Abstract
The diagnosis of acute respiratory diseases in children can be challenging, and no single objective diagnostic test exists for common pediatric respiratory diseases. Previous research has demonstrated that ResAppDx, a cough sound and symptom-based analysis algorithm, can identify common respiratory diseases at the point of care. We present the study protocol for SMARTCOUGH-C 2, a prospective diagnostic accuracy trial of a cough and symptom-based algorithm in a cohort of children presenting with acute respiratory diseases. The objective of the study is to assess the performance characteristics of the ResAppDx algorithm in the diagnosis of common pediatric acute respiratory diseases.
Keywords: smartphone, cough, pneumonia, acute respiratory disease, children
Background and Rationale
Respiratory disorders—including both upper respiratory tract disease (URTD) and lower respiratory tract disease (LRTD)—are the most common reasons for pediatric emergency room visits in the United States.1 LRTDs including pneumonia, bronchiolitis, and asthma are a major cause of childhood severe illness and death both in the United States and globally.2 Pneumonia kills an estimated 800,000 children under the age of 5 years annually worldwide, with the vast majority occurring in low and middle income countries.3
The diagnosis of respiratory diseases—especially LTRDs—in the primary care or emergency department setting can be challenging, even with access to appropriate clinical, radiological and laboratory support. There is no single objective diagnostic test for common pediatric respiratory diseases; as a result, the diagnosis relies on a combination of history, physical examination, laboratory testing, imaging, and clinical judgment.4 Recognizing the limited resources available for evaluation in low and middle-income countries, the World Health Organization (WHO) has developed an algorithm to diagnose pneumonia based on clinical evaluation rather than diagnostic testing.5 A recent systematic review found that fever and tachypnea have high sensitivity (79–92%) but low specificity (47–54%) in diagnosing pneumonia, and hypoxemia has moderate sensitivity and specificity (64% and 77% respectively).6 A WHO-led analysis of 10 studies comprising 15,029 children for whom radiographic diagnoses were available found that the WHO criteria for tachypnea demonstrated a sensitivity of 92% and specificity of 22% for diagnosing radiographic pneumonia, while lower chest indrawing demonstrated a sensitivity of 74% and specificity of 15%.7
Inaccurate diagnosis of respiratory disease can lead to delayed or inappropriate treatment, with antimicrobial overuse driving antimicrobial resistance in both individual patients and in a population.8,9 These challenges have been magnified in the setting of the present COVID-19 epidemic, which has forced many providers to transition to telemedicine and highlighted the dependence of the healthcare system on diagnostic testing. Remote evaluations in telemedicine encounters rely on patient or parent-reported symptoms rather than physical examination findings and testing; as a result, providers frequently are more likely to mitigate risk by prescribing potentially unnecessary antibiotics.10
Respiratory diseases can cause pathophysiologic auscultatory breath sounds by creating turbulent air flow, airway resonance, airway collapse, or alveolar filling.11 While these sounds are traditionally obtained during tidal or deep breathing, the forced exhalation of a cough may provide additional data on respiratory diseases. Cough analytic technology using techniques similar to speech recognition may have the potential to diagnose respiratory tract diseases.12 Previous research has demonstrated that ResAppDx, a cough-based sound analysis algorithm, can distinguish pneumonia and croup from no disease.13–15 A subsequent clinical study (SMARTCOUGH-C) assessed the performance of ResAppDx in a US-based clinical setting. Because of a wide range in performance across the studied indications (positive percent agreement 47–89%, negative percent agreement 47–95%),16 improvements were made based on the findings of this initial study. These improvements included refinements to the ResAppDx algorithms, expanding the focus from pneumonia as a primary outcome to including all common acute respiratory illnesses, revised and expanded clinical case definitions (including detailed criteria for each diagnosis), and improvements in the cough recording procedures. A subsequent study in Australia (Breathe Easy) demonstrated good performance of the refined algorithm among children presenting with acute respiratory illnesses.17
We present the study protocol for SMARTCOUGH-C 2, a prospective diagnostic accuracy trial of an optimized cough- and symptom-based algorithm in a cohort of children presenting with acute respiratory diseases. The objective of the study is to assess the performance characteristics of the ResAppDx algorithm in the diagnosis of common pediatric acute respiratory diseases.
Methods
Study design overview and aims
The SMARTCOUGH-C 2 study is a multi-center prospective, double-blind study comparing the diagnosis of acute pediatric respiratory diseases using the ResAppDx algorithm to clinical diagnosis determined by a committee of independent adjudicators.
