Abstract
Aldosterone is a steroid hormone that regulates blood pressure and cardiovascular function by acting on renal and vascular mineralocorticoid receptors (MRs) to promote sodium retention and modulate endothelial function. Indeed, MRs are expressed in endothelial cells, vascular smooth muscle cells, adipocytes, immune cells, skeletal muscle cells, and cardiomyocytes. Excessive aldosterone and associated MR activation impair insulin secretion, insulin metabolic signaling to promote development of diabetes, and the related cardiometabolic syndrome. These adverse effects of aldosterone are mediated, in part, via increased inflammation, oxidative stress, dyslipidemia, and ectopic fat deposition. Therefore, inhibition of MR activation may have a beneficial effect in prevention of impaired insulin metabolic signaling, type 2 diabetes, and cardiometabolic disorders. This review highlights findings from the recent surge in research regarding MR-related cardiometabolic disorders as well as our contemporary understanding of the detrimental effects of excess MR activation on insulin metabolic signaling.
Keywords: diabetes, insulin resistance, mineralocorticoid receptors
INTRODUCTION
Increased activation of the systemic and tissue renin-angiotensin-aldosterone system (RAAS) and the adrenergic nervous system often exists in states of obesity and insulin resistance, and this activation plays an important role in the development of type 2 diabetes and the cardiometabolic syndrome (1). Data from epidemiological studies and meta-analyses of randomized controlled clinical trials support the notion that elevated aldosterone levels and excessive mineralocorticoid receptor (MR) activation increase the risk for cardiovascular diseases, such as heart failure, hypertension, and stroke as well as diabetic nephropathy, and that MR antagonist administration reduces progression of these diseases (1, 2). For instance, results of clinical trials such as the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) (3) and Randomized Aldactone Evaluation Study (RALES) , using spironolactone in heart failure patients (4), indicate that that MR antagonists reduce the overall mortality and rate of cardiovascular disease-related hospitalization and mortality. Recent studies suggest that components of the cardiometabolic syndrome, including obesity and insulin resistance, are associated with increased plasma aldosterone levels (5–8) and enhanced MR activation (1, 9, 10). Accordingly, MR antagonism should not only lessen cardiovascular diseases but also have the potential to decrease development of the cardiometabolic syndrome and diabetes. Accordingly, we review here the recent evidence that aldosterone/MR signaling is activated in insulin resistance states and type 2 diabetes. Additionally, we explore potential mechanisms by which MR activation impairs insulin metabolic signaling in adipocytes, skeletal muscle cells, and cardiovascular tissue as well as pancreatic β-cell function. We conclude with a discussion of the implications of these studies for the development of pharmacological strategies in prevention and treatment of insulin resistance, the cardiometabolic syndrome and type 2 diabetes.
ALDOSTERONE AND MR ACTIVATION
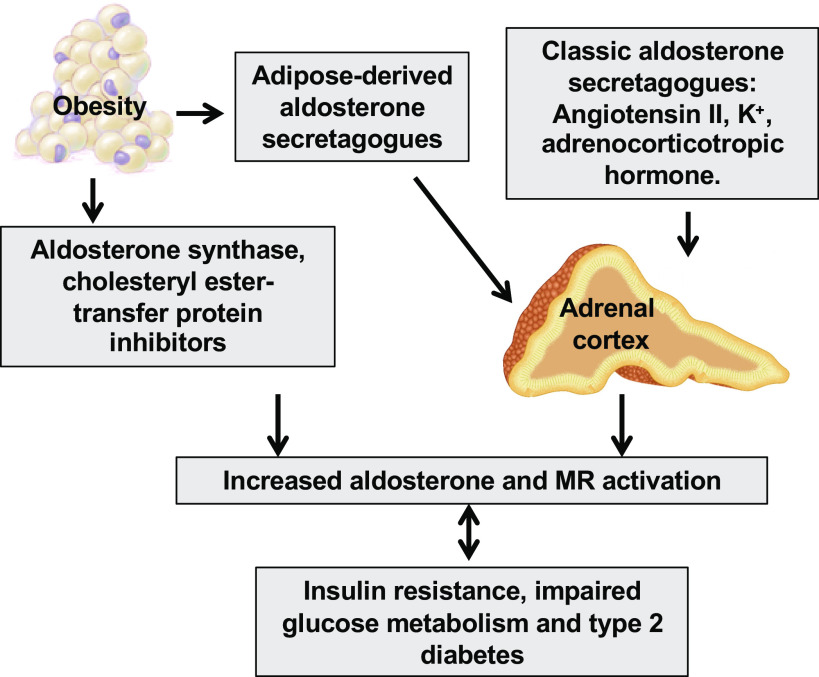
Recent experimental data indicate that diet-induced obesity associated with consumption of a high-fat diet or a diet high in both saturated fat and fructose increases plasma aldosterone levels and tissue MR activity (1, 5–10). Human studies also suggest that plasma aldosterone concentrations are positively correlated with body mass index and indexes of insulin resistance (11). Typically, aldosterone is synthesized in the zona glomerulosa of the adrenal cortex (12), but it is also produced by adipose tissue, including perivascular adipose tissue (13) (Fig. 1). Indeed, mineralocorticoid-releasing factors can increase aldosterone synthase (CYP11B2) activity and thus aldosterone secretion in adipocytes (14, 15). Moreover, other factors, such as cholesteryl ester transfer protein inhibition can promote aldosterone biosynthesis in a nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX)-dependent manner in adipocytes (16). Furthermore, hyperglycemia and hyperinsulinemia further elevate aldosterone levels and associated MR activation and worsen the cardiometabolic syndrome. (Fig. 1).
Figure 1.
Potential mechanisms for increased aldosterone and mineralocorticoid receptor (MR) activation in obesity, insulin resistance, and type 2 diabetes. Aldosterone is classically synthesized in the zona glomerulosa of the adrenal cortex. Aldosterone is also produced from adipose tissue by increased adipose-derived aldosterone secretagogues, aldosterone synthase, and cholesteryl ester-transfer protein inhibitors. Increased aldosterone levels and MR activation promote insulin resistance and type 2 diabetes, which further activate aldosterone/MR signaling and aggravate cardiometabolic syndrome.
Increased salt intake in concert with increased caloric consumption not only leads to elevated blood pressure but also promotes obesity. For instance, there are significant associations between salt intake and sugar-sweetened soft drink consumption in children in a cross-sectional study with 1,688 participants aged 4 to 18 years (17). Moreover, increased salt intake may contribute to a salt-obesity association independent of increased energy intake (18). Furthermore, increased salt intake promotes adipogenesis/lipogenesis and inflammation via increasing the expression of adipokines and activation of systemic and tissue RAAS and increased tissue MR expression (19). Therefore, the interaction of obesity and increased salt intake further induces increases in aldosterone levels, MR activation, insulin resistance, and the associated cardiometabolic syndrome.
Upon being activated by aldosterone, the MR is translocated to the nucleus, where it regulates gene transcription and translation of proteins such as serum- and glucocorticoid-induced protein kinase-1 (10). Aldosterone also exerts rapid nongenomic effects through activation of kinases such as phosphatidylinositol 3-kinase (PI3K), Rho kinase, and protein kinase C (PKC). Activation of Rho kinase and PKC leads to increased cytosolic Ca2+, mitochondrial dysfunction, increased reactive oxygen species (ROS), and associated insulin resistance (20). Furthermore, MR activity is regulated by cortisol as well as aldosterone in certain tissues. In tissues lacking 11β-hydroxysteroid dehydrogenase-2 such as adipose tissue and the heart, cortisol is typically the primary ligand for the MR, due to the fact that blood concentrations of cortisol are 100 to 1,000 times higher than those of aldosterone (21). In contrast, aldosterone is the primary ligand for MRs in vascular tissues, including both endothelial and smooth muscle cells, where there is expression of 11β-hydroxysteroid dehydrogenase-2, which by inactivating cortisol allows aldosterone to selectively activate vascular MRs (22).
BASIC SCIENCE AND CLINICAL EVIDENCE IMPLICATING ENHANCED MR ACTIVATION IN OBESITY, INSULIN RESISTANCE, AND CARDIOMETABOLIC DISORDERS
Preclinical studies have indicated that excessive aldosterone stimulation of MRs is involved in the pathophysiology of obesity, insulin resistance, and the cardiometabolic syndrome. For example, administration of FAD286, an aldosterone synthase inhibitor, decreased plasma aldosterone levels and improved metabolic parameters in Zucker diabetic fatty rats (23). Our research and that of others indicate that inhibition MRs with the MR antagonists spironolactone and eplerenone reduces ROS generation and inflammation, improves insulin-stimulated glucose transport, and insulin sensitivity without blood pressure changes in rodents with increased RAAS activation and insulin resistance (24, 25). Similarly, both spironolactone and drospirenone prevented high-fat diet-induced development of the cardiometabolic syndrome in female obese mice (26). The beneficial metabolic effects of this MR blockade, including reduced body fat and improved glucose tolerance, were associated with “browning” of adipose tissue. Related to this, spironolactone-induced improvement in glucose and lipid metabolism was accomplished, in part, by amelioration of hepatic steatosis and inflammation and suppression of enhanced gluconeogenesis induced by a high-fat and high-fructose diet (27). Furthermore, enhanced MR signaling in immune cells is also implicated in insulin resistance accompanying obesity, as myeloid MR knockout improves glucose intolerance, insulin resistance, and hepatic steatosis in obese mice (28).
