Abstract
Rheumatoid arthritis (RA) is a systemic inflammatory arthritis impacting primarily joints and cardiac and skeletal muscle. RA’s distinct impact on cardiac and skeletal muscle tissue is suggested by studies showing that new RA pharmacologic agents strongly improve joint inflammation, but have little impact on RA-associated mortality, cardiovascular disease, and sarcopenia. Thus, the objective is to understand the distinct effects of RA on cardiac and skeletal muscle, and to therapeutically target these tissues through endurance-based exercise as a way to improve RA mortality and morbidity. We utilize the well-characterized RA mouse model, the K/BxN mouse, to investigate cardiac and skeletal muscle pathologies, including the use of wheel-running exercise to mitigate these pathologies. Strikingly, we found that K/BxN mice, like patients with RA, also exhibit both cardiac and skeletal muscle myopathies that were correlated with circulating IL-6 levels. Three months of wheel-running exercise significantly improved K/BxN joint swelling and reduced systemic IL-6 concentrations. Importantly, there were morphological, gene expression, and functional improvements in both the skeletal muscle and cardiac myopathies with exercise. The K/BxN mouse model of RA recapitulated important RA clinical comorbidities, including altered joint, cardiac and skeletal muscle function. These morphological, molecular, and functional alterations were mitigated with regular exercise, thus suggesting exercise as a potential therapeutic intervention to lessen disease activity in the joint and the peripheral tissues, including the heart and skeletal muscle.
NEW & NOTEWORTHY RA, even when controlled, is associated with skeletal muscle weakness and greater risk of cardiovascular disease (CVD). Using exercise as a therapeutic against, the progression of RA is often avoided due to fear of worsening RA pathology. We introduce the K/BxN mouse as an RA model to study both myocardial and skeletal muscle dysfunction. We show that endurance exercise can improve joint, cardiac, and skeletal muscle function in K/BxN mice, suggesting exercise may be beneficial for patients with RA.
Keywords: arthritis, exercise, heart, inflammation, muscle
INTRODUCTION
Although there have been recent advances in the treatment of rheumatoid arthritis (RA), patients still suffer considerable morbidity and are at risk for increased mortality. A large portion of this morbidity and mortality can be attributed to both cardiac (1–6) and skeletal muscle (7–9) dysfunction: persons with RA have increased rates of both cardiovascular disease (CVD) (10) and sarcopenia or sarcopenic obesity (7–9, 11–13). Notably, even in remission, persons with RA exhibit reduced strength and physical function (14). In the current age of RA treatment, the causes of these morbidities remain unclear, but likely result from a combination of traditional risks—including obesity and inactivity—and disease-specific inflammation and immune cell dysfunction (15, 16).
Peripheral effects of RA could be better understood through use of well-characterized animal models. Currently, there are several mouse models of RA including the collagen-induced arthritis (CIA), collagen antibody-induced arthritis (CAIA), K/BxN, K/BxN serum transfer-induced arthritis (STIA), and inflammatory models (TNFΔARE and the IL-1RA) (17). Although each model has its own advantages and disadvantages, the K/BxN model has some ideal features: consistent, reliable, and rapid development of RA (by ∼6 wk) without a need for continued injections for RA maintenance.
K/BxN mice have a transgenic T cell receptor that recognizes a peptide of glucose-6-phosphate isomerase (G6PI) as an autoantigen in the context of MHC-II (I-Ag7). In this CD4+ T cell-driven model, sustained T cell stimulation of B cells leads to the production of large quantities of high-affinity IgG1 antibodies against G6PI (18). The K/BxN mouse model shows many features common to human RA and other forms of inflammatory arthritis, including leukocyte invasion, synoviocyte proliferation, pannus formation, synovitis and cartilage, and bone erosion (19). It also shares immunological abnormalities with human RA, including polyclonal B cell activation, hypergammaglobulinemia, and autoantibody production (18).
Other mouse models of RA exhibit skeletal muscle dysfunction. Such pathologies include muscle weakness (20), decreased contractile force (21, 22), muscle atrophy (20, 23), and reduced voluntary activity (23, 24). At the molecular level, atrogenes MuRF1 and Atrogin-1 are increased (24), myofilament abnormalities are evident (22), and the generation of reactive oxygen species (ROS) and oxidative post-translational modifications has been shown (22). In the heart, the CAIA RA mouse model shows cardiac hypertrophy, poorer contractile function, and greater ROS accumulation (25). Similarly, the CIA model shows evidence of ROS byproduct accumulation in aortic and heart endothelial tissue (26). Although the K/BxN mouse model shows both valvular disease and inflammatory infiltrates in the myocardium (27), to the best of our knowledge, till date, there have been no reports of the K/BxN mouse, or any RA mouse model showing a phenotype with the combination of both skeletal muscle wasting/weakness and heart failure with preserved ejection fraction (HFpEF).
Exercise can attenuate muscle and heart pathologies under wasting-type diseases including cancer cachexia (28) and diabetes (29). Although some evidence suggests that exercise can attenuate the progression of RA in both human (30) and rodent models of RA (31, 32), it is currently unclear how exercise affects systemic perturbations of RA including systemic inflammation and cardiac and skeletal muscle function. Therefore, the purpose of this study is to utilize the K/BxN mouse model to investigate RA-related cardiac and skeletal muscle pathologies, and how endurance-based exercise can mitigate these conditions. We hypothesize that the K/BxN mouse model will recapitulate the cardiac and skeletal muscle dysfunction observed in RA patients, whereas exercise will be protective against these perturbations.
METHODS
Animals
C57BL/6J (wild-type, Wt) mice were used at 12–15 wk of age. The KRN mice were a generous gift from Diane Mathis (Harvard University). The SCID mice were purchased from Jackson Labs (Cat. No. 001303). The K/BxN mice were generated by crossing the KRN and SCID mice together and using the first generation as experimental cohorts. All animal care followed the guidelines and was approved by the Institutional Animal Care and Use Committees (IACUCs) at Duke University Medical Center.
Ethical Approval
Medical ethical approval was not required for this study.
Echocardiography
Serial echocardiography was performed on conscious mice from all groups with a Vevo 2100 high-resolution imaging system (VisualSonics) as previously described (33).
Pressure-Volume Loop Analysis
In vivo pressure-volume (P-V) analysis was performed as previously described (33, 34). Briefly, ketamine/xylazine (80–100/10 mg kg−1) was administered by an intraperitoneal injection. Proper anesthetization was confirmed by gentle tail pinch. Once anesthetized, the neck and chest were shaved with hair clippers and the mice placed on a heated pad to maintain temperature. Ointment was applied to the eyes to prevent dryness. A midline incision was made in the neck and the tracheal muscles were dissected to expose the trachea. An endotracheal tube was inserted through the mouth while visualizing the trachea to ensure intubation and connection to the respirator. After bilateral vagotomy, the chest was opened and the pericardium was dissected to expose the heart. A 7-0 suture ligature was placed around the transverse aorta to manipulate loading conditions. A 1.4-Fr pressure-conductance catheter (Millar Instruments, Houston, TX) was inserted retroaortically into the left ventricle (LV) to record hemodynamics. Baseline hemodynamic parameters were obtained once the catheter recordings had achieved steady state, usually 3–5 min following conductance catheter placement. Subsequently, parallel conductance (Vp) was determined by 10 µL injection of 15% saline into the right jugular vein to establish the parallel conductance of the blood pool. The derived Vp was used to correct the P-V loop data. Data were recorded digitally at 1,000 Hz and analyzed with pressure volume analysis software (PVAN data analysis software v3.3; Millar Instruments) as previously described (33).
Tissue Histology
Immediately post dissection, the heart was fixed in 4% PFA for 8 h, then cut at the mid-belly and placed in optimal cutting temperature compound (OCT) for later use; 10-μm sections were cut on the cryostat and stained for hematoxylin-eosin (H&E) (35, 36) and Masson’s trichrome.
Cytokine Analysis
Cytokines were measured in plasma using a multiplex pro inflammatory ELISA from Meso Scale Diagnostics (MSD) (Cat. No. K15048D). Cytokines for which the majority of sample had concentrations below the level of detection were excluded.
Gene Expression
mRNA and cDNA were isolated and generated from skeletal muscle and cardiac tissue as previously described (36, 37). RNA sequencing (RNAseq) was performed by the Duke Genomics Core as follows: incoming RNA samples’ QC was performed with an Agilent Fragment Analyzer and a Qubit assay on the PerkinElmer Victor X2. RNA sample quality was assessed based on its concentration and degradation score (RIN > 7 is preferred). Samples with RIN score <4 were not used for library preparation. Illumina Truseq Stranded total RNA-Seq Kit combined with ribo-zero Gold depletion was used to prepare total RNA-seq libraries. Total RNA was first depleted of its rRNA using biotinylated probes that selectively bind rRNA molecules. The rRNA-depleted RNA was then reverse-transcribed. During the second strand synthesis, the cDNA:RNA hybrid was converted into double-stranded cDNA (dscDNA) and dUTP was incorporated into the second cDNA strand, effectively marking the second strand. Illumina sequencing adapters were then ligated to the dscDNA fragments and amplified to produce the final RNA-seq library. The strand marked with dUTP was not amplified, allowing strand-specificity sequencing.
