Abstract
Background:
The SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) coronavirus has emerged as a highly contagious respiratory pathogen causing severe acute lung injury. Extracorporeal membrane oxygenation is a standard tool for the management of life-threatening acute respiratory distress syndrome, but the use of this resource-intensive therapy has come into question due to strained medical systems and limited proven treatments for COVID-19.
Case summary:
A 16-year-old female with obesity presented with fever, myalgias, cough, and tachypnea and was diagnosed with COVID-19. She progressed to severe pediatric acute respiratory distress syndrome requiring intubation on hospital day 4 and cannulation to veno-venous extracorporeal membrane oxygenation on hospital day 6. The patient received remdesivir, steroids, and anakinra. The patient was successfully decannulated on hospital day 12 and was discharged home on hospital day 21.
Conclusion:
We report the use of veno-venous extracorporeal membrane oxygenation as a bridge to lung recovery in a pediatric patient with severe pediatric acute respiratory distress syndrome due to COVID-19.
Keywords: COVID-19, extracorporeal membrane oxygenation, veno-venous extracorporeal membrane oxygenation, pediatric critical care, acute respiratory distress syndrome
Introduction
The use of extracorporeal membrane oxygenation (ECMO) for COVID-19-associated disease is controversial. The limited literature includes a case series from China1 and a recent pooled analysis of ECMO reports.2 Severe disease from COVID-19 in pediatric patients is rare; a case series from China classified only 5.2% of pediatric infections as severe and 0.6% as critical.3 There have been several editorials questioning whether the use of ECMO in pandemic conditions is an appropriate utilization of resources.4,5 Here, we report a rare case of severe pediatric acute respiratory distress syndrome (pARDS) due to COVID-19 with the successful use of veno-venous extracorporeal membrane oxygenation (VV ECMO) as a bridge to lung recovery amid pandemic conditions in New York.
Case report
A 16-year-old female with obesity (body mass index (BMI) = 31 kg/m2, body surface area (BSA) = 1.91 m2) living in New York City presented with 6 days of fever, myalgias, cough, and tachypnea. At the time of admission, New York City was suffering from pandemic conditions due to COVID-19; 56 of our children’s hospital beds were reallocated to the care of patients from our affiliated adult hospital which was operating at 108% census, and 7 of our 14 pediatric intensivists were redeployed to an adult COVID-19 intensive care unit (ICU). On admission, oxygen saturation was 88%, rising to 93% on 1 L nasal cannula. Initial laboratory studies included lymphopenia (absolute lymphocyte count of 3.55k/μL) and transaminitis (aspartate aminotransferase (AST) = 159 U/mL, alanine aminotransferase (ALT) = 284 U/mL). A COVID-19 nasopharyngeal swab was positive. Chest radiograph demonstrated multifocal bilateral patchy opacities. She was admitted to the inpatient floor and treated with hydroxychloroquine (400 mg two times daily day 1, 200 mg two times daily days 2–5) and azithromycin (500 mg day 1, 250 mg days 2–5). Due to worsening hypoxia, she was transferred to the pediatric intensive care unit on hospital day (HOD) 3 and intubated for hypoxemic respiratory failure on HOD 4.
At the time of intubation, she met criteria for severe pARDS with an oxygenation index (OI) of 18 and a P/F ratio of 65. Lab values after intubation were notable for elevated inflammatory markers, including ferritin of 801.6 ng/mL, C-reactive protein of 280.7 mg/L, d-Dimer of 3,618 ng/mL, fibrinogen of 326 mg/dL, and procalcitonin of 1.47 ng/mL (Table 1). For treatment of acute lung injury associated with COVID-19, she was started on methylprednisolone (40 mg twice daily days 1–3) and anakinra (100 mg every 6 hours days 1–5, 100 mg every 12 hours days 5–10, and 100 mg daily days 11–13). Remdesivir was initiated for compassionate use (experimental antiviral agent, 200 mg day 1, 100 mg daily days 2–5)
Table 1.
