Highlights
-
•
Physical exercise reduces cancer risks, enhances quality of life, and improves the prognoses of patients with cancer.
-
•
Physical exercise should be considered an important intervention to prevent and treat cancer and its complications.
-
•
Sensitivity to physical exercise varies in different cancers; we provide evidence for the exercise type and strength in various cancers and in differing stages.
-
•
Inhibiting cancer cell proliferation and inducing apoptosis and regulating metabolism and the immune environment are the main mechanisms of the benefits of physical exercise in cancer prevention and treatment.
Keywords: Cancer development, Cancer treatment, Molecular mechanisms, Physical exercise
Abstract
Exercise can enhance motivation to change lifestyle behaviors, improve aerobic fitness, improve physical function, control fatigue, and enhance quality of life. Studies have demonstrated the benefits to be gained from physical exercise, highlighting the importance of popularizing the concept of physical exercise for individuals and making professional exercise-treatment programs available to patients with cancer. However, the correlation between physical exercise and carcinogenesis is easily overlooked, and exercise interventions are not routinely provided to patients with cancer, especially those with advanced cancer. In this article, we present a literature review of the effects of exercise on cancer development and progression and give recent evidence for the type of exercise best suited for different types of cancer and in different disease stages. Moreover, the molecular mechanisms about regulating metabolism and systemic immune function in cancer are summarized and discussed. In conclusion, physical exercise should be considered as an important intervention for preventing and treating cancer and its complications.
Graphic Abstract
1. Introduction
Exercise can enhance motivation to change lifestyle behaviors, improve aerobic fitness and physical function, control fatigue, and enhance quality of life. As a nondrug intervention and prevention measure, exercise also helps to reduce the risk of cancer. More important, physical exercise plays a role in a variety of cancers during cancer treatment. For example, it can reduce treatment-related toxic and side effects and enhance the curative effect of other treatments. Therefore, it is necessary not only to investigate the influence of physical exercise on all aspects of cancer but also to determine the type, amount, and intensity of exercise that has an effect. Cancer treatment also requires patients to adopt an exercise scheme and follow physical exercise guidelines according to their practical needs. Long-term monitoring of exercise training for cancer survivors can effectively improve their muscle strength, cardiopulmonary function, physical function, and self-reported physical function.1
2. Physical exercise reduces cancer risks
The effect of exercise in reducing cancer risk is different in different kinds of cancer. Although no causal relationship between exercise and risk of cancer has been shown, research does show that improvement in lifestyle can reduce the incidence of cancer. For example, the risk of cancer can be reduced by exercising at least 3–5 h per week, with women benefiting from appropriate high-intensity activities, such as heavy housework and dancing, which are considered to be effective in lowering the risk of breast cancer.2 In a study, those who exercised experienced a decrease in the risk of breast cancer ranging from 15% to 20% and a decrease in colorectal cancer by 24%.3 Exercise intervention has been found to reduce the risk of breast cancer in postmenopausal women4 and has been shown to prevent autonomic nervous dysfunction in patients with breast cancer.5 Lifestyle changes brought about by physical exercise also have a positive impact on the incidence of colorectal cancer. In 126 studies assessing the impact of exercise on colorectal cancer, the risk of colorectal cancer was 19% lower for individuals who had higher levels of exercise than for those who had lower levels.6 In a review of gastric cancer studies that included 10 cohort and 12 case-control studies,7 the risk of cancer was 19% lower among those who had more physical exercise compared with those who had less. Some studies have demonstrated that obesity is one of the high-risk factors for endometrial cancer.8 A review of 33 studies showed that exercise reduced obesity, and women with high levels of exercise were 20% less likely to develop endometrial cancer than women with low levels of exercise.9 Likewise, a review of esophageal cancer studies that included 9 cohort and 15 case-control studies showed that the risk of esophageal cancer for those who had the most physical exercise was 21% lower than for those who had the least.10 In a study with more than 1 million participants, physical exercise carried out in the participants’ spare time reduced the risk of kidney cancer by 23% and lowered the risk of bladder cancer by 13%.11 A review of 4 case-control and 11 cohort studies found that the risk of bladder cancer was reduced by 15% among those who had higher levels of physical exercise compared with those who had lower levels.12 In a review of 25 studies, smokers who exercised had a lower risk of lung cancer than those who did not exercise, but there was no difference in the risk of lung cancer among nonsmokers who exercised compared to those who did not.13 For other cancers, such as blood cancer, pancreatic cancer, ovarian cancer, thyroid cancer, and liver cancer, there is only limited evidence that exercise reduces the risk of carcinogenesis.
The variance of cancer risk based on the type of cancer occurs mainly because different cancers and cancer subtypes have varying sensitivities to physical exercise; for example, the sensitivity of hormone receptor states to physical exercise different. Patients with the subtype of human epidermal growth factor receptor 2 (HER2) –/estrogen receptor (ER) + / progesterone receptor (PR) + / low degree are particularly sensitive to exercise. Their recurrence risk of recurrent breast cancer is reduced, whereas the risks of patients with other gene backgrounds are not.14 In a study using the model of mice with p53-deficient mouse mammary tumor virus-wingless type-1 (MMTV-WNT-1) breast cancer, exercise therapy did not yield a high survival rate or low cancer incidence, which may have been due to the strong effect of the driving gene mutation affecting exercise sensitivity.15 The study demonstrated that sensitivity to exercise was different due to the different hormone receptors and driving genes, and the effect of exercise on inhibiting cancer was restricted by other factors.
In conclusion, exercise can reduce the risk of cancer based on the type of cancer, and the sensitivity response to exercise for different cancer subtypes also varies.
3. Impact of physical exercise on cancer treatments
3.1. Physical exercise reduces adverse events related to cancer treatment
Cancer treatments such as chemotherapy and targeted therapy can produce fatigue, cognitive decline, depression, bone-mass reduction, muscle-mass reduction, cardiac toxicity, and other adverse reactions. These adverse reactions may lead to poor quality-of-life effects and poor treatment effects. Adverse reactions are also one of the reasons that cancer patients cannot adhere to or tolerate the treatment, so the treatment can also affect the survival period of the disease. However, several studies have demonstrated that the maintenance of physical activity can prevent some treatment-related adverse reactions. Exercise training, as it relates to cancer and its treatment, can affect cancer specificity and physiological and psychosocial results.16 In a large-scale clinical research study involving exercise as a treatment method, 301 patients with breast cancer in chemotherapy were divided into several groups, including a high-level (High) group, which participated in nearly 1 h of aerobics; a standard (Stan) group, which participated in aerobics for half an hour; and a combined (Comb) group, which participated in 1 h of exercise that consisted of resistive exercise and aerobics. The research showed that the benefits of the exercise intervention for the Comb group were greater, with muscle strength being significantly better than in the other 2 groups. Compared with the Stan group, the endocrine symptoms in the Comb group were significantly improved, while the exercise intervention for the High group provided multiple positive effects for breast cancer, including improved endocrine symptoms, the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) body pain and SF-36 body composition summary.17
It is thought that patients with cancer who are receiving traditional treatments should have more rest to relieve fatigue related to the treatments, but this is contrary to the view that physical exercise can alleviate cancer-related fatigue. In fact, research shows that physical exercise during chemotherapy can significantly alleviate cancer-related fatigue and that physical exercise and resistance training can prevent lymphedema caused by chemotherapy, thus balancing the damage caused by nerve agents.18 A review of 42 trials involving 3816 subjects showed that exercise alleviated fatigue (standardized mean difference (SMD) = 0.32, 95% confidence interval (95%CI): 0.13–0.52).19 A summary of research involving 56 trials with 4068 patients revealed that aerobic exercise significantly improved the fatigue level of patients in the postoperative and treatment process (SMD = –0.27, 95%CI: –0.37 to –0.17).20 Some studies have confirmed the association between oxidative stress and cancer-related fatigue; it has been shown that a correlation exists between oxidative stress markers in cerebrospinal fluid and treatment-related fatigue in school-aged children with leukemia.21 However, it is not clear whether the level of systemic oxidative stress is caused by cancer, or whether the local cancer microenvironment, including cancer hypoxia, perfusion, cancer cell metabolism, and anticancer immune phenotype, is affected by physical activity.