Libraries were indexed using a single 6bpl indexing approach allowing for multiple libraries to be pooled and sequenced on the same sequencing lane on an Illumina HiSeq 4000 sequencing platform. Before pooling and sequencing, fragment length distribution and library quality were first assessed on a Fragment Analyzer using the High Sensitivity DNA Kit (Agilent Technologies). All libraries were then pooled in equimolar ratio and sequenced. Sequencing was done at 50 bp single-end and ∼55 million reads were generated per library. Once generated, sequence data were demultiplexed and Fastq files were generated using Bcl2Fastq conversion software from Illumina. RNA-seq data were processed using the TrimGalore tool kit, which employs Cutadapt (38), to trim low-quality bases and Illumina sequencing adapters from the 3′ end of the reads. Only reads that were 20 nt or longer after trimming were kept for further analysis. Reads were mapped to the GRCm38v68 version of the mouse genome and transcriptome (39) using the STAR RNA-seq alignment tool (40). Reads were kept for subsequent analysis if they mapped to a single genomic location. Gene counts were compiled using the HTSeq tool. Only genes that had at least 10 reads in any given library were used in subsequent analysis. Normalization and differential expression was performed using the DESeq2 (41) Bioconductor (42) package with the R statistical programming environment. The false discovery rate was calculated to control for multiple hypothesis testing. Pathways enriched for differentially expressed genes, those defined as unadjusted P value < 0.02 were identified using ingenuity pathway analysis. Primer sequences for RT-PCR for each gene are included in Supplemental Table S1; all Supplemental material is available at https://doi.org/10.6084/m9.figshare.13200995).
Grip Strength
Hindlimb and forelimb grip strengths were determined on an automated meter (maximum force applied) as the mouse is removed. Three trials were performed per mouse.
Activity Analysis
Voluntary activity was tested in the open field arena (Omnitech, Columbus, OH) illuminated at 340 lux as described by Deng et al. (43). Mice were placed individually into the open field and baseline locomotion was monitored over 60 min.
RotoRod Analysis
Balance and coordination were examined using an accelerating (4–40 rpm over 5 min) rotorod (Med-Associates) as described previously (44). Mice were given four successive 5-min trials that were separated by 30 min each. Trials were terminated when the mouse fell from the rod or at 300 s and were recorded as latency to fall.
Wheel Running
Mice were housed in individual cages (32 × 14 × 13 cm3, Coulburn Instruments, Whitehall, PA) with 11‐cm-diameter running wheels with 5‐cm path widths. The cages were placed inside a Phenome Technologies ventilated cabinet (Lincolnshire, IL) equipped with 526-nm green wavelength LED lights for illumination during the light cycle and infrared LED lights during the dark cycle. Activity data were collected using the ClockLab data collection and analyses software (Actimetrics, Wilmette, IL). Mice had free access to food and water and were exposed to a 12:12-h light‐dark (LD) cycle (light onset at 0800 h) for 24 h to assess motor activity.
Von Frey Test
The Von Frey hairs test allow detection of mechanical tactile sensation as a measure of local pain. Animals are first acclimated to the Von Frey test apparatus. Each cubicle provides adequate space for the mouse to turn-about and move comfortably. The floor of the platform on which the mice are standing is ventilated with a grid. This allows for urine and feces to fall through, leaving the test cubicle clean, but it also permits the introduction of stimulus fibers to the feet of the mice. Mice are acclimated to the cubicles for three days before testing (20 min/day). Testing is conducted in 1 day. Von Frey filaments (2.44 mm) are applied to the planar region of the fore- or hind-paw of each animal, through the steel grid on which the animal is standing. A filament of given length and diameter is touched at a right angle to the paw just until the fiber bends. The response is paw withdrawal defined as flicking the paw, raising the paw, and/or licking the paw. Filaments of increasing diameter are assessed and the threshold at which paw withdrawal occurs is measured. The procedures are replicated for a given animal with an intertrial interval of at least 10 s.
Statistical Analyses
Wild-type and K/BxN comparisons were made using two-tailed, unpaired t tests, one- and two-way ANOVAs, and regression analysis. Statistical significance with repeated measures (Fig. 1, B and C and Fig. 4, B and C) was determined by repeated-measures ANOVA. Statistical significance was established as P < 0.05.
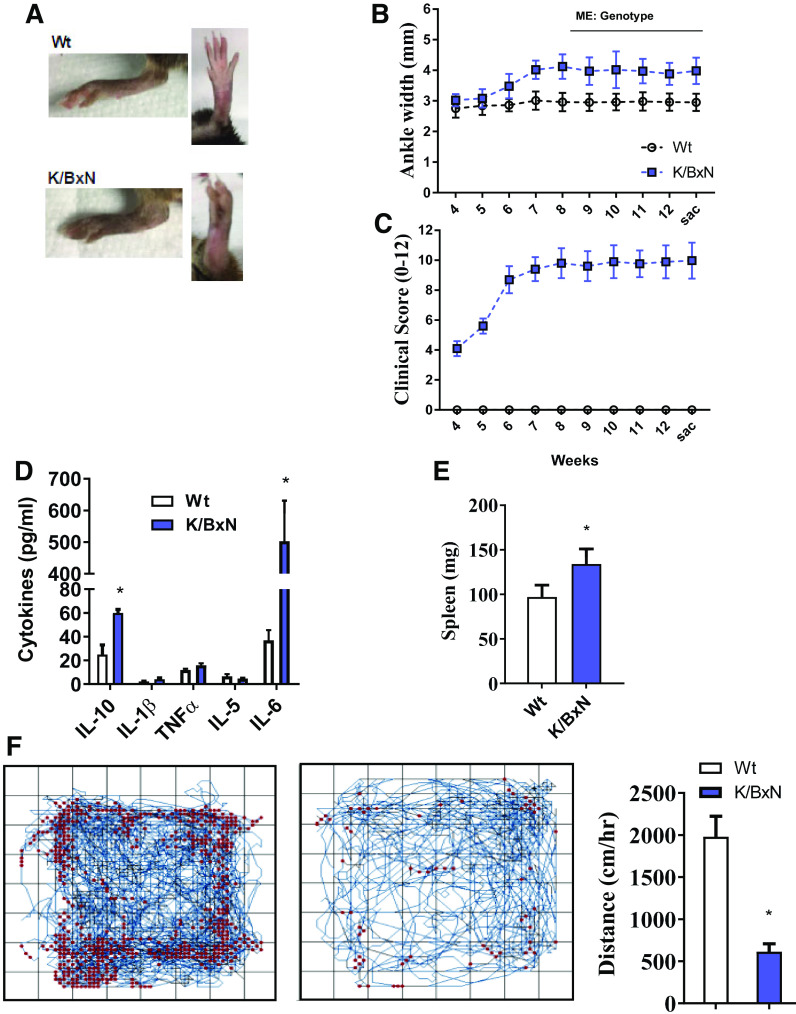
Figure 1.
The K/BxN mouse model of RA shows joint pathology, systemic inflammation, and reductions in physical activity. A: representative image of hind ankle joints in wild-type (Wt) and K/BxN mice. B: time course of ankle width measurements taken throughout the 13-wk study design. C: joint clinical score for front and hind paws through the 13-wk study. (For B and C; n = 10 male mice/group; repeated-measures ANOVA, main effect of genotype, *P < 0.05). Plasma cytokine levels (D) and spleen weights (E) taken at 13 wk from wild-type and K/BxN mice. F: open-field activity measurements taken throughout 60 min in wild-type and K/BxN mice. Representative activity maps for each group (left). Quantification of distance traveled per hour (right). (For D–F; n = 10 male mice/group; two-tailed t test, *P < 0.05). Data are presented as means ± SE. RA, rheumatoid arthritis.
Figure 4.
