Abstract
With the global over 60-year-old population predicted to more than double over the next 35 years, caring for this aging population has become a major global healthcare challenge. In 2016 there were over 1 million deaths in >70 year olds due to lower respiratory tract infections; 13–31% of these have been reported to be caused by viruses. Since then, there has been a global COVID-19 pandemic, which has caused over 2.3 million deaths so far; increased age has been shown to be the biggest risk factor for morbidity and mortality. Thus, the burden of respiratory viral infections in the elderly is becoming an increasing unmet clinical need. Particular challenges are faced due to the interplay of a variety of factors including complex multimorbidities, decreased physiological reserve and an aging immune system. Moreover, their atypical presentation of symptoms may lead to delayed necessary care, prescription of additional drugs and prolonged hospital stay. This leads to morbidity and mortality and further nosocomial spread. Clinicians currently have limited access to sensitive detection methods. Furthermore, a lack of effective antiviral treatments means there is little incentive to diagnose and record specific non-COVID-19 viral infections. To meet this unmet clinical need, it is first essential to fully understand the burden of respiratory viruses in the elderly. Doing this through prospective screening research studies for all respiratory viruses will help guide preventative policies and clinical trials for emerging therapeutics. The implementation of multiplex point-of-care diagnostics as a mainstay in all healthcare settings will be essential to understand the burden of respiratory viruses, diagnose patients and monitor outbreaks. The further development of novel targeted vaccinations as well as anti-viral therapeutics and new ways to augment the aging immune system is now also essential.
The reviews of this paper are available via the supplemental material section.
Keywords: elderly, respiratory viruses, SARS-CoV-2, influenza, RSV, COVID-19, screening, therapeutics, vaccination, polypharmacy
Introduction
With the United Nations (UN) predicting a doubling of the number of over 60-year-olds worldwide from 901 million in 2015 to 2.1 billion in 2050,1 ensuring overall quality of life in our aging population is now a major global public health challenge. Problems arise not only in dealing with an aging immune system and complex multimorbidities, but also in knowing how to identify, distinguish, prevent and treat the infectious diseases that often exacerbate morbidity in the elderly.
The burden of respiratory viral infections in the elderly has been highlighted by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) which has infected millions of people worldwide and had unprecedented impacts on healthcare systems and society as a whole.2–4 Age has been found to be the biggest risk factor for the development of severe COVID-19. Furthermore, a large United Kingdom (UK) prospective sobservation study demonstrated that COVID-19 mortality was over 11 times higher in >80-year-olds than in <50-year-olds.3
Other respiratory viruses, including influenza and respiratory syncytial virus (RSV) however also lead to substantial morbidity and mortality, particularly in the elderly.5 Similarly, there is an increasing understanding of the burden of human metapneumovirus (HMPV), human rhinovirus (HRV) and human parainfluenza virus (HPIV). Respiratory viral infections inhibit the ability of the elderly to function and carry out their everyday life tasks. Thus, having a substantial impact on the elderly individuals and their families. Furthermore, respiratory viruses are key contributors to exacerbation of chronic diseases.6
Epidemiology
SARS-CoV-2
Since the end of 2019, SARS-CoV-2 infection has caused 107.5 million cases of COVID-19 and >2.3 million deaths worldwide.2 A large prospective observational study in the UK found that 72.7% of those presenting to hospital with COVID-19 were >60 years old, highlighting the particular vulnerability of older adults to COVD-19. Numbers of deaths were also shown to increase with increasing age, with 12.8%, 29.2% and 50.4% of deaths seen in 60–69, 70–79 and 80+ year olds, respectively.3 This was mirrored in the United States (US) where 80% of COVID-19 disease was in the >65 years old population.7
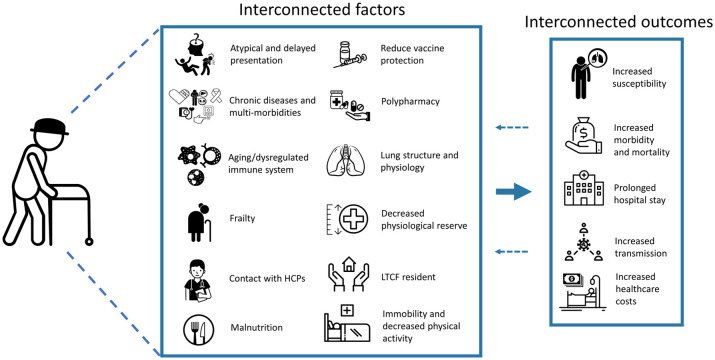
The complex interplay of factors leading to this increased risk is still to be fully delineated (Figure 1). However, the high prevalence of chronic comorbidities and thus risk to COVID-19 in elderly patients is thought to be key (discussed further below). Elderly patients in long-term-care facilities (LTCF) are a particularly vulnerable population and are often frail and have underlying chronic diseases, complex health needs and a requirement for medical support. Due to the COVID-19 transmission dynamics, as well as close contact between care staff and residents and a low availability of testing, there has been a rapid spread of COVID-19 within and between facilities.8 This has been exacerbated by a lack of personal protective equipment, absence of prompt health guidelines and the low ratio of personnel/residents during the epidemic. As a results there has been significant morbidity and mortality as a result of COVID-19 in LTCF.8 In England and Wales, 29.7% (15,018/50,548) deaths involving COVID-19 (registered up to 3 July 2020) were from LTCF residents.9 Furthermore, reports from Belgium have indicated that, as of 17 May 2020, of the 9052 fatal COVID-19 cases, 51% of those were reported to be from LTCFs.10
Figure 1.
Interconnected factors that drive an increased susceptibility and impact of respiratory viral infections in the elderly. Figure was formed from images taken from The Noun Project.159
HCP, healthcare professional; LTCF, long-term-care facility.
The Noun Project, 8800 Venice Blvd., Los Angeles, CA 90034. Work is licensed under the Attribution 3.0 Unported (CC BY 3.0) licence©. Images used were downloaded from https://thenounproject.com on 8 December 2020, and from top left to bottom right were produced by: Confusion by Mohammed Rabiul Alam, BD; falling by Andrew Doane; heart attack by Gan Khoon Lay; heart disease by artworkbean, ID; liver and kidney problem by Gan Khoon Lay; cancer by LAFS, RU; Blood Pressure Cuff by Shiva, IN; Diabetes by Daniel Grohotolski, DE; macrophage by Léa Lortal; natural killer cell by Léa Lortal; neutrophil by Léa Lortal; grandmother by Marie Van den Broeck, BE; Nurse by Llisole; Food by Guilherme Furtado, BR; Vaccine by parkjisun; medicine by UNiCORN; medicine by alvianwijaya, ID; lungs by Saeful Muslim, ID health by StringLabs, ID; home care by Bestdesignmarket, IN; Hospital Bed by Linseed Studio, US; Pneumonia by Gan Khoon Lay; cost by monkik; Hospital by Nociconist, ID; virus transmission by mim studio, ID; Money by Icon Lauk, ID; Hospital Bed by LAFS, RU.
However, the burden of COVID-19 in LTCF is likely underestimated due to the lack of diagnostic testing strategies and capacity for screening.10,11 Particular challenges in diagnosing COVID-19 have been met with a high prevalence of asymptomatic SARS-CoV-2 positive diagnostic results. Of residents and staff in a selection of Belgium LTCFs, 73% (5695/7751) of PCR-positive cases were asymptomatic.12 Implementation of local and national monitoring systems for COVID-19 and other respiratory viral diseases is thus now important to identify infected patients, monitor the disease and fully understand the burden of COVID-19 in LTCFs. Identification of infected patients is also ultimately key in treatment and the prevention of SARS-CoV-2 transmission in these vulnerable patients.
Influenza
Each year, influenza causes an estimated 1.3–2.1 million hospitalisations and associates with an average of 51,203 deaths in the US alone.5,13 The elderly are exposed to influenza A and B throughout their life. However, due to the vast antigenic drift and shift they do not develop immunological immunity against circulating seasonal strains. Due to their aging immune system and co-morbidities, the elderly are particularly vulnerable, with 90% of influenza-related deaths occurring in this population.14 Influenza infection can cause significant functional decline in older adults, and 67% and 25% have been reported to become at least temporarily housebound and bedbound, respectively.15 The presence of common underlying comorbidities in this population, including diabetes and respiratory and cardiovascular diseases, further increases their risk of morbidity and mortality due to influenza (Figure 1).
