Abstract
Carotenoids exert a rich variety of physiological functions in mammals and are beneficial for human health. These lipids are acquired from the diet and metabolized to apocarotenoids, including retinoids (vitamin A and its metabolites). The small intestine is a major site for their absorption and bioconversion. From here, carotenoids and their metabolites are distributed within the body in triacylglycerol-rich lipoproteins to support retinoid signaling in peripheral tissues and photoreceptor function in the eyes. In recent years, much progress has been made in identifying carotenoid metabolizing enzymes, transporters, and binding proteins. A diet-responsive regulatory network controls the activity of these components and adapts carotenoid absorption and bioconversion to the bodily requirements of these lipids. Genetic variability in the genes encoding these components alter carotenoid homeostasis and induce various pathologies in research animals. We here summarize the advanced state of knowledge about intestinal carotenoid metabolism and its impact on carotenoid and retinoid homeostasis of other organ systems, including the eyes, liver, and immune system. The implication of the findings for science-based intake recommendations for these essential dietary lipids is discussed.
Keywords: Carotenoid, Apocarotenoid, Retinoid, Intestine, Metabolism, Abbreviations
Introduction
Carotenoids are a class of terpenoid pigments built from eight isoprene units in plants, fungi, and bacteria [1]. Scientists took great interest in isolating these pigments from nature in the 19th century and referred to them by common names long before their structures were determined (Figure 1). The eponymous β-carotene was the first carotenoid to be isolated from nature by Wackenroder, and its chemical structure was the first of any vitamin or provitamin that was determined in 1930 by Karrer [2], who was awarded a Nobel Prize for his work.
Figure 1.
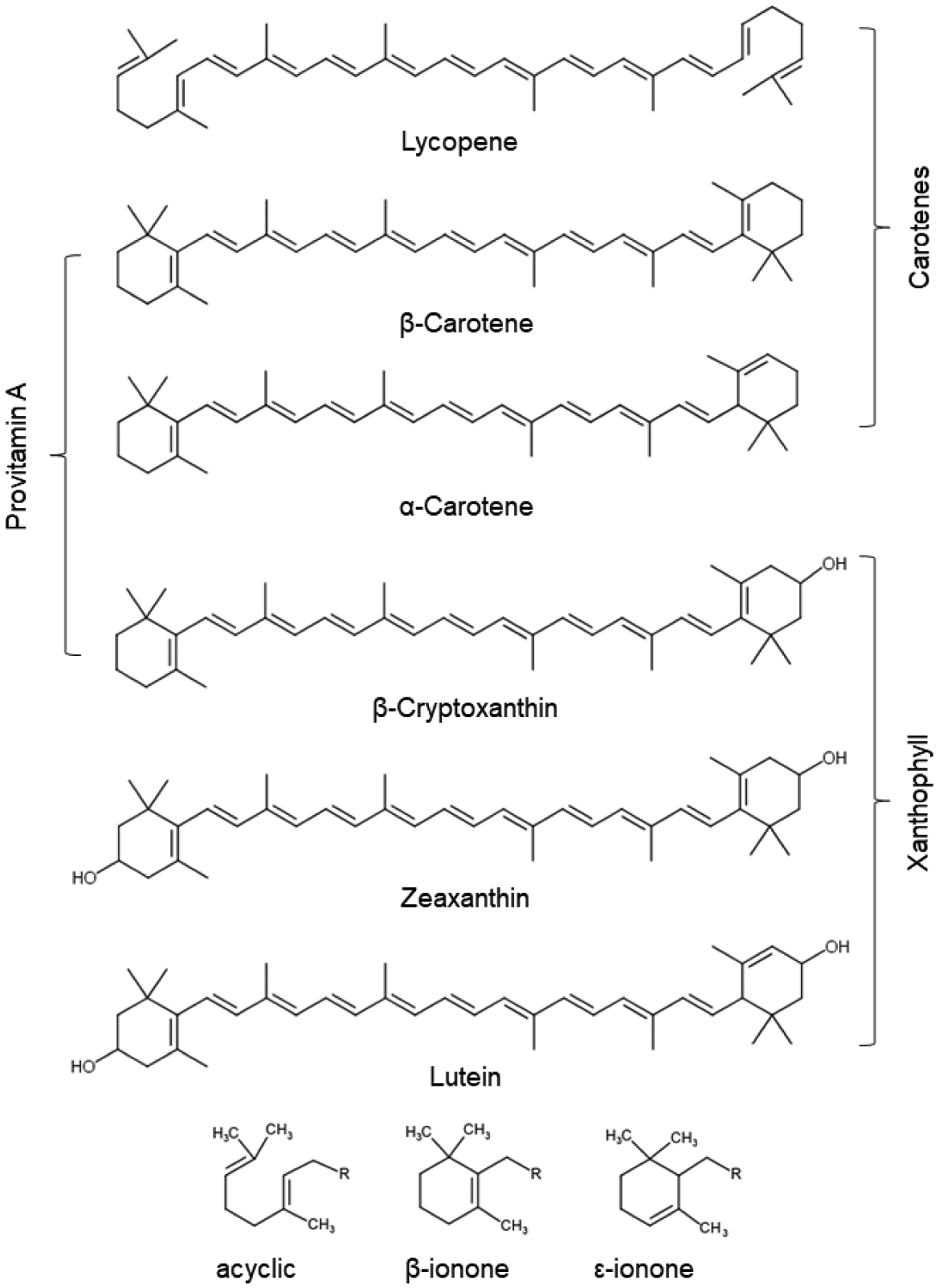
Chemical structures and names of the six major carotenoids in human blood. Their classification into carotenes, xanthophyll, and provitamin A carotenoids is indicated. The chemical characteristics of different end-groups is provided at the bottom of the panel.
Carotenoids possess an extended polyene chromophore with up to eleven double bonds that can carry terminal hexyl-rings. More than 1000 carotenoids occur in nature [3]. Their enormous chemical diversity results from chemical modulations of the core structure, including the shifting of conjugated double bonds and the addition of functional groups to the terminal rings (Figure 1). The short chain apocarotenoids (< C40) also are members of the carotenoid substance class that derive from oxidative cleavage at specific double bonds of the parent carotenoid molecules.
The role of carotenoids in human health is currently a subject of intense investigation. Low status of total plasma carotenoids and individual carotenoids is associated with vitamin A deficiency and a number of degenerative diseases including cardiovascular disease, cognitive impairments, and age-related macular degeneration [4–8]. A number of potential mechanisms through which carotenoids can benefit human health have been proposed. The most commonly cited is their capability of acting as antioxidants, e.g., as free radical scavengers, in lipophilic environments such as membranes and lipoproteins [9]. The antioxidant action of carotenoids may decrease lipid peroxidation and eventually reduce oxidative stress and inflammation responses in cells and tissues [10].
The blue light filtering properties of carotenoids compose another mechanism of protection of cellular components from the environment. Macular pigments have been chemically identified as the carotenoids lutein, zeaxanthin, and meso-zeaxanthin [11]. These carotenoids are enriched in the fovea in primate retinas and confer its yellow colour. Hence, the fovea is traditionally known as the macula lutea, or ‘yellow spot’. The macular pigments can protect the retina against light damage [12, 13] and reduce the adverse impact of light scattering and chromatic aberration, thereby optimizing contrast sensitivity of the retina [14]. The light filtering properties of carotenoids also can provide modest protection against ultraviolet -induced erythema in the skin [15].
Importantly, carotenoids make a crucial contribution to vision and gene transcription. Seminal research in the molecular basis of these actions led to the discovery of visual G protein-coupled receptors and nuclear hormone receptors [16, 17]. Among the carotenoid family are provitamin A carotenoids (β-carotene, α-carotene, and β-cryptoxanthin) that undergo obligate metabolic conversion to retinaldhyde. Retinaldhyde is the chromophore of visual pigments that mediate phototransduction in the retina [18]. Retinaldehyde can be further metabolized to retinol (vitamin A), retinyl esters, and retinoic acid by endogenous enzymes [19]. Retinol and retinyl esters are the transport and storage form of the vitamin, respectively [20]. Retinoic acid binds to nuclear receptors [21], which are ligand activated transcription factors that control the expression of genes which are involved in processes such as cell differentiation, embryonic development, immunity, and metabolism [22–24].
Mammals cannot synthesize carotenoids and must absorb these pigments from the diet. The major site of their absorption and metabolism is the small intestine. A number of studies observed that vitamin A status, individual genetics, and disease states affect this process and contribute to the observed variability of plasma responses to dietary carotenoids in clinical and epidemiological studies [25–27]. In recent years, molecular players of carotenoid metabolism have been identified and their interactions have been studied in animal models. The principles governing this metabolism and the involved transcription factors, metabolizing enzymes, binding proteins, and transporters enzymes are reviewed here. The implications of these studies for recommendations for carotenoid intake in health and disease are discussed.
2. Carotenoid absorption in the intestine
2.1. The formation of mixed micelles
Fifty carotenoids are typically ingested with the diet. Of these, 20 are commonly found in human tissues [28]. These carotenoids are divided into carotenes (pure hydrocarbons) and xanthophyll (oxygenated carotene metabolites). The six main carotenoids circulating in the human blood are β-carotene, α-carotene, β-cryptoxanthin, lutein, zeaxanthin, and lycopene (Figure 1).
Mammals exclusively acquire carotenoids from the diet. The absorption and bioconversion of dietary carotenoids primarily takes place in the intestine, where carotenoids form mixed micelles with amphiphilic and hydrophobic compounds including bile salts, cholesterol, fatty acids, monoacylglycerides and phospholipids. The hydrophobicity of individual carotenoids is the key physiochemical factor that determines their bioaccessibility (octanol-water partition coefficients log PC > 8) in naturally produced mixed micelles as well as their postprandial blood response [29]. Polar side groups of ionone rings play a minor role, though it is often stated in the literature that xanthophylls are more readily bioavailable than carotenes [30]. In many fruits and vegetables, xanthophylls exist as esters of fatty acids [31]. Before carotenoid esters are absorbed, the fatty acid moiety is cleaved by pancreatic carboxyl ester lipase to form free xanthophylls in the gastrointestinal tract [32]. Xanthophyll ester hydrolysis appears to be an efficient process because the absorption of lutein and β-cryptoxanthin esters is comparable to that of the free carotenoid forms [33]. Furthermore, the uptake of zeaxanthin esters may be even greater than that of the parent carotenoid [34].
Additionally, the geometric form of carotenoids, which easily undergo isomerization from the prevalent all-trans-form to cis-isomers following exposure to heat and/or light, affects their bioavailability. The relative absorption of geometric isomers varies between carotenoids. For example, absorption is reduced for β-carotene cis-geometric isomers, whereas uptake is increased for lycopene cis-geometric isomers compared to their respective all-(E)-form [35, 36].
The absorption efficiency of carotenoids is also influenced by food matrix, formulation, and food processing. The context, or matrix, in which carotenoids is provided, is another important factor which determines the bioavailability of these lipids [25, 27]. Carotenoids prepared in oil (e.g. red palm oil), or commercially available water-soluble beadlets, have superior bioavailability compared with those in raw fruits or vegetables [25, 27]. Fat intake, like a modest amount of salad dressing, increases carotenoid uptake by facilitating the formation of mixed micelles [37]. The differences in absorption are likely explained by the occurrence of carotenoids in different cellular compartments (chromoplast versus chloroplast) and aggregate forms in plant tissues. Crystalline or protein-bound carotenoids are less well solubilized in mixed micelles than carotenoids that exist in oil droplets and membranes. Mild heating and food processing elevate the bioavailability of carotenoids by disrupting plant cell walls, binding proteins, and organelles, thus liberating carotenoids for uptake. Fiber, olestra, plant sterol, and stanol esters are dietary compounds that decrease the absorption of carotenoids. Antioxidants, such as vitamins C and E, may increase the stability of the carotenoids in the gastrointestinal tract and thereby facilitate their absorption [38].
2.2. Molecular components facilitating cellular uptake of carotenoids
Upon being made bioaccessible in mixed micelles, carotenoids are absorbed by enterocytes across a membrane bilayer for further metabolic processing within the brush border cells. Studies in polarized CaCo-2 cells, a model for the brush border membrane, revealed that this process is saturable and selective [39], indicating the involvement of proteins. The proteins facilitating carotenoid uptake from mixed micelles were identified as class B scavenger receptors [40–45]. Studies in CaCo-2 cells revealed that scavenger receptor class B type 1 (SR-B1) facilitate carotenoid absorption from synthetic and mixed micelles [46, 47]. The involvement of SR-B1 in the absorption of these micronutrients was later confirmed in knockout mice which display significantly reduced intestinal absorption of carotenes and xanthophylls [40, 43]. Studies in mice also show that SR-B1 facilitates the intestinal absorption of fat soluble vitamins E and K [41, 42]. Later it was demonstrated that cluster determinant 36 (CD36) also contributes to the absorption of carotenoids from mixed micelles [48, 49].
CD36 and SR-B1 are highly expressed in the proximal parts of the mouse intestine and at lower levels in distal parts [50]. CD36 and SR-B1 are glycosylated transmembrane proteins with a large extracellular domain. Structural prediction, based on the crystal structure of the CD36 family member lysosomal membrane protein 2 (LIMP II), [51] indicate the presence of a large cavity traversing the entire length of the protein that serves as a tunnel for lipid transfer from extracellular to cellular compartments [52, 53].
Outside of the intestine, the functions of CD36 and SRB1 are diverse, and the proteins have broad substrate specificity due to their ability to recognize similar molecular patterns rather than specific epitopes. CD36 ligands include carotenoids, long chain fatty, native or modified lipoproteins, thrombospondin-1, collagen, apoptotic cells, amyloid B, and malaria-infected erythrocytes [54]. CD36 is expressed in muscle, adipose tissue, intestine and the capillary endothelium, where it facilitates long chain fatty acid uptake into target cells in capillary beds of tissues [55, 56], [57]. CD36-deficient mice display among other defects, significantly increased levels of circulating fatty acids [58]. SR-B1 binds high-density lipoproteins (HDLs) [59] and facilitates selective cellular uptake of cholesterol in mammalian steroidogenic tissues [60]. SR-B1 is expressed in the liver, intestine, macrophages, adrenal gland, and ovary [58, 61]. SR-B1-deficient mice develop hypercholesterolemia and multiple pathologies, including male sterility [60].
It was proposed that SR-B1 is also involved in intestinal cholesterol absorption [62]. However, these findings could not be confirmed in SR-B1-deficient mice which exhibit relatively normal intestinal cholesterol absorption and trans-intestinal cholesterol efflux [63]. Furthermore, the cholesterol absorption inhibitor ezetimibe effectively inhibits cholesterol absorption in SR-BI-deficient mice [64]. Similarly, CD36 knockout mice display no gross alterations in intestinal fatty acid absorption [65]. The minor role of CD36 in this process might be explained by the relatively high concentration (micromolar) of fatty acids in the intestinal lumen. This allows for protein-independent uptake of dietary fatty acids by brush border cells, whereas absorption of fatty acids in capillary beds (nanomolar concentration) requires facilitators such as CD36 (reviewed in [58]).
The role of class 2 scavenger receptors in the absorption of dietary carotenoids is evolutionarily well conserved. Initial evidence for this function of class 2 scavenger receptors was provided in Drosophila. The neither inactivation nor after potential D (ninaD) protein was identified in a screen of Drosophila mutants that lack functional visual pigments [66]. Nonsense mutations in ninaD render flies deficient in carotenoids, retinoids [44, 66], and tocopherols [45]. Biochemical studies revealed that the ninaD gene encodes a transmembrane protein which facilitates uptake of zeaxanthin and β-carotene from synthetic Tween 40 micelles [45]. In Drosophila larvae, NinaD is expressed in the midgut and its expression is essential for the uptake of carotenoids for retinoid production during compound eye development [67]. In silkworms, a NinaD homologous scavenger receptor has been identified to be critical for acquiring dietary carotenoids for silk coloration [68, 69]. Silkworm mutants display white cocoons because of a lack of yellow carotenoids. In canary birds, SR-B1 is needed for carotenoid coloration of feathers and skin [70]. The white recessive canary bird possesses a splice site mutation which renders SR-B1 inactive [70]. The mutant bird shows white feather coloration and very low levels of carotenoids in blood and tissues. These birds also suffer from severe vitamin A deficiency and depend on supplementation with preformed vitamin A via the diet [71].
3. Carotenoid metabolism in the intestine
Following absorption, carotenoids with pro-vitamin A activity are converted to retinoids and other possible apocarotenoid metabolites (Figure 2). The primary conversion product retinaldehyde is further converted to retinol and retinyl esters. Retinyl esters are incorporated into chylomicrons together with non-cleaved carotenoids and other dietary lipids and then released into the lymph before entering the general circulation [72, 73]. Retinaldehyde, the primary cleavage product, can further be converted to the corresponding acids, alcohols, and esters by endogenous enzymes. The different carotenoid and retinoid processing enzyme classes will now be introduced (Figure 3).
Figure 2.
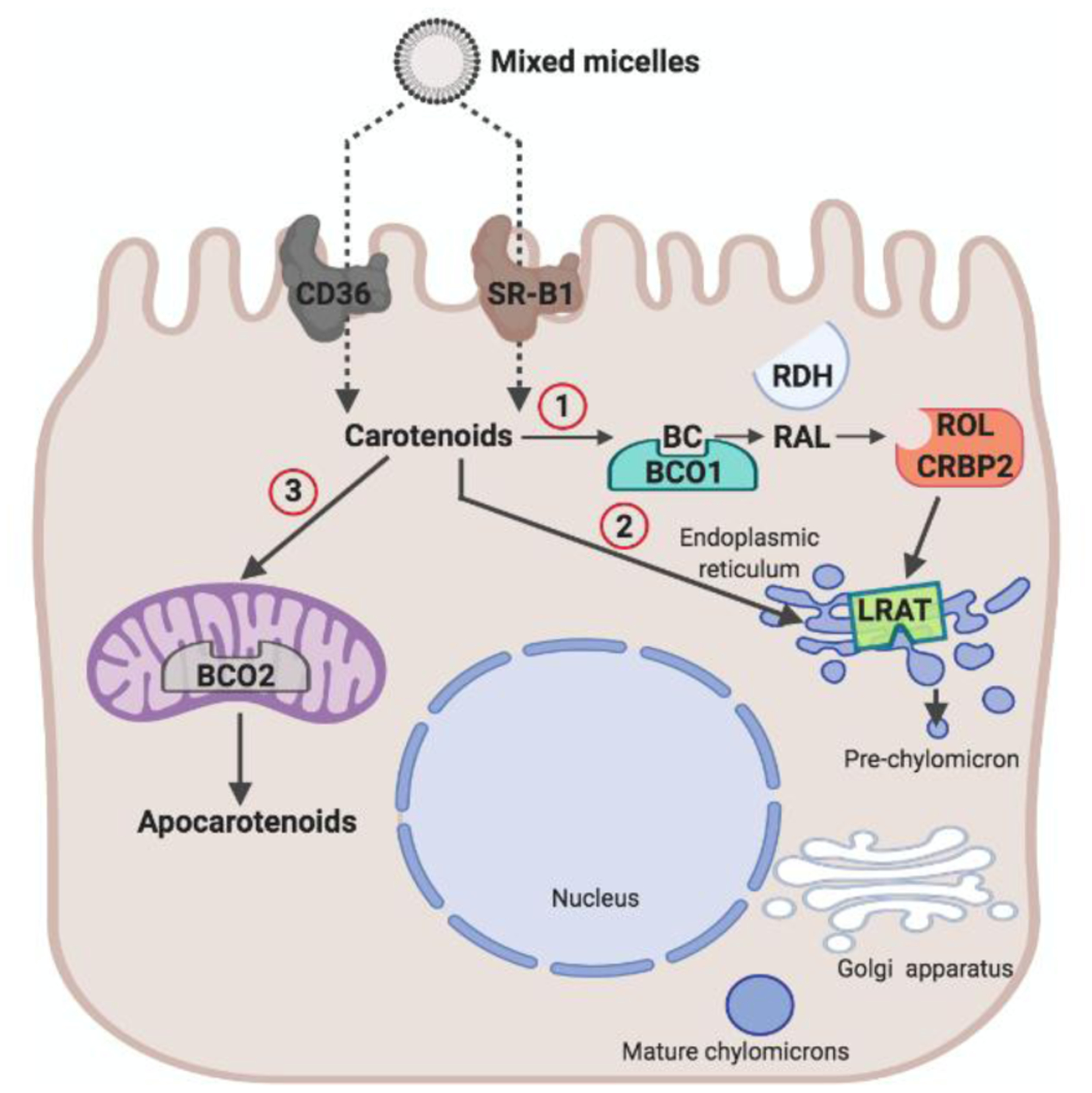
Schematic of carotenoid absorption in an enterocyte. Carotenoid absorption from mixed micelles is facilitated by scavenger receptor class B type 1 (SR-B1) and cluster of differentiation 36 (CD36). Absorbed carotenoids can undergo three metabolic fates. (1) Carotenoids such as β-carotene (BC) can be converted to retinal (RAL). The conversion of BC is catalyzed by β-carotene-15,15’-dioxygenase (BCO1). RAL is reduced to retinol (ROL) by retinal dehydrogenases (RDH) in a process that involves cellular retinol binding protein (CRBP2). ROL is converted to retinyl esters (RE) by lecithin: retinol acyl transferase (LRAT) and packed in chylomicrons for body distribution. (2) Unprocessed carotenoids can be packaged into chylomicrons for body distribution. (3) Carotenoids can be converted by mitochondrial BCO2 (β-carotene-9’,10’-dioxygenase) into apocarotenoids different than retinoids.
Figure 3.