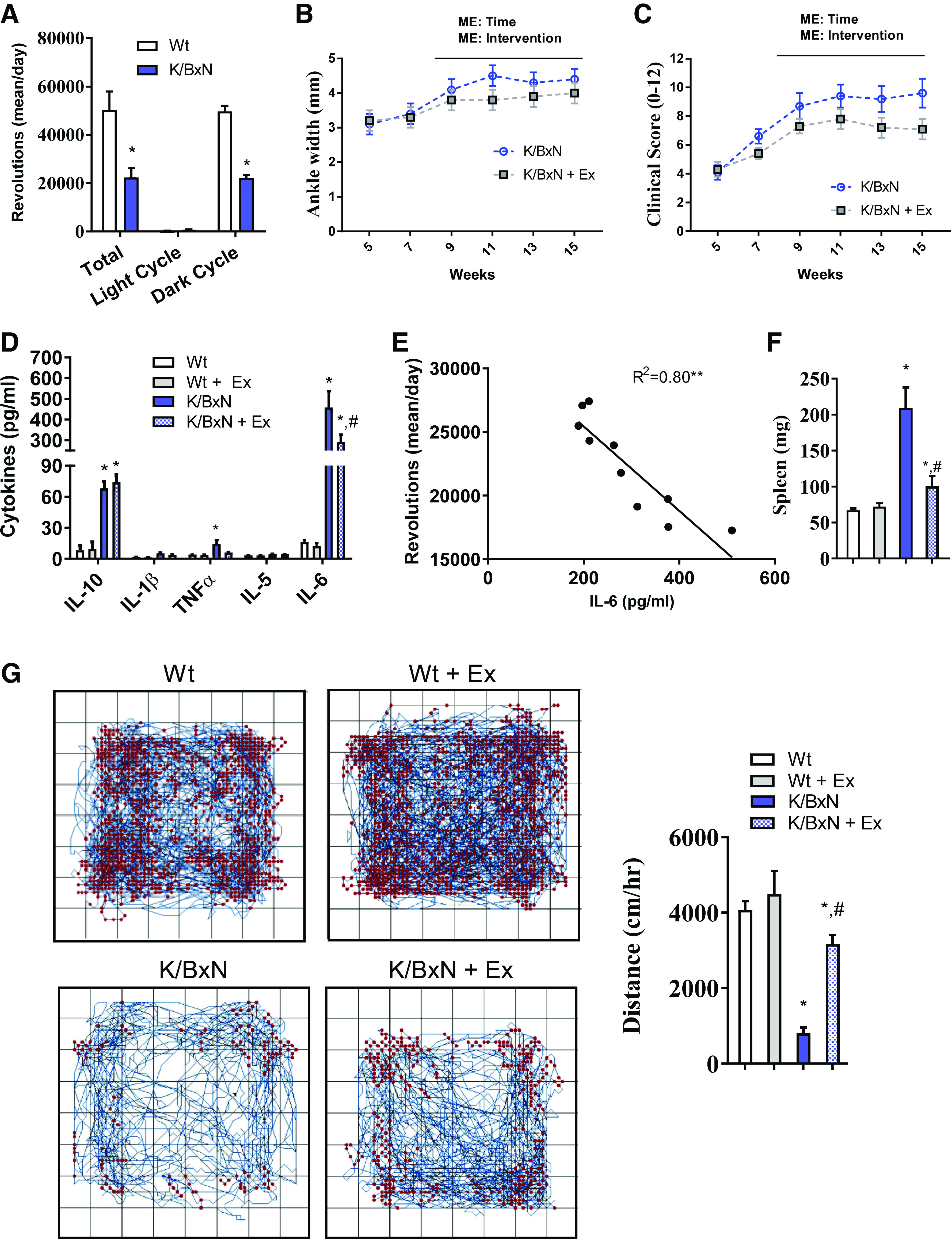
Chronic exercise attenuates disease progression, inflammation, and increases physical activity. A: wheel-running activity shown as the average total revolutions/day and revolutions during the light and dark cycles (n = 10 male mice/group; two-tailed t test, *P < 0.05). Ankle width measurements (B) and clinical scores (C) throughout the 15-wk exercise regimen in sedentary and exercised K/BxN mice. (For B and C; n = 10 male mice/group; repeated-measures ANOVA, main effect of genotype and time, *P < 0.05). D: plasma cytokine levels at 15 wk from sedentary wild-type (Wt), exercised wild-type, sedentary K/BxN, and exercised K/BxN mice (n = 10 male mice/group; ANOVA, *P < 0.05 compared with wild-type #P < 0.05 compared to sedentary K/BxN group). E: correlation between wheel-running activity per day and circulating IL-6 (n = 10 male K/BxN mice; regression analysis, *P < 0.05). F: spleen weight after 15 wk of exercise from sedentary wild-type, exercised wild-type, sedentary K/BxN, and exercised K/BxN mice. G: open-field activity measurements taken after 15 wk of exercise. Representative activity maps for each group (left). Quantification of distance traveled per hour (right). (For F and G, n = 10 male mice/group; ANOVA, *P < 0.05 compared with wild-type #P < 0.05 compared with sedentary K/BxN group). Data are presented as means ± SE.
RESULTS
Consistent with previous reports, K/BxN animals spontaneously developed arthritis as early as 6 wk, and paw swelling was maintained through 12 wk (Fig. 1, A–C) (18, 45). In addition to arthritis, K/BxN was characterized by greater IL-6 and IL-10 concentrations along with splenomegaly (Fig. 1, D and E). Moreover, the K/BxN mouse had a marked reduction in spontaneous cage activity (Fig. 1F).
Cardiac Morphologic, Molecular, and Functional Assessments
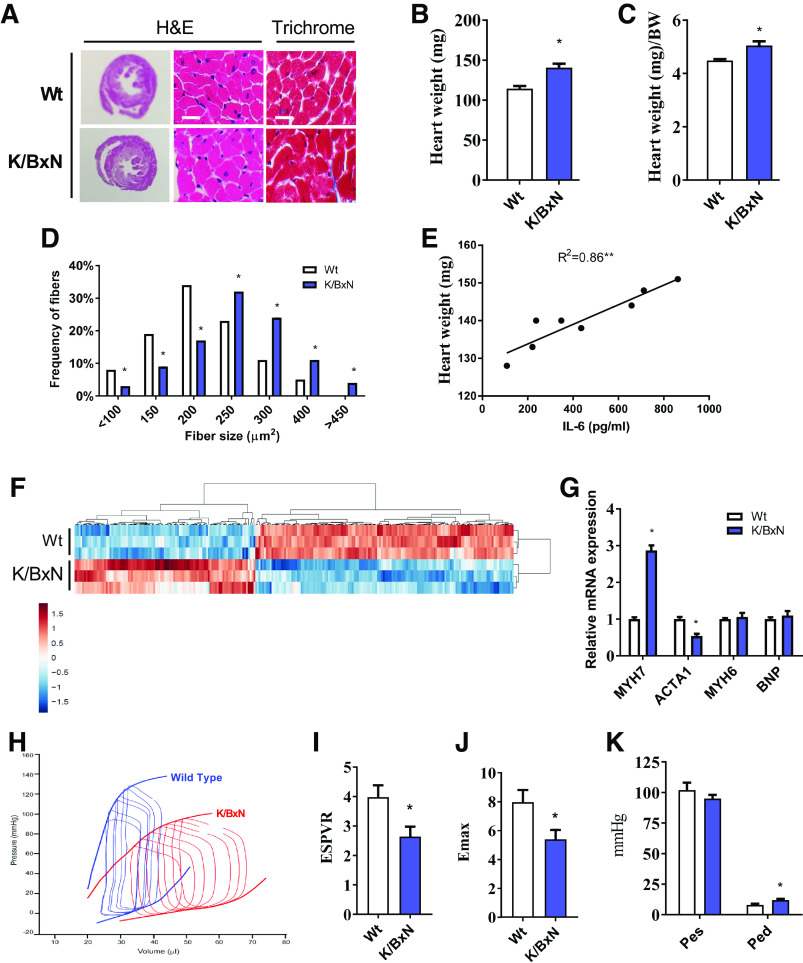
By 3 mo of age, K/BxN mice developed cardiac hypertrophy without any observable fibrosis. The K/BxN hearts had greater gross heart weight, heart weight indexed to body weight, and cardiomyocyte fiber size (Fig. 2, A–D). However, there was no induction of fibrosis, as measured by Masson’s trichrome staining (Fig. 2A). Cardiac hypertrophy was closely correlated with systemic IL-6 (R2 = 0.86, P < 0.05, Fig. 2E). Cardiac tissue gene expression differed between K/BxN and wild-type animals across a broad range of genes (Fig. 2F; see volcano plot in Supplemental Fig. S1 and Supplemental Table S2 for the list of top 25 differentially expressed genes). Gene expression alterations included a reduction in categories related to cardiac function and contractility (unadjusted P < 0.0001 for both; Supplemental Table S3) with 5.3-times greater expression of the fetal heart isoform (2E2.4), MYH7 (unadjusted P = 2E-30, Supplemental Table S2). To confirm these differences, expression of canonical heart failure genes was assessed with RT-PCR; K/BxN tissue had greater MYH7 and less ACTA1 expression (Fig. 2G). K/BxN cardiac gene expression was downregulated in the overlapping canonical pathways, energy production (unadjusted P = 0.00001), and oxidative metabolism (unadjusted P = 0.00002), (Supplemental Table S3). K/BxN gene expression was consistent with a 2.7- to 3.2-times predicted activation of the pathway for migration of inflammatory cells (Supplementary Table S4, unadjusted P < 0.00001 for all). Among autophagy pathway genes, K/BxN tissue had reduced expression of multiple genes, although only LC3ba was significantly downregulated (unadjusted P = 1.06E-5; adjusted P < 0.002) (Supplemental Fig. S2).
Figure 2.
Evidence of cardiomyopathy in the K/BxN mouse model. A: representative heart (H&E) cross-sections at low (left column) and high magnification (middle column) and Masson’s trichrome staining (right column) in wild-type (Wt) and K/BxN mice. Scale bar = 50 µm. B: heart wet weight and heart weight relative to body weight (C). (For B and C; n = 11 male wild-type mice and 16 male K/BxN mice; two-tailed t test, *P < 0.05). D: frequency histogram of cardiomyocyte cross-sectional area. (For D; n = 11 male wild-type mice and 16 male K/BxN mice; chi-square test, *P < 0.05). E: regression analysis showing correlations between heart weight and plasma IL-6 (n = 10 male K/BxN mice; regression analysis, *P < 0.05). F: RNAseq heat map showing differentially expressed genes (FDR < 5%) between wild-type and K/BxN hearts. Gene expression has been z-score-normalized, and the samples and genes are clustered by correlation distance with complete linkage. G: RT-PCR gene expression related to markers of heart disease. H: representative image of pressure-volume loop analysis in the heart of wild-type and K/BxN mice. I: end-systolic pressure volume relationships (ESPVR). J: maximal time-varying elastance (Emax) and K: LV pressure analysis in wild-type and K/BxN hearts. (For G, I–K; n = 11 male wild-type mice and 16 male K/BxN mice; two-tailed t test, *P < 0.05). Data are presented as means ± SE. ESPVR, end-systolic pressure volume relationships; H&E, hematoxylin and eosin; LV, left ventricle; RNAseq, RNA sequencing. MYH, myosin heavy chain; ACTA, actin alpha; BNP, brain natriuretic peptide; Pes, end-systolic pressure; Ped, end-diastolic pressure.