The most detailed studies of the relationship between the adverse effects of cancer treatments and physical exercise have occurred for 2 types of cancer: breast cancer and prostate cancer. Breast cancer is more common among women, and most patients are postmenopausal, hormone-receptor-positive at the time of diagnosis. Aromatase inhibitors are the main types of endocrine therapy, which help to improve the disease-free survival rate and the overall survival rate. However, the main side effects of aromatase inhibition are decreased cognitive ability and bone mass. Quality of life is affected when cognitive decline occurs, with Alzheimer's disease being an example. Exercise is believed to improve cognitive function and reduce cognitive decline caused by disease-related estrogen deficiency22 and, perhaps, can even prevent cognitive impairment caused by estrogen deficiency.23 Exercise is believed to improve cognitive degradation by reducing hormone stimulation of hormone-dependent cancer and the permeability of the vascular wall due to invasion by metastatic cells.24 Interestingly, however, the protection provided by exercise for osteopenia can have a more significant clinical effect for premenopausal women than for postmenopausal women.25 Planned physical activity is recommended during breast cancer treatment because it can protect against heart toxicity caused by treatment.26 Exercise and protein support can provide relief in the precachexia stage of malignant tumors because it lowers amino acid stimulation and protein synthesis resistance, thus increasing muscle mass and strength.27 Similarly, for prostate cancer, physical exercise can improve adverse reactions caused by androgen-deprivation therapy. The prescription of exercise can relieve insulin resistance and metabolic syndrome caused by muscle loss as well as increased risk of cardiovascular events.28 Multimodal exercise training, which includes aerobic training, progressive resistance training, and control training, can attenuate the adverse effects of treatment with androgen deprivation therapy such as cognitive decline.29
Body response to exercise varies for different type of cancers. In a clinical study, 319 survivors of cancer who had had different cancer types were prescribed multimodal exercise 3 days per week in order to evaluate body function. The fatigue index decreased significantly for gynecological cancer, blood cancer, colorectal cancer, and breast cancer; systolic blood pressure decreased significantly for gynecological cancer and breast cancer; heart rate decreased significantly for breast cancer and colorectal cancer; and oxygen uptake peak value improved significantly for prostate cancer, other male urogenital cancers, blood cancer, breast cancer, and adenocarcinoma epithelial neoplasms.30
In conclusion, physical exercise can improve fatigue related to cancer treatment by improving oxidative stress and reducing hormone stimulation, which can significantly improve cognitive function, muscle mass, bone mass, and cardiac toxicity caused by endocrine therapy in prostate cancer and breast cancer.
3.2. Exercise enhances the curative effects of cancer treatment
Cancer patients usually choose traditional treatments, such as surgery, radiotherapy, chemotherapy, targeting, and immunotherapy, and these treatments can inhibit cancer. The benefits of including exercise in the treatment process has been shown; exercise not only reduces the toxic and side effects of treatment but also improves the therapeutic effect of these treatments.31
In terms of first-line treatment of most solid cancers, radical resection of malignant tumors is the most common treatment. Studies have shown that postoperative weakness in patients can be improved with sports training, thus reducing postoperative complications and postoperative hospital-stay days.32 It is known that the proliferation and metastasis of cancer cells during the operation significantly reduces the positive surgical effect, leading to postoperative metastasis and affecting the prognosis and survival of patients.33 The efficiency of radiotherapy as a cancer treatment can also be improved by physical exercise. Some research has shown that the combination of resistance training and radiotherapy can significantly improve the bone density of spinal bone metastases.34 Recent research also shows that radiotherapy can increase the infiltration of natural killer (NK) cells to promote immune response. Although the single factor of exercise training does not affect the NK1.1 staining and gene expression of killer cell lectin-like receptor K1 (KLRk1) and interleukin-2 receptor β (IL-2Rβ), it does increase when radiotherapy is increased and the effect of exercise combined with radiotherapy is enhanced.35 NK cell activation combined with exercise has a positive effect on systemic blood flow perfusion. Cancer cell apoptosis can be improved with exercise,36 which also improves the anticancer effect of radiotherapy. When blood perfusion is sufficient, cytotoxic drugs and immune cells can be transported to the inside. Exercise enhances blood perfusion and the temperature of whole body. Similarly, the specific immune response from immunotherapy also needs to be released in order to play a role. Research shows that the mechanism of blocking the axis of programmed cell death-1 may be induced by the specific immune response released in a specific cancer in order to induce the clinical effect.37 Programmed cell death-1 can increase the infiltration of immune cells in cancer and be regulated by exercise before treatment. Many studies have shown that cancer patients are more likely to respond to treatment when the treatment is characterized by rapid infiltration of immune cells.38 In addition, exercise plus chemotherapy has prolonged the growth delay of breast cancer; exercise reduced hypoxia, increased microvascular density, increased blood perfusion, and normalized the vascular network in the cancer.39 Increasingly, studies have demonstrated the beneficial effects of exercise in improving the curative effect of chemotherapy. It has been found that, compared with sedentary mice with pancreatic duct cancer, the forced exercise mode of gemcitabine with 60%–70% exercise ability can inhibit cancer development; however, the elimination of the suppressive effect was shown in platelet reactive protein 1 gene knockout mice.40 The results indicate that the beneficial effects of combining exercise and chemotherapy can be produced when exercise normalizes blood vessels because of increasing blood perfusion. Exercise can promote normal angiogenesis in cancer, which is an opposite treatment strategy for currently popular anti-angiogenic drugs such as those used for colon cancer. In terms of anti-angiogenic therapy, future studies should determine the effect of exercise on vascular perfusion and angiogenesis and evaluate the final therapeutic effect. As shown in Fig. 1, exercise plays so many different roles in the treatments of cancer, incorporating exercise into cancer treatment is of great significance.
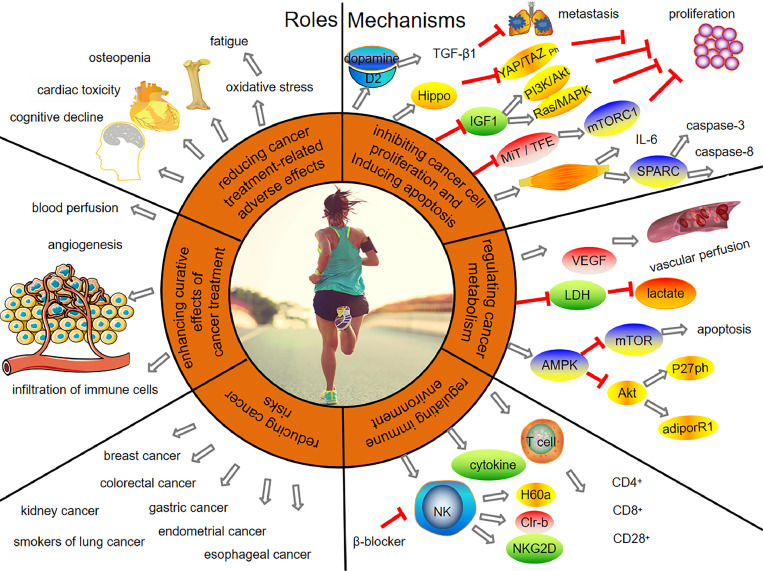
Fig. 1.