Meta-analysis of randomized clinical trials has established the beneficial roles of angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and MR antagonists in patients with insulin resistance, type 2 diabetes, and cardiovascular disease. For instance, data from Heart Outcomes Prevention Evaluation (29), Losartan Intervention For Endpoint (LIFE) reduction in hypertension (30), and the Antihypertensive and Lipid-Lowering treatment to prevent Heart Attack Trial (ALLHAT) (31) demonstrated that RAAS blockade reduced the incidence of new-onset type 2 diabetes. Data from the large prospective Impaired Glucose Tolerance Outcomes Research (NAVIGATOR) trial also showed that treatment with the angiotensin receptor blocker valsartan resulted in a relative reduction of 14% in the incidence of diabetes in patients with impaired glucose tolerance (32). One study also found that angiotensin II infusion increased plasma insulin concentration but suppressed adiponectin production, resulting in impaired insulin sensitivity in rats fed a high-fructose diet (33). Enhanced MR activation is also regarded as an important risk factor for insulin resistance in patients with the cardiometabolic syndrome and heart failure (3, 4). Beneficial effects of MR antagonists in reducing insulin resistance have also been shown in rodent models of genetic and diet-induced obesity (24–27). Although the impact of MR blockade on glucose homeostasis, insulin resistance, and diabetes in patients is under investigation, no definitive data are available. Related to this, one clinical study showed that elevated plasma aldosterone precedes and predicts the development of insulin resistance after 10 years of follow-up (34). Furthermore, patients with primary aldosteronism have impaired pancreatic β-cell function, displaying a negative correlation between impaired insulin metabolism and aldosterone production, suggesting a decrease in insulin secretion secondary to aldosterone excess (35). Moreover, plasma aldosterone levels have been shown to be associated with insulin resistance as characterized by a reduced ability of insulin to inhibit the production of glucose from the liver and to promote glucose uptake in adipose tissue and skeletal muscle in normotensive individuals (36). Spironolactone also prevented chlorthalidone-induced sympathetic activation and insulin resistance in hypertensive patients (37). Indeed, the Framingham Offspring Study demonstrated an association of increased plasma aldosterone levels with the development of longitudinal increases of cardiometabolic syndrome components, suggesting that aldosterone may play a key role in mediating metabolic risk (38). Further supporting this notion, adrenalectomy (39–41) or medical therapy with MR antagonists (42) improves insulin resistance in patients with primary aldosteronism. However, spironolactone administration did not improve insulin sensitivity or glucose metabolism in a small group of subjects with idiopathic hyperaldosteronism (39, 40, 43). A recent study found that treatment of primary aldosteronism after either surgical adrenalectomy (6 patients) or medical treatment with a MR antagonist (3 patients) not only improved insulin secretion and reduced insulin clearance, but also reduced insulin sensitivity (44). Furthermore, eplerenone treatment affected neither basal nor postprandial glucose and lipid levels in a prospective, self-controlled study from 13 healthy adult males (45).
Insulin resistance and hypertension share similar pathophysiological mechanisms in promoting chronic inflammation and vascular dysfunction, and the coexistence of insulin resistance and hypertension is regarded as a cause-effect relationship in patients with hyperaldosteronism (1, 13). For instance, hypertension can cause vascular stiffness and related insulin resistance by altering the delivery of insulin and glucose to skeletal muscle cells, resulting in impaired glucose uptake (1). Therefore, improved glucose metabolism in primary hyperaldosteronism individuals treated with MR imbibition may be related to both lower MR activation and blood pressure reduction. Further clinical studies are required to better understand the definitive role of MR activation in the pathogenesis of insulin resistance.
Aldosterone, MR activation, and resultant effects are subject to racial and sex differences. Blacks in comparison to Whites are at risk for a more serious form of hypertension that is associated with increased aldosterone levels and MR activation, as well as sodium retention. A clinical study from 483 young adult African Americans found that elevated aldosterone is associated with blood pressure and insulin resistance, independent of obesity (46). Furthermore, Blacks often achieve a higher blood pressure without a proportionate increase in the aldosterone level, since they are more sensitive to aldosterone than Caucasians. (47). Furthermore, aldosterone levels are higher in overweight females than in male counterparts, and the elevated plasma aldosterone in women is positively associated with insulin resistance, type 2 diabetes, and cardiometabolic syndrome (48, 49).
MECHANISMS OF EXCESSIVE MR ACTIVATION-INDUCED INSULIN RESISTANCE
Experimental studies have shown that enhanced membrane and nuclear MR activation impairs insulin metabolic signaling (Fig. 2). To this point, our recent studies have shown that that chronic activation of the mammalian target of the rapamycin (mTOR)/S6 kinase-1/(S6K1) signaling pathway, by both excessive nutrient intake and inappropriate activation of the RAAS, promotes insulin resistance in heart, fat, liver, and skeletal muscle tissue through increased serine phosphorylation of the critical insulin-signaling/docking molecule insulin receptor substrate 1 (IRS-1) (25, 50–52). This serine phosphorylation then impairs IRS-1 tyrosine phosphorylation, PI3K engagement, and protein kinase B (Akt) phosphorylation/activation, as well as downstream translocation of glucose transporter 4 (GLUT4) to the cell membrane (25, 50–52). Furthermore, increased aldosterone levels and inappropriate activation of MRs further induce inflammation, oxidative stress, and lipid disorders, leading to impaired insulin metabolic signaling, the cardiometabolic syndrome, and diabetes (Fig. 2).
Figure 2.
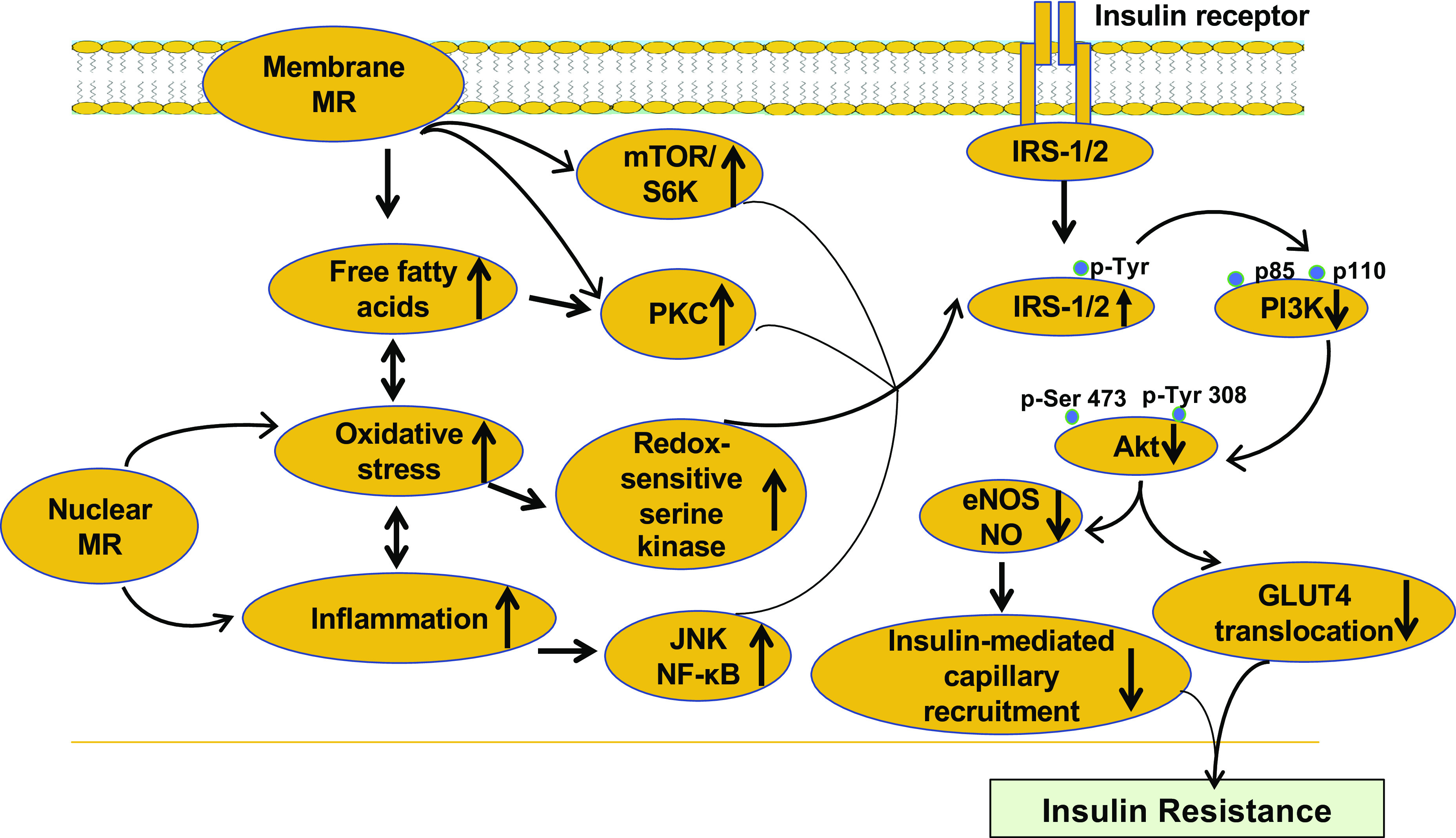
Proposed model for the inappropriate mineralocorticoid receptor (MR) activation in the development of insulin resistance. While membrane MR activation will promote free fatty acid uptake, activation of mammalian target of rapamycin/S6 kinase (mTOR/S6K) and PKC signaling, nuclear MR activation will induce oxidative stress, increased NF-κB signaling, inflammation. These abnormalities lead to impaired insulin metabolic signaling, systemic and tissue insulin resistance. eNOS, endothelial nitric oxide (NO) synthase.
