Abstract
Triggering receptors expressed on myeloid cell-1 (TREM-1) is a superimmunoglobulin receptor expressed on myeloid cells. TREM-1 amplifies the inflammatory response. Epoxyeicosatrienoic acids (EETs), the metabolites of arachidonic acid derived from the cytochrome P450 enzyme, have anti-inflammatory properties. However, the effects of EETs on TREM-1 expression under inflammatory stimulation remain unclear. Therefore, inhibition of soluble epoxide hydrolase (sEH) with a highly selective inhibitor [1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl) urea, TPPU] was used to stabilize EETs. LPS was intratracheally injected into mice to induce pulmonary inflammation, after TPPU treatment for 3 h. Histological examination showed TPPU treatment-alleviated LPS-induced pulmonary inflammation. TPPU decreased TREM-1 expression, but not DAP12 or MyD88 expression. Murine peritoneal macrophages were challenged with LPS in vitro. We found that TPPU reduced LPS-induced TREM-1 expression in a dose-dependent manner, but not DAP12 or MyD88 expression. TPPU also decreased downstream signal from TREM-1, reducing proinflammatory cytokine TNF-α and IL-1β mRNA expression. Furthermore, TPPU treatment inhibited IkB degradation in vivo and in vitro. Our results indicate that the inhibition of sEH suppresses LPS-induced TREM-1 expression and inflammation via inhibiting NF-kB activation in murine macrophage.
Keywords: triggering receptor expressed on myeloid cells-1, epoxyeicosatrienoic acids, soluble epoxide hydrolase inhibitor, macrophage, inflammation
INTRODUCTION
Inflammatory responses mediated by monocyte/macrophages and neutrophil can be stimulated through many receptors with different structures and specificities, such as Toll-like receptors and CD14 for lipopolysaccharide (LPS) [1], or cytokine receptors for tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) [2, 3]. Bouchon A and his colleagues [4] first identified a new Ig superfamily of receptors exclusively expressed on myeloid cells, which were designated triggering receptor expressed on myeloid cells (TREM). TREM-1 is 30-kDa glycoprotein receptor, which stimulates neutrophil- and monocyte-mediated inflammatory responses. Because of the short intracytoplasmic motifs without signaling motifs, TREM-1 must constitutively associate with the adaptor DNAX activation protein 12 (DAP12) for induction of intracellular signals [5]. DAP12 is a transmembrane protein with an immunoreceptor tyrosine-based activation motif (ITAM) and has been proposed to act as the signaling unit. After phosphorylation of DAP12, the production of chemokines and cytokines is induced, such as TNF-α and interleukin-1β (IL-1β) [6–8]. The overexpression and activation of TREM-1 are involved in many inflammatory diseases, including acute myocardial infarction [9], inflammatory bowel disease [10], and lung injury [11]. An increasing number of researchers consider TREM-1 as a key modulator of inflammatory disease and a therapeutic target. For example, triptolide modulates the TREM-1 signal pathway to inhibit the inflammatory response in rheumatoid arthritis [6]. Also, inhibition of TREM-1 alleviates the severity of fungal keratitis by modulating innate immune responses [12]. LPS is an important proinflammatory bacteria-derived product, which significantly increases TREM-1 expression [11, 13] in a myeloid differentiation primary response gene 88 (MyD88)-dependent manner [14].
Cytochrome P450 (CYP) enzyme converts arachidonic acid into epoxyeicosatrienoic acids (EETs), which act as autocrine or paracrine factors in the regulation of inflammation, vascular tone, and hyperalgesia [15, 16]. But, the half-life of EETs in vivo is very short, because the cytosolic enzyme soluble epoxide hydrolase (sEH) catalyzes the rapid hydrolysis of EETs to inactive or less active 1,2-diols [17]. 1-Trifluoromethoxyphenyl-3- (1-propionylpiperidin-4-yl) urea (TPPU) is a potent sEH inhibitor, which stabilizes EETs [18]. We have found that TPPU attenuates bleomycin-induced inflammation and prevents bleomycin-induced pulmonary fibrosis in a mouse model [17]. Other work demonstrated that sEH pharmacological inhibition ameliorates experimental acute pancreatitis in mice [19] and modulates inflammation and autophagy in obese adipose tissue and liver [20]. However, the effect of EETs on TREM-1 expression remains unclear.
The aim of the present study was to investigate the effects of sEH inhibition with TPPU in LPS-induced inflammation and TREM-1 expression. We found that TPPU could attenuate LPS-induced lung injury in a mouse model and inhibit the TREM-1 expression. Furthermore, we found that TPPU suppressed LPS-induced TREM-1 expression and activation in primary murine peritoneal macrophage via inhibiting the degradation of IkB.
MATERIALS AND METHODS
Animal
Adult male C57BL/6 mice were provided by the laboratory animal unit of Central South University. Mice were maintained in controlled temperature (22–24 °C), humidity (55 ± 5 %), and a 12-h dark/light cycle condition. Mice had free access to water and food. Experimental use of mice in the present study was performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The animal protocol was approved by the institutional animal care committee of Xiangya Medical College.
Animal Treatment
Adult male C57BL/6 mice were randomly divided into three groups: control group, LPS + vehicle group, and TPPU + LPS group. The mouse model of acute lung injury (ALI) was established by an intratracheal injection of O111:B4 LPS (5 mg/kg) from Escherichia coli in 50-μL sterile saline according to a previously published protocol [21]. All surgeries were performed under anesthesia with pentobarbital sodium (50 mg/kg). TPPU (1 mg/kg, in 0.5 % EtOH) was administered by intraperitoneal injection 3 h before the LPS injection. The mice were sacrificed 6 h after the LPS injection.