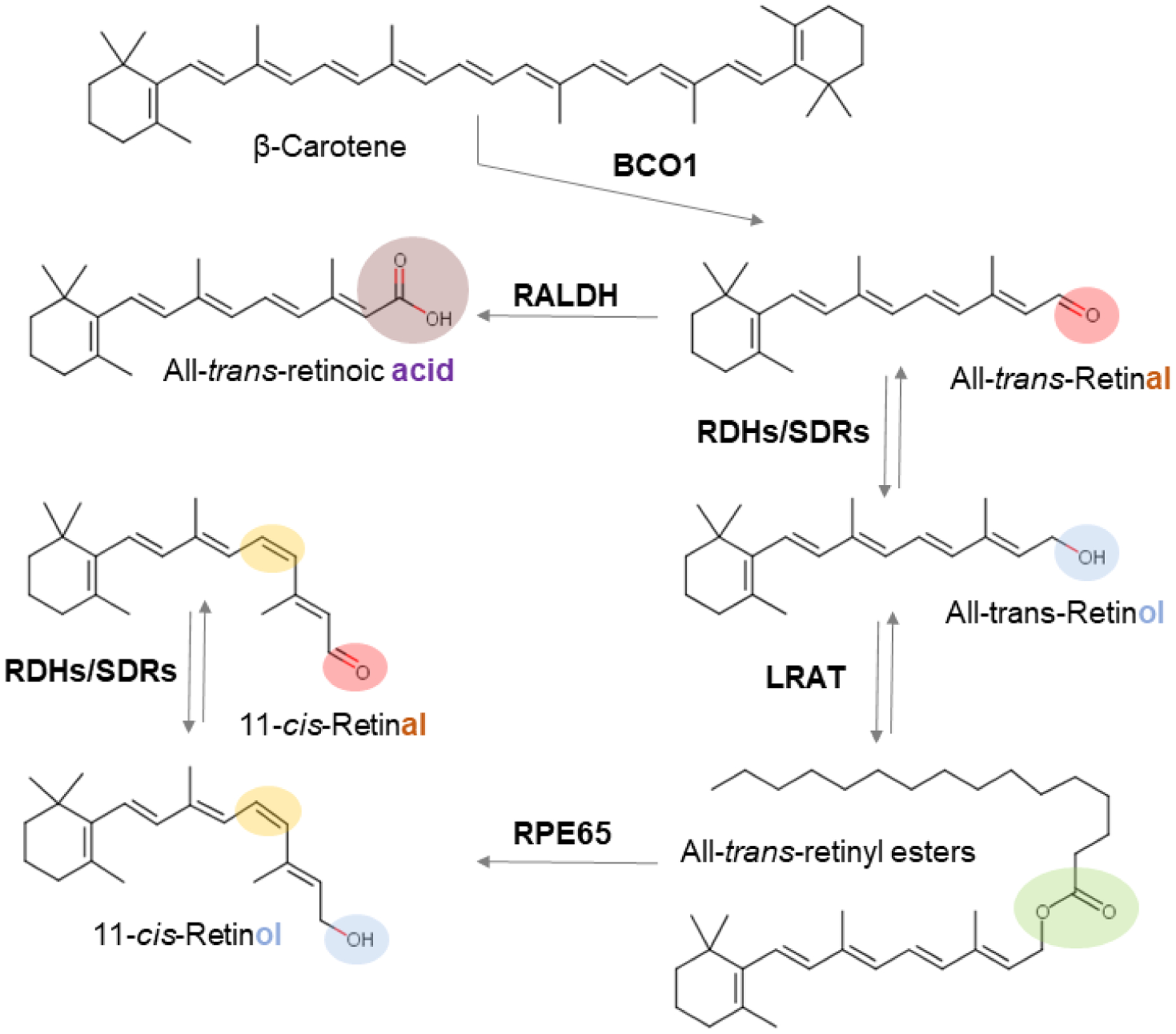
Overview of vitamin A metabolism. The different functional groups of retinoids are indicated by a color code (red, aldehyde; blue, alcohol; green, ester; purple, acid). The 11-cis-retinoid diastereoisomer is indicated by yellow color. Major retinoid metabolizing enzyme classes (see main text) catalyzing the chemical transformations of retinoids are highlighted.
3.1. Carotenoid Cleavage Dioxygenases
In 1930, Moore described the production of vitamin A from β-carotene in the intestine of research animals [74]. In 1965, the respective enzymatic activity was independently characterized by Olson and Goodman [75, 76]. In these initial reports, β-carotene was observed to be cleaved symmetrically at the C15,C15′ double bond to yield two molecules of retinaldehyde. Later on, enzyme activity for the eccentric cleavage of β-carotene was described in cell-free homogenates of the mammalian intestine [77, 78].
The cloning of two genes, encoding carotenoid cleavage dioxygenases (CCDs) - with distinct region selectivity for the cleavage of carotenoids provided the molecular basis of this metabolism [79, 80]. CCDs introduce molecular oxygen at specific double bonds of the carotenoids polyene backbone, resulting in the formation of two apocarotenoid molecules [81–83]. The existence of two CCDs with symmetric and eccentric cleavage modes was subsequently confirmed in many species, including human, macaque, ferret, chicken, bovine, rat, and zebrafish [79, 84–92]. Structural analyses showed that CCDs share a common fold of a seven-bladed propeller covered by a half dome. The active center of the enzymes is accessible through a long tunnel lined with hydrophobic amino acid residues. These non-heme iron oxygenases, with characteristic catalytic centers, catalyze oxidative cleavage and geometric isomerization of carbon double bonds [93–96]. Biochemical analyses with the recombinant BCO1 and BCO2 defined their region specificity of double bond cleavage and substrate specificity [88, 89, 91, 92, 97–99]. BCO1 cleaves across the C15,C15′ double bond adjacent to a canonical β-ionone ring site of carotenoids and β-apocarotenoids [88, 91, 100, 101]. Thus, provitamin A carotenoids and apocarotenoids (> C20) with at least one β-ionone ring are substrates for this enzyme. In fact, β-apo-10’-carotenal and BCO1 can rescue normal embryonic development in mice maintained on a diet free of preformed vitamin A [102]. Additionally, recombinant BCO1 converts the open chain lycopene into acyclic retinoids [86, 88], a reaction that may maintain retinoid signaling under condition of severe vitamin A deficiency [103]
Though originally characterized as β-carotene metabolizing enzyme [79], BCO2 displays broad substrate specificity for carotenoids (Figure 4). The enzyme catalyzes conversion of acyclic lycopene, zeaxanthin, lutein, and cantaxanthin [89, 90, 92, 97–99, 104]. Although BCO2 cleaves across the C9′,C10′ positions adjacent of chemical diverse ionone ring sites [97], it shows higher turnover rates with substrates with hydroxylated ionone ring sites [97]. Mouse and human BCO2 also converts apocarotenoids into dicarbonyl compounds ([97, 99], unpublished). In contrast, chicken BCO2 does not catalyze the latter reaction [89]. On the one hand, the different substrate specificities of mammalian and chicken BCO2s might be attributed to species-specific differences. Birds accumulate 3-hydroxy-dehydro-β-10-apocarotenol (galloxanthin) in oil droplets of their retinas whereas mammals do not accumulate long-chain apocarotenoids when supplemented with xanthophyll [98, 99, 105]. On the other hand, there are potentially methodological limitations with enzymatic assays and product analysis. Dialdehyde cleavage products of apocarotenoids are chemically labile and difficult to detect by standard chromatographic methods when produced in small amounts.
Figure 4.
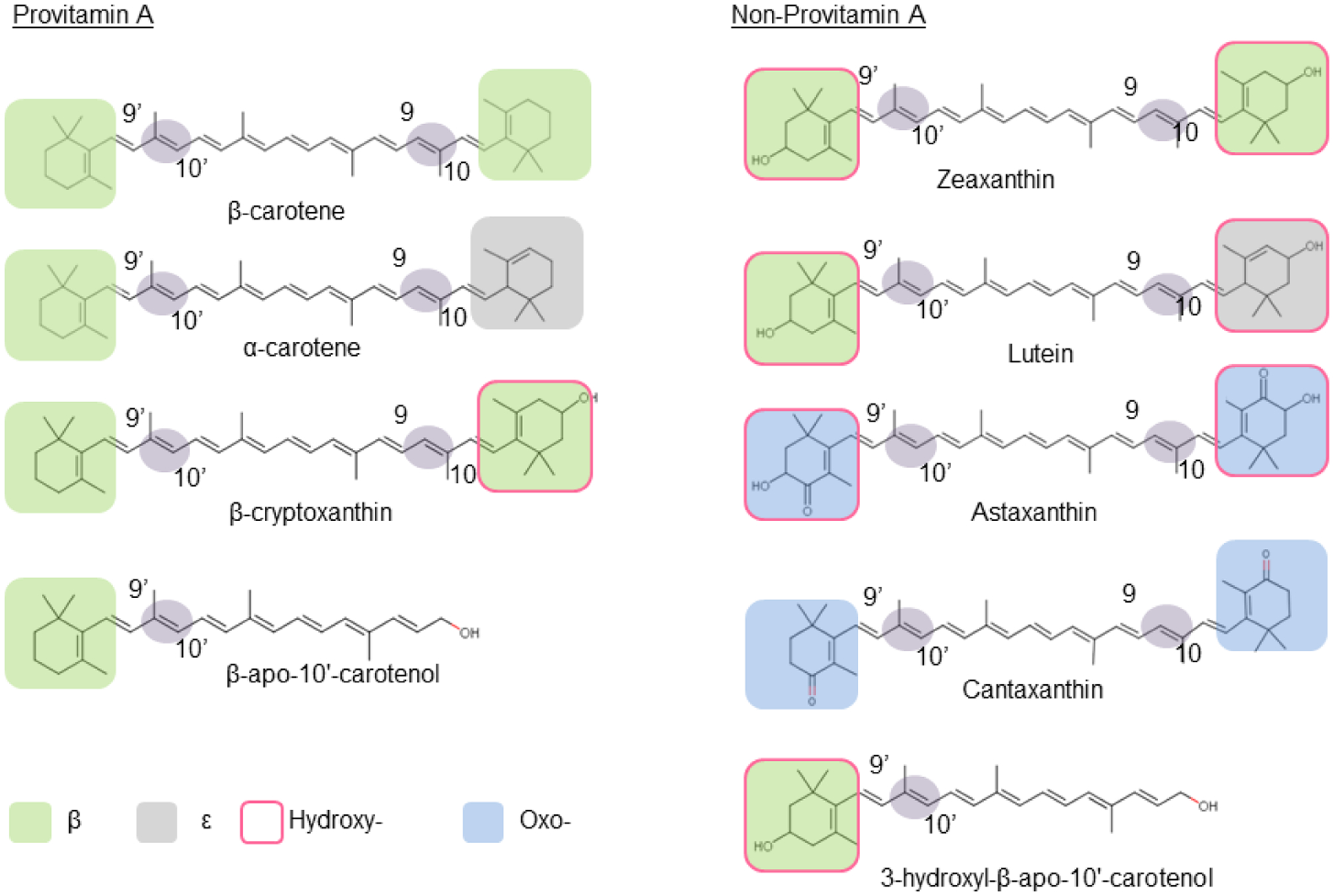
Recombinant BCO2 converts a large panoply of different carotenoids with various ionone ring structures by oxidative cleavage at position 9,10 and 9’,10’ of the carbon skeleton. Confirmed provitamin A and non-provitamin A substrates of recombinant mouse BCO2 are displayed in the panel.
BCO1 and BCO2 are expressed in the intestine and several additional tissues [79, 91, 106, 107]. BCO1 is a monomeric, soluble, and cytosolic enzyme. The cytosolic localization indicates that BCO1 interacts with other cellular components, such as membranes, lipid droplets, and proteins, in order to extract its lipophilic substrates [91, 100, 108]. BCO2 is a mitochondrial enzyme that associates with the inner membrane [89, 99, 104]. This localization of BCO2 was discovered by sub-fractionating of isolated hepatic mitochondria of mice and by electro-microscopy and immunogold staining of human mitochondria [109]. To achieve mitochondrial localization, BCO2 is expressed with a signal peptide sequence in many vertebrate species, including human. The signal peptide is removed during mitochondrial import to yield a mature protein [109]. Rodent BCO2s contain a shorter signal sequence though they also are enriched at the inner membrane of mitochondria [109]. BCO2 substrates such as zeaxanthin and lutein accumulate at the inner mitochondrial membrane in BCO2-deficient hepatic mitochondria of mice [109]. Accumulation of xanthophylls at the inner mitochondrial membrane has also been reported in birds [110], but little is known about how carotenoids are shuttled between different organelles and membranes within in the cell.
The generation of mouse lines, carrying loss-of-function mutations in BCO1 and BCO2 genes, set the stage for an in-depth analysis of the physiological roles of these enzymes. Phenotypically, these mutants are characterized by a yellow fat phenotype [99, 101, 111]. Studies in CCD knockout mice revealed that BCO1 is the major β-carotene metabolizing enzyme and the key enzyme for vitamin A production [101, 109, 111, 112]. In its absence, only trace amounts of β-apo-10’-carotenoids were produced from supplemented β-carotene by BCO2 [101, 113]. Consistent with BCO2’s enzymatic properties and subcellular localization, xanthophylls accumulated in hepatic mitochondria of BCO2-deficient mice [99, 109]. In Bco1 and Bco2 single knockout mice, carotenoid levels were highest in liver and adipose tissue [92, 99, 101, 111]. While β-carotene accumulated unmodified in mouse tissues [114], xanthophyll accumulated in oxidized form [99, 114]. 4’,5’-Didehydro-retro-β-carotene-3,3’-dione (rhodoxanthin) and (6RS,6RS)-ε,ε-Carotene-3,3’-di-one were respectively identified as zeaxanthin oxidation products in white adipose tissues [43] and in blood and liver [99, 114] (Figure 5). Oxidation of xanthophyll has also been reported to occur in wild type mice [115, 116]. Lower levels of oxidized carotenoids were measured in lung and heart of CCD deficient mice [99, 101, 111]. β-Carotene and xanthophyll also accumulate in the eyes of knockout mice with higher levels in the retinal pigment epithelium than in the neuronal retina [43, 117]. However, levels of carotenoids in the eyes were low when compared to other tissues and blood [92]. This observation clearly reflects a lack of a mechanism for ocular accumulation of carotenoids in rodents though the mouse retina expresses high levels of glutathione-S-transferase, GSTP [117]. GSTP1 has been implicated in ocular accumulation of zeaxanthin in the human macula [118]. BCO2-deficient mice also accumulate lycopene in different tissues, whereas levels of supplemented lycopene were indistinguishable between wild type control and BCO1-deficient mice [119].
Figure 5.
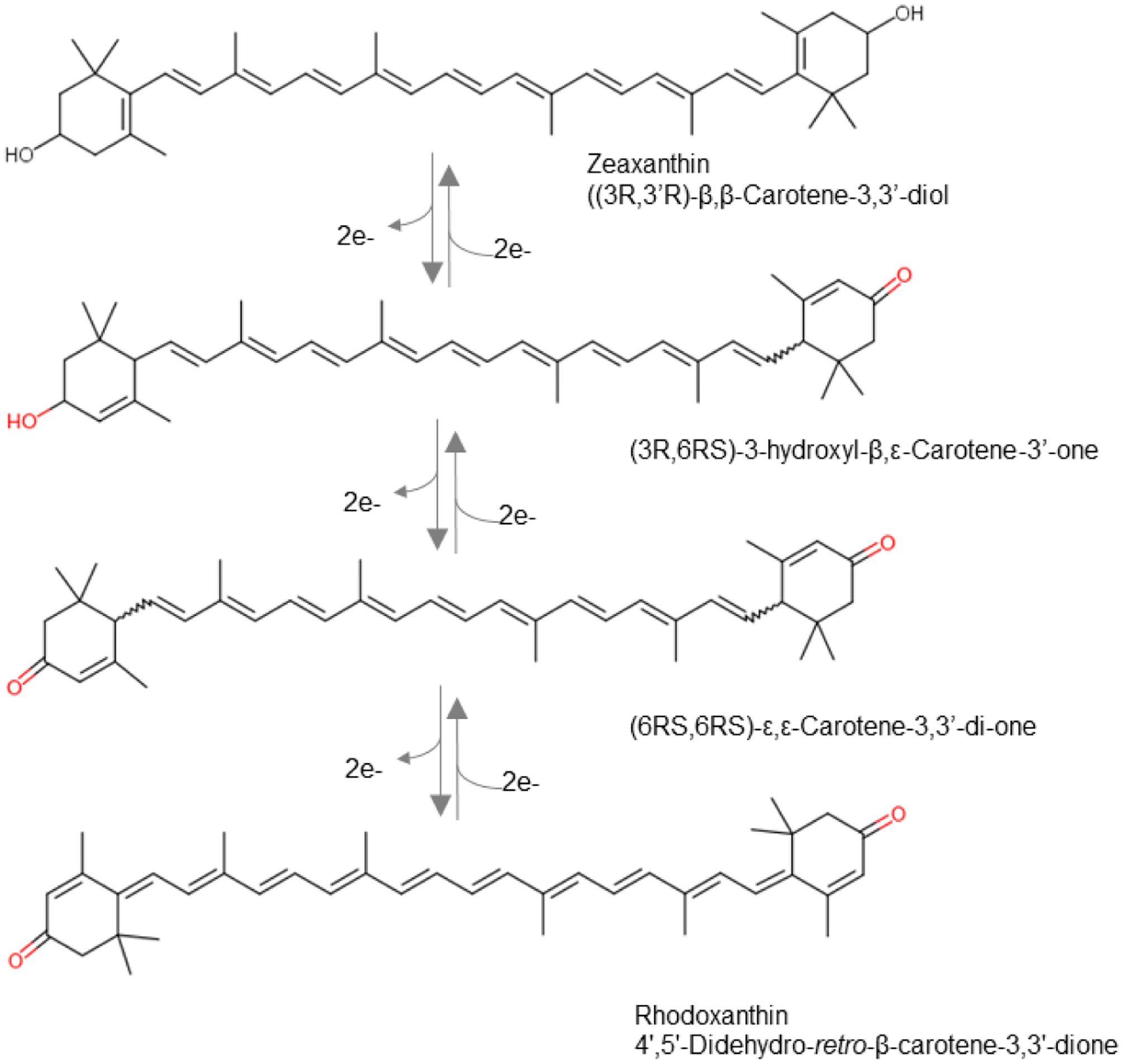
Structures of zeaxanthin and its oxidation products in tissues of mice.
The roles of BCO1 and BCO2 in carotenoid metabolism are well conserved throughout evolution. Similar to mice, humans carrying mutations in the BCO1 gene display elevated β-carotene and reduced vitamin A levels [108]. Mutations in the BCO2 gene have not yet been described in humans but are prevalent in other vertebrates. Mutations in the BCO2 gene associate with a yellow skin phenotype in chicken [120] and reptiles [121]. Loss-of-function mutations in BCO2 gene in cows, rabbits, and sheep cause a yellow fat phenotype [122–125]. It was speculated that the genetic variability in the BCO2 gene in domestic farm animals provides a mechanism to survive in environments with carotenoid shortage [126]. Given the importance of carotenoids in vision, development, reproduction, and immunity, it is possible that BCO2 mutations in domestic animals helped to adapt them to dietary conditions that are vastly different from those of their wild-living ancestors. Notably, Paul Bernstein’s laboratory proposed that a four amino acid long insertion renders human BCO2 inactive and favors the accumulation of the eye protective macula pigment [117]. However, it is controversial whether the human BCO2 gene encodes an enzymatically inactive BCO2 mutant variant. Macaque BCO2 shows overall sequence identity with human BCO2, and the recombinant protein displays robust enzymatic activity [92]. Additionally, it was recently demonstrated that recombinant human BCO2 is enzymatically active when expressed in E. coli cells [127]. Moreover, a recent study provided an alternative explanation for carotenoid accumulation in central parts of the human retina. This study shows that BCO2 mRNA is highly expressed in the peripheral retina and at low level in the central (macula) retina of the human eye. This pattern of expression may explain the selective accumulation of carotenoids in the macula regions of the human retina [128].
The need of tissues to express an enzymatically active BCO2 is indicated by several studies. BCO2-deficiency has been associated with oxidative stress in adult tissues [129–132]. Additionally, studies in zebrafish and mouse show that BCO2 prevents carotenoid toxicity in vertebrate embryos [102, 104]. Accordingly, BCO2-deficient mice exhibit oxidative stress in tissues and reduced mitochondrial respiration rates [99, 114, 129]. In vitro studies in human hepatoma cells indicate that carotenoid accumulation increases ROS production in mitochondria [99, 104]. Oxidative stress is associated with several disease states, including cardiovascular and neurodegenerative disease, type 2 diabetes, cardiovascular disease, and cancer. Interestingly, human BCO2 is expressed as an oxidative stress-inducible gene [92]. This regulation may prevent excessive accumulation of carotenoids in human tissues and explain the observation that carotenoid levels decrease in chronic disease states.
3.2. Metabolism of long chain apocarotenoids
Trace amounts of apocarotenoids were detected in mammalian tissues using modern chromatography techniques and sensitive detection methods, such as mass spectrometry [133–139]. Emerging evidence implicates these compounds as biologically active modulators of physiological processes [103, 140–142]. For instance, pharmacological doses of apo-10’-lycopenoic acid can protect mouse liver from damage [143]. Furthermore, β-13-Apo-carotenone and certain lycopenoids act as retinoic acid receptor (RAR) antagonist and can antagonize vitamin A action [135, 141]. However, it is still not clear whether these apocarotenoids derive from the diet or are synthesized from dietary carotenoids by endogenous enzymatic pathways. A few studies provide evidence that β-10’-apo-carotenol is synthesized from carotenoids in a BCO2-dependent manner from β-carotene [113] and β-cryptoxanthin [97, 101]. Interestingly, it was shown that β-10’-apo-carotenol was transported and metabolized by proteins that were originally characterized in the context of retinoid metabolism [97, 101]. Though β-10’-apo-carotenol accumulated to some extent in mouse tissues upon carotenoid supplementation, no gross transcriptional changes were observed in the liver, fat, and lung under the applied conditions [144]. More recently, a study provided compelling evidence that BCO2 is involved in the production of a bona fide signal molecule that controls lipid transfer in the placenta [145]. While the production and occurrence of β-10’-apo-carotenol is now well-documented in mouse tissues, zeaxanthin, lutein, and lycopene-derived apocartenoid metabolites have yet not been detected [98, 99, 119]. Intervention studies in humans also failed to detect these compounds upon carotenoid supplementation [146, 147]. These observations indicate that apocarotenoid metabolites of carotenoids only exist transiently and are rapidly metabolized. As outlined above, both BCO1 and BCO2 can convert long chain apocarotenoids to smaller chemical entities [88, 97] by pathways that await chemical characterization. Thus, further research is warranted to clarify the metabolism and physiological roles of these molecules in mammalian biology.
3.3. Metabolism of Retinoids
The metabolism of vitamin A and its metabolites has been recently reviewed in several excellent articles [18, 20, 148, 149]. The formal first step in this metabolic pathway is the generation of retinaldehyde from carotenoid precursors by CCDs. Retinaldehyde can be either oxidized to all-trans-retinoic acid (RA) or reduced to all-trans-retinol. All-trans-retinol is converted to retinyl esters. The alcohol and ester form of vitamin A are the predominant retinoids in most tissues, including the intestine. In contrast, the acidic form of the vitamin exists only in very low (nanomolar) concentrations in most tissues [150]. The tissue levels of retinoic acid are tightly controlled, and even small amounts of this hormone-like compound are sufficient to elicit profound cellular responses through the activation of retinoic acid receptors (RARs) [151, 152]. RARs are transcription factors of the nuclear hormone receptor gene family, which in conjunction with retinoid X receptors (RXRs), control transcription by binding to conserved DNA motifs (retinoic acid response elements) in promoter regions of about 500 target genes in the human genome [153]. The amount of retinoic acid in tissues is tightly controlled throughout the mammalian life cycle by cytochrome P450-dependent hydroxylases [154–157].
The conversion of retinol to retinal is catalyzed by cytosolic alcohol dehydrogenases (ADHs) and microsomal retinol dehydrogenases (RDH) [21, 158]. The latter belong to the short chain dehydrogenase/reductase protein family (SDR). Adenine dinucleotide cofactors NAD(H) and NADP(H) are the redox carriers of these reactions. The enzymes bind their cofactors by a conserved sequence motif, the Rossmann-fold, which consists of six to seven parallel β-strands flanked by three to four α-helices [18, 159]. ADHs and RDHs use different catalytic mechanisms with either a zinc atom or a tyrosine in the active center, respectively. In enzymatic assays, ADHs and RDHs catalyze the bidirectional inter-conversion of retinol to retinal, dependent upon the oxidative state of their redox carriers. Under physiological conditions, the ratio of NAD/NADH is around 700 in the cytoplasm, and the ratio of NADP/NADPH is 0.005. Thus, enzymes using NAD as redox carriers catalyze the oxidation while enzymes using NADP catalyze reduction of retinoids.