MR Activation in Inflammation
Excessive MR activation produces proinflammatory responses, which, in turn, promote systemic and tissue insulin resistance. For instance, activation of MRs in adipose tissue increases expression of proinflammatory adipokines, including tumor necrosis factor-α (TNFα), interleukin-6 (IL-6), IL-8, monocyte chemoattractant protein-1 (MCP-1), Toll-like receptor 4 (TLF4), that activate nuclear factor κ-light-chain enhancer of activated B cells (NF-κB) and c-Jun NH2-terminal kinase (JNK) to directly induce serine phosphorylation of IRS-1 and impair insulin metabolic signaling (1, 13). Enhanced MR signaling also induces activation of proinflammatory T helper (Th) cells and macrophage M1 polarization (10, 53, 54), especially in obesity and insulin resistance states (55). Furthermore, MR antagonism has been found to inhibit the expression of TNFα, MCP-1, and IL-6, as well as the macrophage M1 marker markers CD68 and CD11c (24, 56). Our recent data also suggest that low-dose spironolactone inhibits macrophage infiltration and M1 polarization with parallel increases in M2 macrophages in cardiovascular tissue (53, 57). These data suggest that MR activation promotes the expression of proinflammatory adipokines and macrophage recruitment and thus plays an important role in the pathogenesis of the chronic inflammatory responses observed in obese and insulin-resistant states.
MR Activation Causes Oxidative Stress
Aldosterone/MR signaling activates NADPH oxidase, which is one of the important enzymes in the generation of ROS and excessive oxidative stress. In this regard, aldosterone induces expression of NADPH components NOX2, p22phox, and p47phox through a MR-dependent mechanism (58, 59). Meanwhile, both Nox1 and Nox4 are involved in ROS-sensitive aldosterone production (58, 59). Moreover, excessive oxidative stress activates redox-sensitive serine kinases such as JNK and extracellular signal-regulated kinase (ERK)1/2, to induce serine phosphorylation of IRS-1 and impair insulin metabolic signaling (60). Furthermore, MR antagonist administration decreases tissue 3-nitrotyrosine and 4-hydroxynonenal, which are markers of peroxynitrite formation, the lipid peroxidation chain reaction, and excessive tissue oxidative stress (53, 57). These data support a role for enhanced MR activation of NADPH oxidase and resultant oxidative stress in the pathogenesis of insulin resistance.
Both increased inflammation and oxidative stress are associated with and have major roles in the pathogenesis of hypertension. Conversely, hypertension has been shown to cause oxidative stress and inflammation in experimental animals and clinical studies, indicating that oxidative stress, inflammation, and arterial hypertension participate in a self-perpetuating cycle and progressive cardiovascular disease and metabolic disorders (61). Additionally, high-salt intake is also involved in increased tissue oxidative stress and inflammation and the development of metabolic disorders (62). Moreover, increases in aldosterone levels, MR activation, and salt intake act in concert to induce hypertension. Therefore, individual components and/or a combination of elevated aldosterone levels, increased MR activation, and high-salt intake are involved in tissue oxidative stress, inflammation, impaired insulin sensitivity, and hypertension.
MR Activation in Tissue Lipid Disorders
Elevated free fatty acid (FFA) levels activate PKC signaling (63) and induce inflammation, oxidative stress, and insulin resistance that is characterized by reduced phosphorylation of Akt and 5′-monophosphate-activated protein kinase (AMPK), as well as reduced GLUT4 expression (64). MR activation in obesity promotes excessive FFA uptake, intramyocellular lipid droplet accumulation, and insulin resistance in skeletal muscle, liver, and heart. Furthermore, MR activation increases white adipose and decreases brown adipose tissue as well as the ectopic accumulation of lipid droplets and intracellular triglycerides (65). Inhibition of MR activation reduces adipocyte differentiation, adipogenesis, hepatic steatosis, and insulin resistance (28, 66). Furthermore, blockade of MRs with eplerenone prevents palmitic acid-induced skeletal muscle FFA uptake and cardiac accumulation of lipid metabolites (67). Consistent with a role for MR activation in lipid disorders, a clinical study showed that there was a strong negative correlation between plasma aldosterone and high-density lipoprotein cholesterol levels in 30 patients with the cardiometabolic syndrome (11). The association of a higher body mass index with lower high-density lipoprotein levels persisted even after adjustment for body mass index in 356 individuals (68). Another study showed that there was a significant reduction in triglyceride levels after six weeks of eplerenone treatment from 16 patients with type 2 diabetes (69). However, data from the Framingham Heart Study, involving 2,891 persons, indicated that there was no direct correlation between plasma lipids and aldosterone (70). Therefore, the pathophysiological relationship between aldosterone and associated MR activation and serum lipid disorders remains to be clarified.
TISSUE-SPECIFIC MR ACTIVATION IN IMPAIRED INSULIN SECRETION AND INSULIN RESISTANCE
Inappropriate MR activation promotes systemic and tissue insulin resistance through reduced insulin secretion, inflammation, oxidative stress, lipid disorders, decreased insulin, and glucose uptake (Fig. 3).
Figure 3.
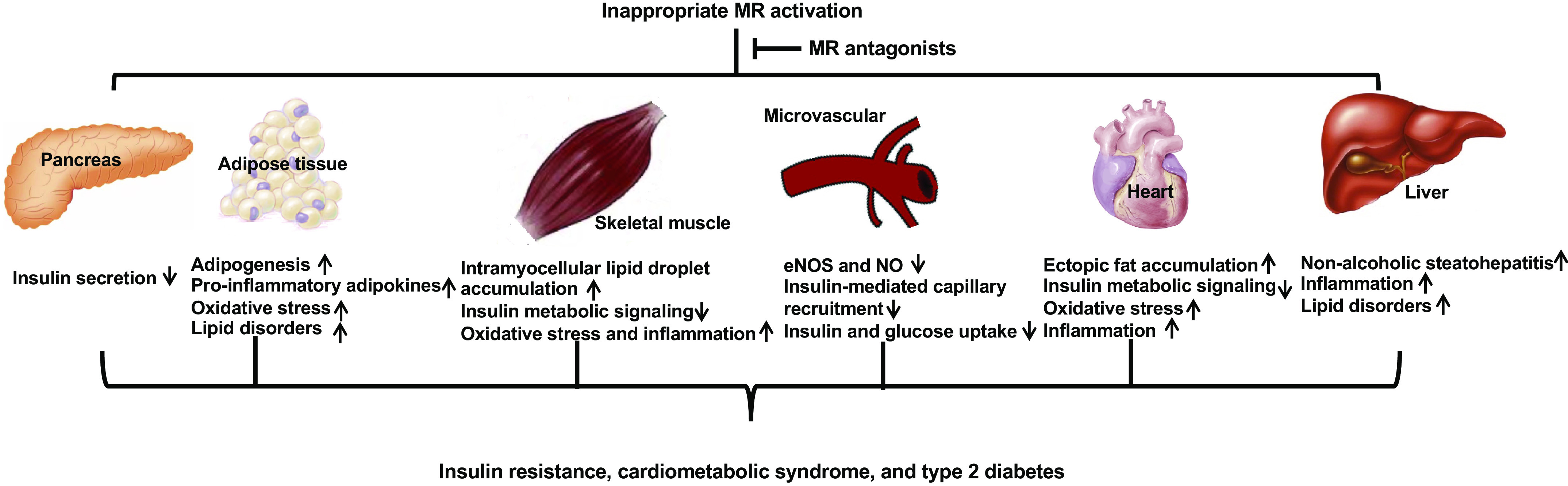
Mechanisms involved in tissue-specific mineralocorticoid receptor (MR) activation in insulin resistance. Inappropriate MR activation promotes systemic and tissue insulin resistance through reduced insulin secretion, inflammation, oxidative stress, lipid disorders, decreased insulin and glucose uptake, as well as impaired insulin metabolic signaling. These abnormalities are prevented by MR antagonists. eNOS, endothelial nitric oxide (NO) synthase.
Pancreatic Islets
Compensatory increases in β-cell insulin secretion are typically seen in patients with insulin resistance, impaired glucose tolerance, and type 2 diabetes (71, 72). In this setting, although insulin concentrations are increased compared with insulin-sensitive individuals, there is a failure to meet the demands of insulin-resistant tissues and failure to maintain normoglycemia (71, 72). Results from two independent genome-wide association studies support the notion that both genetic and dietary components affect insulin-secretory capacity and related increases in type 2 diabetes risk (73, 74). Evidence suggests that excessive angiotensin II signaling through its receptor 1 impairs insulin secretion and that pharmacological inhibition of the angiotensin II receptor 1 could reduce diabetes risk by preserving β-cell l function. (75–78). For instance, angiotensin II may impair insulin secretion indirectly by producing vasoconstriction and reducing islet blood flow (76). Angiotensin II infusion reduces the amplitude of basal insulin oscillation and impairs glucose tolerance and insulin response in human oral glucose tolerance tests (75). To this point, 26 weeks of treatment with the angiotensin receptor blocker valsartan increased glucose-stimulated insulin secretion in patients with impaired glucose tolerance (78). Furthermore, 16 weeks of valsartan treatment also increased insulin responses and reduced glucose excursions in obese patients with hypertension (78).
