Abstract
Objective
To evaluate the short term associations between nitrogen dioxide (NO2) and total, cardiovascular, and respiratory mortality across multiple countries/regions worldwide, using a uniform analytical protocol.
Design
Two stage, time series approach, with overdispersed generalised linear models and multilevel meta-analysis.
Setting
398 cities in 22 low to high income countries/regions.
Main outcome measures
Daily deaths from total (62.8 million), cardiovascular (19.7 million), and respiratory (5.5 million) causes between 1973 and 2018.
Results
On average, a 10 μg/m3 increase in NO2 concentration on lag 1 day (previous day) was associated with 0.46% (95% confidence interval 0.36% to 0.57%), 0.37% (0.22% to 0.51%), and 0.47% (0.21% to 0.72%) increases in total, cardiovascular, and respiratory mortality, respectively. These associations remained robust after adjusting for co-pollutants (particulate matter with aerodynamic diameter ≤10 μm or ≤2.5 μm (PM10 and PM2.5, respectively), ozone, sulfur dioxide, and carbon monoxide). The pooled concentration-response curves for all three causes were almost linear without discernible thresholds. The proportion of deaths attributable to NO2 concentration above the counterfactual zero level was 1.23% (95% confidence interval 0.96% to 1.51%) across the 398 cities.
Conclusions
This multilocation study provides key evidence on the independent and linear associations between short term exposure to NO2 and increased risk of total, cardiovascular, and respiratory mortality, suggesting that health benefits would be achieved by tightening the guidelines and regulatory limits of NO2.
Introduction
Nitrogen dioxide (NO2) is a common air pollutant that has adverse effects on population health, is a precursor to ground level ozone with its own harmful effects on health, and is involved in the secondary formation of fine particulate matter. Most ambient NO2 has an anthropogenic origin, such as fuel combustion and traffic.1 Many studies have reported the effects of short term exposure to NO2 on human mortality or morbidity,2 3 but these are disputed. The Integrated Science Assessment conducted by the United States Environmental Protection Agency reviewed the existing evidence linking NO2 exposure with health outcomes in 20084 and 2016,5 and concluded that there is a causal relation between short term exposure to NO2 and respiratory effects. The review highlighted important gaps in knowledge, resulting in different limits for NO2 in air quality guidelines being adopted by various governmental and intergovernmental institutions.1 6 7 8
Several epidemiological studies have investigated the association between NO2 and total mortality,9 but with important limitations. Firstly, most focused on total non-accidental deaths, with only a few assessing cause specific mortality, such as respiratory and cardiovascular outcomes. Moreover, potential features of associations between NO2 and mortality, such as non-linearity, thresholds, and lag structures, remain to be clarified. More importantly, questions remain about whether NO2 has an independent effect on mortality, or if the observed associations can be explained by confounding effects of co-pollutants. Finally, current publications mainly include studies performed in single cities or countries, with the problem of publication bias and difficulties in integrating results from different analytical approaches. Furthermore, emissions in different contexts, population characteristics, and the reliability of routine health data and vital statistics limited the generalisability of such evidence.
This study aimed to examine these limitations by investigating the short term association between NO2 and mortality across numerous countries/regions worldwide, using data from the Multi-City Multi-Country Collaborative Research Network (https://mccstudy.lshtm.ac.uk/). The assessment takes advantage of a uniform analytical framework based on state-of-the-art epidemiological methodologies to estimate flexible exposure-response associations, to integrate results across cities and countries, and to quantify the excess mortality attributable to short term NO2 exposure.
Methods
Data collection
We obtained health and environmental data from the Multi-City Multi-Country database, which has been described in previous publications.10 11 Our analysis was limited to locations where ground level NO2 measurements were available—a total of 398 cities in 22 countries/regions, with different study periods based on data availability. The geographical distribution of cities with NO2, and the corresponding annual mean concentrations during the study periods are shown in eFigure 1. Mortality data were obtained from local authorities within each country/region. Causes of death were classified according to the international classification of diseases, 9th and 10th revision (ICD-9 and ICD-10) codes, where available. In each location, mortality is represented by daily counts of either non-external causes (ICD-9: 0-799; ICD-10: A0-R99) or, where not available, all cause mortality. We also collected mortality data for two main causes in 16 countries: cardiovascular disease (ICD-10, codes I00-I99) and respiratory disease (ICD-10, codes J00-J99).12
Daily concentrations of particulate matter and other gaseous pollutants, including particulate matter with aerodynamic diameter ≤10 μm or ≤2.5 μm (PM10 and PM2.5, respectively), ozone (O3), sulfur dioxide (SO2), and carbon monoxide (CO), were obtained from the same fixed site monitoring stations as for NO2, where available, to adjust for potential confounding by co-pollutants. We also collected daily data on weather variables, including mean temperature and mean relative humidity from local meteorological bureaux or other statistical authorities. The overall missing rate for all cause mortality, NO2, and temperature time series was 0.13%, 4.4%, and 1.2%, respectively. Detailed information on missing data is summarised in the appendix (eMethod 1.1 and eTable 7).
Statistical analysis
The associations of NO2 with daily total, cardiovascular, and respiratory mortality were assessed using the same analytical protocol in all locations, based on extension of a two stage design widely used in previous multilocation time series studies.13 14
In the first stage, we estimated the city specific associations using a time series quasi-Poisson generalised linear regression model featuring a natural cubic spline function of time with seven degrees of freedom (df) per year to control for unmeasured temporal trends and indicators for day of the week. Temperature was adjusted using a natural spline function with 6 df. Relative humidity was adjusted with the same spline function with 3 df in cities where such data were available. For the potential lag effect of NO2 and temperature, we a priori selected the moving average of lag 0-3 days to control temperature according to modelling choices of many previous studies.14 15 We modelled the association between NO2 and mortality using a distributed lag model with a linear lag response function, inspecting the lag structure on a single lag day of 0 to 3, and moving average of the present and previous day (lag 0-1) to identify the optimal lag choices.
