Abstract
Background:
In recent years, a number of studies have begun to explore the nature of Attention-Deficit/Hyperactivity Disorder (ADHD) in children with Autism Spectrum Disorder (ASD). In this study, we examined the relationship between both symptoms of ADHD and symptoms of ASD on cognitive task performance in a sample of higher-functioning children and adolescents with ASD. Participants completed cognitive tasks tapping aspects of attention, impulsivity/inhibition, and immediate memory.
Aims.
We hypothesized that children with ASD who had higher levels of ADHD symptom severity would be at higher risk for poorer sustained attention and selective attention, greater impulsivity/disinhibition, and weaker memory.
Methods and Procedures:
The sample included 92 children (73 males) diagnosed with ASD (Mean Age=9.41 years; Mean Full Scale IQ=84.2).
Outcomes and Results:
Using regression analyses, more severe ADHD symptomatology was found to be significantly related to weaker performance on tasks measuring attention, immediate memory, and response inhibition. In contrast, increasing severity of ASD symptomatology was not associated with higher risk of poorer performance on any of the cognitive tasks assessed.
Conclusions and Implications:
These results suggest that children with ASD who have more severe ADHD symptoms are at higher risk for impairments in tasks assessing attention, immediate memory, and response inhibition—similar to ADHD-related impairments seen in the general pediatric population. As such, clinicians should assess various aspects of cognition in pediatric patients with ASD in order to facilitate optimal interventional and educational planning.
Keywords: Autism Spectrum Disorder, Attention-Deficit/Hyperactivity Disorder, attention, impulsivity, memory, cognitive task
1. Introduction
Autism Spectrum Disorder (ASD) and Attention-Deficit/Hyperactivity Disorder (ADHD) are lifelong neurodevelopmental disorders with prevalence rates as high as 2% and 8.8%, respectively (Schieve et al., 2012; Pastor & Reuben, 2008; Thomas et al., 2015). ASD and ADHD are also known to co-occur at high rates. Clinically significant levels of ADHD symptoms are prevalent in ASD and range from 28–87% across research samples (Ames & White, 2011; Frazier et al., 2001; Mansour et al., 2017 Ponde, Novaes, & Losapio, 2010; Sinzig, Walter, & Doepfner, 2009; Simonoff et al., 2008). Thus, the frequent co-occurrence of these disorders has prompted much research into the specific features associated with them.
The detrimental impact of comorbid ADHD symptomatology in individuals with ASD has been more widely studied in recent years. Both disorders cause impairments in a number of areas. For instance, children with both ASD and ADHD had higher rates of comorbid psychiatric symptoms compared to those with ASD alone (Jang et al., 2013; Mansour et al., 2017). Additionally, ADHD symptoms have been found to further impair the cognitive functioning and overall psychiatric adjustment in children with ASD (Frazier et al., 2001; Gadow, DeVincent, & Pomeroy, 2006; Lecavalier, et al., 2009; Yoshida & Uchiyama, 2004). ADHD symptoms comorbid with ASD place children at a higher risk for psychiatric hospitalization (Frazier et al., 2001), predict more difficulties with adaptive and daily functioning impairment (Yerys et al., 2009), and are positively correlated with receiving mental health services (Bryson, et al., 2008).
While ASD and ADHD are both categorized as “Neurodevelopmental Disorders” in the DSM-5 (APA, 2013), these two disorders are defined by different core symptom profiles. ADHD is characterized by attention problems, hyperactivity, and impulsivity, while ASD is defined by impaired social functioning, communication, and restricted and stereotyped patterns of behavior (APA, 2013). In addition to these core symptoms, there are additional common features of ASD that are not as common to ADHD (e.g., intellectual disability and speech delay). Yet, despite these distinct developmental features, the high rates of comorbidity and evidence for a shared inherited liability (Musser et al., 2014; Rommelse at al., 2010) continues to prompt investigation into how the individual clinical domains (i.e., ADHD and ASD symptoms) predict exacerbated impairments in the comorbid ASD+ADHD population.
Overall, research findings on possible cognitive deficits in youth with ASD and comorbid ADHD are not entirely consistent. Several past studies examined whether different profiles of cognitive performance were evident between groups with either disorder (i.e., ASD vs ADHD). These studies concluded that both groups tend to perform worse on cognitive tasks than typically developing children; however, children with ADHD exhibited more pronounced problems with inhibitory control and working memory while children with ASD exhibited more problematic cognitive flexibility and planning skills relative to typically-developing youth (Geurts et al., 2004; Happé, Booth, Charlton, Hughes, 2006; Ozonoff & Jensen. 1999; Pennington & Ozonoff, 1996; Sinzig, 2009). In contrast, others have found areas of weak cognitive performance that are shared across both groups, perhaps with more intense impairments in children with ASD, relative to children with ADHD (Corbett, Constantine, Hendren, & Ozonoff, 2009; Geurts et al., 2004). Overall, the literature suggests that problematic cognitive performance is associated with both disorders while evidence for distinct cognitive impairment profiles specific to each disorder are less consistently obtained (Pennington & Ozonoff, 1996; Sergeant, Geurts, Oosterlaan, 2002).
Some studies have emphasized a dimensional approach by examining the potential contribution of relative severity of ADHD and ASD symptomatology to cognitive underperformance. Greater ADHD symptoms in those with ASD have been found to predict greater cognitive deficits, such as inhibitory control (Sinzig et al., 2009; van der Meere et al., 2012). In addition, others have identified deficits with sustained attention as being more linked to ADHD symptomatology than ASD features in those with ASD (Sinzig et al., 2008). Karalunas and colleagues (2018) examined three diagnostically defined groups (ASD, ADHD, typically developing controls) using latent class analysis in a large sample to examine the contributions of different levels of either ASD or ADHD symptoms to several domains of executive functioning. They concluded that both the ADHD and ASD symptom classes exhibited impairments in response inhibition, working memory, and processing speed that could not be differentiated based on the relative severity of symptoms from either disorder separately. They also posited that a single risk for weak executive functioning may contribute to both disorders. Thus, it remains unclear how ADHD symptom severity leads to the commonly observed ADHD cognitive deficits in a sample of individuals with comorbid ASD.
In summary, the literature strongly suggests that ADHD and ASD each contribute risk for suboptimal cognitive functioning, yet given the high rate of comorbidity, it is important to address their unique impact on cognitive outcomes. In this study, we have used a battery of cognitive performance tasks tapping sustained attention, inhibition, selective attention, impulsivity, and immediate memory that our group has used previously and that are sensitive to attentional impairments (i.e., ADHD) in children with developmental disabilities (e.g., Pearson et. al, 2004). We then analyzed whether ASD symptoms or ADHD symptoms were associated with a higher risk for greater performance decrements on these cognitive tasks. It should be noted that our sample did not exclude participants with IQ <70, who were excluded in some previous studies in this area (e.g. Karalunas et al., 2018; Ozonoff & Jensen, 1999). We felt that inclusion of these children created a sample that was more representative of the comorbid ASD+ADHD population in the community The first goal of this study was to examine the risk for cognitive task performance decrements associated with ADHD symptomatology (as measured with a parent report rating scale)—in children with ASD. The second goal of this study was to determine whether greater severity of autistic symptomatology in children with ASD is associated with higher levels of inattention and impulsivity.
2. Method
2.1. Participants
The participants in this study were from a larger NIH-funded study assessing cognitive and behavioral functioning in children with ASD (Mansour et al., 2017; Pearson et al., 2012, Pearson et al., 2013 Participants were recruited from the community, including local schools (special education programs, special needs schools), community agencies/clinics, and community advocacy groups. A screening phone interview was conducted using the Social Communication Questionnaire (SCQ; Rutter, Bailey & Lord, 2003) to screen for symptoms of autism. Participants with SCQ scores greater than or equal to 15 were invited to the clinic for the psychological assessment visit.