Laboratory data.
| Hospital admission (HOD 0) | Time of ICU admission (HOD 3) | Time of intubation (HOD 4) | ECMO cannulation (HOD 6) | ECMO decannulation (HOD 12) | Reference range | |
|---|---|---|---|---|---|---|
| OI | 18 | 43 | 6.7 | |||
| WBC (k/μL) | 6.28 | 6.05 | 6.94 | 14.11 | 3.8–10.5 | |
| Abs lymphocytes (k/μL) | 3.55 | 1.21 | 2.24 | 1.0–3.3 | ||
| AST (U/L) | 159 | 151 | 109 | 42 | 4–32 | |
| ALT (U/L) | 284 | 168 | 175 | 38 | 4–33 | |
| d-Dimer (ng/mL) | 3,618 | 11,084 | 3,756 | <230 | ||
| Ferritin (ng/mL) | 801.6 | 667.7 | 493 | 15–150 | ||
| CRP (mg/L) | 280.7 | 76 | 160.3 | <5 | ||
| Fibrinogen (mg/dL) | 623 | 326 | 483 | 300–520 | ||
| Procalcitonin (ng/mL) | 1.47 | 1.15 | 0.14 | 0.02–0.10 | ||
| Triglycerides (mg/dL) | 122 | 10–149 | ||||
| LDH (U/L) | 1,225 | 692 | 586 | 135–225 |
HOD: hospital day; ICU: intensive care unit; ECMO: extracorporeal membrane oxygenation; OI: oxygenation index; WBC: white blood cells; AST: aspartate aminotransferase; ALT: alanine aminotransferase; CRP: C-reactive protein; LDH: lactate dehydrogenase.
On HOD 6, she experienced an acute deterioration with severe hypoxemia. A blood gas at that time demonstrated a PaO2 of 46 mmHg. She was unable to achieve oxygen saturations above 80% on 100% FiO2, with a positive end expiration pressure (PEEP) of 20 cm H2O, generating ventilator peak pressures of 48 mmHg to achieve tidal volumes 2.8 mL/kg. Nitric oxide was implemented as a rescue therapy without improvement. The OI was 43 and the P/F ratio was 46. She was emergently cannulated to VV ECMO with the right femoral vein (25 French) functioning as the drainage cannula and the right internal jugular (18 French) functioning as the return cannula. A chest tube was placed for a pneumothorax that occurred following cannulation (see Figure 1).
Figure 1.
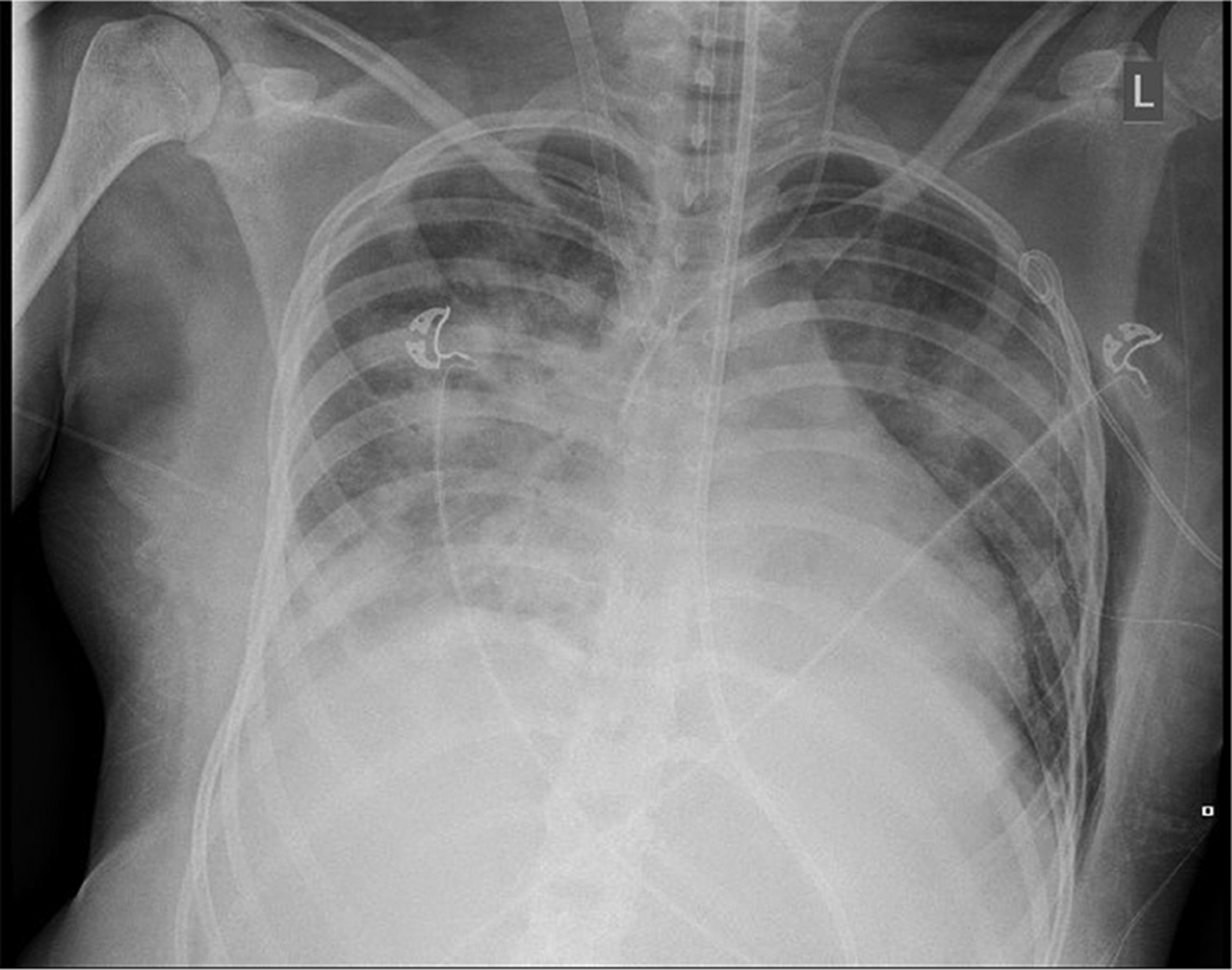
Chest radiograph immediately following ECMO cannulation demonstrating COVID-19-associated lung disease along with ECMO venous cannulas. Additional findings include a left pneumothorax decompressed with left chest tube and associated subcutaneous emphysema.