In the second stage, we used a new multilevel meta-analytical approach to summarise the city specific associations.16 Briefly, this model defines more complex random effects structures that account for the hierarchical structure of the data—namely, cities nested within countries, and provides the best linear unbiased predictors for the associations between NO2 and mortality at both levels.16 We computed the global, country, and city specific estimates and 95% confidence intervals as percentage change in daily mortality per 10 μg/m3 increase of NO2 concentrations. Potential heterogeneity across cities was assessed with Cochran Q tests and I2 statistics. Finally, we computed the proportion of deaths attributable to NO2 exposure above the counterfactual zero level (since there was no obvious threshold level) and presented the population attributable fraction (%) at the country level, which can be calculated as follows:
| RR=exp(â×∆X) |
| PAF%=(RR−1/RR)×100% |
| Where RR is the relative risk of mortality associated with NO2 for each country, ∆X is the difference between the current annual concentration of NO2 and the counterfactual zero level, â is the country specific mortality estimate. |
We extended the main models to assess specific features of the association. Firstly, we fitted models with two pollutants using the other co-pollutants, assessing the robustness of the estimates through likelihood ratio tests. Countries/regions with unavailable data for co-pollutants were excluded accordingly; therefore, the number of countries/regions in eTable 3 varied by co-pollutants. Secondly, we conducted regional analyses (eTable 2 and eTable 4) and explored potential effect modifiers (such as long term air pollution concentrations, temperature, and gross domestic product) on the associations between NO2 and total mortality based on the main models. Finally, we explored potential non-linearity in the associations and pooled the concentration-response relation curve using a meta-smoothing approach applied in previous studies,14 17 modelling NO2 as a non-linear term through a natural spline function with knots at 20 and 40 μg/m3.
We conducted several sensitivity analyses to test the robustness of our estimates, including alternative choices for controlling for temperature, alternative placements of knots for testing non-linear exposure-response between NO2 and mortality, a comparison of models with and without adjustment for humidity, a test for seasonal differences modelled through an interaction with an indicator of warm/cold season (May-September v October-April for northern hemisphere, and vice versa for southern hemisphere), and comparing the associations within different time periods with a cutoff point at year 2000 (around the median year of each country’s time period).
We conducted all statistical analyses in R software (version 3.3.1), using the stats and dlnm packages for fitting first stage models and the mixmeta package for performing multilevel meta-analyses. We presented the percentage change of mortality for a 10 μg/m3 increase in NO2 concentrations. P values less than 0.05 were considered statistically significant in all analyses.
Patient and public involvement
This analysis used health data at the aggregated level, and thus there is no patient or public involvement. No patients were involved in setting the research question or the outcome measures, nor were they involved in developing plans for recruitment, design, or implementation of the study. No patients were asked to advise on interpretation or writing up of results. There are no plans to disseminate the results of the research to study participants or relevant patients.
Results
Descriptive statistics
This analysis included 62.8 million deaths from total or non-external causes, including 19.7 and 5.5 million deaths from cardiovascular diseases and respiratory diseases, respectively (table 1), covering the study period from 1973 to 2018. Cardiovascular deaths accounted for 31.4% of total deaths among all countries, ranging from 19.9% in Thailand to 50.4% in the Czech Republic; while respiratory deaths accounted for 8.8%, ranging from 4.8% in the Czech Republic to 15.7% in Japan. On average, the median annual mean NO2 concentration across 398 cities was 26.9 μg/m3 (25th to 75th centiles 19.5 to 36.2 μg/m3), which was lower than the air quality guidelines for NO2 (annual mean 40 μg/m3) of the World Health Organization.6 The median annual mean temperature was 14.4°C (25th to 75th centiles 7.8°C to 20.6°C). A detailed summary of exposure data is provided in eTable 1. The Spearman correlation coefficients of NO2 were 0.34 with PM10, 0.38 with PM2.5, −0.24 with O3, 0.42 with SO2, and 0.56 with CO. On average, NO2 was negatively correlated with mean temperature (Pearson r=−0.29) and relative humidity (Pearson r=−0.15).
Table 1.