Histological Examination
Right lung lobes were used for histological evaluation. Lungs were fixed in 10 % formalin for 48 h, embedded in paraffin, cut into 5-μm section, and then stained with hematoxylin-eosin (HE). HE staining was done by deparaffinizing and hydrating the slides to water. Slides were stained in Harris hematoxylin for 15 min and eosin for 30 s. Slides were dehydrated, cleared, and mounted with Cytoseal.
RNA Extraction and Real-Time PCR
Total RNA of lung tissues or murine macrophage was extracted using RNAiso Plus (TaKaRa Clontech, Japan) and was quantified by spectrophotometric analysis using an ultraviolet spectrophotometer (Thermo Fisher Scientific, USA). The generation of cDNA from RNA (0.5 μg) was performed using PrimeScript RT reagent Kit with gDNA eraser (TaKaRa Clontech, Japan). Real-Time PCR of cDNA was performed using the SYBR premix Ex Taq (TaKaRa Clontech, Japan) on a deep-well Real-Time PCR Detection System (CFX96 Touch™, Bio-Rad, USA). The sequences of the specific primers were shown in supplementary Table 1. Relative gene expression was measured by the 2−ΔΔCT method. The mouse β-actin housekeeping gene was used as an internal control.
Western Blotting
The lung tissues were homogenized, separated on 10 % SDS-PAGE gel (Bio-Rad, USA), and then transferred onto a PVDF membrane. After blocking, membranes were incubated with anti-TREM-1 antibodies (R&D Systems, USA) or anti-IkB antibodies (Beyotime Biotechnology, China) overnight. Horseradish peroxidase-conjugated secondary antibodies (Santa Cruz, USA) were applied and enhanced chemiluminescence to detect protein content. The mouse β-actin was used as an internal control.
Primary Murine Peritoneal Macrophage Isolation
The C57BL/6 mice were used to isolate peritoneal macrophage. Mice were injected (i.p.) with 3 mL of 3 % thioglycolate (Sigma-Aldrich, St. Louis, MO, USA). After 3 days, peritoneal macrophages were harvested by peritoneal lavage with pre-cooled RPMI 1640. Cells were collected by centrifugation at 1500 rpm for 10 min at 4 °C, and the pellet was resuspended in cell culture medium. Cells were plated in cell culture plates at a density of 1 × 106 cells/mL and 1 × 106 cells/well. After 2 h, culture medium was changed completely for remove of non-adherent cells. Macrophages were cultured in a humified CO2 incubator at 37 °C and rested for 24 h before subsequent experiments.
Flow Cytometry
