be applied in clinical settings.
Perspectives
In our group with relatively low vascular risk, cortical and hippocampal CBF decreased with increasing SBP across the spectrum of normal and high BP. In subjects with hypertension, there appeared to be a window of optimal mid-range SBP where brain perfusion was the highest.
Although more studies are warranted to replicate these findings and confirm generalizability of the BP levels found in our group, our results have the potential to inform treatment goals for general and hypertensive populations.
Supplementary Material
Novelty and significance.
1. What is new?
We performed cross-sectional and longitudinal assessments of relationships between BP and CBF in the neocortex and the hippocampus, a region associated with cognitive decline. Our observations add to earlier findings that high BP is related to reduction in brain perfusion, but also indicate that among subjects with hypertension there may be an optimal BP which maximizes CBF.
2. What is relevant?
Our results contribute to ongoing efforts to establish BP values and treatment goals for optimizing brain function.
3. Summary
Cortical and hippocampal CBF decreased with increasing SBP across the entire BP spectrum and among subject without HTN. Within the HTH group there was a quadratic relationship between SBP and hippocampal CBF (β=−1.55, p=0.03). The CBF was the highest in subjects with mid-range SBP around 125 mmHg. Longitudinally, in hypertensive subjects CBF increased with increased SBP at low baseline SBP, but increased with decreased SBP at high baseline SBP.
Acknowledgments
Sources of Funding
Study funding comes from NIH grants HL111724, AG022374, AG12101, AG08051, HL118624, and Alzheimer’s Association NIRG-09–132490.
Footnotes
Disclosures
None
References list
- 1.Nwankwo T, Yoon SS, Burt V and Gu Q. Hypertension Among Adults in the United States: National Health and Nutrition Examination Survey, 2011–2012. HCHS Data Brief 2013;No.133. [PubMed] [Google Scholar]
- 2.Chobonian A, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr., Jones DW, Matterson BJ, Oparil S, Wright JT Jr. and Rocella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 3.Kennelly SA, Lawlor B and Kenny RA. Blood pressure and the risk for dementia—A double edged sword. Aging Reaserch Reviews 2009;8:61–70. [DOI] [PubMed] [Google Scholar]
- 4.Beason-Held LL, Moghekar A, Zonderman AB, Kraut MA and Resnick SM. Longitudinal Changes in Cerebral Blood Flow in the Older Hypertensive Brain. Stroke 2007;38:1766–1773. [DOI] [PubMed] [Google Scholar]
- 5.Hajjar I, Zhao P, Alsop D, Abduljalil A, Selim M, Novak P and Novak V. Association of Blood Pressure Elevation and Nocturnal Dipping With Brain Atrophy, Perfusion and Functional Measures in Stroke and Nonstroke Individuals. American Journal of Hypertension 2010;23:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strandgaard S, Olesen J, Skinhoj E and Lassen NA. Autoregulation of brain circulation in severe arterial hypertension. British Medical Journal 1973;1:507–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paulson OB, Waldemar G, Schmidt JF and Strandgaard S. Cerebral circulation under normal and pathologic conditions. The American Journal of Cardiology 1989;63:C2–C5. [DOI] [PubMed] [Google Scholar]
- 8.Egan BM and Stevens-Fabry S. Prehypertension-prevalence, health risks, and management strategies. Nat Rev Cardiol 2015;12:289–300. [DOI] [PubMed] [Google Scholar]
- 9.Glodzik-Sobanska L, Rusinek H, Mosconi L, Li Y, Zhan J, De Santi S, Convit A, Rich KE, Brys M and de Leon MJ. The role of quantitative structural imaging in the early diagnosis of Alzheimer’s disease. Neuroimaging Clinics of North America 2005;15:803–826. [DOI] [PubMed] [Google Scholar]
- 10.Birns J and Kalra L. Cognitive function and hypertension. J Hum Hypertens 2009;23:86–96. [DOI] [PubMed] [Google Scholar]
- 11.Kivipelto M, Helkala EL, Hanninen T, Laakso MP, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J and Nissinen A. Midlife vascular risk factors and late-life mild cognitive impairment: A population-based study. Neurology 2001;56:1683–1689. [DOI] [PubMed] [Google Scholar]
- 12.Reisberg B, Sclan SG, Franssen EH, de Leon MJ, Kluger A, Torossian CL, Shulman E, Steinberg G, Monteiro I, McRae T, Boksay I, Mackell JA and Ferris SH. Clinical stages of normal aging and Alzheimer’s disease: The GDS staging system. Neuroscience Research Communications 1993;13 (Suppl. 1):551–554. [Google Scholar]
- 13.Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 14.Ashburner J and Friston KJ. Voxel-based morphometry--the methods. Neuroimage 2000;11:805–821. [DOI] [PubMed] [Google Scholar]
- 15.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B and Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuroimage 2002;33:341–355. [DOI] [PubMed] [Google Scholar]
- 16.Fazekas F, Chawluk JB, Alavi A, Hurtig HI and Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. American Journal of Roentgenology 1987;149:351–356. [DOI] [PubMed] [Google Scholar]