Roles and mechanisms of physical exercise in cancer prevention and treatment. The roles of physical exercise include reducing adverse effects related to cancer treatment, enhancing the curative effects of cancer treatment, and reducing cancer risk. Inhibiting cancer cell proliferation, inducing apoptosis, regulating cancer metabolism, and regulating the immune environment are the main mechanisms by which physical exercise produces benefits in cancer prevention and treatment. adiporR1 = adiponectin receptor 1; Akt = protein kinase B; AMPK = AMP-activated protein kinase; β-blocker = beta-adrenageic receptor block; CD = cluster of differentiation; Clr-b = C-type lectin-related protein B; D2 = dopamine receptor 2; H60a = the minor histocompatibility antigen 60; Hippo = serine/threonine-protein kinase hippo; IGF1 = insulin-like growth factor-1; IL-6 = interleukin 6; LDH = lactate dehydrogenase; MiT-TFE = microphthalmia/transcription factor E; microphthalmia/transcription factor E; mTOR = mammalian target of rapamycin; mTORC1 = mammalian target of rapamycin complex 1; NK = natural killer; NKG2D = natural killer group 2 member D; P27ph = cyclin-dependent kinase inhibitor phosphorylation; PI3K/Akt = phosphatidylinositol 3 kinase/protein kinase B; Ras/MAPK = rat sarcoma/mitogen-activated protein kinase; SPARC = secreted protein acidic and rich in cysteine; T cell = thymus dependent lymphocyte; TGF-β1 = the kinase phosphorylation and transforming growth factor-β1; VEGF = vascular endothelial growth factor; YAP/TAZ ph = phosphorylation of yes-associated protein/transcriptional co-activator with (postsynaptic density-95 (PSD-95), discs-large, zona occludens 1 (ZO-1))PDZ-binding motif.
In conclusion, physical exercise together with surgery, chemotherapy, radiotherapy, immunotherapy, and other cancer treatments can play a role in inhibiting cancer cell growth by improving the cancer microenvironment, including increasing blood-flow perfusion, promoting the formation of normal blood vessels, and activating immune cells in the body.
4. Exercise improves cancer prognosis
Exercise can ameliorate the curative effects and related adverse reactions to cancer treatment, but the core problem for clinicians is whether exercise can affect cancer prognosis and survival. Studies have shown that there is no strong positive correlation between exercise and survival rates for non-Hodgkin's lymphoma, gastric cancer, and other related cancers.41 However, survival rates for breast cancer, prostate cancer, and colorectal cancer may be improved through moderate exercise.42 A number of clinical studies have confirmed that exercise affects cancer survival rates and prognosis to a certain extent. A prospective study investigated 6295 survivors of breast cancer survivors (after 5 years of estrogen receptor (ER) positive I–III), who exercised 4.9–17.4 metabolic equivalent task (METs) h/week and ≥ 17.4 METs. With the increase of exercise (hazard ratio (HR) = 0.81, 95%CI: 0.71–0.93), the all-cause mortality rate decreased (HR = 0.71, 95%CI: 0.61–0.82).43 A clinical trial including 830 patients with Stage II–IV prostate cancer demonstrated that with continuous exercise before and after diagnosis, patients survived at least 2 years, and the all-cause mortality rate was lower than for patients who did not exercise continuously.44 In a prospective study involving 832 patients with Stage III colon cancer, an exercise intervention was implemented 6 months after chemotherapy, with the time dose of exercise related to the prognosis. The 3-year disease-free survival rate for those patients with more than 18 METs of exercise was 84.5%, and the 3-year disease-free survival rate for patients with less than 18 METs was 75.1%.45 The change in lifestyle habits, including diet and exercise, is more significant in colorectal cancer.46 For example, Meyerhardt et al.47 carried out a prospective study in women with Stage I–III colorectal cancer and demonstrated that the patients who self-administered exercise in their leisure time had a decrease in their mortality rate depending on the increase in the number of times they exercised per week (HR = 0.39, 95%CI: 0.18–0.82). Exercise can affect the prognosis by improving body mass index related to diet, which requires fewer than 3 METs before diagnosis and 3–9 METs after diagnosis of colon cancer, but the improvement of prognostic factors was not affected by the additional increase of more than 9 METs.
The effects of diet changes following colorectal cancer diagnosis needs further study. An ongoing multicenter Phase III clinical trial studying the effects of combined high-intensity aerobic and resistance training on metastatic castration-resistant prostate cancer with respect to the overall survival rate is worth paying attention to.48 Most clinical studies have involved patients with intermediate-stage prostate cancer who, with exercise, have more enthusiasm and can tolerate the discomfort and pain caused by advanced cancer. Therefore, it is worth considering whether multiple forms of exercise can yield benefits when patients are in the advanced stages of cancer.
In conclusion, exercise improves cancer prognosis and survival, especially in breast cancer, prostate cancer, and colorectal cancer.
5. Molecular mechanisms of physical exercise in cancer prevention and treatment
5.1. Exercise inhibits cancer cell proliferation and induces apoptosis
Several studies have demonstrated that exercise inhibits, to some extent, cancer cell proliferation. The proliferation of 3 negative breast cancer cells decreased when they were cultured and incubated in serum induced by exercise.49 The study found that the colony-forming ability of triple-negative breast cancer cells in soft agar was significantly reduced.49 It has also been reported that physical exercise in mice can inhibit the malignant transformation of carcinoma in situ because exercise reduces fasting blood glucose and improves glucose response.50 In cancer-bearing mice models, the incidence and proliferation of cancer cells decreased by 60% due to voluntary wheel movement, and the immune system of mice is ready to response after 4 weeks of pretraining before tumor inoculation, so part of the exercise effect may kill cancer cells in the transplanted cancer site.51 It has been shown that physical activity in rats fed with a high-fat diet reduces the proliferation characteristics of multiple Michigan cancer foundation-7 cells and the percentage of S-phase cells.52 Although studies have shown that exercise can inhibit cancer cell proliferation to some extent, no studies have shown that exercise can make malignant tumors disappear.