Flow cytometry was used for testing protein level of TREM-1 on the surface of macrophages. Cells were treated with 100 ng/mL LPS (from Escherichia coli O111:B4; Invivogen, San Diego, CA, USA) for 24 h. After collected, cells were incubated with anti-TREM-1 antibody (Monoclonal Rat IgG, R&D Systems, USA) and chicken anti-rat IgG-FITC antibody (Santa Cruz, USA) for 30 min at 4 °C in the dark. After washed with PBS twice, the cells percentage of positive for TREM-1 was measured using a Mofol XDP type flow cytometry instrument (Beckman Coulter, USA). The data were analyzed with Summit 5.0 or FlowJo 7.6.5 (FolwJo LLC, Ashland, OR, USA).
Statistical Analysis
Statistical analyses were completed using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). Results were expressed as mean ± standard deviation (SD). The difference between multiple groups was tested using one-way analysis of variance, and SNK test was served as the post hoc test for multiple comparisons. A P value <0.05 was considered as statistically significant.
RESULTS
TPPU Attenuated LPS-Induced Pulmonary Inflammation in Mice
HE staining of lung tissues from the control group revealed a well-alveolized pulmonary structure. LPS induced significant alveolar wall thickening and massive infiltration of inflammatory cells in the interstitial tissue. The extent and intensity of the inflammatory injury in TPPU + LPS group were less than those of the LPS group (Fig. 1).
Fig. 1.
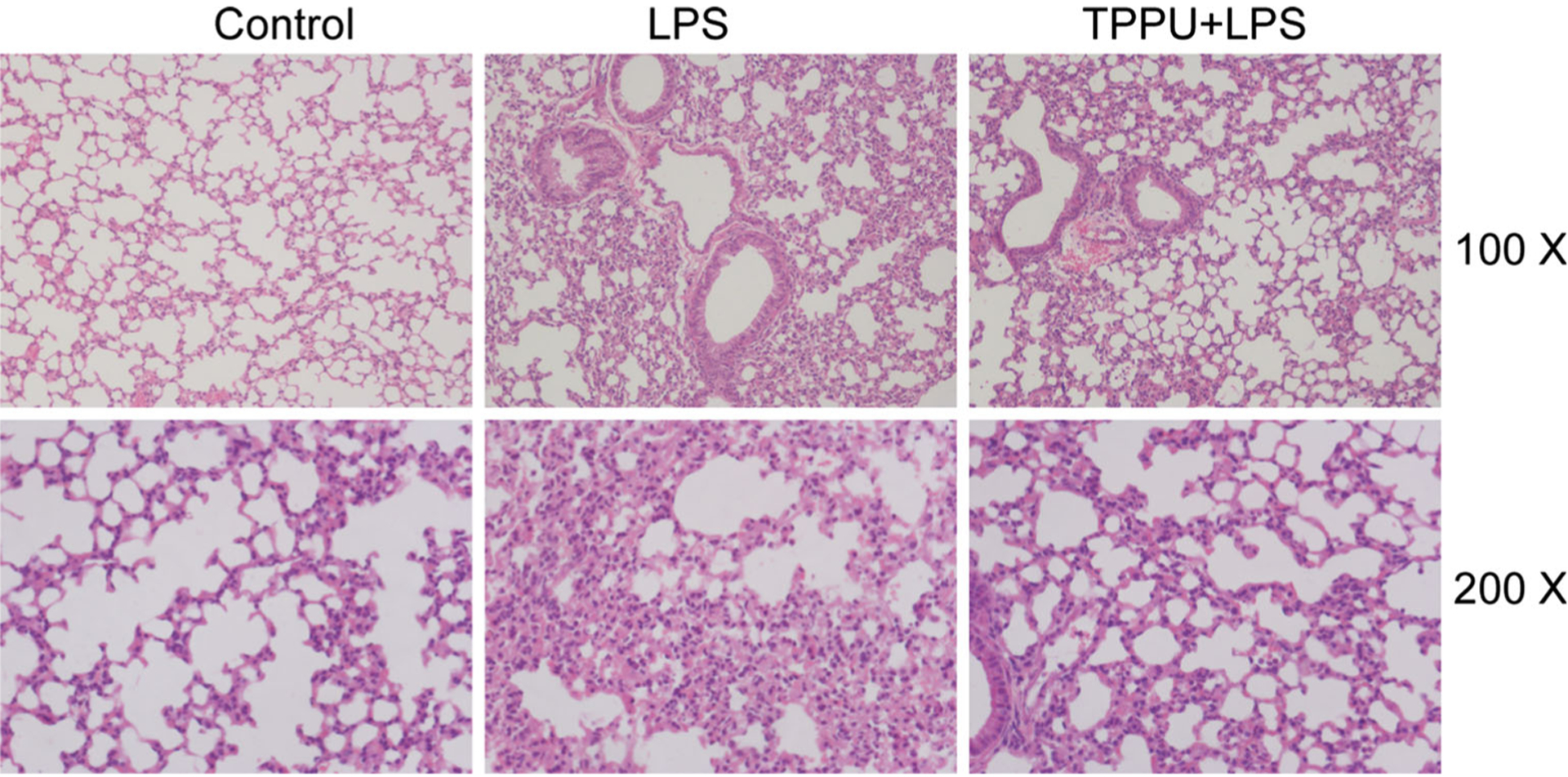
Representative histological lung sections of mice from each group stained with HE.
TPPU Inhibited TREM-1 Expression in LPS-Stimulated Lung Tissue of Mice
Since TREM-1 signaling plays a vital role in the LPS-induced pulmonary inflammation, we detected the TREM-1 expression. We found that LPS (5 mg/kg, i.t.) significantly increased TREM-1 (mRNA and protein) and MyD88 mRNA expression (P < 0.05) but decreased DAP12 mRNA expression (P < 0.05). TPPU (1 mg/kg) inhibited TREM-1 mRNA and protein expression in lung tissue (P < 0.05), but there was no effect on the expression of DAP12 and MyD88 mRNA (Fig. 2, P > 0.05). These results indicate that EETs inhibit TREM-1 signaling via suppressing its expression.
Fig. 2.
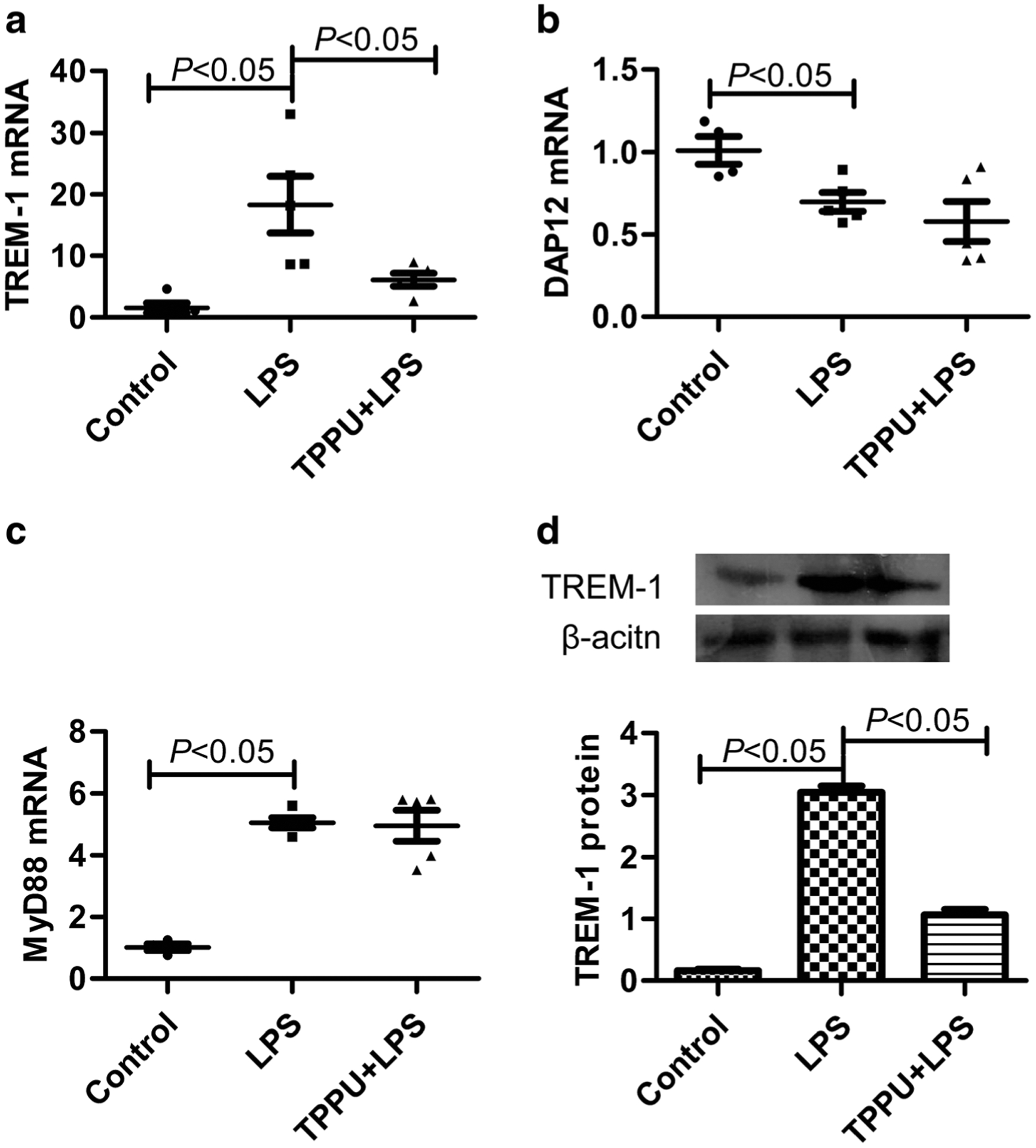
TPPU inhibited TREM-1 expression but not DAP12 or MyD88 in LPS-stimulated lung tissue of mice. a TREM-1 mRNA expression detected by real-time PCR, b DAP12 mRNA expression detected by real-time PCR, c MyD88 mRNA expression detected by real-time PCR, and d TREM-1 protein expression detected by Western blotting (n = 5).
