Abstract
BACKGROUND AND PURPOSE:
Endoluminal reconstruction with the Pipeline Embolization Device is an effective treatment option for select intracranial aneurysms. However, concerns for the patency of eloquent branch arteries covered by the Pipeline Embolization Device have been raised. We aimed to examine the patency of the anterior choroidal artery and clinical sequelae after ICA aneurysm treatment.
MATERIALS AND METHODS:
We prospectively analyzed all patients among our first 157 patients with ICA aneurysms treated by the Pipeline Embolization Device who required placement of at least 1 device across the ostium of the anterior choroidal artery. The primary outcome measure was angiographic patency of the anterior choroidal artery at last follow-up. Age, sex, type of aneurysm, neurologic examination data, number of Pipeline Embolization Devices used, relationship of the anterior choroidal artery to the aneurysm, and completeness of aneurysm occlusion on follow-up angiograms were also analyzed.
RESULTS:
Twenty-nine aneurysms requiring placement of at least 1 Pipeline Embolization Device (median = 1, range = 1–3) across the anterior choroidal artery ostium were identified. At angiographic follow-up (mean = 15.1 months; range = 12–39 months), the anterior choroidal artery remained patent, with antegrade flow in 28/29 aneurysms (96.5%), while 24/29 (82.7%) of the target aneurysms were angiographically occluded by 1-year follow-up angiography. Anterior choroidal artery occlusion, with retrograde reconstitution of the vessel, was noted in a single case. A significant correlation between the origin of the anterior choroidal artery from the aneurysm dome and failure of the aneurysms to occlude following treatment was found.
CONCLUSIONS:
After placement of 36 Pipeline Embolization Devices across 29 anterior choroidal arteries (median = 1 device, range = 1–3 devices), 1 of 29 anterior choroidal arteries was found occluded on angiographic follow-up. The vessel occlusion did not result in persistent clinical sequelae. Coverage of the anterior choroidal artery origin with the Pipeline Embolization Device, hence, may be considered reasonably safe when deemed necessary for aneurysm treatment.
Flow diversion with the Pipeline Embolization Device (PED; Covidien, Irvine, California) has been shown to be an effective treatment option for complex intracranial aneurysms of the internal carotid artery.1–4 Fundamentally, the safety and effectiveness of the device in the cerebral vasculature depends on its ability to differentially facilitate aneurysm occlusion without symptomatically compromising branch vessel patency. Branch vessel flow depends on the arterial-venous pressure gradient and composite impedance of the vascular territory subserved by the branch.5 Although placement of single PEDs across the origins of branch vessels is not expected to affect vascular resistance in the jailed (covered) artery,6,7 the intrinsic thrombogenicity of the implant has caused concern for the patency of jailed branch arteries.5,6,8 Several studies have shown that coverage of the ophthalmic artery is clinically safe,9,10 but coverage of the anterior choroidal artery (AchoA) has not yet been systematically evaluated. Several reports have suggested that >50% compromise of the luminal cross-sectional area is required before flow in branch arteries is diminished significantly,11,12 reflecting a degree of branch ostial coverage lower than that expected from deployment of a single PED (between 18% and 36% surface metal coverage) as determined from benchtop analysis.13 Even when PEDs are overlapped, metal coverage is not reasonably expected to exceed 40% for any 2 devices.14 We therefore hypothesized that coverage of the AchoA with PED is unlikely to cause occlusion of this eloquent vessel territory.
Materials and Methods
This study was an institutional review board–approved retrospective analysis of prospectively acquired data. Inclusion criteria were the following: 1) presence of an intracranial internal carotid artery aneurysm treated by placement of ≥1 PED, and 2) coverage of the AchoA with at least 1 PED.
We recorded the following baseline data: age, sex, neurologic examination before and after the procedure, location of the aneurysm (according to Shapiro et al15), relationship of the AchoA to the aneurysm, and the number of PEDs implanted across the origin of the AchoA. At follow-up, the interval from the index procedure, aneurysm occlusion status, patency of the AchoA, and neurologic examination findings were recorded. Baseline and follow-up neurologic assessments were performed in a nonblinded fashion by 2 board-certified neurologists (T.B., M.S.).