Influenza attack rates in LTCFs have been reported to range widely between 4% and 94%, with mortality rates during outbreaks reaching nearly 55%.16 Influenza-related pneumonia and cardiovascular complications occur substantially more frequently in the elderly. Furthermore, influenza infection can present as a relatively mild respiratory illness in the elderly but set off a sequence of catastrophic events that may be difficult for the clinician to link back to influenza itself.17 Elderly patients may present atypically without fever and simply with a cough, fatigue and confusion, thus making the true burden of influenza hard to estimate.18
Contrasting with seasonal influenza, the majority of symptomatic infections during pandemic influenza occur amongst young adults. This has been hypothesised to be due to prior exposure to the pandemic strain in older adults with persistent immunological memory.19,20 However, in the 2009 H1N1 pandemic, although fewer elderly adults developed clinical influenza, those who did were at high risk of mortality; the Californian >50-year-old population had the highest mortality rate of all age groups of between 18% and 20%.21 Thus, pandemic influenza is also a significant burden in the elderly.
RSV
RSV has been estimated to cause 177,000 adult hospitalisations each year, compared with 294,000 due to influenza.13,22 However, healthy adults with a functioning mature immune system generally develop effective anti-RSV immunity.23 RSV causes significant morbidity in the immunocompromised, babies and the elderly, and is associated with 17,358 deaths in the >65-year-olds in the US each year, which increases with advancing age.5,24 However, geriatric physicians have little incentive to diagnose RSV infections in the elderly due to a lack of available sensitive diagnostic tests and treatments.
Prospective studies have been key in starting to understand the burden of RSV, which first became apparent in LTCFs where RSV has potential to propagate.22 Annual infection rates of 5–10% have been reported in LTCFs, with rates of pneumonia and mortality of 10–20% and 2–5%, respectively.25 However, high variability in incidence between institutions has made it hard to estimate the burden of RSV.26
Looking in the wider community, annual rates of hospitalisation due to RSV in healthy >50 year olds have been reported at 15.01 per 10,000 county residents.27 Furthermore, RSV has been reported to account for 5–15% of community acquired pneumonia and 9–10% of hospital admissions for acute cardiorespiratory diseases.28 A study by Falsey et al. found that 7.4% of elderly patients with influenza-like illness (ILI) were positive for RSV, rising to 8.7% of >75-year-olds and 12.5% of elderly patients requiring hospitalisation.25 Thus, the true impact of RSV is likely underestimated.
HMPV
By the age of 25, nearly all adults have been infected with HMPV. However, infection rarely causes morbidity in young adults. HMPV reinfection does, however, account for a significant burden of morbidity in elderly adults, particularly in institutionalised elderly adults with associated frailty, immunosuppression or chronic cardiopulmonary disease.29,30 HMPV can cause influenza-like symptoms and lower respiratory symptoms such as wheezing and dyspnea.31 However, 40% of HMPV-infected elderly patients reportedly develop pneumonitis,32 and 2–4% of adults admitted for pneumonia are reportedly infected with HMPV.33 One prospective study found an annual hospitalisation rate (in all ⩾50-year-olds) due to HMPV infection of 9.82 per 10,000 residents; this compared with 11.81 for influenza.27 However, further studies are needed to understand the true burden of illness due to HMPV.34
HRV
There are a vast number (>160) of co-circulating HRV serotypes. Therefore, despite previous HRV exposure, older adults are still susceptible to infection. Compared with influenza and RSV, HRV is usually regarded as a more benign pathogen that has comparatively low virulence and may cause asymptomatic infection or mild disease, including common cold symptoms, rhinorrhea, cough and sore throat.35 However, mild infection is less common in older adults, and HRV outbreaks can lead to significant morbidity and mortality.36–38 HRV causes particular burden in elderly patients through exacerbations in those with chronic obstructive pulmonary disease (COPD).39–41 HRV has been detected in 28% of >60-year-olds with upper respiratory tract infection symptoms; 26% of these were unable to perform routine household activities and nearly 20% were confined to bed. HRV has also been found in 9% of patients with radiographic evidence of pneumonia.42 One study looking at hospitalised pneumonia patients surprisingly found more pneumonia complications, requirement for oxygen therapy and a higher mortality rate in HRV-infected patients than in those with influenza.43 It is now important to fully understand the burden of all strains of HRV in the elderly, including emerging strains of HRV-C and D.29
Parainfluenza and other respiratory viruses
HPIV is divided genetically and antigenically into four types, with HPIV-1 to HPIV-3 being major causes of lower respiratory infections in the elderly.44 Unlike most seasonal respiratory pathogens, which peak in the winter period, HPIV can infect throughout the year and cause epidemics amongst institutionalised elderly individuals.45 One serious HPIV3 outbreak in a LTCF with 49 elderly residents had an attack rate of 50%, with 11 radiographic pneumonias and four deaths.46 Although there are reports of HPIV epidemics in LTCFs, the epidemiological evidence of its impact on the wider elderly community is limited. A study using multiplex PCR in patients with ILI detected HPIV infection in 5.8 % of adults, making it the third most prevalent virus detected.47 However, HPIV is often not screened for in patients with acute respiratory infections and may not be reported, resulting in its true impact being underestimated.45
A study looking at respiratory viral infections over 3 years demonstrated that, rather than a single predominating pathogen, a wide range of respiratory viruses co-circulated in LTCFs.48 Further comprehensive prospective studies throughout the year in different settings using sensitive multiplex PCR for all respiratory viruses would be beneficial to try to understand the epidemiology of emerging viruses, including rotavirus, adenovirus, new coronaviruses, human bocavirus and Ki and Wu polyomaviruses.49 This will help to understand the epidemiology and etiological importance of each of these respiratory viruses, both individually and in the context of multiple infections. However, consideration is required about the impact of PCR confirmation of respiratory viral infection on antibiotics use by clinicians, particularly with bacterial and viral coinfection.
Risk factors for increased morbidity and mortality in the elderly
Atypical presentation of respiratory viral infections
Elderly patients have a high rate of complex multimorbidities and symptoms of pre-existing diseases and are more susceptible to respiratory viral infections than healthy young adults due to a multitude of factors (Figure 1). Thus, elderly individuals with a respiratory viral infection may present with respiratory distress or pneumonia. However, elderly patients may also present atypically without fever or respiratory symptoms. Often, elderly adults instead experience an exacerbation of chronic diseases, including heart failure, chronic kidney disease, COPD or diabetes.50,51 C-Reactive protein (CRP) and procalcitonin levels are useful biomarkers of inflammation and respiratory infections. However, difficulties have been found in diagnosing pneumonia or respiratory viral infections in the elderly.52–55
Reports so far have described an increased likelihood of older people with COVID-19 to have an atypical presentation.56 A report from France detailed presentation of the elderly with delirium, postural instability or diarrhoea as opposed to the typical presentation in younger patients.57 This atypical presentation may delay a COVID-19 diagnosis and increase the opportunity for SARS-CoV-2 to be transmitted, thus highlighting the importance of SARS-CoV-2 screening in elderly patients. Computed tomography has been shown to be a useful tool in diagnosing COVID-19 and other respiratory diseases in elderly patients.58–63 However, difficulties have been found in differentiation between viral and bacterial pneumonia.63,64
Elderly patients also present atypically with other respiratory viruses. A recent study by Datta et al. found that only 30% and 7% of hospitalised patients with influenza or RSV infection, respectively, had viral infection listed as their primary diagnosis. Instead, primary diagnoses were frequently found to be dehydration, altered mental status, falls or exacerbations of chronic diseases.65 Patients also presented with confusion, anorexia or a reduction in communication with the environment. This picture is further clouded by patients with obstructive lung diseases presenting with symptoms of respiratory viral infection despite not being infected.66
Difficulty in diagnosing respiratory viral infections in the elderly has implications for patient health as well as the swift introduction of appropriate control measures to contain respiratory viral outbreaks. A study by Gravenstein et al. highlighted that influenza-related morbidity has been greatly underestimated, particularly in frail older adults.67 Thus, the reported morbidity and mortality for respiratory viruses may represent just the tip of the iceberg.68 Although rapid point-of-care multiplex PCR systems for non-SARS-CoV-2 viruses are being piloting in some hospitals, such screening is usually reserved for research, clinical trials and monitoring of epidemics. The importance of accurate rapid diagnostic testing in the hospital setting is now becoming understood. However, due to the frequent atypical presentation of the elderly, accurate rapid diagnostic screening for all respiratory viruses should be routine for both symptomatic and asymptomatic elderly patients.