Though ADHs and RDHs can use retinol and retinal as substrates, most of these enzymes can metabolize other alcohols including sterols [21]. Much of what is known about the physiological roles of different ADHs and RDHs stems from loss-of-function studies in experimental animals, such as knockout mice [21, 158, 160]. The important role of RDHs (RDH5, 8, 10, 11, 12, 13, and 14) in the visual cycle has just been reviewed [18]. For extra-ocular retinoid metabolism, RDH10 plays a critical role for the conversion of retinol to retinal in retinoic acid production [161]. Rdh10 mutations are embryonically lethal [161]. The expression of RDH10 in adult tissues, including the eyes, indicates that the RDH10 contributes to retinoid homeostasis throughout the mammalian life cycle [162]. Evidence from knockout mice suggests that RDH1 contributes to retinoid homeostasis in adult tissues as well [158, 163, 164]. Though not lethal, mutations in the corresponding gene are associated with increased hepatic retinyl ester stores and altered body fat mass [164]. Additionally, a critical retinaldehyde reductase (DHRS3) has been identified. DHRS3 reduces vitamin A aldehyde to retinol, and the levels of this protein are transcriptionally regulated by retinoic acid signaling [165]. Loss-of-function studies in mice indicate that DHRS3 is required to control embryonic retinoic acid levels by limiting the availability of retinal for oxidation to retinoic acid [165]. Because DHRS3 is expressed in adult tissue, the enzyme might be critical for tissue control of retinoic acid levels post-developmentally [162, 166]. Notably, DHRS3 expression is highly increased in ISX null mice with increased rates of intestinal retinaldehyde production and all-trans retinoid acid (RA) levels [167]. Recently, studies suggested RDH11 functions as an retinaldehyde reductase in vivo and is essential for the maintenance of all-trans–retinol steady-state levels in mouse liver and testis [168].
Esterification of retinol also seemingly contributes to the control of retinoid homeostasis in the gut [72]. Early studies demonstrated two independent enzymatic activities for the formation of retinyl esters, namely a lecithin-dependent acyl transfer facilitated by lecithin retinol acyltransferase (LRAT). LRAT is expressed in most tissues except adipocytes, and the acyl-CoA-dependent activity is present in a variety of mammalian tissues such as small intestine, liver, adipocytes, skin, testis, and retina. LRAT has been molecularly cloned from several vertebrate species, and the generation of LRAT-deficient mice confirmed its pivotal role in retinoid metabolism and vitamin A homeostasis [169–171]. LRAT is a 25 kDa protein that localizes to the endoplasmic reticulum and is an integral membrane protein with a single membrane-spanning helix localized at the C-terminus. On the basis of its amino acid sequence and predicted tertiary structure, LRAT is classified as a member of the ancestral NlpC/P60 thiol peptidase protein superfamily [172, 173]. Besides LRAT, the human genome encodes seven genes belonging to this protein family. Their general structural motif is reminiscent of papain-like proteases, and consists of a four-strand antiparallel β-sheet and three α-helices. The conserved catalytic residues Cys161, His60, and His72 define the active site. LRAT adopts an analogous catalytic strategy as thiol peptidases, whereby the deprotonated Cys161 serves as a nucleophile to attack the carbonyl carbon of an ester bond at the SN1 position of phosphatidylcholine, eventually leading to an acyl transfer and trans-esterification of retinol. LRAT-deficient mice lack liver and lung retinyl ester stores and are highly susceptible to vitamin A deficiency [72, 174]. Mice deficient for LRAT display highly elevated hepatic levels of CYP26A1, a major retinoic acid catabolizing enzyme, when maintained on vitamin A sufficient diet It is likely that in the absence of LRAT and retinol esterification, CYP26A1 is activated to metabolize retinoic acid in a compensatory mechanism [174].
In contrast to LRAT, which utilizes lecithin as an acyl-donor, acyl-CoA:retinol acyltransferase (ARAT) takes advantage of a pre-activated acyl-moiety coupled to coenzyme A. ARAT has never been purified or cloned, however, the existence of such vitamin A ester forming enzymes is supported by several lines of evidence. Studies of LRAT-deficient mice revealed that the intestinal absorption decreased to 10% of that in wild type animals after gavage of a physiological dose of the vitamin [72, 73]. Though the critical function of LRAT for intestinal vitamin A metabolism is evident, the consequences of mutations in the LRAT gene for provitamin A metabolism have yet not been investigated.
4. Quality control of vitamin A production in the intestine
Dissection of the carotenoid metabolic pathway using knockout mouse models clearly showed that BCO1 is required for β-carotene metabolism [101, 111], and that BCO2 is required for the metabolism of non-provitamin A carotenoids [99, 119]. Provitamin A carotenoids must contain at least one unsubstituted β-ionone ring and include the symmetric β-carotene as well as asymmetric β,ε-carotene (α-carotene) [175] and 3R-β,β-Caroten-3-ol (β-cryptoxanthin) [176]. In the test tube, recombinant BCO1 splits asymmetric provitamin A precursors into canonical and non-canonical retinaldehyde moieties [88, 91, 97]. Recombinant BCO2, also converts these compounds into various apocarotenoid products [89, 90, 92, 97, 101]. Hence, the enzymatic conversion of a single asymmetric provitamin A carotenoid could result in many apocarotenoid products which can be further metabolized to alcohols, acids, and geometric isomers. Surprisingly, the main apocarotenoids in mammals are retinoids, indicating that there must be a mechanism for their selective production.
Using β-cryptoxanthin as the model carotenoid, evidence has been provided that the enzymatic properties of BCO1 and BCO2 provide a quality control mechanism for vitamin A production [97, 101]. In this pathway, BCO2 removes the non-canonical ionone rings, followed by cleavage of the resulting long chain apocarotenoid into retinaldehyde by BCO1 (Figure 6A). This mechanism produces one molecule of retinoid from one molecule of carotenoid without non-canonical retinoid byproducts. In the mouse intestine, all metabolites of this pathway were identified [97]. Notably, a two-step mechanism for the conversion of the asymmetric provitamins 5,6-epoxy-β-carotene was already proposed in 1966 [177]. These authors proposed a removal of the non-canonical ionone ring site by eccentric cleavage which is then followed by a chain shortening of the β-apo-10’-carotenal by a β-oxidation-like mechanism.
Figure 6.
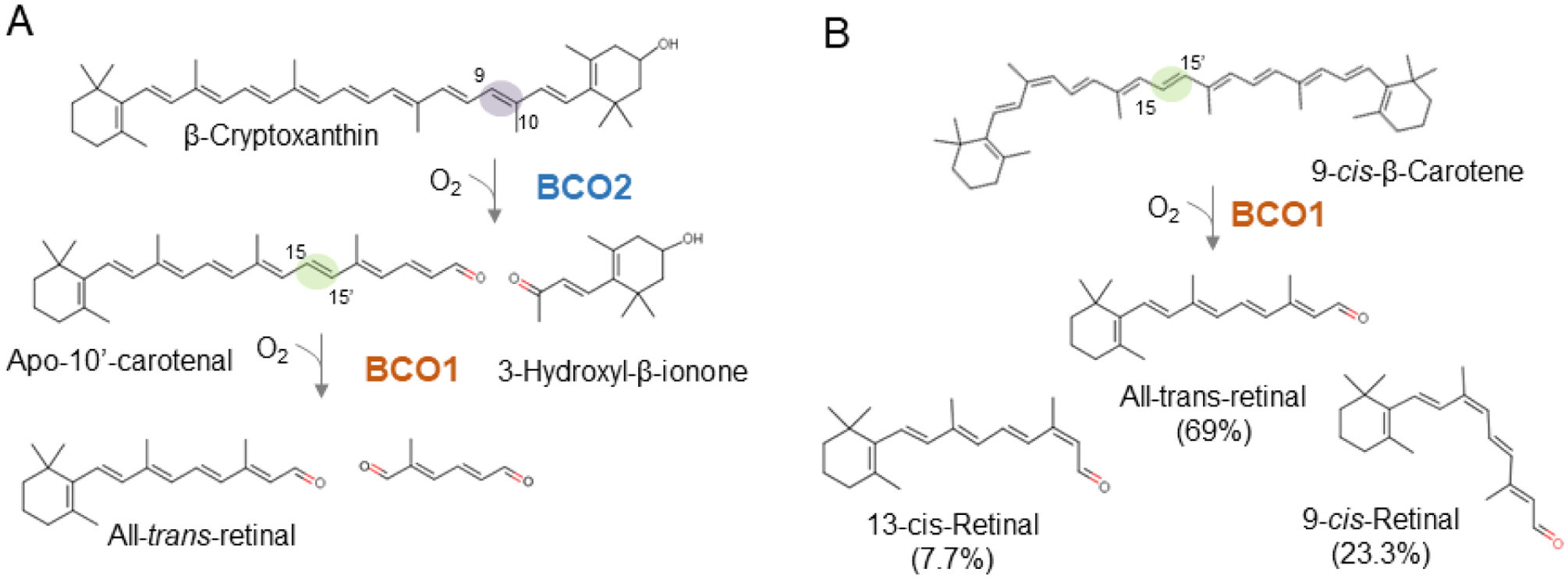
Conversion of asymmetric provitamin A carotenoids into all-trans-retinal. (A) β-cryptoxanthin is converted by a two-step mechanism with a β-10’-apocarotenal intermediate in a pathway that involves BCO1 and BCO2 enzymes. (B) 9-cis-β-carotene is converted by BCO1 into all-trans-, 13-cis, and 9-cis-retinal. Notably, the all-trans-diastereoisomer exist in significantly higher amount than the 9-cis-retinal diastereomer. Trace amounts of 13-cis-retinal are also present. The distribution of the different retinal-diastereomers indicates that BCO1 possesses an intrinsic isomerase activity.
The two-step enzymatic processing of β-cryptoxanthin is made possible by the different ring site selectivity of the two CCDs. The enzymes’ respective centric and eccentric cleavage mode complements this process [79] (Figure 6). Biochemical studies showed that recombinant BCO2 favors non-canonical ionone ring sites over canonical β-ionone ring sites, and that this mode of cleavage primarily leads to the production of β-10’-apocarotenal from asymmetric provitamins [97]. In the test tube, recombinant human BCO1 centrically cleaves β-cryptoxanthin into retinal and 3-hydroxy-retinal [88, 91]. However, 3-hydroxy-retinal was not produced from supplemented β-cryptoxanthin in the mouse intestine [97]. Previously, Lindqvist and Andersson [91] elegantly demonstrated that the catalytic efficiency of human BCO1 for β-crypoxanthin is very poor. This enzymatic property is consistent with the lack of β-cryptoxanthin cleavage in mice [97].
Determination of the structure of CCDs provide an explanation for the substrate selectivity of BCO1 and BCO2. Structural prediction indicates that the enzymes share the common CCD fold that consists of a rigid seven bladed propeller with a long, non-polar substrate tunnel running through the middle [178]. The mouth of this tunnel is surrounded by large hydrophobic patches that aid membrane interaction of the enzyme. In the active site in the tunnel, there is a Fe2+ cofactor, necessarily coordinated by four His residues, which activates oxygen for an otherwise spin-forbidden reaction. Once in the tunnel, the substrate is cleaved at this site [93]. In homology models for BCO1 and BCO2, the retinoid isomerase RPE65 was used as the template for structural prediction [179]. RPE65, a retinoid isomerase in the visual cycle [180–182], is the third family member of the mammalian CCD enzyme family. When comparing the predicted structures of BCO1 and BCO2, researchers reasoned that the diameter of the mouth of the BCO2 tunnel should be wider than that of BCO1 because BCO2 can accommodate bulky hydroxylated ionone ring sites in the substrate tunnel. Structural comparison identified two candidate amino acid residues at the mouth of the substrate tunnel. In BCO1, these amino acids narrow the entrance (Trp270 and a Leu168) and were well conserved in BCO1 across different species. Exchange of these amino acids with the corresponding amino acids of BCO2 (Trp270Phe and Leu168Gly) by site-directed mutagenesis produced a mutant BCO1 enzyme that is able to cleave zeaxanthin with two hydroxylated ionone rings [97]. Thus, a small change in an overall conserved fold provides the structural basis of the ring site specificity of BCO1 and BCO2. In the future, it remains to be clarified whether the described pathway for β-cryptoxanthin metabolism can be applied to the conversion of other asymmetric carotenoids with only one β-ionone ring site.
Distinct enzymatic properties of BCO1 and BCO2 also contribute to the metabolism of β-carotene diastereomers such as 9-cis-β-carotene (Figure 6). Symmetric cleavage of this dietary compound would result in one molecule of all-trans-retinal and one molecule of 9-cis-retinal. The early literature considered 9-cis-retinal as a precursor for 9-cis-retinoic acid that can bind RXRs and control transcriptional activities [183, 184]. Furthermore, 9-cis-retinal can bind to opsin to form isorhodopsin in the eyes [185] and can be used as a chromophore surrogate in treatment of blinding diseases [186]. Additionally, 9-cis-13,14-dihydroretinoic acid has been implicated as a natural endogenous ligand of this nuclear receptor [187]. However, there is presently no consensus as to whether these retinoid diasteromers are produced in meaningful amounts in mammals [24].
Studies in gerbils showed that 9-cis-β-carotene can be used as a dietary source of vitamin A but with only about 38% efficiency as compared to all-trans-β-carotene [188]. The lower efficiency of 9-cis-β-carotene in providing vitamin A might be partially explained by lower intestinal absorption of the 9-cis-geometric isomer when compared to the all-trans-β-carotene isomer [35, 189]. Notably, no increase of 9-cis-retinoid levels was observed in the mouse liver upon 9-cis-β-carotene supplementation [190]. Studies in humans brought up the proposal that the 9-cis-double bond of β-carotene is isomerized to an all-trans-double bond during absorption, and that this mechanism explains the lack of 9-cis-retinoid production [35]. After supplementation with [13C]-labeled 9-cis- β-carotene, the resulting [13C]-retinoids existed mainly in the all-trans-configuration [191]. Upon BCO1 cloning, biochemical studies with the recombinant murine enzyme showed that 9-cis-β-carotene is converted into the all-trans-retinal, 9-cis-retinal, and 13-cis-retinal stereoisomer in a molar ratio of 9:3:1 [190] (Figure 6). Notably, Jim Olson’s group previously obtained a similar result with cell free extracts of rat intestine [192], indicating that BCO1 possesses intrinsic isomerase activity. This finding contrasts with a reports suggesting that 9-cis- β-carotene is converted to 9-cis-retinal and all-trans-retinal in one to one molar ratio [193, 194]. It also contrasts the more recent proposal that 9-cis-β-carotene is not enzymatically converted by recombinant human BCO1 [88]. Isomerase activity has been also described in other CCD family members, including the insect vitamin A forming enzyme NinaB [195, 196] and the mammalian retinoid isomerase RPE65 [180–182]. Biochemical analysis with insect NinaB provided a reaction mechanism for a combined isomerization and oxidative cleavage reaction of double bonds of carotenoid substrates [83]. A similar mode of action of BCO1 would explain why 9-cis-β-carotene is mainly converted to the all-trans-retinoid diastereoisomer.
Taken together, with the large variety of carotenoids available in food, mammals require a reliable and robust mechanism for vitamin A production. Recent biochemical studies indicate that ring site selectivity and intrinsic isomerase activity of involved CCD enzymes play an important role in this process. These enzymatic properties of CCDs are essential for avoiding production of abberant apocarotenoid metabolites which can interfere with vitamin A-dependent physiological processes.
5. Regulation of carotenoid absorption and vitamin production
Several studies indicate that the vitamin A status of the host affects carotenoid absorption [197, 198] and metabolism [199] in the intestine. Early biochemical studies revealed that activity of the vitamin A forming BCO1 is higher in the intestine of vitamin A deficient rats [200]. Van Vliet et al. [201] showed that high doses of vitamin A or its precursor β-carotene decreased intestinal BCO1 enzyme activity, indicating that BCO1 activity is under negative feedback regulation of vitamin A. After the cloning of BCO1 gene, Bachmann and colleagues provided evidence that the negative feedback regulation occurs at the transcriptional level by showing that retinoic acid decreased the intestinal expression of Bco1 mRNA in the gut but not in the liver of chickens [202].
The protein that controls the intestinal Bco1 mRNA expression was discovered by a screening for gut specific transcription factors [203]. The intestine specific homeodomain transcription factor ISX is a 242-amino protein highly expressed in epithelial cells of the brush border membranes. ISX expression initiates early in development just prior to the transition from a gut endoderm to a columnar epithelium [203]. Isx−/− mice are grossly normal and healthy when raised on a standard vitamin A-rich mouse chow [203]. Analysis of their intestinal transcriptome suggested that ISX regulates intestinal expression of SR-B1 [203]. Seino and colleagues then showed that similar to SR-B1, intestinal expression of Bco1 is significantly increased in Isx−/− mice, suggesting that ISX is involved in the regulation of vitamin A production and absorption [204] (Figure 7). This conclusion was supported by the finding that intestinal expression of Isx is low in vitamin A deficiency and high in vitamin A sufficiency [204]. A mechanistic explanation for the vitamin A responsiveness of ISX expression was provided by the identification of an RAR binding element in the promoter of the human ISX gene [205]. It was further shown that RA can induce intestinal Isx expression, and that this induction is accompanied by a down-regulation of SR-B1 and Bco1 expression [205]. The increased expression of Bco1 and SR-B1 in the absence of ISX is driven by other transcription factors that control the activity of these genes [206, 207].
Figure 7.
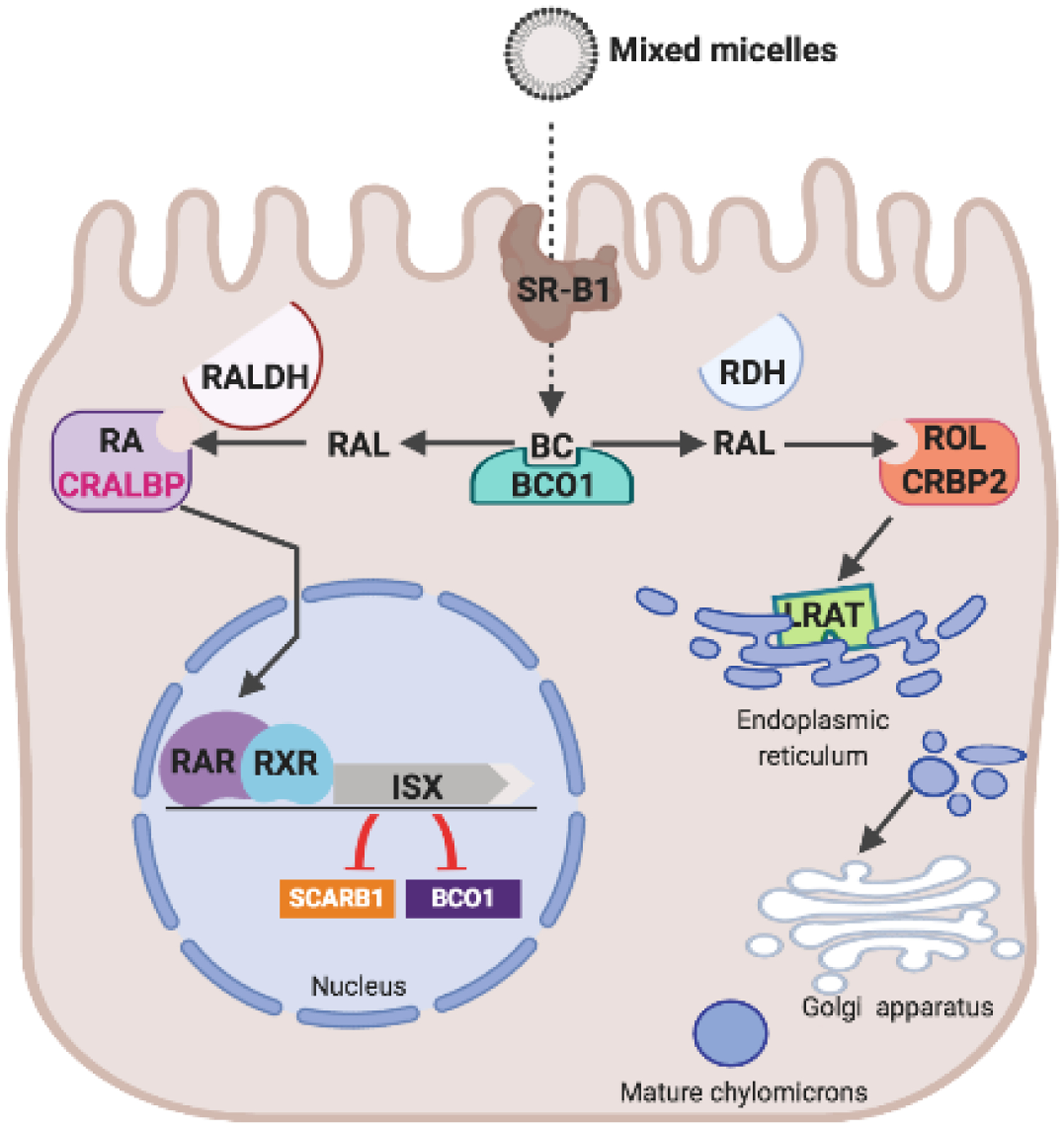
A diet-responsive regulatory network controls intestinal vitamin A production and carotenoid absorption. β-Carotene (BC) absorption is facilitated by scavenger receptor class B type 1 (SR-B1). The conversion of BC by β-carotene-15,15’-dioxygenase (BCO1) is an important branching point in retinoid metabolism. The primary product retinaldehyde (RAL) is reduced to retinol (ROL) by retinal dehydrogenases (RDH) in a process that involves cellular retinol binding protein (CRBP2). ROL is the converted to retinyl esters (RE) by lecithin: retinol acyl transferase (LRAT) and packed in chylomicrons for body distribution. Some RAL is also converted to retinoic acid (RA) by retinal dehydrogenase (RALDH). RA binds to retinoic acid receptors (RARs) that in conjunction with retinoid X receptors (RXR) regulate the expression of intestine specific homeobox (ISX) transcription factor. ISX is a transcriptional repressor of the genes encoding SCRAB1 and BCO1.
For ISX to control Bco1 and Scarb1 expression, it must interact with the promoter region of their genes. Using gel retardation assays, a 21-bp stretch about 1.3 kb upstream of the start ATG of the mouse Bco1 gene was identified as the ISX binding region [208]. ChIP experiments in human CaCo-2 cells indicated that human ISX also interacted with this binding motif. Furthermore, studies in CaCo-2 cells showed that the identified promoter element decreased expression of a luciferase reporter gene in the presence of ISX. A similar binding motif was later identified in the murine SR-B1 promoter [43]. Thus, ISX acts as a transcriptional repressor via binding a conserved DNA motif. Interestingly, the putative ISX binding site in the human BCO1 promoter region is afflicted by a common genetic polymorphism that associates with serum and tissue carotenoid levels [208–210].
The physiological basis of the ISX-dependent regulation of carotenoid absorption and vitamin A production was demonstrated in mice. ISX-deficient mice produce significantly higher amounts of vitamin A from dietary β-carotene than wild type mice [208]. Amounts of stored vitamin A in these animals well exceed that of mice fed with preformed vitamin A. The absolute increase of retinyl esters was most pronounced in the liver, where ~80% of postprandial retinyl esters are stored [20]. Peripheral tissues such as the lung and white adipose tissue also showed a significant increase in retinyl ester accumulation [208]. The vitamin A-dependent regulation of SR-B1 expression by ISX also explains the reported interactions between xanthophyll and β-carotene absorption. For instance, it has been observed that dietary vitamin A reduces xanthophyll absorption in chickens [211]. Studies in mice clearly demonstrated that BCO1 and β-carotene can affect intestinal SR-B1 expression and xanthophyll absorption [43].
In the intestine of vitamin A sufficient mice, ISX is expressed at higher levels in distal rather than in proximal parts [204]. The ISX target genes, encoding BCO1 and SR-B1, reveal a reverse pattern of expression [204]. Thus, in the vitamin A deficient state, the expression of BCO1 and SR-B1 spreads to more distal parts of the intestine. This pattern of expression significantly enlarges the surface of the intestine available for carotenoid and fat soluble vitamin absorption by SR-B1. Similarly, SR-B1 and BCO1 are highly expressed throughout the intestine in ISX-deficient mice. The consequences for intestinal vitamin A production on a diet rich in provitamin A are depicted in Figure 8. In wild type mice, retinol and retinyl esters content is low. In contrast, the levels of these compounds are significantly increased in all parts of the intestine with highest levels in the jejunal parts. Thus, the ISX dependent regulation does not only increase the expression levels of SR-B1 and BCO1 but also the surface area by which carotenoids and fat soluble vitamins can be acquired from the food matrix.