K/BxN heart pressure volume (P-V) loop assessments showed a reduction in load-independent measures of contractility in the K/BxN heart as illustrated with reductions in end-systolic pressure volume relationships (ESPVR) and maximal time-varying elastance (Emax) (Fig. 2, H–J). We also observed an increase in left ventricular diastolic pressure in the K/BxN heart with no differences in left ventricular systolic pressure (Fig. 2K). There were no differences in end-diastolic pressure volume relationships (EDPVR) (Supplemental Fig. S3, ), indicating that K/BxN hearts were not less compliant or stiffer than wild-type controls.
Skeletal Muscle Morphologic, Molecular, and Functional Assessments
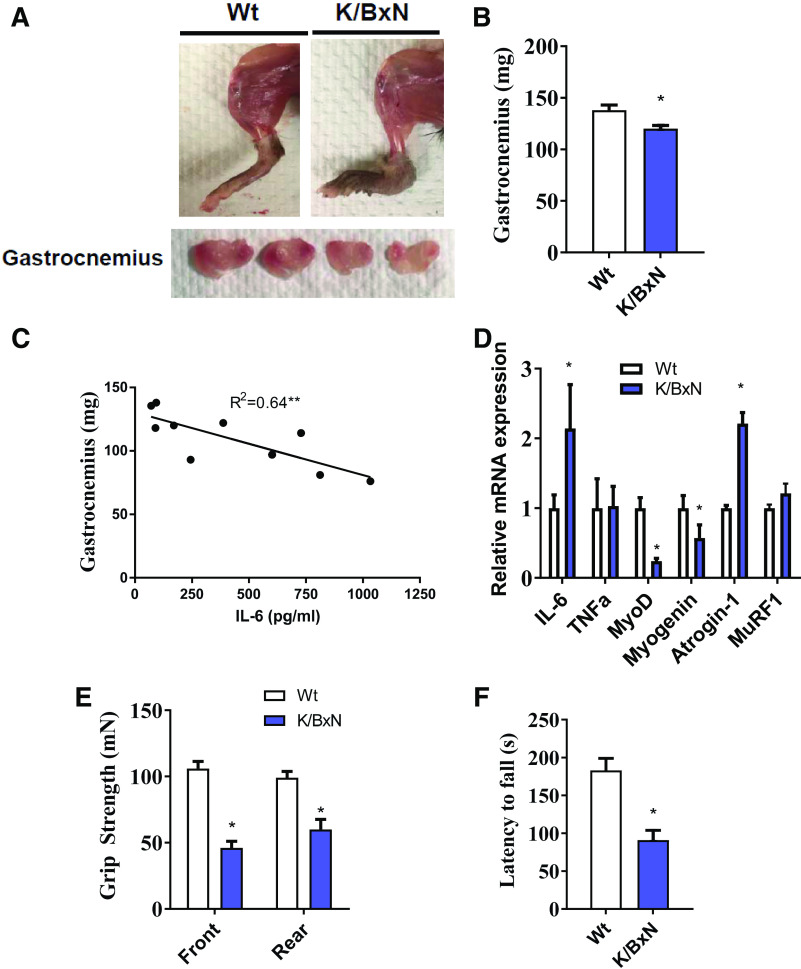
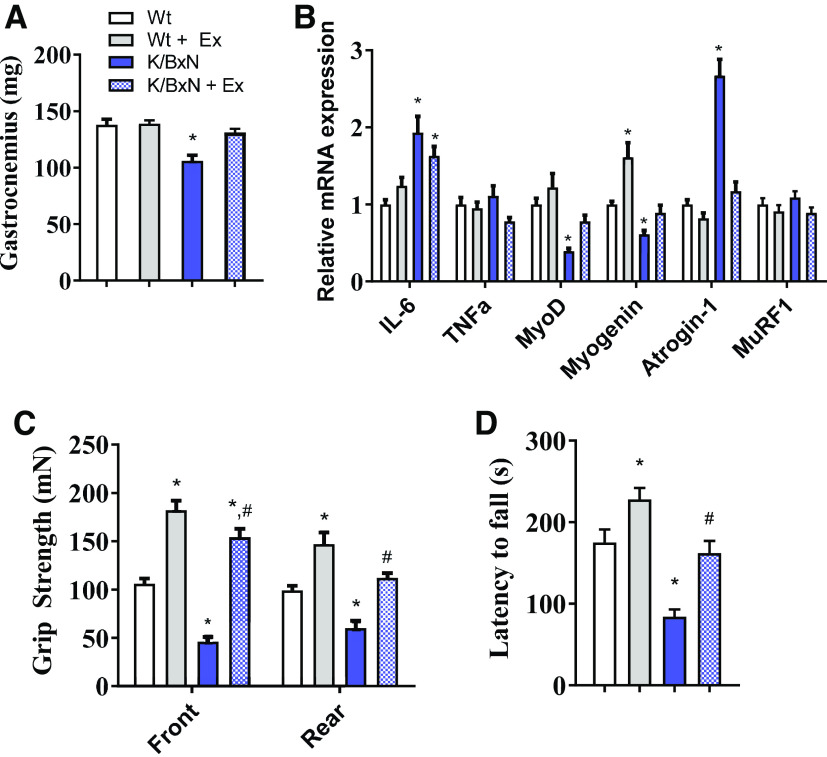
At 3 mo of age, K/BxN animals displayed reduced muscle mass in the gastrocnemius (Fig. 3, A and B), which was highly correlated with systemic IL-6 (R2 = 0.64, P < 0.05, Fig. 3C). To understand transcriptional overview of RA muscle, we assessed expression for genes related to inflammation (IL-6, TNFα), myogenesis (MyoD and myogenin), and proteolysis (atrogin-1 and MuRF1). Compared with wild-type, K/BxN skeletal muscle had greater expression of IL-6 and atrogin-1 and reduced expression of the myogenesis genes, MyoD, and myogenin (Fig. 3D). K/BxN animals had both poorer grip strength and Rotorod stability, indicating reduced muscle function (Fig. 3, E and F).
Figure 3.
Muscle atrophy in the K/BxN mouse model. A: representative images of hindlimb (upper) and gastrocnemius muscle (lower) from wild-type (Wt) and K/BxN mice. B: gastrocnemius muscle weight in wild-type and K/BxN mice (n = 10/group; two-tailed t test, *P < 0.05). C: regression analysis showing a correlation between gastrocnemius weight and plasma IL-6 concentrations (n = 10 male K/BxN mice; regression analysis, *P < 0.05). D: mRNA expression in the gastrocnemius muscle of wild-type and K/BxN mice. Front and rear paw grip strength (E) and time to fall during RotoRod analysis (F). (For D–F, n = 10/group; two-tailed t test, *P < 0.05). Data are presented as means ± SE.
Responses to 3 Mo of Regular Exercise
K/BxN and wild-type animals were provided running wheels from 4 wk of age, before K/BxN arthritis onset, through 15 wk. Both K/BxN and wild-type mice used the running wheels; however, K/BxN amounts (revolutions per day) were approximately half wild-type amounts (Fig. 4A). Over time, exercise significantly reduced K/BxN joint swelling (Fig. 4, B and C). In addition, exercise attenuated the induction of plasma IL-6, whereas the IL-10 levels remained elevated (Fig. 4D). Interestingly, we observed a negative correlation between the amount of wheel-running per day and circulating IL-6 (Fig. 4E). Spleen weight was also reduced with exercise compared with spleen weight of sedentary K/BxN mice (Fig. 4F). The wheel-running regimen did not appear to impact joint pain, as neither the sedentary nor exercised K/BxN mice showed heightened sensitivity by a Von Frey test (Supplemental Fig. S4). After the exercise training, K/BxN animals exhibited more spontaneous cage activity than their sedentary controls (Fig. 4G).