- 17.Rusinek H, Ha J, Yao PL, Storey P, Tirsi A, Tsui WH, Frosch O, Azova S and Convit A. Cerebral perfusion in insulin resistance and type two diabetes. Journal of Cerebral Blood Flow & Metabolism 2015;35(1):95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rusinek H, Brys M, Glodzik L, Switalski R, Tsui WH, Haas F, McGorty K, Chen Q and de Leon MJ. Hippocampal blood flow in normal aging measured with arterial spin labeling at 3T. Magnetic Resonance in Medicine 2011;65:128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zonneveld HI, Loehrer EA, Hofman A, Niessen WJ, van der Lugt A, Krestin GP, Ikram MA and Vernooij MW. The Bidirectional Association between Reduced Cerebral Blood Flow and Brain Atrophy in the General Population. Journal of Cerebral Blood Flow & Metabolism 2015;35:1882–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vernooij MW, van der Lugt A, Ikram MA, Wielopolski PA, Vrooman HA, Hofman A, Krestin GP and Breteler MMB. Total cerebral blood flow and total brain perfusion in the general population: The Rotterdam Scan Study. Cerebral Blood Flow and Metabolism 2008;28:412–418. [DOI] [PubMed] [Google Scholar]
- 21.Huang Y, Wang S, Cai X, Mai W, Hu Y, Tang H and Xu D. Prehypertension and incidence of cardiovascular disease: a meta-analysis. BMC Medicine 2013;11:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foster-Dingley JC, Moonen JEF, de Craen AJM, de Ruijter W, van der Mast RC and van der Grond J. Blood Pressure Is Not Associated With Cerebral Blood Flow in Older Persons. Hypertension 2015;66:954. [DOI] [PubMed] [Google Scholar]
- 23.McGrath ER, Beiser AS, DeCarli C, Plourde KL, Vasan RS, Greenberg SM and Seshadri S. Blood pressure from mid- to late life and risk of incident dementia. Neurology 2017;89:2447–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.den Heijer T, Launer LJ, Prins ND, van Dijk EJ, Vermeer SE, Hofman A, Koudstaal PJ and Breteler MM. Association between blood pressure, white matter lesions, and atrophy of the medial temporal lobe. Neurology 2005;64:263–267. [DOI] [PubMed] [Google Scholar]
- 25.Muller M, Jochemsen HM, Visseren FL, Grool AM, Launer LJ, van der Graaf Y, Geerlings MI and group SM-s. Low blood pressure and antihypertensive treatment are independently associated with physical and mental health status in patients with arterial disease: the SMART study. J Intern Med 2013;274:241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larson EB, Yaffe K and Langa KM. New Insights into the Dementia Epidemic. New England Journal of Medicine 2013;369:2275–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chao LL, Buckley ST, Kornak J, Schuff N, Madison C, Yaffe K, Miller BL, Kramer JH and Weiner MW. ASL Perfusion MRI Predicts Cognitive Decline and Conversion From MCI to Dementia. Alzheimer Dis & Assoc Dis 2010;24:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sorond FA, Khavari R, Serrador JM and Lipsitz LA. Regional cerebral autoregulation during orthostatic stress: age-related differences. J Gerontol A Biol Sci Med Sci 2005;60:1484–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erdem A, Yasargil MG and Roth P. Microsurgical anatomy of the hippocampal arteries. Journal of Neurosurgery 1993;79:256–265. [DOI] [PubMed] [Google Scholar]
- 30.Marinkovic S, Milisavljevic M and Puskas L. Microvascular anatomy of the hippocampal formation. Surgical Neurology 1992;37:339–349. [DOI] [PubMed] [Google Scholar]
- 31.Forster A, Murle B, Kerl HU, Wenz H, Al-Zghloul M, Habich S and Groden C. Sparing of the Hippocampus Indicates Better Collateral Blood Flow in Acute Posterior Cerebral Artery Occlusion. International Journal of Stroke 2015;10:1287–1293. [DOI] [PubMed] [Google Scholar]
- 32.Koller A and Toth P. Contribution of Flow-Dependent Vasomotor Mechanisms to the Autoregulation of Cerebral Blood Flow. Journal of Vascular Research 2012;49:375–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warnert EAH, Rodrigues JCL, Burchell AE, Neumann S, Ratcliffe LEK, Manghat NE, Harris AD, Adams Z, Nightingale AK, Wise RG, Paton JFR and Hart EC. Is High Blood Pressure Self-Protection for the Brain? Circulation research 2016;119:e14–e151. [DOI] [PubMed] [Google Scholar]
- 34.Rane S, Talati P, Donahue MJ and Heckers S. iVASO reproducibility in the hippocampus and cortex at different blood water nulling times. Magn Reson Med 2016;75:2379–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wierenga CE, Hays CC and Zlatar ZZ. Cerebral blood flow measured by arterial spin labeling MRI as a preclinical marker of Alzheimer’s disease. J Alzheimers Dis 2014;42 Suppl 4:S411–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bokkers RP, Bremmer JP, van Berckel BN, Lammertsma AA, Hendrikse J, Pluim JP, Kappelle LJ, Boellaard R and Klijn CJ. Arterial Spin Labeling Perfusion MRI at Multiple Delay Times: A Correlative Study with H215O Positron Emission Tomography in Patients with Symptomatic Carotid Artery Occlusion. Journal of Cerebral Blood Flow & Metabolism 2009;30:222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