The specifics of the psychological assessment have been described previously (Mansour, Dovi, Lane, Loveland, & Pearson 2017; Pearson et al., 2012) (Pearson et al., 2013) and specific instruments are detailed below. Briefly, participants underwent a psychological assessment battery that included the Stanford-Binet, 5th Edition (Roid, 2003), a structured psychiatric interview with a parent, and norm-referenced questionnaires completed by both a parent and teacher. Written informed consent was obtained from parents and assent was obtained from those children who were able to provide it. This study was approved by the Committee for the Protection of Human Subjects at the University of Texas Health Science Center at Houston
Diagnostic measures included the Autism Diagnostic Interview-Revised (ADI-R; Lord, Rutter, & Le Couteur, 1994), the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2002) administered by research-reliable clinicians, and follow-up clinical interview by a licensed psychologist. Children were determined to meet DSM-IV-TR criteria for Autistic Disorder, Asperger’s Disorder, Pervasive Developmental Disorder-Not Otherwise Specified (PDD-NOS) through case review by two licensed psychologists who were highly experienced in assessing autism and related neurodevelopmental disorders (DAP and KAL). Exclusion criteria included having an IQ of less than 40, not having English as the primary language, and sensory and motor limitations that were severe enough to prevent adequate testing on the cognitive tasks.
As seen in Table 1, our sample consisted of 92 children (73 boys) between the ages of 6 and 13 years old (M=9.41) with a mean FSIQ of 84. Within this sample, 54 children met DSM-IV-TR criteria for Autistic Disorder, 21 met criteria for PDD-NOS, and 17 met criteria for Asperger’s Disorder. The children in this sample had a range of symptoms of ADHD severity, with T-scores on the Global Index of the Conners Parent Rating Scale, Revised-Long (CPRS) ranging from 37 to 90, and T-scores on the Global Index of the Conners Teacher Rating Scale, Revised-Long (CTRS) ranging from 45–90. Forty-seven participants (51%) were being treated with one or more psychoactive medications at study entry, including psychostimulants (n=29), antipsychotics (n=1), atypical antipsychotics (n=16), antidepressants (n=13), atomoxetine (n=4), antihypertensives (n=4), a mood stabilizer (n=1), and an anxiolytic (n=1). Nineteen children (19%) were taking two medications, and six children (6%) were receiving three or more. Although we recognized that children taking psychotropic medications were likely to have greater ADHD symptoms, if we had excluded them, we would have created a sample that was not representative of children with ASD (Aman et al, 1995).
Table 1.
Participant Characteristics
| Characteristic | n | % |
| Gender | ||
| Male | 73 | 79.3 |
| Female | 19 | 20.7 |
| Autism Diagnosis | ||
| Autistic Disorder | 54 | 58.7 |
| Asperger’s | 17 | 18.5 |
| PDD-NOS | 21 | 22.8 |
| Race/ethnicity | ||
| Caucasian | 56 | 60.9 |
| African American | 14 | 15.2 |
| Hispanic | 16 | 17.4 |
| Asian | 5 | 5.4 |
| “Other” or no data provided | 1 | 1.1 |
| Comorbid Diagnoses Assessed | ||
| ADHD | 80 | 87 |
| Predominantly Inattentive | 20 | 25 |
| Predominantly Hyperactive-Impulsive | 1 | 1 |
| Combined | 59 | 74 |
| Specific Phobia | 27 | 29 |
| Oppositional Defiant Diagnosis | 18 | 20 |
| Enuresis | 10 | 11 |
| Social Phobia | 6 | 7 |
| Encopresis | 2 | 2 |
| Dysthymic Disorder | 3 | 3 |
| Conduct Disorder | 2 | 2 |
| Generalized Anxiety Disorder | 2 | 2 |
| Major Depressive Disorder | 1 | 1 |
| Separation Anxiety Disorder | 1 | 1 |
| Anorexia Nervosa | 1 | 1 |
| Variable | Mean (SD) | Range |
| Age (years) | 9.41 (1.87) | 6.67–13.50 |
| SB5 Full Scale IQ | 84.18 (19.56) | 46–128 |
| SB5 Mental Age (years) | 7.94 (3.24) | 3.1–21 |
| ADI-R Sum of Subscales | 47.71 (10.90) | 23–66 |
| CPRS Global Index T Score | 68.91 (13.50) | 37–90 |
| CTRS Global Index T Score | 67.99 (12.11) | 45–90 |
2.2. Measures: Clinical Diagnosis/Characterization of the Sample
The Stanford-Binet Intelligence Scale, 5th Edition (SB5).
The SB5 was used to assess intellectual ability. The SB5 is a widely used, individually administered test normed for ages 2 through 80 years. It yields a measure of Full Scale IQ, Verbal IQ, and Nonverbal IQ. The SB5 has excellent reliability and validity (Roid, 2003b).
Diagnostic Interview for Children and Adolescents, 4th Edition-Parent Interview (DICA-IV).
The DICA-IV (Reich, 2000) is a structured psychiatric interview that was administered to parents or primary caregivers to assess major diagnostic categories. The computerized interview was then followed by a diagnostic interview conducted by a licensed psychologist (DAP). The DICA-IV has been found to be sensitive in children with developmental disabilities (Pearson et al., 2013). The DICA-IV, was used to establish diagnoses of ADHD and other comorbid psychiatric diagnoses (the focus of a previous paper from this group, Monsour et al., 2017). As noted previously (Mansour et al., 2017), an ADHD diagnosis was established if the child had sufficient DSM-IV-TR symptoms of ADHD, as reported by the parent on the DICA-IV and in a follow-up clinical interview with a licensed psychologist (disregarding the DSM-IV-TR prohibition of diagnosing ADHD in the context of autism), our behavioral observations, and T ≥ 65 on the Conners Parent and Teacher Rating Scales, Revised-Long (CPRS-R:L) ADHD Indexes (the ADHD Index being the most strongly associated with ADHD diagnosis; Conners, 1997). We found that 86% of sample met all of these criteria for a comorbid diagnosis of ADHD. Table 2 includes a list of additional comorbid conditions.
Table 2.