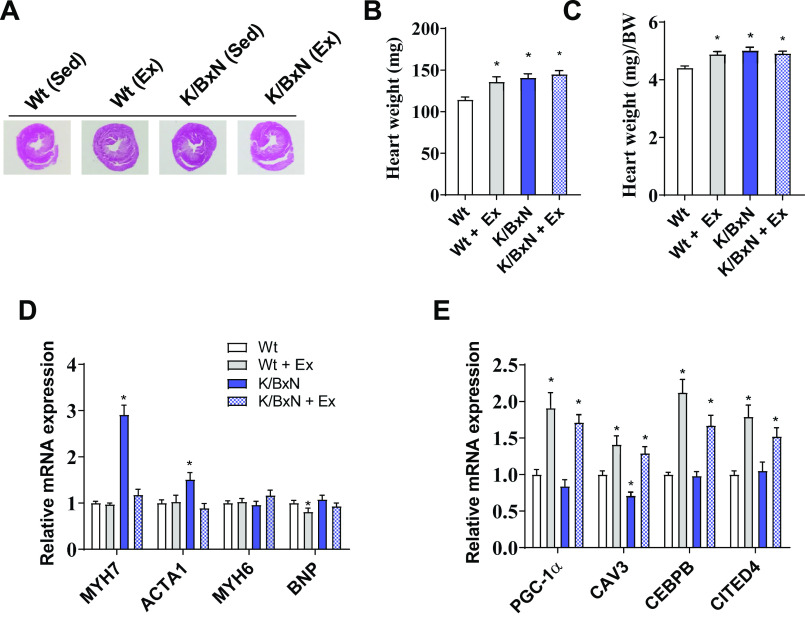
Exercise training resulted in morphological and molecular effects on the K/BxN heart. At baseline, noninvasive echocardiography revealed that K/BxN mice have enhanced concentric hypertrophy (enhanced wall thickness and reduced diastolic dimensions) and relatively preserved systolic function (Table 1). In K/BxN mice treated with exercise, both systolic and diastolic dimensions increased, as did systolic function in comparison with both wild-type and nonexercised K/BxN mice (Table 1). Gross heart weight was not different between sedentary and exercised K/BxN mice (Fig. 5, A–C). Diastolic function was largely unaffected by RA or exercise except for an increase in “E” (peak velocity of early diastolic transmitral flow) with exercise (Table 1). The preservation of systolic function, increase in ventricular dimensions, and unchanged gross heart weight in exercised K/BxN mice suggest an acquired “physiological” hypertrophic phenotype (46). This proposition is supported by our findings that exercise 1) reduced “pathologic” gene signatures (MYH7 and ACTA1) and 2) enhanced “physiologic” exercise-induced gene signatures (PGC1α, CAV3, CEBPβ, and CITED4) (Fig. 5, D and E).
Table 1.
Echocardiography parameters of sedentary wild-type, exercised wild-type, sedentary K/BxN, and exercised K/BxN mice
| Wild-Type | Wild-Type + Ex | K/BxN | K/BxN + Ex | |
|---|---|---|---|---|
| LVDs | 2.21 ±.11 | 2.06 ± 0.11 | 1.73 ± 0.09* | 1.91 ± . 13 |
| LVDd | 3.71 ± 0.12 | 3.51 ± 0.14 | 3.31 ± 0.07* | 3.72 ± 0.13 |
| LVESV | 17.17 ± 2 | 14.42 ± 1.92 | 9.44 ± 1.26* | 12.18 ± 2.09 |
| LVEDV | 59.41 ± 4 | 52.35 ± 4.95 | 44.86 ± 2.4* | 59.84 ± 5 |
| WT | 1.84 ± 0.07 | 2.13 ± 0.08* | 1.919 ± 0.06 | 2.11 ± 0.04* |
| RWT | 0.511 ± 0.03 | 0.63 ± 0.04* | 0.58 ± 0.02 | 0.60 ± 0.03 |
| FS | 40.8 ± 1.8 | 41.58 ± 1.50 | 48.1 ± 1.8* | 49.3 ± 1.9* |
| E | 367 ± 32 | 357 ± 32 | 332 ± 23 | 443 ± 32* |
| A | 235 ± 27 | 202 ± 25 | 246 ± 26 | 250 ± 26 |
| E/A | 1.79 ± 0.3 | 1.98 ± 0.25 | 1.66 ± 0.3 | 2.04 ± 0.3 |
| E/e' | 13.13 ± 1.1 | 14.84 ± 1.94 | 11.8 ± 1.0 | 12.12 ± 1.3 |
| IVRT | 15.2 ± 0.9 | 16.75 ± 1.14 | 15.2 ± 0.6 | 16.5 ± 1.9 |
Data are presented as means ± SE. n = 10 male mice/group; ANOVA, *P < 0.05 compared with sedentary wild‐type mice. A, peak velocity of late transmitral flow; e′, rate of diastole filling; E, peak velocity of early diastolic transmitral flow; E/A, ratio of E to A; E/e′, ratio of E to e′; FS, fractional shortening; IVRT, isovolumetric relaxation time; LVDd, left ventricular diameter during diastole; LVDs, left ventricular diameter during systole; LVEDV, left ventricular end‐diastolic volume; LVESV, left ventricular end‐systolic volume; RWT, relative wall thickness; WT, wall thickness.
Figure 5.
Chronic exercise prevents to the onset of cardiomyopathy in the K/BxN mouse. A: heart cross-sectional morphology in sedentary wild-type (Wt), exercised wild-type, sedentary K/BxN, and exercised K/BxN mice. B: heart weight and heart weight relative to body weight. C: in sedentary wild-type, exercised wild-type, sedentary K/BxN, and exercised K/BxN mice. D: gene expression analysis related to heart failure and E: exercise remodeling. (For B–E, n = 10 male mice/group; ANOVA, *P < 0.05 compared to sedentary wild-type mice). Data are presented as means ± SE. Ex, exercised; Sed, sedentary; PGC, PPARG coactivator; CAV, caveolin; CEPBP, CCAAT enhancer binding protein; CITED, CREB-binding protein/p300-interacting transactivator with Asp/Glu-rich C-terminal domain; MYH, myosin heavy chain; ACTA, actin alpha; BNP, brain natriuretic peptide.
Exercise also mitigated the K/BxN skeletal muscle phenotype. Gastrocnemius mass was returned to wild-type values (Fig. 6A). Muscle IL-6 mRNA expression remained elevated in both sedentary and exercised K/BxN muscle. However, MyoD and myogenin mRNA were increased, whereas atrogin-1 mRNA was suppressed with exercise (Fig. 6B), both toward that of those of wild-type mice. Exercise increased functional measurements including grip strength and Rotorod performance on both wild-type and K/BxN mice (Fig. 6, C and D).
Figure 6.
Chronic exercise preserved the loss of muscle mass and physical function in the K/BxN mouse. A: gastrocnemius muscle weight in sedentary wild-type (Wt), exercised wild-type, sedentary K/BxN, and exercised K/BxN mice. B: mRNA expression in the gastrocnemius muscle from respective groups. (For A and B, n = 10 male mice/group; ANOVA, *P < 0.05 compared to sedentary wild-type mice). C: front and rear paw grip strength and D: time to fall during RotoRod analysis. (For C and D, n = 10 male mice/group; ANOVA, *P < 0.05 compared to sedentary wild-type mice, #P < 0.05 compared with sedentary K/BxN group). Data are presented as means ± SE. ANOVA: analysis of variance.
DISCUSSION
Using a well-characterized genetic model of inflammatory arthritis, we demonstrated abnormalities in both cardiac and skeletal muscle phenotypes. The cardiac phenotype was characterized by hypertrophy, reduced contractility, and greater expression of canonical heart failure genes. However, we did not see any indication of fibrosis. K/BxN skeletal muscle wasting and dysfunction were accompanied by a gene expression pattern of atrophy, inflammation, and impaired regeneration. Three months of exercise significantly improved K/BxN joint swelling and systemic inflammation, as well as cardiac and skeletal muscle morphology, gene expression, and function.
At 3 mo, K/BxN hearts were hypertrophied with reduced contractility in concert with increased systemic inflammation. The cardiac phenotype was characterized by 1) cardiac hypertrophy, 2) a preserved load-dependent measure of cardiac function by echo and reduced load-independent measures of contractility, 3) altered gene expression promoting inflammatory cell migration and, 4) downregulated expression of cardiac function and oxidative phosphorylation. This constellation of findings mimics a subphenotype of human HFpEF that is associated with inflammatory conditions (47) and is observed at increased rates in persons with RA (48). Previously published work in patients with RA has identified diastolic dysfunction as a feature in RA cardiomyopathies (48–50). Although echocardiographic and cardiac magnetic resonance imaging (cMRI) suggest RA-associated HFpEF might be due to patchy fibrosis (51), 3-mo-old K/BxN did not show significant diastolic dysfunction. These findings suggest that enhanced cardiac stiffness may not be a key driver of cardiac pathology, but an indirect or late manifestation of cytokine-induced myocardial injury. Findings such as these emphasize that the “humanized RA” (18) K/BxN mouse is a viable model to study RA-associated cardiomyopathy.
Mouse models of RA are associated with wasting phenotypes, including loss of skeletal muscle mass and function. We observed a similar phenotype in the K/BxN mice, showing marked reductions in muscle mass and physical performance. Interestingly, the extent of muscle atrophy was correlated to circulating IL-6. TNFα was not correlated with either muscle or cardiac alterations in the K/BxN mouse. Moreover, in contrast to IL-6, TNFα had little to no change in circulation or at the muscle mRNA level. In wasting conditions such as cancer cachexia, high circulating IL-6 is typically associated with increased atrogin-1 without changes in MuRF1 (52). Here, we observed similar associations between IL-6 and atrogin-1 induction in the K/BxN mouse, suggesting a possible IL-6-dependent wasting model. In general, IL-6 has divergent biology between skeletal muscle and cardiac tissue. High amounts of circulating IL-6 are associated with myofiber atrophy and wasting, whereas the cardiomyoctye hypertrophies. Although this paradox is still not well understood, it appears that this works in a Stat3-dependent manner. Stat3 signaling is often increased in skeletal muscle wasting, especially under conditions of high circulating IL-6 (65, 66). Moreover, IL-6-induced cardiac hypertrophy is regulated by Stat3 (53). Although this manuscript did not assess cellular signaling mechanisms across muscle tissues in RA, inhibition of IL-6 and/or downstream Stat3 activity are natural therapeutic targets for alleviating RA-related muscle dysfunction.