Exercise intensity in relation to the inhibition of cancer-cell proliferation has been studied frequently. In animal studies, moderate-intensity training can inhibit cancer cell proliferation and induce apoptosis,53 which emphasizes the protective benefits of moderate-intensity training.54 With an increase in exercise intensity, the expression of Ki-67 antigen also increases.55 However, strenuous exercise can show a carcinogenic effect.56 Recent research indicates that low-intensity exercise does not inhibit cancer cell proliferation, while moderate- and high-intensity exercise do, and exercise intensity levels include both those lower than the recommended intensity for cardiovascular health (less than 35% of the maximum exercise intensity, or the recommended intensity for improving cardiovascular health) and 70% of the maximum exercise intensity.57, 58, 59
What is the mechanism behind this inhibiting effect? Studies have shown that moderate swimming exercise can improve the dopamine level in prefrontal cortex, serum, and cancer tissues of mouse models; the dopamine receptor 2 (D2) binding to dopamine regulates the kinase phosphorylation and transforming growth factor-β1 (TGF-β1) by extracellular signal regulation to inhibit cancer cell proliferation and the occurrence of lung metastasis.60 Cancer cell proliferation and other basic biological processes are related to the Hippo signal, which is also the main mechanism of cancer cell inhibition.61 Phosphorylation can activate the Hippo signal to inhibit the 2 homologous transcription factors: the Yes-Associated Protein (YAP) and Transcriptional Co-activator with PDZ-binding Motif (TAZ). Phosphorylation isolates YAP/TAZ from the cytoplasm and inhibits its target genes, which are involved in the induction of genes for cancer cell proliferation and survival.62 However, the Hippo signal is inhibited by most G-coupled receptor ligands, and acute exercise induces catecholamine, which stimulates the Hippo signal through the β-adrenergic receptor, leading to YAP/TAZ inactivation.61,63 The increase in catecholamine was multiplied several times during acute exercise, but it quickly recovered to the baseline level after exercise stopped. The chronic upregulation of stress factors, including catecholamine, was related to the increase in cancer progression.63 As a member of the Interleukin 6 (IL-6) superfamily in breast cancer, leukemia inhibitory factor, as an upper-reaches inhibitor, suppresses breast cancer metastasis through Hippo and YAP signals.64 Studies of weight control through exercise have shown that the main mechanisms involved in repairing DNA are reducing cancer gene expression, enhancing the ability to scavenge reactive oxygen species, and changing hormone levels related to cancer.65 As mentioned in a variety of studies, body weight controlled by exercise brings about changes in body hormones because hormones play a positive role in the cell-growth process. Insulin-like growth factors (IGFs) exert influence on mitosis and anti-apoptosis and also affect the proliferation and differentiation of cancer cells.66 Some studies involving postmenopausal women have shown that after 6 months of walking training, IGF-1 and IGF-3 are significantly lower in exercising women than in non-exercising women.67 IGF binding protein-3 (IGFBP-3) regulates the mitosis of IGF, and its anti-apoptosis effect is inhibited in breast cancer cells.68 Studies have shown that weight loss reduces levels of IGF-1, insulin, and leptin in mouse models of skin cancer, leading to the reduction of IGF-1-related signaling pathways, including rat sarcoma-mitogen-activated protein kinase (Ras-MAPK) proliferation and protein kinase B (known as Akt) -phosphatidylinositol 3 kinase (PKB/AKt-PI3K) anti-apoptosis; IGF-1 targets PKB/Akt and the adenosine monophosphate(AMP)-activated protein kinase pathway, leading to cell cycle inactivation and cancer suppression.69
Studies of the effect of exercise on cancer development have been carried out in humans and in some animal models with various kinds of malignant tumors. In patients with renal cell carcinoma and pancreatic ductal carcinoma and melanoma, exercise can inhibit the up-regulation of the same microphthalmia/transcription factor E (MiT/TFE) gene, and the proliferation of cancer cells are thus ultimately inhibited.70 The inhibitory effect of exercise on cancer cell proliferation is mediated by mammalian target of rapamycin (mTOR) targets, which are expressed in the liver, brain, fat, and skeletal muscle.71 Exercise can also induce apoptosis of tumor cells in skeletal muscle. Related mechanisms for this process are as follows. Studies have shown that after chemotherapy, the muscles of mice are obviously atrophic, and exercise can inhibit muscle reduction, which can be achieved by restoring autophagy or mitosis caused by drugs and restoring mitochondrial function.72 Skeletal muscle produces myogenic IL-6 during the physical exercise process, which can reduce the activity and production of tumor necrosis factor α (TNF-α); in the meantime, IL-6 alleviates the fatigue caused by cancer.73 In mice models and human patients with colon cancer, regular exercise induces skeletal muscle to produce secreted protein acidic and rich in cysteine (SPARC), increases the cleavage of caspase-3 and caspase-8, promotes cell apoptosis, and inhibits colon cancer.74 Fig. 1 shows the mechanisms by which exercise inhibits cancer cell proliferation and induces apoptosis.
In conclusion, only moderate-intensity exercise exerts significant influence on cancer cell proliferation and apoptosis. Exercise can regulate insulin growth factor secretion, target Akt and mTOR passways, regulate skeletal muscle IL-6 activity, and improve mitochondrial function, all of which can inhibit cancer cell proliferation and induce apoptosis.
5.2. Exercise regulates cancer metabolism
Exercise can be used as a target of cancer-suppressor gene therapy to affect cancer metabolism and inhibit Warburg anaerobic glycolysis. The correlation between cancer metabolism and the host depends on the duration, time, intensity, and movement mode of the physical exercise.75 In a hypoxic environment, the decrease of vasoconstriction results in the increase of cancer perfusion and the decrease of cancer hypoxia. Cancer cells are less aggressive through exercise. It has been suggested that hypoxia is very important for cancer cell characteristics and cancer microenvironment invasiveness, and exercise weakens the hypoxia microenvironment in cancer.76 It has been reported that cancer hypoxia, with changes in the microenvironment, is regulated by exercise, which is manifested by the reduction of platelet-derived growth factor receptor β (PDGFRβ) antibody and the increase of the related factor, vascular endothelial growth factor (VEGF), in the microenvironment,39 as represented by the increase in micro-vascular density and micro vascular perfusion. Studies have shown that the levels of lactate, adrenaline, noradrenaline, and cytokines (including IL-6) in the blood increased significantly after 2 h of acute exercise, in which the levels of actin and catecholamine were induced by exercise, which directly regulated the growth of cancer. However, the actual cancer-inhibition effect is the accumulation of multiple bouts of sustained acute exercise.77
This review summarizes the effects of exercise on characteristic cancer cells, including activation of invasion and metastasis, escape from growth inhibitors, cell-death reduction, inhibition of cancer-inflammatory cells, vascular normalization, escape from immune injury, and reprogramming of energy metabolism.78 Lactic acid is the most important end metabolite in the glycolysis pathway. The enhanced aerobic glycolysis in cancer cells can produce a large amount of lactic acid, which can cause a decrease in pH in the microenvironment around cancer cells. Cancer cell proliferation, invasion, and metastasis are related to cancer angiogenesis, all of which are related to the low pH value in the cancer microenvironment. The accumulation of lactic acid in cancer patients suppresses the immune response, which inhibits the T cell response and prevents the output of lactic acid in T cell, thereby interfering with the metabolic pathway.79 It can be observed that lactate, as the immunosuppressive factor of T-cell inhibition, exerts an important effort on cancer escape. It is well known that lactate dehydrogenase (LDH) is related to the prognosis of malignant tumors, and a higher level of LDH often indicates a poor prognosis for patients with cancer. By changing the LDH level, moderate exercise can reduce the level of lactate in cells. Therefore, the above metabolic process plays a role in inhibiting the anaerobic glycolysis of cancer metabolism.80
AMP activated protein kinase (AMPK) is the monitoring point of metabolism. As an important center of homeostasis regulation of whole-body energy metabolism, it can inhibit cell growth in the absence of capacity and biosynthesis.81 In mice fed with a high-fat diet, physical activity stimulated AMPK, inhibited the Akt signal, increased cyclin-dependent kinase inhibitor (p27) phosphorylation and adiponectin receptor 1 (adiporR1) protein levels, led to cell cycle arrest, and reversed the proliferation of breast cancer cells.82 In an experiment that used exercise to induce hepatocarcinoma in mice, exercise reduced the proliferation of cancer cells and reduced the activity of mTOR kinase by stimulating the phosphorylation of AMPK and its substrate raptor.83 AMPK activation promotes the expression of glucose transporter 4 (GLUT4) and the hexokinase 2 (HK2) gene, which are the key intermediates of glucose treatment, and gene expression is realized by regulating the histone deacetylase 5 (HDAC5) and cyclic adenosine monophosphate (cAMP) reaction binding element protein.84,85 It has been found that physical activity can inhibit the occurrence of breast cancer by inhibiting the mTOR signaling pathway; the cancer metabolic reprogramming was inhibited, and physical activity limited the content of glucose and glutamine, thus inducing apoptosis.86 However, research on the relationship between the instability of the exercise genome and the heterogeneity between exercise and cancer has not yet been carried out.78 As shown in Fig. 1 and Fig. 2, physical exercise can regulate cancer metabolism.