Mortality and environmental data in 398 cities of 22 countries/regions
| Country /region |
No of cities | Period | Number of deaths (in thousands)* | Median (interquartile range) | ||||
|---|---|---|---|---|---|---|---|---|
| Total | Cardiovascular | Respiratory | NO2 (μg/m3) | Temperature (°C) | ||||
| Australia | 3 | 1988-2009 | 1178.0 | NA | NA | 21.4 (14.1-27.9) | 18.1 (14.7-21.2) | |
| Brazil | 1 | 1997-2011 | 916.2 | NA | NA | 84.9 (62.6-115.3) | 20.6 (18-22.9) | |
| Canada | 25 | 1986-2015 | 3617.6 | 1220.7 | 307.8 | 23.7 (15.6-33.8) | 7.4 (−0.9-15.7) | |
| Chile | 3 | 2005-13 | 316.8 | NA | NA | 21.6 (13.9-32.4) | 13.7 (10.7-17.2) | |
| China | 15 | 1996-2015 | 1201.7 | 468.0 | 164.7 | 46.5 (36.2-60.4) | 16.3 (6.5-23.5) | |
| Colombia | 1 | 1998-2013 | 426.3 | 123.8 | 46.3 | 30.5 (23.3-37.9) | 13.9 (13.2-14.5) | |
| Czech Republic | 1 | 1994-2015 | 287.5 | 145.0 | 13.9 | 30.8 (24.2-38.7) | 9.2 (2.7-15.3) | |
| Estonia | 4 | 1997-2015 | 133.8 | NA | NA | 11.4 (7.6-16.7) | 6.0 (−0.1-13.6) | |
| Finland | 1 | 1994-2014 | 153.3 | 57.4 | 9.7 | 6.8 (4.3-11.7) | 5.9 (0.0-13.8) | |
| Germany | 12 | 1993-2015 | 3105.9 | NA | NA | 29.6 (21.8-38.4) | 10.5 (4.8-15.9) | |
| Greece | 1 | 2001-10 | 288 | 136.2 | 28.8 | 50.2 (39.6-61.6) | 17.9 (12.9-24.9) | |
| Japan | 47 | 2011-15 | 1885 | 496.7 | 296.0 | 16.7 (12.1-23.4) | 16.1 (7.6-22.7) | |
| Portugal | 5 | 1990-2018 | 1750.7 | 659.8 | 165.9 | 14.9 (10.0-21.7) | 15.4 (11.5-19.9) | |
| Romania | 8 | 1994-2016 | 951.1 | NA | NA | 25.6 (17.7-36.2) | 11.4 (3.4-18.9) | |
| South Korea | 7 | 1992-2015 | 2245.3 | 547.5 | 133.3 | 43.7 (33.0-57.5) | 14.9 (5.7-21.9) | |
| Spain | 48 | 1990-2014 | 2929.6 | 1011.8 | 330.8 | 26.4 (20.2-33.9) | 14.9 (10.2-20.7) | |
| Sweden | 1 | 1990-2010 | 201.2 | 91.3 | 15.9 | 26.8 (20.0-34.8) | 6.8 (1.2-13.9) | |
| Switzerland | 8 | 1995-2013 | 243.6 | 90.7 | 16.0 | 32.3 (24.0-42.0) | 10.7 (4.4-16.5) | |
| Taiwan | 3 | 1994-2014 | 1209.6 | 269.4 | 116.5 | 42.2 (31.6-54.7) | 24.9 (20.4-28.0) | |
| Thailand | 18 | 1999-2008 | 843.4 | 167.8 | 110.2 | 22.0 (16.3-30.4) | 28.0 (26.4-29) | |
| United Kingdom | 39 | 1990-2016 | 5413.4 | 1978.9 | 799.2 | 25.6 (17.9-35.6) | 10.4 (6.6-14.7) | |
| United States | 147 | 1973-2006 | 33 502.8 | 12 303.5 | 2904.9 | 28.7 (20.8-38.6) | 14.7 (7.2-21.8) | |
| Pooled | 398 | 1973-2018 | 62 800.8 | 19 768.5 | 5459.9 | 26.9 (19.5-36.2) | 14.4 (7.8-20.6) | |
NO2=nitrogen dioxide; NA=not available.
Mortality data from cardiovascular and respiratory diseases were not available in Australia, Brazil, Chile, Estonia, Germany, and Romania.
Regression results
Figure 1 illustrates the estimated pooled associations between NO2 and total, cardiovascular, and respiratory mortality on different lag days. Results suggested that the magnitude of associations increased from the current (lag 0) to the previous day (lag 1) and then decreased on lag 2 day for all three endpoints. In addition, considering its causal respiratory effect,5 we used NO2 concentration on lag 1 day as the main lag value for NO2 in subsequent analysis.
Fig 1.
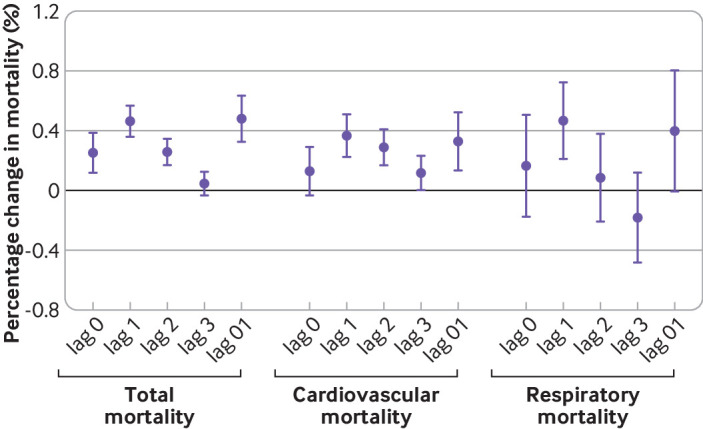
Relative risks of total, cardiovascular, and respiratory mortality associated with a 10 μg/m3 increase in nitrogen dioxide (NO2) on different lag days for NO2. Lags indicate the time difference between the NO2 exposure and the outcome. Lag 0=the present day; lag 1=the previous day; lag 2=the day before lag 1; lag 3=the day before lag 2; lag 0-1=the two day moving average of the present day and the previous day
Table 2 displays the country specific and pooled estimates for the associations of NO2 (lag 1) with total, cardiovascular, and respiratory mortality. Across 398 cities, a 10 μg/m3 increase in NO2 concentrations was associated with an increase of 0.46% (95% confidence interval 0.36% to 0.57%) in total mortality; while across 362 cities with available data, the corresponding increases were 0.37% (95% confidence interval 0.22% to 0.51%) and 0.47% (95% confidence interval 0.21% to 0.72%), respectively, for cardiovascular and respiratory mortality. The country specific estimates showed considerable variations. Consistently positive associations of NO2 with total mortality were seen, ranging from 0.17% to 0.70% for total mortality. Estimates were less precise for cause specific associations, although generally indicating an increased risk.
Table 2.