The MR is primarily localized to pancreatic δ-cells and polypeptide cells in both mouse and human islets (77). A recent study suggests that increased endogenous aldosterone decreases glucose-stimulated insulin secretion (77). Related to this, aldosterone may suppress glucose-stimulated insulin secretion through a MR-independent mechanism (77), and this notion is supported by the observation that surgical excision of aldosterone-producing adenomas reduced circulating glucose concentrations, whereas pharmacological treatment with MR antagonist did not (43). It has also been shown that increased levels of aldosterone can promote renal potassium wasting and resultant hypokalemia, leading to insulin resistance and hyperinsulinemia (79). Other data also indicate that excessive MR activation plays a key role in aldosterone-induced impairment of insulin sensitivity in adipocytes and skeletal muscle tissue (13, 25). Thus, aldosterone may also produce insulin resistance secondarily by altering potassium, increasing inflammatory cytokines, oxidative stress, and reducing beneficial adipokines such as adiponectin (Fig. 3) (60).
Adipocytes
Adipocyte tissue includes white, brown, and beige adipocyte tissues. Whereas white adipocyte tissue is the primary site for its energy storage and expansion, excess increases in this fat are associated with insulin resistance and type 2 diabetes (80). In contrast, brown adipocyte tissue has beneficial functions in production of heat through nonshivering thermogenesis (81). Both aldosterone and MRs are present in greater quantities in white adipose tissue, and enhanced MR activation promotes adipogenesis, especially that of white adipose tissue. For instance, overexpression of MRs in adipocytes increased body weight with increased visceral fat mass, insulin resistance, and features of the cardiometabolic syndrome (82). Indeed, aldosterone induces increases in white adipocyte tissue, characterized by the accumulation of intracytoplasmic lipid droplets and intracellular triglycerides (65). On the other hand, aldosterone inhibits expression and activity of uncoupling protein-1 in adipocytes, which is associated with reduced browning of adipose tissue (83). Conversely, MR antagonists inhibit adipocyte differentiation in both 3T3-L1 cells and primary human adipocytes, and MR knockout mice display defective adipogenesis (66). MR antagonists also induce browning of white adipocyte tissue through impairment of autophagy and conversion of white adipocyte tissue into thermogenic fat (26). Treatment with eplerenone partially alleviates adipocyte dysfunction and insulin resistance in obese mice (24, 84). Typically, adipogenesis is regulated by transcription factors including the peroxisome proliferator-activated receptor-γ (PPARγ) and CCAAT enhancer-binding proteins (85). In diet-induced obesity enhanced MR activation in adipose tissue induces adipogenesis through stimulation of the mTOR/S6K1 signaling pathway which, in turn, activates PPARγ and CCAAT enhancer-binding-related protein signaling, thereby promoting adipogenesis (51). Inhibition of MR activation is associated with increases in brown adipocyte-specific transcripts in visceral and inguinal fat depots (26), indicating that MR signaling decreases o the browning of white adipocyte tissue. Collectively, these studies demonstrate a major role of MR in the regulation of adipose tissue differentiation, adipogenesis, browning of fat, and related insulin resistance.
Excessive fat tissue is not only related to increased aldosterone levels and MR activation but is also associated with abnormalities in the production/action of adipokines, including leptin, which is a direct inducer of aldosterone secretion (86). Indeed, both increased leptin and aldosterone have been implicated in the pathophysiological mechanisms promoting insulin resistance and the cardiometabolic syndrome (87). However, it remains unclear whether aldosterone and leptin production/releasing are intrinsically related and directly interconnected in the pathogenesis of insulin resistance and type 2 diabetes (88). For instance, the basal serum leptin levels were similar in primary aldosteronism patients and healthy subjects, but the serum leptin levels and insulin sensitivity were increased after surgical or pharmacological treatment of hyperaldosteronism (88). Interestingly, aldosterone and leptin levels are characteristically higher in women than in men and increased more so per unit of body mass in women as well (89). This suggests the possibility that elevated aldosterone and leptin may have an especially key role in promoting insulin resistance and the cardiometabolic syndrome in women.
Skeletal Muscle
MRs are also present in skeletal muscle, which is the major site for disposal of ingested glucose, and MR signaling in this tissue has an important role in the maintenance of glucose homeostasis. In this regard, approximately one-third of ingested glucose is taken up by the liver and the remainder by peripheral tissues, primarily skeletal muscle, through an insulin-dependent mechanism (90). Insulin-stimulated skeletal muscle glucose uptake is markedly impaired in diet-induced obesity, insulin resistance, the cardiometabolic syndrome, and type 2 diabetes (90). This abnormality is related to impaired insulin metabolic signaling, impaired glucose transport, and reduced glucose oxidation and glycogen synthesis (90). Excessive MR activation has been found to impair insulin metabolic signaling in skeletal muscle tissue. One study showed that aldosterone inhibited the expression of the insulin receptor in skeletal muscle and that increased aldosterone decreased glucose uptake of skeletal muscles of adult male rats through a reduced translocation of GLUT4 to the plasma membrane, hence contributing to insulin resistance (91). Our recent data also suggest that MR blockade with spironolactone was able to reduce oxidative stress and improve insulin sensitivity in skeletal muscle in TG(mREN2)27 rats, a model of RAAS hyperactivity (25). Therefore, skeletal muscle MR signaling likely plays an important role in the pathogenesis of insulin resistance, the cardiometabolic syndrome, and type 2 diabetes.
Cardiovascular Tissue
Insulin-mediated capillary recruitment is important for increasing the microvascular surface area for both insulin and glucose delivery and subsequent glucose uptake in tissues such as skeletal muscle and fat (92). Clinical disorders characterized by insulin resistance are associated with impaired vasodilatory effects and related reduced capillary recruitment (93). To this point, excess aldosterone and MR activation induce microcirculation dysfunction by reducing nitric oxide (NO) production and increasing oxidative stress and inflammation. Indeed, MR activation with deoxycorticosterone/salt treatment decreases skeletal muscle capillary/fiber ratio and inhibits terminal arteriole capillary vasodilation (94, 95). Meanwhile, enhanced endothelial-specific MR signaling activates serum/glucocorticoid regulated kinase-1 signaling and prompts expression and translocation of the epithelial sodium channel (ENaC) to the endothelial cell surface, which induces microvascular stiffness, impairs insulin-mediated capillary recruitment, and promotes insulin resistance (10). Other factors, including norepinephrine, angiotensin II, and insulin, can also increase ENaC activation through a PI3K-dependent manner (96–98). Furthermore, blockade of the MR with spironolactone and endothelial-specific MR knockout prevents diet-induced aortic and mesenteric arterial stiffness and microvascular dysfunction (10, 53). This prevention is associated with improved insulin-mediated endothelial NO synthase activity and higher bioavailable NO levels and suppression of endothelial ENaC activation (10, 53). Once glucose reaches the tissues and cells, uptake is dependent on the cell membrane glucose transporters, including GLUT2 and GLUT4, to facilitate glucose transport into the cell. Impairment of insulin metabolic signaling inhibits insulin-stimulated endothelial NO synthase activity and NO production, which further increases myosin light chain activation in vascular smooth muscle cells and intracellular Ca2+ levels and Ca2+ sensitization in cardiomyocytes (1). Therefore, inappropriate MR activation inhibits insulin metabolic signaling and induces abnormal glucose metabolism, which is accompanied by cardiovascular dysfunction, fibrosis, and stiffening.
Nonalcoholic Fatty Liver Disease
MR activation has been implicated in the pathogenesis of nonalcoholic fatty liver disease (NAFLD), which includes a broad spectrum of manifestations of fatty liver ranging from simple steatosis to inflammatory nonalcoholic steatohepatitis and insulin resistance. One study has supported that MR antagonism with eplerenone significantly inhibited adipose tissue inflammation, liver steatosis and inflammation, as well as systemic and tissue insulin resistance in an experimental rodent NAFLD model (99). In a clinical single-center, randomized, controlled trial, spironolactone and vitamin E combined therapy improved insulin sensitivity in patients with NAFLD (100). A recent study found that hyperaldosteronism is associated with a higher prevalence of NAFLD (101). Moreover, the status of hepatic steatosis is increased in patients with both idiopathic hyperaldosteronism and aldosterone-producing adenoma (102).
TARGETING MR-BASED THERAPEUTIC STRATEGIES
Two MR antagonists, spironolactone and eplerenone, are currently approved by the FDA for clinical use. Clinical data have confirmed that MR antagonists are beneficial in patients with resistant hypertension, cardiac diastolic dysfunction, and heart failure (3, 4). Accumulating preclinical (24–27) and clinical evidence (35, 36, 38) suggests that obesity is associated with increased MR activation, raising the possibility that inhibition of MR activation will improve insulin metabolic signaling and glucose tolerance. To this point, both eplerenone and spironolactone have been shown to improve glucose and lipid metabolism in animal models of obesity and insulin resistance (24, 27, 84). However, results from the CHARM (Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity) study suggested that spironolactone use may be associated with an increased risk for the development of diabetes (103) and that this may be caused by the compensatory increase in plasma aldosterone associated with MR blockade. Meanwhile, aldosterone synthase inhibitors that reduce aldosterone levels and MR activation are currently in development for human use and may provide an alternative strategy to improve insulin sensitivity (60). However, the blood pressure-lowing effects of aldosterone synthase inhibitors in obesity is significantly less than with MR antagonism (104). One study further indicates that inhibition if aldosterone synthase may exacerbate obesity and hyperinsulinemia (105). Therefore, more research is needed to definitively determine whether aldosterone synthase inhibitors may provide an alternative strategy to improve insulin sensitivity (60).