Recruitment and participants
The study enrolls children age 30 days to 12 years presenting to one of three study sites (Massachusetts General Hospital, Cleveland Clinic Foundation Hospital, and Texas Children’s Hospital) with signs or symptoms of respiratory disease. Enrollment criteria are detailed in Table 1. We selected children age 30 days to 12 years given the high prevalence of acute respiratory illnesses in this age group. Wherever possible, we use World Health Organization criteria for vital sign abnormalities (hypoxemia and tachypnea) in order to maximize generalizability of our findings.5 Because the enrollment sites are all < 1000 ft above sea level, we classify SpO2 < 90% as hypoxemia.18 Bronchodilator responsiveness is defined as improvement in aeration, reduction in wheezing, or reduced work of breathing after administration of a short-acting bronchodilator. At each of the study sites, we enroll children in multiple locations including primary care outpatient facilities, urgent care centers, emergency departments, and inpatient hospital wards within 24 hours of admission. The study protocol is approved by each participating institution’s institutional review board and is registered at ClinicalTrials.gov (NCT03392363). Written informed consent is obtained from the patient’s parent or legal guardian prior to the completion of any study procedures.
Table 1:
Enrollment Criteria
| Inclusion criteria |
Any one of the following:
AND
AND
|
| Exclusion criteria |
Absolute
Relative
|
Cough analytic technology and study procedures
After playing a brief video explaining the study procedures, a proprietary smartphone-based application is used to collect the data analyzed by the ResAppDx algorithm, including the subject’s age, sex, and patient- or parent-reported symptoms (presence of fever, wheeze, runny nose, and duration of illness). Symptom reports are elicited by direct questioning of the child and parent. The built-in smartphone microphone is used to record five spontaneous or voluntary coughs from the subject over a maximum of a one-hour period. Each cough is automatically identified using the ResAppDx proprietary cough identification algorithm. The smartphone (an Apple iPhone 6s) is held by research staff with the screen facing up and the microphone 50 cm from the subject’s mouth at 45 degrees from the path of travel of the cough airflow (Figure 1). Environmental noise is minimized as much as possible.
Figure 1:
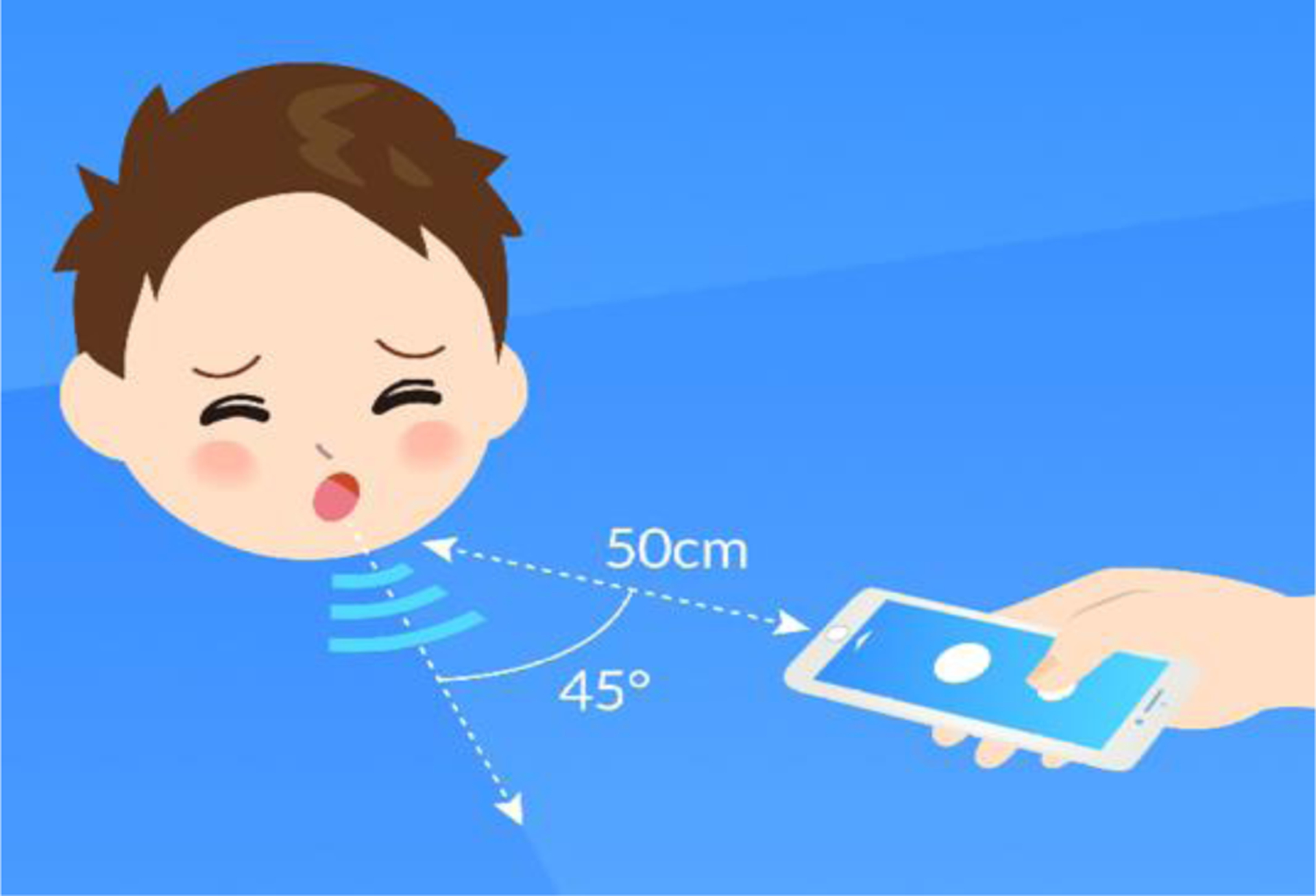
Positioning of the smartphone device relative to the subject
After five coughs are identified by ResAppDx, the audio data is saved to the smartphone with a unique Recording ID number assigned by the software. If the subject is unable to provide a minimum of five coughs recognized by the ResAppDx, the application does not progress past the audio capture screen and the subject is excluded from further analysis. All cough recordings undergo a quality review by study staff to ensure they are free from obvious background noise, coughs by other people in the room, and identifying information (i.e., the subject’s name).