Percentage changes in total, cardiovascular, and respiratory mortality associated with increase of 10 μg/m3 in nitrogen dioxide on lag 1 day. Results are shown as percentage (95% confidence interval)
| Country/region | Total mortality | Cardiovascular mortality | Respiratory mortality | |||||
|---|---|---|---|---|---|---|---|---|
| No* | Mean change (%; 95% CI) | No* | Mean change (%; 95% CI) | No* | Mean change (%; 95% CI) | |||
| Australia | 3 | 0.64 (0.36 to 0.93) | 0 | NA | 0 | NA | ||
| Brazil | 1 | 0.34 (0.22 to 0.47) | 0 | NA | 0 | NA | ||
| Canada | 25 | 0.62 (0.47 to 0.76) | 24 | 0.45 (0.23 to 0.67) | 24 | 0.52 (0.10 to 0.94) | ||
| Chile | 3 | 0.53 (0.39 to 0.67) | 0 | NA | 0 | NA | ||
| China | 15 | 0.57 (0.31 to 0.83) | 13 | 0.54 (0.34 to 0.73) | 13 | 0.76 (0.41 to 1.12) | ||
| Colombia | 1 | 0.31 (0.01 to 0.61) | 1 | 0.28 (−0.09 to 0.66) | 1 | −0.05 (−0.75 to 0.67) | ||
| Czech Republic | 1 | 0.46 (0.16 to 0.76) | 1 | 0.37 (0.01 to 0.73) | 1 | 0.42 (−0.34 to 1.18) | ||
| Estonia | 4 | 0.40 (0.06 to 0.74) | 0 | NA | 0 | NA | ||
| Finland | 1 | 0.41 (0.08 to 0.75) | 1 | 0.39 (−0.01 to 0.79) | 1 | 0.50 (−0.27 to 1.28) | ||
| Germany | 12 | 0.62 (0.47 to 0.78) | 0 | NA | 0 | NA | ||
| Greece | 1 | 0.62 (0.36 to 0.88) | 1 | 0.47 (0.15 to 0.80) | 1 | 0.84 (0.20 to 1.49) | ||
| Japan | 47 | 0.36 (0.19 to 0.54) | 47 | 0.18 (−0.10 to 0.45) | 47 | 0.70 (0.27 to 1.12) | ||
| Portugal | 5 | 0.52 (0.37 to 0.66) | 5 | 0.22 (−0.07 to 0.51) | 5 | 0.13 (−0.40 to 0.66) | ||
| Romania | 8 | 0.27 (0.07 to 0.48) | 0 | NA | 0 | NA | ||
| South Korea | 7 | 0.41 (0.12 to 0.70) | 7 | 0.48 (0.25 to 0.71) | 7 | 0.48 (0.05 to 0.91) | ||
| Spain | 48 | 0.70 (0.51 to 0.88) | 47 | 0.53 (0.25 to 0.81) | 47 | 0.88 (0.40 to 1.35) | ||
| Sweden | 1 | 0.55 (0.27 to 0.83) | 1 | 0.21 (−0.16 to 0.59) | 1 | 0.41 (−0.34 to 1.15) | ||
| Switzerland | 8 | 0.24 (−0.07 to 0.55) | 8 | 0.52 (0.16 to 0.88) | 8 | 0.24 (−0.47 to 0.96) | ||
| Taiwan | 3 | 0.52 (0.28 to 0.76) | 3 | 0.37 (0.07 to 0.67) | 3 | 0.22 (−0.29 to 0.74) | ||
| Thailand | 18 | 0.32 (0.13 to 0.51) | 18 | 0.38 (0.03 to 0.74) | 18 | 0.69 (0.10 to 1.28) | ||
| United Kingdom | 39 | 0.17 (0.04 to 0.30) | 39 | 0.10 (−0.09 to 0.28) | 39 | 0.11 (−0.21 to 0.43) | ||
| United States | 147 | 0.57 (0.46 to 0.68) | 146 | 0.45 (0.30 to 0.61) | 146 | 0.62 (0.34 to 0.90) | ||
| Pooled | 398 | 0.46 (0.36 to 0.57) | 362 | 0.37 (0.22 to 0.51) | 362 | 0.47 (0.21 to 0.72) | ||
NA=not available.
Number of cities with available data within the country or region.
Figure 2 shows the results of the model with non-linear terms for estimating the effect of the association between NO2 and mortality, indicating positive and almost linear concentration-response curves for total, cardiovascular, and respiratory mortality, with no discernible thresholds. Thus a counterfactual scenario at 0 µg/m3 was defined to assess the mortality impacts in each country based on the current study sample (table 3). Although we recognise that this level is not practical from a policy standpoint, our analysis provides insight into the overall health burden from NO2. The population attributable fraction (%) ranged from 0.28% (95% confidence interval 0.05% to 0.51%) in Finland and 3.05% (95% confidence interval 1.79% to 4.29%) in Greece, with a pooled population attributable fraction (%) of 1.23% (95% confidence interval: 0.96% to 1.51%) across the 398 cities.
Fig 2.