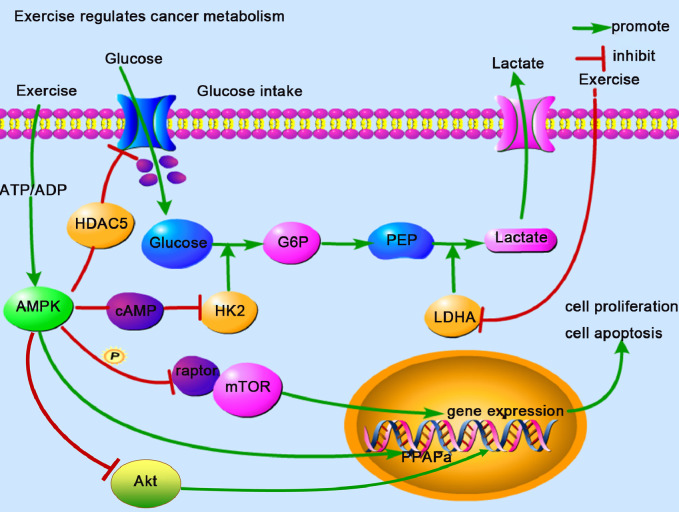
Fig. 2.
Physical exercise regulates cancer metabolism. Physical exercise can increase ADP concentrations, resulting in a reduction in ATP/ADP and downregulation in the expression of GLUT4 and HK2. These are key factors in regulating anaerobic glycolysis, which inhibits cancer cell proliferation and induces apoptosis. Lactate is a critical fuel for metabolites during physical exercise. LDH, especially LDHA, can be inhibited by exercise, which reduces lactate production in anaerobic glycolysis. ADP = adenosine diphosphate; AMPK = AMP activated protein kinase; ATP = adenosine triphosphate; cAMP = cyclic adenosine monophosphate; GLUT4 = glucose transporter 4; G6P = glucose-6-phosphate; HDAC5 = histone deacetylase 5; HK2 = hexokinase 2; LDH = lactate dehydrogenase; LDHA = lactate dehydrogenase A; mTOR = mammalian target of rapamycin; PEP = phosphoenolpyruvate; Akt = protein kinase B; PPAPa = 5′-O-pyrophosphoryladenylyl-(3′-5′)-adenosine.
In conclusion, exercise can affect cancer metabolizing reprogramming by improving cancer internal blood perfusion, angiogenesis, and cancer hypoxia. The direct effect of exercise on LDH in the cancer glycolysis pathway can indirectly affect changes in lactate levels, and exercise can induce apoptosis by activating the AMPK metabolic monitoring point and inhibiting the mTOR signal pathway.
5.3. Exercise regulates the immune environment
One important reason that the elderly are more likely to suffer from cancer is immune aging. Immune aging refers to the decline of NK cell function, the increase in inflammation, the damage to monocyte and dendritic cells in the uptake and presentation of antigens, the increase of aging cells with functional failure, and the decrease in the number of immature T cells responding to the evolving cancer cells. Exercise can prevent immune aging to some extent because it can stimulate the activity of immune NK cells, enhance antigen presentation, reduce inflammation, and prevent the accumulation of aging cells in the elderly.87 Therefore, physical exercise plays a preventive role in malignant tumors caused by immune aging.
In terms of mobilizing an autoimmune response with physical exercise, NK cells are the most sensitive to exercise-dependent mobilization, with T cell, including Cluster of Differentiation (CD) 8 and CD4, being next, and B cells being least sensitive.88 However, 2 kinds of immune cells regulate the effect process through physical exercise. NK cells, as the most sensitive immune mobilization cells, are the key component of innate immune defense, while T cells are cytotoxic cells and participate in the adaptive immune response. The reason NK cells can respond rapidly is not only because they have high reactivity to exercise,51 but also because they activate parts of natural killer group 2 member D (NKG2D) and the minor histocompatibility antigen 60 (H60a), as well as the ligand C-type lectin-related protein B (Clr-b) of natural killer receptor proteins 1 ligand (NKR-P1B).89, 90, 91 A study in mice by Pedersen et al.51 showed that the mobilization of adrenaline-dependent NK cells led to a significant increase in immune-cell infiltration, which plays a role in cancer inhibition. Also, given that the β adrenergic receptor blocker propranolol blocks the immune response of NK cell mobilization, the inhibition of cancer growth in the mice was weakened, the increase of NK cell infiltration was inhibited, and the inhibition of cancer was reduced.51 It has been suggested that the adrenergic signal is inhibited by motor dependence during cancer growth. In addition, cancer immunogenicity can be enhanced through targeted regulation of cancer metabolism.92 It has been shown that NK cell mobilization and activation, as well as blood perfusion and cancer cell apoptosis, can be induced by anti cancer methods assisted by exercise and increasing body core temperature.36 Physical exercise can act as an anti-inflammatory. The change of immune components to an anti-inflammatory state can enhance immunity. Exercise can reduce fat production and increase anti-inflammatory regulatory T cells, which can reduce the regulation of systemic inflammation, thus releasing anti-inflammatory cytokines that play an anti-inflammatory role.93 This process may occur through systemic mechanism changes, including inflammatory state reduction and other mechanisms, such as the release of anti-inflammatory cytokines and catecholamine and the inhibition of pro-inflammatory cytokines during muscle contraction.94 Acute or chronic exercise makes cytokines respond. Cytokines stimulate immune cells in the cancer microenvironment, thus creating different reactions that promote or inhibit cancer. Immunophenotypes in the cancer microenvironment change with the change in cytokines.95
In one study, in the course of systemic immune response, the apoptotic lymphocytes in peripheral blood caused by acute exercise increased significantly, and the lymphocytes of aging and memory, CD4+, CD8+, and T increased.96 As for long-term effects, exercise leads to an increase in CD28 and T lymphocyte levels in young and old patients.96 Research on the T cell action principle of mobilization in immune response showed that the expression of anti-inflammatory cytokines did not change with moderate-intensity exercise, and the expression of pro-inflammatory cytokines and antigen-specific, cell-mediated immunity increased, which played a role in promoting validation reaction and reducing cancer risk. High-intensity exercise could reduce T cell proliferation and antigen sheet cytotoxic reaction. Increasing the expression of inflammatory cytokines and the ratio of CD4+, CD25+, and Treg cells increased the risk of infection.97 High-intensity exercise is not the best way to inhibit cancer development; only moderate-intensity exercise inhibits cancer development. As shown in Fig. 1, physical exercise regulates the immune environment.
In conclusion, exercise can stimulate the immune-cell mobilization response in the cancer microenvironment, including the infiltration of immune NK cells, the immune anti-inflammatory effect of cytokines, and the proliferation of T cell, thus inhibiting cancer cells and preventing immune aging.
6. Conclusion
Studies have shown that physical exercise can reduce cancer incidence. It can inhibit cancer growth and metastasis, improve the side effects resulting from cancer treatment, improve patients’ tolerance to treatment, and improve their quality of life. Only through a deeper understanding of the influence of physical exercise on the growth and development of malignancy, metabolic pathways, and systemic immune functions can we fully understand the correlation between exercise and cancer prevention and treatment and, thus, make personalized exercise treatment plans for patients with cancer. In addition to having positive effects, exercise is of great value in the treatment of other diseases and the heterogeneity of special cancers.
Nevertheless, it is still necessary to study the molecular mechanisms of exercise in more detail. In addition to the mechanisms discussed above, some less well known but important mechanisms need more extensive attention, for example, the effects and mechanisms of exercise training on markers of oxidative status. Moreover, the mechanisms described above need further study in relation to cancer, including the interaction between metabolism and immune regulation during exercise.