Altered immune responses to viral infections
The aging immune system is becoming increasingly recognised as an important factor in driving the susceptibility of the elderly population to respiratory viral infections, with the immune system being further dysregulated in patients with underlying respiratory diseases like COPD (Figure 1).69–73 Immunosenescence and inflammaging are becoming increasingly understood to be driving the immune system becoming imbalanced during aging. Immunosenescence is a multifactorial aging process characterised by the gradual dysregulation and deterioration of the immune system. This impacts its capacity to function and develop long-term immune memory against respiratory viral infections.74 The aging immune system has been reported to have reduced B cell numbers and a dysregulated T cell compartment with thymic involution, reduced naive T cells and a higher proportion of terminally differentiated CD8 T cells in the lung.75 T cells have been shown to have impaired function and express exhaustion markers and lower levels of co-stimulatory molecules.71 A diminished humoral response in the elderly has been linked to T cell function, and waning neutralising antibodies have been found both after RSV infection and months following influenza vaccination.76–78
Inflammaging is a further interwoven process where the aging immune system develops elevated inflammatory mediators. This could potentially drive numerous adverse changes and disease development, including malignancies and autoimmune diseases. Furthermore, these may indirectly increase the susceptibility of the elderly to respiratory viral infections.79 Inflammaging is thought to influence severity of respiratory viral infections and elevated interleukin-6 (IL-6) levels have been correlated with RSV infection severity.80,81 This could also play a role in the predisposition of elderly patients to viral-induced cytokine storm, immunopathology and severe COVID-19 disease.82 New reports also highlight the potential impact of chronic antigen stimulation and persistent cytomegalovirus (CMV) reactivation in driving the aging immune system.83 Further work is now required to fully delineate immunosenescence and inflammaging mechanisms and how they drive susceptibility to respiratory viral infections in the elderly. This could help identify biomarkers for the most vulnerable elderly as well as immunological targets to bolster the elderly anti-viral immune system using novel therapeutics and vaccination approaches.
Multimorbidities
Comorbidities have been found to be a key risk factor for development of severe COVID-19 disease. Docherty et al. demonstrated that only 22.5% of 20,133 UK patients hospitalised with COVID-19 had no comorbidity, and that 30.9% had chronic cardiac disease, 20.7% had diabetes, 17.7% had chronic pulmonary disease excluding asthma, 14.5% had asthma and 16.2% had chronic kidney disease.84 Thus, the high prevalence of these chronic diseases in the elderly likely plays a key role in increased COVID-19 susceptibility. The risk of heart disease on viral susceptibility is well known. The impact of viral infections on exacerbation of atrial fibrillation and heart failure has also been widely reported, as well as the increasing risk of heart attacks and stroke.85–87
The most vulnerable elderly adults are also reportedly 60 times more at risk of hospitalisation due to influenza infection compared with healthy adults aged 65–75.88 Key risk factors include very advanced age, prior hospitalisation admission for influenza infection and chronic health conditions; particularly those requiring monthly medical follow ups. Institutionalisation, immunosuppression and chronic cardiopulmonary illness are also known risk factors for severe illness due to HMPV (Figure 1).89 Thus, with an aging population and increased prevalence of chronic diseases, multi-morbidities may be an important indirect driver for morbidity and mortality due to respiratory viral infections.90 Frailty is characterised by multisystem decline, decreased physiologic reserve, impaired homeostasis, dependency and premature mortality. Frailty has been reported to have a prevalence of 7% in the community dwelling elderly and to be associated with an increased risk of influenza infection through an impaired post-influenza vaccine response.91,92 Infection of these vulnerable patients can thus cause significant functional impairment that can persist for months without returning to baseline or mortality. Actively screening frail patients with exacerbations of chronic disease may be important in diagnosing and treating respiratory viral infections in this vulnerable population.
Decreased mobility and changes to lung physiology, structure and function through aging may also play important roles in impacting the respiratory pump and host responses to a respiratory viral infection. Similarly, decreased functional reserve could reduce their ability to cope with increased airway resistance and decreased lung compliance as a consequence of lower-respiratory-tract infection.93 Malnutrition may increase the susceptibility of elderly individuals to respiratory viral infections.94 Undernutrition in hospitalised patients and risk of nosocomial infections have also been reported to be interrelated.95 Although we understand a lot about the virology of established respiratory viruses, there is still some way to go to fully understand the interplay between different factors that drive elderly susceptibility, as well as their impact on vaccine responses, including for SARS-CoV-2 (Figure 1).
Polypharmacy
The number of elderly patients with multimorbidities is increasing and more patients are being prescribed multiple drugs. A large European study in 2707 elderly home care patients (mean age of 82 years) found that polypharmacy (defined as use of at least six medications) was identified in 51% of participants.96 Upon being admitted to hospital with a respiratory viral infection, these medications may be stopped or altered, potentially impacting the patient’s underlying conditions. Furthermore, morbidity due to viral infection may be accentuated by prescribed medications, including diuretics and nephrotoxic drugs, which could lead to renal failure. Immunosuppressive therapies, including steroids, cancer therapies and drugs for chronic inflammatory diseases have an array of side effects which can exacerbate chronic diseases and may also directly increase viral susceptibility as well as impacting vaccination responses.97,98
Polypharmacy can lead to inappropriate medications being prescribed, drug–drug interactions, an increased risk of nonadherence and adverse drug reactions (ADRs) and is a particular problem in the elderly due to their altered metabolism, reduced drug clearance and therefore greater risk for ADRs.99 ADRs can be multiple and non-specific, particularly in the elderly, and thus may be misinterpreted as either aging or underlying disease, resulting in further treatments, leading to a ‘prescribing cascade’.100 Elderly patients are also more likely to be prescribed antibiotics upon presenting with a respiratory viral infection, leading to the potential of ADRs, drug interactions and complications of underlying diseases.101 With the development of novel anti-viral and immunomodulatory therapeutics, the impact of polypharmacy needs further consideration.
Prevention and treatment of respiratory viral infections
Influenza vaccination
Influenza vaccination has been recommended as the primary prevention method for influenza infection in >65-year-olds since the 1960s,102–105 and not only prevents influenza infection but plays a key role in prevention of secondary events and complications, including cardiovascular morbidity and mortality.106,107 Vaccination of healthcare professionals (HCPs) may also be important in protecting elderly patients from viral infections and complications and has been a consideration during the COVID-19 pandemic. However, the true impact on both influenza and SARS-CoV-2-related infection is yet to be demonstrated conclusively.108
Vaccination has been hugely successful in reducing morbidity and mortality associated with influenza infection in the general population. However, there is limited data about comparative vaccine effectiveness in >65-year-olds (Table 1).109 Serological studies have found lower and waning humoral responses in older adults. Therefore, clinical protection is not likely to persist throughout the whole year, highlighting the requirement for more immunogenic vaccines.110 A new high-dose trivalent inactivated influenza vaccine that contains four times the amount of haemagglutinin has been produced and has been licenced for use in the US since 2009. This has been shown to produce higher antibody responses and be more effective in adults >65 years in preventing overall mortality. However, in a recent meta-analysis, protection with the high-dose trivalent vaccine was found to be only 22.2% [95% confidence interval (CI): –18.2 to 48.8%] against post-influenza mortality.111 A quadrivalent flu vaccine has also been licenced in the US since 2013; this encompasses an additional B-like virus strain and has been shown to provide similar immunogenicity induced by the strains in the trivalent vaccine.112,113 Current influenza vaccines need to be administered annually to cover the circulating strains. Further work for effective vaccines in the elderly is essential in both influenza and other respiratory viruses including RSV and SARS-CoV-2.
Table 1.
Efficacy of influenza vaccinations in the elderly.
| Study (vaccine) | Population (number of participants) | Flu season(s) | Outcome/Comparison | Result | Reference | |
|---|---|---|---|---|---|---|
| 1 | Seasonal flu vaccine | >65 years (3402) 18–50 years: (1131) |
2011–2016 | Vaccine effectiveness >65 years versus 18–49 years |
H3N2: 14% versus 21% H1N1: 49% versus 48% Influenza B: 62% 55% |
Russell et al.114 |
| 2 | Seasonal flu vaccine | >65 years: (4643) 18–58 years: 1151 |
1986–2002 | Vaccine effectiveness >65 years versus 18–58 years |
H3N2: 74% versus 84% H1N1 69% versus 83% Influenza B: 78% versus 67% |
Goodwin et al.115 |
| 3 | QIV versus TIV | >61 years: (989) 18–60 years: (991) |
2015–2016 | Seroconversion rates >61 versus 18–60 years |
⩾73.3% versus ⩾91.6%a | van de Witte et al.113 |
| 4 | TIV-HD versus TIV | >65 years TIV-HD: (6117) >65 years TIV: (3055) |
2009–2010 | Relative efficacy >65 years TIV-HD versus >65 years TIV |
12.6% | DiazGranados et al.116 |
| 5 | TIV-HD versus
TIV |
>65 years TIV-HD: (15,991) >65 years TIV: (15,998) |
2011–2012 | Relative efficacy >65 years TIV-HD versus >65 years TIV |
24.2% | DiazGranados et al.117 |
| 6 | ATIV versus TIV | >65 years ATIV: (165) >65 years TIV: (62) |
2011–2012 | Vaccine effectiveness >65 years ATIV versus >65 TIV |
58% (p < 0.04) versus –7% (0.97) | Van Buynder et al.118 |
Authors reported a non-inferior immunogenicity of QIV versus TIV to the matched influenza strains.