In the intended commercial format, the cough sounds and the clinical symptoms will be analyzed in real time on the smartphone using the ResAppDx algorithm to indicate whether the patient has one or more of the targeted respiratory diseases. However, in the SMARTCOUGH C-2 study to ensure blinding during clinical care, data collection, and quality review, the ResAppDx algorithm is only applied to the cough recordings and symptom data by an independent statistical analysis team after enrollment is complete and the study database is locked.
Additional data is collected directly from the subject or parent, the medical record, and the treating team including inclusion/exclusion criteria, demographic data, baseline characteristics, history of present illness, past medical history, home respiratory medications, physical examination findings, laboratory testing, and imaging. No laboratory testing or imaging is performed as part of the study procedures, but all tests and imaging obtained by the clinical providers during routine care are made available to the adjudication committees. Research staff obtain and document physical exam findings (including auscultation) as reported by the clinical provider at the time of enrollment.
Adjudication procedures
The reference standard against which the ResAppDx diagnosis will be compared is provided by independent adjudication. All cases are reviewed by a Clinical Adjudication Committee, and all chest radiographs are reviewed by a Radiology Adjudication Committee. Both committees are blinded to the ResAppDx diagnosis, and radiologic adjudicators are also blinded to the clinical history.
Case definitions for common pediatric respiratory disorders have been created by the study investigators and representatives of the sponsor based on review of the literature and society guidelines (Table 2). The endpoints are considered independently for the purposes of adjudication (i.e., a subject can be positive for multiple endpoints), though several of the endpoints are related in a hierarchical fashion (Figure 2). It is anticipated that many subjects will have multiple diagnoses (e.g., Upper Respiratory Tract Disease, Lower Respiratory Tract Disease, and Bronchiolitis would be expected to co-exist). To improve consistency in interpretation of chest radiographs, radiologic adjudicators use the WHO protocol for standardized interpretation of chest radiography for pneumonia,19 which allows five possible radiologic conclusions: (1) Primary Endpoint Pneumonia (WHO PEP), (2) Other Infiltrate (OI), (3) WHO PEP and OI, (4) No consolidation, infiltrate or effusion, and (5) Uninterpretable.20,21 Members of both committees have been trained in the relevant case definitions and tested on standardized test subjects/images in order to ensure consistency in applying the case definitions.