Authors’ contributions
WZ generated construction, drafted the main part of the paper, conducted the literature review, and provided comments on the manuscript content; QW generated construction and drafted the paper. Both authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
References
- 1.Rundqvist H, Augsten M, Strömberg A. Effect of acute exercise on prostate cancer cell growth. PLoS One. 2013;8:e67579. doi: 10.1371/journal.pone.0067579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magné N, Melis A, Chargari C. Recommendations for a lifestyle which could prevent breast cancer and its relapse: physical activity and dietetic aspects. Crit Rev Oncol Hematol. 2011;80:450–459. doi: 10.1016/j.critrevonc.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 3.Desnoyers A, Riesco E, Fülöp T, Pavic M. Physical activity and cancer: update and literature review. Rev Med Interne. 2016;37:399–405. doi: 10.1016/j.revmed.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 4.Goncalves AK, Dantas Florencio GL, Maisonnette de Atayde Silva MJ, Cobucci RN, Giraldo PC, Cote NM. Effects of physical activity on breast cancer prevention: a systematic review. J Phys Act Health. 2014;11:445–454. doi: 10.1123/jpah.2011-0316. [DOI] [PubMed] [Google Scholar]
- 5.Dias Reis A, Silva Garcia JB, Rodrigues Diniz R. Effect of exercise training and detraining in autonomic modulation and cardiorespiratory fitness in breast cancer survivors. J Sports Med Phys Fitness. 2017;57:1062–1068. doi: 10.23736/S0022-4707.17.07012-8. [DOI] [PubMed] [Google Scholar]
- 6.Liu L, Shi Y, Li T. Leisure time physical activity and cancer risk: evaluation of the WHO's recommendation based on 126 high-quality epidemiological studies. Br J Sports Med. 2016;50:372–378. doi: 10.1136/bjsports-2015-094728. [DOI] [PubMed] [Google Scholar]
- 7.Psaltopoulou T, Ntanasis-Stathopoulos I, Tzanninis IG, Kantzanou M, Georgiadou D, Sergentanis TN. Physical activity and gastric cancer risk: a systematic review and meta-analysis. Clin J Sport Med. 2016;26:445–464. doi: 10.1097/JSM.0000000000000316. [DOI] [PubMed] [Google Scholar]
- 8.Friedenreich C, Cust A, Lahmann PH. Physical activity and risk of endometrial cancer: the European prospective investigation into cancer and nutrition. Int J Cancer. 2007;121:347–355. doi: 10.1002/ijc.22676. [DOI] [PubMed] [Google Scholar]
- 9.Schmid D, Behrens G, Keimling M, Jochem C, Ricci C, Leitzmann M. A systematic review and meta-analysis of physical activity and endometrial cancer risk. Eur J Epidemiol. 2015;30:397–412. doi: 10.1007/s10654-015-0017-6. [DOI] [PubMed] [Google Scholar]
- 10.Behrens G, Jochem C, Keimling M, Ricci C, Schmid D, Leitzmann MF. The association between physical activity and gastroesophageal cancer: systematic review and meta-analysis. Eur J Epidemiol. 2014;29:151–170. doi: 10.1007/s10654-014-9895-2. [DOI] [PubMed] [Google Scholar]
- 11.Moore SC, Lee IM, Weiderpass E. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med. 2016;176:816–825. doi: 10.1001/jamainternmed.2016.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keimling M, Behrens G, Schmid D, Jochem C, Leitzmann MF. The association between physical activity and bladder cancer: systematic review and meta-analysis. Br J Cancer. 2014;110:1862–1870. doi: 10.1038/bjc.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmid D, Ricci C, Behrens G, Leitzmann MF. Does smoking influence the physical activity and lung cancer relation? A systematic review and meta-analysis. Eur J Epidemiol. 2016;31:1173–1190. doi: 10.1007/s10654-016-0186-y. [DOI] [PubMed] [Google Scholar]
- 14.Jones LW, Kwan ML, Weltzien E. Exercise and prognosis on the basis of clinicopathologic and molecular features in early-stage breast cancer: the LACE and Pathways studies. Cancer Res. 2016;76:5415–5422. doi: 10.1158/0008-5472.CAN-15-3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colbert LH, Westerlind KC, Perkins SN. Exercise effects on tumorigenesis in a p53-deficient mouse model of breast cancer. Med Sci Sports Exerc. 2009;41:1597–1605. doi: 10.1249/MSS.0b013e31819f1f05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christensen JF, Simonsen C, Hojman P. Exercise training in cancer control and treatment. Compr Physiol. 2018;9:165–205. doi: 10.1002/cphy.c180016. [DOI] [PubMed] [Google Scholar]
- 17.Courneya KS, McKenzie DC, Mackey JR. Effects of exercise dose and type during breast cancer chemotherapy: multicenter randomized trial. J Natl Cancer Inst. 2013;105:1821–1822. doi: 10.1093/jnci/djt297. [DOI] [PubMed] [Google Scholar]
- 18.Schmielau J, Rick O, Reuss-Borst M, Kalusche-Bontemps EM, Steimann M. Rehabilitation of cancer survivors with long-term toxicities. Oncol Res Treat. 2017;40:764–771. doi: 10.1159/000485187. [DOI] [PubMed] [Google Scholar]
- 19.Dennett AM, Peiris CL, Shields N, Prendergast LA, Taylor NF. Moderate-intensity exercise reduces fatigue and improves mobility in cancer survivors: a systematic review and meta-regression. J Physiother. 2016;62:68–82. doi: 10.1016/j.jphys.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. 2012;11 doi: 10.1002/14651858.CD006145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodgers C, Sanborn C, Taylor O. Fatigue and oxidative stress in children undergoing leukemia treatment. Biol Res Nurs. 2016;18:515–520. doi: 10.1177/1099800416647794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomez-Pinilla F, Hillman C. The Influence of exercise on cognitive abilities. Compr Physiol. 2013;3:403–428. doi: 10.1002/cphy.c110063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li C, Zhou C, Li R. Can exercise ameliorate aromatase inhibitor-induced cognitive decline in breast cancer patients? Mol Neurobiol. 2016;53:4238–4246. doi: 10.1007/s12035-015-9341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gentry AL, Erickson KI, Sereika SM. Protocol for Exercise Program in Cancer and Cognition (EPICC): a randomized controlled trial of the effects of aerobic exercise on cognitive function in postmenopausal women with breast cancer receiving aromatase inhibitor therapy. Contemp Clin Trials. 2018;67:109–115. doi: 10.1016/j.cct.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fornusek CP, Kilbreath SL. Exercise for improving bone health in women treated for stages I–III breast cancer: a systematic review and meta-analyses. J Cancer Surviv. 2017;11:525–541. doi: 10.1007/s11764-017-0622-3. [DOI] [PubMed] [Google Scholar]
- 26.Ginzac A, Passildas J, Gadea E. Treatment-induced cardiotoxicity in breast cancer: a review of the interest of practicing a physical activity. Oncology. 2019;96:223–234. doi: 10.1159/000499383. [DOI] [PubMed] [Google Scholar]
- 27.Antoun S, Raynard B. Muscle protein anabolism in advanced cancer patients: response to protein and amino acids support, and to physical activity. Annal Oncol. 2018;29:ii10–ii17. doi: 10.1093/annonc/mdx809. [DOI] [PubMed] [Google Scholar]
- 28.Glass OK, Ramalingam S, Harrison MR. Resistance exercise training in patients with genitourinary cancers to mitigate treatment-related skeletal muscle loss. Clin Adv Hematol Oncol. 2016;14:436–446. [PubMed] [Google Scholar]
- 29.Mundell NL, Daly RM, Macpherson H, Fraser SF. Cognitive decline in prostate cancer patients undergoing ADT: a potential role for exercise training. Endocr Relat Cancer. 2017;24:R145–R155. doi: 10.1530/ERC-16-0493. [DOI] [PubMed] [Google Scholar]
- 30.Repka CP, Peterson BM, Brown JM, Lalonde TL, Schneider CM, Hayward R. Cancer type does not affect exercise-mediated improvements in cardiorespiratory function and fatigue. Integr Cancer Ther. 2014;13:473–481. doi: 10.1177/1534735414547108. [DOI] [PubMed] [Google Scholar]