ATIV, adjuvanted trivalent inactivated vaccine; HD, high dose; TIV, trivalent inactivated vaccine; QIV, quadrivalent inactivated vaccine.
Other vaccines and novel vaccination approaches
Vaccination is essential for the prevention of respiratory viral infection and provides not only protection to the individual but herd immunity to others; this is particularly important in elderly patients either in hospital or a LTCF. COVID-19 vaccines are currently under development and promising results have been demonstrated in providing 94% protection in the over-65-year-olds.119,120 However, widespread availability is still some time away and the ability to induce lasting protection in elderly patients is still to be demonstrated.
Although vaccinations exist for influenza there are still no vaccines available for other respiratory viruses such as RSV.121 However, substantial progress has been made in understanding the burden and immunobiology of RSV, and there are now over 60 vaccine candidates currently in preclinical and clinical trials using various platforms.122 Vaccines for HRV have been problematic due to the limited antigenic cross-reactivity between the >160 serotypes of HRV, and it seems unlikely that protection against all serotypes of HRV will be achieved with a single immunogen. New pre-clinical studies have, however, used peptide immunogens successfully to generate cross-reactive antibodies. Furthermore, T cell inducing strategies are also now being explored to generate a broadly cross-reactive vaccine.123 New innovations are under development for the advent of vaccines for HRV and other respiratory viruses such as HMPV. Supplementing these with novel vaccines against key bacterial pathogens that exacerbate lung diseases will be key in caring for the vulnerable older population.124
Early strategies to improve the immune response in elderly individuals following vaccination included increasing the antigen dose or co-administration with an adjuvant. An influenza-adjuvant vaccine using MF59 (an oil-in-water emulsion of squalene oil) has been approved for use in the US since 2015. However, randomized controlled trials comparing the efficacy of high dose and MF59-adjuvanted influenza vaccines have not as yet been undertaken.125 New liposome-based immunostimulant-containing adjuvants may also have potential in improving vaccination efficacy. For example, ASO1 has been approved recently for use in older adults following its efficacy against herpes zoster in clinical trials, and may have broader potential for vaccination against respiratory viruses.
Various promising toll-like receptor (TLR) stimulants are also in development. A synthetic variant of LPS from Salmonella Minnesota acts as a TLR4 agonist. This has been tested in a phase I trial with a RSV F-protein vaccine candidate.126 Other TLR agonists include imiquimod, a TLR7/8 agonist that has been shown to enhance efficacy of the trivalent influenza vaccine in young adults when applied topically.127
There is an increasing understanding that the site of vaccine delivery can influence the resultant immune response and vaccine efficacy.128 Advancements in less invasive delivery systems mean delivery of vaccines in the lungs could be feasible in the future. However, with the challenges of vaccinations in the elderly, other novel approaches for vaccine development in this population are being developed. Senolytic and other immunomodulatory drugs may have potential in enhancing vaccine responses and are starting to be translated into clinical trials.129 For example the mTOR inhibitor RAD001 has been shown to enhance vaccine responses in the elderly in a phase IIa trial, potentially through altering cellular metabolism and increasing expression of interferon (IFN)-related genes.130
With the ever-increasing potential for integrating different omics datasets, systems vaccinology is now being applied widely to understand immunological responses to vaccinations in different populations. Using this approach, and incorporating immunobiography and clinical, microbiome, immunome and other omic data, there is real promise for the development of novel vaccines and adjuvants. Furthermore, there is potential to stratify elderly subpopulations for personalised vaccination approaches in the future using this approach.121,129,131,132
Antiviral therapeutics
The investigation into potential COVID-19 therapeutics is still ongoing, with a variety of novel therapeutics being trialled.133–138 However, exogenous IFN-β has potential in preventing respiratory viral infections in the elderly and early results from a randomised controlled trial demonstrate the efficacy of IFN-β in improving symptoms in hospitalised patients with COVID-19.133,139 Dexamethasone has also been shown to be effective at decreasing mortality in up to a third of hospitalised patients with severe respiratory COVID-19 complications and is now being widely used for COVID-19 patients with a requirement for oxygen.140 However, the relative efficacy of these emerging treatments in elderly patients is still to be delineated.
The mainstay of treating influenza is initiation of anti-viral medication as soon as possible.141 A recent phase III trial demonstrated that baloxavir was effective at reducing high risk influenza complications and decreasing the time to improvement of influenza symptoms.142 Clinical trials have shown efficacy of anti-viral medications when given to patients with uncomplicated influenza infections within 48 h.143 However, there have been no completed randomised, placebo-controlled trials in hospitalised patients. Given the frequency of complicated influenza infections and late diagnosis of influenza in the elderly, the efficacy of anti-viral medications in the elderly needs further evaluation. Antivirals such as Oseltamivir are also known to have various side effects and new drug interactions are coming to light, including an interaction with warfarin.143 Particular consideration should, therefore, be given prior to treatment of vulnerable elderly patients and influenza infection should be confirmed with sensitive diagnostics. Novel antiviral compounds are also being investigated for treatment of influenza;144 these include the development of monoclonal and polyclonal influenza-targeting antibodies, convalescent plasma and small-molecule polymerase inhibitors.
As with influenza infection, management of elderly patients with other acute respiratory viruses is predominantly through supportive care. This is an active process with frequent monitoring as well as hydration, as required supplemental oxygen, treatment of fever and use of bronchodilators until the patient recovers.145 Antihistamines and nonsteroidal anti-inflammatory drugs may relieve some symptoms of viral infection. However, these are often contraindicated in the oldest patients because of underlying kidney disease, risk of gastrointestinal (GI) bleeding and confusion.146 No antiviral drugs that shorten the duration of HRV illness are currently approved for clinical use.147
Although there are promising emerging candidates for the treatment of RSV infection in the elderly, there are also still no effective antiviral treatments on the market. Various novel antiviral strategies are currently being investigated and have previously been reviewed.148 These include targets to prevent fusion of RSV with epithelial cells such as antibodies targeting the RSV fusion protein (including Palivizumab), which have been licenced for prophylactic use in high-risk children. Drugs inhibiting viral replication such as nucleoside analogues (including JNJ-64041575) are also being investigated, and phase II clinical trials have demonstrated their antiviral effects and efficacy in reducing clinical symptoms. Small interfering RNAs have been trialled to downregulate viral protein production. A clinical trial in lung transplant patients used a small interfering (si)RNA (ALN-RSV01), which targets the RSV nucleocapsid protein. This showed that, although it did not show antiviral effects, it did improve the daily total symptom score.149
Immunomodulatory anti-viral agents are currently also under investigation to bolster the immune system for treatment of respiratory viruses. These include a COX-2 inhibitor (celecoxib), statins, pioglitazone and azithromycin.150 Recombinant versions of natural innate immune proteins surfactant proteins A and D may also have potential for development into antiviral therapeutics and have key roles as both broadly selective innate immune molecules and immunomodulators.151–158
Conclusion
COVID-19 has already caused >2.3 million deaths and has highlighted the impact and global burden of respiratory viral infections, particularly in the elderly. Although influenza is also a key respiratory pathogen, the importance of other respiratory viruses is starting to become apparent. Compared with young adults, respiratory viral infection in >65-year-olds is associated with increased morbidity and mortality. Elderly patients may present with severe lower respiratory tract infections, pneumonia or exacerbation of chronic multimorbidities. Further challenges are faced due to elderly patients often presenting without fever or respiratory symptoms but with atypical symptoms including weakness, confusion and falls. This leads to a failure in diagnosis, increased morbidity, prescription of additional medications and further nosocomial spread. It is now essential to introduce sensitive, rapid point-of-care diagnostics to identify all viruses and treat infected elderly patients. This will facilitate an understanding of the true burden of respiratory viruses. To protect the aging immune system, it is now key to couple this with the development of augmented vaccination strategies and novel anti-viral therapeutics.