Table 2:
Case definitions for clinical adjudication
| Disease | Required features |
|---|---|
| Upper Respiratory Tract Disease38,39 |
|
| Croup40,41 |
|
| Lower Respiratory Tract Disease41–43 |
|
| Asth ma/Reactive Airways Disease44,45 |
|
| Viral Lower Respiratory Tract Infection43,46 |
|
| Bronchiolitis46 |
|
| Pneumonia23,24,41 |
|
Figure 2:
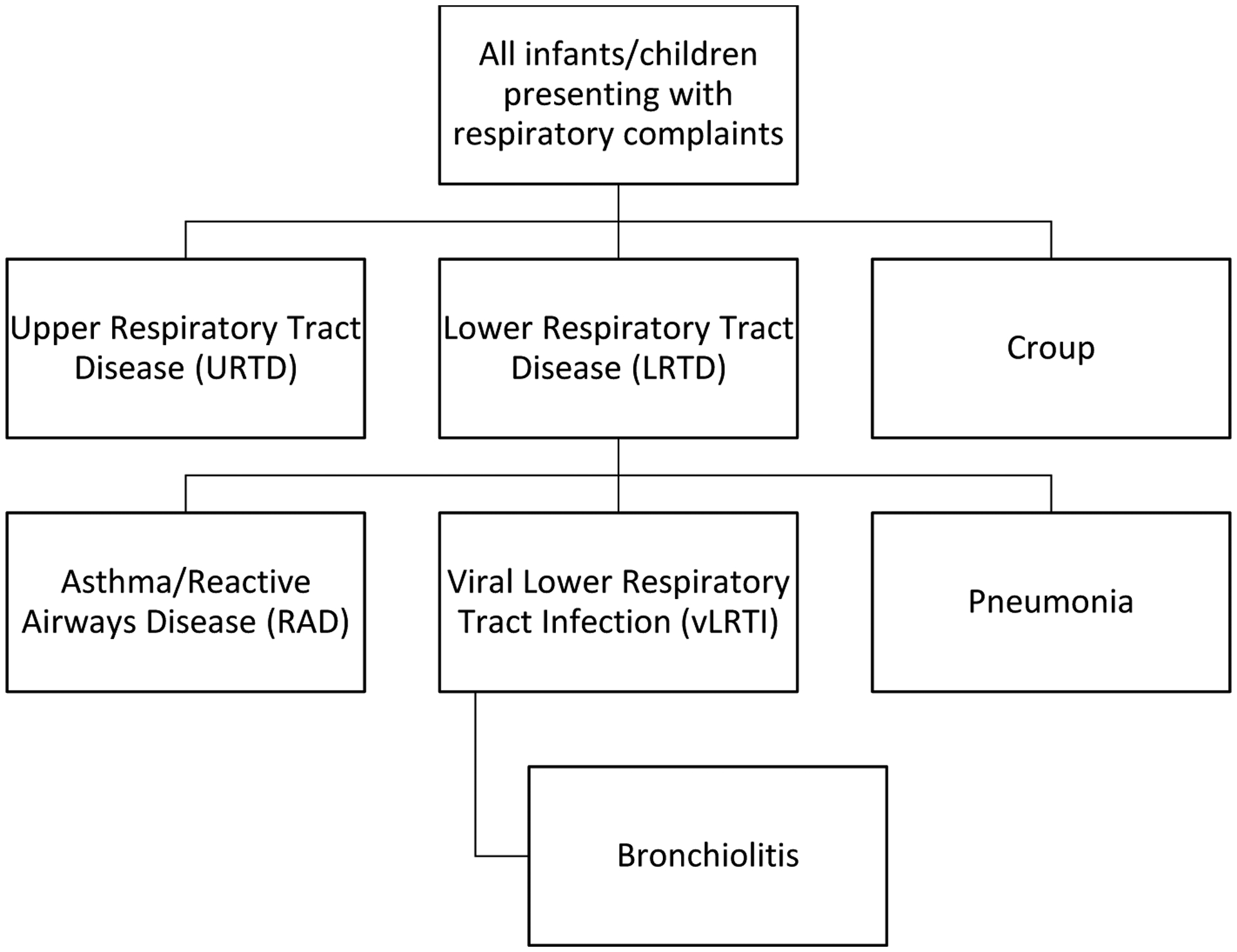
Relationship Between Study Endpoints
Since the identification of pneumonia relies on an imperfect diagnostic test (a chest radiograph),22 three reference standards are used to reflect a range of sensitivity and specificity: (1) clinical pneumonia, a high-sensitivity diagnosis that relies only on clinical signs and symptoms of pneumonia OR chest radiograph with new consolidation, (2) WHO Primary Endpoint Pneumonia plus clinical pneumonia, a high-specificity diagnosis requiring lobar radiographic consolidation plus clinical pneumonia, and (3) WHO Primary Endpoint Pneumonia or Other Infiltrate plus clinical pneumonia, an intermediate specificity diagnosis requiring either lobar consolidation or non-specific interstitial radiographic findings plus clinical pneumonia.23,24 (Clinical adjudicators are asked to review the child’s medical history to determine whether a consolidation that was present on a radiograph obtained during this illness was previously noted to be present; if so, this would not be considered as part of the definition of clinical pneumonia).