- 31.Schmitz KH, Courneya KS, Matthews C. American College of Sports Medicine Roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 32.Cavalheri V, Granger C. Preoperative exercise training for patients with non-small cell lung cancer. Cochrane Database Syst Rev. 2017;6 doi: 10.1002/14651858.CD012020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horowitz M, Neeman E, Sharon E, Ben-Eliyahu S. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat Rev Clin Oncol. 2015;12:213–226. doi: 10.1038/nrclinonc.2014.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rief H, Petersen LC, Omlor G. The effect of resistance training during radiotherapy on spinal bone metastases in cancer patients: a randomized trial. Radiother Oncol. 2014;112:133–139. doi: 10.1016/j.radonc.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Dufresne S, Guéritat J, Chiavassa S. Exercise training improves radiotherapy efficiency in a murine model of prostate cancer. FASEB J. 2020;34:4984–4996. doi: 10.1096/fj.201901728R. [DOI] [PubMed] [Google Scholar]
- 36.Idorn M, Hojman P. Exercise-dependent regulation of NK cells in cancer protection. Trends Mol Med. 2016;22:565–577. doi: 10.1016/j.molmed.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 37.Shin DS, Ribas A. The evolution of checkpoint blockade as a cancer therapy: what's here, what's next? Curr Opin Immunol. 2015;33:23–35. doi: 10.1016/j.coi.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Spranger S, Koblish HK, Horton B, Scherle PA, Newton R, Gajewski TF. Mechanism of tumor rejection with doublets of CTLA-4, PD-1/PD-L1, or IDO blockade involves restored IL-2 production and proliferation of CD8+T cells directly within the tumor microenvironment. J Immunother Cancer. 2014;2:3. doi: 10.1186/2051-1426-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Betof AS, Lascola CD, Weitzel D. Modulation of murine breast tumor vascularity, hypoxia, and chemotherapeutic response by exercise. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schadler KL, Thomas NJ, Galie PA. Tumor vessel normalization after aerobic exercise enhances chemotherapeutic efficacy. Oncotarget. 2016;7:65429–65440. doi: 10.18632/oncotarget.11748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel AV, Friedenreich CM, Moore SC. American College of Sports Medicine roundtable report on physical activity, sedentary behavior, and cancer prevention and control. Med Sci Sports Exerc. 2019;51:2391–2402. doi: 10.1249/MSS.0000000000002117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmitz KH, Campbell AM, Stuiver MM. Exercise is medicine in oncology: engaging clinicians to help patients move through cancer. CA Cancer J Clin. 2019;69:468–484. doi: 10.3322/caac.21579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nechuta S, Chen WY, Cai H. A pooled analysis of post-diagnosis lifestyle factors in association with late estrogen-receptor-positive breast cancer prognosis. Int J Cancer. 2016;138:2088–2097. doi: 10.1002/ijc.29940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friedenreich CM, Wang Q, Neilson HK, Kopciuk KA, McGregor SE, Courneya KS. Physical activity and survival after prostate cancer. Eur Urol. 2016;70:576–585. doi: 10.1016/j.eururo.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 45.Meyerhardt JA, Heseltine D, Niedzwiecki D. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006;24:3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 46.Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc. 2008;67:253–256. doi: 10.1017/S002966510800712X. [DOI] [PubMed] [Google Scholar]
- 47.Meyerhardt JA, Giovannucci EL, Holmes MD. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24:3527–3534. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 48.Newton RU, Kenfield SA, Hart NH. Intense exercise for survival among men with metastatic castrate-resistant prostate cancer (INTERVAL-GAP4): a multicentre, randomised, controlled phase III study protocol. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2018-022899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Santi M, Baldelli G, Lucertini F, Natalucci V, Brandi G, Barbieri E. A dataset on the effect of exercise-conditioned human sera in three-dimensional breast cancer cell culture. Data Brief. 2019;27 doi: 10.1016/j.dib.2019.104704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu J, Yin X, Jiang J. Sports-induced blood sugar utilization prevents development of pancreatic ductal adenocarcinoma. Tumour Biol. 2015;36:663–667. doi: 10.1007/s13277-014-2684-4. [DOI] [PubMed] [Google Scholar]
- 51.Pedersen L, Idorn M, Olofsson GH. Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metab. 2016;23:554–562. doi: 10.1016/j.cmet.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 52.Theriau CF, Shpilberg Y, Riddell MC, Connor MK. Voluntary physical activity abolishes the proliferative tumor growth microenvironment created by adipose tissue in animals fed a high fat diet. J Appl Physiol (1985) 2016;121:139–153. doi: 10.1152/japplphysiol.00862.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Westerlind KC, Mccarty HL, Gibson KJ, Strange R. Effect of exercise on the rat mammary gland: implications for carcinogenesis. Acta Physiol Scand. 2002;175:147–156. doi: 10.1046/j.1365-201X.2002.00980.x. [DOI] [PubMed] [Google Scholar]
- 54.Cohen LA, Boylan E, Epstein M, Zang E. Voluntary exercise and experimental mammary cancer. Adv Exp Med Biol. 1992;322:41–59. doi: 10.1007/978-1-4684-7953-9_5. [DOI] [PubMed] [Google Scholar]
- 55.Siewierska K, Malicka I, Kobierzycki C. The impact of exercise training on breast cancer. In Vivo. 2018;32:249–254. doi: 10.21873/invivo.11231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sáez MDC, Barriga C, García JJ, Rodríguez AB, Ortega E. Exercise-induced stress enhances mammary tumor growth in rats: beneficial effect of the hormone melatonin. Mol Cell Biochem. 2007;294:19–24. doi: 10.1007/s11010-005-9067-5. [DOI] [PubMed] [Google Scholar]
- 57.Thompson HJ. Effect of exercise intensity and duration on the induction of mammary carcinogenesis. Cancer Res. 1994;54(Suppl. 7):S1960–S1963. [PubMed] [Google Scholar]
- 58.Thompson HJ, Westerlind KC, Snedden J, Briggs S, Singh M. Exercise intensity dependent inhibition of 1-methyl-l-nitrosourea induced mammary carcinogenesis in female F-344 rats. Carcinogenesis. 1995;16:1783–1786. doi: 10.1093/carcin/16.8.1783. [DOI] [PubMed] [Google Scholar]
- 59.Thompson HJ, Ronan AM, Ritacco KA, Tagliaferro AR, Meeker LD. Effect of exercise on the induction of mammary carcinogenesis. Cancer Res. 1988;48:2720–2723. [PubMed] [Google Scholar]
- 60.Zhang QB, Zhang BH, Zhang KZ. Moderate swimming suppressed the growth and metastasis of the transplanted liver cancer in mice model: with reference to nervous system. Oncogene. 2016;35:4122–4131. doi: 10.1038/onc.2015.484. [DOI] [PubMed] [Google Scholar]
- 61.Yu FX, Zhang Y, Park HW. Protein kinase A activates the Hippo pathway to modulate cell proliferation and differentiation. Genes Dev. 2013;27:1223–1232. doi: 10.1101/gad.219402.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Badouel C, McNeill H. SnapShot: the Hippo signaling pathway. Cell. 2011;145:484–484.e1. doi: 10.1016/j.cell.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 63.Krizanova O, Babula P, Pacak K. Stress, catecholaminergic system and cancer. Stress. 2016;19:419–428. doi: 10.1080/10253890.2016.1203415. [DOI] [PubMed] [Google Scholar]