Figure 8.
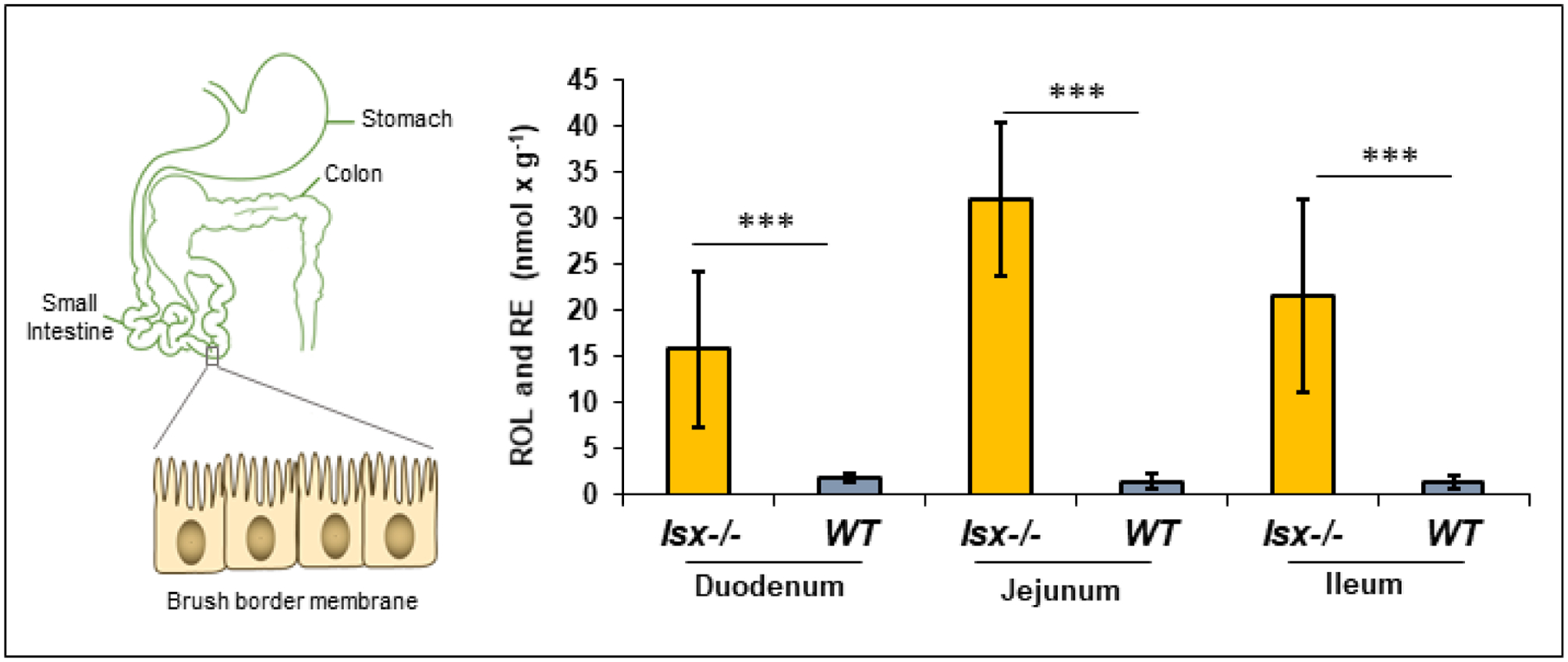
ISX controls vitamin A homeostasis in the intestine. Retinol (ROL) and retinyl ester (RE) levels in different parts of the intestine of Isx−/− and WT mice fed with a β-carotene-rich (25 mg/kg) diet. The values represent five individual animals per genotype. The p values (<0.001) in student T test are indicated by three asterisks.
There is an ongoing debate about the ‘right’ supplementation strategy for vitamin A, especially in early postnatal life [212]. Studies in animal models demonstrate that imbalances in vitamin A supply have significant effects on the offspring. For example, elevated maternal vitamin A affects lymphocyte differentiation in the developing gastrointestinal tract, and too much dietary vitamin A promotes aberrant immune responses and inflammation in the mouse intestine [167, 213, 214]. A recent study reported on the long-term epigenetic effects of preformed vitamin A on early neonatal life [215]. The analyses in rat pups revealed that even modest supplementation of preformed vitamin A during the suckling phase can impact methylation marks in developing white adipose. Physiologically, the amount of preformed vitamin A in breast milk is relatively constant and not affected by dietary fluctuations. The vitamin A-induced genomic imprinting of adipocytes of the pups is in keeping with the finding that neonatal vitamin A supplementation favors body fat gain later in life [216]. Notably, dietary β-carotene does not exert such effects on adipocytes in the same experimental setup [217], most likely because its absorption and bioconversion is tightly controlled by bodily demands via ISX (28, 29).
Taken together, the diet-responsive regulation of vitamin A production provides an elegant mechanism to cope with seasonal fluctuations in food supplies [218] and unbalanced diets [219]. Central to this regulation is the negative feedback mechanism regulated by the transcription factor ISX which controls the expression of the genes encoding the vitamin A forming enzyme BCO1 and the SR-B1 protein in epithelial cells of the intestine [43, 205, 208]. This regulation secures effective carotenoid absorption and vitamin A production when the dietary supply of these nutrients is limited and prevents excessive production of vitamin A when the supply with β-carotene is abundant [43, 208]. This regulation also affects the absorption of xanthophyll and other fat soluble vitamins [43, 208]. In the future, the specific roles of ISX in fat soluble vitamin A and lipid homeostasis needs further elucidation [24].
6. Cross talk between carotenoid metabolism and immunity
The intestine constitutes an effective barrier against toxins, antigens, and enteric flora. Hence, the intestinal mucosa harbors one of the largest populations of lymphocytes in the body. Several studies showed that sufficient intake of dietary vitamin A is critical for maintaining immune homeostasis at the intestinal barrier. The vitamin A metabolite retinoic acid induces the expression of gut-homing receptors on T and B cells, promotes conversion of regulatory T cells, and provokes T cell-independent IgA switches in naϊve B cells [220–222].
Accordingly, vitamin A deficiency compromises intestinal immunity and makes mice more susceptible to infection with bacterial pathogens [223, 224]. Vitamin A deficient mice also display a dramatic increase of interleukin-13 (IL-13)-producing type 2 innate lymphoid cells and display resistance to nematode infection [224]. However, too much dietary vitamin A may also compromise immunity. Increased intestinal retinoic acid in conjunction with IL-15 can act as an adjuvant to enhance secretion of IL-12 and IL-23 [213]. This scenario can promote inflammation [225, 226] and loss of tolerance to dietary antigens [213]. Thus, an increasing amount of studies indicate that imbalances in dietary vitamin A affect immune cell differentiation and function.
A recent study shows that ISX acts as a sensor for dietary vitamin A and has a profound impact on intestinal immune homeostasis [167] (Figure 8). ISX-deficient mice display an increase in the size and number of secondary lymphoid organs in the gut [167]. This increase can already be maternally imprinted in the offspring when ISX-deficient dams are maintained on β-carotene-rich food during pregnancy [167]. The immune phenotype of ISX-deficient mice can be likely attributed to alterations in intestinal carotenoid absorption and bioconversion. ISX-deficient mice show elevated intestinal retinoic acid levels when fed a β-carotene-rich diet. This retinoic acid synthesis can take place in dendritic cells of lymphoid organs [227] as well as in epithelial and stromal cells of the mucosal lymphoid tissues [228–230] where BCO1 is expressed [106]. In wild type mice, excessive retinoic acid production is prevented by the retinoic acid -dependent induction of ISX that regulates the absorption and bioconversion of β-carotene.
Interestingly, ISX-deficient mice develop pancreatic insulitis that is associated with decreased insulin production and systemic deregulation of glucose metabolism [167]. In this context, it is worth mentioning that failure to establish tolerance against commensal flora and food antigens has been implicated in the pathology of type 1 diabetes and other autoimmune diseases [231]. retinoic acid is an important signal for the differentiation of regulatory T-cells in the intestine [23, 232, 233], and the invasion of activated gut-derived T helper cells has been reported in pancreatic islet lesions of diabetic mice [234]. Thus, uncontrolled retinoic acid production in ISX-deficient mice most likely impairs T cell immunity and caused the diabetic phenotype of this mouse mutant.
Taken together, ISX plays an important role in the control of intestinal vitamin A homeostasis as required immunity and tolerance at the intestinal barrier. In vitamin A deficiency, the ISX-dependent regulation of vitamin A production guarantees that even minute amounts of dietary carotenoids are used for vitamin A production to maintain immunity. In periods of excess supplies with carotenoids, ISX prevents excessive vitamin A production that eventually can trigger inflammatory responses in the intestine. In future studies, the ISX-deficient mouse will be a versatile model to study the molecular details of these processes. A better understanding of the factors which control mucosal immune development and tolerance will aid nutritional intervention strategies to improve health in neonatal and adult life.
7. Transport and body distribution of carotenoids and retinoids
7.1. Formation of Chylomicrons
For transport and distribution in the body, intact carotenoids and retinyl esters are packaged into chylomicrons along with other dietary lipids [235] (Figure 10). Pre-chylomicron assembly starts at the endoplasmic reticulum and requires surface proteins apolipoprotein B48 (apoB-48) and apolipoprotein A-IV. For initiation of this assembly, amphipathic surface lipids (phospholipids and cholesterol) and neutral core lipids (triacylglycerol and cholesterol esters) must be present. Microsomal triglyceride transport protein (MTP) catalyzes the lipidation of apoB-48 in the inner leaflet of the ER [236]. The addition of triacylgycerol, cholesterol esters, and phospholipids to apoB-48 generates secretion-competent primordial chylomicrons. The assembled pre-chylomicrons translocate to the Golgi apparatus. In this compartment, pre-chylomicrons acquire apolipoprotein A1, and apoB-48 undergoes significant glycosylation. Mature chylomicrons are then secreted into the lymph and reach the circulation at the level of the subclavian vein. In the circulation, chylomiocrons interact with lipoprotein lipase of peripheral tissues [237]. The resulting chylomicron remnant is taken up by the liver for processing of its remaining lipid cargo. Retinyl esters are cleaved into fatty acid and retinol by a yet not molecularly identified retinyl ester hydrolase(s) and transported to stellate (Ito) cells. Here, they are re-esterified by LRAT and stored in lipid droplets [20, 72].
Figure 10.
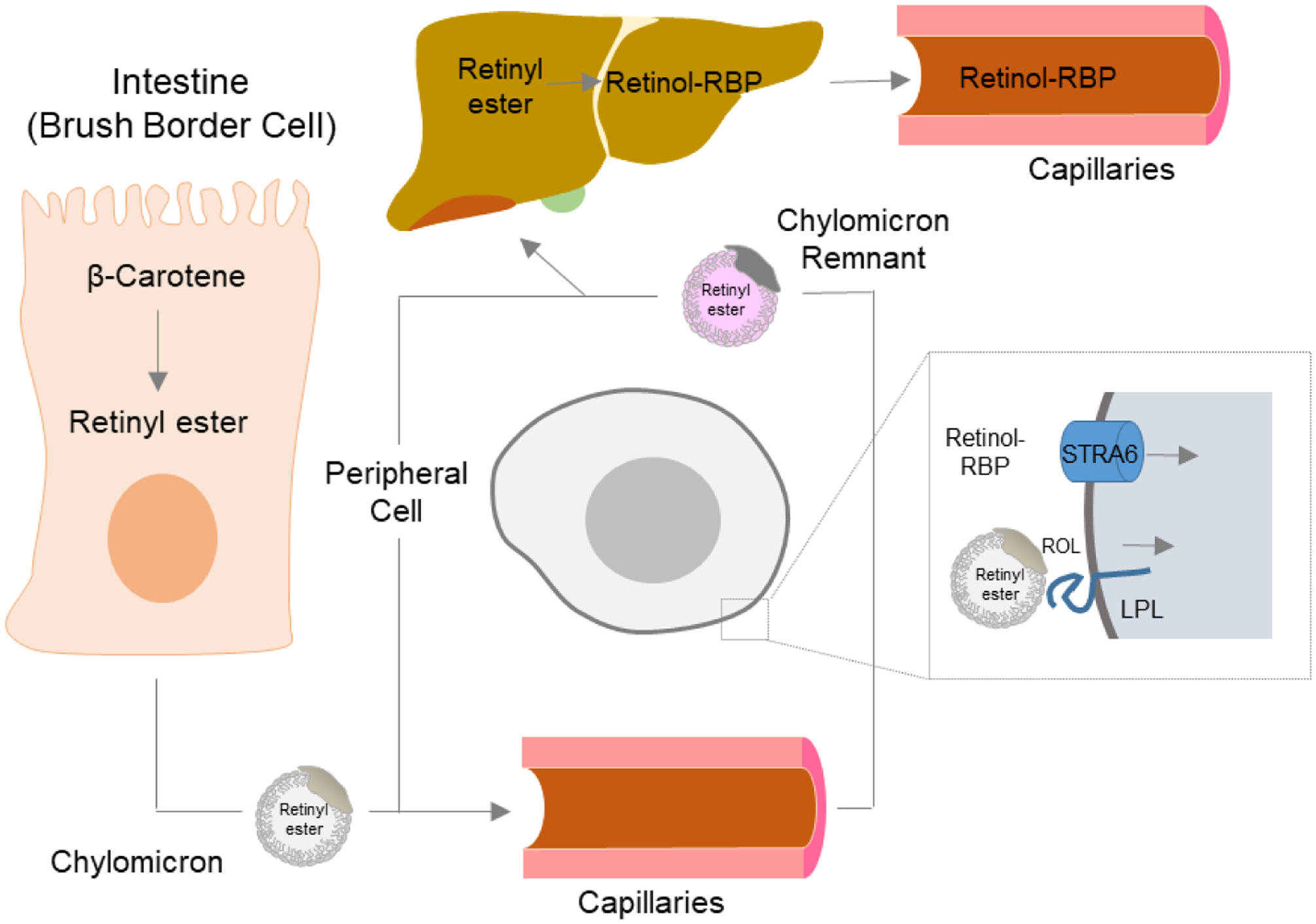
Transport of vitamin A throughout the body. Carotenoids such as β-carotene are absorbed in brush border cells in the intestine, where they are converted into retinyl esters (RE), packaged into chylomicrons, and released into the circulation. The RE in chylomicrons can either be delivered to peripheral cells and taken up in a liproprotein lipase-mediated process or be transported for receptor-mediated endocytosis and storage to the liver. In the liver, stored RE are converted back to retinol which binds to RBP. The retinol-RBP complex is released into the circulation. Vitamin A is taken up from holo-RBP in a STRA6-mediated transport process.
7. 2. Transport of stored vitamin A
Retinol is released from hepatocytes, bound to RBP4, into the circulation [20, 238] (Figure 10). This complex is named holo-RBP4 and is the major retinoid in the fasting circulation. Holo-RBP4 forms a complex with the 55 kDa transthyretin (TTR) homotetramer at a 2:1 molar ratio [239]. The cellular uptake of retinol from holo-RBP4 is mediated by a receptor localized at the cytoplasm membrane of target tissues [240–243]. This receptor is encoded by the Stra6 gene [244, 245]. Structural analysis revealed that STRA6 is assembled as an intricate dimer with 18 transmembrane helices (nine per protomer) and two long horizontal intramembrane helices interacting at the dimer core [246]. The receptor complex displays a lipophilic cleft to which holo-RBP4 binds with high affinity [244, 246]. Studies in cell lines indicate that STRA6 facilitates the bidirectional flux of retinol between RBP4 and cells [247, 248]. Cellular accumulation of retinol is driven by esterification by LRAT [247–250]. STRA6 allows for a higher flux of vitamin A into cells than is possible when this process is reliant on passive diffusion [251]. The evolutionary adaptation to store the fat-soluble vitamin and distribute it in a controlled manner provides a key pro-survival advantage to endure periods with little to no dietary supply of the essential nutrient [248, 249, 251–255]. In healthy subjects, blood holo-RBP4 levels are homeostatic and only decline under conditions of severe vitamin A deficiency (VAD) when body retinoid stores become exhausted [148, 256].
The pathological consequences of mutations in the STRA6, RBP4, or LRAT genes highlight the importance of vitamin A transport for ocular health. To date, 24 missense and nonsense mutations in the STRA6 gene have been identified to cause Matthew-Wood syndrome (MWS) [257–259]. MWS is characterized by severe bilateral microphthalmia, often in combination with pulmonary dysplasia, cardiac defects, and diaphragmatic hernia, among other anomalies and malformations [257]. The symptoms of MWS are consistent with the pivotal role of vitamin A in mammalian embryonic development [260] but are highly variable even within the same family ranging from isolated microphthalmia to fatal syndromes [261]. Similarly, mutations in the RBP4 gene can cause congenital eye malformations, including microphthalmia [262–264]. Again, even within the same family, large variations in the phenotypic manifestation of the RBP4 mutation exist [262]. It has been suggested that there is a maternal mode of inheritance of the phenotype, [262] and that the availability of dietary vitamin A impacts the severity of birth defects in affected patients [252, 262]. Mutations in the LRAT gene are associated with blindness in humans [265]. Currently, there are 11 identified mutations in the LRAT gene that cause Leber congenital amaurosis or retinitis pigmentosa in humans. Although effects of these mutations on LRAT’s activity have not been studied systematically, the severity likely depends on the degree of inactivation of the enzyme [149].
Mouse models for all major players of vitamin A transport have been established and used for an in-depth analysis of this important process: early in life, Stra6−/− mice largely display decreased ocular vitamin A levels and visual impairment including reduced ERG responses and shortened outer segments [252, 255]. Blood and other tissues such as the lungs, fat, and liver display normal vitamin A levels when mice are bred and raised on vitamin A-rich diets [252, 254]. A comparable phenotype has been reported for RBP4-deficient mice [238, 252]. These findings clearly indicate that retinyl ester in chylomicrons, when dietary supply with the vitamin is abundant, can compensate at least in part for the RBP4/STRA6 system. Genetic disruption of the Lrat gene renders mice blind because of the inability to acquire vitamin A and produce retinyl ester for visual chromophore production [169]. LRAT-deficient mice also lack major retinoid stores of the body and are highly susceptible to dietary vitamin A deficiency [72, 174].
7. 3. Transport of carotenoids to the periphery
For hepatic secretion, carotenoids are packaged into very-low-density lipoproteins (VLDL) and secreted into the blood. Carotenoids are not equally distributed among lipoproteins in the fasting circulation. The hydrophobic carotenes are found predominantly in low-density lipoproteins (LDL), while the more hydrophilic xanthophylls tend to be found in higher amounts in high-density lipoproteins (HDL) (Figure 11).
Figure 11.
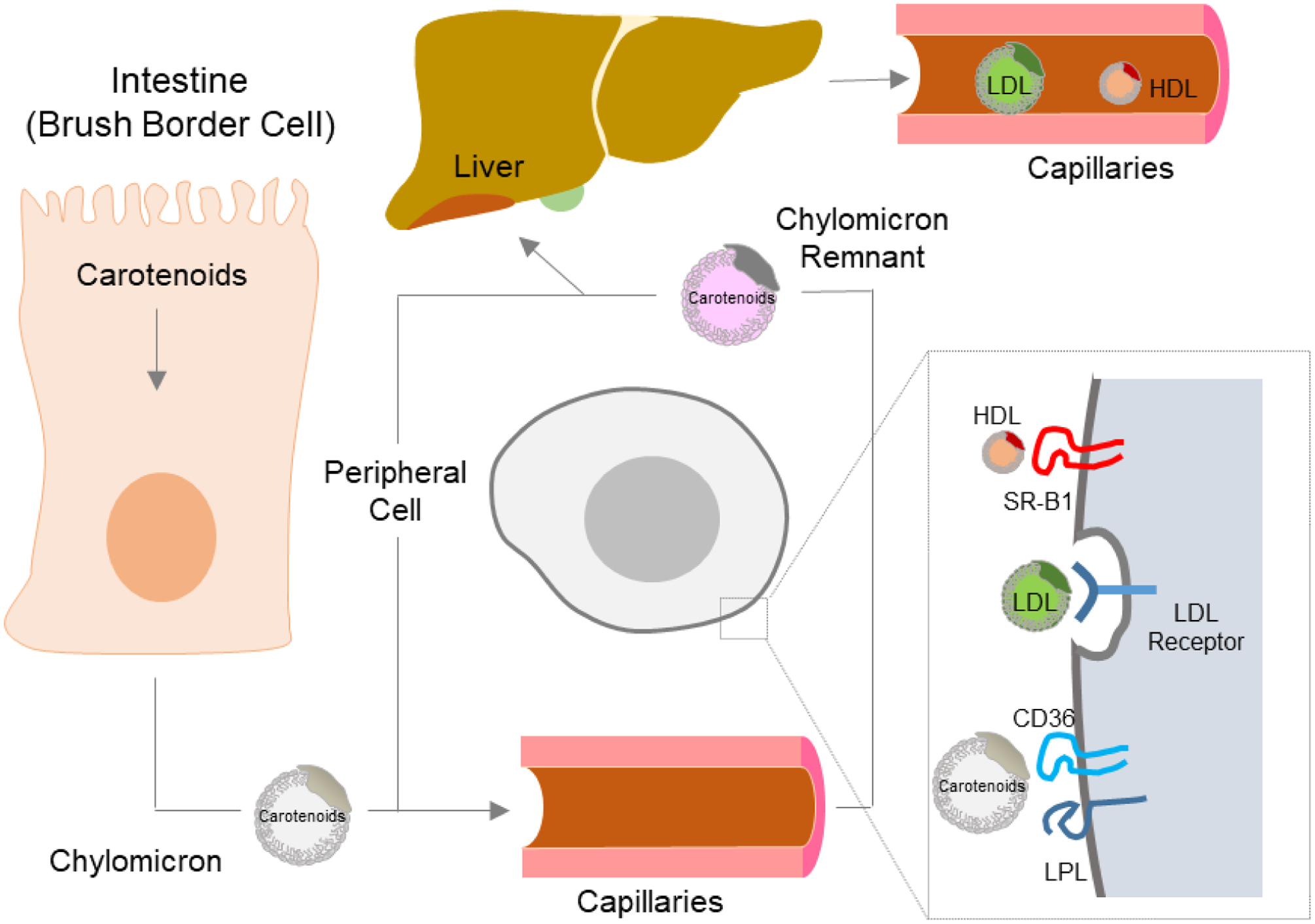
Transport of carotenoids throughout the body. Carotenoids are absorbed in brush border cells in the intestine, where they are packaged into chylomicrons, and released into the circulation. The carotenoids in chylomicrons can be delivered to peripheral cells and taken up in a lipoprotein lipase-mediated process. This uptake may also involve the scavenger receptor CD36. Carotenoids in chylomicron remnants are transported to the liver. In the liver, carotenoids are packaged into Very Low Density Lipoproteins (VLDL). VLDL might be converted to Low Density Lipoproteins (LDL) in the circulation. Carotenoids from LDL are taken up by LDL receptor mediated endocytosis into peripheral cells. Additionally, carotenoids in HDL are taken up by SR-B1 facilitated interactions between HDL and peripheral cells.