CONCLUSIONS
Inappropriate activation of the RAAS, especially MR activation, is related to impaired insulin secretion and insulin metabolic signaling and, thus, development of the cardiometabolic syndrome and diabetes. These abnormalities are mediated by increased inflammation, oxidative stress, and associated vascular dysfunction and lipid metabolic disorders. MR-mediated dysfunction in islet β-cells, adipocytes, skeletal muscle cells, endothelial cells, vascular smooth muscle cells, immune cells, and cardiomyocytes participates in these pathophysiological processes. Patients with obesity, the cardiometabolic syndrome, and type 2 diabetes may especially benefit from MR antagonist therapy. Clinical trials are needed to further determine the long-term insulin metabolic signaling effects of MR antagonists to better enable the development of clinically effective targeting for preventing the cardiometabolic syndrome and diabetes.
Perspectives and Significance
This review examines the role of inappropriate activation of RAAS, especially MR activation in insulin resistance and the cardiometabolic syndrome. The emergence of obesity and insulin resistance as a worldwide epidemic highlights the importance of this relationship. In this regard, increased aldosterone levels and MR activation promote these disorders via well-established effects on inflammation, excessive oxidative stress, and lipid metabolism, as well as islet cell dysfunction. Although most experimental and clinical evidence indicates that elevated aldosterone and increased MR activation impair glucose metabolism and induce insulin resistance, there are some inconsistent results in clinical studies. Small sample sizes, differences in criteria for selection of patients and controls, and different methodologies for assessing insulin sensitivity may help explain these discrepancies in results. Therefore, there is a need for randomized, multiple-center clinical trials recruiting more volunteers and clinal patients to more definitively determine the role of hyperaldosteronism and enhanced MR activation on glucose metabolism, cardiometabolic disorders, and type 2 diabetes. Although the adrenal glands are the primary organ to regulate aldosterone production, increased adipocyte production of aldosterone also plays an important role in excessive aldosterone production and MR activity in states of obesity and insulin resistance. Further research is needed to improve our collective understanding of the significance of the increased aldosterone and MR activation in the pathogenesis of insulin resistance, the cardiometabolic syndrome, and diabetes. The possibility that excessive aldosterone/MR signaling may be especially important in the pathogenesis of insulin resistance and the cardiometabolic syndrome in women is an area that needs to be addressed in basic and clinical research.
GRANTS
This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (DK124329), an American Diabetes Association Innovative Basic Science Award (1-17-IBS-201) to G. Jia, and an award from the Department of Defense/The Henry M. Jackson Foundation for the Advancement of Military Medicine (HU001-18-2-0016) to W. Lockette and J. R. Sowers.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.J., W.L., and J.R.S. conceived and designed research; G.J. prepared figures; G.J., W.L., and J.R.S. drafted manuscript; W.L. and J.R.S. edited and revised manuscript; G.J., W.L., and J.R.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Dr. Stephen Brietzke for support and help.
REFERENCES
- 1.Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res 122: 624–638, 2018. doi: 10.1161/CIRCRESAHA.117.311586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jia G, Jia Y, Sowers JR. Role of mineralocorticoid receptor activation in cardiac diastolic dysfunction. Biochim Biophys Acta Mol Basis Dis 1863: 2012–2018, 2017. doi: 10.1016/j.bbadis.2016.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 348: 1309–1321, 2003. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 4.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 341: 709–717, 1999. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 5.Aroor AR, Habibi J, Nistala R, Ramirez-Perez FI, Martinez-Lemus LA, Jaffe IZ, Sowers JR, Jia G, Whaley-Connell A. Diet-induced obesity promotes kidney endothelial stiffening and fibrosis dependent on the endothelial mineralocorticoid receptor. Hypertension 73: 849 858, 2019. doi: 10.1161/HYPERTENSIONAHA.118.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin N, Wang Y, Liu L, Xue F, Jiang T, Xu M. Dysregulation of the Renin-Angiotensin system and cardiometabolic status in mice fed a long-term high-fat diet. Med Sci Monit 25: 6605–6614, 2019. doi: 10.12659/MSM.914877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schäfer N, Lohmann C, Winnik S, van Tits LJ, Miranda MX, Vergopoulos A, Ruschitzka F, Nussberger J, Berger S, Lüscher TF, Verrey F, Matter CM. Endothelial mineralocorticoid receptor activation mediates endothelial dysfunction in diet-induced obesity. Eur Heart J 34: 3515–3524, 2013. doi: 10.1093/eurheartj/eht095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vecchiola A, Fuentes CA, Solar I, Lagos CF, Opazo MC, Muñoz-Durango N, Riedel CA, Owen GI, Kalergis AM, Fardella CE. Eplerenone implantation improved adipose dysfunction averting RAAS activation and cell division. Front Endocrinol (Lausanne) 11: 223, 2020. doi: 10.3389/fendo.2020.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bender SB, McGraw AP, Jaffe IZ, Sowers JR. Mineralocorticoid receptor-mediated vascular insulin resistance: an early contributor to diabetes-related vascular disease? Diabetes 62: 313–319, 2013. doi: 10.2337/db12-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jia G, Habibi J, Aroor AR, Martinez-Lemus LA, DeMarco VG, Ramirez-Perez FI, Sun Z, Hayden MR, Meininger GA, Mueller KB, Jaffe IZ, Sowers JR. Endothelial mineralocorticoid receptor mediates diet-induced aortic stiffness in females. Circ Res 118: 935–943, 2016. doi: 10.1161/CIRCRESAHA.115.308269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodfriend TL, Egan B, Stepniakowski K, Ball DL. Relationships among plasma aldosterone, high-density lipoprotein cholesterol, and insulin in humans. Hypertension 25: 30–36, 1995. doi: 10.1161/01.HYP.25.1.30. [DOI] [PubMed] [Google Scholar]
- 12.Yamashita K, Ito K, Endo J, Matsuhashi T, Katsumata Y, Yamamoto T, Shirakawa K, Isobe S, Kataoka M, Yoshida N, Goto S, Moriyama H, Kitakata H, Mitani F, Fukuda K, Goda N, Ichihara A, Sano M. Adrenal cortex hypoxia modulates aldosterone production in heart failure. Biochem Biophys Res Commun 524: 184–189, 2020. doi: 10.1016/j.bbrc.2020.01.088. [DOI] [PubMed] [Google Scholar]
- 13.Jia G, Aroor AR, Sowers JR. The role of mineralocorticoid receptor signaling in the cross-talk between adipose tissue and the vascular wall. Cardiovasc Res 113: 1055–1063, 2017. doi: 10.1093/cvr/cvx097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinh Cat AN, Friederich-Persson M, White A, Touyz RM. Adipocytes, aldosterone and obesity-related hypertension. J Mol Endocrinol 57: F7–F21, 2016. doi: 10.1530/JME-16-0025. [DOI] [PubMed] [Google Scholar]
- 15.Ehrhart-Bornstein M, Lamounier-Zepter V, Schraven A, Langenbach J, Willenberg HS, Barthel A, Hauner H, McCann SM, Scherbaum WA, Bornstein SR. Human adipocytes secrete mineralocorticoid-releasing factors. Proc Natl Acad Sci USA 100: 14211–14216, 2003. doi: 10.1073/pnas.2336140100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rios FJ, Neves KB, Nguyen Dinh Cat A, Even S, Palacios R, Montezano AC, Touyz RM. Cholesteryl ester-transfer protein inhibitors stimulate aldosterone biosynthesis in adipocytes through Nox-dependent processes. J Pharmacol Exp Ther 353: 27–34, 2015. doi: 10.1124/jpet.114.221002. [DOI] [PubMed] [Google Scholar]
- 17.He FJ, Marrero NM, MacGregor GA. Salt intake is related to soft drink consumption in children and adolescents: a link to obesity? Hypertension 51: 629–634, 2008. doi: 10.1161/HYPERTENSIONAHA.107.100990. [DOI] [PubMed] [Google Scholar]
- 18.Ma Y, He FJ, MacGregor GA. High salt intake: independent risk factor for obesity? Hypertension 66: 843–849, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05948. [DOI] [PubMed] [Google Scholar]