In humans, physical inactivity is associated with greater RA disease activity (54), poorer immune function (55, 56), CVD (57–60), and skeletal muscle pathology (61–66). Here, in K/BxN animals, regular wheel exercise reduced paw swelling and inflammation in concert with remarkable improvements in cardiac and skeletal muscle phenotypes. These exercise responses reproduce those found in humans, where 6 mo of individualized aerobic and resistance training improved both RA disease activity and cardiovascular risk (67). Further, a pilot 10-wk high-intensity interval-based walking program improves RA disease activity, innate immune function, cardiorespiratory fitness, and physical performance (30). Consistent with exercising K/BxN mice, in exercising humans with RA, improvements in RA body composition are associated with reductions in muscle IL-6, whereas cardiorespiratory fitness improvements are associated with reductions in systemic concentrations of the heart failure biomarker, galectin-3 (68).
Lower amounts of physical activity are a risk factor for developing RA (47). In this study, K/BxN mice started wheel-running exercise 2 wk before onset of systemic autoimmunity. Although exercise training did not prevent disease occurrence, a pre-disease exercise regimen significantly attenuated disease activity and muscle-related morbidity in this animal model of RA. In spontaneous arthritic SKG/Jcl mice, exercise implemented before disease onset delays RA onset and lessens inflammatory arthritis severity (32).
In this study, we found exercise prevented the RA-associated pathologies in both cardiac and skeletal muscle. In the heart, exercise not only prevented pathological gene expression and morphology, but also developed a physiological phenotype, typical of exercise training. Significantly, the exercise stimulus completely dominated the heart biology in the setting of RA. This was evident in morphological, histological, and gene expression responses. In limb skeletal muscle, exercise prevented RA-induced muscle atrophy in association with the reduction in atrogenes atrogin-1 and MuRF1. This is in accordance with the beneficial effects of exercise in other murine models of muscle wasting (28). Moreover, we observed a negative correlation between the extent of wheel-running and circulating IL-6. This supports the potency of exercise as a systemic anti-inflammatory therapeutic. These findings have significant implications for people at risk for developing RA and for those with early incident disease (67). In addition, this adds to our enthusiasm for exercise to be a potent therapeutic for the prevention of RA-associated cardiovascular disease. In humans, further research is needed to assess the benefits of exercise training to prevent or delay RA disease manifestations and to improve outcomes in persons newly diagnosed with RA.
Other animal studies support our main finding that exercise improves inflammatory arthritis, associated cardiometabolic risk, and skeletal muscle atrophy in an RA animal model. In a CIA rat model, 6 wk of swimming improves immune cell metabolism and function (22). Treadmill running in CIA rats reduces synovitis and bone and cartilage destruction (4). In an adjuvant induced model of RA (AIA), eccentric exercise prevents weakness associated with skeletal muscle inflammation (44). However, in CAIA mice, voluntary running conversely promotes arthritis onset and slowed the resolution of inflammation (66). In this CAIA mouse model of arthritis, mechanical strain is implicated in the localization of joint inflammation and erosions (5). We hypothesize that the CAIA model may better approximate the inflammatory arthritis seen in human spondyloarthritis and psoriatic arthritis, where biomechanical stress is implicated in disease pathogenesis, rather than RA (69).
Regardless of the model used, all animal studies in RA share the main limitation of their inability to closely approximate the complex human condition. For example, although pharmacologic inhibition of IL-17 shows great promise in multiple models of RA, including CIA and K/BxN animals, it is ineffective in the management of human RA (70). Interestingly, IL-17 inhibition is therapeutic in persons with spondyloarthritis and psoriatic disease. Although animal models are essential to the study of new therapeutics in RA, including the effects of exercise training, caution is still needed when interpreting the results, and follow-up studies in humans are critical.
In sum, this study further describes the K/BxN mouse phenotype, showing high circulating IL-6 is associated with cardiac and skeletal muscle myopathy. Furthermore, we show chronic endurance exercise is a potent method to slow disease progression and attenuate both cardiomyopathies and peripheral myopathies associated with RA. Future studies are warranted to understand the role of IL-6 in the progression of RA and related systemic effect in the K/BxN mouse. These findings may have implications for the treatment of the cardiac and skeletal muscle pathologies observed in human RA.
GRANTS
This work was supported by NIH Grant R21AR076663 (to K.M.H.); NIH Grant K01AG056664, P30AG028716 and the Mandel Foundation for Cardiovascular Research (to J.P.W.); NIH/NHLBI training Grant T32HL007057 (to D.E.L.); NIH Grant R03AG067949 and the Duke Pepper Center REC Career Development Award (to B.J.A.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.M.H., D.M.A., W.E.K., and J.P.W. conceived and designed research; D.M.A., D.E.L., L.H.K., J.L.H., and J.P.W. performed experiments; K.M.H., B.J.A., D.M.A., A.B., D.E.L., J.L.H., W.E.K., and J.P.W. analyzed data; K.M.H., B.J.A., D.M.A., A.B., L.H.K., W.E.K., and J.P.W. interpreted results of experiments; K.M.H., D.M.A., A.B., and J.P.W. prepared figures; K.M.H., B.J.A., D.M.A., L.H.K., W.E.K., and J.P.W. drafted manuscript; K.M.H., B.J.A., L.H.K., W.E.K., and J.P.W. edited and revised manuscript; K.M.H., B.J.A., D.M.A., A.B., D.E.L., L.H.K., J.L.H., W.E.K., and J.P.W. approved final version of manuscript.
ENDNOTE
All transcriptomic data generated or analyzed during this study are included in this published article (and its supplementary information files). Additional data that support the findings of this study are available from the corresponding author on reasonable request.
ACKNOWLEDGMENTS
The authors acknowledge Duke’s Pathology, Cardiovascular Physiology and Mouse Behavioral Core for assistance with histology, cardiac imaging and physiology and physical function analysis, respectively.
REFERENCES
- 1.Goodson NJ, Wiles NJ, Lunt M, Barrett EM, Silman AJ, Symmons DP. Mortality in early inflammatory polyarthritis: cardiovascular mortality is increased in seropositive patients. Arthritis Rheum 46: 2010–2019, 2002. doi: 10.1002/art.10419. [DOI] [PubMed] [Google Scholar]
- 2.Jacobsson LT, Knowler WC, Pillemer S, Hanson RL, Pettitt DJ, Nelson RG, del Puente A, McCance DR, Charles MA, Bennett PH. Rheumatoid arthritis and mortality. A longitudinal study in Pima Indians. Arthritis Rheum 36: 1045–1053, 1993. doi: 10.1002/art.1780360804. [DOI] [PubMed] [Google Scholar]
- 3.Mutru O, Laakso M, Isomaki H, Koota K. Cardiovascular mortality in patients with rheumatoid arthritis. Cardiology 76: 71–77, 1989. doi: 10.1159/000174474. [DOI] [PubMed] [Google Scholar]
- 4.Symmons DP, Jones MA, Scott DL, Prior P. Longterm mortality outcome in patients with rheumatoid arthritis: early presenters continue to do well. J Rheumatol 25: 1072–1077, 1998. [PubMed] [Google Scholar]
- 5.Wallberg-Jonsson S, Ohman ML, Dahlqvist SR. Cardiovascular morbidity and mortality in patients with seropositive rheumatoid arthritis in Northern Sweden. J Rheumatol 24: 445–451, 1997. [PubMed] [Google Scholar]
- 6.Wolfe F, Mitchell DM, Sibley JT, Fries JF, Bloch DA, Williams CA, Spitz PW, Haga M, Kleinheksel SM, Cathey MA. The mortality of rheumatoid arthritis. Arthritis Rheum 37: 481–494, 1994. doi: 10.1002/art.1780370408. [DOI] [PubMed] [Google Scholar]
- 7.Giles JT, Ling SM, Ferrucci L, Bartlett SJ, Andersen RE, Towns M, Muller D, Fontaine KR, Bathon JM. Abnormal body composition phenotypes in older rheumatoid arthritis patients: association with disease characteristics and pharmacotherapies. Arthritis Rheum 59: 807–815, 2008. doi: 10.1002/art.23719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hakkinen A, Kautiainen H, Hannonen P, Ylinen J, Makinen H, Sokka T. Muscle strength, pain, and disease activity explain individual subdimensions of the Health Assessment Questionnaire disability index, especially in women with rheumatoid arthritis. Ann Rheum Dis 65: 30–34, 2006. doi: 10.1136/ard.2004.034769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roubenoff R. Sarcopenic obesity: does muscle loss cause fat gain? Lessons from rheumatoid arthritis and osteoarthritis. Ann N Y Acad Sci 904: 553–557, 2000. doi: 10.1111/j.1749-6632.2000.tb06515.x. [DOI] [PubMed] [Google Scholar]