Treatment Protocol
Procedures were performed with the patient under general anesthesia, typically by using a triaxial system in a fashion previously described.2 The AchoA was covered by as many PEDs as was thought necessary to achieve a subjectively satisfactory balance between treatment of the aneurysm and AchoA coverage, with the result that in most cases coverage of the AchoA was minimized to a single device. Neurologic status was assessed before treatment, immediately after treatment, at discharge, and at follow-up clinical visits.
All patients received dual antiplatelet coverage. During the intervention, patients additionally underwent anticoagulation by intravenous boluses of heparin sodium, targeting an increase in activated coagulation time of twice baseline. Patients continued with clopidogrel (75 mg daily) and acetylsalicylic acid (325 mg daily) for a minimum of 180 days after treatment. Antiplatelet therapy was not explicitly modified from our standard PED–aneurysm treatment protocol (which uses testing with the VerifyNow P2Y12 assay [Accumetrics, San Diego, California] to establish suitable inhibition at the time of treatment). In those patients in whom the AchoA arose from the aneurysm fundus, posttreatment heparinization at 500–700 IU/h was continued for 4–5 days.
Follow-Up Protocol
As with other patients treated by us with the PED, subjects of this investigation were scheduled for follow-up clinical and angiographic evaluations at 6 months and 1 and 3 years (±2 months) postembolization. We evaluated the longest angiographic follow-up when >1 instance of follow-up was available.
Imaging Evaluation
All pre- and posttreatment angiograms were reviewed by 5 neurointerventionalists in consensus (T.B., P.K.N., E.R., M.S., D.W.Z.). We carefully evaluated the following: the aneurysm site; descriptive features of the AchoA, such as hyperplastic variant16 or origin from the aneurysm dome17; occlusion or patency of the aneurysm; and status of the AchoA at follow-up.
Statistics
The data are expressed as mean ± SD or as median and range. When appropriate, analyses were performed by using a Fisher exact test. A P value < .05 was considered statistically significant.
Results
Between August 2008 and May 2013, we treated 157 patients with ICA aneurysms with PED constructs. Of these, 28 patients with 29 ICA aneurysms requiring placement of at least 1 PED across the origin of the AchoA were identified. None of these patients developed any sustained postprocedural neurologic deficits. Clinical and angiographic follow-up data were available for all patients through at least 1 year. To date, no patient in this cohort has been lost to follow-up. Baseline demographic and clinical data for the 28 identified patients are shown in Table 1. Eighty-two PEDs were implanted successfully in this cohort, of which 36 PEDs were placed across the ostium of the AchoA in developing a therapeutic construct (mean per aneurysm = 1.24 ± 0.57, median = 1, range = 1–3). The mean time to last angiographic follow-up was 15.1 months with a range of 12–39 months. Representative examples of pre- and posttreatment angiograms are shown in Figs 1 and 2.
Table 1:
Study populationa
| No. or Mean (SD) | |
|---|---|
| Age (yr) | 57.6 (13.6) |
| Sex (M/F) | 7/21 |
| Aneurysm side (R/L) | 16/13 |
| Aneurysm site | |
| Paraophthalmic | 17 |
| PcomA | 10 |
| AchoA | 1 (2 bystander) |
| Terminus | 1 |
Note:—R indicates right; L, left; PcomA, posterior communicating artery.
Our study population included 28 patients with 29 aneurysms, with coverage of the anterior choroidal artery with at least 1 PED.
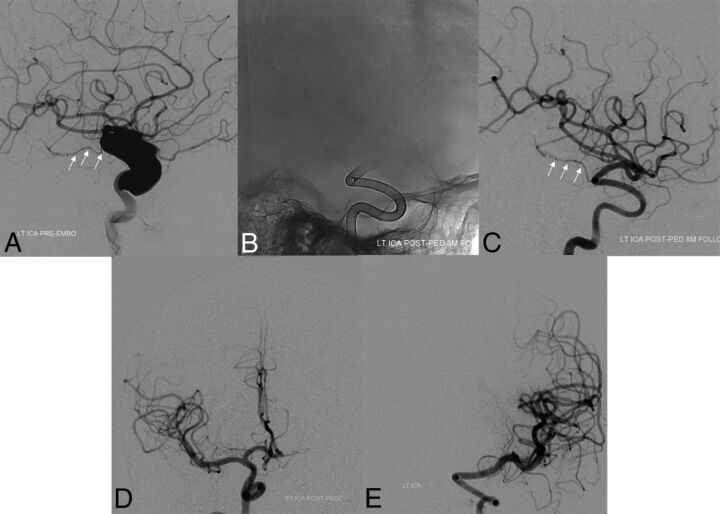
Fig 1.