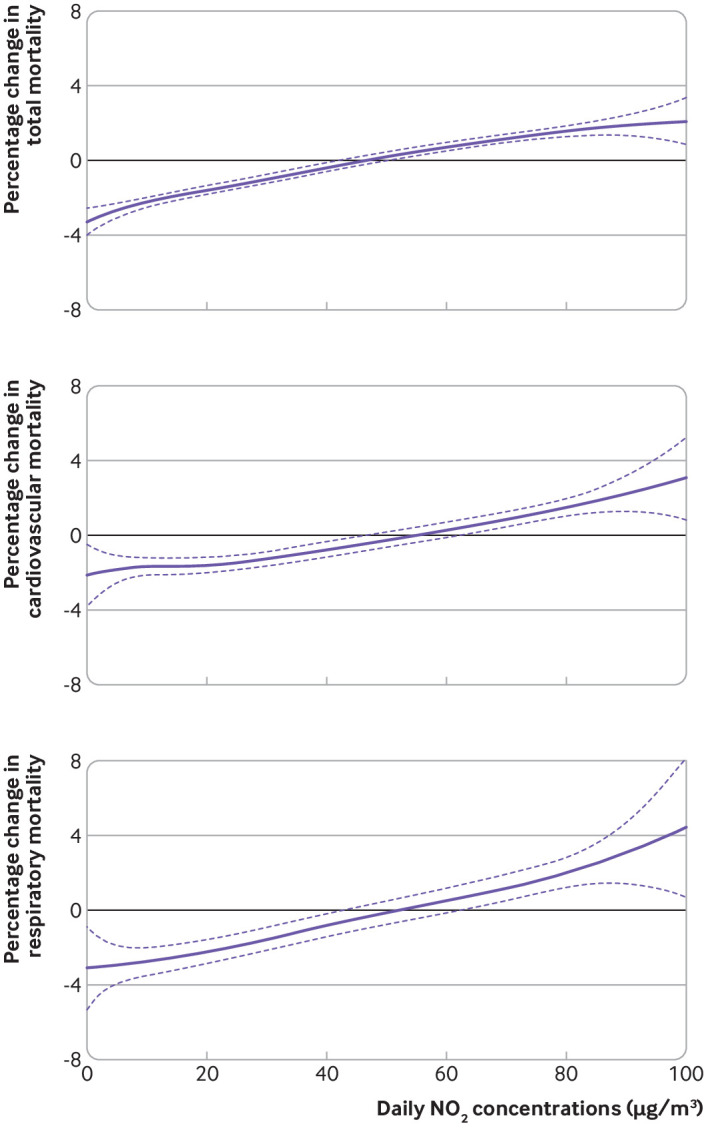
Concentration-response curve between nitrogen dioxide (NO2) concentrations (lag 1) and total, cardiovascular, and respiratory mortality. The vertical scale can be interpreted as the relative change of the mean effect of NO2 on mortality; the fraction of the curve below zero denotes a smaller estimate than the mean effect
Table 3.
Proportion of deaths attributable to reductions in daily NO2 concentrations in each country or region
| Country/region | Cities | Annual deaths (in thousands)* |
Annual NO2 concentrations (μg/m3)† | PAF (%; 95% CI) |
|---|---|---|---|---|
| Australia | 3 | 53.5 | 21.4 | 1.36 (0.76 to 1.96) |
| Brazil | 1 | 61.1 | 84.9 | 2.87 (1.81 to 3.91) |
| Canada | 25 | 115.9 | 23.7 | 1.45 (1.10 to 1.79) |
| Chile | 3 | 35.2 | 21.6 | 1.13 (0.83 to 1.43) |
| China | 15 | 200.3 | 46.5 | 2.61 (1.42 to 3.79) |
| Colombia | 1 | 23.1 | 30.5 | 0.94 (0.04 to 1.84) |
| Czech Republic | 1 | 12.3 | 30.8 | 1.39 (0.48 to 2.30) |
| Estonia | 4 | 7.0 | 11.4 | 0.45 (0.07 to 0.83) |
| Finland | 1 | 6.6 | 6.8 | 0.28 (0.05 to 0.51) |
| Germany | 12 | 135.0 | 29.6 | 1.82 (1.37 to 2.27) |
| Greece | 1 | 28.8 | 50.2 | 3.05 (1.79 to 4.29) |
| Japan | 47 | 377.0 | 16.7 | 0.60 (0.31 to 0.89) |
| Portugal | 5 | 44.9 | 14.9 | 0.76 (0.55 to 0.97) |
| Romania | 8 | 41.4 | 25.6 | 0.70 (0.18 to 1.21) |
| South Korea | 7 | 21.0 | 43.7 | 1.76 (0.51 to 3.00) |
| Spain | 48 | 117.2 | 26.4 | 1.81 (1.34 to 2.29) |
| Sweden | 1 | 9.6 | 26.8 | 1.46 (0.72 to 2.19) |
| Switzerland | 8 | 12.4 | 32.3 | 0.78 (−0.21 to 1.75) |
| Taiwan | 3 | 32.8 | 42.2 | 2.17 (1.17 to 3.15) |
| Thailand | 18 | 84.3 | 22 | 0.70 (0.30 to 1.10) |
| United Kingdom | 39 | 194.0 | 25.6 | 0.45 (0.11 to 0.78) |
| United States | 147 | 985.4 | 28.7 | 1.63 (1.32 to 1.94) |
| Pooled | 398 | 2598.7 | 26.9 | 1.23 (0.96 to 1.51) |
NO2=nitrogen dioxide; PAF%=population attributable fraction.
Annual death numbers summed from the country specific time series data.
Reductions in NO2 concentrations were calculated as the difference between country specific annual NO2 concentrations and the theoretically zero concentration.
In the models with two pollutants (eTable 3), the associations of NO2 with total mortality were robust to the adjustment of co-pollutants. The estimates of NO2 mortality associations increased slightly with adjustment of SO2, O3, and CO. Although the effect estimates decreased by 19% (P=0.08) and 21% (P=0.08) after adjusting for PM10 and PM2.5, respectively, the associations were still positive and statistically significant.
In the sensitivity analyses, compared with main models, the estimates for the associations of NO2 with mortality were generally similar when adjusting for temperature with different lag structures, except for a smaller association when using the lag of the present and mean of the previous three days (eFigure 2). The use of alternative knots did not substantially change the shape of the concentration-response curves (eFigure 3). The associations of NO2 with total and cause specific mortality did not change with or without adjustment of relative humidity (eTable 5). We found no evidence of seasonal difference in the association of NO2 with mortality (P=0.68), with the estimate for the cold season (0.51%, 95% confidence interval 0.41% to 0.60%) similar to that of the warm season (0.43%, 95% confidence interval 0.31% to 0.50%). Finally, the estimates for all three endpoints were nearly the same for different time periods (eTable 6).