Comparative effects of ADHD symptom severity and ASD symptoms severity on Cognitive Tasks (with SB5 Mental Age as a Covariate)
| Task/Variable | Mean (SD) | Transformation Used | F | p | Partial Slope (b) | Incremental R2 |
|---|---|---|---|---|---|---|
| Continuous Performance Task (n=88) | ||||||
| Commissions | 4.19 (5.84) | ln(x+c) | ||||
| ADHD Severity | 4.48 | .037 | .012 | .046 | ||
| ASD Severity | 1.67 | .200 | −.009 | .017 | ||
| SB5 Mental Age (MA) | 7.60 | .007 | −.623 | .078 | ||
| Omissions | 1.59 (2.07) | −(1/x+c) | ||||
| ADHD Severity | 1.83 | .179 | .003 | .016 | ||
| ASD Severity | 0.30 | .585 | −.001 | .003 | ||
| MA | 25.88 | .000 | −.414 | .221 | ||
| Response Time | 557.99 (127.84) | ln(x+c) | ||||
| ADHD Severity | 0.01 | .914 | <001 | <.001 | ||
| ASD Severity | 0.06 | .813 | −.001 | <.001 | ||
| MA | 11.89 | 001 | −.226 | .124 | ||
| Speeded Classification Task (n=86) | ||||||
| Errors | 3.68 (3.16) | ln(x+c) | ||||
| ADHD Severity | 3.40 | .069 | .007 | .023 | ||
| ASD Severity | 0.56 | .455 | −.004 | .004 | ||
| MA | 57.44 | .000 | −1.12 | .397 | ||
| Response Time | 1037.65 (375.93) | N/A | ||||
| ADHD Severity | 3.35 | .071 | −4.860 | .031 | ||
| ASD Severity | 0.04 | .850 | −.646 | <.001 | ||
| MA | 24.08 | .000 | −522.50 | .221 | ||
| Dichotic Listening Task (n=71) | ||||||
| % Correct Responses | 78.08 (20.18) | N/A | ||||
| ADHD Severity | 0.80 | .373 | .006 | .007 | ||
| ASD Severity | 0.04 | .848 | .002 | <.001 | ||
| MA | 52.64 | .000 | −2.15 | .429 | ||
| Omission Errors | 0.13 (0.31) | −(1/(x+1)2) | ||||
| ADHD Severity | 0.15 | .702 | .001 | .002 | ||
| ASD Severity | 0.19 | .668 | .001 | .002 | ||
| MA | 17.90 | .000 | −.336 | .213 | ||
| Intrusion Errors | 0.69 (0.71) | N/A | ||||
| ADHD Severity | 1.83 | .180 | .007 | .019 | ||
| ASD Severity | 0.67 | .797 | .002 | .001 | ||
| MA | 27.00 | .000 | −1.22 | .275 | ||
| Delay of Gratification (n=86) | ||||||
| Overall Efficiency | 0.74 (0.20) | N/A | ||||
| ADHD Severity | 1.41 | .238 | −.002 | .014 | ||
| ASD Severity | 1.31 | .255 | .002 | .013 | ||
| MA | 18.44 | .000 | .244 | .182 | ||
| Correct responses | 46.06 (10.75) | N/A | ||||
| ADHD Severity | 0.04 | .845 | −.015 | <.001 | ||
| ASD Severity | 0.92 | .341 | −.094 | .009 | ||
| MA | 21.36 | .000 | 13.62 | .201 | ||
| Number of Responses | 67.13 (21.31) | N/A | ||||
| ADHD Severity | 0.45 | .503 | .116 | .005 | ||
| ASD Severity | 1.86 | .177 | −.304 | .022 | ||
| MA | .82 | .369 | −6.00 | .010 | ||
| Matching Familiar Figures Task (n=81) | ||||||
| Errors | 44.22 (18.38) | N/A | ||||
| ADHD Severity | 5.96 | .017 | .303 | .052 | ||
| ASD Severity | 0.63 | .429 | .126 | .006 | ||
| MA | 30.92 | .000 | −29.33 | .270 | ||
| Response Time | 6065.16 (3511.43) | N/A | ||||
| ADHD Severity | 2.38 | .127 | −43.875 | .030 | ||
| ASD Severity | 0.12 | .735 | 12.349 | .001 | ||
| MA | 0.01 | .913 | 133.190 | <.001 | ||
| Stop Signal Task (n=79) | ||||||
| Stop Signal RT | 427.20 (204.13) | sqrt(x+c) | ||||
| ADHD Severity | 1.90 | .172 | .051 | .020 | ||
| ASD Severity | 0.39 | .534 | −1.088 | .021 | ||
| MA | 17.77 | .000 | −6.30 | .189 | ||
| Stop Signal Accuracy | 50.85 (10.35) | N/A | ||||
| ADHD Severity | 2.49 | .119 | −.136 | .031 | ||
| ASD Severity | 0.32 | .571 | .061 | .004 | ||
| MA | 3.31 | .073 | 6.36 | .041 | ||
| Go Accuracy | 93.93 (6.11) | x3 | ||||
| ADHD Severity | 6.15 | .015 | −2783.421 | .065 | ||
| ASD Severity | 2.77 | .100 | 2292.715 | .029 | ||
| MA | 12.21 | .001 | 158416.39 | .129 | ||
| Stop Signal Delay | 362.57 (166.92) | N/A | ||||
| ADHD Severity | 0.56 | .456 | −1.045 | .007 | ||
| ASD Severity | 2.70 | .105 | 2.858 | .034 | ||
| MA | 1.36 | .247 | 66.61 | .017 | ||
| Delayed Match to Sample (n=90) | ||||||
| Response time | 1106.23 (567.45) | −(1/x+c) | ||||
| ADHD Severity | 4.87 | .030 | <.001 | .046 | ||
| ASD Severity | 0.28 | .596 | <−.001 | .003 | ||
| MA | 13.67 | .000 | .000 | .143 | ||
| Proportion Correct | 0.61 (0.19) | N/A | ||||
| ADHD Severity | 0.47 | .494 | .001 | .005 | ||
| ASD Severity | 0.02 | .889 | <.001 | <.001 | ||
| MA | 3.23 | .076 | .100 | .036 | ||
Please note: Mental age from the SB-5 served as a covariate in all models. ADHD severity = CPRS-R:L Global Index T Score. ASD severity = Total sum of ADI-R. Means are untransformed, while inferential statistics are reported as transformed data. Incremental R2 = difference between R2 with all variables in the model and the R2 with the variable of interest removed. The partial slope is the unstandardized regression coefficient.
The Autism Diagnostic Interview, Revised (ADI-R).
The ADI-R (Rutter, Le Couteur, & Lord, 2003), a semi-structured interview covering current and historical symptoms of autism, was administered to the primary caregiver of participants. It is based on both DSM-IV and ICD-10 criteria. Domains include: 1) reciprocal social interaction, 2) communication and language, and 3) restricted, repetitive, and interests. The interview yields scores on each of the three major domains, as well as a diagnostic algorithm. The sum of major domain scores served as the measure of ASD symptom severity.
Autism Diagnostic Observation Schedule (ADOS).
The ADOS (Lord, Rutter, DiLavore, & Risi, 2001) consists of a standard series of events (e.g., activities, conversations), observations, and codes of behavior that can yield a formal DSM IV/ICD-10 diagnosis of Autism. Domains that are assessed by the ADOS include social behaviors, communication, and restricted and repetitive behaviors. The ADOS yields subdomain scores as well as a total score reflecting the severity of ASD symptomatology.
Social Communication Questionnaire, Lifetime (SCQ).
The SCQ (Rutter, Bailey & Lord, 2003) is a 40-item, parent-report questionnaire used to screen for symptoms associated with ASD. It provides a total score that ranges from 0–40. Scores exceeding the cut-off score of 15 indicate that an individual may have ASD and suggests that a more comprehensive assessment should be completed.
Conners’ Rating Scales, Revised-Long (CPRS-R:L).
As we did previously ((Mansour et al., 2017), the Global Index from the CPRS-R:L (Conners, Sitarenios, Parker, & Epstein, 1998) was used to assess overall severity of ADHD symptomatology. The CPRS-R: L is a widely used screening measure that assesses symptoms of ADHD and of other behavioral/emotional disorders frequently associated with ADHD (e.g., oppositional behavior, social problems). The Conners scales are normed for children of ages 3–17 years. Only the parent Global Index was used, as we have demonstrated previously that parent and teacher ratings of ADHD symptoms in this population have high concordance (Pearson et al., 2012).
2.3. Measures: Cognitive Tasks
The tasks used in this investigation were selected on the basis of their demonstrated ability to assess sustained attention, selective attention, impulsivity/inhibition, and immediate memory in children with attention deficits, as well as their appropriateness for the cognitive developmental level of the children. (e.g., Pearson et al., 1996; Pearson et al., 2004).
Sustained Attention:
Continuous Performance Test.
On this version of the classic CPT (Rosvold et al., 1956), participants were presented with a series of black and white familiar pictures one at a time. They were instructed to press the response key only when they saw the witch (i.e., the target). There were four blocks of 100 stimuli (including 15% targets); each picture was presented for 200 msec, and the interstimulus interval was 1500 msec. The task lasted approximately 12 minutes. CPT performance was assessed by the number errors (omissions and commissions), and response time. The CPT differentiates children with and without ADHD among typically developing populations (e.g., Sykes, Douglas, & Morgenstern, 1973), as well as among children with intellectual disability (Pearson, Yaffee, Loveland, & Lewis, 1996). It has also shown to be sensitive to stimulant treatment in children with ADHD, with and without ID (Aman, 1991; Aman, Kern, McGhee, & Arnold, 1993; Sykes, Douglas, Weiss, & Minde, 1971).
Selective Attention:
Speeded Classification Task.