TPPU Inhibited TREM-1 Expression in LPS-Challenged Primary Murine Peritoneal Macrophage
Macrophages are the main innate immune cell in inflammatory response. We further investigated the effect of EETs on primary murine peritoneal macrophage under inflammatory stress. We found that LPS (100 ng/mL) treatment for 6 h significantly increased TREM-1 (mRNA and protein) and MyD88 mRNA expression (P < 0.05) but decreased DAP12 mRNA expression (P < 0.05). TPPU (0.1, 1, 10 μM) could dose-dependently inhibit TREM-1 mRNA and protein expression in primary murine peritoneal macrophage (P < 0.05), but no effect on the expression of DAP12 and MyD88 mRNA (Fig. 3, P > 0.05). These data confirm that EETs inhibit TREM-1 expression under inflammatory stress.
Fig. 3.
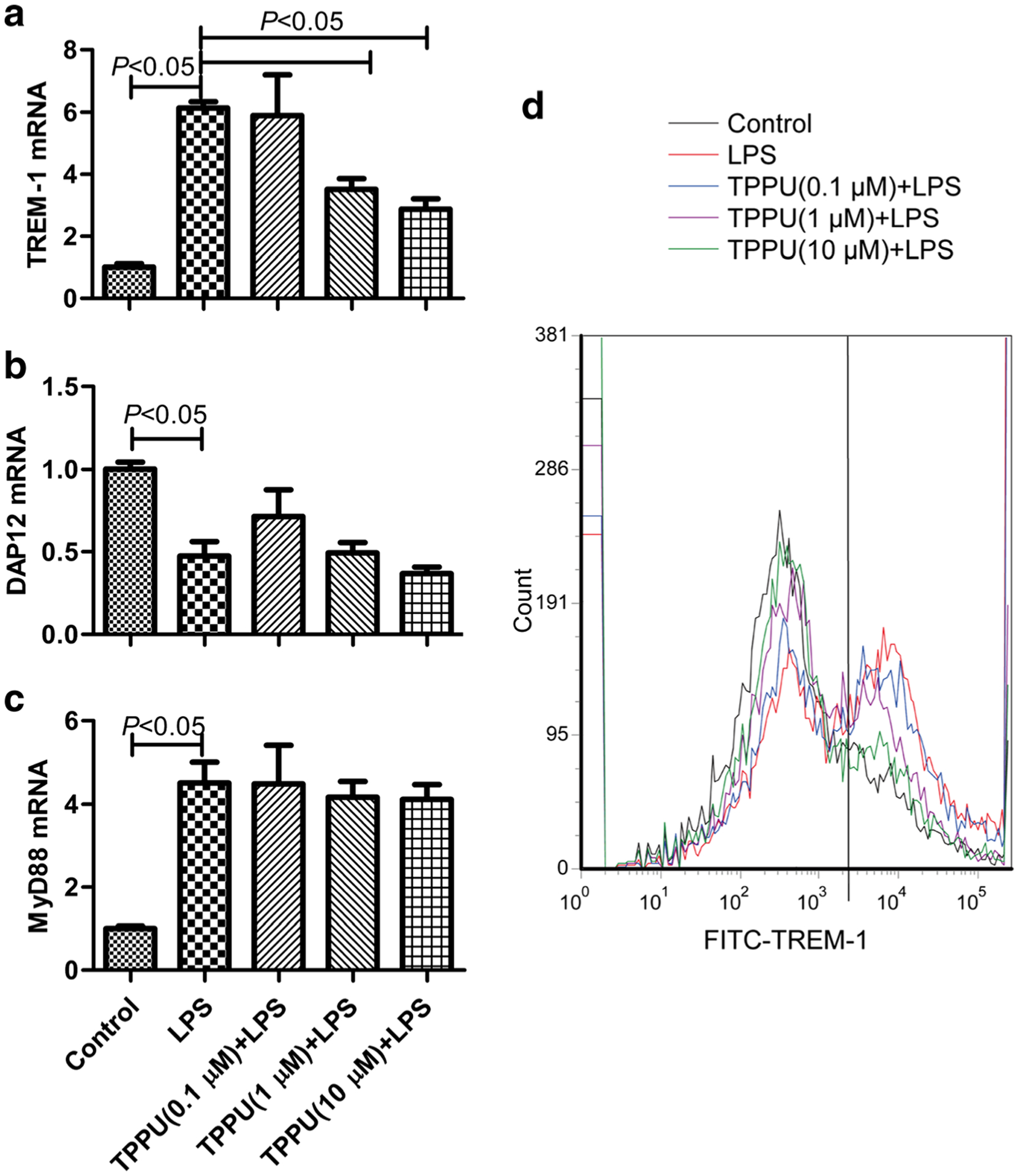
TPPU inhibited TREM-1 expression but not DAP12 or MyD88 in LPS-stimulated primary murine peritoneal macrophage. a TREM-1 mRNA expression detected by real-time PCR, b DAP12 mRNA expression detected by real-time PCR, c MyD88 mRNA expression detected by real-time PCR, and d TREM-1 protein expression detected by flow cytometry (n = 6).