- 19. M, Sorn SR, Lee Y, Kang I. Salt induces adipogenesis/lipogenesis and inflammatory adipocytokines secretion in adipocytes. IJMS 20: 160, 2019. doi: 10.3390/ijms20010160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whaley-Connell A, Johnson MS, Sowers JR. Aldosterone: role in the cardiometabolic syndrome and resistant hypertension. Prog Cardiovasc Dis 52: 401–409, 2010. doi: 10.1016/j.pcad.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chapman K, Holmes M, Seckl J. 11β-Hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action. Physiol Rev 93: 1139–1206, 2013. doi: 10.1152/physrev.00020.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DuPont JJ, Jaffe IZ. 30 Years of the mineralocorticoid receptor: the role of the mineralocorticoid receptor in the vasculature. J Endocrinol 234: T67–T82, 2017. doi: 10.1530/JOE-17-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hofmann A, Brunssen C, Peitzsch M, Martin M, Mittag J, Jannasch A, Engelmann F, Brown NF, Weldon SM, Huber J, Streicher R, Deussen A, Eisenhofer G, Bornstein SR, Morawietz H. Aldosterone synthase inhibition improves glucose tolerance in Zucker diabetic fatty (ZDF) rats. Endocrinology 157: 3844–3855, 2016. doi: 10.1210/en.2016-1358. [DOI] [PubMed] [Google Scholar]
- 24.Guo C, Ricchiuti V, Lian BQ, Yao TM, Coutinho P, Romero JR, Li J, Williams GH, Adler GK. Mineralocorticoid receptor blockade reverses obesity-related changes in expression of adiponectin, peroxisome proliferator-activated receptor-gamma, and proinflammatory adipokines. Circulation 117: 2253–2261, 2008. doi: 10.1161/CIRCULATIONAHA.107.748640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lastra G, Whaley-Connell A, Manrique C, Habibi J, Gutweiler AA, Appesh L, Hayden MR, Wei Y, Ferrario C, Sowers JR. Low-dose spironolactone reduces reactive oxygen species generation and improves insulin-stimulated glucose transport in skeletal muscle in the TG(mRen2)27 rat. Am J Physiol Endocrinol Metab 295: E110–E116, 2008. doi: 10.1152/ajpendo.00258.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armani A, Cinti F, Marzolla V, Morgan J, Cranston GA, Antelmi A, Carpinelli G, Canese R, Pagotto U, Quarta C, Malorni W, Matarrese P, Marconi M, Fabbri A, Rosano G, Cinti S, Young MJ, Caprio M. Mineralocorticoid receptor antagonism induces browning of white adipose tissue through impairment of autophagy and prevents adipocyte dysfunction in high-fat-diet-fed mice. FASEB J 28: 3745–3757, 2014. doi: 10.1096/fj.13-245415. [DOI] [PubMed] [Google Scholar]
- 27.Wada T, Kenmochi H, Miyashita Y, Sasaki M, Ojima M, Sasahara M, Koya D, Tsuneki H, Sasaoka T. Spironolactone improves glucose and lipid metabolism by ameliorating hepatic steatosis and inflammation and suppressing enhanced gluconeogenesis induced by high-fat and high-fructose diet. Endocrinology 151: 2040–2049, 2010. doi: 10.1210/en.2009-0869. [DOI] [PubMed] [Google Scholar]
- 28.Zhang YY, Li C, Yao GF, Du LJ, Liu Y, Zheng XJ, Yan S, Sun JY, Liu Y, Liu MZ, Zhang X, Wei G, Tong W, Chen X, Wu Y, Sun S, Liu S, Ding Q, Yu Y, Yin H, Duan SZ. Deletion of macrophage mineralocorticoid receptor protects hepatic steatosis and insulin resistance through ERα/HGF/Met pathway. Diabetes 66: 1535–1547, 2017. doi: 10.2337/db16-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heart Outcomes Prevention Evaluation Study Investigators; Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med 342: 145–153, 2000. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 30.Devereux RB, DahlöF B, Gerdts E, Boman K, Nieminen MS, Papademetriou V, Rokkedal J, Harris KE, Edelman JM, Wachtell K. Regression of hypertensive left ventricular hypertrophy by losartan compared with atenolol: the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial. Circulation 110: 1456–1462, 2004. doi: 10.1161/01.CIR.0000141573.44737.5A. [DOI] [PubMed] [Google Scholar]
- 31.Barzilay JI, Davis BR, Cutler JA, Pressel SL, Whelton PK, Basile J, Margolis KL, Ong ST, Sadler LS, Summerson J; ALLHAT Collaborative Research Group. Fasting glucose levels and incident diabetes mellitus in older nondiabetic adults randomized to receive 3 different classes of antihypertensive treatment: a report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Arch Intern Med 166: 2191–2201, 2006. doi: 10.1001/archinte.166.20.2191. [DOI] [PubMed] [Google Scholar]
- 32.Navigator Study Group; McMurray JJ, Holman RR, Haffner SM, Bethel MA, Holzhauer B, , et al. Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med 362: 1477–1490, 2010. doi: 10.1056/NEJMoa1001121. [DOI] [PubMed] [Google Scholar]
- 33.Ran J, Hirano T, Fukui T, Saito K, Kageyama H, Okada K, Adachi M. Angiotensin II infusion decreases plasma adiponectin level via its type 1 receptor in rats: an implication for hypertension-related insulin resistance. Metabolism 55: 478–488, 2006. doi: 10.1016/j.metabol.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Kumagai E, Adachi H, Jacobs DR Jr, Hirai Y, Enomoto M, Fukami A, Otsuka M, Kumagae S, Nanjo Y, Yoshikawa K, Esaki E, Yokoi K, Ogata K, Kasahara A, Tsukagawa E, Ohbu-Murayama K, Imaizumi T. Plasma aldosterone levels and development of insulin resistance: prospective study in a general population. Hypertension 58: 1043–1048, 2011. doi: 10.1161/HYPERTENSIONAHA.111.180521. . [DOI] [PubMed] [Google Scholar]
- 35.Mosso LM, Carvajal CA, Maiz A, Ortiz EH, Castillo CR, Artigas RA, Fardella CE. A possible association between primary aldosteronism and a lower beta-cell function. J Hypertens 25: 2125–2130, 2007. doi: 10.1097/HJH.0b013e3282861fa4. [DOI] [PubMed] [Google Scholar]
- 36.Garg R, Hurwitz S, Williams GH, Hopkins PN, Adler GK. Aldosterone production and insulin resistance in healthy adults. J Clin Endocrinol Metab 95: 1986–1990, 2010. doi: 10.1210/jc.2009-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raheja P, Price A, Wang Z, Arbique D, Adams-Huet B, Auchus RJ, Vongpatanasin W. Spironolactone prevents chlorthalidone-induced sympathetic activation and insulin resistance in hypertensive patients. Hypertension 60: 319–325, 2012. doi: 10.1161/HYPERTENSIONAHA.112.194787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ingelsson E, Pencina MJ, Tofler GH, Benjamin EJ, Lanier KJ, Jacques PF, Fox CS, Meigs JB, Levy D, Larson MG, Selhub J, D'Agostino RB Sr, Wang TJ, Vasan RS. Multimarker approach to evaluate the incidence of the metabolic syndrome and longitudinal changes in metabolic risk factors: the Framingham Offspring Study. Circulation 116: 984–992, 2007. doi: 10.1161/CIRCULATIONAHA.107.708537. [DOI] [PubMed] [Google Scholar]
- 39.Giacchetti G, Ronconi V, Turchi F, Agostinelli L, Mantero F, Rilli S, Boscaro M. Aldosterone as a key mediator of the cardiometabolic syndrome in primary aldosteronism: an observational study. J Hypertens 25: 177–186, 2007. doi: 10.1097/HJH.0b013e3280108e6f. [DOI] [PubMed] [Google Scholar]
- 40.Strauch B, Widimský J, Sindelka G, Skrha J. Does the treatment of primary hyperaldosteronism influence glucose tolerance? Physiol Res 52: 503–506, 2003. [PubMed] [Google Scholar]
- 41.Widimský J Jr, Strauch B, Sindelka G, Skrha J. Can primary hyperaldosteronism be considered as a specific form of diabetes mellitus? Physiol Res 50: 603–607, 2001. [PubMed] [Google Scholar]
- 42.Catena C, Lapenna R, Baroselli S, Nadalini E, Colussi G, Novello M, Favret G, Melis A, Cavarape A, Sechi LA. Insulin sensitivity in patients with primary aldosteronism: a follow-up study. J Clin Endocrinol Metab 91: 3457–3463, 2006. doi: 10.1210/jc.2006-0736. [DOI] [PubMed] [Google Scholar]
- 43.Sindelka G, Widimský J, Haas T, Prazny M, Hilgertova J, Skrha J. Insulin action in primary hyperaldosteronism before and after surgical or pharmacological treatment. Exp Clin Endocrinol Diabetes 108: 21–25, 2000. doi: 10.1055/s-0032-1329211. [DOI] [PubMed] [Google Scholar]
- 44.Adler GK, Murray GR, Turcu AF, Nian H, Yu C, Solorzano CC, Manning R, Peng D, Luther JM. Primary aldosteronism decreases insulin secretion and increases insulin clearance in humans. Hypertension 75: 1251–1259, 2020. doi: 10.1161/HYPERTENSIONAHA.119.13922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krug AW, Stelzner L, Rao AD, Lichtman AH, Williams GH, Adler GK. Effect of low dose mineralocorticoid receptor antagonist eplerenone on glucose and lipid metabolism in healthy adult males. Metabolism 62: 386–391, 2013. doi: 10.1016/j.metabol.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huan Y, Deloach S, Keith SW, Goodfriend TL, Falkner B. Aldosterone and aldosterone: renin ratio associations with insulin resistance and blood pressure in African Americans. J Am Soc Hypertens 6: 56–65, 2012. doi: 10.1016/j.jash.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 47.Tu W, Eckert GJ, Hannon TS, Liu H, Pratt LM, Wagner MA, Dimeglio LA, Jung J, Pratt JH. Racial differences in sensitivity of blood pressure to aldosterone. Hypertension 63: 1212–1218, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rutter MK, Parise H, Benjamin EJ, Levy D, Larson MG, Meigs JB, Nesto RW, Wilson PW, Vasan RS. Impact of glucose intolerance and insulin resistance on cardiac structure and function: sex-related differences in the Framingham Heart Study. Circulation 107: 448–454, 2003. doi: 10.1161/01.CIR.0000045671.62860.98. [DOI] [PubMed] [Google Scholar]