Supplemental Material
Supplemental material, sj-pdf-1-tar-10.1177_1753466621995050 for Respiratory viral infections in the elderly by Alastair Watson and Tom M. A. Wilkinson in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-2-tar-10.1177_1753466621995050 for Respiratory viral infections in the elderly by Alastair Watson and Tom M. A. Wilkinson in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-3-tar-10.1177_1753466621995050 for Respiratory viral infections in the elderly by Alastair Watson and Tom M. A. Wilkinson in Therapeutic Advances in Respiratory Disease
Footnotes
Conflict of interest statement: AW has no conflict of interest to declare. TW has received funding for research and consultancy from a range of sources related to the subject area of the review: GlaxoSmithKline, AstraZeneca, Synairgen, Mymhealth Limited.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Alastair Watson  https://orcid.org/0000-0002-6735-2567
https://orcid.org/0000-0002-6735-2567
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Alastair Watson, Faculty of Medicine, Clinical & Experimental Sciences, University of Southampton, Southampton, UK; NIHR Southampton Biomedical Research Centre, University Hospital Southampton NHS Foundation Trust, Southampton, UK; Birmingham Medical School, University of Birmingham, Birmingham, UK.
Tom M. A. Wilkinson, Faculty of Medicine, Clinical and Experimental Sciences, Southampton University, Mailpoint 810, Level F, South Block, Southampton General Hospital, Southampton, Hampshire, SO16 6YD, UK; NIHR Southampton Biomedical Research Centre, University Hospital Southampton NHS Foundation Trust, Southampton, UK.
References
- 1. United Nations, Department of Economic and Social Affairs, Population Division. World population ageing, 1950–2050. New York, NY: UN, 2002. [Google Scholar]
- 2. World Health Organization. COVID-19 weekly epidemiological update, https://www.who.int/docs/default-source/coronaviruse/situation-reports/20201110-weekly-epi-update-13.pdf? (2020, accessed 11 November 2020).
- 3. Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ 2020; 369: m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McKinnon T, Watson A, Richards L, et al. The Volunteers in Research Programme: supporting COVID-19 research and improving medical training in parallel. Clinical Medicine (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289: 179–186. [DOI] [PubMed] [Google Scholar]
- 6. Flamaing J, Engelmann I, Joosten E, et al. Viral lower respiratory tract infection in the elderly: a prospective in-hospital study. Eur J Clin Microbiol Infect Dis 2003; 22: 720–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Centres for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19), https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/older-adults.html (accessed 18 July 2020).
- 8. European Centre for Disease Prevention and Control. Technical report: surveillance of COVID-19 at long-term care facilities in the EU/EEA, https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-long-term-care-facilities-surveillance-guidance.pdf (accessed 18 July 2020).
- 9. Office for National Statistics. Comparison of weekly death occurrences in England and Wales: up to week ending 3 July 2020, https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/causesofdeath/articles/comparisonofweeklydeathoccurrencesinenglandandwales/uptoweekending03july2020 (accessed 18 July 2020).
- 10. Sciensano. Coronavirus COVID-19, overall epidemiological situation 2020, https://covid-19.sciensano.be/fr/covid-19-situation-epidemiologique (accessed 18 July 2020).
- 11. Burke H, Freeman A, Cellura DC, et al. Inflammatory phenotyping predicts clinical outcome in COVID-19. Respiratory Research. 2020; 21: 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sciensano. Coronavirus COVID-19, overall epidemiological situation 2020, https://covid-19.sciensano.be/fr/covid-19-situation-epidemiologique (accessed 4 December 2020).
- 13. Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA 2004; 292: 1333–1340. [DOI] [PubMed] [Google Scholar]
- 14. Govaert TM, Thijs CT, Masurel N, et al. The efficacy of influenza vaccination in elderly individuals. A randomized double-blind placebo-controlled trial. JAMA 1994; 272: 1661–1665. [PubMed] [Google Scholar]
- 15. Talbot HK, Griffin MR, Chen Q, et al. Effectiveness of seasonal vaccine in preventing confirmed influenza-associated hospitalizations in community dwelling older adults. J Infect Dis 2011; 203: 500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Utsumi M, Makimoto K, Quroshi N, et al. Types of infectious outbreaks and their impact in elderly care facilities: a review of the literature. Age Ageing 2010; 39: 299–305. [DOI] [PubMed] [Google Scholar]
- 17. Ferrucci L, Guralnik JM, Pahor M, et al. Hospital diagnoses, medicare charges, and nursing home admissions in the year when older persons become severely disabled. JAMA 1997; 277: 728–734. [PubMed] [Google Scholar]
- 18. Walsh EE, Cox C, Falsey AR. Clinical features of influenza A virus infection in older hospitalized persons. J Am Geriatr Soc 2002; 50: 1498–1503. [DOI] [PubMed] [Google Scholar]
- 19. Hancock K, Veguilla V, Lu X, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med 2009; 361: 1945–1952. [DOI] [PubMed] [Google Scholar]
- 20. Staples KJ, Nicholas B, McKendry RT, et al. Viral infection of human lung macrophages increases PDL1 expression via IFNβ. PLoS One 2015; 10: e0121527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Louie JK, Acosta M, Winter K, et al. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA 2009; 302: 1896–1902. [DOI] [PubMed] [Google Scholar]
- 22. Falsey AR, Hennessey PA, Formica MA, et al. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 2005; 352: 1749–1759. [DOI] [PubMed] [Google Scholar]
- 23. Bagga B, Cehelsky JE, Vaishnaw A, et al. Effect of preexisting serum and mucosal antibody on experimental Respiratory Syncytial Virus (RSV) challenge and infection of adults. J Infect Dis 2015; 212: 1719–1725. [DOI] [PubMed] [Google Scholar]
- 24. Walker E, Ison MG. Respiratory viral infections among hospitalized adults: experience of a single tertiary healthcare hospital. Influenza Other Respir Viruses 2014; 8: 282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Falsey AR, McElhaney JE, Beran J, et al. Respiratory syncytial virus and other respiratory viral infections in older adults with moderate to severe influenza-like illness. J Infect Dis 2014; 209: 1873–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Falsey AR, Walsh EE, Betts RF. Serologic evidence of respiratory syncytial virus infection in nursing home patients. J Infect Dis 1990; 162: 568–569. [DOI] [PubMed] [Google Scholar]
- 27. Widmer K, Zhu Y, Williams JV, et al. Rates of hospitalizations for respiratory syncytial virus, human metapneumovirus, and influenza virus in older adults. J Infect Dis 2012; 206: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Walsh EE. Respiratory syncytial virus infection in adults. Semin Respir Crit Care Med 2011; 32: 423–432. [DOI] [PubMed] [Google Scholar]
- 29. Jartti L, Langen H, Söderlund-Venermo M, et al. New respiratory viruses and the elderly. Open Respir Med J 2011; 5: 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Walsh EE, Peterson DR, Falsey AR. Human metapneumovirus infections in adults: another piece of the puzzle. JAMA Intern Med 2008; 168: 2489–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Falsey AR, Erdman D, Anderson LJ, et al. Human metapneumovirus infections in young and elderly adults. J Infect Dis 2003; 187: 785–790. [DOI] [PubMed] [Google Scholar]
- 32. Boivin G, Abed Y, Pelletier G, et al. Virological features and clinical manifestations associated with human metapneumovirus: a new paramyxovirus responsible for acute respiratory-tract infections in all age groups. J Infect Dis 2002; 186: 1330–1334. [DOI] [PubMed] [Google Scholar]
- 33. Alimi Y, Lim WS, Lansbury L, et al. Systematic review of respiratory viral pathogens identified in adults with community-acquired pneumonia in Europe. J Clin Virol 2017; 95: 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schildgen V, van den Hoogen B, Fouchier R, et al. Human metapneumovirus: lessons learned over the first decade. Clin Microbiol Rev 2011; 24: 734–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Peltola V, Waris M, Osterback R, et al. Rhinovirus transmission within families with children: incidence of symptomatic and asymptomatic infections. J Infect Dis 2008; 197: 382–389. [DOI] [PubMed] [Google Scholar]
- 36. Louie JK, Yagi S, Nelson FA, et al. Rhinovirus outbreak in a long term care facility for elderly persons associated with unusually high mortality. Clin Infect Dis 2005; 41: 262–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hicks LA, Shepard CW, Britz PH, et al. Two outbreaks of severe respiratory disease in nursing homes associated with rhinovirus. J Am Geriatr Soc 2006; 54: 284–289. [DOI] [PubMed] [Google Scholar]