TPPU Attenuated TREM-1 Downstream Proinflammatory Cytokine Expression in Macrophages
Next, we detected the effect of EETs on the activation of TREM-1. TNF-α and IL-1β are the downstream genes of TREM-1 signaling [6–8]. We found that LPS (100 ng/mL) treatment for 6 h significantly increased TNF-α and IL-1β mRNA expression in primary murine peritoneal macrophage (P < 0.05), while TPPU (0.1, 1, 10 μM) could dose-dependently inhibit TNF-α and IL-1β mRNA expression (Fig. 4, P < 0.05). These data indicate that EETs not only inhibit the expression of TREM-1 but also suppress the activation of TREM-1.
Fig. 4.
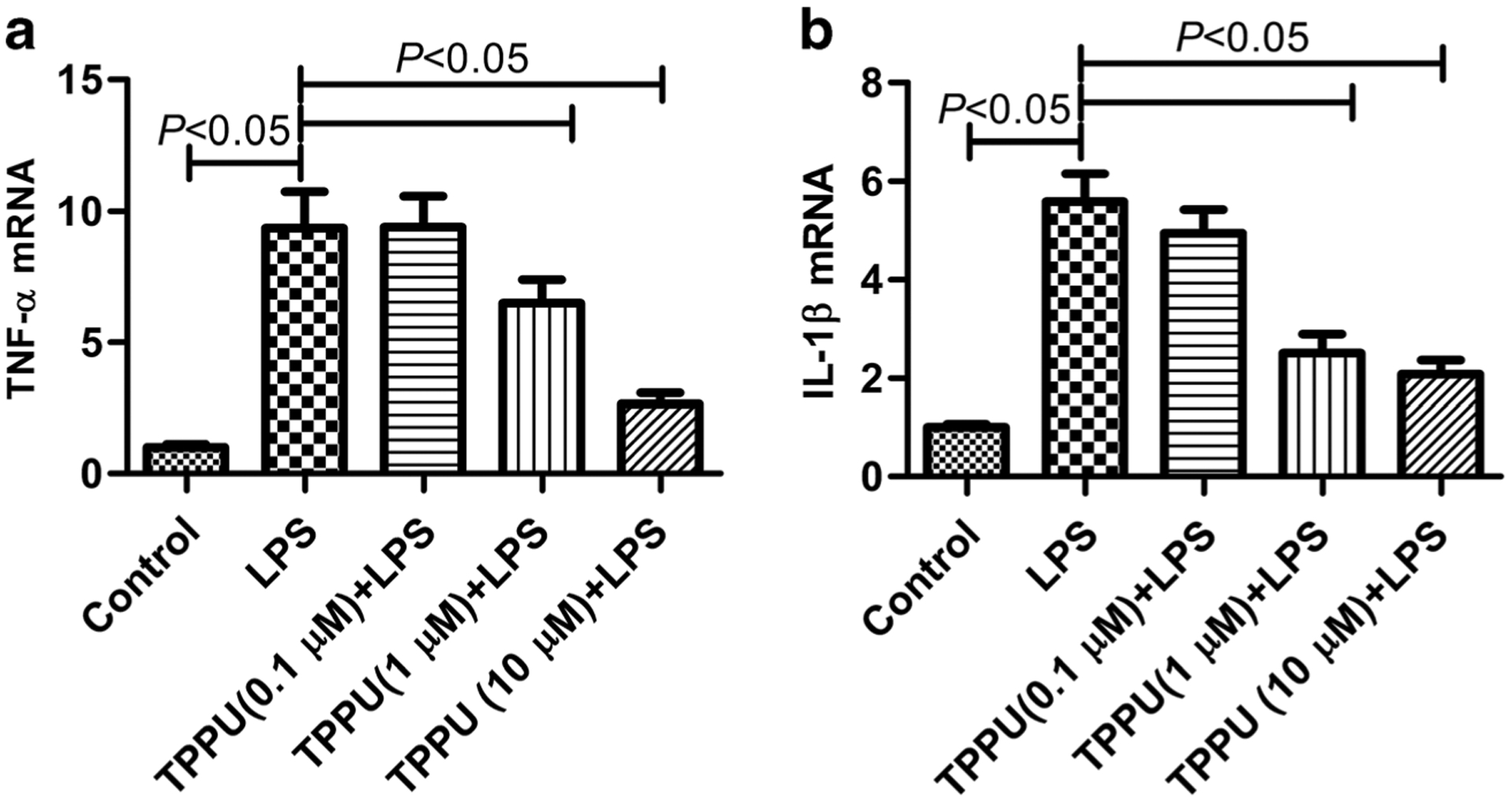
TPPU suppressed TNF-α and IL-1β mRNA expression in primary murine peritoneal macrophage. a TNF-α mRNA expression detected by real-time PCR and b IL-1β mRNA expression detected by real-time PCR (n = 6).