After cannulation, the patient was ventilated using lung-protective settings targeting tidal volumes of 3–4 mL/kg with a PEEP of 14 generating peak pressures of 28 mmHg on 35% FiO2. Nitric oxide was discontinued post cannulation due to a lack of benefit. Initial ECMO flow ranged from a cardiac index of 2.2–2.4 L/min/m2. Sweep gas flow was titrated to normal gas exchange with a range of 2–4 L/min. Pre-membrane pressures remained approximately 140 mmHg, while post-membrane pressures remained approximately 120 mmHg. Epinephrine infusion was initiated peri-cannulation, and discontinued on ECMO day 1. The patient received vancomycin and cefepime for antimicrobial prophylaxis while cannulated on VV ECMO. No bacterial organisms were isolated.
A heparin loading dose was administered at the time of cannulation, bivalirudin was then selected for anticoagulation, which was titrated to a partial thromboplastin time (PTT) of 60–90 seconds with ranges 03–0.4 μg/kg/h. Additional anticoagulation parameters included a goal activated clotting time (ACT) of 180–220 seconds direct thrombin time (DTT) of 60–90 seconds. Antithrombin III assays were checked daily, and thrombate was administered as required to maintain a therapeutic range of 85–125%.
On ECMO day 3, the patient experienced a sudden decrease in circuit flow to a nadir of 0.5 L/min/m2. A clot was noted at the distal end of the femoral venous cannula despite appropriate anticoagulation. A total of 75 mg of alteplase was instilled via her central venous catheter with improvement in circuit flow. No serious bleeding events occurred. Post-thrombolytic therapy circuit flow rose to 0.9 L/min/m2, and by the following day to 1.8 L/min/m2.
By HOD 12, rapid improvement in respiratory mechanics led to successful decannulation. Ventilatory settings after decannulation were notable for tidal volumes 5 mL/kg with a PEEP of 16% and 30% FiO2 generating peak pressures of 29 mmHg and saturating 95%. She was extubated on HOD 17. The patient was successfully discharged home on HOD 21.