The Speeded Classification Task (SCT; Strutt, Anderson, & Well, 1975) is a computerized measure of visual selective attention in which children sort stimuli on the basis of a binary (two-choice) dimension. The dimension could be a shape (circle or square), line orientation (vertical or horizontal), or relative location of a star (above or below the middle of the screen). The relevant dimension could appear by itself (e.g., just a circle), or with one or two distracting dimensions (e.g., a circle with a horizontal line drawn through it and a star above it). Children responded by selecting the best-matching stimulus, using a computer touch screen. SCT performance was assessed by examining sorting errors and response time. The SCT discriminates children with and without ADHD of normal intelligence (Rosenthal and Allen, 1980), as well as children with and without ADHD who have intellectual disabilities (Pearson et al., 1996). It is also sensitive to methylphenidate (MPH) treatment in the latter (Pearson et al., 2004).
Selective Listening Task.
The stimuli used for the Selective Listening Task were taken from the competing sentences subtest of the Dichotic Speech Intelligibility Test (DSI; Jerger, 1987), and were presented from the computer through headphones. Sentences were presented simultaneously in both ears, with one ear being the target channel and the other being the distracter channel. Participants were required to identify the target stimuli from a list while inhibiting information presented in the distractor channel. Sentences were first presented at equal decibel levels in the target and distracter channel. The remainder of the distractor conditions followed with 10db increases in distractor volume per condition. Performance was measured by percent of correct responses, omission errors (when participants failed to make a response), and intrusion errors (i.e., reporting the distractor). All children received a hearing screen before completing the task. Selective listening tasks have been shown to differentiate children with and without ADHD (Prior, Samson, Freethy, & Geffen, 1985; Pearson et al., 1991).
Impulsivity/Inhibition:
Delay of Gratification (DOG) Task.
This task, adapted from the preschool delay task of the Gordon Diagnostic System (Gordon, 1983), measures the ability to suppress or delay impulsive behavioral responses. Children were told that a star would appear on the computer screen if they waited “long enough” to press a response key. If a child responded sooner than four seconds after their previous response, they did not earn a star, and the 4-second counter restarted. Children performed one block as practice, and then four 92-second blocks during the actual test. Performance was measured by the number of correct responses and the efficiency ratio (# correct responses/total # responses). The DOG differentiates children with and without ADHD of normal intelligence (McClure & Gordon, 1984), and is sensitive to MPH treatment in these children (Hall & Kataria, 1992) and in children with ADHD who have intellectual disabilities (Pearson et al., 2004).
Matching Familiar Figures Test (MFFT).
This task consisted of a computerized version of Kagan’s (1964) MFFT, in which the child saw a test picture at the top of the computer screen, along with six alternatives (one of which matched the test stimulus) further down on the screen. Participants were told to select the picture below that matched the test stimulus above. There were two practice items (mug, ruler), followed by 23 experimental items Incorrect responses resulted in a synthesized voice (DML) saying “try again,” while correct responses resulted in the same voice saying “that’s right.” Subjects continue responding on each trial until correct. Performance was measured by number of matching errors and response time. The MFFT discriminates children with and without ADHD in the general pediatric population (Prior et al. 1985; Pearson et al. 1991) and has been found to be sensitive to MPH in children with ADHD in the general pediatric population (e.g., Campbell et al., 1971), and in children with ADHD and intellectual disabilities (Aman et al., 1991b; Pearson et al., 2004).
Stop Signal Task (SST).
The SST (Schachar et al. 1993) is a measure of motor response inhibition, involving a choice reaction time task and a stop task. The choice reaction time task (go task) involves two visual stimuli, either X or O, presented in the middle of a computer screen. On 75% of the trials (the “go trials”), the children were told to respond as quickly and accurately as possible by pressing the correct button corresponding to the letter on screen. On 25% of the trials (the “stop trials”), the child also heard a tone (the “stop signal”) and was told to withhold responding. Short stop signal delays increase the probability of inhibiting (e.g., it is easier to stop a prepotent response earlier than later), while longer delays increase the probability of responding. Total trial time is 3500 msec, with a 500 msec fixation, a 1000 msec go stimulus display (X or O), and a 2000 msec inter-trial interval (ITI). The onset of the stop stimulus (stop signal delay) is modified based on subject performance. On the first trial, the stop signal occurs 250 msec after the onset of the go stimulus. For each correct answer, an additional 50 msec is added to the onset time and for each incorrect answer, 50 msec is removed from the onset time. Participants completed a practice block, followed by 6 experimental blocks with 32 trials per block. The duration of this task is 12 minutes, Primary outcome variables included “Go Accuracy” (correct responses on “go” trials), “Go RT” (response time on “go” trials), “Stop Accuracy” (correct responses on “stop” trials), and “Stop Signal RT” (which is computed based on the “integration method,” as per R.J. Schachar, personal communication, 6/12/2011; also outlined in Verbruggen et al. 2019), and Stop Signal Delay (the time between the visual stimulus and the stop stimulus). Children with ADHD are less able than their non-ADHD peers to successfully inhibit when given the stop signal, as indicated by a longer Stop Signal RT (Lipszyc and Schachar, 2010; Schachar et al. 1993). The SSRT has been used to study inhibition in higher-functioning children with ASD (Ozonoff and Strayer, 1997) and has been found to be sensitive to MPH treatment in children with ADHD (Bedard et al. 2003).
Immediate Memory:
Delayed Match to Sample Task (DMTS).
The DMTS was adapted from the version used by Aman et al. (1991b). During the DMTS participants were first presented with a single color square (target) on the computer screen for 1000 msec and were instructed to commit the figure to memory and then touch the color. After a 1000 msec delay, participants see three colored squares (red, blue, and yellow), one of which was the matching the color they saw in the previous screen. Participants are asked to select the matching color by touching it on the screen. The delay between the target and the choices is increased by 1000 msec for every 3 correct responses to a maximum of a 12000 msec (i.e., 12 sec) delay. For every 3 incorrect responses, the delay is decreased by 1000 msec. There are 36 trials and the task can last up to 12 minutes. Performance was measured by the proportion of correct matches, response time, and the maximum delay. Past research has found the DMTS to be sensitive to MPH treatment in children with ADHD and intellectual disabilities (Aman et al., 1991b).
2.4. Procedure
Parents completed their report measures in a quiet, private room in the clinic. They were interviewed in the office of a licensed psychologist. For children who were taking medication (particularly stimulants), parents and teachers were instructed, to the extent possible, to complete their questionnaires by rating the child’s behavior when he or she was not taking medication. All cognitive tasks were administered in a quiet room in our clinic.
Finally, psychostimulant treatment was discontinued two days prior to testing (with pediatrician approval), in order for children to be tested in their “natural” state. Every effort was made to do this cognitive testing on Mondays, so there was minimal interference with the child’s academic performance (i.e., we strived to make medication-free days fall on weekends). Before each task, the children were given practice trials, during which time we gave feedback on their performance. With the exception of the Matching Familiar Figures Test, no feedback was given during the actual test trials, but the child was redirected to task if s/he looked away or spoke.
3. Results
Primary statistical analyses utilized a multiple regression approach similar to that used in a previous study by this group (Mansour et al., 2017). As expected, mental age (from the SB5 full scale age-equivalent score) was significantly correlated with most dependent variables (mean r=.37); therefore, mental age was included as a covariate predictor in each regression model. As shown in Table 3, no significant correlations emerged among the independent variables (ASD severity, ASD severity, and mental age).
Table 3.
Correlation Matrix of Independent Variables (including associated confidence intervals) associated with each correlation
| Variable | ADHD Severity | ASD Severity | Mental Age (Transformed) |
|---|---|---|---|
| ADHD Severity | 1.00 | −.01* (−0.214 to 0.195) | −.07** (−0.27 to 0.136) |
| ASD Severity | −.01* (−0.214 to 0.195) | 1.00 | −.19* (−0.38 to 0.015) |
| Mental Age (Transformed) | −.07** (−0.27 to 0.136) | −.19* (−0.38 to 0.015) | 1.00 |
Note.
p=.90
p=.50
p=.069.