- 38. Longtin J, Marchand-Austin A, Winter AL, et al. Rhinovirus outbreaks in long-term care facilities, Ontario, Canada. Emerg Infect Dis 2010; 16: 1463–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ko FW, Ip M, Chan PK, et al. Viral etiology of acute exacerbations of COPD in Hong Kong. Chest 2007; 132: 900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Donaldson GC, Seemungal TAR, Bhowmik A, et al. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002; 57: 847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wilkinson TMA, Hurst JR, Perera WR, et al. Effect of interactions between lower airway bacterial and rhinoviral infection in exacerbations of COPD. Chest 2006; 129: 317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015; 373: 415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hung IF, Zhang AJ, To KK, et al. Unexpectedly higher morbidity and mortality of hospitalized elderly patients associated with rhinovirus compared with influenza virus respiratory tract infection. Int J Mol Sci 2017; 18: 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Falsey AR. Noninfluenza respiratory virus infection in long-term care facilities. Infect Control Hosp Epidemiol 1991; 12: 602–608. [DOI] [PubMed] [Google Scholar]
- 45. Markov PV, Crowcroft NS. Modelling the unidentified mortality burden from thirteen infectious pathogenic microorganisms in infants. Epidemiol Infect 2007; 135: 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Todd Faulks J, Drinka PJ, Shult P. A serious outbreak of parainfluenza type 3 on a nursing unit. J Am Geriatr Soc 2000; 48: 1216–1218. [DOI] [PubMed] [Google Scholar]
- 47. Hasman H, Pachucki CT, Unal A, et al. Aetiology of influenza-like illness in adults includes parainfluenzavirus type 4. J Med Microbiol 2009; 58: 408–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Falsey AR, Dallal GE, Formica MA, et al. Long-term care facilities: a cornucopia of viral pathogens. J Am Geriatr Soc 2008; 56: 1281–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kothe H, Bauer T, Marre R, et al. Outcome of community-acquired pneumonia: influence of age, residence status and antimicrobial treatment. Eur Respir J 2008; 32: 139–146. [DOI] [PubMed] [Google Scholar]
- 50. Bellmann-Weiler R, Weiss G. Pitfalls in the diagnosis and therapy of infections in elderly patients—a mini-review. Gerontology 2009; 55: 241–249. [DOI] [PubMed] [Google Scholar]
- 51. Monmany J, Rabella N, Margall N, et al. Unmasking influenza virus infection in patients attended to in the emergency department. Infection 2004; 32: 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gautam S, Cohen AJ, Stahl Y, et al. Severe respiratory viral infection induces procalcitonin in the absence of bacterial pneumonia. Thorax 2020; 75: 974–981. [DOI] [PubMed] [Google Scholar]
- 53. Chang CH, Tsao KC, Hu HC, et al. Procalcitonin and C-reactive protein cannot differentiate bacterial or viral infection in COPD exacerbation requiring emergency department visits. Int J Chron Obstruct Pulmon Dis 2015; 10: 767–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stucker F, Herrmann F, Graf JD, et al. Procalcitonin and infection in elderly patients. J Am Geriatr Soc 2005; 53: 1392–1395. [DOI] [PubMed] [Google Scholar]
- 55. Prendki V, Malézieux-Picard A, Azurmendi L, et al. Accuracy of C-reactive protein, procalcitonin, serum amyloid A and neopterin for low-dose CT-scan confirmed pneumonia in elderly patients: a prospective cohort study. PLoS One 2020; 15: e0239606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lithander FE, Neumann S, Tenison E, et al. COVID-19 in older people: a rapid clinical review. Age Ageing 2020; 49: 501–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. SFGG. Diarrhée, perte d’équilibre, modification du comportement, troubles sanguins sont potentiellement des signes avant-coureurs de l’infection respiratoire du Covid-19 chez la personne âgée fragile, https://sfgg.org/espace-presse/revue-de-presse/diarrhee-perte-dequilibre-modification-du-comportement-troubles-sanguins-sont-potentiellement-des-signes-avant-coureurs-de-linfection-respiratoire-du-covid-19-chez-la-person/ (2020, accessed 24 July 2020).
- 58. Ostridge K, Williams N, Kim V, et al. Distinct emphysema subtypes defined by quantitative CT analysis are associated with specific pulmonary matrix metalloproteinases. Respiratory Research. 2016; 17: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhu T, Wang Y, Zhou S, et al. A comparative study of chest computed tomography features in young and older adults with corona virus disease (COVID-19). J Thorac Imaging 2020; 35: W97–W101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ostridge K, Wilkinson TMA. Present and future utility of computed tomography scanning in the assessment and management of COPD. Eur Respir J 2016; 48: 216–228. [DOI] [PubMed] [Google Scholar]
- 61. Ostridge K, Gove K, Paas KHW, et al. Using novel computed tomography analysis to describe the contribution and distribution of emphysema and small airways disease in chronic obstructive pulmonary disease. Ann Am Thorac Soc 2019; 16: 990–997. [DOI] [PubMed] [Google Scholar]
- 62. Ostridge K, Williams N, Kim V, et al. Relationship between pulmonary matrix metalloproteinases and quantitative CT markers of small airways disease and emphysema in COPD. Thorax 2016; 71: 126–132. [DOI] [PubMed] [Google Scholar]
- 63. Koo HJ, Lim S, Choe J, et al. Radiographic and CT features of viral pneumonia. Radiographics 2018; 38: 719–739. [DOI] [PubMed] [Google Scholar]
- 64. Heath CJ, del Mar Cendra M, Watson A, et al. Co-transcriptomes of initial interactions in vitro between Streptococcus pneumoniae and human pleural mesothelial cells. PloS one. 2015; 10: e0142773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Datta S, Walsh EE, Peterson DR, et al. Can analysis of routine viral testing provide accurate estimates of respiratory syncytial virus disease burden in adults? J Infect Dis 2017; 215: 1706–1710. [DOI] [PubMed] [Google Scholar]
- 66. Neuzil KM, O’Connor TZ, Gorse GJ, et al. Recognizing influenza in older patients with chronic obstructive pulmonary disease who have received influenza vaccine. Clin Infect Dis 2003; 36: 169–174. [DOI] [PubMed] [Google Scholar]
- 67. Gravenstein S, Davidson HE, Taljaard M, et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccination on numbers of US nursing home residents admitted to hospital: a cluster-randomised trial. Lancet Respir Med 2017; 5: 738–746. [DOI] [PubMed] [Google Scholar]
- 68. Falsey AR, Murata Y, Walsh EE. Impact of rapid diagnosis on management of adults hospitalized with influenza. Arch Intern Med 2007; 167: 354–360. [DOI] [PubMed] [Google Scholar]
- 69. Wilkinson TMA. Immune checkpoints in chronic obstructive pulmonary disease. Eur Respir Rev 2017; 26: 170045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Staples KJ, Taylor S, Thomas S, et al. Relationships between Mucosal Antibodies, Non-Typeable Haemophilus influenzae (NTHi) Infection and Airway Inflammation in COPD. PLOS ONE. 2016; 11: e0167250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. McKendry RT, Spalluto CM, Burke H, et al. Dysregulation of antiviral function of CD8+ T cells in the chronic obstructive pulmonary disease lung. Role of the PD-1–PD-L1 axis. Am J Respir Crit Care Med 2016; 193: 642–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wallington JC, Williams AP, Staples KJ, et al. IL-12 and IL-7 synergize to control mucosal-associated invariant T-cell cytotoxic responses to bacterial infection. J Allergy Clin Immunol 2018; 141: 2182–2195.e6. [DOI] [PubMed] [Google Scholar]
- 73. Day K, Ostridge K, Conway J, et al. Interrelationships between small airways dysfunction, neutrophilic inflammation and exacerbation frequency in COPD. Chest. Epub ahead of print 25 November 2020. DOI: 10.1016/j.chest.2020.11.018. [DOI] [PubMed] [Google Scholar]
- 74. Sahin E, Depinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature 2010; 464: 520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fulop T, Pawelec G, Castle S, et al. Immunosenescence and vaccination in nursing home residents. Clin Infect Dis 2009; 48: 443–448. [DOI] [PubMed] [Google Scholar]
- 76. Falsey AR, Singh HK, Walsh EE. Serum antibody decay in adults following natural respiratory syncytial virus infection. J Med Virol 2006; 78: 1493–1497. [DOI] [PubMed] [Google Scholar]
- 77. Saurwein-Teissl M, Lung TL, Marx F, et al. Lack of antibody production following immunization in old age: association with CD8+CD28– T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol 2002; 168: 5893–5899. [DOI] [PubMed] [Google Scholar]
- 78. Huang K-YA, Li CK-F, Clutterbuck E, et al. Virus-specific antibody secreting cell, memory B-cell, and sero-antibody responses in the human influenza challenge model. J Infect Dis 2014; 209: 1354–1361. [DOI] [PubMed] [Google Scholar]