Discussion
Principal findings
A key advantage of this epidemiological study on the short term association of NO2 with daily mortality is the analysis of a large dataset using the same analytical protocol, which allows a valid comparison among countries and regions of the world. We found robust associations of NO2 with daily mortality from total, cardiovascular, and respiratory causes that were independent of concomitant exposures to other air pollutants. More importantly, we pooled a concentration-response curve for NO2 at the global level, suggesting an almost linear association, with no discernible thresholds. This result suggests that NO2 is associated with considerable health risks even at levels below health based standards and guidelines, including the current WHO air quality guidelines.
Among the 398 cities, the risk of total mortality increased by 0.46% for every 10 μg/m3 increase in NO2 concentrations. The magnitude of the association is comparable with the result from one previous systematic review, which included studies in 26 cities worldwide and reported an estimate of 0.78% for all cause mortality with the same NO2 increment as ours.18 The other multilocation study conducted in Europe (known as the Air Pollution on Health: a European Approach (APHEA)2 project) found a smaller effect estimate with an increase of 0.30% (95% confidence interval 0.22% to 0.38%) in all cause mortality associated with a 10 μg/m3 increase in NO2.19 Our estimate is somewhat smaller than some multilocation studies conducted in a single country. For a 10 μg/m3 increase in NO2, a multilocation study in China observed a 0.91% (95% confidence interval 0.70% to 1.12%) increase in total mortality20; another study in Italy reported an estimate of 2.09% (95% confidence interval 0.96% to 3.24%).21 The differences in estimates do not necessarily reflect a diverse effect of NO2 onhealth. Rather, the coverage of regions and time periods, population characteristics, and exposure patterns might contribute to these various findings. Furthermore, as found in our sensitivity analysis, different modelling specification for temperature would introduce fluctuation in the estimated associations.
The 2016 Integrated Science Assessment conducted by the US Environmental Protection Agency determined a causal relation between short term NO2 exposure and respiratory effects, whereas for total mortality and cardiovascular effects, the conclusion was “suggestive of, but not sufficient to infer, a causal relation.” Our findings from this multilocation analysis add to the supporting evidence for causal associations between short term exposure to NO2 and non-respiratory endpoints. Experimental investigations and controlled human exposure studies are needed to understand the non-respiratory effect of NO2.
There has been a long debate on whether exposure to NO2 independently causes health effects, or whether it serves as a marker for a broader mixture of air pollutants, especially those related to traffic.5 Findings from controlled human exposure and animal toxicological studies are scarce, but are key for indicating independent associations.22 23 Although statistically adjusting the NO2 association for another co-pollutant cannot conclusively show an independent effect, gathering evidence might contribute towards understanding this matter. In our analysis, the NO2-mortality association decreased after adjustment of PM10 and PM2.5 but remained positive and statistically significant. A systematic review, including studies in 26 cities worldwide, found similarly that the effect estimates of NO2 on total mortality decreased from 0.78% to 0.60%, but were still statistically significant after adjusting for PM.18 Meanwhile, as found in our previous study, the association between PM and mortality was also influenced by NO2.15 This association might indicate potential mutual confounding in the PM-mortality and NO2-mortality associations. On the other hand, the null estimates found in previous reports of NO2 after adjustment of co-pollutants might be due to the smaller study sample, regional difference, and other uncertainties of study heterogeneity. Our multilocation study and meta-analysis provided robust evidence for the independent association between NO2 and mortality.
Characterising the shape of the concentration-response curve helps to quantify the effect on public health of exposure to NO2 and establish safe concentration limits. The shape of the concentration-response curve between effect on health and short term exposure to NO2, however, has only been examined in a small number of epidemiologic studies with a limited spatial scale. Our study provides pooled estimates of the concentration-response relation curves for short term exposure to NO2 and total, cardiovascular, and respiratory mortality across various cities and countries. Results show that the curves are almost linear with no obvious thresholds, suggesting that the level of NO2 below the current air quality guidelines is still hazardous to public health. We estimated that reduction in daily NO2 concentration to a counterfactual zero level would reduce 1.25% of attributable deaths across the 398 cities. Although reduction of NO2 to zero is infeasible, our analysis provides insight into the public health benefits of substantial reductions in NO2, suggesting considerable health benefits from stricter control of NO2 emissions and tightening of the regulatory limits of NO2 in future revisions of WHO air quality guidelines.
Strengths and limitations of the study
This multilocation study has several advantages. Firstly, the study included 398 cities of 22 countries, providing enormous statistical power and ensuring the stability of the findings. Secondly, we examined the associations of NO2 with not only total, but also cause specific, mortality. Thirdly, we pooled concentration-response curves covering a wide range of ambient NO2 concentrations, the evidence from which can be considered generalisable. Fourthly, the uniform analytical approaches can aid in integrating and comparing the results across different regions and populations. Finally, our results contribute to the increasing evidence that supports independent health effects of NO2 on total, cardiovascular, and respiratory mortality.
We acknowledge some limitations of this study. Firstly, given the vast difference in accessibility, most of the data on health and exposure were obtained from developed areas, such as Europe, North America, and East Asia. Thus global generalisation of the findings should be interpreted with caution, especially for areas with smaller study samples or none (that is, Africa and Latin America).