Variable distributions were examined prior to statistical analyses and transformations were applied as appropriate following the Tukey Ladder of Transformations (Winer et al., 1971; see Table 2). Multiple regression models examined the unique associations between ASD symptom severity (ADI-R Total Score) and ADHD symptom severity (CPRS Global Index) and each cognitive outcome (i.e., ASD severity, ADHD severity, and mental age were predictors in each model predicting a separate cognitive task measure). Table 2 shows results of the regression models; reporting the relationships between the predictor and dependent variables. Although we examined the interaction between ASD and ADHD severity as predictors of each dependent variable, no significant interactions emerged (p>.05). The relationships between predictor and dependent variables are illustrated in separate partial plot figures (Figures 1–4). A partial plot shows the relationship between the predictor variable and the outcome variable with the variance of all the other predictor variables partialled out (i.e., the relationship that is tested by the regression model; see Velleman & Welsch, 1981).
Figure 1.
Partial plot of Continuous Performance Task commission errors and ADHD severity
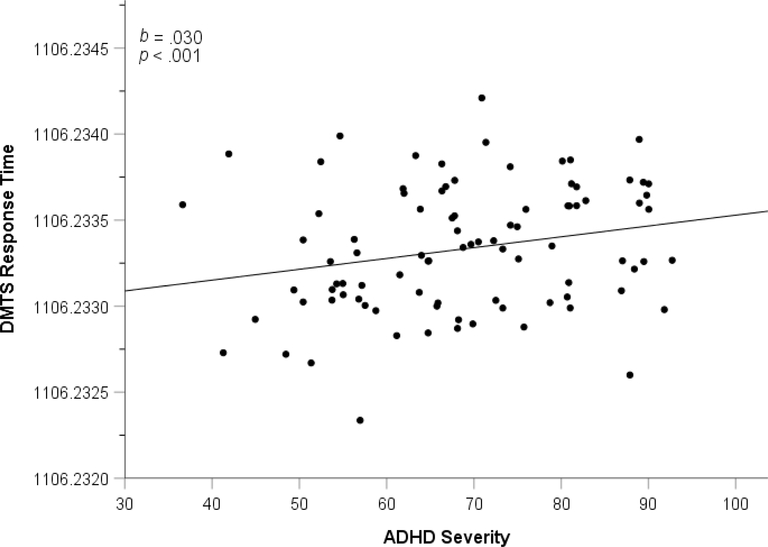
Figure 4.
Partial plot of Delayed Match to Sample Task response time and ADHD severity
3.1. Sustained Attention
Continuous Performance Task.
Figure 1 illustrates the significant relationship between ADHD severity and number of commission errors, b=0.12 (95% CI=.001 to .023), ΔR2 of .046, F(1,84)=4.48, p=.037. In contrast, the relationship between ASD severity and the number of commission errors was not significant, p=.200. Furthermore, there were no significant effects of ADHD severity or ASD severity on the other CPT dependent variables.
3.2. Selective Attention
Speeded Classification Task.
Although there was some evidence of a positive association between ADHD severity and number of errors, this effect was not significant at conventional levels, b=.007 (95% CI= −.001 to .014), ΔR2=.023, F(1,82)=3.40, p=.069. Similarly, there was a pattern suggesting that increasing ADHD severity was associated with decreasing (i.e., faster) response times, but again it fell short of the conventional significance threshold, b=−4.86 (95% CI= −10.14 to .419), ΔR2=.031, F(1,82)=3.35, p=.071. ASD severity was not a significant predictor of errors or response times, all p>.455. Overall, these results may suggest that greater ADHD severity predicted faster response times, but less accurate responding.
Dichotic Listening Task.
The effect of ADHD severity on DSI errors was not significant on any dependent variables (all p≥.180), nor was the effect of ASD severity, all p≥.668.
3.3. Impulsivity/Inhibition
Delay of Gratification (DOG) Task.
Separate models examined efficiency ratio, correct responses, and number of overall responses as dependent variables with ASD severity and ADHD severity as predictors. There were no significant relationships (all p≥.177).
Matching Familiar Figures Test.
The relationship between ADHD severity and total errors on the MFFT was significant and positive, b=0.303 (95% CI=.056 to .551), ΔR2=.052, F(1,77)=5.96, p=.017, suggesting that more severe ADHD symptoms predicted less accuracy on this task (Figure 2). In contrast, the association between ASD severity and total errors was not significant, p=.429. Response time was not significantly predicted by either ADHD severity, p=.127, nor by ASD severity, p=.735.
Figure 2.
Partial plot of Matching Familiar Task errors and ADHD severity
Stop Signal Task (SST).
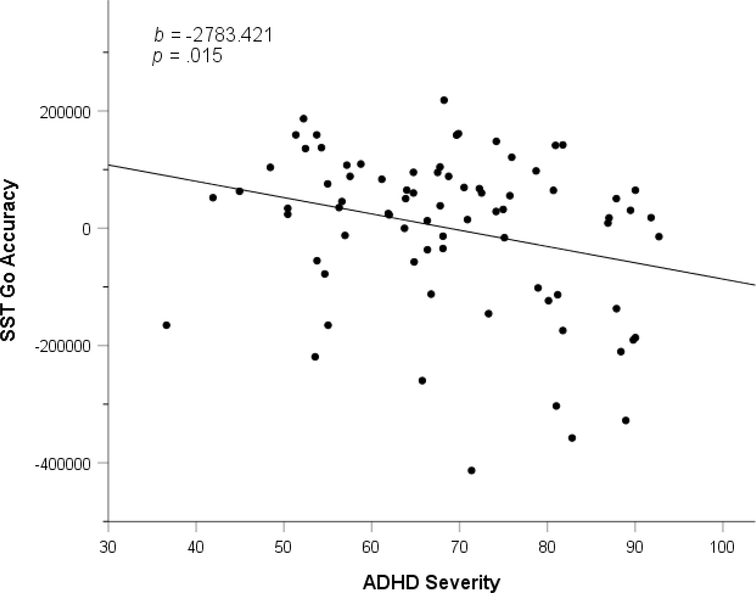
As can be seen in Figure 3, increasing ADHD severity was a significant negative predictor of accuracy during “Go” trials (i.e., trials with no stop tone telling the child to inhibit responding), b=−2783 (95% CI= −5018 to −547), ΔR2=.065, F(1,74)=6.15, p=.015. In contrast, the relationship between ASD severity and accuracy on go trials was not significant, p=.100. Thus, greater ADHD severity predicted worse accuracy on Go trials, while ASD severity did not impact Go trial accuracy.
Figure 3.
Partial plot of Stop Signal Task “Go” trial accuracy and ADHD severity
There were no significant effects of ADHD severity and ASD severity on the other SST dependent variables, including stop signal response time, go response time, stop signal accuracy, and stop signal delay, all p>.110.
3.4. Immediate Memory
Delayed Match to Sample (DMTS) Task.
As can be seen in Figure 4, greater ADHD severity significantly predicted slower response time, b=.006 (95% CI=.00063 to .012), ΔR2=.046, F(1,86)=4.87, p=.030. Please note that b and CI were multiplied by 1000 to make the regression analyses more easily interpretable. In contrast, ASD severity did not significantly predict response time, p=.596. Neither ADHD severity nor ASD severity significantly predicted correct responses (all p>.49).
4. Discussion
Elevated symptoms and diagnoses of ADHD are common among youth with ASD (Gadow, DeVincent & Pomeroy, 2006). Evidence indicates that youth with ASD and comorbid ADHD experience myriad impairments beyond those of youth with ASD alone, including greater variety and intensity of comorbid psychopathology (Mansour et al., 2017). In addition, a growing literature suggests that deficits in executive functioning and related cognitive domains are characteristic of both ASD and ADHD. Yet, it was only within the last decade that research began to earnestly examine how these cognitive differences manifest among this common comorbid profile (i.e., ASD+ADHD). This study suggests that greater ADHD symptom severity was uniquely associated with weaker performance on measures of attention, immediate memory, and response inhibition, over and above what would be predicted on the basis of mental age. In contract, ASD severity did not account for unique variance in any cognitive measures investigated. Taken together, it appears that comorbid ADHD symptomatology may confer additional problems in these cognitive domains beyond what is already associated with ASD.