Genetic studies in Drosophila revealed that the Santa maria gene is required for uptake of carotenoids from circulating insect lipoproteins for visual chromophore production [67]. The Santa maria gene encodes a class two scavenger receptor that is required to render carotenoids available from lipoproteins to support photoreceptor development and vision. Studies in human retinal pigment epithelia cell lines indicate that SR-B1 facilitates uptake of carotenoids from HDL [266, 267]. In the circulation, zeaxanthin and lutein exist in higher concentration in HDL than in low density and very low density lipoproteins [114, 267]. The retinal pigment epithelium cells acquire fat soluble vitamins and other nutrients to deliver them to the adjacent neuronal retina to support photoreceptor function. This mechanism may play an important role in the uptake of macula pigments that are concentrated in the fovea of the macula region in the retina. β-Carotene in circulating LDL is absorbed by LDL receptor mediated endocytosis in to the retinal pigment epithelium [267] (Figure 11). Human RPE cells express relatively high levels of BCO1, the vitamin A forming enzyme, which converts β-carotene to retinaldehyde, and local vitamin A synthesis may contribute to ocular retinoid homeostasis [268].
Carotenoid content and composition is variable between tissues. The high concentration of carotenoids in the fovea region of the retina of the human eyes supports the idea of active uptake and retention mechanism for these lipids. An isoform of glutathione-S-transferase, GSTP1, and a steroidogenic acute regulatory domain protein, StARD3, were suggested as zeaxanthin and lutein binding proteins in the human retina [269, 270]. However, given the very high levels of xanthophylls in the macula, it is unlikely that these pigments predominantly exist in protein-bound form unless GSTP1 and StARD3 would be present in comparable amounts.
8. Implication for carotenoid intake levels and human health benefits
In 2000, the Food and Nutrition Board reviewed the evidence for role(s) of carotenoids as antioxidants and determined that specific functions beyond that related to provitamin A activity had not yet been sufficiently identified [271]. No dietary recommendations for individual non-provitamin A carotenoid intake exist, as it was thought that their absence from the diet causes no specific deficiency symptoms. Recent research challenges this statement and indicates urgent need for action. Studies in primates showed that deficiency of lutein and zeaxanthin can impair vision and make the retina susceptible to light damage [6, 13]. The outcome of the Age-Related Eye Disease Study 2 (AREDS2) study provided compelling evidence for beneficial effects of low dose zeaxanthin and lutein (2 and 10 mg) supplementation for eye health [272, 273]. While the importance of these carotenoids for eye health has been now acknowledged [11], intake of 10 mg/d have been advocated by several researchers [11, 274].
For provitamin A carotenoids, the Food and Nutrition Board of the National Research Council initially established conversion values of 2:1 for purified all-trans-β-carotene from supplements and 6:1 for dietary all-trans-β-carotene from food. Other dietary provitamin A carotenoids (mainly α-carotene and β-cryptoxanthin) were considered to have half the vitamin A activity of dietary all-trans-β-carotene. In 2001, the Food and Nutrition Board revised the original conversion values based on human trials revealing that the bioavailability of all-trans-β-carotene from foods was half of what was originally believed [275]. To decrease confusion in making the changes, a new term was coined: “retinol activity equivalent” (RAE = 1 μg all-trans-retinol). The RAE ratios of conversion for purified all-trans-β-carotene in oil, dietary all-trans-β-carotene, and other dietary provitamin A carotenoids were set at 2:1, 12:1, and 24:1, respectively.
In 2009, a consensus conference on β-carotene in Hohenheim, Germany, concluded that the mean dietary intake of β-carotene in Europe is in the range of 1.5–1.8 mg/d, and that provitamin A intake is <3 mg/d in most European countries. Since provitamin A carotenoids are the major source of vitamin A, it was recommended to increase the β-carotene consumption to 7 mg/d [276]. The role of carotenoids as vitamin A source will become even more important with the growing trend for vegetarian and vegan life styles.
A problem with recommendations for dietary carotenoid intake in health and disease is the variable plasma responses upon supplementation. As reviewed here, many host-related factors affect the bioavailability and bioconversion of carotenoids. It is now generally acknowledged that intestinal absorption of carotenoids is a saturable and protein-mediated process. Moreover, the conversion rate of carotenoids to retinoids is affected by vitamin A status and genetic makeup of the host [26]. The regulation of the bioconversion of carotenoids to vitamin A helps humans to adapt to the fluctuating dietary supplies of these lipids [19]. A study in Zambian children impressively reports the consequences of this regulation under conditions of excessive dietary vitamin A supplies [277]. During mango harvest, a period of high provitamin A supply, these children experienced hypercarotenodermia. This indicate that provitamin A carotenoid bioconversion to vitamin A by BCO1 is inhibited by negative feedback regulation. The hypercarotenodermia is elicited by the uptake of carotenoids by other receptors such as CD36, which are not regulated by ISX. The equivalency ratios for provitamin A carotenoids do not consider vitamin A status and one can assume that the ratio differs significantly between vitamin A sufficient and deficient populations.
The need to control intestinal carotenoid metabolism is also indicated by the critical role of vitamin A in immunity [23]. There is increasing body of evidence that excessive dietary levels of preformed vitamin A can promote inflammation and loss of tolerance at the intestinal barrier [213, 278]. A failure to establish tolerance against commensal flora and food antigens has been implicated in autoimmune disease such as celiac disease, Crohn’s disease, and type 1 diabetes [231]. These diseases are on the rise in many industrialized countries. Studies in mice revealed that mammalian genomes specifically devote a transcription factor to control intestinal vitamin A production from dietary provitamin A [167]. Accordingly, genetic variation in the ISX gene has been associated with inflammatory responses and proliferation of cells [279, 280]. Moreover, ISX gene has been associated with inflammatory bowel disease in genome wide studies [281]. At the state of the present knowledge it can be assumed that the supplementation with preformed vitamin A has a different impact on gastrointestinal immunity than the supplementation with provitamin A which is absorbed and converted to retinoids in a controlled fashion. More research is needed to better understand this crosstalk between diet and immunity in the intestine.
Genome wide association studies and candidate gene studies identified single base pair polymorphisms (SNPs) that affect carotenoid blood and tissue levels [26]. Significant SNPs were found in the genes encoding BCO1, SR-B1, and the transcription factor ISX [198, 209, 282, 283]. Interestingly, SNPs in BCO1 are associated with macula pigment density [284]. This finding is surprising at the first glance since xanthophyll is not a substrate for the vitamin A forming enzymes. The vitamin A-dependent regulation of SR-B1 expression in the intestine explains the interaction between xanthophyll accumulation and β-carotene metabolism. Interestingly, it was previously observed that dietary vitamin A reduces xanthophyll absorption in chickens [211]. Genetic makeup influences the conversion rate of provitamin A carotenoids and may constitute the basis for the low and high responder phenotypes described in the general European male population [285]. Additionally, several SNPs in proteins that affect lipid and lipoprotein metabolism have been associated with carotenoid blood levels [26]. The latter association indicates significant interactions between carotenoid and lipid metabolism. Such interactions have been recently demonstrated in mouse models [114]. Most of the genetic data in humans stem from the analyses of β-carotene, lycopene, and lutein fasting blood levels, but it is assumed that these genetic variations also modulate the bioavailability of the other carotenoids found in human blood and tissue [26].
Additionally, carotenoid absorption and bioconversion can be affected by several chronic disease state of the gut. Atrophic gastritis, a common condition of aging, results in insufficient gastric acid secretion and decreased carotenoid uptake, by disturbing the formation and absorption of mixed micelles [30]. Parasitic infections, prevalent in vitamin A deficient populations, can also have a dramatic negative effect on carotenoid absorption [286]. Pancreatic insufficiency in cystic fibrosis also impacts the absorption of carotenoids and their apocarotenoid metabolites [287]. Additionally, Crohn’s disease and celiac disease have been reported to affect carotenoid levels in blood and tissues of affected individuals [288]. Thus, specific dietary recommendation for carotenoid intake are required in disease states of the gastrointestinal tract.
In conclusion, an increasing body of evidence indicates critical roles of carotenoids in mammalian biology as antioxidants, blue light filters, and vitamin A precursor. Therefore, many nutritionists and clinicians advocate for recommendations for carotenoid intake. The establishment of such recommendations is based on the relationships between carotenoid intake, blood, and tissue concentrations. This relation is not linear and is influenced by many host and environmental factors. As reviewed here, studies in experimental animals provided a molecular framework for how diet and genetics affect the bioavailability and bioconversion of carotenoids. This framework provides a controlled production of vitamin A and prevents excessive accumulation of carotenoids and retinoids. An important aspect in the coming years will be to refine our knowledge about this framework and to determine its specifics in human physiology. Such knowledge will eventually lead to science-based recommendations for the intake of these essential lipids.
Figure 9.
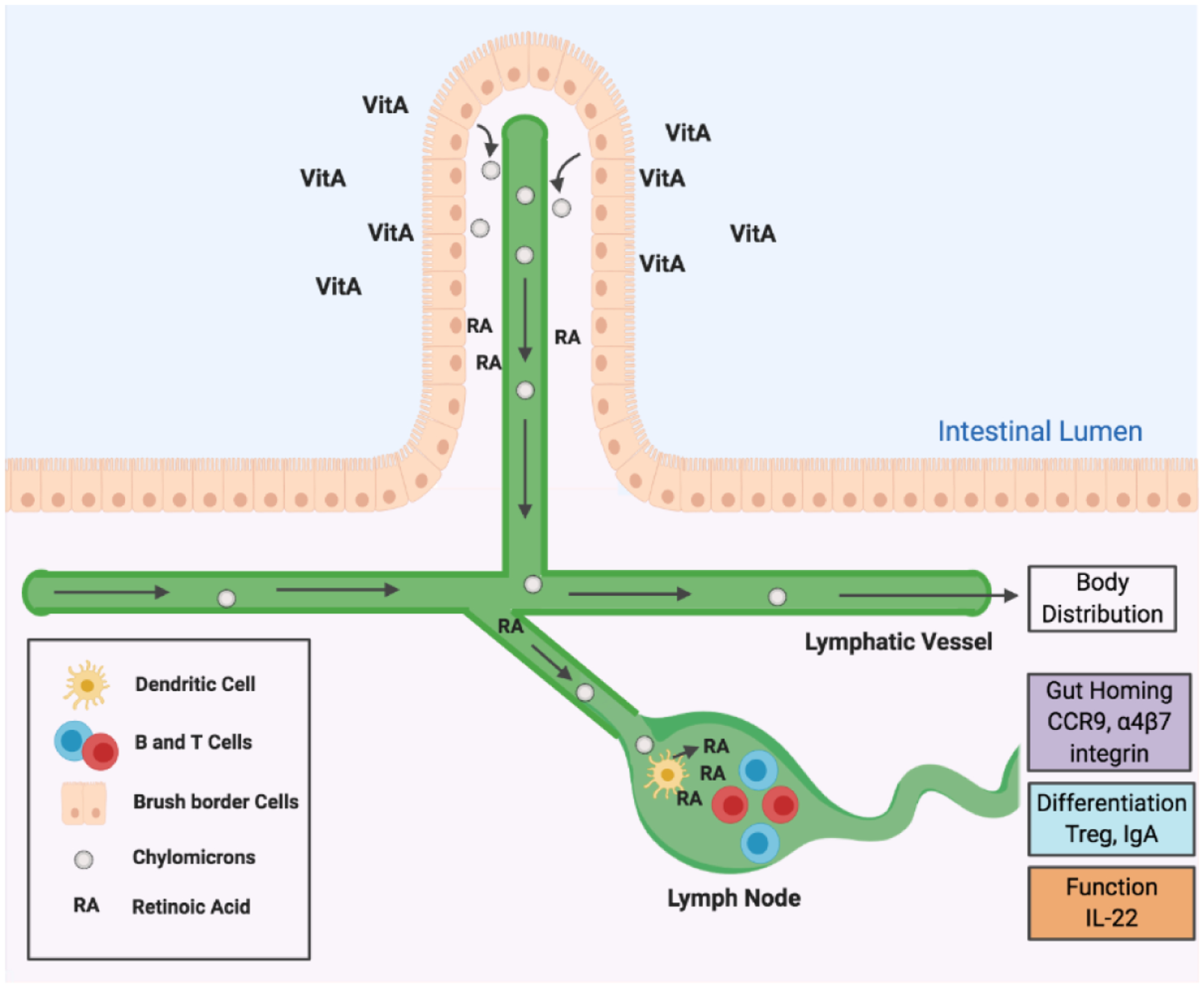
Intestinal vitamin A metabolism and immunity. Brush border cells acquire dietary vitamin A precursors from the intestinal lumen and convert them to retinyl esters. Retinyl esters are packaged into chylomicrons for body distribution. Brush border cells and dendritic cells in the lymphatic system can convert dietary vitamin A also into retinoic acid (RA). RA can promote gut homing, differentiation, and functions of B and T lymphocytes.
Acknowledgments
The authors receive support from National Institutes of Health Grants R01 EY20779 and R01 EY219781. This grant support was responsible for cited research that was carried out in the author’s laboratory and partially allowed for the writing of this review.
Abbreviations
- ADH
alcohol dehydrogenase
- BCO1
β-Carotene-15,15’-Dioxygenase
- BCO2
β-Carotene-9,10’-Dioxygenase
- CCD
Carotenoid Cleavage Dioxygenases
- CD36
Cluster of Differentiation 36
- Cyp26a1
Cytochrome P450, Family 26 A1
- Intestine
Specific Homeobox: Transcription factor
- NinaD
neither inactivation nor afterpotential mutant D
- LRAT
lecithin: retinol acyl transferase
- RPE65
Retinal Pigment Epithelium-Specific Protein 65kDa
- RBP4
Retinol Binding Protein 4
- SR-B1
Scavenger Receptor Class B, Member 1
- STRA6
Stimulated by Retinoic Acid 6
- RALDH1
Retinal Dehydrogenase 1 Family
- RAR
retinoic acid receptor
- RDH
retinol dehydrogenase
- RXR
retinoid X receptor
- SDR
short chain dehydrogenase/reductase
References
- [1].Moise AR, Al-Babili S, Wurtzel ET, Mechanistic aspects of carotenoid biosynthesis, Chemical reviews 114(1) (2014) 164–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Karrer HAP, Wehrli H, Wettstein A, Über die Konstitution des Lycopins und Cartoins, Hel. Chim. Acta 13 (1930) 1084. [Google Scholar]
- [3].Yabuzaki J, Carotenoids Database: structures, chemical fingerprints and distribution among organisms, Database : the journal of biological databases and curation 2017(1) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Krinsky NI, Landrum JT, Bone RA, Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye, Annu Rev Nutr 23 (2003) 171–201. [DOI] [PubMed] [Google Scholar]
- [5].Beatty S, Boulton M, Henson D, Koh HH, Murray IJ, Macular pigment and age related macular degeneration, Br J Ophthalmol 83(7) (1999) 867–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].SanGiovanni JP, Neuringer M, The putative role of lutein and zeaxanthin as protective agents against age-related macular degeneration: promise of molecular genetics for guiding mechanistic and translational research in the field, Am J Clin Nutr 96(5) (2012) 1223S–33S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Johnson EJ, A possible role for lutein and zeaxanthin in cognitive function in the elderly, Am J Clin Nutr 96(5) (2012) 1161S–5S. [DOI] [PubMed] [Google Scholar]
- [8].Semba RD, Lauretani F, Ferrucci L, Carotenoids as protection against sarcopenia in older adults, Arch Biochem Biophys 458(2) (2007) 141–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Krinsky NI, Johnson EJ, Carotenoid actions and their relation to health and disease, Molecular aspects of medicine 26(6) (2005) 459–516. [DOI] [PubMed] [Google Scholar]
- [10].Edge R, Truscott TG, Singlet Oxygen and Free Radical Reactions of Retinoids and Carotenoids-A Review, Antioxidants (Basel) 7(1) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bernstein PS, Li B, Vachali PP, Gorusupudi A, Shyam R, Henriksen BS, Nolan JM, Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease, Prog Retin Eye Res 50 (2016) 34–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Widjaja-Adhi MAK, Ramkumar S, von Lintig J, Protective role of carotenoids in the visual cycle, FASEB J (2018) fj201800467R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Barker FM 2nd, Snodderly DM, Johnson EJ, Schalch W, Koepcke W, Gerss J, Neuringer M, Nutritional manipulation of primate retinas, V: effects of lutein, zeaxanthin, and n-3 fatty acids on retinal sensitivity to blue-light-induced damage, Invest Ophthalmol Vis Sci 52(7) (2011) 3934–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hammond BR Jr., Fletcher LM, Elliott JG, Glare disability, photostress recovery, and chromatic contrast: relation to macular pigment and serum lutein and zeaxanthin, Invest Ophthalmol Vis Sci 54(1) (2013) 476–81. [DOI] [PubMed] [Google Scholar]
- [15].Kopcke W, Krutmann J, Protection from sunburn with beta-Carotene--a meta-analysis, Photochem Photobiol 84(2) (2008) 284–8. [DOI] [PubMed] [Google Scholar]
- [16].Wald G, Molecular basis of visual excitation, Science 162(850) (1968) 230–9. [DOI] [PubMed] [Google Scholar]
- [17].Chambon P, A decade of molecular biology of retinoic acid receptors, Faseb J 10(9) (1996) 940–54. [PubMed] [Google Scholar]
- [18].Kiser PD, Golczak M, Palczewski K, Chemistry of the retinoid (visual) cycle, Chemical reviews 114(1) (2014) 194–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].von Lintig J, Colors with functions: elucidating the biochemical and molecular basis of carotenoid metabolism, Annu Rev Nutr 30 (2010) 35–56. [DOI] [PubMed] [Google Scholar]
- [20].D’Ambrosio DN, Clugston RD, Blaner WS, Vitamin A Metabolism: An Update, Nutrients 3(1) (2011) 63–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kedishvili NY, Enzymology of retinoic acid biosynthesis and degradation, J Lipid Res 54(7) (2013) 1744–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rhinn M, Dollé P, Retinoic acid signalling during development, Development (Cambridge, England) 139(5) (2012) 843–58. [DOI] [PubMed] [Google Scholar]
- [23].Hall JA, Grainger JR, Spencer SP, Belkaid Y, The role of retinoic acid in tolerance and immunity, Immunity 35(1) (2011) 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Blaner WS, Vitamin A signaling and homeostasis in obesity, diabetes, and metabolic disorders, Pharmacol Ther (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Castenmiller JJ, West CE, Bioavailability and bioconversion of carotenoids, Annu Rev Nutr 18 (1998) 19–38. [DOI] [PubMed] [Google Scholar]
- [26].Borel P, Desmarchelier C, Bioavailability of Fat-Soluble Vitamins and Phytochemicals in Humans: Effects of Genetic Variation, Annu Rev Nutr 38 (2018) 69–96. [DOI] [PubMed] [Google Scholar]
- [27].Bohn T, Desmarchelier C, Dragsted LO, Nielsen CS, Stahl W, Ruhl R, Keijer J, Borel P, Host-related factors explaining interindividual variability of carotenoid bioavailability and tissue concentrations in humans, Mol Nutr Food Res 61(6) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Olson JA, Krinsky NI, Introduction: the colorful, fascinating world of the carotenoids: important physiologic modulators, Faseb J 9(15) (1995) 1547–50. [DOI] [PubMed] [Google Scholar]
- [29].Sy C, Gleize B, Dangles O, Landrier JF, Veyrat CC, Borel P, Effects of physicochemical properties of carotenoids on their bioaccessibility, intestinal cell uptake, and blood and tissue concentrations, Mol Nutr Food Res 56(9) (2012) 1385–97. [DOI] [PubMed] [Google Scholar]
- [30].Yeum KJ, Russell RM, Carotenoid bioavailability and bioconversion, Annu Rev Nutr 22 (2002) 483–504. [DOI] [PubMed] [Google Scholar]
- [31].Mariutti LRB, Mercadante AZ, Carotenoid esters analysis and occurrence: What do we know so far?, Arch Biochem Biophys 648 (2018) 36–43. [DOI] [PubMed] [Google Scholar]
- [32].Chitchumroonchokchai C, Failla ML, Hydrolysis of zeaxanthin esters by carboxyl ester lipase during digestion facilitates micellarization and uptake of the xanthophyll by Caco-2 human intestinal cells, J Nutr 136(3) (2006) 588–94. [DOI] [PubMed] [Google Scholar]
- [33].Breithaupt DE, Weller P, Wolters M, Hahn A, Plasma response to a single dose of dietary beta-cryptoxanthin esters from papaya (Carica papaya L.) or non-esterified beta-cryptoxanthin in adult human subjects: a comparative study, The British journal of nutrition 90(4) (2003) 795–801. [DOI] [PubMed] [Google Scholar]
- [34].Breithaupt DE, Weller P, Wolters M, Hahn A, Comparison of plasma responses in human subjects after the ingestion of 3R,3R’-zeaxanthin dipalmitate from wolfberry (Lycium barbarum) and non-esterified 3R,3R’-zeaxanthin using chiral high-performance liquid chromatography, The British journal of nutrition 91(5) (2004) 707–13. [DOI] [PubMed] [Google Scholar]
- [35].Stahl W, Schwarz W, Sies H, Human serum concentrations of all-trans beta- and alpha-carotene but not 9-cis beta-carotene increase upon ingestion of a natural isomer mixture obtained from Dunaliella salina (Betatene), J Nutr 123(5) (1993) 847–51. [DOI] [PubMed] [Google Scholar]
- [36].Boileau AC, Merchen NR, Wasson K, Atkinson CA, Erdman JW Jr., Cis-lycopene is more bioavailable than trans-lycopene in vitro and in vivo in lymph-cannulated ferrets, J Nutr 129(6) (1999) 1176–81. [DOI] [PubMed] [Google Scholar]
- [37].Brown MJ, Ferruzzi MG, Nguyen ML, Cooper DA, Eldridge AL, Schwartz SJ, White WS, Carotenoid bioavailability is higher from salads ingested with full-fat than with fat-reduced salad dressings as measured with electrochemical detection, Am J Clin Nutr 80(2) (2004) 396–403. [DOI] [PubMed] [Google Scholar]
- [38].Tanumihardjo SA, Li J, Dosti MP, Lutein absorption is facilitated with cosupplementation of ascorbic acid in young adults, J Am Diet Assoc 105(1) (2005) 114–8. [DOI] [PubMed] [Google Scholar]
- [39].During A, Hussain MM, Morel DW, Harrison EH, Carotenoid uptake and secretion by CaCo-2 cells: beta-carotene isomer selectivity and carotenoid interactions, J Lipid Res 43(7) (2002) 1086–95. [DOI] [PubMed] [Google Scholar]
- [40].van Bennekum A, Werder M, Thuahnai ST, Han CH, Duong P, Williams DL, Wettstein P, Schulthess G, Phillips MC, Hauser H, Class B scavenger receptor-mediated intestinal absorption of dietary beta-carotene and cholesterol, Biochemistry 44(11) (2005) 4517–25. [DOI] [PubMed] [Google Scholar]
- [41].Goncalves A, Margier M, Roi S, Collet X, Niot I, Goupy P, Caris-Veyrat C, Reboul E, Intestinal scavenger receptors are involved in vitamin K1 absorption, J Biol Chem 289(44) (2014) 30743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Reboul E, Klein A, Bietrix F, Gleize B, Malezet-Desmoulins C, Schneider M, Margotat A, Lagrost L, Collet X, Borel P, Scavenger receptor class B type I (SR-BI) is involved in vitamin E transport across the enterocyte, J Biol Chem 281(8) (2006) 4739–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Widjaja-Adhi MA, Lobo GP, Golczak M, Von Lintig J, A genetic dissection of intestinal fat-soluble vitamin and carotenoid absorption, Human molecular genetics 24(11) (2015) 3206–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kiefer C, Sumser E, Wernet MF, Von Lintig J, A class B scavenger receptor mediates the cellular uptake of carotenoids in Drosophila, Proc Natl Acad Sci 99(16) (2002) 10581–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Voolstra O, Kiefer C, Hoehne M, Welsch R, Vogt K, von Lintig J, The Drosophila class B scavenger receptor NinaD-I is a cell surface receptor mediating carotenoid transport for visual chromophore synthesis, Biochemistry 45(45) (2006) 13429–37. [DOI] [PubMed] [Google Scholar]