- 79. Franceschi C, Capri M, Monti D, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev 2007; 128: 92–105. [DOI] [PubMed] [Google Scholar]
- 80. Walsh EE, Peterson DR, Kalkanoglu AE, et al. Viral shedding and immune responses to respiratory syncytial virus infection in older adults. J Infect Dis 2013; 207: 1424–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kim VL, Coombs NA, Staples KJ, et al. Impact and associations of eosinophilic inflammation in COPD: analysis of the AERIS cohort. Eur Respir J 2017; 50: 1700853. [DOI] [PubMed] [Google Scholar]
- 82. Perrotta F, Corbi G, Mazzeo G, et al. COVID-19 and the elderly: insights into pathogenesis and clinical decision-making. Aging Clin Exp Res 2020; 32: 1599–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wang GC, Kao WH, Murakami P, et al. Cytomegalovirus infection and the risk of mortality and frailty in older women: a prospective observational cohort study. Am J Epidemiol 2010; 171: 1144–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Watson A, Öberg L, Angermann B, et al. COVID-19 Related Gene Expression in the Lung – Insights into the Susceptibility to Infection and Inflammation in COPD (Submitted to Respiratory Research) [Google Scholar]
- 85. Warren-Gash C, Blackburn R, Whitaker H, et al. Laboratory-confirmed respiratory infections as triggers for acute myocardial infarction and stroke: a self-controlled case series analysis of national linked datasets from Scotland. Eur Respir J 2018; 51: 1701794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chang TY, Chao TF, Liu CJ, et al. The association between influenza infection, vaccination, and atrial fibrillation: a nationwide case-control study. Heart Rhythm 2016; 13: 1189–1194. [DOI] [PubMed] [Google Scholar]
- 87. Singanayagam A, Singanayagam A, Elder DHJ, et al. Is community-acquired pneumonia an independent risk factor for cardiovascular disease? Eur Respir J 2012; 39: 187–196. [DOI] [PubMed] [Google Scholar]
- 88. McElhaney JE, Zhou X, Talbot HK, et al. The unmet need in the elderly: how immunosenescence, CMV infection, co-morbidities and frailty are a challenge for the development of more effective influenza vaccines. Vaccine 2012; 30: 2060–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Jartti T, Jartti L, Peltola V, et al. Identification of respiratory viruses in asymptomatic subjects: asymptomatic respiratory viral infections. Pediatr Infect Dis J 2008; 27: 1103–1107. [DOI] [PubMed] [Google Scholar]
- 90. Glezen WP, Greenberg SB, Atmar RL, et al. Impact of respiratory virus infections on persons with chronic underlying conditions. JAMA 2000; 283: 499–505. [DOI] [PubMed] [Google Scholar]
- 91. Yao X, Hamilton RG, Weng NP, et al. Frailty is associated with impairment of vaccine-induced antibody response and increase in post-vaccination influenza infection in community-dwelling older adults. Vaccine 2011; 29: 5015–5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–M156. [DOI] [PubMed] [Google Scholar]
- 93. Janssens J-P, Krause K-H. Pneumonia in the very old. Lancet Infect Dis 2004; 4: 112–124. [DOI] [PubMed] [Google Scholar]
- 94. Kagansky N, Berner Y, Koren-Morag N, et al. Poor nutritional habits are predictors of poor outcome in very old hospitalized patients. Am J Clin Nutr 2005; 82: 784–791; quiz 913–914. [DOI] [PubMed] [Google Scholar]
- 95. Paillaud E, Herbaud S, Caillet P, et al. Relations between undernutrition and nosocomial infections in elderly patients. Age Ageing 2005; 34: 619–625. [DOI] [PubMed] [Google Scholar]
- 96. Fialova D, Topinkova E, Gambassi G, et al. Potentially inappropriate medication use among elderly home care patients in Europe. JAMA 2005; 293: 1348–1358. [DOI] [PubMed] [Google Scholar]
- 97. Hinks TSC, Wallington JC, Williams AP, et al. Steroid-induced deficiency of mucosal-associated invariant T cells in the chronic obstructive pulmonary disease lung. Implications for nontypeable haemophilus influenzae infection. Am J Respir Crit Care Med 2016; 194: 1208–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Agarwal D, Schmader KE, Kossenkov AV, et al. Immune response to influenza vaccination in the elderly is altered by chronic medication use. Immun Ageing 2018; 15: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Dagli RJ, Sharma A. Polypharmacy: a global risk factor for elderly people. J Int Oral Health 2014; 6: i–ii. [PMC free article] [PubMed] [Google Scholar]
- 100. Rochon PA, Gurwitz JH. Optimising drug treatment for elderly people: the prescribing cascade. BMJ 1997; 315: 1096–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Giarratano A, Green SE, Nicolau DP. Review of antimicrobial use and considerations in the elderly population. Clin Interv Aging 2018; 13: 657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices-United States, 2018-19 influenza season. MMWR Recomm Rep 2018; 67: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sanei F, Wilkinson T. Influenza vaccination for patients with chronic obstructive pulmonary disease: understanding immunogenicity, efficacy and effectiveness. Ther Adv Respir Dis 2016; 10: 349–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Pleguezuelos O, Robinson S, Fernández A, et al. A synthetic influenza virus vaccine induces a cellular immune response that correlates with reduction in symptomatology and virus shedding in a randomized phase Ib live-virus challenge in humans. Clin Vaccine Immunol 2015; 22: 828–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Bekkat-Berkani R, Wilkinson T, Buchy P, et al. Seasonal influenza vaccination in patients with COPD: a systematic literature review. BMC Pulm Med 2017; 17: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. LeBras MH, Barry AR. Influenza vaccination for secondary prevention of cardiovascular events: a systematic review. Can J Hosp Pharm 2017; 70: 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Behrouzi B, Araujo Campoverde MV, Liang K, et al. Influenza vaccination to reduce cardiovascular morbidity and mortality in patients with COVID-19: JACC state-of-the-art review. J Am Coll Cardiol 2020; 76: 1777–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Thomas RE, Jefferson T, Lasserson TJ. Influenza vaccination for healthcare workers who care for people aged 60 or older living in long-term care institutions. Cochrane Database Syst Rev 2016; 6: CD005187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Osterholm MT, Kelley NS, Sommer A, et al. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12: 36–44. [DOI] [PubMed] [Google Scholar]
- 110. Young B, Zhao X, Cook AR, et al. Do antibody responses to the influenza vaccine persist year-round in the elderly? A systematic review and meta-analysis. Vaccine 2017; 35: 212–221. [DOI] [PubMed] [Google Scholar]
- 111. Lee JKH, Lam GKL, Shin T, et al. Efficacy and effectiveness of high-dose versus standard-dose influenza vaccination for older adults: a systematic review and meta-analysis. Expert Rev Vaccines 2018; 17: 435–443. [DOI] [PubMed] [Google Scholar]
- 112. Demicheli V, Jefferson T, Ferroni E, et al. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev 201; 2: CD001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. van de Witte S, Nauta J, Montomoli E, et al. A phase III randomised trial of the immunogenicity and safety of quadrivalent versus trivalent inactivated subunit influenza vaccine in adult and elderly subjects, assessing both anti-haemagglutinin and virus neutralisation antibody responses. Vaccine 2018; 36: 6030–6038. [DOI] [PubMed] [Google Scholar]
- 114. Russell K, Chung JR, Monto AS, et al. Influenza vaccine effectiveness in older adults compared with younger adults over five seasons. Vaccine 2018; 36: 1272–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 2006; 24: 1159–1169. [DOI] [PubMed] [Google Scholar]
- 116. DiazGranados CA, Dunning AJ, Jordanov E, et al. High-dose trivalent influenza vaccine compared to standard dose vaccine in elderly adults: safety, immunogenicity and relative efficacy during the 2009–2010 season. Vaccine 2013; 31: 861–866. [DOI] [PubMed] [Google Scholar]
- 117. DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med 2014; 371: 635–645. [DOI] [PubMed] [Google Scholar]
- 118. Van Buynder PG, Konrad S, Van Buynder JL, et al. The comparative effectiveness of adjuvanted and unadjuvanted trivalent inactivated influenza vaccine (TIV) in the elderly. Vaccine 2013; 31: 6122–6128. [DOI] [PubMed] [Google Scholar]
- 119. Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020; 396: 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. New England Journal of Medicine. 2020; 383: 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Crooke SN, Ovsyannikova IG, Poland GA, et al. Immunosenescence: a systems-level overview of immune cell biology and strategies for improving vaccine responses. Exp Gerontol 2019; 124: 110632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Noor A, Krilov LR. Respiratory syncytial virus vaccine: where are we now and what comes next? Expert Opin Biol Ther 2018; 18: 1247–1256. [DOI] [PubMed] [Google Scholar]