With respect to the specific dimensions of attentional impairment, ADHD severity uniquely predicted increased errors of commission as measured by CPT, suggesting poorer ability to inhibit responding to non-targets. Wilson and colleagues (2016) also noted that commission errors can suggest absentmindedness or lower attentional focus in longer tasks with greater proportions of no-go stimuli such as the version used here. Thus, in the present study, commission errors likely reflect poor sustained attention. With regard to visual selective attention, greater ADHD severity predicted faster response time on the SCT above and beyond the effect of ASD severity; however, the relation between ADHD severity and task accuracy fell just short of significance (p=.07). These results seem to suggest faster and less accurate responding overall.
With regard to immediate visual memory on the DMTS, ADHD severity was a unique predictor of slower response time, indicating slower memory processing or slower responding. However, there was no significant added effect of ADHD symptoms on task accuracy. This finding may suggest that, while ADHD severity was not related to accuracy of responding on this short-term memory task, those ASD youth with more severe ADHD symptoms are slower to retrieve/recognize the matching stimulus from short-term memory and/or are slower to generate a response. With regard to the impact of ADHD on short-term memory, previous findings are not consistent. Corbett and colleagues (2009) reported that ASD-diagnosed youth performed worse than a group of youth with ADHD on a spatial working memory measure, but several children in their ASD-diagnosed group also had significant ADHD symptoms, while ASD symptoms were an exclusion criterion for the ADHD group. In a more recent study, more severe ASD symptoms predicted worse working memory performance relative to ADHD-only and typically developing youth (Karalunas et al., 2018). The most consistently supported conclusion is that overall working memory is impacted for both disorders relative to typically developing youth.
Examination of response inhibition with the SST revealed that ADHD severity did not uniquely predict increased problematic response inhibition or slower responding. However, ADHD severity did predict worse accuracy during “Go” trials. These results indicate less efficient perception and execution of the correct response during this forced choice task. In contrast, ADHD severity did predict more errors on the MFFT as well as commission errors on the CPT. These results signify that, among ASD youth, comorbid ADHD symptoms may compound disruptions in response inhibition.
Previous studies have generally concluded that weakened performance in particular cognitive domains are characteristic of both ASD and ADHD (Corbett et al., 2009; Sinzig et al., 2008). Although some studies indicate more pronounced difficulties in one diagnosis or the other, the emerging findings in this small literature are mixed (Boxhoorn et al., 2018; Geurts et al., 2004; Happe et al., 2006). Among youth with comorbid ASD, severity of ADHD has been associated with weaker performance on tasks tapping attention and response inhibition (Sanderson & Allen, 2013; Sinzig et al., 2008). Overall, we obtained support for the hypothesis that comorbid ADHD symptomatology in youth with ASD is associated with more problems on tasks tapping attention, impulsivity, and immediate memory. Across most tasks, elevated ADHD severity also predicted slower responding (with the exception of the SCT, where there was also a trend toward poor accuracy).
As with any study, there are limitations to note. An important interpretive factor is that the current cross-sectional findings cannot establish causality or direction of effect. While these findings may indicated that greater ADHD severity leads to disruptions in underlying cognitive domains, it is possible that the underlying disruptions of these domains are shared across phenotypes and ADHD behavior emerges through a unique process in youth with ASD. Indeed, a recent study with a large sample using empirical methods to define diagnostic “classes” concluded some cognitive impairment was shared across both disorders while other cognitive deficits appeared to be unique to each diagnostic class (Karalunas et al. 2018). Longitudinal examination of cognitive development within the population of individuals with ASD is needed to clarify these processes.
Although it would be of interest, investigating the impact of ADHD presentation types (i.e., combined, predominately inattentive, and predominantly hyperactive/impulsive presentations) and other comorbid psychiatric symptomatology on cognitive task performance was not undertaken in this study due to limitations of statistical power due to our sample size. However, future studies should investigate whether all three ADHD presentations are significantly associated with weaker cognitive performance—and associated areas of decrement. Such studies and could potentially inform intervention for children with comorbid ASD and ADHD. Future studies may also be able to assess the role that attentional factors have on inhibition, and vice versa, to examine additional layers of cognitive performance that may be affected by symptoms of ADHD and ASD in children with ASD.
Another potential limitation of our study is that it did not include a large number of children with ASD who did not have significant ADHD symptoms. Although it was beyond the scope of this investigation to do so, future studies including groups of ASD-diagnosed youth with a wider range of clinical symptoms of ADHD comorbidity are still needed (Rommelse, 2011). Additionally, as noted in Mansour et al. (2017), the children seen in this study were on average somewhat higher functioning (mean IQ=84) relative to the full spectrum of ASD. As others have noted (e.g., Aman, 1991) ADHD may be manifested in a more “cognitive” manner in higher-functioning individuals with developmental disability, and in a more “motoric” manner in lower-functioning individuals. Future studies might include more specific ranges of cognitive profiles (e.g., lower vs. higher IQ levels)—and thus may be able to provide further insight into this question. It is clearly an issue warranting further investigation.
Additional potential limitations include the fact that some children were taking non-stimulant medications when they were tested, and some had other psychiatric conditions. Although these factors may have impacted task performance, we did not have sufficient statistical power to assess these effects in our sample. Although beyond the scope of this study to assess these possible effects, such factors warrant further investigation in future studies with larger sample sizes.
The current results support the conception that comorbid ADHD symptomatology in youth with ASD places them at higher risk for impaired attention and memory on cognitive tasks. It will remain for future investigations to assess the extent to which task performance is associated with cognition in the “real world” in children with ASD+ADHD. Research has also documented that among youth with ADHD, similar cognitive difficulties (as associated with cognitive task performance) are associated with functional impairment in academics, social functioning and behavioral dysregulation (Gropper & Tannock, 2009). Thus, the current results support the need to closely evaluate various aspects of cognitive function in children with ASD +ADHD to inform treatment planning. In doing so, the intervention plan for children with ASD +ADHD may facilitate more optimal developmental outcomes.
What this paper adds:
This paper contributes to the growing literature studying the nature of ADHD in the context of ASD. Our findings are that the cognitive deficits that are typically associated with ADHD in the general pediatric population are also found in higher-functioning children with ASD who also have significant ADHD symptomatology—and that these deficits become more severe as ADHD symptoms become more severe. Our findings also demonstrated that these cognitive task deficits are unrelated to ASD symptomatology. Taken collectively, these findings provides further evidence that ADHD and ASD are two distinct disorders—and that higher-functioning children with ADHD+ASD may have similar profiles on cognitive task performance that are seen in children with ADHD in the general pediatric population.
Acknowledgements
The first and second authors contributed equally to this article. This work was supported by grant number MH072263 from the National Institute of Mental Health (NIMH). A preliminary version of this paper was presented at the International Meeting for Autism Research (IMFAR) in Donastia, Spain, May 4, 2013. The authors wish to express their appreciation to the children, parents, and teachers who participated in this study. We also wish to thank Ms. Ming (Brook) Lu, for her editorial assistance.