- [46].During A, Dawson HD, Harrison EH, Carotenoid transport is decreased and expression of the lipid transporters SR-BI, NPC1L1, and ABCA1 is downregulated in Caco-2 cells treated with ezetimibe, J Nutr 135(10) (2005) 2305–12. [DOI] [PubMed] [Google Scholar]
- [47].Reboul E, Abou L, Mikail C, Ghiringhelli O, Andre M, Portugal H, Jourdheuil-Rahmani D, Amiot MJ, Lairon D, Borel P, Lutein transport by Caco-2 TC-7 cells occurs partly by a facilitated process involving the scavenger receptor class B type I (SR-BI), Biochem J 387(Pt 2) (2005) 455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Borel P, Lietz G, Goncalves A, Szabo de Edelenyi F, Lecompte S, Curtis P, Goumidi L, Caslake MJ, Miles EA, Packard C, Calder PC, Mathers JC, Minihane AM, Tourniaire F, Kesse-Guyot E, Galan P, Hercberg S, Breidenassel C, Gonzalez Gross M, Moussa M, Meirhaeghe A, Reboul E, CD36 and SR-BI are involved in cellular uptake of provitamin A carotenoids by Caco-2 and HEK cells, and some of their genetic variants are associated with plasma concentrations of these micronutrients in humans, J Nutr 143(4) (2013) 448–56. [DOI] [PubMed] [Google Scholar]
- [49].Shyam R, Vachali P, Gorusupudi A, Nelson K, Bernstein PS, All three human scavenger receptor class B proteins can bind and transport all three macular xanthophyll carotenoids, Arch Biochem Biophys 634 (2017) 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Lobo MV, Huerta L, Ruiz-Velasco N, Teixeiro E, de la Cueva P, Celdran A, Martin-Hidalgo A, Vega MA, Bragado R, Localization of the lipid receptors CD36 and CLA-1/SR-BI in the human gastrointestinal tract: towards the identification of receptors mediating the intestinal absorption of dietary lipids, J Histochem Cytochem 49(10) (2001) 1253–60. [DOI] [PubMed] [Google Scholar]
- [51].Neculai D, Schwake M, Ravichandran M, Zunke F, Collins RF, Peters J, Neculai M, Plumb J, Loppnau P, Pizarro JC, Seitova A, Trimble WS, Saftig P, Grinstein S, Dhe-Paganon S, Structure of LIMP-2 provides functional insights with implications for SR-BI and CD36, Nature 504(7478) (2013) 172–6. [DOI] [PubMed] [Google Scholar]
- [52].Rodrigueza WV, Thuahnai ST, Temel RE, Lund-Katz S, Phillips MC, Williams DL, Mechanism of scavenger receptor class B type I-mediated selective uptake of cholesteryl esters from high density lipoprotein to adrenal cells, J Biol Chem 274(29) (1999) 20344–50. [DOI] [PubMed] [Google Scholar]
- [53].Yu M, Lau TY, Carr SA, Krieger M, Contributions of a disulfide bond and a reduced cysteine side chain to the intrinsic activity of the high-density lipoprotein receptor SR-BI, Biochemistry 51(50) (2012) 10044–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Febbraio M, Silverstein RL, CD36: implications in cardiovascular disease, Int J Biochem Cell Biol 39(11) (2007) 2012–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Abumrad NA, el-Maghrabi MR, Amri EZ, Lopez E, Grimaldi PA, Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36, J Biol Chem 268(24) (1993) 17665–8. [PubMed] [Google Scholar]
- [56].Greenwalt DE, Lipsky RH, Ockenhouse CF, Ikeda H, Tandon NN, Jamieson GA, Membrane glycoprotein CD36: a review of its roles in adherence, signal transduction, and transfusion medicine, Blood 80(5) (1992) 1105–15. [PubMed] [Google Scholar]
- [57].Goldberg IJ, Eckel RH, Abumrad NA, Regulation of fatty acid uptake into tissues: lipoprotein lipase- and CD36-mediated pathways, J Lipid Res 50 Suppl (2009) S86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Cifarelli V, Abumrad NA, Intestinal CD36 and Other Key Proteins of Lipid Utilization: Role in Absorption and Gut Homeostasis, Comprehensive Physiology 8(2) (2018) 493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Connelly MA, Klein SM, Azhar S, Abumrad NA, Williams DL, Comparison of class B scavenger receptors, CD36 and scavenger receptor BI (SR-BI), shows that both receptors mediate high density lipoprotein-cholesteryl ester selective uptake but SR-BI exhibits a unique enhancement of cholesteryl ester uptake, J Biol Chem 274(1) (1999) 41–7. [DOI] [PubMed] [Google Scholar]
- [60].Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M, Identification of scavenger receptor SR-BI as a high density lipoprotein receptor, Science 271(5248) (1996) 518–20. [DOI] [PubMed] [Google Scholar]
- [61].Acton SL, Scherer PE, Lodish HF, Krieger M, Expression cloning of SR-BI, a CD36-related class B scavenger receptor, J Biol Chem 269(33) (1994) 21003–9. [PubMed] [Google Scholar]
- [62].Werder M, Han CH, Wehrli E, Bimmler D, Schulthess G, Hauser H, Role of scavenger receptors SR-BI and CD36 in selective sterol uptake in the small intestine, Biochemistry 40(38) (2001) 11643–50. [DOI] [PubMed] [Google Scholar]
- [63].Bura KS, Lord C, Marshall S, McDaniel A, Thomas G, Warrier M, Zhang J, Davis MA, Sawyer JK, Shah R, Wilson MD, Dikkers A, Tietge UJ, Collet X, Rudel LL, Temel RE, Brown JM, Intestinal SR-BI does not impact cholesterol absorption or transintestinal cholesterol efflux in mice, J Lipid Res 54(6) (2013) 1567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Davis HR Jr., Basso F, Hoos LM, Tetzloff G, Lally SM, Altmann SW, Cholesterol homeostasis by the intestine: lessons from Niemann-Pick C1 Like 1 [NPC1L1), Atherosclerosis. Supplements 9(2) (2008) 77–81. [DOI] [PubMed] [Google Scholar]
- [65].Nauli AM, Nassir F, Zheng S, Yang Q, Lo CM, Vonlehmden SB, Lee D, Jandacek RJ, Abumrad NA, Tso P, CD36 is important for chylomicron formation and secretion and may mediate cholesterol uptake in the proximal intestine, Gastroenterology 131(4) (2006) 1197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Stephenson RS, O’Tousa J, Scavarda NJ, Randall LL, Pak WL, Drosophila mutants with reduced rhodopsin content, Symp Soc Exp Biol 36 (1983) 477–501. [PubMed] [Google Scholar]
- [67].Wang T, Jiao Y, Montell C, Dissection of the pathway required for generation of vitamin A and for Drosophila phototransduction, J Cell Biol 177(2) (2007) 305–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Sakudoh T, Iizuka T, Narukawa J, Sezutsu H, Kobayashi I, Kuwazaki S, Banno Y, Kitamura A, Sugiyama H, Takada N, Fujimoto H, Kadono-Okuda K, Mita K, Tamura T, Yamamoto K, Tsuchida K, A CD36-related transmembrane protein is coordinated with an intracellular lipid-binding protein in selective carotenoid transport for cocoon coloration, J Biol Chem 285(10) (2010) 7739–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Sakudoh T, Kuwazaki S, Iizuka T, Narukawa J, Yamamoto K, Uchino K, Sezutsu H, Banno Y, Tsuchida K, CD36 homolog divergence is responsible for the selectivity of carotenoid species migration to the silk gland of the silkworm Bombyx mori, J Lipid Res 54(2) (2013) 482–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Toomey MB, Lopes RJ, Araujo PM, Johnson JD, Gazda MA, Afonso S, Mota PG, Koch RE, Hill GE, Corbo JC, Carneiro M, High-density lipoprotein receptor SCARB1 is required for carotenoid coloration in birds, Proc Natl Acad Sci U S A 114(20) (2017) 5219–5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Preuss SE, Bartels T, Schmidt V, Krautwald-Junghanns ME, Vitamin A requirements of alipochromatic (‘recessive-white’) and coloured canaries (Serinus canaria) during the breeding season, The Veterinary record 160(1) (2007) 14–9. [DOI] [PubMed] [Google Scholar]
- [72].O’Byrne SM, Wongsiriroj N, Libien J, Vogel S, Goldberg IJ, Baehr W, Palczewski K, Blaner WS, Retinoid absorption and storage is impaired in mice lacking lecithin:retinol acyltransferase (LRAT), J Biol Chem 280(42) (2005) 35647–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].O’Byrne SM, Blaner WS, Retinol and retinyl esters: biochemistry and physiology, Journal of lipid research 54(7) (2013) 1731–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Moore T, Vitamin A and carotene VI. The conversion of carotene to vitamin A in vivo, Biochem. J 24 (1930) 692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Olson JA, Hayaishi O, The enzymatic cleavage of beta-carotene into vitamin A by soluble enzymes of rat liver and intestine, Proc Natl Acad Sci U S A 54(5) (1965) 1364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Goodman DS, Huang HS, Biosynthesis of Vitamin a with Rat Intestinal Enzymes, Science 149 (1965) 879–80. [DOI] [PubMed] [Google Scholar]
- [77].Tang GW, Wang XD, Russell RM, Krinsky NI, Characterization of beta-apo-13-carotenone and beta-apo-14’-carotenal as enzymatic products of the excentric cleavage of beta-carotene, Biochemistry 30(41) (1991) 9829–34. [DOI] [PubMed] [Google Scholar]
- [78].Wang XD, Tang GW, Fox JG, Krinsky NI, Russell RM, Enzymatic conversion of beta-carotene into beta-apo-carotenals and retinoids by human, monkey, ferret, and rat tissues, Arch Biochem Biophys 285(1) (1991) 8–16. [DOI] [PubMed] [Google Scholar]
- [79].Kiefer C, Hessel S, Lampert JM, Vogt K, Lederer MO, Breithaupt DE, von Lintig J, Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A, J Biol Chem 276(17) (2001) 14110–6. [DOI] [PubMed] [Google Scholar]
- [80].von Lintig J, Vogt K, Filling the gap in vitamin A research. Molecular identification of an enzyme cleaving beta-carotene to retinal, J Biol Chem 275(16) (2000) 11915–20. [DOI] [PubMed] [Google Scholar]
- [81].Sui X, Golczak M, Zhang J, Kleinberg KA, von Lintig J, Palczewski K, Kiser PD, Utilization of Dioxygen by Carotenoid Cleavage Oxygenases, J Biol Chem 290(51) (2015) 30212–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Dela Sena C, Riedl KM, Narayanasamy S, Curley RW Jr., Schwartz SJ, Harrison EH, The human enzyme that converts dietary provitamin a carotenoids to vitamin a is a dioxygenase, J Biol Chem 289(19) (2014) 13661–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Babino D, Golczak M, Kiser PD, Wyss A, Palczewski K, von Lintig J, The Biochemical Basis of Vitamin A3 Production in Arthropod Vision, ACS Chem Biol 11(4) (2016) 1049–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Wyss A, Wirtz G, Woggon W, Brugger R, Wyss M, Friedlein A, Bachmann H, Hunziker W, Cloning and expression of beta,beta-carotene 15,15’-dioxygenase, Biochem Biophys Res Commun 271(2) (2000) 334–6. [DOI] [PubMed] [Google Scholar]
- [85].Wyss A, Wirtz GM, Woggon WD, Brugger R, Wyss M, Friedlein A, Riss G, Bachmann H, Hunziker W, Expression pattern and localization of beta,beta-carotene 15,15’-dioxygenase in different tissues, Biochem J 354(Pt 3) (2001) 521–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Redmond TM, Gentleman S, Duncan T, Yu S, Wiggert B, Gantt E, Cunningham FX Jr., Identification, expression, and substrate specificity of a mammalian beta-carotene 15,15’-dioxygenase, J Biol Chem 276(9) (2001) 6560–5. [DOI] [PubMed] [Google Scholar]
- [87].Paik J, During A, Harrison EH, Mendelsohn CL, Lai K, Blaner WS, Expression and characterization of a murine enzyme able to cleave beta-carotene. The formation of retinoids, J Biol Chem 276(34) (2001) 32160–8. [DOI] [PubMed] [Google Scholar]
- [88].dela Sena C, Narayanasamy S, Riedl KM, Curley RW Jr., Schwartz SJ, Harrison EH, Substrate specificity of purified recombinant human beta-carotene 15,15’-oxygenase (BCO1), J Biol Chem 288(52) (2013) 37094–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Dela Sena C, Sun J, Narayanasamy S, Riedl KM, Yuan Y, Curley RW Jr., Schwartz SJ, Harrison EH, Substrate Specificity of Purified Recombinant Chicken beta-Carotene 9’,10’-Oxygenase (BCO2), J Biol Chem 291(28) (2016) 14609–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Hu KQ, Liu C, Ernst H, Krinsky NI, Russell RM, Wang XD, The biochemical characterization of ferret carotene-9’,10’-monooxygenase catalyzing cleavage of carotenoids in vitro and in vivo, J Biol Chem 281(28) (2006) 19327–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Lindqvist A, Andersson S, Biochemical properties of purified recombinant human beta-carotene 15,15’-monooxygenase, J Biol Chem 277(26) (2002) 23942–8. [DOI] [PubMed] [Google Scholar]
- [92].Babino D, Palczewski G, Widjaja-Adhi MA, Kiser PD, Golczak M, von Lintig J, Characterization of the Role of beta-Carotene 9,10-Dioxygenase in Macular Pigment Metabolism, J Biol Chem 290(41) (2015) 24844–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Sui X, Kiser PD, Lintig J, Palczewski K, Structural basis of carotenoid cleavage: From bacteria to mammals, Arch Biochem Biophys 539(2) (2013) 203–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Giuliano G, Al-Babili S, von Lintig J, Carotenoid oxygenases: cleave it or leave it, Trends Plant Sci 8(4) (2003) 145–9. [DOI] [PubMed] [Google Scholar]
- [95].Moise AR, von Lintig J, Palczewski K, Related enzymes solve evolutionarily recurrent problems in the metabolism of carotenoids, Trends Plant Sci 10(4) (2005) 178–86. [DOI] [PubMed] [Google Scholar]
- [96].von Lintig J, Kiser PD, Golczak M, Palczewski K, The biochemical and structural basis for trans-to-cis isomerization of retinoids in the chemistry of vision, Trends Biochem Sci 35(7) (2010) 400–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Kelly ME, Ramkumar S, Sun W, Colon Ortiz C, Kiser PD, Golczak M, von Lintig J, The Biochemical Basis of Vitamin A Production from the Asymmetric Carotenoid beta-Cryptoxanthin, ACS Chem Biol 13(8) (2018) 2121–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Mein JR, Dolnikowski GG, Ernst H, Russell RM, Wang XD, Enzymatic formation of apo-carotenoids from the xanthophyll carotenoids lutein, zeaxanthin and beta-cryptoxanthin by ferret carotene-9’,10’-monooxygenase, Arch Biochem Biophys 506(1) (2010) 109–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Amengual J, Lobo GP, Golczak M, Li HN, Klimova T, Hoppel CL, Wyss A, Palczewski K, von Lintig J, A mitochondrial enzyme degrades carotenoids and protects against oxidative stress, FASEB J 25(3) (2011) 948–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Kowatz T, Babino D, Kiser P, Palczewski K, von Lintig J, Characterization of human beta,beta-carotene-15,15’-monooxygenase (BCMO1) as a soluble monomeric enzyme, Arch Biochem Biophys 539(2) (2013) 214–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Amengual J, Widjaja-Adhi MA, Rodriguez-Santiago S, Hessel S, Golczak M, Palczewski K, von Lintig J, Two carotenoid oxygenases contribute to mammalian provitamin A metabolism, J Biol Chem 288(47) (2013) 34081–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Spiegler E, Kim YK, Hoyos B, Narayanasamy S, Jiang H, Savio N, Curley RW Jr., Harrison EH, Hammerling U, Quadro L, beta-apo-10’-carotenoids support normal embryonic development during vitamin A deficiency, Scientific reports 8(1) (2018) 8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Aydemir G, Kasiri Y, Bartok EM, Birta E, Frohlich K, Bohm V, Mihaly J, Ruhl R, Lycopene supplementation restores vitamin A deficiency in mice and possesses thereby partial pro-vitamin A activity transmitted via RAR signaling, Mol Nutr Food Res 60(11) (2016) 2413–2420. [DOI] [PubMed] [Google Scholar]
- [104].Lobo GP, Isken A, Hoff S, Babino D, von Lintig J, BCDO2 acts as a carotenoid scavenger and gatekeeper for the mitochondrial apoptotic pathway, Development 139(16) (2012) 2966–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Toomey MB, Lind O, Frederiksen R, Curley RW Jr., Riedl KM, Wilby D, Schwartz SJ, Witt CC, Harrison EH, Roberts NW, Vorobyev M, McGraw KJ, Cornwall MC, Kelber A, Corbo JC, Complementary shifts in photoreceptor spectral tuning unlock the full adaptive potential of ultraviolet vision in birds, eLife 5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Raghuvanshi S, Reed V, Blaner WS, Harrison EH, Cellular localization of beta-carotene 15,15’ oxygenase-1 (BCO1) and beta-carotene 9’,10’ oxygenase-2 (BCO2) in rat liver and intestine, Arch Biochem Biophys 572 (2015) 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Lindqvist A, He YG, Andersson S, Cell type-specific expression of beta-carotene 9’,10’-monooxygenase in human tissues, J Histochem Cytochem 53(11) (2005) 1403–12. [DOI] [PubMed] [Google Scholar]
- [108].Lindqvist A, Sharvill J, Sharvill DE, Andersson S, Loss-of-function mutation in carotenoid 15,15’-monooxygenase identified in a patient with hypercarotenemia and hypovitaminosis A, J Nutr 137(11) (2007) 2346–50. [DOI] [PubMed] [Google Scholar]
- [109].Palczewski G, Amengual J, Hoppel CL, von Lintig J, Evidence for compartmentalization of mammalian carotenoid metabolism, FASEB J 28(10) (2014) 4457–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Ge Z, Johnson JD, Cobine PA, McGraw KJ, Garcia R, Hill GE, High Concentrations of Ketocarotenoids in Hepatic Mitochondria of Haemorhous mexicanus, Physiological and biochemical zoology : PBZ 88(4) (2015) 444–50. [DOI] [PubMed] [Google Scholar]
- [111].Hessel S, Eichinger A, Isken A, Amengual J, Hunzelmann S, Hoeller U, Elste V, Hunziker W, Goralczyk R, Oberhauser V, von Lintig J, Wyss A, CMO1 deficiency abolishes vitamin A production from beta-carotene and alters lipid metabolism in mice, J Biol Chem 282(46) (2007) 33553–61. [DOI] [PubMed] [Google Scholar]
- [112].Fierce Y, de Morais Vieira M, Piantedosi R, Wyss A, Blaner WS, Paik J, In vitro and in vivo characterization of retinoid synthesis from beta-carotene, Arch Biochem Biophys 472(2) (2008) 126–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Amengual J, Gouranton E, van Helden YG, Hessel S, Ribot J, Kramer E, Kiec-Wilk B, Razny U, Lietz G, Wyss A, Dembinska-Kiec A, Palou A, Keijer J, Landrier JF, Bonet ML, von Lintig J, Beta-Carotene Reduces Body Adiposity of Mice via BCMO1, PloS one 6(6) (2011) e20644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Palczewski G, Widjaja-Adhi MA, Amengual J, Golczak M, von Lintig J, Genetic dissection in a mouse model reveals interactions between carotenoids and lipid metabolism, J Lipid Res 57(9) (2016) 1684–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Nagao A, Maoka T, Ono H, Kotake-Nara E, Kobayashi M, Tomita M, A 3-hydroxy beta-end group in xanthophylls is preferentially oxidized to a 3-oxo epsilon-end group in mammals, J Lipid Res 56(2) (2015) 449–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Yonekura L, Kobayashi M, Terasaki M, Nagao A, Keto-carotenoids are the major metabolites of dietary lutein and fucoxanthin in mouse tissues, J Nutr 140(10) 1824–31. [DOI] [PubMed] [Google Scholar]
- [117].Li B, Vachali PP, Gorusupudi A, Shen Z, Sharifzadeh H, Besch BM, Nelson K, Horvath MM, Frederick JM, Baehr W, Bernstein PS, Inactivity of human beta,beta-carotene-9’,10’-dioxygenase (BCO2) underlies retinal accumulation of the human macular carotenoid pigment, Proc Natl Acad Sci U S A 111(28) (2014) 10173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Bhosale P, Larson AJ, Frederick JM, Southwick K, Thulin CD, Bernstein PS, Identification and characterization of a Pi isoform of glutathione S-transferase (GSTP1) as a zeaxanthin-binding protein in the macula of the human eye, J Biol Chem 279(47) (2004) 49447–54. [DOI] [PubMed] [Google Scholar]
- [119].Ford NA, Clinton SK, von Lintig J, Wyss A, Erdman JW Jr., Loss of carotene-9’,10’-monooxygenase expression increases serum and tissue lycopene concentrations in lycopene-fed mice, J Nutr 140(12) (2010) 2134–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Eriksson J, Larson G, Gunnarsson U, Bed’hom B, Tixier-Boichard M, Stromstedt L, Wright D, Jungerius A, Vereijken A, Randi E, Jensen P, Andersson L, Identification of the yellow skin gene reveals a hybrid origin of the domestic chicken, PLoS Genet 4(2) (2008) e1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Andrade P, Pinho C, Perez I.d.L.G., Afonso S, Brejcha J, Rubin CJ, Wallerman O, Pereira P, Sabatino SJ, Bellati A, Pellitteri-Rosa D, Bosakova Z, Bunikis I, Carretero MA, Feiner N, Marsik P, Pauperio F, Salvi D, Soler L, While GM, Uller T, Font E, Andersson L, Carneiro M, Regulatory changes in pterin and carotenoid genes underlie balanced color polymorphisms in the wall lizard, Proc Natl Acad Sci U S A 116(12) (2019) 5633–5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Berry SD, Davis SR, Beattie EM, Thomas NL, Burrett AK, Ward HE, Stanfield AM, Biswas M, Ankersmit-Udy AE, Oxley PE, Barnett JL, Pearson JF, van der Does Y, Macgibbon AH, Spelman RJ, Lehnert K, Snell RG, Mutation in bovine beta-carotene oxygenase 2 affects milk color, Genetics 182(3) (2009) 923–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Tian R, Pitchford WS, Morris CA, Cullen NG, Bottema CD, Genetic variation in the beta, beta-carotene-9’, 10’-dioxygenase gene and association with fat colour in bovine adipose tissue and milk, Anim Genet 41 (2009) 253–259. [DOI] [PubMed] [Google Scholar]
- [124].Vage DI, Boman IA, A nonsense mutation in the beta-carotene oxygenase 2 (BCO2) gene is tightly associated with accumulation of carotenoids in adipose tissue in sheep (Ovis aries), BMC genetics 11 (2010) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Strychalski J, Brym P, Czarnik U, Gugolek A, A novel AAT-deletion mutation in the coding sequence of the BCO2 gene in yellow-fat rabbits, Journal of applied genetics 56(4) (2015) 535–537. [DOI] [PubMed] [Google Scholar]