Baseline pretreatment digital subtraction angiogram (lateral projection, A) demonstrating a large dysplastic ICA aneurysm with preserved antegrade flow in the anterior choroidal artery (white arrows). In an initial posttreatment follow-up angiogram after placement of a PED, unsubtracted mask (B) and arterial phase DSA in a lateral projection (C) demonstrate complete occlusion of the aneurysm with preservation of antegrade flow within the anterior choroidal artery (white arrows). Follow-up DSAs demonstrate reconstruction of the left ICA with smooth uniform neointimal overgrowth of the minimally porous endoluminal device construct. Eighteen-month follow-up DSAs (frontal projections) demonstrate a contralateral supply of the left anterior cerebral artery from the right ICA (D) and persistent occlusion of the left ICA aneurysm (E).
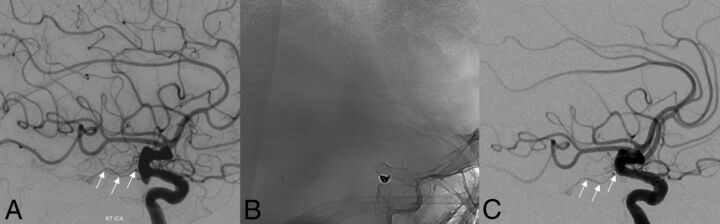
Fig 2.
Baseline lateral DSA projection (A) demonstrating a posterior communicating artery aneurysm and normal flow in the anterior choroidal artery (white arrows). One-year follow-up angiography after placement of a PED and coils, unsubtracted mask (B) and DSA (C), confirm occlusion of the aneurysm with preservation of antegrade flow within the anterior choroidal artery (white arrows).
At follow-up, impairment of the AchoA flow was noted in 2 patients. Asymptomatic occlusion of the AchoA occurred in 1 individual (3.4%), who was continued on clopidogrel for 1 year. In another patient, the angiographic opacification of the AchoA at follow-up was judged sluggish, secondary to an ostium stenosis. In the case of AchoA occlusion (Fig 3), the AchoA originated from the lateral fundus of a large aneurysm, which was covered with 3 PEDs. After treatment, the patient was maintained on heparin, 500 IU/h, for 5 days before being discontinued. Within hours of stopping heparin, the patient experienced a transient episode (5 minutes) of contralateral weakness and hemianopsia, consistent with an AchoA syndrome. An urgent angiogram was obtained, demonstrating occlusion of the aneurysm and the AchoA (Fig 3D) with collateral reconstitution of the AchoA territory via anastomoses with the ipsilateral posterior lateral choroidal and medial lenticulostriate arteries. The patient had no further symptoms and was discharged home on postoperative day 9, neurologically intact. At 6-month and 1-year follow-ups, the aneurysm and AchoA ostium were fully closed (Fig 3E), with the distal AchoA collaterally reconstituted through more robust anastomoses with a medial lenticulostriate branch of the right A1 segment (Fig 3F) and the ipsilateral posterior lateral choroidal artery (Fig 3H). A comparison of baseline features between patients with impaired AchoA flow at follow-up and those with normal flow is shown in Table 2. No occlusion of the AchoA was observed among patients with double-device coverage (n = 3) or in the other 2 patients with triple coverage of the AchoA. No clinical signs or symptoms of AchoA occlusion were present at follow-up in any patients. As of the time of this writing, no new deficits were noted in the 2 patients with compromised AchoAs.
Fig 3.