Secondly, this analysis was inherently a time series design that used ecological data and environmental measurements from fixed site monitors, and we could not completely rule out ecological fallacy and exposure misclassification. In addition, the assumption of this time series study is that the reconstructed daily levels of air pollution are a good representation of the average exposure across the whole population at the city level.24 25 Under this condition, the estimates of aggregated analyses are affected by Berkson’s bias and not measurement error bias, leading therefore to the correct point estimates and only an inflation of the standard errors.26 27 Therefore, although the proximity of people to air quality monitors is unclear, the overall results would not be substantially biased.
Thirdly, there might have been slight changes in air pollution measurements in this multilocation study covering multiple decades, and the health data collection might be subject to diagnostic or coding errors. The effects of these problems on our results are difficult to evaluate, adding more uncertainty to cause specific mortality than to all cause mortality. The internal validity of each location specific dataset is presumed to be high, however, in that the changes in operating protocols, diagnostic practices, and coding have been accounted for by the statistical authorities in the various data gathering and preparation stages.
Fourthly, health outcomes were obtained only from their primary causes, and thus our analysis might underestimate the potential effect of NO2 on total mortality. Finally, missing data were inevitable in such a multilocation study over a long period of time, but the amount of missing data was generally small for both health and exposure data, and its influence on our estimates is unlikely to be substantial (Appendix, eTable 7 and eResults 2.3).
Conclusions and policy implications
In summary, this multilocation time series analysis provides robust evidence for the independent associations of short term exposure to NO2 with increased risk of total, cardiovascular, and respiratory mortality, although the total mortality burden might be underestimated as the study locations did not fully reach global coverage. The associations remained positive and statistically significant after adjusting for co-pollutants. The concentration-response curves were linear without discernible thresholds, suggesting a need to revise and tighten the current air quality guidelines of NO2 for greater public health benefit, and to consider a regulation limit for daily mean NO2 concentration. These findings contribute to a better understanding of how to optimise public health actions and strategies to mitigate air pollution.
What is already known on this topic
Evidence for the short term association between ambient nitrogen dioxide (NO2) and mortality is limited, especially for cause specific associations
Previous investigations have been mostly conducted in a small number of locations that cover limited geographical areas, and use different study designs and modelling approaches that lead to heterogeneous results
What this study adds
This large multilocation study in 398 cities found that each 10 μg/m3 increase in NO2 concentrations on lag 1 day (previous day) was significantly associated with increased risk of total (0.46%), cardiovascular (0.37%), and respiratory (0.47%) mortality
The concentration-response curves were almost linear without discernible thresholds even below the current WHO air quality guidelines, suggesting a need to revise the currently recommended values
The uniform analytical approach allows a direct comparison of estimates across global regions
Web extra.
Extra material supplied by authors
Web appendix: Online only supplements
Contributors: HaK and AG are both senior authors and contributed equally to this work. HaK and AG designed the study. XM,CL, and RC are joint first authors with equal contribution. XM, CL, and RC coordinated the work, conducted the statistical analysis, and took the lead in drafting the manuscript and interpreting the results. FS, AMV-C, ST, EL, IHH, MH, VH, AT, AU, Y-LLG,CI, MLB, and TW provided substantial scientific input in interpreting the results and drafting the manuscript. AM, YG, MdSZSC, PHNS, PMC, NVO, SOG, JK, HO, MM, JJKJ, NR, VH, AS, KK, AA, YH, CFSN, BN, JPT, SF, HoK, BF, CA, MSR, S-CP, SL, AZ, and JS provided the data and contributed to interpretation of the results and to the submitted version of the manuscript. HaK and AG are the guarantors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: HaK was supported by the National Natural Science Foundation of China (92043301, 82030103, and 91843302) and China Medical Board Collaborating Program (16-250). AG and FS were supported by the Medical Research Council, UK (MR/M022625/1), the Natural Environment Research Council, UK (NE/R009384/1), and the European Union’s Horizon 2020 Project Exhaustion (820655). VH was supported by the Spanish Ministry of Science and Innovation (PCIN-2017-046), and the German Federal Ministry of Education and Research (01LS1201A2). YH and MH were supported by the Environment Research and Technology Development Fund (JPMEERF15S11412) of the Environmental Restoration and Conservation Agency, Japan. JK and AU were supported by the Czech Science Foundation (18-22125S). ST was supported by the Shanghai Municipal Science and Technology Commission (18411951600). Y-LLG was supported by a Career Development Fellowship of the Australian National Health and Medical Research Council (APP1163693). SL was supported by an Early Career Fellowship of the Australian National Health and Medical Research Council (APP1109193). JJKJJ and NR were supported by the Academy of Finland (310372). The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the National Natural Science Foundation of China, China Medical Board Collaborating Program, Medical Research Council, UK, Natural Environment Research Council, UK, European Union’s Horizon 2020 Project Exhaustion, Spanish Ministry of Science and Innovation, German Federal Ministry of Education and Research, the Environmental Restoration and Conservation Agency, Japan, the Czech Science Foundation, the Shanghai Municipal Science and Technology Commission, the Australian National Health and Medical Research Council, and the Academy of Finland; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Not required.
Data sharing: Data have been collected within the MCC (Multi-City Multi-Country) Collaborative Research Network (https://mccstudy.lshtm.ac.uk/) under a data sharing agreement and cannot be made publicly available. Researchers can refer to MCC participants listed as coauthors for information on accessing the data for each country.
The lead authors (HaK and AG) affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: The findings will be disseminated through press releases by the research institutions of the contributing authors.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.US EPA. National Ambient Air Quality Standards for six criteria pollutants. 2016.
- 2. Chen R, Yin P, Meng X, et al. Associations Between Ambient Nitrogen Dioxide and Daily Cause-specific Mortality: Evidence from 272 Chinese Cities. Epidemiology 2018;29:482-9. 10.1097/EDE.0000000000000829. [DOI] [PubMed] [Google Scholar]
- 3. Faustini A, Rapp R, Forastiere F. Nitrogen dioxide and mortality: review and meta-analysis of long-term studies. Eur Respir J 2014;44:744-53. 10.1183/09031936.00114713. [DOI] [PubMed] [Google Scholar]
- 4.US-EPA. Integrated science assessment for oxides of nitrogen – health criteria (final report). 2008.