Author Disclosures:
Dr. 1 has received travel reimbursement and research support from Curemark LLC, research support from Biomarin and Novartis, and has served as a consultant to Curemark LLC. Dr. 2 and Ms. 3 have received research support from Curemark LLC. Dr. 4 has received research contracts, consulted with, or served on advisory boards of Biomarin Pharmaceuticals, Bristol-Myers Squibb, Confluence Pharmaceutica, Forest Research, Hoffman LaRoche, Johnson and Johnson, and Supernus Pharmaceutica. Dr. 5 has served as a consultant to Pearson Assessments/Psychological Corporation. Dr. 6 has consulted to Highland Therapeutics, Lilly Corp, Purdue Pharma (research contract) and ehave. The other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aman MG (1991). Assessing psychopathology and behaviour problems in persons with mental retardation: A review of available instruments. Rockville, MD: US Department of Health and Human Services. [Google Scholar]
- Aman MG, Kern RA, McGhee DE, & Arnold LE (1993). Fenfluramine and methylphenidate in children with mental retardation and ADHD: Clinical and side effects. Journal of the American Academy of Child & Adolescent Psychiatry, 32(4), 851–859. [DOI] [PubMed] [Google Scholar]
- Aman MG, Van Bourgondien ME, Wolford PL, & Sarphare G (1995). Psychotropic and anticonvulsant drugs in subjects with autism: prevalence and patterns of use. Journal of the American Academy of Child & Adolescent Psychiatry, 34(12), 1672–1681. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association: Diagnostic and statistical manual of mental disorders, 4th Edition-Text Revision (DSM-IV-TR). Washington, D.C.: Author, 2000. [Google Scholar]
- Ames CS & White SJ (2011). Brief report: Are ADHD traits dissociable from the autistic profile? Links between cognition and behaviour. Journal of Autism and Developmental Disorders, 31, 357–363. [DOI] [PubMed] [Google Scholar]
- Bedard AC, Ickowicz A, Logan GD, Hogg-Johnson S, Schachar R, & Tannock R (2003). Selective inhibition in children with attention-deficit hyperactivity disorder off and on stimulant medication. Journal of Abnormal Child Psychology, 31(3), 315–327. [DOI] [PubMed] [Google Scholar]
- Boxhoorn S, Lopez E, Schmidt C, Schulze D, Hänig S, & Freitag CM (2018). Attention profiles in autism spectrum disorder and subtypes of attention-deficit/hyperactivity disorder. European Child & Adolescent Psychiatry, 27(11), 1433–1447. [DOI] [PubMed] [Google Scholar]
- Bryson SA, Corrigan SK, McDonald TP & Holmes C (2008). Characteristics of children with autism spectrum disorders who received services through community mental health centers. Autism, 12, 65–82. [DOI] [PubMed] [Google Scholar]
- Campbell SB, Douglas VI, & Morgenstern G (1971). Cognitive styles in hyperactive children and the effect of methylphenidate. Journal of Child Psychology and Psychiatry, 12(1), 55–67. [DOI] [PubMed] [Google Scholar]
- Conners CK, Sitarenios G, Parker JD, & Epstein JN (1998). The revised Conners’ Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology, 26(4), 257–268. [DOI] [PubMed] [Google Scholar]
- Corbett BA, Constantine LJ, Hendren R, Rocke D, & Ozonoff S (2009). Examining executive functioning in children with autism spectrum disorder, attention deficit hyperactivity disorder and typical development. Psychiatry Research, 166(2–3), 210–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig F, Margari F, Legrottaglie AR, Palumbi R, De Giambattista C, & Margari L (2016). A review of executive function deficits in autism spectrum disorder and attention-deficit/hyperactivity disorder. Neuropsychiatric Disease and Treatment, 12, 1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier JA, Biederman J, Bellordre CA, Garfield SB, Geller DA, Coffey BJ, et al. (2001). Should the diagnosis of attention-deficit/hyperactivity disorder be considered in children with pervasive developmental disorder? Journal of Attention Disorders, 4, 203–211. [Google Scholar]
- Gadow KD, DeVincent CJ, & Pomeroy J (2006). ADHD symptom subtypes in children with pervasive developmental disorder. Journal of Autism and Developmental Disorders, 36, 271–283. [DOI] [PubMed] [Google Scholar]
- Geurts HM, Verté S, Oosterlaan J, Roeyers H, & Sergeant JA (2004). How specific are executive functioning deficits in attention deficit hyperactivity disorder and autism?. Journal of Child Psychology and Psychiatry, 45(4), 836–854. [DOI] [PubMed] [Google Scholar]
- Gordon M (1983). The Gordon diagnostic system. DeWitt, NY: Gordon Systems. [Google Scholar]
- Gropper RJ, & Tannock R (2009). A pilot study of working memory and academic achievement in college students with ADHD. Journal of Attention Disorders, 12(6), 574–581. [DOI] [PubMed] [Google Scholar]
- Hall CW, & Kataria S (1992). Effects of two treatment techniques on delay and vigilance tasks with attention deficit hyperactive disorder (ADHD) children. The Journal of Psychology, 126(1), 17–25. [DOI] [PubMed] [Google Scholar]
- Happé F, Booth R, Charlton R, & Hughes C (2006). Executive function deficits in autism spectrum disorders and attention-deficit/hyperactivity disorder: examining profiles across domains and ages. Brain and Cognition, 61(1), 25–39. [DOI] [PubMed] [Google Scholar]
- Jang J, Matson JL, Williams LW, Tureck K, Goldin RL, & Cervantes PE (2013). Rates of comorbid symptoms in children with ASD, ADHD, and comorbid ASD and ADHD. Research in Developmental Disabilities, 34(8), 2369–2378. [DOI] [PubMed] [Google Scholar]
- Jerger S (1987). Validation of the pediatric speech intelligibility test in children with central nervous system lesions. Audiology, 26(5), 298–311. [DOI] [PubMed] [Google Scholar]
- Kagan J (1964). Matching familiar figures test. Harvard University. [Google Scholar]
- Karalunas SL, Hawkey E, Gustafsson H, Miller M, Langhorst M, Cordova M, Fair D & Nigg JT (2018). Overlapping and distinct cognitive impairments in attention-deficit/hyperactivity and autism spectrum disorder without intellectual disability. Journal of Abnormal Child Psychology, 46(8), 1705–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecavalier L, Gadow KD, DeVincent CJ, & Edwards MC (2009). Validation of DSM-IV model of psychiatric syndromes in children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 39, 278–289. [DOI] [PubMed] [Google Scholar]
- Lipszyc J, & Schachar R (2010). Inhibitory control and psychopathology: A meta-analysis of studies using the stop signal task. Journal of the International Neuropsychological Society, 16, 1064–1076. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. (2002) Autism Diagnostic Observation Schedule. Los Angeles: Western Psychological Services. [Google Scholar]
- Lord C, Rutter M, & Le Couteur A (1994). Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24(5), 659–685. [DOI] [PubMed] [Google Scholar]
- Mansour R, Dovi AT, Lane DM, Loveland KA, & Pearson DA (2017). ADHD severity as it relates to comorbid psychiatric symptomatology in children with Autism Spectrum Disorders (ASD). Research in Developmental Disabilities, 60, 52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure FD, & Gordon M (1984). Performance of disturbed hyperactive and nonhyperactive children on an objective measure of hyperactivity. Journal of Abnormal Child Psychology, 12(4), 561–571. [DOI] [PubMed] [Google Scholar]
- Musser ED, Hawkey E, Kachan-Liu SS, Lees P, Roullet JB, Goddard K, Steiner RD & Nigg JT (2014). Shared familial transmission of autism spectrum and attention-deficit/hyperactivity disorders. Journal of Child Psychology and Psychiatry, 55(7), 819–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, & Jensen J (1999). Brief report: Specific executive function profiles in three neurodevelopmental disorders. Journal of Autism and Developmental Disorders, 29(2), 171–177. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, & Strayer DL (1997). Inhibitory function in nonretarded children with autism. Journal of Autism and Developmental Disorders, 27(1), 59–77. [DOI] [PubMed] [Google Scholar]
- Pastor PN, Reuben CA (2008) Diagnosed attention deficit hyperactivity disorder and learning disability: United States, 2004–2006. National Center for Health Statistics. Vital Health Stat 10(237). Centers for Disease Control and Prevention. [PubMed] [Google Scholar]