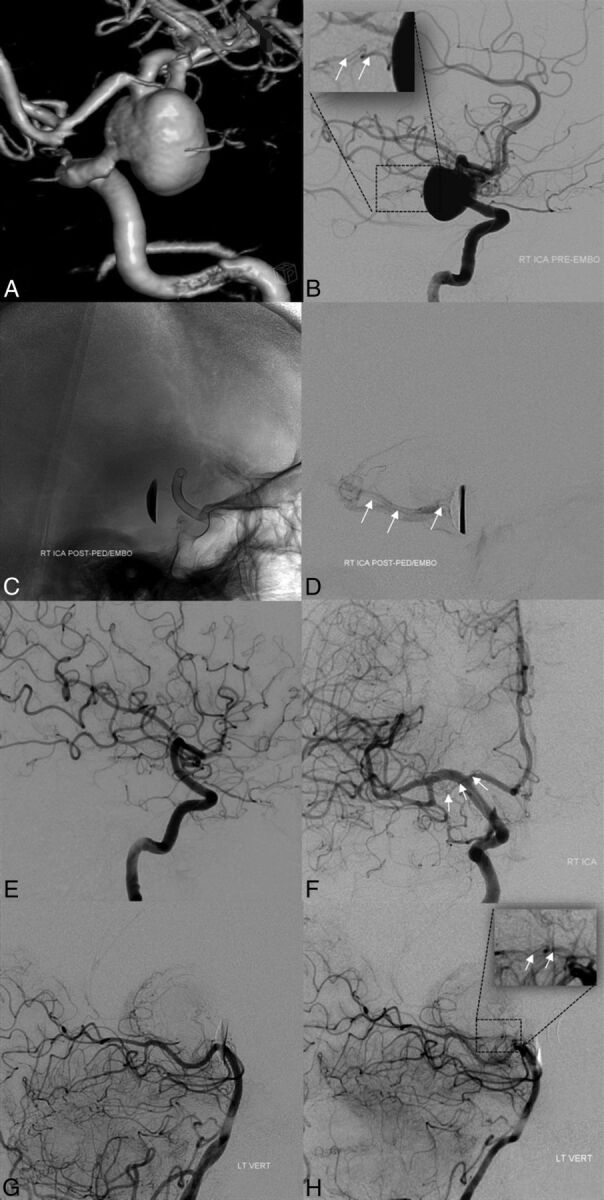
Baseline pretreatment 3D-DSA (A) and lateral projection DSA (B) demonstrating a large anterior choroidal artery aneurysm. Note the origin of the AchoA from the aneurysm fundus (white arrows in inset, B). Immediate posttreatment images, after placement of PED, unsubtracted mask (C) and delayed image DSA (D), demonstrate coverage of the AchoA origin and residual antegrade opacification of the AchoA territory (white arrows, D). Follow-up angiography (E and F) confirms complete occlusion of the aneurysm and AchoA origin; the AchoA is opacified retrogradely (white arrows, F). Baseline pretreatment (G) and follow-up (H) DSAs of the left vertebral artery (lateral projections) demonstrate the collateral opacification of the AchoA through anastomoses with the posterior lateral choroidal artery at follow-up (inset in H, white arrows; compare with the inset in B), not visualized at baseline (G).
Table 2:
AchoA status
| AchoA Status |
||
|---|---|---|
| Open | Impaired | |
| Aneurysms (No.) | 27 (93.1%) | 2 (6.9%) |
| Mean age (SD) (yr) | 57.9 (14.0) | 53.5 (4.9) |
| Sex (M/F) | 7/20 | 1/1 |
| Mean PED covering AchoA (No.) | 1.25 (0.6) | 2 (1.4) |
| Aneurysm occlusion at follow-up (No.) | 24 (88.8%) | 1 (50%) |
| Mean (SD) follow-up (mo) | 15.1 (7.1) | 15.5 (4.9) |
The AchoA originated from the aneurysm fundus in 4 aneurysms (Table 3). Two of these included the aforementioned cases of AchoA occlusion and flow restriction/stenosis. Three of these 4 aneurysms were incompletely occluded at follow-up; this scenario suggests that persistent runoff into the AchoA may be a potential contributing factor for incomplete closure of aneurysms treated with the PED.