- 5.US-EPA. Integrated science assessment for oxides of nitrogen – health criteria (final report). 2016.
- 6. WHO . Air quality guidelines for particulate matter, ozone, nitrogen dioxide and sulfur dioxide: global update 2005. World Health Organization, 2006. [PubMed] [Google Scholar]
- 7. Krzyzanowski M. WHO air quality guidelines for Europe. J Toxicol Environ Health A 2008;71:47-50. 10.1080/15287390701557834 [DOI] [PubMed] [Google Scholar]
- 8. Ministry of Ecology and Environment C. Ambient Air Quality Standards 2012.
- 9. Mills IC, Atkinson RW, Kang S, Walton H, Anderson HR. Quantitative systematic review of the associations between short-term exposure to nitrogen dioxide and mortality and hospital admissions. BMJ Open 2015;5:e006946. 10.1136/bmjopen-2014-006946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gasparrini A, Guo Y, Hashizume M, et al. Temporal Variation in Heat-Mortality Associations: A Multicountry Study. Environ Health Perspect 2015;123:1200-7. 10.1289/ehp.1409070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gasparrini A, Guo Y, Hashizume M, et al. Mortality risk attributable to high and low ambient temperature: a multicountry observational study. Lancet 2015;386:369-75. 10.1016/S0140-6736(14)62114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. WHO . International statistical classification of diseases and related health problems. World Health Organization, 2004. [Google Scholar]
- 13. Bell ML, Dominici F, Samet JM. A meta-analysis of time-series studies of ozone and mortality with comparison to the national morbidity, mortality, and air pollution study. Epidemiology 2005;16:436-45. 10.1097/01.ede.0000165817.40152.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen R, Yin P, Meng X, et al. Fine Particulate Air Pollution and Daily Mortality. A Nationwide Analysis in 272 Chinese Cities. Am J Respir Crit Care Med 2017;196:73-81. 10.1164/rccm.201609-1862OC. [DOI] [PubMed] [Google Scholar]
- 15. Liu C, Chen R, Sera F, et al. Ambient Particulate Air Pollution and Daily Mortality in 652 Cities. N Engl J Med 2019;381:705-15. 10.1056/NEJMoa1817364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sera F, Armstrong B, Blangiardo M, Gasparrini A. An extended mixed-effects framework for meta-analysis. Stat Med 2019;38:5429-44. 10.1002/sim.8362. [DOI] [PubMed] [Google Scholar]
- 17. Samoli E, Analitis A, Touloumi G, et al. Estimating the exposure-response relationships between particulate matter and mortality within the APHEA multicity project. Environ Health Perspect 2005;113:88-95. 10.1289/ehp.7387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mills IC, Atkinson RW, Anderson HR, Maynard RL, Strachan DP. Distinguishing the associations between daily mortality and hospital admissions and nitrogen dioxide from those of particulate matter: a systematic review and meta-analysis. BMJ Open 2016;6:e010751. 10.1136/bmjopen-2015-010751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Samoli E, Aga E, Touloumi G, et al. Short-term effects of nitrogen dioxide on mortality: an analysis within the APHEA project. Eur Respir J 2006;27:1129-38. 10.1183/09031936.06.00143905. [DOI] [PubMed] [Google Scholar]
- 20. Chen R, Yin P, Meng X, et al. Associations Between Ambient Nitrogen Dioxide and Daily Cause-specific Mortality: Evidence from 272 Chinese Cities. Epidemiology 2018;29:482-9. 10.1097/EDE.0000000000000829 [DOI] [PubMed] [Google Scholar]
- 21. Chiusolo M, Cadum E, Stafoggia M, et al. EpiAir Collaborative Group . Short-Term Effects of Nitrogen Dioxide on Mortality and Susceptibility Factors in 10 Italian Cities: The EpiAir Study. Environ Health Perspect 2011;119:1233-8. 10.1289/ehp.1002904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Riedl MA, Diaz-Sanchez D, Linn WS, et al. HEI Health Review Committee . Allergic inflammation in the human lower respiratory tract affected by exposure to diesel exhaust. Res Rep Health Eff Inst 2012;(165):5-43, discussion 45-64. [PubMed] [Google Scholar]
- 23. Thompson AM, Zanobetti A, Silverman F, et al. Baseline repeated measures from controlled human exposure studies: associations between ambient air pollution exposure and the systemic inflammatory biomarkers IL-6 and fibrinogen. Environ Health Perspect 2010;118:120-4. 10.1289/ehp.0900550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Samet JM, Dominici F, Curriero FC, Coursac I, Zeger SL. Fine particulate air pollution and mortality in 20 U.S. cities, 1987-1994. N Engl J Med 2000;343:1742-9. 10.1056/NEJM200012143432401. [DOI] [PubMed] [Google Scholar]
- 25. Bell ML, Samet JM, Dominici F. Time-series studies of particulate matter. Annu Rev Public Health 2004;25:247-80. 10.1146/annurev.publhealth.25.102802.124329. [DOI] [PubMed] [Google Scholar]
- 26. Goldman GT, Mulholland JA, Russell AG, et al. Impact of exposure measurement error in air pollution epidemiology: effect of error type in time-series studies. Environ Health 2011;10:61. 10.1186/1476-069X-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sheppard L, Burnett RT, Szpiro AA, et al. Confounding and exposure measurement error in air pollution epidemiology. Air Qual Atmos Health 2012;5:203-16. 10.1007/s11869-011-0140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Online only supplements