- [126].Fallahshahroudi A, Sorato E, Altimiras J, Jensen P, The Domestic BCO2 Allele Buffers Low-Carotenoid Diets in Chickens: Possible Fitness Increase Through Species Hybridization, Genetics 212(4) (2019) 1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Poliakov E, Soucy J, Gentleman S, Rogozin IB, Redmond TM, Phylogenetic analysis of the metazoan carotenoid oxygenase superfamily: a new ancestral gene assemblage of BCO-like (BCOL) proteins, Scientific reports 7(1) (2017) 13192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Voigt AP, Whitmore SS, Flamme-Wiese MJ, Riker M, Wiley LA, Tucker BA, Stone EM, Mullins RF, Scheetz TE, Molecular characterization of foveal versus peripheral human retina by single-cell RNA sequencing, Exp Eye Res (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Wu L, Guo X, Wang W, Medeiros DM, Clarke SL, Lucas EA, Smith BJ, Lin D, Molecular aspects of beta, beta-carotene-9’, 10’-oxygenase 2 in carotenoid metabolism and diseases, Exp Biol Med (Maywood) 241(17) (2016) 1879–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Wu L, Guo X, Lyu Y, Clarke SL, Lucas EA, Smith BJ, Hildebrand D, Wang W, Medeiros DM, Shen X, Lin D, Targeted Metabolomics Reveals Abnormal Hepatic Energy Metabolism by Depletion of beta-Carotene Oxygenase 2 in Mice, Scientific reports 7(1) (2017) 14624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Guo X, Wu L, Lyu Y, Chowanadisai W, Clarke SL, Lucas EA, Smith BJ, He H, Wang W, Medeiros DM, Lin D, Ablation of beta,beta-carotene-9’,10’-oxygenase 2 remodels the hypothalamic metabolome leading to metabolic disorders in mice, J Nutr Biochem 46 (2017) 74–82. [DOI] [PubMed] [Google Scholar]
- [132].Wu L, Guo X, Hartson SD, Davis MA, He H, Medeiros DM, Wang W, Clarke SL, Lucas EA, Smith BJ, von Lintig J, Lin D, Lack of beta, beta-carotene-9’, 10’-oxygenase 2 leads to hepatic mitochondrial dysfunction and cellular oxidative stress in mice, Mol Nutr Food Res 61(5) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Eroglu A, Harrison EH, Carotenoid metabolism in mammals, including man: formation, occurrence, and function of apocarotenoids, J Lipid Res 54(7) (2013) 1719–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Eroglu A, Hruszkewycz DP, Curley RW Jr., Harrison EH, The eccentric cleavage product of beta-carotene, beta-apo-13-carotenone, functions as an antagonist of RXRalpha, Arch Biochem Biophys 504(1) (2010) 11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Eroglu A, Hruszkewycz DP, dela Sena C, Narayanasamy S, Riedl KM, Kopec RE, Schwartz SJ, Curley RW Jr., Harrison EH, Naturally occurring eccentric cleavage products of provitamin A beta-carotene function as antagonists of retinoic acid receptors, J Biol Chem 287(19) (2012) 15886–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Ford NA, Elsen AC, Zuniga K, Lindshield BL, Erdman JW Jr., Lycopene and apo-12’-lycopenal reduce cell proliferation and alter cell cycle progression in human prostate cancer cells, Nutr Cancer 63(2) (2011) 256–63. [DOI] [PubMed] [Google Scholar]
- [137].Lindshield BL, Canene-Adams K, Erdman JW Jr., Lycopenoids: are lycopene metabolites bioactive?, Arch Biochem Biophys 458(2) (2007) 136–40. [DOI] [PubMed] [Google Scholar]
- [138].Caris-Veyrat C, Garcia AL, Reynaud E, Lucas R, Aydemir G, Ruhl R, A Review About Lycopene-Induced Nuclear Hormone Receptor Signalling in Inflammation and Lipid Metabolism via still Unknown Endogenous Apo-10 -Lycopenoids, Int J Vitam Nutr Res 86(1–2) (2016) 62–70. [DOI] [PubMed] [Google Scholar]
- [139].Aydemir G, Kasiri Y, Birta E, Beke G, Garcia AL, Bartok EM, Ruhl R, Lycopene-derived bioactive retinoic acid receptors/retinoid-X receptors-activating metabolites may be relevant for lycopene’s anti-cancer potential, Mol Nutr Food Res 57(5) (2013) 739–47. [DOI] [PubMed] [Google Scholar]
- [140].Harrison EH, Quadro L, Apocarotenoids: Emerging Roles in Mammals, Annu Rev Nutr 38 (2018) 153–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Narayanasamy S, Sun J, Pavlovicz RE, Eroglu A, Rush CE, Sunkel BD, Li C, Harrison EH, Curley RW Jr., Synthesis of apo-13- and apo-15-lycopenoids, cleavage products of lycopene that are retinoic acid antagonists, J Lipid Res 58(5) (2017) 1021–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Lian F, Smith DE, Ernst H, Russell RM, Wang XD, Apo-10’-lycopenoic acid inhibits lung cancer cell growth in vitro, and suppresses lung tumorigenesis in the A/J mouse model in vivo, Carcinogenesis 28(7) (2007) 1567–74. [DOI] [PubMed] [Google Scholar]
- [143].Ip BC, Hu KQ, Liu C, Smith DE, Obin MS, Ausman LM, Wang XD, Lycopene metabolite, apo-10’-lycopenoic acid, inhibits diethylnitrosamine-initiated, high fat diet-promoted hepatic inflammation and tumorigenesis in mice, Cancer Prev Res (Phila) 6(12) (2013) 1304–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].van Helden YG, Godschalk RW, von Lintig J, Lietz G, Landrier JF, Bonet ML, van Schooten FJ, Keijer J, Gene expression response of mouse lung, liver and white adipose tissue to beta-carotene supplementation, knockout of Bcmo1 and sex, Mol Nutr Food Res 55(10) (2011) 1466–74. [DOI] [PubMed] [Google Scholar]
- [145].Costabile BK, Kim YK, Iqbal J, Zuccaro MV, Wassef L, Narayanasamy S, Curley RW Jr., Harrison EH, Hussain MM, Quadro L, beta-Apo-10’-carotenoids Modulate Placental Microsomal Triglyceride Transfer Protein Expression and Function to Optimize Transport of Intact beta-Carotene to the Embryo, J Biol Chem 291(35) (2016) 18525–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Kopec RE, Caris-Veyrat C, Nowicki M, Gleize B, Carail M, Borel P, Production of asymmetric oxidative metabolites of [13C]-beta-carotene during digestion in the gastrointestinal lumen of healthy men, Am J Clin Nutr 108(4) (2018) 803–813. [DOI] [PubMed] [Google Scholar]
- [147].Cooperstone JL, Novotny JA, Riedl KM, Cichon MJ, Francis DM, Curley RW Jr., Schwartz SJ, Harrison EH, Limited appearance of apocarotenoids is observed in plasma after consumption of tomato juices: a randomized human clinical trial, Am J Clin Nutr 108(4) (2018) 784–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Blaner WS, Li Y, Brun PJ, Yuen JJ, Lee SA, Clugston RD, Vitamin A Absorption, Storage and Mobilization, Sub-cellular biochemistry 81 (2016) 95–125. [DOI] [PubMed] [Google Scholar]
- [149].Chelstowska S, Widjaja-Adhi MA, Silvaroli JA, Golczak M, Molecular Basis for Vitamin A Uptake and Storage in Vertebrates, Nutrients 8(11) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Kane MA, Folias AE, Pingitore A, Perri M, Obrochta KM, Krois CR, Cione E, Ryu JY, Napoli JL, Identification of 9-cis-retinoic acid as a pancreas-specific autacoid that attenuates glucose-stimulated insulin secretion, Proc Natl Acad Sci U S A 107(50) (2010) 21884–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Giguere V, Ong ES, Segui P, Evans RM, Identification of a receptor for the morphogen retinoic acid, Nature 330(6149) (1987) 624–9. [DOI] [PubMed] [Google Scholar]
- [152].Petkovich M, Brand NJ, Krust A, Chambon P, A human retinoic acid receptor which belongs to the family of nuclear receptors, Nature 330(6147) (1987) 444–50. [DOI] [PubMed] [Google Scholar]
- [153].Balmer JE, Blomhoff R, Gene expression regulation by retinoic acid, J Lipid Res 43(11) (2002) 1773–808. [DOI] [PubMed] [Google Scholar]
- [154].Abu-Abed S, Dolle P, Metzger D, Beckett B, Chambon P, Petkovich M, The retinoic acid-metabolizing enzyme, CYP26A1, is essential for normal hindbrain patterning, vertebral identity, and development of posterior structures, Genes Dev 15(2) (2001) 226–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Abu-Abed SS, Beckett BR, Chiba H, Chithalen JV, Jones G, Metzger D, Chambon P, Petkovich M, Mouse P450RAI (CYP26) expression and retinoic acid-inducible retinoic acid metabolism in F9 cells are regulated by retinoic acid receptor gamma and retinoid X receptor alpha, J Biol Chem 273(4) (1998) 2409–15. [DOI] [PubMed] [Google Scholar]
- [156].Niederreither K, Abu-Abed S, Schuhbaur B, Petkovich M, Chambon P, Dolle P, Genetic evidence that oxidative derivatives of retinoic acid are not involved in retinoid signaling during mouse development, Nat Genet 31(1) (2002) 84–8. [DOI] [PubMed] [Google Scholar]
- [157].Zhong G, Hogarth C, Snyder JM, Palau L, Topping T, Huang W, Czuba LC, LaFrance J, Ghiaur G, Isoherranen N, The retinoic acid hydroxylase Cyp26a1 has minor effects on postnatal vitamin A homeostasis, but is required for exogenous atRA clearance, J Biol Chem 294(29) (2019) 11166–11179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Napoli JL, Physiological insights into all-trans-retinoic acid biosynthesis, Biochim Biophys Acta 1821(1) (2012) 152–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Hofmann L, Tsybovsky Y, Alexander NS, Babino D, Leung NY, Montell C, Banerjee S, von Lintig J, Palczewski K, Structural Insights into the Drosophila melanogaster Retinol Dehydrogenase, a Member of the Short-Chain Dehydrogenase/Reductase Family, Biochemistry 55(47) (2016) 6545–6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Kumar S, Sandell LL, Trainor PA, Koentgen F, Duester G, Alcohol and aldehyde dehydrogenases: retinoid metabolic effects in mouse knockout models, Biochim Biophys Acta 1821(1) (2012) 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Sandell LL, Sanderson BW, Moiseyev G, Johnson T, Mushegian A, Young K, Rey JP, Ma JX, Staehling-Hampton K, Trainor PA, RDH10 is essential for synthesis of embryonic retinoic acid and is required for limb, craniofacial, and organ development, Genes Dev 21(9) (2007) 1113–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Adams MK, Belyaeva OV, Wu L, Kedishvili NY, The retinaldehyde reductase activity of DHRS3 is reciprocally activated by retinol dehydrogenase 10 to control retinoid homeostasis, J Biol Chem 289(21) (2014) 14868–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Obrochta KM, Krois CR, Campos B, Napoli JL, Insulin regulates retinol dehydrogenase expression and all-trans-retinoic acid biosynthesis through FoxO1, J Biol Chem 290(11) (2015) 7259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Zhang M, Hu P, Krois CR, Kane MA, Napoli JL, Altered vitamin A homeostasis and increased size and adiposity in the rdh1-null mouse, Faseb J 21(11) (2007) 2886–96. [DOI] [PubMed] [Google Scholar]
- [165].Billings SE, Pierzchalski K, Butler Tjaden NE, Pang XY, Trainor PA, Kane MA, Moise AR, The retinaldehyde reductase DHRS3 is essential for preventing the formation of excess retinoic acid during embryonic development, FASEB J 27(12) (2013) 4877–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Zolfaghari R, Chen Q, Ross AC, DHRS3, a retinal reductase, is differentially regulated by retinoic acid and lipopolysaccharide-induced inflammation in THP-1 cells and rat liver, Am J Physiol Gastrointest Liver Physiol 303(5) (2012) G578–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Widjaja-Adhi MAK, Palczewski G, Dale K, Knauss EA, Kelly ME, Golczak M, Levine AD, von Lintig J, Transcription factor ISX mediates the cross talk between diet and immunity, Proc Natl Acad Sci U S A 114(43) (2017) 11530–11535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Belyaeva OV, Wu L, Shmarakov I, Nelson PS, Kedishvili NY, Retinol dehydrogenase 11 is essential for the maintenance of retinol homeostasis in liver and testis in mice, J Biol Chem 293(18) (2018) 6996–7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Batten ML, Imanishi Y, Maeda T, Tu DC, Moise AR, Bronson D, Possin D, Van Gelder RN, Baehr W, Palczewski K, Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver, J Biol Chem 279(11) (2004) 10422–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Ruiz A, Kuehn MH, Andorf JL, Stone E, Hageman GS, Bok D, Genomic organization and mutation analysis of the gene encoding lecithin retinol acyltransferase in human retinal pigment epithelium, Invest Ophthalmol Vis Sci 42(1) (2001) 31–7. [PubMed] [Google Scholar]
- [171].Ruiz A, Winston A, Lim YH, Gilbert BA, Rando RR, Bok D, Molecular and biochemical characterization of lecithin retinol acyltransferase, J Biol Chem 274(6) (1999) 3834–41. [DOI] [PubMed] [Google Scholar]
- [172].Golczak M, Sears AE, Kiser PD, Palczewski K, LRAT-specific domain facilitates vitamin A metabolism by domain swapping in HRASLS3, Nature chemical biology 11(1) (2015) 26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Pang XY, Cao J, Addington L, Lovell S, Battaile KP, Zhang N, Rao JL, Dennis EA, Moise AR, Structure/function relationships of adipose phospholipase A2 containing a cys-his-his catalytic triad, J Biol Chem 287(42) (2012) 35260–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Liu L, Gudas LJ, Disruption of the lecithin:retinol acyltransferase gene makes mice more susceptible to vitamin A deficiency, J Biol Chem 280(48) (2005) 40226–34. [DOI] [PubMed] [Google Scholar]
- [175].Cooperstone JL, Goetz HJ, Riedl KM, Harrison EH, Schwartz SJ, Kopec RE, Relative contribution of alpha-carotene to postprandial vitamin A concentrations in healthy humans after carrot consumption, Am J Clin Nutr 106(1) (2017) 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Burri BJ, La Frano MR, Zhu C, Absorption, metabolism, and functions of beta-cryptoxanthin, Nutr Rev 74(2) (2016) 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Subbarayan C, Lkshmanan MR, Cama HR, Metabolism and biologicalpotency of 5,-monoepoxy-beta-carotene and 5,6:5’,6’-diepoxy-beta-carotene, Biochem J 99(2) (1966) 308–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Kloer DP, Ruch S, Al-Babili S, Beyer P, Schulz GE, The structure of a retinal-forming carotenoid oxygenase, Science 308(5719) (2005) 267–9. [DOI] [PubMed] [Google Scholar]
- [179].Kiser PD, Zhang J, Badiee M, Li Q, Shi W, Sui X, Golczak M, Tochtrop GP, Palczewski K, Catalytic mechanism of a retinoid isomerase essential for vertebrate vision, Nature chemical biology 11(6) (2015) 409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Redmond TM, Poliakov E, Yu S, Tsai JY, Lu Z, Gentleman S, Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle, Proc Natl Acad Sci 102(38) (2005) 13658–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Jin M, Li S, Moghrabi WN, Sun H, Travis GH, Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium, Cell 122(3) (2005) 449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Moiseyev G, Chen Y, Takahashi Y, Wu BX, Ma JX, RPE65 is the isomerohydrolase in the retinoid visual cycle, Proc Natl Acad Sci 102(35) (2005) 12413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Allenby G, Bocquel MT, Saunders M, Kazmer S, Speck J, Rosenberger M, Lovey A, Kastner P, Grippo JF, Chambon P, et al. , Retinoic acid receptors and retinoid X receptors: interactions with endogenous retinoic acids, Proc Natl Acad Sci U S A 90(1) (1993) 30–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Heyman RA, Mangelsdorf DJ, Dyck JA, Stein RB, Eichele G, Evans RM, Thaller C, 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor, Cell 68(2) (1992) 397–406. [DOI] [PubMed] [Google Scholar]
- [185].Fan J, Rohrer B, Moiseyev G, Ma JX, Crouch RK, Isorhodopsin rather than rhodopsin mediates rod function in RPE65 knock-out mice, Proc Natl Acad Sci 100(23) (2003) 13662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Palczewski K, Retinoids for treatment of retinal diseases, Trends Pharmacol Sci 31(6) (2010) 284–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Ruhl R, Krzyzosiak A, Niewiadomska-Cimicka A, Rochel N, Szeles L, Vaz B, Wietrzych-Schindler M, Alvarez S, Szklenar M, Nagy L, de Lera AR, Krezel W, 9-cis-13,14-Dihydroretinoic Acid Is an Endogenous Retinoid Acting as RXR Ligand in Mice, PLoS Genet 11(6) (2015) e1005213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Deming DM, Baker DH, Erdman JW Jr., The relative vitamin A value of 9-cis beta-carotene is less and that of 13-cis beta-carotene may be greater than the accepted 50% that of all-trans beta-carotene in gerbils, J Nutr 132(9) (2002) 2709–12. [DOI] [PubMed] [Google Scholar]
- [189].Gaziano JM, Johnson EJ, Russell RM, Manson JE, Stampfer MJ, Ridker PM, Frei B, Hennekens CH, Krinsky NI, Discrimination in absorption or transport of beta-carotene isomers after oral supplementation with either all-trans- or 9-cis-beta-carotene, Am J Clin Nutr 61(6) (1995) 1248–52. [DOI] [PubMed] [Google Scholar]
- [190].Maeda T, Perusek L, Amengual J, Babino D, Palczewski K, von Lintig J, Dietary 9-cis-beta,beta-carotene fails to rescue vision in mouse models of leber congenital amaurosis, Mol Pharmacol 80(5) (2011) 943–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].You CS, Parker RS, Goodman KJ, Swanson JE, Corso TN, Evidence of cis-trans isomerization of 9-cis-beta-carotene during absorption in humans, Am J Clin Nutr 64(2) (1996) 177–83. [DOI] [PubMed] [Google Scholar]
- [192].Nagao A, Olson JA, Enzymatic formation of 9-cis, 13-cis, and all-trans retinals from isomers of beta-carotene, FASEB J 8(12) (1994) 968–73. [DOI] [PubMed] [Google Scholar]
- [193].Wang XD, Krinsky NI, Benotti PN, Russell RM, Biosynthesis of 9-cis-retinoic acid from 9-cis-beta-carotene in human intestinal mucosa in vitro, Arch Biochem Biophys 313(1) (1994) 150–5. [DOI] [PubMed] [Google Scholar]
- [194].Hebuterne X, Wang XD, Johnson EJ, Krinsky NI, Russell RM, Intestinal absorption and metabolism of 9-cis-beta-carotene in vivo: biosynthesis of 9-cis-retinoic acid, J Lipid Res 36(6) (1995) 1264–73. [PubMed] [Google Scholar]
- [195].Oberhauser V, Voolstra O, Bangert A, von Lintig J, Vogt K, NinaB combines carotenoid oxygenase and retinoid isomerase activity in a single polypeptide, Proc Natl Acad Sci U S A 105(48) (2008) 19000–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Voolstra O, Oberhauser V, Sumser E, Meyer NE, Maguire ME, Huber A, von Lintig J, NinaB is essential for Drosophila vision but induces retinal degeneration in opsin-deficient photoreceptors, J Biol Chem 285(3) (2010) 2130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Meyers KJ, Mares JA, Igo RP Jr., Truitt B, Liu Z, Millen AE, Klein M, Johnson EJ, Engelman CD, Karki CK, Blodi B, Gehrs K, Tinker L, Wallace R, Robinson J, LeBlanc ES, Sarto G, Bernstein PS, SanGiovanni JP, Iyengar SK, Genetic evidence for role of carotenoids in age-related macular degeneration in the Carotenoids in Age-Related Eye Disease Study (CAREDS), Invest Ophthalmol Vis Sci 55(1) (2014) 587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Borel P, de Edelenyi FS, Vincent-Baudry S, Malezet-Desmoulin C, Margotat A, Lyan B, Gorrand JM, Meunier N, Drouault-Holowacz S, Bieuvelet S, Genetic variants in BCMO1 and CD36 are associated with plasma lutein concentrations and macular pigment optical density in humans, Annals of medicine 43(1) (2011) 47–59. [DOI] [PubMed] [Google Scholar]
- [199].Lala VR, Reddy V, Absorption of beta-carotene from green leafy vegetables in undernourished children, Am J Clin Nutr 23(1) (1970) 110–3. [DOI] [PubMed] [Google Scholar]
- [200].Villard L, Bates CJ, Carotene dioxygenase (EC 1.13.11.21) activity in rat intestine: effects of vitamin A deficiency and of pregnancy, The British journal of nutrition 56(1) (1986) 115–22. [DOI] [PubMed] [Google Scholar]
- [201].van Vliet T, van Vlissingen MF, van Schaik F, van den Berg H, beta-Carotene absorption and cleavage in rats is affected by the vitamin A concentration of the diet, J Nutr 126(2) (1996) 499–508. [DOI] [PubMed] [Google Scholar]
- [202].Bachmann H, Desbarats A, Pattison P, Sedgewick M, Riss G, Wyss A, Cardinault N, Duszka C, Goralczyk R, Grolier P, Feedback regulation of beta,beta-carotene 15,15’-monooxygenase by retinoic acid in rats and chickens, J Nutr 132(12) (2002) 3616–22. [DOI] [PubMed] [Google Scholar]
- [203].Choi MY, Romer AI, Hu M, Lepourcelet M, Mechoor A, Yesilaltay A, Krieger M, Gray PA, Shivdasani RA, A dynamic expression survey identifies transcription factors relevant in mouse digestive tract development, Development 133(20) (2006) 4119–29. [DOI] [PubMed] [Google Scholar]
- [204].Seino Y, Miki T, Kiyonari H, Abe T, Fujimoto W, Kimura K, Takeuchi A, Takahashi Y, Oiso Y, Iwanaga T, Seino S, Isx participates in the maintenance of vitamin A metabolism by regulation of beta-carotene 15,15’-monooxygenase (Bcmo1) expression, J Biol Chem 283(8) (2008) 4905–11. [DOI] [PubMed] [Google Scholar]
- [205].Lobo GP, Hessel S, Eichinger A, Noy N, Moise AR, Wyss A, Palczewski K, von Lintig J, ISX is a retinoic acid-sensitive gatekeeper that controls intestinal beta,beta-carotene absorption and vitamin A production, FASEB J 24(6) (2010) 1656–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Boulanger A, McLemore P, Copeland NG, Gilbert DJ, Jenkins NA, Yu SS, Gentleman S, Redmond TM, Identification of beta-carotene 15, 15’-monooxygenase as a peroxisome proliferator-activated receptor target gene, Faseb J 17(10) (2003) 1304–6. [DOI] [PubMed] [Google Scholar]
- [207].Lietz G, Lange J, Rimbach G, Molecular and dietary regulation of beta,beta-carotene 15,15’-monooxygenase 1 (BCMO1), Arch Biochem Biophys 502(1) (2010) 8–16. [DOI] [PubMed] [Google Scholar]