- 49.Vasan RS, Evans JC, Benjamin EJ, Levy D, Larson MG, Sundstrom J, Murabito JM, Sam F, Colucci WS, Wilson PW. Relations of serum aldosterone to cardiac structure: gender-related differences in the Framingham Heart Study. Hypertension 43: 957–962, 2004. doi: 10.1161/01.HYP.0000124251.06056.8e. [DOI] [PubMed] [Google Scholar]
- 50.Jia G, Aroor AR, Martinez-Lemus LA, Sowers JR. Overnutrition, mTOR signaling, and cardiovascular diseases. Am J Physiol Regul Integr Comp Physiol 307: R1198–R1206, 2014. doi: 10.1152/ajpregu.00262.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim JA, Jang HJ, Martinez-Lemus LA, Sowers JR. Activation of mTOR/p70S6 kinase by ANG II inhibits insulin-stimulated endothelial nitric oxide synthase and vasodilation. Am J Physiol Endocrinol Metab 302: E201–E208, 2012. doi: 10.1152/ajpendo.00497.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lastra G, Habibi J, Whaley-Connell AT, Manrique C, Hayden MR, Rehmer J, Patel K, Ferrario C, Sowers JR. Direct renin inhibition improves systemic insulin resistance and skeletal muscle glucose transport in a transgenic rodent model of tissue renin overexpression. Endocrinology 150: 2561–2568, 2009. doi: 10.1210/en.2008-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DeMarco VG, Habibi J, Jia G, Aroor AR, Ramirez-Perez FI, Martinez-Lemus LA, Bender SB, Garro M, Hayden MR, Sun Z, Meininger GA, Manrique C, Whaley-Connell A, Sowers JR. Low-dose mineralocorticoid receptor blockade prevents western diet-induced arterial stiffening in female mice. Hypertension 66: 99–107, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jia G, Habibi J, DeMarco VG, Martinez-Lemus LA, Ma L, Whaley-Connell AT, Aroor AR, Domeier TL, Zhu Y, Meininger GA, Mueller KB, Jaffe IZ, Sowers JR. Endothelial mineralocorticoid receptor deletion prevents diet-induced cardiac diastolic dysfunction in females. Hypertension 66: 1159–1167, 2015. doi: 10.1161/HYPERTENSIONAHA.115.06015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Orliaguet L, Dalmas E, Drareni K, Venteclef N, Alzaid F. Mechanisms of macrophage polarization in insulin signaling and sensitivity. Front Endocrinol (Lausanne) 11: 62, 2020. doi: 10.3389/fendo.2020.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hirata A, Maeda N, Nakatsuji H, Hiuge-Shimizu A, Okada T, Funahashi T, Shimomura I. Contribution of glucocorticoid-mineralocorticoid receptor pathway on the obesity-related adipocyte dysfunction. Biochem Biophys Res Commun 419: 182–187, 2012. doi: 10.1016/j.bbrc.2012.01.139. [DOI] [PubMed] [Google Scholar]
- 57.Bostick B, Habibi J, DeMarco VG, Jia G, Domeier TL, Lambert MD, Aroor AR, Nistala R, Bender SB, Garro M, Hayden MR, Ma L, Manrique C, Sowers JR. Mineralocorticoid receptor blockade prevents Western diet-induced diastolic dysfunction in female mice. Am J Physiol Heart Circ Physiol 308: H1126–H1135, 2015. doi: 10.1152/ajpheart.00898.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nguyen Dinh Cat A, Montezano AC, Burger D, Touyz RM. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxid Redox Signal 19: 1110–1120, 2013. doi: 10.1089/ars.2012.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sciarretta S, Paneni F, Palano F, Chin D, Tocci G, Rubattu S, Volpe M. Role of the renin-angiotensin-aldosterone system and inflammatory processes in the development and progression of diastolic dysfunction. Clin Sci (Lond) 116: 467–477, 2009. doi: 10.1042/CS20080390. [DOI] [PubMed] [Google Scholar]
- 60.Luther JM. Effects of aldosterone on insulin sensitivity and secretion. Steroids 91: 54–60, 2014. doi: 10.1016/j.steroids.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vaziri ND, Rodriguez-Iturbe B. Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nat Clin Pract Nephrol 2: 582–593, 2006. doi: 10.1038/ncpneph0283. [DOI] [PubMed] [Google Scholar]
- 62.Grillo A, Salvi L, Coruzzi P, Salvi P, Parati G. Sodium intake and hypertension. Nutrients 11: 1970, 2019. doi: 10.3390/nu11091970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pereira S, Park E, Mori Y, Haber CA, Han P, Uchida T, Stavar L, Oprescu AI, Koulajian K, Ivovic A, Yu Z, Li D, Bowman TA, Dewald J, El-Benna J, Brindley DN, Gutierrez-Juarez R, Lam TK, Najjar SM, McKay RA, Bhanot S, Fantus IG, Giacca A. FFA-induced hepatic insulin resistance in vivo is mediated by PKCδ, NADPH oxidase, and oxidative stress. Am J Physiol Endocrinol Metab 307: E34–E46, 2014. doi: 10.1152/ajpendo.00436.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Han L, Liu J, Zhu L, Tan F, Qin Y, Huang H, Yu Y. Free fatty acid can induce cardiac dysfunction and alter insulin signaling pathways in the heart. Lipids Health Dis 17: 185, 2018. doi: 10.1186/s12944-018-0834-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zennaro MC, Le Menuet D, Viengchareun S, Walker F, Ricquier D, Lombes M. Hibernoma development in transgenic mice identifies brown adipose tissue as a novel target of aldosterone action. J Clin Invest 101: 1254–1260, 1998. doi: 10.1172/JCI1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Caprio M, Antelmi A, Chetrite G, Muscat A, Mammi C, Marzolla V, Fabbri A, Zennaro MC, Fève B. Antiadipogenic effects of the mineralocorticoid receptor antagonist drospirenone: potential implications for the treatment of metabolic syndrome. Endocrinology 152: 113–125, 2011. doi: 10.1210/en.2010-0674. [DOI] [PubMed] [Google Scholar]
- 67.Ramírez E, Klett-Mingo M, Ares-Carrasco S, Picatoste B, Ferrarini A, Rupérez FJ, Caro-Vadillo A, Barbas C, Egido J, Tuñon J, Lorenzo Ó. Eplerenone attenuated cardiac steatosis, apoptosis and diastolic dysfunction in experimental type-II diabetes. Cardiovasc Diabetol 12: 172, 2013. doi: 10.1186/1475-2840-12-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bochud M, Nussberger J, Bovet P, Maillard MR, Elston RC, Paccaud F, Shamlaye C, Burnier M. Plasma aldosterone is independently associated with the metabolic syndrome. Hypertension 48: 239–245, 2006. doi: 10.1161/01.HYP.0000231338.41548.fc. [DOI] [PubMed] [Google Scholar]
- 69.Joffe HV, Kwong RY, Gerhard-Herman MD, Rice C, Feldman K, Adler GK. Beneficial effects of eplerenone versus hydrochlorothiazide on coronary circulatory function in patients with diabetes mellitus. J Clin Endocrinol Metab 92: 2552–2558, 2007. doi: 10.1210/jc.2007-0393. [DOI] [PubMed] [Google Scholar]
- 70.Kathiresan S, Larson MG, Benjamin EJ, Corey D, Murabito JM, Fox CS, Wilson PW, Rifai N, Meigs JB, Ricken G, Lifton RP, Levy D, Vasan RS. Clinical and genetic correlates of serum aldosterone in the community: the Framingham Heart Study. Am J Hypertens 18: 657–665, 2005. doi: 10.1016/j.amjhyper.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 71.Luther JM, Brown NJ. The renin-angiotensin-aldosterone system and glucose homeostasis. Trends Pharmacol Sci 32: 734–739, 2011. doi: 10.1016/j.tips.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 104: 787–794, 1999. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Diabetes Genetics Initiative of Broad Institute of Harvard and MIT Lund University, and Novartis Institutes of BioMedical Research; Saxena R, Voight BF, Lyssenko V, Burtt NP, de Bakker PI, Chen H, , et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 316: 1331–1336, 2007. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- 74.Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 445: 881–885, 2007. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 75.Fliser D, Schaefer F, Schmid D, Veldhuis JD, Ritz E. Angiotensin II affects basal, pulsatile, and glucose-stimulated insulin secretion in humans. Hypertension 30: 1156–1161, 1997. doi: 10.1161/01.HYP.30.5.1156. [DOI] [PubMed] [Google Scholar]
- 76.Leung PS. Pancreatic RAS. Adv Exp Med Biol 690: 89–105, 2010. doi: 10.1007/978-90-481-9060-7_6. [DOI] [PubMed] [Google Scholar]