Discussion
SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) is an emergent disease that has caused a rapidly progressing pandemic. Risk factors for mortality in adults include older age, comorbidities, elevated sequential organ failure assessment (SOFA) score, and elevated d-dimer levels.6 Obesity has also been noted to be highly prevalent in patients with COVID-19 requiring intensive care.7 At the time this patient presented New York City was experiencing COVID-19’s ability to spread rapidly with over 33,000 confirmed cases in New York City. There are no proven treatments for COVID-19 and ECMO requires intensive personnel and resources for a single patient, raising the question whether should ECMO be attempted for COVID-19.
Our patient is a 16-year-old previously healthy girl, with obesity as her only possible risk factor for severe disease from COVID-19. ECMO is an accepted therapy for severe ARDS, and historical experience with the use of ECMO for ARDS secondary to H1N1 and MERS showed reduced mortality, supporting the use of ECMO in the setting of novel respiratory infections.8 However, a recent pooled analysis demonstrated high mortality in adult ECMO for COVID-19 (>90%), though the pooled odds of mortality was not significantly different from conventional therapy.2
It appears the patient experienced an overwhelming inflammatory response invoked by SARS-CoV-2, which lead to acute organ dysfunction. We implemented therapy with steroids and anakinra similar to previously reported treatments for secondary hemophagocytic lymphohistiocytosis (HLH). Anakinra is an interleukin-1 receptor antagonist which may blunt the inflammatory response associated with COVID-19. Our patient also received remdesivir, an antiviral agent that may be effective against COVID-19 and currently has emergency approval for treatment of severe COVID-19. Remdesivir is a nucleoside analogue which has demonstrated inhibitory effects on SARS-CoV-1 and SARS-CoV-2 in vitro.9 Utilization of ECMO provided time for resolution of her lung injury and possibly for effectiveness of immunomodulatory therapy, as evidenced by decreasing inflammatory markers (see Figure 2).
Figure 2.
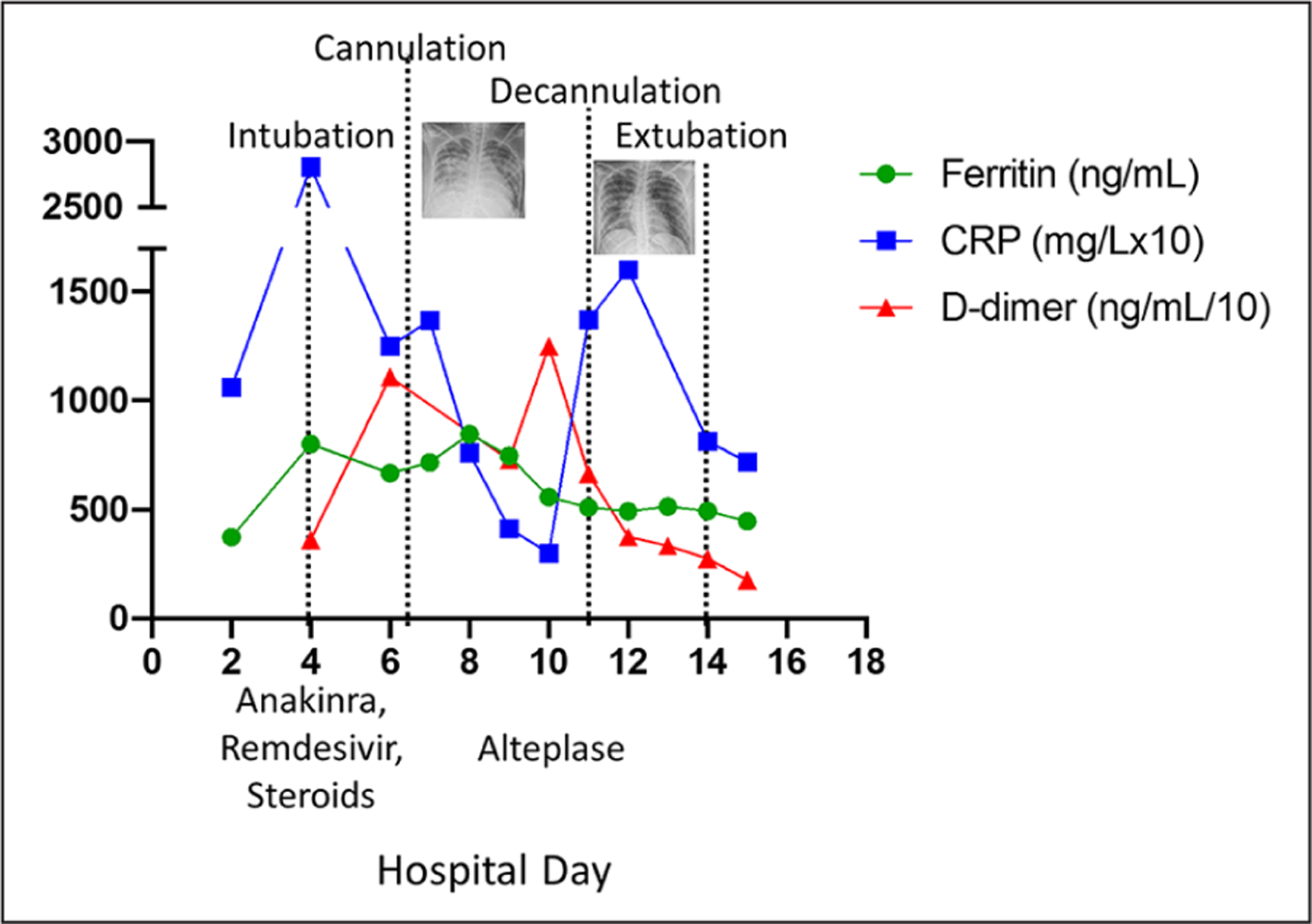
Inflammatory marker trends over hospital course, with significant events noted. CRP multiplied by 10 for scale, d-dimer divided by 10 for scale.