Table 3:
AchoA origin from the dome and association with aneurysm occlusion at follow-upa
| Aneurysm |
Total | ||
|---|---|---|---|
| Closed | Open | ||
| AchoA origin from the aneurysm fundus (No.) | 1 | 3 | 4 |
| AchoA origin outside of the aneurysm fundus (No.) | 24 | 1 | 25 |
| Total | 25 | 4 | 29 |
Fisher exact test: 2-tailed P value = .0043.
Discussion
Minimally porous endoluminal devices such as the PED have provided a conceptual paradigm shift in the treatment of cerebral aneurysms.1–4 Nevertheless, one of the major concerns related to use of such devices is the risk of potential critical branch vessel occlusion.18 Various in vitro and animal model studies have attempted to reproduce human in vivo conditions to explore the effect of PED placement on covered branch status. Jailed lumbar segmental vessels reportedly remained patent18,19 following single-device placement of PEDs within the rabbit aorta. Histologic evaluation of PEDs 6 months after implantation has typically demonstrated near-uniform neointimal overgrowth of the device, interrupted by uncovered “pores” at origins of branch vessels.19 This observation is supported in part by the demonstration of persistent patency in most ophthalmic arteries chronically covered by single PEDs.2,3,9,10
Furthermore, while Puffer et al10 reported that ∼25% of ophthalmic arteries covered by PEDs were occluded on follow-up imaging and ∼18% were occluded in the Budapest PED experience,3 neurologic sequelae of such ophthalmic artery occlusion are demonstrably rare. For the ophthalmic artery, the infrequency of symptomatic occlusions likely reflects the rich collateral support available through anastomoses with orbital branches of the external carotid artery; additionally, the cases in which the ophthalmic artery was occluded serve to illustrate the potential for use of such devices in facilitating asymptomatic vascular remodeling in the treatment of complex aneurysms. Moreover, the apparent infrequency of symptomatic orbital thromboembolic events following coverage with a PED,1 even in cases in which the artery is incorporated into the target aneurysm, is reassuring. Nevertheless, the sufficiency of collateral support for the AchoA20 has been poorly studied and remains disturbingly unpredictable,21 particularly considering the neurologic eloquence of this vascular territory and the knowledge that ischemic infarctions due to perforator occlusion have been described in other territories.22 Although a role for balloon test occlusion, supplemented by simultaneous angiographic assessment of collateral support, might be of use in identifying patients in whom collateral support is immediately sufficient, it is unlikely that much insight into the consequences of slowly progressive, delayed occlusion of the AchoA for individuals with poor collateral support at the time of treatment would be gained. None of our patients underwent test occlusion of the AchoA before treatment.
The present study provides some reassurance that strategically limited PED coverage of the AchoA is infrequently associated with symptomatic flow impairment or vessel occlusion. In this series, no permanent clinical sequelae resulted from coverage of the AchoA by a PED through a mean of 15.1 months of follow-up. A single AchoA occlusion was seen. In this patient, transient symptoms suggestive of an AchoA syndrome developed on postoperative day 5 following discontinuation of heparin (which had been continued during a prolonged period specifically to prevent acute aneurysm thrombosis in this patient). However, the symptoms regressed spontaneously within minutes, coinciding with angiographically confirmed collateral reconstitution of the occluded AchoA by mechanisms previously described.16 Another asymptomatic patient was observed at 1 year postprocedure to have subjectively sluggish antegrade flow within the AchoA, associated with origin stenosis of the vessel; however, this patient remains clinically asymptomatic through 3 years. The median number of PEDs covering the choroidal segment in this series was 1, with double (n = 3) or triple (n = 3) coverage being the exception. In 1 case of triple coverage, the AchoA and its associated aneurysm were occluded. In the remaining 2 triple-coverage cases, antegrade flow was maintained through the 1-year follow-up angiograms. Thus, multidevice coverage may be justified if in the judgment of the operator, such a construct is necessary for aneurysm treatment (Fig 1).