- 77.Luther JM, Luo P, Kreger MT, Brissova M, Dai C, Whitfield TT, Kim HS, Wasserman DH, Powers AC, Brown NJ. Aldosterone decreases glucose-stimulated insulin secretion in vivo in mice and in murine islets. Diabetologia 54: 2152–2163, 2011. doi: 10.1007/s00125-011-2158-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van der Zijl NJ, Moors CC, Goossens GH, Hermans MM, Blaak EE, Diamant M. Valsartan improves {beta}-cell function and insulin sensitivity in subjects with impaired glucose metabolism: a randomized controlled trial. Diabetes Care 34: 845–851, 2011. doi: 10.2337/dc10-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Conn JW. Hypertension, the potassium ion and impaired carbohydrate tolerance. N Engl J Med 273: 1135–1143, 1965. doi: 10.1056/NEJM196511182732106. [DOI] [PubMed] [Google Scholar]
- 80.Pellegrinelli V, Carobbio S, Vidal-Puig A. Adipose tissue plasticity: how fat depots respond differently to pathophysiological cues. Diabetologia 59: 1075–1088, 2016. doi: 10.1007/s00125-016-3933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chouchani ET, Kazak L, Jedrychowski MP, Lu GZ, Erickson BK, Szpyt J, Pierce KA, Laznik-Bogoslavski D, Vetrivelan R, Clish CB, Robinson AJ, Gygi SP, Spiegelman BM. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature 532: 112–116, 2016. [Erratum in Nature 536: 360, 2016]. doi: 10.1038/nature17399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Urbanet R, Nguyen Dinh Cat A, Feraco A, Venteclef N, El Mogrhabi S, Sierra-Ramos C, Alvarez de la Rosa D, Adler GK, Quilliot D, Rossignol P, Fallo F, Touyz RM, Jaisser F. Adipocyte mineralocorticoid receptor activation leads to metabolic syndrome and induction of prostaglandin D2 synthase. Hypertension 66: 149 157, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04981. [DOI] [PubMed] [Google Scholar]
- 83.Marzolla V, Armani A, Zennaro MC, Cinti F, Mammi C, Fabbri A, Rosano GM, Caprio M. The role of the mineralocorticoid receptor in adipocyte biology and fat metabolism. Mol Cell Endocrinol 350: 281–288, 2012. doi: 10.1016/j.mce.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 84.Hirata A, Maeda N, Hiuge A, Hibuse T, Fujita K, Okada T, Kihara S, Funahashi T, Shimomura I. Blockade of mineralocorticoid receptor reverses adipocyte dysfunction and insulin resistance in obese mice. Cardiovasc Res 84: 164–172, 2009. doi: 10.1093/cvr/cvp191. [DOI] [PubMed] [Google Scholar]
- 85.Yoon MS, Zhang C, Sun Y, Schoenherr CJ, Chen J. Mechanistic target of rapamycin controls homeostasis of adipogenesis. J Lipid Res 54: 2166–2173, 2013. doi: 10.1194/jlr.M037705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huby AC, Antonova G, Groenendyk J, Gomez-Sanchez CE, Bollag WB, Filosa JA, Belin de Chantemèle EJ. Adipocyte derived hormone leptin is a direct regulator of aldosterone secretion, which promotes endothelial dysfunction and cardiac fibrosis. Circulation 132: 2134–2145, 2015. doi: 10.1161/CIRCULATIONAHA.115.018226. [DOI] [PubMed] [Google Scholar]
- 87.de Haro Moraes C, Figueiredo VN, de Faria AP, Barbaro NR, Sabbatini AR, Quinaglia T, Ferreira-Melo SE, Martins LC, Demacq C, Júnior HM. High-circulating leptin levels are associated with increased blood pressure in uncontrolled resistant hypertension. J Hum Hypertens 27: 225–230, 2013. doi: 10.1038/jhh.2012.29. [DOI] [PubMed] [Google Scholar]
- 88.Haluzik M, Sindelka G, Widimský J Jr, Prázný M, Zelinka T, Skrha J. Serum leptin levels in patients with primary hyperaldosteronism before and after treatment: relationships to insulin sensitivity. J Hum Hypertens 16: 41–45, 2002. doi: 10.1038/sj.jhh.1001292. [DOI] [PubMed] [Google Scholar]
- 89.Goodfriend TL, Kelley DE, Goodpaster BH, Winters SJ. Visceral obesity and insulin resistance are associated with plasma aldosterone levels in women. Obes Res 7: 355–362, 1999. doi: 10.1002/j.1550-8528.1999.tb00418.x. [DOI] [PubMed] [Google Scholar]
- 90.Abdul-Ghani MA, DeFronzo RA. Pathogenesis of insulin resistance in skeletal muscle. J Biomed Biotechnol 2010: 476279, 2010. doi: 10.1155/2010/476279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Selvaraj J, Sathish S, Mayilvanan C, Balasubramanian K. Excess aldosterone-induced changes in insulin signaling molecules and glucose oxidation in gastrocnemius muscle of adult male rat. Mol Cell Biochem 372: 113–126, 2013. doi: 10.1007/s11010-012-1452-2. [DOI] [PubMed] [Google Scholar]
- 92.Manrique C, Sowers JR. Insulin resistance and skeletal muscle vasculature: significance, assessment and therapeutic modulators. Cardiorenal Med 4: 244–256, 2014. doi: 10.1159/000368423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Womack L, Peters D, Barrett EJ, Kaul S, Price W, Lindner JR. Abnormal skeletal muscle capillary recruitment during exercise in patients with type 2 diabetes mellitus and microvascular complications. J Am Coll Cardiol 53: 2175–2183, 2009. doi: 10.1016/j.jacc.2009.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hernandez N, Torres SH, Finol HJ, Sosa A, Cierco M. Capillary and muscle fiber type changes in DOCA-salt hypertensive rats. Anat Rec 246: 208–216, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 95.Losada M, Torres SH, Hernandez N, Lippo M, Sosa A. Muscle arteriolar and venular reactivity in two models of hypertensive rats. Microvasc Res 69: 142–148, 2005. doi: 10.1016/j.mvr.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 96.Blazer-Yost BL, Esterman MA, Vlahos CJ. Insulin-stimulated trafficking of ENaC in renal cells requires PI 3-kinase activity. Am J Physiol Cell Physiol 284: C1645–C1653, 2003. doi: 10.1152/ajpcell.00372.2002. [DOI] [PubMed] [Google Scholar]
- 97.Ilatovskaya DV, Pavlov TS, Levchenko V, Staruschenko A. ROS production as a common mechanism of ENaC regulation by EGF, insulin, and IGF-1. Am J Physiol Cell Physiol 304: C102–C111, 2013. doi: 10.1152/ajpcell.00231.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mansley MK, Neuhuber W, Korbmacher C, Bertog M. Norepinephrine stimulates the epithelial Na+ channel in cortical collecting duct cells via α2-adrenoceptors. Am J Physiol Renal Physiol 308: F450–F458, 2015. doi: 10.1152/ajprenal.00548.2014. [DOI] [PubMed] [Google Scholar]
- 99.Pizarro M, Solis N, Quintero P, Barrera F, Cabrera D, Rojas-de Santiago P, Arab JP, Padilla O, Roa JC, Moshage H, Wree A, Inzaugarat E, Feldstein AE, Fardella CE, Baudrand R, Riquelme A, Arrese M. Beneficial effects of mineralocorticoid receptor blockade in experimental non-alcoholic steatohepatitis. Liver Int 35: 2129–2138, 2015. [Erratum in Liver Int 36: 314, 2016]. doi: 10.1111/liv.12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Polyzos SA, Kountouras J, Zafeiriadou E, Patsiaoura K, Katsiki E, Deretzi G, Zavos C, Tsarouchas G, Rakitzi P, Slavakis A. Effect of spironolactone and vitamin E on serum metabolic parameters and insulin resistance in patients with nonalcoholic fatty liver disease. J Renin Angiotensin Aldosterone Syst 12: 498–503, 2011. doi: 10.1177/1470320311402110. [DOI] [PubMed] [Google Scholar]
- 101.Fallo F, Dalla Pozza A, Tecchio M, Tona F, Sonino N, Ermani M, Catena C, Bertello C, Mulatero P, Sabato N, Fabris B, Sechi LA. Nonalcoholic fatty liver disease in primary aldosteronism: a pilot study. Am J Hypertens 23: 2–5, 2010. doi: 10.1038/ajh.2009.206. [DOI] [PubMed] [Google Scholar]
- 102.Shibayama Y, Wada N, Baba S, Obara S, Sakai H, Usubuchi H, Terae S, Nakamura A, Atsumi T. The risk factors for hepatic steatosis in patients with primary aldosteronism. Endocr J 67: 623–629, 2020. doi: 10.1507/endocrj.EJ19-0600. [DOI] [PubMed] [Google Scholar]
- 103.Preiss D, Zetterstrand S, McMurray JJ, Ostergren J, Michelson EL, Granger CB, Yusuf S, Swedberg K, Pfeffer MA, Gerstein HC, Sattar N; for the Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity Investigators. Predictors of development of diabetes in patients with chronic heart failure in the Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity (CHARM) program. Diabetes Care 32: 915–920, 2009. doi: 10.2337/dc08-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Calhoun DA, White WB, Krum H, Guo W, Bermann G, Trapani A, Lefkowitz MP, Ménard J. Effects of a novel aldosterone synthase inhibitor for treatment of primary hypertension: results of a randomized, double-blind, placebo- and active-controlled phase 2 trial. Circulation 124: 1945–1955, 2011. doi: 10.1161/CIRCULATIONAHA.111.029892. [DOI] [PubMed] [Google Scholar]
- 105.Liao WH, Suendermann C, Steuer AE, Pacheco Lopez G, Odermatt A, Faresse N, Henneberg M, Langhans W. Aldosterone deficiency in mice burdens respiration and accentuates diet-induced hyperinsulinemia and obesity. JCI Insight 3: e99015, 2018. doi: 10.1172/jci.insight.99015. [DOI] [PMC free article] [PubMed] [Google Scholar]