TPPU Inhibited Degradation of IkB Under LPS Challenge Both In vivo and In vitro
The transcription factor NF-kB plays a vital role in the signaling cascade of the LPS/TLR4 pathway, and IkB inhibits the activation of NF-kB [22]. Herein, we detected IkB to investigate the mechanism of EETs’ inhibitory effect on TREM-1. We found that LPS could promote the degradation of IkB both in lung tissue of mice and primary murine peritoneal macrophage (P < 0.05). And, TPPU treatment partly inhibited the degradation of IkB (Fig. 5, P < 0.05). These data indicate that EETs inhibit TREM-1 expression and function via suppressing NF-kB activation.
Fig. 5.
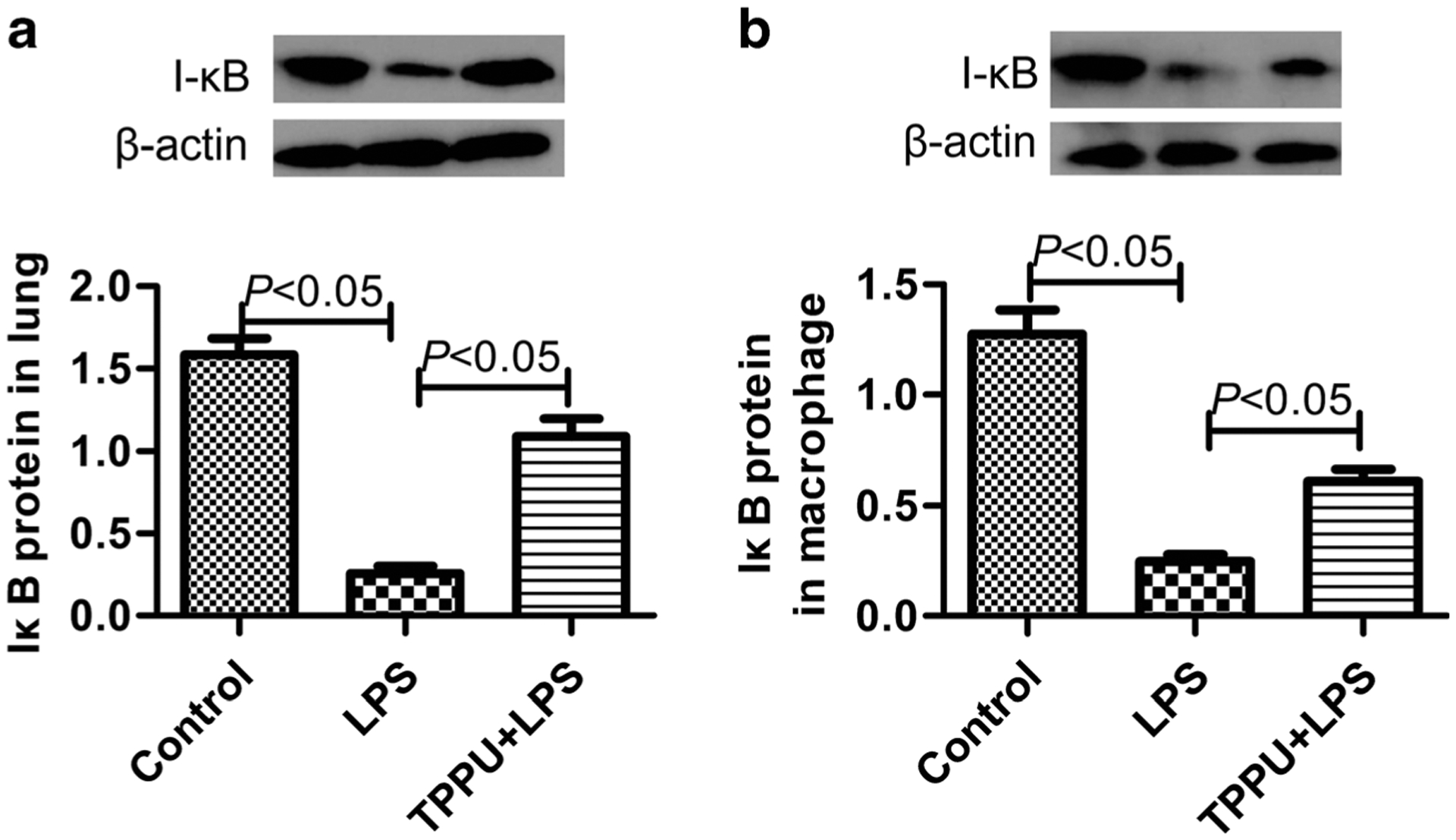
TPPU inhibited degradation of IkB under LPS challenge both in vivo and in vitro. a The IkB protein content in lung tissue of mice (n = 5) and b the IkB protein content in primary murine peritoneal macrophage (n = 6).
DISCUSSION
Inflammatory response is the hallmark of many diseases, including lung injury and acute pancreatitis. Our study firstly reports that the inhibition of sEH with pharmacological drug TPPU suppresses the TREM-1 expression and its downstream pro-inflammatory cytokines synthesis. This provides a new mechanism of the benefit of sEH inhibitors on inflammatory response and suggests a new strategy for inhibiting the TREM-1, a vital target for inflammatory response.
TREM-1 is a membrane receptor that is expressed on the surface of neutrophils and macrophage when they are triggered by pathogens, such as LPS. We have found that transforming growth factor-β1 (TGF-β1) also upregulated expression of TREM-1 in murine lungs [23]. TREM-1 mediates the acute inflammatory response to microbial products. Synergistic activation of TREM-1, toll-like receptor, and nod-like receptor results in the overexpression of pro-inflammatory factors such as TNF-α and IL-1β [24], as well as the downregulation of the anti-inflammatory factor IL-10 [25], and results in excessive inflammatory response. For example, we have reported that TREM-1 amplified the inflammatory responses and aggravated ALI [13]. In this study, we found that LPS induced a significant increase of TREM-1 expression in lung tissue and primary murine peritoneal macrophage, consistent with previous observations [26]. Since TREM-1 is a new target for anti-inflammatory therapeutic strategy, we focused on the strategy in inhibiting TREM-1 expression. We have reported that vasoactive intestinal peptide (VIP) could attenuate TREM-1 expression in LPS-challenge macrophage [13].