- 123. Glanville N, Johnston SL. Challenges in developing a cross-serotype rhinovirus vaccine. Curr Opin Virol 2015; 11: 83–88. [DOI] [PubMed] [Google Scholar]
- 124. Wilkinson TMA, Schembri S, Brightling C, et al. Non-typeable haemophilus influenzae protein vaccine in adults with COPD: a phase 2 clinical trial. Vaccine 2019; 37: 6102–6111. [DOI] [PubMed] [Google Scholar]
- 125. Whitaker JA, von Itzstein MS, Poland GA. Strategies to maximize influenza vaccine impact in older adults. Vaccine 2018; 36: 5940–5948. [DOI] [PubMed] [Google Scholar]
- 126. Falloon J, Ji F, Curtis C, et al. A phase 1a, first-in-human, randomized study of a respiratory syncytial virus F protein vaccine with and without a toll-like receptor-4 agonist and stable emulsion adjuvant. Vaccine 2016; 34: 2847–2854. [DOI] [PubMed] [Google Scholar]
- 127. Hung IF, Zhang AJ, To KK, et al. Topical imiquimod before intradermal trivalent influenza vaccine for protection against heterologous non-vaccine and antigenically drifted viruses: a single-centre, double-blind, randomised, controlled phase 2b/3 trial. Lancet Infect Dis 2016; 16: 209–218. [DOI] [PubMed] [Google Scholar]
- 128. Zhang L, Wang W, Wang S. Effect of vaccine administration modality on immunogenicity and efficacy. Expert Rev Vaccines 2015; 14: 1509–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Kirkland JL, Tchkonia T. Cellular senescence: a translational perspective. EBioMedicine 2017; 21: 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Mannick JB, Morris M, Hockey H-UP, et al. TORC1 inhibition enhances immune function and reduces infections in the elderly. Sci Transl Med 2018; 10: eaaq1564. [DOI] [PubMed] [Google Scholar]
- 131. Ciabattini A, Nardini C, Santoro F, et al. Vaccination in the elderly: the challenge of immune changes with aging. Semin Immunol 2018; 40: 83–94. [DOI] [PubMed] [Google Scholar]
- 132. Burke H, Heinson A, Freeman A, et al. Late breaking abstract - differentially expressed exosomal miRNAs target key inflammatory pathways in COPD. Eur Respir J 2018; 52: OA4922. [Google Scholar]
- 133. Wilkinson T, Dixon R, Page C, et al. ACCORD: a multicentre, seamless, phase 2 adaptive randomisation platform study to assess the efficacy and safety of multiple candidate agents for the treatment of COVID-19 in hospitalised patients: a structured summary of a study protocol for a randomised controlled trial. Trials 2020; 21: 691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Sivaloganathan AA, Nasim-Mohi M, Brown MM, et al. Noninvasive ventilation for COVID-19-associated acute hypoxaemic respiratory failure: experience from a single centre. Br J Anaesth 2020; 125: e368–e371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Burke H, Freeman A, Cellura DC, et al. Inflammatory phenotyping predicts clinical outcome in COVID-19. Respir Res 2020; 21: 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Monk PM, Marsden R, Tear VJ, et al. Safety and efficacy of inhaled SNG001 (IFN-β1a for nebulisation) for the treatment of patients with confirmed SARS-CoV-2 infection: a randomised, double-blind, placebo controlled, pilot trial. Lancet Respir Med (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. van Haren FMP, Page C, Laffey JG, et al. Nebulised heparin as a treatment for COVID-19: scientific rationale and a call for randomised evidence. Critical Care. 2020; 24: 454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Tree JA, Turnbull JE, Buttigieg KR, et al. Unfractionated heparin inhibits live wild type SARS-CoV-2 cell infectivity at therapeutically relevant concentrations. British Journal of Pharmacology. 2021; 178: 626-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Watson A, Spalluto CM, McCrae C, et al. Dynamics of IFN-β responses during respiratory viral infection: insights for therapeutic strategies. Am J Respir Crit Care Med 2020; 201: 83–94. [DOI] [PubMed] [Google Scholar]
- 140. Horby P, Lim WS, Emberson J, et al. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. medRxiv 2020.06.22.20137273. DOI: 10.1101/2020.06.22.20137273. [DOI] [Google Scholar]
- 141. Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical practice guidelines by the infectious diseases society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenzaa. Clin Infect Dis 2019; 68: e1–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Ison MG, Portsmouth S, Yoshida Y, et al. Early treatment with baloxavir marboxil in high-risk adolescent and adult outpatients with uncomplicated influenza (CAPSTONE-2): a randomised, placebo-controlled, phase 3 trial. Lancet Infect Dis 2020; 20: 1204–1214. [DOI] [PubMed] [Google Scholar]
- 143. Shah SP, Patel KM, Subedi R, et al. Review of potential drug interaction between Oseltamivir and Warfarin and why it is important for emergency medicine physicians. Am J Emerg Med 2017; 35: 1207.e3–1207.e4. [DOI] [PubMed] [Google Scholar]
- 144. Nicholas B, Staples KJ, Moese S, et al. A Novel Lung Explant Model for the Ex Vivo Study of Efficacy and Mechanisms of Anti-Influenza Drugs. The Journal of Immunology. 2015; 194: 6144–6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Kodama F, Nace DA, Jump RLP. Respiratory syncytial virus and other noninfluenza respiratory viruses in older adults. Infect Dis Clin North Am 2017; 31: 767–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Wongrakpanich S, Wongrakpanich A, Melhado K, et al. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis 2018; 9: 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Casanova V, Sousa FH, Stevens C, et al. Antiviral therapeutic approaches for human rhinovirus infections. Future Virol 2018; 13: 505–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Xing Y, Proesmans M. New therapies for acute RSV infections: where are we? Eur J Pediatr 2019; 178: 131–138. [DOI] [PubMed] [Google Scholar]
- 149. Astor TL. RNA interference, RSV, and lung transplantation: a promising future for siRNA therapeutics. Am J Respir Crit Care Med 2011; 183: 427–428. [DOI] [PubMed] [Google Scholar]
- 150. Chow EJ, Doyle JD, Uyeki TM. Influenza virus-related critical illness: prevention, diagnosis, treatment. Crit Care 2019; 23: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Watson A, Phipps MJS, Clark HW, et al. Surfactant proteins A and D: trimerized innate immunity proteins with an affinity for viral fusion proteins. J Innate Immun 2019; 11: 13–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Watson A, Sørensen GL, Holmskov U, et al. Generation of novel trimeric fragments of human SP-A and SP-D after recombinant soluble expression in E. coli. Immunobiology 2020; 225: 151953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Watson A, Kronqvist N, Spalluto CM, et al. Novel expression of a functional trimeric fragment of human SP-A with efficacy in neutralisation of RSV. Immunobiology 2017; 222: 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Watson A, Madsen J, Clark H. SP-A and SP-D: dual functioning immune molecules with antiviral and immunomodulatory properties. Front Immunol 2021; 11: 622598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Ujma S, Carse S, Chetty A, et al. Surfactant protein A impairs genital HPV16 pseudovirus infection by innate immune cell activation in a murine model. Pathogens 2019; 8: 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Littlejohn JR, da Silva RF, Neale WA, et al. Structural definition of hSP-D recognition of salmonella enterica LPS inner core oligosaccharides reveals alternative binding modes for the same LPS. PLoS One 2018; 13: e0199175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Watson A. Recombinant expression of functional trimeric fragments of human SP-A and SP-D. Doctoral Thesis, University of Southampton, England, 2016. [Google Scholar]
- 158. Fakih D, Pilecki B, Schlosser A, et al. Protective effects of surfactant protein D treatment in 1, 3-β-glucan-modulated allergic inflammation. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2015; 309: L1333–L1343. [DOI] [PubMed] [Google Scholar]
- 159. The Noun Project. https://thenounproject.com (2020, accessed 8 December 2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tar-10.1177_1753466621995050 for Respiratory viral infections in the elderly by Alastair Watson and Tom M. A. Wilkinson in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-2-tar-10.1177_1753466621995050 for Respiratory viral infections in the elderly by Alastair Watson and Tom M. A. Wilkinson in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-3-tar-10.1177_1753466621995050 for Respiratory viral infections in the elderly by Alastair Watson and Tom M. A. Wilkinson in Therapeutic Advances in Respiratory Disease