- 10.Maradit-Kremers H, Crowson C, Nicola P, Ballman K, Roger V, Jacobsen S, Gabriel S. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis. Arthritis Rheum 52: 402–411, 2005. doi: 10.1002/art.20853. [DOI] [PubMed] [Google Scholar]
- 11.Fraser A, Vallow J, Preston A, Cooper RG. Predicting 'normal' grip strength for rheumatoid arthritis patients. Rheumatology (Oxford ) 38: 521–528, 1999. doi: 10.1093/rheumatology/38.6.521. [DOI] [PubMed] [Google Scholar]
- 12.Helliwell PS, Jackson S. Relationship between weakness and muscle wasting in rheumatoid arthritis. Ann Rheum Dis 53: 726–728, 1994. doi: 10.1136/ard.53.11.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilkinson TJ, Lemmey AB, Jones JG, Sheikh F, Ahmad YA, Chitale S, Maddison PJ, O'Brien TD. Can creatine supplementation improve body composition and objective physical function in rheumatoid arthritis patients? A randomized controlled trial. Arthritis Care Res (Hoboken) 68: 729–737, 2016. [DOI] [PubMed] [Google Scholar]
- 14.Lemmey AB, Wilkinson TJ, Clayton RJ, Sheikh F, Whale J, Jones HS, Ahmad YA, Chitale S, Jones JG, Maddison PJ, O'Brien TD. Tight control of disease activity fails to improve body composition or physical function in rheumatoid arthritis patients. Rheumatology (Oxford) 55: 1736–1745, 2016. doi: 10.1093/rheumatology/kew243. [DOI] [PubMed] [Google Scholar]
- 15.Crowson CS, Nicola PJ, Kremers HM, O'Fallon WM, Therneau TM, Jacobsen SJ, Roger VL, Ballman KV, Gabriel SE. How much of the increased incidence of heart failure in rheumatoid arthritis is attributable to traditional cardiovascular risk factors and ischemic heart disease? Arthritis Rheum 52: 3039–3044, 2005. doi: 10.1002/art.21349. [DOI] [PubMed] [Google Scholar]
- 16.Ruscitti P, Cipriani P, Masedu F, Romano S, Berardicurti O, Liakouli V, Carubbi F, Di Benedetto P, Alvaro S, Penco M, Valenti M, Giacomelli R. Increased cardiovascular events and subclinical atherosclerosis in rheumatoid arthritis patients: 1 year prospective single centre study. PLoS One 12: e0170108, 2017. doi: 10.1371/journal.pone.0170108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caplazi P, Baca M, Barck K, Carano RA, DeVoss J, Lee WP, Bolon B, Diehl L. Mouse models of rheumatoid arthritis. Vet Pathol 52: 819–826, 2015. doi: 10.1177/0300985815588612. [DOI] [PubMed] [Google Scholar]
- 18.Monach PA, Mathis D, Benoist C. The K/BxN arthritis model. Curr Protoc Immunol 15: 22, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Ditzel HJ. The K/BxN mouse: a model of human inflammatory arthritis. Trends Mol Med 10: 40–45, 2004. doi: 10.1016/j.molmed.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Alabarse PVG, Lora PS, Silva JMS, Santo RCE, Freitas EC, de Oliveira MS, Almeida AS, Immig M, Teixeira VON, Filippin LI, Xavier RM. Collagen-induced arthritis as an animal model of rheumatoid cachexia. J Cachexia Sarcopenia Muscle 9: 603–612, 2018. doi: 10.1002/jcsm.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bell RD, Wu EK, Rudmann CA, Forney M, Kaiser CRW, Wood RW, Chakkalakal JV, Paris ND, Klose A, Xiao GQ, Rangel-Moreno J, Garcia-Hernandez ML, Ritchlin CT, Schwarz EM, Rahimi H. Selective sexual dimorphisms in musculoskeletal and cardiopulmonary pathologic manifestations and mortality incidence in the tumor necrosis factor-transgenic mouse model of rheumatoid arthritis. Arthritis Rheumatol 71: 1512–1523, 2019. doi: 10.1002/art.40903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinz MM, Persson M, Aresh B, Olsson K, Cheng AJ, Ahlstrand E, Lilja M, Lundberg TR, Rullman E, Moller KA, Sandor K, Ajeganova S, Yamada T, Beard N, Karlsson BC, Tavi P, Kenne E, Svensson CI, Rassier DE, Karlsson R, Friedman R, Gustafsson T, Lanner JT. Oxidative hotspots on actin promote skeletal muscle weakness in rheumatoid arthritis. JCI Insight 4: e126347, 2019. doi: 10.1172/jci.insight.126347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartog A, Hulsman J, Garssen J. Locomotion and muscle mass measures in a murine model of collagen-induced arthritis. BMC Musculoskelet Disord 10: 59, 2009. doi: 10.1186/1471-2474-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Oliveira Nunes Teixeira V, Filippin LI, Viacava PR, de Oliveira PG, Xavier RM. Muscle wasting in collagen-induced arthritis and disuse atrophy. Exp Biol Med (Maywood) 238: 1421–1430, 2013. doi: 10.1177/1535370213505961. [DOI] [PubMed] [Google Scholar]
- 25.Pironti G, Bersellini-Farinotti A, Agalave NM, Sandor K, Fernandez-Zafra T, Jurczak A, Lund LH, Svensson CI, Andersson DC. Cardiomyopathy, oxidative stress and impaired contractility in a rheumatoid arthritis mouse model. Heart 104: 2026–2034, 2018. doi: 10.1136/heartjnl-2018-312979. [DOI] [PubMed] [Google Scholar]
- 26.Palma Zochio Tozzato G, Taipeiro EF, Spadella MA, Marabini Filho P, de Assis MR, Carlos CP, Girol AP, Chies AB. Collagen-induced arthritis increases inducible nitric oxide synthase not only in aorta but also in the cardiac and renal microcirculation of mice. Clin Exp Immunol 183: 341–349, 2016. doi: 10.1111/cei.12728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Binstadt BA, Hebert JL, Ortiz-Lopez A, Bronson R, Benoist C, Mathis D. The same systemic autoimmune disease provokes arthritis and endocarditis via distinct mechanisms. Proc Natl Acad Sci USA 106: 16758–16763, 2009. doi: 10.1073/pnas.0909132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puppa MJ, White JP, Velazquez KT, Baltgalvis KA, Sato S, Baynes JW, Carson JA. The effect of exercise on IL-6-induced cachexia in the Apc (Min/+) mouse. J Cachexia Sarcopenia Muscle 3: 117–137, 2012. doi: 10.1007/s13539-011-0047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen GQ, Mou CY, Yang YQ, Wang S, Zhao ZW. Exercise training has beneficial anti-atrophy effects by inhibiting oxidative stress-induced MuRF1 upregulation in rats with diabetes. Life Sci 89: 44–49, 2011. doi: 10.1016/j.lfs.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 30.Bartlett DB, Willis LH, Slentz CA, Hoselton A, Kelly L, Huebner JL, Kraus VB, Moss J, Muehlbauer MJ, Spielmann G, Kraus WE, Lord JM, Huffman KM. Ten weeks of high-intensity interval walk training is associated with reduced disease activity and improved innate immune function in older adults with rheumatoid arthritis: a pilot study. Arthritis Res Ther 20: 127, 2018. doi: 10.1186/s13075-018-1624-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kito T, Teranishi T, Nishii K, Sakai K, Matsubara M, Yamada K. Effectiveness of exercise-induced cytokines in alleviating arthritis symptoms in arthritis model mice. Okajimas Folia Anat Jpn 93: 81–88, 2016. doi: 10.2535/ofaj.93.81. [DOI] [PubMed] [Google Scholar]
- 32.Shimomura S, Inoue H, Arai Y, Nakagawa S, Fujii Y, Kishida T, Ichimaru S, Tsuchida S, Shirai T, Ikoma K, Mazda O, Kubo T. Treadmill running ameliorates destruction of articular cartilage and subchondral bone, not only synovitis, in a rheumatoid arthritis rat model. Int J Mol Sci 19: 1653, 2018. doi: 10.3390/ijms19061653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoo B, Lemaire A, Mangmool S, Wolf MJ, Curcio A, Mao L, Rockman HA. Beta1-adrenergic receptors stimulate cardiac contractility and CaMKII activation in vivo and enhance cardiac dysfunction following myocardial infarction. Am J Physiol Heart Circ Physiol 297: H1377–H1386, 2009. doi: 10.1152/ajpheart.00504.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abraham D, Mao L. Cardiac pressure-volume loop analysis using conductance catheters in mice. J Vis Exp 103: e52942 2015. doi: 10.3791/52942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baht GS, Bareja A, Lee DE, Rao RR, Huang R, Huebner JL, Bartlett DB, Hart CR, Gibson JR, Lanza IR, Kraus VB, Gregory SG, Spiegelman BM, White JP. Meteorin-like facilitates skeletal muscle repair through a Stat3/IGF-1 mechanism. Nat Metab 2: 278–289, 2020. [Erratum in Nat Metab 2:794, 2020 ]. doi: 10.1038/s42255-020-0184-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White JP, Wrann CD, Rao RR, Nair SK, Jedrychowski MP, You JS, Martinez-Redondo V, Gygi SP, Ruas JL, Hornberger TA, Wu Z, Glass DJ, Piao X, Spiegelman BM. G protein-coupled receptor 56 regulates mechanical overload-induced muscle hypertrophy. Proc Natl Acad Sci USA 111: 15756–15761, 2014. doi: 10.1073/pnas.1417898111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White JP, Billin AN, Campbell ME, Russell AJ, Huffman KM, Kraus WE. The AMPK/p27(Kip1) axis regulates autophagy/apoptosis decisions in aged skeletal muscle stem cells. Stem Cell Reports 11: 425–439, 2018. doi: 10.1016/j.stemcr.