Two clinical adjudicators independently review all available clinical data (including the clinical record, laboratory testing, imaging, data from the research case report forms, and an audio recording of the subject’s cough) for each subject to determine the presence or absence of each disease at the time of cough sound recording. Because physical examination data are not reliably documented in the clinical record, research staff obtain examination data by direct report from the clinical provider at the time of enrollment. Clinical adjudicators are also asked to determine which subjects have been treated with medication prior to the cough sound recording such that the signs and symptoms of their respiratory disease have likely been altered to the point that they no longer meet criteria for the disease. These pre-treated subjects are excluded from the main analysis for that disease. The possible adjudication outcomes include “Yes,” “No,” and “Unsure.” Similarly, two radiologic adjudicators will independently review each film. In case of disagreement between the first two adjudicators in each review, a third adjudicator is assigned to independently review the case. If there is persistent disagreement, the case is discussed by an adjudication committee until consensus is reached.
Statistical Methods and Analytic Approach
Mathematical features isolated from subject cough sounds and parent- and patient-reported symptoms are applied by the ResAppDx algorithm to determine the presence or absence of each of the study endpoints. Since a non-gold standard test (i.e., adjudication) is used as the comparator standard, sensitivity and specificity (termed Positive Percent Agreement [PPA] and Negative Percent Agreement [NPA], respectively, following FDA guidelines25) will be calculated. We will also calculate positive and negative likelihood ratios, along with 95% confidence intervals for PPA, NPA, and likelihood ratios using the Clopper-Pearson method.26
The overall analysis population will include subjects who meet eligibility criteria, complete the study visit and all study-specific tasks, undergo a physical assessment, produce the required five coughs identified by the ResAppDx algorithm, and pass the cough recording quality review. Because bronchiolitis is defined as a disease only occurring in subjects ≤ 24 months of age, subjects older than 24 months will be excluded from the analysis population for the bronchiolitis PPA and NPA calculations. Similarly, for the radiographic pneumonia endpoints, only subjects in whom a chest radiograph is available will be included in the analysis. In addition, subjects identified by the CAC as being pre-treated such that the signs or symptoms of the endpoint in question were likely to have been altered to the point that they no longer meet the criteria for the disease will be given a CAC final diagnosis of “Unsure” and excluded from the primary analysis. Two sensitivity analyses will be performed, assuming (1) all “Unsure” outcomes as positive, and all “Unsure” outcomes as negative.
While the primary analysis population will include only children who are able to complete study procedures, the study documents and reports all cases of screen failures (not meeting inclusion criteria or no cough attempted) and cough recording failures (subjects who did not provide the required minimum of 5 coughs, subjects whose cough sound recording contained Protected Health Information, subjects in whom the recording failed quality review, and/or the recording was not made as per the study protocol). We will document detailed reasons for both screen failures and cough recording failures, and we will compare the characteristics and outcomes of children who were able to complete the study vs. those were unable to do so. This analysis will aid in understanding the real-world applicability of this device, particularly in low-resource settings.
Sample size justification
Based on data generated in previous studies,27 we estimate the PPA and NPA of the ResAppDx algorithm to be 85% and 80%, respectively. For an assumed N=1500 of evaluable subjects, Table 3 presents the 95% confidence intervals as a function of disease prevalence. Using historical clinical data, we anticipate the prevalence of croup at 5%, pneumonia at 10%, and asthma/RAD at 25% of the eligible study population. The resulting precision with the proposed sample size was determined to be sufficient to establish the proposed test as a useful tool in the aid of diagnosing respiratory disease in children.
Table 3:
Expected 95% Confidence Intervals (CI) for diseases assuming a range of prevalence for an assumed sample size of evaluable subjects of 1500
| Disease prevalence | 0.05 | 0.1 | 0.15 | 0.2 | 0.25 |
|---|---|---|---|---|---|
| # Cases | 75 | 150 | 225 | 300 | 375 |
| PPA | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 |
| 95% CI Lower (PPA) | 0.769 | 0.793 | 0.803 | 0.810 | 0.814 |
| 95% CI Upper (PPA) | 0.931 | 0.907 | 0.897 | 0.890 | 0.886 |
| NPA | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 |
| 95% CI Lower (NPA) | 0.779 | 0.779 | 0.778 | 0.777 | 0.777 |
| 95% CI Upper (NPA) | 0.821 | 0.821 | 0.822 | 0.823 | 0.823 |
Discussion
This paper presents the protocol used in the SMARTCOUGH-C 2 study to measure the diagnostic accuracy of the ResAppDx algorithm in detecting common pediatric respiratory diseases. If the study is successful, the ResAppDx algorithm will be implemented as a novel mobile software application for smartphones intended to be used under the direction of a physician to aid in the diagnosis of childhood respiratory illnesses.