Arachidonic acids (AAs) are metabolized by lipoxygenases (LOXs), cyclooxygenases (COXs), and CYP to eicosanoids which are important regulators of numerous biological processes including inflammation. CYP-derived EETs play an important role in downregulating inflammation [19]. However, EETs are rapidly hydrolyzed by sEH into the less biologically active metabolites. In this study, a potent sEH inhibitor TPPU was used to stabilize EETs. We previously published that TPPU attenuated bleomycin-induced pulmonary fibrosis in a murine model [17], and TPPU also inhibited bleomycin-induced inflammation in lung tissue. The mechanism of these effects still is unclear. Here, we found that TPPU could inhibit TREM-1 expression and activation in lung and macrophage. In addition, TREM-1 is overexpressed in the lung of bleomycin-induced pulmonary fibrosis mouse [23]. So, this study could interpret the mechanism of the protective effect of TPPU on bleomycin-induced pulmonary inflammation in the lung.
sEH pharmacological inhibition impacted several signaling pathways that have been implicated previously [19]. In this study, we found that sEH inhibition was associated with decreased LPS-induced NF-kB inflammatory signaling. NF-kB is one of the main protein complexes mediating the expression of TREM-1. After stimulation of macrophage with LPS, IKK is activated and degrades IkB. Then, the p50/RelA heterodimer is increased and upregulates TREM-1 expression [5]. Inhibition of NF-kB with BAY11–7085 decreases the expression of TREM-1 in TNF-α-treated VSMCs [27].And 1,25-(OH)2D3 upregulates TREM-1 expression dependent on NF-kB signaling pathway in differentiated U937 macrophages [28].
Numerous available sEH inhibitors are potent enough to be moved into the clinic, such as TPPU, AUDA, and APAU [29]. For example, APAU was taken through phase IIA trials. The more metabolically stable and potent, TPPU may represent a better clinical candidate [30]. No adverse reactions were observed in phase I and IIA human clinical trials with sEH inhibitors, even when given at high doses [31]. The therapeutic index of sEH inhibitors appears large.
In summary, these findings establish that sEH pharmacological inhibition suppresses LPS-induced TREM-1 expression and activation, and attenuates inflammatory response in murine macrophages. The NF-kB signaling pathway may be a key target for the beneficial effect of sEH inhibitor. This study suggests that sEH inhibitor may be effective for preventing and treating inflammatory diseases.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (No. 81500065, 81670014), the Specialized Research Fund for the Doctoral Program of Higher Education of China (20130162110052), High School Innovation Fund of Hunan province (15K140), and the Open-End Fund for the Valuable and Precision Instruments of Central South University (CSUCZ201632).
Footnotes
Conflict of Interest. The authors declare no conflict of interest.
REFERENCES
- 1.Yong YK, Shankar EM, Solomon A, Spelman T, Fairley CK, Elliott J, et al. 2016. Polymorphisms in the CD14 and TLR4 genes independently predict CD4+ T-cell recovery in HIV-infected individuals on antiretroviral therapy. AIDS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aghagolzadeh P, Bachtler M, Bijarnia R, Jackson C, Smith ER, Odermatt A, et al. 2016. Calcification of vascular smooth muscle cells is induced by secondary calciprotein particles and enhanced by tumor necrosis factor-alpha. Atherosclerosis. [DOI] [PubMed] [Google Scholar]
- 3.Minutti CM, Garcia-Fojeda B, Saenz A, de Las Casas-Engel M, Guillamat-Prats R, de Lorenzo A, et al. 2016. Surfactant Protein A Prevents IFN-gamma/IFN-gamma Receptor Interaction and Attenuates Classical Activation of Human Alveolar Macrophages. J Immunol. [DOI] [PubMed] [Google Scholar]
- 4.Bouchon A, Dietrich J, and Colonna M. 2000. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. Journal of Immunology 164: 4991–4995. [DOI] [PubMed] [Google Scholar]
- 5.Arts RJ, Joosten LA, van der Meer JW, and Netea MG. 2013. TREM-1: intracellular signaling pathways and interaction with pattern recognition receptors. Journal of Leukocyte Biology 93: 209–215. [DOI] [PubMed] [Google Scholar]
- 6.Fan D, He X, Bian Y, Guo Q, Zheng K, Zhao Y, et al. 2016. Triptolide modulates TREM-1 signal pathway to inhibit the inflammatory response in rheumatoid arthritis. International Journal of Molecular Sciences 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rigo I, McMahon L, Dhawan P, Christakos S, Yim S, Ryan LK, et al. 2012. Induction of triggering receptor expressed on myeloid cells (TREM-1) in airway epithelial cells by 1,25(OH)(2) vitamin D(3). Innate Immunity 18: 250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willi M, Belibasakis GN, and Bostanci N. 2014. Expression and regulation of triggering receptor expressed on myeloid cells 1 in periodontal diseases. Clinical and Experimental Immunology 178: 190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeremie L, Amir B, Marc D, and Sebastien G. 2015. The triggering receptor expressed on myeloid cells-1: a new player during acute myocardial infarction. Pharmacological Research 100: 261–265. [DOI] [PubMed] [Google Scholar]