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.J 17: 10–12, 2011. doi: 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 39.Kersey PJ, Staines DM, Lawson D, Kulesha E, Derwent P, Humphrey JC, Hughes DS, Keenan S, Kerhornou A, Koscielny G, Langridge N, McDowall MD, Megy K, Maheswari U, Nuhn M, Paulini M, Pedro H, Toneva I, Wilson D, Yates A, Birney E. Ensembl genomes: an integrative resource for genome-scale data from non-vertebrate species. Nucleic Acids Res 40: D91–D97, 2012. doi: 10.1093/nar/gkr895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29: 15–21, 2013. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550, 2014. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huber W, Carey VJ, Gentleman R, Anders S, Carlson M, Carvalho BS, Bravo HC, Davis S, Gatto L, Girke T, Gottardo R, Hahne F, Hansen KD, Irizarry RA, Lawrence M, Love MI, MacDonald J, Obenchain V, Oles AK, Pages H, Reyes A, Shannon P, Smyth GK, Tenenbaum D, Waldron L, Morgan M. Orchestrating high-throughput genomic analysis with bioconductor. Nat Methods 12: 115–121, 2015. doi: 10.1038/nmeth.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deng JV, Rodriguiz RM, Hutchinson AN, Kim IH, Wetsel WC, West AE. MeCP2 in the nucleus accumbens contributes to neural and behavioral responses to psychostimulants. Nat Neurosci 13: 1128–1136, 2010. doi: 10.1038/nn.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor GA, Rodriguiz RM, Greene RI, Daniell X, Henry SC, Crooks KR, Kotloski R, Tessarollo L, Phillips LE, Wetsel WC. Behavioral characterization of P311 knockout mice. Genes Brain Behav 7: 786–795, 2008. doi: 10.1111/j.1601-183X.2008.00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rose S, Eren M, Murphy S, Zhang H, Thaxton CS, Chowaniec J, Waters EA, Meade TJ, Vaughan DE, Perlman H. A novel mouse model that develops spontaneous arthritis and is predisposed towards atherosclerosis. Ann Rheum Dis 72: 89–95, 2013. doi: 10.1136/annrheumdis-2012-201431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol 7: 589–600, 2006. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 47.Sharma K, Kass DA. Heart failure with preserved ejection fraction: mechanisms, clinical features, and therapies. Circ Res 115: 79–96, 2014. doi: 10.1161/CIRCRESAHA.115.302922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liang KP, Myasoedova E, Crowson CS, Davis JM, Roger VL, Karon BL, Borgeson DD, Therneau TM, Rodeheffer RJ, Gabriel SE. Increased prevalence of diastolic dysfunction in rheumatoid arthritis. Ann Rheum Dis 69: 1665–1670, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aslam F, Bandeali SJ, Khan NA, Alam M. Diastolic dysfunction in rheumatoid arthritis: a meta-analysis and systematic review. Arthritis Care Res 65: 534–543, 2013. [DOI] [PubMed] [Google Scholar]
- 50.Davis JM, 3rd, Lin G, Oh JK, Crowson CS, Achenbach SJ, Therneau TM, Matteson EL, Rodeheffer RJ, Gabriel SE. Five-year changes in cardiac structure and function in patients with rheumatoid arthritis compared with the general population. Int J Cardiol 240: 379–385, 2017. doi: 10.1016/j.ijcard.2017.03.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kobayshi H, Kobayashi Y, Yokoe I, Akashi Y, Takei M, Jt G. Magnetic resonance-detected myocardial inflammation and fibrosis in rheumatoid arthritis: associations of disease characteristics and n-terminal pro brain natriuretic peptide levels. Arthritis Care Res 69: 1304–1311, 2017. [DOI] [PubMed] [Google Scholar]
- 52.Baltgalvis KA, Berger FG, Pena MM, Davis JM, White JP, Carson JA. Muscle wasting and interleukin-6-induced atrogin-I expression in the cachectic Apc (Min/+) mouse. Pflugers Arch 457: 989–1001, 2009. doi: 10.1007/s00424-008-0574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mir SA, Chatterjee A, Mitra A, Pathak K, Mahata SK, Sarkar S. Inhibition of signal transducer and activator of transcription 3 (STAT3) attenuates interleukin-6 (IL-6)-induced collagen synthesis and resultant hypertrophy in rat heart. J Biol Chem 287: 2666–2677, 2012. doi: 10.1074/jbc.M111.246173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sandberg ME, Wedren S, Klareskog L, Lundberg IE, Opava CH, Alfredsson L, Saevarsdottir S. Patients with regular physical activity before onset of rheumatoid arthritis present with milder disease. Ann Rheum Dis 73: 1541–1544, 2014. doi: 10.1136/annrheumdis-2014-205180. [DOI] [PubMed] [Google Scholar]
- 55.Duggal NA. Reversing the immune ageing clock: lifestyle modifications and pharmacological interventions. Biogerontology 19: 481–496, 2018. [30269199] doi: 10.1007/s10522-018-9771-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duggal NA, Pollock RD, Lazarus NR, Harridge S, Lord JM. Major features of immunesenescence, including reduced thymic output, are ameliorated by high levels of physical activity in adulthood. Aging Cell 17: e12750, 2018. [29517845] doi: 10.1111/acel.12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.AbouAssi H, Connelly MA, Bateman LA, Tune KN, Huebner JL, Kraus VB, Winegar DA, Otvos JD, Kraus WE, Huffman KM. Does a lack of physical activity explain the rheumatoid arthritis lipid profile? Lipids Health Dis 16: 39, 2017. doi: 10.1186/s12944-017-0427-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Byram KW, Oeser AM, Linton MF, Fazio S, Stein CM, Mj O. Exercise is associated with increased small HDL particle concentration and decreased vascular stiffness in rheumatoid arthritis. J Clin Rheumatol 24: 417–421, 2018. [29846271] doi: 10.1097/RHU.0000000000000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Diaz BB, Gonzalez DA, Gannar F, Perez MCR, de Leon AC. Myokines, physical activity, insulin resistance and autoimmune diseases. Immunol Lett 203: 1–5, 2018. doi: 10.1016/j.imlet.2018.09.002 [DOI] [PubMed] [Google Scholar]
- 60.Fenton SAM, Veldhuijzen van Zanten J, Kitas GD, Duda JL, Rouse PC, Yu CA, Metsios GS. Sedentary behaviour is associated with increased long-term cardiovascular risk in patients with rheumatoid arthritis independently of moderate-to-vigorous physical activity. BMC Musculoskelet Disord 18: 131, 2017. doi: 10.1186/s12891-017-1473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Henchoz Y, Bastardot F, Guessous I, Theler JM, Dudler J, Vollenweider P, So A. Physical activity and energy expenditure in rheumatoid arthritis patients and matched controls. Rheumatology (Oxford) 51: 1500–1507, 2012. doi: 10.1093/rheumatology/kes067. [DOI] [PubMed] [Google Scholar]
- 62.Huffman KM, Jessee R, Andonian B, Davis BN, Narowski R, Huebner JL, Kraus VB, McCracken J, Gilmore BF, Tune KN, Campbell M, Koves TR, Muoio DM, Hubal MJ, Kraus WE. Molecular alterations in skeletal muscle in rheumatoid arthritis are related to disease activity, physical inactivity, and disability. Arthritis Res Ther 19: 12, 2017. doi: 10.1186/s13075-016-1215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huffman KM, Pieper CF, Hall KS, St Clair EW, Kraus WE. Self-efficacy for exercise, more than disease-related factors, is associated with objectively assessed exercise time and sedentary behaviour in rheumatoid arthritis. Scand J Rheumatol 44: 106–110, 2015. doi: 10.3109/03009742.2014.931456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Munsterman T, Takken T, Wittink H. Are persons with rheumatoid arthritis deconditioned? A review of physical activity and aerobic capacity. BMC Musculoskelet Disord 13: 202, 2012.doi: 10.1186/1471-2474-13-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev 98: 2133–2223, 2018. doi: 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tierney M, Fraser A, Kennedy N. Physical activity in rheumatoid arthritis: a systematic review. J Phys Act Health 9: 1036–1048, 2012. doi: 10.1123/jpah.9.7.1036. [DOI] [PubMed] [Google Scholar]
- 67.Stavropoulos-Kalinoglou A, Metsios GS, Veldhuijzen van Zanten JJ, Nightingale P, Kitas GD, Koutedakis Y. Individualised aerobic and resistance exercise training improves cardiorespiratory fitness and reduces cardiovascular risk in patients with rheumatoid arthritis. Ann Rheum Dis 72: 1819–1825, 2013. doi: 10.1136/annrheumdis-2012-202075. [DOI] [PubMed] [Google Scholar]
- 68.Andonian BJ, Bartlett DB, Huebner JL, Willis L, Hoselton A, Kraus VB, Kraus WE, Huffman KM. Effect of high-intensity interval training on muscle remodeling in rheumatoid arthritis compared to prediabetes. Arthritis Res Ther 20: 283, 2018. doi: 10.1186/s13075-018-1786-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.White JP. IL-6, cancer and cachexia: metabolic dysfunction creates the perfect storm. Transl Cancer Res 6: S280–S285, 2017. doi: 10.21037/tcr.2017.03.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.White JP, Baynes JW, Welle SL, Kostek MC, Matesic LE, Sato S, Carson JA. The regulation of skeletal muscle protein turnover during the progression of cancer cachexia in the Apc(Min/+) mouse. PLoS One 6: e24650, 2011. doi: 10.1371/journal.pone.0024650. [DOI] [PMC free article] [PubMed] [Google Scholar]