Although a margin of safety may exist in covering neurologically eloquent branches such as the AchoA, judicious treatment should be focused on limiting such coverage. Toward this goal, the degree of absolute metal coverage may be minimized by strategic selection of PED size in developing a therapeutic construct. The functional metal coverage projected by an individual PED varies substantially depending on its size relative to the parent vessel, regional curvature, and other conditions of deployment (with realized values between 18% and 36%). This variation provides the opportunity to minimize judiciously eloquent branch coverage.13,14 From this perspective, the porosities of devices covering the AchoA is likely lower than that expected in a straight-vessel model, due to the relative oversizing of devices for the choroidal segment to match the diameter of the ICA at the more proximal landing zone and because the AchoA typically arises from an outer curvature of the supraclinoid ICA, causing individual device pores of the PED to be further opened.23
In our cohort, 4 AchoAs originated from the aneurysm fundus. Of these, 2 AchoAs were impaired at follow-up and only 1 aneurysm was angiographically occluded at 1-year follow-up. These results raise the possibility that persistent runoff into branches originating from the aneurysm proper may prevent complete aneurysm obliteration by mechanisms similar to those responsible for continued patency of normal side branches. In studies by Raz et al9 and Puffer et al,10 branch origin from the dome was an independent factor associated with lack of aneurysm occlusion at follow-up.
We acknowledge the following limitations of this study: First, the requisite angiographic follow-up was 1 year. Thus, it may be argued that during a longer term, AchoA occlusion may be underestimated. Also, the small number of patients with 2 or 3 PEDs across the artery ostium (n = 5) limits the power of analysis correlating PED number and vessel status.
Conclusions
Our experience demonstrates the overall safety and efficacy of PED placement across the AchoA, with expectation of a few incidences of postprocedural AchoA occlusion. None of the patients in the current series reported permanent symptoms related to AchoA coverage following PED treatment.
ABBREVIATIONS:
- AchoA
anterior choroidal artery
- PED
Pipeline Embolization Device
Footnotes
Disclosures: Maksim Shapiro—UNRELATED: Consultancy: Pipeline proctor and consultant for Covidien; Payment for Development of Educational Presentations: Covidien. Tibor Becske—UNRELATED: Consultancy: Covidien/ev3 (I am a consultant with Covidien, the manufacturer of the device in question); Payment for Lectures (including service on Speakers Bureaus): Covidien/ev3 (I have given a presentation); Payment for Development of Educational Presentations: Covidien/ev3, Comments: I participated in development of FDA-mandated training for US neurointerventionalists in the use of the Pipeline device. Daniel W. Zumofen—UNRELATED: Grants/Grants Pending: personal scholarship from the Fund Helmut Hartweg and the Swiss Academy of Medical Science, Comments: 1-year personal scholarship from the Fund Helmut Hartweg and the Swiss Academy of Medical Science to cofinance my fellowship at the New York University School of Medicine; Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: Swiss Neurosurgery Update, Comments: invited guest speaker at the Swiss Neurosurgery Update in January 2014 in Basel, Switzerland. Travel and accommodation expenses were covered by the meeting organization; not related to present work; no conflict of interest; Other: Stryker, Covidien, Penumbra, Medtronic, Comments: I went to the 2014 national fellowship courses organized by Stryker, Covidien, Penumbra, and Medtronic. Traveling and accommodation expenses were covered by the companies; not related to present work; no conflict of interest. Peter K. Nelson—RELATED: Consulting Fee or Honorarium: Covidien; UNRELATED: Consultancy: Covidien.