- [208].Lobo GP, Amengual J, Baus D, Shivdasani RA, Taylor D, von Lintig J, Genetics and diet regulate vitamin A production via the homeobox transcription factor ISX, J Biol Chem 288(13) (2013) 9017–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Ferrucci L, Perry JR, Matteini A, Perola M, Tanaka T, Silander K, Rice N, Melzer D, Murray A, Cluett C, Fried LP, Albanes D, Corsi AM, Cherubini A, Guralnik J, Bandinelli S, Singleton A, Virtamo J, Walston J, Semba RD, Frayling TM, Common variation in the beta-carotene 15,15’-monooxygenase 1 gene affects circulating levels of carotenoids: a genome-wide association study, Am J Hum Genet 84(2) (2009) 123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Lietz G, Oxley A, Leung W, Hesketh J, Single nucleotide polymorphisms upstream from the beta-carotene 15,15’-monoxygenase gene influence provitamin A conversion efficiency in female volunteers, J Nutr 142(1) (2012) 161S–5S. [DOI] [PubMed] [Google Scholar]
- [211].Thompson SY, Role of carotene and vitamin A in animal feeding, World review of nutrition and dietetics 21 (1975) 224–80. [DOI] [PubMed] [Google Scholar]
- [212].Mason JB, Benn CS, Sachdev H, West KP Jr., Palmer AC, Sommer A, Should universal distribution of high dose vitamin A to children cease?, BMJ 360 (2018) k927. [DOI] [PubMed] [Google Scholar]
- [213].DePaolo RW, Abadie V, Tang F, Fehlner-Peach H, Hall JA, Wang W, Marietta EV, Kasarda DD, Waldmann TA, Murray JA, Semrad C, Kupfer SS, Belkaid Y, Guandalini S, Jabri B, Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens, Nature 471(7337) (2011) 220–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].van de Pavert SA, Ferreira M, Domingues RG, Ribeiro H, Molenaar R, Moreira-Santos L, Almeida FF, Ibiza S, Barbosa I, Goverse G, Labao-Almeida C, Godinho-Silva C, Konijn T, Schooneman D, O’Toole T, Mizee MR, Habani Y, Haak E, Santori FR, Littman DR, Schulte-Merker S, Dzierzak E, Simas JP, Mebius RE, Veiga-Fernandes H, Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity, Nature 508(7494) (2014) 123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Arreguin A, Ribot J, Musinovic H, von Lintig J, Palou A, Bonet ML, Dietary vitamin A impacts DNA methylation patterns of adipogenesis-related genes in suckling rats, Arch Biochem Biophys 650 (2018) 75–84. [DOI] [PubMed] [Google Scholar]
- [216].Granados N, Amengual J, Ribot J, Musinovic H, Ceresi E, von Lintig J, Palou A, Bonet ML, Vitamin A supplementation in early life affects later response to an obesogenic diet in rats, Int J Obes (Lond) 37(9) (2013) 1169–76. [DOI] [PubMed] [Google Scholar]
- [217].Musinovic H, Bonet ML, Granados N, Amengual J, von Lintig J, Ribot J, Palou A, beta-Carotene during the suckling period is absorbed intact and induces retinoic acid dependent responses similar to preformed vitamin A in intestine and liver, but not adipose tissue of young rats, Mol Nutr Food Res 58(11) (2014) 2157–65. [DOI] [PubMed] [Google Scholar]
- [218].Sinha DP, Bang FB, Seasonal variation in signs of vitamin-A deficiency in rural West Bengal children, Lancet 2(7823) (1973) 228–30. [DOI] [PubMed] [Google Scholar]
- [219].Fitzpatrick TB, Basset GJ, Borel P, Carrari F, DellaPenna D, Fraser PD, Hellmann H, Osorio S, Rothan C, Valpuesta V, Caris-Veyrat C, Fernie AR, Vitamin deficiencies in humans: can plant science help?, Plant Cell 24(2) (2012) 395–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, Von Andrian UH, Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells, Nature 424(6944) (2003) 88–93. [DOI] [PubMed] [Google Scholar]
- [221].Uematsu S, Fujimoto K, Jang MH, Yang BG, Jung YJ, Nishiyama M, Sato S, Tsujimura T, Yamamoto M, Yokota Y, Kiyono H, Miyasaka M, Ishii KJ, Akira S, Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5, Nature immunology 9(7) (2008) 769–76. [DOI] [PubMed] [Google Scholar]
- [222].Hammerschmidt SI, Friedrichsen M, Boelter J, Lyszkiewicz M, Kremmer E, Pabst O, Forster R, Retinoic acid induces homing of protective T and B cells to the gut after subcutaneous immunization in mice, J Clin Invest 121(8) (2011) 3051–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Cantorna MT, Nashold FE, Hayes CE, In vitamin A deficiency multiple mechanisms establish a regulatory T helper cell imbalance with excess Th1 and insufficient Th2 function, J Immunol 152(4) (1994) 1515–22. [PubMed] [Google Scholar]
- [224].Spencer SP, Wilhelm C, Yang Q, Hall JA, Bouladoux N, Boyd A, Nutman TB, Urban JF Jr., Wang J, Ramalingam TR, Bhandoola A, Wynn TA, Belkaid Y, Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity, Science 343(6169) (2014) 432–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Crockett SD, Porter CQ, Martin CF, Sandler RS, Kappelman MD, Isotretinoin use and the risk of inflammatory bowel disease: a case-control study, The American journal of gastroenterology 105(9) (2010) 1986–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Reddy D, Siegel CA, Sands BE, Kane S, Possible association between isotretinoin and inflammatory bowel disease, The American journal of gastroenterology 101(7) (2006) 1569–73. [DOI] [PubMed] [Google Scholar]
- [227].Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY, Retinoic acid imprints gut-homing specificity on T cells, Immunity 21(4) (2004) 527–38. [DOI] [PubMed] [Google Scholar]
- [228].Molenaar R, Knippenberg M, Goverse G, Olivier BJ, de Vos AF, O’Toole T, Mebius RE, Expression of retinaldehyde dehydrogenase enzymes in mucosal dendritic cells and gut-draining lymph node stromal cells is controlled by dietary vitamin A, J Immunol 186(4) (2011) 1934–42. [DOI] [PubMed] [Google Scholar]
- [229].Edele F, Molenaar R, Gutle D, Dudda JC, Jakob T, Homey B, Mebius R, Hornef M, Martin SF, Cutting edge: instructive role of peripheral tissue cells in the imprinting of T cell homing receptor patterns, J Immunol 181(6) (2008) 3745–9. [DOI] [PubMed] [Google Scholar]
- [230].Molenaar R, Greuter M, van der Marel AP, Roozendaal R, Martin SF, Edele F, Huehn J, Forster R, O’Toole T, Jansen W, Eestermans IL, Kraal G, Mebius RE, Lymph node stromal cells support dendritic cell-induced gut-homing of T cells, J Immunol 183(10) (2009) 6395–402. [DOI] [PubMed] [Google Scholar]
- [231].Vaarala O, Atkinson MA, Neu J, The “perfect storm” for type 1 diabetes: the complex interplay between intestinal microbiota, gut permeability, and mucosal immunity, Diabetes 57(10) (2008) 2555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Cassani B, Villablanca EJ, De Calisto J, Wang S, Mora JR, Vitamin A and immune regulation: role of retinoic acid in gut-associated dendritic cell education, immune protection and tolerance, Molecular aspects of medicine 33(1) (2012) 63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ, All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation, J Exp Med 204(8) (2007) 1765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].McGuire HM, Vogelzang A, Ma CS, Hughes WE, Silveira PA, Tangye SG, Christ D, Fulcher D, Falcone M, King C, A subset of interleukin-21+ chemokine receptor CCR9+ T helper cells target accessory organs of the digestive system in autoimmunity, Immunity 34(4) (2011) 602–15. [DOI] [PubMed] [Google Scholar]
- [235].Dash S, Xiao C, Morgantini C, Lewis GF, New Insights into the Regulation of Chylomicron Production, Annu Rev Nutr 35 (2015) 265–94. [DOI] [PubMed] [Google Scholar]
- [236].Iqbal J, Rudel LL, Hussain MM, Microsomal triglyceride transfer protein enhances cellular cholesteryl esterification by relieving product inhibition, J Biol Chem 283(29) (2008) 19967–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].He PP, Jiang T, OuYang XP, Liang YQ, Zou JQ, Wang Y, Shen QQ, Liao L, Zheng XL, Lipoprotein lipase: Biosynthesis, regulatory factors, and its role in atherosclerosis and other diseases, Clin Chim Acta 480 (2018) 126–137. [DOI] [PubMed] [Google Scholar]
- [238].Quadro L, Blaner WS, Salchow DJ, Vogel S, Piantedosi R, Gouras P, Freeman S, Cosma MP, Colantuoni V, Gottesman ME, Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein, Embo J 18(17) (1999) 4633–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Episkopou V, Maeda S, Nishiguchi S, Shimada K, Gaitanaris GA, Gottesman ME, Robertson EJ, Disruption of the transthyretin gene results in mice with depressed levels of plasma retinol and thyroid hormone, Proc Natl Acad Sci 90(6) (1993) 2375–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Bok D, Heller J, Transport of retinol from the blood to the retina: an autoradiographic study of the pigment epithelial cell surface receptor for plasma retinol-binding protein, Experimental eye research 22(5) (1976) 395–402. [DOI] [PubMed] [Google Scholar]
- [241].Heller M, Bok D, A specific receptor for retinol binding protein as detected by the binding of human and bovine retinol binding protein to pigment epithelial cells, Am J Ophthalmol 81(1) (1976) 93–7. [DOI] [PubMed] [Google Scholar]
- [242].Kelly M, von Lintig J, STRA6: role in cellular retinol uptake and efflux, Hepatobiliary Surg Nutr 4(4) (2015) 229–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Sivaprasadarao A, Findlay JB, The interaction of retinol-binding protein with its plasma-membrane receptor, Biochem J 255(2) (1988) 561–9. [PMC free article] [PubMed] [Google Scholar]
- [244].Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H, A membrane receptor for retinol binding protein mediates cellular uptake of vitamin a, Science 315(5813) (2007) 820–5. [DOI] [PubMed] [Google Scholar]
- [245].Bouillet P, Sapin V, Chazaud C, Messaddeq N, Decimo D, Dolle P, Chambon P, Developmental expression pattern of Stra6, a retinoic acid-responsive gene encoding a new type of membrane protein, Mech Dev 63(2) (1997) 173–86. [DOI] [PubMed] [Google Scholar]
- [246].Chen Y, Clarke OB, Kim J, Stowe S, Kim YK, Assur Z, Cavalier M, Godoy-Ruiz R, von Alpen DC, Manzini C, Blaner WS, Frank J, Quadro L, Weber DJ, Shapiro L, Hendrickson WA, Mancia F, Structure of the STRA6 receptor for retinol uptake, Science 353(6302) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Kawaguchi R, Yu J, Ter-Stepanian M, Zhong M, Cheng G, Yuan Q, Jin M, Travis GH, Ong D, Sun H, Receptor-Mediated Cellular Uptake Mechanism That Couples to Intracellular Storage, ACS Chem Biol 6(10) (2011) 1041–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Isken A, Golczak M, Oberhauser V, Hunzelmann S, Driever W, Imanishi Y, Palczewski K, von Lintig J, RBP4 disrupts vitamin A uptake homeostasis in a STRA6-deficient animal model for Matthew-Wood syndrome, Cell Metab 7(3) (2008) 258–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Amengual J, Golczak M, Palczewski K, von Lintig J, Lecithin:Retinol Acyltransferase Is Critical for Cellular Uptake of Vitamin A from Serum Retinol-binding Protein, J Biol Chem 287(29) (2012) 24216–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Amengual J, Zhang N, Kemerer M, Maeda T, Palczewski K, von Lintig J, STRA6 is critical for cellular vitamin A uptake and homeostasis, Human molecular genetics (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Kelly M, Widjaja-Adhi MA, Palczewski G, von Lintig J, Transport of vitamin A across blood-tissue barriers is facilitated by STRA6, FASEB J 30(8) (2016) 2985–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Amengual J, Zhang N, Kemerer M, Maeda T, Palczewski K, Von Lintig J, STRA6 is critical for cellular vitamin A uptake and homeostasis, Human molecular genetics 23(20) (2014) 5402–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Muenzner M, Tuvia N, Deutschmann C, Witte N, Tolkachov A, Valai A, Henze A, Sander LE, Raila J, Schupp M, RBP4 and its Membrane Receptor STRA6 Control Adipogenesis by Regulating Cellular Retinoid Homeostasis and RARalpha Activity, Mol Cell Biol (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Berry DC, Jacobs H, Marwarha G, Gely-Pernot A, O’Byrne SM, Desantis D, Klopfenstein M, Feret B, Dennefeld C, Blaner WS, Croniger CM, Mark M, Noy N, Ghyselinck NB, The STRA6 Receptor Is Essential for Retinol-binding Protein-induced Insulin Resistance but Not for Maintaining Vitamin A Homeostasis in Tissues Other Than the Eye, J Biol Chem 288(34) (2013) 24528–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Ruiz A, Mark M, Jacobs H, Klopfenstein M, Hu J, Lloyd M, Habib S, Tosha C, Radu RA, Ghyselinck NB, Nusinowitz S, Bok D, Retinoid content, visual responses, and ocular morphology are compromised in the retinas of mice lacking the retinol-binding protein receptor, STRA6, Invest Ophthalmol Vis Sci 53(6) (2012) 3027–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].de Pee S, Dary O, Biochemical indicators of vitamin A deficiency: serum retinol and serum retinol binding protein, J Nutr 132(9 Suppl) (2002) 2895S–2901S. [DOI] [PubMed] [Google Scholar]
- [257].Chassaing N, Golzio C, Odent S, Lequeux L, Vigouroux A, Martinovic-Bouriel J, Tiziano FD, Masini L, Piro F, Maragliano G, Delezoide A-L, Attié-Bitach T, Manouvrier-Hanu S, Etchevers HC, Calvas P, Phenotypic spectrum of STRA6 mutations: from Matthew-Wood syndrome to non-lethal anophthalmia, Human mutation 30(5) (2009) E673–81. [DOI] [PubMed] [Google Scholar]
- [258].Pasutto F, Sticht H, Hammersen G, Gillessen-Kaesbach G, Fitzpatrick DR, Nurnberg G, Brasch F, Schirmer-Zimmermann H, Tolmie JL, Chitayat D, Houge G, Fernandez-Martinez L, Keating S, Mortier G, Hennekam RC, von der Wense A, Slavotinek A, Meinecke P, Bitoun P, Becker C, Nurnberg P, Reis A, Rauch A, Mutations in STRA6 Cause a Broad Spectrum of Malformations Including Anophthalmia, Congenital Heart Defects, Diaphragmatic Hernia, Alveolar Capillary Dysplasia, Lung Hypoplasia, and Mental Retardation, Am J Hum Genet 80(3) (2007) 550–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Golzio C, Martinovic-Bouriel J, Thomas S, Mougou-Zrelli S, Grattagliano-Bessieres B, Bonniere M, Delahaye S, Munnich A, Encha-Razavi F, Lyonnet S, Vekemans M, Attie-Bitach T, Etchevers HC, Matthew-Wood syndrome is caused by truncating mutations in the retinol-binding protein receptor gene STRA6, Am J Hum Genet 80(6) (2007) 1179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Clagett-Dame M, DeLuca HF, The role of vitamin A in mammalian reproduction and embryonic development, Annu Rev Nutr 22 (2002) 347–81. [DOI] [PubMed] [Google Scholar]
- [261].Casey J, Kawaguchi R, Morrissey M, Sun H, McGettigan P, Nielsen JE, Conroy J, Regan R, Kenny E, Cormican P, Morris DW, Tormey P, Chroinin MN, Kennedy BN, Lynch S, Green A, Ennis S, First implication of STRA6 mutations in isolated anophthalmia, microphthalmia, and coloboma: a new dimension to the STRA6 phenotype, Hum Mutat 32(12) (2011) 1417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Chou CM, Nelson C, Tarle SA, Pribila JT, Bardakjian T, Woods S, Schneider A, Glaser T, Biochemical Basis for Dominant Inheritance, Variable Penetrance, and Maternal Effects in RBP4 Congenital Eye Disease, Cell 161(3) (2015) 634–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Seeliger MW, Biesalski HK, Wissinger B, Gollnick H, Gielen S, Frank J, Beck S, Zrenner E, Phenotype in retinol deficiency due to a hereditary defect in retinol binding protein synthesis, Invest Ophthalmol Vis Sci 40(1) (1999) 3–11. [PubMed] [Google Scholar]
- [264].Khan KN, Carss K, Raymond FL, Islam F, Nihr BioResource-Rare Diseases C, Moore AT, Michaelides M, Arno G, Vitamin A deficiency due to bi-allelic mutation of RBP4: There’s more to it than meets the eye, Ophthalmic genetics (2016) 1–2. [DOI] [PubMed] [Google Scholar]
- [265].den Hollander AI, Roepman R, Koenekoop RK, Cremers FP, Leber congenital amaurosis: genes, proteins and disease mechanisms, Prog Retin Eye Res 27(4) (2008) 391–419. [DOI] [PubMed] [Google Scholar]
- [266].During A, Doraiswamy S, Harrison EH, Xanthophylls are preferentially taken up compared with beta-carotene by retinal cells via a SRBI-dependent mechanism, J Lipid Res 49(8) (2008) 1715–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Thomas SE, Harrison EH, Mechanisms of selective delivery of xanthophylls to retinal pigment epithelial cells by human lipoproteins, J Lipid Res 57(10) (2016) 1865–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Chichili GR, Nohr D, Schaffer M, von Lintig J, Biesalski HK, beta-Carotene conversion into vitamin A in human retinal pigment epithelial cells, Invest Ophthalmol Vis Sci 46(10) (2005) 3562–9. [DOI] [PubMed] [Google Scholar]
- [269].Bhosale P, Bernstein PS, Vertebrate and invertebrate carotenoid-binding proteins, Arch Biochem Biophys 458(2) (2007) 121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Li B, Vachali P, Frederick JM, Bernstein PS, Identification of StARD3 as a lutein-binding protein in the macula of the primate retina, Biochemistry 50(13) (2011) 2541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].I.o. Medicine, Beta-carotene and other carotenoids. In Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids, National Academic Press, Washington DC: (2000) 352–382. [PubMed] [Google Scholar]
- [272].Chew EY, Clemons TE, Sangiovanni JP, Danis RP, Ferris FL 3rd, Elman MJ, Antoszyk AN, Ruby AJ, Orth D, Bressler SB, Fish GE, Hubbard GB, Klein ML, Chandra SR, Blodi BA, Domalpally A, Friberg T, Wong WT, Rosenfeld PJ, Agron E, Toth CA, Bernstein PS, Sperduto RD, Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3, JAMA ophthalmology 132(2) (2014) 142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Wu J, Cho E, Willett WC, Sastry SM, Schaumberg DA, Intakes of Lutein, Zeaxanthin, and Other Carotenoids and Age-Related Macular Degeneration During 2 Decades of Prospective Follow-up, JAMA ophthalmology 133(12) (2015) 1415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Huang YM, Dou HL, Huang FF, Xu XR, Zou ZY, Lin XM, Effect of supplemental lutein and zeaxanthin on serum, macular pigmentation, and visual performance in patients with early age-related macular degeneration, BioMed research international 2015 (2015) 564738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].I.o. Medicine, Dietary Reference Intake for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Maganese, Molybdenum, Nickel, Silicon, Vanadium, and Zink., National Academic Press, Washington DC: (2001). [PubMed] [Google Scholar]
- [276].Grune T, Lietz G, Palou A, Ross AC, Stahl W, Tang G, Thurnham D, Yin SA, Biesalski HK, Beta-carotene is an important vitamin A source for humans, J Nutr 140(12) (2010) 2268S–2285S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].Tanumihardjo SA, Gannon BM, Kaliwile C, Chileshe J, Hypercarotenodermia in Zambia: which children turned orange during mango season?, Eur J Clin Nutr 69(12) (2015) 1346–9. [DOI] [PubMed] [Google Scholar]
- [278].Liao X, Ren J, Wei CH, Ross AC, Cecere TE, Jortner BS, Ahmed SA, Luo XM, Paradoxical effects of all-trans-retinoic acid on lupus-like disease in the MRL/lpr mouse model, PloS one 10(3) (2015) e0118176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Hsu SH, Wang LT, Lee KT, Chen YL, Liu KY, Suen JL, Chai CY, Wang SN, Proinflammatory homeobox gene ISX, regulates tumor growth and survival in hepatocellular carcinoma, Cancer Res 73(2) (2013) 508–18. [DOI] [PubMed] [Google Scholar]
- [280].Sue S, Shibata W, Kameta E, Sato T, Ishii Y, Kaneko H, Miwa H, Sasaki T, Tamura T, Kondo M, Maeda S, Intestine-specific homeobox (ISX) induces intestinal metaplasia and cell proliferation to contribute to gastric carcinogenesis, Journal of gastroenterology (2016). [DOI] [PubMed] [Google Scholar]
- [281].Dinu I, Mahasirimongkol S, Liu Q, Yanai H, Sharaf Eldin N, Kreiter E, Wu X, Jabbari S, Tokunaga K, Yasui Y, SNP-SNP interactions discovered by logic regression explain Crohn’s disease genetics, PloS one 7(10) (2012) e43035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Leung WC, Hessel S, Meplan C, Flint J, Oberhauser V, Tourniaire F, Hesketh JE, von Lintig J, Lietz G, Two common single nucleotide polymorphisms in the gene encoding {beta}-carotene 15,15’-monoxygenase alter {beta}-carotene metabolism in female volunteers, Faseb J (2008). [DOI] [PubMed] [Google Scholar]
- [283].Borel P, Desmarchelier C, Nowicki M, Bott R, A Combination of Single-Nucleotide Polymorphisms Is Associated with Interindividual Variability in Dietary beta-Carotene Bioavailability in Healthy Men, J Nutr 145(8) (2015) 1740–7. [DOI] [PubMed] [Google Scholar]
- [284].Meyers KJ, Johnson EJ, Bernstein PS, Iyengar SK, Engelman CD, Karki CK, Liu Z, Igo RP Jr., Truitt B, Klein ML, Snodderly DM, Blodi BA, Gehrs KM, Sarto GE, Wallace RB, Robinson J, LeBlanc ES, Hageman G, Tinker L, Mares JA, Genetic determinants of macular pigments in women of the Carotenoids in Age-Related Eye Disease Study, Invest Ophthalmol Vis Sci 54(3) (2013) 2333–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Borel P, Grolier P, Mekki N, Boirie Y, Rochette Y, Le Roy B, Alexandre-Gouabau MC, Lairon D, Azais-Braesco V, Low and high responders to pharmacological doses of beta-carotene: proportion in the population, mechanisms involved and consequences on beta-carotene metabolism, J Lipid Res 39(11) (1998) 2250–60. [PubMed] [Google Scholar]
- [286].Jalal F, Nesheim MC, Agus Z, Sanjur D, Habicht JP, Serum retinol concentrations in children are affected by food sources of beta-carotene, fat intake, and anthelmintic drug treatment, Am J Clin Nutr 68(3) (1998) 623–9. [DOI] [PubMed] [Google Scholar]
- [287].Bonifant CM, Shevill E, Chang AB, Vitamin A supplementation for cystic fibrosis, The Cochrane database of systematic reviews (5) (2014) CD006751. [DOI] [PubMed] [Google Scholar]
- [288].Drai J, Borel P, Faure H, Galabert C, Le Moel G, Laromiguiere M, Fayol V, Fasting plasma carotenoids concentrations in Crohn’s and pancreatic cancer patients compared to control subjects, Int J Vitam Nutr Res 79(2) (2009) 87–94. [DOI] [PubMed] [Google Scholar]