Our patient experienced a thrombotic event. While these are common with ECMO, COVID-19 has been associated with the increase in the risk of thrombosis.10 When using therapeutic heparin in adult COVID-19 patients on VV ECMO elsewhere in our 23 hospital healthcare system, severe clotting had been noted despite therapeutic PTTs (Sweberg T, Greco J, personal communication, 4 April 2020). This prompted our use of bivalirudin as an alternative anticoagulant with the aim to minimize thrombotic complications. In spite of appropriate therapy with PTT levels two to three times the upper limit of normal, this patient had a life-threatening thrombotic event. The mechanisms behind this apparent hypercoaguable state warrant further investigation and should be considered when employing ECMO in patients with COVID-19.
Conclusion
ECMO is a resource-intensive therapy used only by experienced centers. During a global pandemic when appropriate resource utilization is critical, ECMO candidates must be carefully selected. Special considerations should be made for young healthy patients. While it is unclear whether our patient’s recovery was hastened by immunomodulatory therapy, without ECMO as a bridge our patient would not have had time to recover. Although the resources required were significant, they were justified as the potential for meaningful recovery in our adolescent patient was high. This case demonstrates that pediatric COVID-19 can present as pARDS, may respond to treatment for secondary HLH, and the use of ECMO for carefully selected patients is warranted even under pandemic conditions.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: M.D.T. is supported by funding from the NIH NIGMS K08GM132794.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Li X, Guo Z, Li B, et al. Extracorporeal membrane oxygenation for coronavirus disease 2019 in Shanghai, China. ASAIO J 2020; 66: 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henry BM, Lippi G. Poor survival with extracorporeal membrane oxygenation in acute respiratory distress syndrome (ARDS) due to coronavirus disease 2019 (COVID-19): pooled analysis of early reports. J Crit Care 2020; 58: 27–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics 2020; 145: e20200702. [DOI] [PubMed] [Google Scholar]
- 4.MacLaren G, Fisher D, Brodie D. Preparing for the most critically ill patients with COVID-19: the potential role of extracorporeal membrane oxygenation. JAMA 2020; 323: 1245–1246. [DOI] [PubMed] [Google Scholar]
- 5.Namendys-Silva SA. ECMO for ARDS due to COVID-19. Heart Lung 2020; 49: 348–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity 2020; 28: 1195–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong X, Xiong J, Feng Z, et al. Extracorporeal membrane oxygenation (ECMO): does it have a role in the treatment of severe COVID-19? Int J Infect Dis 2020; 94: 78–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020; 395: 1569–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020; 191: 145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]