- 64.Chen D, Sun Y, Wei Y. LIFR is a breast cancer metastasis suppressor upstream of the Hippo-YAP pathway and a prognostic marker. Nat Med. 2012;18:1511–1517. doi: 10.1038/nm.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jiang Y, Wang WQ. Potential mechanisms of cancer prevention by weight control. Biophys Rev Lett. 2008;3:421–437. [Google Scholar]
- 66.Hankinson SE, Willett WC, Colditz GA. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. The Lancet. 1998;351:1393–1396. doi: 10.1016/S0140-6736(97)10384-1. [DOI] [PubMed] [Google Scholar]
- 67.Irwin ML, Varma K, Alvarez-Reeves M. Randomized controlled trial of aerobic exercise on insulin and insulin-like growth factors in breast cancer survivors: the Yale Exercise and Survivorship study. Cancer Epidemiol Biomarkers Prev. 2009;18:306–313. doi: 10.1158/1055-9965.EPI-08-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nickerson T, Huynh H, Pollak M. Insulin-like growth factor binding protein-3 induces apoptosis in MCF7 breast cancer cells. Biochem Biophys Res Commun. 1997;237:690–693. doi: 10.1006/bbrc.1997.7089. [DOI] [PubMed] [Google Scholar]
- 69.Xie L, Wang W. Weight control and cancer preventive mechanisms: role of insulin growth factor-1-mediated signaling pathways. Exp Biol Med (Maywood) 2013;238:127–132. doi: 10.1177/1535370213477602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Malta CD, Siciliano D, Calcagni A. Transcriptional activation of RagD GTPase controls mTORC1 and promotes cancer growth. Science. 2017;356:1188–1192. doi: 10.1126/science.aag2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Watson K, Baar K. mTOR and the health benefits of exercise. Semin Cell Dev Biol. 2014;36:130–139. doi: 10.1016/j.semcdb.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 72.Ballaro R, Beltra M, De Lucia S. Moderate exercise in mice improves cancer plus chemotherapy-induced muscle wasting and mitochondrial alterations. FASEB J. 2019;33:5482–5494. doi: 10.1096/fj.201801862R. [DOI] [PubMed] [Google Scholar]
- 73.Wood LJ, Nail LM, Winters KA. Does muscle-derived interleukin-6 mediate some of the beneficial effects of exercise on cancer treatment-related fatigue? Oncol Nurs Forum. 2009;36:519–524. doi: 10.1188/09.ONF.519-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aoi W, Naito Y, Takagi T. A novel myokine, secreted protein acidic and rich in cysteine (SPARC), suppresses colon tumorigenesis via regular exercise. Gut. 2013;62:882–889. doi: 10.1136/gutjnl-2011-300776. [DOI] [PubMed] [Google Scholar]
- 75.Koelwyn GJ, Quail DF, Zhang X, White RM, Jones LW. Exercise-dependent regulation of the tumour microenvironment. Nat Rev Cancer. 2017;17:620–632. doi: 10.1038/nrc.2017.78. [DOI] [PubMed] [Google Scholar]
- 76.McCullough DJ, Stabley JN, Siemann DW, Behnke BJ. Modulation of blood flow, hypoxia, and vascular function in orthotopic prostate tumors during exercise. J Natl Cancer Inst. 2014;106:dju036. doi: 10.1093/jnci/dju036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dethlefsen C, Lillelund C, Midtgaard J. Exercise regulates breast cancer cell viability: systemic training adaptations versus acute exercise responses. Breast Cancer Res Treat. 2016;159:469–479. doi: 10.1007/s10549-016-3970-1. [DOI] [PubMed] [Google Scholar]
- 78.Ruiz-Casado A, Martin-Ruiz A, Perez LM, Provencio M, Fiuza-Luces C, Lucia A. Exercise and the hallmarks of cancer. Trends Cancer. 2017;3:423–441. doi: 10.1016/j.trecan.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 79.Renner K, Singer K, Koehl GE. Metabolic hallmarks of tumor and immune cells in the tumor microenvironment. Front Immunol. 2017;8:248. doi: 10.3389/fimmu.2017.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aveseh M, Nikooie R, Aminaie M. Exercise-induced changes in tumour LDH-B and MCT1 expression are modulated by oestrogen-related receptor alpha in breast cancer-bearing BALB/c mice. J Physiol. 2015;593:2635–2648. doi: 10.1113/JP270463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gwinn DM, Shackelford DB, Egan DF. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Theriau CF, Connor MK. Voluntary physical activity counteracts the proliferative tumor growth microenvironment created by adipose tissue via high-fat diet feeding in female rats. Phy Rep. 2017;5:e13325. doi: 10.14814/phy2.13325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Piguet AC, Saran U, Simillion C. Regular exercise decreases liver tumors development in hepatocyte-specific PTEN-deficient mice independently of steatosis. J Hepatol. 2015;62:1296–1303. doi: 10.1016/j.jhep.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 84.Mcgee SL, van Denderen BJW, Howlett KF. AMP-activated protein kinase regulates GLUT4 transcription by phosphorylating histone deacetylase 5. Diabetes. 2008;57:860–867. doi: 10.2337/db07-0843. [DOI] [PubMed] [Google Scholar]
- 85.Thomson DM, Herway ST, Fillmore N. AMP-activated protein kinase phosphorylates transcription factors of the CREB family. J Appl Physiol. 2008;104:429–438. doi: 10.1152/japplphysiol.00900.2007. [DOI] [PubMed] [Google Scholar]
- 86.Thompson HJ, Jiang W, Zhu Z. Candidate mechanisms accounting for effects of physical activity on breast carcinogenesis. IUBMB Life. 2009;61:895–901. doi: 10.1002/iub.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bigley AB, Spielmann G, LaVoy ECP, Simpson RJ. Can exercise-related improvements in immunity influence cancer prevention and prognosis in the elderly? Maturitas. 2013;76:51–56. doi: 10.1016/j.maturitas.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 88.Walsh NP, Gleeson M, Shephard RJ. Position statement. Part one: immune function and exercise. Exerc Immunol Rev. 2011;17:6–63. [PubMed] [Google Scholar]
- 89.Zimmer P, Bloch W, Schenk A. Exercise-induced natural killer cell activation is driven by epigenetic modifications. Int J Sports Med. 2015;36:510–515. doi: 10.1055/s-0034-1398531. [DOI] [PubMed] [Google Scholar]
- 90.Chen P, Aguilar OA, Rahim MMA. Genetic investigation of MHC-independent missing-self recognition by mouse NK cells using an in vivo bone marrow transplantation model. J Immunol. 2015;194:2909–2918. doi: 10.4049/jimmunol.1401523. [DOI] [PubMed] [Google Scholar]
- 91.Rahim MMA, Chen P, Mottashed AN. The mouse NKR-P1B: Clr-b recognition system is a negative regulator of innate immune responses. Blood. 2015;125:2217–2227. doi: 10.1182/blood-2014-02-556142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Haas R, Smith J, Rocher-Ros V. Lactate regulates metabolic and pro-inflammatory circuits in control of T cell migration and effector functions. PLoS Bio. 2015;13 doi: 10.1371/journal.pbio.1002202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mancuso P. The role of adipokines in chronic inflammation. Immunotargets Ther. 2016;5:47–56. doi: 10.2147/ITT.S73223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Goh J, Kirk EA, Shu XL, Ladiges WC. Exercise, physical activity and breast cancer: the role of tumor-associated macrophages. Exerc Immunol Rev. 2012;18:158–176. [PubMed] [Google Scholar]
- 96.Cao DH, Beyer I, Mets T. Effects of physical exercise on markers of cellular immunosenescence: a systematic review. Calcif Tissue Int. 2017;100:193–215. doi: 10.1007/s00223-016-0212-9. [DOI] [PubMed] [Google Scholar]
- 97.Wang J, Song H, Tang X. Effect of exercise training intensity on murine T-regulatory cells and vaccination response. Scand J Med Sci Sports. 2012;22:643–652. doi: 10.1111/j.1600-0838.2010.01288.x. [DOI] [PubMed] [Google Scholar]