- 10.Genua M, Rutella S, Correale C, and Danese S. 2014. The triggering receptor expressed on myeloid cells (TREM) in inflammatory bowel disease pathogenesis. Journal of Translational Medicine 12: 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan Z, Syed M, Panchal D, Joo M, Bedi C, Lim S, et al. 2016. TREM-1-accentuated lung injury via miR-155 is inhibited by LP17 nanomedicine. American Journal of Physiology - Lung Cellular and Molecular Physiology 310: L426–L438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhong J, Huang W, Deng Q, Wu M, Jiang H, Lin X, et al. 2016. Inhibition of TREM-1 and Dectin-1 alleviates the severity of fungal keratitis by modulating innate immune responses. PLoS One 11: e0150114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun GY, Guan CX, Zhou Y, Liu YP, Li SF, Zhou HF, et al. 2011. Vasoactive intestinal peptide re-balances TREM-1/TREM-2 ratio in acute lung injury. Regulatory Peptides 167: 56–64. [DOI] [PubMed] [Google Scholar]
- 14.Zheng H, Heiderscheidt CA, Joo M, Gao X, Knezevic N, Mehta D, et al. 2010. MYD88-dependent and -independent activation of TREM-1 via specific TLR ligands. European Journal of Immunology 40: 162–171. [DOI] [PubMed] [Google Scholar]
- 15.Huang H, Weng J, and Wang MH. 2016. EETs/sEH in diabetes and obesity-induced cardiovascular diseases. Prostaglandins Other Lipid Mediat. [DOI] [PubMed] [Google Scholar]
- 16.Pillarisetti S, and Khanna I. 2015. A multimodal disease modifying approach to treat neuropathic pain–inhibition of soluble epoxide hydrolase (sEH). Drug Discovery Today 20: 1382–1390. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Y, Sun GY, Liu T, Duan JX, Zhou HF, Lee KS, et al. 2016. Soluble epoxide hydrolase inhibitor 1-trifluoromethoxyphenyl-3- (1-propionylpiperidin-4-yl) urea attenuates bleomycin-induced pulmonary fibrosis in mice. Cell and Tissue Research 363: 399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ren Q, Ma M, Ishima T, Morisseau C, Yang J, Wagner KM, et al. 2016. Gene deficiency and pharmacological inhibition of soluble epoxide hydrolase confers resilience to repeated social defeat stress. Proceedings of the National Academy of Sciences of the United States of America 113: E1944–E1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bettaieb A, Chahed S, Bachaalany S, Griffey S, Hammock BD, and Haj FG. 2015. Soluble epoxide hydrolase pharmacological inhibition ameliorates experimental acute pancreatitis in mice. Molecular Pharmacology 88: 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez-Vicario C, Alcaraz-Quiles J, Garcia-Alonso V, Rius B, Hwang SH, Titos E, et al. 2015. Inhibition of soluble epoxide hydrolase modulates inflammation and autophagy in obese adipose tissue and liver: role for omega-3 epoxides. Proceedings of the National Academy of Sciences of the United States of America 112: 536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi D, He J, Wang D, Deng W, Zhao Y, Ye Y, et al. 2014. 17beta-estradiol suppresses lipopolysaccharide-induced acute lung injury through PI3K/Akt/SGK1 mediated up-regulation of epithelial sodium channel (ENaC) in vivo and in vitro. Respiratory Research 15: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park JS, Park MY, Cho YJ, Lee JH, Yoo CG, Lee CT, et al. 2016. Anti-inflammatory effect of erdosteine in lipopolysaccharide-stimulated RAW 264.7 cells. Inflammation. [DOI] [PubMed] [Google Scholar]
- 23.Peng L, Zhou Y, Dong L, Chen RQ, Sun GY, Liu T, et al. 2016. TGF-beta1 upregulates the expression of triggering receptor expressed on myeloid cells 1 in murine lungs. Science Reports 6: 18946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tao F, Peng L, Li J, Shao Y, Deng L, and Yao H. 2013. Association of serum myeloid cells of soluble triggering receptor-1 level with myocardial dysfunction in patients with severe sepsis. Mediators of Inflammation 2013: 819246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bostanci N, Ozturk VO, Emingil G, and Belibasakis GN. 2013. Elevated oral and systemic levels of soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) in periodontitis. Journal of Dental Research 92: 161–165. [DOI] [PubMed] [Google Scholar]
- 26.Murakami Y, Kohsaka H, Kitasato H, and Akahoshi T. 2007. Lipopolysaccharide-induced up-regulation of triggering receptor expressed on myeloid cells-1 expression on macrophages is regulated by endogenous prostaglandin E2. Journal of Immunology 178: 1144–1150. [DOI] [PubMed] [Google Scholar]
- 27.Rao VH, Rai V, Stoupa S, Subramanian S, and Agrawal DK. 2016. Tumor necrosis factor-alpha regulates triggering receptor expressed on myeloid cells-1-dependent matrix metalloproteinases in the carotid plaques of symptomatic patients with carotid stenosis. Atherosclerosis 248: 160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim TH, Lee B, Kwon E, Choi SJ, Lee YH, Song GG, et al. 2013. Regulation of TREM-1 expression by 1,25-dihydroxyvitamin D3 in human monocytes/macrophages. Immunology Letters 154: 80–85. [DOI] [PubMed] [Google Scholar]
- 29.Morisseau C, and Hammock BD. 2013. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annual Review of Pharmacology and Toxicology 53: 37–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsai HJ, Hwang SH, Morisseau C, Yang J, Jones PD, Kasagami T, et al. 2010. Pharmacokinetic screening of soluble epoxide hydrolase inhibitors in dogs. European Journal of Pharmaceutical Sciences 40: 222–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen DR Whitcomb E MacIntyre V Tran Z.N. Do, Sabry J, et al. 2012. Pharmacokinetics and pharmacodynamics of AR9281, an inhibitor of soluble epoxide hydrolase, in single- and multiple-dose studies in healthy human subjects. Journal of Clinical Pharmacology 52: 319–328. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