There are several potential advantages to a smartphone-based diagnostic test in evaluation of children with acute respiratory diseases. The testing procedure is relatively straightforward, requires minimal expertise, poses minimal risk to a child, and takes advantage of naturally occurring coughs. Smartphones have built-in microphones that meet acoustic requirements for respiratory sound recording, are ubiquitous even in low-resource settings, possess substantial computing power permitting for on-device analyses, and enable non-contact assessments that minimize risk of cross-contamination between patients. The integration of these features allows for an all-in-one data acquisition, analysis, and decision-making device. Particularly relevant to a telemedicine application, a smartphone-based diagnostic test can also provide objective data to assist in the remote evaluation of a sick child. While initially we envision cough sounds would be recorded in the office/clinic/emergency department and a diagnosis would be made available to a provider at the point of care, cough sounds could also be recorded at home and transmitted to a physician as part of remote evaluation in a telehealth setting.
At the same time, the smartphone-based cough analysis platform entails several challenges that must be considered, both in the clinical research and implementation settings. Because the algorithm relies on analysis of audio data, background noise must be limited, which may be difficult in a busy clinical environment. To address this need, the most recent version of the recording software includes a “background noise meter” to warn users if there is excessive ambient noise. The operator must also be aware if other patients or family members are coughing during the test to ensure that the recording represents the patient being tested, and must instruct the child not to cover his/her mouth. Additionally, while the test can utilize either spontaneous or voluntary coughs, infants and young children may not able to provide voluntary coughs, so the test may require additional time to record spontaneous coughs.
Finally, because of the absence of a diagnostic gold standard or even commonly accepted case definitions for common pediatric respiratory illnesses, there are intrinsic limitations to the anticipated accuracy of any diagnostic test. Significant variability has been reported in the interpretation of physical examination findings,6,28 chest radiographs,29–31 and in the clinical definitions of common respiratory diseases.32–34 We have attempted to mitigate the effects of these challenges by applying detailed case definitions, training adjudicators, and testing adjudicators using standardized cases. Nonetheless, adjudicators must still depend on data provided by treating providers, including the assessment of bronchodilator responsiveness, physical examination findings, and decisions regarding diagnostic testing and treatment.
Given the high burden of acute respiratory illnesses in low and middle-income countries, additional large-scale implementation studies beyond this trial will be needed to measure the impact of the ResAppDx algorithms in low-resource settings. These studies will further delineate the effect of recording environment, community respiratory illness epidemiology, and clinical setting on the utility of this application. Additional large-scale studies will also provide insights regarding the best approach to integration in clinical workflow, cost/benefit analyses, and opportunities to further improve the diagnostic algorithms. While the algorithms are being tested initially on an Apple iPhone device in this study, we are conducting additional testing using a variety of other common smartphone platforms, including Android devices, to ensure the generalizability of our findings. Finally, we anticipate that cough sound analysis will have other applications in the management of respiratory diseases, including grading severity of asthma, detecting airway obstruction, and in diagnosis of chronic obstructive pulmonary disease.35–37
Acknowledgments:
The authors wish to acknowledge the study participants, their families, clinical staff, and adjudication committees for their support of the SMARTCOUGH-C-2 study.