REFERENCES
- 1. Becske T, Kallmes DF, Saatci I, et al. Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology 2013;267:858–68 [DOI] [PubMed] [Google Scholar]
- 2. Nelson PK, Lylyk P, Szikora I, et al. The Pipeline embolization device for the intracranial treatment of aneurysms trial. AJNR Am J Neuroradiol 2011;32:34–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Szikora I, Berentei Z, Kulcsar Z, et al. Treatment of intracranial aneurysms by functional reconstruction of the parent artery: the Budapest experience with the Pipeline embolization device. AJNR Am J Neuroradiol 2010;31:1139–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lylyk P, Miranda C, Ceratto R, et al. Curative endovascular reconstruction of cerebral aneurysms with the Pipeline embolization device: the Buenos Aires experience. Neurosurgery 2009;64:632–42; discussion 642–43; quiz N6 [DOI] [PubMed] [Google Scholar]
- 5. Fiorella D, Lylyk P, Szikora I, et al. Curative cerebrovascular reconstruction with the Pipeline embolization device: the emergence of definitive endovascular therapy for intracranial aneurysms. J Neurointerv Surg 2009;1:56–65 [DOI] [PubMed] [Google Scholar]
- 6. Appanaboyina S, Mut F, Löhner, et al. Computational modelling of blood flow in side arterial branches after stenting of cerebral aneurysms. International Journal of Computational Fluid Dynamics 2008;22:669–76 [Google Scholar]
- 7. Cebral JR, Raschi M, Mut F, et al. Analysis of flow changes in side branches jailed by flow diverters in rabbit models. Int J Numer Method Biomed Eng 2014;30:988–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seong J, Wakhloo AK, Lieber BB. In vitro evaluation of flow divertors in an elastase-induced saccular aneurysm model in rabbit. J Biomech Eng 2007;129:863–72 [DOI] [PubMed] [Google Scholar]
- 9. Raz E, Tanweer O, Becske T, et al. Ophthalmic artery patency and clinical follow-up after placement of Pipeline embolization device. In: Proceedings of the Annual Meeting of the American Society of Neuroradiology, San Diego, California. May 18–23, 2013 [Google Scholar]
- 10. Puffer RC, Kallmes DF, Cloft HJ, et al. Patency of the ophthalmic artery after flow diversion treatment of paraclinoid aneurysms. J Neurosurg 2012;116:892–96 [DOI] [PubMed] [Google Scholar]
- 11. Lopes DK, Ringer AJ, Boulos AS, et al. Fate of branch arteries after intracranial stenting. Neurosurgery 2003;52:1275–78; discussion 1278–79 [DOI] [PubMed] [Google Scholar]
- 12. Wakhloo AK, Tio FO, Lieber BB, et al. Self-expanding nitinol stents in canine vertebral arteries: hemodynamics and tissue response. AJNR Am J Neuroradiol 1995;16:1043–51 [PMC free article] [PubMed] [Google Scholar]
- 13. Shapiro M, Raz E, Becske T, et al. Variable porosity of the Pipeline embolization device in straight and curved vessels: a guide for optimal deployment strategy. AJNR Am J Neuroradiol 2014;35:727–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shapiro M, Raz E, Becske T, et al. Building multidevice Pipeline constructs of favorable metal coverage: a practical guide. AJNR Am J Neuroradiol 2014;35:1556–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shapiro M, Becske T, Riina HA, et al. Toward an endovascular internal carotid artery classification system. AJNR Am J Neuroradiol 2014;35:230–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Takahashi S, Suga T, Kawata Y, et al. Anterior choroidal artery: angiographic analysis of variations and anomalies. AJNR Am J Neuroradiol 1990;11:719–29 [PMC free article] [PubMed] [Google Scholar]
- 17. Kang HS, Kwon BJ, Kwon OK, et al. Endovascular coil embolization of anterior choroidal artery aneurysms: clinical article. J Neurosurg 2009;111:963–69 [DOI] [PubMed] [Google Scholar]
- 18. Kallmes DF, Ding YH, Dai D, et al. A second-generation, endoluminal, flow-disrupting device for treatment of saccular aneurysms. AJNR Am J Neuroradiol 2009;30:1153–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kallmes DF, Ding YH, Dai D, et al. A new endoluminal, flow-disrupting device for treatment of saccular aneurysms. Stroke 2007;38:2346–52 [DOI] [PubMed] [Google Scholar]
- 20. Alderazi YJ, Shastri D, Kass-Hout T, et al. Flow diverters for intracranial aneurysms. Stroke Res Treat 2014;2014:415653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee M, Saver JL, Hao Q, et al. Anterior choroidal artery ischaemic patterns predict outcome of carotid occlusion. J Neurol Neurosurg Psychiatry 2012;83:586–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Rooij WJ, Sluzewski M. Perforator infarction after placement of a Pipeline flow-diverting stent for an unruptured A1 aneurysm. AJNR Am J Neuroradiol 2010;31:E43–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bing F, Darsaut TE, Salazkin I, et al. Stents and flow diverters in the treatment of aneurysms: device deformation in vivo may alter porosity and impact efficacy. Neuroradiology 2013;55:85–92 [DOI] [PubMed] [Google Scholar]