- Pearson DA, Aman MG, Arnold LE, Lane DM, Loveland KA, Santos CW, & Factor P (2012). High concordance of parent and teacher attention-deficit/hyperactivity disorder ratings in medicated and unmedicated children with autism spectrum disorders. Journal of Child and Adolescent Psychopharmacology, 22(4), 284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson DA, Lane DM, & Swanson JM (1991). Auditory attention switching in hyperactive children. Journal of Abnormal Child Psychology, 19(4), 479–492. [DOI] [PubMed] [Google Scholar]
- Pearson DA, Santos CW, Aman MG, Arnold LE, Casat CD, Mansour R, Jerger SW, Ezzel S, Factor P, Vanwoerden S, Ye E, Narain P, & Cleveland LA (2013). Effects of extended release methylphenidate treatment on ratings of attention-deficit/hyperactivity disorder (ADHD) and associated behavior in children with autism spectrum disorders and ADHD symptoms. Journal of Child and Adolescent Psychopharmacology, 23(5), 337–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson DA, Santos CW, Casat CD, Lane DM, Jerger SW, Roache JD, Loveland KA, Lachar D, Faria LP, Payne CD, & Cleveland LA (2004). Treatment effects of methylphenidate on cognitive functioning in children with mental retardation and ADHD. Journal of the American Academy of Child and Adolescent Psychiatry, 43, 677–685. [DOI] [PubMed] [Google Scholar]
- Pearson DA, Yaffee LS, Loveland KA, & Lewis KR (1996) Comparison of sustained and selective attention in children who have mental retardation with and without attention deficit hyperactivity disorder. American Journal on Mental Retardation, 100: 592–607. [PubMed] [Google Scholar]
- Pennington BF, & Ozonoff S (1996). Executive functions and developmental psychopathology. Journal of Child Psychology and Psychiatry, 37(1), 51–87. [DOI] [PubMed] [Google Scholar]
- Ponde MP, Novaes CM, & Losapio MF (2010). Frequency of symptoms of attention deficit and hyperactivity disorder in autistic children. Arq Neuropsiquiatr, 68(1), 103–106. [DOI] [PubMed] [Google Scholar]
- Prior M, Sanson A, Freethy C, & Geffen G (1985). Auditory attentional abilities in hyperactive children. Journal of Child Psychology and Psychiatry, 26(2), 289–304. [DOI] [PubMed] [Google Scholar]
- Reich W (2000). Diagnostic interview for children and adolescents (DICA). Journal of the American Academy of Child & Adolescent Psychiatry, 39(1), 59–66. [DOI] [PubMed] [Google Scholar]
- Roid GH (2003). Stanford Binet Intelligence Scales 5th Edition: Examiner’s Manual. Riverside Publishing, Itaska, Illinois. [Google Scholar]
- Rommelse NN, Franke B, Geurts HM, Hartman CA, & Buitelaar JK (2010). Shared heritability of attention-deficit/hyperactivity disorder and autism spectrum disorder. European Child & Adolescent Psychiatry, 19(3), 281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelse NN, Geurts HM, Franke B, Buitelaar JK, & Hartman CA (2011). A review on cognitive and brain endophenotypes that may be common in autism spectrum disorder and attention-deficit/hyperactivity disorder and facilitate the search for pleiotropic genes. Neuroscience & Biobehavioral Reviews, 35(6), 1363–1396. [DOI] [PubMed] [Google Scholar]
- Rosenthal RH, & Allen TW (1980). Intratask distractibility in hyperkinetic and nonhyperkinetic children. Journal of Abnormal Child Psychology, 8(2), 175–187. [DOI] [PubMed] [Google Scholar]
- Rosvold HE, Mirsky AF, Sarason I, Bransome ED Jr, & Beck LH (1956). A continuous performance test of brain damage. Journal of Consulting Psychology, 20(5), 343. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, & Lord C (2003). The Social Communication Questionnaire: Manual. Western Psychological Services. [Google Scholar]
- Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview-Revised. Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- Sanderson C, & Allen ML (2013). The specificity of inhibitory impairments in autism and their relation to ADHD-type symptoms. Journal of autism and developmental disorders, 43(5), 1065–1079. [DOI] [PubMed] [Google Scholar]
- Sergeant JA, Geurts H, & Oosterlaan J (2002). How specific is a deficit of executive functioning for attention-deficit/hyperactivity disorder? Behavioural brain research, 130(1–2), 3–28. [DOI] [PubMed] [Google Scholar]
- Schachar RJ, Tannock R, & Logan G (1993). Inhibitory control, impulsiveness, and attention deficit hyperactivity disorder. Clinical Psychology Review, 13(8), 721–739. [Google Scholar]
- Schieve LA, Rice C, Yeargin-Allsopp M, Boyle CA, Kogan MD, Drews C, & Devine O (2012). Parent-reported prevalence of autism spectrum disorders in US-born children: an assessment of changes within birth cohorts from the 2003 to the 2007 National Survey of Children’s Health. Maternal and child health journal, 16(1), 151–157. [DOI] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, & Baird G (2008). Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, associated factors in a population-derived sample. J American Academy of Child and Adolescent Psychiatry, 47, 921–929. [DOI] [PubMed] [Google Scholar]
- Sinzig J, Walter D, & Doepfner M (2009). Attention deficit/hyperactivity disorder in children and adolescents with autism spectrum disorder: Symptom or syndrome? Journal of Attention Disorders, 13(2), 117–126. [DOI] [PubMed] [Google Scholar]
- Sinzig J, Bruning N, Morsch D, & Lehmkuhl G (2008). Attention profiles in autistic children with and without comorbid hyperactivity and attention problems. Acta Neuropsychiatrica, 20(4), 207–215. [DOI] [PubMed] [Google Scholar]
- Strutt GF, Anderson DR, & Well AD (1975). A developmental study of the effects of irrelevant information on speeded classification. Journal of Experimental Child Psychology, 20(1), 127–135. [Google Scholar]
- Sykes DH, Douglas VI, & Morgenstern G (1973). Sustained attention in hyperactive children. Journal of Child Psychology and Psychiatry, 14(3), 213–220. [DOI] [PubMed] [Google Scholar]
- Sykes DH, Douglas VI, Weiss G, & Minde KK (1971). Attention in hyperactive children and the effect of methylphenidate (Ritalin). Journal of Child Psychology and Psychiatry, 12(2), 129–139. [DOI] [PubMed] [Google Scholar]
- Thomas R, Sanders S, Doust J, Beller E, & Glasziou P (2015). Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics, 135(4), 994–1001. [DOI] [PubMed] [Google Scholar]
- van der Meer JM, Oerlemans AM, van Steijn DJ, Lappenschaar MG, de Sonneville LM, Buitelaar JK, & Rommelse NN (2012). Are autism spectrum disorder and attention-deficit/hyperactivity disorder different manifestations of one overarching disorder? Cognitive and symptom evidence from a clinical and population-based sample. J American Academy of Child & Adolescent Psychiatry, 51(11), 1160–1172. [DOI] [PubMed] [Google Scholar]
- Velleman PF, & Welsch RE (1981). Efficient computing of regression diagnostics. The American Statistician, 35(4), 234–242. [Google Scholar]
- Verbruggen F, Aron AR, Band G, Beste G, Bissett PG, Brockett AT, Grown JW, …Boehler CN (2019). A consensus guide to capturing the ability to inhibit actions and impulsive behaviors in the stop-signal task. eLife, 8, e46323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer BH, Brown DR, & Michel KM (1971). Statistical principles in experimental design. NY: McGraw-Hill, 1971.
- Wilson KM, Finkbeiner KM, de Joux NR, Russell PN, & Helton WS (2016). Go-stimuli proportion influences response strategy in a sustained attention to response task. Experimental brain research, 234(10), 2989–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerys BE, Wallace GL, Sokoloff JL, Shook DA, James JD, & Kenworthy L (2009). Attention deficit/hyperactivity disorder symptoms moderate cognition and behavior in children with autism spectrum disorders. Autism Research, 2, 322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y & Uchiyama T (2004). The clinical necessity for assessing attention deficit/hyperactivity disorder symptoms in children with high-functioning pervasive developmental disorder (PDD). European Child and Adolescent Psychiatry, 13, 307–314. [DOI] [PubMed] [Google Scholar]