Disclosures:
The study is funded by a sponsored research agreement with ResApp Health, Ltd. Drs. Keating and Taylor are employees of ResApp Health and hold options in ResApp Health. Dr. Porter is a scientific advisor and shareholder in ResApp Health. Dr. Abeyratne is the inventor of the ResAppDx algorithm, has been funded by research grants from ResApp Health, is a member of ResApp Health’s scientific advisory board, and holds options in ResApp Health. Dr. Moschovis is funded by NIH grant K23ES030399. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.McDermott KW, Stocks C, Freeman WJ. Overview of Pediatric Emergency Department Visits, 2015 [Internet]. Agency for Healthcare Research and Quality; Available from: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb242-Pediatric-ED-Visits-2015.pdf [PubMed] [Google Scholar]
- 2.Ferkol T, Schraufnagel D. The global burden of respiratory disease. Ann Am Thorac Soc 2014;11(3):404–6. [DOI] [PubMed] [Google Scholar]
- 3.McAllister DA, Liu L, Shi T, et al. Global, regional, and national estimates of pneumonia morbidity and mortality in children younger than 5 years between 2000 and 2015: a systematic analysis. Lancet Glob Health 2019;7(1):e47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rambaud-Althaus C, Althaus F, Genton B, D’Acremont V. Clinical features for diagnosis of pneumonia in children younger than 5 years: a systematic review and meta-analysis. Lancet Infect Dis 2015;15(4):439–50. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Pocket book of hospital care for children: Guidelines for the management of common illnesses with limited resources. 2nd ed. Geneva: WHO Press; 2013. [PubMed] [Google Scholar]
- 6.Shah SN, Bachur RG, Simel DL, Neuman MI. Does This Child Have Pneumonia?: The Rational Clinical Examination Systematic Review. JAMA 2017;318(5):462–71. [DOI] [PubMed] [Google Scholar]
- 7.Rees CA, Basnet S, Gentile A, et al. An analysis of clinical predictive values for radiographic pneumonia in children. BMJ Glob Health 2020;5(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meropol SB, Localio AR, Metlay JP. Risks and benefits associated with antibiotic use for acute respiratory infections: a cohort study. Ann Fam Med 2013;11(2):165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of Inappropriate Antibiotic Prescriptions Among US Ambulatory Care Visits, 2010–2011. JAMA 2016;315(17):1864–73. [DOI] [PubMed] [Google Scholar]
- 10.Ray KN, Shi Z, Gidengil CA, Poon SJ, Uscher-Pines L, Mehrotra A. Antibiotic Prescribing During Pediatric Direct-to-Consumer Telemedicine Visits. Pediatrics 2019;143(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarkar M, Madabhavi I, Niranjan N, Dogra M. Auscultation of the respiratory system. Ann Thorac Med 2015;10(3):158–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kosasih K, Abeyratne UR, Swarnkar V. High frequency analysis of cough sounds in pediatric patients with respiratory diseases. Conf Proc IEEE Eng Med Biol Soc 2012;2012:5654–7. [DOI] [PubMed] [Google Scholar]
- 13.Abeyratne UR, Swarnkar V, Setyati A, Triasih R. Cough sound analysis can rapidly diagnose childhood pneumonia. Ann Biomed Eng 2013;41(11):2448–62. [DOI] [PubMed] [Google Scholar]
- 14.Kosasih K, Abeyratne UR, Swarnkar V, Triasih R. Wavelet augmented cough analysis for rapid childhood pneumonia diagnosis. IEEE Trans Biomed Eng 2015;62(4):1185–94. [DOI] [PubMed] [Google Scholar]
- 15.Sharan RV, Abeyratne UR, Swarnkar VR, Porter P. Automatic Croup Diagnosis Using Cough Sound Recognition. IEEE Trans Biomed Eng 2019;66(2):485–95. [DOI] [PubMed] [Google Scholar]
- 16.ResApp Health, Ltd. ResApp Reports Preliminary Top-line Results from SMARTCOUGH-C Study for Diagnosis of Childhood Respiratory Disease using Cough Sounds | ResApp Health [Internet]. [cited 2020 Dec 30]; Available from: https://www.resapphealth.com.au/resapp-reports-preliminary-top-line-results-from-smartcough-c-study-for-diagnosis-of-childhood-respiratory-disease-using-cough-sounds/
- 17.Porter P, Abeyratne U, Swarnkar V, et al. A prospective multicentre study testing the diagnostic accuracy of an automated cough sound centred analytic system for the identification of common respiratory disorders in children. Respir Res 2019;20:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crocker ME, Hossen S, Goodman D, et al. Effects of high altitude on respiratory rate and oxygen saturation reference values in healthy infants and children younger than 2 years in four countries: a cross-sectional study. Lancet Glob Health 2020;8(3):e362–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahomed N, Fancourt N, de Campo J, et al. Preliminary report from the World Health Organisation Chest Radiography in Epidemiological Studies project. Pediatr Radiol 2017;47(11):1399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cherian T, Mulholland EK, Carlin JB, et al. Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies. Bull World Health Organ 2005;83(5):353–9. [PMC free article] [PubMed] [Google Scholar]
- 21.Fancourt N, Deloria Knoll M, Barger-Kamate B, et al. Standardized Interpretation of Chest Radiographs in Cases of Pediatric Pneumonia From the PERCH Study. Clin Infect Dis Off Publ Infect Dis Soc Am 2017;64(suppl_3):S253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Grady K-AF, Torzillo PJ, Frawley K, Chang AB. The radiological diagnosis of pneumonia in children. Pneumonia 2014;5(1):38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cherian T, Mulholland EK, Carlin JB, et al. Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies. Bull World Health Organ 2005;83(5):353–9. [PMC free article] [PubMed] [Google Scholar]
- 24.Mahomed N, Fancourt N, de Campo J, et al. Preliminary report from the World Health Organisation Chest Radiography in Epidemiological Studies project. Pediatr Radiol 2017;47(11):1399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.FDA Center for Drug Evaluation and Research, FDA Center for Biologics Evaluation and Research. Guidance for Industry: Developing Medical Imaging Drug and Biological Products, Part 3: Design, Analysis, and Interpretation of Clinical Studies. Rockville, MD: Food and Drug Administration; 2004. [Google Scholar]
- 26.Clopper CJ and Pearson ES The Use of Confidence or Fiducial Limits Illustrated in the Case of the Binomial. Biometrika 1934;26:404–13. [Google Scholar]
- 27.Porter P, Abeyratne U, Swarnkar V, et al. A prospective multicentre study testing the diagnostic accuracy of an automated cough sound centred analytic system for the identification of common respiratory disorders in children. Respir Res [Internet] 2019. [cited 2019 Dec 18];20. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6551890/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gajdos V, Beydon N, Bommenel L, et al. Inter-observer agreement between physicians, nurses, and respiratory therapists for respiratory clinical evaluation in bronchiolitis. Pediatr Pulmonol 2009;44(8):754–62. [DOI] [PubMed] [Google Scholar]
- 29.Albaum MN, Hill LC, Murphy M, et al. Interobserver reliability of the chest radiograph in community-acquired pneumonia. PORT Investigators. Chest 1996;110(2):343–50. [DOI] [PubMed] [Google Scholar]
- 30.Elemraid MA, Muller M, Spencer DA, et al. Accuracy of the interpretation of chest radiographs for the diagnosis of paediatric pneumonia. PLoS One 2014;9(8):e106051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson J, Kline JA. Intraobserver and interobserver agreement of the interpretation of pediatric chest radiographs. Emerg Radiol 2010;17(4):285–90. [DOI] [PubMed] [Google Scholar]
- 32.Court SD. The definition of acute respiratory illnesses in children. Postgrad Med J 1973;49(577):771–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Douros K, Everard ML. Time to Say Goodbye to Bronchiolitis, Viral Wheeze, Reactive Airways Disease, Wheeze Bronchitis and All That. Front Pediatr 2020;8:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gardner PS. Virus infections and respiratory disease of childhood. Arch Dis Child 1968;43(232):629–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Porter P, Claxton S, Brisbane J, et al. Diagnosing Chronic Obstructive Airway Disease on a Smartphone Using Patient-Reported Symptoms and Cough Analysis: Diagnostic Accuracy Study. JMIR Form Res 2020;4(11):e24587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharan RV, Abeyratne UR, Swarnkar VR, Claxton S, Hukins C, Porter P. Predicting spirometry readings using cough sound features and regression. Physiol Meas 2018;39(9):095001. [DOI] [PubMed] [Google Scholar]
- 37.Swarnkar V, Abeyratne U, Tan J, et al. Stratifying asthma severity in children using cough sound analytic technology. J Asthma Off J Assoc Care Asthma 2019;1–10. [DOI] [PubMed] [Google Scholar]
- 38.Heikkinen T, Järvinen A. The common cold. Lancet 2003;361(9351):51–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pappas DE, Hendley JO, Hayden FG, Winther B. Symptom profile of common colds in school-aged children. Pediatr Infect Dis J 2008;27(1):8–11. [DOI] [PubMed] [Google Scholar]
- 40.Smith DK, McDermott AJ, Sullivan JF. Croup: Diagnosis and Management. Am Fam Physician 2018;97(9):575–80. [PubMed] [Google Scholar]
- 41.Wilmott RW, Deterding RR, Li A, et al. Kendig’s disorders of the respiratory tract in children. Ninth edition. Philadelphia, PA: Elsevier; 2019. [Google Scholar]
- 42.Davies JC, Bush A. Paediatric Respiratory Disease : Airways and Infection: an Atlas of Investigation and Management. Oxford, U.K.: Clinical Publishing; 2011. [Google Scholar]
- 43.van Woensel JBM, van Aalderen WMC, Kimpen JLL. Viral lower respiratory tract infection in infants and young children. BMJ 2003;327(7405):36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Global Initiative on Asthma. Global Strategy for Asthma Management and Prevention [Internet]. 2019; Available from: http://www.ginaasthma.org
- 45.Papadopoulos NG, Arakawa H, Carlsen K-H, et al. International consensus on (ICON) pediatric asthma. Allergy 2012;67(8):976–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meissner HC. Viral Bronchiolitis in Children. N Engl J Med 2016;374(1):62–72. [DOI] [PubMed] [Google Scholar]


